Abstract
Previous attempts to introduce transposon Tn4001 into Mycoplasma pulmonis and Mycoplasma arthritidis have not been successful, possibly due to functional failure of the transposon's gentamicin resistance determinant. Tn4001C and Tn4001T were constructed, respectively, by insertion of a chloramphenicol acetyltransferase gene and the tetM tetracycline resistance determinant into Tn4001. Both Tn4001C and Tn4001T transposed in M. pulmonis, and Tn4001T transposed in M. arthritidis. The incorporation of a Tn4001T derivative that contained lacZ into either Mycoplasma species resulted in transformants with readily detectable LacZ activity. Tn4001T may be of general utility for use as a mycoplasma cloning vehicle because tetM functions in all species of Mycoplasma examined thus far.
Few strategies exist to genetically manipulate mycoplasmas. There are no plasmids known to replicate in Mycoplasma species other than Mycoplasma mycoides and Mycoplasma capricolum (15, 16). The gram-positive bacterial transposon Tn916 can transpose into the genome of virtually all species of Mycoplasma for which transformation methods have been described, and Tn4001 can transpose into a number of Mycoplasma species, including Mycoplasma pneumoniae, Mycoplasma genitalium, and Mycoplasma gallisepticum (10). Transformation of these species with plasmids containing either transposon results in insertion of the transposon into the mycoplasmal genome at any of numerous sites. Tn916 is large (18 kb) and not amenable to use as a cloning vehicle. In contrast, Tn4001 (4.7 kb) is small enough to be used as a cloning vehicle to insert genes into the mycoplasmal chromosome. SmaI and BamHI cloning sites have been introduced into Tn4001 to create Tn4001mod, which has been used as a vector in M. gallisepticum and M. pneumoniae (17, 20).
Tn4001 does not function in several species of Mycoplasma. Earlier reports of transposition of Tn4001 in Mycoplasma pulmonis were incorrect, and transformation of this species with plasmids containing Tn4001 has not previously been achieved (6, 18). The inability to introduce Tn4001 into M. pulmonis by transformation could result from either lack of transposase activity or failure of the transposon's gentamicin resistance determinant to serve as a selectable marker. In the present study, we inserted alternative antibiotic resistance determinants into Tn4001 to construct transposon derivatives that function in M. pulmonis and in Mycoplasma arthritidis. One of the transposon derivatives (Tn4001T) contains the tetM gene and may be used as a broad-host-range mycoplasma vector, as indicated by the successful expression of a lacZ fusion gene in both M. pulmonis and M. arthritidis.
Transformation of M. pulmonis and M. arthritidis with transposons Tn4001C and Tn4001T.
To determine whether Tn4001 would function in M. pulmonis if an appropriate antibiotic resistance marker was provided and to develop additional antibiotic resistance markers for this species, a chloramphenicol acetyltransferase gene (cat) was developed for use in M. pulmonis. One reason a cat determinant was chosen was that growth of M. pulmonis was found to be highly susceptible to the inhibitory effects of chloramphenicol at a concentration of 15 μg/ml. A chimeric gene consisting of the coding region of the cat gene of Escherichia coli plasmid pACYC184 (5) and the promoter region from the expression locus of the M. pulmonis vsa genes (2) was constructed. The cat coding region was amplified by PCR using the cat forward primer (5′-GGAAGGTACCATGGAGAAAAAAATCAC-3′) and the cat reverse primer (5′-CACTTCTCGAGGCGTAGCACCAGG-3′). A 460-bp portion of the vsa locus encompassing the promoter and the Shine-Dalgarno (SD) sequence was amplified by PCR using the vsa forward primer (5′-CGTTTCTGCAGTTTTTTTGAACC-3′) and the vsa reverse primer (5′-TGCATGGTACCTCCTATTTTAAAATTATG-3′). PCR amplification conditions were as described previously (11). The vsa promoter was chosen for its presumed strength, given that the vsa gene products are major surface proteins of M. pulmonis (i.e., the V-1 antigens) (2, 14, 21). The vsa reverse primer and the cat forward primer were designed with KpnI restriction sites incorporated into their 5′ ends to facilitate cloning such that the correct spacing between the vsa SD sequence and the cat translation start codon would be maintained. Accordingly, the vsa and cat PCR products were digested with KpnI and ligated together to form a hybrid vsa-cat gene. This gene was PCR amplified using the vsa forward primer (which contained an internal PstI site) and the cat reverse primer (which contained an internal XhoI site). The PCR product was digested with PstI and XhoI and inserted into the PstI/XhoI site of plasmid pZErO-1 (Invitrogen). Transformation of E. coli strain JM109 resulted in chloramphenicol-resistant transformants (selected at 25 μg of chloramphenicol/ml), indicating that the vsa-cat gene functions in E. coli. To incorporate the cat determinant into Tn4001, the vsa-cat gene was excised from pZErO-1 by digestion with PstI and XhoI, and the ends of the DNA fragment were made flush by digestion with T4 DNA polymerase. BamHI linkers were attached, and the vsa-cat gene was inserted into the BamHI site of plasmid pISM2062 (17), encoding chloramphenicol resistance, to generate plasmid pIVC-1 (Fig. 1).
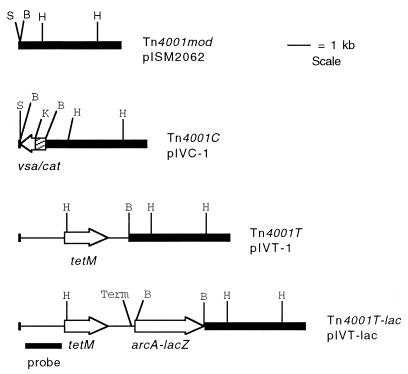
FIG. 1.
Schematic diagram of Tn4001mod in plasmid pISM2062, Tn4001C in pIVC-1, Tn4001T in pIVT-1, and Tn4001T-lac in pIVT-lac. Dark regions indicate Tn4001mod sequences. Unshaded regions indicate the cat, tetM, and arcA-lacZ genes. The hatched region of Tn4001C indicates the vsa promoter region. Thin lines in Tn4001T and Tn4001T-lac indicate streptococcal sequences originating from pJI3 that flank tetM. Arrows represent direction of gene transcription. Abbreviations: S, SmaI; B, BamHI; H, HindIII; K, KpnI; Term, putative transcription terminator derived from M. arthritidis sequences upstream of arcA. The bar represents the 1.5-kb PCR product used to probe transformants containing Tn4001T or Tn4001T-lac.
Transformation of M. pulmonis with pIVC-1 resulted in chloramphenicol-resistant transformants, indicating that vsa-cat functions as a selectable marker in this species of Mycoplasma. M. pulmonis strain KD735-15 (1) was propagated in mycoplasma broth medium consisting of 2.1% PPLO broth without crystal violet (Difco Laboratories, Detroit, Mich.) supplemented with 20% whole horse serum (Gibco BRL Life Technologies, Grand Island, N.Y.), 0.5% IsoVitaleX (VWR), 0.02% degraded free-acid DNA (Sigma), 100 μg of ampicillin/ml, and 0.5% glucose. M. pulmonis was transformed with 10 μg of pIVC-1 by the polyethylene glycol method as described previously (8). After incubation of transformed cells at 37°C for 2 h in nonselective medium, transformants were selected on mycoplasma agar (mycoplasma broth medium supplemented with 1.4% agar) containing 15 μg of chloramphenicol/ml. Transformants were obtained at a frequency of 2 × 10−7 per CFU, which is about 10-fold less efficient than transformation of M. pulmonis with the Tn916-containing plasmid pAM120 (7).
As expected, M. pulmonis cells transformed with pIVC-1 had Tn4001C inserted in the mycoplasmal chromosome. Total DNA was isolated from strains of Mycoplasma as described previously (7). Extrachromosomal plasmid was not detected in mycoplasmal DNA because pIVC-1 does not replicate in mycoplasmas. HindIII-digested genomic DNAs isolated from five independent, Tn4001C-containing M. pulmonis transformants were analyzed on Southern blots probed with the cat gene (obtained by PCR amplification of pACYC184 using the cat forward and reverse primers). Conditions for Southern hybridization and preparation of 32P-labeled probe by the random primer method were as described previously (9). The five transformants exhibited different hybridization banding patterns, indicating that Tn4001C can insert into the chromosome of M. pulmonis at any of numerous sites (Fig. 2A). One of the transformants (Fig. 2A, lane 5) had two DNA fragments that hybridized with the probe, suggesting either that the initial transformant was simultaneously transformed with two copies of pIVC-1 or that Tn4001C was transposed to secondary sites in the chromosome after the initial insertion event. In related hybridization experiments, it was shown that the cat probe did not hybridize with M. pulmonis DNA isolated from wild-type cells that had not been transformed (data not shown). To further explore the issue of whether derivatives of Tn4001 insert into the M. pulmonis chromosome at a diversity of sites, the precise transposon insertion site in about 150 transformants of M. pulmonis was identified by determining the DNA sequence of the mycoplasma-Tn4001 junction region (K. Dybvig, unpublished data). No two transformants had the transposon inserted at the same site.
FIG. 2.
Southern analysis of mycoplasmal transformants. (A) Autoradiogram of a Southern blot hybridized with a cat-specific probe. Lanes 1 through 5 are HindIII-digested genomic DNAs from independent transformants of M. pulmonis containing Tn4001C. (B) Autoradiogram of a Southern blot hybridized with the probe derived from sequences upstream of tetM (Fig. 1). Lanes 1 through 5 are HindIII-digested genomic DNAs from five independent isolates of M. arthritidis transformed with pIVT-lac.
Whether Tn4001 could transpose in M. arthritidis was examined. Attempts to transform M. arthritidis with pISM2062 (which contains Tn4001mod) and pIVC-1 (which contains Tn4001C) were not successful. It is likely that the vsa-cat gene does not function as a selectable marker in this species, suggesting that the vsa promoter may be species specific. In related experiments, attempts to transform M. pulmonis and M. arthritidis with plasmid pKV98 were not successful. pKV98 contains Tn4001 with an alternative cat gene that has been shown to function in M. pneumoniae (12), but this cat gene evidently fails to confer a selectable level of resistance in either M. pulmonis or M. arthritidis. To develop a derivative of Tn4001 that might function in M. arthritidis, the tetM gene was inserted into Tn4001 to generate Tn4001T. tetM was chosen because this gene functions as a selectable marker in many Mycoplasma species, including M. arthritidis (22). A 5-kb HincII fragment from plasmid pJI3 (4) that contained the tetM gene was isolated and inserted into the SmaI site of pISM2062 to generate plasmid pIVT-1 (Fig. 1). M. arthritidis strain H606 was propagated in the same medium as was M. pulmonis except that 0.5% arginine was used instead of glucose. Transformation of M. arthritidis with 10 μg of pIVT-1 was done as described previously (22). Transformation of M. arthritidis (selection at 5 μg of tetracycline/ml) and M. pulmonis (with 3 μg of tetracycline/ml) with pIVT-1 resulted in transformants at frequencies of about 2 × 10−9 and 3 × 10−7, respectively. Southern blot analysis indicated that Tn4001T had transposed into the chromosome of either species at any of numerous sites, as expected. The results for M. arthritidis transformed with pIVT-lac (i.e., pIVT-1 containing lacZ, described below) are shown in Fig. 2B. As was the case for M. pulmonis transformed with pIVC-1, one transformant (Fig. 2B, lane 1) out of five had two DNA fragments that hybridized with the probe.
Although Tn4001C and Tn4001T have an intact gentamicin resistance determinant, transformants of M. pulmonis containing either transposon and transformants of M. arthritidis containing Tn4001T had no change in susceptibility to gentamicin compared to wild-type cells lacking the transposon. The failure of the gentamicin resistance determinant to confer a selectable level of resistance in M. pulmonis and M. arthritidis explains why these species could not be transformed with pISM2062.
M. pulmonis containing Tn4001T could be transformed with pIVC-1 at a relatively high frequency, 3 × 10−6 transformants per CFU. Therefore, the presence of one endogenous copy of Tn4001 in the mycoplasma does not preclude and may actually stimulate transposition of a second copy into the chromosome. Stimulation of transposition may result from the presence of transposase activity in the cell prior to acquisition of the second copy of the transposon. Whether the mechanism of Tn4001C insertion into the genome of cells that already contained Tn4001T was by transposition or by homologous recombination was not examined. Regardless of the mechanism, however, the ability to insert both transposons into the genome should make it possible to complement mutants generated by insertional activation with one of the transposons by using the other transposon as a vehicle to introduce a functional wild-type copy of the inactivated gene.
Use of Tn4001T as a vector in M. pulmonis and M. arthritidis.
A lacZ gene was used to investigate whether Tn4001T can be used as a vector in M. pulmonis and M. arthritidis. Little is known about mycoplasmal gene expression signals, and the choice of a promoter that might drive expression of the lacZ gene was uncertain. Because the vsa promoter evidently did not function in M. arthritidis (i.e., the vsa-cat gene did not function as a selectable marker), a promoter originating from M. arthritidis was sought. We reasoned that the arcA gene (encoding arginine deiminase) promoter might be relatively easy to isolate and have strong activity.
Degenerate oligonucleotide primers were designed to amplify an internal region of arcA. The predicted amino acids of the ArcA proteins of Mycoplasma hominis and Mycoplasma arginini (13, 19), two species of Mycoplasma that are phylogenetically related to M. arthritidis, were aligned, and conserved amino acid regions were identified from which primers with minimal degeneracy could be designed. The arcA forward primer (5′-CGCTCGAGAYTAYATHACNCCNGC-3′) and arcA reverse primer (5′-GCCTCGAGCRTTNCCCATNCC-3′) were used to amplify a PCR product of the expected size (1.1 kb) from M. hominis strain PG21 (3) and M. arthritidis strain PG6 (23). The M. arthritidis PCR product was cloned into the TA cloning vector (Invitrogen), and the amino acids predicted from its nucleotide sequence were significantly similar to ArcA of M. hominis and M. arginini, as expected.
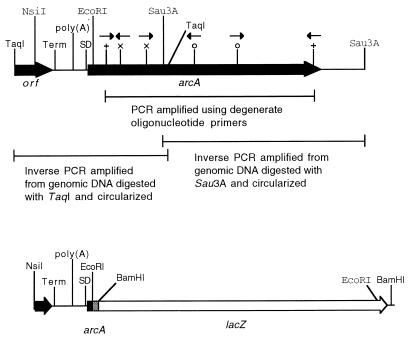
To clone the complete M. arthritidis arcA gene, including the promoter, an inverse PCR strategy was employed using the primers illustrated in Fig. 3. To obtain the 5′ end of arcA, genomic DNA was digested with TaqI, ligated to generate circular molecules for PCR templates, and amplified using primers 5′-TGTGTTCTTTTCTTGCATCGTGGC-3′ and 5′-AGTTCTATCAGACGAACACCGTGC-3′. To obtain the 3′ end of arcA, genomic DNA was digested with Sau3A, ligated, and amplified using primers 5′-CGGCTAAATAATGTTTCACGTTGTC-3′ and 5′-GAACAAACCTAATGCACTTAGACAC-3′. The resulting PCR products were cloned into plasmid pGEM-T (Promega) and their nucleotide sequences were determined, permitting assembly of the sequence of the complete arcA gene of M. arthritidis. The predicted M. arthritidis ArcA protein (arginine deiminase) contains 409 amino acids with extensive overall sequence identity, 87 and 80%, to the ArcA proteins of M. arginini (GenBank accession no. X54141) and M. hominis (GenBank accession no. D13314), respectively.
FIG. 3.
Schematic diagrams of the region of the M. arthritidis chromosome containing the arcA gene (top) and the chimeric arcA-lacZ gene (bottom). Coding regions are indicated by thick lines, with arrows indicating direction. Regions shaded in black originate from the M. arthritidis chromosome. The stippled region is derived from the pZErO vector used for gene construction. The unshaded region is the lacZ coding region derived from pISM2062.2lac. The location and direction of primers used for PCR amplification are illustrated as follows: +, degenerate primers used for the initial amplification of an internal portion of arcA, and × and ○, primers used to amplify the 5′ and 3′ ends, respectively, of arcA by inverse PCR. The location of the putative transcription terminator (Term), the 15-nucleotide poly(A) tract, and the arcA SD sequence are also shown. For clarity, the 3-kb lacZ coding region is drawn at one-half scale.
Sequence analysis of the region upstream of the arcA structural gene failed to reveal a likely promoter candidate (resembling the consensus −10 and −35 sequences characteristic of promoters recognized by ςA). An open reading frame (ORF) was identified upstream of arcA that may represent the 3′ end of another gene. The amino acids predicted from this ORF have no significant similarity to sequences deposited in the protein and nucleotide databases. Immediately downstream of this ORF are sequences capable of forming a stem-loop structure that may serve as a transcription terminator. Thus, it seems likely that an arcA promoter should exist in the area between the putative transcription terminator and the start of the arcA coding region. The sequences in this area are interesting and contain a poly(A) sequence of 15 reiterated nucleotides. Assuming that an arcA promoter is present in this region, its structure would be unusual and worthy of further study.
The putative arcA promoter region was isolated from the cloned inverse PCR product containing the 5′ end of the arcA gene and combined with lacZ (Fig. 3). The plasmid containing the arcA promoter region was linearized by digestion with NsiI, the ends were made flush by digestion with T4 DNA polymerase, XhoI linkers were attached, and a 300-bp region containing the putative promoter and the beginning of the arcA structural gene was excised by digestion with XhoI and EcoRI. This fragment was cloned into the XhoI/EcoRI site of plasmid pZErO-2.1. The promoterless lacZ gene from plasmid pISM2062.2lac (17) was excised as a 3-kb BamHI fragment and inserted into the BamHI site of pZErO-2.1 downstream of the arcA promoter fragment. This resulted in construction of an in-frame fusion gene containing the first 33 nucleotides of the arcA coding region, 31 nucleotides derived from the pZErO-2.1 polylinker, and lacZ. The arcA-lacZ gene was excised from pZErO-2.1 by digestion with XhoI and KpnI, and the ends were made flush by digestion with T4 DNA polymerase. BglII linkers were attached, cohesive ends were generated by digestion with BglII, and the gene was inserted into the BamHI site (BglII and BamHI ends are compatible) of pIVT-1 to generate pIVT-lac. As anticipated, E. coli colonies containing either the arcA-lacZ gene in pZErO-2.1 or pIVT-lac were blue when assayed on agar supplemented with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal), indicating that the fusion gene was functional.
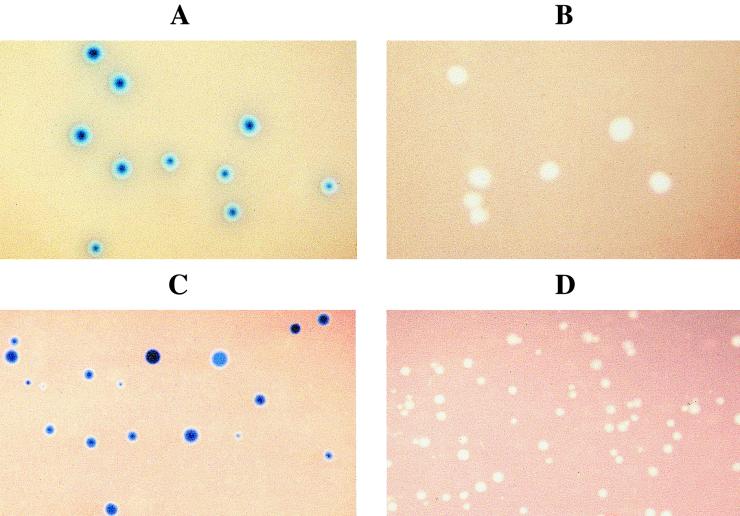
Transformation of M. pulmonis and M. arthritidis with pIVT-lac resulted in tetracycline-resistant colonies that were found to possess β-galactosidase activity when assayed on mycoplasma agar supplemented with X-Gal at a concentration of 150 μg/ml (Fig. 4). Colonies of wild-type mycoplasmas that had not been transformed with pIVT-lac were white, indicating that the β-galactosidase activity resulted from the ArcA-LacZ fusion protein and was not endogenous to the mycoplasmas. Although it has not been determined whether transcription of arcA-lacZ is initiated from an arcA promoter or from outside the arcA promoter region, it is clear that arcA-lacZ is expressed both in M. pulmonis and in M. arthritidis, demonstrating the utility of pIVT-1 as a vector in these species. Because the tetM marker functions in all species of Mycoplasma in which it has been examined, Tn4001T should be used as a general mycoplasmal cloning vector.
FIG. 4.
Blue (LacZ+) M. pulmonis (A) and M. arthritidis (C) colonies transformed with pIVT-lac, assayed on mycoplasma agar plates supplemented with X-Gal. Control colonies (LacZ−) arising from cells that had not been transformed are shown for M. pulmonis and M. arthritidis in panels B and D, respectively.
Nucleotide sequence accession number.
The arcA nucleotide sequence of M. arthritidis has been deposited in GenBank under accession no. AF182646.
Acknowledgments
This work was supported by Public Health Service grants GM51126 and AR44252.
REFERENCES
- 1.Bhugra B, Dybvig K. High-frequency rearrangements in the chromosome of Mycoplasma pulmonis correlate with phenotypic switching. Mol Microbiol. 1992;6:1149–1154. doi: 10.1111/j.1365-2958.1992.tb01553.x. [DOI] [PubMed] [Google Scholar]
- 2.Bhugra B, Voelker L L, Zou N, Yu H, Dybvig K. Mechanism of antigenic variation in Mycoplasma pulmonis: interwoven, site-specific DNA inversions. Mol Microbiol. 1995;18:703–714. doi: 10.1111/j.1365-2958.1995.mmi_18040703.x. [DOI] [PubMed] [Google Scholar]
- 3.Blanchard A, Yáñez A, Dybvig K, Watson H L, Griffiths G, Cassell G H. Evaluation of intraspecies genetic variation within the 16S rRNA gene of Mycoplasma hominis and detection by polymerase chain reaction. J Clin Microbiol. 1993;31:1358–1361. doi: 10.1128/jcm.31.5.1358-1361.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burdett V, Inamine J, Rajagopalan S. Multiple tetracycline resistance determinants in Streptococcus. In: Schlessinger D, editor. Microbiology—1982. Washington, D.C.: American Society for Microbiology; 1982. pp. 155–158. [Google Scholar]
- 5.Chang A C Y, Cohen S N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978;134:1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dybvig K. The genetics and basic biology of Mycoplasma pulmonis: how much is actually Acholeplasma? Plasmid. 1993;30:176–178. doi: 10.1006/plas.1993.1048. [DOI] [PubMed] [Google Scholar]
- 7.Dybvig K, Alderete J. Transformation of Mycoplasma pulmonis and Mycoplasma hyorhinis: transposition of Tn916 and formation of cointegrate structures. Plasmid. 1988;20:33–41. doi: 10.1016/0147-619x(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 8.Dybvig K, Gasparich G E, King K W. Artificial transformation of mollicutes via polyethylene glycol- and electroporation-mediated methods. In: Razin S, Tully J G, editors. Molecular and diagnostic procedures in mycoplasmology. I. Orlando, Fla: Academic Press; 1995. pp. 179–184. [Google Scholar]
- 9.Dybvig K, Sitaraman R, French C T. A family of phase-variable restriction enzymes with differing specificities generated by high-frequency gene rearrangements. Proc Natl Acad Sci USA. 1998;95:13923–13928. doi: 10.1073/pnas.95.23.13923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dybvig K, Voelker L L. Molecular biology of mycoplasmas. Annu Rev Microbiol. 1996;50:25–57. doi: 10.1146/annurev.micro.50.1.25. [DOI] [PubMed] [Google Scholar]
- 11.Dybvig K, Woodard A. Cloning and DNA sequence of a mycoplasmal recA gene. J Bacteriol. 1992;174:778–784. doi: 10.1128/jb.174.3.778-784.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hahn T-W, Mothershed E A, Waldo III R H, Krause D C. Construction and analysis of a modified Tn4001 conferring chloramphenicol resistance in Mycoplasma pneumoniae. Plasmid. 1999;41:120–124. doi: 10.1006/plas.1998.1387. [DOI] [PubMed] [Google Scholar]
- 13.Harasawa R, Koshimizu K, Kitagawa M, Asada K, Kato I. Nucleotide sequence of the arginine deiminase gene of Mycoplasma hominis. Microbiol Immunol. 1992;36:661–665. doi: 10.1111/j.1348-0421.1992.tb02068.x. [DOI] [PubMed] [Google Scholar]
- 14.Horowitz S A, Garret B, Davis J K, Cassell G H. Isolation of Mycoplasma pulmonis membranes and identification of surface antigens. Infect Immun. 1987;55:1314–1320. doi: 10.1128/iai.55.5.1314-1320.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.King K W, Dybvig K. Mycoplasmal cloning vectors derived from plasmid pKMK1. Plasmid. 1993;31:49–59. doi: 10.1006/plas.1994.1006. [DOI] [PubMed] [Google Scholar]
- 16.King K W, Dybvig K. Transformation of Mycoplasma capricolum and examination of DNA restriction and modification in M. capricolum and Mycoplasma mycoides subsp. mycoides. Plasmid. 1994;31:308–311. doi: 10.1006/plas.1994.1033. [DOI] [PubMed] [Google Scholar]
- 17.Knudtson K L, Minion F C. Construction of Tn4001lac derivatives to be used as promoter probe vectors in mycoplasmas. Gene. 1993;137:217–222. doi: 10.1016/0378-1119(93)90009-r. [DOI] [PubMed] [Google Scholar]
- 18.Mahairas G G, Minion F C. Random insertion of the gentamicin resistance transposon Tn4001 in Mycoplasma pulmonis. Plasmid. 1989;21:43–47. doi: 10.1016/0147-619x(89)90085-1. [DOI] [PubMed] [Google Scholar]
- 19.Misawa S, Aoshima M, Takaku H, Matsumoto M, Hayashi H. High-level expression of Mycoplasma arginine deiminase in Escherichia coli and its efficient renaturation as an anti-tumor enzyme. J Biotechnol. 1994;36:145–155. doi: 10.1016/0168-1656(94)90050-7. [DOI] [PubMed] [Google Scholar]
- 20.Romero-Arroyo C E, Jordan J, Peacock S J, Willby M J, Farmer M A, Krause D C. Mycoplasma pneumoniae protein P30 is required for cytadherence and associated with proper cell development. J Bacteriol. 1999;181:1079–1087. doi: 10.1128/jb.181.4.1079-1087.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simmons W L, Zuhua C, Glass J I, Simecka J W, Cassell G H, Watson H L. Sequence analysis of the chromosomal region around and within the V-1-encoding gene of Mycoplasma pulmonis: evidence for DNA inversion as a mechanism for V-1 variation. Infect Immun. 1996;64:472–479. doi: 10.1128/iai.64.2.472-479.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Voelker L L, Dybvig K. Gene transfer in Mycoplasma arthritidis: transformation, conjugal transfer of Tn916, and evidence for a restriction system recognizing AGCT. J Bacteriol. 1996;178:6078–6081. doi: 10.1128/jb.178.20.6078-6081.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Voelker L L, Weaver K E, Ehle L J, Washburn L R. Association of lysogenic bacteriophage MAV1 with virulence of Mycoplasma arthritidis. Infect Immun. 1995;63:4016–4023. doi: 10.1128/iai.63.10.4016-4023.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]