Abstract
Pork is one of the main meats consumed by people, and its nutritional value is closely related to human health. The lipid deposition and composition of pork not only affect the sensory quality but also determine the nutritional quality of pork. The lipids in pork include triglycerides (TAG) and a small amount of cholesterol and phospholipids. TAG are the main lipids in skeletal muscle fat, which is divided into intermuscular fat and intramuscular fat (IMF). In addition to TAG, IMF also contains phospholipids, which are important factors affecting pork flavour. There are three types of fatty acids in TAG: saturated fatty acids (SFA), monounsaturated fatty acids (MUFA), and polyunsaturated fatty acids (PUFA). PUFA, such as n-3 PUFA, have a beneficial effect on health, including the regulation of whole-body energy metabolism and protection against cardiovascular diseases. Therefore, regulating lipid deposition, especially the fatty acid composition, in pork is important for improving the nutritional quality for human health. Notably, several strategies, such as breeding, environmental control, and the nutritional regulation of lipid composition and deposition in pork, have been studied. More recently, faecal transplantation, molecular design breeding and non-coding RNA have been studied and proven useful for regulating lipid deposition in pigs. In this review, we mainly summarized and discussed the research findings to date on the lipid composition and regulation mechanisms of fatty acid deposition and provide new insights into efficient means of improving the lipid composition and lipo-nutritional quality of pork.
Keywords: Fatty acid composition, Intramuscular fat, Dietary factor, Gut microbiota, Non-coding RNA
Graphical abstract
1. Introduction
Pork provides energy and nutrients to the body, and the quality of pork plays an important role in human health. There are two main ways to evaluate the quality of pork: sensory quality and nutritional quality. The evaluation indices of sensory quality include colour, tenderness, pH value, drip loss, flavour, marbling score, and juiciness (Listrat et al., 2016). The marbling score is an important index that reflects intramuscular fat (IMF), which can improve the juiciness, tenderness, and flavour of the meat. The content of IMF is highly correlated with the sensory acceptability of pork (Poklukar et al., 2020). When the IMF content is moderately increased in the range of 2.5% to 3.5%, the flavour and juiciness of pork are enhanced and the impact on consumer acceptability is favourable (Fernandez et al., 1999). Fortin et al. (2005) demonstrated that IMF content is significantly correlated with tenderness (e.g., average shear force, softness, initial tenderness and chewiness). Wu et al. (2022) found that certain fatty acids such as oleic acid (C18:1 n-9), docosahexaenoic acid (C22:6 n-3, DHA) and α-linolenic acid (C18:3 n-3, ALA) might be the precursors to pork flavour, and their contents contribute to the rich aroma of native Chinese pork. Moreover, the IMF content and sensory quality affect consumers’ acceptability and the market value of pork.
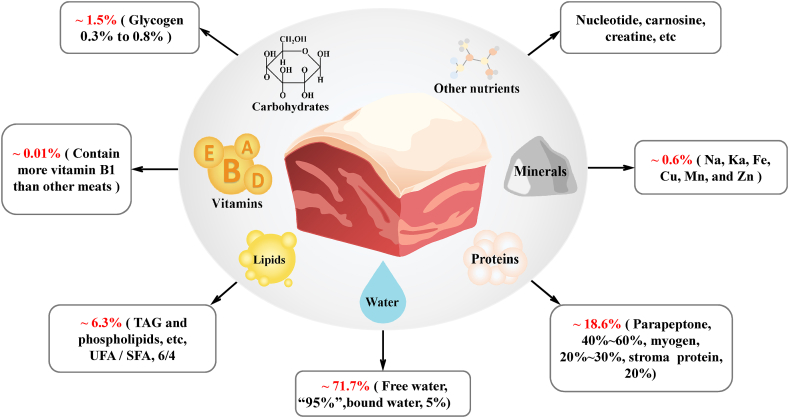
Pork contains many valuable nutrients (Fig. 1), such as biological proteins, lipids, fatty acids, mineral substances, and vitamins (Pereira and Vicente, 2013). The proteins and their building blocks, amino acids, that are found in pork can provide essential amino acids for the human body (Wu et al., 2014). Pork is rich in vitamin B12 and other multivitamins, and vitamin B12 is an important enzyme for amino acid metabolism in the human body. Pork contains several minerals, including zinc, selenium, phosphorous and iron. Haem-iron is a form of iron and is only present in animal foods. Haem-iron promotes certain biological functions involved in the growth and development of children (Pereira and Vicente, 2013). In addition, fat content is an important source of fatty acids in the human diet and is also a considerable factor affecting sensory quality. There are three kinds of fatty acids: saturated fatty acids (SFA), monounsaturated fatty acids (MUFA), and polyunsaturated fatty acids (PUFA). Excessive SFA intake might be associated with obesity, insulin resistance and cardiovascular disease (Li et al., 2019). Interestingly, it is worth noting that some types of fatty acids, especially n-3 fatty acids, are beneficial for human health and protect humans against vascular diseases, cancer, and rheumatoid arthritis (Reyes et al., 2004). In addition to fatty acids, many lipids that have profound effects on human health, such as glycerides, sphingolipids, and glycerophospholipids (Sun et al., 2020; Xu et al., 2021b, 2021c).
Fig. 1.
Chemical composition of lean pork (muscle). Pork muscle tissue contains various chemical components, including water (approximately 71.7%, mainly divided into free water [approximately 95%] and bound water [approximately 5%]), protein (approximately 18.6%, mainly divided into parapeptone [approximately 40% to 60%], myogen [approximately 20% to 30%], and stromal protein [approximately 20%]), lipids (approximately 6.3%, mainly containing TAG and phospholipids, etc., and the ratio of UFA/SFA ≈ 6/4), carbohydrates (approximately 1.5%), vitamins (approximately 0.01%), minerals and other nutrients (mainly nucleotides, carnosine, creatine, etc.). TAG = triglyceride; UFA = unsaturated fatty acids; SFA = saturated fatty acids (Anna Vincent et al., 2020; Yang, 2019).
In recent years, an increasing number of studies have focused on mechanisms that improve the fatty acid composition in pork and developing pork with special nutritional value. Many studies have indicated that fatty acid composition can be regulated in multiple ways. Ren et al. (2020) found that myostatin can act through the MEF2C/miR222/stearoyl-CoA desaturated enzyme 5 (SCD5) cascade to influence fatty acid desaturation. Li et al. (2018) demonstrated that the PUFA contents in pork were significantly increased in fatty acid desaturase 1 (FAT1) knock-in pigs. The researchers transplanted Lactobacillus johnsonii into Duroc × Landrace × Yorkshire (DLY) pigs and found that the SFA content in muscle was significantly increased (Ma et al., 2022). Wang et al. (2020b) identified that intramuscular fat deposition associated long non-coding RNA 1 (IMFlnc1) and miR199a5p regulate IMF accumulation in pigs. In addition, the sex of the pig, the age at slaughter and the temperature of the farm are all factors that influence the fatty acid composition (Guo et al., 2022; Xia et al., 2023; Zhou et al., 2022). In this review, we summarized and discussed the recent findings on lipid composition and the mechanisms and strategies involved in regulating fatty acid composition and deposition in pork.
2. The lipid composition in pork
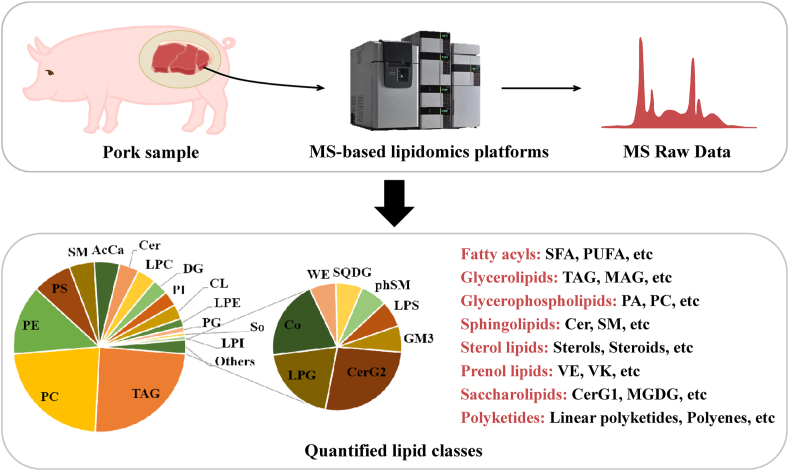
The lipid composition and lipidomic profiles in pork have been recently studied using liquid chromatography tandem mass spectrometry (LC‒MS/MS). Xu et al. (2021b) detected more than 568 different lipid species in skeletal muscle, including 139 triglycerides (TAG), 130 phosphatidylcholines (PC), 75 phosphatidylethanolamines (PE), 42 phosphatidylserines (PS) and other lipid classes (Fig. 2). The quantities of these lipid species have been recently studied, including TAG (approximately 833.3 nmol/mg total lipids), PC (approximately 224.4 nmol/mg total lipids), PE (approximately 41.7 nmol/mg total lipids), and PS (approximately 21.6 nmol/mg total lipids) (Dannenberger et al. (2022)). TAG is the major lipid in pork and is composed of glycerol esterified with three long-chain fatty acids (LCFA) residues. PC, also called lecithin, is the major phospholipid in eukaryotic cell membranes and maintains the integrity of cell membranes (McMaster, 2018). PC is also involved in the formation of nerve myelin (Paoletti et al., 2011) and in the secretion of very low density lipoprotein by hepatocytes (van der Veen et al., 2017). PE is another major phospholipid in eukaryotic cell membranes and has been reported to enhance membrane fusion and the molecular folding of some membrane proteins as a lipid chaperone (Patel and Witt, 2017). The major phospholipids in the endoplasmic reticulum (ER) are PC and PE. An increase in the PC/PE ratio triggers the unfolded protein response (UPR), which promotes apoptosis (Patel and Witt, 2017). PS is an essential inner membrane phospholipid that is synthesized by PC and PE through a “base exchange” reaction catalysed by PS synthase 1 and PS synthase 2, respectively. PS is involved in the formation of protein recruitment sites required for the activation of several key signalling pathways, and it plays a key role in apoptotic cell death (Szondy et al., 2022). In conclusion, phospholipids play an important role in generating secondary messengers for regulating cell division, autophagy, apoptosis, and metabolism. However, the specific functions and pathways of action of these phospholipids and lipokines are not particularly clear and need to be further investigated.
Fig. 2.
Distribution of the lipid classes in pork, as detected by LC‒MS/MS. LC-MS/MS = liquid chromatography tandem mass spectrometry; SFA = saturated fatty acids; PUFA = polyunsaturated fatty acids; TAG = triglyceride; MAG = monoradylglycerols; PA = phosphatidic acid; PC = phosphatidylcholine; Cer = ceramides; SM = sphingomyelin; VE = vitamin E; VK = vitamin K; CerG1 = monogylcosylceramide; MGDG = monogalactosyldiacylglycerol; SQDG = sulfoquinovosyldiacylglycerol; SO = sphingosine; SM = sphingomyelin; PS = phosphatidylserine; PI = phosphatidylinositol; PHSM = phytosphingosine; PG = phosphatidylglycerol; PE = phosphatidylethanolamine; PC = phosphatidylcholine; CL = cardiolipin; LPS = lysophosphatidylserine; LPG = lysophosphatidylglycerol; LPE = lysophosphatidylethanolamine; LPC = lysophosphatidylcholine; DG = diglyceride; CERG2 = simple glc series; WE = wax exters; CER = ceramides; ACCA = acyl carnitine; LPI = lysophosphatidylinositol; CO = coenzyme.
There are more than twenty types of fatty acids in pork. SFA, such as lauric acid (C12:0) and myristic acid (C14:0), can affect the ability of plasma lipoproteins to carry cholesterol. This leads to the deposition of cholesterol in the inner wall of the arteries, which makes the human body prone to various cardiovascular diseases. It is necessary to replace total saturated fat with healthier energy sources since higher dietary intakes of SFA are positively correlated with an increased risk of coronary heart disease (Zong et al., 2016). However, this does not mean that we should completely boycott all SFA because some SFA have positive physiological functions. For example, stearic acid (C18:0) can lower serum cholesterol levels (Cohn et al., 2010). MUFA, mainly oleic acid (C18:1 n-9), are widespread in many plant oils and animal-derived fats. Bermudez et al. (2011) showed that oleic acid (C18:1 n-9) has a mild effect on lowering blood pressure. PUFA are mainly n-3 and n-6 PUFA, both of which have important biological functions in mammalian health. The n-6 PUFA include linoleic acid (C18:2n-6, LA), γ-linolenic acid (C18:3 n-6, GLA), and arachidonic acid (C20:4 n-6, AA), and the n-3 PUFA include ALA, eicosapentaenoic acid (C20:5 n-3, EPA), and DHA. ALA and LA are the precursors of n-3 group and n-6 group PUFA, respectively. They are then derivatized in vivo through a series of carbon chain extension and desaturation processes to produce other PUFA. The intake of moderate amounts of n-3 or n-6 PUFA has been shown to be associated with a relative reduction in the risk of cardiovascular disease (Calder, 2015; Watanabe and Tatsuno, 2017).
There are three types of fatty acid desaturases present in humans: delta-9 (Δ-9), delta-6 (Δ-6), and delta-5 (Δ-5). The Δ-9 desaturase, commonly known as SCD, converts SFA into MUFA. However, humans lack the delta-12 (Δ-12) and delta-15 (Δ-15) desaturases, which are functional for the de novo synthesis of LA and ALA (precursors of n-6 and n-3 group fatty acids). With the presence of Δ-5 and Δ-6 desaturases, LA and ALA can be converted to other n-3 and n-6 groups of fatty acids in vivo, albeit with an inefficiency rate of less than 5% (Jaturasitha et al., 2016; Ma et al., 2016). In contrast to ruminants, pork has a favourable balance of approximately 0.58 to 0.61 between PUFA and SFA, and the fatty acid composition of pork is more easily altered via the diet (Jaturasitha et al., 2016). Overall, decreasing the content of SFA and increasing the content of UFA have become the trend of improving the nutritional quality of pork.
3. The regulatory mechanisms of fatty acid deposition
According to the number of carbon atoms in their hydrocarbon chains, fatty acids are generally divided into three categories: short-chain fatty acids (SCFA), medium-chain fatty acids (MCFA) and LCFA. The different chain lengths of fatty acids also lead to different absorption and digestion rates of fatty acids. In mammals, SCFA and MCFA, with aliphatic tails of fewer than 13 carbons, are absorbed faster than LCFA. They can be directly absorbed by small intestinal mucosal cells and pass through the portal vein to the liver for metabolic energy. LCFA, with aliphatic tails of more than 12 carbons, are absorbed and metabolized slowly in the body, which means they easily accumulate in the liver and fat tissue. LCFA require fatty acid transporters to enter small intestinal mucosal cells and can be used to synthesize TAG. TAG are packaged into chylomicrons, which enter the bloodstream through the lymphatic system (Buttet et al., 2014; Xu et al., 2021a). Therefore, the aim of this section in this review is to highlight recent discoveries related to the synthesis, transportation, and degradation of LCFA, particularly exploring the mechanisms involved in fatty acid deposition.
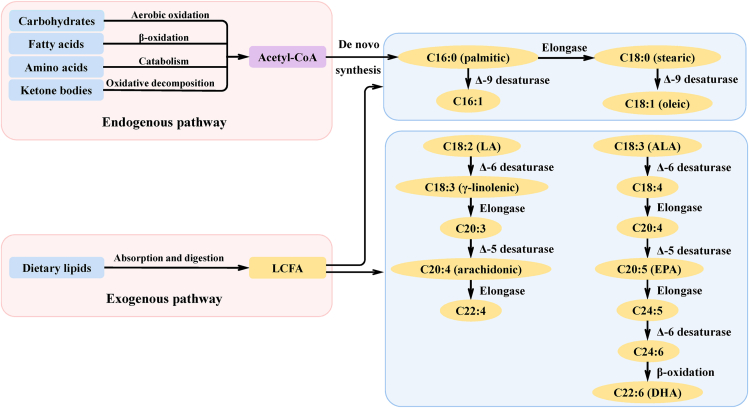
In animals, fat accumulation is the result of an imbalance between synthesis and degradation. When the synthesis of fatty acids is greater than the consumption, fatty acids will be deposited in cells instead of being mobilized to provide energy. The synthesis of fatty acids can be divided into endogenous and exogenous pathways (Fig. 3).
Fig. 3.
Sources of long-chain fatty acids and synthesis of long-chain unsaturated fatty acids in mammals. LCFA = long-chain fatty acids; LA = linoleic acid; ALA = α-linolenic acid; EPA = eicosapentaenoic acid; DHA = docosahexaenoic acid.
Endogenous fatty acids are lipids that are synthesized de novo from intracellular acetyl-CoA. The sources of intracellular acetyl-CoA are mainly the aerobic oxidation of carbohydrates, β-oxidation of fatty acids, catabolism of certain amino acids, and oxidative decomposition of ketone bodies. The main end product of this fatty acid synthesis pathway is palmitic acid (16:0), which can be further extended to stearic acid (C18:0). Both palmitic acid (C16:0) and stearic acid (C18:0) can be converted to MUFA, forming palmitoleic acid (16:1 n-9) and oleic acid (18:1 n-9), respectively. Oleic acid (C18:1 n-9) is the main fatty acid in mammals. Exogenous fatty acids are mainly obtained from dietary lipids, which primarily exist in circulation. They can also be digested and absorbed and used in the synthesis of TAG and phospholipids.
The main method of fat degradation is β-oxidation. β-oxidation is the conversion of free fatty acids into energy, and it occurs in mitochondria (Schenk et al., 2008). The main rate-limiting enzyme for this process is carnitine palmitoyltransferase 1 (CPT1). In recent years, the processes related to oxidation have been studied more clearly, and scientists are likely to pay more attention to the process of fatty acid synthesis. Hence, the aim of this section is to highlight recent discoveries related to the synthesis of TAG, the extension and desaturation of fatty acids, and the transmembrane transport of fatty acids.
3.1. Synthesis of TAG
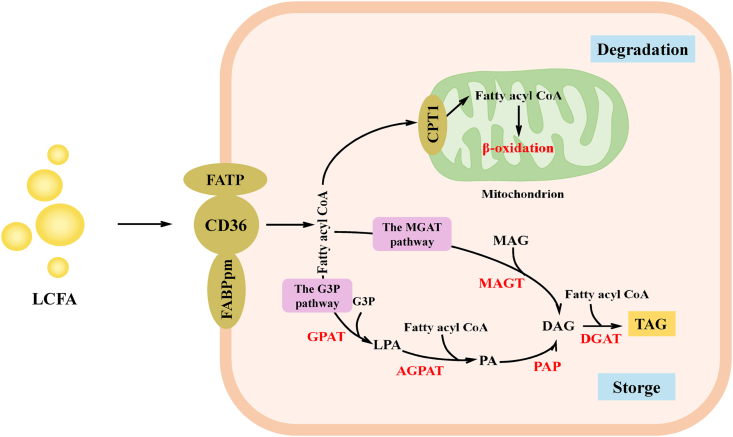
Meat lipids primarily consist of phospholipids and TAG. Phospholipids are structural lipids that constitute the main substance of cell and organelle membranes. TAG are rich in SFA, while phospholipids contain more PUFA (Jaturasitha et al., 2016). After the fatty acids are absorbed by the cells, they are mainly used for β-oxidation in the mitochondria, and the excess fatty acids are used for storage as TAG (Fig. 4). The glycerol 3 phosphate (G3P) pathway and the monoacylglycerol acyl transferase (MGAT) pathway are two crucial ways to synthesize TAG in animals.
Fig. 4.
Free long-chain fatty acid metabolism in cells. Free long-chain fatty acids in the circulation are taken up by cells via membrane-associated fatty acid-binding proteins (CD36, FATP, and FABPpm). Activated fatty acids (fatty acyl-CoA) are mainly “metabolized” by one of two pathways, β-oxidation in mitochondria or storage in the form of synthetic TAG. There are two pathways of TAG synthesis. The MGAT pathway occurs mainly in small intestinal enterocytes, and the G3P pathway occurs mainly in skeletal cells and hepatocytes. LCFA = long-chain fatty acids; FATP = fatty acid transport proteins; CD36 = FAT = fatty acid translocase; FABPpm = plasma membrane-associated fatty acid-binding protein; CPT1 = carnitine palmitoyltransferase 1; G3P = glycerol-3-phosphate; GPAT = glycerol-3-phosphate acyltransferase; LPA = lysophosphatidate; AGPAT = acylglycerol-3-phosphate acyltransferase; PA = phospholipic acid; DAG = diacylglycerol; PAP = phosphatidic acid phosphatases; DAGT = diacylglycerol acyltransferases; MAG = monoacylglycerol; MGAT = monoacylglycerol acyl transferase; TAG = triglyceride.
In most types of cells (mainly skeletal cells and hepatocytes), the G3P pathway is the vital TAG synthesis pathway that facilitates the addition of three fatty acyl CoA to the G3P backbone at the ER membrane (Wang et al., 2017). Glycerol 3 phosphate acyltransferase (GPAT) catalyses the first step of TAG synthesis, converting G3P and fatty acyl CoA to lysophosphatidate (LPA). Then, acylglycerol 3 phosphate acyltransferase (AGPAT) catalyses the conversion of LPA and fatty acyl-CoA to phosphatidic acid (PA). PA is subsequently converted to diacylglycerol (DAG) by phosphatidic acid phosphatase (PAP) enzymes. The last step of TAG synthesis is catalysed by diacylglycerol acyltransferases (DGAT), which convert DAG into TAG (Wang et al., 2017). GPAT is the rate-limiting enzyme that plays a decisive role in the whole process.
In addition, TAG may also be synthesized by the MGAT pathway. In this pathway, two fatty acyl-CoA are esterified to an sn-2 monoacylglycerol (MAG) backbone in succession by MGAT and DGAT enzymes. TAG synthesis by the MGAT pathway mainly occurs in small intestinal enterocytes in the process of dietary fat absorption (D'Aquila et al., 2016). Both the G3P and MGAT pathways add fatty acids to DAG via DGAT1 or DGAT2 in the final step of TAG synthesis (Yen et al., 2015).
3.2. Transmembrane transport of fatty acids
In recent years, many studies on the cellular uptake of fatty acids have shown that membrane-associated fatty acid-binding protein genes are closely related to promoting and regulating the uptake of fatty acids by cells. It is widely believed that fatty acids cross cell membranes through protein-mediated mechanisms. Earlier studies have identified some of these fatty acid transporters, including fatty acid translocase/CD-36 (FAT/CD36), plasma membrane fatty acid-binding protein (FABPpm), and a family of fatty acid transport proteins (FATP1–6). FABPpm, FAT/CD36, and FATP-1 have been determined to be induced by specific stimuli.
CD36 is a heavily glycosylated 88-kDa fatty acid translocase, and it is also a type of fatty acid membrane receptor. CD36 can bind to three categories of ligands outside the cells. One of these ligands is LCFA while the others are proteins that contain the structural homologous repeat domains of thrombospondin proteins and molecules that exhibit a pattern consistent with danger or pathogen-associated molecules (Chen et al., 2022b). Moreover, some studies have indicated that CD36 can influence innate and adaptive immune cell fate and activation by linking signals outside cells with intracellular fatty acid metabolism and redox metabolism (Chen et al., 2022b; Ma et al., 2021; Wang et al., 2020a). Qing et al. (2022) found that overexpression of leptin only leads to enhanced lipolysis of pig subcutaneous fat, while lipid accumulation in mesenteric adipose tissue was associated with increased levels of CD36 expression. Cui et al. (2022) found that dietary supplementation with MCFA promotes lipid deposition in adipose tissue by increasing the expression level of CD36.
FABPpm is a 40-kDa plasma membrane-associated fatty acid-binding protein that can regulate fatty acid transport into skeletal muscle cells (Nickerson et al., 2009). Heart fatty acid-binding protein (H-FABP), also known as FABP3, belongs to the family of intracellular fatty acid-binding proteins that are involved in the transport of LCFA. Shang et al. (2019) found that the mRNA and protein expression levels of H-FABP in back fat, longissimus dorsi muscle (LD) and liver tissues was significantly higher in Tibetan pigs (with high IMF) than in Large White pigs (with low IMF).
FATP is a family of 63- to 70-kDa fatty acid transport proteins, which are classified as members of the solute carrier 27 protein family according to their functions in the transport of exogenous fatty acids. In different fatty acid-metabolizing tissues and cells, different members of the FATP family exhibit specific characteristic expression patterns. FATP1 has been found in the cells of myocardial, skeletal muscle, and adipose tissue. It is the main FATP family member in adipose tissue and plays a very important role in fatty acid metabolism (Pohl et al., 2004). FATP4 is most closely related to FATP1, and it is important for LCFA absorption in the small intestine (Stahl et al., 1999). However, FATP2, FATP3, FATP5, and FATP6 are distantly related to FATP1 and show little or no expression in adipose tissue and muscle (Pohl et al., 2004). FATP6 is the major FATP family member in the heart and is specifically expressed in this organ (Gimeno et al., 2003). A previous study investigated the cDNA structure of porcine FATP1 and their results showed that five FATP1 mRNAs, namely FATP1a, FATP1aV, FATP1b, FATP1c and FATP1cV, could be produced by alternative splicing of primary transcripts. The expression of FATP1 mRNA was much higher in masseter and trapezius muscles than in LD gluteus medius and adipose tissue in pigs (Shu et al., 2009).
3.3. Extension and desaturation of fatty acids
Fatty acid desaturase and elongation of very long-chain fatty acid (ELOVL) protein genes also play a key role; studies have demonstrated that ELOVL and aerobic front-end fatty acid desaturases (FADS) are able to biosynthesize long-chain polyunsaturated fatty acids (LC-PUFA) from preexisting C18 PUFA in vertebrates (Castro et al., 2016). To date, eight members of the ELOVL family have been identified, and they show the specificity of fatty acid substrates. For example, ELOVL1, ELOVL3, ELOVL6, and ELOVL7 prefer to act on the substrates of saturated and monounsaturated fatty acids, while ELOVL2, ELOVL4, ELOVL5, and ELOVL8 act on the substrates of PUFA (Ferraz et al., 2022).
Based on subcellular location, fatty acid desaturases are divided into soluble desaturases and membrane-bound desaturases (Nakamura and Nara, 2004). Stearoyl-ACP desaturase is the only soluble desaturase that only exists in plants. By comparison, membrane-bound desaturases generally exist in eukaryotes and bacteria (Castro et al., 2016). In mammals, the front-end desaturases are named FADS. FADS insert the double bond at a fixed number of carbons from the carboxyl group and are also termed Δ-desaturases. Two vital Δ-desaturases are present in the bioconversion of LA and ALA into longer-chain PUFA: Δ-5 desaturase and Δ-6 desaturase. Δ-5 desaturase is encoded by the FADS1 gene, while the FADS2 gene is responsible for Δ-6 desaturase (Tian et al., 2022). Taniguchi et al. (2015) identified a novel isoform FADS named FADS1b in pigs, and they also found FADS1b and FADS3 isoforms in porcine were predominantly expressed in the inner layer of the subcutaneous adipose tissue.
SCD is also vital in fatty acid metabolism, and it is responsible for the biosynthesis of MUFA from SFA. There are two SCD homologues (SCD1 and SCD5) in humans and four SCD homologues (SCD1, 2, 3, and 4) in mice (Bai et al., 2015). Previous studies showed that liver-specific SCD1 deficiency activates rapamycin complex 1 (mTORC1), induces peroxisome proliferator-activated receptor gamma coactivator 1α (PGC1α) and ER stress, and activates the UPR in vivo mouse models (Aljohani et al., 2019). SCD is a candidate gene for fatty acid composition. Viterbo et al. (2018) identified a significant quantitative trait locus on SSC14 (120 to 124 Mb) in Duroc pigs. Liu et al. (2020) found that SCD and lipogenesis-related genes were induced significantly in differentiated porcine adipocytes, suggesting SCD is also essential for porcine adipocyte differentiation. Moreover, they also revealed that the expression level of SCD in adipose tissue of 7-day-old Bama piglets was much higher than that in adipose tissue of 4-month-old Bama adult pigs.
4. The regulatory role of different factors in regulating fatty acid composition
4.1. Breed difference
There were significant differences in fatty acid composition in different breeds and strains. Analysing the fatty acid composition of different breeds of pigs is of great importance for the application of heterosis. In a previous report by Chen et al. (2022a), their research compared the fatty acid composition of the LD from five breeds, including Jiaxing Black Pig (JBP), Berkshire, Berkshire × JBP (BJBP), Duroc × Berkshire × JBP (DBJBP), and Duroc × Landrace × JBP (DLJBP). The highest PUFA content was found in Berkshire pigs, and the lowest PUFA content was found in JBP pigs. However, it is worth noting that C22:6 was the most abundant PUFA in JBP pigs. Yu et al. (2013) assessed the fatty acid composition of the LD between Lantang and Landrace pigs and found that the proportion of PUFA, including C18:2, C20:2, C20:3, C20:4, and C22:6, in Lantang pigs was significantly higher than that in Landrace pigs. This study also identified the SCD gene as a key gene for increasing PUFA levels. This finding indicates that Lantang pigs have better pork flavour intensity. Touma and Oyadomari (2020) compared the meat quality of Okinawa Agu pigs with those of Large White × Landrace (WL) pigs crossbred with Agu sires (WLA) or Duroc sires (WLD) and found that Agu had the highest proportion of SFA. WLA had the highest proportion of MUFA, and WLD had the highest proportion of PUFA. Huang et al. (2020) compared the fatty acid composition profiles among three Chinese indigenous pig breeds, including Bamaxiang, Erhualian, and Laiwu pigs. They stated that Bamaxiang had a relatively low SFA content and a high PUFA content, including C18:1, C20:1, C20:2, C20:3, and C20:4. Tu et al. (2021) screened for fatty acid differences in Chinese indigenous pork and found that the PUFA level was lower in Dongbei Min pigs and Wuzhishan pigs than in Beijing Black pigs and Duroc × Landrace × Yorkshire pigs.
4.2. Gene editing
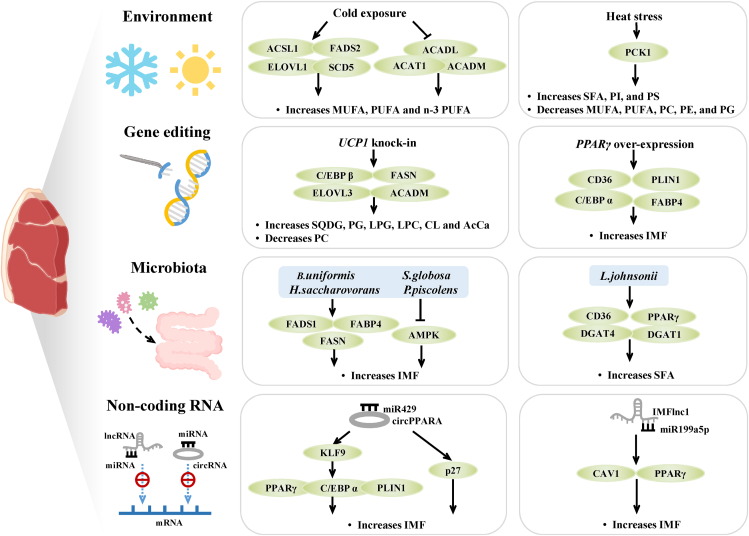
In addition to the common hybrid breeding method, molecular breeding is another famous and popular method in swine breeding (Fig. 5). With the development of clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated 9 (Cas9) technologies, genome editing technology can shorten breeding cycles and reduce production costs. These effects greatly promote the application of molecular breeding technology in improving pork quality. Li et al. (2018) found that using the CRISPR/Cas9 system to insert the FAT1 gene of Caenorhabditis elegans into the porcine Rosa 26 locus can significantly increase the level of PUFA in pigs. The levels of ALA, DHA, and docosapentaenoic acid (DPA) were all significantly increased, and the ratio of n-6/n-3 PUFA was greatly decreased. Gu et al. (2021) established a model of peroxisome proliferator-activated receptor-γ (PPARγ)-overexpressing pigs and found that PPARγ-overexpressing pigs had increased IMF and increased expression levels of adipocyte differentiation genes, including fatty acid-binding protein 4 (FABP4), perilipin 1 (PLIN1), CCAAT/enhancer-binding protein (C/EBP) and CD36. These findings indicate that overexpression of PPARγ may increase the IMF content by promoting the differentiation of muscle preadipocytes. Uncoupling protein 1 (UCP1) is a mitochondrial inner membrane carrier that dissipates energy in the form of heat by transferring protons in ATP synthesis, but pigs are deficient in the functional UCP1 gene. Using the CRISPR/Cas9 system, Pan et al. (2019) constructed adipocyte-specific UCP1 knock-in pigs to study the effect of UCP1 in pigs. The results showed that UCP1 knock-in pigs had significantly increased levels of fatty acyls and saccharolipids, including sulfoquinovosyldiacylglycerols (SQDG), phosphatidylglycerols (PG), lysophosphatidylglycerols (LPG), lysophosphatidylcholines (LPC), acyl carnitines (AcCa) and cardiolipins (CL), possibly through the upregulation of the C/EBPβ, FASN, ELOVL3, and medium-chain acyl-Coenzyme A dehydrogenase (ACADM) genes. Notably, Xu et al. (2021b) investigated the effects of UCP1 knock-in on lipid metabolism in pigs. This result shows that UCP1 also caused elevated levels of sphingolipids and cardiolipin in inguinal white adipose tissue. The increase in the total sphingolipid level may be caused by a higher level of plasma free fatty acids in the circulation. Elevated levels of cardiolipin may be associated with increased mitochondrial function.
Fig. 5.
The regulatory effects of environmental factors, gene editing, gut microbiota transplantation and non-coding RNA on fatty acid composition. SFA = saturated fatty acids; MUFA = monounsaturated fatty acids; PUFA = polyunsaturated fatty acids; PCK1 = phosphoenolpyruvate carboxykinase 1; PI = phosphatidylinositol; PS = phosphatidylserine; PC = phosphatidylcholine; PE = phosphatidylethanolamine; PG = phosphatidylglycerol; ACSL1 = long chain acyl-CoA synthetase 1; FADS2 = fatty acid desaturases 2; ELOVL1 = elongation of very long-chain fatty acids protein 1; SCD5 = stearoyl-CoA desaturated 5; ACADL = long-chain acyl-Coenzyme A dehydrogenase; ACAT1 = acetyl-CoA acetyltransferase 1; ACADM = medium-chain acyl-Coenzyme A dehydrogenase; UCP1 = uncoupling protein 1; C/EBPβ = CCAAT/enhancer-binding protein β; FASN = fatty acid synthase; ELOVL3 = elongation of very long-chain fatty acids protein 3; SQDG = sulfoquinovosyldiacylglycerols; PG = phosphatidylglycerols; LPG = lysophosphatidylglycerols; LPC = lysophosphatidylcholines; CL = cardiolipins; AcCa = acyl carnitines; PPARγ = peroxisome proliferator-activated receptor-γ; C/EBP α = CCAAT/enhancer-binding protein α; FABP4 = fatty acid-binding protein 4; PLIN1 = perilipin 1; IMF = intramuscular fat; CD36 = FAT = fatty acid translocase; FADS1 = fatty acid desaturases 1; AMPK = adenosine monophosphate-activated protein kinase; DGAT1 = diacylglycerol acyltransferases 1; DGAT4 = diacylglycerol acyltransferases 4; KLF9 = kruppel like factor 9; CAV1 = caveolin1; IMFlnc1 = intramuscular fat deposition associated long non-coding RNA 1.
4.3. Environmental factors
The environment is another factor affecting the lipid content (Fig. 5). Heat stress affects lipid metabolism by decreasing β-oxidation and lipolytic enzyme activities. This ultimately leads to porcine adipose tissue-specific reactions and changes in fatty acid content. Qu and Ajuwon found that heat stress increased de novo adipogenesis and TAG storage in porcine adipocytes through the phosphoenolpyruvate carboxykinase 1 (PCK1) pathway, thereby altering fat deposition and fatty acid composition (Qu and Ajuwon, 2018a, 2018b). The SFA content in heat-stressed pigs was increased, and the MUFA and PUFA contents were decreased in these pigs. Heat stress also increased phosphatidylinositol (PI) and PS and decreased PC, PE, and PG. Heng et al. (2019) indicated that heat stress improves the expression of lipid metabolism genes, including fatty acid synthase (FASN), DGAT1, PPARγ, SREBP1c, and FABP4, in the abdominal fat of pigs. Notably, heat stress also affects the mRNA expression of N6-methyl-adenosine (m6A)-related enzymes, such as Wilms’ tumour 1-associating protein, methyltransferase like 14, and obesity-associated protein. This indicates that heat stress might also regulate foetal programming through m6A RNA methylation. Cold expression corresponds to heat stress. Recent studies have indicated that fatty acid levels vary in some tissues when fattened pigs are kept overnight at low temperatures (5–7 °C) (Faure et al., 2013). In skeletal muscle, the contents of SFA, MUFA, PUFA, and n-3 fatty acids were increased, but the proportion of these three fatty acids (SFA, MUFA and PUFA) did not change significantly (Xu et al., 2021c). RNA-seq results suggested that cold exposure significantly increased the expression of fatty acid biosynthesis genes, including long chain acyl-CoA synthetase 1 (ACSL1), FADS2, ELOVL1, and SCD5, and decreased the expression of mitochondrial beta-oxidation genes, including ACADM, long-chain acyl-Coenzyme A dehydrogenase (ACADL), acetyl-CoA acetyltransferase 1 (ACAT1), and long chain acyl-CoA synthetase 4 (ACSL4) (Xu et al., 2021c). In addition to regulating the ambient temperature, the duration of light exposure is also one of the important factors affecting lipid metabolism. However, there are no studies on the effects of light exposure on the fatty acid composition in pork at present. Some researchers found that in rats, continuous light was associated with circadian-clock disruptions that affect the gut microbiota and fatty acid metabolism, ultimately regulating fatty acid profiles (Chu et al., 2019). Yue et al. (2022) showed a decrease in the total amount of MCFA in the colon contents of rats under continuous light.
4.4. Sex and age
Sex is also a major factor affecting the carcass and meat quality of pigs. Xia et al. (2023) showed that castrated male pigs had higher contents of C18:0 and SFA and lower contents of C20:1 and UFA than female pigs in a novel Duroc line pig from the crossbred by French line, American line and Canadian line. Suárez-Belloch et al. (2015) indicated that in crossbred pigs from Large White, Landrace and Duroc pigs, barrows had higher values of SFA in both the IMF and subcutaneous fat. Franco et al. (2014) also reported that castrated males had more SFA (C16:0 and C18:0) and total MUFA contents than gilts in Landrance and Duroc pigs, although these differences were not significant. Moreover, they suggested that the reason for the high SFA content in castrated males may be the high intramuscular and subcutaneous fat contents. The effect of sex on fatty acid composition in pork is not fully understood, and this still deserves further research.
Researchers have also recently investigated whether pork quality would be improved with age. Guo et al. (2022) found that in crossbred (Duroc × Long White × Yorkshire) fattening pigs, the content of ALA is higher in older pigs, but the contents of SFA, MUFA and PUFA were not different. Since ALA is a major essential fatty acid that plays an important role in organic metabolism, this suggests that the nutritional value of pork may also increase with age.
4.5. Non-coding RNA
The regulatory role of non-coding RNA in the organism has been more intensively studied, such as long noncoding RNA (lncRNA), circular RNA (circRNA) and micro-RNA (miRNA). Among them, miRNA plays an important role in regulating adipogenesis, while lncRNA and circRNA mainly indirectly regulate adipogenesis by acting as sponges of miRNA and preventing the binding of target genes (Fig. 5).
Wang et al. (2020b) identified that IMFlnc1 and caveolin1 (CAV1) are both of the target genes of miR199a5p. They revealed that IMFlnc1 sponges miR199a5p, which reduces the suppression of miR199a5p in CAV1 and upregulates CAV1 and PPARγ to promote IMF accumulation. Long intergenic non-coding RNA (lincRNA) are a class of lncRNA, located between genes that encode proteins, which are important regulators and can be involved in various biological processes. Li et al. (2020) identified 22 lincRNA differentially expressed between Wei (fat breed) and Yorkshire (lean breed) pigs. They found that some lincRNAs contribute to the IMF development by regulating their potential target genes.
Circular RNA (circRNA) are closed noncoding RNA molecules. The regulatory mechanisms and functions of circRNA in porcine IMF have been recently studied. Li et al. (2022) identified a novel circRNA, called circPPARA, whose expression was positively correlated with IMF. They found that the upregulation of circPPARA promotes IMF accumulation by adsorbing miR429 and miR200b. Peng et al. (2016) found that kruppel like factor 9 (KLF9) and p27 is the target gene of miR429, which could promote IMF. Therefore, the studies of Li et al. (2022) and Peng et al. (2016) collectively suggest that the upregulation of circPPARA can adsorb miR429 as a sponge, which promotes IMF accumulation via the increase of the expression of KLF9 and p27.
miRNA are small non-coding RNA molecules that bind to the 3' untranslated region (UTR) region of the target RNA, thereby regulating gene expression. Studies have shown that many miRNA are involved in the process of adipogenesis. For example, miR125a5p negatively regulates the target gene kruppel like factor 13 (KLF13), which inhibits IMF deposition (Du et al., 2018). Liu et al. (2021) indicated that miR325p could inhibit KLF3 to promote adipocyte differentiation and IMF deposition. Du et al. (2018) also identified that porcine ELOVL6 is a target gene of miR125a5p, and the upregulation of miR125a5p significantly reduced the total SFA contents.
4.6. Gut microbiota
In recent years, with the development of high-throughput 16S rRNA sequencing and metagenomics, many studies have found that intestinal microorganisms can impact lipid metabolism (Fig. 5). Therefore, some emerging technologies, such as faecal microbiota transplantation and microbiota monocolonization, are playing an increasingly important role in the regulation of fatty acid composition and fat deposition. Ma et al. (2022) compared the differences in lipid metabolism and the gut microbiota between Shaziling pigs and Yorkshire pigs, and they found a higher abundance of probiotic bacteria in the gut of Shaziling pigs. Further screening of the microbial species revealed that L. johnsonii was one of the main microbial species responsible for the differences in lipid content, and this species may have a function in promoting lipid absorption and transport. The researchers transplanted L. johnsonii into DLY pigs. The results showed a significant increase in muscle SFA content, possibly through the upregulation of several lipid-related genes, including DGAT1, DGAT2, CD36, and PPARγ. Xie et al. (2022) compared the gut microbiome of Laiwu pigs with that of DLY pigs to investigate the effect of the microbiota on meat quality. They found that transplantation of the microbiota from the faeces of Laiwu pigs to DLY piglets increased IMF content in the LD. In addition, a multi-omics analysis identified that Bacteroides uniformis, Sphaerochaeta globosa, Hydrogenoanaerobacterium saccharovorans, and Pyramidobacter piscolens may be key species in the regulation of lipid metabolism and deposition. They showed that these four species upregulate fatty acid desaturase 1 (FADS1), FABP4, and FASN and downregulate adenosine monophosphate-activated protein kinase (AMPK), thereby enhancing the IMF content in muscle. Wu et al. (2021) transplanted faecal microbiota from Jinhua and Landrace pigs into mice to verify the effect of the gut microbiota on IMF. They found that mice receiving microbiota from Jinhua pigs had a higher IMF content. Additionally, the expression of lipoprotein lipase (LPL) was increased, and the expression of angiopoietin-like protein 4 (ANGPTL4) was decreased in the gastrocnemius of these mice. The level of SCFA in the colon was reduced. SCFA induce the transcription and secretion of ANGPTL4, which in turn inhibits LPL expression. Therefore, the researchers concluded that the elevation of IMF in these mice was caused by decreasing the abundance of microbiota that can produce SCFA, and the decrease in SCFA content led to a concomitant decrease in ANGPTL4 gene expression. This ultimately promoted LPL expression and fat deposition. Zhao et al. (2022) discovered that archaeal species with methanogenesis functions and butyrate-producing bacterial species have the ability to affect fat deposition and metabolism in Jinhua pigs. This study compared the composition and diversity of the colonic microbiota in high-fat and low-fat Jinhua pigs using metagenomic sequencing. The results demonstrated that the high-fat pigs had a higher abundance of Firmicutes and Tenericutes, and the low-fat pigs had a higher abundance of Ruminococcus, Faecalibacterium, and Oscillibacter. They also found that the expression of FAS, PPARγ, and LPL in abdominal fat was significantly higher in high-fat pigs than in low-fat pigs.
4.7. Nutritional factors
Although molecular breeding or faecal microbiota transplantation can improve the fatty acid composition of pork, none of these techniques can be practically applied in the production line. Notably, an effective way to improve the fatty acid profile is through dietary supplementation, which is also the most acceptable to consumers (Fig. 6).
Fig. 6.
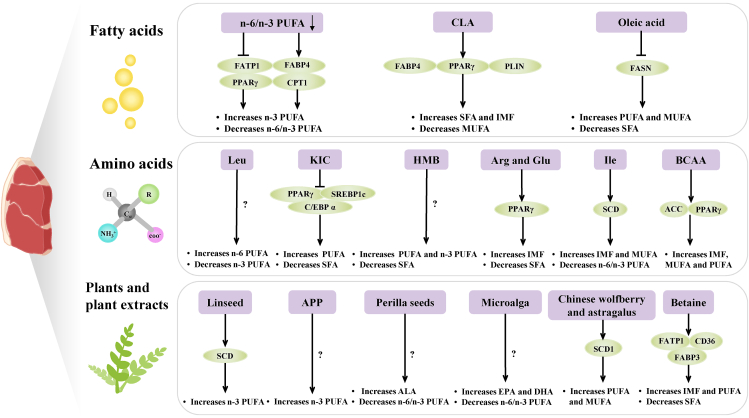
The regulatory role of dietary factors on fatty acid composition. PUFA = polyunsaturated fatty acids; SFA = saturated fatty acids; MUFA = monounsaturated fatty acids; CLA = conjugated linoleic acid; FATP1 = fatty acid transport protein 1; FABP4 = fatty acid-binding protein 4; PPARγ = peroxisome proliferator-activated receptor-γ; CPT1 = carnitine palmitoyltransferase 1; PLIN1 = perilipin 1; FASN = fatty acid synthase; KIC = α-ketoisocaproate; HMB = β-hydroxy-β-methyl butyrate; BCAA = Branched chain amino acids, including Leu, Ile and Val; IMF = intramuscular fat; SCD = stearoyl-CoA desaturated enzyme; APP = apple polyphenols; ALA = α-linolenic acid; DHA = docosahexaenoic acid; EPA = eicosapentaenoic acid; SCD1 = stearoyl-CoA desaturated enzyme 1; FABP3 = fatty acid-binding protein 3; CD36 = FAT = fatty acid translocase; C/EBPα = CCAAT/enhancer-binding protein α; SREBP1c = sterol regulatory element-binding protein 1c; AMPK/mTOR = adenosine monophosphate-activated protein kinase/rapamycin signalling pathway.
4.7.1. Fatty acids
4.7.1.1. The ratio of n-6/n-3 PUFA
Previous studies have shown that the n-6/n-3 PUFA ratio decreases in the LD, subcutaneous adipose tissue (SAT), and longissimus thoracis as the dietary n-6/n-3 PUFA ratio decreases. The n-6/n-3 PUFA ratio in feed can appropriately affect the expression of genes, including PPARγ (Chen et al., 2022), hormone-sensitive lipase (HSL), FABP4, CPT1 (Nong et al., 2020), FATP1 and FATP4 (Li et al., 2015). Therefore, changing the n-6/n-3 ratio in feed is one of the methods to improve meat quality and enhance the deposition of n-3 PUFA in pigs.
4.7.1.2. Conjugated linoleic acid (CLA)
The most commonly used PUFA supplementation is CLA. CLA is a secondary derivative of linoleic acid, which plays an important physiological role in regulating glycolipid metabolism. It affects the immune response, prevents diseases, and improves the quality of animal products. Earlier feeding studies identified that dietary supplementation with CLA can increase the SFA content but decrease the MUFA content in muscle and adipose tissue in pigs (Barnes et al., 2012; Chen et al., 2019; Cordero et al., 2010; Tous et al., 2013). Barnes et al. (2012) reported that the addition of CLA to the diet resulted in changes in the ratio and composition of fatty acids in IMF, and these changes are mainly attributable to the expression of FABP4, ACC and SCD1 and the abundance of Parabacteroides, Bacteroides and Lachnospiraceae_UCG-010. The study by Barnes et al. (2012) also reported that the marbling score tended to increase and the percentage of lipid increased significantly in the CLA-fed pigs.
4.7.1.3. Oleic acid
Oleic acid is a type of MUFA that may affect meat quality and fatty acid composition in muscle.
Martins et al. (2018) have demonstrated that dietary supplementation with high oleic acid can increase the content of MUFA and PUFA, and reduce the SFA content in both femoris and dorsal subcutaneous fat in pigs. They also found high oleic acid diet significantly reduced the expression of FASN, which means less fat is synthesized de novo via the endogenous lipogenic pathway.
4.7.2. Plant extracts
4.7.2.1. Linseed
Linseed is the ripe seed of flax, which contains the precursor ALA. In pigs, most of the deposited n-3 PUFA resulted from the conversion of ALA to EPA and DHA. Some studies have shown that feeding linseed to pigs improves the nutritional quality of pork by raising the n-3 PUFA content in muscle and adipose tissue (Corino et al., 2014; Turner et al., 2014). This result is consistent with earlier study by Kouba et al. (2003), which showed that the changes in fatty acids composition in pigs fed high-linolenic acid could be attributed to a reduction in SCD activity. Wang et al. (2021) indicated that CLA and linseed supplementation increased the IMF content in growing pigs.
4.7.2.2. Perilla seeds
Perilla seeds are an available source of n-3 PUFA. The content of the essential fatty acid ALA in perilla seeds is approximately 52% to 62% (Ahmed, 2018), which is the highest content of ALA in seed oil discovered thus far. It has been discovered that dietary supplementation with perilla cakes significantly increased the content of ALA, whereas the n-6/n-3 PUFA ratio in the backfat, abdominal fat, and LD of growing pigs decreased significantly (Arjin et al., 2021). This result is consistent with the report of Sringarm et al. (2022) that investigated the supplementation of perilla cakes in pigs on a low-lysine diet. Xia et al. (2022a) indicated that the addition of Perilla frutescens seeds to the diet affected the fatty acid composition of Songliao black pigs. It dramatically increased the content of ALA, DPA, EPA, and DHA and reduced the content of SFA.
4.7.2.3. Microalga
Moran et al. (2018) indicated that daily supplementation with DHA-rich microalgae Aurantiochytrium limacinum can also increase the contents of EPA and DHA and decrease the n-6/n-3 PUFA ratio in the Longissimus lumborum in finishing pigs.
4.7.2.4. Chinese herbal medicine
Chinese herbal medicine additives can promote growth and improve the feed conversion rate and meat quality of pigs. Earlier studies indicated that Chinese herbal medicine can improve the content of UFA and essential fatty acids in different pig species (Hu et al., 2018; Shen et al., 2021).
It has been reported that Chinese wolfberry and Astragalus have high medicinal value. They can regulate the content of IMF in pork by affecting the expression of the SCD1 and ELOVL6 genes, which are related to fatty acid metabolism. One study showed that adding wolfberry and Astragalus extract to the diet significantly increased the levels of PUFAs and MUFA, such as palmitic oleic acid, oleic acid, linoleic acid, cis-11-eicosarboxyenoic acid, and cis-11, 14-eicosarboxyenoic acid, in Tibetan fragrant pigs (Hao et al., 2021).
4.7.2.5. Apple polyphenols (APP)
Apple peels and pomace are potential ingredients for pig feed because they contain APP. APP are a group of plant extracts from apple peels and pomace that are the main antioxidants in apples. APP extracts have been shown to improve lipid metabolism and alleviate hepatic steatosis in rats to some extent (Yan et al., 2022). Xu et al. (2019) found that dietary supplementation with 400 mg/kg APP significantly increased the total ALA, DHA and n-3 PUFA levels in the LD in finishing pigs.
4.7.2.6. Betaine
Betaine is a methyl derivative of the amino acid glycine, which is present in most organisms. In animals, betaine can be used as an organic osmoprotectant to protect cells from damage or as a methyl donor by transmethylation. In this way, it can partially replace choline and methionine in feed and take part in lipid metabolism (Huang et al., 2008). Previous studies investigated the mechanism by which betaine can regulate lipid metabolism in pigs. They concluded that betaine might upregulate the FAT/CD36, FATP1, and FABP3 genes. These genes are related to fatty acid transporters and activate the AMPK signalling pathway. Additionally, they upregulate genes related to fatty acid oxidation, including PPARα and CPT1, which affect the composition of muscle fatty acids in pigs (Li et al., 2017a). Zhong et al. (2021) also showed that betaine promotes fatty acid catabolism in skeletal muscle tissue by enhancing the mRNA expression levels of HSL and CPT1β. Some studies have shown that the effects of betaine on fat deposition in different parts of animals are inconsistent; betaine can increase fat oxidation, reduce abdominal fat content, and increase IMF accumulation (Albuquerque et al., 2017; Dong et al., 2020). Furthermore, previous studies found that betaine supplementation increased the PUFA contents and decreased the SFA contents in pig muscle (Dong et al., 2020; Yang et al., 2021; Zhong et al., 2021).
4.7.3. Amino acids
Arg and Glu supplementation have considerable complementary advantages in the pig industry for improving the quality of pork. Recently, research indicated that dietary supplementation with both Arg and Glu can increase IMF deposition and decrease the percentage of SFA in the LD and backfat of pigs (Hu et al., 2017a). Hu et al. (2017b) found that dietary supplementation with Arg and Glu upregulated the mRNA level of PPARγ in LD, leading to an increase in IMF content.
Leu can be converted to acetyl-CoA in vivo and regulates fatty acid metabolism in pigs. Leu and its metabolites α-ketoisocaproate (KIC) and β-hydroxy-β-methyl butyrate (HMB) have been regarded as important feed additives that regulate fatty acid deposition in pork. Dietary Leu supplementation was found to significantly reduce the n-3 PUFA content in the LD. However, dietary KIC and HMB supplementation significantly increased the proportion of total PUFA and decreased the SFA content in the LD (Zhong et al., 2019). In this study, researchers found the expression of PPARγ, SREBP1c and C/EBP α decreased in LD of pigs fed KIC supplemented diets, and hypothesized that HMB supplementation may regulate lipid anabolism and catabolism in oxidized skeletal muscle primarily through the AMPK-mTOR signaling pathway.
The addition of Ile to fattening pig diets stimulates the activity and expression of SCD, increases skeletal muscle lipogenesis, promotes IMF deposition, and promotes the synthesis of MUFA (Luo et al., 2018).
Branched chain amino acids (BCAA), including Leu, Ile and Val, need to be obtained from the diets of mammals. A dietary balance of BCAA in the restricted protein diet can activate acetyl-CoA carboxylase (ACC) and PPARγ via the AMPK-silent information regulator of the transcription 1-PGC1α axis. Additionally, it can reduce mitochondrial biosynthesis in the LD, thereby promoting IMF deposition (Zhang et al., 2021). It has been shown that the addition of BCAA in a 2:1:1 ratio to the restrained protein diet significantly increased the total MUFA content. Moreover, the addition of BCAA in a 2:1:2 ratio significantly increased the ratio of total PUFA and PUFA to SFA, especially increasing the content of EPA and DHA (Zhang et al., 2022).
4.7.4. Carbohydrates
Carbohydrates can serve as important nutrients that have a potentially vital role in lipid metabolism regulation. Carbohydrates are divided into digestible and non-digestible carbohydrates. Digestible carbohydrates include monosaccharides, disaccharides, starches and glycogen. Non-digestible carbohydrates, also known as fibre, include oligosaccharides, resistant starch and non-starch polysaccharides. Yu et al. (2020) found that in pigs fed with a high ratio of dietary amylose, the concentration of C12:0, EPA, DHA and n-3 PUFA in the LD muscle of finishing pigs was increased in response to increasing amylose/amylopectin ratio. They showed that these changes may result from the upregulation of the mRNA expression of lipogenic genes (FAS, LPL and SCD), and down-regulation of CPT1b. Li et al. (2017b) found that a pea starch diet with greater amylose content could decrease back fat depth in finishing pigs. Yang et al. (2015) found that a high amylose content diet is helpful in reducing lipogenesis in muscle.
In addition to starches, there are other carbohydrates that might have effects on growth and health in pigs. Nielsen et al. (2014) suggested that a high level of dietary fibre, either as arabinoxylan or resistant starch, could shift the microbial composition towards butyrogenic species in the faeces and increase the total SCFA and butyrate concentrations in the intestine of pigs. Tan et al. (2021) indicated that using resistant starch as a prebiotic may enhance the gut health of weaned pigs and affect pig growth performance. Nevertheless, the effect of arabinoxylan or resistant starch on lipid metabolism in pigs is not fully understood, and this still deserves further research.
4.7.5. Other nutrients
4.7.5.1. Vitamin E
Vitamin E is a fat-soluble vitamin that is an essential vitamin for pigs. It can be used as an antioxidant to reduce oxidative stimulation, prevent pork fat peroxidation and prolong the fresh shelf life of pork. Vitamin E added to pig diets as a supplement can increase the concentrations of C16:1 and CLA and reduce the contents of C20:0 and C18:0 in backfat and abdominal fat (Wang et al., 2022).
4.7.5.2. Fermented feed
Fermented feeds are also one of the initial factors affecting lipid metabolism. Lu et al. (2021) showed that fermented corn-soybean meal supplementation increased the heptadecanoic acid and EPA but reduced the palmitoleic acid in pigs. Hao et al. (2020) found that dietary supplementation with 8% fermented mixed feed decreased the percentage of SFA and increased the percentage of UFA in LD, and the percentages of C18:1n-9, C18:2n-6, C18:3 n-3, C20:2 and C20:4 n-6 in the LD were also markedly increased in finishing pigs. The effects of fermented feeds on lipid metabolism might be associated with changes in microbiota in pigs. Indeed, Lu et al. (2020) showed that a fermented corn–soybean meal diet significantly increased the percentage of Rikenellaceae and Christensenellaceae but decreased the abundance of Lachnospiraceae in the colonic mucosa-associated microbiota in pigs. Xie et al. (2017) showed that with supplementation of fresh fermented feeds, the relative abundance of butyrate-producing bacteria was higher in both the caecum and colon of piglets. Liu et al. (2023) showed that fermented mixed feed significantly altered the fatty acid profile of the LD of pigs. 10% fermented mixed feed increased the percentage of n-3 PUFA, n-6 PUFA, total PUFA and IMF in the LD and increase the expression of CEBPα, PPARγ and SREBP1 genes which are the key transcription factors for lipid metabolism.
5. Conclusion and perspectives
In recent decades, many studies have found improvements in the lipid composition of pork through dietary supplementation, gene editing, gut microbiota, etc, but there are still many key issues that need to be further studied. First, the comparison of fatty acid composition of different breeds of pigs showed that the fatty acid composition of Chinese indigenous breeds was better than that of typical lean breeds. However, the mechanisms involving in these fatty acid content differences are not clear, and there was no method to use high-quality indigenous breeds to create better hybrid lines. Second, the addition of fish oil, seaweed, and conjugated linoleic acid to pig feed alters the fatty acid content in pork, but some results of different scholars on the regulation of fatty acid content in pork by certain nutrients are inconsistent, revealing that standardization of feed processing and fatty acid detection methods needs to be studied. Third, although some results have been achieved through genetic engineering techniques, including genetic modifications such as overexpression or knockout, most results have been concentrated in animal models. Whether these results can be applied to pork production is still needs to be determined. Fourth, the results of non-coding RNA have increased dramatically in the past three years. However, whether non-coding RNA are useful for regulating lipid deposition and the nutritional value of pork need to be further studied. Fifth, in addition to fatty acids, the regulation of the deposition of other lipids, such as PC and PE, that are beneficial to health still needs to be further studied. Moreover, several lipokines, such as 12,13-dihydroxy-9Z-octadecenoic acid, have been recently identified and have demonstrated crucial roles in metabolic health (Macêdo et al., 2022). However, the content of functional lipokines in pork needs to be determined, and enhancing their content might also improve the lipo-nutritional quality of pork in the future.
Author contributions
Wuzhou Yi: Writing, original draft preparation. Tizhong Shan: Reviewing and editing. Qixin Huang: Writing, original draft preparation.Yizhen Wang: Supervision.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgement
This work was partially supported by the Natural Science Foundation of Zhejiang Province (LZ22C170003), the Zhejiang Provincial Key R&D Program of China (2021C02008), the Fundamental Research Funds for the Central University (226-2022-00113), and the “Hundred Talents Program” funding from Zhejiang University to TZS.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Ahmed H.M. Ethnomedicinal, phytochemical and pharmacological investigations of perilla frutescens (l.) britt. Molecules. 2018;24 doi: 10.3390/molecules24010102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque A., Neves J.A., Redondeiro M., Laranjo M., Félix M.R., Freitas A., Tirapicos J.L., Martins J.M. Long term betaine supplementation regulates genes involved in lipid and cholesterol metabolism of two muscles from an obese pig breed. Meat Sci. 2017;124:25–33. doi: 10.1016/j.meatsci.2016.10.012. [DOI] [PubMed] [Google Scholar]
- Aljohani A., Khan M.I., Syed D.N., Abram B., Lewis S., Neill L.O., Mukhtar H., Ntambi J.M. Hepatic stearoyl-coa desaturase-1 deficiency-mediated activation of mtorc1- pgc-1α axis regulates er stress during high-carbohydrate feeding. Sci Rep. 2019;9 doi: 10.1038/s41598-019-52339-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anna Vincent F.G., Compaoré Ella, Annor George Amponsah, Addy Paulina S., Lilian, Chinelo Aburime D.A., Loh Achu Mercy Bih, Cabia Sergio Dahdouh, Deflache Nina, BD Fatoumata Mariam Dembélé, Edwige Oboulbiga B., Ene-Obong Henrietta N., Nadia Fanou Fogny M.F., Julie Omaghomi Jemide, Pascal Christiant Kouebou, Carmen Muller, Sarah Nájera Espinosa F.O., Doris Rittenschober, Hettie Schönfeldt, Barbara Stadlmayr, Maricia Van Deventer A.R.Y., Ruth Charrondière U., Fao/infoods food composition table for western africa . Food and Agriculture Organization of the United Nations; Rome: 2019. User guide & condensed food composition table; p. 2020. [Google Scholar]
- Arjin C., Souphannavong C., Norkeaw R., Chaiwang N., Mekchay S., Sartsook A., Thongkham M., Yosen T., Ruksiriwanich W., Sommano S.R., Sringarm K. Effects of dietary perilla cake supplementation in growing pig on productive performance, meat quality, and fatty acid profiles. Animals. 2021:11. doi: 10.3390/ani11113213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y., Mccoy J.G., Levin E.J., Sobrado P., Rajashankar K.R., Fox B.G., Zhou M. X-ray structure of a mammalian stearoyl-coa desaturase. Nature. 2015;524:252–256. doi: 10.1038/nature14549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes K.M., Winslow N.R., Shelton A.G., Hlusko K.C., Azain M.J. Effect of dietary conjugated linoleic acid on marbling and intramuscular adipocytes in pork. J Anim Sci. 2012;90:1142–1149. doi: 10.2527/jas.2011-4642. [DOI] [PubMed] [Google Scholar]
- Bermudez B., Lopez S., Ortega A., Varela L.M., Pacheco Y.M., Abia R., Muriana F.J. Oleic acid in olive oil: from a metabolic framework toward a clinical perspective. Curr Pharmaceut Des. 2011;17:831–843. doi: 10.2174/138161211795428957. [DOI] [PubMed] [Google Scholar]
- Buttet M., Traynard V., Tran T.T., Besnard P., Poirier H., Niot I. From fatty-acid sensing to chylomicron synthesis: role of intestinal lipid-binding proteins. Biochimie. 2014;96:37–47. doi: 10.1016/j.biochi.2013.08.011. [DOI] [PubMed] [Google Scholar]
- Calder P.C. Functional roles of fatty acids and their effects on human health. JPEN - J Parenter Enter Nutr. 2015;39:18s–32s. doi: 10.1177/0148607115595980. [DOI] [PubMed] [Google Scholar]
- Castro L.F., Tocher D.R., Monroig O. Long-chain polyunsaturated fatty acid biosynthesis in chordates: insights into the evolution of fads and elovl gene repertoire. Prog Lipid Res. 2016;62:25–40. doi: 10.1016/j.plipres.2016.01.001. [DOI] [PubMed] [Google Scholar]
- Chen J., Cui H., Liu X., Li J., Zheng J., Li X., et al. Effects of dietary n-6:N-3 pufa ratio on growth performance, blood indexes, tissue fatty acid composition and related gene expression in pparγ signaling in finishing pigs. Anim Biosci. 2022;35:730–739. doi: 10.5713/ab.21.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Zhang W., Cai J., Ni Y., Xiao L., Zhang J. Transcriptome analysis in comparing carcass and meat quality traits of jiaxing black pig and duroc × duroc × berkshire × jiaxing black pig crosses. Gene (Amst) 2022;808 doi: 10.1016/j.gene.2021.145978. [DOI] [PubMed] [Google Scholar]
- Chen W., Xu Z., Shan T. Reserach progress on effect and regulation mechanism of conjugated linoleic acid on pork quality Chinese. J Anim Sci. 2019;55:21–29. [Google Scholar]
- Chen Y., Zhang J., Cui W., Silverstein R.L. Cd36, a signaling receptor and fatty acid transporter that regulates immune cell metabolism and fate. J Exp Med. 2022:219. doi: 10.1084/jem.20211314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu W., Zhai J., Xu J., Li S., Li W., Chen Z.J., Du Y. Continuous light-induced pcos-like changes in reproduction, metabolism, and gut microbiota in sprague-dawley rats. Front Microbiol. 2019;10:3145. doi: 10.3389/fmicb.2019.03145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn J.S., Kamili A., Wat E., Chung R.W., Tandy S. Reduction in intestinal cholesterol absorption by various food components: mechanisms and implications. Atherosclerosis Suppl. 2010;11:45–48. doi: 10.1016/j.atherosclerosissup.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Cordero G., Isabel B., Menoyo D., Daza A., Morales J., Piñeiro C., López-Bote C.J. Dietary cla alters intramuscular fat and fatty acid composition of pig skeletal muscle and subcutaneous adipose tissue. Meat Sci. 2010;85:235–239. doi: 10.1016/j.meatsci.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Corino C., Rossi R., Cannata S., Ratti S. Effect of dietary linseed on the nutritional value and quality of pork and pork products: systematic review and meta-analysis. Meat Sci. 2014;98:679–688. doi: 10.1016/j.meatsci.2014.06.041. [DOI] [PubMed] [Google Scholar]
- Cui Z., Wang X., Liao S., Qi M., Zha A., Zuo G., Liao P., Chen Y., Guo C., Tan B. Effects of medium-chain fatty acid glycerides on nutrient metabolism and energy utilization in weaned piglets. Front Vet Sci. 2022;9 doi: 10.3389/fvets.2022.938888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'aquila T., Hung Y.H., Carreiro A., Buhman K.K. Recent discoveries on absorption of dietary fat: presence, synthesis, and metabolism of cytoplasmic lipid droplets within enterocytes. Biochim Biophys Acta. 2016;1861:730–747. doi: 10.1016/j.bbalip.2016.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannenberger D., Eggert A., Kalbe C., Woitalla A., Schwudke D. Are n-3 pufas from microalgae incorporated into membrane and storage lipids in pig muscle tissues?-a lipidomic approach. ACS Omega. 2022;7:24785–24794. doi: 10.1021/acsomega.2c02476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong L., Jin Y., Cui H., Yu L., Luo Y., Wang S., Wang H. Effects of diet supplementation with rumen-protected betaine on carcass characteristics and fat deposition in growing lambs. Meat Sci. 2020;166 doi: 10.1016/j.meatsci.2020.108154. [DOI] [PubMed] [Google Scholar]
- Du J., Xu Y., Zhang P., Zhao X., Gan M., Li Q., Ma J., Tang G., Jiang Y., Wang J., Li X., Zhang S., Zhu L. Microrna-125a-5p affects adipocytes proliferation, differentiation and fatty acid composition of porcine intramuscular fat. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19020501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure J., Lebret B., Bonhomme N., Ecolan P., Kouba M., Lefaucheur L. Metabolic adaptation of two pig muscles to cold rearing conditions. J Anim Sci. 2013;91:1893–1906. doi: 10.2527/jas.2012-5828. [DOI] [PubMed] [Google Scholar]
- Fernandez X., Monin G., Talmant A., Mourot J., Lebret B. Influence of intramuscular fat content on the quality of pig meat - 2. Consumer acceptability of m. Longissimus lumborum. Meat Sci. 1999;53:67–72. doi: 10.1016/s0309-1740(99)00038-8. [DOI] [PubMed] [Google Scholar]
- Ferraz R.B., Paixão R.V., Lopes-Marques M., Machado A.M., Salaro A.L., Castro L.F.C., Ó Monroig, O'sullivan F.L.A. The repertoire of the elongation of very long-chain fatty acids (elovl) protein family is conserved in tambaqui (colossoma macropomum): gene expression profiles offer insights into the sexual differentiation process. Comp Biochem Physiol B Biochem Mol Biol. 2022;261 doi: 10.1016/j.cbpb.2022.110749. [DOI] [PubMed] [Google Scholar]
- Fortin A., Robertson W.M., Tong A.K. The eating quality of canadian pork and its relationship with intramuscular fat. Meat Sci. 2005;69:297–305. doi: 10.1016/j.meatsci.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Franco D., Vazquez J.A., Lorenzo J.M. Growth performance, carcass and meat quality of the celta pig crossbred with duroc and landrance genotypes. Meat Sci. 2014;96:195–202. doi: 10.1016/j.meatsci.2013.06.024. [DOI] [PubMed] [Google Scholar]
- Gimeno R.E., Ortegon A.M., Patel S., Punreddy S., Ge P., Sun Y., Lodish H.F., Stahl A. Characterization of a heart-specific fatty acid transport protein. J Biol Chem. 2003;278:16039–16044. doi: 10.1074/jbc.M211412200. [DOI] [PubMed] [Google Scholar]
- Gu H., Zhou Y., Yang J., Li J., Peng Y., Zhang X., Miao Y., Jiang W., Bu G., Hou L., Li T., Zhang L., Xia X., Ma Z., Xiong Y., Zuo B. Targeted overexpression of pparγ in skeletal muscle by random insertion and crispr/cas9 transgenic pig cloning enhances oxidative fiber formation and intramuscular fat deposition. Faseb J. 2021;35 doi: 10.1096/fj.202001812RR. [DOI] [PubMed] [Google Scholar]
- Guo Z., Chen X., Chen D., Li M., Yin J., Yu B., He J., Huang Z. Effects of slaughter age on carcass traits and meat quality of crossbred (duroc × landrace × yorkshire) finishing pigs. Anim Biotechnol. 2022;33:339–345. doi: 10.1080/10495398.2021.1916512. [DOI] [PubMed] [Google Scholar]
- Hao L., Su W., Zhang Y., Wang C., Xu B., Jiang Z., Wang F., Wang Y., Lu Z. Effects of supplementing with fermented mixed feed on the performance and meat quality in finishing pigs. Anim Feed Sci Technol. 2020;266 [Google Scholar]
- Hao Z., Li Z., Huo J., Chu Y., Li J., Yu X., Liu F., Yin P. Effects of Chinese wolfberry and astragalus extracts on growth performance, pork quality, and unsaturated fatty acid metabolism regulation in Tibetan fragrant pigs. Anim Sci J. 2021;92 doi: 10.1111/asj.13581. [DOI] [PubMed] [Google Scholar]
- Heng J., Tian M., Zhang W., Chen F., Guan W., Zhang S. Maternal heat stress regulates the early fat deposition partly through modification of m(6)a rna methylation in neonatal piglets. Cell Stress Chaperones. 2019;24:635–645. doi: 10.1007/s12192-019-01002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C.J., Jiang Q.Y., Zhang T., Yin Y.L., Li F.N., Deng J.P., Wu G.Y., Kong X.F. Dietary supplementation with arginine and glutamic acid modifies growth performance, carcass traits, and meat quality in growing-finishing pigs. J Anim Sci. 2017;95:2680–2689. doi: 10.2527/jas.2017.1388. [DOI] [PubMed] [Google Scholar]
- Hu C.J., Jiang Q.Y., Zhang T., Yin Y.L., Li F.N., Su J.Y., Wu G.Y., Kong X.F. Dietary supplementation with arginine and glutamic acid enhances key lipogenic gene expression in growing pigs. J Anim Sci. 2017;95:5507–5515. doi: 10.2527/jas2017.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G., Cao X., Cao R., Liu H., Duan Y., Wang H. The effect of Chinese herb feed additives on fatty acid and cholesterol content of pork meat from different swines. Swine Production. 2018:65–68. [Google Scholar]
- Huang Q.C., Xu Z.R., Han X.Y., Li W.F. Effect of dietary betaine supplementation on lipogenic enzyme activities and fatty acid synthase mrna expression in finishing pigs. Anim Feed Sci Technol. 2008;140:365–375. [Google Scholar]
- Huang Y., Zhou L., Zhang J., Liu X., Zhang Y., Cai L., Zhang W., Cui L., Yang J., Ji J., Xiao S., Ai H., Chen C., Ma J., Yang B., Huang L. A large-scale comparison of meat quality and intramuscular fatty acid composition among three Chinese indigenous pig breeds. Meat Sci. 2020;168 doi: 10.1016/j.meatsci.2020.108182. [DOI] [PubMed] [Google Scholar]
- Jaturasitha S., Chaiwang N., Kayan A., Kreuzer M. Nutritional strategies to improve the lipid composition of meat, with emphasis on Thailand and asia. Meat Sci. 2016;120:157–166. doi: 10.1016/j.meatsci.2016.04.014. [DOI] [PubMed] [Google Scholar]
- Kouba M., Enser M., Whittington F.M., Nute G.R., Wood J.D. Effect of a high-linolenic acid diet on lipogenic enzyme activities, fatty acid composition, and meat quality in the growing pig. J Anim Sci. 2003;81:1967–1979. doi: 10.2527/2003.8181967x. [DOI] [PubMed] [Google Scholar]
- Li B., He Y., Wu W., Tan X., Wang Z., Irwin D.M., Wang Z., Zhang S. Circular rna profiling identifies novel circppara that promotes intramuscular fat deposition in pigs. J Agric Food Chem. 2022;70:4123–4137. doi: 10.1021/acs.jafc.1c07358. [DOI] [PubMed] [Google Scholar]
- Li D., Wahlqvist M.L., Sinclair A.J. Advances in n-3 polyunsaturated fatty acid nutrition. Asia Pac J Clin Nutr. 2019;28:1–5. doi: 10.6133/apjcn.201903_28(1).0001. [DOI] [PubMed] [Google Scholar]
- Li F., Duan Y., Li Y., Tang Y., Geng M., Oladele O.A., Kim S.W., Yin Y. Effects of dietary n-6:N-3 pufa ratio on fatty acid composition, free amino acid profile and gene expression of transporters in finishing pigs. Br J Nutr. 2015;113:739–748. doi: 10.1017/S0007114514004346. [DOI] [PubMed] [Google Scholar]
- Li M., Ouyang H., Yuan H., Li J., Xie Z., Wang K., Yu T., Liu M., Chen X., Tang X., Jiao H., Pang D. Site-specific fat-1 knock-in enables significant decrease of n-6pufas/n-3pufas ratio in pigs. G3 (Bethesda) 2018;8:1747–1754. doi: 10.1534/g3.118.200114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Huang Z., Zhao W., Li M., Li C. Transcriptome analysis reveals long intergenic non-coding rnas contributed to intramuscular fat content differences between yorkshire and wei pigs. Int J Mol Sci. 2020:21. doi: 10.3390/ijms21051732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Wang H., Wang X., Wang Y., Feng J. Betaine affects muscle lipid metabolism via regulating the fatty acid uptake and oxidation in finishing pig. J Anim Sci Biotechnol. 2017;8:72. doi: 10.1186/s40104-017-0200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.J., Li J.L., Zhang L., Gao F., Zhou G.H. Effects of dietary starch types on growth performance, meat quality and myofibre type of finishing pigs. Meat Sci. 2017;131:60–67. doi: 10.1016/j.meatsci.2017.04.237. [DOI] [PubMed] [Google Scholar]
- Listrat A., Lebret B., Louveau I., Astruc T., Bonnet M., Lefaucheur L., Picard B., Bugeon J. How muscle structure and composition influence meat and flesh quality. Sci World J. 2016;2016 doi: 10.1155/2016/3182746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Wei W., Lin W., Yu W., Luo W., Niu Y., Zhang L., Chen J. Mir-32-5p regulates lipid accumulation in intramuscular fat of erhualian pigs by suppressing klf3. Lipids. 2021;56:279–287. doi: 10.1002/lipd.12294. [DOI] [PubMed] [Google Scholar]
- Liu L., Wang Y., Liang X., Wu X., Liu J., Yang S., Tao C., Zhang J., Tian J., Zhao J., Wang Y. Stearoyl-coa desaturase is essential for porcine adipocyte differentiation. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21072446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Du M., Tu Y., You W., Chen W., Liu G., et al. Fermented mixed feed alters growth performance, carcass traits, meat quality and muscle fatty acid and amino acid profiles in finishing pigs. Animal Nutrition. 2023;12:87–95. doi: 10.1016/j.aninu.2022.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J., Han Q., Wang S., Wang Z., Li X., Hu J., Yang G., Wang L., Shi X. Effect of fermented corn-soybean meal on carcass and meat quality of grower-finisher pigs. J Anim Physiol Anim Nutr. 2021;105:693–698. doi: 10.1111/jpn.13444. [DOI] [PubMed] [Google Scholar]
- Lu J., Zhu M., Cao H., Zhang X., Wang Z., Zhang X., Li X., Hu J., Yang G., Shi X. Impact of fermented corn-soybean meal on gene expression of immunity in the blood, level of secretory immunoglobulin a, and mucosa-associated bacterial community in the intestine of grower-finisher pigs. Front Vet Sci. 2020;7:246. doi: 10.3389/fvets.2020.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y., Zhang X., Zhu Z., Jiao N., Qiu K., Yin J. Surplus dietary isoleucine intake enhanced monounsaturated fatty acid synthesis and fat accumulation in skeletal muscle of finishing pigs. J Anim Sci Biotechnol. 2018;9:88. doi: 10.1186/s40104-018-0306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Duan Y., Li R., Liang X., Li T., Huang X., Yin Y., Yin J. Gut microbial profiles and the role in lipid metabolism in shaziling pigs. Anim Nutr. 2022;9:345–356. doi: 10.1016/j.aninu.2021.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X., Jiang Z., Lai C. Significance of increasing n-3 pufa content in pork on human health. Crit Rev Food Sci Nutr. 2016;56:858–870. doi: 10.1080/10408398.2013.850059. [DOI] [PubMed] [Google Scholar]
- Ma X., Xiao L., Liu L., Ye L., Su P., Bi E., Wang Q., Yang M., Qian J., Yi Q. Cd36-mediated ferroptosis dampens intratumoral cd8(+) t cell effector function and impairs their antitumor ability. Cell Metabol. 2021;33:1001–1012. doi: 10.1016/j.cmet.2021.02.015. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macêdo A.P.A., Muñoz V.R., Cintra D.E., Pauli J.R. 12,13-dihome as a new therapeutic target for metabolic diseases. Life Sci. 2022;290 doi: 10.1016/j.lfs.2021.120229. [DOI] [PubMed] [Google Scholar]
- Martins J.M., Albuquerque A., Neves J.A., Freitas A.B., Charneca R., Tirapicos J.L. Influence of outdoor rearing and oleic acid supplementation on lipid characteristics of muscle and adipose tissues from obese alentejano pigs. J Anim Physiol Anim Nutr. 2018;102:e578–e590. doi: 10.1111/jpn.12799. [DOI] [PubMed] [Google Scholar]
- Mcmaster C.R. From yeast to humans - roles of the kennedy pathway for phosphatidylcholine synthesis. FEBS Lett. 2018;592:1256–1272. doi: 10.1002/1873-3468.12919. [DOI] [PubMed] [Google Scholar]
- Moran C.A., Morlacchini M., Keegan J.D., Fusconi G. Dietary supplementation of finishing pigs with the docosahexaenoic acid-rich microalgae, aurantiochytrium limacinum: effects on performance, carcass characteristics and tissue fatty acid profile. Asian-Australas J Anim Sci. 2018;31:712–720. doi: 10.5713/ajas.17.0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M.T., Nara T.Y. Structure, function, and dietary regulation of delta6, delta5, and delta9 desaturases. Annu Rev Nutr. 2004;24:345–376. doi: 10.1146/annurev.nutr.24.121803.063211. [DOI] [PubMed] [Google Scholar]
- Nickerson J.G., Alkhateeb H., Benton C.R., Lally J., Nickerson J., Han X.X., Wilson M.H., Jain S.S., Snook L.A., Glatz J.F.C., Chabowski A., Luiken J., Bonen A. Greater transport efficiencies of the membrane fatty acid transporters fat/cd36 and fatp4 compared with fabppm and fatp1 and differential effects on fatty acid esterification and oxidation in rat skeletal muscle. J Biol Chem. 2009;284:16522–16530. doi: 10.1074/jbc.M109.004788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen T.S., Lærke H.N., Theil P.K., Sørensen J.F., Saarinen M., Forssten S., Knudsen K.E. Diets high in resistant starch and arabinoxylan modulate digestion processes and scfa pool size in the large intestine and faecal microbial composition in pigs. Br J Nutr. 2014;112:1837–1849. doi: 10.1017/S000711451400302X. [DOI] [PubMed] [Google Scholar]
- Nong Q., Wang L., Zhou Y., Sun Y., Chen W., Xie J., Zhu X., Shan T. Low dietary n-6/n-3 pufa ratio regulates meat quality, reduces triglyceride content, and improves fatty acid composition of meat in heigai pigs. Animals. 2020;10 doi: 10.3390/ani10091543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J., Tao C., Cao C., Zheng Q., Lam S.M., Shui G., Liu X., Li K., Zhao J., Wang Y. Adipose lipidomics and rna-seq analysis revealed the enhanced mitochondrial function in ucp1 knock-in pigs. Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1864:1375–1383. doi: 10.1016/j.bbalip.2019.06.017. [DOI] [PubMed] [Google Scholar]
- Paoletti L., Elena C., Domizi P., Banchio C. Role of phosphatidylcholine during neuronal differentiation. IUBMB Life. 2011;63:714–720. doi: 10.1002/iub.521. [DOI] [PubMed] [Google Scholar]
- Patel D., Witt S.N. Ethanolamine and phosphatidylethanolamine: partners in health and disease. Oxid Med Cell Longev. 2017;2017 doi: 10.1155/2017/4829180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y., Chen F.F., Ge J., Zhu J.Y., Shi X.E., Li X., Yu T.Y., Chu G.Y., Yang G.S. Mir-429 inhibits differentiation and promotes proliferation in porcine preadipocytes. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17122047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira P.M., Vicente A.F. Meat nutritional composition and nutritive role in the human diet. Meat Sci. 2013;93:586–592. doi: 10.1016/j.meatsci.2012.09.018. [DOI] [PubMed] [Google Scholar]
- Pohl J., Ring A., Hermann T., Stremmel W. Role of fatp in parenchymal cell fatty acid uptake. Biochim Biophys Acta. 2004;1686:1–6. doi: 10.1016/j.bbalip.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Poklukar K., Čandek-Potokar M., Batorek Lukač N., Tomažin U., Škrlep M. Lipid deposition and metabolism in local and modern pig breeds: a review. Animals. 2020;10 doi: 10.3390/ani10030424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing Y., Jamal M.A., Shi D., Zhao S., Xu K., Jiao D., Zhao H., Li H., Jia B., Wang H., Zhao H.Y., Wei H.J. Delayed body development with reduced triglycerides levels in leptin transgenic pigs. Transgenic Res. 2022;31:59–72. doi: 10.1007/s11248-021-00288-1. [DOI] [PubMed] [Google Scholar]
- Qu H., Ajuwon K.M. Adipose tissue-specific responses reveal an important role of lipogenesis during heat stress adaptation in pigs. J Anim Sci. 2018;96:975–989. doi: 10.1093/jas/sky022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu H., Ajuwon K.M. Metabolomics of heat stress response in pig adipose tissue reveals alteration of phospholipid and fatty acid composition during heat stress. J Anim Sci. 2018;96:3184–3195. doi: 10.1093/jas/sky127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren H., Xiao W., Qin X., Cai G., Chen H., Hua Z., Cheng C., Li X., Hua W., Xiao H., Zhang L., Dai J., Zheng X., Zhu Z., Qian C., Yao J., Bi Y. Myostatin regulates fatty acid desaturation and fat deposition through mef2c/mir222/scd5 cascade in pigs. Commun Biol. 2020;3:612. doi: 10.1038/s42003-020-01348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes N., Reyes I., Tiwari R., Geliebter J. Effect of linoleic acid on proliferation and gene expression in the breast cancer cell line t47d. Cancer Lett. 2004;209:25–35. doi: 10.1016/j.canlet.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Schenk S., Saberi M., Olefsky J.M. Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest. 2008;118:2992–3002. doi: 10.1172/JCI34260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang P., Zhang B., Zhang J., Duan M., Wu L., Gong X., Tang K., Zhang H., Chamba Y. Expression and single-nucleotide polymorphisms of the h-fabp gene in pigs. Gene. 2019;710:156–160. doi: 10.1016/j.gene.2019.05.061. [DOI] [PubMed] [Google Scholar]
- Shen X., Li A., Yao M., Xia X. Influence of compound Chinese herb medicine additive on meat quality of growing finishing pigs. Journal of Domestic Animal Ecology. 2021;42:37–42. [Google Scholar]
- Shu G., Zhu X.T., Wang X.Q., Song Y.Z., Bin Y.F., Zhang Y.L., Gao P., Jiang Q.Y. Identification and gene expression of porcine fatty acid transport protein 1 isoforms. J Anim Physiol Anim Nutr. 2009;93:439–446. doi: 10.1111/j.1439-0396.2008.00825.x. [DOI] [PubMed] [Google Scholar]
- Sringarm K., Chaiwang N., Wattanakul W., Mahinchai P., Satsook A., Norkeaw R., Seel-Audom M., Moonmanee T., Mekchay S., Sommano S.R., Ruksiriwanich W., Rachtanapun P., Jantanasakulwong K., Arjin C. Improvement of intramuscular fat in longissimus muscle of finishing Thai crossbred black pigs by perilla cake supplementation in a low-lysine diet. Foods. 2022:11. doi: 10.3390/foods11070907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl A., Hirsch D.J., Gimeno R.E., Punreddy S., Ge P., Watson N., Patel S., Kotler M., Raimondi A., Tartaglia L.A., Lodish H.F. Identification of the major intestinal fatty acid transport protein. Mol Cell. 1999;4:299–308. doi: 10.1016/s1097-2765(00)80332-9. [DOI] [PubMed] [Google Scholar]
- Suárez-Belloch J., Guada J.A., Latorre M.A. Effects of sex and dietary lysine on performances and serum and meat traits in finisher pigs. Animal. 2015;9:1731–1739. doi: 10.1017/S1751731115001111. [DOI] [PubMed] [Google Scholar]
- Sun T., Wang X., Cong P., Xu J., Xue C. Mass spectrometry-based lipidomics in food science and nutritional health: a comprehensive review. Compr Rev Food Sci Food Saf. 2020;19:2530–2558. doi: 10.1111/1541-4337.12603. [DOI] [PubMed] [Google Scholar]
- Szondy Z., Al-Zaeed N., Tarban N., Fige É., Garabuczi É., Sarang Z. Involvement of phosphatidylserine receptors in the skeletal muscle regeneration: Therapeutic implications. J Cachexia Sarcopenia Muscle. 2022;13:1961–1973. doi: 10.1002/jcsm.13024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan F.P.Y., Beltranena E., Zijlstra R.T. Resistant starch: implications of dietary inclusion on gut health and growth in pigs: a review. J Anim Sci Biotechnol. 2021;12:124. doi: 10.1186/s40104-021-00644-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi M., Arakawa A., Motoyama M., Nakajima I., Nii M., Mikawa S. Genomic structural analysis of porcine fatty acid desaturase cluster on chromosome 2. Anim Sci J. 2015;86:369–377. doi: 10.1111/asj.12308. [DOI] [PubMed] [Google Scholar]
- Tian H., Yu H., Lin Y., Li Y., Xu W., Chen Y., Liu G., Xie L. Association between fads gene expression and polyunsaturated fatty acids in breast milk. Nutrients. 2022;14 doi: 10.3390/nu14030457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touma S., Oyadomari M. Comparison of growth performances, carcass characteristics, and meat qualities of okinawan indigenous agu pigs and crossbred pigs sired by agu or duroc boar. Anim Sci J. 2020;91 doi: 10.1111/asj.13362. [DOI] [PubMed] [Google Scholar]
- Tous N., Lizardo R., Vilà B., Gispert M., Font-I-Furnols M., Esteve-Garcia E. Effect of a high dose of cla in finishing pig diets on fat deposition and fatty acid composition in intramuscular fat and other fat depots. Meat Sci. 2013;93:517–524. doi: 10.1016/j.meatsci.2012.10.005. [DOI] [PubMed] [Google Scholar]
- Tu T., Wu W., Tang X., Ge Q., Zhan J. Screening out important substances for distinguishing Chinese indigenous pork and hybrid pork and identifying different pork muscles by analyzing the fatty acid and nucleotide contents. Food Chem. 2021;350 doi: 10.1016/j.foodchem.2021.129219. [DOI] [PubMed] [Google Scholar]
- Turner T.D., Mapiye C., Aalhus J.L., Beaulieu A.D., Patience J.F., Zijlstra R.T., Dugan M.E. Flaxseed fed pork: N-3 fatty acid enrichment and contribution to dietary recommendations. Meat Sci. 2014;96:541–547. doi: 10.1016/j.meatsci.2013.08.021. [DOI] [PubMed] [Google Scholar]
- Van Der Veen J.N., Kennelly J.P., Wan S., Vance J.E., Vance D.E., Jacobs R.L. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim Biophys Acta Biomembr. 2017;1859:1558–1572. doi: 10.1016/j.bbamem.2017.04.006. [DOI] [PubMed] [Google Scholar]
- Viterbo V.S., Lopez B.I.M., Kang H., Kim H., Song C.W., Seo K.S. Genome wide association study of fatty acid composition in duroc swine. Asian-Australas J Anim Sci. 2018;31:1127–1133. doi: 10.5713/ajas.17.0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Jang Y.D., Rentfrow G.K., Azain M.J., Lindemann M.D. Effects of dietary vitamin e and fat supplementation in growing-finishing swine fed to a heavy slaughter weight of 150 kg: ii. Tissue fatty acid profile, vitamin e concentrations, and antioxidant capacity of plasma and tissue. J Anim Sci. 2022:100. doi: 10.1093/jas/skac184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Airola M.V., Reue K. How lipid droplets "tag" along: glycerolipid synthetic enzymes and lipid storage. Biochim Biophys Acta Mol Cell Biol Lipids. 2017;1862:1131–1145. doi: 10.1016/j.bbalip.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Franco F., Tsui Y.C., Xie X., Trefny M.P., Zappasodi R., Mohmood S.R., Fernández-García J., Tsai C.H., Schulze I., Picard F., Meylan E., Silverstein R., Goldberg I., Fendt S.M., Wolchok J.D., Merghoub T., Jandus C., Zippelius A., Ho P.C. Cd36-mediated metabolic adaptation supports regulatory t cell survival and function in tumors. Nat Immunol. 2020;21:298–308. doi: 10.1038/s41590-019-0589-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Chen M.Y., Chen J.F., Ren Q.L., Zhang J.Q., Cao H., Xing B.S., Pan C.Y. Lncrna imflnc1 promotes porcine intramuscular adipocyte adipogenesis by sponging mir-199a-5p to up-regulate cav-1. BMC Mol Cell Biol. 2020;21:77. doi: 10.1186/s12860-020-00324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Huang Y., Wang Y., Shan T. Effects of polyunsaturated fatty acids supplementation on the meat quality of pigs: a meta-analysis. Front Nutr. 2021;8 doi: 10.3389/fnut.2021.746765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Tatsuno I. Omega-3 polyunsaturated fatty acids for cardiovascular diseases: present, past and future. Expet Rev Clin Pharmacol. 2017;10:865–873. doi: 10.1080/17512433.2017.1333902. [DOI] [PubMed] [Google Scholar]
- Wu C., Lyu W., Hong Q., Zhang X., Yang H., Xiao Y. Gut microbiota influence lipid metabolism of skeletal muscle in pigs. Front Nutr. 2021;8 doi: 10.3389/fnut.2021.675445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G., Bazer F.W., Dai Z., Li D., Wang J., Wu Z. Amino acid nutrition in animals: protein synthesis and beyond. Annu Rev Anim Biosci. 2014;2:387–417. doi: 10.1146/annurev-animal-022513-114113. [DOI] [PubMed] [Google Scholar]
- Wu W., Zhan J., Tang X., Li T., Duan S. Characterization and identification of pork flavor compounds and their precursors in Chinese indigenous pig breeds by volatile profiling and multivariate analysis. Food Chem. 2022;385 doi: 10.1016/j.foodchem.2022.132543. [DOI] [PubMed] [Google Scholar]
- Xia J.Q., He X., Wang L., Wang L., Zhang D.J., Wang J.F., Liu D. Evaluation of dietary perilla frutescens seed on performance and carcass quality in finishing castrated male songliao black pigs. Vet Med Sci. 2022;8:598–606. doi: 10.1002/vms3.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J.Q., Liu D.Y., Liu J., Jiang X.P., Wang L., Yang S., Liu D. Sex effects on carcass characteristics, meat quality traits and meat amino acid and fatty acid compositions in a novel duroc line pig. J Anim Physiol Anim Nutr. 2023;107:129–135. doi: 10.1111/jpn.13680. [DOI] [PubMed] [Google Scholar]
- Xie C., Zhu X., Xu B., Niu Y., Zhang X., Ma L., Yan X. Integrated analysis of multi-tissues lipidome and gut microbiome reveals microbiota-induced shifts on lipid metabolism in pigs. Anim Nutr. 2022;10:280–293. doi: 10.1016/j.aninu.2022.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z., Hu L., Li Y., Geng S., Cheng S., Fu X., Zhao S., Han X. Changes of gut microbiota structure and morphology in weaned piglets treated with fresh fermented soybean meal. World J Microbiol Biotechnol. 2017;33:213. doi: 10.1007/s11274-017-2374-7. [DOI] [PubMed] [Google Scholar]
- Xu E., Chen C., Fu J., Zhu L., Shu J., Jin M., Wang Y., Zong X. Dietary fatty acids in gut health: absorption, metabolism and function. Anim Nutr. 2021;7:1337–1344. doi: 10.1016/j.aninu.2021.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Chen X., Chen D., Yu B., Yin J., Huang Z. Effects of dietary apple polyphenol supplementation on carcass traits, meat quality, muscle amino acid and fatty acid composition in finishing pigs. Food Funct. 2019;10:7426–7434. doi: 10.1039/c9fo01304k. [DOI] [PubMed] [Google Scholar]
- Xu Z., Chen W., Wang L., You W., Wang Y., Wang Y., Zhao J., Shan T. Ucp1 knockin induces lipid dynamics and transcriptional programs in the skeletal muscles of pigs. Front Cell Dev Biol. 2021;9 doi: 10.3389/fcell.2021.808095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Chen W., Wang L., Zhou Y., Nong Q., Valencak T.G., Wang Y., Xie J., Shan T. Cold exposure affects lipid metabolism, fatty acids composition and transcription in pig skeletal muscle. Front Physiol. 2021;12 doi: 10.3389/fphys.2021.748801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan B., Chen L., Wang Y., Zhang J., Zhao H., Hua Q., Pei S., Yue Z., Liang H., Zhang H. Preventive effect of apple polyphenol extract on high-fat diet-induced hepatic steatosis in mice through alleviating endoplasmic reticulum stress. J Agric Food Chem. 2022;70:3172–3180. doi: 10.1021/acs.jafc.1c07733. [DOI] [PubMed] [Google Scholar]
- Yang C., Chen D., Yu B., Huang Z., Mao X., Yu J., Zheng P., He J. Effect of dietary amylose/amylopectin ratio on growth performance, carcass traits, and meat quality in finishing pigs. Meat Sci. 2015;108:55–60. doi: 10.1016/j.meatsci.2015.05.026. [DOI] [PubMed] [Google Scholar]
- Yang M., Chen R., Song Y.D., Zhou Y.M., Liu Q., Zhuang S. Effects of dietary betaine supplementation on growth performance, meat quality, muscle fatty acid composition and antioxidant ability in slow-growing broiler chickens. Br Poultry Sci. 2021:1–9. doi: 10.1080/00071668.2021.2008313. [DOI] [PubMed] [Google Scholar]
- Yang Y. 6th ed. Peking University Medical Press; 2019. China food composition tables standard edition. [Google Scholar]
- Yen C.E., Nelson D.W., Yen M.I. Intestinal triacylglycerol synthesis in fat absorption and systemic energy metabolism. J Lipid Res. 2015;56:489–501. doi: 10.1194/jlr.R052902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K., Shu G., Yuan F., Zhu X., Gao P., Wang S., Wang L., Xi Q., Zhang S., Zhang Y., Li Y., Wu T., Yuan L., Jiang Q. Fatty acid and transcriptome profiling of longissimus dorsi muscles between pig breeds differing in meat quality. Int J Biol Sci. 2013;9:108–118. doi: 10.7150/ijbs.5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M., Li Z., Rong T., Wang G., Liu Z., Chen W., Li J., Li J., Ma X. Different dietary starch sources alter the carcass traits, meat quality, and the profile of muscle amino acid and fatty acid in finishing pigs. J Anim Sci Biotechnol. 2020;11:78. doi: 10.1186/s40104-020-00484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue F., Xing L., Wu S., Wei L., Zhou Z., Shi Y., Lam S.M., Shui G., Xiang X., Russell R., Zhang D. Constant light exposure alters gut microbiota and short-/medium-chain fatty acids and aggravates pcos-like traits in hfd-fed rats. Obesity. 2022;30:694–706. doi: 10.1002/oby.23380. [DOI] [PubMed] [Google Scholar]
- Zhang L., Li F., Guo Q., Duan Y., Wang W., Yang Y., Yin Y., Gong S., Han M., Yin Y. Different proportions of branched-chain amino acids modulate lipid metabolism in a finishing pig model. J Agric Food Chem. 2021;69:7037–7048. doi: 10.1021/acs.jafc.1c02001. [DOI] [PubMed] [Google Scholar]
- Zhang L., Li F., Guo Q., Duan Y., Wang W., Yang Y., Yin Y., Gong S., Han M., Yin Y. Balanced branched-chain amino acids modulate meat quality by adjusting muscle fiber type conversion and intramuscular fat deposition in finishing pigs. J Sci Food Agric. 2022;102:3796–3807. doi: 10.1002/jsfa.11728. [DOI] [PubMed] [Google Scholar]
- Zhao G., Xiang Y., Wang X., Dai B., Zhang X., Ma L., Yang H., Lyu W. Exploring the possible link between the gut microbiome and fat deposition in pigs. Oxid Med Cell Longev. 2022;2022 doi: 10.1155/2022/1098892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y., Song B., Zheng C., Li F., Kong X., Duan Y., Deng J. Α-ketoisocaproate and β-hydroxy-β-methyl butyrate regulate fatty acid composition and lipid metabolism in skeletal muscle of growing pigs. J Anim Physiol Anim Nutr. 2019;103:846–857. doi: 10.1111/jpn.13077. [DOI] [PubMed] [Google Scholar]
- Zhong Y., Yan Z., Song B., Zheng C., Duan Y., Kong X., Deng J., Li F. Dietary supplementation with betaine or glycine improves the carcass trait, meat quality and lipid metabolism of finishing mini-pigs. Anim Nutr. 2021;7:376–383. doi: 10.1016/j.aninu.2020.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Xu Z., Wang L., Ling D., Nong Q., Xie J., Zhu X., Shan T. Cold exposure induces depot-specific alterations in fatty acid composition and transcriptional profile in adipose tissues of pigs. Front Endocrinol. 2022;13 doi: 10.3389/fendo.2022.827523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong G., Li Y., Wanders A.J., Alssema M., Zock P.L., Willett W.C., Hu F.B., Sun Q. Intake of individual saturated fatty acids and risk of coronary heart disease in us men and women: two prospective longitudinal cohort studies. BMJ. 2016;355:i5796. doi: 10.1136/bmj.i5796. [DOI] [PMC free article] [PubMed] [Google Scholar]