Abstract
Introduction:
Tumor size(TS) represents a critical parameter in the risk assessment of laparoscopic liver resections(LLR). Moreover, TS has been rarely related to the extent of liver resection. The aim of this study was to study the relationship between tumor size and difficulty of laparoscopic left lateral sectionectomy(L-LLS).
Methods:
The impact of TS cutoffs was investigated by stratifying tumor size at each 10mm- interval. The optimal cut-offs were chosen taking into consideration the number of endpoints which show a statistically significant split around the cut-points of interest and the magnitude of relative risk after correction for multiple risk factors.
Results:
1910 L-LLS were included. Overall, open conversion and intraoperative blood transfusion were 3.1% and 3.3%, respectively. The major morbidity rate was 2.7% and 90-days mortality 0.6%. Three optimal TS cut-offs were identified: 40-mm, 70-mm, and 100-mm. All the selected cut-offs showed a significant discriminative power for the prediction of open conversion, operative time, blood transfusion and need of Pringle manoeuvre. Moreover, 70-mm and 100-mm cut-offs were both discriminative for estimated blood loss and major complications. A stepwise increase in rates of open conversion rate (Z=3.90,p<0.001), operative time (Z=3.84,p<0.001), blood loss (Z=6.50,p<0.001), intraoperative blood transfusion rate (Z=5.15,p<0.001), Pringle manoeuvre use (Z=6.48,p<0.001), major morbidity(Z=2.17,p=0.030) and 30-days readmission (Z=1.99, p=0.047) was registered as the size increased.
Conclusions:
L-LLS for tumours of increasing size was associated with poorer intraoperative and early postoperative outcomes suggesting increasing difficulty of the procedure. We determined 3 optimal TS cutoffs(40-mm, 70-mm and 100-mm) to accurately stratify surgical difficulty after L-LLS.
Keywords: laparoscopic liver, laparoscopic hepatectomy, difficulty, size, left lateral sectionectomy
Introduction
Laparoscopic liver resection (LLR) is now well-accepted globally as a safe and effective surgical procedure. Nonetheless, its implementation in clinical practice is associated with a learning curve and requires a stepwise approach when selecting of cases of increasing complexity. During the Second International Consensus Conference on LLRs held in Morioka in 2014, the panel of experts recommended the use of difficulty scoring systems (DSS) to stratify the technical complexity and risks of LLR, in order to guide surgeons on selecting the appropriate procedure according to their level of experience [1]. Several DSSs have been developed and these are based on parameters such as lesion type, size, location, liver function, extent of liver resection and liver morphology [2–5]. Tumour size is presently well-recognized as having an important impact on the difficulty of LLR and it has been incorporated into most DSSs [2,3,6]. Larger tumours hinder liver mobilization, alter intraparenchymal vascular topographic anatomy and may require wider parenchymal transections and/or extensive vascular dissections. Their manipulation carries an inherent risk of vascular injury or tumour rupture, resulting in major bleeding and tumour seeding [7].
To date, an optimal tumour size cut-off to stratify the complexity of LLR has not been well- established. The Iwate score uses a single cut-off of 30 mm [2], while the Southampton score [3] categorize tumor size according to two thresholds: 30 and 50 mm; respectively. More recently, Kabir et al [4] proposed 30 mm and 70 mm as ideal thresholds for the stratification of the difficulty of LLR. An important limitation of these studies was that the impact of tumor size had not been correlated with the extent of LLR. Intuitively for example, one would expect that a tumor size >3 cm would have a greater impact on the complexity of LLR in the case of a monosegmentectomy but to a lesser extent in the case of a left lateral sectionectomy or right hepatectomy [8].
Hence, we performed the present study with the aim of investigating the impact of tumor size on the surgical complexity of a single specific LLR procedure, i.e laparoscopic left lateral sectionectomy (L-LLSs). L-LLS is presently one of the most common and usually one of the earliest types of LLR that a surgeon attempts during his/her learning curve [79]. L-LLS is widely regarded as a relatively straightforward LLR, thanks to a wide operative field, an easy liver mobilization, and a relatively thin straight parenchymal transection plane. Hence, DSSs specifically tailored to LLS is of particular importance. The primary objective of this study was to examine the relationship between tumor size and difficulty of L-LLS, and to elucidate the optimal tumor size cut-off for this procedure.
Methods
This was a post hoc review of 17680 patients who underwent pure LLR at 50 international centers between 2004–2020. Of these, there were 2698 pure LLS performed. After excluding patients who underwent concomitant major surgical procedures (such as colectomies/gastrectomies/ hilar lymphadenectomies/ bile duct resections), repeat liver resections, multiple liver resections, cysts/ cystic tumors or abscesses; study population included 1913 patients. Tumor size for three patients was not recorded. Finally, 1910 patients were included in this analysis.
All institutions obtained their respective approvals according to their local center’s requirements. This study was approved by the Singapore General Hospital Institution Review Board (CIRB 2020/2802) and the need for patient consent was waived. The anonymized data were collected in the individual centers. These were collated and analyzed centrally at the Singapore General Hospital.
Definitions
Liver resections were defined according to the 2000 Brisbane classification [10]. Left lateral sectionectomies were defined as resections of Segment 2 and 3. Tumor size was measured based on the longest diameter of the tumor on formalin- fixed specimens. Diameter of the largest lesion was used in cases of multiple tumors. Resection difficulty was graded according to the Iwate criteria [2]. Post-operative complications were classified according to the Clavien-Dindo classification and recorded for up to 30 days or during the same hospitalization [11].
Statistical analyses
The impact of tumor size cutoffs in intervals of 10mm was systematically investigated by iteratively dichotomizing the tumor size at each 10mm-interval and computing treatment effect sizes local to that cutoff. This was accomplished using a user-written Stata implementation of the ‘Cutoff_Finder’ R package, with minor modifications to allow the use of Poisson models and quantile regression for computing adjusted relative risks and median differences. To handle baseline imbalances, effect sizes were conditioned on inverse probability-weights, which were estimated from a logistic regression incorporating the following as covariates: age, gender, year of surgery, ASA status, previous abdominal surgery, concomitant minor surgery, cirrhosis, multifocality, difficult posterosuperior segment, malignant pathology, and all components of the Iwate score excluding tumor size. Optimal tumor size cutoffs were then selected by taking into consideration the number of endpoints which show a statistically significant split around the cutoff points of interest, as well as the magnitude of the test statistic (z-score and t-score from modified Poisson and quantile regressions). As a sensitivity analysis, we also estimated empirical cutpoints obtained from maximizing the Youden index in receiver operating characteristics (ROC) analyses of open conversion and use of Pringle’s maneuver.
Within tumor size categories, continuous and categorical variables were summarized as medians (IQR) and proportions respectively. Tests of inequality across tumor size categories were performed using Kruskal-Wallis tests and Fisher’s exact tests respectively for continuous and categorical baseline and surgical characteristics. Finally, we assessed for the presence and strength of monotonic rank ordering using the Jonckheere-Terpstra and Cochran-Armitage trend tests for continuous and binary dependent variables respectively, with the tumor size category regarded as an ordinal independent variable.
Results
Baseline characteristics and perioperative outcomes
The baseline characteristics are summarized in Table 1. The median patient age was 61 years (IQR, 50–71), with a male:female ratio of 1124:786. Cirrhosis was diagnosed in 31.5% of patients and it was complicated by portal hypertension in 7.5% of cases. Malignant lesions were diagnosed in 79.8% of cases with a median tumour size of 35 mm (IQR, 23–58). The median Iwate difficulty score was 5 [IQR, 4–5] corresponding to an intermediate difficulty grade in 92.3% of cases. The median operative time was 160 min (IQR, 112–215) with a median blood loss of 100 cc (IQR, 50– 200); open conversion and intraoperative blood transfusion were required in 3.1% and 3.3% of cases, respectively. Pringle manoeuvre was used in 19.5% of cases. The major morbidity rate, defined by a Clavien-Dindo severity score >2, was 2.7%, while 90-day mortality was 0.6%.
Table 1:
Comparison of baseline clinical and surgical characteristics of patients who underwent laparoscopic left lateral sectionectomy, stratified by tumor size.
| All N = 1910 |
Tumor size ≤39mm N = 1027 |
Tumor size 40- 69mm N = 528 |
Tumor size 70- 99mm N = 212 |
Tumor size ≥100mm N = 143 |
P-value (inequality between groups)† |
|
|---|---|---|---|---|---|---|
| Median age (IQR), yrs | 61 (50–71) | 62 (53–71) | 61 (49–72) | 58 (45–68) | 54 (41–69) | <0.001 |
| Male sex, n (%) | 1124 (58.8%) | 658 (64.1%) | 289 (54.7%) | 115 (54.2%) | 62 (43.4%) | <0.001 |
| Year of surgery 2004–2012 2013–2021 |
414 (21.7%) 1496 (78.3%) |
244 (23.8%) 783 (76.2%) |
97 (17.8%) 434 (82.2%) |
48 (22.6%) 164 (77.4%) |
28 (19.6%) 115 (80.4%) |
0.049 |
| Previous abdominal surgery, n/total (%) |
555/1846 (30.1%) | 317/988 (32.1%) | 141/514 (27.4%) | 55/205 (26.8%) | 42/139 (30.2%) | 0.205 |
| Concomitant minor surgery, n (%) |
70 (3.7%) | 35 (3.4%) | 22 (4.2%) | 10 (4.7%) | 3 (2.1%) | 0.543 |
| ASA score, n/total (%) 1 2 3 4 |
285/1909 (14.9%) 1176/1909 (61.6%) 440/1909 (23.1%) 8/1909 (0.4%) |
136/1027 (13.2%) 630/1027 (61.3%) 258/1027 (25.1%) 3/1027 (0.3%) |
78/528 (14.8%) 326/528 (61.7%) 122/528 (23.1%) 2/528 (0.4%) |
47/212 (22.2%) 126/212 (59.4%) 37/212 (17.5%) 2 (0.9%) |
24/142 (16.9%) 94/142 (66.2%) 23/142 (16.2%) 1/142 (0.7%) |
0.017 |
| Malignant neoplasm, n (%) | 1512 (79.2%) | 922 (89.8%) | 387 (73.3%) | 124 (58.5%) | 79 (55.2%) | <0.001 |
| Cirrhosis, n/total (%) | 602/1909 (31.5%) | 388/1026 (37.8%) | 142/528 (26.9%) | 49/212 (23.1%) | 23/143 (16.1%) | <0.001 |
| Portal hypertension, n/total (%) | 143/1900 (7.5%) | 91/1021 (8.9%) | 37/526 (7.0%) | 14/211 (6.6%) | 1/142 (0.7%) | 0.001 |
| Median tumor size, mm (IQR) |
35 (23–58) | 25 (18–30) | 50 (41–57) | 80 (70–86) | 120 (101–131) | <0.001 |
| Multiple tumors, n (%) | 317 (16.6%) | 177 (17.2%) | 89 (16.9%) | 35 (16.5%) | 16 (11.2%) | 0.335 |
| Median Iwate difficulty score, (IQR) |
5 (4–5) | 4 (4–5) | 5 (5–6) | 5 (5–6) | 5 (5–6) | <0.001 |
| Median Iwate difficulty score excluding tumor size, (IQR) |
4 (4–5) | 4 (4–4) | 4 (4–5) | 4 (4–5) | 4 (4–5) | <0.001 |
| Iwate difficulty, n (%) Low Intermediate High Expert |
131 (6.9%) 1763 (92.3%) 16 (0.8%) 0 (0.0%) |
130 (12.7%) 891 (86.8%) 6 (0.6%) 0 (0.0%) |
1 (0.2%) 519 (98.3%) 8 (1.5%) 0 (0.0%) |
0 (0.0%) 210 (99.1%) 2 (0.9%) 0 (0.0%) |
0 (0.0%) 143 (100.0%) 0 (0.0%) 0 (0.0%) |
<0.001 |
Kruskal-Wallis test for continuous variables and Fisher’s exact test for categorical variables.
IQR interquartile range, ASA physical status classification system,
Optimal tumor size cut-offs analysis
Relative risk (RR) for each outcome after correction for age, gender, year of surgery, ASA status, previous abdominal surgery, concomitant minor surgery, cirrhosis, multifocality, malignant pathology, and all components of the Iwate score excluding tumour size, was shown in Table 2.
Table 2.
Cutoff analysis for nine selected endpoints.
| Tumor size (cm) | Open conversion RR (95% CI) | Operation time (mins) MD (95% CI) | Estimated blood loss (ml) MD (95% CI) | Blood transfusion RR (95% CI) | Pringle maneuver RR (95% CI) | Post-op length of stay MD (95% CI) | Post-opcomplications RR (95% CI) | Major complications RR (95% CI) | 90-day mortality RR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| >1.0 vs ≤1.0 | 0.9 (0.25 to 3.27) | 7 (−11 to 26) | 16 (−28 to 61) | 4.58 (0.28 to 74.81) | 0.96 (0.52 to 1.76) | 0.6 (−0.3 to 1.6) | 0.84 (0.43 to 1.64) | 1.27 (0.25 to 6.57) | 0.28 (0.05 to 1.55) |
| >2.0 vs ≤2.0 | 1.33 (0.7 to 2.54) | 9 (1 to 17) | 11 (−8 to 29) | 2.1 (0.97 to 4.55) | 1.77 (1.3 to 2.4) | 0.2 (−0.2 to 0.6) | 1.27 (0.91 to 1.78) | 1.33 (0.65 to 2.71) | 2.22 (0.4 to 12.23) |
| >3.0 vs ≤3.0 | 1.98 (1.14 to 3.42) | 10 (3 to 16) | 5 (−10 to 21) | 1.84 (1.06 to 3.21) | 1.68 (1.32 to 2.13) | 0 (−0.3 to 0.4) | 1.25 (0.96 to 1.64) | 1.07 (0.61 to 1.85) | 2.14 (0.63 to 7.33) |
| >4.0 vs ≤4.0 | 2.21 (1.33 to 3.67) | 11 (4 to 18) | 7 (−8 to 23) | 2.51 (1.48 to 4.23) | 1.88 (1.49 to 2.36) | −0.1 (−0.4 to 0.3) | 1.2 (0.92 to 1.57) | 1.42 (0.82 to 2.46) | 1.52 (0.51 to 4.54) |
| >5.0 vs ≤5.0 | 2.26 (1.37 to 3.73) | 16 (8 to 23) | 15 (−2 to 31) | 2.86 (1.71 to 4.77) | 2.17 (1.71 to 2.75) | −0.2 (−0.6 to 0.2) | 1.14 (0.86 to 1.52) | 1.67 (0.96 to 2.91) | 1.28 (0.41 to 4.02) |
| >6.0 vs ≤6.0 | 2.33 (1.39 to 3.91) | 18 (10 to 27) | 17 (−1 to 36) | 2.92 (1.74 to 4.91) | 2.13 (1.65 to 2.75) | −0.2 (−0.6 to 0.2) | 1.03 (0.75 to 1.42) | 1.83 (1.02 to 3.27) | 1.94 (0.61 to 6.1) |
| >7.0 vs ≤7.0 | 2.46 (1.43 to 4.26) | 24 (14 to 33) | 30 (8 to 51) | 3.09 (1.8 to 5.3) | 2.11 (1.59 to 2.8) | 0.1 (−0.4 to 0.5) | 1.02 (0.71 to 1.46) | 2.09 (1.13 to 3.87) | 2 (0.58 to 6.86) |
| >8.0 vs ≤8.0 | 2.69 (1.49 to 4.86) | 24 (13 to 35) | 33 (8 to 57) | 3.43 (1.93 to 6.09) | 2.21 (1.61 to 3.05) | 0 (−0.5 to 0.6) | 1.05 (0.7 to 1.59) | 2.57 (1.34 to 4.93) | 1.92 (0.48 to 7.7) |
| >9.0 vs ≤9.0 | 2.44 (1.26 to 4.72) | 26 (14 to 39) | 43 (16 to 71) | 4.28 (2.38 to 7.73) | 2.33 (1.63 to 3.32) | 0.2 (−0.5 to 0.8) | 1.03 (0.65 to 1.65) | 2.41 (1.17 to 4.96) | 1.4 (0.25 to 7.71) |
| >10.0 vs ≤10.0 | 3.35 (1.63 to 6.89) | 38 (23 to 53) | 62 (25 to 98) | 4.58 (2.33 to 8.99) | 2.34 (1.5 to 3.64) | 0.1 (−0.7 to 0.9) | 0.74 (0.39 to 1.42) | 2.61 (1.12 to 6.09) | 2.36 (0.43 to 13.11) |
| >11.0 vs ≤11.0 | 2.81 (1.21 to 6.55) | 42 (25 to 59) | 56 (14 to 98) | 4.15 (1.94 to 8.91) | 1.65 (0.98 to 2.78) | 0.2 (−0.7 to 1.1) | 0.89 (0.45 to 1.79) | 3.5 (1.49 to 8.22) | 3.11 (0.56 to 17.33) |
| >12.0 vs ≤12.0 | 4.59 (1.93 to 10.89) | 34 (13 to 55) | 29 (−28 to 86) | 3.93 (1.56 to 9.92) | 1.31 (0.67 to 2.57) | 0.1 (−1 to 1.2) | 1.12 (0.51 to 2.46) | 3.62 (1.32 to 9.93) | 4.88 (0.87 to |
| >13.0 vs ≤13.0 | 4.95 (1.94 to 12.63) | 45 (21 to 69) | 38 (−24 to 100) | 5.22 (2.04 to 13.35) | 1.82 (0.9 to 3.67) | 0.4 (−0.9 to 1.6) | 1.03 (0.42 to 2.57) | 3.53 (1.14 to 10.97) | 6.31 (1.12 to 35.65) |
| >14.0 vs ≤14.0 | 4.92 (1.55 to 15.68) | 37 (7 to 68) | −22 (−107 to 63) | 5.18 (1.62 to 16.53) | 1 (0.36 to 2.83) | 0.1 (−1.5 to 1.7) | 1.06 (0.34 to 3.29) | 4.05 (1.06 to 15.39) | 10.43 (1.81 to 59.97) |
| >15.0 vs ≤15.0 | 5.15 (1.31 to 20.18) | 44 (6 to 81) | −22 (−129 to 85) | 2.99 (0.55 to 16.28) | 1.26 (0.38 to 4.19) | 0.1 (−1.9 to 2) | 0.62 (0.12 to 3.36) | 3.47 (0.63 to 18.96) | 4.57 (0.26 to 80.46) |
RR relative risk, CI confidence interval, MD median.
Effect sizes were computed by dichotomizing the tumor size at every 10mm-interval (for example, at the tumor size cutoff of 10cm, the effect size of RR 3.35 [95% CI 1.63–6.89] for the outcome of open conversion represents the risk ratio obtained when comparing the rate of open conversion among patients with tumor size >100mm versus patients with tumor size ≤100mm). Effect sizes were adjusted using inverse probability-weights from a logistic regression incorporating the following as covariates: age, gender, year of surgery, ASA status, previous abdominal surgery, concomitant minor surgery, cirrhosis, multifocality, difficult posterosuperior segment, malignant pathology, and all components of the Iwate score excluding tumor size.
Taking into consideration the number of endpoints which show a statistically significant split around the cut-points of interest and the magnitude of RR, three optimal cut-offs were identified: 40 mm, 70 mm, and 100 mm (Table 1). All the selected cut-offs showed a significant discriminative power for the prediction of open conversion, operative time, blood transfusion and need of Pringle manoeuvre. Moreover, 70 mm and 100 mm cut-offs were both discriminative also for estimated blood loss and major complications (Figure 1, Table 2).
Figure 1.
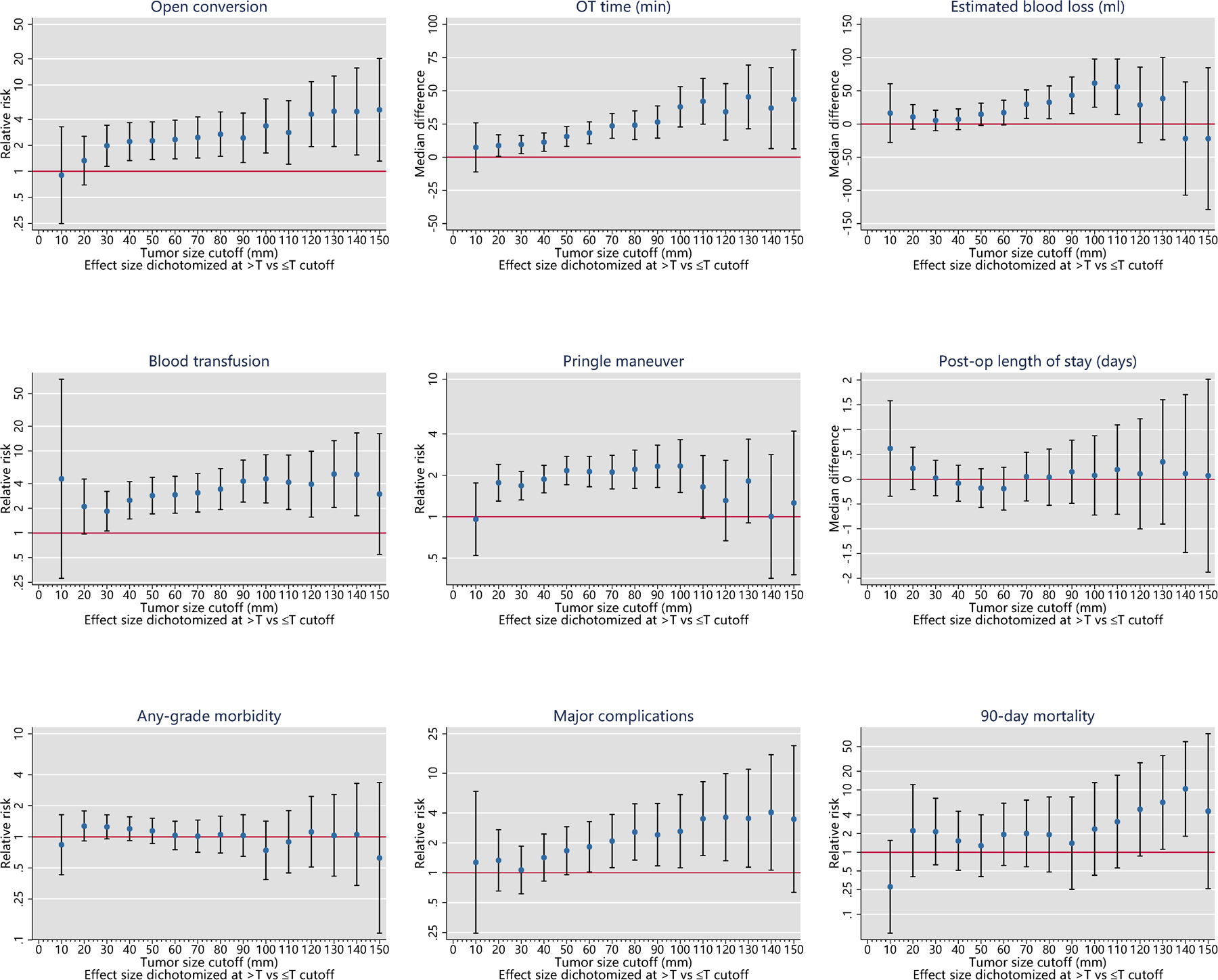
Cutoff analysis
The cut-off of 30 mm was excluded in favour of 40 mm because in equal of number of predictive variables, the significance for blood transfusion was very “weak” (lower bound of the 95% CI 1.06). ROC analyses (Supplementary Figure S1) confirmed that the 40 mm cut-off was able to maximize the Youden index. The 100 mm cut-off was selected instead of 90 mm because associated to notably larger effect sizes, even if discriminated the same perioperative outcomes. Finally, 60 mm (vs 70 mm) and 110 mm (vs 100 mm) were excluded because associated with lower number of predicted outcomes.
According to the selected cut-offs, four study groups were identified: small ≤39mm (n= 1027), intermediate 40–69mm (n=528), large 70–99mm (n=212) and very large ≥100 (n=143) lesions. The 4-level classification system thus established was able to increase the AUC for both open conversion (from 0.59 to 0.62) and application of pringle manoeuvre (from 0.59 to 0.61) compared to 40 mm cut-off alone (Supplementary Figure S1).
Comparison of perioperative characteristics stratified by tumour size (Table 1, 3)
Table 3:
Comparison of perioperative outcomes of patients who underwent laparoscopic left lateral sectionectomy, stratified by tumor size
| All N = 1910 |
Tumor size ≤39mm N = 1027 |
Tumor size 40– 69mm N = 528 |
Tumor size 70– 99mm N = 212 |
Tumor size ≥100mm N = 143 |
Z-statistic & P-value for monotonic trend† |
|
|---|---|---|---|---|---|---|
| Open conversion, n (%) | 63 (3.3%) | 22 (2.1)% | 18 (3.4%) | 13 (6.1%) | 10 (7.0%) |
Z=3.90;
P<0.001 |
| Median operating time (IQR), min | 160 (112–215) | 155 (105–215) | 157 (112–210) | 180 (120–220) | 180 (126–235) |
Z=3.84,
P<0.001 |
| Median blood loss (IQR), ml | 100 (50–200) | 50 (30–153) | 100 (50–200) | 100 (50–250) | 200 (50–300) |
Z=6.50;
P<0.001 |
| Intraoperative blood transfusion, n (%) | 60 (3.1%) | 19 (1.9%) | 16 (3.0%) | 11 (5.2%) | 14 (9.8%) |
Z=5.15;
P<0.001 |
| Pringle maneuver applied, n (%) | 365/1868 (19.5%) | 157/1011 (15.5%) | 99/514 (19.3%) | 60 (28.8%) | 48/135 (35.6%) |
Z=6.48;
P<0.001 |
| Median postoperative stay (IQR), days | 5 (3–6) | 5 (4–6) | 5 (3–6) | 5 (3–6) | 5 (3–6) | Z=0.84; P=0.399 |
| Overall morbidity, n (%) | 255/1909 (13.4%) | 125/1026 (12.2%) | 80/528 (15.2%) | 32/212 (15.1%) | 18/143 (12.6%) | Z=0.97; P=0.330 |
| Major morbidity (Clavien-Dindo grade>2) | 52/1909 (2.7%) | 24/1026 (2.3%) | 12/528 (2.3%) | 8/212 (3.8%) | 8/143 (5.6%) |
Z=2.17;
P=0.030 |
| 30-day readmission, n (%) | 44/1901 (2.3%) | 17/1021 (1.7%) | 15/527 (2.8%) | 7/211 (3.3%) | 5/142 (3.5%) |
Z=1.99;
P=0.047 |
| 30-day mortality, n (%) | 7 (0.4%) | 2 (0.2%) | 3 (0.6%) | 1 (0.5%) | 1 (0.7%) | Z=1.19; P=0.233 |
| 90-day mortality, n (%) | 12 (0.6%) | 4 (0.4%) | 4 (0.8%) | 3 (1.4%) | 1 (0.7%) | Z=1.34; P=0.180 |
| Close margins, n (%) | 110/1902 (5.8%) | 54/1020 (5.3%) | 44/528 (8.3%) | 6/212 (2.8%) | 6/142 (4.2%) | Z=−0.57; P=0.571 |
Two-sided Cochran-Armitage or Jonckheere-Terpstra tests were used to evaluate the presence of a monotonic increasing or decreasing trend over the four tumor size categories, which was treated as an ordinal variable (i.e., Group 1: <40mm, Group 2: 40–69mm, Group 3: 70–99mm, Group 4: ≥100mm).
IQR interquartile range
Comparison of preoperative characteristics of patients who underwent L-LLS stratified by tumour size showed that patients with very large lesions (≥100 mm) were younger (median age 54 years) with benign lesions in slightly less than 50% of cases. The diagnosis of cirrhosis and portal hypertension were less common (55.2% and 16.1%, respectively) compared to others cut-off groups.
The comparison of intraoperative outcomes between the groups showed a stepwise increase in rates of open conversion rate (Z=3.90, p<0.001), operative time (Z=3.84, p<0.001), blood loss (Z=6.50, p<0.001), intraoperative blood transfusion rate (Z=5.15, p<0.001) and Pringle manoeuvre use (Z=6.48, p<0.001) as the tumor size category increased.
A significant worsening trend was noted for Clavien-Dindo score>2 (Z=2.17, p=0.030) and 30-days readmission (Z=1.99, p=0.047) increasing the tumor size categories. Thirty and 90-days mortality rates did not significantly change among the study groups.
Discussion
Since the early experience of LLR, tumour size has been well-recognized as a critical parameter in the assessment of the difficulty of LLR. In the Louisville Statement, it was suggested that LLR should be restricted to lesions < 50 mm. [12]. However, with the accumulation of clinical evidence on the feasibility and safety of the laparoscopic approach for resection of larger tumours, this has culminated in the recent Southampton Guidelines which excluded tumour size as an independent exclusion criterion for LLR [13]. Recent studies have also confirmed the feasibility and safety of LLR for huge (≥ 10 cm) tumors [7, 14].
Today, L-LLS is one of the most frequently performed types of LLR and it has been proposed to be the standard of care for lesions located in the left lateral section [12,13,15–17]. This is due to the favourable anatomical morphology and topography of the left lateral section [18]. Nonetheless, unexpected difficulties may still be encountered during L-LLS and a small proportion of cases may undergo an unplanned open conversion even in expert centers [18]. Not unexpectedly, tumour size has also been shown to be an important predictor of open conversion during L-LLS [18].
We performed this study with the primary objective of examining the relationship between tumor size and difficulty of L-LLS, and to elucidate the optimal tumor size cut-off for stratifying the difficulty of this procedure. Presently, commonly used DSS such as the Iwate Criteria [2] and Southampton score [3] have incorporated tumour size in the calculation of the difficulty score. A size cut-off of 3 cm was used for the Iwate criteria whereas the Southampton score utilized a cut-off of 3 and 5 cm. More recently, Ivanecz et al proposed a tumor size cut-off of 38 mm based on a small series of 142 LLR [19]. Subsequently, Kabir et al performed a robust statistical analysis based on 461 LLR and determined that the optimal size cut-off was 3 and 7 cm [4]. A major limitation of these studies was that the proposed size cut-offs was not tailored to the extent of the LLR performed. Moreover, many of the cut-off values were arbitrarily chosen and not based on robust statistical analysis.
To the best of our knowledge, this is the largest study to date to analyse the correlation between tumour size and the outcomes for a specific type of LLR. In the present study, we identified three cutoffs (40 mm, 70 mm and 100 mm) which consistently discriminated major intraoperative outcomes (open conversion rate, operative time, blood loss and transfusion, Pringle maneuver use) as well as postoperative major complications after L-LLS. These findings suggest that increasing tumor size correlated with higher difficulty of L-LLS. Notably, increased frequency of the use of the Pringle Maneuver may not necessarily be a detrimental outcome but may be a surrogate of surgical difficulty.
Previously, Yang et al. tested a tumor size cutoff of 50 mm specifically in a small series of 103 L-LLS, but found no significant discriminative value for perioperative complications. apart from intraoperative blood loss [20]. An important clinical application of our current findings is that it would help guide surgeons embarking on LLR in selecting L-LLS procedures appropriate to their level of experience based on tumor size. Furthermore, these findings should be taken into account when formulating new DSSs in future to enable more accurate discrimination of the complexity of L-LLS and better comparison between outcomes of LLR during surgical audits.
The main limitation of this study is due to its retrospective nature although many centers had a prospective database. Furthermore, as an international multicenter study, there would be heterogeneity in the surgical technique and perioperative management of L-LLS between the different centers. It is also important to note that tumor size in this study was measured based on postoperative pathological specimens which may vary from measurements made based on preoperative imaging. Nonetheless, its main strength was the large number of patients analysed from an international database which allowed robust statistical analysis and providing a wide generalizable experience reflective of contemporary real-world practice.
Conclusions
In this study, we found that L-LLS for tumours of increasing size was associated with poorer intraoperative and early postoperative outcomes suggesting increasing difficulty of the procedure. We determined 3 optimal tumour size cutoffs (40 mm, 70 mm and 100 mm) which accurately stratified the surgical difficulty of L-LLS.
Supplementary Material
Funding
Dr T. P. Kingham was partially supported by the US National Cancer Institute MSKCC Core Grant number P30 CA008748 for this study
Conflicts of interest
Dr Goh BK has received travel grants and honorarium from Johnson and Johnson, Olympus and Transmedic the local distributor for the Da Vinci Robot.
Dr Marino MV is a consultant for CAVA robotics LLC.
Johann Pratschke reports a research grant from Intuitive Surgical Deutschland GmbH and personal fees or non-financial support from Johnson & Johnson, Medtronic, AFS Medical, Astellas, CHG Meridian, Chiesi, Falk Foundation, La Fource Group, Merck, Neovii, NOGGO, pharma- consult Peterson, and Promedicis.
Moritz Schmelzle reports personal fees or other support outside of the submitted work from Merck, Bayer, ERBE, Amgen, Johnson & Johnson, Takeda, Olympus, Medtronic, Intuitive.
Asmund Fretland reports receiving speaker fees from Bayer.
Fernando Rotellar reports speaker fees and support outside the submitted work from Integra, Medtronic, Olympus, Corza, Sirtex and Johnson & Johnson.
References
- [1].Wakabayashi G, Cherqui D, Geller DA et al. ; Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg. 2015. Apr;261(4):619–29. [DOI] [PubMed] [Google Scholar]
- [2].Wakabayashi G What has changed after the Morioka consensus conference 2014 on laparoscopic liver resection? Hepatobiliary Surg Nutr. Aug 2016;5(4):281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Halls MC, Berardi G, Cipriani F et al. ; Development and validation of a difficulty score to pre- dict intraoperative complications during laparoscopic liver resection. Br J Surg. 2018. Aug;105(9):1182–1191. [DOI] [PubMed] [Google Scholar]
- [4].Kabir T, Syn N, Koh YX, Teo JY, Chung AY, Chan CY, Goh BKP. Impact of tumor size on the difficulty of minimally invasive liver resection. Eur J Surg Oncol. 2022. Jan;48(1):169–176. [DOI] [PubMed] [Google Scholar]
- [5].Russolillo N, Casella M, Langella S, Lo Tesoriere R, Ossola P, Ferrero A. Correlation between anthropometric data and preparatory maneuvers difficulties during laparoscopic right liver resec- tions: a single center prospective study. Surg Endosc. 2022. Feb 24. [DOI] [PubMed] [Google Scholar]
- [6].Goh BK, Prieto M, Syn N, et al. Validation and comparison of the Iwate, IMM, Southampton and Hasegawa difficulty scoring systems for primary laparoscopic hepatectomies. HPB 2021;23:770–76. [DOI] [PubMed] [Google Scholar]
- [7].Cheung TT, Wang X, Efanov M et al. ; International Robotic and Laparoscopic Liver Resection Study Group Collaborators. Minimally invasive liver resection for huge (≥10 cm) tumors: an interna-tional multicenter matched cohort study with regression discontinuity analyses. Hepatobiliary Surg Nutr. 2021. Oct;10(5):587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Goh BK, Kabir T, Syn N. RE: New simple three-level liver resection classification without compromising the performance to predict surgical and postoperative outcomes. Eur J Surg Oncol 2022;46:303–4. [DOI] [PubMed] [Google Scholar]
- [9].Hasegawa Y, Nitta H, Sasaki A, Takahara T, Ito N, Fujita T, Kanno S, Nishizuka S, Waka-bayashi G. Laparoscopic left lateral sectionectomy as a training procedure for surgeons learning lap-aroscopic hepatectomy. J Hepatobiliary Pancreat Sci. 2013. Jun;20(5):525–30. [DOI] [PubMed] [Google Scholar]
- [10].Strasberg SM. Nomenclature of hepatic anatomy and resections: a review of the Brisbane 2000 system. J Hepatobiliary Pancreat Surg. 2005;12(5):351–5. [DOI] [PubMed] [Google Scholar]
- [11].Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004. Aug;240(2):205–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Buell JF, Cherqui D, Geller DA et al. ; World Consensus Conference on Laparoscopic Surgery. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann Surg. 2009. Nov;250(5):825–30. [DOI] [PubMed] [Google Scholar]
- [13].Abu Hilal M, Aldrighetti L, Dagher I et al. : The Southampton Consensus Guidelines for Laparoscopic Liver Surgery: From Indication to Implementation. Ann Surg. 2018. Jul;268(1):11–18. [DOI] [PubMed] [Google Scholar]
- [14].Kabir T, Syn NL, Guo Y, Lim KI, Goh BKP. Laparoscopic liver resection for huge (≥10 cm) hepatocellular carcinoma: A coarsened exact-matched single-surgeon study. Surg Oncol. 2021. Jun;37:101569. [DOI] [PubMed] [Google Scholar]
- [15].Goh BKP, Lee SY, Koh YX, Kam JH, Chan CY. Minimally invasive major hepatectomies: a Southeast Asian single institution contemporary experience with its first 120 consecutive cases. ANZ J Surg. 2020. Apr;90(4):553–557. [DOI] [PubMed] [Google Scholar]
- [16].Goh BKP, Lee SY, Teo JY et al. ; Changing trends and outcomes associated with the adoption of minimally invasive hepatectomy: a contemporary single- institution experience with 400 consecutive resections. Surg Endosc. 2018. Nov;32(11):4658–4665. [DOI] [PubMed] [Google Scholar]
- [17].Kawaguchi Y, Hasegawa K, Wakabayashi G et al. ; Survey results on daily practice in open and laparoscopic liver resections from 27 centers participating in the second International Consensus Con-ference. J Hepatobiliary Pancreat Sci. 2016. May;23(5):283–8. [DOI] [PubMed] [Google Scholar]
- [18].Wang HP, Yong CC, Wu AG, et al. Factors associated with an impact of open conversion on the outcomes of minimally- invasive left lateral sectionectomies: an international multicenter study. Surgery 2022. in press [DOI] [PubMed] [Google Scholar]
- [19].Ivanecz A, Plahuta I, Magdalenic T, et al. Evaluation of the Iwate model for predicting the difficulty of laparoscopic liver resection: does tumor size matter? J Gastrointest Surg 2021;25:1451–60. [DOI] [PubMed] [Google Scholar]
- [20].Yang TH, Chen JL, Lin YJ et al. ; Laparoscopic surgery for large left lateral liver tumors: safety and oncologic outcomes. Surg Endosc. 2018. Oct;32(10):4314–4320. doi: 10.1007/s00464-018-6287-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


