Abstract
Objective:
To propose cut-off points for the TAG–glucose (TyG) index in Brazilian children and evaluate the link to cardiometabolic risk.
Design:
A cross-sectional study with children from a municipality in Minas Gerais, Brazil. Anthropometric (weight, height, waist circumference and waist:height ratio), biochemical (lipid and glucose profile) and blood pressure (BP) tests were performed. Using the receiver operating characteristic curve, cut-off points for the TyG index were proposed according to sex using homoeostasis model of assessment – insulin resistance (IR) as the reference method.
Setting:
Viçosa, MG, Brazil.
Participants:
Children aged 4–9 years (n 515).
Results:
The TyG index cut-off points to identify the risk of IR were 7·9 and 8·1 for boys and girls, respectively. We observed that 48·7 % of the children had an increased TyG index. The increased TyG index was associated with overweight, total body and central fat, increased BP and altered lipid profile. Children with an increased TyG index had a higher accumulation of cardiometabolic risk factors.
Conclusions:
According to the cut-off points proposed by the current study, children at risk of IR estimated by the TyG index presented a higher cardiometabolic risk, including isolated risk factors, as to the higher accumulation of these.
Keywords: Child, Insulin resistance, Receiver operating characteristic curve, Obesity, Hypertension, Dyslipidaemias
Insulin resistance (IR) is the state in which tissues have reduced sensitivity to the metabolic actions of insulin, with decreased glucose uptake and, consequently, reduced muscle and adipose tissue utilisation, impairing suppression of IR(1–5). These metabolic changes increase the risk of chronic diseases during adulthood, such as type 2 diabetes mellitus, the metabolic syndrome and CVD. That being the case, the identification of IR at the earliest age has great clinical and epidemiological relevance(5,6).
In this context, direct diagnostic methods of IR, such as the gold standard technique of hyperinsulinaemic–euglycaemic glucose clamp, are costly, invasive and complex, with limited use in younger age groups(7). Because of the practicality and the large scale of studies demonstrating its validity, including in children, the most used indirect method is the homoeostasis model of assessment – insulin resistance (HOMA-IR), which uses insulin values and fasting glucose. However, the examination of insulin is still expensive in certain countries, making the method inaccessible(4).
Thus, some alternative methods, derived from simple and universally available tests, have been proposed to detect early IR in children of developing countries, thus preventing the onset of other chronic diseases(8). One of these emerging methods is the TAG–glucose (TyG) index, which is extensively researched in adults(4,8–14).
The TyG index has been useful in assessing the risk of IR in comparison with the gold standard (glucose clamp technique) and the HOMA-IR in adults(4,9,13,15,16). In addition, it functions as a risk marker for the development of type 2 diabetes mellitus, the metabolic syndrome and CVD, such as atherosclerosis in adults(10,15–17). In view of the above, the current study aimed to propose cut-off points for the TyG index in Brazilian children and to evaluate their association with cardiometabolic risk. Our hypothesis is that children with increased TyG index present higher prevalence of cardiometabolic risk factors, as well as greater accumulation of these.
Methodology
Population and study design
This is a cross-sectional study, with data from two surveys carried out with children aged 4–9 years, in the municipality of Viçosa, MG, Brazil in 2015 and 2016: The Lactation Support Program and the School Health Assessment Survey.
The first is a cross-sectional analysis of a retrospective cohort of the birth of children in the only maternity in the municipality of Viçosa, MG, who were monitored during their 1st year of life and evaluated between 4 and 7 years of age in the years 2015 and 2016. The information about the calculation of sample size, selection and data collection was previously published(18). The sample consisted of 141 children.
The second survey is School Health Assessment Survey, which is a cross-sectional study with a representative sample of 8- and 9-year-old children enrolled in seventeen public and seven private schools in the urban area of Viçosa, MG, in 2015, whose objective was to evaluate the cardiovascular health of these children. The information on the calculation and sample size, children’s selection and data collection was further detailed in other studies(19–21). The School Health Assessment Survey sample consisted of 376 children.
After the data collection, the power of the study was calculated in the OpenEpi online software (www.openepi.com), considering as an end point of the IR by TyG index between two groups: exposed (altered waist circumference (WC)) and not exposed (normal WC). Based on the average and sd of the TyG index group of children with a normal circumference (7·89 ± 0·38) and with an altered WC (8·13 ± 0·43), a sample size of 515 children with 100 % power was estimated at a significance level of 5 %.
Anthropometry and body composition
The anthropometric measures were performed by a member of the trained team (nutritionists), with the children without shoes, wearing light clothing and accompanied by their guardians. Height (with vertical stadiometer measured in centimetres and millimetres) and weight (in electronic digital scale with capacity for 150 kg and accuracy of 10 g) were measured for the calculation of BMI. The nutritional status was classified according to BMI for age in z-score, according to sex(22,23), and overweight and obesity were grouped as ‘overweight’ for purposes of analysis. The numbers for WC and waist:height ratio were classified according to cut-off points proposed by Filgueiras et al. (21).
Body composition was assessed by dual-energy X-ray absorptiometry. The children remained in supine position, and the rays were emitted and measured by an energy discriminating detector, while wearing light clothes, no earrings, a bracelet or any metal ornament. Body fat (%) and android fat (%), stratified by sex, higher than the 85th percentile of the sample, were considered excessive(21,24). All evaluations were performed at the Federal University of Viçosa Health Division.
Biochemical and blood pressure data
Blood samples were collected in children fasting for 12 h at the Clinical Analysis Laboratory of Federal University of Viçosa’s Health Division. Fasting serum glucose was performed by the glucose oxidase enzymatic method using the Cobas Mira Plus automation equipment (Roche Corp.) or by the enzymatic colorimetric method without deproteinisation (Bioclin). Fasting insulin was dosed by the electrochemiluminescence method or analysed in serum using Access Immunoassay Systems. TAG, total cholesterol (TC) and lipid fractions were evaluated (LDL-cholesterol, HDL-cholesterol and TAG). TC, HDL-cholesterol and TAG were measured by the enzymatic colorimetric method, with automation by a Cobas Mira Plus equipment (Roche Corp.), and LDL-cholesterol concentration was calculated by Friedwald’s ‘formula(25) in the Lactation Support Program study. In the School Health Assessment Survey study, TC, HDL-cholesterol and LDL-cholesterol were measured by the enzymatic colorimetric test (Bioclin) and analysed by the BS-200 apparatus (Bioclin). The ratios TC:HDL-cholesterol, LDL-cholesterol:HDL-cholesterol and TAG:HDL-cholesterol were also calculated.
The following values were deemed altered: fasting serum glucose ≥ 100 mg/dl, TC ≥ 170 mg/dl, LDL-cholesterol ≥ 110 mg/dl, HDL-cholesterol < 45 mg/dl and TAG ≥ 75 mg/dl(26,27). Due to the absence of cut-off points, values higher than the 85th percentile of the sample, according to sex and age, were considered increased for TC/HDL-cholesterol, LDL-cholesterol/HDL-cholesterol and TAG/HDL-cholesterol.
The children’s blood pressure (BP) was assessed and classified according to the protocol established by the VII Brazilian Guideline for Hypertension using an automatic BP monitor(28).
Insulin resistance
To assess the risk of IR, the TyG index was calculated using the formula: Ln(fasted TAG (mg/dl) × fasting serum glucose (mg/dl)/2) with values expressed in logarithmic scale(8). IR was also evaluated by HOMA-IR according to the formula: Fasting insulin (μU/ml) × fasting serum glucose (mmol/l)/22·5(29). HOMA-IR was categorised according to the 75th percentile of the sample, by sex, and was adopted as a reference to define the TyG index cut-off point(30) for the children in the current study.
Cardiometabolic risk factors
The number of cardiometabolic risk factors was obtained by the sum of the following present changes: overweight (overweight and obesity), body fat (%) > 85th percentile, increased WC and waist:height ratio, BP ≥ 90th percentile, fasting serum glucose ≥ 100 mg/dl; TC ≥ 170 mg/dl, LDL-cholesterol ≥ 110 gm/dl; HDL-cholesterol < 45 mg/dl, TAG ≥ 75 mg/dl/l, as well as android fat and increased TC:HDL-cholesterol, LDL-cholesterol:HDL-cholesterol and TAG:HDL-cholesterol ratios (>85th percentile, according to sex).
Statistical analyses
Statistical analyses were performed on STATA 13.0 and SPSS version 23.0 (SPSS Inc.), adopting a significance level of 5 % in all analyses. The Shapiro–Wilk normality test, histograms, kurtosis and asymmetry were used to evaluate the distribution of quantitative variables. The data’s descriptive analysis was performed through measures of frequency distribution, central tendency (average or median) and dispersion (sd or interquartile range).
To determine the TyG index cut-off point in predicting the risk of IR in children, we analysed the receiver operating characteristic, considering the cut-off point with the best balance between sensitivity and specificity values, according to sex. HOMA-IR was used as a reference method for the identification of the TyG index cut-off point. The analysis of the receiver operating characteristic curve and the identification of cut-off points were performed by MedCalc software version 9.4.2.0.
The sample was stratified according to the TyG index (normal and increased), according to the cut-off point identified by the receiver operating characteristic curve. The χ 2 test was used to compare the distribution of anthropometric, body composition, BP and biochemical variables among the groups according to the risk of IR by the TyG index. Student’s t test was used to compare the average number of cardiometabolic risk factors, according to the TyG index (normal and increased).
To verify the association between the risk of IR, defined by the TyG index, and the cardiometabolic risk factors, we performed Poisson regression models. Cardiometabolic risk factors were considered as independent variables and the increased TyG index as a dependent variable. The variables that were not categorised according to cut-off points specific for sex and age were also adjusted by sex and age. Variables with a lower level of significance P ≥ 0·05 were excluded one by one from the model until the definition of the final model. The Hosmer–Lemeshow test was used to verify the fit of the final model. The association measure used was the prevalence ratio and their respective 95 % CI.
Results
The sample consisted of children with a mean age of 7·84 ± 1·28 years, of which 50·3 % were girls. It was observed that girls had a higher percentage of high LDL-cholesterol (61·7 %), hypertriglyceridaemia (55·9 %) and a high TC:HDL-cholesterol ratio (60·5 %) when compared with boys (Table 1). The mean of TyG index was 7·98 ± 0·42 in the total sample. There was no statistical difference in TyG values between the sexes (P = 0·057).
Table 1.
Distribution of cardiometabolic risk factors in children aged 4–9 years, according to sex, Viçosa, MG, Brazil (2015/2016)
| Variables | All | Male | Female | P | ||
|---|---|---|---|---|---|---|
| n | n | % | n | % | ||
| Excess weight | ||||||
| Yes | 162 | 78 | 48·1 | 84 | 51·9 | 0·632 |
| No | 353 | 178 | 50·4 | 175 | 49·6 | |
| ↑ WC | ||||||
| Yes | 184 | 86 | 46·7 | 98 | 53·3 | 0·358 |
| No | 327 | 167 | 51·1 | 160 | 48·9 | |
| ↑ WtHR | ||||||
| Yes | 96 | 40 | 41·7 | 56 | 58·3 | 0·088 |
| No | 415 | 213 | 51·3 | 202 | 48·7 | |
| ↑ % Body fat | ||||||
| Yes | 80 | 40 | 50·0 | 40 | 50·0 | 0·940 |
| No | 434 | 215 | 49·5 | 219 | 50·5 | |
| ↑ % Android fat | ||||||
| Yes | 95 | 47 | 49·5 | 48 | 50·5 | 0·976 |
| No | 419 | 208 | 49·6 | 211 | 50·4 | |
| ↑ Blood pressure | ||||||
| Yes | 63 | 29 | 46·0 | 34 | 54·0 | 0·545 |
| No | 449 | 225 | 50·1 | 224 | 49·9 | |
| ↑ FSG | ||||||
| Yes | 8 | 4 | 50·0 | 4 | 50·0 | 0·631* |
| No | 507 | 252 | 49·7 | 255 | 50·3 | |
| ↑ Total cholesterol | ||||||
| Yes | 396 | 53 | 44·5 | 66 | 55·5 | 0·199 |
| No | 119 | 203 | 51·3 | 193 | 48·7 | |
| ↓ HDL-cholesterol | ||||||
| Yes | 160 | 75 | 46·9 | 85 | 53·1 | 0·388 |
| No | 355 | 181 | 51·0 | 174 | 49·0 | |
| ↑ LDL-cholesterol | ||||||
| Yes | 81 | 31 | 38·3 | 50 | 61·7 | 0·026† |
| No | 433 | 224 | 51·7 | 209 | 48·3 | |
| ↑ TAG | ||||||
| Yes | 222 | 98 | 44·1 | 124 | 55·9 | 0·029† |
| No | 293 | 158 | 53·9 | 135 | 46·1 | |
| ↑ TC/HDL-cholesterol | ||||||
| Yes | 56 | 21 | 37·5 | 35 | 62·3 | 0·053 |
| No | 459 | 235 | 51·2 | 224 | 48·8 | |
| ↑ LDL-cholesterol/HDL-cholesterol | ||||||
| Yes | 56 | 23 | 41·1 | 33 | 58·9 | 0·171 |
| No | 459 | 233 | 50·8 | 226 | 49·2 | |
| ↑ TAG/HDL-cholesterol | ||||||
| Yes | 86 | 34 | 39·5 | 52 | 60·5 | 0·039† |
| No | 429 | 222 | 51·7 | 207 | 48·3 | |
WC, waist circumference; WtHR, waist:height ratio; FSG, fasting serum glucose; TC, total cholesterol.
Fisher’s exact test.
χ 2 test (P < 0·05).
The cut-off points identified that depict the best balance between the values of sensitivity (S) and specificity (E) were 7·9 for boys and 8·1 for girls. These cut-offs presented satisfactory values of sensitivity and negative predictive values, with specificity values being moderate and the predictive values being low (Table 2). Considering these cut-off points, the prevalence of IR risk by the TyG index was 48·7 % (n 251). The prevalence of IR by HOMA-IR was 21·2 %.
Table 2.
AUC, cut-off points, sensitivity, specificity and predictive values of the TAG–glucose index to identify the risk of insulin resistance in children aged 4–9 years, Viçosa, MG, Brazil (2015/2016)
| AUC | 95 % CI | P | Cut-off point | S | E | PPV | PNV | |||
|---|---|---|---|---|---|---|---|---|---|---|
| All (n 515) | 0·717 | 0·676, 0·756 | <0·001 | 7·9 | 80 | 72·1, 86·5 | 55·3 | 50·2, 60·4 | 37·7 | 89·1 |
| Male (n 256) | 0·713 | 0·653, 0·768 | <0·001 | 7·9 | 78·1 | 66, 87·5 | 56·3 | 48·9, 63·4 | 37·3 | 88·5 |
| Female (n 259) | 0·722 | 0·666, 0·776 | <0·001 | 8·1 | 72·7 | 60·6, 83 | 65·3 | 58·1, 72 | 41·7 | 87·5 |
S, sensitivity; E, specificity; PPV, positive predictive value; PNV, negative predictive value.
Children with higher TYG index had an increased prevalence of total excess weight and central adiposity, hypertension and altered lipid profile (P ≤ 0·05) (Table 3).
Table 3.
Distribution of cardiometabolic risk factors, according to the TAG–glucose (TyG) index, in children aged 4–9 years, Viçosa, MG, Brazil (2015/2016)
| Variable | Normal TyG | High TyG* | |||
|---|---|---|---|---|---|
| n | % | N | % | P | |
| Excess weight | 59 | 36·4 | 103 | 63·6 | <0·001† |
| ↑ WC | 66 | 35·9 | 118 | 64·1 | <0·001† |
| ↑ WtHR | 36 | 37·5 | 60 | 62·5 | 0·003† |
| ↑ Body fat (%) | 26 | 32·5 | 54 | 67·5 | <0·001† |
| ↑ Android fat (%) | 26 | 27·4 | 69 | 72·6 | <0·001† |
| ↑ Blood pressure | 25 | 39·7 | 38 | 60·3 | 0·048† |
| ↑ TC | 45 | 37·8 | 74 | 62·6 | 0·001† |
| ↓ HDL-cholesterol | 65 | 40·6 | 95 | 59·4 | 0·001† |
| ↑ LDL-cholesterol | 29 | 35·8 | 52 | 64·2 | 0·003† |
| ↑ TC/HDL-cholesterol | 18 | 24·7 | 55 | 75·3 | <0·001† |
| ↑ LDL-cholesterol/HDL-cholesterol | 23 | 30·7 | 52 | 69·3 | <0·001† |
WC, waist circumference; WtHR, waist:height ratio; TC, total cholesterol.
Cut-off point proposed by the current study (male ≥ 7·9; female ≥ 8·1).
χ 2 test.
In the Poisson regression model, the prevalence of increased TyG was higher in overweight children, with high WC, increased waist:height ratio, excess body fat, android excess fat and hypertension, with increased values of TC, LDL-cholesterol and TC:HDL-cholesterol and LDL-cholesterol:HDL-cholesterol ratios, as well as in children with low HDL-cholesterol (Table 4).
Table 4.
Association between cardiometabolic risk (independent variables) and increased TAG–glucose (TyG) index (dependent variable) in children aged 4–9 years, Viçosa, MG, Brazil, 2015/2016
| Variable | High TyG index | ||
|---|---|---|---|
| PR | 95 % CI | P | |
| Excess weight* | 1·51 | 1·28, 1·79 | <0·001 |
| ↑ WC* | 1·60 | 1·34, 1·89 | <0·001 |
| ↑ WtHR* | 1·37 | 1·13, 1·65 | 0·001 |
| ↑ Total body fat* | 1·51 | 1·26, 1·81 | <0·001 |
| ↑ Android fat* | 1·58 | 1·33, 1·89 | <0·001 |
| ↑ Blood pressure* | 1·28 | 1·02, 1·60 | 0·028 |
| TC ≥ 170 mg/dl† | 1·40 | 1·18, 1·66 | <0·001 |
| HDL-cholesterol < 45 mg/dl† | 1·39 | 1·17, 1·65 | <0·001 |
| LDL-cholesterol ≥ 110 mg/dl† | 1·39 | 1·17, 1·65 | <0·001 |
| TC/HDL ≥ p85† | 1·71 | 1·45, 2·02 | <0·001 |
| LDL/HDL ≥ p85† | 1·55 | 1·30, 1·86 | <0·001 |
WC, waist circumference; WtHR, waist:height ratio; TC, total cholesterol; PR, prevalence ratio.
Poisson regression.
Variables classified according to sex and adjusted for age.
Adjusted by sex and age.
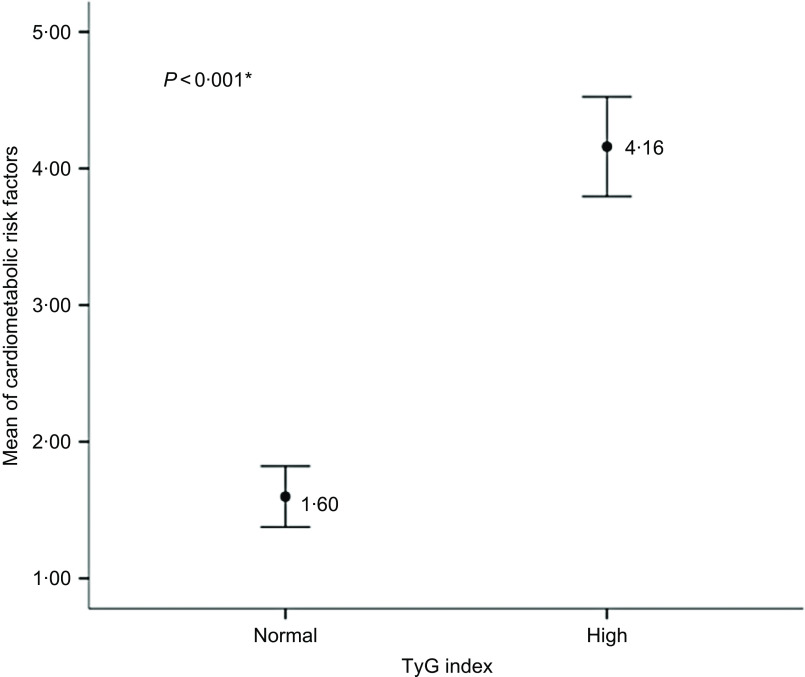
Children with an increased TyG index had a higher rate of cardiometabolic risk factors than those with normal TyG index (Fig. 1).
Fig. 1.
Number of cardiometabolic risk factors according to TAG–glucose (TyG) index in children, Viçosa, MG, Brazil, 2015/2016. *Student’s t test
Discussion
Our results demonstrated that the cut-off points of the TyG index to identify the risk of IR were 7·9 and 8·1 for boys and girls, respectively. Analysis of the receiver operating characteristic curve showed that the TyG index may be useful to identify the risk of IR, since it has AUC of 0·71 for boys and 0·72 for girls. It is known that the highest possible value for the AUC is 1·0, and the closer this value, the greater the accuracy of the test. Since these with AUC close to 0·75 are very useful clinically, it can be considered that the TyG index, in the current study, presented moderate accuracy to identify IR risk in Brazilian children from 4 to 9 years of age(31,32).
Considering the best balance between S and E, the test had a moderate accuracy in the identification of the risk of IR, since for the cut-off points, the numbers were S = 78·1 % and E = 56·2 % for boys and S = 72·7 % E = 65·3 % for girls; therefore, it can be a useful method for metabolic screening of children, since it has an easy calculation and uses common biochemical tests(32). Due to its higher sensitivity, the TyG index was more appropriate to identify children with IR and, consequently, had a reduced number of false negatives.
The low positive predictive value can be determined by the low specificity and the low prevalence of the outcome in the sample(33). We can observe in the current study that the positive predictive value was influenced by the moderate value of specificity. Due to the lack of cut-off points for HOMA-IR for the age group of the current study, we adopted the value of the 75th percentile of the sample, resulting in a proportion of 25 % of children with inadequate HOMA-IR. Hence, future studies that will adopt other criteria for the HOMA-IR may find different predictive values for the cut-off points proposed here.
In children and adolescents aged 9–13 years old from South Korea, a cut-off point of the TyG index for IR diagnosis of 8·2 was identified, with S and E similar to the current study: 77·3 and 68·3 %, respectively(13). Furthermore, it should be considered that children with a TyG index greater than these cut-off points should be submitted to more precise and specific methods to confirm the diagnosis(18). In addition, it is necessary to validate the cut-off points proposed in the current study for children of other age groups and places of residence.
We observed that children with cardiometabolic risk factors, such as with excess weight and total and central body adiposity, hypertension and altered lipid profile, presented higher prevalence of increased TyG index. Similar results were found by other authors who observed a positive association of high TyG index with overweight, high BP and low HDL-cholesterol in children and teenagers(18,34).
It is worth noting that childhood obesity may lead to increased hepatic TAG production and decreased HDL-cholesterol, and these abnormalities may be observed mainly in children with abdominal obesity(13). The accumulation of visceral fat is a key factor in the development of IR. Visceral fat has higher lipolysis rates and increases the supply of NEFA to the liver through the portal vein, which is associated with changes in glucose and lipid metabolism. As a consequence of the expansion of adipose tissue, which also has an endocrinologic function, there is an increase in tissue hypoxia, infiltration of inflammatory cells and changes in the cytokine profile, which is also associated with IR(3).
The state of IR can also increase BP through several mechanisms(35). Among them, by causing a failure in NO-mediated vasodilatory activity produced by insulin in the endothelial cells, leading to endothelial dysfunction and hypertension(35,36). On the other hand, as IR increases the risk of hypertension, factors related to this increase in BP influence the occurrence of IR, such as increased activation of the renin–angiotensin–aldosterone system and the sympathetic nervous system, oxidative stress, inflammation and functional mitochondrial abnormalities(37).
Another factor that associates IR to the occurrence of CVD is dyslipidaemia, characterised by hypertriglyceridaemia, low levels of HDL-cholesterol and/or increased LDL-cholesterol levels(26). In IR, the decrease in insulin function increases lipolysis, with the formation of NEFA, and there is less activity of lipoprotein lipase, generating a remnant of TAG-rich chylomicrons, causing elevation of hepatic free fatty acids (FFA) and secretion of TAG-rich VLDL, processes that also affect the metabolism of HDL-cholesterol(38).
In addition, in the current study, the accumulation of cardiometabolic risk factors in children with high TyG index was higher in relation to the others. Simental-Mendía et al. (39), in a study with children and adolescents aged 6–15 years, observed that those distributed in the highest quintile of the TyG index had higher prevalence of cardiometabolic risk in relation to the others, suggesting a direct association between cardiovascular risk and the increased TyG index.
Some positive points of the current study should be considered. This is one of the few studies in developing countries that proposed cut-off points for the TyG index in childhood, being the first Brazilian study with children, according to our knowledge. Our sample is homogeneous in relation to the physiological characteristics, which contributes to the reduction of possible influences of the body composition. As IR increases the risk of CVD in the future, this is an important step in assessing the risk of this metabolic change, for greater monitoring and prevention throughout life. It is worth noting that the cut-off points established in the current study are specific to the population studied and should not be generalised to other ethnic groups. It is necessary to carry out multi-centred studies with children from other locations and age groups(12).
However, some limitations must be mentioned. First, there was an imbalance between the sensitivity and specificity of the cut-off points found, which could be false positive, and the positive predictive value was low, indicating caution in its use in clinical practice. However, the method correlated well with other risk factors and cardiometabolic alterations and has a relatively high sensitivity, making its use interesting for screening services, with the need for more specific tests to confirm diagnosis. Secondly, we highlight the absence of a well-established cut-off point for classifying IR from HOMA-IR, which was the reference method used in the current study. Due to the sample size, we were unable to stratify the analyses by age. Nevertheless, the sample consists of prepubertal children, which is a slightly heterogeneous group without major biological changes that could affect results.
We concluded that the cut-off points of the TyG index for identifying the risk of IR were 7·9 for boys and 8·1 for girls aged 4–9 years. The children at risk of IR estimated by the TyG index, according to the cut-off points proposed by the current study, presented a higher cardiometabolic risk. The proposed cut-off points can be used as a routine to assess children at risk of developing IR, provided that they are validated for the age group in which they will be applied, in order to help in the early identification of IR and associated cardiometabolic risk.
Acknowledgements
Acknowlegements: The authors thank the children and their parents for participating in the study, the Coordination for the Improvement of Higher Education Personnel, the National Council for Scientific and Technological Development and Quibasa/BioClin laboratory. Financial support: The current work was supported by a scholarship granted by the Coordination for the Improvement of Higher Education Personnel (code 001), by the National Council for Scientific and Technological Development (number of cases: 478 910/2013–2014 and 407 547/2012–2016). Conflict of interest: There are no conflicts of interest. Authorship: A.D.M.d.B.: conception of the study, accomplishment, analysis of the data and writing of the article; M.D.S.F.: data analysis and critical manuscript review; H.H.M.H. and S.d.C.C.F.: critical manuscript review; S.A.V.R.: study design, data analysis and critical manuscript review; J.F.d.N.: study design, supervision and critical manuscript review. Ethics of human subject participation: The current study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all the procedures involving human subjects were approved by the Ethics Committee on Human Research of the Federal University of Viçosa, cases no. 892476/2014 and 663.171/2014. Moreover, the current project was presented to the Municipal Department of Education, the Regional Superintendent of Education and principals of schools. All participants, as well as their responsible, were informed about the objectives of the research, and informed consent was obtained from all children’s parents.
References
- 1. Muniyappa R, Lee S, Chen H et al. (2007) Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab 294, E15–E26. [DOI] [PubMed] [Google Scholar]
- 2. Moreira SR, Ferreira AP, Lima RM et al. (2008) Predicting insulin resistance in children: anthropometric and metabolic indicators. J Pediatr 84, 47–52. [DOI] [PubMed] [Google Scholar]
- 3. Sinaiko AR & Caprio S (2012) Insulin resistance. J Pediatr 161, 11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vasques ACJ, Novaes FS, de Oliveira MS et al. (2011) TyG index performs better than HOMA in a Brazilian population: a hyperglycemic clamp validated study. Diabetes Res Clin Pract 93, 8–10. [DOI] [PubMed] [Google Scholar]
- 5. Vieira SA (2017) Dietary pattern, body adiposity and cardiometabolic risk factors in children from 4 to 7 years of age. PhD Thesis, Universidade Federal de Viçosa.
- 6. Moon S, Park JS & Ahn Y (2017) The cut-off values of triglycerides and glucose index for metabolic syndrome in American and Korean adolescents. J Korean Med Sci 32, 427–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim JW, Park SH & Kim Y et al. (2016) The cutoff values of indirect indices for measuring insulin resistance for metabolic syndrome in Korean children and adolescents. Ann Pediatr Endocrinol Metab 21, 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Simental-Mendía LE, Rodriguéz-Morán M & Guerrero-Romero F (2008). The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord 6, 299–304. [DOI] [PubMed] [Google Scholar]
- 9. Guerrero-Romero F, Simental-Mendía LE, González-Ortís M et al. (2010) The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab 95, 3347–3351. [DOI] [PubMed] [Google Scholar]
- 10. Unger G, Benozzi SF, Peruzza F et al. (2014) Triglycerides and glucose index: a useful indicator of insulin resistance. Endocrinol Nutr 61, 533–540. [DOI] [PubMed] [Google Scholar]
- 11. Lee SH, Han K, Yang HK et al. (2015) A novel criterion for identifying metabolically obese but normal weight individuals using the product of triglycerides and glucose. Nutr Diabetes 5, 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Er LK, Wu S, Chou HH et al. (2016) Triglyceride glucose-body mass index is a simple and clinically useful surrogate marker for insulin resistance in nondiabetic individuals. PLoS One 11, 0149731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kang B, Yang Y, Lee EY et al. (2017) Triglycerides/glucose index is a useful surrogate marker of insulin resistance among adolescents. Int J Obes 41, 789–792. [DOI] [PubMed] [Google Scholar]
- 14. Zheng R & Mao Y (2017) Triglyceride and glucose (TyG) index as a predictor of incident hypertension: a 9-year longitudinal population-based study. Lipids Health Dis 16, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Irace C, Carallo C, Scavelli FB et al. (2013) Markers of insulin resistance and carotid atherosclerosis. A comparison of the homeostasis model assessment and triglyceride glucose index. Int J Clin Pract 67, 665–672. [DOI] [PubMed] [Google Scholar]
- 16. Du T, Yang G, Zhang M et al. (2014) Clinical usefulness of lipid ratios, visceral adiposity indicators, and the triglycerides and glucose index as risk markers of insulin resistance. Cardiovasc Diabetol 13, 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nor NSM, Lee S, Bacha F et al. (2015) Triglyceride glucose index as a surrogate measure of insulin sensitivity in obese adolescents with normoglycemia, prediabetes, and type 2 diabetes mellitus: comparison with the hyperinsulinemic–euglycemic clamp. Pediatr Diabetes 17, 458–465. [DOI] [PubMed] [Google Scholar]
- 18. Ribeiro SAV, Fonseca PCA, Andreoli CS et al. (2018). The TyG index cutoff point and its association with body adiposity and lifestyle in children. J Pediatr 95, 217–223. [DOI] [PubMed] [Google Scholar]
- 19. Milagres LC, Rocha NP, Filgueiras MS et al. (2017) Vitamin D insufficiency/deficiency is associated with insulin resistance in Brazilian children, regardless of body fat distribution. Public Health Nutr 20, 2878–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Suhett LG, Silveira BKS, Filgueiras MS et al. (2018) Inverse association of calcium intake with abdominal adiposity and C-reactive protein in Brazilian children. Public Health Nutr 21, 1912–1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Filgueiras MS, Vieira SA, Fonseca PCA et al. (2018) Waist circumference, waist-to-height ratio and conicity index to evaluate android fat excess in Brazilian children. Public Health Nutr 22, 140–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Friedewald WT, Levy RI & Fredrickson DS (1972) Estimation of the concentration of low- density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18, 499–502. [PubMed] [Google Scholar]
- 23. World Health Organization (2007) Growth reference data for 5–19. http://www.who.int/growthref/en/ (accessed February 2020).
- 24. World Health Organization (2006) WHO child growth standards length/height-for-age, weight-for-age, weight-for-length, weight-forheight and body mass index-for-age: methods and development. https://www.who.int/childgrowth/standards/Technical_report.pdf (accessed February 2020).
- 25. McCarthy HD & Ashwell M (2006) A study of central fatness using waist-to-height ratios in UK children and adolescents over two decades supports the simple message – ‘keep your waist circumference to less than half your height’. Int J Obes 30, 988–992. [DOI] [PubMed] [Google Scholar]
- 26. Faludi A, Izar MCO, Saraiva JFK et al. (2017) Updated Brazilian guideline for dyslipidemia and atherosclerosis prevention – 2017. Arq Bras Cardiol 109, 1–76. [Google Scholar]
- 27. Oliveira JEP, Foss-Freitas MC, Junior RMM et al. (2018) Guidelines of the Brazilian Diabetes Society 2017–2018. São Paulo: Clannad. [Google Scholar]
- 28. Sociedade Brasileira de Cardiologia (2016) 7th Brazilian guideline of arterial hypertension. Arq Bras Cardiol 107, 1–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Matthews DR, Hosker JP, Rudenski AS et al. (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–419. [DOI] [PubMed] [Google Scholar]
- 30. Shashaj B, Luciano R, Contoli B et al. (2015) Reference ranges of HOMA-IR in normal-weight and obese young Caucasians. Acta Diabetol 53, 251–260. [DOI] [PubMed] [Google Scholar]
- 31. Faraggi D & Reiser B (2002) Estimation of the area under the ROC curve. Stat Med 21, 3093–3106. [DOI] [PubMed] [Google Scholar]
- 32. Angoorani P, Heshmet R, Ejtahed HS et al. (2018). Validity of triglyceride–glucose index as an indicator for metabolic syndrome in children and adolescents: the CASPIAN-V study. Eat Weight Disord 23, 877–883 [DOI] [PubMed] [Google Scholar]
- 33. Akobeng AK (2006) Understanding diagnostic tests 1: sensitivity, specificity and predictive values. Acta Pædiatrica 96, 338–341. [DOI] [PubMed] [Google Scholar]
- 34. Silva CC (2013) Index TyG (triglycerides/glucose) in the assessment of insulin resistance in adolescents: validation study front of the hyperglycemic clamp. Masters Dissertation, Universidade Federal de Campinas, SP.
- 35. Ferreira AP, Nóbrega OT & França NM (2009) Association of body mass index and insulin resistance with metabolic syndrome in Brazilian children. Arq Bras Cardiol 93, 147–153. [DOI] [PubMed] [Google Scholar]
- 36. Carvalho MHC, Colaço AL & Fortes ZB (2006) Cytokines, endothelial dysfunction, and insulin resistance. Arq Bras Endocrinol Metabol 50, 304–312. [DOI] [PubMed] [Google Scholar]
- 37. Whaley-Connell A & Sowers JR (2009) Hypertension and insulin resistance. Hypertension 54, 462–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tangvaarasittichai S (2015) Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J Diabetes 6, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Simental-Mendía LE, Hernández-Ronquillo G, Gómez-Diaz R et al. (2017) The triglycerides and glucose index is associated with cardiovascular risk factors in normal-weight children and adolescents. Pediatr Res 82, 920–925. [DOI] [PubMed] [Google Scholar]