Abstract
The Janus kinase (JAK) signal transducer and activator of transcription (JAK-STAT) pathway is an evolutionarily conserved mechanism of transmembrane signal transduction that enables cells to communicate with the exterior environment. Various cytokines, interferons, growth factors, and other specific molecules activate JAK-STAT signaling to drive a series of physiological and pathological processes, including proliferation, metabolism, immune response, inflammation, and malignancy. Dysregulated JAK-STAT signaling and related genetic mutations are strongly associated with immune activation and cancer progression. Insights into the structures and functions of the JAK-STAT pathway have led to the development and approval of diverse drugs for the clinical treatment of diseases. Currently, drugs have been developed to mainly target the JAK-STAT pathway and are commonly divided into three subtypes: cytokine or receptor antibodies, JAK inhibitors, and STAT inhibitors. And novel agents also continue to be developed and tested in preclinical and clinical studies. The effectiveness and safety of each kind of drug also warrant further scientific trials before put into being clinical applications. Here, we review the current understanding of the fundamental composition and function of the JAK-STAT signaling pathway. We also discuss advancements in the understanding of JAK-STAT–related pathogenic mechanisms; targeted JAK-STAT therapies for various diseases, especially immune disorders, and cancers; newly developed JAK inhibitors; and current challenges and directions in the field.
Subject terms: Tumour immunology, Biomarkers
Introduction
The Janus kinase (JAK) signal transducer and activator of transcription (JAK-STAT) pathway is an evolutionarily conserved signaling pathway that functions in several crucial physiological processes, including hematopoiesis, differentiation, metabolism, and immune modulation.1–4 Structurally, the JAK-STAT pathway involves transmembrane receptors, receptor-associated cytosolic tyrosine kinases (i.e. JAKs), and signal transducers and activators of transcription (i.e., STATs).5 The JAK protein family contains four members: JAK1, JAK2, JAK3, and TYK2.6–9 The STAT family consists of seven proteins: STAT1, STAT2, STAT3, STAT4, STAT5A, STAT5B, and STAT6.10–12 The JAK-STAT signaling pathway was first discovered in investigations of interferon-related transcriptional activation. Subsequently, a general outline of the components and pathogenesis of the JAK-STAT signaling pathway was gradually completed over a period of about 20 years.13–15 More than 50 types of cytokines, including interferons (IFNs), interleukins (ILs), and growth factors, have been shown to play roles in JAK-STAT signaling to fulfill regulatory functions in cell differentiation, metabolism, survival, homeostasis, and immune response. Once receptors bind to an extracellular ligand, JAKs initiate tyrosine phosphorylation of the receptors and recruit corresponding STATs.16–18 The phosphorylated STATs then dimerize and enter the nucleus to regulate specific gene transcription. This process enables the rapid transmission of external signals to the nucleus to regulate biological and pathological processes. Genome-wide association studies for disease exploration have identified more than 200 somatic mutations and single-nucleotide polymorphisms of JAK-STAT pathway genes that are functionally correlated with human diseases, including rheumatoid arthritis (RA), hematological malignancies, and atopic dermatitis (AD).19–21 Abnormal activation of JAK-STAT signaling has been identified in diverse immune-mediated conditions and cancers, including melanomas, glioblastomas, and head, neck, lung, pancreatic, breast, rectal, and prostate cancers.22–27 Based on the ‘double-edged sword’ function of the JAK-STAT pathway in disease pathogenesis, numerous agents targeting the JAK-STAT pathway have been developed and tested in preclinical and clinical trials.28–31 As a result, first-generation JAK inhibitors, such as ruxolitinib, tofacitinib, and baricitinib, have been approved for clinical use.32–34 Furthermore, a new generation of selective JAK inhibitors has emerged as a promising option for drug development and has shown success in preclinical trials. Despite this progress, some studies have reported adverse effects of JAK inhibitors, including infection, hematologic events, Wernicke encephalopathy, and even cancer, emphasizing the need for follow-up studies and in-depth investigations on drug thresholds, the mechanisms of adverse events, and drug resistance.35–37
In this review, we have expanded and updated the comprehensive research on the components, classical activation, and negative regulation of JAK-STAT signaling based on prior research. We have placed a specific emphasis on the immunomodulatory role of JAK-STAT signaling and the genetic associations between the JAK-STAT signaling pathway and certain diseases. We also discuss in detail the application of JAK inhibitors for the treatment of immune-related and malignant diseases and summarize their efficacy and safety in a series of clinical and preclinical trials.
JAK, STAT, and the JAK-STAT pathway
The JAK-STAT pathway is an evolutionarily conserved signaling pathway activated by cytokine stimulation that enables extracellular signals to be transmitted across the cell membrane to the nucleus, causing changes in DNA transcription.38,39 JAK-STAT signaling regulates a variety of cellular functions, including proliferation, migration, differentiation, and apoptosis.40,41 Importantly, JAK-STAT signaling also has an important regulatory role in immune function.42–44
The JAK family
The JAK family of non-receptor tyrosine kinases consists of four proteins: JAK1, JAK2, JAK3, and TYK2.6,45–47 Each kinase functions as an intracellular adaptor protein for cytokine signaling.48–53 JAK3 is expressed at high levels predominantly in hematopoietic cells, whereas the other members are widely expressed in many tissues.54,55 The JAK proteins are composed of the FERM (the complex of four point one, ezrin, radixin, and moesin), Src homology domain (SH2), pseudokinase, and kinase domains.56–58 The FERM domain has a clover structure composed of F1, F2, and F3 substructures.59 The FERM and SH2 domains are primarily responsible for JAK binding to receptors. The pseudokinase domain regulates the activity of the kinase domain, which is essential for the phosphorylation of receptor tyrosine which leads to further phosphorylation of downstream molecules.60 The four domains can be further divided into seven partitions referred to as JH1–7.45,61,62 JH1 and JH2 are located at the C-terminal end of the protein, and JH3–7 is located at the N-terminal end.63–67 JH1 encodes a kinase that phosphorylates an important component of the kinase domain of the substrate.68–70 The main function of JH2, also known as the pseudokinase domain without kinase activity, is to enhance the kinase function of JH1. JH3 and JH4 maintain the stability of the kinase structure.71–76 JH5, JH6, and JH7 are responsible for the attachment of JAK to corresponding receptors. Cytokines such as interferons, interleukins, and growth factors and their receptors are the main activators of JAK.77 The receptor-ligand complex activates receptor-bound JAK, which catalyzes the phosphorylation of a receptor tyrosine. The four known members of the JAK family each interact with specific cytokine receptors and recruit corresponding STATs to exert diverse biological functions.78–80 JAK1, JAK3, and TYK2 are responsible for immune system development and immune regulation, whereas JAK2 mainly participates in hematopoiesis.81–90
The STAT family
STAT proteins are signaling molecules downstream of JAK. The STAT family members are STAT1, STAT2, STAT3, STAT4, STAT5A, STAT5B, and STAT6.91–94 STATs consist of an N-terminal domain and coil, a helix domain, a DNA-binding domain, a connection domain, an SH2 domain, and a transcription-activation domain.95–100 The N-terminal domain and coil promote the formation of STAT dimers. The helix domain regulates the processes of nuclear import and export.101–104 The DNA-binding domain enables STATs to bind DNA as transcription factors.105 The SH2 domain recognizes phosphorylated tyrosine on specific cytokine receptors.106 After the receptor tyrosine is phosphorylated, cytosolic STATs are recruited to the activated receptor, and a STAT tyrosine is phosphorylated, leading to the formation of STAT dimers.107–109 STAT dimers then enter the nucleus as a component of transcription factor complexes to promote the transcription of specific target genes.110,111 STATs are then dephosphorylated in the nucleus and returned to the cytoplasm. Within the STAT family, STAT3 has been acknowledged to play a central role in signal transmission from the plasma membrane to the nucleus, making it a promising target for drug development.112–114
Negative regulation of JAK-STAT signaling
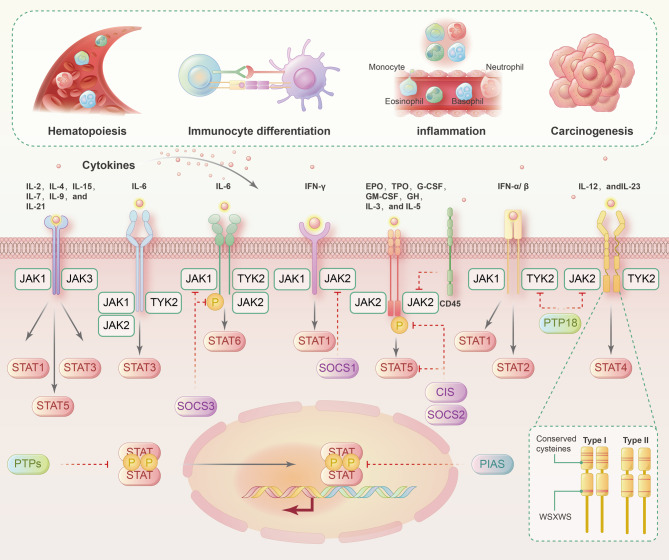
Many regulators inhibit the activation of the JAK-STAT signaling pathway. There are three main types of JAK-STAT regulators: suppressors of cytokine signaling (SOCSs), protein inhibitors of activated STATs (PIASs), and protein tyrosine phosphatases.115–119 The SOCS family are the major signaling molecules that attenuate the JAK-STAT pathway and include CIS, SOCS1, SOCS2, SOCS3, SOCS4, SOCS5, SOCS6, and SOCS7.120–122 Structurally, all the SOCSs contain an SH2 domain and a SOCS cassette. SOCSs can be induced by cytokines such as IL-2, IL-3, and IFN-γ. The entry of activated STATs into the nucleus enhances the transcription of SOCSs, which exert negative regulatory effects on JAK-STAT signaling by blocking STAT-receptor binding, inactivating JAKs via the N-terminal kinase inhibitory structure, or binding and ubiquitinating JAKs or STATs for proteasomal degradation.123,124 A negative feedback loop is driven by the positive effect of activated STATs on the transcription of SOCSs.125 The PIAS family includes PIAS1, PIAS3, PIASx, and PIASy.126–128 PIASs can interact with STAT to prevent STAT dimerization or prevent STAT dimers from binding to DNA. As phosphatases, protein tyrosine phosphatases can interact with receptors to dephosphorylate JAK. Protein tyrosine phosphatases can also directly dephosphorylate STAT dimers to inhibit JAK-STAT signaling (Fig. 1).129,130
Fig. 1.
Canonical activation and negative regulation of JAK-STAT signaling pathways. Canonical activation of the JAK-STAT signaling pathway: cytokines bind to their corresponding receptors, which undergo a conformational change and recruit related JAKs. The JAKs undergo phosphorylation, which leads to tyrosine phosphorylation of the receptors to create docking sites for STATs. STATs are phosphorylated, dissociate from the receptor, and enter the nucleus as homodimers or heterodimers to bind specific DNA sites and regulate cytokine-related gene transcription. Negative regulation of the JAK-STAT signaling pathway: the CIS/SOCS (cytokine signaling inhibitor), PIAS (protein inhibitor of activated STAT), and PTP (protein tyrosine phosphatase) families are participants in the negative regulation of the JAK-STAT signaling pathway. The CIS/SOCS family negatively regulates the JAK-STAT pathway through direct binding of JAKs or JAK receptors to inhibit JAK kinase activity, binding of receptor tyrosine kinase sites to intercept STAT-receptor binding, or formation of enzyme complexes to degrade JAKs or STATs. The PIAS family mainly interacts with STAT dimers to inhibit STAT binding to DNA, thereby blocking JAK-STAT signal transduction. The PTP family negatively regulates the JAK/STAT pathway mainly by dephosphorylating activated receptors, JAKs, and STATs. Solid lines indicate the activation process. The dotted lines represent negative regulation
The JAK-STAT pathway, immunoregulation, and lineage plasticity
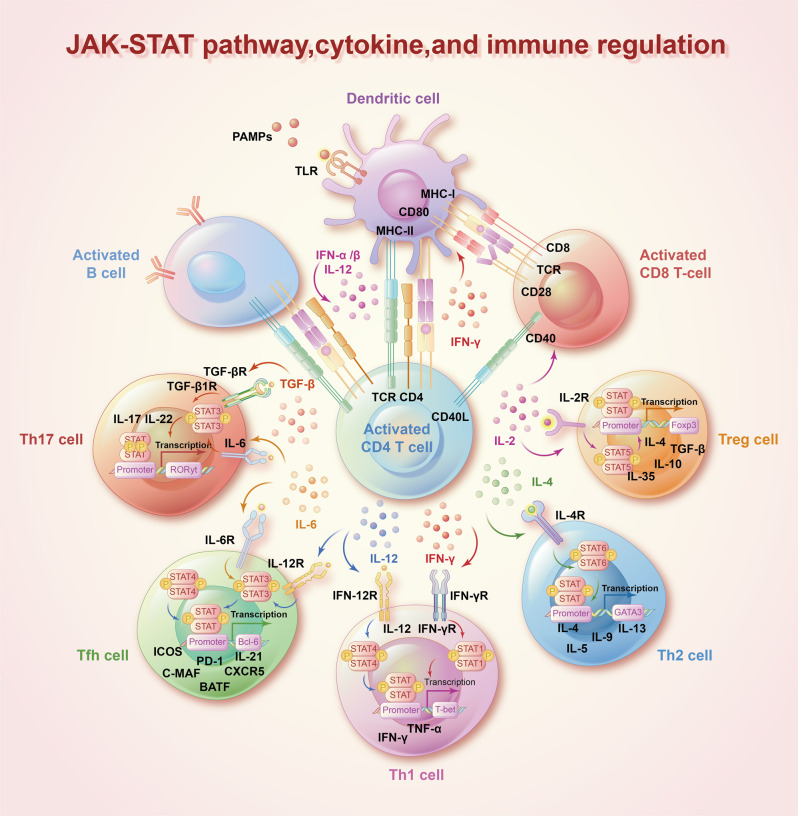
Cytokines are crucial for humoral and cellular immune responses.44,131,132 A wide range of cytokines associated with autoimmune diseases, including IFNs, ILs, and colony-stimulating factors, exert pleiotropic effects primarily through a combination of type I and type II cytokine receptors.133–135 The interaction of type I and type II cytokine receptors can activate JAKs and subsequently recruit STATs to transduce signals from cytokines (Table 1).1,136,137 Numerous studies have shown that cytokine-induced activation of the JAK-STAT pathway plays instrumental roles in the differentiation and development of immune cells and the homeostasis of the immune system (Fig. 2).138–140 Under stimulation by different cytokines, the JAK-STAT signaling pathway performs complex functions in immune regulatory events, these functions include not only cancer cell recognition, triggered mainly by IFNs and STAT1 and STAT2 signaling, but also immune escape triggered mainly by IL-6-STAT3 signaling.23 JAK-STAT signaling has been widely shown to drive antitumor immune surveillance in response to cytokines (like IL-2, IL-15, and IFNs) by inducing the activation, cytotoxicity, and function of natural killer (NK) cells.141–144 On the other hand, the JAK-STAT pathway has been implicated in the pathogenesis of numerous autoimmune diseases such as RA, inflammatory bowel disease, and AD.145,146 For example, IL-6 induced the phosphorylation of STAT3 and upregulated the differentiation of Th17 cells, contributing to RA development.147 Ma, C. S. et al. also showed that STAT3 deficiency resulted in the production of incomplete T follicular helper cells and reduction of B-cell helper activity.148 In animal models of spondyloarthritis (SpA), TYK2 was shown to be an essential mediator of IL-22–induced STAT3 phosphorylation to enhance type 3 immunity and accelerate SpA progression.149 Furthermore, multiple genome-wide association analyzes have clearly demonstrated that polymorphisms and mutations in the JAK-STAT pathway are associated with autoimmune diseases and immune-mediated cancers (Fig. 3).19,150,151 Casaca, V. I. et al. reported that the rs324011 polymorphism in STAT6 resulted in early immune dysregulation with depressed Treg function and increased Th1 response at birth.152 Fabre, A. et al. reported links between STAT3 gain-of-function mutations and early-onset polyautoimmunity.153 Mutated STAT3 was also reported to participate in the pathogenesis of immune-mediated aplastic anemia by altering the T-cell phenotype and giving cytotoxic properties to CD8+ T cells.154 Additionally, JAK mutations were revealed to block IFN-γ signal transmission, contributing to immune evasion and insensitivity to anti-PD-1/PD-L1 immunotherapy.155 Recent clinical trials have shown that targeting JAKs and preventing their phosphorylation suppresses abnormal immune and inflammatory responses caused by cytokines, providing reasonable and solid evidence to support the use of JAK inhibitors as therapies to treat autoimmune diseases and cancers.
Table 1.
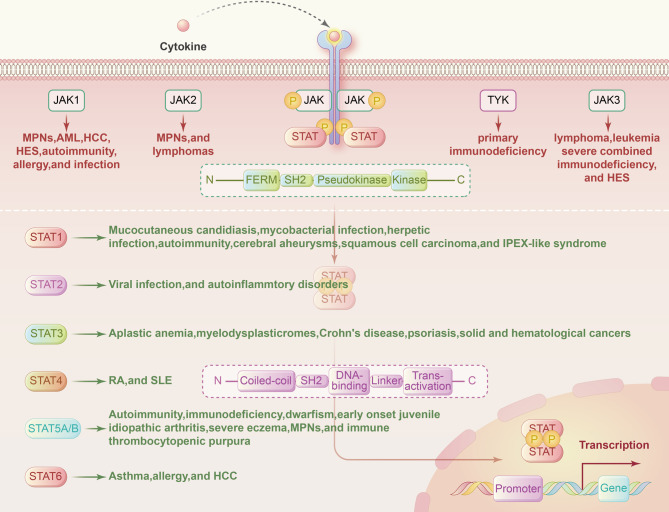
The associated cytokines and diseases of JAKs and STATs
| JAKs and STATs | Associated cytokine | Associated diseases | Ref. |
|---|---|---|---|
| JAK1 | IL-2, IL-4, IL-6, IL-7, IL-9, IL-10, IL-11, IL-15, IL-19, IL-20, IL-21, IL-22, IL-24, IL-28, IL-29, CNTF, OSM, LIF, CT-1, IFNα/β, and IFNγ | MPNs, AML, HCC, HES, autoimmunity, allergy, and infection | 63–67 |
| JAK2 | IL-3, IL-5, IL-6, IL-10, IL-11, IL-12, IL-13, IL-19, IL-20, IL-22, IL-23, IL-27, GH, EPO, TPO, PRL, Leptin, G-CSF, and GM-CSF | MPNs, and lymphomas | 71–75 |
| JAK3 | IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21 | lymphoma, leukemia, severe combined immunodeficiency, and HES | 48–53 |
| Tyk2 | IL-6, IL-10, IL-11, IL-12, Il-13, IL-23, IL-27, IL-19, IL-20, IL-22, IL-28, IL-29, IFN-α/β, and IFNγ | primary immunodeficiency | 9,64,85–90 |
| STAT1 | IL-2, IL-6, IL-10, IL-11, IL-22, IL-27, IL-28, IL-29, PDGF, EGF, HGF, TNF, TPO, angiotensin II, IFN-α/β, and IFNγ | mucocutaneous candidiasis, mycobacterial infection, herpetic infection, autoimmunity, cerebral aneurysms, squamous cell carcinoma, and IPEX-like syndrome | 95–100 |
| STAT2 | IL-28, IL-29, and IFNα/β | viral infection, and autoinflammatory disorders | 42,76,110,111 |
| STAT3 | IL-2, IL-3, IL-5, IL-6, IL-7, IL-9, IL-10, IL-11, IL-15, IL-19, IL-20, IL-21, IL-22, IL-23, IL-24, IL-26, IL-27, IL-28, IL-29, IL-31, LIF, CNTF, CT-1, OSM, CLCF1, GH, TPO, G-CSF, GM-CSF, Leptin, and IFNα/β | aplastic anemia, myelodysplastic syndromes, Crohn’s disease, psoriasis, solid and hematological cancers | 321,371–374 |
| STAT4 | IL-12, IL-23, IL-27, and IFNα/β | RA and SLE | 355–358 |
| STAT5a, STAT5b | IL-2, IL-3, IL-4, IL-5, IL-7, IL-9, IL-10, IL-15, IL-21, IL-22, IL-27, IL-28, IL-29, EGF, EPO, G-CSF, GM-CSF, TPO, GH, PDGF, Prolactin, and Leptin | autoimmunity, immunodeficiency, dwarfism, early-onset juvenile idiopathic arthritis, severe eczema, MPNs, and immune thrombocytopenic purpura | 362–367 |
| STAT6 | IL-3, IL-4, IL-5, and IL-13 | asthma, allergy, and HCC | 101–104 |
Fig. 2.
The JAK-STAT signaling pathway and immune. Interactions between a large number of cytokines and the JAK-STAT pathway influence immune cell differentiation and development and exert immunoregulatory effects. IFN and IL-12 are crucial for Th1 cell differentiation and drive T-bet gene expression through STAT1 and STAT4, respectively. IL-4 upregulates GATA3 genes via STAT6 to activate Th2 cell differentiation. IL-6 and TGF play an essential role in Th17 cell differentiation via STAT3 to trigger RORt gene expression. IL-6 and IL-12 influence T follicular helper cell differentiation via STAT3 to increase Bcl-6 transcription. IL-2 induces Treg cell differentiation via the direct combination of STAT5A/B with the Foxp3 gene
Fig. 3.
Mutations of the JAK-STAT pathway in human disease. Genetic mutations and polymorphisms of genes in the JAK-STAT pathway are widely involved in the pathogenesis of human diseases. The most frequent site of disease-causing mutations is within the SH2 domain in both JAKs and STATs
The effects of the JAK-STAT pathway on disease progression are complex. Cellular plasticity enables cells to adopt new phenotypes in response to environmental changes.156–158 Cancer cells amplify this plasticity to cause tumor heterogeneity, metastasis, and therapy resistance.156,159,160 The histologic transformation of lung cancers harboring epidermal growth factor receptor (EGFR) mutations from adenocarcinoma to aggressive neuroendocrine cancer is a notable example of lineage plasticity in cancer.161,162 Numerous lines of evidence suggest that JAK-STAT signaling is tightly connected to lineage plasticity and resistance through stem cell self-renewal modulation and multilineage differentiation.163–165 One of the most outstanding examples of this is the contribution of activated JAK-STAT signaling to the lineage transition of prostate cancer from adenocarcinoma to neuroendocrine cancer.31,166 In liver cancer, JAK-STAT3 is involved in the RAS-induced, malignancy-associated trans-differentiation of hepatocytes into intrahepatic cholangiocarcinoma cells.167 Moreover, activation of the IL-6-JAK-STAT pathway by WNT5A is known to facilitate epithelial-mesenchymal transition (EMT) in keloid scarring.160 A more comprehensive understanding of the effects of the JAK-STAT signaling pathway on lineage plasticity will provide a plausible molecular basis for the development of new therapies for malignant diseases.168
The JAK-STAT pathway and diseases
There is emerging evidence that JAK-STAT activation can play dual roles in diseases. Hyperactivation of the JAK-STAT pathway has been implicated in the poor outcomes of many diseases including melanomas, glioblastomas, and head, neck, lung, pancreatic, breast, rectal, and prostate cancers.23,125,169 Conversely, favorable regulatory roles of the JAK-STAT pathway have been demonstrated in head and neck squamous cell carcinomas and prostate and colorectal cancers. In the next sections, we have highlighted the critical roles of the JAK-STAT pathway in some common diseases, including rheumatoid arthritis, myeloproliferative neoplasms, kidney diseases, and prostate, breast, and lung cancers.
Rheumatoid arthritis
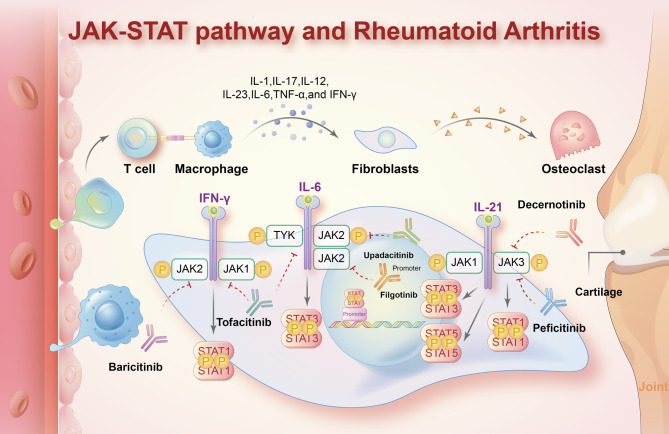
RA is a chronic inflammatory joint disease marked by progressive synovitis, leading to irreversible cartilage and bone erosion, eventual joint destruction, and functional disability.170–172 A central feature of RA pathogenesis is the production of inflammatory cytokines such as TNF, IL-1, and IL-6 by synovial cells.173–175 Several studies have reported that multiple proinflammatory cytokines drive RA progression by activating JAK-STAT signaling (Fig. 4).176–179 Fibroblasts, the key drivers of inflammation in the RA-afflicted synovium, were confirmed to produce IL-6 in the presence of STAT4 activation to trigger continuous joint destruction.180 Mori T. et al. pointed out that the IL-6–STAT3 cytokine loop is activated by inflammatory cytokines that are highly expressed in RA, causing chronic and persistent inflammation and joint destruction.181 Fibroblast-like synoviocytes from patients with active RA display elevated STAT1 expression and activity.182,183 Increased STAT3 activity in synovial CD4+ T cells in RA results in increased numbers of Th17 cells and reduced numbers of Treg cells and is closely related to the severity of synovitis. JAK3, STAT4, and STAT6 are also highly expressed in CD1a+ dendritic cells of patients with RA and may help to identify RA at the synovium level.184–188 Mouse models of proteoglycan-induced arthritis revealed that IL-4 controls inflammatory responses in RA by inhibiting IL-12-STAT4 signaling. Additionally, a gene ontology and pathway analysis revealed increased expression of type I IFN-inducible genes in the peripheral blood of patients with RA.189 Higgs, B. W. et al. demonstrated that an overactive type I IFN pathway is involved in the disease activity of RA.190 Another study showed that miR-17 suppressed the secretion of proinflammatory cytokines such as IL-6 and IL-1β and bound to STAT3 and JAK1 to play an anti-inflammatory and anti-erosive role in RA development. Wang, J. et al. reported that long intergenic non-protein-encoding long-chain RNA p53-induced transcript (LncRNA LINC-PINT) suppressed TNF-α–induced synovial fibroblasts in RA by upregulating SOCS1 levels.191
Fig. 4.
Roles of the JAK-STAT pathway in the pathogenesis of rheumatoid arthritis and recently approved JAK inhibitors. Abnormal JAK-STAT signaling induced by multiple cytokines is regarded as the essential pathogenesis of RA. The interaction of many cytokines (including IL-1, IL-17, IL-12, IL-23, IL-6, TNF-α, and IFN-γ) and the JAK-STAT pathway mediates inflammatory responses in the synovium and causes joint destruction. There are several JAK inhibitors approved for RA treatment, including tofacitinib, baricitinib, filgotinib, upadacitinib, peficitinib, and decernotinib. The dotted lines indicate negative regulation
JAK inhibitors were recently introduced as a novel type of disease-modifying antirheumatic drugs (DMARDs), which reduce synovitis and systemic inflammation and improve function in RA (Table 2). The European Society of Rheumatology (EULAR) in 2019 equated the application of JAK inhibitors with that of biological DMARDs (bDMARDs) in circumstances where conventional synthetic DMARDs (csDMARDs) are ineffective.192–194 A large observational study of the efficacy of commonly used second-line RA drugs confirmed that JAK inhibitors were more effective than methotrexate.195–198 Chen, C. et al. recently proposed that the JAK3 inhibitor Z583 irreversibly combines with cysteine residue 909 (Cys909) of JAK3 to prevent activation of JAK-STAT signaling, exerting powerful suppressive effects on RA progression.199 Given the expression and influence of JAK-STAT signaling in RA, JAK inhibitors are regarded as appropriate agents for RA management, although further large-scale studies are required to define more specific clinical applications of agents targeting JAK-STAT signaling in RA.200–205
Table 2.
Four licensed JAKis for RA treatment
| JAKis | Main trials | Chemical structure | T1/2 | Excretion | Clinically used doses | ref. |
|---|---|---|---|---|---|---|
| Baricitinib | Phase III, Genovese et al. (RA-BEACON); Phase III, Dougados et al. (RA-BUILD); Phase III, Fleischmann et al. (RA-BEGIN); and Phase III, Taylor et al. (RA-BEAM) | 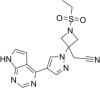 |
9–13 h | Urine75%, and Stool20% | 2 mg/4 mg | 475–478 |
| Tofacitinib | ORAL program: Phase III, Fleischmann et al. (ORAL solo); Phase III, Burmester et al. (ORAL step); Phase III, Vollenhoven et al. (ORAL-standard); Phase III, Kremer et al. (ORAL sync); Phase III, van der Heijde et al. (ORAL scan); and Phase III, Lee et al. (ORAL start) | 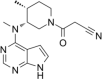 |
3 h | Urine51%, and Stool13% | 5 mg/10 mg | 200–205 |
| Filgotinib | Phase III, Genovese et al,(FINCH 2 trial), and Extension Study of Phase II, Kavanaugh et al. (DARWIN 3) | 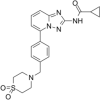 |
6 h | Urine87%, and Stool15% | 100 mg/200 mg | 177,178,196,197 |
| Upadacitinib | Phase III, Cohenet et al. (SELECT program) | 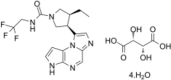 |
8–14 h | Urine24%, and Stool38% | 15 mg/30 mg | 261,262,265,266 |
Myeloproliferative neoplasms
Within hematology, JAK-STAT signaling has been explored in the context of myeloproliferative neoplasms (MPNs).206–209 MPNs are clonal hematopoietic conditions characterized by excessive proliferation of myeloid lineage cells, which contributes to abnormal numbers and morphology of peripheral blood cells and a high-risk of acute myeloid leukemia development.210–213 High-resolution genome-wide genotyping has identified acquired somatic mutations that are associated with increased JAK-STAT activity in various types of MPNs.214–216 Numerous studies reported that JAK2 mutations are frequent in MPNs and exert disruptive effects on the regulation of diverse biological processes.217–222 Rampal R et al. employed an integrated genomic analysis and found that the distinctive upregulation of JAK-STAT target genes helped distinguish different subtypes of MPNs, and the degree of valine-to-phenylalanine substitution at amino acid position 617 of JAK2 (JAK2V617F) influenced the disease severity.223 JAK2V617F results in constitutive tyrosine phosphorylation activity and is the most prevalent gain-of-function alteration in the majority of MPNs.61,224–226 Experiments with Pf4-Cre transgenic mice indicated that activated JAK-STAT signaling in megakaryocytes with the JAK2V617F mutation promoted the induction and maintenance of myeloproliferation in MPN through the production of proinflammatory cytokines and chemokines.227 Based on the association between JAK2V617F and MPN, the World Health Organization Classification and the International Consensus Classification both proposed a novel definition of “JAK2 mutation-prevalent MPNs”, which mainly consist of polycythemia vera, essential thrombocythemia, primary myelofibrosis, and unclassifiable MPN.219,228 Genomic analysis revealed frequent JAK2V617F mutations in over 90% of polycythemia vera cases and about 50% of essential thrombocythemia and primary myelofibrosis cases.
Calreticulin (CALR), a chaperone protein that localizes in the endoplasmic reticulum and participates in protein folding, is the second most frequently mutated protein in MPNs after JAK2. It is well established that CALR mutation is mutually exclusive with other MPN driver mutations, including JAK2V617F and the thrombopoietin receptor (MPL) mutation MPLW515L.229–231 Whole-exome sequencing identified CALR mutations in ~70% to 80% of JAK2V617F-negative ETs and primary myelofibrosis.232,233 In vivo research demonstrated that CALR mutation alone was strong enough to initiate an MPN phenotype and increased JAK-STAT signaling activity. The cellular model revealed that CALR mutants specifically activated MPL to drive the pathogenesis of MPN. Elf, S. et al. further demonstrated that the malignant transformation driven by mutant CALR in MPN required interaction with MPL.234,235
Thrombopoietin and its receptor MPL are primary regulators of megakaryocyte growth and differentiation.236–238 The pathogenetic mutation MPLW515L induces abnormal activation of JAK-STAT signaling and participates in MPN development.231,239 MPLW515L promotes hematopoietic cell proliferation and cellular responses to thrombopoietin. Lnk, the negative regulator of thrombopoietin, can reverse the growth-stimulating signals in cells carrying MPLW515L and cause malignant cells to die. Moreover, a study of a cohort of 1182 patients with MPN and the exploration of bone marrow-derived DNA from different disease courses consistently showed that MPLW515L and JAK2V617F occurred concurrently in MPNs, suggesting that the functions of these driver mutations may be relatively complementary in the course of MPN pathogenesis.240,241
Atopic dermatitis
Atopic dermatitis (AD) is one of the most common chronic immune-mediated skin conditions. Patients with AD suffer recurrent symptoms and impaired quality of life.242–244 Existing therapies for AD relieve the symptoms but cannot delay disease progression, and all of these therapies have limitations in clinical applications.245,246 The pathogenesis of AD has a multifactorial origin and is considered to predominantly result from T helper 2 (Th2) cell-mediated inflammation and the gradual upregulation of Th1, Th17, and Th22 cell-mediated inflammation at the later stages of disease progression.247 A variety of mediators, including IL-4, IL-13, IL-31, and TSLP, bind specific transmembrane receptors and stimulate the JAK-STAT pathway, thereby initiating intracellular signaling and exerting proinflammatory effects in AD.248,249 In-depth explorations of AD pathogenesis have suggested that the JAK-STAT pathway is involved in Th2 immune polarization, eosinophil activation, and skin barrier destruction during AD progression.250
A greater understanding of the roles of the JAK-STAT pathway in AD has enabled the application of JAK inhibitors as a new approach to AD treatment.251–253 Baricitinib, an oral selective inhibitor of JAK1 and JAK2, has been approved for the treatment of moderate-to-severe AD.254 The results of two phase 3 trials (BREEZE-AD1 and BREEZE-AD2) have shown that baricitinib significantly relieves unbearable pruritus and skin lesions in patients with moderate-to-severe AD.255 Topical ruxolitinib has also shown anti-inflammatory and antipruritic effects in experimental models and phase 3 clinical trials (TRuE-AD1 and TRuE-AD2).256–258 Given the close connection between JAK1 and the pathogenesis of AD, selective JAK1 inhibitors are promising therapeutic candidates for AD. Upadacitinib, was a highly specific JAK1 inhibitor previously explored in RA and further approved by the FDA in 2019 for the treatment of moderate-to-severe refractory AD in patients aged ≥12 years.259–268 Mechanistically, upadacitinib relieves chronic dermatitis by potently attenuating the cytokines related to JAK1 inflammatory signaling (including IL-6 and IFNs).269–272 The minimal effects of upadacitinib on JAK2 and JAK3 result in a few side effects, especially in the hemopoietic system.273–278 Several phase 3 trials (Measure Up 1, Measure Up 2, and AD Up) have shown that upadacitinib as a monotherapy or in combination with topical corticosteroids has favorable efficacy and tolerability in patients with moderate-to-severe AD.259,279 In 2022, abrocitinib, a selective JAK1 inhibitor, was also approved for the treatment of moderate-to-severe AD.280–283 Phase 3 studies including JADE MONO-1, MONO-2, and TEEN have shown that 200 or 100 mg abrocitinib was more effective than placebo or dupilumab in terms of the early alleviation of itch and reduction of the eczema area as well as the severity index (EASI-75) and Investigator’s Global Assessment (IGA) scores.284–288 A phase 2 study (NCT02201524) also suggested that abrocitinib resulted in favorable symptom improvement in patients with moderate-to-severe psoriasis.289
Hepatocellular carcinoma
Several studies have indicated that the JAK-STAT signaling pathway is widely involved in multiple types of solid cancers. Hepatocellular carcinoma (HCC) is a lethal and heterogeneous tumor with consistently high incidence and mortality rates, contributing to more than 700,000 deaths annually.290–292 Chronic hepatitis B virus (HBV) infection occurs in more than 400 million people across the globe and is the most frequent cause of HCC. Despite improvements in the management of HCC, the clinical prognosis of HCC remains unfavorable, with a 5-year survival rate of 10%. The process of hepatic carcinogenesis is thought to involve a series of genetic alterations and abnormal changes in signaling pathways.293–297 The JAK-STAT signaling pathway has been extensively explored for potential roles in HCC pathogenetic progress. The use of interferon-alfa (IFN-α) to drive host antiviral responses is the current first-line treatment for chronic hepatitis B and has been verified to slow the progression of liver fibrosis and even the occurrence of HCC. As a crucial immune-related cytokine, IFN-α activates JAK-STAT signaling to induce various IFN-stimulated genes with antiviral and immunomodulatory functions.298 Han M et al. found that the expression and activation of IFN-stimulated genes including STAT1, myxovirus resistance (Mx), and SOCS3 in the peripheral blood of patients with chronic hepatitis B were closely correlated with IFN-induced antiviral outcomes.299,300 SOCS3 overexpression following HBV infection caused the inactivation of IFN signaling and was correlated with the severity of liver inflammation.301,302 In an HBV-producing HepG2 cell line, Hepatitis B Virus X protein weakened IFN-α signaling by inactivating STAT3 to increase SOCS3 and protein phosphatase 2 A expression. Gao D et al. also observed downregulation of SOCS3 by miR-122 increased the anti-HBV effectiveness of IFN.303 Numerous studies reported that SOCS3 is downregulated in HCC tissues and is negatively implicated in HCC malignant transformation.304,305 SOCS3 methylation was discovered to be more frequent in HBV-positive HCC than in normal liver tissues and was associated with poor HCC outcomes. Eyes absent homolog 2, which transcriptionally upregulates SOCS3 expression to block JAK-STAT signaling, was identified as a potential tumor suppressor in HCC and found to inhibit HCC progression.306 Conversely, CircSOD2 suppressed SOCS3 expression and triggered JAK2–STAT3 signaling to drive HCC progression.307 Furthermore, as a member of the B7 family of immune checkpoint proteins, HHLA2 was demonstrated to activate the JAK-STAT pathway and accelerate HCC progression by binding to TMIGD2.308
Prostate cancer
Androgen receptor (AR)-targeted therapies such as enzalutamide have achieved considerable clinical success in patients with prostate cancer, mainly by attacking the glandular luminal cells. Unfortunately, advanced prostate cancer usually undergoes lineage conversion into squamous cell carcinoma or neuroendocrine cancer, resulting in drug resistance. Several studies have found associations between excessive JAK-STAT signaling activation in prostate cancer and the regulation of lineage plasticity, resistance to AR-targeted therapies, and poor clinical outcomes.309,310 JAK1, JAK2, STAT1, and STAT3 are essential drivers of lineage plasticity in cancer cells. In particular, constitutively activated STAT3 promotes lineage plasticity and associated cell migration and invasion.311,312 In mouse organoids, genetically engineered mouse models (GEMMs), and human cell lines, combined with fibroblast growth factor receptor (FGFR) signaling, the JAK-STAT pathway was shown to trigger luminal-basal phenotype conversion and subsequent castration-resistant prostate cancer (CRPC) formation.31 The pharmacological inhibition of JAK-STAT signaling in vitro and in vivo led to the re-sensitization of resistant cancer cells to enzalutamide and effectively reversed the undesirable phenotypic plasticity.166 These results provide vital theoretical support for new approaches to overcome drug resistance in CRPC and shed light on the association between lineage plasticity and carcinogenesis.
Breast cancer
Breast cancer is the most prevalent cancer in women worldwide. There are three general categories of breast cancer: hormone receptor-positive (HR+), human epidermal growth factor receptor-positive (HER2+), and triple-negative (TN).313–315 Distant metastasis and therapy resistance are the root cause of the poor prognosis of the majority of patients with breast cancer.316,317 Accumulating evidence suggests that all the STAT family members are closely associated with breast cancer, having either pro-tumorigenic or antitumorigenic characteristics.318 Therefore, breast cancer provides an example of the double-edged sword role of JAK-STAT in carcinogenesis.
As an essential regulator of cell transformation and apoptosis during breast development, STAT3 has been extensively explored in the context of breast cancer.319,320 Several studies have shown that STAT3 plays a central role in the proliferation, invasion, metastasis, and immune escape of breast cancer cells.321,322 Moreover, STAT3 helps maintain the phenotype and function of breast cancer stem cells (BCSCs), which display pluripotent properties, resistance to chemotherapy, and high capacity for self-renewal.323–326 The classical IL-6-JAK-STAT3 pathway upregulates genes related to breast cancer progression, induces resistance to aromatase inhibitor (AI) chemotherapy, and correlates with poor survival in HR+ breast cancer.327–330 In patients with HER2+ breast cancer, alone or in combination with trastuzumab, the selective JAK1/2 inhibitor ruxolitinib weakens cancer cell viability and improves the clinical outcomes by inhibiting the IL-6–JAK2–STAT3–calprotectin axis.331
Despite the pro-tumorigenic effects of JAK-STAT signaling, some preclinical studies have shown that JAK inhibitors impair the antitumor immunity of NK cells and increase the metastatic burden in breast cancer.332 Furthermore, ruxolitinib, a JAK1 and JAK2 inhibitor, can induce the generation of proinflammatory mediators by macrophages, resulting in the establishment of a pro-tumorigenic microenvironment and drug resistance in breast cancer.333 The diverse effects of the JAK-STAT pathway in breast cancer highlight the importance of JAK-STAT signaling and offer insights into the development of effective therapeutic strategies.
Kidney diseases
With the notable exceptions of RA, hematological malignancies, and solid cancers, it’s also reported by the transcriptome analysis that increased STAT1 and STAT3 expression in kidney glomerular and tubulointerstitial sections were associated with disease progression in focal segmental glomerulosclerosis.334 Pang et al. confirmed that STAT3 activation mediated renal fibrosis in unilateral ureteral obstruction models, and the application of the novel STAT3 inhibitor S3I-201 suppressed renal interstitial fibroblast activation and fibrosis.335 By contrast, Koike, K. et al. observed that activated JAK-STAT3 signaling in unilateral ureteral obstruction models induced matrix metalloproteinase-2 expression in proximal tubular cells to relieve renal fibrosis and promote tissue repair.336 The conflicting findings regarding the functions of JAK-STAT signaling in renal tissue repair and fibrosis may result from differences in animal models and the interventions applied to regulate the JAK-STAT pathway.
STAT3 activation induced by hyperuricemia in tubular and interstitial cells was accompanied by kidney fibrosis and dysfunction, and the STAT3 inhibitor S3I-201 was confirmed to suppress the JAK-STAT pathway to achieve anti-fibrotic effects. In an exploration of kidney tubulointerstitial fibrosis, miR-150–based RNA interference was confirmed to reverse activation of the SOCS1-JAK-STAT pathway and alleviate tubulointerstitial fibrosis.337 In addition, overactive STAT1 and STAT3 expression and activated JAK-STAT signaling were demonstrated to accompany progressive kidney inflammation and proteinuria in IgA nephropathy.338
Activation of JAK-STAT signaling was also found to play an essential pathogenic role in renal damage caused by diabetes mellitus through the stimulation of excessive glomerular mesangial cell growth.339–341 Increased activity of the JAK-STAT pathway in mice with diabetic nephropathy prevented podocyte autophagy and enhanced disease progression.342 A remarkable increase in JAK2 level was also integrally linked to a series of deteriorating clinical symptoms of diabetic nephropathy, including albuminuria, glomerulosclerosis, and reduced numbers of podocytes.343 In STZ-induced diabetic rat models, hyperglycemia triggered the JAK-STAT pathway in glomerular mesangial cells through angiotensin II, and treatment with angiotensin II blockade reversed pathological changes to improve kidney function.
Autosomal dominant polycystic kidney disease (ADPKD), the most common congenital kidney disorder, is commonly accompanied by abnormal activity of JAK-STAT pathways.344,345 Forced STAT5 expression in ADPKD causes aberrant proliferation by transcriptionally upregulating cyclin D1 in a growth hormone-dependent manner. Pkd1nl/nl murine models revealed heterotopic JAK2 expression in cyst-lining cells and interstitium and validated that JAK2 inhibition could postpone cystic growth in ADPKD.346
Experiments using an MRL/lpr mouse model indicated that renal CD8+ tissue-resident memory T cells required JAK/STAT signaling for self-renewal and effector functions, which were related to lupus nephritis activity. The STAT3 level was also dramatically elevated in lupus nephritis and linked to unfavorable clinical parameters.347 In non-human primate allograft models based on calcineurin-inhibitor-free regimens, use of the JAK3 inhibitor CP-690,550 in combination with mycophenolate mofetil lowered the incidence of kidney acute rejection and prolonged allograft survival.348–350 Additionally, JAK-STAT signaling triggered by IFN-γ was demonstrated to be inhibited by the FGFR pathway in renal cell carcinoma cells, and treatment with lenvatinib, a targeted FGFR receptor tyrosine kinase inhibitor, recovered antitumor immunity and enhanced the efficacy of immune checkpoint inhibitors.351
JAK-STAT pathway and treatment
The JAK-STAT signaling pathway is the essential intracellular route through which diverse extracellular soluble molecules bind membrane receptors and transfer signals to the nucleus. Given its primary roles in a series of cancers and autoimmune diseases, JAK-STAT signaling has emerged as a prominent target for drug development. Drugs that target the JAK-STAT pathway can be classified into three groups according to how they affect the signal transduction process: cytokine or receptor antibodies, STAT inhibitors, and JAK inhibitors.352–354
Upstream cytokines and receptors are pivotal for modulating the functions of the JAK-STAT signaling pathway.355–358 Drugs that manipulate JAK-STAT-dependent cytokines and receptors (e.g., siltuximab and tocilizumab for the classical blockade of IL-6) inhibit JAK-STAT signal transduction and have therefore been used as therapeutic interventions in many diseases.359–362
Most STAT inhibitors function by restricting STAT phosphorylation, inhibiting SH2-mediated dimerization, or inducing STAT degradation.363–367 Given the crucial functions of activated STAT3 and STAT5 in signal transduction and pathogenic progression, multiple inhibitors targeting STAT3 and STAT5, including peptides, peptidomimetics, oligonucleotides, siRNAs, small molecules, and metal-based complexes, have shown promising preclinical efficacy.368–374 Hundreds of research articles have described the various underlying mechanisms of STATs in disease progression and the efficacies of dozens of STAT inhibitors in preclinical and clinical trials.
STAT3, the most widely studied STAT protein, is frequently mutated and overactivated in diverse cancers and promotes cancer cell proliferation, invasion, metastasis, and immune evasion.318 Napabucasin (BBI608), the first direct STAT3 inhibitor to enter phase 3 clinical trials and the first-in-class cancer stemness inhibitor, has been shown to restrain diverse malignant processes in multiple cancers. Moreover, napabucasin was found to decrease the expression of critical stemness-related genes, deplete cancer stem cell (CSC) populations and re-sensitize chemo-resistant cells to cisplatin in cisplatin-resistant NSCLC models.375 Napabucasin also possesses anti-acute myeloid leukemia (AML) properties and enhanced the Bcl-2 inhibitor efficacy by inhibiting STAT3 and inducing DNA damage, as revealed in in vitro and in vivo experiments.376 In addition, napabucasin eliminated the immunosuppressive functions of both murine and human myeloid-derived suppressor cells (MDSCs) in malignant melanoma and was found to be associated with the patients’ clinical outcomes.377 A recent phase 3 trial (NCT01830621) of napabucasin monotherapy in advanced colorectal cancer showed that its potential outcomes in terms of patient prognosis were better in the napabucasin group than in the placebo group.378 Moreover, napabucasin has been recently approved by the FDA as an orphan drug for gastric and pancreatic cancer treatment. And OPB-31121, a novel STAT3 inhibitor, induces cell apoptosis and was shown to exert synergism with 5-fluorouracil and cisplatin in both gastric cancer cells and xenograft models.379 Additionally, OPB-51602, a selective inhibitor of STAT3 phosphorylation, reduces complex I activity and increases ROS concentrations to maintain high toxicity to cancer cells.380 OPB-51602 was observed to improve acquired drug resistance by preventing the reliance of cancer cells on mitochondrial oxidative phosphorylation (OXPHOS).381,382 In a first-in-human phase I clinical trial (NCT01184807), OPB-51602 exhibited promising antitumor activity among patients with refractory solid tumors, especially NSCLC.383 Moreover, TTI-101 (C188-9), another STAT3 inhibitor, was found to attenuate the activation of the phosphotyrosine (pY) peptide-binding site within the SH2 domain of STAT3, without the influence of mitochondrial function.384 Multiple studies conducted in animal models have demonstrated effective therapeutic potential and favorable safety profiles in a series of autoimmune diseases and cancers, including SSc, Crohn’s disease, head and neck cancer, and lung, breast, colorectal, and liver cancers.385–389 Most recently, TTI-101 has been granted the ‘fast track’ designation by the FDA for applying relapsed/refractory locally advanced, unresectable, or metastatic hepatocellular carcinoma. An ongoing phase 1 clinical trial of TTI-101 (NCT03195699) has reported that patients with advanced solid tumors tolerated the administration of TTI-101 well.
Moreover, emerging studies have revealed the potential of STAT5 inhibitors. A prior study has demonstrated that STAT5 programs the new generation of GM-CSF-producing T helper (TH) cells (TH-GM), which cause more severe neuroinflammation than TH1 and TH17 cells.390 The preliminary result of a STAT5 inhibitor clinflamozyde in a recent COVID-19 clinical trial approved by the FDA observed that clinflamozyde selectively repressed the STAT5-TH-GM pathway to relieve severe cytokine storms and significantly improved clinical outcomes of patients with COVID-19.
Moreover, increasing strategies integrating STAT3 with immune checkpoint inhibitors (e.g., anti-CTLA-4 and PD-1/PD-L1 antibodies), CAR-T-cell therapy (NCT02906371), stimulator of interferon genes (STING) agonists, and even cancer vaccines have shown encouraging synergistic antitumor efficacy in diverse preclinical trails.319,391–394
Based on the previous exploration, STAT inhibitors have demonstrated promising value in their antitumor effects. Unfortunately, most STAT inhibitors are still in the preclinical stages of development, and few have been approved for clinical application owing to the lack of intrinsic catalytic activity and selectivity shown by most candidates. A large number of clinical trials targeting STATs as monotherapies and combination therapies are currently underway, offering an attractive drug target for disease treatment.
Despite this, natural products and their derivatives have served as sources of novel antitumor agents based on the JAK-STAT pathway.395–397 Over the past several decades, in vitro and in vivo experiments have shown that various natural products have inhibitory effects on the STAT3 signaling pathway and exhibit promising anticancer activities.398 For example, the phytochemical curcumin was shown to effectively inhibit STAT3 and play anticancer roles in breast, ovarian, lung cancer, and esophageal squamous cell carcinoma.399–401 Moreover, the natural flavonoid myricetin was found to downregulate the levels of programmed death ligand-1 (PD-L1) and indoleamine 2, 3-dioxygenase 1 (IDO1) and restore T-cell activity and antitumor immunity by inhibiting the IFN-γ-activated JAK-STAT–IRF1 axis in lung A549, breast MDA-MB-231 and colon HCT116 cancer cells.402,403 Runtsch et al. proposed that the natural metabolite itaconate and its derivatives inhibit M2 polarization by blocking JAK1-STAT6 pathway phosphorylation, providing a novel perspective on M2 macrophage-driven diseases.404 As a new natural STAT3 inhibitor, XYA-2 was found to bind the SH2 domain of STAT3 and synergistically downregulate the levels of MYC and SLC39A10 in human gastric cancer cell lines and patient-derived xenograft (PDX) mouse models to perform its anticancer roles.405
In addition, SOCS proteins, as part of a negative feedback loop in the JAK-STAT pathway, and peptides targeting SOCS interactors have also shown promise as inhibitors of disease progression.117,406,407
Recently, several JAK inhibitors have been clinically approved for the treatment of various diseases, and a growing body of innovative drug candidates are in preclinical and clinical trials (Table 3). Here, we review the critical applications and adverse events of the major JAK inhibitors in human diseases.
Table 3.
The applications and safety of main JAK inhibitors
| JAK inhibitors | Selectivity | Approved indications | Indications under the clinical trials | Reported adverse events | Ref. |
|---|---|---|---|---|---|
| Tofacitinib | JAK1 and JAK3 | rheumatoid arthritis, ulcerative colitis, juvenile idiopathic arthritis, and psoriatic arthritis | psoriasis, Crohn’s disease, COVID-19, alopecia areata, dermatomyositis, atopic dermatitis, keratoconjunctivitis sicca, relapsing polychondritis, ankylosing spondylitis, inflammatory bowel disease, and transplant rejection | infections, malignancies, anemia, neutropenia, elevated in serum creatinine and transaminases, hypercholesterolemia, gastrointestinal symptoms, and thromboembolism | 202,203,205,445–448,451–459,465–467 |
| Peficitinib | JAK3, JAK1, TYK2 and, JAK2 | rheumatoid arthritis | ulcerative colitis and psoriasis | infections, malignancies, elevated creatine kinase, elevated creatinine, and hyperlipidemia | 185–188,192–194 |
| Ruxolitinib | JAK1 and JAK2 | myelofibrosis and polycythaemia vera | psoriasis, polycythemia, vitiligo, malignancies, acute graft-versus-host disease, rheumatoid arthritis, essential thrombocythemia, alopecia areata, atopic dermatitis, COVID-19 | anemia, thrombocytopenia, neutropenia, hypokalemia, infections, and peripheral edema | 206,411–417,419–425,437–442 |
| Baricitinib | JAK1 and JAK2 | rheumatoid arthritis, atopic dermatitis, and COVID-19 | lupus erythematosus, juvenile dermatomyositis, psoriasis, diabetic nephropathy, alopecia areata, and autoinflammatory diseases | infections, malignancies, and hyperlipidemia | 475,477–490 |
| Delgocitinib | JAK1, JAK2, TYK2, and JAK3 | atopic dermatitis | eczema, discoid lupus erythematosus, psoriasis, and alopecia areata | nasopharyngitis, Kaposi’s varicella, contact dermatitis, and acne | 498–503 |
| Momelotinib | JAK1 and JAK2 | / | myelofibrosis and multiple myeloma | diarrhea, cough, nausea, anemia, neutropenia, thrombocytopenia, and treatment-emergent peripheral neuropathy | 206–209,220–222 |
| Filgotinib | JAK1 | rheumatoid arthritis | inflammatory bowel disease, psoriatic arthritis, and ankylosing spondylitis | infections, nasopharyngitis, and headache | 177–179,196–198 |
| Upadacitinib | JAK1 | rheumatoid arthritis, ankylosing spondylitis, non-radiographic axial spondyloarthritis, and psoriatic arthritis | inflammatory bowel disease, and atopic dermatitis | infections, malignancies, elevated lipid parameters, increased creatine phosphokinase, increased hepatic aminotransferase, low blood cell counts, stroke, and venous thromboembolisms | 260–272,275–278 |
| Fedratinib | JAK2 | myelofibrosis | thrombocytopenia and solid tumors | fatal encephalopathies, anemia, gastrointestinal symptoms, increased liver transaminases, increased serum creatinine, and increased pancreatic enzymes | 417,507–511 |
| Abrocitinib | JAK1 | atopic dermatitis | psoriasis, systemic lupus erythematosus, and arthritis | nasopharyngitis, nausea, vomiting, acne, herpes zoster, increased blood creatine phosphokinase, dizziness, and headache | 283–287,289,528–531 |
| Decernotinib | JAK3 | / | rheumatoid arthritis | headache, nausea, infections, elevated transaminases, lipoproteins, and creatinine, reduced lymphocyte and neutrophil | 513,515–517 |
| Itacitinib | JAK1 | / | graft-versus-host disease and lung cancer | reduced platelet and neutrophil, anemia, and hyperglycemia | 518,519 |
| Deunavacitinib | TYK2 | plaque psoriasis | psoriatic arthritis, systemic lupus erythematosus, and inflammatory bowel diseases | nasopharyngitis and upper respiratory tract infections | 534–537 |
JAK inhibitors are small-molecule inhibitors that cause immunosuppression, reduce the pathological production of proinflammatory cytokines driven by JAK-STAT signaling, and inhibit gain-of-function JAK mutants.408,409 Various JAK inhibitors are currently in preclinical and clinical studies to treat a series of autoimmune diseases and cancers.410–417
The first-generation oral JAK inhibitors revolutionized the treatment of a group of heterogeneous disorders.418 The first clinically approved JAK inhibitor was the JAK1 and JAK2 inhibitor ruxolitinib, which was approved by the U.S. Food and Drug Administration (FDA) in 2011 to treat myelofibrosis.206,419–425 The COMFORT-I trial conducted by Verstovsek, S. et al. demonstrated the clinical benefits of ruxolitinib for patients with intermediate-2 or high-risk myelofibrosis; the ruxolitinib group showed improved manifestations, reduced spleen size, and elevated overall survival compared with the placebo group.420 Later, the COMFORT-II trial showed that patients with myelofibrosis benefited from continuous ruxolitinib therapy in comparison with patients that received the best available therapy, highlighting the long-term effectiveness and overall survival benefits of ruxolitinib.426 Kvasnicka, H. M. et al. reported that long-term administration of ruxolitinib for 48 months or 60 months ameliorated and stabilized the progression of bone marrow fibrosis.427 Zeiser et al. conducted a phase 3 trial to assess the efficacy of ruxolitinib in patients with glucocorticoid-refractory or -dependent chronic graft-versus-host disease. The results showed that 165 patients that received ruxolitinib manifested more favorable overall responses at 24 weeks, a longer duration of response at 12 months, and better failure-free survival than patients that received common second-line therapies.428 In a single-center retrospective study of patients with inflammatory bowel disease, 6 months of ruxolitinib treatment resulted in improved extraintestinal symptoms, stool frequency, steroid taper, and nutritional status.429 A case report suggested that the use of ruxolitinib early in the disease courses of refractory systemic idiopathic juvenile arthritis and interstitial lung diseases may block IFN-γ signaling to postpone disease-related deterioration.430 More recently, the RUXCOVID phase 3 trial randomly divided 432 patients into a ruxolitinib group (n = 287) and a placebo group (n = 145) plus standard of care to estimate the efficacy of ruxolitinib for the treatment of coronavirus disease 2019 (COVID-19). The results indicated that the median recovery time was 1 day less for patients treated with ruxolitinib compared with patients that received placebo.431 However, Fisher, D. et al. recently pointed out that multi-cytokine overproduction persisted in patients with myelofibrosis after treatment with ruxolitinib.432 Notably, a 75-year-old man with myelofibrosis was reported to develop progressive multifocal leukoencephalopathy after receiving ruxolitinib treatment.433 Several studies have also reported that the rapid discontinuation of ruxolitinib administration induced life-threatening withdrawal symptoms, which may be attributed to robust changes in the activities of inflammatory cytokines.434–442
The preferentially selective JAK3 and JAK1 inhibitor tofacitinib was the first JAK inhibitor approved for patients with RA that had poor responses to conventional drugs like methotrexate.443,444 Several studies showed that tofacitinib was consistently more efficacious and safer compared with other DMARDs in patients with RA.445–448 In recent years, tofacitinib exhibited promising efficacy in clinical trials for diverse autoimmune diseases.203,449–459 Tofacitinib was approved by the FDA for the treatment of active ulcerative colitis in 2018 and was also later validated in numerous studies to be safe and effective for the treatment of inflammatory bowel diseases.460–467 Sandborn, W. J. et al. performed three phase 3 trials and confirmed the favorable efficacy of tofacitinib as an induction and maintenance therapy in patients with moderately to severely active ulcerative colitis. Then, they further integrated several global clinical trials and validated the long-term safety and effectiveness of tofacitinib for patients with ulcerative colitis.468 In an open-label trial involving 10 patients with sarcoidosis, tofacitinib ameliorated skin and internal-organ symptoms in all 10 patients chiefly by suppressing type 1 immunity.469 You, H. et al. indicated that tofacitinib was the same or better than conventional immunosuppressants for improving the modified Rodnan skin score of patients with refractory skin thickening in diffuse cutaneous systemic sclerosis.470 Changelian, P. S. et al. demonstrated the potential benefit of tofacitinib for preventing allograft rejection in vivo by showing that tofacitinib mediated immunosuppression to prevent organ rejection in a murine model of heart transplantation and in a non-human primate model of kidney transplantation.350 To further explore the safety of tofacitinib in a real-world setting, Khosrow-Khavar, F. et al. enrolled two cohorts of patients with RA that received either tofacitinib or tumor necrosis factor (TNF) inhibitor and found a statistically insignificant increase in adverse cardiovascular outcomes in patients after tofacitinib treatment.471 However, the ORAL Surveillance reported that tofacitinib caused higher rates of infection and cardiovascular events compared with tumor necrosis factor inhibitors in patients with RA that were more than 50 years of age and had at least one additional cardiovascular risk factor. In preclinical trials and preclinical genetic studies, ruxolitinib was found to exhibit potential cardiotoxicity that may present as toxic myocardial damage, decreased left ventricular systolic function, or cardiac failure.472–474
The selective JAK1 and JAK2 inhibitor baricitinib was approved for the treatment of RA in 2017 and has been widely confirmed to improve clinical symptoms of RA in patients that have an inadequate response to methotrexate.475,476 The RA-BEAM trial involving patients with moderately to severely active RA and an inadequate response to methotrexate showed that baricitinib was superior to placebo with respect to signs and symptoms, physical function, and joint structural damage.477–484 Baricitinib also produced better improvements than adalimumab according to the criteria of the American College of Rheumatology (ACR20 response) and Disease Activity Score 28-joint count C reactive protein (DAS28-CRP).485–490 The RA-BEACON trials explored the efficacy of baricitinib in patients with RA that were refractory to bDMARDs. In two phase 3 trials in patients with severe alopecia areata, baricitinib was superior to placebo in producing hair regrowth.487. Baricitinib was also found to have a high affinity for numb-associated kinase (NAK) which enabled it to suppress NAK activity and block clathrin-mediated endocytosis to reduce viral infection in COVID-19.491,492 In addition, a pilot study showed that baricitinib improved respiratory symptoms and did not cause serious adverse events in 12 patients with mild-to moderate COVID-19 pneumonia.493 Cantini, F. et al. also performed a retrospective multicenter study of 113 consecutive patients hospitalized with moderate pneumonia and validated the beneficial effects of baricitinib in terms of reduced viral burden, mortality, and hospitalization rates.494 The ongoing RECOVERY trial further confirmed the effectiveness of baricitinib in reducing mortality due to COVID-19. An integrated study of 3,492 patients with RA that received baricitinib found no correlation between baricitinib use and major adverse cardiovascular events, arterial thrombotic events, or congestive heart failure.495
Peficitinib, a pan-JAK inhibitor that significantly inhibits JAK3, was approved for the treatment of RA in Japan in 2019.496 Numerous phase 2b (e.g., RAJ1, RA21, and RA22) trials have shown that peficitinib, as a monotherapy or combined with csDMARDs, achieved a rapid and statistically significant American College of Rheumatology (ACR) response rate over 12 weeks in patients with moderate-to-severe RA.187,188,192 The phase 3 trial (RAJ3 and RAJ4) also observed that peficitinib performs better in alleviating RA symptoms and joint destruction after 12 weeks of treatment. In addition, a phase 2b trial showed that patients with ulcerative colitis receiving ≥75 mg of peficitinib exhibited higher rates of clinical response, remission, and mucosal healing at week 8.194 Based on the outcomes of a phase 2a multicenter placebo-controlled study, peficitinib also exhibited dose-dependent improvements in clinical and histological lesions among patients with moderate-to-severe psoriasis.193 Furthermore, the safety profile of peficitinib is acceptable, commonly including nasopharyngitis, herpes zoster, diarrhea, increased blood creatine phosphokinase levels, and lymphopenia.497 Delgocitinib, a pan-JAK inhibitor with broad inhibitory effects on proinflammatory cytokines, is effective against diverse inflammatory skin conditions, including AD, eczema, discoid lupus erythematosus, psoriasis, and alopecia areata.498–500 In phase 3 trials, the topical administration of delgocitinib produced clinically meaningful improvements in efficacy endpoints for patients with moderate-to-severe AD, resulting in the approval of delgocitinib for AD treatment. Moreover, throughout the study periods in both children and adults with AD, the majority of adverse events were mild and not relevant to the use of delgocitinib.501–504
The first-generation JAK inhibitors are considered to be pan-JAK inhibitors that target multiple JAK isoforms. Given their highly conserved ATP-binding sites, JAK inhibitors produce a wide range of effects but also cause side effects including cardiovascular events, thromboembolic events, infection, and oncogenic complications.505 Therefore, the development of more selective JAK inhibitors with an emphasis on enhanced specificity and fewer adverse effects has become a focus of drug research.506 Fedratinib is a selective JAK2 inhibitor that was approved by the FDA in 2019 to treat patients with intermediate-2 or high-risk myelofibrosis.507–511 The National Comprehensive Care Network also classified fedratinib as a category 1 recommendation for higher-risk patients with myelofibrosis and platelet counts ≥50 × 109/L.512 The randomized JAKARTA phase 3 trial and nonrandomized JAKARTA-2 phase 2 trial consistently showed that fedratinib attenuated symptoms and splenomegaly in patients with ruxolitinib-resistant or intolerant intermediate-1, intermediate-2, or high-risk myelofibrosis.508 In addition, Harrison, C. N. et al. conducted a phase 2 multicenter study and reported that treatment with 400 mg fedratinib resulted in remarkable reductions in spleen volume and clinical symptoms in ~30% of patients with myelofibrosis that was resistant or intolerant to ruxolitinib.417 However, fedratinib was reported to cause Wernicke’s encephalopathy, confirmed by magnetic resonance imaging, in 8 of 670 patients that received fedratinib treatment and in 4 women who received 500 mg/day fedratinib in the JAKARTA trial, which raised concerns and hampered the further clinical development of fedratinib.510 Anemia and thrombocytopenia were also observed in patients that received fedratinib treatment in both the JAKARTA trial and the JAKARTA-2 trial. Decernotinib, a JAK3-selective inhibitor, is currently under development and considered efficacious against RA.513,514 The previous phase 2a and 2b trials both reported that decernotinib, as a monotherapy or in combination with methotrexate, contributed to the improved signs and symptoms of RA patients with inadequate response to methotrexate.515–517 The major adverse effects seen in those trials included headache; nausea; elevated levels of transaminases, lipoproteins, and creatinine; and reduced numbers of lymphocytes and neutrophils.516 Itacitinib, a novel selective JAK1 inhibitor, has a wide range of anti-inflammatory activities. Thus far, itacitinib has been primarily used for the treatment of graft-versus-host disease (GVHD). A multicenter phase 2 trial (NCT03846479) recently reported that patients with low-risk acute GVHD administered 28 days of itacitinib without the conventional administration of systemic corticosteroids (SCSs) achieved effective drug response rates and reduced symptomatic flares.518 A phase 3 trial (GRAVITAS-301, NCT03139604) demonstrated that itacitinib, in combination with corticosteroids, did not attain the expected improvement in the overall response rate (ORR) at day 28 among patients with acute GVHD.519 The European Society for Blood and Bone Marrow Transplantation (EBMT 2022) reported the preliminary results of a phase 2 trial (NCT 04071366), stating that itacitinib exhibited good performance in the prevention of cytokine release syndrome (CRS) following by immunotherapies. Moreover, the World Congress of lung cancer (WCLC 2022) presented that patients with NSCLC with high PD-L1 expression (≥50%) had a favorable profile of efficacy and tolerance after 6 weeks of treatment with a combination of itacitinib and pembrolizumab. The most common adverse events mainly included decreased platelet and neutrophil counts, anemia, and hyperglycemia. Further studies are needed to obtain further insights into the use of itacitinib for the treatment of acute GVHD.
Currently, several JAK inhibitors in different phases of clinical trials are being explored to expand potential indications, increase drug adherence and achieve better safety. For example, upadacitinib (ABT‐494) is a potent and selective JAK1 inhibitor that received FDA approval for clinical use in patients with moderate-to-severe RA, psoriatic arthritis (PsA), ankylosing spondylitis (AS) and non-radiographic axial spondylarthritis (nr-axSpA).520–522 The SELECT-MONOTHERAPY study found that upadacitinib monotherapy improved the clinical symptoms when administered in combination with stable background csDMARDs in patients with moderate-to-severe active RA.523 Two classical phase 3 trials (SELECT-PsA 1 and SELECT-PsA 2) conducted in patients with PsA who had an inadequate or intolerant response to one or more TNF inhibitors found that upadacitinib reduced the PsA disease severity compared with placebo or adalimumab.272,277 Furthermore, upadacitinib was found to be effective in patients with active AS who had a bDMARD-inadequate response during a 14-week treatment period.275 In the SELECT-AXIS 2 trial, upadacitinib alleviated the painful symptoms of nr-axSpA as compared with placebo at week 14.524 Recently, the European Union passed approval for upadacitinib treatment to be developed for patients with inflammatory bowel disease based on positive clinical outcomes in two induction studies (U-ACHIEVE and U-ACCOMPLISH) and a maintenance study (U-ACHIEVE).525 Nonetheless, additional trials with large population samples are required to validate the long-term safety of upadacitinib, because other JAK inhibitors with similar mechanisms have reported adverse events such as cardiovascular events, embolism and thrombosis, cancer and even death.
Abrocitinib, another FDA-approved JAK1 selective inhibitor being developed for AD treatment, functions by blocking several critical cytokines, including IL-4, IL-13, IL-22, and IL-31, that participate in the pathology of AD.526,527 In a series of phase 3 trials (JADE Mono-1, MONO-2, TEEN, COMPARE, EXTEND, and REGIMEN), abrocitinib monotherapy achieved marked amelioration of pruritus and AD disease severity, resulting in the expansion of indications for abrocitinib administration to adolescents aged 12–17 years having moderate-to-severe AD.284,285,287,528–530 Furthermore, the rapid improvement of pruritus produced by abrocitinib supports treatment compliance, which further validates the safety and tolerability of abrocitinib in patients with moderate-to-severe AD.531 Abrocitinib has manageable safety, and its common adverse events include nausea, headache, acne and reduced platelet counts.532,533 Therefore, its benefit-to-harm ratio needs to be thoroughly investigated before it can be used for the long-term treatment of AD. Strikingly, deucravacitinib (BMS-986165), by producing a distinctively allosteric mechanism to combine with the TYK2 regulatory domain and not influence the functions of JAK1/2/3, became the first oral agent that was used to treat psoriasis with a favorable safety profile.534 Deucravacitinib is also the first approved TYK2 selective inhibitor for the treatment of moderate-to-severe plaque psoriasis; it was approved in September 2022 by the USA FDA and then approved in Japan for plaque, generalized pustular, and erythrodermic psoriasis.535 The two crucial phase 3 POETYK PSO-1 and POETYK PSO-2 trials have proven the superior performance of deucravacitinib against moderate-to-severe plaque psoriasis in terms of multiple efficacy endpoints compared with placebo or apremilast.536,537 Moreover, enduring symptom remission was still maintained at 52 weeks of continuous treatment with deucravacitinib. The most common adverse events of deucravacitinib include mild-to-moderate nasopharyngitis and upper respiratory tract infections. The recently announced POETYK PSO-3 trial broadens the exploration of the efficacy of deucravacitinib in Asian populations for the first time. The oral results of the 30th European Academy of Dermatology and Venereology (EADV) Congress demonstrated that the administration of deucravacitinib to patients with moderate-to-severe Asian plaque psoriasis results in sustained efficacy and even favorable efficacy for refractory scalp psoriasis. Meanwhile, the efficacy and safety of deucravacitinib in a variety of immune-related diseases, including psoriatic arthritis (PsA), systemic lupus erythematosus, and inflammatory bowel disease, is also under exploration in multiple large clinical trials.538
In recent years, several independently developed Chinese JAK inhibitors (e.g., including jaktinib, golidocitinib, ivarmactinib, and itacitinib) under different phases of clinical trials are being considered to have great clinical potential.539–543 In October 2022, Jaktinib, a deuterated compound of momelotinib that widely inhibits JAK1, JAK2, JAK3, TYK2 and ACVR1, became the first approved domestic JAK inhibitor for the treatment of moderate-to-severe myelofibrosis.544,545 A published phase 2 trial has shown that jaktinib reduced the spleen size and alleviated the clinical symptoms of patients with moderate-to-severe myelofibrosis.546 It was evident that jaktinib was well-tolerated and that anemia and thrombocytopenia were the most common adverse events. The phase 3 trial (NCT04617028) further reported the encouraging efficacy of jaktinib in the treatment of myelofibrosis, with remarkable improvement of the symptoms, anemia and spleen volume when compared with hydroxyurea. Based on the reported existing efficacy and safety, approvals were needed for conducting clinical trials on the use of jaktinib in a series of diseases, such as COVID-19, AD, systemic lupus erythematosus, alopecia areata, and mild-to-moderate psoriasis.547
Overall, JAK inhibitors offer great promise as targeted therapies. However, sufficient real-world evidence is required to fully explore their long-term safety, durability and effectiveness.
Current challenges and directions in the field
A sophisticated understanding of JAK-STAT signaling has provided many insights into the development of novel drugs.548–550 However, many challenges remain for improving target selectivity in JAK-STAT signaling, and exploration of the long-term safety of the developed agents is still required. Each JAK inhibitor impedes the combination of a JAK enzyme with ATP through the highly selective ATP-binding pocket.551,552 The Michaelis equilibrium of ATP, JAK enzyme and JAK inhibitor shows that the combination of JAK with its substrate is affected by the intracellular drug concentration and drug selectivity.553 However, drug concentrations depend on multiple factors, such as the patient’s age, body weight, liver and renal function and drug interactions, and it has been challenging to achieve absolute selectivity in terms of JAK inhibitors.140,554 More selective JAK inhibitors are being developed to reduce their unwanted effects on cytokine functions and further improve the overall safety and efficacy.555–557 For example, the JAK inhibitors abrocitinib and delgocitinib are currently being further developed to optimize treatment efficiency with minimum off-target effects.
Combination therapies using JAK inhibitors combined with other immunomodulatory or anti-inflammatory agents are an emerging approach; these are also being explored to achieve maximum treatment response with few adverse events.558,559 The simultaneous inhibition of the JAK-STAT signaling pathway and other potential targets appears to be another feasible option for drug development. For example, fedratinib, an innovative JAK inhibitor that simultaneously targets the activities of JAK2 and Fms-like tyrosine kinase 3 (FLT3) with crucial effects on the survival and proliferation of primitive hematopoietic progenitor cells, has been approved as an oral treatment for intermediate or high-risk myelofibrosis.560–562
The topical or inhalational application of JAK inhibitors is another exciting area of research in multiple ongoing animal models and larger clinical trials.548,563 The intestinally restricted pan-JAK inhibitor TD-1473 is expected to soon become available to treat inflammatory bowel disease with fewer systemic adverse side effects than other treatment options.564 The ongoing development and approval of emerging JAK inhibitors in preclinical studies and clinical settings will provide optimized JAK inhibitors as potent therapeutic options for a range of diseases in the future.
Conclusions
As a primarily canonical signaling pathway, the JAK-STAT signaling pathway transduces cytokine-activated extracellular signals to the nucleus to mediate gene expression, thus exerting indispensable functions in a series of cellular processes, particularly ones with immunomodulatory effects. Abnormal activation of JAK-STAT signaling is central to the initiation and progression of many diseases, especially immune-related diseases, and cancers. Drugs that target the JAK-STAT pathway have been approved by the FDA as alternative treatments for certain diseases and have exhibited powerful clinical benefits. A substantial number of novel agents targeting the JAK-STAT pathway are currently under development, yet there is still little evidence of differences in efficacy between selective JAK inhibitors and pan-selective JAK inhibitors. Explorations of the use of JAK inhibitors in early disease stages and in combination with other conventional drugs are also currently in progress. Recent studies have shown that a portion of patients are unresponsive to JAK inhibitors and have tried to unveil the relevant drug resistance mechanisms. Future research should offer comprehensive insights into the physiological and pathogenic mechanisms of the JAK-STAT pathway in disease development and aim to identify biomarkers to assess the long-term efficacy and safety of JAK inhibitors and to optimize the therapeutic efficacy of JAK inhibitors in patients with different stages and severity of disease to achieve individualized treatment. Overall, understanding the associations of the JAK-STAT pathway with immune regulation and disease progression will provide new therapeutic strategies to treat diverse diseases, particularly immune-related diseases, and cancer.
Acknowledgements
This work was funded by the National Key Research and Development Program of China (2021YFC2301800), and the National Nature Science Foundation of China (82200673 and U20A20343).
Author contributions
L.L. and J.L. designed the study, and reviewed and edited the manuscript; C.X., Q.Y., and X.G. participated in the original draft preparation; Q.S., X.Y., Q.C., and Z.B. collected the references and help with reviewing the manuscript. All authors have read and approved the article.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Chen Xue, Qinfan Yao, and Xinyu Gu
Contributor Information
Juan Lu, Email: lujuanzju@zju.edu.cn.
Lanjuan Li, Email: ljli@zju.edu.cn.
References
- 1.Leonard WJ, O’Shea JJ. Jaks and STATs: biological implications. Annu. Rev. Immunol. 1998;16:293–322. doi: 10.1146/annurev.immunol.16.1.293. [DOI] [PubMed] [Google Scholar]
- 2.O’Shea JJ. Jaks, STATs, cytokine signal transduction, and immunoregulation: are we there yet? Immunity. 1997;7:1–11. doi: 10.1016/S1074-7613(00)80505-1. [DOI] [PubMed] [Google Scholar]
- 3.Hou XS, Perrimon N. The JAK-STAT pathway in drosophila. Trends Genet. 1997;13:105–110. doi: 10.1016/S0168-9525(97)01006-8. [DOI] [PubMed] [Google Scholar]
- 4.Barrat FJ, Crow MK, Ivashkiv LB. Interferon target-gene expression and epigenomic signatures in health and disease. Nat. Immunol. 2019;20:1574–1583. doi: 10.1038/s41590-019-0466-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meraz MA, et al. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell. 1996;84:431–442. doi: 10.1016/S0092-8674(00)81288-X. [DOI] [PubMed] [Google Scholar]
- 6.Yamaoka K, et al. The Janus kinases (Jaks) Genome Biol. 2004;5:253. doi: 10.1186/gb-2004-5-12-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harpur AG, Andres AC, Ziemiecki A, Aston RR, Wilks AF. JAK2, a third member of the JAK family of protein tyrosine kinases. Oncogene. 1992;7:1347–1353. [PubMed] [Google Scholar]
- 8.Rane SG, Reddy EP. JAK3: a novel JAK kinase associated with terminal differentiation of hematopoietic cells. Oncogene. 1994;9:2415–2423. [PubMed] [Google Scholar]
- 9.Minegishi Y, et al. Human tyrosine kinase 2 deficiency reveals its requisite roles in multiple cytokine signals involved in innate and acquired immunity. Immunity. 2006;25:745–755. doi: 10.1016/j.immuni.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 10.Levy DE, Darnell JE., Jr Stats: transcriptional control and biological impact. Nat. Rev. Mol. Cell Biol. 2002;3:651–662. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- 11.Darnell JE., Jr. STATs and gene regulation. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 12.Arimoto KI, et al. STAT2 is an essential adaptor in USP18-mediated suppression of type I interferon signaling. Nat. Struct. Mol. Biol. 2017;24:279–289. doi: 10.1038/nsmb.3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen W, Daines MO. Khurana Hershey, G. K. Turning off signal transducer and activator of transcription (STAT): the negative regulation of STAT signaling. J. Allergy Clin. Immunol. 2004;114:476–489. doi: 10.1016/j.jaci.2004.06.042. [DOI] [PubMed] [Google Scholar]
- 14.Darnell JE., Jr Transcription factors as targets for cancer therapy. Nat. Rev. Cancer. 2002;2:740–749. doi: 10.1038/nrc906. [DOI] [PubMed] [Google Scholar]
- 15.McBride KM, Banninger G, McDonald C, Reich NC. Regulated nuclear import of the STAT1 transcription factor by direct binding of importin-alpha. EMBO J. 2002;21:1754–1763. doi: 10.1093/emboj/21.7.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Shea JJ, Gadina M, Schreiber RD. Cytokine signaling in 2002: new surprises in the Jak/Stat pathway. Cell. 2002;109:S121–S131. doi: 10.1016/S0092-8674(02)00701-8. [DOI] [PubMed] [Google Scholar]
- 17.Ihle JN, Kerr IM. Jaks and Stats in signaling by the cytokine receptor superfamily. Trends Genet. 1995;11:69–74. doi: 10.1016/S0168-9525(00)89000-9. [DOI] [PubMed] [Google Scholar]
- 18.Ihle JN, et al. Signaling by the cytokine receptor superfamily: JAKs and STATs. Trends Biochem. Sci. 1994;19:222–227. doi: 10.1016/0968-0004(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 19.Koskela HL, et al. Somatic STAT3 mutations in large granular lymphocytic leukemia. N. Engl. J. Med. 2012;366:1905–1913. doi: 10.1056/NEJMoa1114885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jerez A, et al. STAT3 mutations unify the pathogenesis of chronic lymphoproliferative disorders of NK cells and T-cell large granular lymphocyte leukemia. Blood. 2012;120:3048–3057. doi: 10.1182/blood-2012-06-435297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Constantinescu SN, Girardot M, Pecquet C. Mining for JAK-STAT mutations in cancer. Trends Biochem. Sci. 2008;33:122–131. doi: 10.1016/j.tibs.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Mirtti T, et al. Nuclear Stat5a/b predicts early recurrence and prostate cancer-specific death in patients treated by radical prostatectomy. Hum. Pathol. 2013;44:310–319. doi: 10.1016/j.humpath.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Owen, K. L., Brockwell, N. K., Parker, B. S. JAK-STAT signaling: a double-edged sword of immune regulation and cancer progression. Cancers (Basel). 11, 2002 (2019). [DOI] [PMC free article] [PubMed]
- 24.Meissl K, Macho-Maschler S, Müller M, Strobl B. The good and the bad faces of STAT1 in solid tumours. Cytokine. 2017;89:12–20. doi: 10.1016/j.cyto.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 25.Spiotto MT, Chung TD. STAT3 mediates IL-6-induced neuroendocrine differentiation in prostate cancer cells. Prostate. 2000;42:186–195. doi: 10.1002/(SICI)1097-0045(20000215)42:3<186::AID-PROS4>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 26.Rojas A, et al. IL-6 promotes prostate tumorigenesis and progression through autocrine cross-activation of IGF-IR. Oncogene. 2011;30:2345–2355. doi: 10.1038/onc.2010.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee SO, et al. RNA interference targeting Stat3 inhibits growth and induces apoptosis of human prostate cancer cells. Prostate. 2004;60:303–309. doi: 10.1002/pros.20072. [DOI] [PubMed] [Google Scholar]
- 28.Tzeng HT, Chyuan IT, Lai JH. Targeting the JAK-STAT pathway in autoimmune diseases and cancers: a focus on molecular mechanisms and therapeutic potential. Biochem. Pharm. 2021;193:114760. doi: 10.1016/j.bcp.2021.114760. [DOI] [PubMed] [Google Scholar]
- 29.Lai SY, Johnson FM. Defining the role of the JAK-STAT pathway in head and neck and thoracic malignancies: implications for future therapeutic approaches. Drug Resist. Updat. 2010;13:67–78. doi: 10.1016/j.drup.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 30.Burmester GR, et al. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet. 2013;381:451–460. doi: 10.1016/S0140-6736(12)61424-X. [DOI] [PubMed] [Google Scholar]
- 31.Chan JM, et al. Lineage plasticity in prostate cancer depends on JAK/STAT inflammatory signaling. Science. 2022;377:1180–1191. doi: 10.1126/science.abn0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boyle DL, et al. The JAK inhibitor tofacitinib suppresses synovial JAK1-STAT signalling in rheumatoid arthritis. Ann. Rheum. Dis. 2015;74:1311–1316. doi: 10.1136/annrheumdis-2014-206028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palmroth M, et al. Tofacitinib suppresses several JAK-STAT pathways in rheumatoid arthritis in vivo and baseline signaling profile associates with treatment response. Front. Immunol. 2021;12:738481. doi: 10.3389/fimmu.2021.738481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tucci G, et al. Baricitinib therapy response in rheumatoid arthritis patients associates to STAT1 phosphorylation in monocytes. Front. Immunol. 2022;13:932240. doi: 10.3389/fimmu.2022.932240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verstovsek S, et al. A phase I, open-label, dose-escalation, multicenter study of the JAK2 inhibitor NS-018 in patients with myelofibrosis. Leukemia. 2017;31:393–402. doi: 10.1038/leu.2016.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benucci M, et al. Cardiovascular safety, cancer and Jak-inhibitors: differences to be highlighted. Pharm. Res. 2022;183:106359. doi: 10.1016/j.phrs.2022.106359. [DOI] [PubMed] [Google Scholar]
- 37.Desai RJ, Pawar A, Weinblatt ME, Kim SC. Comparative risk of venous thromboembolism in rheumatoid arthritis patients receiving tofacitinib versus those receiving tumor necrosis factor inhibitors: an observational cohort study. Arthritis Rheumatol. 2019;71:892–900. doi: 10.1002/art.40798. [DOI] [PubMed] [Google Scholar]
- 38.O’Shea JJ, et al. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu. Rev. Med. 2015;66:311–328. doi: 10.1146/annurev-med-051113-024537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh SR, Chen X, Hou SX. JAK/STAT signaling regulates tissue outgrowth and male germline stem cell fate in Drosophila. Cell Res. 2005;15:1–5. doi: 10.1038/sj.cr.7290255. [DOI] [PubMed] [Google Scholar]
- 40.Fahmideh H, et al. The role of natural products as inhibitors of JAK/STAT signaling pathways in glioblastoma treatment. Oxid. Med Cell Longev. 2022;2022:7838583. doi: 10.1155/2022/7838583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gordon, M. J., Smith, M. R., Nastoupil, L. J. Follicular lymphoma: the long and winding road leading to your cure? Blood Rev. 57, 100992 (2022). [DOI] [PubMed]
- 42.Banerjee S, Biehl A, Gadina M, Hasni S, Schwartz DM. JAK-STAT signaling as a target for inflammatory and autoimmune diseases: current and future prospects. Drugs. 2017;77:521–546. doi: 10.1007/s40265-017-0701-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Villarino AV, Kanno Y, O’Shea JJ. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat. Immunol. 2017;18:374–384. doi: 10.1038/ni.3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Waldmann TA, Chen J. Disorders of the JAK/STAT pathway in T cell lymphoma pathogenesis: implications for immunotherapy. Annu. Rev. Immunol. 2017;35:533–550. doi: 10.1146/annurev-immunol-110416-120628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roskoski R., Jr Janus kinase (JAK) inhibitors in the treatment of inflammatory and neoplastic diseases. Pharm. Res. 2016;111:784–803. doi: 10.1016/j.phrs.2016.07.038. [DOI] [PubMed] [Google Scholar]
- 46.Yin T, Yang L, Yang YC. Tyrosine phosphorylation and activation of JAK family tyrosine kinases by interleukin-9 in MO7E cells. Blood. 1995;85:3101–3106. doi: 10.1182/blood.V85.11.3101.bloodjournal85113101. [DOI] [PubMed] [Google Scholar]
- 47.Choudhury GG, Marra F, Kiyomoto H, Abboud HE. PDGF stimulates tyrosine phosphorylation of JAK 1 protein tyrosine kinase in human mesangial cells. Kidney Int. 1996;49:19–25. doi: 10.1038/ki.1996.3. [DOI] [PubMed] [Google Scholar]
- 48.Miyazaki T, et al. Functional activation of Jak1 and Jak3 by selective association with IL-2 receptor subunits. Science. 1994;266:1045–1047. doi: 10.1126/science.7973659. [DOI] [PubMed] [Google Scholar]
- 49.Russell SM, et al. Interaction of IL-2R beta and gamma c chains with Jak1 and Jak3: implications for XSCID and XCID. Science. 1994;266:1042–1045. doi: 10.1126/science.7973658. [DOI] [PubMed] [Google Scholar]
- 50.Saijo K, Park SY, Ishida Y, Arase H, Saito T. Crucial role of Jak3 in negative selection of self-reactive T cells. J. Exp. Med. 1997;185:351–356. doi: 10.1084/jem.185.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Das Gupta D, et al. IRF4 deficiency vulnerates B-cell progeny for leukemogenesis via somatically acquired Jak3 mutations conferring IL-7 hypersensitivity. Cell Death Differ. 2022;29:2163–2176. doi: 10.1038/s41418-022-01005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liao W, et al. Opposing actions of IL-2 and IL-21 on Th9 differentiation correlate with their differential regulation of BCL6 expression. Proc. Natl. Acad. Sci. USA. 2014;111:3508–3513. doi: 10.1073/pnas.1301138111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Malka Y, et al. Ligand-independent homomeric and heteromeric complexes between interleukin-2 or -9 receptor subunits and the gamma chain. J. Biol. Chem. 2008;283:33569–33577. doi: 10.1074/jbc.M803125200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Degryse S, et al. JAK3 mutants transform hematopoietic cells through JAK1 activation, causing T-cell acute lymphoblastic leukemia in a mouse model. Blood. 2014;124:3092–3100. doi: 10.1182/blood-2014-04-566687. [DOI] [PubMed] [Google Scholar]
- 55.Barcia Durán JG, et al. Endothelial Jak3 expression enhances pro-hematopoietic angiocrine function in mice. Commun. Biol. 2021;4:406. doi: 10.1038/s42003-021-01846-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Glassman CR, et al. Structure of a Janus kinase cytokine receptor complex reveals the basis for dimeric activation. Science. 2022;376:163–169. doi: 10.1126/science.abn8933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ferrao R, Lupardus PJ. The Janus Kinase (JAK) FERM and SH2 Domains: Bringing Specificity to JAK-Receptor Interactions. Front. Endocrinol. (Lausanne) 2017;8:71. doi: 10.3389/fendo.2017.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou YJ, et al. Unexpected effects of FERM domain mutations on catalytic activity of Jak3: structural implication for Janus kinases. Mol. Cell. 2001;8:959–969. doi: 10.1016/S1097-2765(01)00398-7. [DOI] [PubMed] [Google Scholar]
- 59.Goult BT, et al. Structure of a double ubiquitin-like domain in the talin head: a role in integrin activation. EMBO J. 2010;29:1069–1080. doi: 10.1038/emboj.2010.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lupardus PJ, et al. Structure of the pseudokinase-kinase domains from protein kinase TYK2 reveals a mechanism for Janus kinase (JAK) autoinhibition. Proc. Natl Acad. Sci. USA. 2014;111:8025–8030. doi: 10.1073/pnas.1401180111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bandaranayake RM, et al. Crystal structures of the JAK2 pseudokinase domain and the pathogenic mutant V617F. Nat. Struct. Mol. Biol. 2012;19:754–759. doi: 10.1038/nsmb.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barahmand-Pour F, Meinke A, Groner B, Decker T. Jak2-Stat5 interactions analyzed in yeast. J. Biol. Chem. 1998;273:12567–12575. doi: 10.1074/jbc.273.20.12567. [DOI] [PubMed] [Google Scholar]
- 63.Guschin D, et al. A major role for the protein tyrosine kinase JAK1 in the JAK/STAT signal transduction pathway in response to interleukin-6. EMBO J. 1995;14:1421–1429. doi: 10.1002/j.1460-2075.1995.tb07128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Finbloom DS, Winestock KD. IL-10 induces the tyrosine phosphorylation of tyk2 and Jak1 and the differential assembly of STAT1 alpha and STAT3 complexes in human T cells and monocytes. J. Immunol. 1995;155:1079–1090. doi: 10.4049/jimmunol.155.3.1079. [DOI] [PubMed] [Google Scholar]
- 65.Yang H, et al. The influenza virus PB2 protein evades antiviral innate immunity by inhibiting JAK1/STAT signalling. Nat. Commun. 2022;13:6288. doi: 10.1038/s41467-022-33909-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shao T, et al. Treatment with a JAK1/2 inhibitor ameliorates murine autoimmune cholangitis induced by IFN overexpression. Cell Mol. Immunol. 2022;19:1130–1140. doi: 10.1038/s41423-022-00904-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gerlach K, et al. The JAK1/3 inhibitor tofacitinib suppresses T cell homing and activation in chronic intestinal inflammation. J. Crohns Colitis. 2020;18:jjaa162. doi: 10.1093/ecco-jcc/jjaa162. [DOI] [PubMed] [Google Scholar]
- 68.Lee TS, et al. Mechanisms of constitutive activation of Janus kinase 2-V617F revealed at the atomic level through molecular dynamics simulations. Cancer. 2009;115:1692–1700. doi: 10.1002/cncr.24183. [DOI] [PubMed] [Google Scholar]
- 69.Roskoski R., Jr Janus kinase (JAK) inhibitors in the treatment of neoplastic and inflammatory disorders. Pharm. Res. 2022;183:106362. doi: 10.1016/j.phrs.2022.106362. [DOI] [PubMed] [Google Scholar]
- 70.Liosi ME, et al. Insights on JAK2 modulation by potent, selective, and cell-permeable pseudokinase-domain ligands. J. Med. Chem. 2022;65:8380–8400. doi: 10.1021/acs.jmedchem.2c00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rai S, et al. Inhibition of interleukin-1β reduces myelofibrosis and osteosclerosis in mice with JAK2-V617F driven myeloproliferative neoplasm. Nat. Commun. 2022;13:5346. doi: 10.1038/s41467-022-32927-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rahman MF, et al. Interleukin-1 contributes to clonal expansion and progression of bone marrow fibrosis in JAK2V617F-induced myeloproliferative neoplasm. Nat. Commun. 2022;13:5347. doi: 10.1038/s41467-022-32928-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Neubauer H, et al. Jak2 deficiency defines an essential developmental checkpoint in definitive hematopoiesis. Cell. 1998;93:397–409. doi: 10.1016/S0092-8674(00)81168-X. [DOI] [PubMed] [Google Scholar]
- 74.Mu W, Ao J, Li Y, Zhang J, Duan C. Exploring the protective mechanisms of total tannins from Geum japonicum var. chinense F.Bolle in mice with hematopoietic dysfunction via the JAK2/STAT3/5 signaling pathway. J. Ethnopharmacol. 2022;296:115507. doi: 10.1016/j.jep.2022.115507. [DOI] [PubMed] [Google Scholar]
- 75.Rubio T, Viana R, Moreno-Estellés M, Campos-Rodríguez Á, Sanz P. TNF and IL6/Jak2 signaling pathways are the main contributors of the glia-derived neuroinflammation present in Lafora disease, a fatal form of progressive myoclonus epilepsy. Neurobiol. Dis. 2023;176:105964. doi: 10.1016/j.nbd.2022.105964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hu X, Li J, Fu M, Zhao X, Wang W. The JAK/STAT signaling pathway: from bench to clinic. Signal Transduct. Target Ther. 2021;6:402. doi: 10.1038/s41392-021-00791-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dodington DW, Desai HR, Woo M. JAK/STAT - emerging players in metabolism. Trends Endocrinol. Metab. 2018;29:55–65. doi: 10.1016/j.tem.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 78.DiSanto JP. Cytokines: shared receptors, distinct functions. Curr. Biol. 1997;7:R424–R426. doi: 10.1016/S0960-9822(06)00208-9. [DOI] [PubMed] [Google Scholar]
- 79.Schindler C, Darnell JE., Jr Transcriptional responses to polypeptide ligands: the JAK-STAT pathway. Annu. Rev. Biochem. 1995;64:621–651. doi: 10.1146/annurev.bi.64.070195.003201. [DOI] [PubMed] [Google Scholar]
- 80.Ihle JN, Witthuhn BA, Quelle FW, Yamamoto K, Silvennoinen O. Signaling through the hematopoietic cytokine receptors. Annu. Rev. Immunol. 1995;13:369–398. doi: 10.1146/annurev.iy.13.040195.002101. [DOI] [PubMed] [Google Scholar]
- 81.Thomis DC, Gurniak CB, Tivol E, Sharpe AH, Berg LJ. Defects in B lymphocyte maturation and T lymphocyte activation in mice lacking Jak3. Science. 1995;270:794–797. doi: 10.1126/science.270.5237.794. [DOI] [PubMed] [Google Scholar]
- 82.Karaghiosoff M, et al. Partial impairment of cytokine responses in Tyk2-deficient mice. Immunity. 2000;13:549–560. doi: 10.1016/S1074-7613(00)00054-6. [DOI] [PubMed] [Google Scholar]
- 83.Rusiñol L, Puig L. Tyk2 targeting in immune-mediated inflammatory diseases. Int J. Mol. Sci. 2023;24:3391. doi: 10.3390/ijms24043391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liau NPD, et al. Enzymatic characterization of wild-type and mutant janus kinase 1. Cancers (Basel) 2019;11:1701. doi: 10.3390/cancers11111701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gauzzi MC, et al. The amino-terminal region of Tyk2 sustains the level of interferon alpha receptor 1, a component of the interferon alpha/beta receptor. Proc. Natl Acad. Sci. USA. 1997;94:11839–11844. doi: 10.1073/pnas.94.22.11839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Velazquez L, et al. Distinct domains of the protein tyrosine kinase tyk2 required for binding of interferon-alpha/beta and for signal transduction. J. Biol. Chem. 1995;270:3327–3334. doi: 10.1074/jbc.270.7.3327. [DOI] [PubMed] [Google Scholar]
- 87.Stahl N, et al. Association and activation of Jak-Tyk kinases by CNTF-LIF-OSM-IL-6 beta receptor components. Science. 1994;263:92–95. doi: 10.1126/science.8272873. [DOI] [PubMed] [Google Scholar]
- 88.Bacon CM, et al. Interleukin 12 (IL-12) induces tyrosine phosphorylation of JAK2 and TYK2: differential use of Janus family tyrosine kinases by IL-2 and IL-12. J. Exp. Med. 1995;181:399–404. doi: 10.1084/jem.181.1.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Welham MJ, Learmonth L, Bone H, Schrader JW. Interleukin-13 signal transduction in lymphohemopoietic cells. Similarities and differences in signal transduction with interleukin-4 and insulin. J. Biol. Chem. 1995;270:12286–12296. doi: 10.1074/jbc.270.20.12286. [DOI] [PubMed] [Google Scholar]
- 90.Watford WT, O’Shea JJ. Human tyk2 kinase deficiency: another primary immunodeficiency syndrome. Immunity. 2006;25:695–697. doi: 10.1016/j.immuni.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 91.Verhoeven Y, et al. The potential and controversy of targeting STAT family members in cancer. Semin. Cancer Biol. 2020;60:41–56. doi: 10.1016/j.semcancer.2019.10.002. [DOI] [PubMed] [Google Scholar]
- 92.Furqan M, et al. STAT inhibitors for cancer therapy. J. Hematol. Oncol. 2013;6:90. doi: 10.1186/1756-8722-6-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dorritie KA, McCubrey JA, Johnson DE. STAT transcription factors in hematopoiesis and leukemogenesis: opportunities for therapeutic intervention. Leukemia. 2014;28:248–257. doi: 10.1038/leu.2013.192. [DOI] [PubMed] [Google Scholar]
- 94.Zhang T, Kee WH, Seow KT, Fung W, Cao X. The coiled-coil domain of Stat3 is essential for its SH2 domain-mediated receptor binding and subsequent activation induced by epidermal growth factor and interleukin-6. Mol. Cell Biol. 2000;20:7132–7139. doi: 10.1128/MCB.20.19.7132-7139.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kwock JT, et al. IL-27 signaling activates skin cells to induce innate antiviral proteins and protects against Zika virus infection. Sci. Adv. 2020;6:eaay3245. doi: 10.1126/sciadv.aay3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Léger T, Balaguer P, Le Hégarat L, Fessard V. Fate and PPARγ and STATs-driven effects of the mitochondrial complex I inhibitor tebufenpyrad in liver cells revealed with multi-omics. J. Hazard Mater. 2023;442:130083. doi: 10.1016/j.jhazmat.2022.130083. [DOI] [PubMed] [Google Scholar]
- 97.Lu, C. et al. IFNGR/STAT1 signaling in recipient hematopoietic antigen presenting cells suppresses graft-versus-host disease. J. Clin. Invest.133, e125986 (2022). [DOI] [PMC free article] [PubMed]
- 98.Wang J, et al. Leukemia inhibitory factor protects against graft-versus-host disease while preserving graft-versus-leukemia activity. Blood. 2022;140:2076–2090. doi: 10.1182/blood.2022015677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Qin Y, et al. Age-associated B cells contribute to the pathogenesis of rheumatoid arthritis by inducing activation of fibroblast-like synoviocytes via TNF-α-mediated ERK1/2 and JAK-STAT1 pathways. Ann. Rheum. Dis. 2022;81:1504–1514. doi: 10.1136/ard-2022-222605. [DOI] [PubMed] [Google Scholar]
- 100.Pelham SJ, et al. STAT5B restrains human B-cell differentiation to maintain humoral immune homeostasis. J. Allergy Clin. Immunol. 2022;150:931–946. doi: 10.1016/j.jaci.2022.04.011. [DOI] [PubMed] [Google Scholar]
- 101.Duetsch G, et al. STAT6 as an asthma candidate gene: polymorphism-screening, association and haplotype analysis in a Caucasian sib-pair study. Hum. Mol. Genet. 2002;11:613–621. doi: 10.1093/hmg/11.6.613. [DOI] [PubMed] [Google Scholar]
- 102.Schindler C, Levy DE, Decker T. JAK-STAT signaling: from interferons to cytokines. J. Biol. Chem. 2007;282:20059–20063. doi: 10.1074/jbc.R700016200. [DOI] [PubMed] [Google Scholar]
- 103.Chen H, et al. Activation of STAT6 by STING is critical for antiviral innate immunity. Cell. 2011;147:436–446. doi: 10.1016/j.cell.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 104.Drube, S. et al. Interleukin-3 stabilizes CD124/IL-4α surface expression in mast cells via Tyk2 and STAT6. Immunology.169, 102–112 (2022). [DOI] [PubMed]
- 105.Hoey T, Schindler U. STAT structure and function in signaling. Curr. Opin. Genet. Dev. 1998;8:582–587. doi: 10.1016/S0959-437X(98)80015-4. [DOI] [PubMed] [Google Scholar]
- 106.Shuai K. The STAT family of proteins in cytokine signaling. Prog. Biophys. Mol. Biol. 1999;71:405–422. doi: 10.1016/S0079-6107(98)00051-0. [DOI] [PubMed] [Google Scholar]
- 107.Li WX. Canonical and non-canonical JAK-STAT signaling. Trends Cell Biol. 2008;18:545–551. doi: 10.1016/j.tcb.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Baldini C, Moriconi FR, Galimberti S, Libby P, De Caterina R. The JAK-STAT pathway: an emerging target for cardiovascular disease in rheumatoid arthritis and myeloproliferative neoplasms. Eur. Heart J. 2021;42:4389–4400. doi: 10.1093/eurheartj/ehab447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xin P, et al. The role of JAK/STAT signaling pathway and its inhibitors in diseases. Int. Immunopharmacol. 2020;80:106210. doi: 10.1016/j.intimp.2020.106210. [DOI] [PubMed] [Google Scholar]
- 110.Hong J, et al. PRC2-mediated epigenetic suppression of Type I IFN-STAT2 signaling impairs antitumor immunity in luminal breast cancer. Cancer Res. 2022;82:4624–4640. doi: 10.1158/0008-5472.CAN-22-0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gothe F, et al. Aberrant inflammatory responses to type I interferon in STAT2 or IRF9 deficiency. J. Allergy Clin. Immunol. 2022;150:955–964.e916. doi: 10.1016/j.jaci.2022.01.026. [DOI] [PubMed] [Google Scholar]
- 112.Li YJ, Zhang C, Martincuks A, Herrmann A, Yu H. STAT proteins in cancer: orchestration of metabolism. Nat. Rev. Cancer. 2023;23:115–134. doi: 10.1038/s41568-022-00537-3. [DOI] [PubMed] [Google Scholar]
- 113.Shih PC. The role of the STAT3 signaling transduction pathways in radioresistance. Pharm. Ther. 2022;234:108118. doi: 10.1016/j.pharmthera.2022.108118. [DOI] [PubMed] [Google Scholar]
- 114.Zhang L, et al. Signal transducer and activator of transcription 3 signaling in tumor immune evasion. Pharm. Ther. 2022;230:107969. doi: 10.1016/j.pharmthera.2021.107969. [DOI] [PubMed] [Google Scholar]
- 115.Yoshimura A, Yasukawa H. JAK’s SOCS: a mechanism of inhibition. Immunity. 2012;36:157–159. doi: 10.1016/j.immuni.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 116.Linossi EM, et al. Discovery of an exosite on the SOCS2-SH2 domain that enhances SH2 binding to phosphorylated ligands. Nat. Commun. 2021;12:7032. doi: 10.1038/s41467-021-26983-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Durham GA, Williams JJL, Nasim MT, Palmer TM. Targeting SOCS proteins to control JAK-STAT signalling in disease. Trends Pharm. Sci. 2019;40:298–308. doi: 10.1016/j.tips.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 118.Liau NPD, et al. The molecular basis of JAK/STAT inhibition by SOCS1. Nat. Commun. 2018;9:1558. doi: 10.1038/s41467-018-04013-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rico-Bautista E, Flores-Morales A, Fernández-Pérez L. Suppressor of cytokine signaling (SOCS) 2, a protein with multiple functions. Cytokine Growth Factor Rev. 2006;17:431–439. doi: 10.1016/j.cytogfr.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 120.Yoshimura A, Naka T, Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nat. Rev. Immunol. 2007;7:454–465. doi: 10.1038/nri2093. [DOI] [PubMed] [Google Scholar]
- 121.Starr R, et al. A family of cytokine-inducible inhibitors of signalling. Nature. 1997;387:917–921. doi: 10.1038/43206. [DOI] [PubMed] [Google Scholar]
- 122.Liang Y, Xu WD, Peng H, Pan HF, Ye DQ. SOCS signaling in autoimmune diseases: molecular mechanisms and therapeutic implications. Eur. J. Immunol. 2014;44:1265–1275. doi: 10.1002/eji.201344369. [DOI] [PubMed] [Google Scholar]
- 123.Alexander WS, et al. Suppressors of cytokine signaling (SOCS): negative regulators of signal transduction. J. Leukoc. Biol. 1999;66:588–592. doi: 10.1002/jlb.66.4.588. [DOI] [PubMed] [Google Scholar]
- 124.Kishimoto T, Kikutani H. Knocking the SOCS off a tumor suppressor. Nat. Genet. 2001;28:4–5. doi: 10.1038/ng0501-4. [DOI] [PubMed] [Google Scholar]
- 125.Thomas SJ, Snowden JA, Zeidler MP, Danson SJ. The role of JAK/STAT signalling in the pathogenesis, prognosis and treatment of solid tumours. Br. J. Cancer. 2015;113:365–371. doi: 10.1038/bjc.2015.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Seif F, et al. The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun. Signal. 2017;15:23. doi: 10.1186/s12964-017-0177-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wu CS, Zou L. The SUMO (small ubiquitin-like modifier) ligase PIAS3 primes ATR for checkpoint activation. J. Biol. Chem. 2016;291:279–290. doi: 10.1074/jbc.M115.691170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rytinki MM, Kaikkonen S, Pehkonen P, Jääskeläinen T, Palvimo JJ. PIAS proteins: pleiotropic interactors associated with SUMO. Cell Mol. Life Sci. 2009;66:3029–3041. doi: 10.1007/s00018-009-0061-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Frankson R, et al. Therapeutic targeting of oncogenic tyrosine phosphatases. Cancer Res. 2017;77:5701–5705. doi: 10.1158/0008-5472.CAN-17-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pike KA, Tremblay ML. Protein tyrosine phosphatases: regulators of CD4 T cells in inflammatory bowel disease. Front. Immunol. 2018;9:2504. doi: 10.3389/fimmu.2018.02504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Long D, Chen Y, Wu H, Zhao M, Lu Q. Clinical significance and immunobiology of IL-21 in autoimmunity. J. Autoimmun. 2019;99:1–14. doi: 10.1016/j.jaut.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 132.Yan Z, Gibson SA, Buckley JA, Qin H, Benveniste EN. Role of the JAK/STAT signaling pathway in regulation of innate immunity in neuroinflammatory diseases. Clin. Immunol. 2018;189:4–13. doi: 10.1016/j.clim.2016.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Roxburgh CS, McMillan DC. Therapeutics targeting innate immune/inflammatory responses through the interleukin-6/JAK/STAT signal transduction pathway in patients with cancer. Transl. Res. 2016;167:61–66. doi: 10.1016/j.trsl.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 134.O’Shea JJ, Plenge R. JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity. 2012;36:542–550. doi: 10.1016/j.immuni.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.O’Shea JJ, Murray PJ. Cytokine signaling modules in inflammatory responses. Immunity. 2008;28:477–487. doi: 10.1016/j.immuni.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Darnell JE, Jr., Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 137.Schwartz DM, Bonelli M, Gadina M, O’Shea JJ. Type I/II cytokines, JAKs, and new strategies for treating autoimmune diseases. Nat. Rev. Rheumatol. 2016;12:25–36. doi: 10.1038/nrrheum.2015.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.O’Shea JJ, Holland SM, Staudt LM. JAKs and STATs in immunity, immunodeficiency, and cancer. N. Engl. J. Med. 2013;368:161–170. doi: 10.1056/NEJMra1202117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Costa-Pereira AP, et al. Mutational switch of an IL-6 response to an interferon-gamma-like response. Proc. Natl Acad. Sci. USA. 2002;99:8043–8047. doi: 10.1073/pnas.122236099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.O’Shea JJ, Kontzias A, Yamaoka K, Tanaka Y, Laurence A. Janus kinase inhibitors in autoimmune diseases. Ann. Rheum. Dis. 2013;72:ii111–ii115. doi: 10.1136/annrheumdis-2012-202576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kang YH, Biswas A, Field M, Snapper SB. STAT1 signaling shields T cells from NK cell-mediated cytotoxicity. Nat. Commun. 2019;10:912. doi: 10.1038/s41467-019-08743-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Putz EM, et al. CDK8-mediated STAT1-S727 phosphorylation restrains NK cell cytotoxicity and tumor surveillance. Cell Rep. 2013;4:437–444. doi: 10.1016/j.celrep.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Watford WT, et al. Signaling by IL-12 and IL-23 and the immunoregulatory roles of STAT4. Immunol. Rev. 2004;202:139–156. doi: 10.1111/j.0105-2896.2004.00211.x. [DOI] [PubMed] [Google Scholar]
- 144.Boudreau JE, et al. IL-15 and type I interferon are required for activation of tumoricidal NK cells by virus-infected dendritic cells. Cancer Res. 2011;71:2497–2506. doi: 10.1158/0008-5472.CAN-10-3025. [DOI] [PubMed] [Google Scholar]
- 145.Cordell HJ, et al. An international genome-wide meta-analysis of primary biliary cholangitis: novel risk loci and candidate drugs. J. Hepatol. 2021;75:572–581. doi: 10.1016/j.jhep.2021.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Goropevšek A, Holcar M, Avčin T. The role of STAT signaling pathways in the pathogenesis of systemic lupus erythematosus. Clin. Rev. Allergy Immunol. 2017;52:164–181. doi: 10.1007/s12016-016-8550-y. [DOI] [PubMed] [Google Scholar]
- 147.Park JS, et al. STA-21, a promising STAT-3 inhibitor that reciprocally regulates Th17 and Treg cells, inhibits osteoclastogenesis in mice and humans and alleviates autoimmune inflammation in an experimental model of rheumatoid arthritis. Arthritis Rheumatol. 2014;66:918–929. doi: 10.1002/art.38305. [DOI] [PubMed] [Google Scholar]
- 148.Ma CS, et al. Functional STAT3 deficiency compromises the generation of human T follicular helper cells. Blood. 2012;119:3997–4008. doi: 10.1182/blood-2011-11-392985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gracey E, et al. TYK2 inhibition reduces type 3 immunity and modifies disease progression in murine spondyloarthritis. J. Clin. Invest. 2020;130:1863–1878. doi: 10.1172/JCI126567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sigurdsson S, et al. Polymorphisms in the tyrosine kinase 2 and interferon regulatory factor 5 genes are associated with systemic lupus erythematosus. Am. J. Hum. Genet. 2005;76:528–537. doi: 10.1086/428480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ellinghaus D, et al. Combined analysis of genome-wide association studies for Crohn disease and psoriasis identifies seven shared susceptibility loci. Am. J. Hum. Genet. 2012;90:636–647. doi: 10.1016/j.ajhg.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Casaca VI, et al. STAT6 polymorphisms are associated with neonatal regulatory T cells and cytokines and atopic diseases at 3 years. Allergy. 2013;68:1249–1258. doi: 10.1111/all.12220. [DOI] [PubMed] [Google Scholar]
- 153.Fabre A, et al. Clinical aspects of STAT3 gain-of-function germline mutations: a systematic review. J. Allergy Clin. Immunol. Pr. 2019;7:1958–1969.e1959. doi: 10.1016/j.jaip.2019.02.018. [DOI] [PubMed] [Google Scholar]
- 154.Lundgren S, et al. Somatic mutations in lymphocytes in patients with immune-mediated aplastic anemia. Leukemia. 2021;35:1365–1379. doi: 10.1038/s41375-021-01231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Marabelle A, Aspeslagh S, Postel-Vinay S, Soria JC. JAK mutations as escape mechanisms to anti-PD-1 therapy. Cancer Disco. 2017;7:128–130. doi: 10.1158/2159-8290.CD-16-1439. [DOI] [PubMed] [Google Scholar]
- 156.Jehanno C, Vulin M, Richina V, Richina F, Bentires-Alj M. Phenotypic plasticity during metastatic colonization. Trends Cell Biol. 2022;32:854–867. doi: 10.1016/j.tcb.2022.03.007. [DOI] [PubMed] [Google Scholar]
- 157.Westneat DF, Potts LJ, Sasser KL, Shaffer JD. Causes and consequences of phenotypic plasticity in complex environments. Trends Ecol. Evol. 2019;34:555–568. doi: 10.1016/j.tree.2019.02.010. [DOI] [PubMed] [Google Scholar]
- 158.Bakir B, Chiarella AM, Pitarresi JR, Rustgi AK. EMT, MET, plasticity, and tumor metastasis. Trends Cell Biol. 2020;30:764–776. doi: 10.1016/j.tcb.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Burkhardt DB, et al. Mapping phenotypic plasticity upon the cancer cell state landscape using manifold learning. Cancer Discov. 2022;12:1847–1859. doi: 10.1158/2159-8290.CD-21-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lee YI, et al. WNT5A drives interleukin-6-dependent epithelial-mesenchymal transition via the JAK/STAT pathway in keloid pathogenesis. Burns Trauma. 2022;10:tkac023. doi: 10.1093/burnst/tkac023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Quintanal-Villalonga Á, et al. Lineage plasticity in cancer: a shared pathway of therapeutic resistance. Nat. Rev. Clin. Oncol. 2020;17:360–371. doi: 10.1038/s41571-020-0340-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yu HA, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin. Cancer Res. 2013;19:2240–2247. doi: 10.1158/1078-0432.CCR-12-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Beebe K, Lee WC, Micchelli CA. JAK/STAT signaling coordinates stem cell proliferation and multilineage differentiation in the Drosophila intestinal stem cell lineage. Dev. Biol. 2010;338:28–37. doi: 10.1016/j.ydbio.2009.10.045. [DOI] [PubMed] [Google Scholar]
- 164.Lo UG, et al. The driver role of JAK-STAT signalling in cancer stemness capabilities leading to new therapeutic strategies for therapy- and castration-resistant prostate cancer. Clin. Transl. Med. 2022;12:e978. doi: 10.1002/ctm2.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chatterjee D, et al. Cell polarity opposes Jak/STAT-mediated Escargot activation that drives intratumor heterogeneity in a Drosophila tumor model. Cell Rep. 2023;42:112061. doi: 10.1016/j.celrep.2023.112061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Deng S, et al. Ectopic JAK-STAT activation enables the transition to a stem-like and multilineage state conferring AR-targeted therapy resistance. Nat. Cancer. 2022;3:1071–1087. doi: 10.1038/s43018-022-00431-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sun L, et al. Modelling liver cancer initiation with organoids derived from directly reprogrammed human hepatocytes. Nat. Cell Biol. 2019;21:1015–1026. doi: 10.1038/s41556-019-0359-5. [DOI] [PubMed] [Google Scholar]
- 168.Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12:31–46. doi: 10.1158/2159-8290.CD-21-1059. [DOI] [PubMed] [Google Scholar]
- 169.Baratchian M, et al. H3K9 methylation drives resistance to androgen receptor-antagonist therapy in prostate cancer. Proc. Natl Acad. Sci. USA. 2022;119:e2114324119. doi: 10.1073/pnas.2114324119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–361. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 171.Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376:1094–1108. doi: 10.1016/S0140-6736(10)60826-4. [DOI] [PubMed] [Google Scholar]
- 172.Scherer HU, Häupl T, Burmester GR. The etiology of rheumatoid arthritis. J. Autoimmun. 2020;110:102400. doi: 10.1016/j.jaut.2019.102400. [DOI] [PubMed] [Google Scholar]
- 173.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 2011;365:2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 174.Noack M, Miossec P. Selected cytokine pathways in rheumatoid arthritis. Semin. Immunopathol. 2017;39:365–383. doi: 10.1007/s00281-017-0619-z. [DOI] [PubMed] [Google Scholar]
- 175.Pieringer H, Studnicka-Benke A. What is causing my arthritis, doctor? A glimpse beyond the usual suspects in the pathogenesis of rheumatoid arthritis. QJM. 2013;106:219–228. doi: 10.1093/qjmed/hcs205. [DOI] [PubMed] [Google Scholar]
- 176.Lubberts E. The IL-23-IL-17 axis in inflammatory arthritis. Nat. Rev. Rheumatol. 2015;11:415–429. doi: 10.1038/nrrheum.2015.53. [DOI] [PubMed] [Google Scholar]
- 177.Genovese MC, et al. Effect of filgotinib vs placebo on clinical response in patients with moderate to severe rheumatoid arthritis refractory to disease-modifying antirheumatic drug therapy: the FINCH 2 randomized clinical trial. Jama. 2019;322:315–325. doi: 10.1001/jama.2019.9055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kavanaugh A, et al. Filgotinib (GLPG0634/GS-6034), an oral selective JAK1 inhibitor, is effective as monotherapy in patients with active rheumatoid arthritis: results from a randomised, dose-finding study (DARWIN 2) Ann. Rheum. Dis. 2017;76:1009–1019. doi: 10.1136/annrheumdis-2016-210105. [DOI] [PubMed] [Google Scholar]
- 179.Combe B, et al. Filgotinib versus placebo or adalimumab in patients with rheumatoid arthritis and inadequate response to methotrexate: a phase III randomised clinical trial. Ann. Rheum. Dis. 2021;80:848–858. doi: 10.1136/annrheumdis-2020-219214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Nguyen HN, et al. Autocrine loop involving IL-6 family member LIF, LIF Receptor, and STAT4 drives sustained fibroblast production of inflammatory mediators. Immunity. 2017;46:220–232. doi: 10.1016/j.immuni.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mori T, et al. IL-1β and TNFα-initiated IL-6-STAT3 pathway is critical in mediating inflammatory cytokines and RANKL expression in inflammatory arthritis. Int. Immunol. 2011;23:701–712. doi: 10.1093/intimm/dxr077. [DOI] [PubMed] [Google Scholar]
- 182.Kasperkovitz PV, et al. Activation of the STAT1 pathway in rheumatoid arthritis. Ann. Rheum. Dis. 2004;63:233–239. doi: 10.1136/ard.2003.013276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.van der Pouw Kraan TC, et al. Rheumatoid arthritis is a heterogeneous disease: evidence for differences in the activation of the STAT-1 pathway between rheumatoid tissues. Arthritis Rheum. 2003;48:2132–2145. doi: 10.1002/art.11096. [DOI] [PubMed] [Google Scholar]
- 184.Walker JG, et al. Expression of Jak3, STAT1, STAT4, and STAT6 in inflammatory arthritis: unique Jak3 and STAT4 expression in dendritic cells in seropositive rheumatoid arthritis. Ann. Rheum. Dis. 2006;65:149–156. doi: 10.1136/ard.2005.037929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Tanaka Y, et al. Efficacy and safety of peficitinib (ASP015K) in patients with rheumatoid arthritis and an inadequate response to conventional DMARDs: a randomised, double-blind, placebo-controlled phase III trial (RAJ3) Ann. Rheum. Dis. 2019;78:1320–1332. doi: 10.1136/annrheumdis-2019-215163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Takeuchi T, et al. Efficacy and safety of peficitinib (ASP015K) in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase III randomised, double-blind, placebo-controlled trial (RAJ4) in Japan. Ann. Rheum. Dis. 2019;78:1305–1319. doi: 10.1136/annrheumdis-2019-215164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Takeuchi T, et al. Efficacy and safety of the oral Janus kinase inhibitor peficitinib (ASP015K) monotherapy in patients with moderate to severe rheumatoid arthritis in Japan: a 12-week, randomised, double-blind, placebo-controlled phase IIb study. Ann. Rheum. Dis. 2016;75:1057–1064. doi: 10.1136/annrheumdis-2015-208279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Genovese MC, et al. Peficitinib, a JAK inhibitor, in combination with limited conventional synthetic disease-modifying antirheumatic drugs in the treatment of moderate-to-severe rheumatoid arthritis. Arthritis Rheumatol. 2017;69:932–942. doi: 10.1002/art.40054. [DOI] [PubMed] [Google Scholar]
- 189.van der Pouw Kraan TC, et al. Rheumatoid arthritis subtypes identified by genomic profiling of peripheral blood cells: assignment of a type I interferon signature in a subpopulation of patients. Ann. Rheum. Dis. 2007;66:1008–1014. doi: 10.1136/ard.2006.063412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Higgs BW, et al. Patients with systemic lupus erythematosus, myositis, rheumatoid arthritis and scleroderma share activation of a common type I interferon pathway. Ann. Rheum. Dis. 2011;70:2029–2036. doi: 10.1136/ard.2011.150326. [DOI] [PubMed] [Google Scholar]
- 191.Wang J, Zhao Q. LncRNA LINC-PINT increases SOCS1 expression by sponging miR-155-5p to inhibit the activation of ERK signaling pathway in rheumatoid arthritis synovial fibroblasts induced by TNF-α. Int. Immunopharmacol. 2020;84:106497. doi: 10.1016/j.intimp.2020.106497. [DOI] [PubMed] [Google Scholar]
- 192.Kivitz AJ, et al. Peficitinib, a JAK inhibitor, in the treatment of moderate-to-severe rheumatoid arthritis in patients with an inadequate response to methotrexate. Arthritis Rheumatol. 2017;69:709–719. doi: 10.1002/art.39955. [DOI] [PubMed] [Google Scholar]
- 193.Papp K, et al. A phase 2a randomized, double-blind, placebo-controlled, sequential dose-escalation study to evaluate the efficacy and safety of ASP015K, a novel Janus kinase inhibitor, in patients with moderate-to-severe psoriasis. Br. J. Dermatol. 2015;173:767–776. doi: 10.1111/bjd.13745. [DOI] [PubMed] [Google Scholar]
- 194.Sands BE, et al. Peficitinib, an oral janus kinase inhibitor, in moderate-to-severe ulcerative colitis: results from a randomised, phase 2 study. J. Crohns Colitis. 2018;12:1158–1169. doi: 10.1093/ecco-jcc/jjy085. [DOI] [PubMed] [Google Scholar]
- 195.Lauper K, et al. Effectiveness of TNF-inhibitors, abatacept, IL6-inhibitors and JAK-inhibitors in 31 846 patients with rheumatoid arthritis in 19 registers from the ‘JAK-pot’ collaboration. Ann. Rheum. Dis. 2022;81:1358–1366. doi: 10.1136/annrheumdis-2022-222586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Westhovens R, et al. Filgotinib (GLPG0634/GS-6034), an oral JAK1 selective inhibitor, is effective in combination with methotrexate (MTX) in patients with active rheumatoid arthritis and insufficient response to MTX: results from a randomised, dose-finding study (DARWIN 1) Ann. Rheum. Dis. 2017;76:998–1008. doi: 10.1136/annrheumdis-2016-210104. [DOI] [PubMed] [Google Scholar]
- 197.Vanhoutte F, et al. Efficacy, safety, pharmacokinetics, and pharmacodynamics of filgotinib, a selective JAK-1 inhibitor, after short-term treatment of rheumatoid arthritis: results of two randomized phase IIa trials. Arthritis Rheumatol. 2017;69:1949–1959. doi: 10.1002/art.40186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Westhovens R, et al. Filgotinib in combination with methotrexate or as monotherapy versus methotrexate monotherapy in patients with active rheumatoid arthritis and limited or no prior exposure to methotrexate: the phase 3, randomised controlled FINCH 3 trial. Ann. Rheum. Dis. 2021;80:727–738. doi: 10.1136/annrheumdis-2020-219213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Chen C, et al. A highly selective JAK3 inhibitor is developed for treating rheumatoid arthritis by suppressing γc cytokine-related JAK-STAT signal. Sci. Adv. 2022;8:eabo4363. doi: 10.1126/sciadv.abo4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Charles-Schoeman C, et al. Efficacy and safety of tofacitinib following inadequate response to conventional synthetic or biological disease-modifying antirheumatic drugs. Ann. Rheum. Dis. 2016;75:1293–1301. doi: 10.1136/annrheumdis-2014-207178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Fleischmann R, et al. Phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) or adalimumab monotherapy versus placebo in patients with active rheumatoid arthritis with an inadequate response to disease-modifying antirheumatic drugs. Arthritis Rheum. 2012;64:617–629. doi: 10.1002/art.33383. [DOI] [PubMed] [Google Scholar]
- 202.van Vollenhoven RF, et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N. Engl. J. Med. 2012;367:508–519. doi: 10.1056/NEJMoa1112072. [DOI] [PubMed] [Google Scholar]
- 203.Kremer J, et al. Tofacitinib in combination with nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis: a randomized trial. Ann. Intern. Med. 2013;159:253–261. doi: 10.7326/0003-4819-159-4-201308200-00006. [DOI] [PubMed] [Google Scholar]
- 204.van der Heijde D, et al. Tofacitinib (CP-690,550) in patients with rheumatoid arthritis receiving methotrexate: twelve-month data from a twenty-four-month phase III randomized radiographic study. Arthritis Rheum. 2013;65:559–570. doi: 10.1002/art.37816. [DOI] [PubMed] [Google Scholar]
- 205.Lee EB, et al. Tofacitinib versus methotrexate in rheumatoid arthritis. N. Engl. J. Med. 2014;370:2377–2386. doi: 10.1056/NEJMoa1310476. [DOI] [PubMed] [Google Scholar]
- 206.Mesa RA, et al. SIMPLIFY-1: a phase III randomized trial of momelotinib versus ruxolitinib in janus kinase inhibitor-naïve patients with myelofibrosis. J. Clin. Oncol. 2017;35:3844–3850. doi: 10.1200/JCO.2017.73.4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Mesa R, et al. Overall survival in the SIMPLIFY-1 and SIMPLIFY-2 phase 3 trials of momelotinib in patients with myelofibrosis. Leukemia. 2022;36:2261–2268. doi: 10.1038/s41375-022-01637-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Meyer SC, et al. CHZ868, a type II JAK2 inhibitor, reverses type I JAK inhibitor persistence and demonstrates efficacy in myeloproliferative neoplasms. Cancer Cell. 2015;28:15–28. doi: 10.1016/j.ccell.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Harrison CN, et al. Momelotinib versus best available therapy in patients with myelofibrosis previously treated with ruxolitinib (SIMPLIFY 2): a randomised, open-label, phase 3 trial. Lancet Haematol. 2018;5:e73–e81. doi: 10.1016/S2352-3026(17)30237-5. [DOI] [PubMed] [Google Scholar]
- 210.Tefferi A, Vardiman JW. Myelodysplastic syndromes. N. Engl. J. Med. 2009;361:1872–1885. doi: 10.1056/NEJMra0902908. [DOI] [PubMed] [Google Scholar]
- 211.Zeidan AM, Shallis RM, Wang R, Davidoff A, Ma X. Epidemiology of myelodysplastic syndromes: why characterizing the beast is a prerequisite to taming it. Blood Rev. 2019;34:1–15. doi: 10.1016/j.blre.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 212.Aul C, Bowen DT, Yoshida Y. Pathogenesis, etiology and epidemiology of myelodysplastic syndromes. Haematologica. 1998;83:71–86. [PubMed] [Google Scholar]
- 213.Cazzola M. Myelodysplastic syndromes. N. Engl. J. Med. 2020;383:1358–1374. doi: 10.1056/NEJMra1904794. [DOI] [PubMed] [Google Scholar]
- 214.Bejar R, et al. Clinical effect of point mutations in myelodysplastic syndromes. N. Engl. J. Med. 2011;364:2496–2506. doi: 10.1056/NEJMoa1013343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Tefferi A. Novel mutations and their functional and clinical relevance in myeloproliferative neoplasms: JAK2, MPL, TET2, ASXL1, CBL, IDH and IKZF1. Leukemia. 2010;24:1128–1138. doi: 10.1038/leu.2010.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Quentmeier H, et al. SOCS2: inhibitor of JAK2V617F-mediated signal transduction. Leukemia. 2008;22:2169–2175. doi: 10.1038/leu.2008.226. [DOI] [PubMed] [Google Scholar]
- 217.Plo I, et al. JAK2 stimulates homologous recombination and genetic instability: potential implication in the heterogeneity of myeloproliferative disorders. Blood. 2008;112:1402–1412. doi: 10.1182/blood-2008-01-134114. [DOI] [PubMed] [Google Scholar]
- 218.Dawson MA, et al. JAK2 phosphorylates histone H3Y41 and excludes HP1alpha from chromatin. Nature. 2009;461:819–822. doi: 10.1038/nature08448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Thiele J, et al. The international consensus classification of myeloid neoplasms and acute Leukemias: myeloproliferative neoplasms. Am. J. Hematol. 2023;98:166–179. doi: 10.1002/ajh.26751. [DOI] [PubMed] [Google Scholar]
- 220.Chifotides HT, Bose P, Verstovsek S. Momelotinib: an emerging treatment for myelofibrosis patients with anemia. J. Hematol. Oncol. 2022;15:7. doi: 10.1186/s13045-021-01157-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Tefferi A, et al. Momelotinib for myelofibrosis: 12-year survival data and retrospective comparison to ruxolitinib. Am. J. Hematol. 2022;97:E433–e435. doi: 10.1002/ajh.26714. [DOI] [PubMed] [Google Scholar]
- 222.Geyer HL, Mesa RA. Therapy for myeloproliferative neoplasms: when, which agent, and how? Blood. 2014;124:3529–3537. doi: 10.1182/blood-2014-05-577635. [DOI] [PubMed] [Google Scholar]
- 223.Rampal R, et al. Integrated genomic analysis illustrates the central role of JAK-STAT pathway activation in myeloproliferative neoplasm pathogenesis. Blood. 2014;123:e123–e133. doi: 10.1182/blood-2014-02-554634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Jones AV, et al. Widespread occurrence of the JAK2 V617F mutation in chronic myeloproliferative disorders. Blood. 2005;106:2162–2168. doi: 10.1182/blood-2005-03-1320. [DOI] [PubMed] [Google Scholar]
- 225.Ungureanu D, et al. The pseudokinase domain of JAK2 is a dual-specificity protein kinase that negatively regulates cytokine signaling. Nat. Struct. Mol. Biol. 2011;18:971–976. doi: 10.1038/nsmb.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Kralovics R, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N. Engl. J. Med. 2005;352:1779–1790. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 227.Woods B, et al. Activation of JAK/STAT signaling in megakaryocytes sustains myeloproliferation in vivo. Clin. Cancer Res. 2019;25:5901–5912. doi: 10.1158/1078-0432.CCR-18-4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Arber DA, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–2405. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- 229.Kollmann K, et al. A novel signalling screen demonstrates that CALR mutations activate essential MAPK signalling and facilitate megakaryocyte differentiation. Leukemia. 2017;31:934–944. doi: 10.1038/leu.2016.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Tefferi A, et al. CALR vs JAK2 vs MPL-mutated or triple-negative myelofibrosis: clinical, cytogenetic and molecular comparisons. Leukemia. 2014;28:1472–1477. doi: 10.1038/leu.2014.3. [DOI] [PubMed] [Google Scholar]
- 231.Pikman Y, et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med. 2006;3:e270. doi: 10.1371/journal.pmed.0030270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Nangalia J, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N. Engl. J. Med. 2013;369:2391–2405. doi: 10.1056/NEJMoa1312542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Klampfl T, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N. Engl. J. Med. 2013;369:2379–2390. doi: 10.1056/NEJMoa1311347. [DOI] [PubMed] [Google Scholar]
- 234.Elf S, et al. Mutant calreticulin requires both its mutant C-terminus and the thrombopoietin receptor for oncogenic transformation. Cancer Discov. 2016;6:368–381. doi: 10.1158/2159-8290.CD-15-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Mullally A, Lane SW, Brumme K, Ebert BL. Myeloproliferative neoplasm animal models. Hematol. Oncol. Clin. North Am. 2012;26:1065–1081. doi: 10.1016/j.hoc.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Debili N, et al. The Mpl-ligand or thrombopoietin or megakaryocyte growth and differentiative factor has both direct proliferative and differentiative activities on human megakaryocyte progenitors. Blood. 1995;86:2516–2525. doi: 10.1182/blood.V86.7.2516.2516. [DOI] [PubMed] [Google Scholar]
- 237.Kaushansky K, et al. Thrombopoietin, the Mp1 ligand, is essential for full megakaryocyte development. Proc. Natl Acad. Sci. USA. 1995;92:3234–3238. doi: 10.1073/pnas.92.8.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Ku H, Yonemura Y, Kaushansky K, Ogawa M. Thrombopoietin, the ligand for the Mpl receptor, synergizes with steel factor and other early acting cytokines in supporting proliferation of primitive hematopoietic progenitors of mice. Blood. 1996;87:4544–4551. doi: 10.1182/blood.V87.11.4544.bloodjournal87114544. [DOI] [PubMed] [Google Scholar]
- 239.Lussana F, et al. Driver mutations (JAK2V617F, MPLW515L/K or CALR), pentraxin-3 and C-reactive protein in essential thrombocythemia and polycythemia vera. J. Hematol. Oncol. 2017;10:54. doi: 10.1186/s13045-017-0425-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Lasho TL, et al. Concurrent MPL515 and JAK2V617F mutations in myelofibrosis: chronology of clonal emergence and changes in mutant allele burden over time. Br. J. Haematol. 2006;135:683–687. doi: 10.1111/j.1365-2141.2006.06348.x. [DOI] [PubMed] [Google Scholar]
- 241.Pardanani AD, et al. MPL515 mutations in myeloproliferative and other myeloid disorders: a study of 1182 patients. Blood. 2006;108:3472–3476. doi: 10.1182/blood-2006-04-018879. [DOI] [PubMed] [Google Scholar]
- 242.Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet. 2020;396:345–360. doi: 10.1016/S0140-6736(20)31286-1. [DOI] [PubMed] [Google Scholar]
- 243.Eckert L, et al. The burden of atopic dermatitis in US adults: health care resource utilization data from the 2013 National Health and Wellness Survey. J. Am. Acad. Dermatol. 2018;78:54–61.e51. doi: 10.1016/j.jaad.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 244.Drucker AM, Wang AR, Qureshi AA. Research gaps in quality of life and economic burden of atopic dermatitis: the national eczema association burden of disease audit. JAMA Dermatol. 2016;152:873–874. doi: 10.1001/jamadermatol.2016.1978. [DOI] [PubMed] [Google Scholar]
- 245.Eichenfield LF, et al. Guidelines of care for the management of atopic dermatitis: section 1. Diagnosis and assessment of atopic dermatitis. J. Am. Acad. Dermatol. 2014;70:338–351. doi: 10.1016/j.jaad.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Freitas E, Gooderham M, Torres T. New topical therapies in development for atopic dermatitis. Drugs. 2022;82:843–853. doi: 10.1007/s40265-022-01722-2. [DOI] [PubMed] [Google Scholar]
- 247.Tsoi LC, et al. Progression of acute-to-chronic atopic dermatitis is associated with quantitative rather than qualitative changes in cytokine responses. J. Allergy Clin. Immunol. 2020;145:1406–1415. doi: 10.1016/j.jaci.2019.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Huang IH, Chung WH, Wu PC, Chen CB. JAK-STAT signaling pathway in the pathogenesis of atopic dermatitis: an updated review. Front. Immunol. 2022;13:1068260. doi: 10.3389/fimmu.2022.1068260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Makowska, K. et al. Immunopathogenesis of atopic dermatitis: focus on interleukins as disease drivers and therapeutic targets for novel treatments. Int. J. Mol. Sci. 24, 781 (2023). [DOI] [PMC free article] [PubMed]
- 250.Cotter DG, Schairer D, Eichenfield L. Emerging therapies for atopic dermatitis: JAK inhibitors. J. Am. Acad. Dermatol. 2018;78:S53–s62. doi: 10.1016/j.jaad.2017.12.019. [DOI] [PubMed] [Google Scholar]
- 251.Nakashima C, Yanagihara S, Otsuka A. Innovation in the treatment of atopic dermatitis: emerging topical and oral Janus kinase inhibitors. Allergol. Int. 2022;71:40–46. doi: 10.1016/j.alit.2021.10.004. [DOI] [PubMed] [Google Scholar]
- 252.Kopalli SR, Annamneedi VP, Koppula S. Potential natural biomolecules targeting JAK/STAT/SOCS signaling in the management of atopic dermatitis. Molecules. 2022;27:4660. doi: 10.3390/molecules27144660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Klaeschen AS, et al. JAK1/2 inhibition impairs the development and function of inflammatory dendritic epidermal cells in atopic dermatitis. J. Allergy Clin. Immunol. 2021;147:2202–2212.e2208. doi: 10.1016/j.jaci.2020.11.041. [DOI] [PubMed] [Google Scholar]
- 254.Reich K, et al. Efficacy and safety of baricitinib combined with topical corticosteroids for treatment of moderate to severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:1333–1343. doi: 10.1001/jamadermatol.2020.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Simpson EL, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis and inadequate response to topical corticosteroids: results from two randomized monotherapy phase III trials. Br. J. Dermatol. 2020;183:242–255. doi: 10.1111/bjd.18898. [DOI] [PubMed] [Google Scholar]
- 256.Papp K, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: results from 2 phase 3, randomized, double-blind studies. J. Am. Acad. Dermatol. 2021;85:863–872. doi: 10.1016/j.jaad.2021.04.085. [DOI] [PubMed] [Google Scholar]
- 257.Papp, K. et al. Long-term safety and disease control with ruxolitinib cream in atopic dermatitis: results from two phase 3 studies. J. Am. Acad. Dermatol.88, 1008–1016 (2022). [DOI] [PubMed]
- 258.Kim BS, et al. Treatment of atopic dermatitis with ruxolitinib cream (JAK1/JAK2 inhibitor) or triamcinolone cream. J. Allergy Clin. Immunol. 2020;145:572–582. doi: 10.1016/j.jaci.2019.08.042. [DOI] [PubMed] [Google Scholar]
- 259.Reich K, et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD Up): results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021;397:2169–2181. doi: 10.1016/S0140-6736(21)00589-4. [DOI] [PubMed] [Google Scholar]
- 260.Rubbert-Roth A, et al. Trial of upadacitinib or abatacept in rheumatoid arthritis. N. Engl. J. Med. 2020;383:1511–1521. doi: 10.1056/NEJMoa2008250. [DOI] [PubMed] [Google Scholar]
- 261.Cohen SB, et al. Safety profile of upadacitinib in rheumatoid arthritis: integrated analysis from the SELECT phase III clinical programme. Ann. Rheum. Dis. 2021;80:304–311. doi: 10.1136/annrheumdis-2020-218510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Fleischmann RM, et al. Safety and effectiveness of upadacitinib or adalimumab plus methotrexate in patients with rheumatoid arthritis over 48 weeks with switch to alternate therapy in patients with insufficient response. Ann. Rheum. Dis. 2019;78:1454–1462. doi: 10.1136/annrheumdis-2019-215764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Mendes-Bastos P, et al. Characterization of acne associated with upadacitinib treatment in patients with moderate-to-severe atopic dermatitis: A post hoc integrated analysis of 3 phase 3 randomized, double-blind, placebo-controlled trials. J. Am. Acad. Dermatol. 2022;87:784–791. doi: 10.1016/j.jaad.2022.06.012. [DOI] [PubMed] [Google Scholar]
- 264.Deodhar A, et al. Safety and efficacy of upadacitinib in patients with active ankylosing spondylitis and an inadequate response to nonsteroidal antiinflammatory drug therapy: one-year results of a double-blind, placebo-controlled study and open-label extension. Arthritis Rheumatol. 2022;74:70–80. doi: 10.1002/art.41911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.van Vollenhoven R, et al. Efficacy and safety of upadacitinib monotherapy in methotrexate-naive patients with moderately-to-severely active rheumatoid arthritis (SELECT-EARLY): a multicenter, multi-country, randomized, double-blind, active comparator-controlled trial. Arthritis Rheumatol. 2020;72:1607–1620. doi: 10.1002/art.41384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Fleischmann R, et al. Upadacitinib versus placebo or adalimumab in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase iii, double-blind, randomized controlled trial. Arthritis Rheumatol. 2019;71:1788–1800. doi: 10.1002/art.41032. [DOI] [PubMed] [Google Scholar]
- 267.Silverberg JI, et al. Upadacitinib plus topical corticosteroids in atopic dermatitis: week 52 AD Up study results. J. Allergy Clin. Immunol. 2022;149:977–987.e914. doi: 10.1016/j.jaci.2021.07.036. [DOI] [PubMed] [Google Scholar]
- 268.Guttman-Yassky E, et al. Upadacitinib in adults with moderate to severe atopic dermatitis: 16-week results from a randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 2020;145:877–884. doi: 10.1016/j.jaci.2019.11.025. [DOI] [PubMed] [Google Scholar]
- 269.Forbes GM. In active UC, upadacitinib induced and maintained remission. Ann. Intern. Med. 2022;175:Jc113. doi: 10.7326/J22-0073. [DOI] [PubMed] [Google Scholar]
- 270.Sandborn WJ, et al. Efficacy of upadacitinib in a randomized trial of patients with active ulcerative colitis. Gastroenterology. 2020;158:2139–2149.e2114. doi: 10.1053/j.gastro.2020.02.030. [DOI] [PubMed] [Google Scholar]
- 271.Sandborn WJ, et al. Efficacy and safety of upadacitinib in a randomized trial of patients with Crohn’s disease. Gastroenterology. 2020;158:2123–2138.e2128. doi: 10.1053/j.gastro.2020.01.047. [DOI] [PubMed] [Google Scholar]
- 272.Mease PJ, et al. Upadacitinib for psoriatic arthritis refractory to biologics: SELECT-PsA 2. Ann. Rheum. Dis. 2021;80:312–320. doi: 10.1136/annrheumdis-2020-218870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.He H, Guttman-Yassky E. JAK inhibitors for atopic dermatitis: an update. Am. J. Clin. Dermatol. 2019;20:181–192. doi: 10.1007/s40257-018-0413-2. [DOI] [PubMed] [Google Scholar]
- 274.Mohamed MF, et al. Pharmacokinetics, safety and tolerability of ABT-494, a novel selective JAK 1 inhibitor, in healthy volunteers and subjects with rheumatoid arthritis. Clin. Pharmacokinet. 2016;55:1547–1558. doi: 10.1007/s40262-016-0419-y. [DOI] [PubMed] [Google Scholar]
- 275.van der Heijde D, et al. Efficacy and safety of upadacitinib for active ankylosing spondylitis refractory to biological therapy: a double-blind, randomised, placebo-controlled phase 3 trial. Ann. Rheum. Dis. 2022;81:1515–1523. doi: 10.1136/ard-2022-222608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Fleischmann RM, et al. Switching between Janus kinase inhibitor upadacitinib and adalimumab following insufficient response: efficacy and safety in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2021;80:432–439. doi: 10.1136/annrheumdis-2020-218412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.McInnes IB, et al. Trial of upadacitinib and adalimumab for psoriatic arthritis. N. Engl. J. Med. 2021;384:1227–1239. doi: 10.1056/NEJMoa2022516. [DOI] [PubMed] [Google Scholar]
- 278.Ghosh S, et al. Upadacitinib treatment improves symptoms of bowel urgency and abdominal pain, and correlates with quality of life improvements in patients with moderate to severe ulcerative colitis. J. Crohns Colitis. 2021;15:2022–2030. doi: 10.1093/ecco-jcc/jjab099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Guttman-Yassky E, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397:2151–2168. doi: 10.1016/S0140-6736(21)00588-2. [DOI] [PubMed] [Google Scholar]
- 280.Vazquez, M. L. et al. Identification of N-{cis-3-[Methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino]cyclobutyl}propane-1-sulfonamide (PF-04965842): a selective JAK1 clinical candidate for the treatment of autoimmune diseases. J. Med. Chem. 61, 1130–1152 (2018). [DOI] [PubMed]
- 281.Mikhaylov, D., Ungar, B., Renert-Yuval, Y. & Guttman-Yassky, E. Oral Janus kinase inhibitors for atopic dermatitis. Ann. Allergy Asthma Immunol.130, 577–592 (2023). [DOI] [PubMed]
- 282.De, S. K. Abrocitinib: first globally approved selective janus kinase-1 inhibitor for the treatment of atopic dermatitis. Curr. Med. Chem. 30, 10.2174/0929867330666230216123419, E-pub Ahead of Print (2023). [DOI] [PubMed]
- 283.Deeks ED, Duggan S. Abrocitinib: first approval. Drugs. 2021;81:2149–2157. doi: 10.1007/s40265-021-01638-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Bieber T, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N. Engl. J. Med. 2021;384:1101–1112. doi: 10.1056/NEJMoa2019380. [DOI] [PubMed] [Google Scholar]
- 285.Blauvelt A, et al. Abrocitinib induction, randomized withdrawal, and retreatment in patients with moderate-to-severe atopic dermatitis: results from the JAK1 atopic dermatitis efficacy and safety (JADE) REGIMEN phase 3 trial. J. Am. Acad. Dermatol. 2022;86:104–112. doi: 10.1016/j.jaad.2021.05.075. [DOI] [PubMed] [Google Scholar]
- 286.Weidinger S, Schreiber S. Abrocitinib for atopic dermatitis: a step forward. Lancet. 2020;396:215–217. doi: 10.1016/S0140-6736(20)31284-8. [DOI] [PubMed] [Google Scholar]
- 287.Simpson EL, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020;396:255–266. doi: 10.1016/S0140-6736(20)30732-7. [DOI] [PubMed] [Google Scholar]
- 288.Uppal SK, Chat VS, Kearns DG, Wu JJ. Abrocitinib for atopic dermatitis. Lancet. 2021;397:195–196. doi: 10.1016/S0140-6736(21)00036-2. [DOI] [PubMed] [Google Scholar]
- 289.Schmieder GJ, et al. Efficacy and safety of the Janus kinase 1 inhibitor PF-04965842 in patients with moderate-to-severe psoriasis: phase II, randomized, double-blind, placebo-controlled study. Br. J. Dermatol. 2018;179:54–62. doi: 10.1111/bjd.16004. [DOI] [PubMed] [Google Scholar]
- 290.Villanueva A. Hepatocellular carcinoma. N. Engl. J. Med. 2019;380:1450–1462. doi: 10.1056/NEJMra1713263. [DOI] [PubMed] [Google Scholar]
- 291.Sung H, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 292.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J. Clin. Oncol. 2009;27:1485–1491. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Llovet JM, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. doi: 10.1038/s41572-020-00240-3. [DOI] [PubMed] [Google Scholar]
- 294.European Association For The Study Of The Liver & European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J. Hepatol. 56, 908–943 (2012). [DOI] [PubMed]
- 295.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 296.Schafer DF, Sorrell MF. Hepatocellular carcinoma. Lancet. 1999;353:1253–1257. doi: 10.1016/S0140-6736(98)09148-X. [DOI] [PubMed] [Google Scholar]
- 297.Parikh ND, Pillai A. Recent advances in hepatocellular carcinoma treatment. Clin. Gastroenterol. Hepatol. 2021;19:2020–2024. doi: 10.1016/j.cgh.2021.05.045. [DOI] [PubMed] [Google Scholar]
- 298.Caraglia M, et al. Alpha-interferon and its effects on signalling pathways within cells. Curr. Protein Pept. Sci. 2004;5:475–485. doi: 10.2174/1389203043379378. [DOI] [PubMed] [Google Scholar]
- 299.Wang YX, et al. Interferon-inducible MX2 is a host restriction factor of hepatitis B virus replication. J. Hepatol. 2020;72:865–876. doi: 10.1016/j.jhep.2019.12.009. [DOI] [PubMed] [Google Scholar]
- 300.Han M, et al. Altered expression of interferon-stimulated genes is strongly associated with therapeutic outcomes in hepatitis B virus infection. Antivir. Res. 2017;147:75–85. doi: 10.1016/j.antiviral.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 301.Koeberlein B, et al. Hepatitis B virus overexpresses suppressor of cytokine signaling-3 (SOCS3) thereby contributing to severity of inflammation in the liver. Virus Res. 2010;148:51–59. doi: 10.1016/j.virusres.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 302.Robek MD, Boyd BS, Wieland SF, Chisari FV. Signal transduction pathways that inhibit hepatitis B virus replication. Proc. Natl Acad. Sci. USA. 2004;101:1743–1747. doi: 10.1073/pnas.0308340100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Gao D, et al. Down-regulation of suppressor of cytokine signaling 3 by miR-122 enhances interferon-mediated suppression of hepatitis B virus. Antivir. Res. 2015;118:20–28. doi: 10.1016/j.antiviral.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 304.Khan MGM, et al. Prognostic significance of SOCS1 and SOCS3 tumor suppressors and oncogenic signaling pathway genes in hepatocellular carcinoma. BMC Cancer. 2020;20:774. doi: 10.1186/s12885-020-07285-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Li H, et al. C1QTNF1-AS1 regulates the occurrence and development of hepatocellular carcinoma by regulating miR-221-3p/SOCS3. Hepatol. Int. 2019;13:277–292. doi: 10.1007/s12072-019-09944-5. [DOI] [PubMed] [Google Scholar]
- 306.Liu ZK, et al. EYA2 suppresses the progression of hepatocellular carcinoma via SOCS3-mediated blockade of JAK/STAT signaling. Mol. Cancer. 2021;20:79. doi: 10.1186/s12943-021-01377-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Zhao Z, et al. CircSOD2 induced epigenetic alteration drives hepatocellular carcinoma progression through activating JAK2/STAT3 signaling pathway. J. Exp. Clin. Cancer Res. 2020;39:259. doi: 10.1186/s13046-020-01769-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Guo H, et al. HHLA2 activates the JAK/STAT signaling pathway by binding to TMIGD2 in hepatocellular carcinoma cells. Inflammation. 2022;45:1585–1599. doi: 10.1007/s10753-022-01644-x. [DOI] [PubMed] [Google Scholar]
- 309.Mora LB, et al. Constitutive activation of Stat3 in human prostate tumors and cell lines: direct inhibition of Stat3 signaling induces apoptosis of prostate cancer cells. Cancer Res. 2002;62:6659–6666. [PubMed] [Google Scholar]
- 310.Gao L, et al. Down-regulation of signal transducer and activator of transcription 3 expression using vector-based small interfering RNAs suppresses growth of human prostate tumor in vivo. Clin. Cancer Res. 2005;11:6333–6341. doi: 10.1158/1078-0432.CCR-05-0148. [DOI] [PubMed] [Google Scholar]
- 311.Liu C, et al. Niclosamide suppresses cell migration and invasion in enzalutamide resistant prostate cancer cells via Stat3-AR axis inhibition. Prostate. 2015;75:1341–1353. doi: 10.1002/pros.23015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Liu C, et al. Inhibition of constitutively active Stat3 reverses enzalutamide resistance in LNCaP derivative prostate cancer cells. Prostate. 2014;74:201–209. doi: 10.1002/pros.22741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Onkar SS, et al. The Great Immune Escape: Understanding the Divergent Immune Response in Breast Cancer Subtypes. Cancer Discov. 2023;13:23–40. doi: 10.1158/2159-8290.CD-22-0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J. Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 315.Feng R, et al. Cancer situation in China: what does the China cancer map indicate from the first national death survey to the latest cancer registration? Cancer Commun. (Lond.) 2023;43:75–86. doi: 10.1002/cac2.12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Luen, S. J. et al. Genomic characterisation of hormone receptor-positive breast cancer arising in very young women. Ann Oncol, (2023). [DOI] [PMC free article] [PubMed]
- 317.Chlebowski RT, et al. Ethnicity and breast cancer: factors influencing differences in incidence and outcome. J. Natl Cancer Inst. 2005;97:439–448. doi: 10.1093/jnci/dji064. [DOI] [PubMed] [Google Scholar]
- 318.Wong GL, Manore SG, Doheny DL, Lo HW. STAT family of transcription factors in breast cancer: Pathogenesis and therapeutic opportunities and challenges. Semin. Cancer Biol. 2022;86:84–106. doi: 10.1016/j.semcancer.2022.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Zou S, et al. Targeting STAT3 in cancer immunotherapy. Mol. Cancer. 2020;19:145. doi: 10.1186/s12943-020-01258-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Bromberg J. Signal transducers and activators of transcription as regulators of growth, apoptosis and breast development. Breast Cancer Res. 2000;2:86–90. doi: 10.1186/bcr38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Yu H, Lee H, Herrmann A, Buettner R, Jove R. Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nat. Rev. Cancer. 2014;14:736–746. doi: 10.1038/nrc3818. [DOI] [PubMed] [Google Scholar]
- 322.Johnson DE, O’Keefe RA, Grandis JR. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018;15:234–248. doi: 10.1038/nrclinonc.2018.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Wang T, et al. JAK/STAT3-regulated fatty acid β-oxidation is critical for breast cancer stem cell self-renewal and chemoresistance. Cell Metab. 2018;27:136–150.e135. doi: 10.1016/j.cmet.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Li J, et al. Tumor cell-intrinsic CD96 mediates chemoresistance and cancer stemness by regulating mitochondrial fatty acid β-oxidation. Adv. Sci. (Weinh.) 2023;10:e2202956. doi: 10.1002/advs.202202956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Zhu Y, et al. Natural product preferentially targets redox and metabolic adaptations and aberrantly active STAT3 to inhibit breast tumor growth in vivo. Cell Death Dis. 2022;13:1022. doi: 10.1038/s41419-022-05477-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Liu C, et al. A highly potent small-molecule antagonist of exportin-1 selectively eliminates CD44(+)CD24(-) enriched breast cancer stem-like cells. Drug Resist. Updat. 2023;66:100903. doi: 10.1016/j.drup.2022.100903. [DOI] [PubMed] [Google Scholar]
- 327.Meraviglia-Crivelli D, et al. IL-6/STAT3 signaling in tumor cells restricts the expression of frameshift-derived neoantigens by SMG1 induction. Mol. Cancer. 2022;21:211. doi: 10.1186/s12943-022-01679-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Dinakar YH, et al. Role of STAT3 in the initiation, progression, proliferation and metastasis of breast cancer and strategies to deliver JAK and STAT3 inhibitors. Life Sci. 2022;309:120996. doi: 10.1016/j.lfs.2022.120996. [DOI] [PubMed] [Google Scholar]
- 329.DeMichele A, et al. Host genetic variants in the interleukin-6 promoter predict poor outcome in patients with estrogen receptor-positive, node-positive breast cancer. Cancer Res. 2009;69:4184–4191. doi: 10.1158/0008-5472.CAN-08-2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Dethlefsen C, Højfeldt G, Hojman P. The role of intratumoral and systemic IL-6 in breast cancer. Breast Cancer Res. Treat. 2013;138:657–664. doi: 10.1007/s10549-013-2488-z. [DOI] [PubMed] [Google Scholar]
- 331.Rodriguez-Barrueco R, et al. Inhibition of the autocrine IL-6-JAK2-STAT3-calprotectin axis as targeted therapy for HR-/HER2+ breast cancers. Genes Dev. 2015;29:1631–1648. doi: 10.1101/gad.262642.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Bottos A, et al. Decreased NK-cell tumour immunosurveillance consequent to JAK inhibition enhances metastasis in breast cancer models. Nat. Commun. 2016;7:12258. doi: 10.1038/ncomms12258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Irey EA, et al. JAK/STAT inhibition in macrophages promotes therapeutic resistance by inducing expression of protumorigenic factors. Proc. Natl Acad. Sci. USA. 2019;116:12442–12451. doi: 10.1073/pnas.1816410116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Tao J, et al. JAK-STAT signaling is activated in the kidney and peripheral blood cells of patients with focal segmental glomerulosclerosis. Kidney Int. 2018;94:795–808. doi: 10.1016/j.kint.2018.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Pang M, et al. A novel STAT3 inhibitor, S3I-201, attenuates renal interstitial fibroblast activation and interstitial fibrosis in obstructive nephropathy. Kidney Int. 2010;78:257–268. doi: 10.1038/ki.2010.154. [DOI] [PubMed] [Google Scholar]
- 336.Koike K, et al. Protective role of JAK/STAT signaling against renal fibrosis in mice with unilateral ureteral obstruction. Clin. Immunol. 2014;150:78–87. doi: 10.1016/j.clim.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 337.Luan J, et al. miR-150-based RNA interference attenuates tubulointerstitial fibrosis through the SOCS1/JAK/STAT pathway in vivo and in vitro. Mol. Ther. Nucleic Acids. 2020;22:871–884. doi: 10.1016/j.omtn.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Tao J, et al. JAK-STAT activity in peripheral blood cells and kidney tissue in IgA nephropathy. Clin. J. Am. Soc. Nephrol. 2020;15:973–982. doi: 10.2215/CJN.11010919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Wang X, Shaw S, Amiri F, Eaton DC, Marrero MB. Inhibition of the Jak/STAT signaling pathway prevents the high glucose-induced increase in tgf-beta and fibronectin synthesis in mesangial cells. Diabetes. 2002;51:3505–3509. doi: 10.2337/diabetes.51.12.3505. [DOI] [PubMed] [Google Scholar]
- 340.Marrero MB, Banes-Berceli AK, Stern DM, Eaton DC. Role of the JAK/STAT signaling pathway in diabetic nephropathy. Am. J. Physiol. Ren. Physiol. 2006;290:F762–F768. doi: 10.1152/ajprenal.00181.2005. [DOI] [PubMed] [Google Scholar]
- 341.Sengupta U, Ukil S, Dimitrova N, Agrawal S. Expression-based network biology identifies alteration in key regulatory pathways of type 2 diabetes and associated risk/complications. PLoS One. 2009;4:e8100. doi: 10.1371/journal.pone.0008100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Chen D, et al. JAK/STAT pathway promotes the progression of diabetic kidney disease via autophagy in podocytes. Eur. J. Pharm. 2021;902:174121. doi: 10.1016/j.ejphar.2021.174121. [DOI] [PubMed] [Google Scholar]
- 343.Zhang H, et al. Podocyte-specific JAK2 overexpression worsens diabetic kidney disease in mice. Kidney Int. 2017;92:909–921. doi: 10.1016/j.kint.2017.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Casarella A, et al. Autosomic dominant polycystic kidney disease and metformin: old knowledge and new insights on retarding progression of chronic kidney disease. Med. Res. Rev. 2022;42:629–640. doi: 10.1002/med.21850. [DOI] [PubMed] [Google Scholar]
- 345.Fragiadaki M, et al. STAT5 drives abnormal proliferation in autosomal dominant polycystic kidney disease. Kidney Int. 2017;91:575–586. doi: 10.1016/j.kint.2016.10.039. [DOI] [PubMed] [Google Scholar]
- 346.Patera F, Cudzich-Madry A, Huang Z, Fragiadaki M. Renal expression of JAK2 is high in polycystic kidney disease and its inhibition reduces cystogenesis. Sci. Rep. 2019;9:4491. doi: 10.1038/s41598-019-41106-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Arakawa T, et al. Activation of signal transducer and activator of transcription 3 correlates with cell proliferation and renal injury in human glomerulonephritis. Nephrol. Dial. Transpl. 2008;23:3418–3426. doi: 10.1093/ndt/gfn314. [DOI] [PubMed] [Google Scholar]
- 348.Borie DC, et al. Combined use of the JAK3 inhibitor CP-690,550 with mycophenolate mofetil to prevent kidney allograft rejection in nonhuman primates. Transplantation. 2005;80:1756–1764. doi: 10.1097/01.tp.0000184634.25042.ea. [DOI] [PubMed] [Google Scholar]
- 349.Borie DC, et al. Immunosuppression by the JAK3 inhibitor CP-690,550 delays rejection and significantly prolongs kidney allograft survival in nonhuman primates. Transplantation. 2005;79:791–801. doi: 10.1097/01.TP.0000157117.30290.6F. [DOI] [PubMed] [Google Scholar]
- 350.Changelian PS, et al. Prevention of organ allograft rejection by a specific Janus kinase 3 inhibitor. Science. 2003;302:875–878. doi: 10.1126/science.1087061. [DOI] [PubMed] [Google Scholar]
- 351.Adachi Y, et al. Inhibition of FGFR reactivates IFNγ signaling in tumor cells to enhance the combined antitumor activity of lenvatinib with anti-PD-1 antibodies. Cancer Res. 2022;82:292–306. doi: 10.1158/0008-5472.CAN-20-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Favoino E, et al. Working and safety profiles of JAK/STAT signaling inhibitors. Are these small molecules also smart? Autoimmun. Rev. 2021;20:102750. doi: 10.1016/j.autrev.2021.102750. [DOI] [PubMed] [Google Scholar]
- 353.Podewski EK, et al. Alterations in Janus kinase (JAK)-signal transducers and activators of transcription (STAT) signaling in patients with end-stage dilated cardiomyopathy. Circulation. 2003;107:798–802. doi: 10.1161/01.CIR.0000057545.82749.FF. [DOI] [PubMed] [Google Scholar]
- 354.Panés J, Vermeire S. JAK inhibitors: back to small molecules for the treatment of IBD. J. Crohns Colitis. 2020;14:S711–s712. doi: 10.1093/ecco-jcc/jjaa109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Miyagi T, et al. High basal STAT4 balanced by STAT1 induction to control type 1 interferon effects in natural killer cells. J. Exp. Med. 2007;204:2383–2396. doi: 10.1084/jem.20070401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Thieu VT, et al. Signal transducer and activator of transcription 4 is required for the transcription factor T-bet to promote T helper 1 cell-fate determination. Immunity. 2008;29:679–690. doi: 10.1016/j.immuni.2008.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Weinstein JS, et al. STAT4 and T-bet control follicular helper T cell development in viral infections. J. Exp. Med. 2018;215:337–355. doi: 10.1084/jem.20170457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Guenterberg KD, et al. Interleukin-29 binds to melanoma cells inducing Jak-STAT signal transduction and apoptosis. Mol. Cancer Ther. 2010;9:510–520. doi: 10.1158/1535-7163.MCT-09-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Kroon P, et al. JAK-STAT blockade inhibits tumor initiation and clonogenic recovery of prostate cancer stem-like cells. Cancer Res. 2013;73:5288–5298. doi: 10.1158/0008-5472.CAN-13-0874. [DOI] [PubMed] [Google Scholar]
- 360.Tanaka T, Narazaki M, Ogata A, Kishimoto T. A new era for the treatment of inflammatory autoimmune diseases by interleukin-6 blockade strategy. Semin. Immunol. 2014;26:88–96. doi: 10.1016/j.smim.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 361.Chen LYC, et al. Soluble interleukin-6 receptor in the COVID-19 cytokine storm syndrome. Cell Rep. Med. 2021;2:100269. doi: 10.1016/j.xcrm.2021.100269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Refaeli Y, Van Parijs L, London CA, Tschopp J, Abbas AK. Biochemical mechanisms of IL-2-regulated Fas-mediated T cell apoptosis. Immunity. 1998;8:615–623. doi: 10.1016/S1074-7613(00)80566-X. [DOI] [PubMed] [Google Scholar]
- 363.Liu X, et al. Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes Dev. 1997;11:179–186. doi: 10.1101/gad.11.2.179. [DOI] [PubMed] [Google Scholar]
- 364.Lin JX, Leonard WJ. The role of Stat5a and Stat5b in signaling by IL-2 family cytokines. Oncogene. 2000;19:2566–2576. doi: 10.1038/sj.onc.1203523. [DOI] [PubMed] [Google Scholar]
- 365.Mui AL, Wakao H, O’Farrell AM, Harada N, Miyajima A. Interleukin-3, granulocyte-macrophage colony stimulating factor and interleukin-5 transduce signals through two STAT5 homologs. EMBO J. 1995;14:1166–1175. doi: 10.1002/j.1460-2075.1995.tb07100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Wakao H, Gouilleux F, Groner B. Mammary gland factor (MGF) is a novel member of the cytokine regulated transcription factor gene family and confers the prolactin response. EMBO J. 1995;14:854–855. doi: 10.1002/j.1460-2075.1995.tb07064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Azam M, et al. Interleukin-3 signals through multiple isoforms of Stat5. EMBO J. 1995;14:1402–1411. doi: 10.1002/j.1460-2075.1995.tb07126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Buettner R, Mora LB, Jove R. Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin. Cancer Res. 2002;8:945–954. [PubMed] [Google Scholar]
- 369.Page BD, et al. Small molecule STAT5-SH2 domain inhibitors exhibit potent antileukemia activity. J. Med Chem. 2012;55:1047–1055. doi: 10.1021/jm200720n. [DOI] [PubMed] [Google Scholar]
- 370.Lis C, et al. Development of Erasin: a chromone-based STAT3 inhibitor which induces apoptosis in Erlotinib-resistant lung cancer cells. Sci. Rep. 2017;7:17390. doi: 10.1038/s41598-017-17600-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Caldenhoven E, et al. STAT3beta, a splice variant of transcription factor STAT3, is a dominant negative regulator of transcription. J. Biol. Chem. 1996;271:13221–13227. doi: 10.1074/jbc.271.22.13221. [DOI] [PubMed] [Google Scholar]
- 372.Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat. Rev. Immunol. 2007;7:41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- 373.Hillmer EJ, Zhang H, Li HS, Watowich SS. STAT3 signaling in immunity. Cytokine Growth Factor Rev. 2016;31:1–15. doi: 10.1016/j.cytogfr.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Xiao, L. et al. IL-9/STAT3/fatty acid oxidation-mediated lipid peroxidation contributes to Tc9 cell longevity and enhanced antitumor activity. J. Clin. Invest. 132, e153247 (2022). [DOI] [PMC free article] [PubMed]
- 375.MacDonagh L, et al. BBI608 inhibits cancer stemness and reverses cisplatin resistance in NSCLC. Cancer Lett. 2018;428:117–126. doi: 10.1016/j.canlet.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 376.Bi S, et al. Napabucasin (BBI608) eliminate AML cells in vitro and in vivo via inhibition of Stat3 pathway and induction of DNA damage. Eur. J. Pharm. 2019;855:252–261. doi: 10.1016/j.ejphar.2019.05.020. [DOI] [PubMed] [Google Scholar]
- 377.Bitsch R, et al. STAT3 inhibitor Napabucasin abrogates MDSC immunosuppressive capacity and prolongs survival of melanoma-bearing mice. J. Immunother. Cancer. 2022;10:e004384. doi: 10.1136/jitc-2021-004384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Jonker DJ, et al. Napabucasin versus placebo in refractory advanced colorectal cancer: a randomised phase 3 trial. Lancet Gastroenterol. Hepatol. 2018;3:263–270. doi: 10.1016/S2468-1253(18)30009-8. [DOI] [PubMed] [Google Scholar]
- 379.Kim MJ, et al. OPB-31121, a novel small molecular inhibitor, disrupts the JAK2/STAT3 pathway and exhibits an antitumor activity in gastric cancer cells. Cancer Lett. 2013;335:145–152. doi: 10.1016/j.canlet.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 380.Brambilla L, Lahiri T, Cammer M, Levy DE. STAT3 inhibitor OPB-51602 is cytotoxic to tumor cells through inhibition of complex I and ROS induction. iScience. 2020;23:101822. doi: 10.1016/j.isci.2020.101822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Hirpara J, et al. Metabolic reprogramming of oncogene-addicted cancer cells to OXPHOS as a mechanism of drug resistance. Redox Biol. 2019;25:101076. doi: 10.1016/j.redox.2018.101076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Genini D, et al. Mitochondrial dysfunction induced by a SH2 domain-targeting STAT3 inhibitor leads to metabolic synthetic lethality in cancer cells. Proc. Natl Acad. Sci. USA. 2017;114:E4924–e4933. doi: 10.1073/pnas.1615730114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Wong AL, et al. Phase I and biomarker study of OPB-51602, a novel signal transducer and activator of transcription (STAT) 3 inhibitor, in patients with refractory solid malignancies. Ann. Oncol. 2015;26:998–1005. doi: 10.1093/annonc/mdv026. [DOI] [PubMed] [Google Scholar]
- 384.Kasembeli MM, et al. TTI-101: a competitive inhibitor of STAT3 that spares oxidative phosphorylation and reverses mechanical allodynia in mouse models of neuropathic pain. Biochem. Pharm. 2021;192:114688. doi: 10.1016/j.bcp.2021.114688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Zhao Y, et al. Analysis and validation of human targets and treatments using a hepatocellular carcinoma-immune humanized mouse model. Hepatology. 2021;74:1395–1410. doi: 10.1002/hep.31812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Jung KH, et al. Multifunctional effects of a small-molecule STAT3 inhibitor on NASH and hepatocellular carcinoma in mice. Clin. Cancer Res. 2017;23:5537–5546. doi: 10.1158/1078-0432.CCR-16-2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Di JX, Zhang HY. C188-9, a small-molecule STAT3 inhibitor, exerts an antitumor effect on head and neck squamous cell carcinoma. Anticancer Drugs. 2019;30:846–853. doi: 10.1097/CAD.0000000000000783. [DOI] [PubMed] [Google Scholar]
- 388.Pedroza M, et al. Role of STAT3 in skin fibrosis and transforming growth factor beta signalling. Rheumatology. 2018;57:1838–1850. doi: 10.1093/rheumatology/kex347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Robinson P, et al. Genetic and small-molecule modulation of stat3 in a mouse model of Crohn’s disease. J. Clin. Med. 2022;11:7020. doi: 10.3390/jcm11237020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Sheng W, et al. STAT5 programs a distinct subset of GM-CSF-producing T helper cells that is essential for autoimmune neuroinflammation. Cell Res. 2014;24:1387–1402. doi: 10.1038/cr.2014.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Luo F, et al. Niclosamide, an antihelmintic drug, enhances efficacy of PD-1/PD-L1 immune checkpoint blockade in non-small cell lung cancer. J. Immunother. Cancer. 2019;7:245. doi: 10.1186/s40425-019-0733-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Guha P, et al. STAT3 inhibition induces Bax-dependent apoptosis in liver tumor myeloid-derived suppressor cells. Oncogene. 2019;38:533–548. doi: 10.1038/s41388-018-0449-z. [DOI] [PubMed] [Google Scholar]
- 393.Pei J, et al. STAT3 inhibition enhances CDN-induced STING signaling and antitumor immunity. Cancer Lett. 2019;450:110–122. doi: 10.1016/j.canlet.2019.02.029. [DOI] [PubMed] [Google Scholar]
- 394.Moreira D, et al. STAT3 inhibition combined with CpG immunostimulation activates antitumor immunity to eradicate genetically distinct castration-resistant prostate cancers. Clin. Cancer Res. 2018;24:5948–5962. doi: 10.1158/1078-0432.CCR-18-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Jamieson S, Wallace CE, Das N, Bhattacharyya P, Bishayee A. Guava (Psidium guajava L.): a glorious plant with cancer preventive and therapeutic potential. Crit. Rev. Food Sci. Nutr. 2023;63:192–223. doi: 10.1080/10408398.2021.1945531. [DOI] [PubMed] [Google Scholar]
- 396.Sun Q, et al. Flavonoids regulate tumor-associated macrophages - from structure-activity relationship to clinical potential (Review) Pharm. Res. 2022;184:106419. doi: 10.1016/j.phrs.2022.106419. [DOI] [PubMed] [Google Scholar]
- 397.Kalick LS, et al. Mangosteen for malignancy prevention and intervention: current evidence, molecular mechanisms, and future perspectives. Pharm. Res. 2023;188:106630. doi: 10.1016/j.phrs.2022.106630. [DOI] [PubMed] [Google Scholar]
- 398.Yang J, Wang L, Guan X, Qin JJ. Inhibiting STAT3 signaling pathway by natural products for cancer prevention and therapy: In vitro and in vivo activity and mechanisms of action. Pharm. Res. 2022;182:106357. doi: 10.1016/j.phrs.2022.106357. [DOI] [PubMed] [Google Scholar]
- 399.Mahata S, et al. In-silico and in-vitro investigation of STAT3-PIM1 heterodimeric complex: Its mechanism and inhibition by curcumin for cancer therapeutics. Int. J. Biol. Macromol. 2022;208:356–366. doi: 10.1016/j.ijbiomac.2022.03.137. [DOI] [PubMed] [Google Scholar]
- 400.Patel SS, et al. Cellular and molecular mechanisms of curcumin in prevention and treatment of disease. Crit. Rev. Food Sci. Nutr. 2020;60:887–939. doi: 10.1080/10408398.2018.1552244. [DOI] [PubMed] [Google Scholar]
- 401.Tian M, Tian D, Qiao X, Li J, Zhang L. Modulation of Myb-induced NF-kB -STAT3 signaling and resulting cisplatin resistance in ovarian cancer by dietary factors. J. Cell Physiol. 2019;234:21126–21134. doi: 10.1002/jcp.28715. [DOI] [PubMed] [Google Scholar]
- 402.Chen YC, et al. Myricetin inhibits interferon-γ-induced PD-L1 and IDO1 expression in lung cancer cells. Biochem. Pharm. 2022;197:114940. doi: 10.1016/j.bcp.2022.114940. [DOI] [PubMed] [Google Scholar]
- 403.Liu K, et al. Focus on immune checkpoint PD-1/PD-L1 pathway: new advances of polyphenol phytochemicals in tumor immunotherapy. Biomed. Pharmacother. 2022;154:113618. doi: 10.1016/j.biopha.2022.113618. [DOI] [PubMed] [Google Scholar]
- 404.Runtsch MC, et al. Itaconate and itaconate derivatives target JAK1 to suppress alternative activation of macrophages. Cell Metab. 2022;34:487–501.e488. doi: 10.1016/j.cmet.2022.02.002. [DOI] [PubMed] [Google Scholar]
- 405.Guan X, et al. Dual inhibition of MYC and SLC39A10 by a novel natural product STAT3 inhibitor derived from Chaetomium globosum suppresses tumor growth and metastasis in gastric cancer. Pharm. Res. 2023;189:106703. doi: 10.1016/j.phrs.2023.106703. [DOI] [PubMed] [Google Scholar]
- 406.Cianciulli A, Calvello R, Porro C, Trotta T, Panaro MA. Understanding the role of SOCS signaling in neurodegenerative diseases: current and emerging concepts. Cytokine Growth Factor Rev. 2017;37:67–79. doi: 10.1016/j.cytogfr.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 407.Ahmed CM, Larkin J, 3rd, Johnson HM. SOCS1 mimetics and antagonists: a complementary approach to positive and negative regulation of immune function. Front. Immunol. 2015;6:183. doi: 10.3389/fimmu.2015.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.McLornan DP, Pope JE, Gotlib J, Harrison CN. Current and future status of JAK inhibitors. Lancet. 2021;398:803–816. doi: 10.1016/S0140-6736(21)00438-4. [DOI] [PubMed] [Google Scholar]
- 409.Liu C, Kieltyka J, Fleischmann R, Gadina M, O’Shea JJ. A decade of JAK inhibitors: what have we learned and what may be the future? Arthritis Rheumatol. 2021;73:2166–2178. doi: 10.1002/art.41906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.De Vries LCS, Wildenberg ME, De Jonge WJ, D’Haens GR. The future of janus kinase inhibitors in inflammatory bowel disease. J. Crohns Colitis. 2017;11:885–893. doi: 10.1093/ecco-jcc/jjx003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Verstovsek S, et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N. Engl. J. Med. 2010;363:1117–1127. doi: 10.1056/NEJMoa1002028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Rosmarin D, et al. Ruxolitinib cream for treatment of vitiligo: a randomised, controlled, phase 2 trial. Lancet. 2020;396:110–120. doi: 10.1016/S0140-6736(20)30609-7. [DOI] [PubMed] [Google Scholar]
- 413.Zeiser R, et al. Ruxolitinib for glucocorticoid-refractory acute graft-versus-host disease. N. Engl. J. Med. 2020;382:1800–1810. doi: 10.1056/NEJMoa1917635. [DOI] [PubMed] [Google Scholar]
- 414.Hurwitz HI, et al. Randomized, double-blind, phase ii study of ruxolitinib or placebo in combination with capecitabine in patients with metastatic pancreatic cancer for whom therapy with gemcitabine has failed. J. Clin. Oncol. 2015;33:4039–4047. doi: 10.1200/JCO.2015.61.4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Mesa RA, et al. Effect of ruxolitinib therapy on myelofibrosis-related symptoms and other patient-reported outcomes in COMFORT-I: a randomized, double-blind, placebo-controlled trial. J. Clin. Oncol. 2013;31:1285–1292. doi: 10.1200/JCO.2012.44.4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Kiladjian JJ, et al. Long-term efficacy and safety of ruxolitinib versus best available therapy in polycythaemia vera (RESPONSE): 5-year follow up of a phase 3 study. Lancet Haematol. 2020;7:e226–e237. doi: 10.1016/S2352-3026(19)30207-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Harrison CN, et al. Janus kinase-2 inhibitor fedratinib in patients with myelofibrosis previously treated with ruxolitinib (JAKARTA-2): a single-arm, open-label, non-randomised, phase 2, multicentre study. Lancet Haematol. 2017;4:e317–e324. doi: 10.1016/S2352-3026(17)30088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Roskoski R., Jr. Properties of FDA-approved small molecule protein kinase inhibitors: a 2020 update. Pharm. Res. 2020;152:104609. doi: 10.1016/j.phrs.2019.104609. [DOI] [PubMed] [Google Scholar]
- 419.Vannucchi AM, et al. Ruxolitinib versus standard therapy for the treatment of polycythemia vera. N. Engl. J. Med. 2015;372:426–435. doi: 10.1056/NEJMoa1409002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Verstovsek S, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N. Engl. J. Med. 2012;366:799–807. doi: 10.1056/NEJMoa1110557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Passamonti F, et al. Ruxolitinib for the treatment of inadequately controlled polycythaemia vera without splenomegaly (RESPONSE-2): a randomised, open-label, phase 3b study. Lancet Oncol. 2017;18:88–99. doi: 10.1016/S1470-2045(16)30558-7. [DOI] [PubMed] [Google Scholar]
- 422.Harrison CN, et al. Addition of navitoclax to ongoing ruxolitinib therapy for patients with myelofibrosis with progression or suboptimal response: phase ii safety and efficacy. J. Clin. Oncol. 2022;40:1671–1680. doi: 10.1200/JCO.21.02188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Dao KT, et al. Efficacy of ruxolitinib in patients with chronic neutrophilic leukemia and atypical chronic myeloid leukemia. J. Clin. Oncol. 2020;38:1006–1018. doi: 10.1200/JCO.19.00895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Harrison C, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N. Engl. J. Med. 2012;366:787–798. doi: 10.1056/NEJMoa1110556. [DOI] [PubMed] [Google Scholar]
- 425.Tefferi A, Litzow MR, Pardanani A. Long-term outcome of treatment with ruxolitinib in myelofibrosis. N. Engl. J. Med. 2011;365:1455–1457. doi: 10.1056/NEJMc1109555. [DOI] [PubMed] [Google Scholar]
- 426.Harrison CN, et al. Long-term findings from COMFORT-II, a phase 3 study of ruxolitinib vs best available therapy for myelofibrosis. Leukemia. 2016;30:1701–1707. doi: 10.1038/leu.2016.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Kvasnicka HM, et al. Long-term effects of ruxolitinib versus best available therapy on bone marrow fibrosis in patients with myelofibrosis. J. Hematol. Oncol. 2018;11:42. doi: 10.1186/s13045-018-0585-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Zeiser R, et al. Ruxolitinib for glucocorticoid-refractory chronic graft-versus-host disease. N. Engl. J. Med. 2021;385:228–238. doi: 10.1056/NEJMoa2033122. [DOI] [PubMed] [Google Scholar]
- 429.Rudra S, et al. Ruxolitinib: targeted approach for treatment of autoinflammatory very early onset inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 2022;20:1408–1410.e1402. doi: 10.1016/j.cgh.2021.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Bader-Meunier B, et al. Effectiveness and safety of ruxolitinib for the treatment of refractory systemic idiopathic juvenile arthritis like associated with interstitial lung disease: a case report. Ann. Rheum. Dis. 2022;81:e20. doi: 10.1136/annrheumdis-2020-216983. [DOI] [PubMed] [Google Scholar]
- 431.Han MK, et al. Ruxolitinib in addition to standard of care for the treatment of patients admitted to hospital with COVID-19 (RUXCOVID): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Rheumatol. 2022;4:e351–e361. doi: 10.1016/S2665-9913(22)00044-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Fisher DAC, et al. Cytokine production in myelofibrosis exhibits differential responsiveness to JAK-STAT, MAP kinase, and NFκB signaling. Leukemia. 2019;33:1978–1995. doi: 10.1038/s41375-019-0379-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Wathes R, Moule S, Milojkovic D. Progressive multifocal leukoencephalopathy associated with ruxolitinib. N. Engl. J. Med. 2013;369:197–198. doi: 10.1056/NEJMc1302135. [DOI] [PubMed] [Google Scholar]
- 434.Coltro G, et al. A life-threatening ruxolitinib discontinuation syndrome. Am. J. Hematol. 2017;92:833–838. doi: 10.1002/ajh.24775. [DOI] [PubMed] [Google Scholar]
- 435.Tvorogov D, et al. Accumulation of JAK activation loop phosphorylation is linked to type I JAK inhibitor withdrawal syndrome in myelofibrosis. Sci. Adv. 2018;4:eaat3834. doi: 10.1126/sciadv.aat3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Houthuys JF, Wilmer AP, Peetermans M, Meersseman P, Devos T. Severe ARDS due to ruxolitinib discontinuation syndrome: case presentation and literature review. Heliyon. 2022;8:e11782. doi: 10.1016/j.heliyon.2022.e11782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Jagasia M, et al. Ruxolitinib for the treatment of steroid-refractory acute GVHD (REACH1): a multicenter, open-label phase 2 trial. Blood. 2020;135:1739–1749. doi: 10.1182/blood.2020004823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Guglielmelli P, et al. Impact of mutational status on outcomes in myelofibrosis patients treated with ruxolitinib in the COMFORT-II study. Blood. 2014;123:2157–2160. doi: 10.1182/blood-2013-11-536557. [DOI] [PubMed] [Google Scholar]
- 439.Passamonti F, et al. Impact of ruxolitinib on the natural history of primary myelofibrosis: a comparison of the DIPSS and the COMFORT-2 cohorts. Blood. 2014;123:1833–1835. doi: 10.1182/blood-2013-12-544411. [DOI] [PubMed] [Google Scholar]
- 440.Verstovsek S, et al. Long-term survival in patients treated with ruxolitinib for myelofibrosis: COMFORT-I and -II pooled analyses. J. Hematol. Oncol. 2017;10:156. doi: 10.1186/s13045-017-0527-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Verstovsek S, et al. Long-term treatment with ruxolitinib for patients with myelofibrosis: 5-year update from the randomized, double-blind, placebo-controlled, phase 3 COMFORT-I trial. J. Hematol. Oncol. 2017;10:55. doi: 10.1186/s13045-017-0417-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Marconi VC, et al. Randomized trial of ruxolitinib in antiretroviral-treated adults with human immunodeficiency virus. Clin. Infect. Dis. 2022;74:95–104. doi: 10.1093/cid/ciab212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Scott LJ. Tofacitinib: a review of its use in adult patients with rheumatoid arthritis. Drugs. 2013;73:857–874. doi: 10.1007/s40265-013-0065-8. [DOI] [PubMed] [Google Scholar]
- 444.Dhillon S. Tofacitinib: a review in rheumatoid arthritis. Drugs. 2017;77:1987–2001. doi: 10.1007/s40265-017-0835-9. [DOI] [PubMed] [Google Scholar]
- 445.Guimarães PO, et al. Tofacitinib in patients hospitalized with Covid-19 pneumonia. N. Engl. J. Med. 2021;385:406–415. doi: 10.1056/NEJMoa2101643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Sandborn WJ, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2017;376:1723–1736. doi: 10.1056/NEJMoa1606910. [DOI] [PubMed] [Google Scholar]
- 447.Sandborn WJ, et al. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N. Engl. J. Med. 2012;367:616–624. doi: 10.1056/NEJMoa1112168. [DOI] [PubMed] [Google Scholar]
- 448.Ytterberg SR, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N. Engl. J. Med. 2022;386:316–326. doi: 10.1056/NEJMoa2109927. [DOI] [PubMed] [Google Scholar]
- 449.Wollenhaupt J, et al. Safety and efficacy of tofacitinib, an oral janus kinase inhibitor, for the treatment of rheumatoid arthritis in open-label, longterm extension studies. J. Rheumatol. 2014;41:837–852. doi: 10.3899/jrheum.130683. [DOI] [PubMed] [Google Scholar]
- 450.Kremer JM, et al. The safety and efficacy of a JAK inhibitor in patients with active rheumatoid arthritis: results of a double-blind, placebo-controlled phase IIa trial of three dosage levels of CP-690,550 versus placebo. Arthritis Rheum. 2009;60:1895–1905. doi: 10.1002/art.24567. [DOI] [PubMed] [Google Scholar]
- 451.Sandborn WJ, et al. Efficacy and safety of extended induction with tofacitinib for the treatment of ulcerative colitis. Clin. Gastroenterol. Hepatol. 2022;20:1821–1830.e1823. doi: 10.1016/j.cgh.2020.10.038. [DOI] [PubMed] [Google Scholar]
- 452.Sandborn WJ, et al. Efficacy and safety of tofacitinib in ulcerative colitis based on prior tumor necrosis factor inhibitor failure status. Clin. Gastroenterol. Hepatol. 2022;20:591–601.e598. doi: 10.1016/j.cgh.2021.02.043. [DOI] [PubMed] [Google Scholar]
- 453.Sandborn WJ, et al. Safety of tofacitinib for treatment of ulcerative colitis, based on 4.4 years of data from global clinical trials. Clin. Gastroenterol. Hepatol. 2019;17:1541–1550. doi: 10.1016/j.cgh.2018.11.035. [DOI] [PubMed] [Google Scholar]
- 454.Gladman D, et al. Tofacitinib for psoriatic arthritis in patients with an inadequate response to TNF inhibitors. N. Engl. J. Med. 2017;377:1525–1536. doi: 10.1056/NEJMoa1615977. [DOI] [PubMed] [Google Scholar]
- 455.Khosrow-Khavar F, Kim SC, Lee H, Lee SB, Desai RJ. Tofacitinib and risk of cardiovascular outcomes: results from the safety of TofAcitinib in routine care patients with Rheumatoid Arthritis (STAR-RA) study. Ann. Rheum. Dis. 2022;81:798–804. doi: 10.1136/annrheumdis-2021-221915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Deodhar A, et al. Tofacitinib for the treatment of ankylosing spondylitis: a phase III, randomised, double-blind, placebo-controlled study. Ann. Rheum. Dis. 2021;80:1004–1013. doi: 10.1136/annrheumdis-2020-219601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Krueger J, et al. Tofacitinib attenuates pathologic immune pathways in patients with psoriasis: a randomized phase 2 study. J. Allergy Clin. Immunol. 2016;137:1079–1090. doi: 10.1016/j.jaci.2015.12.1318. [DOI] [PubMed] [Google Scholar]
- 458.Sandborn WJ, et al. A phase 2 study of tofacitinib, an oral Janus kinase inhibitor, in patients with Crohn’s disease. Clin. Gastroenterol. Hepatol. 2014;12:1485–1493.e1482. doi: 10.1016/j.cgh.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 459.Bissonnette R, et al. Topical tofacitinib for atopic dermatitis: a phase IIa randomized trial. Br. J. Dermatol. 2016;175:902–911. doi: 10.1111/bjd.14871. [DOI] [PubMed] [Google Scholar]
- 460.Ma C, et al. Innovations in oral therapies for inflammatory bowel disease. Drugs. 2019;79:1321–1335. doi: 10.1007/s40265-019-01169-y. [DOI] [PubMed] [Google Scholar]
- 461.Colombel JF, et al. Maintenance of remission with tofacitinib therapy in patients with ulcerative colitis. Clin. Gastroenterol. Hepatol. 2022;20:116–125.e115. doi: 10.1016/j.cgh.2020.10.004. [DOI] [PubMed] [Google Scholar]
- 462.Kotwani P, Terdiman J, Lewin S. Tofacitinib for rescue therapy in acute severe ulcerative colitis: a real-world experience. J. Crohns Colitis. 2020;14:1026–1028. doi: 10.1093/ecco-jcc/jjaa018. [DOI] [PubMed] [Google Scholar]
- 463.Takeuchi T. Cytokines and cytokine receptors as targets of immune-mediated inflammatory diseases-RA as a role model. Inflamm. Regen. 2022;42:35. doi: 10.1186/s41232-022-00221-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Smolen, J. S. et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann. Rheum. Dis. 82, 3–18 (2022). [DOI] [PubMed]
- 465.Fleischmann R, et al. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N. Engl. J. Med. 2012;367:495–507. doi: 10.1056/NEJMoa1109071. [DOI] [PubMed] [Google Scholar]
- 466.Mease P, et al. Tofacitinib or adalimumab versus placebo for psoriatic arthritis. N. Engl. J. Med. 2017;377:1537–1550. doi: 10.1056/NEJMoa1615975. [DOI] [PubMed] [Google Scholar]
- 467.Panés J, et al. Tofacitinib for induction and maintenance therapy of Crohn’s disease: results of two phase IIb randomised placebo-controlled trials. Gut. 2017;66:1049–1059. doi: 10.1136/gutjnl-2016-312735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Sandborn, W. J. et al. Tofacitinib for the treatment of ulcerative colitis: an integrated summary of up to 7.8 years of safety data from the global clinical program. J. Crohns Colitis17, 338–351 (2022). [DOI] [PMC free article] [PubMed]
- 469.Damsky W, et al. Inhibition of type 1 immunity with tofacitinib is associated with marked improvement in longstanding sarcoidosis. Nat. Commun. 2022;13:3140. doi: 10.1038/s41467-022-30615-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.You H, et al. Tofacitinib as a possible treatment for skin thickening in diffuse cutaneous systemic sclerosis. Rheumatology. 2021;60:2472–2477. doi: 10.1093/rheumatology/keaa613. [DOI] [PubMed] [Google Scholar]
- 471.Khosrow-Khavar F, Desai RJ, Lee H, Lee SB, Kim SC. Tofacitinib and risk of malignancy: results from the safety of tofacitinib in routine care patients with rheumatoid arthritis (STAR-RA) study. Arthritis Rheumatol. 2022;74:1648–1659. doi: 10.1002/art.42250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Miyawaki H, et al. Long-term effects of the janus kinase 1/2 inhibitor ruxolitinib on pulmonary hypertension and the cardiac function in a patient with myelofibrosis. Intern. Med. 2020;59:229–233. doi: 10.2169/internalmedicine.3528-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Karpov AA, et al. Inhibition of JAK1,2 prevents fibrotic remodeling of pulmonary vascular bed and improves outcomes in the rat model of chronic thromboembolic pulmonary hypertension. Int. J. Mol. Sci. 2022;23:15646. doi: 10.3390/ijms232415646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Hoisnard L, et al. Adverse events associated with JAK inhibitors in 126,815 reports from the WHO pharmacovigilance database. Sci. Rep. 2022;12:7140. doi: 10.1038/s41598-022-10777-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Genovese MC, et al. Baricitinib in patients with refractory rheumatoid arthritis. N. Engl. J. Med. 2016;374:1243–1252. doi: 10.1056/NEJMoa1507247. [DOI] [PubMed] [Google Scholar]
- 476.Taylor PC, et al. Safety of baricitinib for the treatment of rheumatoid arthritis over a median of 4.6 and up to 9.3 years of treatment: final results from long-term extension study and integrated database. Ann. Rheum. Dis. 2022;81:335–343. doi: 10.1136/annrheumdis-2021-221276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Tanaka Y, et al. Clinical outcomes in patients switched from adalimumab to baricitinib due to non-response and/or study design: phase III data in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2019;78:890–898. doi: 10.1136/annrheumdis-2018-214529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Smolen JS, et al. Patient-reported outcomes from a randomised phase III study of baricitinib in patients with rheumatoid arthritis and an inadequate response to biological agents (RA-BEACON) Ann. Rheum. Dis. 2017;76:694–700. doi: 10.1136/annrheumdis-2016-209821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Lei Y, et al. A multicenter blinded preclinical randomized controlled trial on Jak1/2 inhibition in MRL/MpJ-Fas(lpr) mice with proliferative lupus nephritis predicts low effect size. Kidney Int. 2021;99:1331–1341. doi: 10.1016/j.kint.2021.01.024. [DOI] [PubMed] [Google Scholar]
- 480.Wallace DJ, et al. Baricitinib for systemic lupus erythematosus: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet. 2018;392:222–231. doi: 10.1016/S0140-6736(18)31363-1. [DOI] [PubMed] [Google Scholar]
- 481.King B, et al. Efficacy and safety of the oral Janus kinase inhibitor baricitinib in the treatment of adults with alopecia areata: Phase 2 results from a randomized controlled study. J. Am. Acad. Dermatol. 2021;85:847–853. doi: 10.1016/j.jaad.2021.05.050. [DOI] [PubMed] [Google Scholar]
- 482.Simpson EL, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis: results from a randomized monotherapy phase 3 trial in the United States and Canada (BREEZE-AD5) J. Am. Acad. Dermatol. 2021;85:62–70. doi: 10.1016/j.jaad.2021.02.028. [DOI] [PubMed] [Google Scholar]
- 483.Guttman-Yassky E, et al. Baricitinib in adult patients with moderate-to-severe atopic dermatitis: a phase 2 parallel, double-blinded, randomized placebo-controlled multiple-dose study. J. Am. Acad. Dermatol. 2019;80:913–921.e919. doi: 10.1016/j.jaad.2018.01.018. [DOI] [PubMed] [Google Scholar]
- 484.Bieber T, et al. Efficacy and safety of baricitinib in combination with topical corticosteroids in patients with moderate-to-severe atopic dermatitis with inadequate response, intolerance or contraindication to ciclosporin: results from a randomized, placebo-controlled, phase III clinical trial (BREEZE-AD4) Br. J. Dermatol. 2022;187:338–352. doi: 10.1111/bjd.21630. [DOI] [PubMed] [Google Scholar]
- 485.Taylor PC, et al. Baricitinib versus placebo or adalimumab in rheumatoid arthritis. N. Engl. J. Med. 2017;376:652–662. doi: 10.1056/NEJMoa1608345. [DOI] [PubMed] [Google Scholar]
- 486.Kalil AC, et al. Baricitinib plus remdesivir for hospitalized adults with Covid-19. N. Engl. J. Med. 2021;384:795–807. doi: 10.1056/NEJMoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.King B, et al. Two phase 3 trials of baricitinib for alopecia areata. N. Engl. J. Med. 2022;386:1687–1699. doi: 10.1056/NEJMoa2110343. [DOI] [PubMed] [Google Scholar]
- 488.Wolfe CR, et al. Baricitinib versus dexamethasone for adults hospitalised with COVID-19 (ACTT-4): a randomised, double-blind, double placebo-controlled trial. Lancet Respir. Med. 2022;10:888–899. doi: 10.1016/S2213-2600(22)00088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Ely EW, et al. Efficacy and safety of baricitinib plus standard of care for the treatment of critically ill hospitalised adults with COVID-19 on invasive mechanical ventilation or extracorporeal membrane oxygenation: an exploratory, randomised, placebo-controlled trial. Lancet Respir. Med. 2022;10:327–336. doi: 10.1016/S2213-2600(22)00006-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Marconi VC, et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir. Med. 2021;9:1407–1418. doi: 10.1016/S2213-2600(21)00331-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Stebbing J, et al. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect. Dis. 2020;20:400–402. doi: 10.1016/S1473-3099(20)30132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Richardson P, et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020;395:e30–e31. doi: 10.1016/S0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Cantini F, et al. Baricitinib therapy in COVID-19: A pilot study on safety and clinical impact. J. Infect. 2020;81:318–356. doi: 10.1016/j.jinf.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Cantini F, et al. Beneficial impact of Baricitinib in COVID-19 moderate pneumonia; multicentre study. J. Infect. 2020;81:647–679. doi: 10.1016/j.jinf.2020.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Taylor PC, et al. Cardiovascular safety during treatment with baricitinib in rheumatoid arthritis. Arthritis Rheumatol. 2019;71:1042–1055. doi: 10.1002/art.40841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Markham A, Keam SJ. Peficitinib: first global approval. Drugs. 2019;79:887–891. doi: 10.1007/s40265-019-01131-y. [DOI] [PubMed] [Google Scholar]
- 497.Lee YH, et al. Comparative efficacy and safety of peficitinib 25, 50, 100, and 150 mg in patients with active rheumatoid arthritis: a bayesian network meta-analysis of randomized controlled trials. Clin. Drug Investig. 2020;40:65–72. doi: 10.1007/s40261-019-00863-9. [DOI] [PubMed] [Google Scholar]
- 498.Dhillon S. Delgocitinib: first approval. Drugs. 2020;80:609–615. doi: 10.1007/s40265-020-01291-2. [DOI] [PubMed] [Google Scholar]
- 499.Worm M, et al. The pan-JAK inhibitor delgocitinib in a cream formulation demonstrates dose response in chronic hand eczema in a 16-week randomized phase IIb trial. Br. J. Dermatol. 2022;187:42–51. doi: 10.1111/bjd.21037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Worm M, et al. Efficacy and safety of topical delgocitinib in patients with chronic hand eczema: data from a randomized, double-blind, vehicle-controlled phase IIa study. Br. J. Dermatol. 2020;182:1103–1110. doi: 10.1111/bjd.18469. [DOI] [PubMed] [Google Scholar]
- 501.Nakagawa H, et al. Long-term safety and efficacy of delgocitinib ointment, a topical Janus kinase inhibitor, in adult patients with atopic dermatitis. J. Dermatol. 2020;47:114–120. doi: 10.1111/1346-8138.15173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Nakagawa H, et al. Delgocitinib ointment, a topical Janus kinase inhibitor, in adult patients with moderate to severe atopic dermatitis: A phase 3, randomized, double-blind, vehicle-controlled study and an open-label, long-term extension study. J. Am. Acad. Dermatol. 2020;82:823–831. doi: 10.1016/j.jaad.2019.12.015. [DOI] [PubMed] [Google Scholar]
- 503.Nakagawa H, et al. Delgocitinib ointment in pediatric patients with atopic dermatitis: a phase 3, randomized, double-blind, vehicle-controlled study and a subsequent open-label, long-term study. J. Am. Acad. Dermatol. 2021;85:854–862. doi: 10.1016/j.jaad.2021.06.014. [DOI] [PubMed] [Google Scholar]
- 504.Nakagawa H, et al. Phase 2 clinical study of delgocitinib ointment in pediatric patients with atopic dermatitis. J. Allergy Clin. Immunol. 2019;144:1575–1583. doi: 10.1016/j.jaci.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 505.Garrido-Trigo A, Salas A. Molecular structure and function of janus kinases: implications for the development of inhibitors. J. Crohns Colitis. 2020;14:S713–s724. doi: 10.1093/ecco-jcc/jjz206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Nielsen OH, Boye TL, Chakravarti D, Gubatan J. Selective tyrosine kinase 2 inhibitors in inflammatory bowel disease. Trends Pharm. Sci. 2022;43:424–436. doi: 10.1016/j.tips.2022.02.008. [DOI] [PubMed] [Google Scholar]
- 507.Pardanani A, et al. Safety and efficacy of TG101348, a selective JAK2 inhibitor, in myelofibrosis. J. Clin. Oncol. 2011;29:789–796. doi: 10.1200/JCO.2010.32.8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Pardanani A, et al. Safety and efficacy of fedratinib in patients with primary or secondary myelofibrosis: a randomized clinical trial. JAMA Oncol. 2015;1:643–651. doi: 10.1001/jamaoncol.2015.1590. [DOI] [PubMed] [Google Scholar]
- 509.Harrison CN, et al. Fedratinib in patients with myelofibrosis previously treated with ruxolitinib: an updated analysis of the JAKARTA2 study using stringent criteria for ruxolitinib failure. Am. J. Hematol. 2020;95:594–603. doi: 10.1002/ajh.25777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Pardanani A, et al. A phase 2 randomized dose-ranging study of the JAK2-selective inhibitor fedratinib (SAR302503) in patients with myelofibrosis. Blood Cancer J. 2015;5:e335. doi: 10.1038/bcj.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Harrison CN, et al. Safety and efficacy of fedratinib, a selective oral inhibitor of Janus kinase-2 (JAK2), in patients with myelofibrosis and low pretreatment platelet counts. Br. J. Haematol. 2022;198:317–327. doi: 10.1111/bjh.18207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Gerds AT, et al. Myeloproliferative neoplasms, version 3.2022, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2022;20:1033–1062. doi: 10.6004/jnccn.2022.0046. [DOI] [PubMed] [Google Scholar]
- 513.Conigliaro P, et al. Challenges in the treatment of rheumatoid arthritis. Autoimmun. Rev. 2019;18:706–713. doi: 10.1016/j.autrev.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 514.Mahajan S, et al. VX-509 (decernotinib) is a potent and selective janus kinase 3 inhibitor that attenuates inflammation in animal models of autoimmune disease. J. Pharm. Exp. Ther. 2015;353:405–414. doi: 10.1124/jpet.114.221176. [DOI] [PubMed] [Google Scholar]
- 515.Fleischmann RM, et al. A randomized, double-blind, placebo-controlled, twelve-week, dose-ranging study of decernotinib, an oral selective JAK-3 inhibitor, as monotherapy in patients with active rheumatoid arthritis. Arthritis Rheumatol. 2015;67:334–343. doi: 10.1002/art.38949. [DOI] [PubMed] [Google Scholar]
- 516.Genovese MC, van Vollenhoven RF, Pacheco-Tena C, Zhang Y, Kinnman N. VX-509 (decernotinib), an oral selective JAK-3 inhibitor, in combination with methotrexate in patients with rheumatoid arthritis. Arthritis Rheumatol. 2016;68:46–55. doi: 10.1002/art.39473. [DOI] [PubMed] [Google Scholar]
- 517.Genovese MC, Yang F, Østergaard M, Kinnman N. Efficacy of VX-509 (decernotinib) in combination with a disease-modifying antirheumatic drug in patients with rheumatoid arthritis: clinical and MRI findings. Ann. Rheum. Dis. 2016;75:1979–1983. doi: 10.1136/annrheumdis-2015-208901. [DOI] [PubMed] [Google Scholar]
- 518.Etra A, et al. Effective treatment of low-risk acute GVHD with itacitinib monotherapy. Blood. 2023;141:481–489. doi: 10.1182/blood.2022017442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Zeiser R, et al. Efficacy and safety of itacitinib versus placebo in combination with corticosteroids for initial treatment of acute graft-versus-host disease (GRAVITAS-301): a randomised, multicentre, double-blind, phase 3 trial. Lancet Haematol. 2022;9:e14–e25. doi: 10.1016/S2352-3026(21)00367-7. [DOI] [PubMed] [Google Scholar]
- 520.Burmester GR, et al. Safety and efficacy of upadacitinib in patients with rheumatoid arthritis and inadequate response to conventional synthetic disease-modifying anti-rheumatic drugs (SELECT-NEXT): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018;391:2503–2512. doi: 10.1016/S0140-6736(18)31115-2. [DOI] [PubMed] [Google Scholar]
- 521.Duggan S, Keam SJ. Upadacitinib: first approval. Drugs. 2019;79:1819–1828. doi: 10.1007/s40265-019-01211-z. [DOI] [PubMed] [Google Scholar]
- 522.Kavanaugh A, et al. Safety and efficacy of filgotinib: up to 4-year results from an open-label extension study of phase II rheumatoid arthritis programs. J. Rheumatol. 2021;48:1230–1238. doi: 10.3899/jrheum.201183. [DOI] [PubMed] [Google Scholar]
- 523.Smolen JS, et al. Upadacitinib as monotherapy in patients with active rheumatoid arthritis and inadequate response to methotrexate (SELECT-MONOTHERAPY): a randomised, placebo-controlled, double-blind phase 3 study. Lancet. 2019;393:2303–2311. doi: 10.1016/S0140-6736(19)30419-2. [DOI] [PubMed] [Google Scholar]
- 524.Deodhar A, et al. Upadacitinib for the treatment of active non-radiographic axial spondyloarthritis (SELECT-AXIS 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2022;400:369–379. doi: 10.1016/S0140-6736(22)01212-0. [DOI] [PubMed] [Google Scholar]
- 525.Danese S, et al. Upadacitinib as induction and maintenance therapy for moderately to severely active ulcerative colitis: results from three phase 3, multicentre, double-blind, randomised trials. Lancet. 2022;399:2113–2128. doi: 10.1016/S0140-6736(22)00581-5. [DOI] [PubMed] [Google Scholar]
- 526.Gooderham MJ, et al. Efficacy and safety of oral janus kinase 1 inhibitor abrocitinib for patients with atopic dermatitis: a phase 2 randomized clinical trial. JAMA Dermatol. 2019;155:1371–1379. doi: 10.1001/jamadermatol.2019.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.Ständer S, et al. High threshold efficacy responses in moderate-to-severe atopic dermatitis are associated with additional quality of life benefits: pooled analyses of abrocitinib monotherapy studies in adults and adolescents. J. Eur. Acad. Dermatol. Venereol. 2022;36:1308–1317. doi: 10.1111/jdv.18170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Silverberg JI, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:863–873. doi: 10.1001/jamadermatol.2020.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.Eichenfield LF, et al. Efficacy and safety of abrocitinib in combination with topical therapy in adolescents with moderate-to-severe atopic dermatitis: the JADE TEEN randomized clinical trial. JAMA Dermatol. 2021;157:1165–1173. doi: 10.1001/jamadermatol.2021.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530.Shi VY, et al. Phase 3 efficacy and safety of abrocitinib in adults with moderate-to-severe atopic dermatitis after switching from dupilumab (JADE EXTEND) J. Am. Acad. Dermatol. 2022;87:351–358. doi: 10.1016/j.jaad.2022.04.009. [DOI] [PubMed] [Google Scholar]
- 531.Napolitano M, et al. The efficacy and safety of abrocitinib as a treatment option for atopic dermatitis: a short report of the clinical data. Drug Des. Devel. Ther. 2021;15:1135–1147. doi: 10.2147/DDDT.S240866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Reich K, et al. Magnitude and time course of response to abrocitinib for moderate-to-severe atopic dermatitis. J. Allergy Clin. Immunol. Pract. 2022;10:e3222. doi: 10.1016/j.jaip.2022.08.042. [DOI] [PubMed] [Google Scholar]
- 533.Simpson EL, et al. Integrated safety analysis of abrocitinib for the treatment of moderate-to-severe atopic dermatitis from the phase II and phase III clinical trial program. Am. J. Clin. Dermatol. 2021;22:693–707. doi: 10.1007/s40257-021-00618-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Chimalakonda A, et al. Selectivity profile of the tyrosine kinase 2 inhibitor deucravacitinib compared with janus kinase 1/2/3 inhibitors. Dermatol. Ther. 2021;11:1763–1776. doi: 10.1007/s13555-021-00596-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Hoy SM. Deucravacitinib: first approval. Drugs. 2022;82:1671–1679. doi: 10.1007/s40265-022-01796-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536.Armstrong AW, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J. Am. Acad. Dermatol. 2023;88:29–39. doi: 10.1016/j.jaad.2022.07.002. [DOI] [PubMed] [Google Scholar]
- 537.Strober B, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 Program fOr Evaluation of TYK2 inhibitor psoriasis second trial. J. Am. Acad. Dermatol. 2023;88:40–51. doi: 10.1016/j.jaad.2022.08.061. [DOI] [PubMed] [Google Scholar]
- 538.Morand E, et al. Deucravacitinib, a tyrosine kinase 2 inhibitor, in systemic lupus erythematosus: a phase ii, randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol. 2023;75:242–252. doi: 10.1002/art.42391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Chen B, et al. Efficacy and safety of ivarmacitinib in patients with moderate-to-severe, active, ulcerative colitis: a phase II study. Gastroenterology. 2022;163:1555–1568. doi: 10.1053/j.gastro.2022.08.007. [DOI] [PubMed] [Google Scholar]
- 540.Wu H, et al. JAK1-STAT3 blockade by JAK inhibitor SHR0302 attenuates inflammatory responses of adjuvant-induced arthritis rats and decreases Th17 and total B cells. Jt. Bone Spine. 2016;83:525–532. doi: 10.1016/j.jbspin.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 541.Sun X, et al. Preventive and therapeutic effects of a novel JAK inhibitor SHR0302 in acute graft-versus-host disease. Cell Transpl. 2021;30:9636897211033778. doi: 10.1177/09636897211033778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542.Zhao Y, et al. Efficacy and safety of SHR0302, a highly selective janus kinase 1 inhibitor, in patients with moderate to severe atopic dermatitis: a phase II randomized clinical trial. Am. J. Clin. Dermatol. 2021;22:877–889. doi: 10.1007/s40257-021-00627-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543.Su, Q. et al. Discovery of (2R)-N-[3-[2-[(3-Methoxy-1-methyl-pyrazol-4-yl)amino]pyrimidin-4-yl]-1H-indol-7-yl]-2-(4-methylpiperazin-1-yl)propenamide (AZD4205) as a potent and selective janus kinase 1 inhibitor. J. Med. Chem. 63, 4517–4527 (2020). [DOI] [PubMed]
- 544.Tefferi A, Gangat N, Pardanani A. Jaktinib (JAK1/2 inhibitor): a momelotinib derivative with similar activity and optimized dosing schedule. Am. J. Hematol. 2022;97:1507–1509. doi: 10.1002/ajh.26712. [DOI] [PubMed] [Google Scholar]
- 545.Liu J, et al. A phase i, randomized, double-blind, placebo-controlled, single ascending dose, multiple ascending dose and food effect study to evaluate the tolerance, pharmacokinetics of jaktinib, a new selective janus kinase inhibitor in healthy chinese volunteers. Front. Pharm. 2020;11:604314. doi: 10.3389/fphar.2020.604314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 546.Zhang Y, et al. Safety and efficacy of jaktinib in the treatment of Janus kinase inhibitor-naïve patients with myelofibrosis: results of a phase II trial. Am. J. Hematol. 2022;97:1510–1519. doi: 10.1002/ajh.26709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 547.Meng X, et al. Potential for jaktinib hydrochloride to treat cytokine storms in patients with COVID-19. Biosci. Trends. 2020;14:161–167. doi: 10.5582/bst.2020.03106. [DOI] [PubMed] [Google Scholar]
- 548.Philips RL, et al. The JAK-STAT pathway at 30: much learned, much more to do. Cell. 2022;185:3857–3876. doi: 10.1016/j.cell.2022.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549.Thoidingjam LK, et al. Small Molecule Inhibitors of Interferon-Induced JAK-STAT Signalling. Angew. Chem. Int. Ed. Engl. 2022;61:e202205231. doi: 10.1002/anie.202205231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Pohóczky K, et al. Discovery of novel targets in a complex regional pain syndrome mouse model by transcriptomics: TNF and JAK-STAT pathways. Pharm. Res. 2022;182:106347. doi: 10.1016/j.phrs.2022.106347. [DOI] [PubMed] [Google Scholar]
- 551.Patterson H, Nibbs R, McInnes I, Siebert S. Protein kinase inhibitors in the treatment of inflammatory and autoimmune diseases. Clin. Exp. Immunol. 2014;176:1–10. doi: 10.1111/cei.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 552.Choy EH. Clinical significance of Janus Kinase inhibitor selectivity. Rheumatology. 2019;58:953–962. doi: 10.1093/rheumatology/key339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553.Lin CM, Cooles FA, Isaacs JD. Basic mechanisms of JAK inhibition. Mediterr. J. Rheumatol. 2020;31:100–104. doi: 10.31138/mjr.31.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Cook N, Hansen AR, Siu LL, Abdul Razak AR. Early phase clinical trials to identify optimal dosing and safety. Mol. Oncol. 2015;9:997–1007. doi: 10.1016/j.molonc.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 555.Pérez-Jeldres T, et al. Targeting cytokine signaling and lymphocyte traffic via small molecules in inflammatory bowel disease: JAK inhibitors and S1PR agonists. Front. Pharm. 2019;10:212. doi: 10.3389/fphar.2019.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.Sabino J, Verstockt B, Vermeire S, Ferrante M. New biologics and small molecules in inflammatory bowel disease: an update. Ther. Adv. Gastroenterol. 2019;12:1756284819853208. doi: 10.1177/1756284819853208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.Chen CX, et al. JAK-inhibitors for coronavirus disease-2019 (COVID-19): a meta-analysis. Leukemia. 2021;35:2616–2620. doi: 10.1038/s41375-021-01266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 558.Kleppe M, et al. Dual targeting of oncogenic activation and inflammatory signaling increases therapeutic efficacy in myeloproliferative neoplasms. Cancer Cell. 2018;33:29–43.e27. doi: 10.1016/j.ccell.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 559.Pastore F, et al. PRMT5 inhibition modulates E2F1 methylation and gene-regulatory networks leading to therapeutic efficacy in JAK2(V617F)-mutant MPN. Cancer Discov. 2020;10:1742–1757. doi: 10.1158/2159-8290.CD-20-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560.Blair HA. Fedratinib: first approval. Drugs. 2019;79:1719–1725. doi: 10.1007/s40265-019-01205-x. [DOI] [PubMed] [Google Scholar]
- 561.Talpaz M, Kiladjian JJ. Fedratinib, a newly approved treatment for patients with myeloproliferative neoplasm-associated myelofibrosis. Leukemia. 2021;35:1–17. doi: 10.1038/s41375-020-0954-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562.Zhang W, et al. Mutant FLT3: a direct target of sorafenib in acute myelogenous leukemia. J. Natl. Cancer Inst. 2008;100:184–198. doi: 10.1093/jnci/djm328. [DOI] [PubMed] [Google Scholar]
- 563.Fridman JS, et al. Preclinical evaluation of local JAK1 and JAK2 inhibition in cutaneous inflammation. J. Invest. Dermatol. 2011;131:1838–1844. doi: 10.1038/jid.2011.140. [DOI] [PubMed] [Google Scholar]
- 564.Al-Bawardy B, Shivashankar R, Proctor DD. Novel and emerging therapies for inflammatory bowel disease. Front. Pharm. 2021;12:651415. doi: 10.3389/fphar.2021.651415. [DOI] [PMC free article] [PubMed] [Google Scholar]