Abstract
Rift Valley fever is an important yet ignored viral hemorrhagic fever claiming many lives of African and Arabian countries over the past decade. Unfortunately, a recent outbreak of Rift Valley fever is currently ravaging in Mauritania. Death toll is rising continuously with 23 deaths reported in the month of October, 2022. Our article aims to shed light on the ongoing Rift Valley fever outbreak and recommendations to eradicate this potential threat to public health. Online databases including PubMed, the Lancet, and Science Direct as well as conferences, news, and press releases were used to for data collection. All the available medical literature related to Rift Valley fever in Mauritania were taken into consideration while writing the manuscript. As of October 17, 2022, 47 cases have been documented out of which 23 are dead. Case fatality rate has been reached to 49% which has given a wakeup call to the authorities. Efforts are being made by the concerned authorities and World Health Organization to halt the progression of this outbreak. Further investigations are required to completely eradicate the recurrent outbreaks in Mauritania especially in the area of vaccine development. Active involvement of public with the government authorities is of extreme significance in combating this disease.
Keywords: infectious disease, public health, Rift Valley fever
1. INTRODUCTION AND BACKGROUND
Rift Valley fever (RVF) is an important yet neglected illness that causes serious consequences for human and animal health. 1 During the past decades, several human lives have been lost due to this disease all over the African and Arabian countries. Seven hundred thirty‐eight cases, including 230 deaths were claimed by RVF during the 2007 epidemic in Sudan. Six hundred eighty‐four cases, including 234 deaths were recorded in Kenya from 2006 to 2007. One thousand eighty‐seven suspected cases, including 121 deaths were reported in Yemen in 2000 owing to RVF. 2 Graphical representation of these outbreaks is shown in Figure 1. During the preceding five outbreaks in Mauritania, the death toll was 224 in 1987, 13 in 2010, 17 in 2012, 8 in 2015, and 25 in 2020 3 , 4 , 5 as shown in Figure 2. Although the overall mortality of RVF is less than 1%, case‐fatality ratio for individuals with the hemorrhagic form of RVF disease is almost 50%, according to the World Health Organization (WHO). 2 RVF virus (RVFV) is an arthropod‐borne virus that is responsible for increased morbidity and mortality in humans as well as hoofed herbivores. The name of the disease is based on the Rift Valley Province of Kenya, where it was first identified in 1931 in a sheep. 6 The virus belongs to the family Phenuiviridae genus Phlebovirus. 7 It consists of large (L), small (S), and medium (M) segments. 8 The viral polymerase is encoded by L‐segment, 9 nucleoprotein by S‐segment, 10 and two envelop glycoproteins by M‐segment. 11 Human cases result from direct contact with the blood and organs of infected animals. 12 Unpasteurized milk products also contribute to the transmission of the virus to humans. 12 Among mosquitoes, Aedes and Culex are the main vectors for RVFV. 13 Fortunately, human‐to‐human contact is not responsible for viral transmission; however, nosocomial infections and outbreaks in communities do occur due to aerosol transmission. 14 Socioeconomic and climate factors also contribute to recurrent outbreaks of RVF. 15 The incubation period of the virus is from 2 to 6 days. 2 Symptoms vary from person to person ranging from mild fever, headaches, malaise, and myalgias, to serious manifestations like ocular symptoms (0.5%−2%), hemorrhagic fever (<1%), and meningoencephalitis (<1%). 16 RVF is difficult to diagnose on basis of clinical symptoms alone. Reverse transcriptase polymerase chain reaction (RT‐PCR), enzyme‐linked immunoassay for antibodies, and cell culture viral isolation are the laboratory tests used for establishing diagnosis. 17 Most RVF cases are self‐limiting and are treated by over‐the‐counter medications; however, serious cases require hospitalization. 17 Ribavirin has long been considered an efficient antiviral drug choice for the management of RVF due to its in vitro as well as some in vivo efficacy against some other hemorrhagic fever viruses as well. 18 , 19 , 20 No vaccine is currently available for human beings. However, an inactivated vaccine has been developed but is neither licensed nor commercially available. 2
Figure 1.
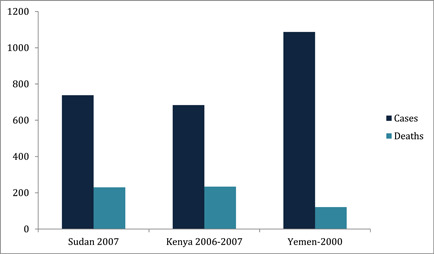
RVF cases and deaths in different regions during previous years. RVF, Rift Valley fever.
Figure 2.
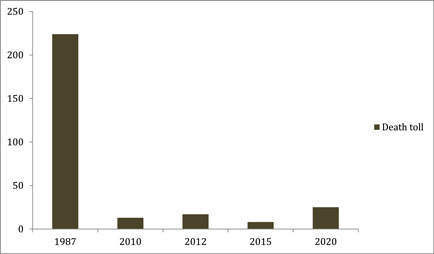
Death toll of Rift Valley fever disease in Mauritania during previous outbreaks.
2. CURRENT STATUS OF OUTBREAK
Mauritania witnessed the first RVF case of 2022 on August 25. A 25‐year‐old male, an animal breeder by profession, from Hodh El Gharbi wilaya (region), presented with the hemorrhagic syndrome to the healthcare center on August 25 and was transferred to the regional hospital the next day, where he died on August 29, 2022. The case was confirmed by PCR on August 29 and WHO was notified on August 30, 2022. From then till October 17, 2022, 47 confirmed cases have been identified in 9 out of 15 Wilayas (regions). The total death toll during this duration is 23. Among the 47 confirmed cases, most of the people are animal breeders (Table 1). The male‐to‐female ratio for cases up till October 17 is 4.4:1. 5
Table 1.
Showing confirmed cases of Rift Valley fever disease in Mauritania in 2022.
| Wilayas (regions) | Number of confirmed RVF cases | Number of deaths | Case fatality rate (CFR) (%) |
|---|---|---|---|
| Hodh Echargui | 12 | 4 | 33 |
| Hodh El Gharbi | 13 | 7 | 54 |
| Assaba | 4 | 2 | 50 |
| Adrar | 9 | 3 | 33 |
| Tagant | 3 | 2 | 67 |
| Nouakchott Ouest | 2 | 1 | 50 |
| Nouakchott Sud | 1 | 1 | 100 |
| Nouakchott Nord | 2 | 2 | 100 |
| Dakhlet Nouadhibou | 1 | 1 | 100 |
| Total | 47 | 23 | 49 |
Abbreviation: RVF, Rift Valley fever.
3. WHAT ARE THE CURRENT CHALLENGES FACED BY MAURITANIA?
RVF has caused recurrent outbreaks in Africa and the Arabian Peninsula. Heavy seasonal rainfalls and proximity of the Senegal river make this region damp, creating a perfect environment where mosquitoes (vectors) can flourish easily, thus posing a major challenge. 21 Many people living in Mauritania and nearby African areas are farm workers. Direct contact with body fluids, blood, and meat of the infected animals are major risk factors for the RVF infection among farm workers, capable of initiating a vicious cycle of RVFV transmission. Some studies also evidently suggest that contact with newly born livestock and aborted animals are responsible for the rapid spread of this disease. 22 One fearsome aspect that especially needs to be addressed is that infection in livestock is silent in many cases, and thus it is not easy to separate the infected and unaffected animals from each other. Moreover, the virus remains in the blood of the infected animals in detectable ranges for only 7 days. Therefore, in a large number of cases, the viremia has already subsided when the animal is tested for the infection. 23 Adding to the already alarming concerns; scientists are still unable to identify the animal host that helps to amplify the virus in its enzootic phase. 24 Early symptoms of RVFV are very similar to seasonal flu and other viral infections, which makes it difficult to detect early. Moreover, Congo‐ and Dengue‐viral coinfections were also found in patients with RVF. This overlapping of signs and symptoms with other viral illness make it quite challenging to diagnose RVF timely and accurately. 25 Differential diagnoses of RVF are shown in Table 2. While the world is still fighting hard from the after‐effects of COVID‐19 pandemic which came down as a huge load on already burdened and economically unstable areas of Africa including Mauritania, this new outbreak has affected the already troubled health system of Mauritania.
Table 2.
Showing differential diagnoses of Rift Valley fever disease.
| Differential diagnosis | Causative organism | Some common clinical features |
|---|---|---|
| Common cold | Rhinovirus | Headache, fever, rhinitis, malaise, cough, muscle‐aches |
| Influenza | Influenza virus | Headache, fever, rhinitis, sore throat, cough, malaise, backache, generalized muscle‐ache |
| COVID‐19 | Corona virus | Headache, fever, cough, muscle‐ache, sore‐throat |
| Dengue hemorrhagic fever | Dengue virus | Headache, muscle ache, cough, retro‐orbital pain, low platelets, hemorrhage, petechiae |
| Crimean−Congo hemorrhagic fever | Congo virus (nairo virus) | Headache, malaise, hemorrhage, petechial rash |
4. WHAT ARE THE RECOMMENDATIONS AT HAND?
Though the healthcare departments and healthcare personnel of Mauritania are utilizing every possible endeavor to cope with the current outbreak of RVF and alleviate the risks to prevent future outbreaks, there is still an urgent need to establish primary healthcare centers where symptomatic treatment can be given to patients in time. Since RVF outbreak is not new for Mauritania, previously recommended policies are being implemented there. How well the disease can be controlled depends on how efficiently the recommendations are being implemented by public. The clinicians must consider RVF as a differential in every febrile illness in the Mauritania. Javelle et al. proposed an algorithm for the management of the patients based on the recommendations of the Centers for Disease Control and Prevention and using some models of outbreaks of dengue hemorrhagic fever. 26 This model can be used at the bedside to decide the management approach of the specific patient under consideration. Warning signs and the risk of severe disease should be checked on the list and patients should be sieved toward the specific management plans accordingly. Patients suffering from comorbid conditions should be put on the high‐risk list as they are more prone to the development of severe disease. 27 Such triage of patients would alleviate the already over‐burdened healthcare system of Mauritania. Standard precautionary protocols including Personal Protective Equipment should be provided to the health staff to reduce the risk of in‐hospital transmission of the virus. 28 Preventive measures including using long‐sleeved clothing, chemicals to repel mosquitoes, bed‐nets at the night, to reduce the incidence of mosquito bites should be strictly implemented. Use of light‐colored clothing instead of darker colors is also advised. Stored water in open containers should either be emptied or covered to decrease the breeding of mosquito larvae. 29 To interrupt the transmission of the virus directly from the animal hosts, healthy practices while handling animals ought to be ensured. Using gloves when milking the livestock, implementing safer animal slaughter techniques with proper hand‐hygiene, and avoiding contact with the tissues of the affected animals hold crucial significance in breaking the chain of transmission. The movement of livestock must be restricted to the areas facing outbreak, so that there is no circulation of virus from the affected to the unaffected areas. 5 WHO recommends that the animals should be vaccinated against the RVFV, so that the virus does not proliferate in animals and may not eventually become a potential risk for human beings. However, vaccination is not recommended during an epidemic, as it increases the risk of formation of new viral strains, that may be more virulent than the previous one. Uncomplicated cases should be treated at homes or in outpatient departments to alleviate the burden of already stricken healthcare system. 26 Mauritania cannot be RVFV‐free region unless mass public awareness is taken into account. Any false theory among public regarding the virus should be eliminated on rational grounds. The salvation of Mauritania from RVFV depends upon implementing standard guidelines and making energetic aforementioned endeavors to end this lethal disease. Current challenges and future recommendations to deal with RVFV are presented in Figure 3.
Figure 3.
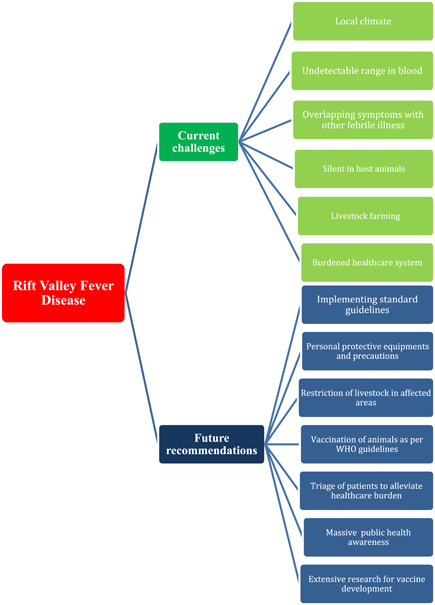
Illustrating infographics about challenges and recommendations regarding Rift Valley fever disease.
5. CONCLUSION
RVFV is not a new virus in Mauritania. It is worth appreciating that efforts are being mitigated by the government and public health authorities to put an end to recurrent outbreaks of RVFV in Mauritania. However, there is a dire need of extensive research to develop an effective vaccine for human beings and strict implementing of standard protocols before the situation flare‐up further.
AUTHOR CONTRIBUTIONS
Shehroze Tabassum: Conceptualization; writing—original draft; writing—review and editing. Farhan Naeem: Writing—original draft; writing—review and editing. Masood Azhar: Writing—original draft; writing—review and editing. Aroma Naeem: Writing—original draft; writing—review and editing. Malik O. Oduoye: Writing—original draft; writing—review and editing. Tirth Dave: Writing—original draft; writing—review and editing.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
TRANSPARENCY STATEMENT
The lead author Shehroze Tabassum affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Tabassum S, Naeem F, Azhar M, Naeem A, Oduoye MO, Dave T. Rift Valley fever virus outbreak in Mauritania yet again in 2022: no room for complacency. Health Sci Rep. 2023;6:e1278. 10.1002/hsr2.1278
DATA AVAILABILITY STATEMENT
The data used in this study are publicly available from the National Library of Medicine https://www.ncbi.nlm.nih.gov/. All relevant data are included in the manuscript. Additional data related to this study may be requested from the corresponding author upon reasonable request.
REFERENCES
- 1. Himeidan YE, Kweka EJ, Mahgoub MM, El Rayah EA, Ouma JO. Recent outbreaks of Rift Valley fever in East Africa and the Middle East. Front Public Health. 2014;2:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rift Valley fever [Internet] . 2022. https://www.who.int/news-room/fact-sheets/detail/rift-valley-fever
- 3. Caminade C, Ndione J, Diallo M, et al. Rift Valley fever outbreaks in Mauritania and related environmental conditions. Int J Environ Res Public Health. 2014;11(1):903‐918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. CDC [Internet] . Outbreak summaries. Rift Valley fever. 2020. https://www.cdc.gov/vhf/rvf/outbreaks/summaries.html
- 5. Rift Valley fever—Mauritania [Internet] . 2022. https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON417
- 6. Enzootic hepatitis or Rift Valley fever . An undescribed virus disease of sheep, cattle and man from East Africa [Internet]. 2022. https://www.cabdirect.org/cabdirect/abstract/19312701811
- 7. Pepin M, Bouloy M, Bird BH, Kemp A, Paweska J. Rift Valley fever virus (Bunyaviridae: Phlebovirus): an update on pathogenesis, molecular epidemiology, vectors, diagnostics and prevention. Vet Res. 2010;41(6):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ikegami T, Makino S. The pathogenesis of Rift Valley fever. Viruses. 2022;3:493‐519. https://pubmed.ncbi.nlm.nih.gov/21666766/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Muller R, Poch O, Delarue M, Bishop DHL, Bouloy M. Rift Valley fever virus L segment: correction of the sequence and possible functional role of newly identified regions conserved in RNA‐dependent polymerases. J Gen Virol. 1994; 75(Pt 6):1345‐1352. [DOI] [PubMed] [Google Scholar]
- 10. Billecocq A, Spiegel M, Vialat P, et al. NSs protein of Rift Valley fever virus blocks interferon production by inhibiting host gene transcription. J Virol. 2004;78(18):9798‐9806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Suzich JA, Kakach LT, Collett MS. Expression strategy of a phlebovirus: biogenesis of proteins from the Rift Valley fever virus M segment. J Virol. 1990;64(4):1549‐1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Helmy Y, El‐Adawy H, Abdelwhab E. A comprehensive review of common bacterial, parasitic and viral zoonoses at the human‐animal interface in Egypt. Pathogens. 2017;6(3):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bird BH, Ksiazek TG, Nichol ST, Maclachlan NJ. Rift Valley fever virus. J Am Vet Med Assoc. 2009;234(7):883‐893. [DOI] [PubMed] [Google Scholar]
- 14. Borio L, Inglesby T, Peters CJ, et al. Hemorrhagic fever viruses as biological weapons: medical and public health management. JAMA. 2002;287(18):2391‐2405. [DOI] [PubMed] [Google Scholar]
- 15. Drake JM, Hassan AN, Beier JC. A statistical model of Rift Valley fever activity in Egypt. J Vector Ecol. 2013;38(2):251‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hassan OA, Affognon H, Rocklöv J, et al. The One Health approach to identify knowledge, attitudes and practices that affect community involvement in the control of Rift Valley fever outbreaks. PLoS Neglected Trop Dis. 2017;11(2):e0005383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hartman A. Rift Valley fever. Clin Lab Med. 2017;37(2):285‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huggins JW. Prospects for treatment of viral hemorrhagic fevers with ribavirin, a broad‐spectrum antiviral drug. Clin Infect Dis. 1989;11(suppl 4):S750‐S761. 10.1093/clinids/11.supplement_4.s750 [DOI] [PubMed] [Google Scholar]
- 19. McCormick JB, King IJ, Webb PA, et al. Lassa fever. N Engl J Med. 1986;314(1):20‐26. 10.1056/NEJM198601023140104 [DOI] [PubMed] [Google Scholar]
- 20. van Eeden PJ, van Eeden SF, Joubert JR, King JB, van de Wal BW, Michell WL. A nosocomial outbreak of Crimean‐Congo haemorrhagic fever at Tygerberg Hospital. Part II. Management of patients. South African Med J = Suid‐Afrikaanse tydskrif vir geneeskunde. 1985;68(10):718‐721. [PubMed] [Google Scholar]
- 21. Mint Mohamed Lemine A, Ould Lemrabott MA, Hasni Ebou M, et al. Mosquitoes (Diptera: Culicidae) in Mauritania: a review of their biodiversity, distribution and medical importance. Parasit Vectors. 2017;10(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sow A, Faye O, Ba Y, et al. Rift Valley fever outbreak, Southern Mauritania, 2012. Emerging Infect Dis. 2014;20:296‐299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wilson W, Davis A, Gaudreault N, et al. Experimental infection of calves by two genetically‐distinct strains of Rift Valley fever virus. Viruses. 2016;8(5):145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Olive MM, Goodman SM, Reynes JM. The role of wild mammals in the maintenance of Rift Valley fever virus. J Wildl Dis. 2012; 48(2):241‐266. [DOI] [PubMed] [Google Scholar]
- 25. Soumaré POL, Freire CCM, Faye O, et al. Phylogeography of Rift Valley fever virus in Africa reveals multiple introductions in Senegal and Mauritania. PLoS One. 2012;7(4):e35216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Javelle E, Lesueur A, Pommier de Santi V, et al. The challenging management of Rift Valley fever in humans: literature review of the clinical disease and algorithm proposal. Ann Clin Microbiol Antimicrob. 2020;19:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. El‐Din abdel‐Wahab KS, El Baz LM, El‐Tayeb EM, Omar H, Moneim Ossman MA, Yasin W. Rift Valley fever virus infections in Egypt: pathological and virological findings in man. Trans R Soc Trop Med Hyg. 1978;72(4):392‐396. [DOI] [PubMed] [Google Scholar]
- 28. Al‐Hamdan NA, Panackal AA, Al Bassam TH, et al. The risk of nosocomial transmission of Rift Valley fever. PLoS Neglected Trop Dis. 2015; 9(12):e0004314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Paweska JT. Rift Valley fever. Revue Scientifique et Technique de l'OIE. 2015;34(2):375‐389. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in this study are publicly available from the National Library of Medicine https://www.ncbi.nlm.nih.gov/. All relevant data are included in the manuscript. Additional data related to this study may be requested from the corresponding author upon reasonable request.


