Abstract
Objectives
The present study builds on recent findings suggesting that the stress of institutional and interpersonal racism may contribute to African Americans’ elevated risk for dementia. We investigated the extent to which 2 consequences of racism—low socioeconomic status (SES) and discrimination—predict self-reported cognitive decline (SCD) 19 years later. Further, we examined potential mediating pathways that might link SES and discrimination to cognitive decline. Potential mediators included depression, accelerated biological aging, and onset of chronic illnesses.
Methods
Hypotheses were tested using a sample of 293 African American women. SCD was assessed using the Everyday Cognition Scale. Structural equation modeling was used to assess the effects of SES and racial discrimination, both measured in 2002, on SCD reported in 2021. Turning to the mediators, midlife depression was assessed in 2002, accelerated aging in 2019, and chronic illness in 2019. Age and prodrome depression were included as covariates.
Results
There were direct effects of SES and discrimination on SCD. In addition, these 2 stressors showed a significant indirect effect on SCD through depression. Finally, there was evidence for a more complex pathway where SES and discrimination accelerate biological aging, with accelerated aging, in turn leading to chronic illness, which then predicted SCD.
Discussion
Results of the present study add to a growing literature indicating that living in a racialized society is a central factor in explaining the high risk for dementia among Black Americans. Future research should continue to emphasize the various ways that exposure to racism over the life course effects cognition.
Keywords: Cognitive impairment, Health disparities, Racism, Stress pathways
Although Black Americans have been seriously underrepresented in dementia-related research, there is substantial evidence from population-based samples and meta-analyses (Mehta et al., 2017; Steenland et al., 2016) indicating that they are at elevated risk for cognitive decline compared to non-Hispanic White Americans (NHWA). Indeed, their increased risk may be as much as twice that of NHWA (Barnes, 2022; Kornblith et al., 2022). Although the factors that account for this greater risk are not well understood (Barnes, 2022; Gleason et al., 2021), these observed racial differences do not appear to be solely a consequence of genetic or biological differences. Indeed, recent evidence suggests that institutional and interpersonal racism play an important role in the greater risk of dementia among Black Americans (Barnes, 2022; Gleason et al., 2021). Black Americans are members of a racialized group. They are racialized as “Black” based on skin color, hair texture, and facial features (Omi & Winant, 2014), and this racialization influences how they are treated by other social groups and institutions. It increases the probability of trauma and chronic stress in the form of racial discrimination, socioeconomic hardship, substandard education, neighborhood disadvantage, and both micro and macro aggressions. Importantly, there is now evidence suggesting that persistent exposure to such adversity is associated with cognitive impairment and dementia (Coogan et al., 2020; Letang et al., 2021; Zuelsdorff et al., 2020).
The present study elaborates on this line of research. We examine the extent to which two consequences of racism—low socioeconomic status (SES) and discrimination—predict self-reported cognitive decline 19 years later. Further, we examine the extent to which various mediating pathways link low SES and discrimination to cognition changes. Our potential mediators include psychological depression, accelerated biological aging (weathering), and the onset of chronic illnesses. We test our study hypotheses using a sample of 293 African American women.
The Present Study
The present study uses the Everyday Cognition Scale (ECog) to assess self-reported cognitive changes (Farias et al., 2008, 2011). This instrument was developed to assess functional abilities in older adults across a wide range of neuropsychological domains, including everyday memory, language, visuospatial abilities, planning, organization, and divided attention. While objective neuropsychological assessments of cognition are not available in the present data set, past research has reported that personal awareness of memory problems is predictive of the onset of dementia (Jonker et al., 2000) and is related to objective memory measures (Burmester et al., 2016). Further, self-reports of memory problems have been associated with brain pathology such as amyloid beta (Aβ), neurodegeneration (Amariglio et al., 2015), as well as reduced hippocampal volume (van der Flier et al., 2004) and gray matter (Peter et al., 2014). The individual items that comprise the ECog have been shown to function similarly across racial and ethnic groups (Filshtein et al., 2020).
The ECog asks respondents to report the extent that they are experiencing more difficulty with regard to various cognitive tasks than they did 10 years earlier. The emphasis is on whether there has been a decline in the past decade. Research on brain pathology suggests that neuropathology (e.g., amyloid plaques, neurofibrillary tangles, cerebrovascular disease, Lewy bodies) begins to accrue decades before cognitive symptoms become apparent (Maruszak et al., 2014a). Therefore, the present study uses assessments of race-related stressors collected in 2002 to predict ECog scores obtained in 2021.
Race-Related Stressors
Two race-related stressors—low SES and personal experiences of racial discrimination—are included as predictors of cognitive decline. Low SES might be seen as a proxy for a number of chronic stressors and Black Americans are more likely than other ethnoracial groups to be of low SES. One of the consequences of institutional racism is that Black Americans suffer lower quality of education, job attainment, and income than most other racial/ethnic groups. Nationwide, 43% of Black students attend schools that fail to offer the full range of necessary college preparatory courses (Clasp, 2015). Economically, approximately 1/3 of Black families live near or below the poverty line, and the ratio of Black to White median family income has remained constant (roughly 56%) since the late 1960s (Manduca, 2018). Thus, Black Americans are much more likely than other racial/ethnic groups to experience SES-related stressors such as difficulty paying bills, working at a low-status job, periods of unemployment, substandard housing conditions, and having to negotiate the challenges of food desserts and high crime (Massey, 2007). Given the variety of chronic strains, pressures, and hassles associated with low SES, it is hardly a surprise that it is a robust predictor of early onset of chronic illness and mortality (Cockerham et al., 2017).
Importantly, based on past research, low SES, with its profusion of attendant stressors, would also be expected to increase risk for dementia (Zuelsdorff et al., 2013, 2020). There is strong evidence that high levels of perceived stress are associated with declines in working (Oumohand et al., 2020) and episodic memory (Zaheed et al., 2021). These changes occur, at least in part, because chronic stress fosters reductions in the volume of key brain regions such as the hippocampus (Piccolo & Noble, 2018; Schoenfeld et al., 2017) and prefrontal cortex (Ansell et al., 2012). These brain structures are critical for episodic memory and executive function and are among the first to show neurodegenerative changes in Alzheimer’s disease (Maruszak & Thuret, 2014b). Thus, low SES, with all of its associated chronic stressors, might be viewed as a contributor to the higher prevalence of dementia seen among Black Americans.
In addition to low SES with its attendant stressors, Black Americans often experience personal incidents of racial discrimination, ranging from subtle insults to flagrantly racist incidents such as harassment by police. While most Americans think of themselves as “color blind,” job application studies (Pager et al., 2009) and implicit bias research (Sawyer & Gampa, 2018) continue to document widespread prejudice and discrimination. Frequent exposure to such treatment is another chronic stressor that likely negatively effects the brain and elevates the risk for dementia. Consonant with this idea, Barnes et al. (2012) presented cross-sectional evidence that older Black Americans who experienced greater stress due to racial discrimination demonstrated more impairment in episodic memory, perceptual speed, and global cognition. And, Zahodne et al. (2019) published longitudinal data showing that greater racial discrimination predicted lower memory scores 6 years later in their sample of African Americans. Finally, Coogan et al. (2020) recently reported that incidents of racial discrimination were associated with subjective cognitive function among a sample of Black women. In the present study, we expect to find that midlife reports of racial discrimination, like midlife reports of low SES, will predict reports of everyday cognitive function assessed 19 years later.
Psychological Depression
A wide variety of longitudinal studies have reported that depression during midlife is associated with dementia several years later (Barnes et al., 2012; Brenowitz et al., 2021; Nabe-Nielsen et al., 2020). Importantly, these studies find that midlife depression continues to predict cognitive decline after controlling for late-life depression. This is an important finding as depression assessed close to the onset of dementia may be a component of the disease (a part of the prodrome) rather than a cause of the cognitive decline. In addition to disturbances in mood and affect, the major depressive disorder often includes impairment of memory, attention, and visuospatial processing. Such symptoms appear to be a consequence of the neurobiological changes that accompany depression. Scores of neuroimaging studies have established that depression leads to roughly a 10% diminution of hippocampal volume (Nolan et al., 2020). In many cases there is some restoration of volume following treatment (Arnone et al., 2013), but it appears that recurrent depression may be etiologically associated with subsequent development of dementia.
Based upon such findings, we expect to find that depression assessed in midlife (2002) will predict self-assessments of cognitive decline obtained 19 years later (2021). Further, we hypothesize that this association will remain after controlling for prodromal depression (i.e., depression that occurring close to onset of dementia). We will do this by controlling for depression assessed in 2019, just a few months prior to obtaining self-reports of cognitive decline in 2021. Finally, we anticipate that part of the effect of low SES and exposure to discrimination on cognitive decline will be indirect through depression. Prior studies (Barnes et al., 2012; Coogan et al., 2020) have reported that depression mediates much of the effect of racism on dementia, and we expect to find this mediation effect in the present study as well.
Accelerated Aging (Weathering) and Chronic Illness
Geronimus and colleagues (Geronimus, 2001) have argued that the elevated rates of illness, disability, and mortality seen among Black Americans are a consequence of the biological “weathering” produced by the structural barriers, material hardships, stereotypes, and other threats associated with life in a racially charged society. They define weathering as the general physiological wear and tear or deterioration that occurs in response to cumulative social and economic adversity. This deterioration, or accelerated aging, is viewed as a consequence of chronic stimulation of the sympathetic nervous system, the hypothalamic-pituitary-adrenal axis (HPA) axis, the endocrine system, and the various biological and methylation changes that they foster. While past research has used allostatic load (Geronimus et al., 2006) or inflammation (Simons et al., 2018) as indicators of weathering, recently, various epigenetic indices, referred to as epigenetic clocks, have been developed specifically to assess accelerated aging (see Supplementary Materials).
The present study uses GrimAge, a relatively new epigenetic clock based on methylation at 1,030 CPG sites scattered around the genome (Lu et al., 2019). Like its predecessors, the residuals obtained by regressing GrimAge on chronological age indicate, in years, the extent to which an individual is older or younger that their chronological age. This instrument has been shown to be a robust predictor of morbidity and mortality (Hillary et al., 2020; Lu et al., 2019). It shows, for example, strong associations with shorter life span, coronary heart disease, diabetes, and comorbidity, four health outcomes more common among African Americans. Further, GrimAge has been validated with a large African American sample (Lu et al., 2019).
Finally, studies using an African American sample have shown that accelerated Grim Age is predicted by discrimination and low SES (Simons et al., 2021). These findings, coupled with the strong evidence that accelerated aging predicts chronic illness, suggest a pathway whereby low SES and racial discrimination indirectly contribute to cognitive decline. We expect that these two stressors assessed at midlife will predict accelerated biological aging several years later, and this accelerated aging, in turn, will elevate the risk for the onset of various chronic illnesses (e.g., Cardiovascular disease (CVD), High blood pressure (HBP), diabetes, and stroke) that have been shown to increase the probability of cognitive impairment.
Summary of Hypotheses:
(a)Low SES and experiences of racial discrimination at midlife (2002) will predict greater cognitive decline in 2021.
(b)Midlife depression (2002) will predict greater cognitive decline (2021) and this relationship will remain after controlling for late-life depression (2019). Further, midlife depression is expected to mediate a significant proportion of the effect of low SES and discrimination on cognitive decline.
(c)Indirect or mediating pathways are expected where both low SES (2002) and experiences of discrimination (2002) predict increased accelerated aging (2019) which, in turn, predicts chronic illness (2019), which then predicts greater cognitive decline (2020).
Method
Participants and Procedures
We investigated our research questions using data collected at Wave 3 (2002), Wave 8 (2019), and Wave 8.5 (2021) from the caregivers in the Family and Community Health Study (FACHS). FACHS is a longitudinal study of several hundred African American families that was initiated in 1997. All the families had a 5th grader at the study inception. Using a stratified random sampling procedure, the sampling strategy was designed to generate families representing a range of socioeconomic statuses and neighborhood settings. Details regarding the recruitment are described by Gibbons and colleagues (2004) and Simons and colleagues (2011). Lengthy psychosocial interviews were conducted with the primary caregiver (usually the mother) and the target child. Shorter interviews were completed with the secondary caregiver (usually a father, stepfather, or grandmother) when one was present. At Wave 1, about half of the sample resided in Georgia (n = 422) and the other half in Iowa (n = 467). The present study uses data from Wave 3, which occurred in 2002, the 8th Wave, which occurred in 2019, and Wave 8.5, which occurred in 2021. Most of all of the primary caregivers included in the study were women (mothers and grandmothers) who completed the full battery of instruments included in the present study. In contrast, the secondary caregivers included in the study, many of whom were men, completed a shorter interview schedule that did not include the Wave 3 variables utilized in the present study. Hence, our analyses only focus upon women. The mean primary caregiver age at Wave 3 was 40.71 (SD = 5.96), while the mean age at Wave 8.5 was 59.29 (SD = 6.03). Slightly over 40% had family incomes below the federal poverty level, a high school degree was the most common level of education, although 5.1% had less than a 12th-grade education, and 34.7% were married. The majority (69.1%) lived in large urban areas, 13.7% lived in the suburbs, and 17.2% lived in rural areas.
The Wave 8 interview included blood draws utilized to perform various biological assays, including the genome-wide DNA methylation assays used to assess accelerated aging. Given the logistics of scheduling home visits by phlebotomists, only the roughly 600 members of the sample still residing in Georgia or Iowa were identified as eligible for the Wave 8 interview and blood draw; approximately 2/3 of these individuals agreed to participate. Within 2 weeks of the psychosocial interview, a certified phlebotomist visited the home and collected four tubes of blood (30 ml) from each consenting participant. Interviews and blood samples were obtained from 389 respondents (96 men and 293 women). Given the absence of wave 3 data for the men, analyses for the present study focus on 293 women. Comparisons of these participants with those who participated only in Wave 1 did not reveal significant differences with regard to either demographic characteristics or the independent variables (e.g., household income, education, or chronological age). Those rare instances of missing data were handled by full information maximum likelihood (Lee & Shi, 2021). Details regarding the blood draw and handling of the samples are presented in Supplementary Materials. The Illumina EPIC 850 BeadChip was used to assay genome-wide DNA methylation (see Supplementary Materials). The protocol and all study procedures were approved by the Institutional Review Board at the University of Georgia.
Measures
Outcome variable
Subjective cognitive decline (ECog).—
Ten items from the 12-item short-version of the ECog (Farias et al., 2011) were used to assess self-perceived cognitive decline. Two items, “reading a map” and “keeping living and work space organized,” were not included as pretesting showed them to be problematic with our study population. The items ask participants to report the extent to which there has been a decline in their cognitive functioning over the past 10 years. The items focus on functions such as remembering where they placed objects, the current date or day of the week, and thinking ahead with responses ranging from 1 (better or no change) to 4 (consistently much worse). The higher the scores, the greater the functional limitations. The short-form version of the ECog correlates with established functional measures and neuropsychological scores and shows excellent discrimination (Area under the curve (AUC) = 0.95) between normal adults and those with any cognitive impairment (Farias et al., 2011). Coefficient alpha in the present study was 0.847, indicating strong internal consistency.
Predictor variables
Socioeconomic status.—
Respondents reported their highest level of education in 2002. In addition, they reported on their household income in 2002 from all family members and all sources. Scales were standardized and then averaged to form a measure of SES.
Discrimination.—
Respondents completed 11 items from the Schedule of Racist Events (Landrine & Klonoff, 1996) in 2002. The items assess the frequency (1 = never, 4 = frequently) with which various discriminatory events have been experienced. The items focus on events such as being the victim of disrespectful treatment by a store owner or sales clerks, racial slurs, being hassled by the police, exclusion from social activities, and not being expected to do well because of being African American. Coefficient alpha for the scale was .894.
Depression.—
Respondents completed five items from the Mini-Mood and Anxiety Symptom Questionnaire (Clark & Watson, 1997) in 2002 and 2019. This scale asks respondents to indicate how much of the time during the past week they had felt depressed, discouraged, hopeless, like a failure, and worthless with responses range from 1 (not at all) to 3 (extremely). The Cronbach alpha was 0.84 for both 2002 and 2019.
Index of chronic illness.—
In 2019, respondents reported whether a doctor had ever told them (no = 0, yes = 1) that they were suffering from each of five chronic diseases (cardiovascular problems, diabetes, hypertension, stroke, and cancer) that have been associated with elevated inflammation and dementia. Preliminary analyses showed that the five were significantly correlated with ECog with correlations ranging from 0.11 to 0.19. Items were summed to form an index of chronic illness, ranging from 0 to 5.
Accelerated GrimAge.—
Using blood collected in 2019, the Illumina EPIC 850 BeadChip was used to assay genome-wide DNA methylation. Details regarding this procedure, as well the data cleaning and restructuring methods used to prepare this data for analysis, are presented in Supplementary Materials. Scores were calculated for our respondents using the algorithm specified by Lu et al. (2019). The correlation between GrimAge and chronological age in the study sample was 0.730, which is roughly comparable to findings reported in prior studies with other racial/ethnic groups (Crimmins et al., 2021). To transform GrimAge into an accelerated aging score (i.e., GrimAccelAging), we regressed GrimAge on chronological age (Lu et al., 2019). A positive value on this variable indicates, in years, accelerated epigenetic aging, while a negative value indicates, in years, decelerated aging.
Analytic Strategy
Structural equation modeling (SEM) in Mplus 8 (Muthén & Muthén, 2017) was used to depict the effect of psychosocial stress, accelerated aging, and chronic illness on self-reported cognitive decline. To evaluate the goodness of fit of the model, we report the root mean square error of approximation < 0.05 and the comparative fit index > 0.90 as well as Chi-square test and degrees of freedom. The significance of direct and indirect effects was assessed by examining the 95% confidence interval (CI) estimated with bias-corrected and accelerated bootstrapping with 1,000 replications.
Results
As expected, the majority of the participants reported little change in cognitive function. The three items with the most reported change were “remembering where I placed an object,” “remembering the date or day of the week,” and “communicating thoughts.” The percent reporting their functioning was either “consistently a little worse” or ‘consistently much worse” was 28%, 11%, and 10%, respectively, on these three items. The percentages for the other seven items were around 4%–6%.
Table 1 presents the zero-order correlations for the study variables, along with their means and standard deviations. Column 1 shows that the total ECog score is significantly related to all of the study variables. SES is significantly related to all other variables except chronic illness, and racial discrimination is related to all of the study variables except depression (2019) and chronic illness. Depression in 2002 and 2019 are also not related to chronic illness. Finally, GrimAccelAging is significantly associated with all of the other variables except depression 2002.
Table 1.
Zero-Order Correlation Matrix for Study Variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|
| 1. ECog 2021 | — | |||||||
| 2. Socioeconomic status 2002 | −0.151** | — | ||||||
| 3. Racial discrimination 2002 | 0.198** | 0.199** | — | |||||
| 4. Depression 2002 | 0.288** | −0.177** | 0.132* | — | ||||
| 5. Depression 2019 | 0.294** | −0.216** | 0.067 | 0.414** | — | |||
| 6. Chronic illness 2019 | 0.246** | −0.084 | 0.007 | −0.006 | 0.067 | — | ||
| 7. Grim accelerated aging 2019 | 0.124* | −0.211** | 0.158* | 0.082 | 0.126* | 0.170** | — | |
| 8. Age | 0.194** | 0.116* | 0.137* | −0.047 | −0.032 | 0.266** | 0.053 | — |
| Mean | 1.281 | −0.019 | 1.875 | 1.277 | 1.324 | 1.261 | −0.739 | 59.290 |
| SD | 0.423 | 0.781 | 0.572 | 0.370 | 0.417 | 1.024 | 4.326 | 6.035 |
Notes: SD = standard deviation.
*p < .05. **p < .01 (two-tailed tests).
Table 2 shows that mean differences on the various study variables differ for those who reported “no change” in response to all 10 ECog questions (N = 121) versus those who reported “consistently much worse” or “consistently a little worse” on at least three questions (N = 51). This dichotomization is somewhat arbitrary but serves the purpose of distinguishing those who report no decline in cognition from those who report some decline in at least two domains. The two groups show significant differences in mean levels of discrimination, depression 2002, and chronic illness. In addition, there are nonsignificant differences in descriptive interest: mean income is lower, and aging is faster for those reporting cognitive impairment.
Table 2.
Mean Scores on the Psychosocial Variables for Those Who Reported “No Change” in Any Domain Versus Those Who Reported “Consistently a Little Worse” or “Consistently Much Worse” on at Least Three Cognitive Domains
| No change (N = 121) | A little or much worse (N = 51) | t Value | p Value | |
|---|---|---|---|---|
| Income 2002 | 31,617.69 | 25,208.17 | −1.820† | .071 |
| Education 2002 | 12.09 | 11.80 | −0.587 | .558 |
| Discrimination 2002 | 1.77 | 2.01 | 2.452* | .015 |
| Depression 2002 | 1.21 | 1.40 | 2.602* | .012 |
| Chronic illness 2019 | 1.06 | 1.74 | 3.631** | .001 |
| Grim accelerated aging 2019 | −1.08 | −0.27 | 1.072 | .285 |
Notes: †p < .1. *p < .05. **p < .01 (two-tailed tests).
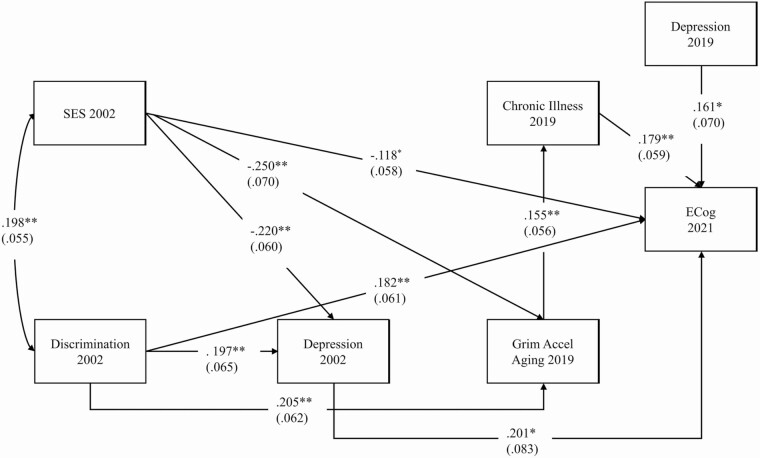
Next, SEM was used to analyze the direct effects and patterns of mediation that might be evident in the data. We began by running the fully recursive model (i.e., all possible paths were included). The results obtained are presented in Supplementary Materials. Some of the paths were not statistically significant, and the fit statistics indicated only marginal goodness of fit for the model. To obtain a better fit, we trimmed the model by dropping all paths with a p value greater than .20. As shown in Figure 1, this more parsimonious model provided a good fit to the data. The model shows that SES (β = −0.118, p ≤ .05) and discrimination (β = 0.182, p ≤ .01), both assessed in 2002, significantly predict cognitive decline reported in 2021, even after controlling for the other variables in the model. In addition, depression measured in 2002 predicts cognitive decline (β = 0.201, p ≤ .01), as does chronic illness (β = 0.179, p ≤ .01). All of these results remain with depression assessed in 2019 included in the model to control any prodromal effects. Depression assessed in 2019 is significantly related to cognitive decline (β = 0.161, p ≤ .05). However, the fact that SES, discrimination, early depression, and chronic illness remain significant with 2019 depression in the model indicates that these effects are not simply a consequence of these variables being confounded with prodromal depression.
Figure 1.
Trimmed structural equation model depicting the effect of psychosocial stress, accelerated aging, and chronic illness on self-reported cognitive decline. N = 293. Values are standardized parameter estimates and standard errors are in parentheses. Age is controlled in the analyses. Model fit: root mean square error of approximation = 0.026 and comparative fit index = 0.986; Chi-square = 14.456, df = 12, p = .2725. †p < .1, *p < .05, **p < .01.
In addition to these direct effects, Figure 1 also depicts indirect or mediating pathways. First, both SES (β = −0.220, p ≤ .01) and discrimination (β = 0.197, p ≤ .01) are related to 2002 depression which, in turn, is related to ECog. Second, both SES (β = −0.250, p ≤ .01) and discrimination (β = 0.205, p ≤ .01) are related to accelerated aging, which, consonant with prior research, predicts chronic illness (β = 0.155, p ≤ .01). Chronic illness, in turn, predicts change in ECog. The statistical significance of these indirect effects was assessed by examining 95% CIs estimated with bias-corrected and accelerated bootstrapping with 1,000 replications (Hayes, 2009). As shown in Table 3, all four of these mediating pathways are significant at the 0.05 level. The findings show that depression partially mediates the effect of SES and discrimination on ECog. And, the indirect effect of SES and discrimination through accelerated aging and chronic illness is also significant.
Table 3.
Direct and Indirect Effects From Socioeconomic Status and Discrimination to Everyday Cognition Through Depression, Grim Accelerated Aging, and Chronic Illness
| Paths | Direct effect | Indirect effect | 95% CI |
|---|---|---|---|
| SES → ECog | −0.118* | [−0.234, −0.009] | |
| SES → depression (2002) → ECog | −0.044* | [−0.101, −0.008] | |
| SES → GrimAccelAging → chronic illness → ECog | −0.007* | [−0.020, −0.001] | |
| DISC → ECog | 0.182** | [0.067, 0.310] | |
| DISC → depression (2002) → ECog | 0.040* | [0.006, 0.103] | |
| DISC → GrimAccelAging → chronic illness → ECog | 0.006* | [0.001, 0.015] |
Notes: CI = confidence interval; DISC = discrimination 2002; ECog = Everyday Cognition; SES = socioeconomic status 2022. Age is controlled in these analyses.
*p < .05. **p < .01 (two-tailed tests).
Referring back to Figure 1, it is interesting to note that there is no direct effect of accelerated aging on ECog. One might expect that accelerated biological aging affects the brain just as it does the other organs of the body. In the present study, however, the effect of accelerated aging was indirect through onset of chronic illness. It might be, however, that our finding is specific to the Grim Index and that a different measure of epigenetic aging would show a direct effect on ECog in addition to an indirect effect through chronic illness. In order to address this possibility, we re-estimated our model substituting the recently developed measure of accelerated aging—DunedinPACE. Analyses based on several data sets have shown that this biomarker of aging predicts morbidity, frailty, and mortality as well, or in some cases better, than GrimAge (Belsky et al., 2022). Further, there is some evidence that it predicts cognitive decline (Belsky et al., 2022). However, our findings using DunedinPACE paralleled those obtained with GrimAccelAging. Both SES and discrimination were related to DunedinPACE, but the effect of DunedinPACE on ECog was limited to its indirect effect through chronic illness. Thus, the same pattern of results was obtained using either of two recently developed, robust, measures of epigenetic aging. Our findings indicate that accelerated epigenetic aging increases the risk for cognitive impairment because it promotes the development of chronic illnesses that contribute to cognitive decline.
Discussion
Research to date has made little progress in identifying the factors that explain the high rates of dementia experienced by Black Americans compared to most other ethnic/racial groups. In response, some researchers have asserted that the pervasive stress of racism may be central to explaining Black Americans elevated risk for cognitive impairment (Coogan et al., 2020; Gleason et al., 2021; Zuelsdorff et al., 2020). Consonant with this assertion, the present study of Black American women found that two race-related stressors—low SES and racial discrimination—predicted reports of SCD assessed 19 years later even after controlling for several other variables. This finding is consistent with research showing that stress disrupts cognition (Zaheed et al., 2020) and over time may foster a diminution of key brain regions such as the hippocampus (Piccolo & Noble, 2018).
In addition to these direct effects, the analyses identified two statistically significant pathways whereby these two stressors were linked indirectly to SCD. First, depression, which has been found to affect the hippocampus and elevate risk of dementia, mediated a portion of the effect of SES and discrimination on SCD. Finally, there was evidence for a more complex pathway where SES and discrimination accelerate biological aging, with accelerated aging, in turn, leading to chronic illness. Chronic Illness then predicts SCD. The first portion of this pathway is consistent with the weathering argument, as our two race-related stressors are not directly related to chronic illness. Rather, they increase the probability of chronic illness by accelerating biological aging.
The finding that SES and discrimination evince the same direct and indirect pathways to SCD might be considered an internal replication. It indicates an internal coherence to the model tested where race-related stressors are linked to SCD in similar ways. Also, the finding that SES and discrimination make independent contributions to these pathways suggests that there may be additional racialized contexts, such as living in a disadvantaged neighborhood, that contribute to these same pathways.
Some have suggested that in addition to its indirect effect through chronic illness, accelerated biological aging may directly increase the probability of dementia (Belsky et al., 2022). We found no support for this idea. We performed our analyses using two of the most powerful epigenetic measures currently available, and neither of them showed a direct effect on SCD. Rather, both of these indices exerted their influence on SCD through their impact on chronic illness. It may be, however, that these aging indicators would show a direct effect if biomarkers such Aβ or P-Tau were used to assess Alzheimer’s disease. Likewise, future research could examine the possibility of specific moderators of the association between accelerated biological aging and SCD.
A major strength of the present study was its use of longitudinal data. Our longitudinal data allowed us to investigate the long-term consequences of exposure to race-related stressors. Further, it enabled us to differentiate the effect of depression occurring a few months prior to our assessment of SCD and depression that occurred 19 years earlier, thereby demonstrating that the effect of midlife depression was not attributable to its being a part of the prodrome of dementia. Of course, a major limitation of the study is the absence of either neuropsychological tests or biomarkers of Alzheimer’s disease and neurodegeneration. Such measures are invaluable and will be incorporated into the current data set in the coming months. Another limitation of the study is that the sample only consisted of women. Unfortunately, given the focus of the FACHS study when it was first initiated, males did not complete many of the instruments used in the present investigation. In our view, the results we found for women are likely to hold for men as well. Future studies need to establish the extent to which this is the case.
In recent years, several studies have reported that race-related stressors are associated with risk of dementia among Black Americans. For example, cross-sectional studies have reported that discrimination is associated with reports of SCD (Coogan et al., 2020) and with neuropsychological assessments of cognitive functioning (Barnes, 2012). Further, using 6 years of longitudinal data, Zahodne et al. (2019) published findings showing that discrimination predicted lower memory scores, and a longitudinal study by Zuelsdorff et al. (2020) found that Black Americans reported more stressful events than Whites and that higher stress partially explained racial differences in cognitive speed and flexibility. The present study provides further evidence regarding a potential link between race-related stressors and cognition. Future research should continue to emphasize the various ways in which exposure to racism over the life course influences the cognitive health of Black Americans.
Supplementary Material
Contributor Information
Ronald L Simons, Department of Sociology, University of Georgia, Athens, Georgia, USA.
Mei Ling Ong, Center for Family Research, University of Georgia, Athens, Georgia, USA.
Steven R H Beach, Department of Psychology, University of Georgia, Athens, Georgia, USA.
Man-Kit Lei, Department of Sociology, University of Georgia, Athens, Georgia, USA.
Robert Philibert, Department of Psychiatry, University of Iowa School of Medicine, Iowa City, Iowa, USA.
Michelle M Mielke, Department of Epidemiology and Prevention, Wake Forest University, School of Medicine, Winston-Salem, North Carolina, USA.
Funding
National Institute on Aging (RF1 AG077386).
Conflict of Interest
The authors declare no conflict of interest.
References
- Amariglio, R. E., Mormino, E. C., Pietras, A. C., Marshall, G. A., Vannini, P., Johnson, K. A., Sperling, R. A. & Rentz, D. M. (2015). Subjective cognitive concerns, amyloid-β, and neurodegeneration in clinically normal elderly. Neurology, 85, 56–62. doi: 10.1212/WNL.0000000000001712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansell, E. B., Rando, K., Tuit, K., Guarnaccia, J., & Sinha, R. (2012). Cumulative adversity and smaller gray matter volume in medial prefrontal, anterior cingulate, and insula regions. Biological Psychiatry, 72, 57–64. doi: 10.1016/j.biopsych.2011.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnone, D., McKie, S., Elliott, R., Juhász, G., Thomas, E. J., Downey, D., Williams, S. J., Deakin, J. F. W., & Anderson, I. M. (2013). State-dependent changes in hippocampal grey matter in depression. Molecular Psychiatry, 18, 1265–1272. doi: 10.1038/mp.2012.150 [DOI] [PubMed] [Google Scholar]
- Barnes, L. L. (2022). Alzheimer disease in African American individuals: Increased incidence or not enough data? Nature Reviews Neurology, 18, 56–62. https://www.nature.com/articles/s41582-021-00589-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes, L. L., Lewis, T. T., Begeny, C. T., Yu, L., Bennett, D. A., & Wilson, R. S. (2012). Perceived discrimination and cognition in older African Americans. Journal of the International Neuropsychological Society, 18, 856–865. doi: 10.1017/S1355617712000628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky, D. W., Caspi, A., Corcoran, D. L., Sugden, K., Poulton, R., Arseneault, L., Caspi, A., Corcoran, D. L., Sugden, K., Poulton, R., Arseneault, L., Baccarelli, A., Chamart, K. Gao, X. Hannon, E., Harrington, H. L, Houts, R., Kothari, M., Kwon, D., Mill, J., Schartz, J., Vokonas, P., Wang, C., Williams, B. S., & Moffitt, T. E. (2022). DunedinPACE, a DNA methylation biomarker of the pace of aging. Elife, 11, e73420. doi: 10.7554/eLife.73420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenowitz, W. D., Zeki Al Hazzouri, A., Vittinghoff, E., Golden, S. H., Fitzpatrick, A. L., & Yaffe, K. (2021). Depressive symptoms imputed across the life course are associated with cognitive impairment and cognitive decline. Journal of Alzheimer’s Disease, 83, 1379–1389. doi: 10.3233/jad-210588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmester, B., Leathem, J., & Merrick, P. (2016). Subjective cognitive complaints and objective cognitive function in aging: A systematic review and meta-analysis of recent cross-sectional findings. Neuropsychology Review, 26, 376–393. doi: 10.1007/s11065-016-9332-2 [DOI] [PubMed] [Google Scholar]
- Clark, L. A., & Watson, D. (1997). The Mini Mood and Anxiety Symptom Questionnaire (Mini-MASQ). Unpublished manuscript. University of Iowa, Iowa City, Iowa. [Google Scholar]
- Clasp. (2015). College preparation for African American students. https://www.clasp.org/ [Google Scholar]
- Cockerham, W. C., Hamby, B. W., & Oates, G. R. (2017). The social determinants of chronic disease. American Journal of Preventive Medicine, 52, S5–S12. doi: 10.1016/j.amepre.2016.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coogan, P., Schon, K., Li, S., Cozier, Y., Bethea, T., & Rosenberg, L. (2020). Experiences of racism and subjective cognitive function in African American women. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring, 12, e12067. doi: 10.1002/dad2.12067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins, E. M., Thyagarajan, B., Levine, M. E., Weir, D. R., & Faul, J. (2021). Associations of age, sex, race/ethnicity, and education with 13 epigenetic clocks in a nationally representative US sample: The Health and Retirement Study. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 76, 1117–1123. doi: 10.1093/gerona/glab016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias, S. T., Mungas, D., Harvey, D. J., Simmons, A., Reed, B. R., & DeCarli, C. (2011). The measurement of everyday cognition: Development and validation of a short form of the Everyday Cognition scales. Alzheimer’s & Dementia, 7, 593–601. doi: 10.1016/j.jalz.2011.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias, S. T., Mungas, D., Reed, B. R., Cahn-Weiner, D., Jagust, W., Baynes, K., & DeCarli, C. (2008). The measurement of everyday cognition (ECog): Scale development and psychometric properties. Neuropsychology, 22, 531–544. doi: 10.1037/0894-4105.22.4.531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filshtein, T., Chan, M., Mungas, D., Whitmer, R., Fletcher, E., DeCarli, C., & Farias, S. (2020). Differential item functioning of the everyday cognition (ECoG) scales in relation to racial/ethnic groups. Journal of the International Neuropsychological Society, 26(5), 515–526. doi: 10.1017/s1355617719001437 [DOI] [PubMed] [Google Scholar]
- Geronimus, A. T. (2001). Understanding and eliminating racial inequalities in women’s health in the United States: The role of the weathering conceptual framework. Journal of the American Medical Women’s Association (1972), 56, 133–136. https://europepmc.org/article/med/11759779 [PubMed] [Google Scholar]
- Geronimus, A. T., Hicken, M., Keene, D., & Bound, J. (2006). “Weathering” and age patterns of allostatic load scores among Blacks and Whites in the United States. American Journal of Public Health, 96, 826–833. doi: 10.2105/ajph.2004.060749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason, C. E., Zuelsdorff, M., Gooding, D. C., Kind, A. J., Johnson, A. L., James, T. T., Zueksdorff, M., Gooding, D. C., Kind, AmJ. H., Johnson, A., James, T. T. Lambrou, N. H., Wyman, M. F., Ketchum, F. B., Gee, A., Johnson, S. C., Bendin, B. B., & Zetterberg, H. (2021). Alzheimer’s disease biomarkers in Black and non-Hispanic White cohorts: A contextualized review of the evidence. Alzheimer’s & Dementia, 18, 1545–1564. doi: 10.1002/alz.12511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes, A. F. (2009). Beyond Baron and Kenny: Statistical mediation analysis in the new millennium. Communication Monographs, 76, 408–420. doi: 10.1080/03637750903310360 [DOI] [Google Scholar]
- Hillary, R. F., Stevenson, A. J., McCartney, D. L., Campbell, A., Walker, R. M., Howard, D. M., ... Marioni, R. E. (2020). Epigenetic measures of ageing predict the prevalence and incidence of leading causes of death and disease burden. Clinical Epigenetics, 12, 1–12. doi: 10.1186/s13148-020-00905-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonker, C., Geerlings, M. I., & Schmand, B. (2000). Are memory complaints predictive for dementia? A review of clinical and population-based studies. International Journal of Geriatric Psychiatry, 15, 983–991. doi: [DOI] [PubMed] [Google Scholar]
- Kornblith, E., Bahorik, A., Boscardin, W. J., Xia, F., Barnes, D. E., & Yaffe, K. (2022). Association of race and ethnicity with incidence of dementia among older adults. Journal of American Medical Association, 327, 1488–1495. doi: 10.1001/jama.2022.3550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrine, H., & Klonoff, E. A. (1996). The schedule of racist events: A measure of racial discrimination and a study of its negative physical and mental health consequences. Journal of Black Psychology, 22, 144–168. doi: 10.1177/00957984960222002 [DOI] [Google Scholar]
- Lee, T., & Shi, D. (2021). A comparison of full information maximum likelihood and multiple imputation in structural equation modeling with missing data. Psychological Methods, 26, 466–485. doi: 10.1037/met0000381 [DOI] [PubMed] [Google Scholar]
- Letang, S. K., Lin, S. S. H., Parmelee, P. A., & McDonough, I. M. (2021). Ethnoracial disparities in cognition are associated with multiple socioeconomic status-stress pathways. Cognitive Research: Principles and Implications, 6, 1–17. doi: 10.1186/s41235-021-00329-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, A. T., Quach, A., Wilson, J. G., Reiner, A. P., Aviv, A., Raj, K., Quach, A., Wilson, J. G., Reiner, A., Aviv, A., Raj, K., Hou, L., Baccrelli, A. A., Li, Y., Stewart, J. D., Whitsel, E. A., Assimes, T. L., Ferrucci, L., & Horvath, S. (2019). DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging (Albany NY), 11, 303–327. doi: 10.18632/aging.101684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manduca, R. (2018). Income inequality and the persistence of racial economic disparities. Sociological Science, 5, 182–205. doi: 10.15195/v5.a8 [DOI] [Google Scholar]
- Maruszak, A., Pilarski, A., Murphy, T., Branch, N., & Thuret, S. (2014a). Hippocampal neurogenesis in Alzheimer’s disease: Is there a role for dietary modulation? Journal of Alzheimer’s Disease, 38, 11–38. doi: 10.3233/JAD-131004 [DOI] [PubMed] [Google Scholar]
- Maruszak, A., & Thuret, S. (2014b). Why looking at the whole hippocampus is not enough—A critical role for anteroposterior axis, subfield and activation analyses to enhance predictive value of hippocampal changes for Alzheimer’s disease diagnosis. Frontiers in Cellular Neuroscience, 8, 1–11. doi: 10.3389/fncel.2014.00095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey, D. S. (2007). Categorically unequal: The American stratification system. Russell Sage Foundation. [Google Scholar]
- Mehta, K. M., & Yeo, G. W. (2017). Systematic review of dementia prevalence and incidence in United States race/ethnic populations. Alzheimer’s & Dementia, 13, 72–83. doi: 10.1016/j.jalz.2016.06.2360 [DOI] [PubMed] [Google Scholar]
- Muthén, L. K., & Muthén, B. (2017). Mplus user’s guide: Statistical analysis with latent variables (8th ed.). Muthén & Muthén. [Google Scholar]
- Nabe-Nielsen, K., Rod, N. H., Hansen, M., Prescott, E., Grynderup, M. B., Islamoska, S., Rod, N. H., Hansen, A. M., Prescott, E., Grynderup, M. B., Islamoska, S., Ishtiak-Ahmed, K., Garde, A. H., Gytelberg, F. Mortensen, E. L., Phung, T. K., Waldemar, G., Westendorp, R. G. J. (2020). Perceived stress and dementia: Results from the Copenhagen city heart study. Aging & Mental Health, 24, 1828–1836. doi: 10.1080/13607863.2019.1625304 [DOI] [PubMed] [Google Scholar]
- Nolan, M., Roman, E., Nasa, A., Levins, K. J., O’Hanlon, E., O’Keane, V., & Willian Roddy, D. (2020). Hippocampal and amygdalar volume changes in major depressive disorder: A targeted review and focus on stress. Chronic Stress, 4, 1–19. doi: 10.1177/2470547020944553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omi, M., & Winant, H. (2014). Racial formation in the United States. Routledge/Taylor and Francis. [Google Scholar]
- Oumohand, S. E., Ward, D. D., Boenniger, M. M., Merten, N., Kirschbaum, C., & Breteler, M. M. (2020). Perceived stress but not hair cortisol concentration is related to adult cognitive performance. Psychoneuroendocrinology, 121, 104810. doi: 10.1016/j.psyneuen.2020.104810 [DOI] [PubMed] [Google Scholar]
- Pager, D., Bonikowski, B., & Western, B. (2009). Discrimination in a low-wage labor market: A field experiment. American Sociological Review, 74, 777–799. doi: 10.1177/000312240907400505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter, J., Scheef, L., Abdulkadir, A., Boecker, H., Heneka, M., Wagner, M., Koppara, A., Kloppel, S., & Jessen, F. Alzheimer’s Disease Neuroimaging Initiative. (2014). Gray matter atrophy pattern in elderly with subjective memory impairment. Alzheimer’s & Dementia, 10, 99–108. doi: 10.1016/j.jalz.2013.05.1764 [DOI] [PubMed] [Google Scholar]
- Piccolo, L. R., Noble, K. G., & Pediatric Imaging, Neurocognition, and Genetics Study. (2018). Perceived stress is associated with smaller hippocampal volume in adolescence. Psychophysiology, 55, e13025. doi: 10.1111/psyp.13025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer, J., & Gampa, A. (2018). Implicit and explicit racial attitudes changed during Black lives matter. Personality and Social Psychology Bulletin, 44, 1039–1059. doi: 10.1177/0146167218757454 [DOI] [PubMed] [Google Scholar]
- Schoenfeld, T. J., McCausland, H. C., Morris, H. D., Padmanaban, V., & Cameron, H. A. (2017). Stress and loss of adult neurogenesis differentially reduce hippocampal volume. Biological Psychiatry, 82, 914–923. doi: 10.1016/j.biopsych.2017.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons, R. L., Lei, M. K., Beach, S. R., Barr, A. B., Simons, L. G., Gibbons, F. X., & Philibert, R. A. (2018). Discrimination, segregation, and chronic inflammation: Testing the weathering explanation for the poor health of Black Americans. Developmental Psychology, 54, 1993–2006. doi: 10.1037/dev0000511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons, R. L., Lei, M. K., Beach, S. R., Brody, G. H., Philibert, R. A., & Gibbons, F. X. (2011). Social environment, genes, and aggression: Evidence supporting the differential susceptibility perspective. American Sociological Review, 76(6), 883–912. doi: 10.1177/0003122411427580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons, R. L., Lei, M. K., Klopach, E., Berg, M., Zhang, Y., & Beach, S. S. (2021). (Re) Setting epigenetic clocks: An important avenue whereby social conditions become biologically embedded across the life course. Journal of Health and Social Behavior, 62, 436–453. doi: 10.1177/00221465211009309 [DOI] [PubMed] [Google Scholar]
- Steenland, K., Goldstein, F. C., Levey, A., & Wharton, W. (2016). A meta-analysis of Alzheimer’s disease incidence and prevalence comparing African-Americans and Caucasians. Journal of Alzheimer’s Disease, 50, 71–76. doi: 10.3233/jad-150778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Flier, W. M., Van Buchem, M. A., Weverling-Rijnsburger, A. W., Mutsaers, E. R., Bollen, E. L., Admiraal-Behloul, F., Westendorp, R. G. J., Middelkoop, H. A. M. (2004). Memory complaints in patients with normal cognition are associated with smaller hippocampal volumes. Journal of Neurology, 251, 671–675. doi: 10.1007/s00415-004-0390-7 [DOI] [PubMed] [Google Scholar]
- Zahodne, L. B., Kraal, A. Z., Sharifian, N., Zaheed, A. B., & Sol, K. (2019). Inflammatory mechanisms underlying the effects of everyday discrimination on age-related memory decline. Brain, Behavior, and Immunity, 75, 149–154. doi: 10.1016/j.bbi.2018.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuelsdorff, M., Okonkwo, O. C., Norton, D., Barnes, L. L., Graham, K. L., Clark, L. R., & Gleason, C. E. (2020). Stressful life events and racial disparities in cognition among middle-aged and older adults. Journal of Alzheimer’s Disease, 73, 671–682. doi: 10.3233/JAD-190439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuelsdorff, M. L., Engelman, C. D., Friedman, E. M., Koscik, R. L., Jonaitis, E. M., Rue, A. L., & Sager, M. A. (2013). Stressful events, social support, and cognitive function in middle-aged adults with a family history of Alzheimer’s disease. Journal of Aging and Health, 25, 944–959. doi: 10.1177/0898264313498416 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.