Abstract
Objective:
To analyse the presence of cardiometabolic risk factors in adolescents with normal-weight obesity (NWO), as well as to investigate health behaviours related to the phenotype.
Design:
The study was conducted according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses guidelines and the bibliographic search was carried out in the PubMed, Scielo and ScienceDirect databases.
Setting:
School, university and population.
Participants:
Adolescents between 10 and 19 years old.
Results:
A total of eight papers were included. Most studies have found a relationship between NWO and the presence of cardiometabolic risk factors, such as high waist circumference, unfavourable lipid and glycid profile. As for health behaviours, three of the eight studies included evaluated eating habits; however, the results were not conclusive. In addition, four studies analysed the practice of physical activity or physical fitness, which was lower in NWO.
Conclusions:
The available evidence indicates that NWO is related to the early development of cardiometabolic changes, physical inactivity and less physical fitness in adolescents. The results also reveal the importance of early detection of the phenotype, as well as the need for further research on the associated factors to prevent future diseases. Registration (PROSPERO: CRD42020161204).
Keywords: Normal-weight obese, Normal-weight obesity, Obesity, Teenager, Adolescent, Adiposity
Adolescence is a complex stage, in which the transition from childhood to adulthood takes place, that promotes puberty-related body changes, as well as social adaptations(1). The most common indicator to assess the nutritional state of adolescents is the BMI, obtained on dividing the weight by the square of the height, and analysed according to sex and age(2). However, the isolated use of BMI has its limitations, as it cannot differ muscle, bone and fat mass(3–5). Thus, more than a quarter of children and adolescents with a high percentage body fat may be misclassified as eutrophic when only BMI is used(6).
Therefore, literature has investigated other obesity phenotypes that affect individuals with normal weight, such as ‘normal-weight obesity’ (NWO). The expression was introduced by De Lorenzo et al. (2006)(7) who adopted it as a criterion for identifying individuals with normal weight, yet with high percentage body fat. Studies carried out in adults indicate positive associations between NWO and cardiometabolic deregulation(8–10).
Thus, regardless of a normal BMI, NWO presents a low-grade pro-inflammatory state, increased oxidative stress, insulin resistance and dyslipidemia, which lead to an increased risk of metabolic syndrome, CVD and death related to the cardiovascular system, due to the accumulation of body fat(7,11–18). Furthermore, modifiable behavioural factors, such as physical inactivity(19,20), smoking and low fibre diet(19), have been associated with this phenotype.
In adolescents, NWO may go unnoticed for years, due to the young age and normal body weight(21). Hence, the identification of NWO may uncover an unknown risk group, and understanding which factors are related to this phenomenon is key to assist in building effective prevention and intervention strategies. To our knowledge, to date, no systematic review has assessed NWO exclusively in adolescents and the association of this phenotype with cardiometabolic risk remains uncertain, as well as the health behaviours practised by this public. Thus, this systematic review aims to analyse the presence of cardiometabolic risk factors in adolescents with NWO, as well as to investigate the health behaviours associated with the phenotype.
Methods
Data sources and research strategy
The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA)(22) guidelines were followed for this systematic review, which was registered in the International prospective register of systematic reviews (PROSPERO: CRD42020161204). The research was based on the components of the PECO acronym, with the following guiding question: “What evidence is available about the relationship between NWO in adolescence and the presence of cardiometabolic risk factors? What are the health behaviours related to the phenotype in adolescents? The literature search was performed through the National Library of Medicine (PubMed), Scientific Electronic Library Online (Scielo) and the ScienceDirect.
The selection of studies was carried out between December 2019 and September 2020, the last search being carried out in September 2020. The references were stored in the Zotero bibliographic software, version 5.0. The steps were carried out in duplicate, without the use of filters, using descriptors from Medical Subject Headings (MeSH), Descritores em Ciências da Saúde (DecS) and other search terms related to the theme, with the following strategy: (adolescent OR adolescence OR teen OR teenager OR youth OR children) AND (‘normal weight obesity’ OR ‘normal weight obese’). The complete search strategy is available in the supplementary file.
Study selection
The steps of inclusion and exclusion of papers (Fig. 1) began with the identification of the papers in the databases. Then, duplicates were excluded. Afterwards, title and abstract screening was performed by two independent reviewers (B. C. C. and L. G. S.) in order to identify studies that met the inclusion criteria. Finally, reading and assessment of the full texts were also performed by the aforementioned reviewers, and divergences were solved through discussions until an agreement was reached. These steps were performed using the Rayyan QCRI software(23).
Fig. 1.
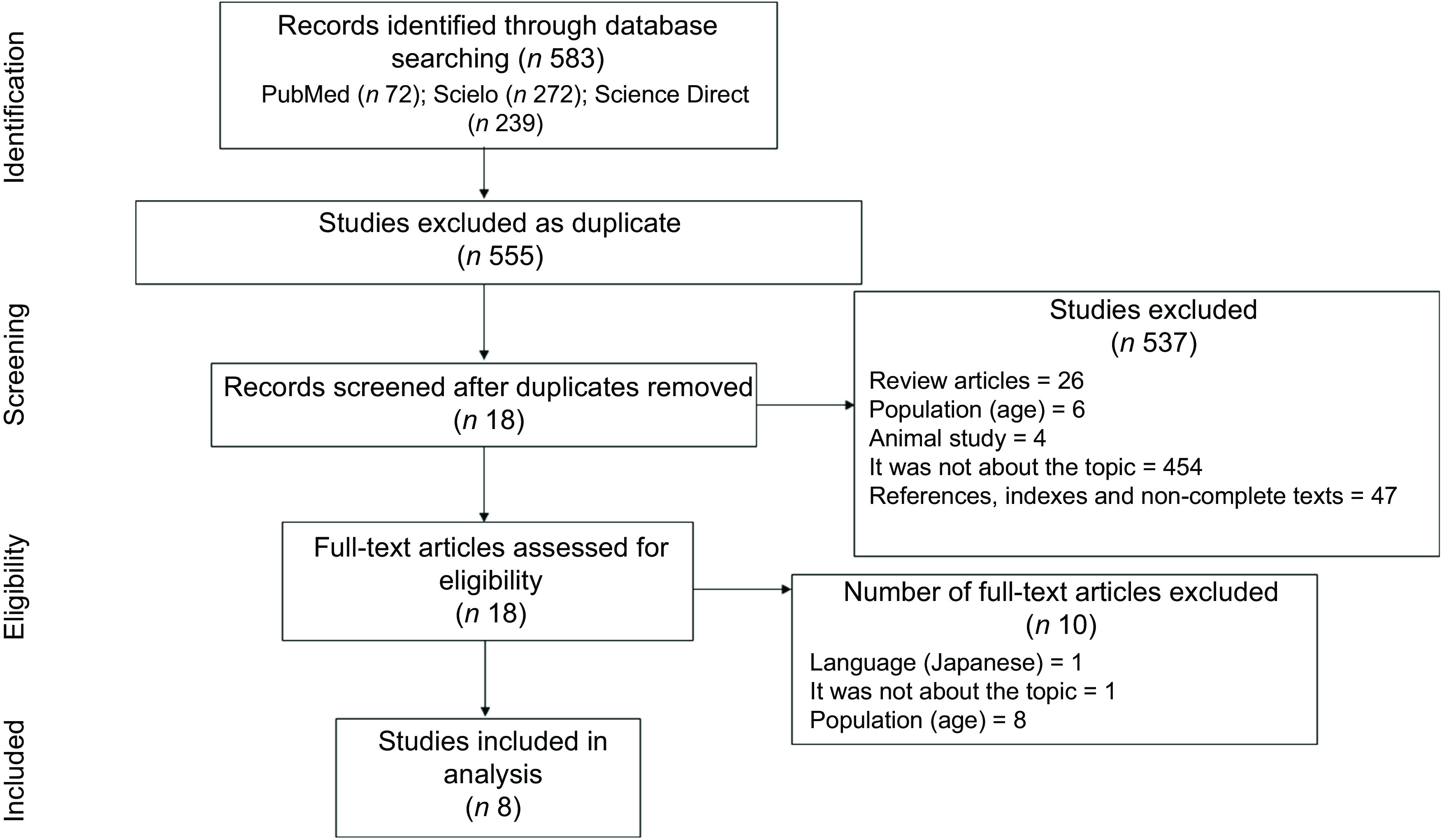
Flow chart of the literature search and study selection procedures, according to PRISMA recommendation. PRISMA, The Preferred Reporting Items for Systematic reviews and Meta-Analyses
Definition of the normal-weight obese phenotype
Adolescents with adequate weight for height, but with excess body fat, were called NWO. The specific criteria for defining the phenotype, such as the BMI normal range and adiposity considered excessive, were different, according to the authors (Table 1).
Table 1.
Studies characteristics that investigated normal-weight obesity in adolescents
| Study (author, location and year) | Sample size | Desing | Age range (years) | Body fat assessment method | Diagnostic criteria for NWO | NWO prevalence | Objective |
|---|---|---|---|---|---|---|---|
| Bragança et al., Brazil, 2020(29) | 328 M and 205 F | CS | 18–19 | ADP | BMI < 25·0 kg/m2 and %BF ≥ 25·0 in males and ≥ 30·0 in females | 6·8 % of the total sample (or 8·9 % of eutrophic individuals by BMI) | To compare biomarkers in adolescentes classified simultaneously by BMI and body fat percentage |
| García-Hermoso et al., Colombia, 2020(30) | 902 M and 1017 F | CS | 9–17 | BIA | BMI < 25·0 kg/m2 and %BF over the sex- and age-specific 90·0th percentile for Colombian children and adolescents (boys > 23·4–28·3 % and girls > 31·0–34·1 %) | 46·0 % (sample with only eutrophic by BMI) | Investigate if Colombian youth with NWO have a poorer cardiometabolic profile and physical fitness performance than their normal-weight peers and to determine if physical fitness levels are related to prevalence of NWO |
| Musálek et al., Czech Republic, 2018(28) | 100 M and 110 F | CS | 9–12 | Skinfolds * | BMI 25·0–60·0th percentile, along with average values from three skinfolds > 85·0th of Czech national reference | † | Investigate the difference in skeletal robustness and lean-fat ratio on the extremities as indicators of health development that are important for: muscular competence, physical fitness and bone health |
| Cheng and Wiklund, Finland, 2018(27) | 236 F | L | 9–18 | DXA | Relative weight between –10·0 % and +20·0 %‡ and %BF ≥ 30·0 | 39·0 % of the total sample (or 51·4 % of eutrophic individuals by BMI) | To study whether NWO in childhood and adolescence is associated with increased cardiometabolic risk in early adulthood |
| Wiklund et al., Finland, 2017(26) | |||||||
| Olafsdottir, Torfadottir and Arngrimsson, Iceland, 2016(20) | 96 M and 86 F | CS | 17–18 | DXA | BMI 18·5–24·9 kg/m2 and %BF above 17·6 % in males and above 31·6 % in females | 42·0 % (sample with only eutrophic by BMI) | To explore health behaviours and metabolic risk factors in NWO adolescents |
| Yaguchi-Tanaka et al., Japan, 2013(32) | 72 F | CS | 18 § | BIA | BMI 18·5–24·9 kg/m2 and %BF ≥ 30·0 | 55·6 % (sample with only eutrophic by BMI) | Examine the relationship between NWO and dietary habits |
| Serrano et al., Brazil, 2010(31) | 113 F | CS | 14–18 | BIA | BMI > 10·0 to < 85·0th percentile and %BF ≥ 28·0 | 33·6 % of the total sample (or 48·7 % of those eutrophic by BMI) | Assess body composition, anthropometric, biochemical and clinical changes |
NWO, normal-weight obesity; M, male; F, female; CS, cross-sectional study; ADP, air displacement plethysmography; %BF, body fat percentage; BIA, bioelectrical impedance analysis; L, longitudinal study; DXA, dual-energy X-ray absorptiometry.
Values of the three skinfolds (over triceps, subscapular and suprailliac) were compared with anthropometric references for Czech children.
Research with a sample of balanced groups, it is not possible to define actual prevalence of NWO.
Growth charts of each participant were obtained from the Finnish School Health Care System. To be able to compare growth at certain time points, the weight per cent (%) and height z-score were extrapolated from growth charts using a form that was created by the Finnish Paediatric Research Association and accepted by the Finnish National Health Administration (Form No. 7466:92). On the basis of their growth chart data, participants were classified into underweight (relative weight to height from growth chart under –10 %), normal weight (relative weight between –10 and + 20 %) and overweight + obesity (relative weight greater than + 20 %).
Age group not informed, but average age of participants was 18 years.
Inclusion and exclusion criteria
The inclusion criteria were based on the selection of original articles carried out with humans published in English, Spanish or Portuguese that assessed the relationship of NWO with the presence of cardiometabolic risk factors or with health behaviours. Participants should be between 10 and 19 years old, that is, be considered a teenager, according to the WHO definition(24). As many papers in the literature are not performed only with the age group of interest, the inclusion criteria were studies in which the majority of participants were between 10 and 19 years old, allowing a variation of +/–1 year of age in sample (9–20 years), as long as the mean/median age is between 10 and 19 years. There was no restriction on the date of publication of the articles. Revisions, book chapters, publications whose full texts were not available, studies with animals, studies with adults and manuscripts that did not meet the inclusion criteria mentioned above were excluded.
Quality assessment
The included studies underwent an analysis, performed in duplicate by independent reviewers (B. C. C. and N. N. L.), using the Quality Assessment Tool for Observational Cohort and Cross-sectional Studies, by National Heart, Lung, and Blood Institute(25). Discrepancies were resolved by consensus.
Data analysis and extraction
Information from each study regarding the author(s), year of publication, study design, country, sample size and according to sex, age, authors’ criteria for defining NWO, method used to evaluate the variables, main objectives, NWO prevalence and main results were extracted.
Results
Included studies
A total of 583 papers were identified following the search through the databases. After removing duplicates, the title and abstract of 555 papers were read. Of these, 537 were excluded, of which 26 were reviews, 6 were not carried out with NWO adolescents, 4 were related to animal study, 454 did not address the topic and 47 were references, indexes or non-complete texts. Thus, 18 were selected for complete reading. After reading the full text, 10 were excluded for not meeting the inclusion criteria, of which 1 was not in an English, Portuguese or Spanish version, 1 did not address the NWO theme and 8 were not conducted with NWO adolescents (Fig. 1) (Supplementary file).
The overall characteristics of the included studies
The overall characteristics of the studies that investigated factors related to NWO are displayed in Table 1. It was found that the articles by Wiklund et al. (2017)(26) and Cheng and Wiklund (2018)(27) come from the same population, that is, from the same study. Thus, for counting the sample size, prevalence of the phenotype, criteria adopted to define NWO, study design and place of performance, they were considered only once, as a study, in order to avoid duplication.
Among the selected studies, three were conducted in Europe(20,26–28), three in South America(29–31) and one in Asia(32). Most articles had a cross-sectional design, except one that was longitudinal(26,27). The year of publication of the studies varied from 2010(31) to 2020(29,30), revealing that it is recent theme (Table 1).
The total sample size was 3265 individuals, mostly females (56·3 %; n 1839). It is valid to consider that total sample is made up of both underweight and overweight, as well as normal-weight individuals. It is important to emphasise that these four studies(26–29,31) were not performed only with eutrophic individuals. From the entire sample (n 3265), 1240 subjects were classified as NWO (40·0 %). And, among those who had adequate weight (n 2864), 43·3 % (n 1240) had NWO, that is, a relevant percentage of people classified as eutrophic according to the BMI had NWO.
The most used method for assessing body fat was bioelectrical impedance analysis(30–32), followed by dual-energy X-ray absorptiometry(20,26,27). The cut-off points adopted, both for BMI and for excess body fat, in the definition of NWO, were very diverse (Table 1).
Cardiometabolic risk and normal-weight obesity
Most of the studies observed that NWO in adolescents is related to the presence of cardiometabolic risk factors (Table 2). In this context, in the study by Yaguchi-Tanaka et al. (2013)(32), individuals with the NWO phenotype displayed higher values of BMI and weight, compared to the eutrophic individuals without the phenotype.
Table 2.
Main results of the included studies
| Author and year | Statistical analysis and adjustment | Main conclusions (NWO in relation to eutrophic individuals without NWO) |
|---|---|---|
| Bragança et al., 2020(29) | Chi-square | NWO were more sedentary (66·7 % v. 32·5 %; P = 0·006). |
| ANOVA | NWO with higher values of waist circumference (cm) (82·8 [sd 6·0] v. 78·5 [sd 6·2]; P < 0·05), total cholesterol (mg/dl) (172·5 [SD 29·9] v. 149·6 [sd 26·5]; P < 0·05), LDL (mg/dl) (103·5 [sd 26·8] v. 84·9 [sd 22·9]; P < 0·05) and HDL (49·7 [SD 11·7] v. 46·9 [SD 10·9]; P < 0·05). In addition to lower muscle mass in kg (34·2 [sd 5·3] v. 43·0 [sd 9·3]; P < 0·05). | |
| Kruskal–Wallis | NWO with higher BMI values (kg/m2) (22·6 [IQ 21·2–23·5] v. 20·0 [IQ 18·6–2·0]; P < 0·05) and IL 6 (pg/ml) (2·4 [IQ 1·3–3·5] v. 1·5 [IQ 1·0–2·7]; P < 0·05). | |
| García-Hermoso et al., 2020(30) | Mann–Whitney | NWO with higher mean BMI (kg/m2) (19·67 [sd 1·91] v. 17·86 [sd 1·85]; P < 0·001), height (m) (1·55 [sd 1·12] v. 1·50 [sd 0·12]; P < 0·001), weight (kg) (47·79 [sd 9·66] v. 41·09 [sd 9·32]; P < 0·001), fat mass (kg) (9·89 [sd 3·56] v. 7·24 [sd 3·09]; P < 0·001) and age (13·86 [sd 2·21] v. 12·82 [sd 2·11]; P < 0·001). In addition, less adherence to the Mediterranean diet assessed by the average score using the KIDMED questionnaire (3·4 [sd 1·6] v. 4·0 [sd 1·7]; P < 0·001). |
| ANCOVA stratified by sex and adjusted for age, BMI, pubertal stage and Mediterranean diet adherence | NWO was related to boys (65·85 [sd 6·13] v. 61·78 [sd 5·12]; P = 0·042) and girls (63·94 [sd 5·89] v. 59·12 [sd 5·07]; P < 0·001) with greater waist circumference. In addition, boys (–0·15 [sd 2·31] v. –0·97 [sd 2·10]; P = 0·019) and girls (–0·12 [sd 2·38] v. –1·08 [sd 2·31]; P = 0·044) with the highest mean cardiometabolic risk score*. Boys also had a higher mean TAG value (85·32 [sd 38·13] v. 76·72 [sd 30·8]; P = 0·017). | |
| Logistic regression model† stratified by sex and adjusted for age, pubertal stage and Mediterranean diet adherence | For both sexes, the prevalence of NWO was lower in young people classified as having healthy levels of cardiorespiratory fitness (boys: OR = 0·54, 95 % CI 0·37, 0·78; girls: OR = 0·35, 95 % CI 0·24, 0·50) (P < 0·001). | |
| Musálek et al., 2018(28) | One-way ANOVA with Bonferroni corrections (post hoc comparisons were used using a Fisher’s Partial Least Significant Difference) or Kruskal–Wallis non-parametric ANOVA with Z-value test after Kruskal–Wallis Multiple-Comparison Z-Value Test (Dunn’s Test) | Analysis of skeletal robustness indicated that NWO had a significantly lower index of z-score in the lower extremity for males (–0·85 ± 0·61 v. 0·07 ± 1·0; P < 0·001) and females (–0·43 ± 0·98 v. 0·08 ± 0·88 ; P < 0·01). In addition, the NWO boys had twice poorer robustness of the lower limbs z-score (–0·85) than NWO girls z-score (–0·43) (P < 0·001; Hays’ ω 2 = 0·81). The evaluation of the muscle area showed that the NWO had significantly smaller muscle area in the upper arm (in z-score) for males (–1·15 ± 0·71 v. 0·47 ± 0·88; P < 0·001) and females (–0·95 ± 0·64 v. 0·64 ± 0·51; P < 0·001), in addition to a smaller calf muscle area for males (–1·34 ± 0·45 v. 0·89 ± 0·54; P < 0·001) and females (–0·85 ± 0·58 v. 0·86 ± 0·55; P < 0·001). |
| Cheng and Wiklund, 2018(27) | A hierarchical (multilevel) non-linear model with random effects adjusted for whole body fat mass or android abdominal fat mass | The cardiometabolic risk, measured by a score‡, is significantly higher in NWO when compared to eutrophic patients without NWO before menarche and this difference persisted in early adulthood (P < 0·01). |
| Wiklund et al., 2017(26) | ANOVA with the least significant difference post hoc test | NWO with higher cardiometabolic risk, measured by a score§, already in childhood and the difference that persisted in early adulthood (P < 0·001) |
| Olafsdottir, Torfadottir and Arngrimsson, 2016(20) | Student;s t test (adjusted for sex) when variables had a normal distribution or by the Mann–Whitney nonparametric test otherwise. Statistical differences in categorical variables were calculated using the chi-square test. | NWO participants with greater waist circumference (cm) (male: 80·3 ± 5·4 v. 76·0 ± 3·5 [P < 0·001]; female: 77·5 ± 5·1 v. 71·7 ± 4·7 [P < 0·001]), BMI (kg/m2) (male: 22·2 ± 1·8 v. 21·2 ± 1·5 [P = 0·001]; female: 22·5 ± 1·8 v. 21·0 ± 1·5 [P < 0·001]) and weight (kg) (male: 73·7 ± 9·1 v. 70·5 ± 7·7 [P = 0·046]; female: 64·3 ± 5·7 v. 59·9 ± 5·2; P = 0·001]). In addition, a higher serum insulin value (μU/ml) among female participants (8·8 ± 3·7 v. 6·9 ± 3·1; P = 0·023). NWO women also had more insulin resistance (HOMA) (1·6 ± 0·6 v. 1·2 ± 0·6; P = 0·020). In addition, male (50·9 ± 5·7 v. 55·8 ± 5·5; P < 0·001) and female (37·8 ± 4·3 v. 43·2 ± 4·9; P < 0·001) participants had lower aerobic fitness measured in the laboratory (VO2 ml/kg/min), in addition to being more sedentary (28 % v. 15·2 %; P = 0·014) by analysing a questionnaire on physical activity. As for eating habits, NWO have a lower frequency of breakfast (56·0 % v. 73·3 %; P = 0·015) and vegetables (37·0 % v. 57·1 %; P = 0·008). |
| Logistic regression models adjusted for sex | NWO was positively associated with a 2·2-fold increased risk of having one or more risk factors for metabolic syndrome|| (OR = 2·2; 95 % CI: 1·2, 3·9). | |
| Yaguchi-Tanaka et al., 2013(32) | Student’s t test | Individuals with the NWO phenotype with higher BMI (22·3 ± 1·3 v. 20·2 ± 1·0; P < 0·01) and heavier (55·8 ± 5·0 v. 50·1 ± 3·8; P < 0·01). |
| Student’s t test using energy-adjusted density model (amount of nutrients or food consumed in grams/1000 kcal) | Lower intake of saturated fat (6·5 ± 1·6 v. 7·4 ± 1·8; P < 0·05) and sugar and confectionery products (31·9 ± 19 v. 47·3 ± 22·3; P < 0·01) by NWO individuals. | |
| Logistic regression model adjusted for food variables¶ | NWO spend less time to eat their meals, and the NWO chance in individuals who eat their meals in an average time (“medium”) is eight times the chance of those who consume ‘very slow/relatively slow’ (OR = 8·16; 95 % CI: 1·83, 36·44). In addition, the chance of NWO in individuals who eat meals ‘very fast/relatively fast’ is 11 times the chance of those who eat ‘very slow/relatively slow’ (OR = 11·48; 95 % CI: 2·55, 51·72). | |
| Serrano et al., 2010(31) | Student’s t test, Mann–Whitney or chi-square test | NWO showed, with greater frequency, elevated HOMA (15·78 % v. 2·5 %; P < 0·05) and elevated TAG (18·42 % v. 10 %; P < 0·05). In addition, higher insulin values (μm/ml) (11·64 [6·82] v. 8·36 [4·18]; P < 0·05), HOMA (μm/ml) (2·43 [1·8] v.1·63 [0·85]; P < 0·05), leptin (ng/ml) (12·45 [5·55] v. 12·10 [22·86]; P < 0·05), systolic blood pressure (mmHg) (104·15 [7·87] v. 99·65 [7·78]; P < 0·05) and diastolic (70·63 [7·20 ] v. 66·57 [6·39]; P < 0·05). |
NWO, normal weight obesity; KIMED, Mediterranean Diet Quality Index in Children and Adolescents; HOMA, homoeostasis model assessment.
A cardiometabolic risk score was created from the sum of the z-scores values of systolic blood pressure, serum TAG, waist circumference, HDL-C (multiplied by –1), and fasting glucose z-score. A higher cardiometabolic risk z-score is indicative of an unhealthier risk profile.
Logistic regression model was employed to determine the odds of being classified as NWO according to cardiorespiratory fitness categories using unhealthy cardiorespiratory fitness as a reference. Evaluated the cardiorespiratory fitness by the 20-m shuttle run test (20 mSRT) which was grouped into two categories: healthy and unhealthy.
Cardiometabolic risk score (MetS score) was calculated separately for each time point by standardising and then summing the following continuously distributed metabolic traits to create a z-score: mean arterial pressure ([(2 × diastolic blood pressure) + systolic blood pressure]/3); HOMA-IR; serum HDL cholesterol × –1; and fasting serum TAG z-score. A higher score indicates a worse cardiometabolic profile.
The risk score was calculated by standardising and then summing the following continuously distributed metabolic traits to create a z-score: mean arterial pressure ([(2 × diastolic blood pressure)+systolic blood pressure]/3); abdominal fat mass; fasting plasma glucose; serum HDL cholesterol × –1; and fasting serum TAG z-score. A higher score indicates a less favourable cardiometabolic risk profile.
Metabolic syndrome defined according to the Joint Interim Statement (JIS) of the IDF Task Force on Epidemiology and Prevention, National Heart, Lung and Blood Institute, American Heart Association, World Heart Federation, International Atherosclerosis Society and International Association for the Study of Obesity.
Adjustment for rate of eating, eating breakfast every day, eating afternoon snack every day, eating snack after dinner more than once per week, use of dietary supplements, intentional change of dietary habits, experience of dieting, diet control under direction of physician or dietician, exercise habit. Note: a rate of eating was assessed using a questionnaire in which participants were allowed to mark their meals ‘very slowly’, ‘relatively slowly’, ‘medium’, ‘relatively fast’ and ‘very fast’. For statistical analysis, there was a combination of ‘very slow’ and ‘relatively slow’ into a single category. The same was done for ‘very fast’ and ‘relatively fast’. Thus, there were three categories for assessing the rate of ingestion, with ‘very slow/relatively slow’ being a reference for calculating the OR.
In another study recently published in Brazil(29), it was found that individuals with NWO displayed higher values of BMI and waist circumference when compared to the eutrophic individuals without the phenotype. Besides, lower muscle mass (in kg) was observed in NWO individuals. When analysing the biomarkers, researchers also observed that NWO individuals had higher values of total cholesterol, LDL, HDL and IL-6.
García-Hermoso et al. (2020)(30) recently published a study in Colombia, comparing NWO individuals and eutrophic individuals without NWO, and reported that NWO individuals displayed higher values of BMI, weight and fat mass. Furthermore, through the ANCOVA, NWO was associated with higher values of TAG for males, and, in both sexes, to higher waist circumference and higher average cardiometabolic risk score. The cardiometabolic risk score was proposed using the sum of the values of the z-scores of the systolic blood pressure, serum TAG, waist circumference, HDL (multiplied by –1) and the z-score of fasting blood glucose, calculated separately according to sex and to each age group (on a yearly basis), whereas a higher z-score of cardiometabolic risk indicates a non-healthy profile.
In a similar fashion, a study conducted in Iceland(20) showed that NWO participants of both sexes displayed higher waist circumference, BMI and weight compared to eutrophic individuals without the phenotype. In addition, with respect to females, higher values were found for serum insulin and insulin resistance evaluated through the insulin resistance index (homoeostasis model assessment; HOMA). The authors also reported that NWO individuals displayed 2·2 times more likely to manifest one or more metabolic syndrome risk factors (OR = 2·2; CI 95 %: 1·2, 3·9) compared to eutrophic individuals.
In a longitudinal study carried out in Finland, Wiklund et al. (2017)(26) and Cheng and Wiklund (2018)(27) observed that cardiometabolic risk was significantly higher in NWO individuals at the beginning of the study, with younger participants, and that this difference persisted until the early adulthood (P < 0·01). To assess cardiometabolic risk, Wiklund et al. (2017)(26) proposed a risk score, which was calculated by standardising and subsequently adding up the following metabolic traits continually distributed to generate a z-score: average blood pressure ([2 × diastolic blood pressure) + systolic blood pressure]/3); abdominal fat; fasting plasma glucose; HDL × –1; and z-score of fasting serum TAG. By their part, Cheng and Wiklund (2018)(27) calculated the score as follows: average blood pressure ([2 × diastolic blood pressure) + systolic blood pressure]/3); homoeostasis model assessment of insulin resistance (HOMA-IR); HDL × –1; and z-score of fasting serum TAG. In both cases, higher scores implied poorer cardiometabolic profiles.
In the study by Serrano et al. (2010)(31), elevated HOMA and TAG were more frequent in NWO than in eutrophic individuals without excess body fat. Besides, higher values of insulin, HOMA, leptin, and systolic and diastolic blood pressure were found among adolescents with NWO, compared to the eutrophic group without NWO.
Furthermore, Serrano et al. (2010)(31) found that the NWO group displayed similar behaviour compared to overweight group, with respect to blood pressure, HDL fraction and blood glucose. This finding led authors to conclude that excess adiposity in eutrophic adolescents (NWO) may be related to biochemical and clinical changes similar to those found in overweight adolescents.
Health behaviours and normal-weight obesity
With respect to health behaviours, a recently published study(30) assessed the adherence to the Mediterranean diet, characterised by the rich consumption of plant foods, olive oil, fresh, unprocessed products and fish(33). The assessment was carried out through the Mediterranean Diet Quality Index in Children and Adolescents (KIDMED) questionnaire, which is highly (α = 0·79) consistent to determine this adherence. KIDMED includes 16 questions based on the assessment of food habits, according to the principles that sustain and weaken the food patterns of this diet. The sum of all the values of the administered questionnaire was categorised into two levels: (1) 0–7, low/moderate adherence and (2) 8–12, good adherence. By resorting to this instrument, García-Hermoso et al. (2020)(30) showed a low adherence to the Mediterranean diet among the NWO individuals, in comparison to eutrophic individuals without NWO.
Furthermore, García-Hermoso et al. (2020)(30) assessed the cardiorespiratory fitness through 20-m running test, in which the peak oxygen consumption was estimated through the equation proposed by Barnett et al. (1993)(34) that enables the classification of cardiometabolic risk into ‘healthy’ and ‘non-healthy’ in Colombian children and adolescents, according to sex and age. In both sexes, the authors observed that the NWO prevalence was lower in young individuals with healthy levels of cardiorespiratory fitness (boys: OR = 0·54, CI 95 % = 0·37, 0·78; girls: OR = 0·35, CI95 % = 0·24, 0·50) (P < 0·001).
Likewise, another study(20) identified that NWO individuals of both sexes displayed lower aerobic fitness measured in laboratory (maximum oxygen uptake was assessed through open-circuit spirometry with a treadmill exercise test protocol), also being more sedentary, according to a questionnaire about lifestyle and health behaviours that included physical activity. Besides, the assessment of food habits through a 24-h recall and a FFQ showed that NWO individuals displayed lower frequency of breakfast and vegetables intake than eutrophic individuals without NWO.
Bragança et al. (2020)(29), through the 24-h Physical Activity Recall, which was developed based on an adaptation of the Self-Administered Physical Activity Checklist(35), measured the physical activity level, obtained by the calculation of the weekly number of metabolic equivalents of task. The metabolic equivalents of task for each activity were obtained in the Compendium of Physical Activities(36). To categorise the physical activity levels, the following cut-off values of the International Physical Activity Questionnaires in metabolic equivalents of task/week were applied: sedentary (0), low (1 to < 600), moderate (600 to < 3000) and high (≥ 3000)(37), whereas the authors found that NWO individuals were more sedentary than eutrophic individuals (66·7 % v. 32·5 %; P = 0·006).
Musálek et al. (2018)(28) investigated the skeletal robustness and muscle areas on the upper arm and calf as indicators of muscle strength, physical fitness and bone age, taking into account that the higher levels of physical activity positively affects lean mass and bone development. To perform the skeletal breadth measurements, measures of humeral and femoral epicondyle were taken using a T520 thoracometer. After obtaining these measures, the researchers calculated the skeletal robustness indices according to the formula proposed by Frisancho (1990)(38), from humerus and femur breadth epicondyles. Musálek et al. (2018)(28) concluded that NWO individuals displayed significantly lower skeletal robustness in the lower extremities, according to the Frame index (z-score) from the femur. Furthermore, NWO individuals displayed lower z-scores for the muscle areas on the upper arm and calf.
In the study by Yaguchi-Tanaka et al. (2013)(32) that investigated food habits, NWO individuals displayed lower intake of saturated fat, sugar and bakery products, assessed through the brief self-administered diet history questionnaire. Yaguchi-Tanaka et al. (2013)(32) also assessed the speed of ingestion of foods through a questionnaire to which participants should point out whether they eat their meals ‘very slowly’, ‘relatively slowly’, ‘moderate’, ‘relatively quickly’ and ‘very quickly’. For statistical analysis, the ‘very slowly’ and ‘relatively slowly’ classifications were combined into a single category. The same was done for the ‘very quickly’ and ‘relatively quickly’ classifications. The authors observed that the likelihood of NWO in individuals who eat their meals in a moderate time is eight times greater than in those who eat very slowly or relatively slowly (OR = 8·16; CI 95 %: 1·83, 36·44). Also, the likelihood of NWO in individuals who eat their meals very quickly or relatively quickly is 11 times greater than in those who eat very slowly or relatively slowly (OR = 11·48; CI 95 %: 2·55, 51·72).
Risk of bias
All the selected studies underwent duplicate evaluation using a critical appraisal tool from the National Heart, Lung, and Blood Institute (2014)(25). The main limitations, according to what was assessed in the Checklist items, include the fact that the exposure was not assessed prior to outcome measurement and there was not sufficient time frame to see an effect, in six studies. However, this can be justified by the cross-sectional design of most studies included in this review. Four studies were not clear with respect to the criteria for identification and definition of confounding factors. One study did not provide enough details on the sample and resorted to a self-referred measure for assessment of a variable but did not establish objective criteria (Table 3). Despite these limitations, the papers selected allowed to raise important questions regarding NWO and also showed good quality.
Table 3.
Quality assessment of observational cohort and cross-sectional studies
| National Heart Lung and Blood Institute Checklist | Bragança et al., 2020(29) | García-Hermoso et al., 2020(30) | Musálek et al., 2018(28) | Olafsdottir, Torfadottir and Arngrimsson, 2016(20) | Yaguchi-Tanaka et al., 2013(32) | Serrano et al., 2010(31) | Cheng and Wiklund, 2018(27) | Wiklund et al., 2017(26) |
|---|---|---|---|---|---|---|---|---|
| Was the research question or objective in this paper clearly stated? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Was the study population clearly specified and defined? | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes |
| Was the participation rate of eligible persons at least 50 %? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Were all the subjects selected or recruited from the same or similar populations? Were inclusion and exclusion criteria for being in the study prespecified and applied uniformly to all participants? | Yes | Yes | Yes | Yes | NR | Yes | Yes | Yes |
| Was a sample size justification, power description, or variance and effect estimates provided? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| For the analyses in this paper, were the exposure(s) of interest measured prior to the outcome(s) being measured? | No | No | No | No | No | No | Yes | Yes |
| Was the time frame sufficient so that one could reasonably expect to see an association between exposure and outcome if it existed? | No | No | No | No | No | No | Yes | Yes |
| For exposures that can vary in amount or level, did the study examine different levels of the exposure as related to the outcome? | NA | Yes | Yes | NA | NA | NA | NA | NA |
| Were the exposure measures (independent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes |
| Was the exposure(s) assessed more than once over time? | NA | NA | NA | NA | NA | NA | Yes | Yes |
| Were the outcome measures (dependent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Were the outcome assessors blinded to the exposure status of participants? | CD | CD | CD | CD | CD | CD | CD | CD |
| Was loss to follow-up after baseline 20 % or less? | NA | NA | NA | NA | NA | NA | Yes | Yes |
| Were key potential confounding variables measured and adjusted statistically for their impact on the relationship between exposure(s) and outcome(s)? | No | Yes | No | Yes | Yes | No | Yes | No |
NR, not reported; NA, not applicable; CD, cannot determine.
Discussion
NWO has been regarded as an important risk factor for metabolic dysregulation and CVD, especially in adults(39). This is, to our knowledge, the first systematic review that addresses the presence of cardiometabolic risk factors in NWO adolescents, as well as the health behaviours related to the phenotype. The available evidence has shown that, although NWO adolescents are within the normal range of BMI or have an appropriate weight, they have higher values of anthropometric measurements, such as weight, BMI and waist circumference. Furthermore, they have more changes in the biochemical markers of cardiometabolic risk, such as insulin resistance and hypertriglyceridemia, besides being more sedentary and having less physical fitness, than eutrophic individuals without NWO.
The prevalence of the NWO phenotype is not uncommon, ranging from 5 % to 45 %, due to ethnic differences in populations, the use of different cut-off points for excess body fat and the lack of consensus on the diagnostic criteria(20,40,41). In the present study, the prevalence of NWO in adolescents also varied between studies, from 6·8 % to 55·6 %, with high values, revealing the importance of adopting measures to prevent and control this phenotype. Anthropometric markers of cardiometabolic risk, such as weight and waist circumference, are associated with insulin resistance, type 2 diabetes mellitus, CVD and premature death(42–44). In addition, biochemical markers, such as plasma cholesterol and TAG levels, directly correlate with the chance of CVD(45,46).
In this sense, considering the young age group in question, the identification of cadiometabolic risk factors in NWO adolescents in this study is worrisome because, in addition to influencing the occurrence of metabolic syndrome and CVD in the future, these changes tend to persist into adulthood(47–53).
As for health behaviours, some factors, such as physical activity and eating habits, are fundamental for health promotion and quality of life(54,55). Thus, sedentary lifestyle and inappropriate eating habits contribute to the accumulation of adipose tissue and are also related to chronic degenerative diseases(56–58).
In this review, it was evidenced that NWO are more sedentary and have less physical fitness. Sedentary behaviour is related to body fat accumulation(59,60), metabolic syndrome(61) and diabetes(62), thus influencing the onset of NWO. In contrast, regular physical activity generates numerous health benefits, such as cardiovascular strengthening and integrity, increased insulin sensitivity and additional energetic expenditure(63). Besides these positive physiological effects, participation in physical activity can improve physical self-perceptions and enhance self-esteem in young people(64).
Although conclusive results on NWO eating habits have not been reached in this review, since studies have evaluated different parameters and by different methods, it is valid to consider that the quality of the diet is an important factor for health promotion(65). Therefore, the appropriate consumption of fibres may be associated to a decrease in glucose levels, blood pressure, serum lipids(66) and inflammatory markers, thus contributing to reducing noncommunicable chronic diseases(67). García-Hermoso et al. (2020)(30) observed that NWO adolescents have less adherence to the Mediterranean diet, which is characterised by high consumption of plant foods(33) and, in the study by Olafsdottir, Torfadottir and Arngrimsson (2016)(20), NWO presented less frequency of consumption of vegetables.
Regarding the risk of bias in individuals studies, it was observed that six studies did not evaluate the exposure prior to outcome measurement and there was not sufficient time frame to see an effect; four studies were not clear with respect to the criteria for identification and definition of confounding factors; one study did not provide enough details on the sample and resorted to a self-referred measure for the assessment of a variable. Despite these limitations, the papers selected allowed to raise important questions regarding NWO and also showed good quality.
Some limitations need to be taken into consideration in the present review. Different procedures for the assessment of body composition were used. In addition, there was no standardisation regarding the NWO definition criteria, with different cut-off points considered to be adequate for BMI and body fat. The papers were restricted to those published in Portuguese, English or Spanish, and there was a heterogeneity of the data, which ruled out the possibility of conducting a meta-analysis. Most papers, except one, were transversal, which makes an inference about the causal relation between the factors and NWO infeasible. More studies are necessary to clarify these associations and potential mechanisms involved with NWO.
The strengths of this review included its systematic approach based on the PRISMA(22) guidelines, peer-reviewed studies and evaluation of risk of bias in articles by the National Heart, Lung, and Blood Institute(25). The studies exhibit good quality and all had comparison groups – the healthy eutrophics – for data analysis. Furthermore, the studies were conducted with different populations, in seven countries across three continents, emphasising the consistency of the findings. Besides, it is worth to highlight that the present study provides the literature with innovative knowledge and stresses the need for further epidemiological studies.
Conclusions
In conclusion, the available evidences suggest that NWO may be identified, with high prevalence, in adolescents. Furthermore, the phenotype is related to the early development of cardiometabolic risk factors, sedentary lifestyle and lower physical fitness. Further investigations are still needed to better clarify these relations and support the implementation of prevention and control measures regarding the phenotype.
Acknowledgements
Acknowledgements: Coordination for the Improvement of Higher Education Personnel (CAPES, Brazil). Financial support: This research received no specific grant from any funding agency, commercial or not-for-profit sectors. Conflict of interest: None. Authorship: BCC participated in the research, selection and evaluation process of the included articles, wrote the paper and analysed data. In addition, it was ultimately responsible for the content. LGS participated in the research and selection process of the included articles, as well as the critical review of the paper. NNL participated in the evaluation of the included articles, as well as the critical review of the paper. PFP, SAVR and SCCF participated in the critical review of the paper and discussions about the included articles. All authors have read and approved the final manuscript. Ethics of human subject participation: Not applicable (ethical approval was not required for the study).
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980020004863.
click here to view supplementary material
References
- 1. WHO (2020) Health Topics: Adolescent Health. Geneva: World Health Organization; available at https://www.who.int/westernpacific/health-topics/adolescent-health (accessed July 2020). [Google Scholar]
- 2. WHO (2007) Growth Reference Data for 5–19 Years. Geneva: World Health Organization; available at https://www.who.int/growthref/en/ (accessed July 2020). [Google Scholar]
- 3. Krebs NF, Himes JH, Jacobson D et al. (2007) Assessment of child and adolescent overweight and obesity. Pediatrics 120, S193–S228. [DOI] [PubMed] [Google Scholar]
- 4. Kelishadi R, Gouya MM, Ardalan G et al. (2007) First reference curves of waist and hip circumferences in an Asian population of youths: CASPIAN study. J Trop Pediatr 53, 158–164. [DOI] [PubMed] [Google Scholar]
- 5. Okorodudu DO, Jumean MF, Montori VM et al. (2010) Diagnostic performance of body mass index to identify obesity as defined by body adiposity: a systematic review and meta-analysis. Int J Obes 34, 791–799. [DOI] [PubMed] [Google Scholar]
- 6. Javed A, Jumean M, Murad MH et al. (2015) Diagnostic performance of body mass index to identify obesity as defined by body adiposity in children and adolescents: a systematic review and meta-analysis. Pediatr Obes 10, 234–244. [DOI] [PubMed] [Google Scholar]
- 7. De Lorenzo A, Martinoli R, Vaia F et al. (2006) Normal weight obese (NWO) women: an evaluation of a candidate new syndrome. Nutr Metab Cardiovasc Dis 16, 513–523. [DOI] [PubMed] [Google Scholar]
- 8. Kapoor N, Furler J, Paul TV et al. (2019) Normal weight obesity: an underrecognized problem in individuals of South Asian Descent. Clin Ther 41, 1638–1642. [DOI] [PubMed] [Google Scholar]
- 9. Correa-Rodríguez M, González-Ruíz K, Rincón-Pabón D et al. (2020) Normal-weight obesity is associated with increased cardiometabolic risk in young adults. Nutrients 12, 1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee HS, Park YM, Han K et al. (2020) Obesity-related hypertension: findings from The Korea National Health and Nutrition Examination Survey 2008–2010. PLoS One 15, e0230616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. De Lorenzo A, Del Gobbo V, Premrov MG et al. (2007) Normal-weight obese syndrome: early inflammation? Am J Clin Nutr 85, 40–45. [DOI] [PubMed] [Google Scholar]
- 12. Di Renzo L, Bigioni M, Del Gobbo V et al. (2007) Interleukin-1 (IL-1) receptor antagonist gene polymorphism in normal weight obese syndrome: relationship to body composition and IL-1 alpha and beta plasma levels. Pharmacol Res 55, 131–138. [DOI] [PubMed] [Google Scholar]
- 13. Romero-Corral A, Somers VK, Sierra-Johnson J et al. (2010) Normal weight obesity: a risk factor for cardiometabolic dysregulation and cardiovascular mortality. Eur Heart J 31, 737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shea JL, King MTC, Yi Y et al. (2012) Body fat percentage is associated with cardiometabolic dysregulation in BMI-defined normal weight subjects. Nutr Metab Cardiovasc Dis 22, 741–747. [DOI] [PubMed] [Google Scholar]
- 15. Madeira FB, Silva AA, Veloso HF et al. (2013) Normal weight obesity is associated with metabolic syndrome and insulin resistance in young adults from a middle-income country. PLoS One 8, e60673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marques-Vidal P, Pécoud A, Hayoz D et al. (2010) Normal weight obesity: relationship with lipids, glycaemic status, liver enzymes and inflammation. Nutr Metab Cardiovasc Dis 20, 669–675. [DOI] [PubMed] [Google Scholar]
- 17. Kang S, Kyung C, Park JS et al. (2014) Subclinical vascular inflammation in subjects with normal weight obesity and its association with body fat: an 18 F-FDG-PET/CT study. Cardiovasc Diabetol 13, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Oliveros E, Somers VK, Sochor O et al. (2014) The concept of normal weight obesity. Prog Cardiovasc Dis 56, 426–433. [DOI] [PubMed] [Google Scholar]
- 19. Männistö S, Harald K, Kontto J et al. (2014) Dietary and lifestyle characteristics associated with normal-weight obesity: the National FINRISK 2007 Study. Br J Nutr 111, 887–894. [DOI] [PubMed] [Google Scholar]
- 20. Olafsdottir AS, Torfadottir JE & Arngrimsson SA (2016) Health behavior and metabolic risk factors associated with normal weight obesity in adolescents. PLoS One 11, e0161451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Karelis AD, St-Pierre DH, Conus F et al. (2004) Metabolic and body composition factors in subgroups of obesity: what do we know? J Clin Endocrinol Metab 89, 2569–2575. [DOI] [PubMed] [Google Scholar]
- 22. Moher D, Liberati A, Tetzlaff J et al. (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6, e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ouzzani M, Hammady H, Fedorowicz Z et al. (2016) Rayyan-a web and mobile app for systematic reviews. Syst Rev 5, 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. WHO (2005) Nutrition in Adolescence: Issues and Challenges for the Health Sector: Issues in Adolescent Health and Development. Geneva: World Health Organization; available at https://apps.who.int/iris/handle/10665/43342 (accessed July 2020). [Google Scholar]
- 25. Study Quality Assessment Tools (2020) NHLBI, NIH. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed September 2020).
- 26. Wiklund P, Törmäkangas T, Shi Y et al. (2017) Normal-weight obesity and cardiometabolic risk: a 7-year longitudinal study in girls from prepuberty to early adulthood. Obesity 25, 1077–1082. [DOI] [PubMed] [Google Scholar]
- 27. Cheng S & Wiklund P (2018) The effects of muscle mass and muscle quality on cardio-metabolic risk in peripubertal girls: a longitudinal study from childhood to early adulthood. Int J Obes 42, 648–654. [DOI] [PubMed] [Google Scholar]
- 28. Musálek M, Pařízková J, Godina E et al. (2018) Poor skeletal robustness on lower extremities and weak lean mass development on upper arm and calf: normal weight obesity in middle-school-aged children (9 to 12). Front Pediatr 6, 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bragança MLBM, Oliveira BR, Fonseca JM et al. (2020) Assessment of blood biomarkers in adolescents classified by body mass index and body fat percentage. Cadernos de Saúde Pública 36, e00084719. [DOI] [PubMed]
- 30. García-Hermoso A, Agostinis-Sobrinho C, Camargo-Villalba GE et al. (2020) Normal-weight obesity is associated with poorer cardiometabolic profile and lower physical fitness levels in children and adolescents. Nutrients 12, 1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Serrano HMS, Carvalho GQ, Pereira PF et al. (2010) Body composition, biochemical and clinical changes of adolescents with excessive adiposity. Arquivos Brasileiros Cardiologia 95, 464–472. [DOI] [PubMed] [Google Scholar]
- 32. Yaguchi-Tanaka Y, Kawagoshi Y, Sasaki S et al. (2013) Cross-sectional study of possible association between rapid eating and high body fat rates among female Japanese college students. J Nutr Sci Vitaminol 59, 243–249. [DOI] [PubMed] [Google Scholar]
- 33. Graça P, Mateus MP & Lima RM (2013) The Mediterranean diet concept and the promotion of healthy eating in Portuguese schools. Revista Nutrícias 13, 6–9. [Google Scholar]
- 34. Barnett A, Chan LYS & Bruce LC (1993) A preliminary study of the 20-m multistage shuttle run as a predictor of peak VO2 in Hong Kong Chinese Students. Pediatr Exerc Sci 5, 42–50. [Google Scholar]
- 35. Sallis JF, Strikmiller PK, Harsha DW et al. (1996) Validation of interviewer- and self-administered physical activity checklists for fifth grade students. Med Sci Sports Exerc 28, 840–851. [DOI] [PubMed] [Google Scholar]
- 36. Ainsworth BE, Haskell WL, Leon AS et al. (1993) Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc 25, 71–80. [DOI] [PubMed] [Google Scholar]
- 37. Benedetti TRB, Antunes PD, Rodriguez-Añez CR et al. (2007) Reproducibility and validity of the International Physical Activity Questionnaire (IPAQ) in elderly men. Rev Brasileira Med do Esporte 13, 11–16. [Google Scholar]
- 38. Frisancho AR (1990) Anthropometric Standards for the Assessment of Growth and Nutritional Status. Michigan: University of Michigan Press. [Google Scholar]
- 39. Jean N, Somers VK, Sochor O et al. (2014) Normal-weight obesity: implications for cardiovascular health. Curr Atheroscler Rep 16, 464. [DOI] [PubMed] [Google Scholar]
- 40. Conus F, Rabasa-Lhoret R & Péronnet F (2007) Characteristics of metabolically obese normal-weight (MONW) subjects. Appl Physiol Nutr Metab 32, 4–12. [DOI] [PubMed] [Google Scholar]
- 41. Marques-Vidal P, Chiolero A & Paccaud F (2008) Large differences in the prevalence of normal weight obesity using various cut-offs for excess body fat. E Spen Eur E J Clin Nutr Metab 3, e159–e162. [Google Scholar]
- 42. Katzmarzyk PT, Srinivasan SR, Chen W et al. (2004) Body mass index, waist circumference, and clustering of cardiovascular disease risk factors in a biracial sample of children and adolescents. Pediatrics 114, e198–e205. [DOI] [PubMed] [Google Scholar]
- 43. Nechuta SJ, Shu X-O, Li H-L et al. (2010) Combined impact of lifestyle-related factors on total and cause-specific mortality among Chinese women: prospective cohort study. PLoS Med 7, e1000339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Balagopal PB, de Ferranti SD, Cook S et al. (2011) Nontraditional risk factors and biomarkers for cardiovascular disease: mechanistic, research, and clinical considerations for youth: a scientific statement from the American Heart Association. Circulation 123, 2749–2769. [DOI] [PubMed] [Google Scholar]
- 45. Schulte H, Cullen P & Assmann G (1999) Obesity, mortality and cardiovascular disease in the Münster Heart Study (PROCAM). Atherosclerosis 144, 199–209. [DOI] [PubMed] [Google Scholar]
- 46. Kannel WB (2000) The Framingham study: ITS 50-year legacy and future promise. J Atheroscler Thromb 6, 60–66. [DOI] [PubMed] [Google Scholar]
- 47. Lauer RM, Clarke WR, Mahoney LT et al. (1993) Childhood predictors for high adult blood pressure. Muscatine Study Pediatr Clin North Am 40, 23–40. [DOI] [PubMed] [Google Scholar]
- 48. Berenson GS, Wattigney WA, Bao W et al. (1994) Epidemiology of early primary hypertension and implications for prevention: the Bogalusa Heart Study. J Hum Hypertens 8, 303–311. [PubMed] [Google Scholar]
- 49. Cook NR, Gillman MW, Rosner BA et al. (1997) Prediction of young adult blood pressure from childhood blood pressure, height, and weight. J Clin Epidemiol 50, 571–579. [DOI] [PubMed] [Google Scholar]
- 50. Raitakari OT, Juonala M, Kähönen M et al. (2003) Cardiovascular risk factors in childhood and carotid artery intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study. JAMA 290, 2277–2283. [DOI] [PubMed] [Google Scholar]
- 51. Hartiala O, Magnussen CG, Kajander S et al. (2012) Adolescence risk factors are predictive of coronary artery calcification at middle age: the cardiovascular risk in young Finns study. J Am Coll Cardiol 60, 1364–1370. [DOI] [PubMed] [Google Scholar]
- 52. Jakubowski KP, Cundiff JM & Matthews KA (2018) Cumulative childhood adversity and adult cardiometabolic disease: a meta-analysis. Health Psychol 37, 701–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Weihrauch-Blüher S, Schwarz P & Klusmann J-H (2019) Childhood obesity: increased risk for cardiometabolic disease and cancer in adulthood. Metab Clin Exp 92, 147–152. [DOI] [PubMed] [Google Scholar]
- 54. Brazil & Departamento de Atenção Básica (2014) Strategies for the Care of People with Chronic Disease. Brasília: Ministério da Saúde. [Google Scholar]
- 55. Brazil (2011) Strategic Action Plan for Tackling Chronic Non-Communicable Diseases (NCDs) in Brazil: 2011–2022, 1a edição. Brasília, DF: Ministério da Saúde. [Google Scholar]
- 56. Barnes AS (2012) Obesity and sedentary lifestyles. Tex Heart Inst J 39, 224–227. [PMC free article] [PubMed] [Google Scholar]
- 57. Power C, Pinto Pereira SM, Law C et al. (2014) Obesity and risk factors for cardiovascular disease and type 2 diabetes: Investigating the role of physical activity and sedentary behaviour in mid-life in the 1958 British cohort. Atherosclerosis 233, 363–369. [DOI] [PubMed] [Google Scholar]
- 58. Barroso TA, Marins LB, Alves R et al. (2017) Association of Central Obesity with The Incidence of Cardiovascular Diseases and Risk Factors. Int J Cardiovasc Sci 30, 416–424. [Google Scholar]
- 59. Mac Ananey O, McLoughlin B, Leonard A et al. (2015) Inverse relationship between physical activity, adiposity, and arterial stiffness in healthy middle-aged subjects. J Phys Act Health 12, 1576–1581. [DOI] [PubMed] [Google Scholar]
- 60. Garcia-Pastor T, Salinero JJ, Sanz-Frias D et al. (2016) Body fat percentage is more associated with low physical fitness than with sedentarism and diet in male and female adolescents. Physiol Behav 165, 166–172. [DOI] [PubMed] [Google Scholar]
- 61. Edwardson CL, Gorely T, Davies MJ et al. (2012) Association of sedentary behaviour with metabolic syndrome: a meta-analysis. PLoS One 7, e34916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hu FB, Li TY, Colditz GA et al. (2003) Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. JAMA 289, 1785–1791. [DOI] [PubMed] [Google Scholar]
- 63. Mahan LK & Raymond JL (2012) Israel weight management nutrition. In Krause’s Food & Nutrition Therapy, pp. 477–478. Rio de Janeiro: Elsevier Editora Ltda. [Google Scholar]
- 64. Lubans D, Richards J, Hillman C et al. (2016) Physical activity for cognitive and mental health in youth: a systematic review of mechanisms. Pediatrics 138, e20161642. [DOI] [PubMed]
- 65. de Souza TP, Ferreira FC & Barbosa MR (2016) Ingestão alimentar e excesso de adiposidade de mulheres sedentárias [Food intake and excess of adiposity in healthy women]. Braspen J 31, 316–321. [Google Scholar]
- 66. Gallagher ML (2012) Ingestion: nutrients and their metabolism. In Krause’s Food & Nutrition Therapy, pp. 36–38. Rio de Janeiro: Elsevier Editora Ltda. [Google Scholar]
- 67. Bernaud FSR & Rodrigues TC (2013) Fibra alimentar: ingestão adequada e efeitos sobre a saúde do metabolismo [Dietary fiber – adequate intake and effects on metabolism health]. Arq Bras Endocrinol Metab 57, 397–405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980020004863.
click here to view supplementary material


