Abstract
Background
Implant-based breast augmentation remains popular, but the controversy over the safety and longevity of implants has continued. An event-based analysis of reasons for implant explantation may provide us with some insight into the controversy.
Methods
Data from May 1994 to October 2022 of explantation cases from aesthetic breast augmentation in three medical centers were retrospectively reviewed. Patient characteristics, time to explantation, reasons for visit, the major reason for explantation and intraoperative findings were analyzed.
Results
A total of 522 patients with 1004 breasts were included in our study. Objective explantation reasons accounted for 34.0% in primary augmentation breasts and 47.6% in revision augmentation breasts, which were significantly different (p = 0.006). The most common complaint was dissatisfaction with breast appearance, followed by concerns about implant safety, poor hand feeling and pain. 43.5% of the implants worn for more than 10 years were removed for objective reasons, which was found significantly different with the proportion of objective reasons in implants removed within 1 year and 1–5 years postoperatively (p < 0.008).
Conclusion
The proportion of different reasons for implant explantation varies across the times of surgeries and the years that the implant had been worn. As the years of implant wearing increase, the proportion of subjective reasons decreases in implant removal cases and objective reasons increase among them.
Level of Evidence III
This journal requires that authors assign a level of evidence to each article. For a full description of these Evidence-Based Medicine ratings, please refer to the Table of Contents or the online Instructions to Authors www.springer.com/00266.
Introduction
Breast augmentation surgery is one of the most popular aesthetic breast surgery procedures in the world. According to ISAPS global statistics, from 2018 to 2020 breast augmentation has been the top surgical procedures in the world, even during the COVID-19 pandemic [1]. With the increased number of the breast prosthesis implanted, debates about the implant safety and its relation to illnesses never stopped since the implant was introduced in 1960s [2]. Implant rupture, capsular contracture and other complications have long been receiving attention. The very current events, including breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) [3], breast implant illness (BII) [4] and primary squamous cell carcinoma (SCC) [5], which have recognized to be related to breast implant have prompted increased public attention surrounding breast implant safety. Besides, in November 2021 the US Food and Drug Administration (FDA) has placed a black box warning on breast implant package in order to make sure that patients make informed decisions, and tells women breast implants are not considered lifetime devices, which suggests that breast implant has a certain lifespan and thus has led to lively discussions about implant longevity [6, 7].
Implant explantation is indicated for a variety of reasons, including not only objective reasons which refers to complications associated with breast implants, but also subjective reasons referring to the patient’s desire for a change for cosmetic reason [8, 9]. According to a core study conducted by FDA, the cumulative rate of breast implant removal at 8–10 years postoperatively is 7.3–32.4%, with subjective and objective reasons almost equally weighted [10]. Although studies have focused on implant removal cases and sought to learn from them, the time span of the currently available studies is relatively short; in particular the reasons for removal in cases where the prosthesis has been worn for more than 10 years are missing. Besides, the relatively small sample size is also a shortcoming of the current data. In this study, we summarized the retrospective data of 1004 implant explantations in 522 patients across 28 years from three different medical centers, including cases where the prosthesis was worn for more than 10 years, hoping to fill the above research gaps and provide a reference for clinical practice.
Patients and Methods
A multicenter, event-based retrospective review was conducted of patients who underwent breast implant explantation after cosmetic augmentation mammoplasty in Plastic Surgery Hospital, Bravou Plastic Surgery Hospital and Dalian Metime Medical Cosmetic Hospital from May 1994 to October 2022. Patients with both silicone gel implants originally placed for aesthetic reasons were included in our study, while patients with saline, gel–saline implants, and any patient received implantation for reconstructive purposes were excluded. The following information was obtained from patients’ electronic medical records: age at explantation, medical history and smoking status, implant type, implant placement plane, incision, reasons for visit, date of implantation and explantation, main reason for explantation, intraoperative findings.
In our study, the main reasons for implant explantation were divided into six categories: ①device problem: the integrity of the implant was damaged, including implant rupture or implant leakage; ②pathological reasons: pathological phenomena were found preoperatively or intraoperatively, including Baker grade III/IV capsular contracture, late seroma, hematoma and infection; ③aesthetic reasons: patients were dissatisfied with the post-augmentation breast shape, but no pathological changes were found intraoperatively; ④psychological reasons: patients were worried about the safety of the implant and requested implant removal; ⑤physical symptoms: patients had complaints (such as breast pain) without pathological finding pre- or intraoperatively; and ⑥involved implants: neither complaint nor pathological finding but involved by the contralateral implant. The first two groups were summarized as objective reasons, while the third and fourth were summarized as subjective reasons.
Continuous variables were summarized using mean ± standard deviation (SD) and compared using the two-sided t test or Mann–Whitney test. Categorical data were presented as percentages or proportions and analyzed using the chi-square or Fisher’s exact test. Statistical analysis was performed with SPSS 26.0 statistical package (IBM, Armonk, NY, USA). A value of p < 0.05 was considered statistically significant, while the pairwise comparisons in the multiple chi-squared test were adjusted using Bonferroni correction. This study was approved by the Ethics Committee of the Plastic Surgery Hospital of the Chinese Academy of Medical Sciences and Peking Union Medical College.
Result
During the 28-year study period, 522 patients with 1004 breasts with implants removed met our inclusion criteria, among which 463 patients with 903 breasts experienced primary augmentation, while the remaining 59 patients with 101 breasts experienced revision augmentation. Of the 1004 implants explanted, 40 were removed unilaterally, and the remaining cases were removed bilaterally. As shown in Table 1, the mean patient age was similar between the primary augmentation and revision augmentation group (38 ± 9 years versus 39 ± 9 years; p = 0.43), whereas the mean time to explantation was higher in the primary augmentation cohort (8 ± 6 years versus 5 ± 6 years, p = 0.0002). No difference regarding the smoking history between the two groups was identified (8 versus 0, p = 0.61). The distribution of implant type used and implant placement plane in both groups was similar (p = 0.65 and 0.57, respectively), whereas the incisions utilized in the two groups were significantly different (p < 0.001).
Table 1.
Characteristics of included patients
| Characteristic | Groups | ||
|---|---|---|---|
| Primary augmentation (%) | Revision augmentation (%) | p value | |
| Total | 463 (89) | 59 (11) | |
| Mean age at explantation ± SD, year | 38 ± 9 | 39 ± 9 | 0.43 |
| Mean time to explantation ± SD, year | 8 ± 6 | 5 ± 6 | 0.0002 |
| Tobacco use | 8 (2) | 0 (0) | 0.61 |
| Implant type | 0.65 | ||
| Smooth round | 58 (13) | 7 (12) | |
| Textured round | 102 (22) | 11 (19) | |
| Textured anatomical | 47 (10) | 8 (14) | |
| Unknown | 256 (55) | 33 (56) | |
| Implant placement plane | 0.57 | ||
| Subpectoral | 344 (74) | 41 (69) | |
| Dual plane | 16 (3) | 1 (2) | |
| Subglandular | 93 (20) | 15 (25) | |
| Unknown | 10 (2) | 2 (3) | |
| Incision | < 0.001 | ||
| Axillary | 241 (52) | 15 (25) | |
| Peri-areolar | 93 (20) | 26 (44) | |
| Inframammary fold | 48 (10) | 8 (10) | |
| Unknown | 81 (17) | 10 (17) | |
Table 2 shows that objective reasons for explantation by patient counted 47.8% of the total 463 primary augmentation patients and 61.0% of 59 revision augmentation patients, with no statistical difference identified (p = 0.084). For reasons differentiated by breast, objective reasons for removal accounted for 34% of primary augmentation breasts and 47.6% of the revision augmentation breasts, with a statistically significant difference between the two (p = 0.006) (Table 3, Fig. 1).
Table 2.
Implant explantation reasons by patient
| Reasons for removal | Groups | ||
|---|---|---|---|
| Primary augmentation (%) | Revision augmentation (%) | p value | |
| Objective | 221(47.7) | 36(61.0) | 0.084 |
| Device problem | 80(17.3) | 3(5.1) | |
| Pathological | 141(30.5) | 33(55.9) | |
| Subjective | 221(47.7) | 22(37.3) | |
| Aesthetic | 126(27.2) | 20(33.9) | |
| Psychological | 95(20.5) | 2(3.4) | |
| Physical symptoms | 21(4.5) | 1(1.7) | |
| Total | 463(100) | 59(100) | |
Significant difference comparing distribution of objective and subjective reasons between revision augmentation implants to primary augmentation patients as determined by the χ2 test; p < 0.05 was considered significant
Table 3.
Implant explantation reasons by breast
| Reasons for removal | Groups | ||
|---|---|---|---|
| Primary augmentation (%) | Revision augmentation (%) | p value | |
| Objective | 307 (34.0) | 48 (47.6) | 0.006 |
| Device problem | 111 (12.3) | 4 (4.0) | |
| Pathological | 196 (21.7) | 44 (43.6) | |
| Capsular contracture | 149 (16.5) | 41(40.6) | |
| Subjective | 483 (43.5) | 41 (41.0) | |
| Aesthetic | 276 (30.6) | 38 (37.6) | |
| Psychological | 207 (22.9) | 3 (3.0) | |
| Physical symptoms | 38 (4.2) | 2 (2.0) | |
| Involved | 75 (8.3) | 10 (9.9) | |
| Total | 903 (100) | 101 (100) | |
Significant difference comparing distribution of objective and subjective reasons between revision augmentation implants to primary augmentation implants as determined by the χ2 test; p < 0.05 was considered significant
Fig. 1.
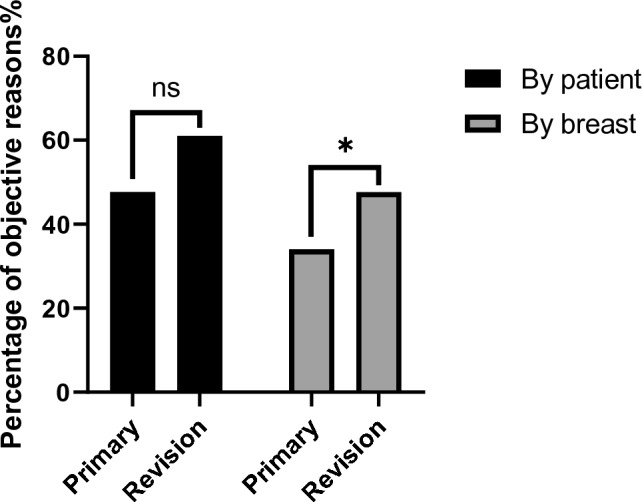
Comparison of the occurrence of objective reasons by patient or breast between primary and revision augmentation
To understand the frequent reasons for patient visits, we further investigated the incidence of the top occurrences of patient complaints by patients experienced primary or revision augmentation, the results of which are shown in Table 4. Among all patient complaints, the most frequent one was dissatisfaction with the appearance of the breast, which occurred in 37% of primary augmentation group and 31% revision augmentation group, followed by concerns about implant safety, poor hand feeling and pain. It is worth noting that 61 of all patients initially sought for treatment with non-objective factors but then found to have objective lesions. Such a mismatch between complaint and real cause accounted for 21.6% of all patients (data not shown).
Table 4.
Frequency of common complaints among reasons for patient visits
| Complaints | Groups | |
|---|---|---|
| Primary augmentation (%) | Revision augmentation (%) | |
| Dissatisfaction with breast appearance | 172 (37) | 18 (31) |
| Concern about safety | 129( 28) | 2 (3) |
| Poor hand feeling | 57 (12) | 20 (34) |
| Pain | 35 (8) | 7 (12) |
| Breast asymmetry | 14 (3) | 9 (15) |
For the purpose of understanding whether there is a trend in the distribution of time to explantation, we divided explanted implants into four groups. As shown in Table 5, 175 implants were removed within 1 year after implantation, accounting for 17.4% of the total implants, while 308 implants remained in the body for more than 10 years, accounting for 30.7% of the total implants. Subgroup analysis was performed in groupings based on time to explantation, data showed in implants removed within 1 year, subjective reasons accounted for the most (56%), while objective reasons accounted for only 26.3%. Of the portion of implants removed more than 10 years after implantation, 43.5% were removed for objective reasons, while the percentage of subjective reasons decreased to 45.1%. In the pairwise comparison of the four groups, the percentage of objective or subjective reasons for removal of the implants at more than 10 years was statistically different from 0–1 to 5–10 years (p < 0.008, respectively) (Table 5, Fig. 2).
Table 5.
Distribution of reasons for implant explantation in different times to explantation
| Reasons | Groups | |||
|---|---|---|---|---|
| 0–1 years | 1–5 years | 5–10 years | 10+ years | |
| Objective | 46 (26.3)a | 92 (35.0) | 83 (32.2)b | 134 (43.5)a,b |
| Device problem | 6 (3.4) | 17 (6.5) | 20 (7.8) | 72 (23.4) |
| Pathological | 40 (22.9) | 75 (28.5) | 63 (24.4) | 62 (20.1) |
| Subjective | 98 (56.0)a | 143 (54.3) | 144 (55.8)b | 139 (45.1)a,b |
| Aesthetic | 84 (48.0) | 89 (33.8) | 83 (32.2) | 58 (18.8) |
| Psychological | 14 (8.0) | 54 (20.5) | 61 (23.6) | 81 (26.3) |
| Physical symptoms | 15 (8.6) | 5 (1.9) | 12 (4.7) | 8 (2.6) |
| Involved | 16 (9.1) | 23 (8.7) | 19 (7.4) | 27 (8.8) |
| Total | 175 (17.4) | 263 (26.2) | 258 (25.7) | 308 (30.7) |
Numbers (%)
Significant difference comparing revision augmentation implants to primary augmentation implants as determined by the χ2 test; p < 0.008 was considered significant applying Bonferroni correction. The identical superscript letters indicate statistical significance between these two groups
Fig. 2.
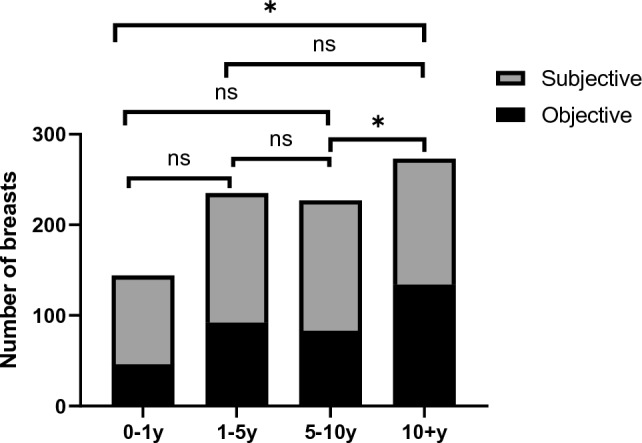
Distribution of objective and subjective reasons for implant explantation in different time to explantation
Discussion
Breast implants are used in nearly 300,000 augmentations annually in the USA and show a continuous upward trend [11, 12]. An increase in these surgeries necessitates a greater emphasis on clinician and patient awareness of the possible risk after breast prosthesis implantation and the cause of implant removal. In 2018, Van Slyke et al. [13] reviewed 539 explanted breast implants, comparing the longevity of Biocell and other types of implants and their proportion in implant performance failure. Tanna et al. [14] summarizes the possible causes of prosthesis removal, its clinical manifestations and treatment. Although efforts have been made to study the relationship between implant removal and implant type as well as treatment strategies after removing implants, the distribution of the causes of removal in these patients is still lacking. However, understanding the causes of implant explantation based on the event is particularly important for optimizing breast implant surgery. To our knowledge, this is the first investigation concerning the reasons for breast implant explantation in aesthetic breast augmentations based on a sample size greater than 1000 breasts. In this study, we analyzed 1004 implants removed from 522 patients and summarized the reasons for implant removal along with the distribution pattern of time to explantation and found that objective reasons occurred in higher rates in patients experienced revision breast augmentations and implants carried more than ten years.
Rupture of silicone implants has come under scrutiny in recent years, especially since the onset of the Poly Implant Prothèse crisis [15–17] and efforts have been made to prevent intraoperative implant rupture [18, 19]. According to published data, silicone implants rupture incidence is estimated to be 8% in asymptomatic women [20] and 33% in symptomatic women [21–24]. The US Food and Drug Administration currently recommends that patients with silicone gel implants undergo magnetic resonance imaging screening three years after surgery and every two years thereafter. It reflects that the risk of implant rupture is constant with increasing age. Implant rupture accounted for an increasing proportion of removal cases as the implant was worn for longer periods of time, especially among cases with implant worn for up to 10 years or more. From our study data, it is evident that implant rupture accounted for a greater proportion of cases worn for more than 10 years (23.4% versus 3.4–7.8%), while subjective reasons accounted for a smaller proportion than in cases less than 10 years (45.1% versus 54.3–56.0%). This phenomenon may be related to the aging of the prosthesis and the accumulation of mechanical damage, but no statistical conclusions could be drawn from our study.
Pathological reasons included capsular contracture, late seroma, infection, hematoma, etc., among which capsular contracture (CC) accounted for 79.1% of the included cases. Capsular contracture, with an reported incidence rate ranging from 2.8 to 18.9% [25–28], is considered to results from the immune response to a foreign body which causes pain and discomfort to patients. Over the years, many efforts have been made to reduce the incidence of CC [29]. In our study, CC accounted for a higher proportion in revision augmentation implant removed cases, the possible reason for this being that the repeated implant removal and placement affects local tissue conditions, leading to a greater susceptibility to CC (40.6% versus 16.5%). The published literature so far shows a higher incidence of CC in revision breast augmentation than in primary breast augmentation, with an incidence of 18.9% in primary augmentation and 28.9% in revision augmentation [26, 30–33], which is consistent with our speculation. A comparison of primary breast augmentation versus revision augmentation on CC incidence is not achievable from our data, but it provides strong support for such conclusion. Published data show that the highest incidence of CC occurs within 1 year after surgery [34], with an increasing number of studies showing that the risk of CC evolves over time [28, 35, 36]. There was no apparent increase in the proportion of CC among the reasons for implant removal over time according to our data; however, it should be noted that this does not represent the incidence of CC, but only be seen as a reference for the proportion of CC in the causes of implant removal.
Subjective reasons, consisting of aesthetic and psychological reasons, also account for an important part of the reasons for prosthesis removal. As can be seen from Table 5 and Fig. 2, the percentage of subjective reasons was reduced in removal cases where the prosthesis had been worn for more than 10 years (45.5% versus 54.3–56.0%), which may be explained by the fact that these patients had a higher tolerance for the prosthesis and were not as sensitive to the appearance of the breast for a short period of time after augmentation. Fullness, superior/inferior pole proportion and orientation and size of the nipple areolar complex are considered key elements in patient postoperative aesthetic satisfaction [37, 38]. Dissatisfaction with the appearance of the breast was the most frequent of all complaints for consultation, higher than aesthetic reasons among the main reasons for implant removal by patient (N = 190 versus N = 146). Since many pathological changes, such as capsular contracture, can lead to changes in breast morphology, the frequency of this complaint is inclusive of the portion of people who have had their implants removed for objective reasons. Of particular note is that 21.6% of the included patients initially visited doctors due to subjective reasons, while the presence of objective causes was found after the visit. This suggests that plastic surgeons should think more and pay extra attention to the presence of device problem as well as pathological factors when encountering patients who were seen for subjective reasons in their clinical work.
The usual cause of pain following breast augmentation includes capsular contracture, muscle spasm, neuroma and chest wall irritation. Besides, there are also common causes of breast pain that are not at all related to breast implants which include cyclic changes in the menstrual cycle, pain from breastfeeding and mastitis [39]. Pain occurred in 9.1% of our included patients; however, many of their implants were not originally placed by us and, as a result, there is a lack of information on how many patients had breast pain before their initial implant insertion and we were therefore unable to determine the cause of these patients’ symptoms.
BII is the term used to describe the emergence of a group of women who present with a variety of systemic symptoms believed to be connected to breast implants which has gained increasing attention recently. Based on the available evidence, BII can cause localized breast pain [40], along with systemic symptoms such as those described in chronic fatigue syndrome [41]. Forty of our included implants had presented with somatic symptoms, but no intraoperative or postoperative pathological manifestation was found. These patients cannot be excluded from having BII, but unfortunately since there are no clear diagnostic criteria for BII, we were unable to confirm the proportion of BII in the included cases, and we hope to analyze and categorize such a group of patients in the future when the diagnosis becomes clearer.
There are several limitations worth highlighting. Firstly, this is an event-based study, and we can only analyze the percentage of reasons to which implants have been explanted but not conclude the incidence rate of events after breast augmentation. Secondly, many of patients showed up for removing implants placed by other surgeons, and the year of the prosthesis placement surgery was old. Thus, there was missing information in the medical records. Thirdly, this study spans 28 years, and there may have been overlapping generations of silicone gel-filled breast implants during this period that were not well represented in the medical record and may have caused some bias to the results.
In the future, we hope to provide a higher level of evidence for clinical work by conducting prospective randomized controlled trials to explore the longevity and safety of different brands and types of breast implants.
Conclusion
The proportion of different reasons for implant explantation varies across the times of surgeries and the years that the implant had been worn. As the years of implant wearing increase, the proportion of subjective reasons decreases in implant removal cases and objective reasons increase among them.
Funding
The authors have no financial interest to declare in relation to the content of this study. No funding was received for this article.
Declarations
Conflict of interest
The authors declare that they have no conflicts of interest to disclose.
Ethical Approval
This study was approved by the Ethics Committee of the Plastic Surgery Hospital of the Chinese Academy of Medical Sciences and Peking Union Medical College.
Informed Consent
For this type of study, informed consent is not required.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ziying Zhang and Jun Qi have contributed equally to this manuscript.
Reference
- 1.The International Society of Aesthetic Plastic Surgery (2021) ISAPS international survey on aesthetic/cosmetic procedures performed in 2020. Available at: https://www.isaps.org/wp-content/uploads/2022/01/ISAPS-Global-Survey_2020.pdf
- 2.Melmed EP. A review of explantation in 240 symptomatic women: a description of explantation and capsulectomy with reconstruction using a periareolar technique. Plast Reconstr Surg. 1998;101:1364–1373. doi: 10.1097/00006534-199804050-00036. [DOI] [PubMed] [Google Scholar]
- 3.Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–2390. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magnusson MR, Cooter RD, Rakhorst H, et al. Breast implant illness: a way forward. Plast Reconstr Surg. 2019;143:74s–81s. doi: 10.1097/prs.0000000000005573. [DOI] [PubMed] [Google Scholar]
- 5.Zomerlei TA, Samarghandi A, Terando AM. Primary squamous cell carcinoma arising from a breast implant capsule. Plast Reconstr Surg Glob Open. 2015;3:e586. doi: 10.1097/gox.0000000000000567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanne JH. Breast implants: US regulator issues “black box” warning and requires more patient information. BMJ (Clin Res Ed) 2021;375:n2639. doi: 10.1136/bmj.n2639. [DOI] [PubMed] [Google Scholar]
- 7.Luan J. Discussion on controversial issues of prosthetic breast augmentation. Chin J Plast Surg. 2022 doi: 10.3760/cma.j.cn114453-20220601-00170. [DOI] [Google Scholar]
- 8.Spear SL, Parikh PM, Goldstein JA. History of breast implants and the food and drug administration. Clin Plast Surg. 2009;36:15–21. doi: 10.1016/j.cps.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Cole NM. Consequences of the U.S. food and drug administration-directed moratorium on silicone gel breast implants: 1992 to 2006. Plast Reconstr Surg. 2018;141:1137–1141. doi: 10.1097/prs.0000000000004284. [DOI] [PubMed] [Google Scholar]
- 10.Center for Devices and Radiological Health, U.S. Food and Drug Administration (2011) FDA update on the safety of silicone gel-filled breast implants. https://www.fda.gov/media/80685/download. Accessed 31 May 2022
- 11.Loyo-Berrios N (2013) Building national infrastructure for postmarket surveillance of silicone breast implants. Available at: www.fda.gov/downloads/MedicalDevices/NewsEvents/WorkshopsConferences/UCM360961.pdf. Accessed Nov 10 2017
- 12.The American Society for Aesthetic Plastic Surgery Cosmetic surgery national data bank statistics. Aesthet Surg J. 2017;37:1–29. doi: 10.1093/asj/sjx076. [DOI] [PubMed] [Google Scholar]
- 13.Van Slyke AC, Carr M, Carr NJ. Not all breast implants are equal: a 13-year review of implant longevity and reasons for explantation. Plast Reconstr Surg. 2018;142:281e–289e. doi: 10.1097/prs.0000000000004678. [DOI] [PubMed] [Google Scholar]
- 14.Tanna N, Calobrace MB, Clemens MW, et al. Not all breast explants are equal: contemporary strategies in breast explantation surgery. Plast Reconstr Surg. 2021;147:808–818. doi: 10.1097/prs.0000000000007784. [DOI] [PubMed] [Google Scholar]
- 15.Hillard C, Fowler JD, Barta R, et al. Silicone breast implant rupture: a review. Gland Surg. 2017;6:163–168. doi: 10.21037/gs.2016.09.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR) (2012) Safety of poly implant prothèse (PIP) silicone breast implant. European Commission, 1 February. Available at: http://ec.europa.eu/health/scientific_committees/emerging/docs/scenihr_o_034.pdf. Accessed Sept 17 2012
- 17.Handel N, Garcia ME, Wixtrom R. Breast implant rupture: causes, incidence, clinical impact, and management. Plast Reconstr Surg. 2013;132:1128–1137. doi: 10.1097/PRS.0b013e3182a4c243. [DOI] [PubMed] [Google Scholar]
- 18.Tsao SB, Wu CC. Silicone breast implant injector: a retooled breast augmentation device. Aesthet Plast Surg. 2021;45:95–99. doi: 10.1007/s00266-020-01966-x. [DOI] [PubMed] [Google Scholar]
- 19.Panczel G, Munhoz AM. A simple and low-cost method of sleeve to insert silicone gel breast implants. Plast Reconstr Surg Glob Open. 2019;7:e2389. doi: 10.1097/gox.0000000000002389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hedén P, Nava MB, van Tetering JP, et al. Prevalence of rupture in Inamed silicone breast implants. Plast Reconstr Surg. 2006;118:303–308. doi: 10.1097/01.prs.0000233471.58039.30. [DOI] [PubMed] [Google Scholar]
- 21.Coroneos CJ, Selber JC, Offodile AC, II, et al. US FDA breast implant postapproval studies: long-term outcomes in 99,993 patients. Ann Surg. 2019;269:30–36. doi: 10.1097/sla.0000000000002990. [DOI] [PubMed] [Google Scholar]
- 22.Goodman CM, Cohen V, Thornby J, et al. The life span of silicone gel breast implants and a comparison of mammography, ultrasonography, and magnetic resonance imaging in detecting implant rupture: a meta-analysis. Ann Plast Surg. 1998;41:577–585. doi: 10.1097/00000637-199812000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Kessler DA. The basis of the FDA’s decision on breast implants. N Engl J Med. 1992;326:1713–1715. doi: 10.1056/nejm199206183262525. [DOI] [PubMed] [Google Scholar]
- 24.Netscher DT, Weizer G, Malone RS, et al. Diagnostic value of clinical examination and various imaging techniques for breast implant rupture as determined in 81 patients having implant removal. South Med J. 1996;89:397–404. doi: 10.1097/00007611-199604000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Headon H, Kasem A, Mokbel K. Capsular contracture after breast augmentation: an update for clinical practice. Arch Plast Surg. 2015;42:532–543. doi: 10.5999/aps.2015.42.5.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spear SL, Murphy DK. Natrelle round silicone breast implants: core study results at 10 years. Plast Reconstr Surg. 2014;133:1354–1361. doi: 10.1097/prs.0000000000000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu X, Zhou L, Pan F, et al. Comparison of the postoperative incidence rate of capsular contracture among different breast implants: a cumulative meta-analysis. PLoS ONE. 2015;10:e0116071. doi: 10.1371/journal.pone.0116071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calobrace MB, Stevens WG, Capizzi PJ, et al. Risk factor analysis for capsular contracture: a 10-year Sientra study using round, smooth, and textured implants for breast augmentation. Plast Reconstr Surg. 2018;141:20s–28s. doi: 10.1097/prs.0000000000004351. [DOI] [PubMed] [Google Scholar]
- 29.Shin BH, Kim BH, Kim S, et al. Silicone breast implant modification review: overcoming capsular contracture. Biomaterials research. 2018;22:37. doi: 10.1186/s40824-018-0147-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kühn S, Georgijewitsch MA, Wehle A, et al. Implant replacement or removal: What happens after capsular contracture? A German study examining breast implant revision surgery and patient choices in 946 cases. Breast care (Basel, Switzerland) 2021;16:350–357. doi: 10.1159/000509598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caplin DA. Indications for the use of MemoryShape breast implants in aesthetic and reconstructive breast surgery: long-term clinical outcomes of shaped versus round silicone breast implants. Plast Reconstr Surg. 2014;134:27s–37s. doi: 10.1097/prs.0000000000000609. [DOI] [PubMed] [Google Scholar]
- 32.Maxwell GP, Van Natta BW, Bengtson BP, et al. Ten-year results from the Natrelle 410 anatomical form-stable silicone breast implant core study. Aesthet Surg J. 2015;35:145–155. doi: 10.1093/asj/sju084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stevens WG, Calobrace MB, Harrington J, et al. Nine-year core study data for Sientra’s FDA-approved round and shaped implants with high-strength cohesive silicone gel. Aesthet Surg J. 2016;36:404–416. doi: 10.1093/asj/sjw015. [DOI] [PubMed] [Google Scholar]
- 34.Adams WP, Jr, Rios JL, Smith SJ. Enhancing patient outcomes in aesthetic and reconstructive breast surgery using triple antibiotic breast irrigation: six-year prospective clinical study. Plast Reconstr Surg. 2006;117:30–36. [PubMed] [Google Scholar]
- 35.Zeplin PH, Corduff N. Influence of patient age on capsular contracture after aesthetic breast augmentation. Plast Surg (Oakville, Ont) 2015;23:67–69. doi: 10.4172/plastic-surgery.1000915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marques M, Brown SA, Oliveira I, et al. Long-term follow-up of breast capsule contracture rates in cosmetic and reconstructive cases. Plast Reconstr Surg. 2010;126:769–778. doi: 10.1097/PRS.0b013e3181e5f7bf. [DOI] [PubMed] [Google Scholar]
- 37.Mejia Jimenez N, Patrón Gómez AS. Breast aesthetic preferences: analysis of 1294 surveys. Aesthetic Plast Surg. 2021;45:2088–2093. doi: 10.1007/s00266-021-02253-z. [DOI] [PubMed] [Google Scholar]
- 38.Atiye B, Chahine F. Metrics of the aesthetically perfect breast. Aesthetic Plast Surg. 2018;42:1187–1194. doi: 10.1007/s00266-018-1154-6. [DOI] [PubMed] [Google Scholar]
- 39.Nahabedian MY. Discussion: not all breast implants are equal: a 13-year review of implant longevity and reasons for explantation. Plast Reconstr Surg. 2018;142:290e–292e. doi: 10.1097/prs.0000000000004679. [DOI] [PubMed] [Google Scholar]
- 40.Cohen Tervaert JW, Mohazab N, Redmond D, et al. Breast implant illness: scientific evidence of its existence. Expert Rev Clin Immunol. 2022;18:15–29. doi: 10.1080/1744666x.2022.2010546. [DOI] [PubMed] [Google Scholar]
- 41.U.S. Food and Drug Administration (2022) Medical device reports for systemic symptoms in women with breast implants. Available at: https://www.fda.gov/medical-devices/breast-implants/medical-device-reports-systemic-symptoms-women-breast-implants


