Abstract
Maribavir was approved by the US Food and Drug Administration for the treatment of patients aged ≥12 years and weighing ≥35 kg with posttransplant cytomegalovirus infection/disease refractory (with/without resistance) to valganciclovir, ganciclovir, cidofovir, or foscarnet, with an oral dose of 400 mg twice daily. With no pediatric clinical data available and difficulty in trial recruitment, population pharmacokinetic modeling and simulations were conducted to predict the pharmacokinetics and inform maribavir dosing in adolescents.
METHODS
Development of a population pharmacokinetic model for adults
A population pharmacokinetic (PopPK) model 1 was developed using nonlinear mixed effects modeling (NONMEM, Versions 7.4.3 and 7.5.0; ICON plc) to describe the time course of maribavir plasma concentrations, administered orally, in participants enrolled in nine phase I studies, two phase II studies (NCT01611974, EudraCT 2010‐024247‐32, 400/800/1200 mg twice daily [b.i.d.]), and one phase III study (NCT02931539, 400 mg b.i.d.; Table S1). This analysis included healthy volunteers and hematopoietic stem cell transplant or solid organ transplant recipients with cytomegalovirus (CMV) infection.
The effects of covariates on maribavir pharmacokinetics (PK) were evaluated. The baseline body weight distribution of the participants is shown in Figure S1. The body weight exponents on clearance and volume of distribution parameters were evaluated by either fixing (0.75 for clearance parameters, one for volume of distribution parameters) or estimating from the PK data. The model with estimated weight exponents returned imprecise estimates for both apparent total clearance (CL/F) ~ and peripheral volume of distribution ~ weight and thus was not considered as sufficiently robust for subsequent simulation of adolescent exposure based on body weight. The model with fixed allometric exponents was used to derive individual estimates of steady‐state area under the plasma concentration curve over a dosing interval (AUC0–τ), peak plasma concentration (Cmax), and trough plasma concentration (Cmin) values for the 400 or 1200 mg b.i.d. regimen for transplant recipients with CMV infection.
Adolescent PopPK model simulations
The adult PopPK model was used to perform simulations for virtual adolescent transplant recipients with CMV infection or diseases. Virtual adolescents (aged 12–18 years) were represented by simulating weights at 25 to <100 kg; 1000 virtual participants were sampled from a uniform distribution of weight within each 2.5‐kg increment from 25 to <30 kg and within each 5‐kg increment from 30 to <100 kg. This range of body weight is wider than the range from the third percentile weight in 12‐year‐old males to the 97th percentile weight in 18‐year‐old males. 2 Concentration‐time profiles were simulated (sampling at 0, 1, 2, 3, 4, 6, 8, and 12 h) following 400 mg b.i.d. steady‐state doses for every individual in each weight group using the PopPK model with fixed weight exponents and including interindividual variability. No residual variability was included in the predictions. Steady‐state AUC0–τ (linear‐up/log‐down trapezoidal rule), Cmax at steady state (Cmax,ss), and Cmin at steady state (Cmin,ss) were then calculated by noncompartmental methods.
Steady‐state exposures in virtual adolescents following 400 mg b.i.d. doses were summarized and compared with corresponding exposures in an adult population with CMV. The geometric means and percentiles of estimated steady‐state AUC0–τ and Cmin,ss following 400 mg b.i.d. maribavir dosing in adult transplant recipients in the phase III study were used as benchmarks for efficacy purposes. The geometric mean, 75th percentile, and 95th percentile values of estimated steady‐state AUC0–τ and Cmax,ss following 1200 mg b.i.d. maribavir in adult transplant recipients in the phase II studies were used as the PK comparators for safety, as this dose showed similar efficacy and safety to 400 mg b.i.d. The percentages of participants in each weight group with exposures above or below these benchmarks were also summarized.
RESULTS
Adult PopPK model
The final PopPK model for adults was a two‐compartment model with first‐order absorption and elimination and an absorption lag‐time. Final covariates included body weight for clearance and volume terms, patients with CMV as a categorical covariate for CL/F, and maribavir dose for the first‐order absorption rate (Ka). The initially estimated weight exponents on CL/F and apparent peripheral volume of distribution were deemed imprecise; thus, in the final model the weight effects were fixed rather than estimated. The parameter estimates for the PopPK model with fixed weight exponents are presented in Table S2. Notably, CL/F was estimated to be lower in transplant recipients with CMV (0.756× of that in healthy subjects), and Ka decreased with an increase in maribavir dose.
The AUC0–τ, Cmax,ss, and Cmin,ss values following maribavir 400 or 1200 mg b.i.d. doses in adult transplant recipients with CMV infection estimated using the PopPK model with fixed weight exponents are shown in Table S3.
PopPK simulations for a virtual adolescent population with CMV
The simulated steady‐state exposures for maribavir 400 mg b.i.d. in virtual adolescent patients with CMV infection using a PopPK model with fixed weight exponents are shown in Figure 1a (AUC0–τ), Figure 1b (Cmax,ss), and Figure 1c (Cmin,ss). Clear trends of exposure versus weight relationships in the PopPK model were observed. Maribavir exposure generally increased as body weight decreased.
FIGURE 1.
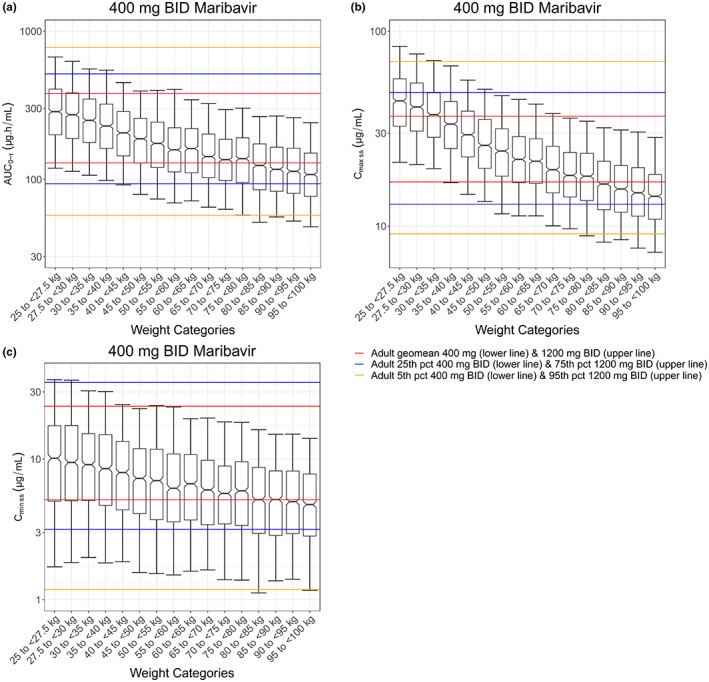
Simulations of maribavir exposure in virtual adolescents by body weights with the fixed weight effect population pharmacokinetics model. (a) AUC0–τ, (b) Cmax,ss, (c) Cmin,ss. Black center line of each box represents the median, and the top and base of the box represent the first and third quartiles (interquartile range). Whiskers represent the 5th and 95th percentiles. The lower and upper red horizontal lines represent the geometric means for 400 and 1200 mg b.i.d. doses in adult patients with cytomegalovirus, respectively. The lower and upper yellow horizontal lines denote the 5th percentile exposure at the 400 mg b.i.d. dose and the 95th percentile exposure at the 1200 mg b.i.d. dose in adult patients with cytomegalovirus, respectively. The lower and upper blue horizontal lines denote the 25th percentile exposure at the 400 mg b.i.d. dose and the 75th percentile exposure at the 1200 mg b.i.d. dose in adult patients with cytomegalovirus, respectively. AUC0–τ, area under the plasma concentration curve over a dosing interval; Cmax,ss, peak plasma concentration at steady state; Cmin,ss, trough plasma concentration at steady state; b.i.d., twice daily; pct, percentile
For exposure–efficacy considerations, at 400 mg b.i.d., AUC0–τ and Cmin,ss in virtual adolescents were generally at or above their respective geometric means in adult patients with CMV receiving 400 mg b.i.d. At the population level, for all weight groups at 400 mg b.i.d., ≥50% of the virtual adolescent population had AUC0–τ and Cmin,ss values above the respective geometric means in adult patients with CMV receiving 400 mg b.i.d. (Table 1).
TABLE 1.
Select summary of percentages of virtual adolescents under 40 kg with maribavir exposure from 400 mg b.i.d. compared with exposures in adults
| PopPK model with fixed weight‐effect exponents, with body weight range (kg) | ||||||
|---|---|---|---|---|---|---|
| 25–27.5 | 27.5–30 | 30–35 | 35–40 | |||
| Variable | Adult metric | Dose | Percentage of patients | |||
| AUC0–τ | >Geometric mean AUC0–τ | 400 mg b.i.d. | 94 | 92 | 92 | 85 |
| Cmin,ss | >Geometric mean Cmin,ss | 400 mg b.i.d. | 74 | 74 | 74 | 71 |
| AUC0–τ | ≤Geometric mean AUC0–τ | 1200 mg b.i.d. | 69 | 74 | 80 | 83 |
| AUC0–τ | ≤75th percentile AUC0–τ | 1200 mg b.i.d. | 87 | 91 | 93 | 94 |
| AUC0–τ | ≤95th percentile AUC0–τ | 1200 mg b.i.d. | 97 | 98 | 99 | 99 |
| Cmax,ss | ≤Geometric mean Cmax,ss | 1200 mg b.i.d. | 34 | 40 | 48 | 60 |
| Cmax,ss | ≤75th percentile Cmax,ss | 1200 mg b.i.d. | 60 | 64 | 75 | 82 |
| Cmax,ss | ≤95th percentile Cmax,ss | 1200 mg b.i.d. | 87 | 91 | 95 | 96 |
Abbreviations: AUC0–τ, area under the plasma concentration curve over a dosing interval; b.i.d., twice daily; Cmax,ss, peak plasma concentration at steady state; Cmin,ss, trough plasma concentration at steady state; PopPK, population pharmacokinetics.
For exposure–safety considerations, simulated maribavir exposures in virtual adolescents <40 kg receiving 400 mg b.i.d. were higher than those in adults receiving the same dose. Nevertheless, the simulated geometric means of AUC0–τ and Cmax,ss in virtual adolescents <40 kg were lower than the 75th percentile of exposures in adults at 1200 mg b.i.d. For population percentages, >50% of virtual adolescents in each weight group had simulated AUC0–τ below the adult geometric mean of AUC0–τ at 1200 mg b.i.d. For Cmax,ss, all except the <35 kg groups had >50% virtual individuals with Cmax,ss below the adult geometric mean Cmax,ss at 1200 mg b.i.d. (Table 1).
DISCUSSION
Although the currently available anti‐CMV agents such as ganciclovir, foscarnet, and cidofovir may be considered in clinical practice to treat refractory or resistant CMV infections, they are not approved for this indication, and their use is limited by their respective toxicities. 3 In adults, maribavir demonstrated a relatively wide therapeutic window, with similar anti‐CMV efficacy from 400 to 1200 mg b.i.d. and dysgeusia as the most common treatment‐emergent adverse event at doses up to 1200 mg b.i.d. 3 , 4 and dose‐proportional PK from 400 to 1200 mg b.i.d. 5 However, the efficacy and safety of maribavir in children, including adolescents, have not yet been reported. The unmet need in adolescent transplant recipients with refractory or resistant CMV, combined with difficulty in adolescent patient recruitment in clinical trials, warrants the use of PopPK modeling and simulation to provide information on dosing for this population.
The authors believe that for maribavir, a “full extrapolation” (i.e., PK matching only 6 ) paradigm is appropriate for comparison between adults and adolescents, as the target for maribavir (CMV UL97 protein) and its inhibitory potency is identical in all age groups. In addition, the presentation, progression, and response to intervention are similar for CMV infection in adult and pediatric patients. 7 Therefore, a similar exposure–response relationship is expected between adults and adolescents, justifying the PK‐matching approach for the selection of maribavir dosing regimen in adolescents.
From physiological and developmental perspectives, the overall absorption and elimination of maribavir is expected to be similar between adults and adolescents. Maribavir has high intestinal permeability and is rapidly and highly absorbed after oral administration. 8 It is mainly cleared through metabolism by cytochrome P450 (CYP; CYP3A4 and CYP1A2, both of which mature well before the age of 12 9 ). Therefore, similar maribavir disposition profiles are expected between adults and adolescents at a similar body weight range.
To project maribavir exposures in adolescents while considering uncertainties in the PK–body weight relationship due to lack of pediatric PK data, a PopPK model with fixed weight components was developed from adult data. At weights ≥35 kg, medians of AUC0–τ, Cmax,ss, and Cmin,ss for 400 mg b.i.d. in virtual adolescents were generally within the geometric means of respective exposures at 400 and 1200 mg b.i.d. doses in adults. In addition, at the 400 mg b.i.d. dose, ≥50% of virtual adolescents in all ≥35 kg weight groups were projected to have AUC0–τ, Cmax,ss, and Cmin,ss values between the geometric means of corresponding exposures at 400 and 1200 mg b.i.d. doses in adults. At weights <35 kg with 400 mg b.i.d., the model‐simulated geometric mean Cmax was higher than the geometric mean Cmax at 1200 mg b.i.d. in adults, and >50% of virtual adolescents would have individual Cmax higher than the geometric mean Cmax at 1200 mg b.i.d. in adults. Therefore, 400 mg b.i.d. in adolescents weighing ≥35 kg is expected to show similar safety and efficacy profiles to those in adult patients with CMV.
This adolescent population simulation study used a full extrapolation methodology to predict maribavir exposures in virtual adolescents by matching with therapeutically relevant exposures of maribavir in adults. Combined with the cumulative safety and efficacy data in adults, the PopPK modeling and simulations supported the expansion of the maribavir indication to adolescents (12 years of age and older) with a body weight of ≥35 kg at a regimen of 400 mg b.i.d. Methods detailed herein set an example that can be applied to other therapeutics for potential indication expansion from adults to adolescents where “full extrapolation” (PK‐based matching) criteria apply.
AUTHOR CONTRIBUTIONS
K.S. and I.H.S. wrote the manuscript. K.S. and I.H.S. designed the research. K.S., S.H., and I.H.S. performed the research. K.S., S.H., C.F., and I.H.S. analyzed the data. K.S., S.H., and C.F. contributed analytical tools.
FUNDING
This research was funded by Takeda Development Center Americas, Inc.
CONFLICT OF INTEREST STATEMENT
Kefeng Sun and Ivy H. Song are employees of Takeda Development Center Americas, Inc. and Takeda stock owners. Siobhan Hayes and Colm Farrell are employees of ICON, funded by Takeda Development Center Americas, Inc., for contracted research.
Supporting information
Appendix S1
Appendix S2
Appendix S3
Appendix S4
Appendix S5
ACKNOWLEDGMENTS
Under the direction of the authors, Lauren Gwynne, PhD of Caudex, Oxford, UK and Sweta Rathore, PhD of Caudex, Toronto, ON, Canada, provided writing assistance, and Michael Rowlands, PhD of Caudex, London, UK, provided editorial assistance. Caudex was funded by Takeda Development Center Americas, Inc. for these medical writing and editorial services.
Sun K, Hayes S, Farrell C, Song IH. Population pharmacokinetic modeling and simulation of maribavir to support dose selection and regulatory approval in adolescents with posttransplant refractory cytomegalovirus. CPT Pharmacometrics Syst Pharmacol. 2023;12:719‐723. doi: 10.1002/psp4.12943
REFERENCES
- 1. Song I, Chen G, Hayes S, Farrell C, Jomphe C, Gosselin NH. Population pharmacokinetics and exposure–response relationships of maribavir in transplant recipients with cytomegalovirus infection. Transplant Cell Therapy. 2022;28(3[suppl.]):S368‐S369. [Google Scholar]
- 2. Centers for Disease Control and Prevention (CDC) . Clinical Growth Charts. Accessed March 11, 2022. https://www.cdc.gov/growthcharts/clinical_charts.htm
- 3. Papanicolaou GA, Silveira FP, Langston AA, et al. Maribavir for refractory or resistant cytomegalovirus infections in hematopoietic‐cell or solid‐organ transplant recipients: a randomized, dose‐ranging, double‐blind, phase 2 study. Clin Infect Dis. 2019;68(8):1255‐1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Avery RK, Alain S, Alexander BD, et al. Maribavir for refractory cytomegalovirus infections with or without resistance post‐transplant: results from a phase 3 randomized clinical trial. Clin Infect Dis. 2021;75(10):690‐701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang LH, Peck RW, Yin Y, Allanson J, Wiggs R, Wire MB. Phase I safety and pharmacokinetic trials of 1263W94, a novel oral anti‐human cytomegalovirus agent, in healthy and human immunodeficiency virus‐infected subjects. Antimicrob Agents Chemother. 2003;47(4):1334‐1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dunne J, Rodriguez WJ, Murphy MD, et al. Extrapolation of adult data and other data in pediatric drug‐development programs. Pediatrics. 2011;128(5):e1242‐e1249. [DOI] [PubMed] [Google Scholar]
- 7. Vora SB, Englund JA. Cytomegalovirus in immunocompromised children. Curr Opin Infect Dis. 2015;28(4):323‐329. [DOI] [PubMed] [Google Scholar]
- 8. Song I, Ilic K, Sun K, Martin P. Clinical pharmacology of maribavir (SHP620): a comprehensive overview. Biol Blood Marrow Transplant. 2019;25(Suppl 3):S342. [Google Scholar]
- 9. Anderson GD. Children versus adults: pharmacokinetic and adverse‐effect differences. Epilepsia. 2002;43(Suppl 3):53‐59. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Appendix S2
Appendix S3
Appendix S4
Appendix S5


