Abstract
Objective:
To investigate homocysteine (Hcy) and folate levels, prevalence of hyperhomocysteinaemia (HHcy) and folate deficiency, which are affected by lifestyles in urban, agricultural and stock-raising populations.
Design:
This is a cross-sectional study.
Setting:
Urban, agricultural and stock-raising regions in Emin, China.
Participants:
Totally 1926 subjects – 885 (45·9 %) from urban, 861 (44·7 %) from agricultural and 180 (9·4 %) from stock-raising regions – were obtained using multistage stratified random sampling. Inclusion criteria encompassed inhabitants aged ≥15 years who resided at the current address for ≥6 months and agreed to participate in the study. Surveys on health behaviour questionnaires and physical examinations were conducted and blood samples collected.
Results:
The folate level of subjects from the stock-raising region was the lowest, followed by those from the agricultural region, and the highest in those from the urban region (3·48 v. 6·50 v. 7·12 ng/ml, P < 0·001), whereas mean Hcy showed no significant difference across regions. The OR for HHcy in stock-raising regions was 1·90 (95 % CI 1·11, 3·27) compared with the urban region after adjusting for all possible covariates. The OR for folate deficiency in stock-raising and agriculture regions was 11·51 (95 % CI 7·09, 18·67) and 1·91 (95 % CI 1·30, 2·82), respectively, compared with the urban region after adjusting for all possible covariates.
Conclusions:
HHcy and folate deficiency are highly prevalent in stock-raisers, which is of important reference for HHcy control in Xinjiang, with a possibility of extension to others with approximate lifestyles.
Keywords: Folate, Homocysteine, Stock-raising region, Hyperhomocysteinaemia, Prevalence
CVD has a high prevalence and huge burden worldwide, and has become one of the leading causes of human death, and 80 % of CVD takes place in the developing world(1). Numerous factors are related to CVD, of which circulating homocysteine (Hcy) and folate levels are the focus of much attention in recent years. Related research shows that folate deficiency and elevated Hcy levels are associated with human health, CVD and mortality(2–4). Hcy can also be used to predict diabetes, dementia and pregnancy disorders(5–7). Folate deficiency is also associated with chronic diseases such as CVD, cancer and cognitive dysfunction; maternal folate nutritional status is related to the risk of neural tube defects in the offspring(8). Folate is also one of the main determinants of circulating Hcy(9). Folate supplementation may prevent CVD and some cancers and lower circulating Hcy(10).
For this purpose, some developed countries have carried out several large-scale population-based surveys to evaluate circulating Hcy and folate status and taken evidence-based actions such as folate fortification(11). They have established policies for folate fortification of cereals and flour to decrease the prevalence of hyperhomocysteinaemia (HHcy), and observed some plausible changes including in CVD prevention(12–14), which is of valuable reference for developing countries. Folate is a vitamin that cannot be synthesised by human body and only obtained from microbes that break down food during digestion(15). Therefore, folate and Hcy levels vary widely due to high geographical, lifestyle, dietary and racial/ethnic diversity(16–18). Accurate estimates of the prevalence of a condition and relevant determinants are essential as a source of primary information and for rational planning of health services, and would allow public health policymakers to assign sufficient priority and resources to its management and prevention(19), which is also the same for folate deficiency and HHcy, especially in resource-constricted areas and developing countries. Nonetheless, there is a paucity of equivalent data from developing countries.
Based on previous studies, the prevalence of HHcy in Chinese population is 27·5 %(20 ), and the prevalence and the risk factors may also vary due to geographical, ethnic, social and dietary diversity(20). Xinjiang, a multi-ethnic province with a population of various lifestyles and dietary habits living in urban, agricultural and stock-raising regions(21), is located in northwest China and Central Asia and provides an ideal setting for this type of study, where folate fortification is not mandatory as well. Local nomads have been residing in stock-raising areas like villages, forests and mountains; move around depending on seasonal changes; and spend most of their time in remote areas, and thus their living is characterised by much dependence on animal products, including animal oil, meat, milk and dairy products, and by limited access to fresh fruit and vegetables(21,22). Only an existing study conducted in rural Xinjiang showed a high prevalence of HHcy, but failed to analyse the effects of different living environments and folate concentrations on Hcy(23).
In countries where folate fortification is not mandatory and where resource is constricted, it is of significant value to identify the target population that would most benefit from folate supplementation. Therefore, the main purpose of the current study was to comprehend folate and Hcy levels and their potential determinants in agricultural, stock-raising and urban regions using a population-based, cross-sectional survey, and to provide clues to other regions with approximate conditions.
Subjects and methods
Site
Emin county, northern Xinjiang, is home to >133 000 people aged ≥15 years from urban, agricultural and stock-raising regions, which makes it an ideal setting for a study of the effects of different lifestyles on Hcy and folate metabolism. The survey was conducted from January to December 2014.
Study population
Multistage stratified random sampling was used to obtain a sample of the population aged ≥15 years. At the first stage, the whole county was divided into three regions as urban, agricultural and stock-raising regions, based on the urban and rural area code issued by the National Bureau of Statistics of the People’s Republic of China, as well as on the regional production mode and the main source of economic income. At the second stage, two townships were randomly selected in each region using simple random sampling. At the third stage, two villages were randomly selected as survey villages in each of the extracted townships. In the final stage of sampling, a given number of participants from each site were selected from communities or villages using lists compiled from local government registers of households. Inclusion criteria encompassed: (i) local inhabitants aged ≥15 years; (ii) residing at current address for ≥6 months; (iii) agreeing to participate and sign an informed consent form. Exclusion criteria included renal insufficiency; pernicious anaemia; malignant tumours; pregnancy; and taking anticonvulsants, anti-tuberculosis drugs, birth control pills, dopamine drugs, folate and/or vitamin B12 supplementation in the past month.
Data collection
Questionnaire, physical examination and biochemical examination
Population health behaviour questionnaires and physical examinations were conducted using onsite surveys to collect detailed information from all participants via a face-to-face interview by trained investigators, which included demographic characteristics (name, gender, age, ethnicity and current address), socioeconomic status (educational status), lifestyle risk factors (cigarette consumption and alcohol intake) and individual and family medical history. Physical examination included measurements of height, body weight, waist circumference (WC) and blood pressure (BP). Laboratory examination included measurement of plasma Hcy and folate.
Measurement of blood pressure, height, weight and waist circumference
BP was presented as the mean of three measurements using an Omron HEM-1000 electronic sphygmomanometer via a standardised procedure(24). All participants were advised to avoid cigarette smoking, alcohol, caffeinated beverages, tea and exercise for at least 30 min prior to measurements. Three BP measurements were taken, after a rest of at least 5 min, from the unclothed right arm of the person in a sitting position at an interval of at least 1 min. Body weight, height and WC were measured using standard methods(25). Height and weight were measured to the nearest 0·1 cm and 0·1 kg, respectively, with the participants in lightweight clothing and without shoes. WC was measured at the midpoint between the lower rib and upper margin of the iliac crest to the nearest 0·1 cm at the end of a normal expiration. BMI was calculated by dividing weight by height-squared (kg/m2).
Laboratory measurements
Venous blood samples were obtained by the same trained nurses in the morning after overnight fasting. Plasma was separated within 30 min of collection by centrifugation (4°C, 20 min, at 1509·3 g ); stored at onsite refrigerators temporarily; transported in a cooler to the clinical laboratory at the People’s Hospital of Xinjiang Uygur Autonomous Region; and stored at –80°C until assayed. Plasma Hcy and folate were measured by the same staff blinded to the aim and design of the study. Plasma Hcy was measured by an enzymatic cycling assay method using a DIRUI equipment of the type CS-T300, with a plasma Hcy precision of 0·1 μmol/l. The kit purchased from Shenzhen Tailed Medical Co. (batch number 20190104) was used. Folate was measured by chemiluminescence method with an UniCel D×I 800 automatic biochemical analyser (Beckman Coulter), with a plasma folate precision of 0·01 ng/ml and with the detectable concentration ranging from 1·0 to 24·2 ng/ml. The folate assay kit was purchased from Beckman Coulter, Inc. (batch number 832212). All measurements were performed in strict accordance with the instrument and reagent instructions.
Diagnostic criteria
HHcy is defined as circulating Hcy ≥10 µmol/l(26). Folate deficiency is defined as a concentration <3 ng/ml(12). Hypertension is defined as systolic BP ≥140 mmHg, and/or diastolic BP ≥90 mmHg, and/or use of antihypertensive medication within previous 2 weeks. Overweight and obesity is defined as BMI ≥25 kg/m2 according to WHO classifications(27). Abdominal obesity is defined as WC ≥90 cm for men and ≥85 cm for women. Cigarette consumption is defined as cumulative smoking in the past reaching more than twenty packs of cigarettes and is currently smoking. Alcohol intake is defined as at least once a week within the previous 30 d of the survey.
Statistical analysis
Continuous variables, if normally distributed, were presented as means ± sd and analysed using t test or ANOVA, followed by post hoc analysis (least significant difference test). Categorical variables were expressed as proportion (%) and frequency (n) and analysed using the χ2 test. Hcy and folate levels were compared by covariance analysis among and between groups after adjusting for gender, age, BMI, alcohol intake, cigarette consumption, hypertension, education and ethnicity. Partial correlation analysis was performed between Hcy and folic acid. Univariate logistic regression analysis was performed to select the potential factors that have been speculated to influence Hcy and folate concentrations with P < 0·1 to enter the equation (data not reported). Multivariable logistic regression was performed for Hcy after adjusting for significant variables in univariate regression analysis such as gender, age, BMI, alcohol intake, cigarette consumption, hypertension, folate tertile and regions; and for folate deficiency by adjusting for gender, age, ethnicity, education and regions. OR and 95 % CI were calculated. Tertile of folate was used in the multivariable logistic regression analysis. A P value <0·05 was considered statistically significant. All statistical analyses were performed with SPSS, version 23.0.
Results
Characteristics of the study populations
A total of 1946 participants volunteered to complete the study, of whom 1934 provided blood samples. An additional eight subjects were excluded from the analysis because of insufficient blood sample. Finally, data of 1926 participants with 885 (45·9 %) from urban, 861 (44·7 %) from agricultural and 180 (9·4 %) from stock-raising regions were evaluated. The proportion of subjects enrolled is approximate to that of the local population(28).
As in Table 1, study populations from urban, agricultural and stock-raising regions were similar in terms of age and gender. The prevalence of hypertension, systolic BP and diastolic BP levels, education attainment, cigarette consumption, alcohol intake, overweight and obesity proportion, and ethnicity were significantly different among the three regions (P for all <0·05). In the stock-raising region, Kazakh subjects accounted for 79·1 %, and the prevalence of hypertension was higher compared with agricultural and urban regions (38·3 v. 24·8 v. 18·5 %, P < 0·001). In the urban region, the majority was Han ethnicity, accounting for 63 %, and the proportion of cigarette consumers, alcohol intake and overweight and obese subjects were significantly higher than in rural and pastoral regions (P for all <0·05). In the agricultural region, the majority was also Han ethnicity, accounting for 56·2 %, and the prevalence of hypertension was lower than in urban and stock-raising regions (18·5 v. 24·8 v. 38·3 %, P < 0·001).
Table 1.
Characteristics of the study population*
| Characteristics | Urban | Agriculture | Stock-raising | P | P1 | P2 | P3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||||||||
| 885 | 45·9 | 861 | 44·7 | 180 | 9·4 | ||||||||
| Age (years) | |||||||||||||
| Mean | 51·7 | 50·7 | 50·6 | 0·316 | 0·159 | 0·933 | 0·363 | ||||||
| sd | 14·1 | 14·4 | 14·2 | ||||||||||
| ≤44 | 285 | 32·2 | 276 | 32·1 | 61 | 33·9 | 0·884 | 0·635 | 0·827 | 0·897 | |||
| 45–59 | 323 | 36·5 | 331 | 38·4 | 65 | 36·1 | |||||||
| ≥60 | 277 | 31·3 | 254 | 29·5 | 54 | 30·3 | |||||||
| Gender | |||||||||||||
| Men | 382 | 43·2 | 388 | 45·1 | 72 | 40·0 | 0·420 | 0·424 | 0·213 | 0·434 | |||
| Ethnicity | |||||||||||||
| Han | 469 | 63·0 | 455 | 56·2 | 26 | 15·1 | <0·001 | <0·001 | <0·001 | <0·001 | |||
| Kazakh | 171 | 23·0 | 262 | 32·3 | 136 | 79·1 | |||||||
| Mongolian | 30 | 4·0 | 32 | 4·0 | 8 | 4·7 | |||||||
| Others | 75 | 10·1 | 61 | 7·5 | 2 | 1·2 | |||||||
| Education | |||||||||||||
| Primary and lower | 560 | 86·7 | 399 | 52·2 | 50 | 29·1 | <0·001 | <0·001 | <0·001 | <0·001 | |||
| Junior | 76 | 11·8 | 310 | 40·5 | 118 | 68·6 | |||||||
| Senior and higher | 10 | 1·5 | 56 | 7·3 | 4 | 2·3 | |||||||
| Cigarette consumption | 180 | 28·3 | 169 | 23·2 | 34 | 21·1 | 0·046 | 0·032 | 0·572 | 0·068 | |||
| Cigarette consumption in men | 173 | 60·7 | 160 | 48·5 | 34 | 54·0 | 0·010 | 0·002 | 0·425 | 0·325 | |||
| Alcohol intake | 175 | 27·5 | 157 | 21·5 | 36 | 22·4 | 0·033 | 0·011 | 0·818 | 0·189 | |||
| Alcohol intake in men | 174 | 61·1 | 151 | 45·8 | 35 | 55·6 | 0·001 | <0·001 | 0·153 | 0·420 | |||
| BMI (kg/m2) | |||||||||||||
| Mean | 25·7 | 25·2 | 25·5 | 0·011 | 0·003 | 0·232 | 0·571 | ||||||
| sd | 3·6 | 3·5 | 4·2 | ||||||||||
| BMI ≥25 kg/m2 | 462 | 54·9 | 414 | 49·1 | 90 | 51·1 | 0·049 | 0·015 | 0·615 | 0·349 | |||
| Abdominal obesity | 499 | 59·7 | 452 | 53·6 | 98 | 54·7 | 0·035 | 0·011 | 0·783 | 0·216 | |||
| Women’s WC ≥85 cm | 268 | 56·4 | 246 | 53·6 | 60 | 56·1 | 0·652 | 0·366 | 0·643 | 0·930 | |||
| Men’s WC ≥ 90 cm | 231 | 64·0 | 206 | 53·6 | 38 | 52·8 | 0·011 | 0·004 | 0·892 | 0·073 | |||
| Prevalence of hypertension | 219 | 24·8 | 159 | 18·5 | 69 | 38·3 | <0·001 | 0·001 | <0·001 | <0·001 | |||
| Systolic blood pressure (mmHg) | |||||||||||||
| Mean | 128·7 | 125·0 | 129·5 | <0·001 | <0·001 | 0·002 | 0·572 | ||||||
| sd | 17·8 | 17·5 | 17·4 | ||||||||||
| Diastolic blood pressure (mmHg) | |||||||||||||
| Mean | 79·1 | 78·0 | 81·4 | <0·001 | 0·038 | <0·001 | 0·010 | ||||||
| sd | 11·2 | 10·7 | 9·8 | ||||||||||
WC, waist circumference.
We conducted among-group and between-group comparisons using ANOVA followed by post hoc analysis (least significant difference test) and χ2 test and provided specific P-values as P for among-group comparison and as P1 (urban v. agriculture), P2 (agriculture v. stock-raising) and P3 (stock-raising v. urban) for between-group comparisons.
Level of homocysteine and folate
As shown in Table 2, after adjusting for gender, age, BMI, alcohol intake, cigarette consumption, hypertension, education and ethnicity, the folate level of subjects from the stock-raising region was the lowest, followed by those from the agricultural region, and the highest in those from the urban region with statistical significance (3·48 v. 6·50 v. 7·12 ng/ml, P < 0·001). Nonetheless, the mean concentration of plasma Hcy showed no significant differences among subjects from different regions.
Table 2.
Homocysteine and folate levels in different populations*
| Urban | Agriculture | Stock-raising | F/P | P1 | P2 | P3 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | sd | Mean | sd | Mean | sd | |||||
| Hcy (µmol/l) | 13·48 | 8·92 | 13·48 | 7·92 | 14·37 | 7·91 | 1·001/0·368 | 0·994 | 0·170 | 0·193 |
| Folate (ng/ml) | 7·12 | 4·46 | 6·50 | 3·81 | 3·48 | 4·02 | 54·058/<0·001 | 0·003 | <0·001 | <0·001 |
Hcy, homocysteine.
Adjusted for gender, age, BMI, alcohol intake, cigarette consumption, hypertension, education and ethnicity. F is the statistic value of covariance analysis; P represents the P value for among-group comparison; P1, urban v. agriculture; P2, agriculture v. stock-raising; P3, stock-raising v. urban.
Prevalence of hyperhomocysteinaemia and folate deficiency
As given in online Supplemental Table S1, the prevalence of HHcy in the stock-raising region was significantly higher than in agricultural and urban regions (82·2 v. 69·7 and 68·4 %, P < 0·001). The prevalence of HHcy in female, Kazakh, non-drinking, non-smoking, overweight and obese subjects and those with lower education attainment from the stock-raising region was significantly higher than those of other regions (P for all <0·05).
The prevalence of folate deficiency in subjects from the stock-raising region was significantly higher than in those from the agricultural and urban regions (54·8 v. 8·3 and 2·6 %, P < 0·001), which was also the same in the subgroup analysis (P for all <0·001) as shown in online Supplemental Table S2.
Relationship between homocysteine and folate
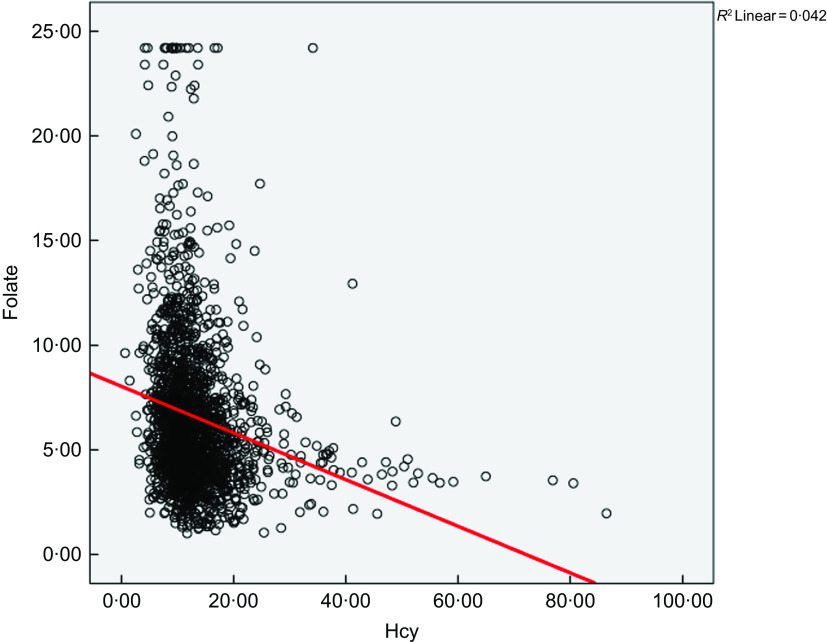
The partial correlation analysis showed a significant inverse relationship between Hcy and folate concentration (r = –0·200, P < 0·001) after controlling for age, gender, alcohol and cigarette consumption, hypertension and region as shown in Fig. 1.
Fig. 1.
Relationship between circulating homocysteine and folate in the study population, adjusted for age, gender, cigarette consumption, alcohol intake and BMI
Factors associated with hyperhomocysteinaemia and folate deficiency
Univariable and multivariate logistic regression analyses of risk factors for HHcy and folate deficiency are given in Table 3. A multivariate logistic regression analysis of risk factors for HHcy was conducted after adjusting for gender, age, BMI, alcohol and cigarette consumption, hypertension, folate tertile and regions. The OR for HHcy in the stock-raising region was 1·90 (95 % CI 1·11, 3·27) compared with the urban region after adjusting for all possible covariates. A multivariate logistic regression analysis of risk factors for folate deficiency was conducted after adjusting for gender, age, region, ethnicity and education. The OR for folate deficiency in the stock-raising and agriculture regions were 11·51 (95 % CI 7·09, 18·67) and 1·91 (95 % CI 1·30, 2·82), respectively, compared with the urban region after adjusting for all possible covariates.
Table 3.
Univariable and multivariate logistic regression analyses of risk factors for HHcy and folate deficiency*
| Univariable analysis | P | Multivariable analysis | P | |||
|---|---|---|---|---|---|---|
| OR | 95 % CI | OR | 95 % CI | |||
| HHcy deficiency | ||||||
| Urban | 1 | 1 | ||||
| Agriculture | 1·064 | 0·869, 1·303 | 0·550 | 0·949 | 0·731, 1·231 | 0·691 |
| Stock-raising | 2·140 | 1·424, 3·217 | <0·001 | 1·902 | 1·106, 3·271 | 0·020 |
| Folate deficiency | ||||||
| Urban | 1 | 1 | ||||
| Agriculture | 2·684 | 1·928, 3·735 | <0·001 | 1·909 | 1·295, 2·815) | 0·001 |
| Stock-raising | 23·168 | 15·456, 34·727 | <0·001 | 11·511 | 7·091, 18·686) | <0·001 |
Hyperhomocysteinaemia (HHcy) deficiency was adjusted for gender, age, BMI, alcohol intake, cigarette consumption, hypertension, folate tertile and region. Folate deficiency was adjusted for gender, age, region, ethnicity and education.
Discussion
CVD is the leading cause of human death(1), for which folate deficiency and elevated Hcy are independent risk factors(2,3,8). Hcy is also a sensitive marker of folate deficiency(2,29). Folate and Hcy levels vary widely due to geographical, racial/ethnic, lifestyle and dietary diversity. Xinjiang in northwest China provides an ideal setting for a study on the effects of diverse lifestyles and dietary habits across populations from urban, agricultural and stock-raising regions. The main observations of the current study encompassed(1): levels of circulating folate were the lowest in the stock-raising region(2); the prevalence of HHcy and folate deficiency was higher in the stock-raising region(3); stock-raising and agricultural regions were the major related factors for elevated Hcy and folate deficiency.
The current study extended previous findings to stock-raising regions(23). That is, the stock-raising region showed a significantly higher HHcy prevalence and higher OR for the presence of HHcy and folate deficiency. Indeed, it may be indicative of the dietary habits of the population living in the stock-raising region. As evidenced previously, stock-raisers reside in villages, forests and mountains and move around based on seasonal changes, which makes them dependent on animal products with limited access to enough vegetables and fruits(21). Therefore, populations from stock-raising regions may benefit most from Hcy lowering possibly by folate supplementation.
The current study also provides a direct evidence on the coexistence of HHcy and folate deficiency in populations with different lifestyles, especially in stock-raisers, from developing countries. Folate fortification is not mandatory in China, making the results possibly representative of this type of study. Consistent with previous studies(29), coexistence of elevated Hcy and folate deficiency and a significant inverse relationship between the two in a population from the same background may further confirm the importance of folate in terms of lowering elevated Hcy. Hcy is an intermediate product in the metabolism of methionine and cysteine. The circulation of methionine requires the participation of folate, a vitamin that human body cannot synthesise. Methylenetetrahydrofolate reductase catalyses the irreversible conversion of 5, 10-methylene tetrahydrofolate to 5-methyl-tetrahydrofolate, committing one-carbon units to the methionine cycle. When folate and/or B vitamins are deficient, methionine cycle is blocked, generating the accumulation of plasma Hcy in the blood. HHcy further induces oxidative stress, endothelium dysfunction, inflammation, smooth muscle cell proliferation and endoplasmic reticulum stress, which are the key pathogenesis of CVD(30). At present, most scholars are optimistic for the trend of folate intervention on the risk of HHcy and CVD reduction(31). With folate fortification implemented in the United States and Canada since 1998, circulating folate concentrations in the population increased from 4·6 to 10·0 ng/ml, and Hcy decreased from 10·1 to 9·4 μmol/l(32). Folate supplementation reduces the risk of stroke by about 10 %, and the risk of CVD by about 4 %, which is more effective in populations with lower baseline folate and at a high risk(33,34). Consistent with previous studies(35–37), the presence of HHcy is also associated with male gender and older age. In contrast, current alcohol intake is not associated with elevated Hcy in our data, which may be due to some bias in population selection. Therefore, folate fortification, cigarette cessation and alcohol abstinence may be still the target lifestyle modification measures to lower elevated Hcy(20,23,36).
Moreover, this observation could be extended to settings of populations with approximate lifestyles and dietary habits. Xinjiang, located in northwest China, is close to Central Asian and Eastern European countries such as Kazakhstan, Kyrgyzstan, Mongolia and Russia. Most of the population there still share similar lifestyles (stock-raising) and dietary habits (more animal products and limited fruit and vegetables)(38,39), where the burden of CVD seems to be huge. For instance, CVD is estimated to account for more than a half (53 %) of all deaths in Kazakhstan. Age-standardised CVD mortality was 636 in Kazakhstan and 531 in the Russia Federation per 100 000 population in 2010, almost 5–6 times higher than in the United Kingdom (112 per 100 000)(40).
The current study has following innovations: First, it extended previous findings to stock-raising regions. The prevalence of HHcy and folate deficiency was higher in stock-raisers and so they are likely the target population for folate supplementation, which may be of valuable reference to other stock-raising regions. Second, the current data included both Hcy and folate analyses, which may provide a direct evidence for disease prevention. Nevertheless, some limitations to the current study need to be acknowledged. This is a cross-sectional study that did not show a causal relationship between Hcy and folate, whereas it has been well established. Thus, our data provide a direction for the prevention and control of public health-related diseases. In addition, we failed to assess the contribution of vitamin B12 on Hcy, which may have brought some bias to our results and explanations.
In conclusion, the prevalence of HHcy and folate deficiency is unacceptably high in underdeveloped regions, particularly among stock-raisers and thus a possible target for folate supplementation. The results may be of important reference for the prevention and control of HHcy in Xinjiang, with a possibility of extension to populations that share lifestyles and dietary habits.
Acknowledgements
Acknowledgements: We thank all study participants for their support and thank volunteers for providing great help. We also thank the leaders of health bureaus of Emin county. We express our gratefulness to the Department of Science and Technology for funding this work. Financial support: The current study was funded by Department of Science and Technology of Xinjiang Uygur Autonomous Region of China (grant number 2017B03015). Conflict of interest: All the authors declared no conflict of interest. Authorship: N.L. put forward, designed and implemented the investigation, analysed the data and drafted the manuscript. F.P., M.H., L.W., L.Z, J.H., D.Z., G.C., Q.L., L.S. and N.Y. participated in investigation design and implementation, data analysis and manuscript writing. All authors have read and approved the final manuscript. Ethics of human subject participation: The current study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving study participants were approved by the ethics committee at People’s Hospital of Xinjiang Uygur Autonomous Region China. Written informed consent was obtained from all participants.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980019004841.
click here to view supplementary material
References
- 1. Zhou M, Wang H, Zhu J et al. (2016) Cause-specific mortality for 240 causes in China during 1990–2013: a systematic subnational analysis for the global burden of disease study 2013. Lancet 387, 251–272. [DOI] [PubMed] [Google Scholar]
- 2. Veeranna V, Zalawadiya SK, Niraj A et al. (2011) Homocysteine and reclassification of cardiovascular disease risk. J Am Coll Cardiol 58, 1025–1033. [DOI] [PubMed] [Google Scholar]
- 3. Wang Y, Li X, Qin X et al. (2013) Prevalence of hyperhomocysteinaemia and its major determinants in rural Chinese hypertensive patients aged 45–75 years. Br J Nutr 109, 1284–1293. [DOI] [PubMed] [Google Scholar]
- 4. Zhong C, Xu T, Xu T et al. (2016) Plasma homocysteine and prognosis of acute ischemic stroke: a gender-specific analysis from CATIS randomized clinical trial. Mol Neurobiol 54, 1–9. [DOI] [PubMed] [Google Scholar]
- 5. Xu C, Wu Y, Liu G et al. (2014) Relationship between homocysteine level and diabetic retinopathy: a systematic review and meta-analysis. Diagn Pathol 9, 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hainsworth AH, Yeo NE, Weekman EM et al. (2016) Homocysteine, hyperhomocysteinemia and vascular contributions to cognitive impairment and dementia (VCID). Biochim Biophys Acta 1862, 1008–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boyama BA, Cepni I, Imamoglu M et al. (2016) Homocysteine in embryo culture media as a predictor of pregnancy outcome in assisted reproductive technology. Gynecol Endocrinol 32, 193–195. [DOI] [PubMed] [Google Scholar]
- 8. Ebara S (2017) Nutritional role of folate. Congenit Anom 57, 138–141. [DOI] [PubMed] [Google Scholar]
- 9. Zappacosta B, Mastroiacovo P, Persichilli S et al. (2013) Homocysteine lowering by folate-rich diet or, pharmacological supplementations in subjects with, moderate hyperhomocysteinemia. Nutrients 5, 1531–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Anderson CA, Jee SH, Charleston J et al. (2010) Effects of folic acid supplementation on serum folate and plasma homocysteine concentrations in older adults: a dose-response trial. Am J Epidemiol 172, 932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Refsum H, Nurk E, Smith AD et al. (2006) The Hordaland Homocysteine Study: a community-based study of homocysteine, its determinants, and associations with disease. J Nutr 136, 1731–1740. [DOI] [PubMed] [Google Scholar]
- 12. Jacques PF, Selhub J, Bostom AG et al. (1999) The effect of folic acid fortification on plasma folate and total homocysteine concentrations. N Engl J Med 340, 1449–1454. [DOI] [PubMed] [Google Scholar]
- 13. Hickling S, Hung J, Knuiman M et al. (2005) Impact of voluntary folate fortification on plasma homocysteine and serum folate in Australia from 1995 to 2001: a population based cohort study. J Epidemiol Community Health 59, 371–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tucker KL, Mahnken B, Wilson PW et al. (1996) Folic acid fortification of the food supply: potential benefits and risks for the elderly population. JAMA 276, 1879–1885. [DOI] [PubMed] [Google Scholar]
- 15. https://wenku.baidu.com/view/e1e632cd03d8ce2f0166233b.html (accessed January 2016).
- 16. Monteagudo C, Scander H, Nilsen B et al. (2017) Folate intake in a Swedish adult population: food sources and predictive factors. Food Nutr Res 61, 1328960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Teixeira JA, Steluti J, Gorgulho BM et al. (2020) Prudent dietary pattern influences homocysteine level more than folate, vitamin B12, and docosahexaenoic acid: a structural equation model approach. Eur J Nutr 59, 81–91. [DOI] [PubMed] [Google Scholar]
- 18. Calcaterra V, Larizza D, De Giuseppe R et al. (2019) Diet and lifestyle role in homocysteine metabolism in Turner’s syndrome. Med Princ Pract 28, 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kearney PM, Whelton M, Reynolds K et al. (2005) Global burden of hypertension: analysis of worldwide data. Lancet 365, 217–223. [DOI] [PubMed] [Google Scholar]
- 20. Yang B, Fan S, Zhi X et al. (2015) Prevalence of hyperhomocysteinemia in China: a systematic review and meta-analysis. Nutrients 7, 74–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhou L, Zhao X, Heizhati M et al. (2019) Trends in lipids and lipoproteins among adults in Northwestern Xinjiang, China, from 1998 through 2015. J Epidemiol 59, 257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Han W, Hu Y, Tang Y et al. (2017) Relationship between urinary sodium with blood pressure and hypertension among a Kazakh community population in Xinjiang, China. J Hum Hypertens 31, 333–340. [DOI] [PubMed] [Google Scholar]
- 23. Guo S, Pang H, Guo H et al. (2015) Ethnic differences in the prevalence of high homocysteine levels among low-income rural Kazakh and Uyghur adults in far western China and its implications for preventive public health. Int J Environ Res Public Health 12, 5373–5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Perloff D, Grim C, Flack J et al. (1993) Human blood pressure determination by sphygmomanometry. Circulation 88, 2460–2470. [DOI] [PubMed] [Google Scholar]
- 25. World Health Organization (2000) Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser, 894. [PubMed]
- 26. Sacco RL, Adams R, Albers G et al. (2006) Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Circulation 113, e409–449. [PubMed] [Google Scholar]
- 27. Zhao S, Wang Y, Mu Y et al. (2014) Prevalence of dyslipidaemia in patients treated with lipid-lowering agents in China: results of the DYSlipidemia International Study (DYSIS). Atherosclerosis 235, 463–469. [DOI] [PubMed] [Google Scholar]
- 28. https://baike.baidu.com/item/%E9%A2%9D%E6%95%8F%E5%8E%BF/135402?fr=aladdin. http://city.funonglu.com/xinjiang/tcdq/em/jbqk.html (accessed September 2019).
- 29. Lonn E, Yusuf S, Arnold MJ et al. (2006) Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med 354, 1567–1577. [DOI] [PubMed] [Google Scholar]
- 30. Moretti R & Caruso P (2019) The controversial role of homocysteine in neurology: from labs to clinical practice. Int J Mol Sci 20, 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang X, Qin X, Demirtas H et al. (2007) Efficacy of folic acid supplementation in stroke prevention: a meta-analysis. Lancet 369, 1876–1882. [DOI] [PubMed] [Google Scholar]
- 32. Qin X & Huo Y (2016) H-type hypertension, stroke and diabetes in China: opportunities for primary prevention. J Diabetes 8, 38–40. [DOI] [PubMed] [Google Scholar]
- 33. Li Y, Huang T, Zheng Y et al. (2016) Folic acid supplementation and the risk of cardiovascular diseases: a meta-analysis of randomized controlled trials. J Am Heart Assoc 5, e003768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Huo Y, Li J, Qin X et al. (2015) Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: the CSPPT randomized clinical trial. JAMA 313, 1325–1335. [DOI] [PubMed] [Google Scholar]
- 35. Qin YY, Wang P, Qin JQ et al. (2018) Prevalence of hyperhomocysteinemia during routine physical examination in Guangxi Province, China and related risk factors. J Clin Lab Anal 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang J, Liu TT, Zhang W et al. (2016) Hyperhomocysteinemia is associated with vitamin B-12 deficiency: a cross-sectional study in a rural, elderly population of Shanxi China. J Nutr Health Aging 20, 594–601. [DOI] [PubMed] [Google Scholar]
- 37. González-Gross M, Benser J, Breidenassel C et al. (2012) Gender and age influence blood folate, vitamin B12, vitamin B6, and homocysteine levels in European adolescents: the Helena Study. Nutr Res 32, 817–826. [DOI] [PubMed] [Google Scholar]
- 38. Stefler D & Bobak M (2015) Does the consumption of fruits and vegetables differ between Eastern and Western European populations? Systematic review of cross-national studies. Arch Public Health 73, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Goryakin Y, Rocco L, Suhrcke M et al. (2015) Fruit and vegetable consumption in the former Soviet Union: the role of individual- and community-level factors. Public Health Nutr 18, 2825–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. WHO (2015) Global Health Observatory Data Repository. http://apps.who.int/ghodata/ (accessed March 2016).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980019004841.
click here to view supplementary material