Abstract
The rapid development of synthetic biology has enabled the production of compounds with revolutionary improvements in biotechnology. DNA manipulation tools have expedited the engineering of cellular systems for this purpose. Nonetheless, the inherent constraints of cellular systems persist, imposing an upper limit on mass and energy conversion efficiencies. Cell-free protein synthesis (CFPS) has demonstrated its potential to overcome these inherent constraints and has been instrumental in the further advancement of synthetic biology. Via the removal of the cell membranes and redundant parts of cells, CFPS has provided flexibility in directly dissecting and manipulating the Central Dogma with rapid feedback. This mini-review summarizes recent achievements of the CFPS technique and its application to a wide range of synthetic biology projects, such as minimal cell assembly, metabolic engineering, and recombinant protein production for therapeutics, as well as biosensor development for in vitro diagnostics. In addition, current challenges and future perspectives in developing a generalized cell-free synthetic biology are outlined.
Keywords: Cell-free protein synthesis, Synthetic biology, Minimal cell, Metabolic engineering, Biomanufacturing, In vitro diagnostics
Graphical Abstract
1. Introduction
The call to move towards a more sustainable, green industry has been a catalyst for the growth of biotechnology, shifting our economy away from industries dependent on petroleum and toward bio-based industries. Synthetic biology is an interdisciplinary field that combines biology, engineering, and technology to create products and services through living systems. Its rapid growth has the potential to revolutionize biotechnology, medicine, and the environment [1], [2], [3]. Recent breakthroughs in the genomic editing tools of the CRISPR/Cas systems [4], [5], [6], in conjunction with the earlier molecular tools of synthetic biology, have allowed the precise and efficient engineering of biological systems to generate a wide range of products, including renewable energy sources, crop varieties, food and medical products, and other environmentally friendly products. Although the ongoing development of such gene modification techniques has provided powerful programming tools for cell factories, there are still inherent constraints [7]. The coupling of the maintenance of the living system and the synthesis of desired products defines the upper-limit efficiency of the whole synthetic system. In addition, the complex cellular system and chassis behavior of different model host cells result in inconsistency from case to case.
With its distinct advantages for applications in synthetic biology, the Cell-free protein synthesis (CFPS) approach is emerging to solve the aforementioned general constraints of cells [8], [9], [10], [11]. The CFPS system employs a minimum enzymatic apparatus for transcription, translation, and energy regeneration, derived either from cell extracts or pure enzymes [12], [13]. Born as a simple and streamlined reconstituted system, CFPS naturally overcomes this inherent limitation of a living cell and provides direct access to its essential activities. Such an open nature of the CFPS enables the first-ever programming of modular cellular mimicking processes with active transcription and translation support. In addition, non-native chemicals can be introduced directly into the system, allowing greater flexibility in the selection of regulating reagents [14], [15].
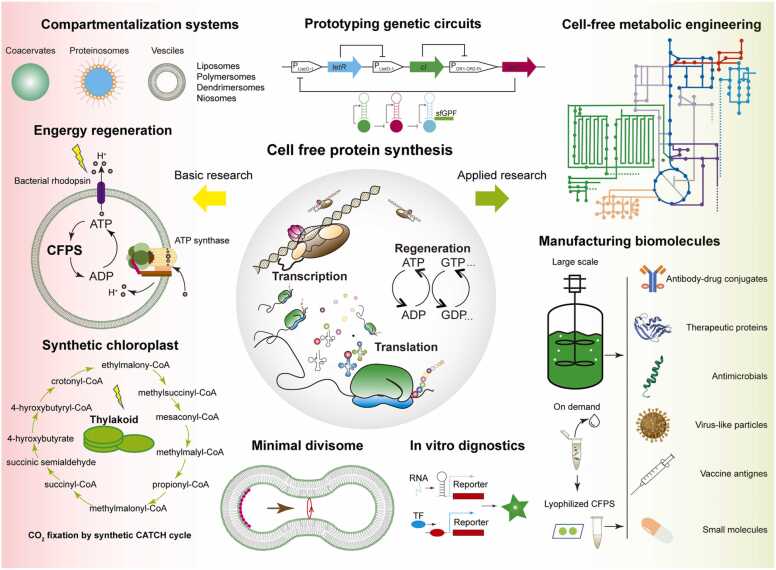
The application of the CFPS technique came much later to the field of synthetic biology than its application as tools for discovering and illustrating basic principles of biological systems. The first well-known application of the CFPS technique was done by Nirenberg and Matthaei [16], [17], [18] to decipher the genetic codes. Later, with continuous improvement in efficiency, it gradually developed as a complementary tool for the production of recombinant proteins, in particular, for membrane proteins and toxins that are not well expressed in cells [15], [19], [20], [21], [22], [23]. In addition to protein production, the application of the CFPS technique to the broad field of synthetic biology probably came first to the field of synthetic cell construction via a bottom-up strategy [24], [25], [26], [27]. This distinct field seeks to obtain a plausible route to the origin of life from the bottom up, which starts with simple molecules, such as fatty acids, DNA or RNA molecules, to establish cell mimicry systems [28], [29]. Such a system can possess certain essential characteristics of a living cell or carry out certain basic activities [30], [31]. However, when the CFPS technique is applied, more complex modular systems can be established beyond simple enzyme-catalyzed reactions, such as energy generation, metabolism, template-guided self-replication, growth, and division, which can be further integrated with additional regulatory machinery (Fig. 1) [32], [33]. In addition, CFPS also shows great potential to revolutionize cell-based synthetic biology with product-driven applications [33], [34]. The fast cycle of CFPS has greatly shortened the period of the typical design-build-test (DBT) circle, in particular for tasks such as metabolic engineering [35], protein-directed evolution [36], [37], [38], [39], and de novo protein design [40]. Last but not least, the combination of the CFPS technique and DNA manipulation tools, such as DNA amplification and editing, further extends its application in biomedical applications of in vitro diagnostic and portable analytical tools (Fig. 1, Table 1).
Fig. 1.
Applications of cell-free protein synthesis in both fundamental and applied research in synthetic biology. With reconstituted transcription and translation machinery, the CFPS system (adapted from Ref. [25] with permission from Copyright 2017, Elsevier B.V.) is extensively utilized in the construction of minimal cellular mimicry systems within different compartmentalization systems (adapted from Ref. [7] with permission from Copyright 2019, WILEY-VCH), such as energy regeneration, metabolism (photosynthesis via the synthetic metabolic pathway: the crotonyl-CoA/ethylmalonyl-CoA/hydroxybutyryl-CoA (CATCH) cycle), and the reconstitution of minimal divisome. CFPS synthesis has been accepted as a basic method for prototyping genetic circuits (adapted from Ref. [109] under Creative Commons CC BY license 2019) metabolic engineering, and large- and small-scale biomanufacturing in the applied sector. The lyophilization of CFPS offers a long shelf life and non-cold chain shipping, allowing on-demand production of antibody-drug conjugates, therapeutic proteins, antimicrobial peptides, virus-like particles, vaccine antigens, and small-molecule medicines. Furthermore, the coupling of CFPS with other biosensors offers considerable diagnostic potential in vitro.
Table 1.
Summary of the major applications of CFPS in synthetic biology.
| Applications | Specifications | References |
|---|---|---|
| Construction of minimal cellular mimicry systems | Compartmentalization | Liposomes [111], polymersomes [113], proteinosomes [120], [121], dentrimersomes [122], [123], [124], encapsulins [125], [126] and niosomes [127], [128] were developed for compartmentalization |
| Energy regeneration | ATP regeneration via a light-driven proton pump and an ATP synthase [138], [139] | |
| Metabolism | Semi-synthetic chloroplast [140] | |
| Self-replication | Replication via phi29 polymerase and PURE system [141,142] | |
| Growth and Division | De novo synthesis phospholipids [144]; reconstitution of proto-ring within a GUV [155] | |
| Communication, motility and evolution | See references [156], [157], [158] | |
| Products-oriented applications | Metabolic engineering | Achieve 1.5 g/L production of n-butanol [165], 17.6 g/L of mevalonate [164] |
| Manufacturing biomolecules | Antibody-drug conjugates, therapeutic proteins, antimicrobials, virus-like particles, vaccine antigens, small molecules [168], [169] | |
| In vitro diagnostics | Develop a portable device for the detection of anhydrotetracycline, Ebola virus RNA, and various small molecules [183], [184] |
2. Constant evolution of CFPS systems
Since its inception in the 1960 s, the CFPS system has undergone extensive systematic optimization to increase its efficiency. This optimization has been extended to all major components, including extract [41], [42], [43], [44], [45], [46], template [47], [48], [49], energy source [50], [51], [52], [53], buffer [53], [54], [55], [56], [57], and incubation and reaction settings [41], [58]. Productivity continues to increase from around 0.1 mg/mL to above 4.0 mg/mL in batch configurations for crude lysate-based CFPS [59]. In terms of the fully reconstituted CFPS system, protein synthesis using recombinant elements (PURE) has also been systematically optimized, resulting in an improvement in efficiency from micrograms to sub-milligrams per mL in a batch configuration [60]. The pioneering work by Spirin and co-workers greatly improved the efficiency of the resulting CFPS system by a gradient-driven passive compound exchange through a dialysis membrane, which provided not only a large pool of substrate precursors, but also a sink for removal of inhibitory by products such as free phosphate. Besides in solution, the CFPS reaction can also be lyophilized as pellets or on porous matrices such as filter paper or cellulose matrices, which allowed the storage under room temperature and could be activated later via rehydration (see the section ‘Manufacturing Biomolecules’ and ‘New Trends in In Vitro Diagnostics’) [61], [62], [63]. The detailed development of various CFPS was reviewed in a set of review papers by us and other groups [22], [25], [64], [65], [66].
In addition to the PURE system, CFPS relies on crude cell lysate to provide the enzymatic pool to support gene expression [67]. Currently, the sources of the extract are extremely diverse, including prokaryotic, eukaryotic, and archaeal cells, from model host cells such as E. coli [41], [42], Bacillus subtilis [68], yeast [69], insect cells [70], [71], rabbit reticulocyte [72], wheat germ [73], [74], [75], tobacco cell [76], rice callus cells [77], CHO cells [78], [79], Hela [80], to non-model organisms such as Thermococcus kodakaraensis [81], Klebsiella pneumoniae [82], Vibrio natriegens [83], Clostridium thermocellum [84] and Streptomyces [85], which were reviewed with regard to their advantages and disadvantages by Kubick et al. [86]. In conclusion, prokaryotic systems have the advantage of high productivities but no inherited post-translational modifications, whereas eukaryotic systems have the advantage of inherited post-translational modifications but low total expression yields. Furthermore, the limited modification machinery from eukaryotic cell extract may result in the heterogeneity of the proteins that are produced. Due to the accessibility of most molecular tools, E. coli, the most thoroughly researched model cell, remained the predominant source for synthetic biology. Furthermore, recent studies have shown that some post-translational modifications, such as phosphorylation [87], [88], glycosylation [89], and lipidation [90], could be performed successfully with E. coli-based CFPS systems by incorporating functional extracts or pure enzymes containing the associated modification machinery. Therefore, we focus on the E. coli-based CFPS system in the following parts.
Within a cell, gene expression is a carefully regulated process that ensures the spatial-temporal distribution of hundreds of proteins to perform their required activities. The evolution of genetic circuits is focused on the regulation of gene expression networks, which have been studied intensively in vivo using numerous approaches [91]. With inheritance from the crude cell lysate, the CFPS system has the ability to activate in vivo validated genetic circuits. However, relatively fewer incidents have been reported compared to in vivo. For example, experiments to control CFPS transcription rates were conducted using different polymerases, such as T7 and bacterial E. coli RNA polymerases [92], [93]. Additionally, different T7 promoters were used to regulate transcription and translation rates in CFPS [94]. To further engineer cell-free synthetic gene circuits, a cell-free expression toolbox was designed with 13 different E. coli sigma factors, two bacteriophage RNA polymerases, and a set of repressors. This toolbox allowed the design and testing of various circuit motifs, such as multiple-stage cascades, an AND gate, negative and positive feedback loops, transcriptional cascades with a protein-regulated incoherent feedforward loop, and in vitro ring oscillators [95]. In addition to proteins, RNA molecules can also regulate transcription, translation, and catalysis and provide an attractive alternative to regulatory elements [96], [97], [98], [99], [100], [101]. Riboswitches, for example, are located in the non-translated region of mRNA and can up- or down-regulate gene expression in response to ligand binding [102], [103], [104], [105], [106], [107]. Synthetic theophylline-responsive riboswitch and natural adenine-sensing B. subtilis riboswitch have been established for robust in vitro on-off switching in water-in-oil emulsions and vesicles [108].
3. Application of CFPS to the construction of minimal cells
3.1. Compartmentalization
The prerequisite to distinguish a living cell from the non-living environment is a physical boundary that offers the basic control of mass exchange between the internal compartment and the outer space. Anything that stabilizes phase separation can be used as a material to form synthetic compartments [110]. Inspired by nature, extracted or synthetic phospholipids are often used for their ability to form cell-like versicles by self-assembly into a bilayer [111]. Similar to the structure of a phospholipid molecule, synthetic polymers, such as block copolymers [112], can also self-assemble into vesicles [113] and can be tuned to control the basic properties of the formed vesicles, such as size, membrane thickness [114], rigidity and permeability [115], surface properties [116], and selectivity of the encapsulated materials [117]. Hybrid vesicles that form homogeneous or phase-separated membranes have been reported to depend on the specific application requirement [118], [119]. In addition, alternative compartmentalization systems such as proteinosomes [120], [121], dentrimersomes [122], [123], [124], encapsulins [125], [126], and niosomes [127], [128] were reported. Following the basic principles for forming a self-assembled compartment, they offer superior properties, such as stability, encapsulation efficiency, and biocompatibility with natural cells. Moreover, other phase-forming materials, such as hydrogel [129], [130] and coacervate [131], [132], by liquid-liquid phase separation, can also be used as membraneless compartmentalization methods, mimicking the membrane organelles in cells [133], [134], [135]. However, in addition to the physical boundary, the cell membrane functions as the matrices and catalytic interfaces for many processes like signaling, which certainly require addition factors like membrane proteins, as discussed in the ‘Summary and Perspective’ section.
3.2. Integration of individual synthetic modules
Before the application of the CFPS technique in the construction of minimal cells, individual modules such as energy regeneration, self-replication, growth and division, metabolism, motility, and communication could be carried out by a few simple molecules. However, the success of encapsulating the CFPS system in a synthetic microcompartment would already be a multifunctional synthetic cell that at least contains modules for energy regeneration, transcription, and translation machinery. In 2004 Noireaux et al. successfully encapsulated CFPS within phospholipid vesicles by emulsion transfer, which could produce 30 µM protein in 4 days [136]. Furthermore, expression could be tuned by introducing regulatory molecules, such as transcription factors (see the above section on genetic circuits). The CFPS system contains a number of molecules to fuel the reaction, which are normally the energy precursor within glycolysis pathways [53], or high-energy phosphate compounds such as acetyl phosphate [137], creatine phosphate [43]. Besides the energy precursors used in the CFPS system, the light-induced proton gradient was adopted to fuel gene expression in the CFPS system through a light-activated proton pump (bacteria rhodopsin) and an ATP synthase within the membrane of the synthetic cell [138], [139]. In addition to energy regeneration, the cell uses metabolism to obtain building blocks required for self-reproduction. The beauty of the cell metabolism network is that the entire metabolism network is auto-regulated to maintain homeostatic control while providing continuous materials and energy. Recently, a show-off case was reported in which light-driven CO2 fixation could be carried out by a synthetic chloroplast consisting of natural and synthetic parts [140]. As the ultimate goal of building a living cell, self-reproduction allows the generation of offspring that have the same genetic materials, which is the critical step for continuous existence and evolution. In order to be able to self-reproduce, a cell would need several essential characters including: 1) self-replication of information molecule (genetic material); 2) growth and division. Therefore, these functions are the first goal to be achieved toward a minimal cell. In one case, the Danelon group showed [141] a self-replicated artificial cell that was able to self-replicate by co-encapsulating the DNA template encoded with the phi29 polymerase via the PURE system. In this case, the DNA of phi29 could be replicated when the coding proteins were expressed by the PURE system. In another case, Libicher et al. [142] demonstrated a replication of the 116 kb multipartite genome (distributed in 11 plasmids) via the PURE system. The size of 116 kb already matched the minimum genome. However, further efforts are still needed to check if this could be achieved in any synthetic microcompartments. Being able to self-replicate, cells need to be able to grow and divide to reproduce in a sustainable way. As the first physical boundary of a cell, the materials that hold the compartment define the volume of the system and need to be reproduced. Several examples have shown the growth of different microcompartments without enzymes [143]; however, we envision that de novo synthesis of lipid molecules is vital for autonomous self-reproduction. As the critical step, recent studies showed the successful synthesis of PE and PG within the synthetic cell by activating the synthesis of correlated enzymes by the encapsulated CFPS system [144]. Finally, a division step would complete the self-reproduction process. Although division could be achieved through many mechanical processes by introducing appropriate shear forces [145], self-autonomous division would require several key steps to ensure a symmetric distribution of materials in the resulting daughter cells [146]. In bacterial cells, such as E. coli, binary division is initiated by forming a proto-ring, which ultimately leads to complete division by invagination [147], [148], [149], [150]. Pioneering work by the Schwille group has shown that positioning the proto-ring at the middle of the cell only required three self-organized proteins-Min C, D, and E from E. coli [151], [152], [153], [154]. Furthermore, one of their recent studies has shown a successful positioning of the proto-ring with Min C, D, E, FtsA, and FtsZ by de novo synthesis via a PURE system within a GUV [155]. More interestingly, a ring-induced shape transformation of the formed GUVs was observed, which might be the leading forces to trigger further deformation until the GUVs were divided. Many other synthetic systems have been successfully established that could enable communication [156], mobility [157], and evolution [158] with the integration of the CFPS technique. Therefore, we would envision a more mature multifunctional minimal cell, merging with the continuous efforts in the near future.
4. Application of CFPS to products-oriented synthetic biology
4.1. Cell-free metabolic engineering (CFME)
Unlike cell metabolic engineering, cell-free metabolic engineering involves the engineering of metabolic pathways in a cell-free environment through purified enzymes or crude cell lysates [159]. The major advantage of CFME is that it is much faster and more efficient, since it eliminates the need for iteration and selection steps when producing a desired product. CFME also avoids problems associated with cell-based approaches, such as cell toxicity and genetic instability. In addition, it is much easier to optimize and troubleshoot in CFME, as the reactions occur in vitro, allowing more flexibility. Finally, CFME enables the development of more complex metabolic pathways and the production of more diverse products. Long before the introduction of CFPS, enormous efforts have been made to synthesize valuable chemical compounds and natural products in bioreactors using enzymes [160], [161], [162]. However, it became extremely challenging and labor intensive to produce individual purified enzymes and combine them with proper stoichiometry for the best conversion efficiency. Recent cases gave us an overview of the current extremes of what could be achieved by purified enzymes. The Bowie group [163] reconstituted a cell-free monoterpene pathway with 27 purified enzymes, which achieved more than 95 % conversion yield and 15 g/L titer. Such a higher titer is more than an order of magnitude higher than the lethal concentrations for cell-based systems.
Pioneered by the group of Jewett [35], they proposed a novel CFPS-based metabolic engineering framework to build biosynthetic pathways. This process involved directly synthesizing each enzyme of a biosynthetic pathway in vitro using cell-free lysates and combining multiple crude lysates to initiate the DBT cycle. Enzyme-rich lysate performed catalytic tasks in place of separately isolated enzymes. Applying this strategy to mevalonate biosynthesis [164], the CFPS system produced 17.6 g/L (119 mM) of mevalonate in 20 h, in contrast to the initial titer of 1.6 g/L produced in 9 h. Using the same mechanism, a prototype of n-butanol biosynthesis was developed. The system produced 1.2 g/L of n-butanol by using natural glycolytic enzymes to convert glucose to acetyl-CoA and heterologous enzymes to convert acetoacetyl-CoA to n-butanol. In less than a day, the researchers studied four Ter homologs and three AdhE homologs that replaced some of the initial Ter and AdhE enzymes. They also demonstrated the use of linear DNA templates, which eliminated the need for laborious cloning procedures and resulted in an increase of up to 1.5 g/L in the synthesis of n-butanol [165]. In conclusion, CFPS-based metabolic engineering provides significantly faster DBT cycles than cell-based metabolic engineering and eliminates enzyme purification processes.
4.2. Manufacturing biomolecules
Proteins are major macromolecules that are essential for the structure and function of living cells, providing structure to cells, acting as enzymes to catalyze biochemical reactions, and performing or regulating a variety of other metabolic processes. Therefore, as an important recombinant protein production tool with unique advantages, the CFPS system has been applied to produce various therapeutic protein products, covering different cytokines, cytotoxins, antibodies, vaccine antigens, virus-like particles and antimicrobials, which were reviewed elsewhere [8], [166], [167]. What we want to emphasize is the flexibility of deploying the CFPS system in a variety of application environments, which suits both large-centralized industrial-scale production and small-batch production of therapeutic and laboratory reagents. Large-scale CFPS has been utilized in commercial efforts, such as Sutro Biopharma, Inc., which was able to produce up to 1000 liters of cell-free reactions [167]. This method took advantage of fast synthesis outside the cell, which greatly sped up the drug development process. Different from large centralized infrastructures, the CFPS can provide a flexible on demand protein production. As pioneered by Pardee et al., CFPS can be lyophilized to allow convenient storage and transportation conditions [65]. Lyophilized CFPS systems can be used for the decentralized, small-batch production of therapeutics and reagents, with potential uses in global health and emergency response. Furthermore, they demonstrated the production of more than 50 compounds, including vaccines, antibodies, antimicrobial peptides, and small molecules, using the same approach [168]. Recently, the Jewett group managed to provide another example of protective conjugate vaccines [169]. These products can be created outside of the laboratory setting and have the potential to revolutionize the field of bio-manufacturing.
Because of the limited amount of proteinogenic or canonical amino acids, the engineering of proteins with distinct chemical activities has been hampered. Non-canonical amino acids (NCAAs) and amino acid analogs could offer new functionalities to proteins, such as altered activity, enhanced stability, alternative post-translational modifications and more, which is particularly useful in the fields of drug discovery, bio-catalysis, and protein design when site-specific incorporation of NCAAs is possible [170]. The introduction of an NCAA at a specific position within the protein sequence requires the presence of an aminoacyl-tRNA synthetase specific for the desired NCAA, a tRNA molecule with an anticodon complementary to the NCAA's codon, and a genetically encoded amino acid specific enzyme, such as a codon-specific ribosome [171], [172], [173], [174], [175]. Several groups have done pioneering work [176]. However, difficulties persist in selecting NCAAs, which must be able to easily traverse membrane barriers. Although CPFS provided an open environment and unique advantages for selective multiplexed inclusion, the development of multidomain proteins or protein complexes would benefit more from the use of this technique [177], [178], [179].
4.3. New trends in in vitro diagnostics
In combination with DNA manipulation techniques, in particular the CRISPR/Cas-based system, the CFPS systems could be further developed as molecular diagnostic tools [180], [181]. In a recent case, Collins and co-authors developed a flexible three-layer device using silicone elastomers and cellulose matrices, containing freeze-dried insets with genetic circuits. The circuits were configured to express the lacZ β-galactosidase operon, hydrolyzing a substrate that caused a colorimetric output when exposed to a target analyte [182]. The authors optimized the device materials and reaction kinetics to complete the colorimetric output in less than 60 min. Using the same principles, they built prototype devices with freeze-dried circuits for the detection of anhydrotetracycline, Ebola virus RNA, and small molecules [183]. Jung et al. developed a cell-free biosensor for water contamination via RNA output sensors activated by ligand induction [184]. Such a system consisted of highly processive RNA polymerase, allosteric protein transcription factors and synthetic DNA templates, which contained a fluorescence-activation RNA aptamer sequence. The binding of a target contaminant could trigger the release of allosteric transcription factors and allow the transcription of the synthetic DNA template. The resulting product, which contained a fluorescence activation RNA aptamer, could be detected via its florescence. The further development of various sensors for small molecules has been reviewed elsewhere [166], [180], [185].
5. Summary and perspectives
With continuous effort to improve the efficiency of the CFPS system, a new higher record of 4 mg/mL was achieved with an E. coli lysate-based batch CFPS system [59] and up to 6 mg/mL with continuous exchange configurations [186]. In the case of a complete reconstituted PURE system, the productivity of a continuous exchange setup reached around 30 % of the total protein contained in the optimized PURE system, comparable to the level of overexpressed protein in E. coli cells [60]. However, one would still wonder: Is the current productivity upper limit of the CFPS system catching up to the efficiency within a living cell? It would be challenging to directly answer this question due to the lack of accurate data on the overall performance of a cell. Nevertheless, we could get a close estimate. During the 20-minute doubling time of an active growing E. coli cell, an average ribosome would need to synthesize around 55,000 peptide bonds, which is at least 1–2 orders of magnitude higher than the current performance of CFPS systems [25], [187]. Taking into account the rate of synthesis, current CFPS systems are even less efficient, considering the highest protein synthesis rate at around 5000 peptide bonds per hour per ribosome [188]. Therefore, further efforts are still needed to improve overall efficiency. This would require a more efficient energy regeneration system and a homeostatic environment to maintain the efficiency of ribosomes [25]. A set of systematic studies have revealed the limiting factors for the overall yield of the corresponding CFPS system, including fast depletion of substrates (energy precursors, NTPs and certain amino acids) [22], degradation of DNA templates, transcribed mRNAs and target proteins [190] and accumulation of inhibitory biproducts, such as free inorganic phosphates, which cause the fast decay of ribosomes [189]. Furthermore, optimizations based on the fully reconstituted PURE system revealed certain transcription and translation factors would be benefit for improving the final yield [191], [192]. In addition, molecular crowding agents and chaperons also showed beneficial effects on the overall yields [53], [193]. Finally, recent proteomic analysis could give valuable information of limiting factors for extract-based CFPS systems, which might vary depending on the individual strains and preparation procedures [194], [195], [196]. As stated above, ribosome, as the core translational apparatus, plays a vital role in the overall performance of the CFPS system. Therefore, achieving ribosome biogenesis in cell-free environment not only reveals the fundamental principles but also has many potential applications in synthetic biology. Pioneering work from Jewett’s group in ribosome synthesis, namely integrated synthesis, assembly, and translation (iSAT), has shown the generation of functional 30 S, 50 S subunits with in vitro transcribed rRNAs [197]. Moreover, individual purified ribosomal proteins that form the 30 s and 50 s subunits could be assembled with native rRNAs into a functional ribosome [187], [198]. Furthermore, such iSAT process could be validated within a double emulsion templated vesicle [199]. However, despite the successful assembly of functional ribosomes with in vitro transcribed rRNA for 30 S subunits, post-translational modifications of 23 S rRNA might be crucial [200], which certainly need to be further investigated to generate full reconstituted ribosomes. Despite the rapid development and expansion of CFPS technique, it is still not a straight forward lab routine, which might require certain optimization work to establish the best suitable system for particular applications. Commercially available CFPS kits, together with a set of detailed protocols [42], [201], [202], [203], might be a good starting point.
The current fast development of different compartmentalization methods and materials for the formation of different cell membrane mimicries. The functional membranes are essential for a minimal cell, including selective permeability, responsive to different environmental signals, which are fulfilled by a large group of integral membrane proteins [204], [205]. Even before application in the field of synthetic biology, CFPS system was intensively tuned to express membrane proteins, which were difficult to overexpress in vivo, due to their cytotoxicity. Devoid of living cells, CFPS system possess unique advantages for membrane proteins and has successfully overexpressed a large number of membrane proteins for both functional and structural characterization, prepared with different hydrophobic reagents, such as detergent, lipids (i.e., liposomes, nanodiscs or other model lipid bilayers) [206], [207], [208]. The main effort in membrane protein expression using CFPS system shifts towards the optimization of co-translational hydrophobic environments, which is certainly target dependent [209]. In sum, such advantage of CFPS system on membrane protein expression will certainly be beneficial for minimal cell projects, as exemplified in our recent effort to reconstitute a reversible membrane switch direct on the supported lipid bilayers [90]. Furthermore, efforts to improve encapsulation efficiency are still needed, especially when a protein-rich and highly viscous solution is used [210], [211], [212], [213]. As noted above, the current CFPS system is still far less efficient than the cellular system. The encapsulation process would further challenge the performance of the CPFS system. Parameters and conditions optimized from bulk solutions would need to be curated and validated in the microcompartment, taking into account the molecular crowding effect and stochasticity therein [214], [215], [216]. Taken together, these factors would have a direct impact if we move towards an autonomous self-reproduction system, which would require the self-replication of genetic materials, the de novo synthesis of ribosomes, the minimal unit for protein production, phospholipids, and the minimal divisome [154], [217], [218]. In this regard, the research toward a minimal cell has just begun.
Despite the fact that sustainable and ecologically friendly biomaterials have a clear benefit in the product-driven sector of synthetic biology, they nonetheless cost more than the material obtained by conventional chemical refining techniques. Therefore, there is a continuous demand to reduce the overall cost, which is certainly critical for the application of CFPS systems. Although there have been ongoing efforts to reduce the price of CFPS systems, only a small number of high-value protein products have been successfully produced using this method so far. There is still some uncertainty about the issue. How far does the CFPS method go beyond prototyping in the realm of synthetic biology? The development of CFPS pathways for certain metabolites would also benefit from a quantitative model [219]. However, the one of the current barriers is the lack of standardized data that quantitatively explain the performance of the in vitro metabolic network. Another factor making standardization difficult is the wide variety of host strain backgrounds. Recent results from proteome analysis on multiple E. coli lysates may offer broad directions for a possible metabolic network [194], [195], [196]. In addition, the convergence of technological advancements in artificial intelligence will accelerate the process of constructing mathematical and computational models as a corollary. As previously stated, the CFPS system is rapidly growing into a valuable production tool for protein-based drugs and in vitro diagnostics. Real-time on-demand protein synthesis could be particularly beneficial in instances with limited resources, such as the current global SARS-Cov-2 outbreak.
Despite the hurdles that now exist, the rapid advancement in synthetic biology via CFPS has gained widespread interest from the scientific communities, which will surely result in a more diverse application and may soon be a game-changer in the area.
CRediT authorship contribution statement
All authors contributed to the conceptualization, writing, reviewing and editing this manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
L.K. is thankful for the support of the Natural Science Research of Jiangsu Higher Education Institutions of China, China (Grant No. 17KJB180003), the Natural Science Foundation of Jiangsu Normal University, China (Grant No. 17XLR037), Priority Academic Program Development of Jiangsu Higher Education Institutions, China, and the Jiangsu Specially-Appointed Professor program, China.
References
- 1.Clarke L., Kitney R. Developing synthetic biology for industrial biotechnology applications. Biochem Soc Trans. 2020;48 doi: 10.1042/BST20190349. 113-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cubillos-Ruiz A., Guo T., Sokolovska A., Miller P.F., Collins J.J., et al. Engineering living therapeutics with synthetic biology. Nat Rev Drug Disco. 2021;20 doi: 10.1038/s41573-021-00285-3. 941-60. [DOI] [PubMed] [Google Scholar]
- 3.Mccarty N.S., Ledesma-Amaro R. Synthetic biology tools to engineer microbial communities for biotechnology. Trends Biotechnol. 2019;37 doi: 10.1016/j.tibtech.2018.11.002. 181-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doudna J.A., Charpentier E. The new frontier of genome engineering with CRISPR-Cas9. Science. 2014;346 doi: 10.1126/science.1258096. [DOI] [PubMed] [Google Scholar]
- 5.Doudna J.A. The promise and challenge of therapeutic genome editing. Nature. 2020;578:229–236. doi: 10.1038/s41586-020-1978-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cox D.B., Platt R.J., Zhang F. Therapeutic genome editing: prospects and challenges. Nat Med. 2015;21:121–131. doi: 10.1038/nm.3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kai L., Schwille P. Cell-free protein synthesis and its perspectives for assembling cells from the bottom-up. Adv Biosyst. 2019;3 doi: 10.1002/adbi.201800322. [DOI] [PubMed] [Google Scholar]
- 8.Li J., Zhang L., Liu W. Cell-free synthetic biology for in vitro biosynthesis of pharmaceutical natural products. Synth Syst Biotechnol. 2018;3:83–89. doi: 10.1016/j.synbio.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris D.C., Jewett M.C. Cell-free biology: exploiting the interface between synthetic biology and synthetic chemistry. Curr Opin Biotechnol. 2012;23:672–678. doi: 10.1016/j.copbio.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang L., Zhao J., Lian J., Xu Z. Cell-free protein synthesis enabled rapid prototyping for metabolic engineering and synthetic biology. Synth Syst Biotechnol. 2018;3:90–96. doi: 10.1016/j.synbio.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perez J.G., Stark J.C., Jewett M.C. Cell-free synthetic biology: engineering beyond the cell. Cold Spring Harb Perspect Biol. 2016;8:a023853. doi: 10.1101/cshperspect.a023853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao W., Bu N., Lu Y. Efficient incorporation of unnatural amino acids into proteins with a robust cell-free system. Methods Protoc. 2019;2:16. doi: 10.3390/mps2010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kohno T., Endo Y. Production of protein for nuclear magnetic resonance study using the wheat germ cell-free system. Methods Mol Biol. 2007;375:257–272. doi: 10.1007/978-1-59745-388-2_13. [DOI] [PubMed] [Google Scholar]
- 14.Lu Y. Cell-free synthetic biology: engineering in an open world. Synth Syst Biotechnol. 2017;2:23–27. doi: 10.1016/j.synbio.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roos C., Kai L., Proverbio D., Ghoshdastider U., Filipek S., et al. Co-translational association of cell-free expressed membrane proteins with supplied lipid bilayers. Mol Membr Biol. 2013;30:75–89. doi: 10.3109/09687688.2012.693212. [DOI] [PubMed] [Google Scholar]
- 16.Nirenberg M.W., Matthaei J.H. The dependence of cell-free protein synthesis in E. coli upon naturally occurring or synthetic polyribonucleotides. Proc Natl Acad Sci U S A. 1961;47:1588–1602. doi: 10.1073/pnas.47.10.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matthaei J.H., Nirenberg M.W. Characteristics and stabilization of DNAase-sensitive protein synthesis in E. coli extracts. Proc Natl Acad Sci U S A. 1961;47:1580–1588. doi: 10.1073/pnas.47.10.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matthaei H., Nirenberg M.W. The dependence of cell-free protein synthesis in E. coli upon RNA prepared from ribosomes. Biochem Biophys Res Commun. 1961;4:404–408. doi: 10.1016/0006-291x(61)90298-4. [DOI] [PubMed] [Google Scholar]
- 19.Smolskaya S., Logashina Y.A., Andreev Y.A. Escherichia coli extract-based cell-free expression system as an alternative for difficult-to-obtain protein biosynthesis. Int J Mol Sci. 2020;21:928. doi: 10.3390/ijms21030928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kimura-Soyema T., Shirouzu M., Yokoyama S. Cell-free membrane protein expression. Methods Mol Biol. 2014;1118:267–273. doi: 10.1007/978-1-62703-782-2_18. [DOI] [PubMed] [Google Scholar]
- 21.Ramm F., Jack L., Kaser D., Schlosshauer J.L., Zemella A., et al. Cell-free systems enable the production of AB(5) toxins for diagnostic applications. Toxins (Basel) 2022;14:233. doi: 10.3390/toxins14040233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carlson E.D., Gan R., Hodgman C.E., Jewett M.C. Cell-free protein synthesis: applications come of age. Biotechnol Adv. 2012;30:1185–1194. doi: 10.1016/j.biotechadv.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schneider B., Junge F., Shirokov V.A., Durst F., Schwarz D., et al. Membrane protein expression in cell-free systems. Methods Mol Biol. 2010;601:165–186. doi: 10.1007/978-1-60761-344-2_11. [DOI] [PubMed] [Google Scholar]
- 24.Laohakunakorn N., Grasemann L., Lavickova B., Michielin G., Shahein A., et al. Bottom-up construction of complex biomolecular systems with cell-free synthetic biology. Front Bioeng Biotechnol. 2020;8:213. doi: 10.3389/fbioe.2020.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jia H., Heymann M., Bernhard F., Schwille P., Kai L. Cell-free protein synthesis in micro compartments: building a minimal cell from biobricks. N Biotechnol. 2017;39:199–205. doi: 10.1016/j.nbt.2017.06.014. [DOI] [PubMed] [Google Scholar]
- 26.Schwille P., Spatz J., Landfester K., Bodenschatz E., Herminghaus S., et al. MaxSynBio: avenues towards creating cells from the bottom up. Angew Chem Int Ed Engl. 2018;57:13382–13392. doi: 10.1002/anie.201802288. [DOI] [PubMed] [Google Scholar]
- 27.Matsubayashi H., Ueda T. Purified cell-free systems as standard parts for synthetic biology. Curr Opin Chem Biol. 2014;22:158–162. doi: 10.1016/j.cbpa.2014.09.031. [DOI] [PubMed] [Google Scholar]
- 28.Szostak J.W., Bartel D.P., Luisi P.L. Synthesizing life. Nature. 2001;409:387–390. doi: 10.1038/35053176. [DOI] [PubMed] [Google Scholar]
- 29.Luisi P.L., Ferri F., Stano P. Approaches to semi-synthetic minimal cells: a review. Naturwissenschaften. 2006;93:1–13. doi: 10.1007/s00114-005-0056-z. [DOI] [PubMed] [Google Scholar]
- 30.Luisi P.L. Toward the engineering of minimal living cells. Anat Rec. 2002;268:208–214. doi: 10.1002/ar.10155. [DOI] [PubMed] [Google Scholar]
- 31.Stano P., Luisi P.L. Semi-synthetic minimal cells: origin and recent developments. Curr Opin Biotechnol. 2013;24:633–638. doi: 10.1016/j.copbio.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Guindani C., Da Silva L.C., Cao S., Ivanov T., Landfester K. Synthetic cells: from simple bio-inspired modules to sophisticated integrated systems. Angew Chem Int Ed Engl. 2022;61 doi: 10.1002/anie.202110855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zawada J.F., Burgenson D., Yin G., Hallam T.J., Swartz J.R., et al. Cell-free technologies for biopharmaceutical research and production. Curr Opin Biotechnol. 2022;76 doi: 10.1016/j.copbio.2022.102719. [DOI] [PubMed] [Google Scholar]
- 34.Swartz J. Developing cell-free biology for industrial applications. J Ind Microbiol Biotechnol. 2006;33:476–485. doi: 10.1007/s10295-006-0127-y. [DOI] [PubMed] [Google Scholar]
- 35.Karim A.S., Jewett M.C. A cell-free framework for rapid biosynthetic pathway prototyping and enzyme discovery. Metab Eng. 2016;36:116–126. doi: 10.1016/j.ymben.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 36.Miller O.J., Bernath K., Agresti J.J., Amitai G., Kelly B.T., et al. Directed evolution by in vitro compartmentalization. Nat Methods. 2006;3:561–570. doi: 10.1038/nmeth897. [DOI] [PubMed] [Google Scholar]
- 37.Hestericova M., Heinisch T., Alonso-Cotchico L., Marechal J.D., Vidossich P., et al. Directed evolution of an artificial imine reductase. Angew Chem Int Ed Engl. 2018;57:1863–1868. doi: 10.1002/anie.201711016. [DOI] [PubMed] [Google Scholar]
- 38.Dodevski I., Markou G.C., Sarkar C.A. Conceptual and methodological advances in cell-free directed evolution. Curr Opin Struct Biol. 2015;33:1–7. doi: 10.1016/j.sbi.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y., Xue P., Cao M., Yu T., Lane S.T., et al. Directed evolution: methodologies and applications. Chem Rev. 2021;121:12384–12444. doi: 10.1021/acs.chemrev.1c00260. [DOI] [PubMed] [Google Scholar]
- 40.Chen Z., Kibler R.D., Hunt A., Busch F., Pearl J., et al. De novo design of protein logic gates. Science. 2020;368:78–84. doi: 10.1126/science.aay2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zubay G. In vitro synthesis of protein in microbial systems. Annu Rev Genet. 1973;7:267–287. doi: 10.1146/annurev.ge.07.120173.001411. [DOI] [PubMed] [Google Scholar]
- 42.Schwarz D., Junge F., Durst F., Frolich N., Schneider B., et al. Preparative scale expression of membrane proteins in Escherichia coli-based continuous exchange cell-free systems. Nat Protoc. 2007;2:2945–2957. doi: 10.1038/nprot.2007.426. [DOI] [PubMed] [Google Scholar]
- 43.Kigawa T., Yabuki T., Matsuda N., Matsuda T., Nakajima R., et al. Preparation of Escherichia coli cell extract for highly productive cell-free protein expression. J Struct Funct Genom. 2004;5:63–68. doi: 10.1023/B:JSFG.0000029204.57846.7d. [DOI] [PubMed] [Google Scholar]
- 44.Kwon Y.C., Jewett M.C. High-throughput preparation methods of crude extract for robust cell-free protein synthesis. Sci Rep. 2015;5:8663. doi: 10.1038/srep08663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim T.W., Keum J.W., Oh I.S., Choi C.Y., Park C.G., et al. Simple procedures for the construction of a robust and cost-effective cell-free protein synthesis system. J Biotechnol. 2006;126:554–561. doi: 10.1016/j.jbiotec.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 46.Yamane T., Ikeda Y., Nagasaka T., Nakano H. Enhanced cell-free protein synthesis using a S30 extract from Escherichia coli grown rapidly at 42 degrees C in an amino acid enriched medium. Biotechnol Prog. 2005;21:608–613. doi: 10.1021/bp0400238. [DOI] [PubMed] [Google Scholar]
- 47.Haberstock S., Roos C., Hoevels Y., Dotsch V., Schnapp G., et al. A systematic approach to increase the efficiency of membrane protein production in cell-free expression systems. Protein Expr Purif. 2012;82:308–316. doi: 10.1016/j.pep.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 48.Mureev S., Kovtun O., Nguyen U.T., Alexandrov K. Species-independent translational leaders facilitate cell-free expression. Nat Biotechnol. 2009;27:747–752. doi: 10.1038/nbt.1556. [DOI] [PubMed] [Google Scholar]
- 49.Endo Y., Sawasaki T. High-throughput, genome-scale protein production method based on the wheat germ cell-free expression system. Biotechnol Adv. 2003;21:695–713. doi: 10.1016/s0734-9750(03)00105-8. [DOI] [PubMed] [Google Scholar]
- 50.Kim D.M., Swartz J.R. Prolonging cell-free protein synthesis with a novel ATP regeneration system. Biotechnol Bioeng. 1999;66:180–188. [PubMed] [Google Scholar]
- 51.Kim D.M., Swartz J.R. Regeneration of adenosine triphosphate from glycolytic intermediates for cell-free protein synthesis. Biotechnol Bioeng. 2001;74:309–316. [PubMed] [Google Scholar]
- 52.Calhoun K.A., Swartz J.R. Energizing cell-free protein synthesis with glucose metabolism. Biotechnol Bioeng. 2005;90:606–613. doi: 10.1002/bit.20449. [DOI] [PubMed] [Google Scholar]
- 53.Jewett M.C., Swartz J.R. Mimicking the Escherichia coli cytoplasmic environment activates long-lived and efficient cell-free protein synthesis. Biotechnol Bioeng. 2004;86:19–26. doi: 10.1002/bit.20026. [DOI] [PubMed] [Google Scholar]
- 54.Liu Y., Fritz B.R., Anderson M.J., Schoborg J.A., Jewett M.C. Characterizing and alleviating substrate limitations for improved in vitro ribosome construction. ACS Synth Biol. 2015;4:454–462. doi: 10.1021/sb5002467. [DOI] [PubMed] [Google Scholar]
- 55.Caschera F., Karim A.S., Gazzola G., D'aquino A.E., Packard N.H., et al. High-throughput optimization cycle of a cell-free ribosome assembly and protein synthesis system. ACS Synth Biol. 2018;7:2841–2853. doi: 10.1021/acssynbio.8b00276. [DOI] [PubMed] [Google Scholar]
- 56.Rasor B.J., Chirania P., Rybnicky G.A., Giannone R.J., Engle N.L., et al. Mechanistic insights into cell-free gene expression through an integrated -omics analysis of extract processing methods. ACS Synth Biol. 2023 doi: 10.1021/acssynbio.2c00339. [DOI] [PubMed] [Google Scholar]
- 57.Jewett M.C., Calhoun K.A., Voloshin A., Wuu J.J., Swartz J.R. An integrated cell-free metabolic platform for protein production and synthetic biology. Mol Syst Biol. 2008;4:220. doi: 10.1038/msb.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spirin A.S., Baranov V.I., Ryabova L.A., Ovodov S.Y., Alakhov Y.B. A continuous cell-free translation system capable of producing polypeptides in high yield. Science. 1988;242:1162–1164. doi: 10.1126/science.3055301. [DOI] [PubMed] [Google Scholar]
- 59.Garenne D., Thompson S., Brisson A., Khakimzhan A., Noireaux V. The all-E. coliTXTL toolbox 3.0: new capabilities of a cell-free synthetic biology platform. Synth Biol (Oxf) 2021;6:ysab017. doi: 10.1093/synbio/ysab017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kazuta Y., Matsuura T., Ichihashi N., Yomo T. Synthesis of milligram quantities of proteins using a reconstituted in vitro protein synthesis system. J Biosci Bioeng. 2014;118:554–557. doi: 10.1016/j.jbiosc.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 61.Smith M.T., Berkheimer S.D., Werner C.J., Bundy B.C. Lyophilized Escherichia coli-based cell-free systems for robust, high-density, long-term storage. Biotechniques. 2014;56:186–193. doi: 10.2144/000114158. [DOI] [PubMed] [Google Scholar]
- 62.Wilding K.M., Zhao E.L., Earl C.C., Bundy B.C. Thermostable lyoprotectant-enhanced cell-free protein synthesis for on-demand endotoxin-free therapeutic production. N Biotechnol. 2019;53:73–80. doi: 10.1016/j.nbt.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 63.Yang J., Cui Y., Cao Z., Ma S., Lu Y. Strategy exploration for developing robust lyophilized cell-free systems. Biotechnol Notes. 2021;2:44–50. doi: 10.1016/j.biotno.2021.08.004. [DOI] [Google Scholar]
- 64.Endo Y., Sawasaki T. Cell-free expression systems for eukaryotic protein production. Curr Opin Biotechnol. 2006;17:373–380. doi: 10.1016/j.copbio.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 65.Pardee K. Perspective: Solidifying the impact of cell-free synthetic biology through lyophilization. Biochem Eng J. 2018;138:91–97. doi: 10.1016/j.bej.2018.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lu Y. Textile-embedded cell-free biosensors. Nat Biomed Eng. 2022;6:225–226. doi: 10.1038/s41551-022-00869-3. [DOI] [PubMed] [Google Scholar]
- 67.Borkowski O., Bricio C., Murgiano M., Rothschild-Mancinelli B., Stan G.B., et al. Cell-free prediction of protein expression costs for growing cells. Nat Commun. 2018;9:1457. doi: 10.1038/s41467-018-03970-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kelwick R., Webb A.J., Macdonald J.T., Freemont P.S. Development of a Bacillus subtilis cell-free transcription-translation system for prototyping regulatory elements. Metab Eng. 2016;38:370–381. doi: 10.1016/j.ymben.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 69.Hodgman C.E., Jewett M.C. Optimized extract preparation methods and reaction conditions for improved yeast cell-free protein synthesis. Biotechnol Bioeng. 2013;110:2643–2654. doi: 10.1002/bit.24942. [DOI] [PubMed] [Google Scholar]
- 70.Ezure T., Suzuki T., Higashide S., Shintani E., Endo K., et al. Cell-free protein synthesis system prepared from insect cells by freeze-thawing. Biotechnol Prog. 2006;22:1570–1577. doi: 10.1021/bp060110v. [DOI] [PubMed] [Google Scholar]
- 71.Kubick S., Schacherl J., Fleischer-Notter H., Royall E., Roberts L.O., Stiege W. In vitro translation in an insect-based cell-free system. Cell-Free Protein Expr. 2003:209–217. doi: 10.1007/978-3-642-59337-6. [DOI] [Google Scholar]
- 72.Schweet R., Lamfrom H., Allen E. The synthesis of hemoglobin in a cell-free system. Proc Natl Acad Sci USA. 1958;44:1029–1035. doi: 10.1073/pnas.44.10.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Roberts B.E., Paterson B.M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci USA. 1973;70:2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Anderson C.W., Straus J.W., Dudock B.S. Preparation of a cell-free protein-synthesizing system from wheat germ. Methods Enzym. 1983;101:635–644. doi: 10.1016/0076-6879(83)01044-7. [DOI] [PubMed] [Google Scholar]
- 75.Takai K., Sawasaki T., Endo Y. Practical cell-free protein synthesis system using purified wheat embryos. Nat Protoc. 2010;5:227–238. doi: 10.1038/nprot.2009.207. [DOI] [PubMed] [Google Scholar]
- 76.Buntru M., Vogel S., Stoff K., Spiegel H., Schillberg S. A versatile coupled cell-free transcription-translation system based on tobacco BY-2 cell lysates. Biotechnol Bioeng. 2015;112:867–878. doi: 10.1002/bit.25502. [DOI] [PubMed] [Google Scholar]
- 77.Suzuki K., Inoue H., Matsuoka S., Tero R., Hirano-Iwata A., et al. Establishment of a cell-free translation system from rice callus extracts. Biosci Biotechnol Biochem. 2020;84:2028–2036. doi: 10.1080/09168451.2020.1779024. [DOI] [PubMed] [Google Scholar]
- 78.Brodel A.K., Sonnabend A., Kubick S. Cell-free protein expression based on extracts from CHO cells. Biotechnol Bioeng. 2014;111:25–36. doi: 10.1002/bit.25013. [DOI] [PubMed] [Google Scholar]
- 79.Martin R.W., Majewska N.I., Chen C.X., Albanetti T.E., Jimenez R.B.C., et al. Development of a CHO-based cell-free platform for synthesis of active monoclonal antibodies. ACS Synth Biol. 2017;6:1370–1379. doi: 10.1021/acssynbio.7b00001. [DOI] [PubMed] [Google Scholar]
- 80.Mikami S., Masutani M., Sonenberg N., Yokoyama S., Imataka H. An efficient mammalian cell-free translation system supplemented with translation factors. Protein Expr Purif. 2006;46:348–357. doi: 10.1016/j.pep.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 81.Endoh T., Kanai T., Sato Y.T., Liu D.V., Yoshikawa K., et al. Cell-free protein synthesis at high temperatures using the lysate of a hyperthermophile. J Biotechnol. 2006;126:186–195. doi: 10.1016/j.jbiotec.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 82.Yang C., Yang M., Zhao W., Ding Y., Wang Y., et al. Establishing a Klebsiella pneumoniae-based cell-free protein synthesis system. Molecules. 2022;27:4684. doi: 10.3390/molecules27154684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Des Soye B.J., Davidson S.R., Weinstock M.T., Gibson D.G., Jewett M.C. Establishing a high-yielding cell-free protein synthesis platform derived from vibrio natriegens. ACS Synth Biol. 2018;7:2245–2255. doi: 10.1021/acssynbio.8b00252. [DOI] [PubMed] [Google Scholar]
- 84.Cui J., Stevenson D., Korosh T., Amador-Noguez D., Olson D.G., et al. Developing a cell-free extract reaction (CFER) System in clostridium thermocellum to identify metabolic limitations to ethanol production. Front Energy Res. 2020:8. doi: 10.3389/fenrg.2020.00072. [DOI] [Google Scholar]
- 85.Xu H., Yang C., Tian X., Chen Y., Liu W.Q., et al. Regulatory part engineering for high-yield protein synthesis in an all-streptomyces-based cell-free expression system. ACS Synth Biol. 2022;11:570–578. doi: 10.1021/acssynbio.1c00587. [DOI] [PubMed] [Google Scholar]
- 86.Zemella A., Thoring L., Hoffmeister C., Kubick S. Cell-free protein synthesis: pros and cons of prokaryotic and eukaryotic systems. Chembiochem. 2015;16:2420–2431. doi: 10.1002/cbic.201500340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Katsura K., Tomabechi Y., Matsuda T., Yonemochi M., Mikuni J., et al. Phosphorylated and non-phosphorylated HCK kinase domains produced by cell-free protein expression. Protein Expr Purif. 2018;150:92–99. doi: 10.1016/j.pep.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 88.Oza J.P., Aerni H.R., Pirman N.L., Barber K.W., Ter Haar C.M., et al. Robust production of recombinant phosphoproteins using cell-free protein synthesis. Nat Commun. 2015;6:8168. doi: 10.1038/ncomms9168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jaroentomeechai T., Stark J.C., Natarajan A., Glasscock C.J., Yates L.E., et al. Single-pot glycoprotein biosynthesis using a cell-free transcription-translation system enriched with glycosylation machinery. Nat Commun. 2018;9:2686. doi: 10.1038/s41467-018-05110-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kai L., Sonal, Heermann T., Schwille P. Reconstitution of a reversible membrane switch via prenylation by one-pot cell-free expression. ACS Synth Biol. 2023;12:108–119. doi: 10.1021/acssynbio.2c00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schaerli Y., Jimenez A., Duarte J.M., Mihajlovic L., Renggli J., et al. Synthetic circuits reveal how mechanisms of gene regulatory networks constrain evolution. Mol Syst Biol. 2018;14 doi: 10.15252/msb.20178102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shin J., Noireaux V. Efficient cell-free expression with the endogenous E. coli RNA polymerase and sigma factor 70. J Biol Eng. 2010;4:8. doi: 10.1186/1754-1611-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mcmanus J.B., Emanuel P.A., Murray R.M., Lux M.W. A method for cost-effective and rapid characterization of engineered T7-based transcription factors by cell-free protein synthesis reveals insights into the regulation of T7 RNA polymerase-driven expression. Arch Biochem Biophys. 2019;674 doi: 10.1016/j.abb.2019.07.010. [DOI] [PubMed] [Google Scholar]
- 94.Senda N., Enomoto T., Kihara K., Yamashiro N., Takagi N., et al. Development of an expression-tunable multiple protein synthesis system in cell-free reactions using T7-promoter-variant series. Synth Biol (Oxf) 2022;7:ysac029. doi: 10.1093/synbio/ysac029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shin J., Noireaux V., An E. coli cell-free expression toolbox: application to synthetic gene circuits and artificial cells. ACS Synth Biol. 2012;1:29–41. doi: 10.1021/sb200016s. [DOI] [PubMed] [Google Scholar]
- 96.Mattick J.S. RNA regulation: a new genetics. Nat Rev Genet. 2004;5:316–323. doi: 10.1038/nrg1321. [DOI] [PubMed] [Google Scholar]
- 97.Takahashi M.K., Chappell J., Hayes C.A., Sun Z.Z., Kim J., et al. Rapidly characterizing the fast dynamics of RNA genetic circuitry with cell-free transcription-translation (TX-TL) systems. ACS Synth Biol. 2015;4:503–515. doi: 10.1021/sb400206c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Debroy S., Gebbie M., Ramesh A., Goodson J.R., Cruz M.R., et al. Riboswitches. A riboswitch-containing sRNA controls gene expression by sequestration of a response regulator. Science. 2014;345:937–940. doi: 10.1126/science.1255091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rhea K.A., Mcdonald N.D., Cole S.D., Noireaux V., Lux M.W., et al. Variability in cell-free expression reactions can impact qualitative genetic circuit characterization. Synth Biol (Oxf) 2022;7:ysac011. doi: 10.1093/synbio/ysac011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jia H., Sun X., Sun H., Li C., Wang Y., et al. Intelligent microbial heat-regulating engine (IMHeRE) for improved thermo-robustness and efficiency of bioconversion. ACS Synth Biol. 2016;5:312–320. doi: 10.1021/acssynbio.5b00158. [DOI] [PubMed] [Google Scholar]
- 101.Chappell J., Takahashi M.K., Lucks J.B. Creating small transcription activating RNAs. Nat Chem Biol. 2015;11:214–220. doi: 10.1038/nchembio.1737. [DOI] [PubMed] [Google Scholar]
- 102.Chushak Y., Harbaugh S., Zimlich K., Alfred B., Chavez J., et al. Characterization of synthetic riboswitch in cell-free protein expression systems. RNA Biol. 2021;18:1727–1738. doi: 10.1080/15476286.2020.1868149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lins M.R.D.C.R., Correa G.G., Amorim L.A.D.S., Franco R.A.L., Ribeiro N.V., et al. Characterization of five purine riboswitches in cellular and cell-free expression systems. Curr Microbiol. 2022;79:207. doi: 10.1007/s00284-022-02902-9. [DOI] [PubMed] [Google Scholar]
- 104.Espah Borujeni A., Mishler D.M., Wang J., Huso W., Salis H.M. Automated physics-based design of synthetic riboswitches from diverse RNA aptamers. Nucleic Acids Res. 2016;44:1–13. doi: 10.1093/nar/gkv1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Weigand J.E., Suess B. Aptamers and riboswitches: perspectives in biotechnology. Appl Microbiol Biotechnol. 2009;85:229–236. doi: 10.1007/s00253-009-2194-2. [DOI] [PubMed] [Google Scholar]
- 106.Wieland M., Hartig J.S. Artificial riboswitches: synthetic mRNA-based regulators of gene expression. Chembiochem. 2008;9:1873–1878. doi: 10.1002/cbic.200800154. [DOI] [PubMed] [Google Scholar]
- 107.Grundy F.J., Henkin T.M. From ribosome to riboswitch: control of gene expression in bacteria by RNA structural rearrangements. Crit Rev Biochem Mol Biol. 2006;41:329–338. doi: 10.1080/10409230600914294. [DOI] [PubMed] [Google Scholar]
- 108.Martini L., Mansy S.S. Cell-like systems with riboswitch controlled gene expression. Chem Commun (Camb) 2011;47:10734–10736. doi: 10.1039/c1cc13930d. [DOI] [PubMed] [Google Scholar]
- 109.Yue K., Zhu Y., Kai L. Cell-free protein synthesis: chassis toward the minimal cell. Cells. 2019;8:315. doi: 10.3390/cells8040315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li M., Huang X., Tang T.Y., Mann S. Synthetic cellularity based on non-lipid micro-compartments and protocell models. Curr Opin Chem Biol. 2014;22:1–11. doi: 10.1016/j.cbpa.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 111.Buddingh B.C., Van Hest J.C.M. Artificial cells: synthetic compartments with life-like functionality and adaptivity. Acc Chem Res. 2017;50:769–777. doi: 10.1021/acs.accounts.6b00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kita-Tokarczyk K., Grumelard J., Haefele T., Meier W. Block copolymer vesicles—using concepts from polymer chemistry to mimic biomembranes. Polymer. 2005;46:3540–3563. doi: 10.1016/j.polymer.2005.02.083. [DOI] [Google Scholar]
- 113.Discher D.E., Eisenberg A. Polymer vesicles. Science. 2002;297:967–973. doi: 10.1126/science.1074972. [DOI] [PubMed] [Google Scholar]
- 114.Ma L., Eisenberg A. Relationship between wall thickness and size in block copolymer vesicles. Langmuir. 2009;25:13730–13736. doi: 10.1021/la9012729. [DOI] [PubMed] [Google Scholar]
- 115.Rodríguez-García R., Mell M., López-Montero I., Netzel J., Hellweg T., et al. Polymersomes: smart vesicles of tunable rigidity and permeability. Soft Matter. 2011;7:1532. doi: 10.1039/c0sm00823k. [DOI] [Google Scholar]
- 116.Egli S., Schlaad H., Bruns N., Meier W. Functionalization of block copolymer vesicle surfaces. Polymers. 2011;3:252–280. doi: 10.3390/polym3010252. [DOI] [Google Scholar]
- 117.Fu Z., Ochsner M.A., De Hoog H.P., Tomczak N., Nallani M. Multicompartmentalized polymersomes for selective encapsulation of biomacromolecules. Chem Commun (Camb) 2011;47:2862–2864. doi: 10.1039/c0cc03971c. [DOI] [PubMed] [Google Scholar]
- 118.Le Meins J.F., Schatz C., Lecommandoux S., Sandre O. Hybrid polymer/lipid vesicles: state of the art and future perspectives. Mater Today. 2013;16:397–402. doi: 10.1016/j.mattod.2013.09.002. [DOI] [Google Scholar]
- 119.Kleineberg C., Wolfer C., Abbasnia A., Pischel D., Bednarz C., et al. Light-driven ATP regeneration in diblock/grafted hybrid vesicles. Chembiochem. 2020;21:2149–2160. doi: 10.1002/cbic.201900774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Huang X., Patil A.J., Li M., Mann S. Design and construction of higher-order structure and function in proteinosome-based protocells. J Am Chem Soc. 2014;136:9225–9234. doi: 10.1021/ja504213m. [DOI] [PubMed] [Google Scholar]
- 121.Huang X., Li M., Mann S. Membrane-mediated cascade reactions by enzyme-polymer proteinosomes. Chem Commun (Camb) 2014;50:6278–6280. doi: 10.1039/c4cc02256d. [DOI] [PubMed] [Google Scholar]
- 122.Torre P., Xiao Q., Buzzacchera I., Sherman S.E., Rahimi K., et al. Encapsulation of hydrophobic components in dendrimersomes and decoration of their surface with proteins and nucleic acids. Proc Natl Acad Sci USA. 2019;116:15378–15385. doi: 10.1073/pnas.1904868116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Percec V., Wilson D.A., Leowanawat P., Wilson C.J., Hughes A.D., et al. Self-assembly of Janus dendrimers into uniform dendrimersomes and other complex architectures. Science. 2010;328:1009–1014. doi: 10.1126/science.1185547. [DOI] [PubMed] [Google Scholar]
- 124.Wagner A.M., Eto H., Joseph A., Kohyama S., Haraszti T., et al. Dendrimersome synthetic cells harbor cell division machinery of bacteria. Adv Mater. 2022;34 doi: 10.1002/adma.202202364. [DOI] [PubMed] [Google Scholar]
- 125.Jones J.A., Giessen T.W. Advances in encapsulin nanocompartment biology and engineering. Biotechnol Bioeng. 2021;118:491–505. doi: 10.1002/bit.27564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sutter M., Boehringer D., Gutmann S., Gunther S., Prangishvili D., et al. Structural basis of enzyme encapsulation into a bacterial nanocompartment. Nat Struct Mol Biol. 2008;15:939–947. doi: 10.1038/nsmb.1473. [DOI] [PubMed] [Google Scholar]
- 127.Tavano L., De Cindio B., Picci N., Ioele G., Muzzalupo R. Drug compartmentalization as strategy to improve the physico-chemical properties of diclofenac sodium loaded niosomes for topical applications. Biomed Micro. 2014;16:851–858. doi: 10.1007/s10544-014-9889-6. [DOI] [PubMed] [Google Scholar]
- 128.Baillie A.J., Florence A.T., Hume L.R., Muirhead G.T., Rogerson A. The preparation and properties of niosomes--non-ionic surfactant vesicles. J Pharm Pharm. 1985;37:863–868. doi: 10.1111/j.2042-7158.1985.tb04990.x. [DOI] [PubMed] [Google Scholar]
- 129.Park N., Um S.H., Funabashi H., Xu J., Luo D. A cell-free protein-producing gel. Nat Mater. 2009;8:432–437. doi: 10.1038/nmat2419. [DOI] [PubMed] [Google Scholar]
- 130.Allen M.E., Hindley J.W., Baxani D.K., Ces O., Elani Y. Hydrogels as functional components in artificial cell systems. Nat Rev Chem. 2022;6:562–578. doi: 10.1038/s41570-022-00404-7. [DOI] [PubMed] [Google Scholar]
- 131.Ghosh B., Bose R., Tang T.Y.D. Can coacervation unify disparate hypotheses in the origin of cellular life. Curr Opin Colloid Interface Sci. 2021;52 doi: 10.1016/j.cocis.2020.101415. [DOI] [Google Scholar]
- 132.Gao N., Mann S. Membranized coacervate microdroplets: from versatile protocell models to cytomimetic materials. Acc Chem Res. 2023;56:297–307. doi: 10.1021/acs.accounts.2c00696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kato S., Garenne D., Noireaux V., Maeda Y.T. Phase separation and protein partitioning in compartmentalized cell-free expression reactions. Biomacromolecules. 2021;22:3451–3459. doi: 10.1021/acs.biomac.1c00546. [DOI] [PubMed] [Google Scholar]
- 134.Chen Y., Yuan M., Zhang Y., Liu S., Yang X., et al. Construction of coacervate-in-coacervate multi-compartment protocells for spatial organization of enzymatic reactions. Chem Sci. 2020;11:8617–8625. doi: 10.1039/d0sc03849k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Aufinger L., Simmel F.C. Artificial gel-based organelles for spatial organization of cell-free gene expression reactions. Angew Chem Int Ed Engl. 2018:17245–17248. doi: 10.1002/anie.201809374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Noireaux V., Libchaber A. A vesicle bioreactor as a step toward an artificial cell assembly. Proc Natl Acad Sci USA. 2004;101:17669–17674. doi: 10.1073/pnas.0408236101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ryabova L.A., Vinokurov L.M., Shekhovtsova E.A., Alakhov Y.B., Spirin A.S. Acetyl phosphate as an energy source for bacterial cell-free translation systems. Anal Biochem. 1995;226:184–186. doi: 10.1006/abio.1995.1208. [DOI] [PubMed] [Google Scholar]
- 138.Berhanu S., Ueda T., Kuruma Y. Artificial photosynthetic cell producing energy for protein synthesis. Nat Commun. 2019;10:1325. doi: 10.1038/s41467-019-09147-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Biner O., Fedor J.G., Yin Z., Hirst J. Bottom-up construction of a minimal system for cellular respiration and energy regeneration. ACS Synth Biol. 2020;9:1450–1459. doi: 10.1021/acssynbio.0c00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Miller T.E., Beneyton T., Schwander T., Diehl C., Girault M., et al. Light-powered CO2 fixation in a chloroplast mimic with natural and synthetic parts. Science. 2020;368:649–654. doi: 10.1126/science.aaz6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Van Nies P., Westerlaken I., Blanken D., Salas M., Mencia M., et al. Self-replication of DNA by its encoded proteins in liposome-based synthetic cells. Nat Commun. 2018;9:1583. doi: 10.1038/s41467-018-03926-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Libicher K., Hornberger R., Heymann M., Mutschler H. In vitro self-replication and multicistronic expression of large synthetic genomes. Nat Commun. 2020;11:904. doi: 10.1038/s41467-020-14694-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Exterkate M., Driessen A.J.M. Synthetic minimal cell: self-reproduction of the boundary layer. ACS Omega. 2019;4:5293–5303. doi: 10.1021/acsomega.8b02955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Blanken D., Foschepoth D., Serrao A.C., Danelon C. Genetically controlled membrane synthesis in liposomes. Nat Commun. 2020;11:4317. doi: 10.1038/s41467-020-17863-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Deshpande S., Spoelstra W.K., Van Doorn M., Kerssemakers J., Dekker C. Mechanical division of cell-sized liposomes. ACS Nano. 2018;12:2560–2568. doi: 10.1021/acsnano.7b08411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Canman J.C., Cabernard C. Mechanics of cell division and cytokinesis. Mol Biol Cell. 2018;29:685–686. doi: 10.1091/mbc.E17-11-0671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Micali G., Grilli J., Osella M., Cosentino Lagomarsino M. Concurrent processes set E. coli cell division. Sci Adv. 2018;4:eaau3324. doi: 10.1126/sciadv.aau3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gray A.N., Egan A.J., Van't Veer I.L., Verheul J., Colavin A., et al. Coordination of peptidoglycan synthesis and outer membrane constriction during Escherichia coli cell division. Elife. 2015;4 doi: 10.7554/eLife.07118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bisson-Filho A.W., Hsu Y.P., Squyres G.R., Kuru E., Wu F., et al. Treadmilling by FtsZ filaments drives peptidoglycan synthesis and bacterial cell division. Science. 2017;355:739–743. doi: 10.1126/science.aak9973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yang X., Lyu Z., Miguel A., Mcquillen R., Huang K.C., et al. GTPase activity-coupled treadmilling of the bacterial tubulin FtsZ organizes septal cell wall synthesis. Science. 2017;355:744–747. doi: 10.1126/science.aak9995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Jia H., Schwille P. Bottom-up synthetic biology: reconstitution in space and time. Curr Opin Biotechnol. 2019;60:179–187. doi: 10.1016/j.copbio.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 152.Loose M., Kruse K., Schwille P. Protein self-organization: lessons from the min system. Annu Rev Biophys. 2011;40:315–336. doi: 10.1146/annurev-biophys-042910-155332. [DOI] [PubMed] [Google Scholar]
- 153.Kretschmer S., Schwille P. Pattern formation on membranes and its role in bacterial cell division. Curr Opin Cell Biol. 2016;38:52–59. doi: 10.1016/j.ceb.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 154.Martos A., Jimenez M., Rivas G., Schwille P. Towards a bottom-up reconstitution of bacterial cell division. Trends Cell Biol. 2012;22:634–643. doi: 10.1016/j.tcb.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 155.Kohyama S., Merino-Salomon A., Schwille P. In vitro assembly, positioning and contraction of a division ring in minimal cells. Nat Commun. 2022;13:6098. doi: 10.1038/s41467-022-33679-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Dubuc E., Pieters P.A., Van Der Linden A.J., Van Hest J.C., Huck W.T., et al. Cell-free microcompartmentalised transcription-translation for the prototyping of synthetic communication networks. Curr Opin Biotechnol. 2019;58:72–80. doi: 10.1016/j.copbio.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bartelt S.M., Steinkuhler J., Dimova R., Wegner S.V. Light-guided motility of a minimal synthetic cell. Nano Lett. 2018;18:7268–7274. doi: 10.1021/acs.nanolett.8b03469. [DOI] [PubMed] [Google Scholar]
- 158.Fallah-Araghi A., Baret J.C., Ryckelynck M., Griffiths A.D. A completely in vitro ultrahigh-throughput droplet-based microfluidic screening system for protein engineering and directed evolution. Lab Chip. 2012;12:882–891. doi: 10.1039/c2lc21035e. [DOI] [PubMed] [Google Scholar]
- 159.Dudley Q.M., Karim A.S., Jewett M.C. Cell-free metabolic engineering: biomanufacturing beyond the cell. Biotechnol J. 2015;10:69–82. doi: 10.1002/biot.201400330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Suresh A., Shravan Ramgopal D., Panchamoorthy Gopinath K., Arun J., Sundarrajan P., et al. Recent advancements in the synthesis of novel thermostable biocatalysts and their applications in commercially important chemoenzymatic conversion processes. Bioresour Technol. 2021;323 doi: 10.1016/j.biortech.2020.124558. [DOI] [PubMed] [Google Scholar]
- 161.Thompson M.P., Peñafiel I., Cosgrove S.C., Turner N.J. Biocatalysis using immobilized enzymes in continuous flow for the synthesis of fine chemicals. Org Process Res Dev. 2018;23:9–18. doi: 10.1021/acs.oprd.8b00305. [DOI] [Google Scholar]
- 162.Guo W., Sheng J., Feng X. Mini-review: in vitro metabolic engineering for biomanufacturing of high-value products. Comput Struct Biotechnol J. 2017;15:161–167. doi: 10.1016/j.csbj.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Korman T.P., Opgenorth P.H., Bowie J.U. A synthetic biochemistry platform for cell free production of monoterpenes from glucose. Nat Commun. 2017;8:15526. doi: 10.1038/ncomms15526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Dudley Q.M., Anderson K.C., Jewett M.C. Cell-free mixing of escherichia coli crude extracts to prototype and rationally engineer high-titer mevalonate synthesis. ACS Synth Biol. 2016;5:1578–1588. doi: 10.1021/acssynbio.6b00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Karim A.S., Jewett M.C. A cell-free framework for rapid biosynthetic pathway prototyping and enzyme discovery. Metab Eng. 2016;36:116–126. doi: 10.1016/j.ymben.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 166.Silverman A.D., Karim A.S., Jewett M.C. Cell-free gene expression: an expanded repertoire of applications. Nat Rev Genet. 2020;21:151–170. doi: 10.1038/s41576-019-0186-3. [DOI] [PubMed] [Google Scholar]
- 167.Tinafar A., Jaenes K., Pardee K. Synthetic biology goes cell-free. BMC Biol. 2019;17:64. doi: 10.1186/s12915-019-0685-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Pardee K., Slomovic S., Nguyen P.Q., Lee J.W., Donghia N., et al. Portable, on-demand biomolecular manufacturing. Cell. 2016;167:248–259. doi: 10.1016/j.cell.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 169.Stark J.C., Jaroentomeechai T., Moeller T.D., Hershewe J.M., Warfel K.F., et al. On-demand biomanufacturing of protective conjugate vaccines. Sci Adv. 2021;7:eabe9444. doi: 10.1126/sciadv.abe9444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hohsaka T., Sisido M. Incorporation of non-natural amino acids into proteins. Curr Opin Chem Biol. 2002;6(6):809–815. doi: 10.1016/s1367-5931(02)00376-9. [DOI] [PubMed] [Google Scholar]
- 171.Liu D.R., Magliery T.J., Pastrnak M., Schultz P.G. Engineering a tRNA and aminoacyl-tRNA synthetase for the site-specific incorporation of unnatural amino acids into proteins in vivo. Proc Natl Acad Sci U S A. 1997;94(19):10092–10097. doi: 10.1073/pnas.94.19.10092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ibba M., Hennecke H. Towards engineering proteins by site-directed incorporation in vivo of non-natural amino acids. Biotechnol (N Y) 1994;12:678–682. doi: 10.1038/nbt0794-678. [DOI] [PubMed] [Google Scholar]
- 173.Ryu Y., Schultz P.G. Efficient incorporation of unnatural amino acids into proteins in Escherichia coli. Nat Methods. 2006;3:263–265. doi: 10.1038/nmeth864. [DOI] [PubMed] [Google Scholar]
- 174.Young T.S., Schultz P.G. Beyond the canonical 20 amino acids: expanding the genetic lexicon. J Biol Chem. 2010;285:11039–11044. doi: 10.1074/jbc.R109.091306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Benner S.A. Expanding the genetic lexicon: incorporating non-standard amino acids into proteins by ribosome-based synthesis. Trends Biotechnol. 1994;12:158–163. doi: 10.1016/0167-7799(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 176.O'donoghue P., Ling J., Wang Y.S., Soll D. Upgrading protein synthesis for synthetic biology. Nat Chem Biol. 2013;9:594–598. doi: 10.1038/nchembio.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ranji Charna A., Des Soye B.J., Ntai I., Kelleher N.L., Jewett M.C. An efficient cell-free protein synthesis platform for producing proteins with pyrrolysine-based noncanonical amino acids. Biotechnol J. 2022;17 doi: 10.1002/biot.202200096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Martin R.W., Des Soye B.J., Kwon Y.C., Kay J., Davis R.G., et al. Cell-free protein synthesis from genomically recoded bacteria enables multisite incorporation of noncanonical amino acids. Nat Commun. 2018;9:1203. doi: 10.1038/s41467-018-03469-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hong S.H., Ntai I., Haimovich A.D., Kelleher N.L., Isaacs F.J., et al. Cell-free protein synthesis from a release factor 1 deficient Escherichia coli activates efficient and multiple site-specific nonstandard amino acid incorporation. ACS Synth Biol. 2014;3:398–409. doi: 10.1021/sb400140t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zhang L., Guo W., Lu Y. Advances in cell-free. Biosens: Princ Mech Appl Biotechnol J. 2020;15 doi: 10.1002/biot.202000187. [DOI] [PubMed] [Google Scholar]
- 181.Soltani M., Davis B.R., Ford H., Nelson J.A.D., Bundy B.C. Reengineering cell-free protein synthesis as a biosensor: Biosensing with transcription, translation, and protein-folding. Biochem Eng J. 2018;138:165–171. doi: 10.1016/j.bej.2018.06.014. [DOI] [Google Scholar]
- 182.Nguyen P.Q., Soenksen L.R., Donghia N.M., Angenent-Mari N.M., De Puig H., et al. Wearable materials with embedded synthetic biology sensors for biomolecule detection. Nat Biotechnol. 2021;39:1366–1374. doi: 10.1038/s41587-021-00950-3. [DOI] [PubMed] [Google Scholar]
- 183.Pardee K., Green A.A., Ferrante T., Cameron D.E., Daleykeyser A., et al. Paper-based synthetic gene networks. Cell. 2014;159:940–954. doi: 10.1016/j.cell.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Jung J.K., Alam K.K., Verosloff M.S., Capdevila D.A., Desmau M., et al. Cell-free biosensors for rapid detection of water contaminants. Nat Biotechnol. 2020;38:1451–1459. doi: 10.1038/s41587-020-0571-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Voyvodic P.L., Bonnet J. Cell-free biosensors for biomedical applications. Curr Opin Biomed Eng. 2020;13:9–15. doi: 10.1016/j.cobme.2019.08.005. [DOI] [Google Scholar]
- 186.Kigawa T., Yabuki T., Yoshida Y., Tsutsui M., Ito Y., et al. Cell-free production and stable-isotope labeling of milligram quantities of proteins. FEBS Lett. 1999;442:15–19. doi: 10.1016/s0014-5793(98)01620-2. [DOI] [PubMed] [Google Scholar]
- 187.Li J., Haas W., Jackson K., Kuru E., Jewett M.C., et al. Cogenerating synthetic parts toward a self-replicating system. ACS Synth Biol. 2017;6:1327–1336. doi: 10.1021/acssynbio.6b00342. [DOI] [PubMed] [Google Scholar]
- 188.Jewett M.C., Forster A.C. Update on designing and building minimal cells. Curr Opin Biotechnol. 2010;21:697–703. doi: 10.1016/j.copbio.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Failmezger J., Nitschel R., Sanchez-Kopper A., Kraml M., Siemann-Herzberg M. Site-specific cleavage of ribosomal RNA in Escherichia coli-based cell-free protein synthesis systems. PLoS One. 2016;11 doi: 10.1371/journal.pone.0168764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Garamella J., Marshall R., Rustad M., Noireaux V. The all E. coli TX-TL Toolbox 2.0: a platform for cell-free synthetic biology. ACS Synth Biol. 2016;5:344–355. doi: 10.1021/acssynbio.5b00296. [DOI] [PubMed] [Google Scholar]
- 191.Li J., Gu L., Aach J., Church G.M. Improved cell-free RNA and protein synthesis system. PLoS One. 2014;9 doi: 10.1371/journal.pone.0106232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Doerr A., Foschepoth D., Forster A.C., Danelon C. In vitro synthesis of 32 translation-factor proteins from a single template reveals impaired ribosomal processivity. Sci Rep. 2021;11:1898. doi: 10.1038/s41598-020-80827-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Niwa T., Kanamori T., Ueda T., Taguchi H. Global analysis of chaperone effects using a reconstituted cell-free translation system. Proc Natl Acad Sci USA. 2012;109:8937–8942. doi: 10.1073/pnas.1201380109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Foshag D., Henrich E., Hiller E., Schafer M., Kerger C., et al. The E. coli S30 lysate proteome: a prototype for cell-free protein production. N Biotechnol. 2018;40:245–260. doi: 10.1016/j.nbt.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 195.Hurst G.B., Asano K.G., Doktycz C.J., Consoli E.J., Doktycz W.L., et al. Proteomics-based tools for evaluation of cell-free protein synthesis. Anal Chem. 2017;89:11443–11451. doi: 10.1021/acs.analchem.7b02555. [DOI] [PubMed] [Google Scholar]
- 196.Garenne D., Beisel C.L., Noireaux V. Characterization of the all-E. coli transcription-translation system myTXTL by mass spectrometry. Rapid Commun Mass Spectrom. 2019;33:1036–1048. doi: 10.1002/rcm.8438. [DOI] [PubMed] [Google Scholar]
- 197.Jewett M.C., Fritz B.R., Timmerman L.E., Church G.M. In vitro integration of ribosomal RNA synthesis, ribosome assembly, and translation. Mol Syst Biol. 2013;9:678. doi: 10.1038/msb.2013.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Aoyama R., Masuda K., Shimojo M., Kanamori T., Ueda T., et al. In vitro reconstitution of the Escherichia coli 70S ribosome with a full set of recombinant ribosomal proteins. J Biochem. 2022;171:227–237. doi: 10.1093/jb/mvab121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Caschera F., Lee J.W., Ho K.K., Liu A.P., Jewett M.C. Cell-free compartmentalized protein synthesis inside double emulsion templated liposomes with in vitro synthesized and assembled ribosomes. Chem Commun (Camb) 2016;52:5467–5469. doi: 10.1039/c6cc00223d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Green R., Noller H.F. In vitro complementation analysis localizes 23S rRNA posttranscriptional modifications that are required for Escherichia coli 50S ribosomal subunit assembly and function. RNA. 1996;2:1011–1021. [PMC free article] [PubMed] [Google Scholar]
- 201.Sun Z.Z., Hayes C.A., Shin J., Caschera F., Murray R.M., et al. Protocols for implementing an Escherichia coli based TX-TL cell-free expression system for synthetic biology. J Vis Exp. 2013 doi: 10.3791/50762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kuruma Y., Ueda T. The PURE system for the cell-free synthesis of membrane proteins. Nat Protoc. 2015;10:1328–1344. doi: 10.1038/nprot.2015.082. [DOI] [PubMed] [Google Scholar]
- 203.Levine M.Z., Gregorio N.E., Jewett M.C., Watts K.R., Oza J.P. Escherichia coli-based cell-free protein synthesis: protocols for a robust, flexible, and accessible platform technology. J Vis Exp. 2019 doi: 10.3791/58882. [DOI] [PubMed] [Google Scholar]
- 204.Sachs J.N., Engelman D.M. Introduction to the membrane protein reviews: the interplay of structure, dynamics, and environment in membrane protein function. Annu Rev Biochem. 2006;75:707–712. doi: 10.1146/annurev.biochem.75.110105.142336. [DOI] [PubMed] [Google Scholar]
- 205.Cho W., Stahelin R.V. Membrane-protein interactions in cell signaling and membrane trafficking. Annu Rev Biophys Biomol Struct. 2005;34:119–151. doi: 10.1146/annurev.biophys.33.110502.133337. [DOI] [PubMed] [Google Scholar]
- 206.Klammt C., Schwarz D., Lohr F., Schneider B., Dotsch V., et al. Cell-free expression as an emerging technique for the large scale production of integral membrane protein. FEBS J. 2006;273:4141–4153. doi: 10.1111/j.1742-4658.2006.05432.x. [DOI] [PubMed] [Google Scholar]
- 207.Junge F., Haberstock S., Roos C., Stefer S., Proverbio D., et al. Advances in cell-free protein synthesis for the functional and structural analysis of membrane proteins. N Biotechnol. 2011;28:262–271. doi: 10.1016/j.nbt.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 208.Levin R., Kock Z., Martin J., Zangl R., Gewering T., et al. Cotranslational assembly of membrane protein/nanoparticles in cell-free systems. Biochim Biophys Acta Biomembr. 2022;1864 doi: 10.1016/j.bbamem.2022.184017. [DOI] [PubMed] [Google Scholar]
- 209.Bernhard F., Tozawa Y. Cell-free expression--making a mark. Curr Opin Struct Biol. 2013;23:374–380. doi: 10.1016/j.sbi.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 210.De Souza T.P., Fahr A., Luisi P.L., Stano P. Spontaneous encapsulation and concentration of biological macromolecules in liposomes: an intriguing phenomenon and its relevance in origins of life. J Mol Evol. 2014;79:179–192. doi: 10.1007/s00239-014-9655-7. [DOI] [PubMed] [Google Scholar]
- 211.Sun B., Chiu D.T. Determination of the encapsulation efficiency of individual vesicles using single-vesicle photolysis and confocal single-molecule detection. Anal Chem. 2005;77:2770–2776. doi: 10.1021/ac048439n. [DOI] [PubMed] [Google Scholar]
- 212.Pereira De Souza T., Steiniger F., Stano P., Fahr A., Luisi P.L. Spontaneous crowding of ribosomes and proteins inside vesicles: a possible mechanism for the origin of cell metabolism. Chembiochem. 2011;12:2325–2330. doi: 10.1002/cbic.201100306. [DOI] [PubMed] [Google Scholar]
- 213.Luisi P.L., Allegretti M., Pereira De Souza T., Steiniger F., Fahr A., et al. Spontaneous protein crowding in liposomes: a new vista for the origin of cellular metabolism. Chembiochem. 2010;11:1989–1992. doi: 10.1002/cbic.201000381. [DOI] [PubMed] [Google Scholar]
- 214.Elowitz M.B., Levine A.J., Siggia E.D., Swain P.S. Stochastic gene expression in a single cell. Science. 2002;297:1183–1186. doi: 10.1126/science.1070919. [DOI] [PubMed] [Google Scholar]
- 215.Karig D.K., Jung S.Y., Srijanto B., Collier C.P., Simpson M.L. Probing cell-free gene expression noise in femtoliter volumes. ACS Synth Biol. 2013;2:497–505. doi: 10.1021/sb400028c. [DOI] [PubMed] [Google Scholar]
- 216.Nishimura K., Tsuru S., Suzuki H., Yomo T. Stochasticity in gene expression in a cell-sized compartment. ACS Synth Biol. 2015;4:566–576. doi: 10.1021/sb500249g. [DOI] [PubMed] [Google Scholar]
- 217.Levy M., Falkovich R., Daube S.S., Bar-Ziv R.H. Autonomous synthesis and assembly of a ribosomal subunit on a chip. Sci Adv. 2020;6:eaaz6020. doi: 10.1126/sciadv.aaz6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Luisi P.L., Stano P. Synthetic biology: minimal cell mimicry. Nat Chem. 2011;3:755–756. doi: 10.1038/nchem.1156. [DOI] [PubMed] [Google Scholar]
- 219.Martin J.P., Rasor B.J., Debonis J., Karim A.S., Jewett M.C., et al. A dynamic kinetic model captures cell-free metabolism for improved butanol production. Metab Eng. 2023;76:133–145. doi: 10.1016/j.ymben.2023.01.009. [DOI] [PubMed] [Google Scholar]