Abstract
Chronic kidney disease (CKD) is becoming a major public health problem worldwide. This study aimed to explore whether peanut skin extract (PSE) has protective effects against high-fat and high-fructose (HF) diet-induced kidney injury. Rats were fed HF diet in the whole experiment, while rats in PSE-treated groups were supplemented with PSE. Finally, PSE reduced kidney tissue weight, perinephric fat weight, and levels of serum ammonia, creatinine, and urea nitrogen, along with decreases of renal IL-1β and TNF-α level. Histological examination indicated that PSE alleviated renal tubular dilatation, and degeneration and partial exfoliation of renal tubular epithelial cells. In addition, PSE decreased serum and urinary uric acid level, together with reductions of XOD production and XOD activity both in serum and liver, and down-regulated expressions of renal NLRP3 and ERS proteins. Thus, PSE may be a potential functional food for protecting against renal injury in high energy intake.
Keywords: Peanut skin, Chronic kidney disease (CKD), Xanthine oxidase, High fat diet
Introduction
Nowadays, red and processed meat, and soft drinks are popularly consumed in many countries. With the increase in the consumption of these food, not only obesity, type 2 diabetes, nonalcoholic fatty liver disease (NAFLD), and cardiovascular disease (CVD) have truly become big epidemic problems around the word, but also the prevalence of chronic kidney disease (CKD) is increasing in recent years (Asghari et al., 2018). The major risk factor for the development of CKD is associated with obesity induced by the intake of a large number of saturated fat, fructose and cholesterol in these food (He et al., 2021). Considerable number of cross-sectional studies have suggested that a high fat and high sugar dietary pattern is directly associated with the progression of CKD in human (Hossein-Rouhani et al., 2019), and more evidences from animals have indicated that the overconsumption of diet containing abundant saturated fat, fructose and cholesterol can induce renal injuries (Thongnak et al., 2020). Long term and excessive intake of these food can lead to deposition of these component in our body, and thereby, disturb the normal metabolism, ultimately resulting in the development and progression of CKD. Since the sharp rise in the prevalence of CKD, and CKD can develop end-stage renal disease, there is a cause for great concern, and need for protection against the disease. It is noteworthy that dietary polyphenol-rich food has been proved to be beneficial for the prevention of CKD (Kataoka et al., 2022).
Peanut skin (the seed coat of peanuts) is a by-product of peanuts (Arachis hypogaea Linn.), and generally consider a waste product. Notably, it often acts as a traditional medicine for treating diseases in some area around the world. Not only peanut skin contains protein, fat, and carbohydrate, but also it is rich in phenolics (Mingrou et al., 2022). The total phenolics of peanut skin extract (PSE) was reported to be 9–15% of the dry skin (Nepote et al., 2002; Yu et al., 2005), which includes phenolic acids, resveratrol, catechins, epicatechins, procyanidin dimers, procyanidins trimers, tetramers, and oligomers with higher degree of polymerization (Mingrou et al., 2022). Study have indicated that phenolics in PSE shows strong antioxidant effect (Yu et al., 2006, 2010; Yu et al., 2005). Additionally, animals experimental studies support a role of PSE in hepatoprotective effect, antibacterial activity, and anti-melanogenesis (Helmy et al., 2017; Ibrahim and Al-Azawi, 2018; Yu et al., 2010; Tatsuno et al., 2012). Particularly, recent studies on animals have shown that PSE exerts anti-diabetes, anti-obesity, and hypolipidemic effects (Elhardallou et al., 2015; Kang et al., 2014; Xu et al., 2022; Bansode et al., 2012; Toomer et al., 2019). These advance studies might suggest the potential role of PSE in the protection against high-fat diet-induced renal injury. Therefore, the objective of the present study was to investigate the protective effects of PSE against renal injury in a high-fat and high-fructose (HF) diet-fed rats.
Materials and methods
PSE preparation
The peanut skin was obtained from the Bozhou Xingrui Trade Co., Ltd. (Anhui, China). The preparation of PSE was referenced by the method described previously (Yu et al., 2005): briefly, the ultrasound-assisted method was used to extract the components of the skin using 30% ethanol (material/solvent = 1:5, w/w) at a room temperature; The solvent were removed by evaporation under reduced pressure at 50 °C, and then, PSE was collected for further experiment.
Animal and treatment
Twenty-four healthy adult male Sprague–Dawley rats (200 ± 30 g) were obtained from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). Rats were housed at a 12 h/12 h light/dark cycle at 23 °C, and 30 –70% humidity. The experimental procedures were carried out in accordance with the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978). Use of the rats was approved by both Shaanxi Normal University, and the local animal Ethics Committee. After 1 week of acclimation, all rats were divided into 4 groups with 6 animals per cage, as follows: control group, HF group, HF + PSE (0.5 mg/mL) group, and HF + PSE (1 mg/mL) group. The dosage of PSE used in this study was selected based on a previous study (Xu et al., 2022). During the 12 weeks of experimental period, the experiment was carried out by the following procedure: rats in the control group received standard diet (standard diet: 53% carbohydrate, 23.9% protein and 5% fat) without PSE supplementation; rats in the HF group were fed HF diet (HF diet: 76.3% standard diet, 10% lard, 10% fructose and 3% cholesterol) without PSE supplementation; rats in these two HF + PSE treated groups were fed HF diet, and supplemented with PSE in the drinking water (0.5 mg/mL, and 1 mg/mL). At the end, rats were fasted for 12 h, and urine was collected in this period, and then anesthetized. The blood was collected from heart by using syringe. Serum was obtained by centrifugation at 4 ℃. Renal tissues and liver tissue were collected, and weighed. One part of renal tissues was fixed in 4% paraformaldehyde solution for histological examination. The other part of renal tissues, and liver tissues were stored at -80℃ for biochemical analyses.
Histological examination
Renal tissues were treated with paraformaldehyde solution for 48 h, and then the paraffin-embedded renal tissues were cut at 4 μm, and stained with hematoxylin and eosin (H&E) staining solution (Servicebio Technology Co., Ltd., Wuhan, China) according to the operating instructions. The severity of histological alterations in renal tissues was examined under a light microscope (Thermo Fisher, USA).
Analyses of biochemical parameters
Serum uric acid, ammonia, creatinine, and urea nitrogen, and urinary uric acid were analyzed with commercially available kits (Solarbio, Beijing, China). Renal tumor necrosis factor-α (TNF-α) and interleukin 1β (IL-1β) were measured by using ELISA kits (Solarbio, Beijing, China). Urinary pH value was determined by a BPHPOCKET pH meter (BELL Analytical Instruments Co., Ltd., Daliang, China). Xanthine oxidase (XOD) activity both in serum and liver were detected by commercially available kit (Solarbio, Beijing, China).
Western blotting analysis
Primary antibody against XOD, NOD-like receptor thermal protein domain associated protein 3 (NLRP3), immunoglobulin heavy chain binding protein (GRP78), inositol-requiring enzyme 1 (IRE1), and PKR-like ER kinase (PERK) were obtained from Sino Biological (Sino Biological, Inc., China). Protein was obtained by homogenizing the tissues in RIPA buffer, and quantified with a BCA assay kit (Solarbio, Beijing, China). Then, the sample was separated by 10% SDS-PAGE, and electrophoretically transferred to PVDF membranes. After blocking with 5% skim milk in TBST buffer, the membranes were probed with primary antibodies (Dilution 1:1000). After removing unbound primary antibody with TBST buffer, the membrane was incubated with the secondary horseradish peroxidase-conjugated antibodies (dilution 1:2000). The signal was detected with a Ultra High Sensitivity ECL kit (Med Chem Express, Shanghai, China). Quantitation of protein expression was evaluated by the ImageJ software version k1.47 (NIH, Bethesda, MD, USA).
Statistical analysis
All results are presented as mean ± standard error of mean (SEM). Statistical significance were performed using one-way ANOVA for multiple group comparison followed by Tukey’s test with GraphPad Prism 5 software (GraphPad software, San Diego CA, USA). P ≤ 0.05 was considered statistically significant.
Results and discussion
PSE improves body weight gain, fat tissue weight, and renal functional parameters
Obesity is a major risk factor for the development of renal injury (Hall, et al., 2004). Studies on animals have indicated that excessive consumption of the HF diet cause obesity. In the present study, there is no significant difference in energy intake among all groups (Table 1). Moreover, although changes in the absolute body weight among all HF diet-fed groups showed no significant difference, the average body weight gain of rats (compared to the body weight at week 0) in the PSE treated-rats at dose of 1 mg/mL showed significant decrease compared with rats in the HF group. Additionally, as shown in Table 1, the perinephric fat weight, and the relative perinephric fat weight in the HF group showed significant increases. However, the perinephric fat weight, and the relative weight in the PSE-treated group showed significant decreases (Table 1). The above data suggest that PSE shows anti-obesity effect, which is consistent with the previous study (Kang et al., 2014).
Table 1.
Changes in body weight, perinephric fat weight, kidney weight, food intake, and energy intake in HF diet-fed rats
| Group | Control | HF | HF + PSE (0.5 mg/mL) | HF + PSE (1 mg/mL) |
|---|---|---|---|---|
| Final body weight (g) | 224.1 ± 9.55 b | 268.7 ± 10.75 a | 248.7 ± 17.87 ab | 231.2 ± 16.20 ab |
| Final body weight gain (g) | 30.26 ± 9.21 c | 77.79 ± 7.58 a | 62.74 ± 14.50 ab | 38.89 ± 15.18 bc |
| Perinephric fat weight (g) | 0.94 ± 0.13 c | 3.25 ± 0.30 a | 2.52 ± 0.38 b | 2.12 ± 0.15 b |
|
Relative perinephric fat weight (/body weight) |
0.92 ± 0.09 b | 1.26 ± 0.15 a | 1.03 ± 0.12 ab | 0.98 ± 0.13 b |
| Kidney weight (g) | 1.76 ± 0.13 b | 2.29 ± 0.13 a | 1.89 ± 0.13 ab | 1.95 ± 0.09 b |
|
Relative kidney weight (/body weight) |
0.64 ± 0.03 b | 0.80 ± 0.03 a | 0.70 ± 0.03 ab | 0.70 ± 0.03 b |
| Food intake (g/rat/day) | 11.25 ± 0.00 a | 10.07 ± 0.64 b | 10.09 ± 0.51 b | 9.84 ± 0.69 b |
| Energy intake (kcal/rat/day) | 41.13 ± 0.00 a | 40.44 ± 2.57 a | 40.51 ± 2.03 a | 39.49 ± 2.77 a |
Values are shown as mean ± SEM (n = 6). Different letters have significant differences, p < 0.05
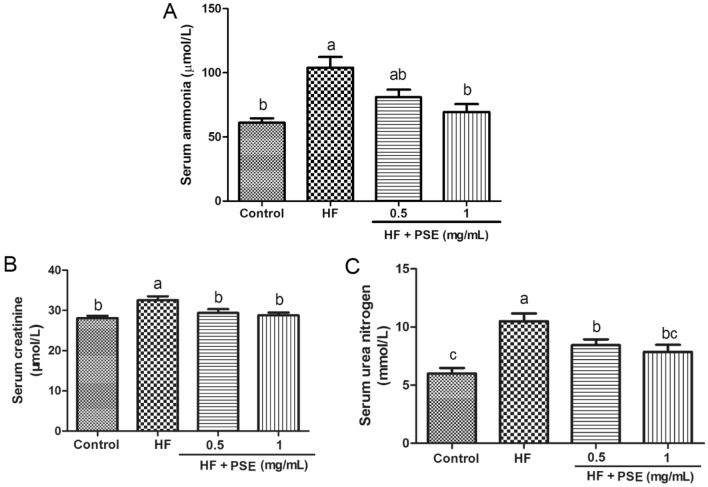
It is well known that excessive consumption of HF diet lead to obesity-associated liver injury. Mounting evidence connects liver injury to kidney injury in high energy intake, where the liver injury can affect the development of renal injury through regulating some cell proteins, such as insulin-like growth factor-1 and syndecan-1 (Musso et al., 2015). Serum ammonia is a strong sensitive test of liver failure (Hu et al., 2020). In this study, rats from the HF group showed a higher level of serum ammonia than rats in the control group, whereas PSE reversed this alteration, suggesting that PSE may has hepatoprotective effect in HF diet-fed rats (Fig. 1A). In fact, previous study has demonstrated that PSE shows protective effect against liver injury in a high fat diet-fed animal (Toomer et al., 2019). Based on these studies, PSE might contribute to inhibiting the development of renal injury in HF diet-fed rats.
Fig. 1.
PSE decreased serum parameters in HF diet-fed rats. (A) Serum ammonia, (B) creatinine, and (C) urea nitrogen. Results are presented as mean ± SEM (n = 6). Different letters have significant differences, p < 0.05
To evaluate the renal function in HF diet-fed rats, the kidney weight, serum urea nitrogen and creatinine were analyzed. Commonly, long term intake of HF diet causes obesity, along with the significant increases in kidney weight, and serum urea nitrogen and creatinine (Ren, et al., 2018; Kaur, et al., 2016). Urea nitrogen and creatinine are valuable screening tests in evaluating renal disease, in which they essentially reflect renal function (Walker et al., 1990). In this study, the consumption of the HF diet significantly increased the absolute kidney weight, relative kidney weight, and levels of urea nitrogen and creatinine in rats from the HF group (Table 1 and Fig. 1B, C), suggesting the reduction of renal function. However, these indicators in rats supplemented with PSE trend to obvious decreases compared with rats in the HF group (Table 1 and Fig. 1B, C), indicating that PSE might improve renal function.
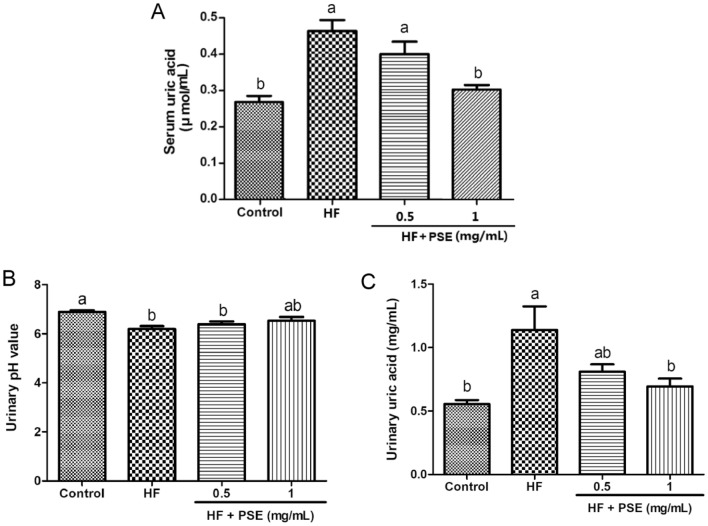
In addition, numerous studies have indicated that a higher uric acid concentration shows both in humans and animals with high energy consumption (Chengfu, et al., 2015; Kaur, et al., 2016). Not only the high serum uric acid concentration is a valuable indicator in reflecting the renal function, but also it is a major risk factor for the development of kidney injury (Su et al., 2020). It is well known that both a high uric acid concentration and a low pH value can result in the formation of urate crystal on renal tubular epithelial cells, which can stimulate renal inflammatory response, leading to the progression of renal tubular epithelial cells damages (William Poore et al., 2020). In this study, the significant increases in serum uric acid, and urinary uric acid, and a lower urinary pH value showed in rats from the HF group (Fig. 2A–C). However, PSE supplementation increased urinary pH value, but no significant difference showed between the HF group and the PSE-treated groups. Moreover, PSE supplementation decreased serum and urinary uric acid concentration ( Fig. 2A–C). These results indicate that PSE might prevent against the risk of the urate crystal formation on renal tubular epithelial cells, and thereby, reducing the damages in renal tubular epithelial cells. According to all the above results, PSE might improve renal dysfunction in HF diet-fed obese rats.
Fig. 2.
PSE decreases the level of uric acid in HF diet-fed rats. (A) Serum uric acid, (B) urinary pH value, and (C) urinary uric acid. Results are presented as mean ± SEM (n = 6). Different letters have significant differences, p < 0.05
PSE alleviates renal injuries, and pro-inflammatory response
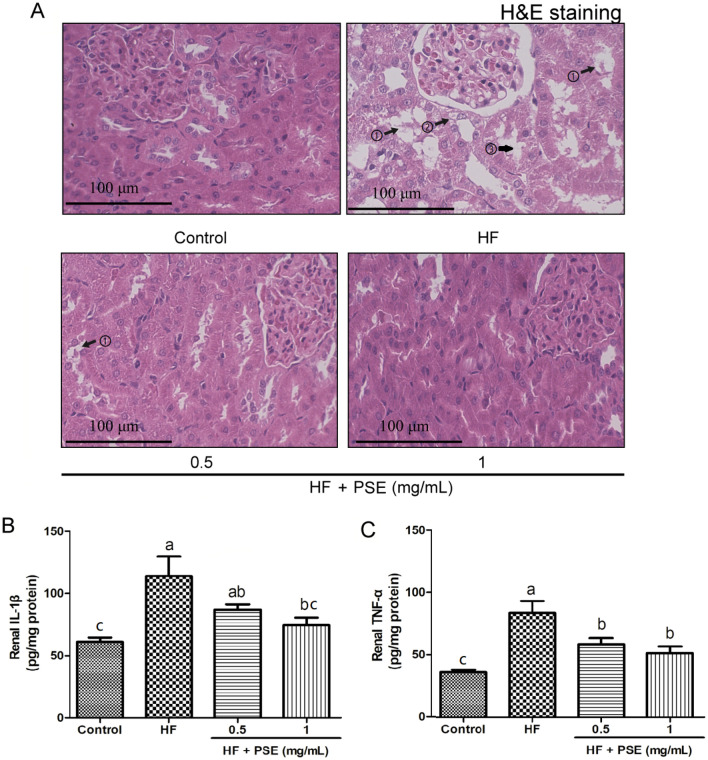
To further examine the morphological changes in renal tissues among all groups, the renal tissues were stained with H&E. Many studies have suggested that people with obesity shows some morphological changes in kidney (Hossein-Rouhani et al., 2019), and more evidences from animals indicated that long term intake of high-fat diet can induce pathological alterations in renal tissue (Thongnak et al., 2020). Clearly, renal tissues stained with H&E showed that excessive consumption of the HF diet caused renal tubular dilatation, and degeneration and partial exfoliation of renal tubular epithelial cells (Fig. 3A), which is consistent with the previous reported study (Thongnak et al., 2020).
Fig. 3.
PSE alleviates kidney injuries and pro-inflammatory response in HF diet-fed rats. (A) representative images of renal tissues stained with H&E: ①, partial exfoliation of renal tubular epithelial cells; ②, degeneration of renal tubular epithelial cells; ③, renal tubular dilatation. (B) Renal IL-1β, and (C) TNF-α. Results are presented as mean ± SEM (n = 6). Different letters have significant differences, p < 0.05
Notably, dietary polyphenol-rich foods are beneficial for the prevention of renal injury (Kataoka et al., 2018). PSE contains a large number of phenolic compounds, such as catechins, epicatechins, procyanidin B2, and procyanidin A2. A paper has well reviewed phenolic compounds in PSE (Mingrou et al., 2022). Importantly, studies also have indicated that phenolic compounds in PSE exerts anti-diabetes, anti-obesity, and hypolipidemic effects, and are powerful weapons against oxidative stress (Mingrou et al., 2022). In this study, as shown in Fig. 3A, PSE supplementation alleviated renal tubular dilatation, and degeneration and partial exfoliation of renal tubular epithelial cells, suggesting that PSE supplementation truly alleviates renal injuries, which might be benefit from the effects of those phenolic compounds in PSE.
IL-1β and TNF-α are two important pro-inflammatory cytokines, which play a central role in the progression of renal injury (Andronovici et al., 2022). TNF-α exhibits both the pro-inflammatory and immunoregulatory properties. IL-1β, a member of IL-1 cytokine family, is a pleiotropic and immunoregulatory cytokine. The increases in the secretions of IL-1β and TNF-α can stimulate the production of other cytokines, leading to inflammatory cells recruitment, and increase of ROS generation, where they are all risk factors for exacerbating renal injury. In our study, rats fed the HF diet without the treatment showed the increases in the levels of renal IL-1β and TNF-α (Fig. 3B, C). However, PSE decreased the levels of renal IL-1β and TNF-α (Fig. 3B, C). Interestingly, some active compounds in PSE, such as resveratrol, have a strong ant-inflammatory effects (Mingrou et al., 2022), suggesting that the protective effect of PSE against kidney injury might be associated with those active compounds on the inhibition of TNF-α and IL-1β production in HF diet-fed rats.
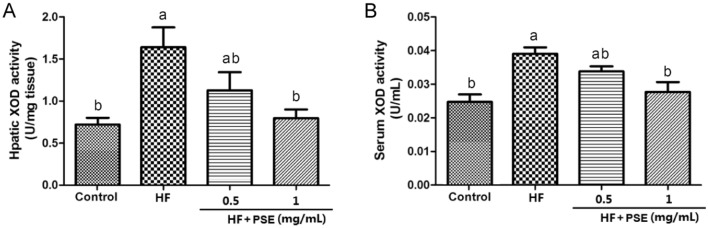
PSE inhibits XOD activity and expression
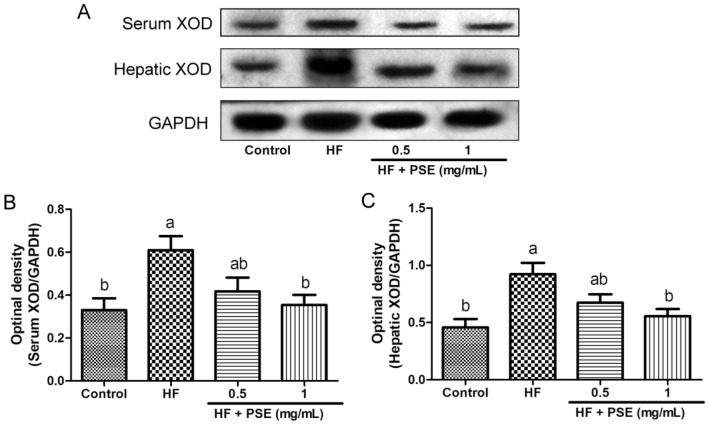
XOD is a key enzyme for purine metabolism, which catalyzes the generations of uric acid and ROS. Notably, studies have revealed that the increase of uric acid level, and the XOD activity are closely associated with the progression of kidney injury (Su et al., 2020; Liu et al., 2015). Since liver is a primary organ for XOD production, in addition to serum XOD activity, the hepatic XOD activity was also determined in this study. Compared with rats in the control group, serum and hepatic XOD activities in rats from the HF group significantly increased (Fig. 4A, B). However, PSE supplementation lead to the significant decreases in serum and hepatic XOD activities (Fig. 4A, B). Further experiment indicated that PSE supplementation reduced XOD expression both in serum and liver, compared with rats in the HF group (Fig. 5A–C). Therefore, the decreases in the serum and urinary uric level by PSE treatment might be closely associated with the inhibition of XOD activity, and/or XOD production.
Fig. 4.
PSE decreases XOD activity in HF diet-fed rats. (A) hepatic XOD activity, (B) serum XOD activity. Results are presented as mean ± SEM (n = 6). Different letters have significant differences, p < 0.05
Fig. 5.
PSE inhibits XOD expression in HF diet-fed rats. (A) representative images of western blotting analysis from serum XOD, and hepatic XOD. Semi-quantitative analysis of hepatic expressions in (B) serum XOD, and C hepatic XOD. Results are presented as mean ± SEM (n = 3). Different letters have significant differences, p < 0.05
PSE decreases NLRP3 expression, and endoplasmic reticulum stress (ERS)
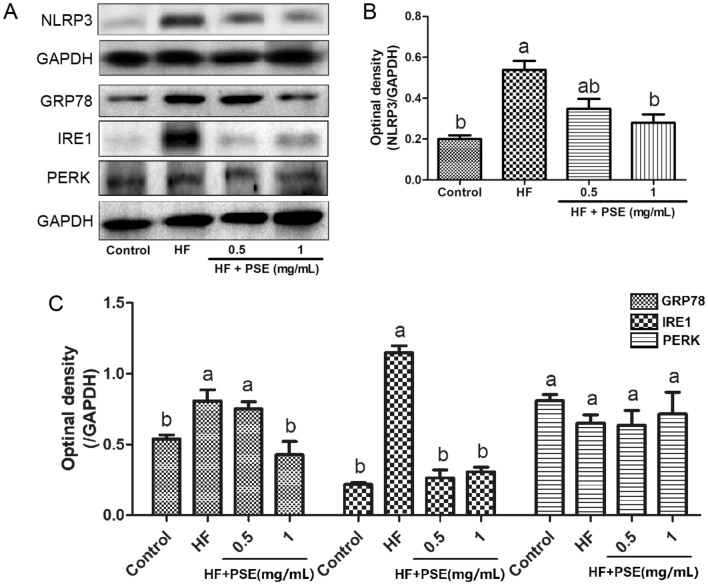
One of important reasons for the renal injury induced by high uric acid concentration has been proved to be involved in NLRP3 inflammasome. NLRP3 inflammasome has been indicated to a higher expression in patients with uric acid nephropathy (Hu et al., 2019). High uric acid level can lead to the activation of NLRP3 inflammasome, resulting in secretion of IL-1β, causing the development of renal parenchymal cells damages (Mulay, 2019). However, the inhibition of NLRP3 inflammasome contributes to the reduction of renal damage (Krishnan et al., 2019). In the present study, compared with rats in the control group, the renal NLRP3 expression in rats from the HF group was up-regulated, whereas PSE supplementation down-regulated renal NLRP3 expression (Fig. 6A, B).
Fig. 6.
PSE decreases expression of renal NLRP3 inflammasome and ERS proteins in HF diet-fed rats. (A) representative images of western blotting analysis from renal NLRP3, GRP78, IRE1, and PERK. Semi-quantitative analysis of hepatic expressions in (B) NLRP3, and (C) GRP78, IRE1, and PERK. Results are presented as mean ± SEM (n = 3). Different letters have significant differences, p < 0.05
Moreover, the increase of uric acid concentration was proved to be associated with the occurrence of ERS (Li et al., 2013). Study has indicated that ERS participates in both acute and chronic renal damages (Cybulsky, 2017). GRP78 is one of important ERS biomarkers. Upon ERS, ERS proteins, including IRE1 and PERK, is divorced from GRP78, which can lead to a up-regulation of GRP78 (Hotamisligil, 2010). At the same time, the divorced IRE1 and PERK are activated, and thereby, can result in the activation of IκB/NF-κB and IKK signaling pathways, insulin resistance, NLRP3-mediated crosstalk, increases of mitochondrial ROS, ultimately accelerating the progression of cell damages (Hotamisligil, 2010). In this study, although there is no significant difference in the expression of renal PERK, the expressions of renal GRP78 and IRE1 in rats from the HF group were up-regulated (Fig. 6A and C). However, compared to rats in the HF group, PSE supplementation down-regulated the expressions of GRP78 and IRE1 (Fig. 6A and C), indicating that PSE supplementation might decrease ERS in the HF diet-fed rats, which contributes to the inhibition of renal injury progression.
In conclusion, PSE showed strong protective effects against renal injury in HF diet-fed rats. In addition, PSE supplementation decreased the expressions of NLRP3 inflammasome and ERS proteins, which might be associated with the effects of PSE on the decreases of XOD activity, and uric acid production. According to the results, PSE may be a potential functional food for protecting against renal injury in HF diet consumption.
Acknowledgements
This work was financially supported by the Fundamental Research Funds for the Central Universities (Nos. GK202103069, GK202103074), National Natural Science Foundation of China (Nos. 82003928, 82071348), and General Financial Grant from the China postdoctoral science foundation (No. 2020M683422).
Declarations
Conflict of interest
None of the authors of this study has any financial interest or conflict with industries or parties.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yang Yang, Email: 2020017@snnu.edu.cn.
Jing Yu, Email: yujing151617@163.com.
Jiaoyao Huo, Email: huojiayao@snnu.edu.cn.
Luting Yang, Email: yangluting@snnu.edu.cn.
Yaping Yan, Email: yaping.yan@snnu.edu.cn.
References
- Andronovici AM, Caruntu ID, Onofriescu M, Hurjui LL, Giusca SE, Covic AS, Braescu R, Foia LG. TNF-α, IL-1β, MMP-8 Crevicular profile in patients with chronic kidney disease and periodontitis. Applied Sciences. 2022;12(2):736. doi: 10.3390/app12020736. [DOI] [Google Scholar]
- Asghari G, Momenan M, Yuzbashian E, Mirmiran P, Azizi F. Dietary pattern and incidence of chronic kidney disease among adults: a population-based study. Nutrition & Metabolism. 2018;15:1–11. doi: 10.1186/s12986-018-0322-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansode RR, Randolph P, Hurley S, Ahmedna M. Evaluation of hypolipidemic effects of peanut skin-derived polyphenols in rats on Western-diet. Food Chemistry. 2012;135:1659–1666. doi: 10.1016/j.foodchem.2012.06.034. [DOI] [PubMed] [Google Scholar]
- Chengfu X, Xingyong W, Lei X, Honglei W, Ming Y, Min M, Yan S, Genyun X, Steven D, Youming L, Chaohui Y. Xanthine oxidase in non-alcoholic fatty liver disease and hyperuricemia: One stone hits two birds. Journal of Hepatology. 2015;62:1412–1419. doi: 10.1016/j.jhep.2015.01.019. [DOI] [PubMed] [Google Scholar]
- Cybulsky AV. Endoplasmic reticulum stress, the unfolded protein response and autophagy in kidney diseases. Nature Reviews Nephrology. 2017;13:681–696. doi: 10.1038/nrneph.2017.129. [DOI] [PubMed] [Google Scholar]
- Elhardallou SB, Babiker WA, Sulieman AME, Gobouri AA. Effect of diet supplementation with food industry by-products on diabetic rats. Food and Nutrition Sciences. 2015;6:875–882. doi: 10.4236/fns.2015.610092. [DOI] [Google Scholar]
- He LQ, Wu XH, Huang YQ, Zhang XY, Shu L. Dietary patterns and chronic kidney disease risk: a systematic review and updated meta-analysis of observational studies. Nutrition Journal. 2021;20:1–11. doi: 10.1186/s12937-020-00661-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JE, Henegar JR, Dwyer TM, Liu J, Da Silva AA, Kuo JJ, Tallam L. Is obesity a major cause of chronic kidney disease? Advances in Renal Replacement Therapy. 2004;11(1):41–54. doi: 10.1053/j.arrt.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Helmy HM, Kamel MM, Hagag K, El-Hawary N, El-Shemy NS. Antimicrobial activity of dyed wool fabrics with peanut red skin extract using different heating techniques. Egyptian Journal of Chemistry. 2017;60:103–116. doi: 10.21608/ejchem.2017.1601.1129. [DOI] [Google Scholar]
- Hong Y, Hu Y, Sun YA, Shi JQ, Xu J. High-fat diet caused renal damage in ApoE−/− mice via the activation of RAGE-mediated inflammation. Toxicology Research. 2021;10:1171–1176. doi: 10.1093/toxres/tfab102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;6:900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossein-Rouhani M, Mortazavi-Najafabadi M, Esmaillzadeh A, Feiz A, Azadbakht L. Direct association between high fat dietary pattern and risk of being in the higher stages of chronic kidney disease. Journal International De Vitaminologie Et De Nutrition. 2019;89:261–270. doi: 10.1024/0300-9831/a000260. [DOI] [PubMed] [Google Scholar]
- Hu C, Huang K, Zhao L, Zhang F, Wu Z, Li L. Serum ammonia is a strong prognostic factor for patients with acute-on-chronic liver failure. Scientific Reports. 2020;10:16970. doi: 10.1038/s41598-020-73603-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Wu H, Wang D, Yang Z, Dong J. LncRNA ANRIL promotes NLRP3 inflammasome activation in uric acid nephropathy through miR-122-5p/BRCC3 axis. Biochimie. 2019;157:102–110. doi: 10.1016/j.biochi.2018.10.011. [DOI] [PubMed] [Google Scholar]
- Ibrahim AK, Al-Azawi AH. Hepatoprotective effect of (Arachis hypogea L.) peanut skin extracts on CCl4 induced liver damage in mice. Bioscience Research. 15: 3415-3428 (2018)
- Kang NE, Ha AW, Woo HW, Kim WK. Peanut sprouts extract (Arachis hypogaea L.) has anti-obesity effects by controlling the protein expressions of PPARγ and adiponectin of adipose tissue in rats fed high-fat diet. Nutrition research and practice. 8: 158-164 (2014). [DOI] [PMC free article] [PubMed]
- Kataoka S, Norikura T, Sato S. Maternal green tea polyphenol intake during lactation attenuates kidney injury in high-fat-diet-fed male offspring programmed by maternal protein restriction in rats. The Journal of Nutritional Biochemistry. 2018;56:99–108. doi: 10.1016/j.jnutbio.2018.01.012. [DOI] [PubMed] [Google Scholar]
- Kaur T, Kaur A, Singh M, Buttar HS, Pathak D, Singh AP. Mast cell stabilizers obviate high fat diet-induced renal dysfunction in rats. European Journal of Pharmacology. 2016;777:96–103. doi: 10.1016/j.ejphar.2016.02.066. [DOI] [PubMed] [Google Scholar]
- Krishnan SM, Ling YH, Huuskes BM, Ferens DM, Saini N, Chan CT, Diep H, Kett MM, Samuel CS, Kemp-Harper BK, Robertson A, Cooper MA, Peter K, Latz E, Mansell AS, Sobey CG, Drummond GR, Vinh A. Pharmacological inhibition of the NLRP3 inflammasome reduces blood pressure, renal damage, and dysfunction in salt-sensitive hypertension. Cardiovascular Research. 2019;115:776–787. doi: 10.1093/cvr/cvy252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Zhao F, Cheng S, Wang X, Hao Y. Uric acid-induced endoplasmic reticulum stress triggers phenotypic change in rat glomerular mesangial cells. Nephrology. 2013;18:682–689. doi: 10.1111/nep.12127. [DOI] [PubMed] [Google Scholar]
- Liu J, Wang C, Liu F, Lu Y, Cheng J. Metabonomics revealed xanthine oxidase-induced oxidative stress and inflammation in the pathogenesis of diabetic nephropathy. Analytical and Bioanalytical Chemistry. 2015;407:2569–2579. doi: 10.1007/s00216-015-8481-0. [DOI] [PubMed] [Google Scholar]
- Mingrou L, Guo S, Ho CT, Bai N. Review on chemical compositions and biological activities of peanut (Arachis hypogeae L.). Journal of food biochemistry. 46: e14119 (2022) [DOI] [PubMed]
- Mulay SR. Multifactorial functions of the inflammasome component NLRP3 in pathogenesis of chronic kidney diseases. Kidney International. 2019;96:58–66. doi: 10.1016/j.kint.2019.01.014. [DOI] [PubMed] [Google Scholar]
- Musso G, Cassader M, Cohney S, Pinach S, Gambino R. Emerging liver-kidney interactions in nonalcoholic fatty liver disease. Trends in Molecular Medicine. 2015;21(10):645–662. doi: 10.1016/j.molmed.2015.08.005. [DOI] [PubMed] [Google Scholar]
- Nepote V, Grosso NR, Guzman CA. Extraction of antioxidant components from peanut skins. Grasas Y Aceites. 54: 391-395 (2002)
- Ren Y, Wang D, Lu F, Zou X, Xu L, Wang K, HuangW, Su H, Zhang C, Gao Y, Dong H, Dong H. Coptidis Rhizoma inhibits NLRP3 inflammasome activation and alleviates renal damage in early obesity-related glomerulopathy. Phytomedicine. 49: 52-65 (2018) [DOI] [PubMed]
- Su HY, Yang C, Liang D, Liu HF. Research advances in the mechanisms of hyperuricemia-induced renal injury. BioMed Research International. 2020;2020:5817348. doi: 10.1155/2020/5817348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsuno T, Jinno M, Arima Y, Kawabata T, Hasegawa T, Yahagi N, Takano F, Ohta T. Anti-inflammatory and anti-melanogenic proanthocyanidin oligomers from peanut skin. Biological & Pharmaceutical Bulletin. 2012;35:909–916. doi: 10.1248/bpb.35.909. [DOI] [PubMed] [Google Scholar]
- Thongnak L, Chatsudthipong V, Lungkaphin A. Mitigation of renal inflammation and endoplasmic reticulum stress by vildagliptin and statins in high-fat high-fructose diet-induced insulin resistance and renal injury in rats. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids. 1865: 158755 (2020) [DOI] [PubMed]
- Toomer OT, Vu T, Pereira M, Williams K. Dietary supplementation with peanut skin polyphenolic extracts (PSPE) reduces hepatic lipid and glycogen stores in mice fed an atherogenic diet. Journal of Functional Foods. 2019;55:362–370. doi: 10.1016/j.jff.2019.02.041. [DOI] [Google Scholar]
- Walker HK, Hall WD, Hurst JW. Clinical methods: the history, physical, and laboratory examinations. Boston: Butterworths. Chapter 192. (1990) [PubMed]
- William Poore BS, Carter J. Boyd BS, Nikhi P. Singh BS, Kyle Wood MD, Barbara Gower PhD, Dean G. Assimos MD. Obesity and Its Impact on Kidney Stone Formation. Reviews in Urology. 22(1): 17-23 (2020) [PMC free article] [PubMed]
- Xu M, Lv C, Wang H, Lu Q, Ye M, Zhu X, Liu R. Peanut skin extract ameliorates high-fat diet-induced atherosclerosis by regulating lipid metabolism, inflammation reaction and gut microbiota in ApoE−/− mice. Food Research International. 2022;154:111014. doi: 10.1016/j.foodres.2022.111014. [DOI] [PubMed] [Google Scholar]
- Yu J, Ahmedna M, Goktepe I. Effects of processing methods and extraction solvents on concentration and antioxidant activity of peanut skin phenolics. Food Chemistry. 2005;90:199–206. doi: 10.1016/j.foodchem.2004.03.048. [DOI] [Google Scholar]
- Yu J, Ahmedna M, Goktepe I, Dai J. Peanut skin procyanidins: Composition and antioxidant activities as affected by processing. Journal of Food Composition and Analysis. 2006;19:364–371. doi: 10.1016/j.jfca.2005.08.003. [DOI] [Google Scholar]
- Yu J, Ahmedna M, Goktepe I. Potential of peanut skin phenolic extract as antioxidative and antibacterial agent in cooked and raw ground beef. International Journal of Food Science & Technology. 2010;45:1337–1344. doi: 10.1111/j.1365-2621.2010.02241.x. [DOI] [Google Scholar]