Abstract
Background
in COVID-19 patients, older age (sixty or older), comorbidities, and frailty are associated with a higher risk for mortality and invasive mechanical ventilation (IMV) failure. It therefore seems appropriate to suggest limitations of care to older and vulnerable patients with severe COVID-19 pneumonia and a poor expected outcome, who would not benefit from invasive treatment. HFNO (high flow nasal oxygen) is a non-invasive respiratory support device already used in de novo acute respiratory failure. The main objective of this study was to evaluate the survival of patients treated with HFNO outside the ICU (intensive care unit) for a severe COVID-19 pneumonia, otherwise presenting limitations of care making them non-eligible for IMV. Secondary objectives were the description of our cohort and the identification of prognostic factors for HFNO failure.
Methods
we conducted a retrospective cohort study. We included all patients with limitations of care making them non-eligible for IMV and treated with HFNO for a severe COVID-19 pneumonia, hospitalized in a COVID-19 unit of the pulmonology department of Lyon Sud University Hospital, France, from March 2020 to March 2021. Primary outcome was the description of the vital status at day-30 after HFNO initiation, using the WHO (World Health Organization) 7-points ordinal scale.
Results
fifty-six patients were included. Median age was 83 years [76.3-87.0], mean duration for HFNO was 7.5 days, 53% had a CFS score (Clinical Frailty Scale) >4. At day-30, 73% of patients were deceased, one patient (2%) was undergoing HFNO, 9% of patients were discharged from hospital. HFNO failure occurred in 66% of patients. Clinical signs of respiratory failure before HFNO initiation (respiratory rate >30/min, retractions, and abdominal paradoxical breathing pattern) were associated with mortality (p=.001).
Conclusions
we suggest that HFNO is an option in non-ICU skilled units for older and frail patients with a severe COVID-19 pneumonia, otherwise non-suitable for intensive care and mechanical ventilation. Observation of clinical signs of respiratory failure before HFNO initiation was associated with mortality.Background
Keywords: COVID-19, high flow nasal oxygen, HFNO, frailty
Cases of COVID-19 were first reported in late-December 2019 in Wuhan, China [1]. SARS-CoV-2 virus, the pathogen responsible for the COVID-19 infection spread globally to generate a pandemic in 2020 [2]. Most of COVID-19 patients develop mild symptoms or are asymptomatic [3], while 20% have a severe disease [4], [5], [6] which manifestations include respiratory failure, ARDS (acute respiratory distress syndrome), sepsis and septic shock, thromboembolism, and/or multiple organ failure [7]. Admission in intensive care units (ICUs) concerns 21% of COVID-19 inpatients [8] with mean lengths of stay from 8 to 19 days [8,9]. In COVID-19 patients treated with invasive ventilation, mortality ranges from 35.7% to 43% [8,10,11].
Since 2020, the SARS-CoV-2 infection has emerged as a global health crisis and led to an overwhelming of ICUs. Data from the firsts COVID-19 waves permitted to determine risk factors for poor outcomes in COVID-19, and to identify patients who benefit least from ICU and invasive treatments. As such, clinical frailty, an older age (sixty or older) and cardiovascular comorbidities, arterial hypertension, chronic kidney diseases and obesity are associated with a higher risk of in-hospital and ICU mortality, and invasive mechanical ventilation failure in severe COVID-19 [12], [13], [14]. Interestingly, admission of frail COVID-19 patients in ICU seemed to be lower in later COVID-19 waves than if the first wave [15]. Moreover, COVID-19 survivors tend to suffer limitations in daily living, reduced quality of life and persistent symptoms, especially those who have undergone critical care [16]. Therefore, it seems appropriate to discuss limitations of care with those patients and consider a less invasive management.
High Flow Nasal Oxygen (HFNO) is a validated, non-invasive respiratory support to manage hypoxemia in de novo acute respiratory failure (ARF) [17,18]. Compared with standard oxygen therapy (SO), the main advantage of HFNO is the ability to deliver a high and precise FiO2 (fraction of inspired dioxygen) [19]. Its nasal interface and the capacity to deliver heated and humidified gases make it a very well-tolerated respiratory support, as compared to SO and NIV (non-invasive ventilation). HFNO devices commonly used can generate up to 60 L/min oxygen (pure or mixed with room air), with interesting physiological implications, such as washout of nasopharyngeal dead space [20] or a possible PEEP-effect (positive end-expiratory pressure) [21].
In other indication than COVID-19, HFNO is recommended as a first-intention treatment in de novo ARF [18]. Previous data suggest a possible superiority versus SO, essentially on preventing invasive mechanical ventilation [22], [23], [24] but the level of evidence remains low. On the wards, HFNO is already commonly used in several indications [25], sometimes in a context of palliative care [26].
In this context of health care crisis, some authors recommended using HFNO for severe COVID-19 in medical units [27,28], in selected patients not eligible to be hospitalized in critical care. The efficacy of HFNO outside of ICU and in these patients have been poorly evaluated. Moreover, predictors of HFNO efficacy in COVID-19, in this population are mostly unknown. Improving knowledge on the use of HFNO in this indication could allow a rational allocation of medical resources in this context of health emergency.
In our center, we decided early to use HFNO in severe COVID-19 pneumonia, in patients with limitations of care making them non-eligible for IMV and critical care after a multidisciplinary discussion including critical care physicians.
We aimed to evaluate HFNO in this indication through a monocentric, retrospective cohort. Our main objective was to evaluate the survival of patients admitted for a severe COVID-19 pneumonia, non-suitable for ICU and treated with HFNO. Secondary objectives were the description of our cohort and the identification of prognostic factors for HFNO failure.
Methods
Study design
This is an observational monocentric cohort. Data were retrospectively collected from electronic medical records. This study was approved by the Ethical Committee of the HCL (CSE-HCL - IRB00013204) and is conform to MR004 methodology (Commission Nationale Informatique et Liberté, 21_5398).
Population
We screened for inclusion all patients hospitalized in one of the COVID-19 units of the pulmonology department of Lyon Sud University Hospital, France, from March 2020 to March 2021.
According to the WHO guidelines [7], we defined severe COVID-19 pneumonia as the association of clinical signs of pneumonia (fever, cough, dyspnea) plus one of the following: tachypnea, severe respiratory distress, or SpO2 <90% on room air.
Inclusion criteria were: (i) patients with severe COVID-19 pneumonia, according to WHO definition, (ii) requirement of HFNO treatment, and (iii) limitations of care with ineligibility to critical care (especially invasive ventilation). Other inclusion criteria were (iv) an age of 18 years or older and (v) a proven SARS-CoV-2 pneumonia (with a positive nose swab RT-PCR). All patients were followed-up to May 10, 2021.
Exclusion criterion was HFNO lasting less than 24 hours (temporary assistance).
Objectives and outcomes
The primary outcome was the description of the vital status, thirty days after the initiation of HFNO, using the WHO 7-points ordinal scale (v3.0, March 3, 2020, Master Protocol). The 7 points are:
-
1
Not hospitalized, no limitation on activities,
-
2
Not hospitalized, limitation on activities,
-
3
Hospitalized, not requiring supplemental oxygen therapy,
-
4
Hospitalized, requiring supplemental oxygen therapy,
-
5
Hospitalized, on non-invasive ventilation or high flow oxygen devices,
-
6
Hospitalized, on invasive mechanical ventilation or ECMO.
-
7
Deceased.
All patients were categorized “five” at inclusion. Because of the retrospective nature of the study, it was difficult to differentiate level two from one. Thus, we decided to only use score two to qualify patients discharged from hospital (corresponding to the least favorable hypothesis).
Secondary objectives were the description of the epidemiological and clinical characteristics of the cohort including clinical frailty assessed by the CFS (Clinical Frailty Score, supplemental), a description of the non-respiratory complications of COVID-19 in our patients, a description of their medication during hospitalization, a description of adherence to HFNO with a distinction between “good” (no cannula removal by patient), “moderate” (cannula removal by patient with no desaturation/other complication), “poor” (cannula removal by patient with desaturation/other complication) and “non-adherent” (refusal of HFNO). We also aimed to determine predictive factors for mortality, both in-hospital and at day-30 and day-90 from HFNO initiation.
In our study, we considered as clinical signs of respiratory failure the following: respiratory rate >30/min, retractions, and abdominal paradoxical breathing pattern. HFNO failure was defined within the week after HFNO initiation as the persistence or occurrence of signs of respiratory failure (see above) and/or low SpO2 <90% despite maximum treatment (flow 60 L/min and FiO2 100%).
COVID-19 units
Our COVID-19 units were organized as medical wards with frequent paramedical surveillance (vital signs monitoring once every four hours). The patient/caregiver ratio was five patients per paramedic. Patients had a continuous and centralized monitoring of pulse oximetry with telemetry. They were hospitalized in separate rooms, and isolated with containment procedures. In these units, we had the possibility to use HFNO, SO, NIV or CPAP. We admitted COVID-19 patients not severe enough for ICU (admission before a possible transfer in ICU (step-up)), or not eligible for ICU because of limitations of care, or discharged from ICU after stabilization of their condition (step-down).
High flow nasal oxygen
The HFNO device used was AIRVOTM2 (Fisher & Paykel Healthcare, Auckland, New Zealand). Criteria for initiation and weaning, as the modification of the parameters of the HFNO during the hospitalization were left to the discretion of the physician.
ROX index
The ROX index is a prognostic index evaluating the short-term probability of HFNO failure, and thus the need for intubation [29]. It was recently suggested in hypoxemic ARF management in a prospective multicenter study [30]. The main objective of this index is to avoid intubation delay in patients treated with HFNO for an ARF, which is associated with an increased mortality [31]. The ROX index is calculated by the following formula ROX = (SpO2/FiO2) / RR (SpO2 is oxygen pulse saturation; and RR is respiratory rate). It is validated in the 24 hours following HFNO initiation and it is suggestive of HFNO success when greater than or equal to 4.88. In our study, the ROX index was calculated retrospectively, in the first 24 hours after HFNO initiation (D0), at day one (D1), three (D3) and five (D5) after the initiation of HFNO.
Level of care assessment scale (LCAS)
During the pandemic, the Lyon University Hospitals spread the use of a level of care assessment scale (LCAS). Initially developed by our team to determine the level of care assigned to a patient, it was already validated by the Ethical Committee of Lyon University Hospitals. LCAS is only used in medical and surgical departments of the Lyon University Hospitals. This four-level scale is a medical prescription. For each hospitalized patient, the level of care was determined at admission after ethical discussion with the patient, his family, and the medical staff (including ICU physicians if needed). It was reassessed every day and could be adjusted.
The four LCAS levels are
-
1
No treatment limitations, especially no limitations to invasive mechanical ventilation or cardiopulmonary resuscitation.
-
2
mission in ICU possible but limitations to invasive mechanical ventilation and on cardiopulmonary resuscitation.
-
3
No admission in ICU but maximal care in intermediate/conventional units.
-
4
Exclusive palliative care, focused on the patient's well-being.
Statistics
Continuous variables are expressed as mean (± standard deviation), or median (and interquartile range IQR) if not normally distributed (tested with Kolmogorov-Smirnov test). Means were compared using Student's “t” test. Medians were compared by Mann-Whitney test as appropriate. Categorical variables were compared by the Chi-squared or the Fisher Test as appropriate. Survival data were represented by the Kaplan Meier method and were compared by the Log-Rank test. The univariate analysis used regression techniques: binary linear regression (“enter” method) and Cox regression for survival times. All tests were two-sided. Missing variables are presented as such. To consider the inflation of the alpha risk in the event of multiple tests, we applied a Bonferroni correction (N=15 independent tests). With 15 independent tests performed, the threshold to consider is 0.0033.
Results
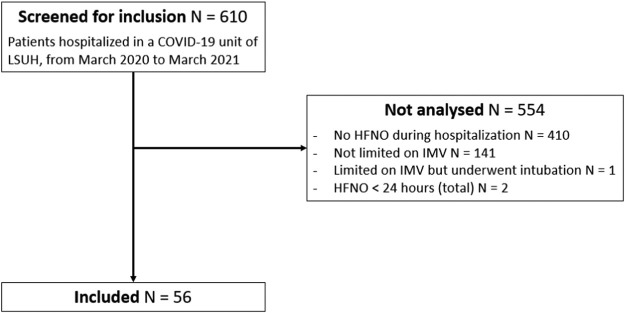
A total of 610 patients were admitted to the COVID-19 unit of the pulmonology department at Lyon Sud University Hospital, France, between March 2020 and March 2021. Among them, 554 patients were not analyzed. A total of 56 patients hospitalized from March 23, 2020, to February 8, 2021, were included in the cohort (figure 1 ).
Figure 1.
flow chart.
LSUH: Lyon Sud University Hospital; HFNO: high flow nasal oxygen; IMV: invasive mechanical ventilation.
Characteristics at inclusion
The median age was 83.0 years [76.3-87.0] (table 1 ), with age ranging from 60 to 95 years old. The mean Body Mass Index (BMI) was 27.1 kg/m2 (± 5.3). The most frequently reported comorbidities were arterial hypertension (75%), chronic heart failure (50%), chronic obstructive lung disease and cancer (29% each). Twenty-nine patients (52%) presented with a CFS greater than 4 at admission and 13% were institutionalized. Polypharmacy, defined by a daily prescription of 5 drugs or more, occurred in 79% of patients.
Table 1.
patients characteristics at admission.
| Variable | Modality | N = 56 |
| Sex | Female | 24 (43%) |
| Male | 32 (57%) | |
| Age (years) | Median (IQR) | 83.0 [76.3-87.0] |
| Weight (kg) | Mean (SD) | 73.7 (15.2) |
| BMI (kg/m2) | Mean (SD) | 27.1 (5.3) |
| Underweight (<22) | 10 (18%) | |
| Normal (22-24.9) | 8 (14%) | |
| Overweight (25-29.9) | 22 (39%) | |
| Obesity (>30) | 13 (23%) | |
| Missing | 3 (5%) | |
| Smoking status | Non-smoker | 28 (50%) |
| Former smoker | 20 (36%) | |
| Active smoker | 5 (9%) | |
| Missing | 3 (5%) | |
| Comorbidities | Hypertension | 42 (75%) |
| Cardiac arrhythmia | 20 (36%) | |
| Chronic heart failure | 28 (50%) | |
| Coronaropathy | 9 (16%) | |
| Cardiac valve disease | 7 (13%) | |
| OLD | 16 (29%) | |
| OSA | 4 (7%) | |
| Active cancer | 16 (29%) | |
| Diabetes (w/ complications) | 10 (18%) | |
| Immunosuppression | 7 (13%) | |
| Severe CKD | 3 (5%) | |
| NCD (excluding dementia) | 9 (16%) | |
| Dementia | 12 (21%) | |
| Treatments | ACE inhibitors | 17 (30%) |
| ARB | 14 (25%) | |
| Number of daily treatments | 1 - 4 | 9 (16%) |
| 5 - 10 | 26 (46%) | |
| 11 - 20 | 18 (32%) | |
| Missing | 3 (5%) | |
| CFS at admission | 2 | 2 (4%) |
| 3 | 11 (20%) | |
| 4 | 13 (23%) | |
| 5 | 10 (18%) | |
| 6 | 12 (21%) | |
| 7 | 7 (13%) | |
| Missing | 1 (2%) | |
| Living conditions | Institutionalized | 7 (13%) |
| Alone | 21 (38%) | |
| Accompanied | 26 (46%) | |
| Missing | 2 (4%) | |
| LCAS at admission | 2 | 21 (37%) |
| 3 | 35 (63%) |
IQR: interquartile range; SD: standard deviation; BMI: body mass index; OLD: obstructive lung disease; OSA: obstructive sleep apnea; w/: with; CKD: chronic kidney diseases; NCD: neurological chronic diseases; ACE: angiotensin-converting enzyme; ARB: angiotensin receptor blockers; CFS: Clinical Frailty Scale; LCAS: level of care assessment scale.
Regarding COVID-19 (table S1), the mean time from first symptoms to admission was 5.4 days (± 4.1).
Six patients were admitted to the COVID-19 unit under HFNO; in the 50 other patients, median SO flow rate was 5 L/min [2.75-10]. Thirty-two patients underwent a CT-scan during the stay. Among them, 15 patients (46.9%) showed an interstitial pattern at CT-scan. Bacterial co-infection was demonstrated in 17.9% of patients, and all patients received at least one antibiotic during their hospitalization (>24 hours of treatment). Fifty-five patients (98.2%) received corticosteroids during the stay. The median daily dose of corticosteroids was 40 mg prednisone equivalent [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50]. The median duration of corticosteroid treatment was 9.0 days [6.0-12.8].
Concerning HFNO settings at initiation, the median flow rate was 48 L/min [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50] and the median FiO2 was 0.75 [0.5-1]. Mean duration for HFNO was 7.5 days (± 9.5). During the stay, HFNO failure occurred in 37 patients (66%). Adherence to HNFO was considered good for 44 patients (78.6%), moderate for 11 patients (19.6%) and poor for one patient (1.8%). No patient was considered non-adherent to HFNO. A total of 13 patients (23.2%) presented with a non-respiratory COVID-19 complication (table S2), with 7 patients (12.5%) undergoing confusion.
Thirty days survival (primary outcome) and three months survival
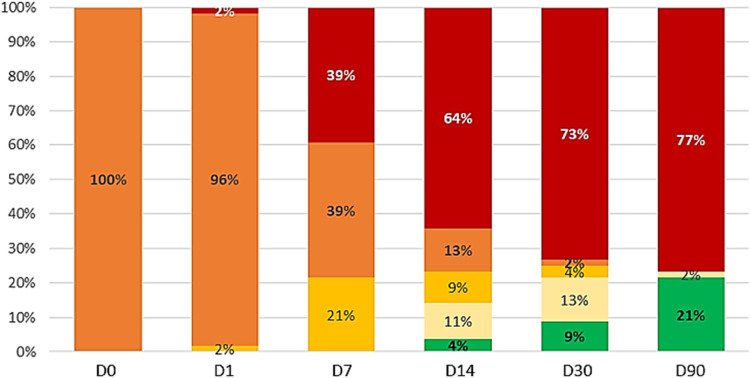
At day-30 from the beginning of HFNO, 41 patients were dead (73%), one patient was still under HFNO (2%), two patients (4%) were receiving SO and seven (13%) were hospitalized without oxygen therapy. Five patients (9%) were discharged from hospital (figure 2 ). According to CFS, 90%, 83% and 86% of patients with CFS 5, 6 and 7 on admission respectively were dead at day-30 (table S3).
Figure 2.
changes in vital status over time (WHO 7-points ordinal scale).
DX: day-X; HFNO: high flow nasal oxygen.
At day-90, 43 patients (77%) were dead, one patient (2%) was hospitalized without oxygen therapy, and twelve patients (21%) were discharged.
Prognostic factors
Univariate risk factors associated with day-30 and day-90 mortality are presented in table 2 and table 3 , respectively. Observation of signs of respiratory failure before HFNO initiation was associated with day-30 mortality (p=0.003), whereas ROX index <4.88 at D0 and a CFS between 5 and 7 (mildly to severely frail) were not (p=0.44 and 0.018 respectively). Other variables, including those associated with the severity of the COVID-19 infection, were not associated with mortality.
Table 2.
factors associated with 30-day mortality (univariate analysis).
| Alive | Dead | Total | p value | ||
| Sex | Female | 7 (47%) | 17 (41%) | 24 (43%) | 0.728 |
| Male | 8 (53%) | 24 (59%) | 32 (57%) | ||
| BMI (kg/m2) | Underweight (<22) | 0 (0%) | 10 (26.3%) | 10 (17.9%) | NC |
| Normal (22-24.9) | 2 (13.3%) | 6 (15.8%) | 8 (14.3%) | ||
| Overweight (25-29.9) | 7 (46.6%) | 15 (39.5%) | 22 (39.3%) | ||
| Obesity (>30) | 6 (40%) | 7 (18.4%) | 13 (23.2%) | ||
| Hypertension | Yes | 1 (6.7%) | 13 (31.7%) | 14 (25%) | 0.052† |
| No | 14 (93.3%) | 28 (68.3%) | 42 (75%) | ||
| Lung damage (GGO) | No | 0 (0%) | 1 (5%) | 1 (3.1%) | NC |
| Mild (0-33%) | 2 (16.7%) | 2 (10%) | 4 (12.5%) | ||
| Moderate (33-66%) | 8 (66.7%) | 7 (35%) | 15 (46.9%) | ||
| Major (66-100%) | 2 (16.7%) | 10 (50%) | 12 (37.5%) | ||
| Lung damage (consolidation) | No | 3 (25%) | 5 (25%) | 8 (25%) | NC |
| Mild (0-33%) | 6 (50%) | 4 (20%) | 10 (31.3%) | ||
| Moderate (33-66%) | 3 (25%) | 8 (40%) | 11 (34.4%) | ||
| Major (66-100%) | 0 (0%) | 3 (15%) | 3 (9.4%) | ||
| Lung damage (interstitial changes) | No | 6 (54.5%) | 10 (50%) | 16 (51.6%) | NC |
| Mild (0-33%) | 5 (45.5%) | 7 (35%) | 12 (38.7%) | ||
| Moderate (33-66%) | 0 (0%) | 3 (15%) | 3 (9.7%) | ||
| ROX DO (threshold)‡ | ROX D0 <4.88 | 3 (21.4%) | 21 (52.5%) | 24 (44.4%) | 0.044 |
| ROX D0 ≥4.88 | 11 (78.6%) | 19 (47.5%) | 30 (55.6%) | ||
| Clinical signs of respiratory failure before HFNO initiation§ | No | 14 (93.3%) | 19 (50%) | 33 (62.3%) | 0.003 |
| Yes | 1 (6.7%) | 19 (50%) | 20 (37.7%) | ||
| Number of daily treatments (2 categories) | <10 | 11 (73.3%) | 22 (57.9%) | 33 (62.3%) | 0.296 |
| 10 and more | 4 (26.7%) | 16 (42.1%) | 20 (37.7%) | ||
| CFS at admission (2 categories) | Well – vulnerable (2-4) | 11 (73.3%) | 15 (37.5%) | 26 (47.3%) | 0.018 |
| Mildly frail – severely frail (5-7) | 4 (26.7%) | 25 (62.5%) | 29 (52.7%) | ||
| Early rehabilitation¶ | No | 1 (0.1%) | 36 (0.9%) | 37 (66.1%) | <0.0001 |
| Yes | 14 (0.9%) | 5 (0.1%) | 19 (33.9%) |
† Fisher test; ‡ ROX index after HFNO initiation (D0), compared to threshold (4.88); § include respiratory rate >30/min and/or retractions and/or abdominal paradoxical breathing pattern; ¶ includes respiratory and functional physiotherapy, daily walk, active sitting position; BMI: body mass index; NC: not calculable; GGO: ground-glass opacities; HFNO: high flow nasal oxygen therapy; CFS: clinical frailty scale.
Table 3.
factors associated with day-90 mortality (univariate analysis).
| Alive | Dead | Total | p value | ||
| CFS at admission (2 categories) | Well – vulnerable (2-4) | 9 (69.2%) | 17 (40.5%) | 26 (47.3%) | 0.07 |
| Mildly frail – severely frail (5-7) | 4 (30.8%) | 25 (59.5%) | 29 (52.7%) | ||
| ROX D0 (threshold)† | ROX D0 <4.88 | 3 (25%) | 21 (50.0%) | 24 (44.4%) | 0.124 |
| ROX J0 ≥4.88 | 9 (75%) | 21 (50.0%) | 30 (55.6%) | ||
| Clinical signs of respiratory failure before HFNO initiation‡ | No | 12 (92.3%) | 21 (52.5%) | 33 (62.3%) | 0.01 |
| Yes | 1 (7.7%) | 19 (47.5%) | 20 (37.7%) | ||
| Early rehabilitation§ | No | 0 (0%) | 37 (0.9%) | 37 (66.1%) | <0.0001 |
| Yes | 13 (100%) | 6 (0.1%) | 19 (33.9%) |
† ROX index after HFNO initiation (D0), compared to threshold (4.88); ‡ include respiratory rate > 30/min and/or retractions and/or abdominal paradoxical breathing pattern; § includes respiratory and functional physiotherapy, daily walk, active sitting position; CFS: clinical frailty scale; HFNO: high flow oxygen.
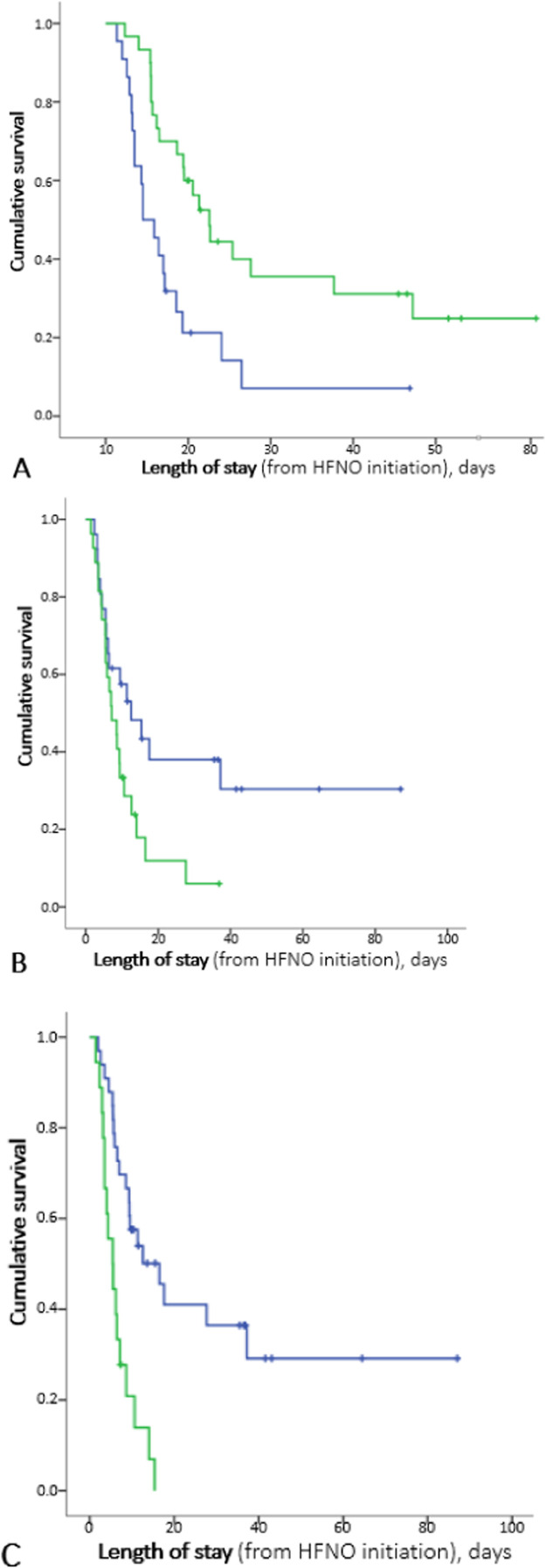
We evaluated in-hospital mortality in time-dependent univariate analysis (figure 3 ). We found a significant association with ROX index: median survival of patients with a ROX index at D0 <4.88 was 4.50 days [2.11-6.89], as compared to 12.58 days [9.34-15.83] for those with an index ≥4.88 (p=0.002). The median survival of patients with signs of respiratory failure before HFNO initiation was 5.46 days [3.03-7.88] versus 16.50 days [7.67-25.33] for those without such signs (p <10−4). Concerning frailty, no significant association was found. Patients assessed with a CFS ≤4 at admission had a median survival of 12.58 days [4.27-20.89], as compared to 7.17 days [3.63-10.70] for a higher score (p=0.031).
Figure 3.
in-hospital cumulative survival. A: depending on the value of ROX index at D0, compared to threshold (4.88); B: depending on the presence of a CFS score low (2-4) or high (5-7) at admission; C: depending on the presence or absence of signs of respiratory failure before HFNO introduction.
ROX index is calculated by (SpO2/FiO2) / RR (SpO2 is oxygen pulse saturation, FiO2 is fraction of inspired dioxygen, and RR is respiratory rate); HR: hazard ratio; HFNO: high flow nasal oxygen; CFS: clinical frailty scale; clinical signs of respiratory failure include respiratory rate > 30/min, retractions, and abdominal paradoxical breathing pattern.
Discussion
In this publication, we describe the clinical course of a cohort of patients with limitations of care treated with HFNO outside the ICU for a severe COVID-19 pneumonia.
This is a retrospective and monocentric observational study including 56 patients aged sixty or older, with frailty. Fifteen patients (27%) were alive at day-30, and thirteen patients (23%) were still alive at day-90. Interestingly, the evolutional pattern seems defined from day-14.
Observation of clinical signs of respiratory failure before HFNO initiation was the only factor showing a significant association with day-30 mortality, with 95% mortality in univariate analysis. There was an increased risk of in-hospital mortality in patients with a ROX index lower than 4.88 at D0 or showing signs of respiratory failure before HFNO initiation. There was a tendency for a worse outcome in frailer patients (admission CFS >4), without significant results. Our data highlight the importance of clinical and pragmatic criteria for the selection of patients who will benefit more of HFNO among frail COVID-19 patients.
Consistent with previous publications [32,33], we found a possible association between clinical frailty attested by the CFS score and mortality in patients treated with HFNO. The absence of statistical significance in our cohort might be related to our small sample size. An association between CFS score and mortality [34] or a greater length of stay [35] has already been suggested in previous publications. In an international prospective study including 1133 hospitalized patients, 72% of patients screened as frail were either dead or dependent on hospital care at 30 days [36]. Additionally, Hewitt and colleagues [32] suggested that frailty was a good predictor of COVID-19 outcomes. In their multicentric observational study conducted on 1564 patients hospitalized during the first wave of COVID-19, death and day-7 mortality were better predicted by the CFS score on admission than by age or comorbidity.
In our study, the presence of clinical signs of respiratory failure before initiating HFNO was associated with a higher risk of in-hospital and day-30 mortality. As mentioned in previous publications, the absence of dyspnea despite severe hypoxemia was a remarkable feature of COVID-19 pneumonia. This phenomenon is called “silent” or “happy hypoxia” [37], and contrasts with more usual presentation of ALI. In a cohort of 67 hypoxemic COVID-19 patients hospitalized in ICU, only 18.7% presented dyspnea on admission [38]. Various pathophysiological hypotheses have been reported, including direct or indirect viral damage to the mechanoreceptors and chemoreceptors regulating ventilation [39], or a COVID-19 associated encephalopathy with damage to the central respiratory drive [37]. According to some authors, the initial absence of dyspnea is to be considered as a loss of an alarm signal, which can be followed by a rapid clinical worsening [40]. We observed an increase in mortality in patients presenting with signs of respiratory failure before initiating HFNO. This may reflect the depletion of respiratory reserve capacities in patients with advanced ventilatory impairment and/or occurrence of a medical complication. The limit of this assertion is the link between dyspnea, which is a subjective impression, with respiratory failure, which corresponds to the clinical manifestation of disturbed respiratory mechanic.
Finally, the adherence to HFNO in our cohort is excellent, with 78.6% of patients compliant to the treatment. Only one patient expressed a poor adherence with removal of cannulas leading to a desaturation down to 75% SpO2, in a context of delirium. None of our patients refused HFNO. The excellent adherence to HFNO is often reported [25], especially when compared to SO [26] or to NIV, as well as in palliative care.
It is important to note that our data show an inverse relationship between mortality and early rehabilitation during the stay (respiratory and functional physiotherapy, daily walk, active sitting position). We consider that there is a probable confusion bias insofar as the patients in whom this early rehabilitation is possible are most likely to be in a better clinical condition. Nevertheless, we emphasize early rehabilitation in our management of patients with respiratory failure, whether they have COVID-19 or not, because its impact on the survival and functional status of patients is clearly established [41].
In the current literature, age [11,13,42], comorbidities such as cardiovascular diseases, arterial hypertension, chronic kidney diseases, obesity [4,6,43], and clinical frailty are risk factors for death from COVID-19 and for invasive mechanical ventilation failure [14,44]. Therefore, and in a context of COVID-19 associated ARF, it is legitimate to suggest HFNO to this vulnerable population, for whom a more invasive intervention does not seem to modify the overall survival.
In our study, HFNO failure occurred in 66% of patients. Several clinical trials reported data on HFNO efficacy in ICU for severe COVID-19. In those publications, patients were younger and less frail than in our cohort.
An Italian study [45] randomized 109 ICU patients to receive either 48 hours NIV then HFNO or CPAP or HFNO alone for a severe COVID-19. Failure, defined as the need for endotracheal intubation, occurred in 51% of patients of the HFNO group, with a median time of 21 hours between enrollment and intubation. These results are close to our findings. In a multicentric study [46], 1273 ICU patients with severe COVID-19 were randomized to receive either CPAP or HFNO versus SO. Respiratory support failure, defined as the composite outcome intubation or mortality within 30 days, occurred in 44% of the HFNO group. Failure was significantly lower in the CPAP group (36%) as compared to the SO group (44%), but not in HFNO versus SO. Interestingly, the decrease in the incidence of the outcome with CPAP was attributable to a decrease in the need for invasive ventilation, but neither HFNO nor CPAP reduced mortality as compared to SO. Moreover, CPAP was associated with an increase in adverse events.
In a multicentric prospective cohort study of the COVID-ICU group [47], HFNO failure (defined as either intubation or death in ICU) occurred in 48% of patients receiving HFNO for a severe COVID-19. HFNO was also associated with a 40% reduction in oxygenation failure versus SO. In the cohort, 10% of HFNO patients had a do not intubate (DNI) order.
The SOHO trial [48] is a multicenter trial randomizing HFNO versus SO in critically ill patients with respiratory failure due to COVID-19. Patients with DNI order were excluded. There was no statistical difference on mortality rate at day-28, which was the primary endpoint (10% in HFNO and 11% in SO). In secondary outcomes, the intubation rate by day-28 was 45% in the high-flow group and 53% in the standard oxygen group (p=.04).
No prospective clinical trial evaluated HFNO efficacy in frail COVID-19 patients, only retrospective studies. In a Dutch retrospective cohort study including 32 older frail patients treated with HFNO in the wards for a severe COVID-19 (median age 79.0 years), the survival rate was 25% at hospital discharge [49]. Interestingly, a retrospective study described the clinical course of a cohort of 537 COVID-19 patients treated with CPAP (continuous positive airway pressure) outside ICU for a hypoxemic ARF [50]. Results were quite similar. Among the 537 patients, 26% had a DNI order. In the DNI subgroup, 60-day in-hospital mortality was 73%, with a median duration of CPAP of 4 days [1], [2], [3], [4], [5], [6], [7], [8]. However, there were no data on adherence to CPAP.
Concerning the identification of prognostic factors to estimate the probability of HFNO failure, there is a major interest in patients with no limitation to invasive mechanical ventilation, for whom the postponement of intubation leads to an increased mortality [31]. In COVID-19 patients, several prognostic factors have been identified, such as the presence of comorbidities, dyspnea on admission or the ROX index [51,52]. Our results are consistent with previous findings.
In a population of patients hospitalized for a severe COVID-19, with limitations of care making them ineligible for ICU, the clinical implication of an early and reliable evaluation of the probability of HFNO failure would rather be to determine which subgroup of patients would benefit from a more interventionist strategy. In our study, we looked for the presence of clinical signs of respiratory failure (that we defined by observation of respiratory rate >30/min, retractions, and/or abdominal paradoxical breathing pattern). Our data suggest that patients presenting those signs before HFNO initiation have an increased risk of mortality (both in-hospital and day-30 mortality). This subgroup of patients would probably not benefit from HFNO but could be offered early comfort or palliative care.
Regarding the use of antibiotics or corticosteroids, the decision was made for each patient by the medical team in charge. For corticosteroids, our patients received a median daily dose of 40 mg prednisone equivalent for a median duration of 9.0 days. The use of corticosteroid treatments in our first-wave patients was pragmatic; management of steroids in our COVID-19 patients was then in accordance with the findings of the RECOVERY trial [53].
The limitations of our study are its retrospective nature, the lack of power inherent in the number of patients included, and its monocentric nature, which constrains the extrapolation of our data to other centers. In addition, the absence of randomization with a control arm does not allow a comparison of HFNO with another respiratory support method. We decided to perform univariate analysis because evaluation of prognostic factors was exploratory. Moreover, due to the small sample size, we would have risked not having enough events for multivariate analysis. Confirmation of our findings would require a dedicated study, with multivariate analysis taking confusing factors into account. Finally, the lack of data on the exact functional status of COVID-19 survivors after discharge from our units makes it difficult to extrapolate on the level of functioning of outpatients, especially their degree of autonomy at home.
Our study has several strengths. Firstly, the primary endpoint is robust and pragmatic, recommended by the WHO for this type of study. To our knowledge, this is the first cohort of this scale to describe the evolution of severe COVID-19 patients with frailty treated with HFNO outside the ICU because of limitations of care making them non-eligible for IMV. The use of the Bonferroni correction considers the alpha risk inflation associated with multiple testing.
Conclusions
We suggest that HFNO is an option in non-ICU skilled units for older and frail patients with a severe COVID-19 pneumonia, otherwise non-suitable for intensive care and mechanical ventilation. In our cohort, the observation of clinical signs of respiratory failure (respiratory rate >30/min, retractions, and abdominal paradoxical breathing pattern) was associated with an increased mortality and patients showing such manifestations would probably not benefit from HFNO.
Declaration of Competing Interest
None
Acknowledgements
Jean-Christophe Richard.
Affiliations: Medical Intensive Care Department, Croix-Rousse University Hospital, Hospices Civils de Lyon, Lyon, France.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.resmer.2023.101026.
Appendix. Supplementary materials
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munster VJ, Koopmans M, van Doremalen N, van Riel D, de Wit E. A Novel Coronavirus Emerging in China - Key Questions for Impact Assessment. N Engl J Med. 2020;382:692–694. doi: 10.1056/NEJMp2000929. [DOI] [PubMed] [Google Scholar]
- 3.Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 4.Du R-H, Liang L-R, Yang C-Q, Wang W, Cao T-Z, Li M, et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. 2020;55 doi: 10.1183/13993003.00524-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO. Clinical management of COVID-19: Living guideline, 15 September 2022 n.d. https://www.who.int/publications/i/item/WHO-2019-nCoV-Clinical-2022.2 (accessed September 15, 2022).
- 8.Chang R, Elhusseiny KM, Yeh Y-C, Sun W-Z. COVID-19 ICU and mechanical ventilation patient characteristics and outcomes-A systematic review and meta-analysis. PLoS One. 2021;16 doi: 10.1371/journal.pone.0246318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vekaria B, Overton C, Wiśniowski A, Ahmad S, Aparicio-Castro A, Curran-Sebastian J, et al. Hospital length of stay for COVID-19 patients: Data-driven methods for forward planning. BMC Infect Dis. 2021;21:700. doi: 10.1186/s12879-021-06371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Auld SC, Caridi-Scheible M, Blum JM, Robichaux C, Kraft C, Jacob JT, et al. ICU and Ventilator Mortality Among Critically Ill Adults With Coronavirus Disease 2019. Crit Care Med. 2020;48:e799–e804. doi: 10.1097/CCM.0000000000004457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.COVID-ICU Group on behalf of the REVA Network and the COVID-ICU Investigators. Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med. 2021;47:60–73. doi: 10.1007/s00134-020-06294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dres M, Hajage D, Lebbah S, Kimmoun A, Pham T, Béduneau G, et al. Characteristics, management, and prognosis of elderly patients with COVID-19 admitted in the ICU during the first wave: insights from the COVID-ICU study : Prognosis of COVID-19 elderly critically ill patients in the ICU. Ann Intensive Care. 2021;11:77. doi: 10.1186/s13613-021-00861-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pijls BG, Jolani S, Atherley A, Derckx RT, Dijkstra JIR, Franssen GHL, et al. Demographic risk factors for COVID-19 infection, severity, ICU admission and death: a meta-analysis of 59 studies. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-044640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verduri A, Short R, Carter B, Braude P, Vilches-Moraga A, Quinn TJ, et al. Comparison between first and second wave of COVID-19 outbreak in older people. The COPE multicentre European observational cohort study. Eur J Public Health. 2022:ckac108. doi: 10.1093/eurpub/ckac108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gamberini L, Mazzoli CA, Prediletto I, Sintonen H, Scaramuzzo G, Allegri D, et al. Health-related quality of life profiles, trajectories, persistent symptoms and pulmonary function one year after ICU discharge in invasively ventilated COVID-19 patients, a prospective follow-up study. Respir Med. 2021;189 doi: 10.1016/j.rmed.2021.106665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frat J-P, Thille AW, Mercat A, Girault C, Ragot S, Perbet S, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015;372:2185–2196. doi: 10.1056/NEJMoa1503326. [DOI] [PubMed] [Google Scholar]
- 18.Oczkowski S, Ergan B, Bos L, Chatwin M, Ferrer M, Gregoretti C, et al. ERS clinical practice guidelines: high-flow nasal cannula in acute respiratory failure. Eur Respir J. 2022;59 doi: 10.1183/13993003.01574-2021. [DOI] [PubMed] [Google Scholar]
- 19.Suffredini DA, Allison MG. A Rationale for Use of High Flow Nasal Cannula for Select Patients With Suspected or Confirmed Severe Acute Respiratory Syndrome Coronavirus-2 Infection. J Intensive Care Med. 2021;36:9–17. doi: 10.1177/0885066620956630. [DOI] [PubMed] [Google Scholar]
- 20.Möller W, Feng S, Domanski U, Franke K-J, Celik G, Bartenstein P, et al. Nasal high flow reduces dead space. J Appl Physiol. 2017;122:191–197. doi: 10.1152/japplphysiol.00584.2016. 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pinkham M, Tatkov S. Effect of flow and cannula size on generated pressure during nasal high flow. Crit Care. 2020;24:248. doi: 10.1186/s13054-020-02980-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ou X, Hua Y, Liu J, Gong C, Zhao W. Effect of high-flow nasal cannula oxygen therapy in adults with acute hypoxemic respiratory failure: a meta-analysis of randomized controlled trials. CMAJ. 2017;189:E260–E267. doi: 10.1503/cmaj.160570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, Jing G, Scott JB. Year in Review 2019: High-Flow Nasal Cannula Oxygen Therapy for Adult Subjects. Respir Care. 2020;65:545–557. doi: 10.4187/respcare.07663. [DOI] [PubMed] [Google Scholar]
- 24.Roca O, de Acilu MG, Caralt B, Sacanell J, Masclans JR, collaborators ICU. Humidified high flow nasal cannula supportive therapy improves outcomes in lung transplant recipients readmitted to the intensive care unit because of acute respiratory failure. Transplantation. 2015;99:1092–1098. doi: 10.1097/TP.0000000000000460. [DOI] [PubMed] [Google Scholar]
- 25.Zemach S, Helviz Y, Shitrit M, Friedman R, Levin PD. The Use of High-Flow Nasal Cannula Oxygen Outside the ICU. Respir Care. 2019;64:1333–1342. doi: 10.4187/respcare.06611. [DOI] [PubMed] [Google Scholar]
- 26.Ruangsomboon O, Dorongthom T, Chakorn T, Monsomboon A, Praphruetkit N, Limsuwat C, et al. High-Flow Nasal Cannula Versus Conventional Oxygen Therapy in Relieving Dyspnea in Emergency Palliative Patients With Do-Not-Intubate Status: A Randomized Crossover Study. Ann Emerg Med. 2020;75:615–626. doi: 10.1016/j.annemergmed.2019.09.009. [DOI] [PubMed] [Google Scholar]
- 27.Franco C, Facciolongo N, Tonelli R, Dongilli R, Vianello A, Pisani L, et al. Feasibility and clinical impact of out-of-ICU noninvasive respiratory support in patients with COVID-19-related pneumonia. Eur Respir J. 2020;56 doi: 10.1183/13993003.02130-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rabec C, Gonzalez-Bermejo J, Mercy M, Grassion L, Pontier S, Patout M, et al. Respiratory support in patients with COVID-19 (outside intensive care unit). A position paper of the Respiratory Support and Chronic Care Group of the French Society of Respiratory Diseases. Respiratory Medicine and Research. 2020;78 doi: 10.1016/j.resmer.2020.100768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roca O, Messika J, Caralt B, García-de-Acilu M, Sztrymf B, Ricard J-D, et al. Predicting success of high-flow nasal cannula in pneumonia patients with hypoxemic respiratory failure: The utility of the ROX index. J Crit Care. 2016;35:200–205. doi: 10.1016/j.jcrc.2016.05.022. [DOI] [PubMed] [Google Scholar]
- 30.Roca O, Caralt B, Messika J, Samper M, Sztrymf B, Hernández G, et al. An Index Combining Respiratory Rate and Oxygenation to Predict Outcome of Nasal High-Flow Therapy. Am J Respir Crit Care Med. 2019;199:1368–1376. doi: 10.1164/rccm.201803-0589OC. [DOI] [PubMed] [Google Scholar]
- 31.Kang BJ, Koh Y, Lim C-M, Huh JW, Baek S, Han M, et al. Failure of high-flow nasal cannula therapy may delay intubation and increase mortality. Intensive Care Med. 2015;41:623–632. doi: 10.1007/s00134-015-3693-5. [DOI] [PubMed] [Google Scholar]
- 32.Hewitt J, Carter B, Vilches-Moraga A, Quinn TJ, Braude P, Verduri A, et al. The effect of frailty on survival in patients with COVID-19 (COPE): a multicentre, European, observational cohort study. Lancet Public Health. 2020;5 doi: 10.1016/S2468-2667(20)30146-8. e444–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sablerolles RSG, Lafeber M, van Kempen JAL, van de Loo BPA, Boersma E, Rietdijk WJR, et al. Association between Clinical Frailty Scale score and hospital mortality in adult patients with COVID-19 (COMET): an international, multicentre, retrospective, observational cohort study. Lancet Healthy Longev. 2021;2 doi: 10.1016/S2666-7568(21)00006-4. e163–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brummel NE, Bell SP, Girard TD, Pandharipande PP, Jackson JC, Morandi A, et al. Frailty and Subsequent Disability and Mortality among Patients with Critical Illness. Am J Respir Crit Care Med. 2017;196:64–72. doi: 10.1164/rccm.201605-0939OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lujic S, Randall DA, Simpson JM, Falster MO, Jorm LR. Interaction effects of multimorbidity and frailty on adverse health outcomes in elderly hospitalised patients. Sci Rep. 2022;12:14139. doi: 10.1038/s41598-022-18346-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.So RKL, Bannard-Smith J, Subbe CP, Jones DA, van Rosmalen J, Lighthall GK, et al. The association of clinical frailty with outcomes of patients reviewed by rapid response teams: an international prospective observational cohort study. Crit Care. 2018;22:227. doi: 10.1186/s13054-018-2136-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Breville G, Accorroni A, Allali G, Adler D. [Pathophysiology of COVID-19 related happy hypoxemia] Rev Med Suisse. 2021;17:831–834. [PubMed] [Google Scholar]
- 38.Guan W-J, Ni Z-Y, Hu Y, Liang W-H, Ou C-Q, He J-X, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.U R A, Verma K. Happy Hypoxemia in COVID-19-A Neural Hypothesis. ACS Chem Neurosci. 2020;11:1865–1867. doi: 10.1021/acschemneuro.0c00318. [DOI] [PubMed] [Google Scholar]
- 40.Dhont S, Derom E, Van Braeckel E, Depuydt P, Lambrecht BN. The pathophysiology of “happy” hypoxemia in COVID-19. Respir Res. 2020;21:198. doi: 10.1186/s12931-020-01462-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomas P, Baldwin C, Bissett B, Boden I, Gosselink R, Granger CL, et al. Physiotherapy management for COVID-19 in the acute hospital setting: clinical practice recommendations. J Physiother. 2020;66:73–82. doi: 10.1016/j.jphys.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Falandry C, Bitker L, Abraham P, Subtil F, Collange V, Balança B, et al. Senior-COVID-Rea Cohort Study: A Geriatric Prediction Model of 30-day Mortality in Patients Aged over 60 Years in ICU for Severe COVID-19. Aging Dis. 2022;13:614–623. doi: 10.14336/AD.2021.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roger C, Collange O, Mezzarobba M, Abou-Arab O, Teule L, Garnier M, et al. French multicentre observational study on SARS-CoV-2 infections intensive care initial management: the FRENCH CORONA study. Anaesth Crit Care Pain Med. 2021;40 doi: 10.1016/j.accpm.2021.100931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simonnet A, Chetboun M, Poissy J, Raverdy V, Noulette J, Duhamel A, et al. High Prevalence of Obesity in Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) Requiring Invasive Mechanical Ventilation. Obesity (Silver Spring) 2020;28:1195–1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grieco DL, Menga LS, Cesarano M, Rosà T, Spadaro S, Bitondo MM, et al. Effect of Helmet Noninvasive Ventilation vs High-Flow Nasal Oxygen on Days Free of Respiratory Support in Patients With COVID-19 and Moderate to Severe Hypoxemic Respiratory Failure: The HENIVOT Randomized Clinical Trial. JAMA. 2021;325:1731–1743. doi: 10.1001/jama.2021.4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perkins GD, Ji C, Connolly BA, Couper K, Lall R, Baillie JK, et al. Effect of Noninvasive Respiratory Strategies on Intubation or Mortality Among Patients With Acute Hypoxemic Respiratory Failure and COVID-19: The RECOVERY-RS Randomized Clinical Trial. JAMA. 2022;327:546–558. doi: 10.1001/jama.2022.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.COVID-ICU group, for the REVA network, COVID-ICU investigators Benefits and risks of noninvasive oxygenation strategy in COVID-19: a multicenter, prospective cohort study (COVID-ICU) in 137 hospitals. Crit Care. 2021;25:421. doi: 10.1186/s13054-021-03784-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frat J-P, Quenot J-P, Badie J, Coudroy R, Guitton C, Ehrmann S, et al. Effect of High-Flow Nasal Cannula Oxygen vs Standard Oxygen Therapy on Mortality in Patients With Respiratory Failure Due to COVID-19: The SOHO-COVID Randomized Clinical Trial. JAMA. 2022;328:1212–1222. doi: 10.1001/jama.2022.15613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Steenkiste J, van Herwerden MC, Weller D, van den Bout CJ, Ruiter R, den Hollander JG, et al. High-flow Nasal Cannula therapy: A feasible treatment for vulnerable elderly COVID-19 patients in the wards. Heart Lung. 2021;50:654–659. doi: 10.1016/j.hrtlng.2021.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vaschetto R, Barone-Adesi F, Racca F, Pissaia C, Maestrone C, Colombo D, et al. Outcomes of COVID-19 patients treated with continuous positive airway pressure outside the intensive care unit. ERJ Open Res. 2021;7:00541–02020. doi: 10.1183/23120541.00541-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferrer S, Sancho J, Bocigas I, Bures E, Mora H, Monclou E, et al. ROX index as predictor of high flow nasal cannula therapy success in acute respiratory failure due to SARS-CoV-2. Respir Med. 2021;189 doi: 10.1016/j.rmed.2021.106638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Polydora E, Alexandrou M, Tsipilis S, Athanasiou N, Katsoulis M, Rodopoulou A, et al. Predictors of high flow oxygen therapy failure in COVID-19-related severe hypoxemic respiratory failure. J Thorac Dis. 2022;14:851–856. doi: 10.21037/jtd-21-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.RECOVERY Collaborative Group. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.