Keywords: Nutrition transition, China, Animal products, Processed foods, Nutrient intakes
Abstract
Objective:
To extend analyses of nutrition transition in developed countries to China within the framework of the 3Vs rule considering degree of processing starting with plant/animal calorie ratio (Rule 1), industrially processed foods (IPFs, Rule 2), and food diversity through nutrient intakes (Rule 3).
Design:
Total and main food group (n 13) calorie intakes, percentages of animal and IPF calories, adequacy of the Dietary Reference Intake (DRI) and prevalence of chronic diseases were retrieved from scientific literature and international databases.
Setting:
China, 1990–2019.
Participants:
Overall population.
Results:
The total calorie intake decreased by 9 % over 30 years while the prevalence of chronic diseases substantially increased. Percentages of IPFs (Rule 1) and animal (Rule 2) calorie intake shifted from 9 to 30 % and 2 to 30 %, respectively. Meanwhile, the overall DRI adequacy (Rule 3) did not improve, with calcium and retinol deficiencies in 2019, and, although remaining above DRI, iron, copper, magnesium, and vitamins E, C and B1–B9 intakes regularly decreased. Notably, the prevalence of obesity increased five-fold, paralleling the exponential increase in IPF calorie intake. Both sources of calories were highly correlated with prevalence of main chronic diseases.
Conclusions:
Despite a slight decreased of total calorie consumption and small variations of adequacy with DRI, the farther the Chinese population moved away from the 3Vs rule during the 1990–2019 period, the more the prevalence of chronic diseases increased. Further analyses on foods’ transitions will be better assessed when advocating sources/quality of calories (Rules 1/2), rather than only nutrient composition (Rule 3).
Since the 1950s, the nutrition transition has occurred in many countries worldwide in different periods and at different rates(1). Progressive and long lasting in developed countries(2), it is more rapid and recent in emerging and developing countries and is associated with increasing urbanisation, industrialization and population growth(3). In developed countries, it marks the passage from a diet rich in minimally processed plant-based foods with limited variety (with a significant part of the diet composed of homemade dishes) before the Second World War to a diet richer in animal-based products and increasingly industrially processed foods (IPFs), and latter ultra-processed foods (UPFs)(4,5), showing peak sales in Western countries in the 1980s(5). Therefore, this nutrition transition paralleled the industrial one, bringing together with an increase of non-communicable diseases(6).
Indeed, current analyses of developed countries indicate that excess animal and UPF calories are associated with increased risks of chronic diseases(4,7), especially overweight, obesity, metabolic syndrome, type 2 diabetes and hepatic steatosis(8–11), cardiovascular diseases, renal function decline and total cancers(10,12,13), all-cause mortality(14), and with the degradation of food system sustainability as well(2,15,16). Notably, UPFs supply empty calories and numerous xenobiotic substances foreign to human body(4), and are mainly hyperglycaemic and poorly satiating food matrices(17).
Thus, numerous studies have focused on the relationship between nutrition and health to alleviate the pandemic level of overweight and obesity, and their associated chronic diseases, these latter being addressed by medical advances and public health policies, but leading to either decrease or stagnation of healthy life years in Western countries from 1990 to 2017(18). Starting from calories(19), these studies included successively the nutrients, the food groups, the dietary patterns and the level of processing of industrial foods as potential inputs(20,21), together with sedentary lifestyles and low level of physical activities(22). Finally, the nutrition transition, which initially included food security and safety, should now include nutritional and environmental securities relative to food systems underlying the developed countries(23).
From these observations in developed countries, the issue arises whether or not a similar chronic diseases-related nutritional transition is happening in developing and emerging countries where nutritional transition came latter. Notably, emerging countries, beginning nutrition transition in the early 1990s(3), offer a unique model to address the diet-chronic diseases relationship, these latter progressively replacing micronutrient deficiencies and critical infectious diseases(24). Among emerging countries, China is the one with the largest population in the world, i.e. approximately >1·4 million people in 2020 (≈18·5 % of the total world population), only closely followed in number by India with 1·38 million people. Thus, China appears as a relevant study laboratory for this recent nutritional transition. However, although studied many times in the past, the Chinese nutritional transition has not been studied as much through the lens of both human health and a sustainability perspective (i.e. global health), especially including the degree of food processing(25,26).
For this, from all data provided by these successive studies and nutrition transitions observed in Western countries, an innovative holistic framework has been proposed under the name of 3Vs rule to address both health and environmental securities(16,27). This rule is based on three inclusive and interconnected metrics that governs the diet-global health relationship, namely the plant/animal calorie ratio (Rule 1 in French: ‘Végétal’ for plant, 85 % optimum daily calories), the food degree of processing (Rule 2: ‘Vrai’ for real foods, 85 % minimum daily calories), and food diversity, if possible organic, local and/or seasonal (Rule 3: ‘Varié’ for Varied). This simple tool theoretically and potentially suggests that the more a dietary pattern deviates from these rules, the less global health is preserved, for example with excess animal-based foods, and/or excess UPFs and/or an overly monotonous diet. The 3Vs rule therefore constitutes a holistic indicator to robustly determine whether the overall dietary pattern of a specific country/region deviate or not from global health sustainability.
Through this new lens, the objective of this study was therefore to evaluate the evolution of the prevalence of chronic diseases during the Chinese nutrition transition in the last three decades (1990–2019), based on calories and nutrient intakes (Rule 3), and on food sources (Rule 1) and their industrial processing (Rule 2).
Materials and methods
Data sources and collection
In order to target the widest possible population, the main sources of data were the OECD.Stat(28), Statista(29), FAO.Stat(30) and Our World in Data(31) web platforms. Notably, the Our World in Data platform retrieves data from the FAO food supply database (since 1961), The Complex Emergency Database (CE-DAT, since 1998), and the Inequality of Food Consumption database (for developing countries only; since 1990); Statista provides statistics and facts on retail and trades, and statistics and market data on consumer goods and Fast-Moving Consumer Goods (FMCG); and OECD.Stat includes data and metadata relating to OECD countries and select non-member economies (including China). In addition to these generic crude data, and to complete them when necessary, the other main source was The China Health and Nutrition Survey (that began in 1989) designed to provide representation of rural, urban and suburban areas varying substantially in geography, economic development, public resources and health indicators(32), and from which food consumptions, and chronic disease risks were collected during the 1989–2015 period based on several epidemiological studies(33–40). Other data were extracted from original articles or review papers about China and/or nutrition transitions through the ISI Web of Science database, with the following topic fields and Boolean operators: ‘Animal product* OR processed food*’ OR ‘Chronic disease*’ AND ‘Consumption* OR intake* OR sale*’ AND ‘China OR Chinese’, and the affiliated keywords for each lexical field.
The total, food group-specific, animal, and IPF calorie intakes/d in the Chinese population during the 1990–2019 period were extracted from the collected data. The following thirteen food groups were considered: fruits, sweetened beverages, vegetables, plain and fortified cereals, legumes, tubers, meats, dairy products, eggs, fishes and seafood, oils and fats, nuts and seeds, and sugars.
For the first part of the 3Vs rule, i.e. ‘Végétal/Plant’, animal-based foods encompassed white and red meat, dairy products, eggs, and seafood and fishes. For the second part of the 3Vs rule, i.e. ‘Vrai/Real’, due to the virtual absence of data for UPF consumption in China, only IPFs were retrieved (even if all IPFs are not UPFs): data concerned product vector categories for sugar, fat and salt consumption for the years 1999, 2006, 2012 and 2017(41), i.e. oils and fat, fruit/vegetable juices, frozen processed foods, dried processed foods, dairy, confectionary, biscuits, chilled processed foods, carbonated soft drinks, baked goods and other processed foods. For the lacking data during the 1990–2019 period, IPF data were extrapolated from these 4 years. For the third part of the 3V rule, i.e. ‘Varié/Varied’, information about access to detailed food diversity in the Chinese population during the 1990–2019 period was not available in the literature; therefore, this was approached as follows: (1) the change in the calorie shares accounted for by the main food groups, i.e. diminishing calorie shares by overly dominant food groups during 1990–2019 indirectly suggests an increasing variety (provided these changes were not to the benefits of IPF calories); and (2) the evaluation of the supply of macro- and micronutrients and fibre and the corresponding adequacy with regard to the international DRI(42), based on food group quantities consumed and the median food group composition.
Data processing, calculations and analyses
The basis of the calculations was the average calorie intake (kcal/d/capita) during the 1990–2019 period calculated from the main food groups consumed. When it was necessary to convert grams to kcal (except for sweetened beverages), we used all ‘as eaten’ food products within each food group and retrieved them from the recently updated French Ciqual database(43) (due to the absence of an available online Chinese food database). After checking for normality of the distribution of the data (Shapiro-Wilk’s test, non-significant), the non-parametric median calorie content for one gram of each food group (see the number of foods considered within each food group in Table 1) was determined. Then, fish and seafood were aggregated for calculations. For cereal intake, the 2016–2019 values were only available for the urban population. The values were corrected based on data before 2016 in urban and rural populations with a ratio of 0·67, rural populations consuming more cereals. For sugar intake, the 2014–2019 values were also extrapolated from the 1990–2013 data. Finally, concerning sweetened beverages, available data only covered the 2009–2014 period, with an increase each year of +1·14 %(44); this rate was applied to extrapolate to the previous 1990–2008 and subsequent 2015–2019 periods. However, for the extrapolation of either sugar or sweetened beverage data, due to their low contributions to calorie intake among all other food groups, we estimated that these extrapolations were rather reliable.
Table 1.
Median calorie content by main food groups
| Food groups | Calorie/g* |
|---|---|
| Eggs (n 9)† | 1·42 |
| Dairy products (n 247) | 2·40 |
| Meats (n 284) | 2·33 |
| Fishes (n 63) | 1·25 |
| Seafood (n 40) | 0·88 |
| Fruits (n 69) | 0·54 |
| Vegetables (n 204) | 0·35 |
| Cereals (n 78) | 3·59 |
| Tubers (n 35) | 1·29 |
| Legumes (n 25) | 1·06 |
| Nuts and seeds (n 49) | 6·03 |
| Oils and fat (n 26) | 8·98 |
| Sugar (n 1) | 4·00 |
Median calorie contents within each food group were based on all ready-to-eat foods (therefore including IPFs) as retrieved from the Ciqual database on 17 August 2020(43).
Number of ready-to-eat foods considered for the calculation of the median calorie content.
For the first dimension of the 3Vs rule (‘Végétal’/Plant), data regarding the consumption of animal food groups were in most cases expressed in g/d or in kg/year/capita and were more rarely expressed in calories. Therefore, when necessary, data were converted into calories to determine the median percentages of calories consumed for each animal group (Table 1). For the second dimension of the 3V rule (‘Vrai’/Real), we first considered the total amount of IPFs consumed in 1999, 2006, 2012 and 2017(41) and, based on the quasi linear growth rates, extrapolated the data to other years between 1990–1999, 2000–2006, 2007–2012, 2013–2017 and 2018–2019. For these first two dimensions of the 3V rule, the total calories from animal and IPF sources were reported with regard to the total daily consumed calories for a Chinese adult, leading to daily animal and IPF calorie percentages (%). The same calculations were also performed for other calorie sources from other food groups.
For the third dimension of the 3Vs rule (‘Varié’/Varied), we calculated the contribution in calorie percentages of each of the thirteen main food groups and their evolution during the 1990–2019 period. From the DRI(42,45), and the supplies of macro- and micro-nutrients in each of these thirteen food groups, we also evaluated the DRI adequacy (%) in the 1990–2019 period for each micronutrient (fibre, vitamins, minerals and trace elements: see the number of ‘as eaten’ foods considered within each food group in Table 1 (43)) and the evolution of the protein, carbohydrate, and lipid calorie percentages. Due to the lack of data for vitamin K1 content(43), no data were reported for this vitamin. Finally, β-carotene was converted to vitamin A with 12 mg β-carotene = 1 mg vitamin A/retinol equivalent.
For chronic disease prevalence, percentages were also extracted from the scientific literature as described above. Because the prevalence of CVD was not available, the data were based on the percentage of cardiovascular death out of all deaths. Then, these percentages were correlated with food group, animal and IPF calorie shares (%) during the 1990–2019 period, and the best fitted equations (i.e. with the highest correlation coefficient, R 2, as calculated by Excel software, Microsoft Office 2016©, USA) were identified for each association.
Instead of means, median values were always used in this study (e.g. median calorie and nutrient contents for food groups) due to the non-significant result for Shapiro-Wilk’s test, indicating a non-normal distribution of the data (SPAD 9.1 software, Coheris©).
Results
Calorie intake by food group
Calorie intake was calculated for each food group and then summed (Fig. 1). Overall, total calorie intake tended to decrease during the 1990–2019 period from 2991 to 2725 kcal/d (approximately -9 %). When we looked at changes over time in food groups there was an approximately two-fold decrease in cereal calorie intake from 2440 to 1220 kcal/d. This decrease was notably accompanied by increases in calorie intake from meat-based products (+220 %, i.e. from 205 to 450 kcal/d), dairy products (+455 %, i.e. from 33 to 150 kcal/d) and oils and fats (+7120 %, i.e. from 5 to 356 kcal/d). The intakes of legumes, tubers, nuts and seeds, sugars, sweet beverages and fruits and vegetables were consistently below 80 kcal/d during the 1990–2019 period (Fig. 1).
Fig. 1.
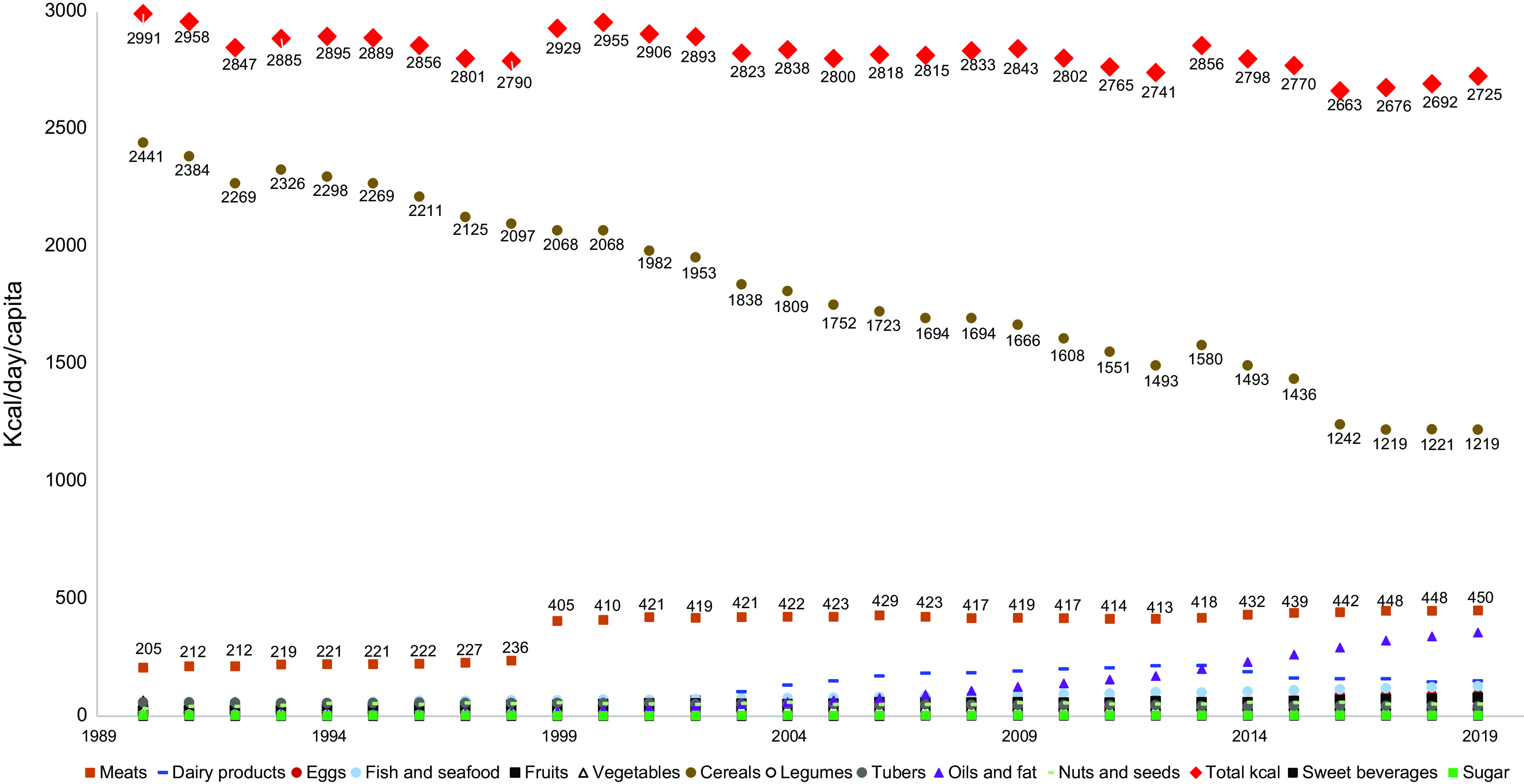
Changes in calorie intake by food group during the 1990–2019 period
Percentages of animal and industrially processed calorie intakes
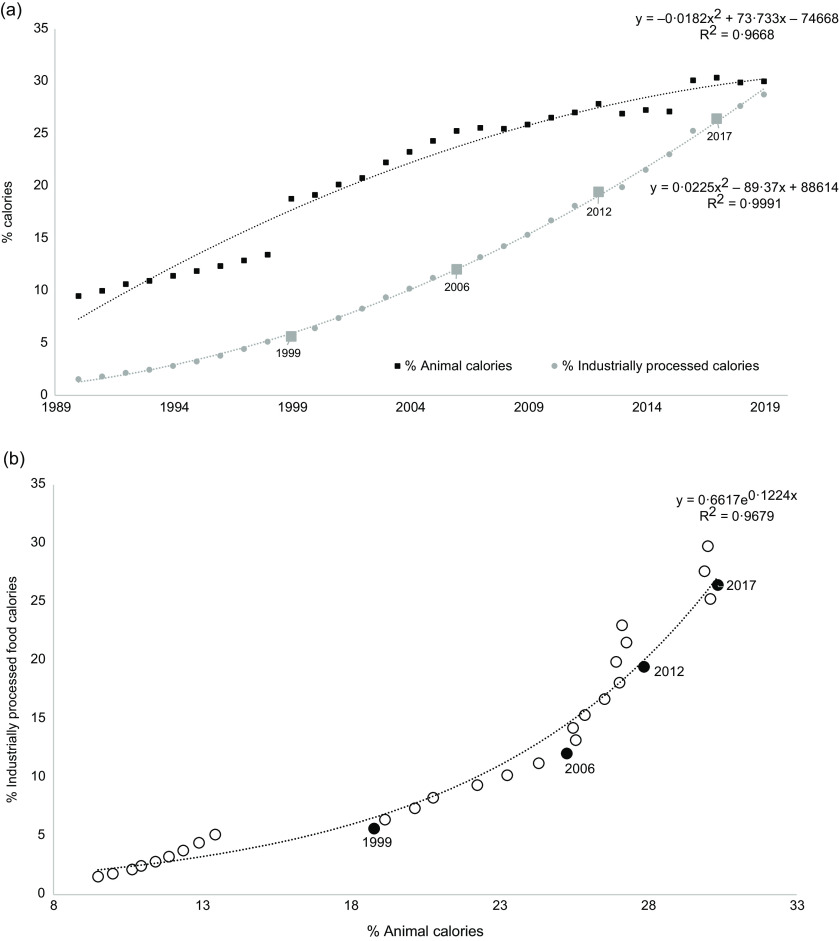
The percentages of animal and IPF calorie intakes substantially increased during the 1990–2019 period (Fig. 2(a)). Estimated data from 1990 to 2019 indicate that the IPF calorie share increased from 1·5 to 28·7 %, according to a polynomial curve (R 2 = 1·00). Animal calorie intake also increased from 9·5 % in 1990 to 30·0 % in 2019, i.e. a three-fold increase with two more acute increases in 1999 (+6 %) and 2016 (+3 %) (polynomial curve, R 2 = 0·97). Animal calorie intake was strongly and exponentially correlated with IPF calorie intake (R 2 = 0·97, Fig. 2(b)), meaning that the percentage of IPF calorie intake increased more rapidly than the percentage of animal calorie intake.
Fig. 2.
During the 1990–2019 period: (a) Changes (%) in animal and industrially processed food calorie intakes (large squares are real data in 1999, 2006, 2012 and 2017 for IPF, and small points are extrapolated data); (b) Correlations between percentages of animal and industrially processed calorie intakes. Fitted curves were chosen based on the highest R 2
Percentages of variety among food groups and DRI adequacy
Overall, calorie shares among the thirteen food groups importantly changed during the 1990–2019 period, with a more balanced calorie percentage shares among them in 2019 (Fig. 3). In 1990, cereals constituted 82 % of the total calorie intake, while they only accounted for 45 % in 2019, i.e. a decrease by approximately 50 %. At the same time, total meat calorie intake (including processed meats) more than doubled in 30 years, from 7 to 17 %. The most striking increase was observed for oil and fat calorie intake, which changed from 0·2 to 13·1 %, and for dairy products which changed from 1·1 to 5·5 %. Finally, the calorie shares of less processed plant-based food groups other than cereals did not increase that much: thus, fruits, vegetables, legumes, tubers, and nuts and seeds calorie shares increased from 0·4–2·2 % to only 1·1–2·8 % (Fig. 3). These changes in food group intake were reflected in the evolution of the protein/carbohydrate/lipid calorie ratios, which changed from 13/72/15 % to 16/45/39 % (Fig. 4). While the protein share remained quite stable, an important portion of carbohydrate calories was replaced by lipid calories.
Fig. 3.
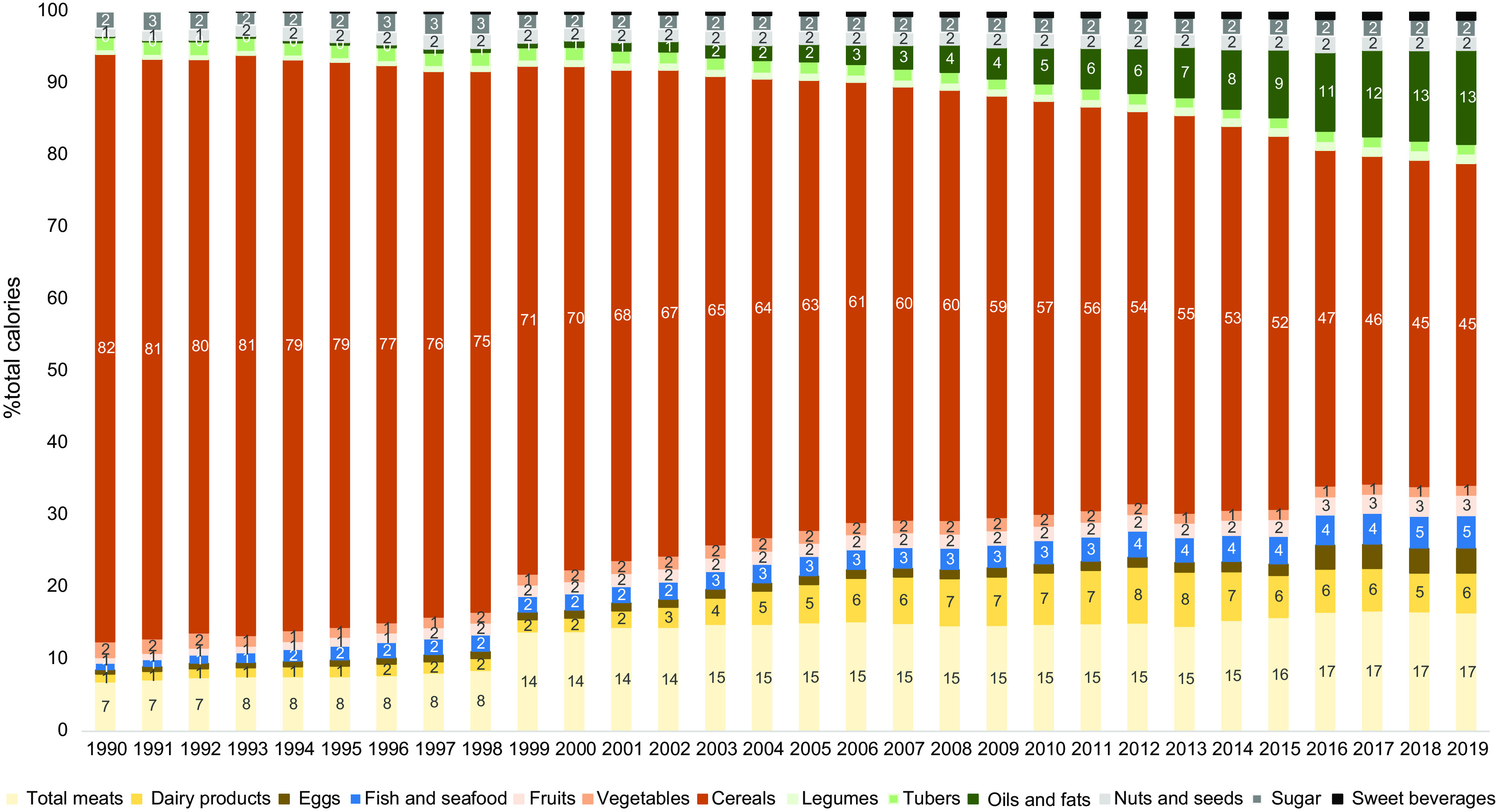
Percentages of food group (n 11) calorie shares during the 1990–2019 period
Fig. 4.
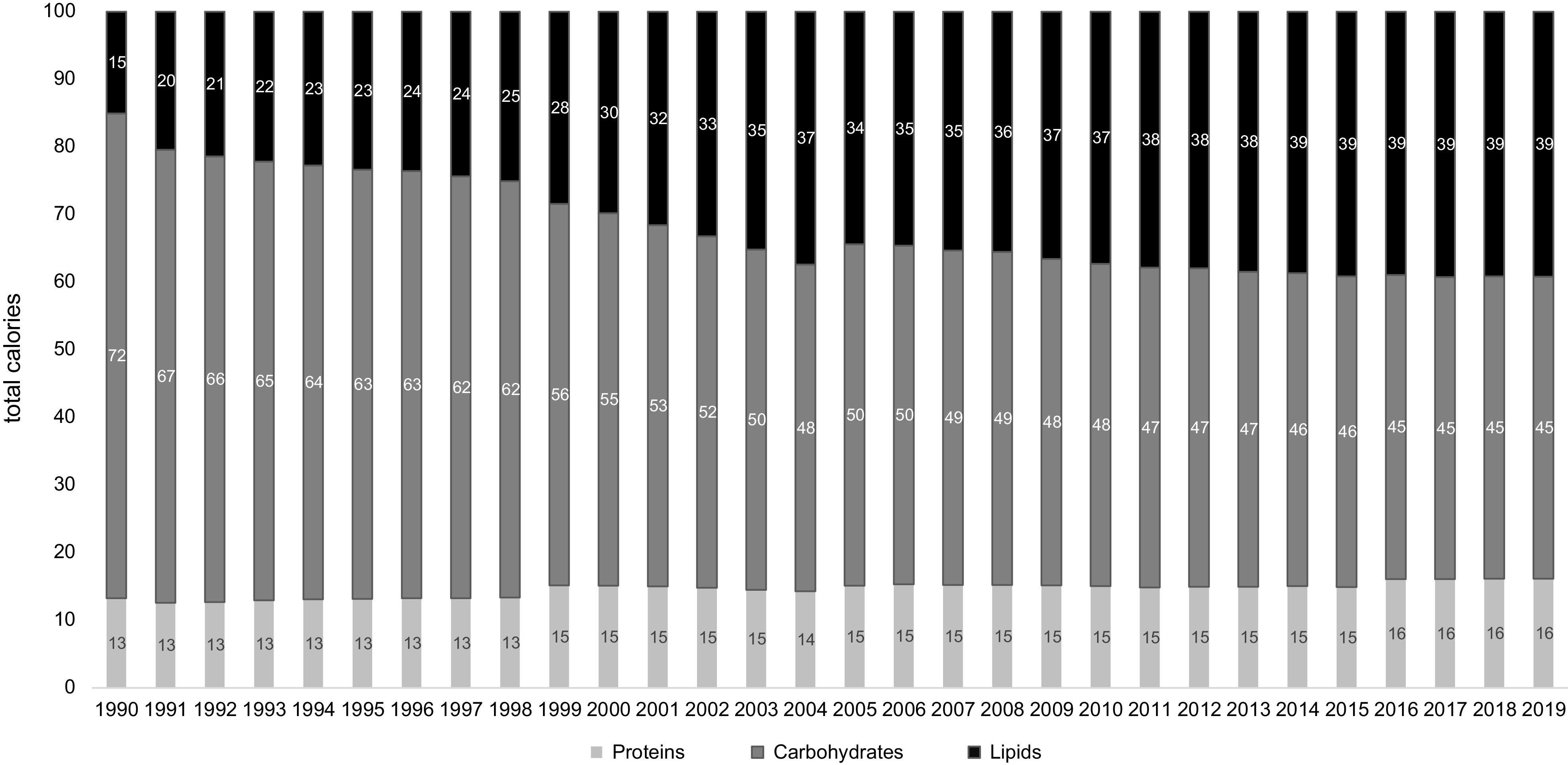
Percentages of macronutrient calorie shares during the 1990–2019 period
The changes in some food group shares with regard to calorie intake was accompanied with different evolutions of fibre, minerals and trace elements (Fig. 5(a)) or vitamins (Fig. 5(b)) intakes. Although above DRI during the 1990–2019 period, the intakes of iron, copper, magnesium, vitamin E, C, B1, B2, B3, B5, B6 and B9 decreased. In the same time, calcium, retinol, vitamins E and D (but most of vitamin D is supposed to be supplied through sun exposure) remained below DRI while iodine intake increased to levels above DRI, and fibre intake decreased below DRI. Vitamin B12 and selenium intakes remained above DRI and increased during this period. In 2019, fibre, retinol and calcium intakes were insufficient, addressing only 74, 37 and 44 % of DRI, respectively.
Fig. 5.

The adequacy of the diet with regard to the DRI (%) for: (a) fibre, minerals and trace elements; and (b) vitamins during the 1990–2019 period. A negative percentage indicates a deficiency
Chronic disease prevalence during the 1990–2019 period
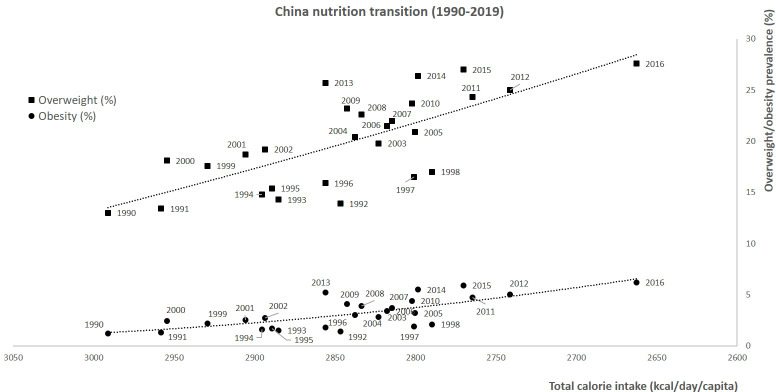
First, total calorie intake was inversely correlated with the overweight/obesity prevalence during the 1990–2016 period (Fig. 6, polynomial curve, R 2 = 0·53–0·55). During this period, the prevalence of overweight increased from 13·0 to 27·6 %, and the prevalence of obesity increased from 1·2 to 6·2 %. Then, the prevalence of overweight was plotted against food group, animal and IPF calorie shares during the 1990–2019 period (i.e. n 15 calorie groups). For the fifteen correlations, the R 2 ranged from 0·21 (vegetable) to 0·99 (oils and fats), with the highest correlations found for sweet beverages (R 2 = 0·997), fish and seafood (R 2 = 0·966), oils and fats (R 2 = 0·994), IPF (R 2 = 0·992), and animal (R 2 = 0·952) calorie shares (Fig. 7(a)). Concerning the first two dimensions of the 3Vs rule, the evolutions of animal and IPF calories followed those of the prevalence of overweight and obesity during the 1990–2019 period, with linear, exponential or polynomial curves (R 2 range = (0·97–1·00)) (Fig. 7(b)). Notably, the prevalence of overweight linearly increased at least two-fold during the 1990–2019 period (R 2 = 1·00), while the prevalence of obesity increased exponentially (R 2 = 1·00), ultimately increasing six-fold over the 30 years (Fig. 7(b)). In addition, the IPF calorie share (%) was linearly correlated with the prevalence of obesity (%) (R 2 = 1·00, Fig. 8(a)), and the animal calorie share (%) was exponentially correlated with the prevalence of obesity (R 2 = 0·943, Fig. 8(b)).
Fig. 6.
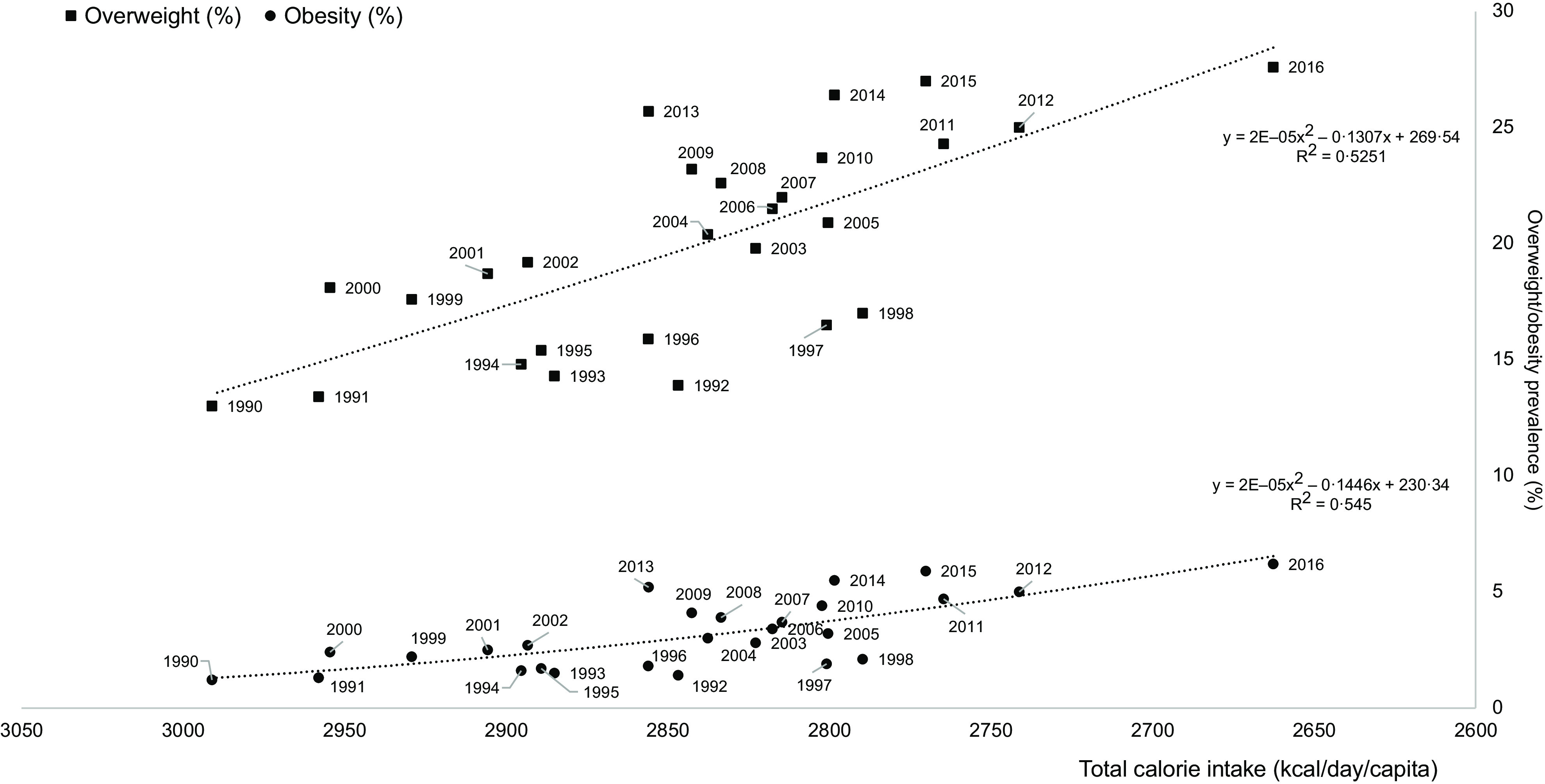
Associations between total calorie intake (kcal/d/capita) and the prevalence of overweight and obesity during the 1990–2019 period
Fig. 7.
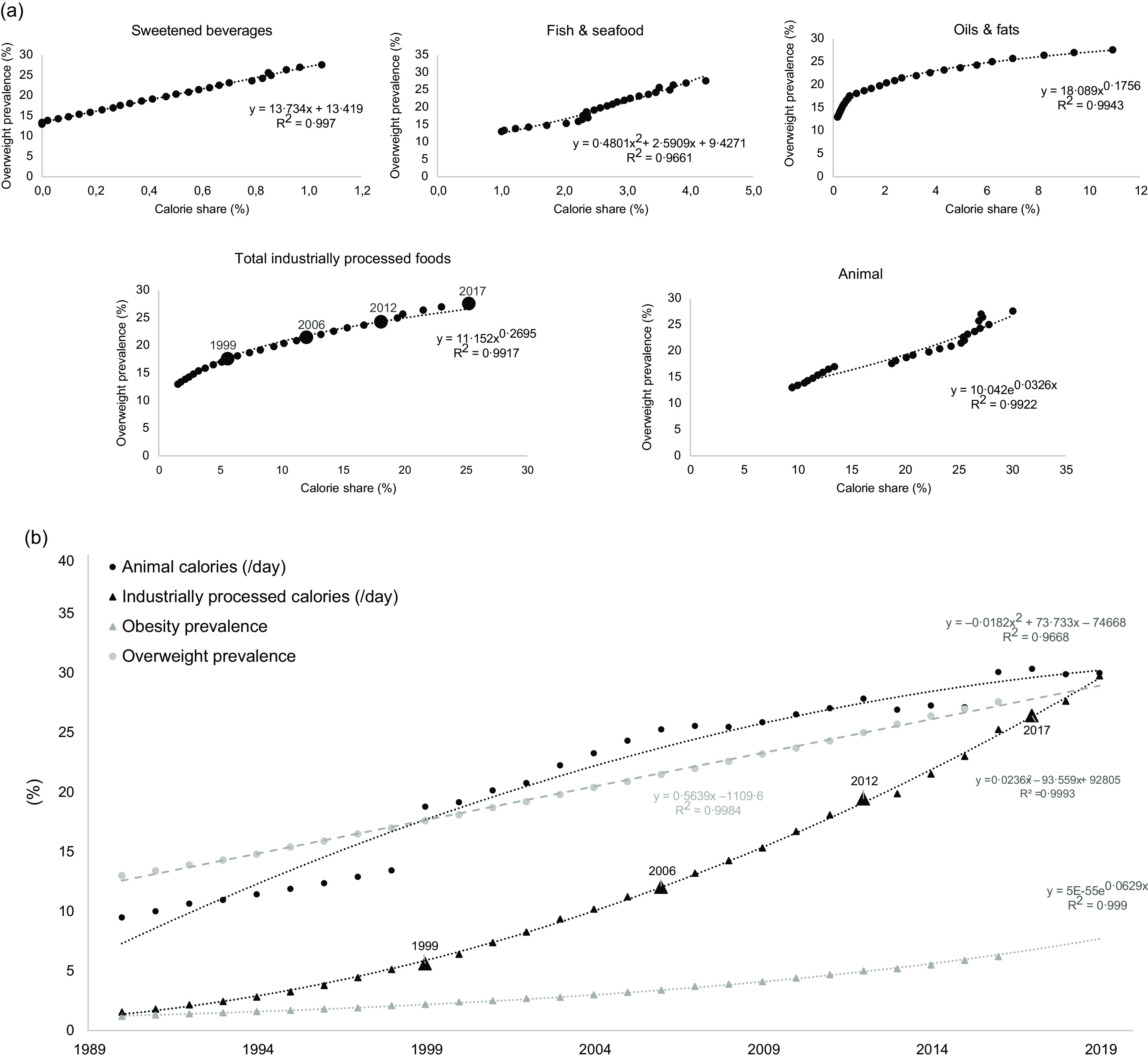
During the 1990–2019 period: (a) percentages of food group calorie share and the prevalence of overweight (%); and (b) changes in animal and industrially processed calorie shares (%) and the prevalence of overweight and obesity (%) (larger triangles are real data in 1999, 2006, 2012 and 2017 for IPF, and small triangles are extrapolated data). Fitted curves were chosen based on the highest R 2
Fig. 8.
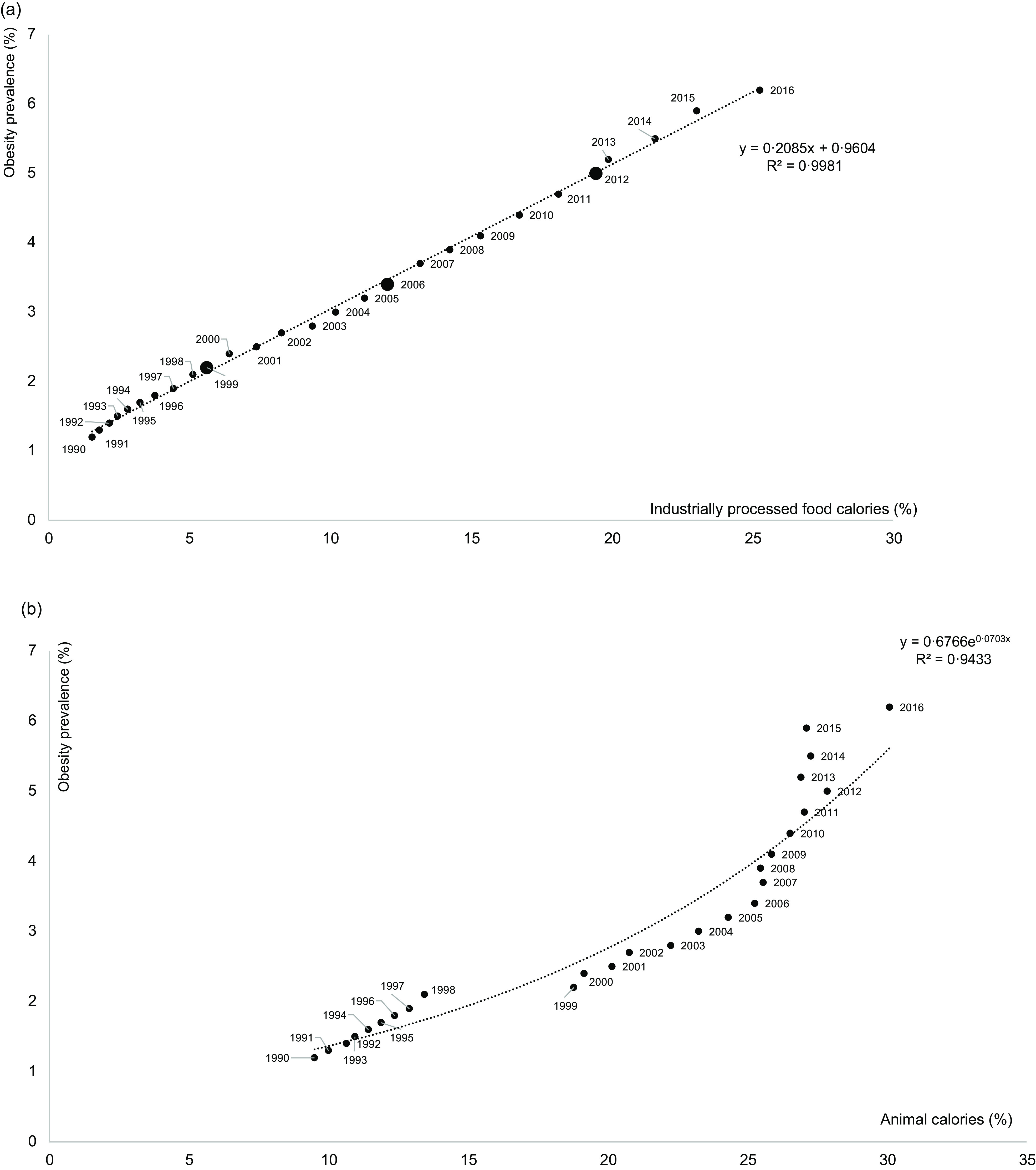
During the 1990–2016 period: percentages of: (a) industrially processed food calorie share; and (b) animal calorie shares and the prevalence of obesity (%) (larger circles are real data in 1999, 2006 and 2012 for IPF, and small triangles are extrapolated data). Fitted curves were chosen based on the highest R 2
Concerning other chronic diseases, significant correlations were found between animal (Fig. 9(a)) and IPF (Fig. 9(b)) calorie intakes (%) and type 2 diabetes, metabolic syndrome, hepatic steatosis and total cancer prevalence and with the CVD mortality rate (among all deaths), i.e. all prevalence increased with increased consumption. Due to the lack of data for hepatic steatosis before 2001, the adjusted curves were less well fitted (lowest R 2, Fig. 9(a) and (b)). The types of correlations differed according to the considered chronic diseases: for the animal calorie share (%), the best fitted curves are linear; for metabolic syndrome, all cancers and hepatic steatosis, the best fitted curves are exponential (Fig. 9(a)); and for IPF calorie share (%), except diabetes, all other chronic disease prevalence follow a polynomial curve with a high R 2, i.e. above 0·96 (Fig. 9(b)).
Fig. 9.

During the 1990–2019 period, chronic disease prevalence (other than overweight/obesity) correlated with percentages of: (a) animal calorie consumption; and (b) industrially processed calorie consumption (larger symbols are real data in 1999, 2006 and 2012 for IPF, and small triangles are extrapolated data). Fitted curves were chosen based on the highest R 2
Discussion
Calorie intake and overweight/obesity
The first result showed a slight decrease in total calorie intake of approximately 9 % during the last three decades. Based on data from the China Statistical Yearbook (1980–2011)(46), a decrease had been previously observed during the 1992–2010 period (-20 % total calories/d), with disparities between rural and urban populations, the latter consuming fewer calories on average than rural populations who perform physical work on farms and therefore need to consume more calories(47). The urban population has increased dramatically in the last three decades, and the proportion of the population accounted for by rural residents decreased from 80 % (796 million) in 1980 to 50 % (671 million) in 2010(47). The overall observed decrease in calorie intake(47) during dietary transition could have resulted from demographic transition and the associated urban migration, which was estimated to be 30 % in 2010. In the same study, the overall changes in the consumption of the main food groups during the period 1980–2010(47) is similar to that described in the present study also showing further continuation until 2019. According to a recent model to project food demand between 2010 and 2050, this trend will continue due to the fact that the highest income per capita in China is associated with a higher animal-based product consumption in kcal/capita/d(48).
In addition, the present data indicate that the total calorie intake was negatively associated with the prevalence of overweight and obesity highlighting that the explanation might be found elsewhere than the only total calorie intake. Obviously, the decrease in physical activity and increase in sedentary lifestyle have probably played relevant roles, especially for urban populations(49). Beyond this first explanation, concerning diets and food factors, two hypotheses may also be proposed: (1) despite the decrease in physical activity, the total calorie intake did not sufficiently decrease, making the calorie intake remains too high, i.e. an average of 2700 kcal/d/capita in 2019; and/or (2) the quality of the calorie predominates over the quantity consumed(50). This second hypothesis, previously proposed by Camacho and Ruppel(50), is supported by our data, which show strong correlations between increased prevalence of overweight/obesity during the 1990–2019 period and increased shares of calories from plant oils and fat, sweetened beverages, animal and IPF products, the access to which has likely increased with a higher industrialisation and income per capita of the urban population(48). A similar observation was previously made by Dr. Campbell (2004), looking at mortality rates from cancer and other chronic diseases from 1973–1975 in 65 counties in China(51). The data showed that people living in the countryside with a diet based on minimally processed plant-based foods did not suffer from overweight or obesity, unlike city dwellers who nevertheless consumed nearly 800 fewer calories but obtained their calories from more IPFs(51).
Adequacy to the 3Vs rule
Rule 1: 85 % plant calories
The animal calorie share increased from 9 % in 1990 to 30 % in 2019, which is 15 % above the optimum value determined in the 3Vs rule. This increase was strongly correlated with the increased prevalence of the main chronic diseases, especially mortality due to CVDs, which increased from 28 % of all deaths in 1990 to 42 % in 2017. Notably, it was reported in 2019 that China had the highest age-standardized rates of diet-related CVD deaths (299 (275–324) deaths per 100 000 population)(52). Animal-based product intake was also significantly correlated with the prevalence of obesity (R 2 = 0·94), which is in agreement with a previous ecological study showing that meat availability contributes to the global prevalence of obesity and that this contribution is comparable to that of sugar(53). Therefore, a decrease in total dietary calories, but with more than 15 % of the calorie intake provided by animal-based foods appears to be associated with a higher prevalence of chronic diseases. Correlations obviously do not mean a strict causal link with increased animal-based product consumption. Other dietary factors (i.e. a diet low in whole grains, nuts, seeds, seafood, fruits, legumes and/or fibre)(54), decreased physical activity(55) with the enormous increases in urbanization and population density(56) (but also in the rural population(57)), and environmental pollution, which is especially high in China (e.g. chronic obstructive pulmonary disease was the fourth leading cause of years of life lost in 2013, just behind CVDs and road injuries)(58) can also be involved in the observed association.
Rule 2: 85 % minimum real foods calories
Concerning this second dimension, which is linked to the degree of processing, IPF calorie intake (%) was strongly correlated with increased risks of the main chronic diseases, excepting CVD mortality, which plateaued after 20 % was reached for IPF calories. Notably, the prevalence of overweight, which is observed before obesity, increased two-fold in 30 years (from 13 to 28 %). This constant increase led us to consider that IPFs have been progressively substituted with UPF. In France, UPFs were evaluated to correspond to 67 % of packaged IPFs(59). And, the increase in the UPF calorie share has been consistently associated with the prevalence of overweight and obesity and with the prevalence of chronic diseases(4,8,9), e.g. +9 % risk of obesity for an absolute increase of 10 % of UPFs in the French diet(60). This suggests that in China, if UPF calorie shares continue to increase from the reported values in 2013 (≈11 % calorie share)(61) and 2019 (≈18 %)(2), the prevalence of obesity prevalence will rapidly exceed 6 %, reaching a prevalence similar to that in Western countries, e.g. between 15 and 25 % in Europe(62). Between 2013 and 2019, the UPF calorie share therefore increased by +7 % while, at the same time, the prevalence of obesity increased from 5·2 to 6·2 %. Today, several countries have UPF calorie intake levels far above 15 %(2,61), even reaching more than 30 %, and the prevalence of obesity in these countries is generally above 10 %. One can then predict that the tendency for an increased UPF intake will be associated with a still higher prevalence of obesity in China in 2030, as already predicted in 2008(63).
Concerning other chronic diseases, the increased prevalence of cardiovascular diseases, type 2 diabetes, and metabolic syndrome has been shown to be also associated with increased consumption of UPFs(64–67). Regarding diabetes during the transition period of 1990–2019, Chinese people were used to consume a high level of cereals, which still constitute 45 % of their total calorie intake today. Consuming wholegrain cereal is usually considered a healthy dietary habit, particularly with regard to preventing diabetes(68). However, through the lens of the 3Vs rule, the important question is whether these cereals are refined or not. In the USA, a previous study reported that people who consumed high levels of refined white rice had a higher risk of type 2 diabetes than those who consumed high levels of unrefined brown rice, which highlights the main role played by the outer layers of the grains in health(69). More generally, whole grain cereals have a stronger protective effect than refined cereals(70), especially with regard to diabetes(71). Therefore, the high level of consumption of white rice-based foods in China(72) might also explain the increased prevalence of type 2 diabetes – although studies give contradictory results(73,74), which substantially increased between 1990 and 2019 from ≈2 to ≈14 %. A high intake of processed meats is also associated with an increased risk of type 2 diabetes(70,75). Finally, the substantially increased prevalence rates of hepatic steatosis – also called the ‘soda disease’(76) – can also be related to increased consumption of UPFs, notably those with added fructose.
Rule 3: varied among real foods
This third dimension appears independent of the two first ones since the variations of adequacy of the Chinese diet to the DRI (possibly overestimated due to fortified cereals) did not prevent the increases in the prevalence of chronic diseases – despite deficiencies observed for some nutrients in 2019 (i.e. calcium, vitamin E and retinol) and a still slightly too high lipid share as regards to the protein/carbohydrate/protein ratio(77). This statement in general population was in agreement with the one observed in rural China from 1991 to 2011(78) and with those found in the China Health and Nutrition Survey (1991–2015) for 40 088 females aged 18–64 years(35) and other data reported in 2017(79). This analysis at the level of food nutrients suggests an apparent more diversified dietary pattern during this nutrition transition, but not really reflected by an overall improvement of the DRI, except for iodine, selenium, and vitamins D and B12, probably due to the increase in the consumption of animal products. Other decreases in fibre and micronutrients intakes during the 1990–2019 period are very likely to be due to the low level of consumption of minimally-processed plant-based food groups together with a sharp increase in IPF, and oils and fats intake. This evolution corresponds to the previously described shift from a traditional to a modern dietary pattern (with more IPFs) during the 1991–2011 period in The China Health and Nutrition Survey, and associated with increase cardio metabolic risks(37).
Nutrition transition towards a Western dietary pattern
With regard to nutrition transition in developed countries, there is therefore an underestimated sub-transition from IPF to UPF(5,80), with peak sales in Western countries occurring in the 1980s(5,80). In emerging countries, this transition is more recent, beginning in the early 1990s(3), with increasing rates of sales of UPF, with the sales peak yet to be reached(61,81). Thus, the main large emerging countries, such as Brazil, Russia, India and China (i.e. BRIC), are facing a rapid nutritional transition, notably characterised by an important increase in animal and IPF/UPF energy consumption(82–84), e.g. greater than 4 % of the compounding annual UPF growth rate for sales occurred in China and India(2). There was a 115 % growth rate of UPF sales between 2000 and 2013 in Asia and the Pacific region, while in the same period, the growth rates were only 2·3 % in the saturated market of the USA, 18·5 % in Western Europe and 25 % in Australia, compared with a global growth rate of 44 %(61). This rapid increase in UPF sales, both in China and Western countries, appears linked to rapid increases in chronic disease prevalence. The trends emphasised in this study support the previous analyses of the nutrition transition in developing and emerging countries, with a pattern of degenerative disease that is accelerating(85). Notably, we observed that UPF sales in China increased from 138·3 g/d/capita in 2013(61) to 218·3 g/d/capita in 2019(2), i.e. +58 %, a percentage close to the increase in IPF consumption between 2013 and 2019 (+50 %).
Limitations of the study
First, the ecological nature of our study may involve inference fallacies because correlations are not causalities, and chronic diseases are multifactorial. Other factors are obviously involved, such as the rapid migration from the countryside to cities, accelerating the transition from an active to a sedentary lifestyle(56,57), as well as environmental pollution, which reaches very high levels in China, notably leading to chronic obstructive pulmonary disease(86) and CVD(87). However, the observations made in this study are in agreement to what is observed in other countries where the prevalence of chronic diseases parallels the industrialisation of diets and foods, e.g. the Western countries(88) and Brazil(84).
Second, another potential limitation of this study comes from some of the extrapolations made from the original crude data, notably for calculating the food group and IPF energy intakes. Although several sources showed a decrease in total energy intake during the 1990–2019 period, total energy intake was not consistent in those sources. For example, Li et al. found a total energy intake of approximately 2000/d in 2010(47), while we found, based on food groups, 2700 kcal/d. Despite discrepancies in absolute values, the relative tendencies to a lower energy intake shown in this study are in agreement with the previous literature. In addition, the IPF energy intake was extrapolated from only four data points(2). However, the change in the intake of IPF was quasi linear between 1999 and 2017 (R 2 = 0·99, P < 0·05), which suggests that the extrapolation for the missing period was quite reliable. Otherwise, it should not be excluded, especially in overweight/obese individuals, that participants in surveys tend to underreport their food intakes and can contribute to the observed decreased energies intake during the 1990–2019 period.
Finally, our data are very broad in scope and obviously do not reflect important disparities between urban and rural populations or between different Chinese regions, counties and/or ethnicities. Therefore, results of this study are only indicative and only reflect a very global tendency of the nutrition transition in China.
Conclusions and perspectives
As regard to the increased rates of diet-associated chronic disease prevalence, China is undoubtedly joining the ranks of developed countries. Despite a slight decreased total energy consumption and small variations of adequacy with DRI, the farther the Chinese population moved away from the 3Vs rule during the 1990–2019 period, the more the prevalence of chronic diseases increased. Therefore, the types of food groups and their degree of processing, rather than their only nutrient content, are very likely to be involved in the relationship between calorie consumption and health, showing that addressing only one dimension of the 3Vs rule is insufficient for achieving a healthy diet and preventing chronic diseases. This may show that the adequacy of the diet with regard to the DRI is an insufficient indicator of a healthy diet, especially when animal and UPF energy intakes drastically increase, as has occurred in many Western countries. Thus, food health potential cannot be summarised as the sum of nutrients and/or energies, and the quality of energies – and the complex food matrix environment in which they are embedded – matters more than macro- and micro-nutrient and total energetic intakes as such, i.e. the composition of foods. In the end, analysing food group-based diets by including the degree of processing may better reflect their real overall nutritional quality. Therefore, to conform to the 3Vs rule, it appears more relevant to consume mildly processed nutrient-dense foods than micronutrient-enriched IPF or UPF.
Such correlations between the 3Vs rule and chronic disease prevalence deserve attention, and this analysis should be carried out in other emerging countries, such as Brazil and Russia, as well as in less developed countries, notably in South-Asia and Africa. In the latter regions, national recommendations are still based on satisfying the nutrients’ need while the population tend to adopt Western dietary patterns, increasing energies provided by animal-based foods, IPF and UPF, and sedentary lifestyles. Therefore, the 3Vs rule appears as a relevant lever to ‘drive’ the nutrition transitions in emerging countries, and those beginning in developing countries. It means improving the already existing traditional food systems while developing minimal food processing and avoiding industrialisation of traditional foods that irremediably leads to an economy of scale of food system in favour of food security and safety, but detrimental for sustainable health and environmental securities.
Acknowledgements
Acknowledgements: None. Financial support: This review article has been funded by the INRAE/Cirad’s GloFoodS (‘Transitions for World Food Security’) metaprogram. Conflict of interest: Anthony Fardet has been a member of the scientific committee of the Siga and Wuji & Co. societies since 2017. He is also co-president of the scientific committee of the Complexus Care Association. Edmond Rock declares no conflicts of interest. The INRAE/Cirad GloFoodS metaprogram funder had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript or in the decision to publish the results. Authorship: The present study was developed by A.F. and E.R., who formulated the research question and designed the study. Data collection and formatting according to the 3Vs rule was carried out by K.A. and A.F. A.F. took the lead on writing the manuscript. A.F. and K.A. designed the figures and tables and analysed the data. All authors reviewed and approved the final manuscript. Ethics of human subject participation: Not applicable.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980021003311.
click here to view supplementary material
References
- 1. Popkin BM, Corvalan C & Grummer-Strawn LM (2019) Dynamics of the double burden of malnutrition and the changing nutrition reality. Lancet 395, 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baker P, Machado P, Santos T et al. (2020) Ultra-processed foods and the nutrition transition: Global, regional and national trends, food systems transformations and political economy drivers. Obes Rev 21, e13126. [DOI] [PubMed] [Google Scholar]
- 3. Popkin BM (2001) Nutrition in transition: the changing global nutrition challenge. Asia Pac J Clin Nutr 10, S13–S18. [PubMed] [Google Scholar]
- 4. FAO, Monteiro CA, Cannon G et al. (2019) Ultra-Processed Foods, Diet Quality, and Health Using the NOVA Classification System. Rome, Italy: FAO. [Google Scholar]
- 5. Monteiro CA, Cannon G, Levy RB et al. (2019) Ultra-processed foods: what they are and how to identify them? Public Health Nutr 22, 936–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Popkin BM (2011) Contemporary nutritional transition: determinants of diet and its impact on body composition. Proc Nutr Soc 70, 82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Elizabeth L, Machado P, Zinöcker M et al. (2020) Ultra-processed foods and health outcomes: a narrative review. Nutrients 12, 1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Askari M, Heshmati J, Shahinfar H et al. (2020) Ultra-processed food and the risk of overweight and obesity: a systematic review and meta-analysis of observational studies. Int J Obes 44, 2080–2091. [DOI] [PubMed] [Google Scholar]
- 9. Pagliai G, Dinu M, Madarena MP et al. (2020) Consumption of ultra-processed foods and health status: a systematic review and meta-analysis. Br J Nutr 125, 308–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lane MM, Davis JA, Beattie S et al. (2020) Ultraprocessed food and chronic noncommunicable diseases: a systematic review and meta-analysis of 43 observational studies. Obes Rev 22, e13146. [DOI] [PubMed] [Google Scholar]
- 11. Wijarnpreecha K, Thongprayoon C, Edmonds PJ et al. (2016) Associations of sugar- and artificially sweetened soda with nonalcoholic fatty liver disease: a systematic review and meta-analysis. QJM 109, 461–466. [DOI] [PubMed] [Google Scholar]
- 12. Rey-García J, Donat-Vargas C, Sandoval-Insausti H et al. (2021) Ultra-processed food consumption is associated with renal function decline in older adults: a prospective cohort study. Nutrients 13, 428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fiolet T, Srour B, Sellem L et al. (2018) Consumption of ultra-processed foods and cancer risk: results from NutriNet-Santé prospective cohort. BMJ 360, k322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rico-Campà A, Martínez-González MA, Alvarez-Alvarez I et al. (2019) Association between consumption of ultra-processed foods and all cause mortality: SUN prospective cohort study. BMJ 365, l1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fardet A & Rock E (2020) Ultra-processed foods and food system sustainability: what are the links? Sustainability 12, 6280. [Google Scholar]
- 16. Fardet A & Rock E (2020) How to protect both health and food system sustainability? A holistic ‘global health’-based approach via the 3V rule proposal. Public Health Nutr 23, 3028–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fardet A & Rock E (2019) Ultra-processed foods: a new holistic paradigm? Trends Food Sci Technol 93, 174–184. [Google Scholar]
- 18. Kyu HH, Abate D, Abate KH et al. (2018) Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392, 1859–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Scrinis G (2013) Nutritionism – The Science and Politics of Dietary Advice. New York, USA: Columbia University Press. [Google Scholar]
- 20. Monteiro CA, Geoffrey C, Jean-Claude M et al. (2018) The UN decade of nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutr 21, 5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fardet A & Rock E (2020) Exclusive reductionism, chronic diseases and nutritional confusion: degree of processing as a lever for improving public health. Crit Rev Food Sci Nutr, 1–16. doi: 10.1080/10408398.2020.1858751. [DOI] [PubMed] [Google Scholar]
- 22. Murray CJL, Aravkin AY, Zheng P et al. (2020) Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396, 1223–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Johnston JL, Fanzo JC & Cogill B (2014) Understanding sustainable diets: a descriptive analysis of the determinants and processes that influence diets and their impact on health, food security, and environmental sustainability. Adv Nutr 5, 418–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Forouzanfar MH, Afshin A, Alexander LT et al. (2016) Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388, 1659–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yin J, Zhang X, Huang W et al. (2021) The potential benefits of dietary shift in China: synergies among acceptability, health, and environmental sustainability. Sci Total Environ 779, 146497. [DOI] [PubMed] [Google Scholar]
- 26. Lei L & Shimokawa S (2017) Promoting dietary guidelines and environmental sustainability in China. China Econ Rev 59, 101087. [Google Scholar]
- 27. Fardet A & Rock E (2018) Reductionist nutrition research has meaning only within the framework of holistic thinking. Adv Nutr 9, 655–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. OECD & FAO (2019) OECD Statistics. https://stats.oecd.org/viewhtml.aspx?datasetcode=HIGH_AGLINK_2019&lang=fr# (accessed September 2020).
- 29. Statista (2020) Insights and Facts across 170 Industries and 150+ Countries. https://www.statista.com/ (accessed September 2020).
- 30. FAO (2020) FAOSTAT. http://www.fao.org/faostat/fr/#home (accessed September 2020).
- 31. Roser M & Ritchie H (2013) Our World in Data – Food Supply. https://ourworldindata.org/food-supply (accessed September 2020).
- 32. Popkin BM, Du SF, Zhai FY et al. (2010) Cohort profile: the China Health and Nutrition Survey-monitoring and understanding socio-economic and health change in China, 1989–2011. Int J Epidemiol 39, 1435–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang Y, Su C, Ouyang YF et al. (2020) Secular trends in sedentary behaviors and associations with weight indicators among Chinese reproductive-age women from 2004 to 2015: findings from the China Health and Nutrition Survey. Int J Obes 44, 2267–2278. [DOI] [PubMed] [Google Scholar]
- 34. Huang LN, Wang HJ, Wang ZH et al. (2019) Regional disparities in the association between cereal consumption and metabolic syndrome: results from the China Health and Nutrition Survey. Nutrients 11, 764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhao J, Su C, Wang HJ et al. (2018) Secular trends in energy and macronutrient intakes and distribution among adult females (1991–2015): results from the China Health and Nutrition Survey. Nutrients 10, 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shen X, Fang AP, He JJ et al. (2017) Trends in dietary fat and fatty acid intakes and related food sources among Chinese adults: a longitudinal study from the China Health and Nutrition Survey (1997–2011). Public Health Nutr 20, 2927–2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li M & Shi ZM (2017) Dietary pattern during 1991–2011 and its association with cardio metabolic risks in Chinese adults: the China Health and Nutrition Survey. Nutrients 9, 1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Han LL, Wang YX, Li J et al. (2014) Gender differences in associations of serum ferritin and diabetes, metabolic syndrome, and obesity in the China Health and Nutrition Survey. Mol Nutr Food Res 58, 2189–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Xi B, He D, Hu YH et al. (2013) Prevalence of metabolic syndrome and its influencing factors among the Chinese adults: the China Health and Nutrition Survey in 2009. Prev Med 57, 867–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yan S, Li J, Li S et al. (2012) The expanding burden of cardiometabolic risk in China: the China Health and Nutrition Survey. Obes Rev 13, 810–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Baker P & Friel S (2014) Processed foods and the nutrition transition: evidence from Asia. Obes Rev 15, 564–577. [DOI] [PubMed] [Google Scholar]
- 42. World Health Organization & Food and Agriculture Organization of the United Nations (2004) Vitamin and Mineral Requirements in Human Nutrition, 2nd ed. Bangkok, Thailand: WHO, FAO. [Google Scholar]
- 43. ANSES (2017) Table CIQUAL. Composition nutritionnelle des aliments (Nutritional composition of foods). http://wwwafssafr/TableCIQUAL/ (accessed September 2020).
- 44. Malik VS & Hu FB (2019) Sugar-sweetened beverages and cardiometabolic health: an update of the evidence. Nutrients 11, 1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chinese Nutrition Society (2013) Dietary Reference Intake for Chinese. Beijing, China: People’s Medical Publishing House. [Google Scholar]
- 46. The Editorial Committee of China Agricultural Statistical Yearbook (ECCASY) (2012) China Agriculture Yearbook. Beijing, China: China Agriculture Press. [Google Scholar]
- 47. Jian Ping L & Zhou Ping S (2012) Food consumption patterns and per-capita calorie intake of China in the past three decades. J Food Agric Environ 10, 201–206. [Google Scholar]
- 48. Gouel C & Guimbard H (2018) Nutrition Transition and the Structure of Global Food Demand. Paris, France: Centre d’Etudes Prospectives et d’Informations Internationales. [Google Scholar]
- 49. Ding CC, Feng GY, Yuan F et al. (2020) Temporal trends and recent correlates in sedentary behaviors among Chinese adults from 2002 to 2010–2012. Int J Environ Res Public Health 17, 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Camacho S & Ruppel A (2017) Is the calorie concept a real solution to the obesity epidemic? Glob Health Action 10, 1289650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Campbell TC (2004) The China Study: The Most Comprehensive Study of Nutrition Ever Conducted and the Startling Implications for Diet, Weight Loss and Long-Term Health. Dallas, Texas, USA: BenBella Books. [Google Scholar]
- 52. Afshin A, Sur PJ, Fay KA et al. (2019) Health effects of dietary risks in 195 countries, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 393, 1958–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. You W & Henneberg M (2016) Meat in modern diet, just as bad as sugar, correlates with worldwide obesity: an ecological analysis. J Nutr Food Sci 6, 1000517. [Google Scholar]
- 54. Meier T, Gräfe K, Senn F et al. (2019) Cardiovascular mortality attributable to dietary risk factors in 51 countries in the WHO European Region from 1990 to 2016: a systematic analysis of the Global Burden of Disease Study. Eur J Epidemiol 34, 37–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shi R, Cai YM, Qin R et al. (2020) Dose-response association between physical activity and clustering of modifiable cardiovascular risk factors among 26,093 Chinese adults. BMC Cardiovasc Disord 20, 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wang ZY, Qin ZZ, He J et al. (2019) The association between residential density and physical activity among urban adults in regional China. BMC Public Health 19, 1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tu RQ, Li YQ, Shen LJ et al. (2019) The prevalence and influencing factors of physical activity and sedentary behaviour in the rural population in China: the Henan Rural Cohort Study. BMJ Open 9, e029590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. GBD 2013 Mortality and Causes of Death Collaborators (2015) Global, regional, and national age – sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 385, 117–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Davidou S, Christodoulou A, Fardet A et al. (2020) The holistico-reductionist Siga classification according to degree of food processing: an evaluation of ultra-processed foods in French supermarkets. Food Funct 11, 2026–2039. [DOI] [PubMed] [Google Scholar]
- 60. Beslay M, Srour B, Méjean C et al. (2020) Ultra-processed food intake in association with BMI change and risk of overweight and obesity: a prospective analysis of the French NutriNet-Santé cohort. PLoS Med 17, e1003256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pan American Health Organization (2015) Ultra-Processed Food and Drink Products in Latin America: Trends, Impact on Obesity, Policy Implications. Washington, DC, USA: PAHO. [Google Scholar]
- 62. Monteiro CA, Moubarac J-C, Levy RB et al. (2017) Household availability of ultra-processed foods and obesity in nineteen European countries. Public Health Nutr 21, 18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kelly T, Yang W, Chen CS et al. (2008) Global burden of obesity in 2005 and projections to 2030. Intl J Obes 32, 1431–1437. [DOI] [PubMed] [Google Scholar]
- 64. Srour B, Fezeu LK, Kesse-Guyot E et al. (2019) Ultra-processed food intake and risk of cardiovascular disease: prospective cohort study (NutriNet-Santé). BMJ 365, l1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Srour B, Fezeu LK, Kesse-Guyot E et al. (2019) Ultraprocessed food consumption and risk of type 2 diabetes among participants of the NutriNet-Santé prospective cohort. JAMA Intern Med 180, 283–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Steele EM, Juul F, Neri D et al. (2019) Dietary share of ultra-processed foods and metabolic syndrome in the US adult population. Prev Med 125, 40–48. [DOI] [PubMed] [Google Scholar]
- 67. Tavares LF, Fonseca SC, Garcia Rosa ML et al. (2012) Relationship between ultra-processed foods and metabolic syndrome in adolescents from a Brazilian Family Doctor Program. Public Health Nutr 15, 82–87. [DOI] [PubMed] [Google Scholar]
- 68. Wu W, Qiu J, Wang A et al. (2019) Impact of whole cereals and processing on type 2 diabetes mellitus: a review. Crit Rev Food Sci Nutr 60, 1447–1474. [DOI] [PubMed] [Google Scholar]
- 69. Sun Q, Spiegelman D, van Dam RM et al. (2010) White rice, brown rice, and risk of type 2 diabetes in US men and women. Arch Intern Med 170, 961–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Fardet A & Boirie Y (2014) Associations between food and beverage groups and major diet-related chronic diseases: an exhaustive review of pooled/meta-analyses and systematic reviews. Nutr Rev 72, 741–762. [DOI] [PubMed] [Google Scholar]
- 71. Della Pepa G, Vetrani C, Vitale M et al. (2018) Wholegrain intake and risk of type 2 diabetes: evidence from epidemiological and intervention studies. Nutrients 10, 1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Liang W, Lee AH & Binns CW (2010) White rice-based food consumption and ischemic stroke risk: a case-control study in Southern China. J Stroke Cerebrovasc Dis 19, 480–484. [DOI] [PubMed] [Google Scholar]
- 73. Li MZ, Su L, Liang BY et al. (2013) Trends in prevalence, awareness, treatment, and control of diabetes mellitus in mainland china from 1979 to 2012. Int J Endocrinol 2013, 753150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Dong F, Howard A-G, Herring AH et al. (2015) White rice intake varies in its association with metabolic markers of diabetes and dyslipidemia across region among Chinese adults. Ann Nutr Metab 66, 209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kouvari M, Notara V, Kalogeropoulos N et al. (2016) Diabetes mellitus associated with processed and unprocessed red meat: an overview. Int J Food Sci Nutr 67, 735–743. [DOI] [PubMed] [Google Scholar]
- 76. Bray GA, Nielsen SJ & Popkin BM (2004) Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr 79, 537–543. [DOI] [PubMed] [Google Scholar]
- 77. Joint WHO/FAO Expert Consultation (2003) Diet, Nutrition and the Prevention of Chronic Diseases. WHO Technical Report Series 916. Geneva, Switzerland: WHO. [PubMed] [Google Scholar]
- 78. Su C, Zhao J, Wu Y et al. (2017) Temporal trends in dietary macronutrient intakes among adults in rural China from 1991 to 2011: findings from the CHNS. Nutrients 9, 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wan Y, Wang F, Yuan J et al. (2017) Optimal dietary macronutrient distribution in China (ODMDC): a randomised controlled-feeding trial protocol. Asia Pac J Clin Nutr 26, 972–980. [DOI] [PubMed] [Google Scholar]
- 80. Monteiro CA, Moubarac JC, Cannon G et al. (2013) Ultra-processed products are becoming dominant in the global food system. Obes Rev 14, 21–28. [DOI] [PubMed] [Google Scholar]
- 81. PAHO & WHO (2019) Ultra-Processed Food and Drink Products in Latin America: Sales, Sources, Nutrient Profiles, and Policy Implications. Washington, DC, USA: PAHO, WHO. [Google Scholar]
- 82. Burggraf C, Kuhn L, Zhao QR et al. (2015) Economic growth and nutrition transition: an empirical analysis comparing demand elasticities for foods in China and Russia. J Integ Agric 14, 1008–1022. [Google Scholar]
- 83. Law C, Fraser I & Piracha M (2020) Nutrition transition and changing food preferences in India. J Agric Econ 71, 118–143. [Google Scholar]
- 84. Conde WL & Monteiro CA (2014) Nutrition transition and double burden of undernutrition and excess of weight in Brazil. Am J Clin Nutr 100, 1617S–1622S. [DOI] [PubMed] [Google Scholar]
- 85. Popkin BM (2001) Nutrition in transition: the changing global nutrition challenge. Asia Pac J Clin Nutr 10, S13–S18. [PubMed] [Google Scholar]
- 86. Chen X, Wang T, Qiu XH et al. (2020) Susceptibility of individuals with chronic obstructive pulmonary disease to air pollution exposure in Beijing, China: a case-control panel study (COPDB). Sci Total Environ 717, 137285. [DOI] [PubMed] [Google Scholar]
- 87. Xu JX, Geng WF, Geng XY et al. (2020) Study on the association between ambient air pollution and daily cardiovascular death in Hefei, China. Environ Sci Pollut Res Int 27, 547–561. [DOI] [PubMed] [Google Scholar]
- 88. Raj S (2020) Influences of the nutrition transition on chronic disease. In Integrative and Functional Medical Nutrition Therapy: Principles and Practices, pp. 17–29 [Noland D, Drisko JA and Wagner L, editors]. New York, USA; Cham: Springer International Publishing. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980021003311.
click here to view supplementary material