Abstract
Objective:
To compare the Mediterranean and Dietary Approaches to Stop Hypertension (DASH) diets in deterring 10-year CVD.
Design:
Prospective cohort (n 2020) with a 10-year follow-up period for the occurrence of combined (fatal or non-fatal) CVD incidence (International Classification of Diseases (ICD)-10). Baseline adherence to the Mediterranean and DASH diets was assessed via a semi-quantitative FFQ according to the MedDietScore and DASH scores, respectively.
Setting:
Attica, Greece.
Participants:
Two thousand twenty individuals (mean age at baseline 45·2 (sd 14·0) years).
Results:
One-third of individuals in the lowest quartile of Mediterranean diet consumption, as compared with 3·1 % of those in the highest quartile, developed 10-year CVD (P < 0·0001). In contrast, individuals in the lowest and highest DASH diet quartiles exhibited similar 10-year CVD rates (n (%) of 10-year CVD in DASH diet quartiles 1 v. 4: 79 (14·7 %) v. 75 (15·3 %); P = 0·842). Following adjustment for demographic, lifestyle and clinical confounding factors, those in the highest Mediterranean diet quartile had a 4-fold reduced 10-year CVD risk (adjusted hazard ratio (HR) 4·52, 95 % CI 1·76, 11·63). However, individuals with highest DASH diet quartile scores did not differ from their lowest quartile counterparts in developing such events (adjusted HR 1·05, 95 % CI 0·69, 1·60).
Conclusions
High adherence to the Mediterranean diet, and not to the DASH diet, was associated with a lower risk of 10-year fatal and non-fatal CVD. Therefore, public health interventions aimed at enhancing adherence to the Mediterranean diet, rather than the DASH diet, may most effectively deter long-term CVD outcomes particularly in Mediterranean populations.
Keywords: Mediterranean diet, Dietary Approaches to Stop Hypertension diet, CVD
CVD constitute one of the prevailing causes of morbidity and mortality in developed countries(1,2). Several non-modifiable and modifiable demographic, lifestyle and clinical factors (including obesity (especially abdominal obesity), dyslipidaemia, the metabolic syndrome and/or type 2 diabetes mellitus)(3,4) alike are implicated in the onset, further progression and, ultimately, attributable mortality of CVD(5). Although not constituting the initial triggers in all cases, both the aforementioned non-modifiable and modifiable factors are hypothesised to be implicated via a complex interplay in disease onset and propagation through the positive feedback mechanisms of the dysregulated renin–angiotensin–aldosterone system, leading to the activation of an inflammatory cascade and lipid dysregulation(6). Of ever-mounting public health interest, though, are modifiable lifestyle factors, such as dietary patterns, which may be altered via related interventions so as to deter CVD onset and adverse outcomes, including attributable morbidity and mortality rates(1,2, 7). To this effect, the Mediterranean and/or the Dietary Approaches to Stop Hypertension (DASH) dietary patterns are most often recommended for CVD prevention and deterrence of its associated health outcomes. However, limited evidence(8) and lack of international consensus often lead to conflicting public health nutrition recommendations regarding the most appropriate dietary pattern for CVD prevention, particularly in settings where the Mediterranean diet is either readily available or adopted due to target populations’ socio-cultural characteristics.
Both the Mediterranean and DASH diets are rich in fruits and vegetables, cereals, pulses and nuts, entailing dietary intakes characterised by a low glycaemic load and rich antioxidant content(9,10) The Mediterranean diet has a higher fat content as compared with the DASH diet which has a low fat content, ideally around 27 %. On the one hand, the Mediterranean diet(11) is associated with numerous health benefits, including CVD prevention(12) and diminished all-cause mortality(10,13), particularly in Mediterranean populations where it is readily adopted for socio-cultural purposes. To this effect, it appears that particular constituents of the Mediterranean diet, such as olive oil which is rich in oleic acid and polyphenols, along with physical activity(14,15), moderate energetic intake(16) and optimal body weight(17), deter biological pathways implicated in CVD onset and progression, such as vascular degeneration(18 ,19). The antioxidant, anti-inflammatory and antithrombotic properties of the Mediterranean diet are primarily attributed to its richness in foods of plant origin, olive oil and wine. On the other hand, the DASH diet, which was originally conceived for non-Mediterranean Western populations, is primarily composed of lean meats, low-fat dairy products and whole grains, as well as plentiful fruits and vegetables(20,21), moderate energetic intake(16) and optimal body weight(17), deters biological pathways implicated in CVD onset and progression, such as vascular degeneration(18 ,19). As of such, it entails a dietary intake low in saturated fats and cholesterol, albeit rich in fibre and micronutrients, such as Ca, Mg and K, which are essential for preserving normal blood pressure levels and, consequently, CVD prevention. Similarly to the Mediterranean diet, recent meta-analytic findings reveal that high adherence to the DASH diet, combined with reduced Na intake, is associated with diminished CVD incidence and attributable mortality rates(7). However, the above findings are limited to Western populations where the Mediterranean diet does not prevail. To date, a comparison of the efficacies of the above dietary patterns in preventing CVD events has not been conducted, particularly in populations where either of the above diets may be readily adopted and/or proposed for public(19). It is anticipated that such findings would provide essential insights for developing optimal public health interventions for preventing CVD(22).
Therefore, the aim of the current study was to compare the efficacy of the Mediterranean and DASH diets in deterring 10-year fatal and non-fatal CVD events in a Mediterranean adult population.
Materials and methods
Setting
A population-based cohort study, with 10-year follow-up period. The study was implemented in Athens metropolitan area, the capital province of Greece, in the Attica region, which includes 78 % urban municipalities.
Design
The ATTICA Study design and population recruitment procedures have been previously detailed(23). A random multistage sampling was implemented based on the age and gender distribution of the population, in accordance with the 2001 National Census Survey. One participant per household was enrolled, while institutionalised individuals were excluded from participation. Of the initially invited 4056 individuals, 3042 agreed to participate (75 % participation rate); 1514 of the participants were male (aged 46 ± 13 years; range 18–87 years) and 1528 were female (aged 45 ± 13 years; range 18–89 years). The study sample did not differ from the general population with respect to the distribution of age and gender. All participants were interviewed by trained personnel (including cardiologists, nutritionists and nurse practitioners) who used standard questionnaires.
Baseline socio-demographic and lifestyle measurements
The information about participants’ characteristics was based on face-to-face interviews; collected information included demographic characteristics (e.g. age, gender and highest attained educational level), dietary and lifestyle habits (including smoking status and habitual/leisure time physical activity), as well medical examination. In particular, based on the years of education completed and income, participants were categorised into the following educational categories: (i) low (i.e. <12 years education), (ii) moderate (i.e. including 13–16 years education) and (iii) high (i.e. >16 years education).
Dietary habits were assessed based on a validated semi-quantitative FFQ, the European Prospective Investigation into Cancer (EPIC) FFQ that was kindly provided by the Unit of Nutrition Epidemiology of Athens University Medical School(24), according to which participants reported average weekly or daily intakes of 156 food items and drinks during the past year, as well as a Mediterranean diet questionnaire(25). Subsequently, the approximate monthly and/or weekly frequency of food item consumption was calculated. Composite scores were employed to describe the overall dietary patterns and to evaluate the level of adherence to the Mediterranean dietary pattern or the DASH pattern. Specifically, of the Mediterranean foods recorded, a diet score (i.e. MedDietScore©) was calculated (range 0–55) to depict adherence to dietary patterns most proximal to those of the Mediterranean diet(11,25). Based on the derived MedDietScore, the following quartiles were constructed: (i) first quartile: MedDietScore <25), (ii) second quartile: 25·0–26·9, (iii) third quartile: 27·0–27·9 and (iv) fourth quartile: 28·0–55·0. The median MedDietScore was 26·5 (sem 0·1). Subsequently, low adherence to the Mediterranean diet was deemed as MedDietScore ≤27·0, while scores greater were considered as high adherence to the Mediterranean diet. Moreover, of the food and drinks recorded in the questionnaire, those most proximately associated with the DASH dietary pattern were grouped and adjusted to the scoring used in the Nurses’ Health Study cohort(26). A DASH-style score was then developed for the participants (n 699 with available nutrition information through the FFQ and the rest through the Mediterranean diet questionnaire) assessing the consumption of nine food groups, including fruits, vegetables, nuts, legumes, low-fat dairies, all cereals, red and processed meats, sugary drinks and sweets(27). The consumption of each food group was classified into quintiles, allocating a value from 1 to 5, so that a higher value reflected the consumption proposed by the DASH dietary pattern(26). Therefore, the total DASH diet scores ranged from 9 to 45, with higher scores reflecting greater adherence to the DASH-style dietary pattern. Subsequently, based on the DASH diet scores in the current analysis, the following quartiles of consumption were constructed: (i) first quartile: DASH diet score <24, (ii) second quartile: 24·0–26·9, (iii) third quartile: 27·0–30·9 and (iv) fourth quartile: 31·0–45·0. The median DASH score was 27·0 (sem 0·1). Low adherence to the DASH diet was deemed as scores ≤27·0, while those higher were indicative of high adherence.
As regards smoking habits, at baseline, ‘current smokers’ included those who smoked at least one cigarette per day, ‘never smokers’ included those who have never smoked and ‘former smokers’ included those who had ceased smoking ≥1 year prior to evaluation. For the assessment of physical activity status, the International Physical Activity Questionnaire(28) was used as an index of weekly energy expenditure. Physical activity was defined as >1 d/week leisure-time activity, of specific intensity and duration, during the past year. Alternatively, subjects were identified as physically inactive.
Baseline anthropometric and clinical measurements’ assessment
Weight (in kg) and standing height (in m2) were used to calculate BMI; those with BMI >29·9 kg/m2 were defined as obese. Waist and hip circumferences (in cm) were used to calculate waist-to-hip ratio, based on standard procedures. At the end of the physical examination, following >30 min at rest and while in a sitting position, subjects’ arterial blood pressure was measured blindly three times by a trained cardiologist on participants’ right arm which was relaxed and well supported by a table, at 45° from the trunk (ELKA aneroid manometric sphygmomanometer; Von Schlieben Co.). Systolic and diastolic blood pressure levels were determined by the first perception of sound (of tapping quality) and phase V (fully muffed repetitive sounds), respectively. Individuals with systolic blood pressure ≥ 140 mmHg or diastolic blood pressure > 90 mmHg, or under antihypertensive medication, were classified as hypertensive. Furthermore, following 12 h of fasting, morning blood samples were collected from participants’ antecubital vein and blood lipid examinations (including serum total cholesterol) were measured using the chromatographic enzymic method in a RA-1000 Technicon automatic analyzer (Dade Behring). Hypercholesterolaemia was defined as >220 mg/dl (or 5·70 mmol/l) total cholesterol levels or the use of hypolipidaemic medication. Blood glucose levels (in mg/dl) were measured with a Beckman Glucose Analyzer (Beckman Instruments). Fasting blood sugar levels >125 mg/dl, or the use of antidiabetic medication, were indicative of diabetes mellitus. The metabolic syndrome was defined by the National Cholesterol Education Program Adult Treatment Panel III (revised) definition(29).
Follow-up assessment, 2002–2012
Follow-up assessments were conducted 10 years following initial recruitment, that is, 2012–2013. Of the initially enrolled 3042 participants at baseline, 10-year follow-up was achieved in 2583 participants (85 % participation rate; of those lost to follow-up, 224 could not be traced due to missing or erroneous contact information and 235 denied to participate). A complete CVD assessment was achieved in 2020 participants. The follow-up examination included the retrieval of detailed information from participants’ medical records. When lacking accurate records, participants were evaluated by face-to-face interview by trained study investigators. The re-examination included (i) vital status (death from any cause or due to CVD) or (ii) development of CHD (including myocardial infarction, angina pectoris, other identified forms of ischemia – WHO-ICD coding 410–414·9, 427·2, 427·6 – heart failure of different types and chronic arrhythmias – WHO-ICD coding 400·0–404·9, 427·0–427·5, 427·9) or development of stroke (WHO-ICD coding 430–438).
Some differences in the baseline characteristics were observed between those who participated to follow-up and those who did not participate regarding the distribution of age (46 ± 14 v. 41 ± 11 years, P < 0·001), history of hypertension (31 v. 24 %, P = 0·001), diabetes (8 v. 3 %, P = < 0·001), hypercholesterolaemia (41 v. 33 %, P = 0·004) and smoking status (55 v. 61 %, P < 0·001), whereas no differences were reported regarding the distribution of gender (P = 0·613), obesity status (P = 0·208) and eating habits (P = 0·560).
Statistical analysis
Categorical variables are presented as absolute and relative frequencies (n, %). Continuous variables are presented as mean (M) ± standard deviation; the normality of their distributions was assessed with P–P plots. The baseline characteristics between CVD-free and CVD event groups were compared overall and stratified according to gender. The frequencies of categorical variables were compared with Pearson’s χ 2 test. The Student’s t test and Mann–Whitney U test were used to compare normally and non-normally distributed continuous variables between groups, respectively. Ordinal variables were compared between groups with the likelihood-ratio test. The probability of time to CVD fatal or non-fatal events was depicted with Kaplan–Meier curves. CVD survival rates, according to baseline quartiles of dietary consumption for the MedDietScore and DASH diet scores, were compared between groups with the Log-rank test. Cox proportional hazard models were used to explore the effects of baseline quartiles of MedDietScore and DASH diet scores on 10-year fatal and non-fatal CVD events. Unadjusted and adjusted hazard ratios (HR) and the corresponding 95 % CI were calculated separately for MedDietScore and DASH diet quartile scores (independent factors) in relation to 10-year incidence of CVD (outcome)(30). Multivariable models included adjustment for potential confounding factors, that is, age, gender, physical activity status, weight category, smoking, history of hypertension, hypercholesterolaemia, diabetes and the metabolic syndrome. The proportionality assumption for the Cox models was assessed graphically. The level of significance was set to P < 0·05. STATA 15 software was used for all analyses (M Psarros and Associates/StataCorp LLC).
Results
Baseline Mediterranean diet and DASH diet scores in relation to 10-year incidence of fatal and non-fatal CVD
Among the study sample (n 2020, 49·8 % (n 1006) males) with an overall mean age at a baseline of 45·2 (sd 14·0) years (Table 1), the 10-year combined (fatal or non-fatal) CVD incidence rate was 15·7 % (n 317 cases).
Table 1.
Baseline characteristics of ATTICA study participants (n 2020), according to the level of adherence to the Mediterranean and Dietary Approaches to Stop Hypertension (DASH) diets*
| Overall | Low adherence to the Mediterranean diet (≤27·0) | High adherence to the Mediterranean diet (>27·0) | P | Low adherence to the DASH diet (≤27·0) | High adherence to the DASH diet (>27·0) | P | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |||
| Age (years) | <0·0001a | <0·008a | ||||||||||
| Mean | 45·2 | 51·0 | 36·2 | 44·3 | 46·0 | |||||||
| sd | 14·0 | 12·6 | 10·7 | 14·6 | 13·2 | |||||||
| Gender (men) | 1006 | 49·8 | 854 | 69·8 | 152 | 19·1 | <0·0001b | 500 | 51·8 | 506 | 48·0 | <0·084b |
| Educational status | <0·0001c | 0·793c | ||||||||||
| Low | 1237 | 62·4 | 807 | 67·0 | 430 | 55·4 | 595 | 62·7 | 642 | 62·2 | ||
| Moderate | 714 | 36·0 | 383 | 31·8 | 331 | 42·7 | 338 | 35·6 | 376 | 36·4 | ||
| High | 30 | 1·5 | 15 | 1·2 | 15 | 1·9 | 16 | 1·7 | 14 | 1·4 | ||
| Average daily energetic intake (kJ) | 0·379d | 0·985d | ||||||||||
| Mean | 9949 | 10 058 | 9799 | 9949 | 9954 | |||||||
| sd | 3975 | 3975 | 3983 | 4125 | 3845 | |||||||
| Physically active | 825 | 40·8 | 493 | 40·3 | 332 | 41·7 | 0·548b | 375 | 38·9 | 450 | 42·7 | 0·954b |
| BMI (kg/m2) | <0·0001a | <0·051a | ||||||||||
| Mean | 26·3 | 28·4 | 23·0 | 26·1 | 26·5 | |||||||
| sd | 4·5 | 4·1 | 2·7 | 4·4 | 4·6 | |||||||
| Weight category | <0·0001c | 0·116c | ||||||||||
| Normal/underweight | 842 | 41·7 | 231 | 18·9 | 611 | 77·0 | 423 | 44·0 | 419 | 39·7 | ||
| Overweight | 811 | 40·2 | 637 | 52·1 | 174 | 21·9 | 378 | 39·3 | 433 | 41·9 | ||
| Obese | 364 | 18·0 | 355 | 29·0 | 9 | 1·1 | 161 | 16·7 | 203 | 19·2 | ||
| Abnormal waist-to-hip ratio | 777 | 40·5 | 867 | 70·9 | 217 | 27·3 | <0·0001b | 505 | 52·5 | 579 | 54·9 | 0·283b |
| Current smokers | 857 | 42·6 | 528 | 43·3 | 329 | 41·4 | 0·414b | 409 | 42·5 | 448 | 42·6 | 0·975b |
| Systolic blood pressure (mmHg) | <0·0001d | 0·913d | ||||||||||
| Mean | 123·0 | 129·5 | 113·0 | 123·0 | 123·0 | |||||||
| sd | 18·4 | 17·7 | 14·4 | 18·2 | 18·5 | |||||||
| Diastolic blood pressure (mmHg) | 0·038d | 0·166d | ||||||||||
| Mean | 79·0 | 82·7 | 73·3 | 78·6 | 79·4 | |||||||
| sd | 11·6 | 11·0 | 10·1 | 11·2 | 11·9 | |||||||
| Blood glucose (mg/dl) | <0·0001d | 0·857d | ||||||||||
| Mean | 93·3 | 97·2 | 87·2 | 93·2 | 93·4 | |||||||
| sd | 24·1 | 27·2 | 16·8 | 24·2 | 24·1 | |||||||
| Hypercholesterolaemia | 860 | 42·6 | 649 | 53·1 | 211 | 26·6 | <0·0001b | 391 | 40·6 | 469 | 44·5 | 0·084b |
| Hypertension | 598 | 31·5 | 516 | 44·6 | 82 | 11·1 | <0·0001b | 273 | 29·9 | 325 | 33·0 | 0·147b |
| Diabetes | 145 | 7·2 | 134 | 11·0 | 11 | 1·4 | <0·0001b | 71 | 7·4 | 74 | 7·0 | 0·765b |
| Metabolic syndrome | 399 | 19·8 | 365 | 29·8 | 34 | 4·3 | <0·0001b | 178 | 18·4 | 221 | 20·9 | 0·158b |
Continuous variables are presented as mean ± sd and categorical variables as absolute and relative frequencies (n and %).
P-values referring to differences between CVD events and CVD-free events during the 10-year follow-up, derived using the following: aStudent’s t test for normally distributed continuous variables, b χ 2 test for categorical variables, cLikelihood-ratio test for ordinal categorical variables and dMann–Whitney test for non-normally distributed continuous variables.
As shown in Table 2, as compared with their CVD-event free counterparts, at baseline, individuals who subsequently developed 10-year CVD events were of older mean age, more often male, of lower educational status, and with lower mean MedDietScore. However, it is of note that they did not differ with respect to mean baseline DASH diet scores. In addition, they more often presented at baseline with several well-established CVD risk factors, including abnormal waist-to-hip ratio, hypertension and hypercholesterolaemia, as well as diabetes and the metabolic syndrome.
Table 2.
Baseline characteristics among ATTICA study participants (n 2020) in relation to the 10-year fatal or non-fatal incidence of CVD*
| CVD-free events (n 1703) | CVD events (n 317) | P | |||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | ||||
| Age (years) | <0·0001a | ||||||
| Mean | 42·8 | 57·8 | |||||
| sd | 12·8 | 13·2 | |||||
| Gender (men) | 808 | 47·4 | 198 | 62·5 | <0·0001b | ||
| Educational status | <0·0001c | ||||||
| Low | 1003 | 59·9 | 234 | 76·2 | |||
| Moderate | 645 | 38·5 | 69 | 22·5 | |||
| High | 26 | 1·6 | 4 | 1·3 | |||
| Average daily energetic intake (kJ) | 0·451d | ||||||
| Mean | 9912 | 10 238 | |||||
| sd | 3866 | 4724 | |||||
| MedDietScore (0–55) | <0·0001d | ||||||
| Mean | 26·4 | 22·8 | |||||
| sd | 6·3 | 6·5 | |||||
| DASH score (0–45) | 0·197d | ||||||
| Mean | 27·1 | 27·5 | |||||
| sd | 5·1 | 5·2 | |||||
| Physically active | 696 | 40·9 | 129 | 40·7 | 0·954b | ||
| BMI (kg/m2) | <0·0001a | ||||||
| Mean | 26·0 | 27·9 | |||||
| sd | 4·4 | 4·5 | |||||
| Weight category | <0·0001c | ||||||
| Normal/underweight | 762 | 44·8 | 80 | 25·2 | |||
| Overweight | 660 | 38·8 | 151 | 47·6 | |||
| Obese | 278 | 16·4 | 86 | 27·1 | |||
| Abnormal waist-to-hip ratio | 611 | 37·6 | 166 | 56·8 | <0·0001b | ||
| Current smokers | 740 | 43·6 | 117 | 37·1 | 0·035b | ||
| Systolic blood pressure (mmHg) | <0·0001d | ||||||
| Mean | 121·3 | 132·6 | |||||
| sd | 17·6 | 19·6 | |||||
| Diastolic blood pressure (mmHg) | <0·0001d | ||||||
| Mean | 78·4 | 82·3 | |||||
| sd | 11·4 | 11·8 | |||||
| Blood glucose (mg/dl) | <0·0001d | ||||||
| Mean | 91·4 | 103·5 | |||||
| sd | 21·7 | 33·0 | |||||
| Hypercholesterolaemia | 679 | 39·9 | 181 | 57·1 | <0·0001b | ||
| Hypertension | 448 | 28·0 | 150 | 50·7 | <0·0001b | ||
| Diabetes | 77 | 4·5 | 68 | 21·5 | <0·0001b | ||
| Metabolic syndrome | 283 | 16·6 | 116 | 36·6 | <0·0001b | ||
DASH, Dietary Approaches to Stop Hypertension.
Continuous variables are presented as mean (M) ± sd and categorical variables as absolute and relative frequencies (n and %).
P-values referring to differences between CVD events and CVD-free events during the 10-year follow-up, derived using the following: aStudent’s t test for normally distributed continuous variables, b χ 2 test for categorical variables, cLikelihood-Ratio test for ordinal categorical variables and dMann–Whitney test for non-normally distributed continuous variables.
Comparisons of the proportion of study participants who developed 10-year CVD events according to their adherence to the Mediterranean and DASH diets at baseline are illustrated in Fig. 1. As shown in Table 3, among individuals in the lowest quartile of Mediterranean diet score, one-third (n 168) developed 10-year fatal or non-fatal CVD events. In contrast, among those adhering to the highest quartile of Mediterranean diet consumption (namely MedDietScore ranging between 28·0 and 55·0), only 3·1 % (n 15) subsequently developed 10-year CVD events. As of such, it is inferred that individuals with highest adherence to the Mediterranean diet had a 15-fold lower risk than their lowest adherence counterparts to manifest 10-year CVD events (Table 4; unadjusted HR 15·75, 95 % CI 9·12, −27·20). Similar trends were observed in both genders, albeit documented most prominently among women (n of 10-year CVD events in MedDietScore quartiles 1 v. 4: 53 (36 %) v. 11 (2·5 %); P < 0·0001) rather than men (n of 10-year CVD events in MedDietScore quartiles 1 v. 4: 115 (32·2 %) v. 4 (9·3 %); P < 0·0001). On the other hand, when assessed according to the DASH diet, individuals in the lowest and highest quartiles of consumption exhibited similar rates of manifesting 10-year CVD (n of 10-year CVD events in DASH diet quartiles 1 v. 4: 79 (14·7 %) v. 75 (15·3 %); P = 0·842). Furthermore, the occurrence of 10-year CVD did not significantly differ among those with lowest, as opposed to highest, quartile DASH scores in either men (n of 10-year CVD events in DASH diet quartiles 1 v. 4: 54 (20·0 %) v. 51 (22·2 %); P = 0·599) or women (n of 10-year CVD events in DASH Diet quartiles 1 v. 4: 25 (9·4 %) v. 24 (9·3 %); P = 0·086).
Fig. 1.
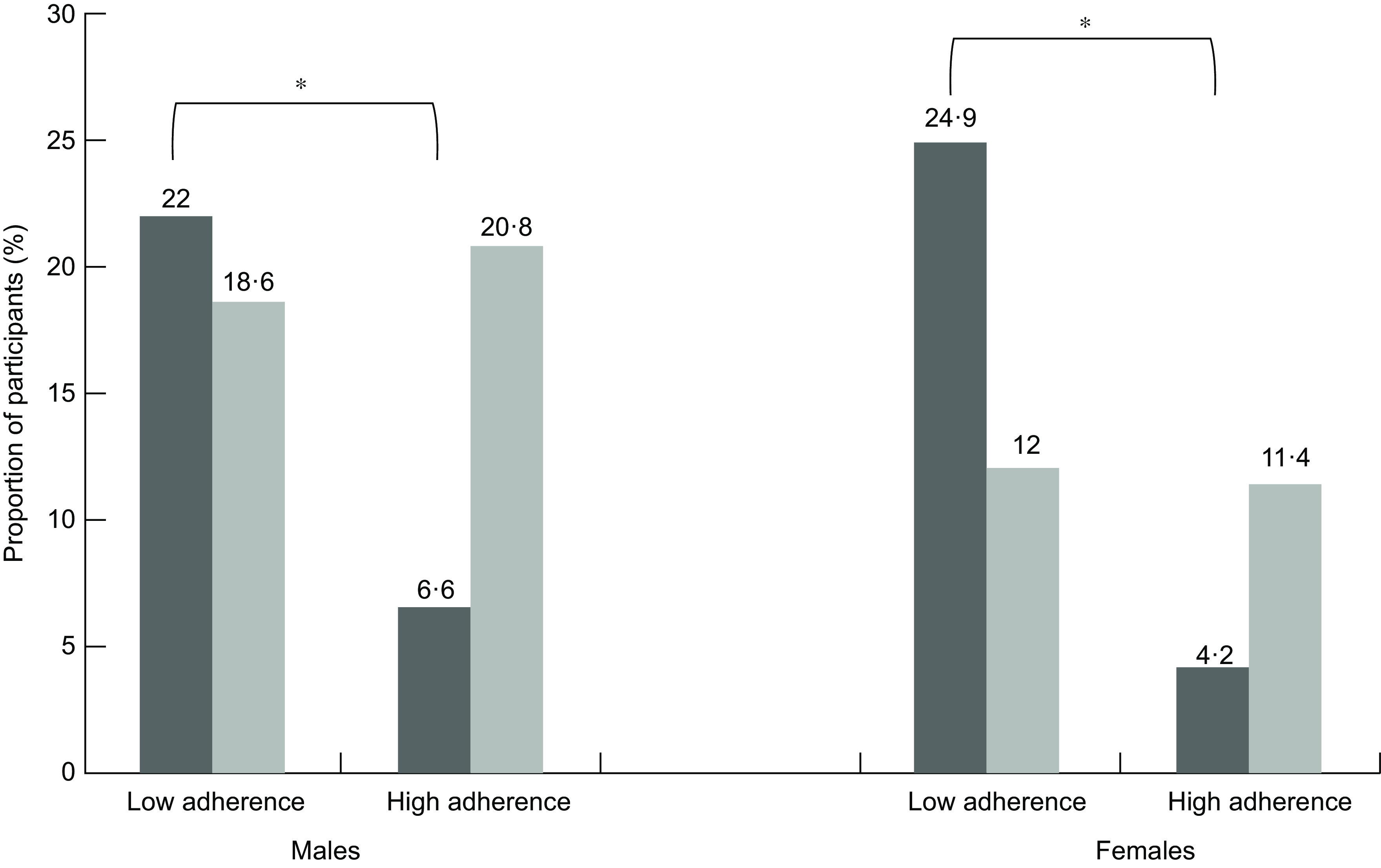
Comparisons of the proportion of ATTICA study participants (n 2020) who developed 10-year CVD events according to their adherence to the Mediterranean and Dietary Approaches to Stop Hypertension (DASH) diets at baseline. At baseline, low (namely quartiles 1 and 2) and high (quartiles 3 and 4) adherence to the Mediterranean and DASH diets were assessed separately. According to the baseline level of adherence, the proportion of individuals with 10-year CVD events is depicted. As shown, both males and females with higher baseline adherence to the Mediterranean diet were significantly less likely to develop 10-year CVD events (P < 0·0001). Such an association was not sustained when evaluated according to baseline adherence to the DASH diet among either males (P = 0·599) or females (P = 0·086). *P < 0·0001.  , Mediterranean diet;
, Mediterranean diet;  , DASH diet
, DASH diet
Table 3.
Baseline characteristics of dietary intake based on the Mediterranean and Dietary Approaches to Stop Hypertension (DASH) diets (expressed in quartiles of diet scores) among ATTICA study participants in relation to the 10-year fatal or non-fatal incidence of CVD, according to gender*
| CVD-free events | CVD events | P | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Overall (n 2020) | |||||
| Mediterranean diet score | <0·0001 | ||||
| First quartile (scores <25·0) | 337 | 19·8 | 168 | 53·0 | |
| Second quartile (scores 25–26·9) | 606 | 35·6 | 112 | 35·3 | |
| Third quartile (scores 27·0–27·9) | 286 | 16·8 | 22 | 6·9 | |
| Fourth quartile (scores 28·0–55·0) | 474 | 27·8 | 15 | 4·7 | |
| DASH score | 0·842 | ||||
| First quartile (scores <23·9) | 457 | 26·8 | 79 | 24·9 | |
| Second quartile (scores 24·0–26·9) | 359 | 21·1 | 70 | 22·1 | |
| Third quartile (scores 27·0–30·9) | 471 | 27·7 | 93 | 29·3 | |
| Fourth quartile (scores 31·0–45·0) | 416 | 24·4 | 75 | 23·7 | |
| Males (n 1006) | |||||
| Mediterranean diet score | <0·0001 | ||||
| First quartile (scores <25·0) | 242 | 30·0 | 115 | 58·1 | |
| Second quartile (scores 25–26·9) | 424 | 52·5 | 73 | 36·9 | |
| Third quartile (scores 27·0–27·9) | 103 | 12·7 | 6 | 3·0 | |
| Fourth Quartile (scores 28·0–55·0) | 39 | 4·8 | 4 | 2·0 | |
| DASH score | 0·599 | ||||
| First quartile (scores <23·9) | 216 | 26·7 | 54 | 27·3 | |
| Second quartile (scores 24·0–26·9) | 191 | 23·6 | 39 | 19·7 | |
| Third quartile (scores 27·0–30·9) | 220 | 27·2 | 54 | 27·3 | |
| Fourth quartile (scores 31·0–45·0) | 181 | 22·4 | 51 | 25·8 | |
| Females (n 1014) | |||||
| Mediterranean diet score | <0·0001 | ||||
| First quartile (scores <25·0) | 95 | 10·6 | 53 | 44·5 | |
| Second quartile (scores 25–26·9) | 182 | 20·3 | 39 | 32·8 | |
| Third quartile (scores 27·0–27·9) | 183 | 20·4 | 16 | 13·4 | |
| Fourth quartile (scores 28·0–55·0) | 435 | 48·6 | 11 | 9·2 | |
| DASH score | 0·086 | ||||
| First quartile (scores <23·9) | 241 | 26·9 | 25 | 21·0 | |
| Second quartile (scores 24·0–26·9) | 168 | 18·8 | 31 | 26·1 | |
| Third quartile (scores 27·0–30·9) | 251 | 28·0 | 39 | 32·8 | |
| Fourth quartile (scores 31·0–45·0) | 235 | 26·3 | 24 | 20·2 | |
Categorical variables are expressed as absolute and relative frequencies (n and %).
P-values refer to differences between CVD events and CVD-free events during the 10-year follow-up, derived using the likelihood-ratio test.
Table 4.
Unadjusted and adjusted hazard ratios (HR, 95 % CI) of Mediterranean and Dietary Approaches to Stop Hypertension (DASH) diet quartile scores in relation to 10-year incidence of fatal and non-fatal CVD events (outcome) among ATTICA study participants*
| Unadjusted HR | 95 % CI | Age-adjusted HR | 95 % CI | Age- and gender- adjusted HR | 95 % CI | Fully adjusted HR† | 95 % CI | |
|---|---|---|---|---|---|---|---|---|
| Mediterranean diet score | ||||||||
| First quartile (scores <25·0) | 1·00 | 1·00 | 1·00 | 1·00 | ||||
| Second quartile (scores 25–26·9) | 2·70 | 2·05, 3·55 | 1·57 | 1·08, 2·02 | 1·48 | 1·08, 2·02 | 1·44 | 0·98, 2·11 |
| Third quartile (scores 27·0–27·9) | 6·48 | 4·04, 10·38 | 2·61 | 1·55, 4·41 | 2·06 | 1·14, 3·72 | 1·92 | 0·92, 3·98 |
| Fourth quartile (scores 28·0–55·0) | 15·75 | 9·12, 27·20 | 3·85 | 2·04, 7·26 | 2·91 | 1·44, 5·85 | 4·52 | 1·76, 11·63 |
| DASH score | ||||||||
| First quartile (scores <23·9) | 1·00 | 1·00 | 1·00 | 1·00 | ||||
| Second quartile (scores 24·0–26·9) | 0·89 | 0·63, 1·26 | 0·87 | 0·59, 1·28 | 0·87 | 0·59, 1·29 | 0·88 | 0·59, 1·33 |
| Third quartile (scores 27·0–30·9) | 0·88 | 0·63, 1·21 | 0·93 | 0·65, 1·34 | 0·91 | 0·64, 1·31 | 1·02 | 0·69, 1·50 |
| Fourth quartile (scores 31·0–45·0) | 0·96 | 0·68, 1·35 | 0·98 | 0·67, 1·42 | 0·97 | 0·66, 1·42 | 1·05 | 0·69, 1·60 |
HR, hazard ratios.
All comparisons are conducted in relation to the corresponding first quartile score.
Adjusted HR and 95 % CI: adjusted HR for age, gender, physical activity, educational status, weight category, smoking status, hypertension, hypercholesterolaemia, diabetes and the metabolic syndrome at baseline.
Time to fatal and non-fatal CVD events according to baseline Mediterranean diet and Dietary Approaches to Stop Hypertension diet scores
Figure 2 presents the Kaplan–Meier curve for differences in the probability and time to developing combined (fatal or non-fatal) CVD events, according to baseline quartile consumption levels of the MedDietScore and DASH diet scores. As illustrated, individuals within the highest quartile of the MedDietScore were significantly less likely than their lowest quartile counterparts to subsequently develop CVD events (Log-rank test P < 0·001). In contrast, when assessed according to baseline DASH diet scores, no significant associations were detected between the corresponding lowest and highest quartiles of consumption in relation to 10-year CVD (Log-rank test P = 0·788).
Fig. 2.
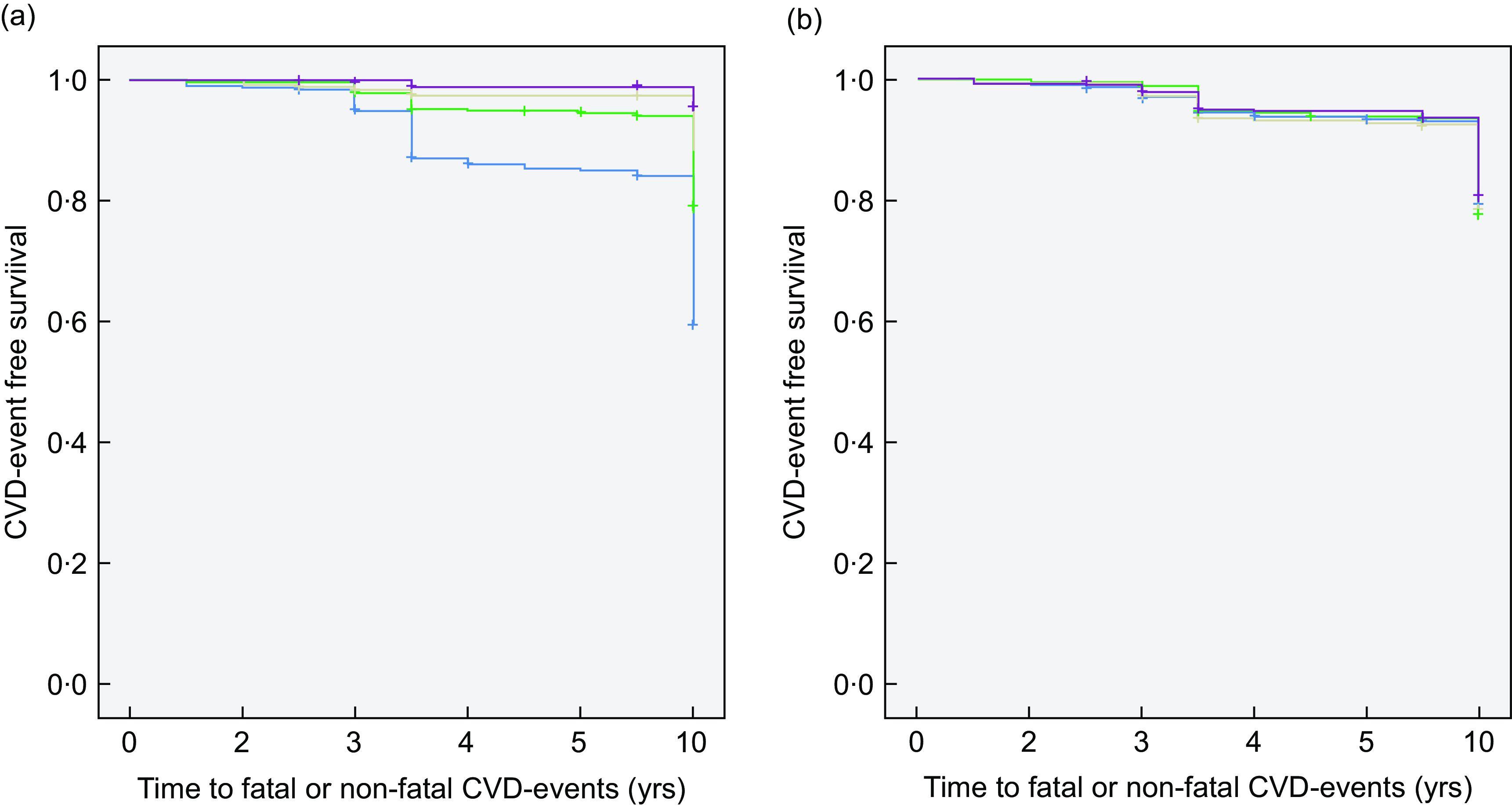
Kaplan–Meier curves for depicting the probability and time to occurrence of combined (fatal or non-fatal) CVD events according to the Mediterranean diet and Dietary Approaches to Stop Hypertension (DASH) diet scores at baseline in the ATTICA study population (n 2020). Individuals with higher baseline Mediterranean diet quartile scores were significantly less likely to develop 10-year CVD events ((a) Log-rank test P < 0·0001;  , quartile 1 (scores <25·0);
, quartile 1 (scores <25·0);  , quartile 2 (scores 25·0–26·9);
, quartile 2 (scores 25·0–26·9);  , quartile 3 (scores 27·0–27·9);
, quartile 3 (scores 27·0–27·9);  , quartile 4 (scores 28·0–55·0)). This observed association was not sustained when evaluated according to baseline DASH diet quartile scores ((b) Log-rank test P = 0·788;
, quartile 4 (scores 28·0–55·0)). This observed association was not sustained when evaluated according to baseline DASH diet quartile scores ((b) Log-rank test P = 0·788;  , quartile 1 (scores <24·0);
, quartile 1 (scores <24·0);  , quartile 2 (scores 24·0–26·9);
, quartile 2 (scores 24·0–26·9);  , quartile 3 (scores 27·0–30·9);
, quartile 3 (scores 27·0–30·9);  , quartile 4 (scores 31·0–45·0))
, quartile 4 (scores 31·0–45·0))
Risk of 10-year fatal and non-fatal CVD events according to the Mediterranean diet and Dietary Approaches to Stop Hypertension diet scores
As shown in Table 4, following the adjustment for several known confounding factors (including age, gender, physical activity, weight category, smoking, hypertension, hypercholesterolaemia, diabetes and the metabolic syndrome), as compared with individuals within the lowest quartile of MedDietScore consumption, those within the highest quartile of consumption had a 4-fold risk to not develop 10-year fatal or non-fatal CVD events (adjusted HR 4·52, 95 % CI 1·76, 11·63). It is of interest that although it was initially observed that persons within the third quartile of the MedDietScore consumption revealed an approximately 2-fold age- and gender-adjusted differential risk for developing 10-year CVD events, this association was not sustained following comprehensive adjustment for all of the aforementioned demographic, lifestyle and clinical confounding factors (adjusted HR 1·92, 95 % CI 0·92, 3·98). Finally, it is noteworthy that when assessed according to baseline DASH diet score, individuals with highest quartile scores did not significantly differ from their lowest quartile counterparts in developing 10-year combined fatal and non-fatal CVD events (adjusted HR 1·05, 95 % CI 0·69, 1·60).
Discussion
With an ever-ageing population in developed countries, the elucidation of the most effective dietary pattern for preventing CVD is an emerging public health priority for securing both citizens’ quality of life and concomitantly minimising healthcare-associated costs(31). Such evidence is anticipated to best inform optimal and effective public health strategies(32) for deterring CVD and, ultimately, diminishing attributable hospitalisation costs. The current study compared the efficacy of the Mediterranean and DASH dietary patterns in deterring 10-year fatal and non-fatal CVD events in a Mediterranean adult population. The main study findings revealed that one-third of individuals at the lowest quartile of Mediterranean diet consumption at baseline (as compared with 3 % of those at the highest quartile) developed 10-year fatal or non-fatal CVD events. Furthermore, with regard to time to CVD events, individuals within the highest quartile of the MedDietScore were significantly less likely than their lowest quartile counterparts to subsequently develop CVD events. In contrast, when assessed according to the DASH diet, individuals in the lowest and highest quartiles of consumption exhibited similar rates of manifesting 10-year CVD. Moreover, no significant associations were detected between the corresponding lowest and highest quartiles of DASH consumption in relation to time to occurrence of CVD events. Following the adjustment for several known confounding factors, those within the highest quartile of consumption were in excess of 4-fold more likely to not develop 10-year fatal or non-fatal CVD events. When assessed according to baseline DASH diet score, individuals with highest quartile scores did not significantly differ from their lowest quartile counterparts in developing 10-year combined fatal and non-fatal CVD events. Hence, in the Mediterranean population examined, high adherence to the Mediterranean diet, and not the DASH diet, was associated with a reduction in the frequency and time to occurrence of 10-year fatal and non-fatal CVD. Therefore, the study findings support that in similar populations and settings, public health interventions aimed at enhancing adherence to the Mediterranean diet, rather than the DASH diet, may most effectively deter long-term CVD outcomes.
There exists mounting evidence regarding the cardioprotective effects of adhering to the Mediterranean diet in populations residing both within its indigenous region and beyond(33–35). Similarly, the DASH diet was originally developed and further adopted for deterring CVD, particularly in Western (i.e. non-Mediterranean) populations(7). In the present investigation regarding the comparison of the adoption of these diets in a Mediterranean population in relation to long-term (namely 10-year) fatal and non-fatal CVD outcomes, high adherence to the Mediterranean diet apparently deterred adverse health outcomes. In contrast, adoption of the DASH diet did not apparently protect participants from the manifestation of long-term CVD.
The DASH diet, as originally conceived for non-Mediterranean Western populations, is primarily composed of lean meats, low-fat dairy products and whole grains, including an abundance of fruits and vegetables(20,21). It entails a dietary intake low in total fat (around 27 % of daily energy intake), saturated fats and cholesterol, nevertheless rich in fibre and micronutrients (namely Ca, Mg and K), which is pivotal for maintaining and/or achieving normotension, particularly in individuals with high blood pressure(36), and preventing hypertension(37). It has been shown that adherence to the DASH diet ultimately deters CVD, since such dietary practices are associated with diminished CVD incidence and attributable mortality rates(7). Recent meta-analytic findings arising from prospective international cohort studies(38) show that high adherence to the DASH diet, as evident by comparing individuals of the highest v. lowest intake categories, results in a 20 % reduction in CVD occurrence(7), as well as mortality(38). In addition, findings arising from the Atherosclerosis Risk in Communities Study in the USA entailing an extensive 25-year follow-up period reveal that, as compared with their counterparts with the lowest quintiles of dietary intakes, adults aged 45–64 years with the highest quintiles of either the alternative Mediterranean diet or DASH diet had significantly lower risks, of comparable magnitudes, of incident CVD and attributable mortality(39). However, the above findings are limited to Western populations where the Mediterranean diet does not prevail, and as of such the alternative Mediterranean diet is adopted and evaluated instead.
The current study confirms previous reports demonstrating that adherence to the DASH diet is not associated with diminished rates of CVD incidence(4) and/or mortality(4,40). These findings have been primarily confirmed not only in Mediterranean populations but also in Western populations which are not indigenous to the Mediterranean region. Specifically, in postmenopausal women aged 55–69 years, adherence to the DASH diet was not associated with mortality attributable to CVD, including specifically CHD or stroke(41). In addition, findings arising from the Women’s Health Initiative displayed that the Mediterranean diet, as opposed to the DASH diet, was solely associated with the occurrence of sudden cardiac death in postmenopausal women(42). Furthermore, among older adults participating in the Cardiovascular Health Study and evaluated over 21·5 years, adherence to the DASH diet was not associated with the occurrence of heart failure(43). As of such, there is mounting evidence both within and beyond Mediterranean populations that the adoption of the DASH diet may deter hypertension, albeit without conferring protection from long-term adverse CVD outcomes and mortality rates(44).
Several plausible hypotheses exist for explicating why adoption of the Mediterranean diet, as opposed to the DASH diet, may incur enhanced protection from long-term adverse CVD outcomes.
First, due to its inherent nature and design, it is upheld that the DASH diet is most likely to have greatest blood pressure lowering effects among individuals with hypertension(45) and/or other adverse metabolic profiles associated with CVD(27), posing them at baseline at greater risk for developing CVD outcomes and hence biasing study findings towards the null hypothesis. Second, as also entailed in the current study, the methods employed for assessing Na intake often lack accuracy since Na intake, which is a crucial component of the DASH dietary pattern, is often not well characterised by an FFQ(27,42). Since the reduction in Na intake induces blood pressure lowering effects particularly among hypertensive individuals(46), study findings may be further biased in favour of the null hypothesis. Nevertheless, withstanding the aforementioned considerations regarding the assessment of Na intake, adherence to the DASH diet has been shown to be associated with CVD outcomes, including coronary artery disease(47), stroke(48,49), and overall with a wide array of non-Mediterranean populations. Even so, it is upheld that beyond nutrient intake, the Mediterranean diet confers additional widespread attributes for preventing CVD. Particularly in Mediterranean settings, this dietary pattern is associated with the daily adoption of a constellation of lifestyle practices, such as moderate body weight, physical activity and active social networks which enhance psychosocial and cognitive function, including social interaction, participation in leisure activities and physical activities and sleep quality(50,51). A concomitant lack of adherence to the Mediterranean diet and physical activity leads to overweight/obesity and ultimately CVD. Overweight and obesity(52), as well as waist circumference and central obesity(53), have been previously documented as risk factors for CVD as they increase adipokine- and leptin-mediated adrenergic tone and trigger dysregulation of the renin–angiotensin–aldosterone system(6). Central obesity and non-adherence to the Mediterranean diet are associated with a chronic inflammation(54) and CVD-related inflammatory markers(55), respectively. While the exact underlying beneficial mechanisms by which social networks additionally drive this interplay remain to be elucidated(56,57), given the aforementioned positive impacts of the above constellation of lifestyle factors on CVD health(58), the above cumulatively emphasise the importance of adopting a comprehensive lifestyle, rather than solely a dietary pattern, which may comprehensively prevent the onset and further progression of CVD.
Collectively, the current study findings reveal that targeted primary and secondary prevention strategies for deterring CVD are hence most likely favoured through the recommended adoption of the Mediterranean diet, rather than DASH diet, particularly in Mediterranean populations. Such streamlined strategies would likely benefit most if a global approach, encompassing both dietary and lifestyle modifications as entailed in the Mediterranean diet, was adopted. Ultimately, particularly in Mediterranean populations, such preemptive interventions may diminish the disease burden and associated healthcare costs attributable to CVD.
Strengths and limitations
The study strengths include the prospective cohort study design employed among a representative randomly selected, population-based sample residing in the most densely populated urban district of Greece, wherein high background prevalence rates of CVD have been documented(59,60). The duration of follow-up extended 10 years, allowing for sufficient evaluation of the outcomes of interest, while simultaneously deterring a misclassification bias secondary to disease latency. Even so, the study limitations include that the study shares all the limitations associated with observational investigations encompassing single baseline measurements. Specifically, while adherence to the Mediterranean diet (MedDietScore) and DASH diets was measured, either changes in dietary patterns over time and/or complete dietary analysis for nutrient components was not evaluated within the context of the current investigation. Even so, due to the extended follow-up period entailed, it is upheld that dietary patterns more accurately predict CVD risk as they provide a more comprehensive understanding of how dietary factors cumulatively affect the risk of disease. Finally, initiation of pharmaceutical treatment was not evaluated within the context of the current analysis, as could potentially mediate CVD outcomes. However, since fatal and non-fatal CVD events were assessed as a combined outcome, this limitation was overcome. Moreover, it is upheld that such treatment effects would only bias towards the null hypothesis, and as of such our findings are an underestimation of true effect sizes.
Conclusions
Since particularly Mediterranean populations may benefit most from adhering to the Mediterranean, rather than DASH, diet for preventing 10-year fatal and non-fatal CVD, the implementation of corresponding streamlined public health nutritional interventions may deter both CVD and related adverse health outcomes. Further investigations are necessary to provide insights regarding the potential added value of implementing such an approach in other non-Mediterranean populations.
Acknowledgements
Acknowledgements: The authors would like to thank the field investigators of the study: M. Toutouza (biochemical evaluation), I. Papaioannou (physical examination), E. Tsetsekou (physical examination), A. Zeimbekis (physical examination), K. Masoura (physical examination), A. Katinioti (physical examination), S. Vellas (physical examination), E. Kambaxis (nutritional evaluation), K. Paliou (nutritional evaluation), C. Tselika (technical support), S. Poulopoulou (technical support), M. Koukoura (technical support), K. Vassiliadou (genetic evaluation) and M. Toutouza (data management). Financial support: The Hellenic Cardiology Society, the Hellenic Atherosclerosis Society, the Graduate Program in Applied Nutrition and Dietetics of Harokopio University and the Coca-Cola SA funded the current study by research grants (KE252/ELKE/HUA). The ATTICA Study is funded by research grants from the Hellenic Society of Cardiology (grant – 1, 2002). Conflicts of interest: There are no conflicts of interest. Authorship: D.B.P. and E.C. formulated the research questions. M.D.K., C.C., C.P. and D.B.P. contributed to the design of the study. M.D.K., C.C., D.T., C.P. and D.B.P. carried out the study. E.C., M.D.K. and D.B.P. analysed the data and interpreted findings. Additionally, C.C., D.T. and C.P. contributed to the interpretation of findings. E.C., M.D.K. and D.B.P. drafted the manuscript, while C.C., D.T. and C.P. revised it critically for important intellectual content. Ethics of human subject participation: The current study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving study participants were approved by the Medical Research Ethics Committee of the University of Athens Medical School. Written informed consent was obtained from all subjects/patients.
References
- 1. Wall HK, Ritchey MD, Gillespie C et al. (2018) Vital signs: prevalence of key cardiovascular disease risk factors for million hearts 2022 – United States, 2011–2016. MMWR Morb Mortal Wkly Rep 67, 983–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ritchey MD, Wall HK, Gillespie C et al. (2014) Million hearts: prevalence of leading cardiovascular disease risk factors: United States, 2005–2012. MMWR Morb Mortal Wkly Rep 63, 462–467. [PMC free article] [PubMed] [Google Scholar]
- 3. Okosun IS, Boltri JM, Anochie LK et al. (2004) Racial/ethnic differences in prehypertension in American adults: population and relative attributable risks of abdominal obesity. J Hum Hypertens 18, 849–855. [DOI] [PubMed] [Google Scholar]
- 4. Khosravi A, Gharipour M, Nezafati P et al. (2017) Pre-hypertension, pre-diabetes or both: which is best at predicting cardiovascular events in the long term? J Hum Hypertens 31, 382–387. [DOI] [PubMed] [Google Scholar]
- 5. Lenfant C, Chobanian AV, Jones DW et al. (2003) Seventh report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7): resetting the hypertension sails. Hypertension 41, 1178–1179. [DOI] [PubMed] [Google Scholar]
- 6. Kachur S, Morera R, De Schutter A et al. (2018) Cardiovascular risk in patients with prehypertension and the metabolic syndrome. Cur Hypertens Rep 20, 15. [DOI] [PubMed] [Google Scholar]
- 7. Schwingshackl L, Bogensberger B & Hoffmann G (2018) Diet quality as assessed by the healthy eating index, alternate healthy eating index, dietary approaches to stop hypertension score, and health outcomes: an updated systematic review and meta-analysis of cohort studies. J Acad Nutr Diet 118, 74–100 e111. [DOI] [PubMed] [Google Scholar]
- 8. Sanches Machado d’Almeida K, Ronchi Spillere S, Zuchinali P et al. (2018) Mediterranean diet and other dietary patterns in primary prevention of heart failure and changes in cardiac function markers: a systematic review. Nutrients 10, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Willcox DC, Scapagnini G & Willcox BJ (2014) Healthy aging diets other than the Mediterranean: a focus on the Okinawan diet. Mech Ageing Dev 136–137, 148–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Trichopoulou A & Critselis E (2004) Mediterranean diet and longevity. Eur J Cancer Prev 13, 453–456. [DOI] [PubMed] [Google Scholar]
- 11. Willett WC, Sacks F, Trichopoulou A et al. (1995) Mediterranean diet pyramid: a cultural model for healthy eating. Am J Clin Nutr 61, 1402S–1406S. [DOI] [PubMed] [Google Scholar]
- 12. Grosso G, Marventano S, Yang J et al. (2017) A comprehensive meta-analysis on evidence of Mediterranean diet and cardiovascular disease: are individual components equal? Crit Rev Food Sci Nutr 57, 3218–3232. [DOI] [PubMed] [Google Scholar]
- 13. Di Daniele N, Noce A, Vidiri MF et al. (2017) Impact of Mediterranean diet on metabolic syndrome, cancer and longevity. Oncotarget 8, 8947–8979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rebelo-Marques A, De Sousa Lages A, Andrade R et al. (2018) Aging Hallmarks: the benefits of physical exercise. Front Endocrinol 9, 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Garatachea N, Pareja-Galeano H, Sanchis-Gomar F et al. (2015) Exercise attenuates the major hallmarks of aging. Rejuvenation Res 18, 57–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Michan S (2014) Calorie restriction and NAD(+)/sirtuin counteract the hallmarks of aging. Front Biosci (Landmark Ed) 19, 1300–1319. [DOI] [PubMed] [Google Scholar]
- 17. Perez LM, Pareja-Galeano H, Sanchis-Gomar F et al. (2016) ‘Adipaging’: ageing and obesity share biological hallmarks related to a dysfunctional adipose tissue. J Physiol 594, 3187–3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fernandez del Rio L, Gutierrez-Casado E, Varela-Lopez A et al. (2016) Olive oil and the Hallmarks of aging. Molecules 21, 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Critselis E & Panagiotakos D (2019) Adherence to the Mediterranean diet and healthy ageing: current evidence, biological pathways, and future directions. Crit Rev Food Sci Nutr 60, 2148–2157. [DOI] [PubMed] [Google Scholar]
- 20. Sacks FM (1995) Dietary approaches to stop hypertension. Ann Epidemiol 5, 502. [DOI] [PubMed] [Google Scholar]
- 21. Sacks FM, Obarzanek E, Windhauser MM et al. (1995) Rationale and design of the Dietary Approaches to Stop Hypertension trial (DASH). A multicenter controlled-feeding study of dietary patterns to lower blood pressure. Ann Epidemiol 5, 108–118. [DOI] [PubMed] [Google Scholar]
- 22. Ferreira AS (2015) Immunity, inflammation, and prehypertension: in what order? J Clin Hypertens (Greenwich) 17, 775–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Panagiotakos DB, Georgousopoulou EN, Pitsavos C et al. (2015) Ten-year (2002–2012) cardiovascular disease incidence and all-cause mortality, in urban Greek population: the ATTICA Study. Int J Cardiol 180, 178–184. [DOI] [PubMed] [Google Scholar]
- 24. Katsouyanni K, Rimm EB, Gnardellis C et al. (1997) Reproducibility and relative validity of an extensive semi-quantitative food frequency questionnaire using dietary records and biochemical markers among Greek schoolteachers. Int J Epidemiol 26, Suppl. 1, S118–S127. [DOI] [PubMed] [Google Scholar]
- 25. Panagiotakos DB, Pitsavos C & Stefanadis C (2006) Dietary patterns: a Mediterranean diet score and its relation to clinical and biological markers of cardiovascular disease risk. Nutr Metab Cardiovasc Dis 16, 559–568. [DOI] [PubMed] [Google Scholar]
- 26. Fung TT, Chiuve SE, McCullough ML et al. (2008) Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med 168, 713–720. [DOI] [PubMed] [Google Scholar]
- 27. Bathrellou E, Kontogianni MD, Chrysanthopoulou E et al. (2019) Adherence to a DASH-style diet and cardiovascular disease risk: the 10-year follow-up of the ATTICA study. Nutr Health 25, 225–230. [DOI] [PubMed] [Google Scholar]
- 28. Papathanasiou G, Georgoudis G, Papandreou M et al. (2009) Reliability measures of the short International Physical Activity Questionnaire (IPAQ) in Greek young adults. Hell J Cardiol 50, 283–294. [PubMed] [Google Scholar]
- 29. Lipsy RJ (2003) The national cholesterol education program adult treatment panel III guidelines. J Manag Care Pharm 9, 2–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.(2008) Population Attributable Risk (PAR). In Encyclopedia of Public Health, pp. 1131–1132 [Kirch W, editor]. Dordrecht: Springer. [Google Scholar]
- 31. Panagiotakos D, Sitara M, Pitsavos C et al. (2007) Estimating the 10-year risk of cardiovascular disease and its economic consequences, by the level of adherence to the Mediterranean diet: the ATTICA study. J Med Food 10, 239–243. [DOI] [PubMed] [Google Scholar]
- 32. Chang AY, Skirbekk VF, Tyrovolas S et al. (2019) Measuring population ageing: an analysis of the global burden of disease study 2017. Lancet Public Health 4, e159–e167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Razquin C & Martinez-Gonzalez MA (2019) A traditional Mediterranean diet effectively reduces inflammation and improves cardiovascular health. Nutrients 11, 1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen GC, Neelakantan N, Martin-Calvo N et al. (2019) Adherence to the Mediterranean diet and risk of stroke and stroke subtypes. Europ J Epidemiol 34, 337–349. [DOI] [PubMed] [Google Scholar]
- 35. Martinez-Gonzalez MA, Gea A & Ruiz-Canela M (2019) The Mediterranean diet and cardiovascular health. Circ Res 124, 779–798. [DOI] [PubMed] [Google Scholar]
- 36. Appel LJ, Champagne CM, Harsha DW et al. (2003) Effects of comprehensive lifestyle modification on blood pressure control: main results of the PREMIER clinical trial. JAMA 289, 2083–2093. [DOI] [PubMed] [Google Scholar]
- 37. Whelton PK, Carey RM, Aronow WS et al. (2018) 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation 138, e484–e594. [DOI] [PubMed] [Google Scholar]
- 38. Schwingshackl L & Hoffmann G (2015) Diet quality as assessed by the healthy eating index, the alternate healthy eating index, the dietary approaches to stop hypertension score, and health outcomes: a systematic review and meta-analysis of cohort studies. J Acad Nutr Diet 115, 780–800 e785. [DOI] [PubMed] [Google Scholar]
- 39. Hu EA, Steffen LM, Coresh J et al. (2019) Adherence to the healthy eating index-2015 and other dietary patterns may reduce risk of cardiovascular disease, cardiovascular mortality, and all-cause mortality. J Nutr 150, 312–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mokhtari Z, Sharafkhah M, Poustchi H et al. (2019) Adherence to the dietary approaches to stop hypertension (DASH) diet and risk of total and cause-specific mortality: results from the Golestan cohort study. Int J Epidemiol 48, 1824–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Folsom AR, Parker ED & Harnack LJ (2007) Degree of concordance with DASH diet guidelines and incidence of hypertension and fatal cardiovascular disease. Am J Hypertens 20, 225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bertoia ML, Triche EW, Michaud DS et al. (2014) Mediterranean and dietary approaches to stop hypertension dietary patterns and risk of sudden cardiac death in postmenopausal women. Am J Clin Nutr 99, 344–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Del Gobbo LC, Kalantarian S, Imamura F et al. (2015) Contribution of major lifestyle risk factors for incident heart failure in older adults: the cardiovascular health study. JACC Heart Fail 3, 520–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bloch MJ (2017) The Dietary Approaches to Stop Hypertension (DASH) diet-promise unmet. J Am Soc Hypertens 11, 323–324. [DOI] [PubMed] [Google Scholar]
- 45. Appel LJ, Moore TJ, Obarzanek E et al. (1997) A clinical trial of the effects of dietary patterns on blood pressure: DASH collaborative research group. New Engl J Med 336, 1117–1124. [DOI] [PubMed] [Google Scholar]
- 46. Sacks FM, Svetkey LP, Vollmer WM et al. (2001) Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet: DASH-sodium collaborative research group. New Engl J Med 344, 3–10. [DOI] [PubMed] [Google Scholar]
- 47. Djousse L, Ho YL, Nguyen XT et al. (2018) DASH score and subsequent risk of coronary artery disease: the findings from million veteran program. J Am Heart Assoc 7, e008089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Larsson SC, Wallin A & Wolk A (2016) Dietary approaches to stop hypertension diet and incidence of stroke: results from 2 prospective cohorts. Stroke 47, 986–990. [DOI] [PubMed] [Google Scholar]
- 49. Lin PH, Yeh WT, Svetkey LP et al. (2013) Dietary intakes consistent with the DASH dietary pattern reduce blood pressure increase with age and risk for stroke in a Chinese population. Asia Pac J Clin Nutr 22, 482–491. [PubMed] [Google Scholar]
- 50. Yannakoulia M, Kontogianni M & Scarmeas N (2015) Cognitive health and Mediterranean diet: just diet or lifestyle pattern? Ageing Res Rev 20, 74–78. [DOI] [PubMed] [Google Scholar]
- 51. Feart C, Samieri C & Barberger-Gateau P (2015) Mediterranean diet and cognitive health: an update of available knowledge. Curr Opin Clin Nutr Metab Care 18, 51–62. [DOI] [PubMed] [Google Scholar]
- 52. Booth JN 3rd, Li J, Zhang L et al. (2017) Trends in prehypertension and hypertension risk factors in US adults: 1999–2012. Hypertension 70, 275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Meng XJ, Dong GH, Wang D et al. (2012) Epidemiology of prehypertension and associated risk factors in urban adults from 33 communities in China: the CHPSNE study. Circ J 76, 900–906. [DOI] [PubMed] [Google Scholar]
- 54. Festa A, D’Agostino R Jr, Williams K et al. (2001) The relation of body fat mass and distribution to markers of chronic inflammation. Int J Obes Relat Metab Disord 25, 1407–1415. [DOI] [PubMed] [Google Scholar]
- 55. Chrysohoou C, Pitsavos C, Panagiotakos DB et al. (2004) Association between prehypertension status and inflammatory markers related to atherosclerotic disease: the ATTICA Study. Am J Hypertens 17, 568–573. [DOI] [PubMed] [Google Scholar]
- 56. Anastasiou CA, Yannakoulia M, Kosmidis MH et al. (2017) Mediterranean diet and cognitive health: initial results from the Hellenic Longitudinal Investigation of Ageing and Diet. PLoS One 12, e0182048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wade AT, Davis CR, Dyer KA et al. (2017) A Mediterranean diet to improve cardiovascular and cognitive health: protocol for a randomised controlled Intervention study. Nutrients 9, 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wahid A, Manek N, Nichols M et al. (2016) Quantifying the association between physical activity and cardiovascular disease and diabetes: a systematic review and meta-analysis. J Am Heart Assoc 5, e002495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Aunan JR, Watson MM, Hagland HR et al. (2016) Molecular and biological hallmarks of ageing. Br J Surg 103, e29–e46. [DOI] [PubMed] [Google Scholar]
- 60. Panagiotakos DB, Fitzgerald AP, Pitsavos C et al. (2007) Statistical modelling of 10-year fatal cardiovascular disease risk in Greece: the HellenicSCORE (a calibration of the ESC SCORE project). Hell J Cardiol 48, 55–63. [PubMed] [Google Scholar]


