Abstract
We reported previously that artificial overexpression of the flhDC operon in liquid-grown Serratia liquefaciens resulted in the formation of filamentous, multinucleated, and hyperflagellated cells that were indistinguishable from surface-induced swarm cells (L. Eberl, G. Christiansen, S. Molin, and M. Givskov, J. Bacteriol. 178:554–559, 1996). In the present report we show by means of reporter gene measurements, Northern analysis, and in situ reverse transcription-PCR that the amount of flhDC mRNA in surface-grown swarm cells does not exceed the maximum level found in nondifferentiated, vegetative cells. This suggests that surface-induced S. liquefaciens swarm cell differentiation, although dependent on flhDC gene expression, does not occur through elevated flhDC mRNA levels.
Swarming motility is an intrinsically linked surface and cell density phenomenon. Surface exposure leads to a differentiation process that transforms cells into multinucleate, aseptate, and profusely flagellated swarm cells that are highly elongated (see reference 16 for a review). The cells of a swarming colony have the ability to migrate coordinately in rafts away from the center of the colony. The combined action of cells involved in motility and cells involved in cell division and growth results in colony expansion and rapid colonization of all of the available surface. In the uropathogen Proteus mirabilis it has been demonstrated that swarming behavior is closely associated with modulation of virulence characteristics and the ability to invade human urothelial cells (1–3). In Vibrio parahaemolyticus differentiation into swarm cells plays an important role in adsorption to and colonization of chitinaceous shells of crustaceans (4). For the opportunistic pathogen Serratia liquefaciens, evidence that expression of the phospholipase gene (phlA), which encodes a potential virulence determinant, is differentially expressed in swarm cells has been presented (6). In S. liquefaciens MG1, the swarming phenomenon is linked to high cell density by means of a quorum-sensing mechanism (7, 14). Quorum sensing controls production of the extracellular biosurfactant serrawettin W2, which is required for swarm cells to travel atop the agar surface and which enables swarm colony expansion (21).
The flhDC operon appears to play a crucial role in the swarm cell differentiation process. In both S. liquefaciens and P. mirabilis artificial and prolonged overexpression of the flhDC operon dramatically enhances cell elongation and causes increased flagellation (6, 10). Moreover, for P. mirabilis it has been demonstrated (by Northern analysis) that the amount of flhDC mRNA is increased more than 30-fold in swarm cells compared to the amount found in vegetative cells (10). Previously it was shown that S. liquefaciens swarm cells carry more flagella and express higher levels of phospholipase (PhlA) than their vegetative counterparts (6). Since artificial overexpression of flhDC in S. liquefaciens also leads to cell elongation and increased phospholipase and flagellar expression (6, 13), the apparent synchronization of swarm cell differentiation with phlA and flagellum expression would be most readily explained by assuming that, as in P. mirabilis, the level of flhDC mRNA is specifically increased in swarm cells. However, here we present evidence that the level of flhDC mRNA in S. liquefaciens swarm cells is within the range of levels found in vegetative cells, which suggests that surface-induced S. liquefaciens swarm cell differentiation, although dependent on flhDC gene expression, does not occur through elevated flhDC mRNA levels.
MATERIALS AND METHODS
Strains and plasmids.
The following isogenic S. liquefaciens strains were used: MG1, the wild type (12); MG3 (flhD), in which the flhDC operon is inactivated by insertion of a 1.5-kbp DNA fragment carrying the streptomycin resistance marker (13); and TAK69 (flhD::luxAB), which carries a luxAB transposon (18) insertion in the flhD gene localized 120 bases from the translation start site. Plasmid pMG600 carries a Ptac-flhDC transcriptional fusion and lacI so that expression of the flhDC operon can be controlled by IPTG (isopropyl-β-d-thiogalactopyranoside) addition to the medium (13). pMG600 confers kanamycin resistance to the host. Plasmid pAC33 is a derivative of pMG600 that carries the aacC1 gene which confers gentamicin resistance to the host.
Medium and growth conditions.
Luria-Bertani (LB) medium (5) was used throughout. LB medium solidified with 0.6% agar was used as the medium for swarming bacteria. In the experiments involving cells harboring plasmids, kanamycin at a concentration of 20 μg/ml and gentamicin at 15 μg/ml was used to select for plasmid maintenance. IPTG was supplied to the growth medium as indicated below. The temperature of all incubations was 30°C.
Measurements of bioluminescence.
Bioluminescence was quantified in a Bio-Orbit 1253 luminometer by measuring 1-ml samples of cultures appropriately diluted to give a linear response. One microliter of n-decanal was mixed with each sample prior to measuring. Luminescence is reported in specific light units, which are calculated as relative light units per second per unit of optical density at 450 nm (OD450).
Electrophoresis and immunoblotting.
Cells were harvested and resuspended to an OD450 of 1.0. The proteins were heat denatured in sodium dodecyl sulfate (SDS)-containing sample buffer and separated by a standard SDS-polyacrylamide gel electrophoresis procedure (19). The proteins were transferred to an Immobilon-P membrane (Millipore) by means of a semidry blotting apparatus and subjected to immunoblotting analysis (Western blotting) using rabbit antibodies directed against S. liquefaciens flagellar protein followed by visualization by the binding of secondary alkaline phosphatase-labeled anti-rabbit immunoglobulin G using p-nitroblue tetrazolium and 5-bromo-4-chloro-3-indolylphosphate.
Oligonucleotides.
Two sets of primers for seminested PCR amplification of the S. liquefaciens phlA and flhDC genes were designed based on the published sequences (11, 13): PhlA902F (forward primer targeting nucleotide positions 902 to 922), 5′-CCGGTGGCCTCTCCCTCTCAG-3′; PhlA1056i (internal forward primer targeting nucleotide positions 1056 to 1079), 5′-TGGCCAAGGACGTTTACTCACTCA-3′; PhlA1629R (reverse primer targeting nucleotide positions 1629 to 1606), 5′-ATGCCGGTCAGGGTATCGTTGTTG-3′; FlhDC295F (forward primer targeting nucleotide positions 295 to 319), 5′-CGATGTTCCGC CTTGGTATTGATGA-3′; FlhDC529i (internal forward primer targeting nucleotide positions 529 to 552), 5′-GCCCGACGAAGAAAAGAGCCTGAT-3′; FlhDC1052R (reverse primer targeting nucleotide positions 1052 to 1036), 5′-CGCGGGATGGGGGTTGG-3′. The two primers PhlA1056i and FlhDC529i were labeled with biotin in the 5′ end during automated synthesis and were subsequently purified by reverse-phase high-performance liquid chromatography. All primers were tested by PCR with S. liquefaciens MG1 DNA as the template and were found to give PCR products of the expected sizes.
Northern analysis.
The PCR product generated by use of the FlhDC295F and FlhDC1052R primers, with S. liquefaciens MG1 DNA as the template, was used as a probe in Northern analysis to target the flhDC mRNA molecules. Preparation of total RNA from 1-ml cell suspensions (OD450 = 1), preparation of [α-32P]dCTP-labeled probe, Northern blotting, and quantification of radioactive signals from specific bands were done as previously described (17).
Detection of specific mRNA in individual cells.
Cell fixation, cell wall permeabilization, in situ reverse transcription-PCR (RT-PCR), and detection of intracellular PCR products were done essentially as described earlier (26). Cell wall permeabilization was done by the use of 1 mg (instead of 0.5 mg) of lysozyme/ml. Since biotin-labeled internal primers were used (instead of fluorescein-labeled internal primers), biotinylated intracellular PCR products were detected using a streptavidin-horseradish peroxidase conjugate (DuPont, NEN Research Products).
Microscopy and image processing.
Microscopic examinations were done with an Axioplan microscope (Carl Zeiss, Oberkochen, Germany), using a Ph3 Plan-NEOFLUAR 63×/1.25 oil objective (Carl Zeiss). Digital images were captured with a slow-scan charge-coupled device CH250 camera (Photometrics, Tucson, Ariz.) mounted on the microscope. The exposure time was 25 ms for phase-contrast micrographs and 1,000 ms for epifluorescence micrographs. Captured digital images were processed with PMIS software, and cell size distributions were obtained by the use of an image analysis program written in MATLAB, version 5.1 (MathWorks Inc., Natick, Mass.).
RESULTS AND DISCUSSION
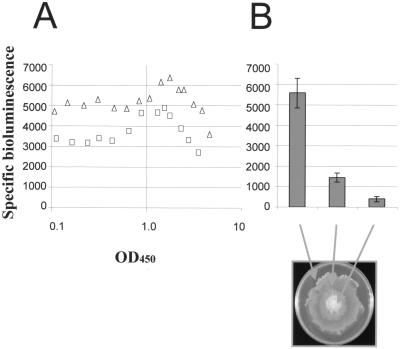
In order to test the hypothesis that flhDC expression is specifically increased in S. liquefaciens swarm cells, we employed the reporter strain TAK69, which carries a chromosomal insertion of the promoterless luxAB genes in flhD (see Materials and Methods for details). When monitored through the growth cycle, the transcriptional activity of the flhD-luxAB fusion was found to be constitutive during the log phase of growth. As the growth rate decreased, the transcriptional activity gradually increased until it reached its maximum value at a cell density of approximately 1.5 OD450 units. Following this, activity declined during the entry into stationary phase (Fig. 1A). Due to the luxAB insertion in flhD the TAK69 strain is unable to synthesize flagella and unable to swim and swarm. However, IPTG-induced expression of a Ptac-flhDC fusion carried on the plasmid pAC33 complemented the inactivated chromosomal flhDC operon. As judged from the expansion rate of swarming colonies, the presence of 20 μM IPTG restored the swarming motility of TAK69/pAC33 to the level of the MG1 wild-type strain (not shown). Microscopic inspection of the cells present in the outer swirling layer of the swarming colony revealed the presence of elongated cells similar to those from a wild-type colony (data not shown). In liquid culture, induction of the flhDC operon with 20 μM IPTG did not affect growth, nor did the bacteria differentiate into swarm cells (data not shown). Transcriptional activity of the chromosomal flhD-luxAB fusion was slightly upregulated (1.3-fold) in the presence of the IPTG-induced flhDC operon (Fig. 1A). However, the transcriptional activity of the flhD-luxAB fusion followed the same pattern in the presence or absence of the plasmid-borne IPTG-induced Ptac-flhDC fusion, demonstrating that the flhDC operon of S. liquefaciens is not subject to autogenous control. The finding that swarm cell differentiation takes place in surface-grown cells with constitutive Ptac-driven flhDC expression but apparently does not take place in liquid-grown cells with similar induction of the Ptac-flhDC fusion is not in agreement with the hypothesis that swarm cells have increased levels of flhDC transcription. Furthermore, expression from the flhDC promoter was not specifically increased in the surface-grown cells relative to that in liquid-grown cells (Fig. 1B).
FIG. 1.
Expression of bioluminescence from a chromosomal flhD::luxAB transcriptional fusion in strain TAK69. (A) Samples of TAK69 (squares) and TAK69 carrying plasmid pAC33 (ptac-flhDC) (triangles) were taken from liquid cultures throughout the growth cycle; (B) samples taken from a swarming colony of TAK69 carrying plasmid pAC33 (mean values of eight individual samples with error bars indicating deviations). All media were supplemented with 20 μM IPTG.
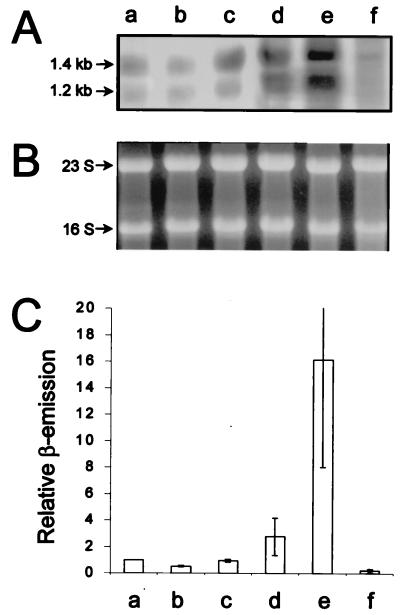
Complementation of the inactivated chromosomal flhDC operon in the reporter strain with a plasmid-borne IPTG-inducible flhDC operon is an artificial system, which might give rise to artifacts. We therefore analyzed the content of flhDC mRNA in S. liquefaciens MG1 (wild type). RNA extracted from cells isolated from the outer layer of a swarming colony, or from a liquid culture at the mid-log (OD450 = 0.4) and late log (OD450 = 1.2) phases was subjected to flhDC mRNA Northern analysis. In concurrence with the results obtained with the reporter strain, Fig. 2 shows that the late-log-phase cells and the cells isolated from the edge of a swarming colony had similar flhDC mRNA levels. The RNA in each lane was isolated from samples normalized to identical OD450 values (as described in Materials and Methods). As shown in Fig. 2B this normalization resulted in samples containing similar amounts of rRNA. The finding that P. mirabilis swarm cells have an flhDC mRNA content more than 30-fold higher than that of vegetative cells was also based on a Northern analysis of samples with the same amounts of rRNA (10). Our results therefore strongly suggest that surface-induced swarm cells of S. liquefaciens differ considerably from those of P. mirabilis with respect to flhDC transcription.
FIG. 2.
Northern analysis of flhDC mRNA in S. liquefaciens (performed as described in Materials and Methods). (A) The Northern blot contains RNA from MG1 cells isolated from the edge of a swarming colony (lane a), liquid-grown MG1 mid-log-phase (OD450 = 0.4) cells (lane b), liquid-grown MG1 late-log-phase (OD450 = 1.2) cells (lane c), liquid-grown MG3/pMG600 mid-log-phase cells sampled 20 min after addition of 50 μM IPTG (lane d), liquid-grown MG3/pMG600 mid-log-phase cells sampled 20 min after addition of 200 μM IPTG (lane e), and liquid-grown MG3/pMG600 mid-log-phase cells grown in the absence of IPTG (lane f). The sizes of the two transcripts are indicated at the left. (B) Photograph of the ethidium-stained gel used for Northern blotting. 16S and 23S rRNA bands are indicated. (C) Radioactive signals emitted from specific bands and quantified by the use of an electronic instant-imager device. The relative values are averages of two independent Northern blots. Bars, standard deviations.
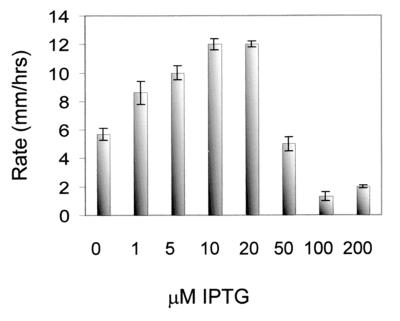
The original hypothesis that S. liquefaciens swarm cells have an increased level of flhDC mRNA was put forward because artificial overexpression of flhDC leads to elongation of liquid-grown cells so that they resemble swarm cells (6). Using strain MG3 and plasmid pMG600, which are similar to strain TAK69 and plasmid pAC33 (see Materials and Methods), it was shown that, for liquid-grown cells, induction with 100 μM IPTG caused a slight cell elongation, while induction with 1 mM IPTG was required in order for the liquid-grown cells to differentiate into fully elongated cells resembling swarm cells (6). In order to assess the amount of flhDC mRNA that causes liquid-grown cells to differentiate, samples for Northern analysis were taken from an exponential-phase MG3/pMG600 culture at OD450 = 0.3, 20 min after the culture had received IPTG to a final concentration of 50 or 200 μM. As shown in Fig. 2, the level of flhDC mRNA in cells grown in liquid culture in the presence of 50 μM IPTG was approximately 3-fold higher than the level found in the wild-type swarm cells, while the amount of flhDC mRNA in the cells induced with 200 μM IPTG was approximately 16-fold higher than the level in the wild-type swarm cells. These results suggest that differentiation of liquid-grown cells occurs only in response to artificially high flhDC mRNA levels, which are not found in surface-grown swarm cells. Figure 3 shows that the swarming motility of MG3/pMG600 is inhibited at IPTG concentrations above 50 μM, indicating that high expression of flhDC is not compatible with swarming motility on agar surfaces.
FIG. 3.
Expansion rates of MG3/pMG600 colonies on 0.6% agar medium supplemented with different IPTG concentrations.
As shown in Fig. 2, flhDC transcripts of two different sizes (approximately 1.4 and 1.2 kb) were detected in the Northern analysis. For lanes a to c the flhDC mRNA is transcribed in the wild-type strain, while for lanes d to f the level of flhDC mRNA is controlled from the IPTG-inducible Ptac promoter in the MG3/pMG600 strain. Since the intensities of both bands are increased by IPTG induction in strain MG3/pMG600, we believe that the smaller transcript (1.2 kb) originates from posttranscriptional processing of the larger transcript (1.4 kb).
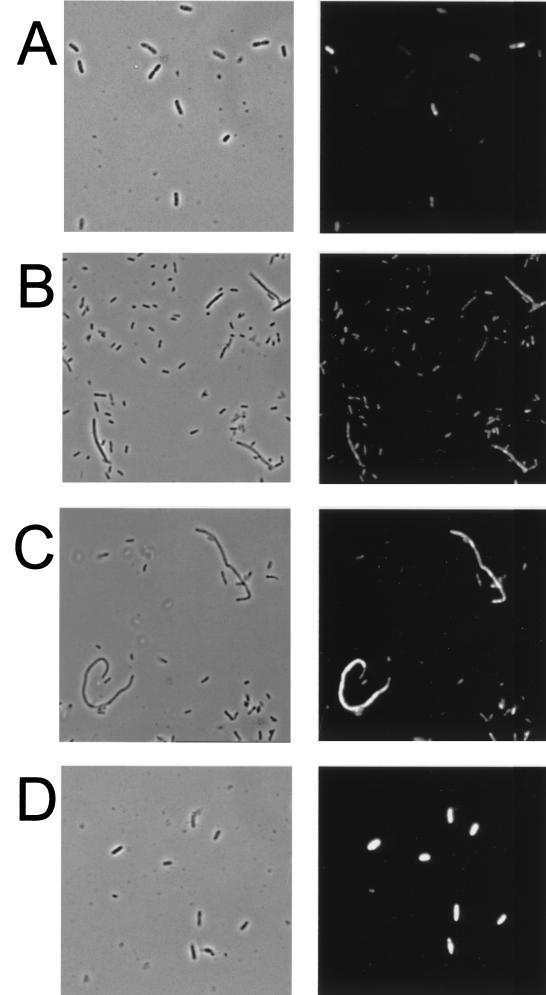
The presence of differentiated and nondifferentiated cells in the outer region of a swarming S. liquefaciens colony (see below) raises the question of whether the determined levels of flhDC transcription (Fig. 1) and flhDC mRNA content (Fig. 2) represent an average of a high level in the swarm cells and a low level in the nondifferentiated cells. However, since swarm cells can be distinguished from nondifferentiated cells by their morphology, the problems associated with population-level analysis can be overcome by monitoring gene expression in individual cells. Using Salmonella enterica serovar Typhimurium as a model organism, we have previously demonstrated the ability of in situ RT-PCR to monitor the presence or absence of specific mRNA in individual cells (26, 27) and have shown that this method can be used to monitor different levels of specific mRNA in individual bacterial cells semiquantitatively (17). In order to monitor flhDC mRNA levels in individual S. liquefaciens bacteria, MG1 cells sampled from the outermost layer of a swarming colony and from a mid-log-phase (OD450 = 0.4) culture were subjected to flhDC mRNA-targeted in situ RT-PCR. The in situ RT-PCR was carried out with reporter molecule-labeled primers, and subsequently intracellular reporter molecules were detected by the use of a fluorogenic assay. As shown in Fig. 4A and B, the swarm cells and vegetative cells did not exhibit significant differences in fluorescence intensity after flhDC mRNA-targeted in situ RT-PCR, suggesting that the swarm cells contained about the same amount of flhDC mRNA as the vegetative cells. To exclude the possibility that in situ RT-PCR is inhibited in the swarm cells, we performed in situ RT-PCR targeting the phlA mRNA. Expression of the phospholipase, determined as transcription of a phlA-lacZ fusion, was previously shown to be specifically increased in swarm cells (13), and, as shown in Fig. 4C, this was also visualized using in situ RT-PCR.
FIG. 4.
In situ RT-PCR targeting flhDC mRNA (A, B, and D) or plhA mRNA (C) in S. liquefaciens. (A) Liquid-grown MG1 mid-log-phase (OD450 = 0.4) cells. (B and C) MG1 cells isolated from the edge of a swarming colony. (D) Liquid-grown MG3/pMG600 mid-log-phase cells sampled 20 min after addition of 200 μM IPTG. Phase-contrast photomicrographs (left) and epifluorescence photomicrographs (right) of the same viewing fields are shown.
The cell populations taken from the growing culture or from the edge of a swarming colony were heterogeneous with respect to the intensity of the fluorescence emitted from the cells after flhDC mRNA-targeted in situ RT-PCR (Fig. 4A and B). This could mean that the flhDC mRNA levels in the cells were at the limits of detection. However, in general the dividing cells and the small daughter cells emitted little fluorescence, while the nondividing (growing) cells emitted more fluorescence. This pattern of fluorescence heterogeneity indicates cell cycle-regulated expression of the flhDC operon, with lowest levels in dividing and newborn cells, which is in concurrence with the results of Prüss and Matsumura (25) demonstrating that expression of the flhDC operon in liquid-grown Escherichia coli cells peaks in the middle of the cell cycle and is lowest at the time of cell division. Nevertheless, we found it worthwhile to ensure that different levels of flhDC mRNA could be visualized using the in situ RT-PCR method. Furness et al. (10) reported that P. mirabilis swarm cells contained at least 30-fold more flhDC mRNA than vegetative cells. Since the Northern analysis indicated that liquid-grown S. liquefaciens MG3/pMG600 cells induced with 200 μM IPTG contain approximately 16 times more flhDC mRNA than wild-type vegetative cells (Fig. 2, lanes a and e), we could determine whether such differences in flhDC mRNA levels could be detected by use of the in situ RT-PCR method. After being subjected to flhDC mRNA-targeted in situ RT-PCR, liquid-grown S. liquefaciens MG3/pMG600 cells induced with 200 μM IPTG did indeed fluoresce much more than wild-type vegetative and swarm cells (Fig. 4A, B, and D).
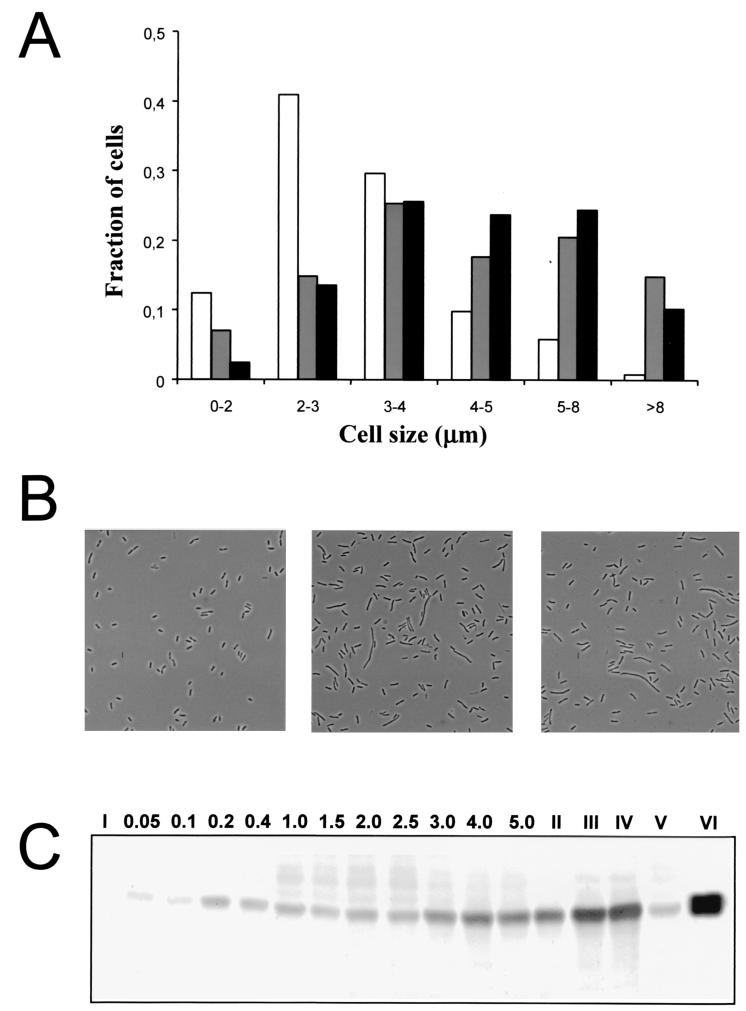
It was of interest to investigate the extent of flagellation of S. liquefaciens swarm and swim cells. Cell samples taken from the outer region of a swarming S. liquefaciens colony contain a mixture of differentiated swarm cells and nondifferentiated cells as shown in Fig. 5. This renders collection of pure populations of S. liquefaciens swarm cells difficult. However, approximately 65% of the cells isolated from the edge of a swarming colony consisted of elongated cells, and Western analysis demonstrated that the average content of flagella per cell mass (OD450) in this population was approximately threefold higher than the content of liquid-grown cells in the late log phase (Fig. 5). We also investigated liquid-grown S. liquefaciens MG3/pMG600 cells induced with different IPTG concentrations (0, 10, 25, 50, 100, 250, 500, and 1,000 μM). The cell size distribution and the content of flagella per cell mass were determined in samples taken from these cultures, which were all grown from an OD450 of 0.02 to 0.3 (data not shown). The cell size analysis supported our previous report (6) that liquid-grown S. liquefaciens MG3/pMG600 cells induced with 1 mM IPTG closely resemble wild-type S. liquefaciens swarm cells isolated from the edge of a swarming colony (Fig. 5A and B). However, the Western analysis suggested that the population of liquid-grown S. liquefaciens MG3/pMG600 cells induced with 1 mM IPTG had approximately twice the amount of flagella found on the wild-type swarm cell population (Fig. 5C).
FIG. 5.
Cell size and flagellar content in liquid cultures and a population isolated from the edge of a swarming colony. (A) Cell size distributions of an S. liquefaciens MG1 population from a mid-log-phase (OD450 = 0.4) culture (white bars), an S. liquefaciens MG1 population from the edge of a swarming colony (gray bars), and an S. liquefaciens MG3/pMG600 population from an IPTG-induced (1 mM) mid-log-phase culture (black bars). (B) Micrographs of MG1 cells from a mid-log-phase culture (left), MG1 cells from the edge of a swarming colony (middle), and MG3/pMG600 cells from an IPTG-induced (1 mM) mid-log-phase culture (right). (C) Western blot with flagellum-specific antibodies. Lane I, MG3 (flhD); lanes II, III, and IV, samples from the edge of a swarming MG1 colony loaded 3-fold diluted, 1.5-fold diluted, and undiluted, respectively; lane V, sample from the center of a swarming MG1 colony; lane VI, sample from an IPTG-induced (1 mM) MG3/pMG600 mid-log-phase culture; lanes 0.05 to 5.0, samples from an exponentially growing MG1 culture at OD450 values equal to the lane numbers.
Taken together the results presented here demonstrate that surface-induced swarm cell differentiation in S. liquefaciens is not accompanied by a substantial increase in flhDC transcription or flhDC mRNA content. Cells do, however, elongate, and therefore the average swarm cell carries many more flagella than a swim or vegetative cell, but the cells are, strictly speaking, not hyperflagellated. In contrast to this, P. mirabilis swarm cell differentiation is evidently accompanied by a 30-fold increase in flhDC mRNA content and a 50-fold increase in flagellation (10, 20). Our results obtained with IPTG-controlled flhDC expression demonstrate that a similar increase in the level of S. liquefaciens flhDC mRNA would switch off the swarming motility of surface-grown cultures. Since differentiation of liquid-grown S. liquefaciens into swarm cells requires a substantially higher flhDC mRNA content than that found in wild-type (surface-grown) swarm cells, it might be speculated that swarm cell differentiation on the surface occurs through posttranscriptional regulation of the flhDC operon. If posttranscriptional modification occurs specifically in response to stimuli present in a swarming colony (for example, surface exposure), then liquid-grown cells may differentiate only in response to artificially elevated flhDC mRNA contents, resulting in an increased FlhDC amount and activity that under physiological conditions is found only in swarm cells.
The ability of the cells comprising the swim and the swarm subpopulations to go through cycles of differentiation and dedifferentiation is considered a major factor determining expansion of the moving culture (8, 9). However, it must be emphasized that Serratia and Proteus display different kinds of swarming behavior. In P. mirabilis the swarm-swim interconversion cycles occur in a synchronized fashion, and as a result the colony either grows or expands. Unlike Proteus, Serratia secretes serrawettins, making continuous spreading of the growing culture possible (21–24). Gfp tagging and single-cell analysis were recently used to dissect a swarming Serratia colony (8). This analysis suggested that the behavior of the differentiated rafted Serratia swarm cells causes the formation and spreading of a surface-conditioning film and circulates cells between the subcultures of swarm and vegetative cells present at the border and the more central parts of the colony, respectively (8). This continuously creates new zones of growth and abolishes the formation of distinct consolidation-motility phases, as seen with P. mirabilis. On the other hand, P. mirabilis produces a capsular polypeptide that reduces surface friction and enables the differentiated cells to move on growth media with high agar content (15). Proteus swarms on agar concentrations from 0.5 to 2.5%, whereas Serratia swarming takes place in the narrow range of 0.5 to 1% agar. The difference in control of flhDC gene expression and cell differentiation probably relates to the obvious mechanistic and behavioral differences in swarming motility of the two related enteric bacteria Serratia and Proteus.
ACKNOWLEDGMENTS
Work on biofilms is supported by grants from the Danish Biotechnology Program and the Danish Medical Research Council to M.G. and S.M. and from the Deutsche Forschungsgemeinschaft (EB 2051/1-1) to L.E.
REFERENCES
- 1.Allison C, Hughes C. Bacterial swarming: an example of prokaryotic differentiation and multicellular behaviour. Sci Prog. 1991;75:403–422. [PubMed] [Google Scholar]
- 2.Allison C, Coleman N, Jones P L, Hughes C. Ability of Proteus mirabilis to invade human urothelial cells is coupled with to motility and swarming differentiation. Infect Immun. 1992;60:4740–4746. doi: 10.1128/iai.60.11.4740-4746.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allison C, Lai H-C, Hughes C. Co-ordinate expression of virulence genes during swarm-cell differentiation and population migration of Proteus mirabilis. Mol Microbiol. 1992;6:1583–1591. doi: 10.1111/j.1365-2958.1992.tb00883.x. [DOI] [PubMed] [Google Scholar]
- 4.Belas M R, Colwell R R. Adsorption kinetics of laterally and polarly flagellated Vibrio. J Bacteriol. 1982;151:1568–1580. doi: 10.1128/jb.151.3.1568-1580.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertani G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951;62:293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eberl L, Christiansen G, Molin S, Givskov M. Differentiation of Serratia liquefaciens into swarm cells is controlled by the expression of the flhDC master operon. J Bacteriol. 1996;178:554–559. doi: 10.1128/jb.178.2.554-559.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eberl L, Winson M K, Sternberg C, Stewart G S A B, Christiansen G, Chhabra S R, Bycroft B, Williams P, Molin S, Givskov M. Involvement of N-acyl-L-homoserine lactone autoinducers in controlling the multicellular behaviour of Serratia liquefaciens. Mol Microbiol. 1996;20:127–136. doi: 10.1111/j.1365-2958.1996.tb02495.x. [DOI] [PubMed] [Google Scholar]
- 8.Eberl L, Molin S, Givskov M. Surface motility in Serratia liquefaciens. J Bacteriol. 1999;181:1703–1712. doi: 10.1128/jb.181.6.1703-1712.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esipov S E, Shapiro J A. Kinetic model of Proteus mirabilis swarm colony development. J Math Biol. 1998;36:249–268. [Google Scholar]
- 10.Furness R B, Fraser G M, Hay N A, Hughes C. Negative feedback from a Proteus class II flagellum export defect to the flhDC master operon controlling cell division and flagellum assembly. J Bacteriol. 1997;179:5585–5588. doi: 10.1128/jb.179.17.5585-5588.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Givskov M, Molin S. Expression of the extracellular phospholipase from Serratia liquefaciens is growth-phase dependent, catabolite repressed and regulated by anaerobiosis. Mol Microbiol. 1992;6:1363–1374. doi: 10.1111/j.1365-2958.1992.tb00857.x. [DOI] [PubMed] [Google Scholar]
- 12.Givskov M, Olsen L, Molin S. Cloning and expression in Escherichia coli of the gene for extracellular phospholipase A1 from Serratia liquefaciens. J Bacteriol. 1988;170:5855–5862. doi: 10.1128/jb.170.12.5855-5862.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Givskov M, Eberl L, Christiansen G, Benedik M J, Molin S. Induction of phospholipase and flagellar synthesis in Serratia liquefaciens is controlled by expression of the flagellar master operon flhDC. Mol Microbiol. 1995;15:445–454. doi: 10.1111/j.1365-2958.1995.tb02258.x. [DOI] [PubMed] [Google Scholar]
- 14.Givskov M, Östling J, Lindum P W, Eberl L, Christiansen A B, Christiansen G, Molin S, Kjelleberg S. The participation of two separate regulatory systems in controlling swarming motility of Serratia liquefaciens. J Bacteriol. 1998;180:742–745. doi: 10.1128/jb.180.3.742-745.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gygi D, Bailey M J, Allison C, Hughes C. Requirement for FlhA in flagella assembly and swarm-cell differentiation by Proteus mirabilis. Mol Microbiol. 1995;15:761–770. doi: 10.1111/j.1365-2958.1995.tb02383.x. [DOI] [PubMed] [Google Scholar]
- 16.Harshey R M. Bees aren't the only ones: swarming in gram-negative bacteria. Mol Microbiol. 1994;13:389–394. doi: 10.1111/j.1365-2958.1994.tb00433.x. [DOI] [PubMed] [Google Scholar]
- 17.Holmstrøm K, Tolker-Nielsen T, Molin S. Physiological states of individual Salmonella typhimurium cells monitored by in situ reverse transcription-PCR. J Bacteriol. 1999;181:1733–1738. doi: 10.1128/jb.181.6.1733-1738.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kristensen C S, Eberl L, Sanchez-Romero J M, Givskov M, Molin S, deLorenzo V. Site-specific deletions of chromosomally located DNA segments with the multimer resolution system of broad-host-range plasmid RP4. J Bacteriol. 1995;177:52–58. doi: 10.1128/jb.177.1.52-58.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 20.Lai H-C, Gygi D, Fraser G M, Hughes C. A swarming-defective mutant of Proteus mirabilis lacking a putative cation-transporting membrane P-type ATPase. Microbiology. 1998;144:1957–1961. doi: 10.1099/00221287-144-7-1957. [DOI] [PubMed] [Google Scholar]
- 21.Lindum P W, Anthoni U, Christoffersen C, Eberl L, Molin S, Givskov M. N-Acyl-l-homoserine lactone autoinducers control production of an extracellular lipopeptide biosurfactant required for swarming motility of Serratia liquefaciens MG1. J Bacteriol. 1998;180:6384–6388. doi: 10.1128/jb.180.23.6384-6388.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuyama T, Murakami T, Fujita M, Fujita S, Yano I. Extracellular vesicle formation and biosurfactant production by Serratia marcescens. J Gen Microbiol. 1986;132:865–875. [Google Scholar]
- 23.Matsuyama T, Kaneda K, Ishizuka I, Toida T, Yano I. Surface-active novel glycolipid and linked 3-hydroxy fatty acids produced by Serratia rubidaea. J Bacteriol. 1990;172:3015–3022. doi: 10.1128/jb.172.6.3015-3022.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsuyama T, Kaneda K, Nakagawa Y, Isa K, Hara-Hotta H, Yano I. A novel extracellular cyclic lipopeptide which promotes flagellum-dependent and -independent spreading growth of Serratia marcescens. J Bacteriol. 1992;174:1769–1776. doi: 10.1128/jb.174.6.1769-1776.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prüss B M, Matsumura P. Cell cycle regulation of flagellar genes. J Bacteriol. 1997;179:5602–5604. doi: 10.1128/jb.179.17.5602-5604.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tolker-Nielsen T, Holmstrøm K, Molin S. Visualization of specific gene expression in Salmonella typhimurium by in situ PCR. Appl Environ Microbiol. 1997;63:4196–4203. doi: 10.1128/aem.63.11.4196-4203.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tolker-Nielsen T, Holmstrøm K, Boe L, Molin S. Non-genetic population heterogeneity studied by in situ polymerase chain reaction. Mol Microbiol. 1998;27:1099–1105. doi: 10.1046/j.1365-2958.1998.00760.x. [DOI] [PubMed] [Google Scholar]