Abstract
Introduction
Cognition refers to brain functions including memory, learning, and thought processing and is increasingly important to individuals. However, impairment of cognitive function is a concern among North American adults. Therefore, effective and reliable treatments are needed.
Methods
This randomized, double-blind, placebo-controlled study examined the effects of 42 days of Neuriva® supplementation, a whole coffee cherry extract and phosphatidylserine supplement, on memory, accuracy, focus and concentration and learning among 138 healthy adults (40–65 years) with self-reported memory problems. Plasma brain-derived neurotrophic factor (BDNF) levels, Computerized Mental Performance Assessment System (COMPASS) tasks, the Everyday Memory Questionnaire (EMQ), and Go/No-Go tests were assessed at baseline and day 42.
Results
As compared to placebo, Neuriva® supplementation elicited greater improvements at day 42 in numeric working memory COMPASS task accuracy outcomes (p ≤ 0.024) which assessed memory, accuracy, and focus and concentration, and reaction time outcomes (p ≤ 0.031) which assessed memory as well as focus and concentration. Neuriva® supplementation improved overall accuracy (p = 0.035) in the picture recognition task that assessed memory, accuracy, and learning compared to placebo. No significant differences between groups were observed for BDNF, the EMQ, or Go/No-Go tests.
Conclusion
Results suggest 42 days of Neuriva® supplementation was safe, well tolerated, and beneficial in improving memory, accuracy, focus and concentration, and learning in a healthy adult population with self-reported memory problems.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40120-023-00454-z.
Keywords: Cognition, Dietary supplement, Memory, Nutraceutical
Key Summary Points
| Why carry out this study? |
| Improving and maintaining cognition are increasingly important because of the aging population, increase in life expectancy, and impact of cognitive decline on quality of life |
| Effective and reliable options to support cognition are warranted |
| This study investigated the effects of Neuriva®, a supplement containing whole coffee cherry extract and phosphatidylserine, on cognitive performance in healthy adults with self-reported memory problems |
| What was learned from the study? |
| Neuriva® supplementation was safe well tolerated, and significantly improved measures of memory, accuracy, focus and concentration, and learning compared to placebo in a healthy adult population with self-reported memory problems |
| Future larger studies of longer duration are needed to confirm the current study’s findings |
Introduction
Improving and maintaining brain health and cognition, including memory, learning, and thought processing [1], are increasingly important among individuals and public health agencies because of the aging population, increase in life expectancy, and impact of cognitive decline on quality of life [2–4]. A recent systematic review showed a range in North American prevalence of cognitive impairment of 7.1–28.3% among community-dwelling adults aged 50 years or older [5]. An important brain protein that stimulates neurogenesis and is essential for neuronal plasticity, central nervous system development [6], and supports overall cognitive function [7] is brain-derived neurotrophic factor (BDNF). Aging may be associated with a decline in BDNF levels, and this decline may be one of the many factors contributing to cognitive impairment [8].
Currently, interventions to manage cognitive decline include pharmacological agents, cognitive training, and physical exercises [9]. However, there are limitations to their use or inconsistencies in study findings [10–18]. The brain health supplement market size was valued at US $7.68 billion in 2021 and projections indicate a compound annual growth rate of 8.3% from 2022 to 2030 [3] as individuals are increasingly interested in brain health supplements. Therefore, efficacious natural treatment options are warranted to support and improve cognition with aging.
Whole coffee cherry extract (WCCE) is rich in polyphenols which may contribute to its potential to improve cognition [19, 20]. A WCCE-containing beverage was reported to improve cognition related to alertness and attenuate fatigue after an acute dose in healthy adults [21]. Moreover, a post hoc analysis revealed significant reductions in reaction time after 7 and 28 days of WCCE supplementation, compared to placebo, in older adults with mild cognitive impairment who were otherwise healthy [22]. Reaction time was also significantly improved from baseline after acute supplementation with WCCE in adults with memory complaints [23]. Cotter et al. [24] summarized findings of studies related to WCCE and BDNF, which included two randomized, placebo-controlled trials that showed an increase in BDNF levels compared to placebo [25] and baseline values [23].
Phosphatidylserine (PS) is an important phospholipid in the brain that is needed for healthy nerve cell membranes and myelination and plays an important role in signaling pathways [26, 27]. PS comprises 13–15% of total phospholipids in the cerebral cortex [27]; however, physiological changes associated with aging result in a reduction of PS [26]. Previous research in animal models [28] and humans [29–31] have shown beneficial effects of PS supplementation on cognitive function.
While WCCE and PS supplementation have independently been found to improve cognition, there may be differences in their potential mechanisms. Therefore, research investigating the efficacy of these active ingredients in combination is warranted. The objective of this study was to investigate the efficacy of a combination of WCCE and PS on cognitive performance in healthy adults with self-reported memory problems.
Methods
Study Design and Approvals
This was a randomized, double-blind, placebo-controlled, parallel study conducted at KGK Science Inc. in London, ON, Canada from February 14, 2020 to May 15, 2021. Participants consumed either Neuriva® or placebo for 42 days and visited the clinic at screening, baseline (day 0), and day 42.
The study was approved by the Natural and Non-Prescription Health Products Directorate, Health Canada, Ottawa, Ontario on December 18, 2019. Research ethics board approval was granted on December 24, 2019, from the Institutional Review Board Services, Aurora, Ontario (Pro00040971). The study was conducted in compliance with the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) Guideline for Good Clinical Practice (GCP) and in accordance with the Declaration of Helsinki guidelines and its subsequent amendments. The trial followed the Consolidated Standards of Reporting Trials (CONSORT) guidelines for randomized controlled trials [32] (Supplementary Material Table S1). Written informed consent was obtained from all participants prior to any study procedures being initiated.
Participants
Eligible participants were 40–65 years old, had a body mass index (BMI) between 18.5 and 34.9 kg/m2, self-reported memory problems as assessed by questions 1, 2, and 18 of the Everyday Memory Questionnaire (EMQ; a score sum of ≥ 6 was deemed eligible as it indicated memory problems were experienced once or twice a week or they had a deficit in one question area), and did not have dementia or other significant cognitive impairments as assessed by the Mini Mental State Exam-2 (MMSE-2) Standard Version (score ≥ 24). Exclusion criteria were women who were pregnant, breastfeeding, or planning to become pregnant during the trial, allergy to the study products, self-reported confirmation of neurophysiological condition and/or cognitive impairment that could have interfered with participation, medical or chronic use of cannabinoid products, tobacco use, alcohol intake of more than two standard drinks per day or more than 10 standard drinks per week, alcohol or drug abuse within the last 12 months, any unstable chronic disease or condition, or any prescribed or over-the-counter medications or supplements that could have interfered with the study outcomes or participant safety.
Participants were instructed to maintain their lifestyle habits including physical activity and diet as well as medications and supplement routines which were monitored through use of a daily study diary.
Investigational Product and Placebo
The investigational product, Neuriva®, contained 100 mg of WCCE and 100 mg of PS. Excipients included microcrystalline cellulose, silica, water, ascorbyl palmitate, mixed tocopherols, titanium dioxide, pectin, soy lecithin, rice bran, carrageenan, and hypromellose. The placebo comprised all the investigational product’s excipient ingredients.
Participants were instructed to consume one serving of Neuriva® or placebo daily for 42 days in the morning with water, with or without food, and record consumption in their study diary. If a dose was missed, participants were instructed to consume the dose as soon as possible and advised not to exceed one serving per day.
Randomization and Blinding
Participants were assigned a randomization number according to the order of the randomization list (www.randomization.com) and randomized at baseline to each study arm in a 1:1 ratio by a blinded investigator. A randomization schedule was created and provided to the investigator indicating the order of randomization.
The Neuriva® and placebo capsules, and the bottles they were sealed in, were identical in appearance. The bottles were labelled according to the requirements of the ICH-GCP guidelines and applicable local regulatory guidelines by unblinded personnel who were not involved in any study assessments. Investigators, other site personnel, statistician, and participants were blinded to the product.
Compliance
Participants were instructed to record daily study product consumption and return all unused capsules for compliance assessment. Compliance was calculated by dividing the total number of capsules consumed by the total number of capsules expected to be consumed multiplied by 100. In the event of discrepancy between the information in the study diary and the number of capsules returned, compliance was based on the product returned unless an explanation for loss of product was provided.
Outcomes
The objective of this study was to investigate the efficacy of Neuriva® on cognitive performance in healthy adults with self-perceived memory problems. To address the study objective, two co-primary outcomes were identified including the difference in the change from baseline to day 42 in (1) plasma BDNF and (2) memory, accuracy, focus and concentration, and learning as assessed by the Computerized Mental Performance Assessment System (COMPASS). Secondary outcomes included the difference in the change in (1) everyday memory as assessed by the EMQ and (2) sustained attention using the Go/No-Go assessment. Safety outcomes included incidence of adverse events (AEs), vital signs, clinical chemistry, and hematology.
Laboratory Analysis
Blood samples were collected by a qualified phlebotomist at baseline and day 42 to assess the change in plasma BDNF and safety outcomes. Plasma BDNF was analyzed using the Milliplex MAP Human Myokine Magnetic Bead Panel (HMYOMAG-56K, Millipore) by Mount Sinai Services Laboratory (Toronto, ON, Canada). Safety outcomes of clinical chemistry (alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin, creatinine, electrolytes (sodium, potassium, chloride), and estimated glomerular filtration rate) and hematology (white blood cell count with differential (neutrophils, lymphocytes, monocytes, eosinophils, basophils), red blood cell count, hemoglobin, hematocrit, platelet count, and red blood cell indices, namely mean platelet volume, mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, red blood cell distribution width) were analyzed by Dynacare (London, ON, Canada) using standardized procedures.
COMPASS
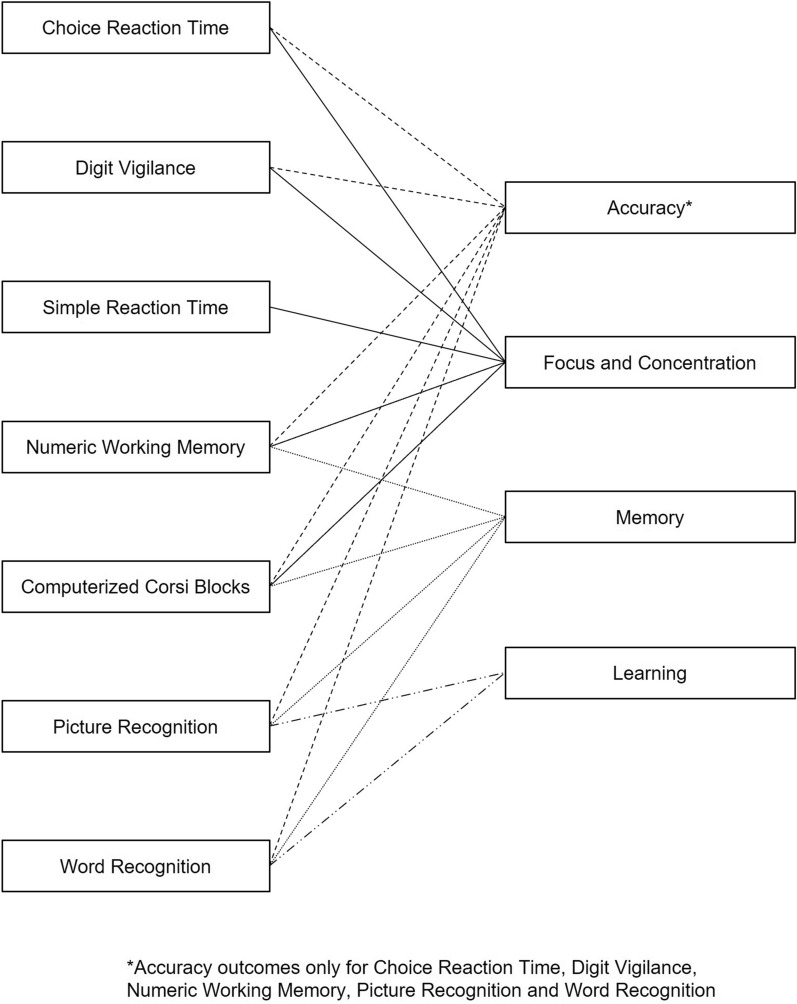
COMPASS (Northumbria University, Newcastle-upon-Tyne, UK) was used to assess cognition including memory, accuracy, focus and concentration, and learning. This tool was selected for cognitive assessment as it has been shown to be sensitive to nutritional interventions [33, 34]. COMPASS is a pre-programmed cognitive testing system that delivers randomized stimuli for every assessment performed by each participant. COMPASS tasks used in the current study included choice reaction time, digit vigilance, simple reaction time, numeric working memory, computerized Corsi blocks, picture recognition, and word recognition. A combination of COMPASS tasks and corresponding outcomes assessed the different cognitive domains (Table 1). The individual COMPASS tasks in relation to the cognitive domains assessed are mapped in Fig. 1 [35–37]. Participants received instructions from a clinic coordinator on how to perform the cognitive tasks at baseline and day 42.
Table 1.
COMPASS tasks, descriptions, and outcomes with corresponding cognitive domains
| COMPASS task | Description | Outcomes | Cognitive domains assessed |
|---|---|---|---|
| Choice reaction time | Participants were required to indicate whether a left- or right-pointing arrow appeared on the screen as quickly as possible after it appeared |
Overall accuracy (%) Correct reaction time (ms) |
Accuracy (accuracy outcomes only) Focus and concentration |
| Digit vigilance | On the right side of the screen a number appeared while on the left side of the screen a series of numbers were displayed and when the left side number matched the right side, participants were required to respond |
Overall accuracy (%) False alarms (count) Correct reaction time (ms) |
Accuracy (accuracy outcomes only) Focus and concentration |
| Simple reaction time | As soon as an arrow pointing up appeared on the screen, participants were required to respond as quickly as possible | Overall reaction time (ms) | Focus and concentration |
| Numeric working memory | Numbers were displayed on the screen one at a time and participants were required to memorize the numbers. Following this, numbers appeared one at a time on the screen and participants were required to indicate whether or not each number appeared in the previous set |
Overall accuracy (%) “No” accuracy (%) “Yes” accuracy (%) Correct reaction time (ms) “No” reaction time (ms) “Yes” reaction time (ms) |
Memory Accuracy (accuracy outcomes only) Focus and concentration |
| Computerized Corsi blocks | Nine blue squares were displayed on the screen. Some squares turned red then back to blue. Participants were required to memorize the sequence of red squares. This task was repeated five times at each level with increasing levels of difficulty until the participant could not recall the sequence | Span score |
Memory Accuracy Focus and concentration |
| Picture recognition | A series of pictures were displayed on the screen. Participants were required to memorize the pictures. Following this, those pictures plus decoys were displayed on the screen one at a time and the participant was required to indicate whether each picture was displayed previously |
Overall accuracy (%) “No” accuracy (%) “Yes” accuracy (%) Overall reaction time (ms) Correct reaction time (ms) “No” reaction time (ms) “Yes” reaction time (ms) |
Memory Accuracy (accuracy outcomes only) Learning |
| Word recognition | A series of words were displayed on the screen. Participants were required to memorize the words. Following this, those words plus decoys were displayed on the screen one at a time and the participant was required to indicate whether each word was displayed previously |
Overall accuracy (%) “No” accuracy (%) “Yes” accuracy (%) Overall reaction time (ms) Correct reaction time (ms) “No” reaction time (ms) “Yes” reaction time (ms) |
Memory Accuracy (accuracy outcomes only) Learning |
Fig. 1.
Mapping of COMPASS tasks to cognitive domains
Go/No-Go Assessment
The Go/No-Go task, part of the Test of Attentional Performance, is a computerized test that assesses sustained attention and inhibitory control, an executive cognitive process, that allows the participant to stop motor activity even after quick initiation [38]. Participants had to react using simple keypresses to discriminable non-verbal stimuli. The response accuracy of each No-Go trial was used as a measure for inhibitory control [39]. Both forms, “1 of 2” and “2 of 5” were used and involved participants responding as quick as possible to one correct stimuli among two and two correct stimuli among five, respectively, that appeared on a screen in varying sequence. These forms were completed by participants at baseline and day 42.
EMQ
The EMQ is a subjective measure of memory failure in everyday life [40]. Questions are grouped into five categories including speech, reading and writing, faces and places, action, and learning new things. Participants completed this questionnaire at baseline and day 42.
Adverse Events
Participants were instructed to record any AEs in their study diary which were reviewed at each study visit. AEs were classified on the basis of the description, duration, intensity, frequency, and outcome and were assessed for causality and intensity by the qualified investigator (QI). The Medical Dictionary for Regulatory Activities terminology version 23.0 was used for coding.
Statistical Analysis
A sample size calculation was completed for each co-primary outcome. The larger of the two sample sizes computed was used to ensure the study was sufficiently powered for each co-primary outcome. On the basis of previous studies [25, 29], a total of 138 participants (n = 69 per group) were required to enable detection of a difference in mean change in memory score of 5.64 and mean difference of 76% in BDNF between Neuriva® and placebo groups with a 5% significance level, 80% power, and accounting for a 20% attrition rate.
Baseline demographic summary statistics were computed and presented as mean ± standard deviation (SD) for quantitative variables and frequency with percentage for qualitative variables. Baseline variables were compared between groups using the t test and the test of independence. All data were evaluated for normality using the Shapiro–Wilk’s test. The change in plasma BDNF, EMQ scores, and Go/No-Go scores, from baseline at day 42, were compared between groups using Wilcoxon’s rank sum test. Change in COMPASS scores were compared between groups using a two-sample t test. Changes from baseline at day 42 were evaluated using a paired t test for all measures except plasma BDNF which was evaluated using Wilcoxon’s signed-rank test.
The intention-to-treat (ITT) population included all participants who received either study product, and had post-randomization efficacy data available. The per protocol (PP) population included all participants who consumed at least 80% of either study product, did not have any major protocol violations, and completed all study visits and procedures associated with both co-primary outcomes. Results presented are for the PP population unless otherwise stated. All statistical analyses were completed using R Statistical Software Package Version 3.5.2 or newer for Microsoft Windows [41] with p < 0.05 considered statistically significant.
Results
Study Population
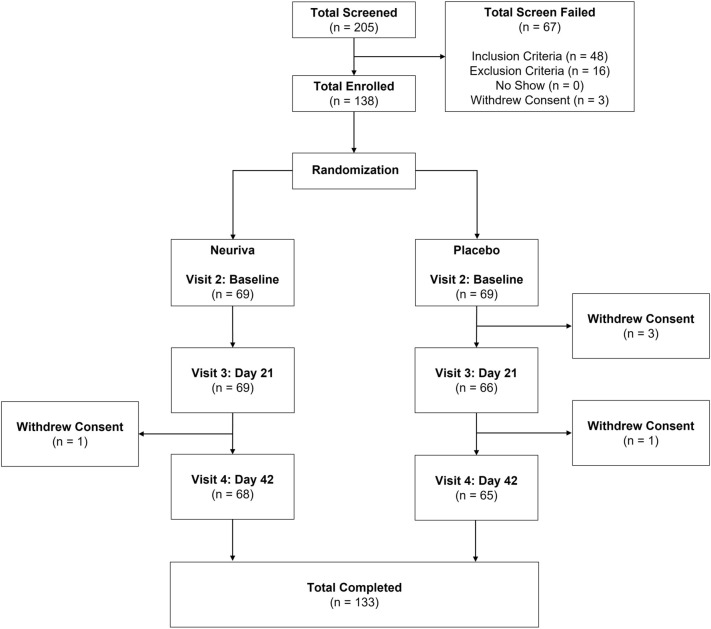
A total of 205 individuals were screened and 138 were enrolled in the study (Fig. 2). There were 138 participants included in the ITT population and 128 in the PP population (Table 2). Five participants in the Neuriva® group were excluded from the PP population because of early termination (n = 1) and major protocol deviations (n = 4). Five participants in the placebo group were excluded from the PP population because of early termination (n = 4) and less than 80% compliance (n = 1). Participant treatment compliance rates in the Neuriva® and placebo groups for the PP population were 99.7% ± 3.35% and 100.5% ± 2.62% (mean ± SD), respectively. In the PP population, six participants in the Neuriva® group and one participant in the placebo group reported they quit smoking within the last 10 years.
Fig. 2.
Disposition of study participants
Table 2.
Baseline demographic characteristics for the PP population (n = 128)
| Characteristic | Neuriva® (n = 64) |
Placebo (n = 64) |
P value |
|---|---|---|---|
| Age, mean ± SD | 53.92 ± 6.08 | 54.66 ± 6.95 | 0.53a |
| Gender, n (%) | |||
| Female | 47 (73.4) | 39 (61.0) | 0.21b |
| Male | 17 (26.6) | 25 (39.0) | |
| Alcohol use, n (%) | |||
| None | 9 (14.0) | 19 (30.0) | |
| Daily | 2 (3.0) | 0 (0.0) | 0.19b |
| Weekly | 32 (50.0) | 28 (43.0) | |
| Occasionally | 21 (33.0) | 17 (27.0) | |
| Tobacco use, n (%) | |||
| Ex-smoker | 14 (22.0) | 5 (8.0) | 0.020b |
| No | 50 (78.0) | 59 (92.0) | |
| Recreational cannabis use, n (%) | |||
| No | 60 (94.0) | 63 (98.0) | 0.15b |
| Yes | 4 (6.0) | 1 (2.0) | |
n number, SD standard deviation
aP value generated by the t test
bP values generated using the test of independence on untransformed data. p < 0.05 considered statistically significant
Plasma BDNF
There was no significant difference in plasma BDNF between Neuriva® and placebo groups at day 42. There was a significant decrease in plasma BDNF concentration from baseline at day 42 in the placebo group (− 427.7 ± 1937.8 pg/mL; p = 0.041). No significant changes in BDNF were observed with Neuriva® supplementation (− 480.7 ± 2296.0 pg/mL; p = 0.306).
COMPASS
Various combinations of COMPASS tasks and corresponding outcomes assessed cognitive domains (memory, accuracy, focus and concentration, learning) (Table 1, Fig. 1).
Memory
There were 5/21 significant differences between Neuriva® and placebo groups across the numeric working memory and picture recognition tasks. Participants supplemented with Neuriva® had significantly greater improvements in overall accuracy (p = 0.024), “yes” accuracy (p = 0.01), reaction time for correct responses (p = 0.031), and “yes” reaction time (p = 0.016) when tasked with memorizing numbers from a previous list compared to those on placebo (Fig. 3; Supplementary Material Table S2). The Neuriva® group also had a significantly greater improvement (p = 0.035) in overall accuracy in identifying whether they had seen a picture in the previous display from baseline at day 42 (Fig. 4; Supplementary Material Table S2).
Fig. 3.
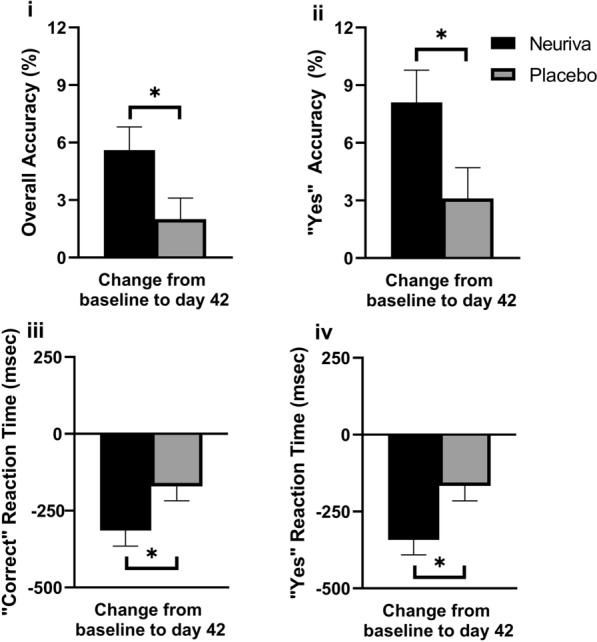
Numeric working memory change in scores for (i) overall accuracy (%; p = 0.024), (ii) “yes” accuracy (%; p = 0.01), (iii) reaction time for correct responses (ms; p = 0.031), and (iv) “yes” reaction time (ms; p = 0.016) from baseline at day 42 for Neuriva® and placebo in the PP population (n = 128). Memory, accuracy, focus and concentration were assessed by (i) and (ii), and memory and focus and concentration were assessed by (iii) and (iv). All values presented are mean ± SEM. Between group differences were compared using a two-sample t test with p < 0.05 considered statistically significant. Asterisk indicates a statistically significant difference between Neuriva® and placebo
Fig. 4.
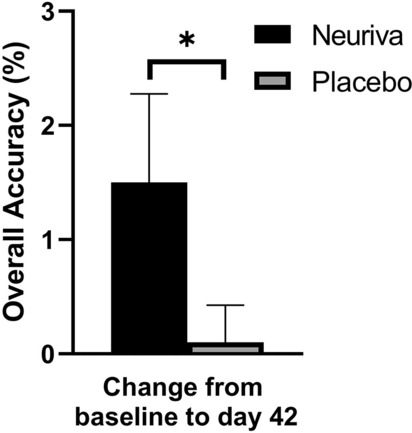
Picture recognition change in scores for overall accuracy (%; p = 0.035) from baseline at day 42 for Neuriva® and placebo in the PP population (n = 128). Memory, accuracy, and learning were assessed by overall accuracy by the picture recognition task. All values presented are mean ± SEM. Between group changes were compared using a two-sample t test with p < 0.05 considered statistically significant. Asterisk indicates a statistically significant difference between Neuriva® and placebo
The Neuriva® and placebo groups had 17/21 (p ≤ 0.033) and 11/21 (p ≤ 0.009), respectively, changes in COMPASS scores from baseline at day 42 (Table 3). There were 11 outcomes wherein both groups had significant improvements from baseline at day 42, with those supplemented with Neuriva® having reported greater score improvements than placebo for six of these outcomes.
Table 3.
Change in COMPASS scores from baseline at day 42 for memory, accuracy, focus and concentration, and learning for Neuriva® and placebo in the PP population (n = 128)
| Task | Neuriva® (n = 64) Mean ± SD Within-group P value |
Placebo (n = 64) Mean ± SD Within-group P value |
|---|---|---|
| Choice reaction time (focus and concentration) | ||
| Reaction time: correct (ms) |
− 388.8 ± 591.1 < 0.001 |
− 382.5 ± 584.0 < 0.001 |
| Digit vigilance (accuracy, focus and concentration) | ||
| Accuracy: overall (%) |
7.0 ± 13.9 < 0.001 |
4.1 ± 13.3 0.049 |
| False alarm (count) |
− 1.1 ± 2.9 0.003 |
− 1.2 ± 2.1 < 0.001 |
| Numeric working memory (memory, accuracy*, focus and concentration) | ||
| Accuracy: overall (%) |
5.6 ± 9.7 < 0.001 |
2.0 ± 8.8 0.219 |
| Accuracy: yes (%) |
8.1 ± 13.5 < 0.001 |
3.1 ± 12.8 0.096 |
| Reaction time: correct (ms) |
− 314.8 ± 402.7 < 0.001 |
− 170.7 ± 376.7 0.001 |
| Reaction time: no (ms) |
− 280.1 ± 497.3 < 0.001 |
− 220.3 ± 448.5 < 0.001 |
| Reaction time: yes (ms) |
− 341.9 ± 393.5 < 0.001 |
− 166.8 ± 388.0 0.006 |
| Computerized Corsi blocks (memory, accuracy, focus and concentration) | ||
| Span score |
0.4 ± 1.4 0.033 |
0.5 ± 1.3 0.003 |
| Picture recognition (memory, accuracy*, learning) | ||
| Accuracy: overall (%) |
1.5 ± 6.2 0.012 |
0.1 ± 2.6 0.994 |
| Accuracy: yes (%) |
1.9 ± 8.2 0.022 |
0.2 ± 4.4 0.952 |
| Reaction time: overall (ms) |
− 217.6 ± 390.3 < 0.001 |
− 140.6 ± 240.2 0.001 |
| Reaction time: correct (ms) |
− 206.0 ± 370.5 < 0.001 |
− 144.3 ± 231.1 < 0.001 |
| Reaction time: no (ms) |
− 167.8 ± 332.1 < 0.001 |
− 155.4 ± 253.8 < 0.001 |
| Reaction time: yes (ms) |
− 267.4 ± 495.1 < 0.001 |
− 125.8 ± 264.9 0.095 |
| Word recognition (memory, accuracy*, learning) | ||
| Accuracy: yes (%) |
5.4 ± 15.3 0.012 |
1.5 ± 16.1 0.716 |
| Reaction time: overall (ms) |
− 179.6 ± 303.8 < 0.001 |
− 192.2 ± 453.6 < 0.001 |
| Reaction time: correct (ms) |
− 156.3 ± 294.6 < 0.001 |
− 187.1 ± 427.1 < 0.001 |
| Reaction time: no (ms) |
− 130.9 ± 372.3 0.020 |
− 144.0 ± 474.1 0.009 |
| Reaction time: yes (ms) |
− 228.3 ± 419.7 < 0.001 |
− 240.4 ± 519.9 < 0.001 |
*Accuracy was assessed by accuracy outcomes only
ms milliseconds, n number, SD standard deviation
Within-group changes were evaluated using a paired t test with p < 0.05 considered statistically significant
Data not shown for non-significant within group changes
Focus and Concentration
The numeric working memory task also assessed focus and concentration wherein 4/13 changes in task outcome scores were significantly different between Neuriva® and placebo. Participants supplemented with Neuriva® had significantly (p ≤ 0.031) greater score improvements in all four cases compared to placebo (Fig. 3; Supplementary Material Table S2).
Neuriva® and placebo groups had 9/13 (p ≤ 0.033) and 7/13 (p ≤ 0.049) score improvements, respectively, from baseline at day 42 (Table 3). There were seven outcomes wherein both groups had score improvements from baseline at day 42 with Neuriva® having greater score improvements than placebo in five of these outcomes.
Accuracy
There were 3/13 significant differences between Neuriva® and placebo across the numeric working memory (accuracy outcomes only) and picture recognition tasks. The Neuriva® group had significantly greater improvements in score change for overall accuracy and “yes” accuracy (p ≤ 0.024) compared to placebo in numeric working memory (Fig. 3; Supplementary Material Table S2). The Neuriva® group also had a significantly greater improvement in score change from baseline at day 42 for overall accuracy (p = 0.035) for the picture recognition task (Fig. 4; Supplementary Material Table S2).
There were 8/13 (p ≤ 0.033) and 3/13 (p ≤ 0.049) improvements in accuracy tasks for the Neuriva® and placebo groups, respectively, from baseline at day 42 (Table 3). Of the three task outcomes wherein both groups observed significant improvements at day 42 compared to baseline, the Neuriva® group had greater score improvements than placebo in one outcome.
Learning
There was one (1/14) significant difference between Neuriva® and placebo in learning-related outcomes which occurred in the picture recognition task. The Neuriva® group had a significantly greater improvement in score for overall accuracy (p = 0.035) compared to placebo at day 42 (Fig. 4; Supplementary Material Table S2).
There were 11/14 (p ≤ 0.022) and 7/14 (p ≤ 0.009) changes for the Neuriva® and placebo groups, respectively, from baseline at day 42 (Table 3). There were seven outcomes wherein both groups had significant improvements from baseline at day 42 with three of those improvements greater for the Neuriva® group than placebo.
Go/No-Go Test
There were no significant differences between the Neuriva® and placebo groups in either Go/No-Go test forms (Supplementary Material Table S3). In test form “1 of 2”, participants supplemented with Neuriva® and those on placebo had one significant improvement (− 1.0 ± 3.9 and − 0.8 ± 3.0, respectively; p ≤ 0.002) in number of false alarms/errors from baseline at day 42 with a greater score reduction in the Neuriva® group. The Neuriva® and placebo groups had 6/7 (p ≤ 0.022) and 4/7 (p ≤ 0.015) significant changes, respectively, from baseline at day 42 for test form “2 of 5” with greater score improvements observed with Neuriva® supplementation (Supplementary Material Table S3).
Everyday Memory Questionnaire
There were no significant differences between Neuriva® and placebo groups in the EMQ (data not shown); however, there were 26/35 significant improvements for both groups from baseline at day 42. The Neuriva® group reported greater score improvements than placebo in 4/23 questions wherein both groups showed improvements from baseline at day 42. The Neuriva® and placebo groups had 5/6 (p ≤ 0.032) and 3/6 (p ≤ 0.014) significant improvements, respectively, at day 42 compared to baseline in learning new things. There were significant (p ≤ 0.041) improvements in all question scores of the speech and reading and writing categories for both groups from baseline at day 42, and for 1/6 questions for faces and places (p < 0.001). For questions related to actions, there were 3/6 and 5/6 significant (p ≤ 0.042) score improvements at day 42 compared to baseline for Neuriva® and placebo groups, respectively.
Adverse Events and Safety Measures
There were a total of 72 adverse events reported by 48 unique participants, 23 participants in the Neuriva® group and 25 in the placebo group. There was one report each of dry mouth and anxiety categorized as possibly related to Neuriva® and placebo, respectively. All adverse events were resolved by the end of study.
Any clinically relevant potassium values were repeated and no longer clinically relevant except for one participant who was lost to follow-up. All other participants’ clinical chemistry and hematology as well as vital signs for all participants were deemed healthy as assessed by the Qualified Investigator.
Discussion
Forty-two days of Neuriva® supplementation was shown to improve memory outcomes in a population with self-reported memory problems. Memory was objectively and subjectively assessed using COMPASS and the EMQ, respectively. Neuriva® supplementation elicited greater score improvements compared to placebo in five objective assessments of memory using COMPASS. Both groups showed significant improvements from baseline in the subjective assessment of memory using the EMQ. The improvements in memory-related COMPASS outcomes in the current study are consistent with a previous study that showed significant improvements in memory outcomes after 12 weeks of supplementation with 300 mg/day PS among elderly participants [29]. In contrast, Kato-Kataoka et al. [31] found 100 mg/day of PS, the same amount used in the current study, significantly improved memory after 6 months whereas the current study found improvements after 6 weeks [31]. It is possible that the combination of PS and WCCE in the current study contributed to the favorable effects on memory seen earlier.
Improving memory is important given declines in working memory have been shown to begin as early as 30–40 years of age [42]. In addition, working memory is a subdomain of executive function, as defined in the Diagnostic and Statistical Manual of Mental Disorders-5 [43], which has an important role in everyday life including future planning, problem-solving, and focus attention [42]. It is possible to suggest that the improvements in memory outcomes observed with Neuriva® supplementation may help attenuate the age-related decline in memory and support cognition. This is particularly important for individuals with self-reported memory problems who may be at risk of cognitive impairment [44].
The current study showed no trade-off between accuracy and speed, important factors in everyday decision-making, for memory or focus and concentration with Neuriva® supplementation. This was supported by a greater number of improvements from baseline in the Go/No-Go tests for Neuriva® than placebo. The speed–accuracy trade-off involves either speed or accuracy being sacrificed when making decisions, depending on which factor is more favorable in a given situation [45]. Typically, decisions made quicker are less accurate and decisions made slower are more accurate [45]. However, Neuriva® supplementation improved both accuracy and speed when participants were challenged with a numeric memory task. Given decision-making is important for executive cognitive functioning and declines with age [46] interventions to help support and maintain factors involved in decision-making are important during the aging process.
Despite the improvements in cognitive domains, there was no significant difference in plasma BDNF concentration, a growth factor part of the neurotrophin family, between Neuriva® and placebo at day 42. This is consistent with a previous nutraceutical study [47] suggesting other mechanisms of action exerted by WCCE and PS may be contributing to their cognitive benefits. While previous studies have shown supplementation with 100 mg WCCE significantly increased BDNF levels compared to placebo [25] and baseline [23], inter-individual variation in plasma BDNF concentration and response to supplementation could be factors contributing to the absence of a significant difference in the current study. This variation is comparable to previous studies reported in the literature [48, 49] which may be attributed to potential confounding factors such as age, cognitive state, lifestyle including exercise, and disease state [50–54]. Genetic polymorphisms of the BDNF gene have been identified as a contributor to high variability of BDNF concentrations [55, 56] and future studies should consider this factor when examining changes in BDNF. There was, however, a significant mean decrease in BDNF concentration in the placebo group at day 42 that was not observed in the Neuriva® group. This suggests a possible protective effect of Neuriva® on BDNF which may be due to the presence of PS in the current study’s product, but more research is warranted. Studies of longer duration will further the understanding of Neuriva® supplementation on BDNF concentrations and mechanisms of action.
The current study showed 42 days of Neuriva® supplementation was safe in healthy adults. Only one adverse event, dry mouth, was reported in the Neuriva® group which was deemed possibly related to the study product but was resolved by the end of the study. Supplementation tolerability was also favorable given the low rate of attrition (1.5%) and high rate of compliance in the Neuriva group.
It is relevant to consider the potential for the placebo effect in cognition studies as it has been previously reported in the literature [57, 58]. The current study showed the placebo effect accounted for 6–100% of the response for memory-related COMPASS tasks. This is comparable to what has been reported previously in the literature for memory-related outcomes [31, 35]. Moreover, a previous study showed positive effects on memory and attention performance after 2-week consumption of a placebo pill compared to no pill among healthy older adults [57]. Another study showed university students who thought they were consuming a cognitive-enhancing drug had improved cognitive performance compared to those who thought they were consuming a placebo [58]. Therefore, it is important to consider the presence of a placebo effect when interpreting results from the current study.
Further, physical activity has a positive impact on cognition [59, 60]; however, though participants were instructed to maintain their usual physical activity level during the study, detailed information on participant activity was not captured. The interaction between physical activity, particularly cardiovascular exercise [60], and Neuriva® could be explored in future studies. In addition, education level is an important factor to consider when evaluating cognition which was not controlled for and is a limitation of the current study that should be considered in future randomized controlled trials.
Conclusions
Results of the current study showed 42 days of Neuriva® supplementation significantly improved measures of memory, accuracy, focus and concentration, and learning compared to placebo in healthy adults with self-reported memory problems. These findings were supported by improvements in these cognitive domains from baseline with Neuriva® supplementation. Neuriva® supplementation was found to be safe and well tolerated in the population studied. Future larger randomized controlled trials with longer durations are needed to further explore the efficacy of Neuriva® on cognition, and confirm the current study’s findings. Moreover, research is warranted in more vulnerable populations such as those with mild cognitive impairment and advanced age.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors wish to thank the participants for their compliance to the conduct of the study. We also thank Foram Patel for her assistance with formatting.
Funding
This research and rapid service fee was funded by Reckitt.
Author Contributions
Conceptualization, Malkanthi Evans, Jane M. Barracato, Alejandra A. Gratson; methodology, Erin D. Lewis, Jane M. Barracato, Alejandra A. Gratson, David C. Crowley, and Malkanthi Evans; investigation, David C. Crowley; data curation, David C. Crowley; formal analysis, Erin D. Lewis, and Malkanthi Evans; writing—original draft preparation, Katarina M. Doma, Erin D. Lewis, and Malkanthi Evans; writing—review and editing, Katarina M. Doma, Erin D. Lewis, Jane M. Barracato, Lauren R. Brink, Alejandra A. Gratson, Neeraj Pandey, and Malkanthi Evans; visualization, Katarina M. Doma, Erin D. Lewis, and Malkanthi Evans; supervision, David C. Crowley, and Malkanthi Evans; funding acquisition, Jane M. Barracato, Lauren R. Brink, and Alejandra A. Gratson. All authors have read and agreed to the published version of the manuscript.
Disclosures
Jane M. Barracato, Lauren R. Brink, Neeraj Pandey, and Alejandra A. Gratson are employees of Reckitt; Katarina M. Doma, Erin D. Lewis, David C. Crowley, and Malkanthi Evans have nothing to disclose.
Compliance with Ethics Guidelines
Research ethics board approval was granted on December 24, 2019, from the Institutional Review Board Services, Aurora, Ontario (Pro00040971). Written informed consent was obtained from all participants prior to any study procedures being initiated. The study was conducted in accordance with the Declaration of Helsinki and its later amendments.
Data Availability
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.NIH National Institute on Aging. Cognitive health and older adults [updated 2020]. https://www.nia.nih.gov/health/cognitive-health-and-older-adults. Accessed 14 Nov 2022.
- 2.Centers for Disease Control and Prevention. Healthy Brain Initiative 2020. https://www.cdc.gov/aging/healthybrain/index.htm. Accessed 6 May 2022.
- 3.Grand View Research. Brain health supplements market size, share & trends analysis report by product (natural molecules, herbal extract), by application, by region, and segment forecasts, 2022–2030. 2021. Report No. GVR-2-68038-866-4.
- 4.Wang Y, Pan Y, Li H. What is brain health and why is it important? BMJ. 2020;371:m3683. doi: 10.1136/bmj.m3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pais R, Ruano L, P Carvalho O, Barros H. Global cognitive impairment prevalence and incidence in community dwelling older adults—a systematic review. Geriatrics. 2020;5(4):84. [DOI] [PMC free article] [PubMed]
- 6.Bathina S, Das UN. Brain-derived neurotrophic factor and its clinical implications. Arch Med Sci AMS. 2015;11(6):1164. doi: 10.5114/aoms.2015.56342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andero R, Choi DC, Ressler KJ. BDNF–TrkB receptor regulation of distributed adult neural plasticity, memory formation, and psychiatric disorders. Prog Mol Biol Transl Sci. 2014;122:169–192. doi: 10.1016/B978-0-12-420170-5.00006-4. [DOI] [PubMed] [Google Scholar]
- 8.Miranda M, Morici JF, Zanoni MB, Bekinschtein P. Brain-derived neurotrophic factor: a key molecule for memory in the healthy and the pathological brain. Front Cell Neurosci. 2019;13:363. doi: 10.3389/fncel.2019.00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brodziak A, Wolinska A, Kolat E, Rozyk-Myrta A. Guidelines for prevention and treatment of cognitive impairment in the elderly. Med Sci Monit. 2015;21:585–597. doi: 10.12659/MSM.892542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlier J, Giorgetti R, Varì M, Pirani F, Ricci G, Busardò F. Use of cognitive enhancers: methylphenidate and analogs. Eur Rev Med Pharmacol Sci. 2019;23(1):3–15. doi: 10.26355/eurrev_201901_16741. [DOI] [PubMed] [Google Scholar]
- 11.Cassilhas RC, Viana VA, Grassmann V, et al. The impact of resistance exercise on the cognitive function of the elderly. Med Sci Sports Exerc. 2007;39(8):1401. doi: 10.1249/mss.0b013e318060111f. [DOI] [PubMed] [Google Scholar]
- 12.Naqvi R, Liberman D, Rosenberg J, Alston J, Straus S. Preventing cognitive decline in healthy older adults. CMAJ. 2013;185(10):881–885. doi: 10.1503/cmaj.121448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rebok GW, Ball K, Guey LT, et al. Ten-year effects of the advanced cognitive training for independent and vital elderly cognitive training trial on cognition and everyday functioning in older adults. J Am Geriatr Soc. 2014;62(1):16–24. doi: 10.1111/jgs.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ricci G. Pharmacological human enhancement: an overview of the looming bioethical and regulatory challenges. Front Psychiatry. 2020;11:53. 10.3389/fpsyt.2020.00053. [DOI] [PMC free article] [PubMed]
- 15.Thompson TW, Waskom ML, Garel K-LA, et al. Failure of working memory training to enhance cognition or intelligence. PloS One. 2013;8(5):e63614. [DOI] [PMC free article] [PubMed]
- 16.Gates NJ, Rutjes AW, Di Nisio M, et al. Computerised cognitive training for 12 or more weeks for maintaining cognitive function in cognitively healthy people in late life. Cochrane Database Syst Rev. 2020;2(2):CD012277. [DOI] [PMC free article] [PubMed]
- 17.Pergher V, Shalchy MA, Pahor A, Van Hulle MM, Jaeggi SM, Seitz AR. Divergent research methods limit understanding of working memory training. J Cognit Enhanc. 2020;4:100–120. doi: 10.1007/s41465-019-00134-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen F-T, Hopman RJ, Huang C-J, et al. The effect of exercise training on brain structure and function in older adults: a systematic review based on evidence from randomized control trials. J Clin Med. 2020;9(4):914. doi: 10.3390/jcm9040914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tajik N, Tajik M, Mack I, Enck P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: a comprehensive review of the literature. Eur J Nutr. 2017;56(7):2215–2244. doi: 10.1007/s00394-017-1379-1. [DOI] [PubMed] [Google Scholar]
- 20.Vauzour D. Dietary polyphenols as modulators of brain functions: biological actions and molecular mechanisms underpinning their beneficial effects. Oxid Med Cell Longev. 2012;2012:914273. [DOI] [PMC free article] [PubMed]
- 21.Reed RA, Mitchell ES, Saunders C, O’Connor PJ. Acute low and moderate doses of a caffeine-free polyphenol-rich coffeeberry extract improve feelings of alertness and fatigue resulting from the performance of fatiguing cognitive tasks. J Cognit Enhanc. 2018;3(2):193–206. doi: 10.1007/s41465-018-0118-8. [DOI] [Google Scholar]
- 22.Robinson JL, Hunter JM, Reyes-Izquierdo T, et al. Cognitive short- and long-term effects of coffee cherry extract in older adults with mild cognitive decline. Aging Neuropsychol Cogn. 2020;27(6):918–934. doi: 10.1080/13825585.2019.1702622. [DOI] [PubMed] [Google Scholar]
- 23.Robinson JL, Yanes JA, Reid MA, et al. Neurophysiological effects of whole coffee cherry extract in older adults with subjective cognitive impairment: a randomized, double-blind, placebo-controlled, cross-over pilot study. Antioxidants. 2021;10(2):144. doi: 10.3390/antiox10020144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cotter J, Fawkes N, Shah N. Coffee fruit extract–a nutritional stimulator of endogenous BDNF. Nutr Neurosci. 2022;25(9):2008–2010. doi: 10.1080/1028415X.2021.1913953. [DOI] [PubMed] [Google Scholar]
- 25.Reyes-Izquierdo T, Nemzer B, Shu C, et al. Modulatory effect of coffee fruit extract on plasma levels of brain-derived neurotrophic factor in healthy subjects. Br J Nutr. 2013;110(3):420–425. doi: 10.1017/S0007114512005338. [DOI] [PubMed] [Google Scholar]
- 26.Glade MJ, Smith K. Phosphatidylserine and the human brain. Nutrition. 2015;31(6):781–786. doi: 10.1016/j.nut.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 27.Kim HY, Huang BX, Spector AA. Phosphatidylserine in the brain: metabolism and function. Prog Lipid Res. 2014;56:1–18. doi: 10.1016/j.plipres.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee B, Sur B-J, Han J-J, et al. Krill phosphatidylserine improves learning and memory in Morris water maze in aged rats. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(6):1085–1093. doi: 10.1016/j.pnpbp.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 29.Richter Y, Herzog Y, Lifshitz Y, Hayun R, Zchut S. The effect of soybean-derived phosphatidylserine on cognitive performance in elderly with subjective memory complaints: a pilot study. Clin Interv Aging. 2013;8:557–563. doi: 10.2147/CIA.S40348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yong T, Qianyong Z, Mantian M, Gang H, Jing W. Research on human memory enhancement by phosphatidylserine fortified milk. Chongqing Med. 2011;30.
- 31.Kato-Kataoka A, Sakai M, Ebina R, Nonaka C, Asano T, Miyamori T. Soybean-derived phosphatidylserine improves memory function of the elderly Japanese subjects with memory complaints. J Clin Biochem Nutr. 2010;47(3):246–255. doi: 10.3164/jcbn.10-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Int J Surg. 2012;10(1):28–55. doi: 10.1016/j.ijsu.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 33.Steiner GZ, Yeung A, Liu J-X, et al. The effect of Sailuotong (SLT) on neurocognitive and cardiovascular function in healthy adults: a randomised, double-blind, placebo controlled crossover pilot trial. BMC Complement Altern Med. 2015;16(1):1–13. [DOI] [PMC free article] [PubMed]
- 34.Wightman EL, Haskell-Ramsay CF, Thompson KG, et al. Dietary nitrate modulates cerebral blood flow parameters and cognitive performance in humans: a double-blind, placebo-controlled, crossover investigation. Physiol Behav. 2015;149:149–158. doi: 10.1016/j.physbeh.2015.05.035. [DOI] [PubMed] [Google Scholar]
- 35.Wightman EL, Jackson PA, Spittlehouse B, Heffernan T, Guillemet D, Kennedy DO. The acute and chronic cognitive effects of a sage extract: a randomized, placebo controlled study in healthy humans. Nutrients. 2021;13(1):218. doi: 10.3390/nu13010218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wightman EL, Jackson PA, Forster J, et al. Acute effects of a polyphenol-rich leaf extract of Mangifera indica l.(zynamite) on cognitive function in healthy adults: a double-blind, placebo-controlled crossover study. Nutrients. 2020;12(8):2194. [DOI] [PMC free article] [PubMed]
- 37.Haskell-Ramsay CF, Jackson PA, Forster JS, Dodd FL, Bowerbank SL, Kennedy DO. The acute effects of caffeinated black coffee on cognition and mood in healthy young and older adults. Nutrients. 2018;10(10):1386. doi: 10.3390/nu10101386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zimmermann P, Fimm B. A test battery for attentional performance. Applied neuropsychology of attention. London: Psychology Press; 2004. p. 124–65.
- 39.Georgiou G, Essau CA. Go/No-Go task. In: Goldstein S, Naglieri JA, editors. Encyclopedia of child behavior and development. Boston: Springer; 2011. pp. 705–706. [Google Scholar]
- 40.Tate RL, Cameron ID. A compendium of tests, scales and questionnaires: The practitioner’s guide to measuring outcomes after acquired brain impairment. London: Psychology Press; 2010.
- 41.R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2019.
- 42.Ferguson HJ, Brunsdon VEA, Bradford EEF. The developmental trajectories of executive function from adolescence to old age. Sci Rep. 2021;11(1):1382. doi: 10.1038/s41598-020-80866-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sachdev PS, Blacker D, Blazer DG, et al. Classifying neurocognitive disorders: the DSM-5 approach. Nat Rev Neurol. 2014;10(11):634–642. doi: 10.1038/nrneurol.2014.181. [DOI] [PubMed] [Google Scholar]
- 44.Mitchell A, Beaumont H, Ferguson D, Yadegarfar M, Stubbs B. Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: meta-analysis. Acta Psychiatr Scand. 2014;130(6):439–451. doi: 10.1111/acps.12336. [DOI] [PubMed] [Google Scholar]
- 45.Standage D, Blohm G, Dorris MC. On the neural implementation of the speed-accuracy trade-off. Front Neurosci. 2014;8:236. doi: 10.3389/fnins.2014.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murman DL. The impact of age on cognition. Semin Hear. 2015;36(3):111–121. doi: 10.1055/s-0035-1555115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lopresti AL, Smith SJ, Majeed M, Drummond PD. Effects of an Oroxylum indicum Extract (Sabroxy®) on cognitive function in adults with self-reported mild cognitive impairment: a randomized, double-blind, placebo-controlled study. Aging Neurosci. 2021;13:728360. doi: 10.3389/fnagi.2021.728360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gourgouvelis J, Yielder P, Clarke ST, Behbahani H, Murphy B. You can’t fix what isn’t broken: eight weeks of exercise do not substantially change cognitive function and biochemical markers in young and healthy adults. PeerJ. 2018;6:e4675. doi: 10.7717/peerj.4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tarassova O, Ekblom MM, Moberg M, Lövdén M, Nilsson J. Peripheral BDNF response to physical and cognitive exercise and its association with cardiorespiratory fitness in healthy older adults. Front Physiol. 2020;11:1080. doi: 10.3389/fphys.2020.01080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Komulainen P, Pedersen M, Hänninen T, et al. BDNF is a novel marker of cognitive function in ageing women: the DR's EXTRA Study. Neurobiol Learn Mem. 2008;90(4):596–603. doi: 10.1016/j.nlm.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 51.Naegelin Y, Dingsdale H, Säuberli K, Schädelin S, Kappos L, Barde Y-A. Measuring and validating the levels of brain-derived neurotrophic factor in human serum. eNeuro. 2018;5(2):ENEURO.0419–17.2018. [DOI] [PMC free article] [PubMed]
- 52.Rahmani M, Rahmani F, Rezaei N. The brain-derived neurotrophic factor: missing link between sleep deprivation, insomnia, and depression. Neurochem Res. 2020;45(2):221–231. doi: 10.1007/s11064-019-02914-1. [DOI] [PubMed] [Google Scholar]
- 53.Shimada H, Makizako H, Doi T, et al. A large, cross-sectional observational study of serum BDNF, cognitive function, and mild cognitive impairment in the elderly. Front Aging Neurosci. 2014;6:69. [DOI] [PMC free article] [PubMed]
- 54.Zoladz J, Pilc A, Majerczak J, Grandys M, Zapart-Bukowska J, Duda K. Endurance training increases plasma brain-derived neurotrophic factor concentration in young healthy men. J Physiol Pharmacol. 2008;59(Suppl 7):119–132. [PubMed] [Google Scholar]
- 55.Toh YL, Ng T, Tan M, Tan A, Chan A. Impact of brain-derived neurotrophic factor genetic polymorphism on cognition: a systematic review. Brain Behav. 2018;8(7):e01009. doi: 10.1002/brb3.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tudor L, Konjevod M, Nikolac Perkovic M, et al. Genetic variants of the brain-derived neurotrophic factor and metabolic indices in veterans with posttraumatic stress disorder. Front Psychiatry. 2018;9:637. [DOI] [PMC free article] [PubMed]
- 57.Oken BS, Flegal K, Zajdel D, Kishiyama S, Haas M, Peters D. Expectancy effect: impact of pill administration on cognitive performance in healthy seniors. J Clin Exp Neuropsychol. 2008;30(1):7–17. doi: 10.1080/13803390701775428. [DOI] [PubMed] [Google Scholar]
- 58.Colagiuri B, Boakes RA. Perceived treatment, feedback, and placebo effects in double-blind RCTs: an experimental analysis. Psychopharmacology. 2010;208(3):433–441. doi: 10.1007/s00213-009-1743-9. [DOI] [PubMed] [Google Scholar]
- 59.Fernandes J, Arida RM, Gomez-Pinilla F. Physical exercise as an epigenetic modulator of brain plasticity and cognition. Neurosci Biobehav Rev. 2017;80:443–456. doi: 10.1016/j.neubiorev.2017.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schmolesky MT, Webb DL, Hansen RA. The effects of aerobic exercise intensity and duration on levels of brain-derived neurotrophic factor in healthy men. J Sports Sci Med. 2013;12(3):502–511. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.