Abstract
Novel Cu-nitrogen doped graphene nanocomposite catalysts are developed to investigate the Cu-nitrogen doped fuel cell cathode catalyst. Density functional theory calculations are performed using Gaussian 09w software to study the oxygen reduction reaction (ORR) on Cu-nitrogen doped graphene nanocomposite cathode catalyst in low-temperature fuel cells. Three different nanocomposite structures Cu2–N6/Gr, Cu2–N8/Gr and Cu–N4/Gr were considered in the acidic medium under standard conditions (298.15 K, 1 atm) in order to explore the properties of the fuel cell. The results showed that all structures are stable at the potential range 0–5.87 V. Formation energy, Mulliken charge and HOMO-LUMO energy calculations showed that Cu2–N6/Gr and Cu2–N8/Gr are more stable structure-wise, while free energy calculations showed that only Cu2–N8/Gr and Cu–N4/Gr structures support spontaneous ORR. The maximum cell potential under standard conditions was shown at 0.28 V and 0.49 V for Cu2–N8/Gr and Cu–N4/Gr respectively. From the calculations, the Cu2–N6/Gr and Cu2–N8/Gr structures are less favorable in H2O2 generation; however, Cu–N4/Gr showed the potential for H2O2 generation. In conclusion, Cu2–N8/Gr and Cu–N4/Gr are more favorable to ORR than Cu2–N6/Gr.
Keywords: DFT, Fuel cell, Oxygen reduction reaction, Cathode catalyst, H2O2 generation
Graphical abstract
Highlights
-
•
Novel Cu2–N6/Gr, Cu2–N8/Gr motifs investigated.
-
•
Cu2–N6/Gr, Cu2–N8/Gr are more stable than Cu–N4/Gr.
-
•
Cu2–N8/Gr, Cu–N4/Gr showed spontaneous ORR.
-
•
Inferior probability of H2O2 formation.
-
•
Maximum cell potential reached 0.49 V.
1. Introduction
Energy security and sustainability are the impetus for modern economics. Transformation of the energy ecosystem requires the search for cost-effective, secure, and sustainable energy technologies to replace incumbent energy sources. Fuel cells contribute well to this transformation as they evidently have their merits, particularly for compactness and zero emissions, such as proton exchange membrane fuel cells (PEMFCs) [1].
Low-temperature fuel cells operate at temperatures below 200 °C and a carbon support catalyst is deployed to improve the oxygen reduction reaction (ORR). Carbon support catalysts are not conducive to high temperature operations, leading to an increased rate of carbon corrosion and, therefore, degrading the carbon support [2]. Corrosion of the carbon support will directly affect the catalyst layer, causing the catalyst materials to agglomerate.
Generally, low-temperature commercial fuel cells use noble metals, such as Pt and Pd, as a catalyst. These noble metal catalysts can efficiently adsorb and split hydrogen into hydrogen ions [3,4] at the anode and provide a better ORR at the cathode [5]. The damage to the catalyst via carbon corrosion, where the carbon support starts to oxidize, the catalyst particles coated on top of the carbon support then lose their support and start to agglomerate, hence, resulting in diminished fuel cell performance over a short operation period.
To reduce the cost due to carbon corrosion, cheaper alternatives are used to replace the catalyst layer. One such example is the non-platinum group metal (non-PGM) catalysts. Non-PGM catalysts are usually produced using nitrogen atoms doped onto a graphite layer [[6], [7], [8]] or a layer of transition metals [9]. These are easy to produce and are much less expensive than PGM or PGM alloy catalysts such as Pt/Ni/Ir and Pt/Ni [[10], [11], [12]]. In addition, non-PGM have better electron conductivity. Fe/N/Gr, Co/N/Gr, and Mn/N/Gr are the most commonly used dopant [13,14] and are studied. The Mn/N/Gr type catalyst has a more stable potential cycle than Fe; however, the catalyst is not stable after the cathode potential exceeds 0.53 V [15]. Furthermore, the Mn–N4/Gr catalyst has OOH dissociation at 0.38 eV [15].
For Co-based non-PGM catalysts, Co–N4 has electrode stability between 0 and 1.23 V, and Co–N2 is only stable below 0.45 V [16]. Furthermore, its OOH binding energy is −1.02 eV for Co–N4 and −1.75 eV for Co–N2 without solvent, which are more favorable than Mn. Even with the best stability of the Co–N4 catalyst, research showed that Co–N2 has a stronger trend towards the ORR two-electron pathway and produces H2O2 as an intermediate [16]. H2O2 causes degeneration of the electrolyte membrane.
Compared to Mn and Co, Fe-based non-PGM catalysts produce less H2O2 and this H2O2 generation can be reduced by increasing the Fe content to 1.0 wt %. The generation of H2O2 in the ORR process at 0.1 wt % for common transition metals is as follows [17], N/Gr (39.4%) > N/Gr/Ni (39.0%) > N/Gr/Mn (23.9%) > N/Gr/Co (16.8%) > N/Gr/Fe (9.6%).
The formation of Fe(OH)2 during the ORR process [18] can remove Fe atoms from the carbon layer, which reduces the ORR efficiency, and the N/Gr/Fe catalyst can degenerate by oxidation [19].
Research on copper is mainly based on a single metal with nitrogen atoms as dopants [20,21], which gives good ORR performance, and spontaneous ORR makes them more favorable for catalysts. A single metal Cu–N2/Gr structure shows that it has a higher probability of following two-electron pathways to generate H2O2. In addition, there was no indication of the cathode potential of the Cu-nitrogen doped catalyst. A single structure of Cu–N2/Gr cannot describe the H2O2 generation capability or the cell potential of the Cu-nitrogen doped catalyst. Therefore, other structures, such as dual-metal structures, should be considered. Research shows that Fe-based non-PGM catalysts Fe2–N6/Gr and Fe2–N8/Gr have good formation energies and ORR performance [22,23]. Since Cu has shown better ORR performance steps than Fe [24], the Cu2–N6/Gr and Cu2–N8/Gr structures should have considerably better ORR performance than the single metal Cu-nitrogen doped catalyst or Fe-based non-PGM catalysts. Nørskov et al. reported that Cu as a dopant with nitrogen in graphite gives ORR performance almost as Pt (111) [24] catalyst surface for the binding energy of O and OH. Furthermore, Cu shows a good binding energy of OH and OOH, indicating stable catalysts for ORR [25,26].
The DFT method is an effective technique to study ORR performance and catalyst properties [27]. Therefore, in this study, DFT calculations were performed to determine the ORR mechanism and stability of Cu2–N6/Gr, Cu2–N8/Gr, and Cu–N4/Gr electrocatalysts in an acidic medium with different potentials at 298.15 K. From these calculations, the structural stability, the binding ability of ORR steps, the break-free ability of H2O, evidence of H2O2 generation, and the maximum cell potential were predicted.
2. Methodology
DFT calculations were performed with Gaussian 09w software using the B3LYP/3-21G basis set with non-periodic and non-dispersion interaction as Bhatt et al. [21]. Defect structure is visualized using GaussView 6.0. All atoms in every structure were relaxed by optimization. A common single pristine graphene layer was developed, and it is used to develop other structures (Cu2–N6/Gr, Cu2–N8/Gr, and Cu–N4/Gr) to investigate ORR considering the defect structure at the catalyst surface. All structures were developed to contain only pyridinic nitrogen. After optimization, zero imaginary frequency for dual atom structures and one (1) imaginary frequency for the Cu–N4/Gr structure. Additionally, the optimized charges of all three catalysts were zero and singlet spin for all dual atom structures. The molecules used in the ORR steps were individually optimized. Bond lengths and bond angles were measured and compared with those of previous research.
Catalyst structures based on Cu confirmed their stability with the cathode potential. Stability was inspected using the formation energy (ΔE) defined accordingly in the Eq. (1) [16,21],
| (1) |
Here, is energy of optimized graphene layer with Cu–N defect. The ‘a’ and ‘b’ are positive integers defining the selected Cu–N defects ( = 1,2 and = 4,6,8). M and N are Cu and nitrogen, respectively. The and are chemical potential of carbon defined as total energy per carbon atom for defect-free graphene, and the chemical potential of nitrogen defined as half of the total energy of N2 molecule, respectively. x and y are the number of nitrogen atoms added and the carbon atoms removed during the defect formation, respectively. is the energy of optimized pristine graphene layer. is the total energy of Mn + defined as Eq. (2),
| (2) |
where, is the total energy of isolated M (M = Cu) in the gas phase and, n, e, U are the number of electron transfer (+2), electron charge, and external potential in order.
Binding energies (BE) were calculated from the ORR steps in an acidic medium, as defined by Eq. (3) [16,20,21],
| (3) |
Here, is the total energy of molecules adsorbed by defect graphene. is the total energy of defect graphene configuration, and is isolated molecule species (O2, O, H2O, OOH, OH, H2O2). Negative signed binding energies () indicate it is more favorable for molecules to be attached to the defect configuration. The formation of H2O2 during ORR is also considered.
ORR steps in H+ medium are defined as follows [16], * indicates the configuration of the defect.
| * + O2 + 4H+ + 4e− → *O2 + 4H+ + 4e− |
| *O2 + 4H+ + 4e− → *OOH + 3H+ + 3e− |
| *OOH + 3H+ + 3e− → *O + H2O + 2H+ + 2e− |
| *O + H2O + 2H++ 2e− → *OH + H2O + H+ + e− |
| *OH + H2O + H+ + e− → 2H2O |
The free energies were calculated for each ORR step of all defects configurations as defined by Eq. (4) [16],
| (4) |
is the energy from the DFT calculation to the relevant reaction step, and is the correction of zero-point energy is obtained from the NIST database and DFT calculations. T and S are the absolute temperature and entropy, respectively. Here, T = 298.15 K, and the entropy value was obtained from the NIST database. ΔGU = -eU where U and e are the electrode potential and the charge transferred, respectively. , where KB is Boltzmann's constant and T = 298.15 K is a contribution of the interaction of adsorbate with the local electric field in the electric double layer formed in the vicinity of the cathode, which is negligible according to a Nørskov et al. [24]. Corrections of zero-point energy and entropy values were obtained from frequency calculations for the molecule adsorb by defect graphene. The free energy vs. reaction coordinate graphs were drawn with different potentials (U) for each structure. Additionally, the free energy of O2, 4.92 eV, was obtained from the literature under standard conditions with reaction O2 + 2H2 → 2H2O [24,28].
The Mulliken charge population was applied to molecules as defined by Eq. (5) [29],
| ΔQx = Qafter – Qbefore | (5) |
The Qafter and Qbefore are charges of the molecule (X) after and before adsorption, respectively.
The energy gap (Eg), chemical hardness(η), chemical potential (μ), and electrophilicity index (ω) were calculated using the Koopman's principle for optimized structures as defined by Eqs. (6), (7), (8), (9), respectively [[30], [31], [32], [33]],
| (6) |
| (7) |
| (8) |
| (9) |
Here, I and A are the ionization potential (≅−EHOMO) and the electron affinity (≅−ELUMO), respectively.
3. Results and discussion
3.1. Formation energy
The formation energies of the Cu2–N6/Gr, Cu2–N8/Gr, and Cu–N4/Gr structures were −23.60, −23.49 and −13.22 eV in order at zero potential (U = 0). These values were only applied under open circuit conditions, and they changed with different cathode potentials according to Eq. (1) [16,21]. Table 1 shows that the formation energy increased with increasing external potentials. A negative sign means energy value gain when a structure forms. Therefore, the negative formation energies are favorable for the formation of stable structures, and the formation energies of all the structures studied remain negative between the potential range of 0–5.87 V. The critical cell potential of negative formation energies was observed at 5.89, 5.87, and 6.60 V for Cu2–N6/Gr, Cu2–N8/Gr, and Cu–N4/Gr, respectively, see Table 1. The critical U value at zero formation energy demarcates the stability/instability of the cathode. The structures forming stability follow the increasing order of Cu–N4/Gr < Cu2–N8/Gr < Cu2–N6/Gr, as indicated by the external potential, U, at zero.
Table 1.
Formation energies for Cu2–N6/Gr, Cu2–N8/Gr, and Cu–N4/Gr structures with different external potentials (U).
| Defect | External potential (U)/V | Formation energy/eV | Structure |
|---|---|---|---|
| CU2–N6/GR | 0.00 | −23.60 |  |
| 1.00 | −19.60 | ||
| 2.00 | −15.60 | ||
| 5.89 | −0.04 | ||
| 5.90 | 0.00 | ||
| CU2–N8/GR | 0.00 | −23.49 |  |
| 1.00 | −19.49 | ||
| 3.00 | −11.49 | ||
| 5.87 | −0.01 | ||
| 5.88 | 0.03 | ||
| CU-N4/GR | 0.00 | −13.22 |  |
| 1.00 | −11.22 | ||
| 4.00 | −5.22 | ||
| 6.60 | −0.02 | ||
| 6.61 | 0.00 |
3.2. Binding ability
The binding energies of ORR intermediates were calculated in an acidic medium involving O2, O, H2O, OOH, OH and H2O2 molecules (Table 2), and with and without the water solvent energies obtained from the work of Sha et al. [34]. Kattel et al. and Bhatt et al. reported that the binding energies of O2 to the structure of Co–N4/Gr and Cu–N2/Gr were −0.67 and −2.90 eV, respectively. As a first ORR step, O2 binding to the defect shown in this study is significantly high, except for the Cu–N4/Gr structure in relation to Cu–N2/Gr [16,21]. Structure-wise Cu–N2/Gr has a higher O2 binding energy than Cu–N4/Gr, also, Cu2–N6/Gr has a higher binding energy compared to the Cu2–N8/Gr structure. This indicates that adding more nitrogen atoms to Cu did not create a good O2 adsorbent defect. The efficiency of the O2 adsorbent in decreasing order is as follows, Cu2–N6/Gr > Cu2–N8/Gr > Cu–N4/Gr.
Table 2.
Binding energies (BE) of Cu2–N6/Gr, Cu2–N8/Gr, Cu–N4/Gr structures with and without solvent energies (SE) [34] and shortest distance (d) between Cu–O, O–O atoms in angstroms (Å). The Mulliken charge of the adsorbate (Qx) and the Mulliken charge of the Cu site (QCu) for the shortest distance of the optimized structure. (01) and (02) in the Cu2–N6/Gr defect represent the different ORR paths 03-04-06-08 and 01-05-08 in Fig. 1, respectively. Two Mulliken charge values represent the atom order of the molecule column. Here, e = 1.602 × 10−19 C.
| Defect | Molecule | BE (without solvent)/eV | SE/eV | BE (with solvent)/eV | dO-O/Å | dCU-O/Å | Qx/e | QCu/e |
|---|---|---|---|---|---|---|---|---|
| CU2–N6/GR (01) | O + O | −3.54 | −0.41 | −3.95 | 1.50 | 1.82 | −0.223, −0.365 | 0.979 |
| OOH | −4.13 | −0.47 | −4.60 | 1.55 | 1.84 | −0.328 | 0.934 | |
| O | −7.53 | −0.70 | −8.23 | – | 1.74 | −0.592 | 1.087 | |
| OH | −5.12 | −0.38 | −5.50 | – | 1.89 | −0.643 | 0.952 | |
| H2O | −1.30 | – | – | – | 2.09 | −0.573 | 0.937 | |
| OH + OH (H2O2) | −5.44 | – | – | 2.48 | 1.75,1.85 | −0.618, −0.602 | 0.947, 0.744 | |
| CU2–N6/GR (02) | O + O | −3.78 | −0.41 | −4.19 | 1.49 | 1.82 | −0.367, −0.220 | 1.010 |
| O + OH | −9.52 | −1.08 | −10.60 | 2.52 | 1.83 | −0.368, −0.661 | 0.631, 1.051 | |
| OH + OH | −6.52 | −0.76 | −7.28 | 2.45 | 1.79 | −0.665, −0.605 | 1.060, 0.897 | |
| OH | −5.13 | −0.38 | −5.51 | – | 1.89 | −0.640 | 0.949 | |
| H2O | −1.30 | – | – | – | 2.09 | −0.573 | 0.937 | |
| CU2–N8/GR | O2 | −2.79 | −0.41 | −3.20 | 1.46 | 1.89 | −0.273 | 0.967 |
| OOH | −3.27 | −0.47 | −3.74 | 1.55 | 1.93 | −0.303 | 0.993 | |
| OH + OH | −5.34 | −0.76 | −6.10 | 2.66 | 1.81,1.86 | −0.606, −0.612 | 0.919,0.845 | |
| OH | −3.81 | −0.38 | −4.19 | – | 2.05 | −0.615 | 1.015 | |
| H2O | −0.98 | – | – | – | 2.20 | −0.599 | 1.068 | |
| CU-N4/GR | O2 | −2.76 | −0.41 | −3.17 | 1.38 | 1.98 | −0.195 | 0.996 |
| OOH | −1.70 | −0.47 | −2.17 | 1.52 | 1.87 | −0.312 | 0.922 | |
| O | −5.15 | −0.70 | −5.85 | – | 1.88 | −0.312 | 0.905 | |
| OH | −1.99 | −0.38 | – | – | 1.84 | −0.581 | 0.857 | |
| H2O | −1.23 | – | – | – | 2.08 | −0.587 | 1.011 | |
| H2O2 | −0.92 | – | – | 1.54 | 2.18 | −0.319 | 0.999 |
The binding energies of OH and OOH on the Cu2–N6/Gr structure are shown to be −5.12 and −4.13 eV and −3.81 and −3.27 eV, respectively, for binding onto the Cu2–N8/Gr structure. The binding energies for OH and OOH to the Cu–N2/Gr structure were reported to be −2.68 and −1.81 eV, respectively, while for the Cu–N4/Gr structure were −1.99 and −1.70 eV [21]. The OH and OOH binding energies also signify the above N atoms and the idea of a stable defect. The energy values, shown in Table 2, also suggest that the Cu2–N6/Gr defect supports high ORR molecule binding.
There are two possibilities of ORR pathways for Cu2–N6/Gr as shown in Fig. 1 and Table 2. Both pathways show that the O2 adsorbent is high relative to the other structures considered in this study. For the structure of Cu2–N6/Gr, the 1-5-8 pathway has a higher O2 adsorbent affinity than the 3-4-6-8 pathway. For both the 1-5-8 and 3-4-6-8 pathways, the initial step is the same, but having different binding energies may cause the ORR to take two different paths. Fig. 2(a)–(e) shows all five ORR steps of the 1-5-8 pathway with intermediates of *O2, *O + OH, *OH + OH, *OH, *H2O and Fig. 3(a)–(e) shows all five ORR steps of the 3-4-6-8 pathway with intermediates of *O2,*OOH, *O, *OH, *H2O respectively. Fig. 3(f) shows the result of trying to form H2O2 from the *OOH intermediate.
Fig. 1.
ORR paths for Cu2–N6/Gr, Cu2–N8/Gr, Cu–N4/Gr structures.
Fig. 2.
Top and side view of optimized ORR steps for Cu2–N6/Gr (02) structure. (a) O2 (b) O + OH (c) OH + OH (d) OH (e) H2O molecules binding on Cu2–N6/Gr (02) defect. (White, grey, blue, orange and red sphere represents hydrogen, carbon, nitrogen, copper and oxygen atoms, respectively). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 3.
Top and side view of optimized ORR steps for Cu2–N6/Gr (01) structure. (a) O2 (b) OOH (c) O (d) OH (e) H2O (f) dual OH molecules form and binding instead of H2O2 on Cu2–N6/Gr (01) defect. (White, grey, blue, orange and red sphere represents hydrogen, carbon, nitrogen, copper and oxygen atoms, respectively). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The difference in free energy (ΔG) for O2 binding was −3.59 and −3.58 eV for Cu2–N6/Gr (01) and Cu2–N6/Gr (02), respectively. These ΔG values indicate that the Cu2–N6/Gr structure favors both pathways, as shown by the O2 adsorption step.
The Cu–O bond length of O2 was 1.82, 1.89, and 1.98 Å for Cu2–N6/Gr, Cu2–N8/Gr, and Cu–N4/Gr, respectively. The results show that the most important first step of ORR favors the Cu2–N6/Gr structure. Xiao et al. and Isaacs et al. reported Cu–O bond lengths ranging from 2.04 to 2.59 Å [20,35]. However, in this study, the distance of Cu–O bond lengths is less than 2 Å for both the Cu2–N6/Gr and Cu2–N8/Gr structures, and this may be due to the binding of the O atom to dual Cu atoms as shown in Fig. 2, Fig. 3, Fig. 4. Here, Fig. 4(a)–(e) shows optimized ORR steps for Cu2–N8/Gr structure with *O2, *OOH, *OH + OH, *OH, *H2O intermediates respectively. For the Cu–N4/Gr structure, the Cu–O bond length is comparable to the minimum value of the Cu–O bond range studied previously. Furthermore, O–O bond length of O2 binding to the Cu–N4/Gr structure shows similarity to the Cu–N2/Gr structure [21]. Additionally, a previous investigation using VASP (Vienna ab-initio Simulation Package) [20] on Cu–N4/Gr indicated that the Cu–O bond lengths for the O2 and O intermediates were 2.22 Å and 1.67 Å, respectively, with O2 exhibiting weaker binding compared to O, potentially attributed to varying basis set configurations employed in the study. The O–O bond length of O2 binding in the Cu2–N6/Gr and Cu2–N8/Gr structures shows a slight increase in the bond length. This increase in the bond length may be due to π bonding electron on the adsorption side of O weakened by the Cu–O bond.
Fig. 4.
Top and side view of optimized ORR steps for Cu2–N8/Gr structure. (a) O2 (b) OOH (c) OH + OH (d) OH (e) H2O molecules binding on Cu2–N8/Gr defect. (White, grey, blue, orange and red sphere represents hydrogen, carbon, nitrogen, copper and oxygen atoms, respectively). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
When molecule binding occurs, the Cu atom protrudes from the graphene layer. According to the optimized structures, most of the protruding Cu atom occur due to the binding of the O atom or the OH molecule (Fig. 3, Fig. 5, Fig. 6), and this is consistent with the work reported by Xiao et al. and Bhatt et al. [20,21]. The final ORR step involves the binding of H2O. The H2O binding strength follows a decreasing order with respect to the defect structures: Cu2–N6/Gr > Cu–N4/Gr > Cu2–N8/Gr. Based on the results of this study, it has been confirmed that the attachment of H2O to the Cu atom on the Cu2–N8/Gr defect structure is weakest, as evidenced by the 2.20 Å Cu–O bond length, when compared to the other defect structures investigated. On the other hand, Cu2–N6/Gr is shown to have a high H2O adsorbent possibility but also to have a better adsorption ability for O2 than H2O. The binding energies indicate that Cu–N4/Gr has less ability to adsorb H2O when compared to Cu2–N6/Gr. The bond length to H2O is similar for both defects. The binding energies of *OH, *OOH and *O have significantly higher values, indicating that all three defects could be considered as possible stable active sites for ORR.
Fig. 5.
Cu2–N6/Gr (01) structure (a) H atom binds with O1 atom of OOH to form H2O2 (b) Middle state of optimization (c) Final optimization. (White, grey, blue, orange and red sphere represents hydrogen, carbon, nitrogen, copper and oxygen atoms, respectively). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 6.
Cu2–N8/Gr structure (a) H atom (01) bind with second O atom of O1O2H molecule to form H2O (b) H atom (01) binding to second O atom and previously bonded H atom (02) break its bond and travel to first O atom (c) Final optimization, forming dual OH molecules instead of H2O2. (White, grey, blue, orange and red sphere represents hydrogen, carbon, nitrogen, copper and oxygen atoms, respectively). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.3. Effects of the Mulliken charge
The Mulliken charge for the adsorbate of the optimized structures is shown in Table 2. The Mulliken charge population (ΔQx) was applied to both O atoms of the O2 intermediate as Eq. (5) for all structures. ΔQx values for Cu2–N6/Gr (01) −0.063 e and 0.239 e, Cu2–N6/Gr (02) −0.141 e and −0.077 e, Cu2–N8/Gr 0.02 e and −0.151 e, Cu–N4/Gr −0.024 e. Here, the negative sign indicates an electron loss of the intermediate [29]. Therefore, the electron loss of the O atom could be considered as the electron gain of the Cu atom, which could explain the increased binding strength. The decreasing order of the charge population bonding strength is Cu2–N6/Gr > Cu2–N8/Gr > Cu–N4/Gr, suggesting that Cu2–N6/Gr has the highest O2 adsorption ability. Additionally, this observation is consistent with the decreasing order of the O2 adsorption efficiency predicted using the binding energy. For H2O, the Mulliken charge population values for are 0.027 e, 0.058 e, 0.008 e and −0.054 e for Cu2–N6/Gr (01), Cu2–N6/Gr (02), Cu2–N8/Gr and Cu–N4/Gr, respectively. These values signify the binding strength of H2O in decreasing order as Cu–N4/Gr > Cu2–N8/Gr > Cu2–N6/Gr (01) > Cu2–N6/Gr (02). Only Cu–N4/Gr and Cu2–N8/Gr are compared with the above H2O adsorbent strength decreasing order explained by the binding energy. The difference in H2O adsorbent strength order between the Mulliken charge population and the binding energy may be due to the O atom drawing electrons from H atoms of H2O, altering the Mulliken charge values of the O atom. Based on the Mulliken charges of Cu2–N6/Gr, it has the weakest bond strength on H2O, allowing H2O to break free from the catalyst easily. The Mulliken charges of the optimized structures in Table 2 indicate that the bonding capability is dependent on the difference of Qx and QCu and the bond length dCu-O. Strong bonding is favored by a large difference in Qx and QCu and a short bond length dCu-O.
The Cu2–N6/Gr structure follows the ORR steps on the 01-05-08 and 03-04-06-08 paths (Fig. 1). The 03-04-06-08 path has the possibility of generating H2O2 due to the formation of *OOH intermediate with an additional H atom (Fig. 5(a)), but optimization shows instead of H2O2 generation, it generates a separate *OH intermediates (Fig. 5(c)) and follows the 01-05-08 path from step 05 (Fig. 3, Fig. 5 and (f)).
Separate generation of the OH molecule may be due to the attraction of electrons by the Cu atoms. According to Mulliken charges, N atoms lose fewer electrons to Cu atoms of Cu2–N6/Gr on demand of electrons compared to Cu–N4/Gr. Therefore, the Cu atom of Cu2–N6/Gr may try to replenish its electron demand from O atom charges by forming a strong Cu–O bond (Fig. 5(b) and (c)) unlike Cu–N4/Gr which form H2O2. Furthermore, this scenario could be explained as π bonding electron on O atoms that attracts to each Cu atom. If the O atoms largely lose their electron charge density, that could cause the O–O bond to break and generate *2OH instead of H2O2 from *OOH.
Nallathambi et al. reported that H2O2 generation could stop the ORR four-electron path halfway [36]. The ORR steps of an acidic medium show that a two-electron pathway is needed for the generation of H2O2. However, the optimization did not produce a two-electron pathway for the Cu2–N6/Gr structure. Cu2–N8/Gr structures only follow the ORR 02-04-05-08 path (Fig. 1). It is a four-electron pathway without H2O2 generation. The *OOH intermediate on its ORR path is more favorable to form dual OH over H2O (Figs. 4(c) and 6(a)–(c)). Binding of the H atom (01) to the second O atom (Fig. 6(a)) causes the breakage of the O–O bond due to π electron deviation. This situation creates the H2O molecule short time. However, the formation of *2OH is made possible by the attraction of electrons from the O atom of H2O by the Cu atom caused by weak Cu–N bonding. This in turn would weaken one of the O–H bonds and lead to the release of H to react to form OH (Fig. 6(b)). Since the *OOH of Cu2–N8/Gr structure creates the *2OH configuration by H atoms binding to both O atoms (Fig. 6(c)), the possibility of H2O2 formation is reduced.
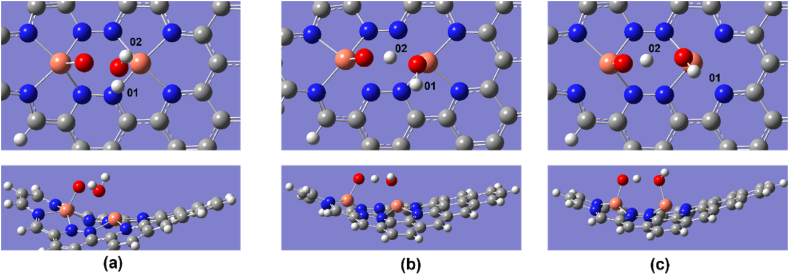
The ORR associated with the Cu–N4/Gr structure follows only the 02-04-06-08 path (Fig. 1) with *O2, *OOH, *O, *OH, *H2O intermediates respectively (Fig. 7(a)–(e)). The optimized structures show the possibility of H2O2 generation (Fig. 7(f)). With a binding energy of −0.92 eV and a Cu–O bond length of 2.18 Å, H2O2 can easily break free from the Cu–N4/Gr defect (Fig. 7(f)). The O–O bond in the simulation is shown to be stable, as suggested by Bhatt et al. [21]. The Mulliken charge population value of H2O2, 0.026 e, indicates that H2O2 has the ability to break free from the catalyst surface as H2O. Since H2O2 directly participates in the degeneration of the fuel cell membrane [37,38], the structure of Cu–N4/Gr can significantly reduce the efficiency of the fuel cell. Additionally, the two-electron pathway of H2O2 generation can reduce the current density of the fuel cell.
Fig. 7.
Top and side view of optimized ORR steps for Cu–N4/Gr structure. (a) O2 (b) OOH (c) O (d) OH (e) H2O (f) H2O2 molecules binding on Cu–N4/Gr defect. (White, grey, blue, orange and red sphere represents hydrogen, carbon, nitrogen, copper and oxygen atoms, respectively). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.4. HOMO and LUMO energy calculations
The HOMO and LUMO energies were calculated for every step of the ORR and structural defects to further investigate the binding energy. The energy gap (Eg), chemical hardness (η), chemical potential (μ), and electrophilicity index (ω) of *O2, *H2O, *H2O2, intermediates and defect structures were calculated as shown in Table 3 using Eqs. (6), (7), (8), (9)) [29,31,32].
Table 3.
Energy gap (Eg), chemical hardness (η), chemical potential (μ), and electrophilicity index (ω) values of *O2, *H2O, *H2O2 intermediates and defect structures.
| Defect | Intermediate/structure | Eg/eV | η/eV | μ/eV | ω/eV |
|---|---|---|---|---|---|
| CU2–N6/GR (01) | Cu2–N6/Gr | 0.04 | 0.04 | −0.13 | 0.23 |
| O + O | 0.03 | 0.03 | −0.14 | 0.33 | |
| H2O | 0.03 | 0.03 | −0.12 | 0.23 | |
| H2O2 | – | – | – | – | |
| CU2–N6/GR (02) | O + O | 0.03 | 0.03 | −0.14 | 0.35 |
| H2O | 0.03 | 0.03 | −0.12 | 0.22 | |
| H2O2 | – | – | – | – | |
| CU2–N8/GR | Cu2–N8/Gr | 0.05 | 0.05 | −0.12 | 0.15 |
| O2 | 0.04 | 0.04 | −0.13 | 0.19 | |
| H2O | 0.05 | 0.05 | −0.11 | 0.13 | |
| H2O2 | – | – | – | – | |
| CU-N4/GR | Cu–N4/Gr | 0.03 | 0.03 | −0.13 | 0.27 |
| O2 | 0.04 | 0.04 | −0.13 | 0.19 | |
| H2O | 0.04 | 0.04 | −0.11 | 0.16 | |
| H2O2 | 0.03 | 0.03 | −0.12 | 0.21 |
According to Eqs. (6), (7)) η and Eg are directly associated with each other. The chemical hardness elucidates the stability and reactivity of the structure. Therefore, stable structures are less reactive, and high-reactive structures are less stable [8,29,31]. Defects can be ranked in decreasing order of chemical hardness as Cu2–N8/Gr > Cu2–N6/Gr > Cu–N4/Gr. This suggests that the reactivity of the Cu–N4/Gr structure is higher than that of other structures, but it is also less stable. This reactivity could cause electrons in the valence band to be easily excited to occupy the conduction band. The reactivity order is confirmed by the formation energy shown in Table 1. The Cu2–N8/Gr structure is more stable than others and is the least reactive of the three defect structures, and Cu2–N6/Gr has medium structural stability and reactivity according to chemical hardness values.
The chemical hardness of the oxygen bonding decreasing order Cu2–N8/Gr = Cu–N4/Gr > Cu2–N6/Gr indicates that Cu2–N6/Gr is less stable and more reactive than the other two structures. If the ORR first step is less stable and has high reactivity, the probability of achieving the next ORR step is high. Therefore, the Cu2–N6/Gr defect is more suitable to complete the ORR first step. From previous calculations, the binding energy of the second step for ORR of Cu2–N6/Gr structure is higher than that of the first step, and this collaborates well with the chemical hardness data. The chemical hardness of the H2O bond in decreasing order is as follows: Cu2–N8/Gr > Cu–N4/Gr > Cu2–N6/Gr. This indicates that the H2O bonded to the Cu2–N8/Gr defects is more stable than the other defects. DFT calculations also show that the longest bond length of Cu–O and the highest binding energy would increase the probability of breaking H2O from the Cu2–N8/Gr defect sites. In the case of H2O bonding to the highly reactive Cu2–N6/Gr defect, this would result in an oxygen evolution reaction (OER) [20,39].
The chemical potential is directly related to Mulliken's electronegativity [31]. The chemical reactivity of the structure increases with decreasing chemical potential. The chemical potential decreases in the following order of defects: Cu2–N8/Gr > Cu2–N6/Gr = Cu–N4/Gr. This shows that the chemical reactivity of the Cu2–N6/Gr and Cu–N4/Gr structures is slightly higher than that of Cu2–N8/Gr. Furthermore, the chemical reactivity of the oxygen-bonded structures is the highest for Cu2–N6/Gr, confirming the previous chemical hardness evaluation of Cu2–N6/Gr.
Since electrophilicity is the electron accepting capacity from the surrounding atoms to form a stable energy state, and this relates directly to the structural stability. The electrophilicity of the studied defects has the following decreasing order, Cu–N4/Gr > Cu2–N6/Gr > Cu2–N8/Gr, and this also confirms with the reactivity order of the structures in terms of chemical hardness. The oxygen-bonded Cu2–N6/Gr shows a higher electron acceptation than the other structures, hence, indicating that the Cu2–N6/Gr structure favors electron adsorption to form a stable structure. Two ORR steps of Cu2–N6/Gr that generate different paths could occur to this reactivity. The Cu2–N8/Gr and Cu–N4/Gr structures show similar electrophilicity values for the O2 bonding. The stability of the Cu–N4/Gr structure is attributed to the four nitrogen atoms that donate electrons to the Cu atom. Compared to the Cu2–N6/Gr structure, which has six nitrogen atoms, the Cu–N4/Gr structure is more stable (Table 1). The Cu2–N8/Gr structure has twice as many nitrogen atoms as Cu–N4/Gr, but its electrophilicity value is likely to be low, similar to that of Cu–N4/Gr. Furthermore, H2O-bonded Cu2–N8/Gr showed the lowest electrophilicity value for the last ORR step, indicating a low probability that a further reaction will take place.
3.5. Free energy
Free energy values were calculated for the three defect structures with possible ORR pathways in an acidic medium as shown in Fig. 8(a–d). Cu2–N6/Gr shows a spontaneous reaction (downhill) for the first two steps of ORR with cell potential (U) 0–1.00 V (Fig. 8(a) and (b)). The second to the third steps of the ORR for all potentials show uphill. From the third step to the last downhill, the configuration was found again for 0 V–1.00 V. This indicates that a large energy barrier is present between the second step and the third step, which prevents the spontaneous reaction of ORR from being completed. The highest reaction step barrier, 5.34 eV and 5.11 eV, are shown by Cu2–N6/Gr, for both (01) and (02) paths at U = 0 V, respectively, in *O–O hydrogenate by attaching the H atom to form *OOH and *O–OH. In summary, even with good binding energies, both ORR paths of Cu2–N6/Gr structure did not show spontaneity.
Fig. 8.
Free energy of possible ORR paths for Cu2–N6/Gr, Cu2–N8/Gr and Cu–N4/Gr defect structures with different potentials in acidic medium. (a) Cu2–N6/Gr (01) (b) Cu2–N6/Gr (02) (c) Cu2–N8/Gr (d) Cu–N4/Gr.
The Cu2–N8/Gr structure shows spontaneous reaction for all ORR steps for cell potential ranges from 0 to 0.28 V. With cell potential >0.28 V, the fifth step of ORR becomes uphill, showing the *2OH → *OH + H2O reaction process is not spontaneous (Fig. 8(c)). A further increase in the cell potential caused an increase in the uphill processes. The Cu–N4/Gr structure shows a spontaneous process for all ORR steps until the cell potential reaches 0.49 V. After the cell potential exceeds 0.49 V (U > 0.49 V), the ORR step of forming *O + H2O becomes uphill (Fig. 8(d)). Then, with the enhancement of the potential, the uphill process also increased. The maximum cell potentials for spontaneous reactions are shown to be 0.49 and 0.28 V for Cu–N4/Gr and Cu2–N8/Gr, respectively. These results show that the open circuit voltage (OCV) is relatively better than the Pt catalyst cathode, which initially shows 0.9 V, as reported by Kim et al. [40].
A comparison of the formation energies and chemical hardness indicates that the Cu–N4/Gr structure has a lower probability of forming and is less stable than other structures. Therefore, generating 0.49 V as cell voltage is a low possibility. Furthermore, the Cu–N4/Gr defect structure has a greater possibility of forming H2O2, as indicated in Table 2. The free energy diagram (Fig. 9) shows that the formation of H2O2 was spontaneous within the range of 0–0.49 V. After exceeding 0.49 V, the H2O2 formation step becomes uphill. Both the free energy and the maximum potential of the downhill process to form H2O2 and H2O from *OOH are the same. Furthermore, the Mulliken charges of the O atoms of *OOH are approximately equal (−0.312 e and −0.331 e, respectively), indicating that the final step of ORR of Cu–N4/Gr is solely dependent on the binding of the H atom to the O atom of *OOH (Fig. 7(b)). If the H atom binds to the Cu–O side O atom of *OOH, it could generate H2O2, and binding to the other O atom would generate 2H2O in the final step. Since the H2O2 formation path supplies only two electrons through an external path, from the anode to the cathode, this could result in a decrease in current density.
Fig. 9.
Free energy diagram of H2O2 generation in Cu–N4/Gr defect structures with different potentials in acidic medium.
However, this study has several limitations that should be acknowledged. First, the 3-21G basis set used in this study can be considered small. However, it was selected based on Bhatt et al.'s study [21], which compared Cu–N2/Gr results with the current study. Furthermore, this study did not include periodic boundary conditions or dispersion interactions, unlike the work by Xiao et al. [20] on Cu–N4/Gr. However, these studies cannot be used as a basis for a comparison with the current study due to their use of different DFT simulation software. The use of Gaussian09 instead of VASP-like simulation software was due to financial constraints. Although VASP-like software is better suited for surface simulation and PBC approach calculations, this study employed a single layer of graphene to examine the impact of defect structures on the surface of the catalyst in relation to ORR, similar to the approaches taken by Bhatt et al. [21] and Kattel et al. [16]. Future studies related to this research will employ dispersion correction with the PBC approach to provide a basis for comparison with the current study.
4. Conclusion
Computational calculations predicted the Cu2–N8/Gr and Cu–N4/Gr motifs, showing promising spontaneous ORR with maximum cell potential of 0.28 and 0.49 V under standard conditions. The Cu2–N6/Gr motif is not favorable for spontaneous ORR. From the formation energy calculations, Cu2–N8/Gr, Cu–N4/Gr, and Cu2–N6/Gr are stable for potential range (U) 0–5.87, 0–6.6, and 0–5.89 V, respectively. The maximum cell potentials of Cu2–N8/Gr and Cu–N4/Gr are within the potential range of formation energy, showing structural stability. Based on the chemical hardness, chemical potential, electrophilicity, and Mulliken charge calculations, the Cu2–N8/Gr shows a higher stability and favors ORR than Cu2–N6/Gr. Cu2–N6/Gr is more favorable for ORR than Cu–N4/Gr by binding energy values. The Cu2–N6/Gr motif is not recognized as a spontaneous catalyst by free energy calculations. Therefore, considering these three motifs, only Cu2–N8/Gr and Cu–N4/Gr show promising ORR ability. Consequently, the possibility of cell voltage solely depends on Cu2–N8/Gr could occur due to its high stability. According to DFT optimization, H2O2 is generated only from Cu–N4/Gr. The other structures follow four-electron transfer pathways to generate high electron density. The Cu-Nitrogen doped non-PGM catalyst with the considered motifs has a relatively high current density even with low cell voltage when comparing to Pt-like catalysts. The amount of H2O2 generated by Cu–N4/Gr could be limited by its low stability. Bhatt et al. have also shown that the Cu–N2/Gr motif has a high probability of generating H2O2, but the −5.68 eV formation energy [21] indicates that it has lower stability than Cu–N4/Gr. In this study, a novel Cu2–N8/Gr catalyst is a good candidate for fuel cell application as it has good cell potential and non-H2O2 formation. Furthermore, the three structures presented in this study show a better O2 binding energy than the Pt catalyst and PGM alloys [41].
Author contribution statement
Yashas Balasooriya: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Pubudu Samarasekara, Muhammad Raziq Rahimi Kooh: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Chee Ming Lim, Yuan-Fong Chou Chau: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Roshan Thotagamuge: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Data availability statement
Data included in article/supplementary material/referenced in article.
Funding
The work described in this article was supported by the Universiti Brunei Darussalam Research Grant UBD/RSCH/1.9/FICBF(b)/2022/017.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
We, the authors, thank the Government of Brunei Darussalam, Universiti Brunei Darussalam and the Wayamba University of Sri Lanka for their continuous support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e15989.
Contributor Information
Muhammad Raziq Rahimi Kooh, Email: raziq.kooh@ubd.edu.bn.
Roshan Thotagamuge, Email: roshan@wyb.ac.lk.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Fuel Cells. [cited 2022 8th January 2022]; Available from: https://www.energy.gov/eere/fuelcells/fuel-cells.
- 2.Antolini E. Carbon supports for low-temperature fuel cell catalysts. Appl. Catal. B Environ. 2009;88(1–2):1–24. [Google Scholar]
- 3.Roland U.B., Roessner Thomas, Frank On the nature of spilt-over hydrogen. J. Mol. Catal. Chem. 1997;127(1–3):61–84. [Google Scholar]
- 4.Conner W.C., Jr., Pajonk G., Teichner S. Advances in Catalysis. Elsevier; 1986. Spillover of sorbed species; pp. 1–79. [Google Scholar]
- 5.Lin R., et al. Rapid microwave-assisted solvothermal synthesis of shape-controlled Pt-Ni alloy nanoparticles for PEMFC. Electrochim. Acta. 2018;283:764–771. [Google Scholar]
- 6.Reyimjan A., Sidik A.B.A. O2 reduction on graphite and nitrogen-doped graphite experiment and theory. J. Phys. Chem. 2006;110:1787–1793. doi: 10.1021/jp055150g. [DOI] [PubMed] [Google Scholar]
- 7.Lee K.R., et al. Electrochemical oxygen reduction on nitrogen doped graphene sheets in acid media. Electrochem. Commun. 2010;12(8):1052–1055. [Google Scholar]
- 8.Zhang L., Xia Z. Mechanisms of oxygen reduction reaction on nitrogen-doped graphene for fuel cells. J. Phys. Chem. C. 2011;115(22):11170–11176. [Google Scholar]
- 9.Rao C.V., Cabrera C.R., Ishikawa Y. In search of the active site in nitrogen-doped carbon nanotube electrodes for the oxygen reduction reaction. J. Phys. Chem. Lett. 2010;1(18):2622–2627. [Google Scholar]
- 10.Lin R., et al. High durability of Pt-Ni-Ir/C ternary catalyst of PEMFC by stepwise reduction synthesis. Electrochim. Acta. 2020:330. [Google Scholar]
- 11.Beermann V., et al. Real-time imaging of activation and degradation of carbon supported octahedral Pt–Ni alloy fuel cell catalysts at the nanoscale using in situ electrochemical liquid cell STEM. Energy Environ. Sci. 2019;12(8):2476–2485. [Google Scholar]
- 12.Alexei L.N., Pinheiro A.O.-N., de Souza Elki C., Joelma Perez V.A.P., Ticianelli Edson A., Gonzalez A.E.R. Electrocatalysis-on-Noble-Metal-and-Noble-Metal-Alloys-Dispersed-on-High-Surface-Area-Carbon. J. New Mater. Electrochem. Syst. 2003;6:1–8. [Google Scholar]
- 13.Orellana W. Catalytic properties of transition metal–N4 moieties in graphene for the oxygen reduction reaction: evidence of spin-dependent mechanisms. J. Phys. Chem. C. 2013;117(19):9812–9818. [Google Scholar]
- 14.Khotseng L. Electrocatalysts for Fuel Cells and Hydrogen Evolution - Theory to Design. 2018. Oxygen reduction reaction; p. 27. [Google Scholar]
- 15.Liu K., et al. Mn- and N- doped carbon as promising catalysts for oxygen reduction reaction: theoretical prediction and experimental validation. Appl. Catal. B Environ. 2019;243:195–203. [Google Scholar]
- 16.Kattel S., Atanassov P., Kiefer B. Catalytic activity of Co-N(x)/C electrocatalysts for oxygen reduction reaction: a density functional theory study. Phys. Chem. Chem. Phys. 2013;15(1):148–153. doi: 10.1039/c2cp42609a. [DOI] [PubMed] [Google Scholar]
- 17.Masa J., et al. Metal-free catalysts for oxygen reduction in alkaline electrolytes: influence of the presence of Co, Fe, Mn and Ni inclusions. Electrochim. Acta. 2014;128:271–278. [Google Scholar]
- 18.Na Yang l.P., Li Li, Jing Li, Liao Qiang, Shao Minhua, Wei Zidong. Theoretically probing the possible degradation mechanisms of an FeNC catalyst during the oxygen reduction reaction. Chem. Sci. 2021:12476–12484. doi: 10.1039/d1sc02901k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie X., et al. Performance enhancement and degradation mechanism identification of a single-atom Co–N–C catalyst for proton exchange membrane fuel cells. Nat. Catal. 2020;3(12):1044–1054. [Google Scholar]
- 20.Xiao Y., Zhang W. DFT analysis elementary reaction steps of catalytic activity for ORR on metal-, nitrogen- co-doped graphite embedded structure. SN Appl. Sci. 2020;2(2):1–11. [Google Scholar]
- 21.Bhatt M.D., Lee G., Lee J.S. Density functional theory (DFT) calculations for oxygen reduction reaction mechanisms on metal-, nitrogen- co-doped graphene (M-N2-G (M = Ti, Cu, Mo, Nb and Ru)) electrocatalysts. Electrochim. Acta. 2017;228:619–627. [Google Scholar]
- 22.Chen C., et al. Dual‐metal single‐atomic catalyst: the challenge in synthesis, characterization, and mechanistic investigation for electrocatalysis. SmartMat. 2022:1–32. [Google Scholar]
- 23.Zhang N., et al. High-density planar-like Fe2N6 structure catalyzes efficient oxygen reduction. Matter. 2020;3(2):509–521. [Google Scholar]
- 24.Nørskov J.K., R J., Logadottir A., Lindqvist L. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B. 2004;108:17886–17892. [Google Scholar]
- 25.Calle-Vallejo F., Martinez J.I., Rossmeisl J. Density functional studies of functionalized graphitic materials with late transition metals for Oxygen Reduction Reactions. Phys. Chem. Chem. Phys. 2011;13(34):15639–15643. doi: 10.1039/c1cp21228a. [DOI] [PubMed] [Google Scholar]
- 26.Rongrong Chen H.L., Chu Deryn, Wang Guofeng. Unraveling oxygen reduction reaction mechanisms on carbon-supported Fe-phthalocyanine and Co-phthalocyanine catalysts in alkaline solutions. J. Phys. Chem. 2009;113:20689–20697. [Google Scholar]
- 27.Stephen Walch A.D. Masoud Aryanpour, and Heinz pitsch, mechanism of molecular oxygen reduction at the cathode of a PEM fuel cell non-electrochemical reactions on catalytic Pt particles. J. Phys. Chem. 2008;112:8464–8475. [Google Scholar]
- 28.Yu L., et al. Oxygen reduction reaction mechanism on nitrogen-doped graphene: a density functional theory study. J. Catal. 2011;282(1):183–190. [Google Scholar]
- 29.Syaahiran M.A., et al. Theoretical study of CO adsorption interactions with Cr-doped tungsten oxide/graphene composites for gas sensor application. ACS Omega. 2022;7(1):528–539. doi: 10.1021/acsomega.1c04936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glossman-Mitnik D. Computational study of the chemical reactivity properties of the rhodamine B molecule. Procedia Comput. Sci. 2013;18:816–825. [Google Scholar]
- 31.Chattaraj P.K., Giri S. Electrophilicity index within a conceptual DFT framework. Annu. Rep. Sect. C: Phys. Chem. 2009;105 [Google Scholar]
- 32.Parr R.G., Szentpaly L.v., Liu S. Electrophilicity index. J. Am. Chem. Soc. 1999;121:1922–1924. [Google Scholar]
- 33.Syaahiran A., et al. A theoretical insight of Cr dopant in tungsten oxide for gas sensor application. Mater. Today Commun. 2021;28 [Google Scholar]
- 34.Sha Y., et al. Theoretical study of solvent effects on the platinum-catalyzed oxygen reduction reaction. J. Phys. Chem. Lett. 2010;1(5):856–861. [Google Scholar]
- 35.Isaacs M., C J.C., Aguirre M.J., Estiú G., Caruso F., Ferraudi G., Costamagna J. Electrocatalytic reduction of CO2 by aza-macrocyclic complexes of Ni(II), Co(II), and Cu(II). Theoretical contribution to probable mechanisms. Inorg. Chim. Acta. 2002;339:224–232. [Google Scholar]
- 36.Nallathambi V., et al. Development of high performance carbon composite catalyst for oxygen reduction reaction in PEM Proton Exchange Membrane fuel cells. J. Power Sources. 2008;183(1):34–42. [Google Scholar]
- 37.Satoru Hommura K.K., Shimohira Tetsuji, Teraoka Y. Development of a method for clarifying the perfluorosulfonated membrane degradation mechanism in a fuel cell environment. J. Electrochem. Soc. 2008;155(1):A29–A33. [Google Scholar]
- 38.Cheng Chen T.F.F. H2O2 formation under fuel-cell conditions. J. Electrochem. Soc. 2007;11(1):1127–1137. [Google Scholar]
- 39.Chai G.-L., et al. Active sites engineering leads to exceptional ORR and OER bifunctionality in P,N Co-doped graphene frameworks. Energy Environ. Sci. 2017;10(5):1186–1195. [Google Scholar]
- 40.Kim J., Lee J., Tak Y. Relationship between carbon corrosion and positive electrode potential in a proton-exchange membrane fuel cell during start/stop operation. J. Power Sources. 2009;192(2):674–678. [Google Scholar]
- 41.Qi X., et al. DFT study on ORR catalyzed by bimetallic Pt-skin metals over substrates of Ir, Pd and Au. Nano Mater. Sci. 2021 doi: 10.1016/j.nanoms.2021.06.002. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supplementary material/referenced in article.