Abstract
Background
TFE3-rearranged renal cell carcinoma (TFE3-rearranged RCC) is a type of kidney cancer with a low incidence, with no consensus about whether it has a worse prognosis than clear cell renal cell carcinoma (ccRCC). This study attempted to elucidate the impact of TFE3-rearranged RCC by analyzing its clinical features and prognosis.
Methods
Patients treated in Sun Yat-sen Memorial Hospital (SYSMH) who were suspected to be diagnosed with TFE3-rearranged RCC were divided into two groups, TFE3-rearranged RCC and ccRCC with positive TFE3 protein expression on immunohistochemistry [TFE3(+) ccRCC], by dual-color, break-apart fluorescence in situ hybridization (FISH). After balancing the baseline characteristics with TFE3(+) ccRCC using the propensity score matching (PSM) method in a ratio of 2, we selected patients diagnosed with ccRCC with negative TFE3 protein expression on immunohistochemistry [TFE3(−) ccRCC]. The impact of TFE3 gene rearrangement and protein expression on renal cell carcinoma was determined by feature comparison with a nonparametric test and survival analysis with the Kaplan‒Meier method.
Results
Among 37 patients suspected of having TFE3-rearranged RCC, 13 patients were diagnosed with TFE3-rearranged RCC, and 24 patients had TFE3(+) ccRCC. The recurrence and new metastasis of TFE3-rearranged RCC was relatively common, even if the tumor stage was early at the first diagnosis. Through feature comparison and survival analysis, we found that TFE3-rearranged RCC was quite similar to TFE3(+) ccRCC. Compared with TFE3(−) ccRCC, TFE3(+) ccRCC tended to have a larger tumor diameter (P = 0.011), higher neutrophil/lymphocyte ratio (NLR) (P = 0.017) and metastatic potential (P = 0.022), and worse overall survival (OS) (P = 0.043) and PFS (P = 0.016). The survival analysis showed that TFE3-rearranged RCC had a worse PFS than ccRCC (P = 0.002), and TFE3(+) RCC had a worse PFS than TFE3(−) RCC (P = 0.001). According to the stratification system based on the combination of TFE3 and lymphovascular invasion (LVI), we further found that the prognosis from good to poor was TFE3(−) LVI(−), TFE3(+) LVI(−), TFE3(+) LVI(+) and TFE3(−) LVI(+), with statistically significant differences in both OS (P = 0.001) and PFS (P < 0.001). In addition, we also reported two cases with poor prognosis, of which one was TFE3-rearranged RCC and the other was TFE3(+) ccRCC.
Conclusions
This is a novel finding that both FISH confirmed TFE3 gene rearrangement-mediated TFE3-rearranged RCC and IHC confirmed positive TFE3 protein expression [TFE3(+)] contribute to a poor prognosis in RCC, suggesting more active treatment and careful follow-up for TFE3(+) RCC patients. The combination of TFE3 and LVI may be a new risk stratification system for RCC.
Keywords: Renal cell carcinoma, TFE3, TFE3-Rearranged RCC, LVI, Prognosis
1. Introduction
TFE3-rearranged renal cell carcinomas (TFE3-rearranged RCC) were originally described by Argani [[1], [2], [3]]. It was identified as a renal cancer subtype in the World Health Organization (WHO) classification in 2004, which used to be named Xp11.2 translocation/TFE3 gene fusion renal cell carcinoma, and was identified as MiT family translocation renal cell carcinoma together with t(6; 11) translocation/TFEB gene fusion renal cell carcinoma in the WHO classification in 2016 [4]. In the latest WHO classification in 2022, it was classified as a separate entity again and eventually changed its name to TFE3-rearranged RCC, which was characterized by fusions of TFE3 with multiple partner genes enriched in chromosomes 1, 17, and X [5,6]. TFE3-rearranged RCC accounts for approximately 20–75% of pediatric renal cell carcinomas [7] and approximately 4% of adult renal cell carcinomas [8]. Similar to clear cell renal cell carcinoma (ccRCC) and papillary renal cell carcinoma (pRCC), most TFE3-rearranged RCC patients present with hematuria, lumbago, abdominal mass or are only found by physical examination [9].
Immunohistochemistry (IHC) of TFE3 was initially considered the method for the diagnosis of TFE3-rearranged RCC [10], and some studies had attempted to diagnose it by combining cathepsin K, HMB45 and other immunohistochemical markers [1,11,12], but the accuracy of these IHC markers was not high enough [13]. The current gold standard for the diagnosis of TFE3-rearranged RCC is dual-color and break-apart fluorescence in situ hybridization (FISH) to detect gene rearrangement of TFE3 [[14], [15], [16]]. However, FISH is not routinely performed due to its high cost and inconvenience of operation [17].
The prognosis of TFE3-rearranged RCC patients shows high heterogeneity, ranging from indolence to rapid invasion [7,18,19]. Some studies have shown that TFE3-rearranged RCC progresses slowly in children, has a poor prognosis in adults, and has a worse prognosis in elderly patients than in children [9], but other studies have noted a higher rate of aggressive course in children, which indicates a poor prognosis [20]. Older age and advanced stage were associated with a poor prognosis [7,19], and larger tumor size and the presence of tumor necrosis were associated with aggressiveness [21]. In general, there is no unified understanding of the prognosis of TFE3-rearranged RCC. In regard to the prognosis of TFE3-rearranged RCC, some studies found that it was worse than that of pRCC and similar to that of ccRCC [7,19], while some other studies suggested that it was worse than that of non-TFE3-rearranged RCC, including ccRCC [17,22]. The lack of understanding of TFE3-rearranged RCC reflected the current unclear role of TFE3 gene rearrangement and TFE3 protein in RCC.
In this study, we identified patients diagnosed with TFE3-rearranged RCC at Sun Yat-sen Memorial Hospital (SYSMH) to describe their characteristics, analyzed their prognosis, and compared them with ccRCC to elucidate the impact of TFE3 gene rearrangement and protein expression in RCC.
2. Materials and methods
2.1. Data source
All patients involved in the study were treated in SYSMH and received relevant puncture biopsy or surgical treatment. Corresponding pathological results were obtained to confirm the pathological type.
2.2. Study population
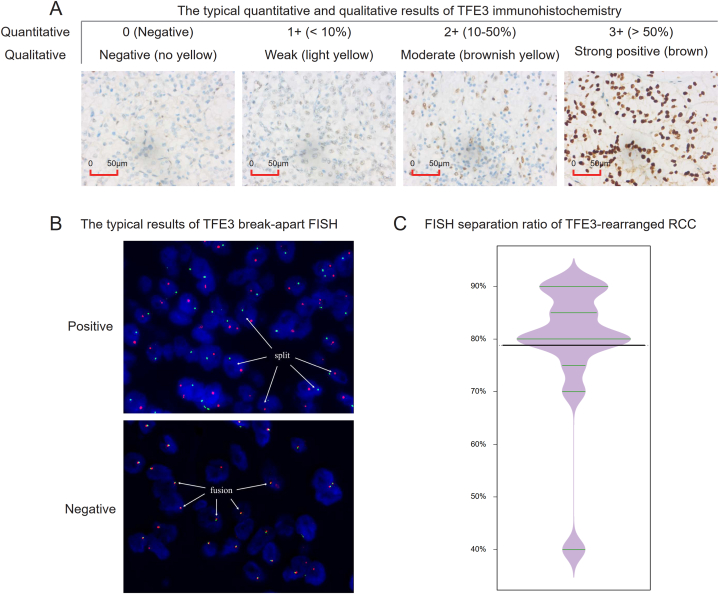
We collected all recorded patients who had been hospitalized in SYSMH because of renal mass from October 2016 to October 2022, and after excluding patients without renal pathological results and patients diagnosed with renal pelvic tumors and non-ccRCC, we identified patients considered ccRCC but who needed to first undergo differential diagnosis with TFE3-rearranged RCC and patients definitively diagnosed with ccRCC. Furthermore, patients suspected of TFE3-rearranged RCC were recognized, and they were positive for TFE3 protein expression on IHC [TFE3(+)]. The staining of TFE3 primary antibody was located in the nucleus. The quantitative immunohistochemical results were recorded as 0 (negative), 1+ (positive cell proportion <10%), 2+ (positive cell proportion 10–50%) and 3+ (positive cell proportion >50%). Qualitatively, it can be divided into negative (no yellow), weak positive (light yellow), moderate positive (brownish yellow) and strong positive (brown) according to the degree of nuclear coloring. Finally, the cases of moderate or strong positivity combined with 2+ or 3+ positivity were regarded as positive for TFE3 protein expression on IHC [TFE3(+)] (Fig. 2A).
Fig. 2.
Results of TFE3 immunohistochemistry and TFE3 break-apart FISH. (A) Typical results of TFE3 immunohistochemistry. The quantitative results were recorded as 0 (negative), 1+ (<10%), 2+ (10–50%) and 3+ (>50%) based on the positive cell proportion; and the qualitative results were divided into negative (no yellow), weak positive (light yellow), moderate positive (brownish yellow) and strong positive (brown) according to the degree of TFE3 primary antibody nuclear coloring. (B) The typical results of TFE3 break-apart FISH. The positive result, corresponding to TFE3-rearranged RCC, showed that the red signal was separated from the green signal by more than one signal point, and the separation ratio was more than 15%; the negative result, corresponding to TFE3(+) ccRCC, showed that the separation ratio of red and green signals was not high, and most of them showed overlap. (C) Violin plot of the FISH segregation ratio of 13 TFE3-rearranged RCC patients, in which the mean value was 78.85%.
FISH was used to diagnose TFE3-rearranged RCC in the study, and the typical results are shown in Fig. 2B. The positive result, corresponding to TFE3-rearranged RCC, presented that the red signal was separated from the green signal by more than one signal point, and the separation ratio was more than 15%; the negative result, corresponding to TFE3(+) ccRCC, presented that the separation ratio of red and green signals was not high, and most of them showed overlap. Based on the FISH results detecting TFE3 gene rearrangement, these patients were finally divided into FISH confirmed TFE3-rearranged RCC and TFE3(+) ccRCC. In addition, patients diagnosed with TFE3(−) ccRCC were also included in our study. The following were the inclusion criteria: (1) date of hospital visit was the same range as TFE3-rearranged RCC and TFE3(+) ccRCC; and (2) negative TFE3 protein expression on IHC. The exclusion criteria were as follows: (1) patients without complete baseline characteristics and (2) patients without survival status and follow-up information.
The propensity score matching (PSM) method was performed to balance the baseline characteristics between TFE3(+) ccRCC and TFE3(−) ccRCC. Specifically, the “MatchIt” R package was used for PSM, in which the covariate variables included age, sex, height, weight, body mass index (BMI), hypertension, diabetes, chief complaint divided into symptoms and examination findings, and surgery divided into partial nephrectomy (RN) and nonRN, with the matching method of nearest neighbor matching and the ratio of 1:2 matching [23]. After PSM was completed, we also performed a statistical analysis of the above covariates between the two groups to ensure that there was no significant difference in the baseline characteristics between them (Supplementary Table 1).
2.3. Measures
The primary endpoints of this study were overall survival (OS) and progression-free survival (PFS), and 31 October 2022 was set as the cutoff date for follow-up. All patients’ follow-up information was obtained by telephone consultation combined with a re-examination imaging review. The survival time was determined by the cutoff date and the time of death, thereby calculating the OS; the time of recurrence and new metastasis were determined by the last examination time; thus, PFS was calculated by combining these two and the time of death. Other variables included in the study were as follows: age, sex, height, weight, BMI, hypertension, diabetes, chief complaint, laterality, maximum diameter, TNM stage and stage group (American Joint Committee on Cancer [AJCC] eighth edition), WHO ISUP grade, surgery type, drug therapy, neutrophil/lymphocyte ratio (NLR), serum creatinine (Scr), urea, albumin/globulin ratio (AGR), shape, capsule sign, cystic degeneration, hemorrhage, necrosis, calcification, positive margin, renal capsule aggression, perirenal fat invasion, pelvis invasion, renal sinus invasion, renal venous invasion, FISH detection result, FISH separation ratio, lymphovascular invasion (LVI), IHC markers including TFE3, Ki67, Vimentin, CK7, CA9, CD117, RCC, CD10, P504S, PAX8, CK, and HMB45. FISH and IHC of markers were performed in the department of pathology following the routine procedure.
2.4. Statistical analysis
Since the number of patients involved in this study was not large enough, when comparing the numerical variables, we used the nonparametric test method to evaluate whether there were significant differences, and the results were expressed as medians [IQR (interquartile range)]. The chi-squared test was used for statistical analysis of categorical variables, and the results are shown as numbers (percentages). If the variable of the patient in the tumor group was missing, the corresponding value was set to not available (NA), and then the nonmissing value was used to calculate median (IQR) or number (percentage). The Kaplan‒Meier method and log-rank test were applied to calculate OS and PFS. We set a significance level of 0.05 (two-sided). All analyses were performed by R statistics software (version 4.1.1, Institute for Statistics and Mathematics, Vienna, Austria; www.r-project.org).
3. Results
3.1. The baseline characteristics of TFE3-rearranged RCC patients
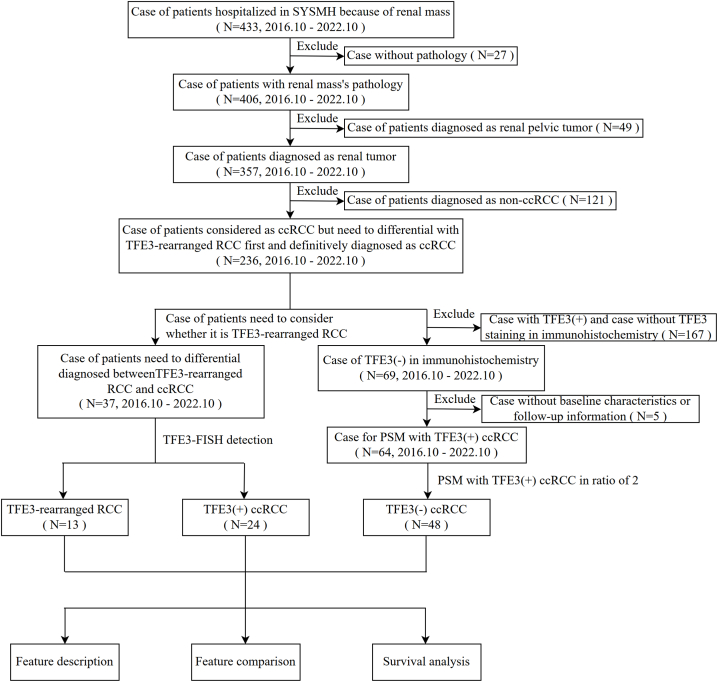
The research flow chart is shown in Fig. 1. Among all 433 recorded patients with renal masses, from October 2016 to October 2022, 236 patients were considered to have ccRCC but needed to be first differentiated from TFE3-rearranged RCC and definitively diagnosed with ccRCC. After excluding 27 patients without renal pathological results, 49 patients were diagnosed with renal pelvic tumors, and 121 patients were diagnosed with non-ccRCC. Furthermore, 37 patients suspected of TFE3-rearranged RCC were recognized, and they were divided into 13 TFE3-rearranged RCC and 24 TFE3(+) ccRCC based on the FISH result detecting TFE3 gene rearrangement. In addition, 48 out of 64 TFE3(−) ccRCC patients who met the inclusion and exclusion criteria were finally included in the study after PSM, while the baseline characteristics before and after PSM are presented in Supplementary Table 1, and the jitter and histogram plot evaluating the matching effect are shown in Supplementary Fig. 1.
Fig. 1.
Flow chart of this study. The three groups of patients involved in the study were identified: TFE3-rearranged renal cell carcinoma (TFE3-rearranged RCC), ccRCC with positive TFE3 protein expression on immunohistochemistry [TFE3(+) ccRCC], and ccRCC with negative TFE3 protein expression on immunohistochemistry [TFE3(−) ccRCC]. The role of TFE3 gene rearrangement and protein expression in renal cell carcinoma was evaluated by feature description, feature comparison and survival analysis.
Detailed baseline characteristics of 13 TFE3-rearranged RCC patients were as follows: 6 males and 7 females, with a median age of 33.00 years old, of which 6 cases were found by examination and 7 cases were due to hematuria, lumbago or other symptoms. Five cases were on the left, and 8 were on the right, with a median maximum diameter of 5.30 cm. One case was unknown for the initial TNM staging because the initial surgery was performed in an external hospital, and the TNM staging of the other 12 patients at the initial diagnosis was T1, T2 and T3, accounting for 7, 3 and 2 cases, respectively; most patients did not involve the lymph nodes or distinct sites, except for 1 case with N1 and 2 cases with M1. Postoperative pathology indicated that the median FISH separation ratio was 80.00%, while the mean value was 78.85% (Fig. 2C). The median Ki67 value was 8.00%, and 3 cases had LVI, which was a marker indicating poor prognosis [24,25]. Ignoring 2 out of 13 cases with an unknown state of LVI, because one performed initial surgery in the external hospital and the other was diagnosed by puncture, the positive rate of LVI was 27.27%.
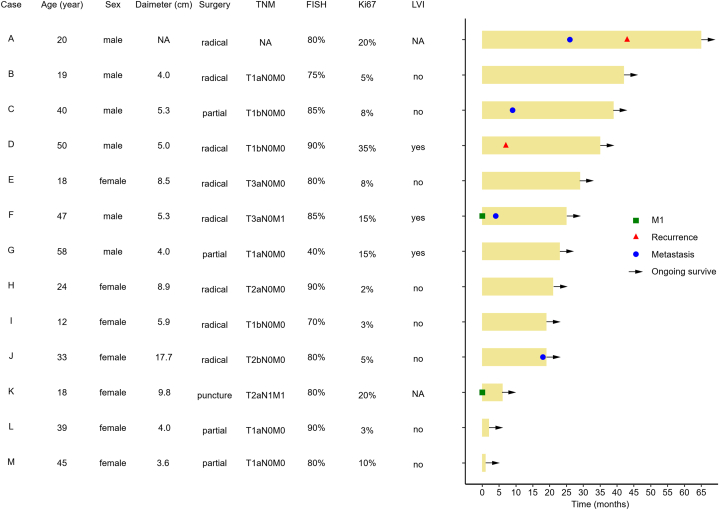
At present, all 13 patients survived, of which 1 case had recurrence, 3 cases had new metastasis, and 1 case had both recurrence and new metastasis during the follow-up (Fig. 3). The recurrence rate was 15.38%, the new metastasis rate was 30.77%, and the progression rate was 38.46%. Even if the tumor stage of TFE3-rearranged RCC patients is early at the first diagnosis, recurrence and new metastasis are relatively common. More detailed baseline characteristics of TFE3-rearranged RCC patients are shown in Table 1 and Supplementary Table 2.
Fig. 3.
The swimming plot depicting the clinical course of 13 TFE3-rearranged RCC patients. Two cases had tumor metastasis at the initial diagnosis (M1), and during the follow-up, one case developed simple recurrence, three cases developed simple new metastasis, and one case developed recurrence and new metastasis. All 13 TFE3-rearranged RCC patients are currently surviving.
Table 1.
Patients and tumor characteristics of TFE3-rearranged RCC and TFE3(+) ccRCC and comparison between them.
| TFE3(+) RCC (n = 37) | TFE3-rearranged RCC (n = 13) | TFE3(+) ccRCC (n = 24) | P | |
|---|---|---|---|---|
| Age | 45.00 [28.00, 53.00] | 33.00 [19.00, 45.00] | 48.50 [39.50, 56.25] | 0.011* |
| Sex | 0.541 | |||
| Male | 21 (56.76) | 6 (46.15) | 15 (62.50) | |
| Female | 16 (43.24) | 7 (53.85) | 9 (37.50) | |
| BMI | 21.94 [20.07, 24.91] | 20.08 [18.96, 22.72] | 22.22 [21.65, 27.15] | 0.056 |
| Chief complaint | 0.904 | |||
| Examination | 19 (51.35) | 6 (46.15) | 13 (54.17) | |
| Symptom | 18 (48.65) | 7 (53.85) | 11 (45.83) | |
| Laterality | 0.570 | |||
| Left | 18 (48.65) | 5 (38.46) | 13 (54.17) | |
| Right | 19 (51.35) | 8 (61.54) | 11 (45.83) | |
| Diameter (cm) | 5.90 [4.00, 8.72] | 5.30 [4.00, 8.60] | 5.95 [4.00, 8.72] | 0.906 |
| T | 0.688 | |||
| T1 | 24 (66.67) | 7 (58.33) | 17 (70.83) | |
| T2 | 7 (19.44) | 3 (25.00) | 4 (16.67) | |
| T3 | 4 (11.11) | 2 (16.67) | 2 (8.33) | |
| T4 | 1 (2.78) | 0 (0.00) | 1 (4.17) | |
| N | 1.000 | |||
| N0 | 32 (88.89) | 11 (91.67) | 21 (87.50) | |
| N1 | 4 (11.11) | 1 (8.33) | 3 (12.50) | |
| M | 0.253 | |||
| M0 | 33 (91.67) | 10 (83.33) | 23 (95.83) | |
| M1 | 3 (8.33) | 2 (16.67) | 1 (4.17) | |
| AJCC group | 0.591 | |||
| Stage I | 24 (66.67) | 7 (58.33) | 17 (70.83) | |
| Stage II | 5 (13.89) | 2 (16.67) | 3 (12.50) | |
| Stage III | 4 (11.11) | 1 (8.33) | 3 (12.50) | |
| Stage IV | 3 (8.33) | 2 (16.67) | 1 (4.17) | |
| Surgery | 0.744 | |||
| RN | 20 (54.1) | 8 (61.54) | 12 (50.00) | |
| nonRN | 17 (45.9) | 5 (38.46) | 12 (50.00) | |
| Drug therapy | 0.262 | |||
| Yes | 8 (24.24) | 5 (38.46) | 3 (15.00) | |
| No | 25 (75.76) | 8 (61.54) | 17 (85.00) | |
| NLR | 2.15 [1.72, 3.14] | 1.62 [1.35, 1.93] | 2.74 [2.13, 3.42] | 0.001** |
| Scr | 77.50 [65.25, 89.50] | 68.00 [62.75, 84.25] | 81.50 [71.75, 90.25] | 0.202 |
| FISH detection | <0.001*** | |||
| Positive | 13 (35.14) | 13 (100.00) | 0 (0.00) | |
| Negative | 24 (64.86) | 0 (0.00) | 24 (100.00) | |
| LVI | 0.361 | |||
| Yes | 5 (14.71) | 3 (27.27) | 2 (8.70) | |
| No | 29 (85.29) | 8 (72.73) | 21 (91.30) | |
| IHC_TFE3 | NA | |||
| + | 37 (100.00) | 13 (100.00) | 24 (100.00) | |
| - | 0 (0.00) | 0 (0.00) | 0 (0.00) | |
| Ki67 | 9.00 [5.00, 15.00] | 8.00 [5.00, 15.00] | 10.00 [5.00, 15.00] | 0.973 |
| Recurrence | 0.951 | |||
| Yes | 4 (11.11) | 2 (15.38) | 2 (8.70) | |
| No | 32 (88.89) | 11 (84.62) | 21 (91.30) | |
| Metastasis | 0.841 | |||
| Yes | 9 (25.00) | 4 (30.77) | 5 (21.74) | |
| No | 27 (75.00) | 9 (69.23) | 18 (78.26) | |
| Survival status | 0.315 | |||
| Alive | 33 (89.19) | 13 (100.00) | 20 (83.33) | |
| Dead | 4 (10.81) | 0 (0.00) | 4 (16.67) |
TFE3-rearranged RCC, TFE3-rearranged renal cell carcinoma; TFE3(+) ccRCC, ccRCC with positive TFE3 immunohistochemistry; BMI, body mass index; AJCC, American Joint Committee on Cancer; RN, partial nephrectomy; NLR, neutrophil/lymphocyte ratio; Scr, serum creatinine; LVI, lymphovascular invasion; IHC, immunohistochemistry. *, 0.01 < P < 0.05; **, 0.001 < P < 0.01; ***, P < 0.001.
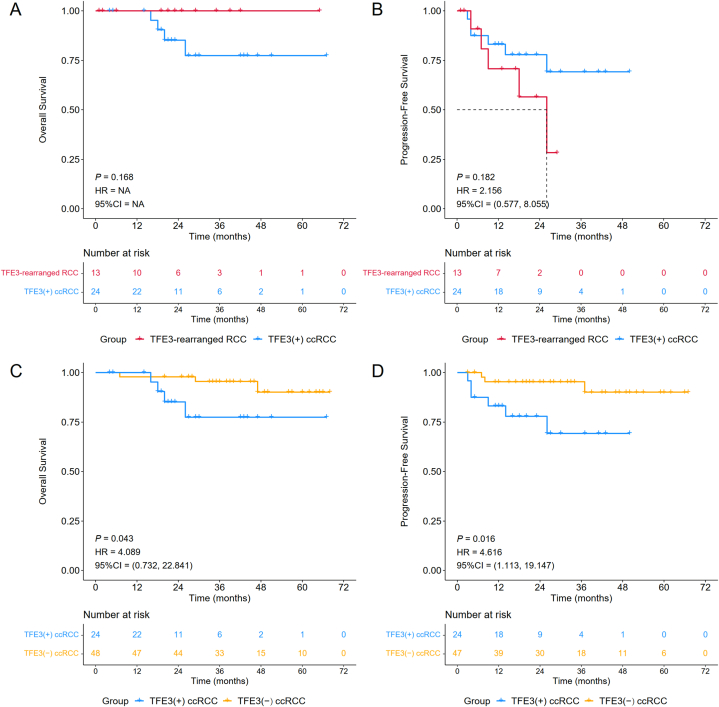
3.2. No survival differences between TFE3-rearranged RCC and TFE3(+) ccRCC patients
To compare the differences in two groups of patients with positive TFE3 protein expression, we carried out characteristic comparison and survival analysis for TFE3-rearranged RCC and TFE3(+) ccRCC. The results of stratified analysis are presented in Table 1 and Supplementary Table 2, which show that patients diagnosed with TFE3-rearranged RCC tended to be younger (P = 0.011) and have a smaller NLR (P = 0.001). Specifically, the median age of TFE3-rearranged RCC patients was 33.00, while that of TFE3(+) ccRCC was 48.50, suggesting that TFE3-rearranged RCC might prefer younger patients. The median preoperative NLR of TFE3-rearranged RCC was 1.62, which was smaller than the median preoperative NLR of TFE3(+) ccRCC (2.74). No statistically significant difference existed between the two groups for tumor stage, LVI, Ki67, recurrence, metastasis, or survival status. Correspondingly, there was no significant difference in OS [P = 0.168, HR = NA, 95% CI = NA] or PFS [P = 0.182, HR = 2.156, 95% CI = (0.577, 8.055)] between TFE3-rearranged RCC and TFE3(+) ccRCC (Fig. 4A and B), although the PFS of TFE3-rearranged RCC exhibited a worse trend. Since no TFE3-rearranged RCC patient died during the follow-up period, its OS was 100%, while the OS of TFE3(+) ccRCC patients at 1 year, 2 years and 3 years was 100%, 85.2% (95% CI, 70.9%–100.0%) and 77.4% (95% CI, 59.6%–100.0%), respectively. The PFS of TFE3-rearranged RCC patients at 1 and 2 years was 70.7% (95% CI, 47.6%–100.0%) and 56.6% (95% CI, 31.3%–100.0%), while the 1-, 2- and 3-year PFS of TFE3(+) ccRCC patients was 83.1% (95% CI, 69.3%–99.7%), 77.9% (95% CI, 62.5%–97.2%), and 69.3% (95% CI, 50.3%–95.4%), respectively. Overall, there were no differences in survival rates between TFE3-rearranged RCC and TFE3(+) ccRCC patients.
Fig. 4.
Subgroup analysis of Kaplan‒Meier survival curves stratified by cancer type: TFE3-rearranged RCC vs TFE3(+) ccRCC (A–B) and TFE3(+) ccRCC vs TFE3(−) ccRCC (C–D).
3.3. TFE3(+) ccRCC patients have higher metastatic potential and worse survival probability than TFE3(−) ccRCC patients
There was no significant difference in prognosis between TFE3-rearranged RCC and TFE3(+) ccRCC in our study, while previous studies have shown that the prognosis of TFE3-rearranged RCC patients is worse than that of ccRCC patients [17,22]. We hypothesized that TFE3(−) ccRCC might have a better prognosis than TFE3(+) ccRCC, thus making the overall prognosis of ccRCC relatively better. We were interested in the difference between the two groups, and 24 cases of TFE3(+) ccRCC and 48 cases of TFE3(−) ccRCC were finally included in the study for comparison after performing the PSM method at a ratio of 2 to balance their baseline characteristics.
As shown in Table 2 and Supplementary Table 3, TFE3(+) ccRCC patients showed a larger tumor diameter (P = 0.011) and higher NLR (P = 0.017) and metastatic potential (P = 0.022), which indicated a poor prognosis. The median maximum tumor diameter and NLR of TFE3(+) ccRCC patients were 5.95 cm and 2.74, respectively, while those of TFE3(−) ccRCC patients were smaller, only 3.90 cm and 2.01, respectively. After each exclusion of one patient who could not obtain metastasis information, 5 of 23 TFE3(+) ccRCC patients and only 1 of 47 TFE3(−) ccRCC patients had new metastasis, and the new metastatic rate was 21.74% vs 2.13%. Notably, compared to TFE3(−) ccRCC patients, TFE3(+) ccRCC patients had a higher proportion of irregular tumor shape (33.33% vs 10.42% P = 0.040) and tumor hemorrhage (41.67% vs 6.25%, P = 0.001).
Table 2.
Patients and tumor characteristics of TFE3(+) ccRCC and TFE3(−) ccRCC and comparison between them.
| ccRCC (n = 72) | TFE3(+) ccRCC (n = 24) | TFE3(−) ccRCC (n = 48) | P | |
|---|---|---|---|---|
| Age | 51.50 [42.75, 61.00] | 48.50 [39.50, 56.25] | 53.50 [45.00, 61.00] | 0.075 |
| Sex | 1.000 | |||
| Male | 46 (63.89) | 15 (62.50) | 31 (64.58) | |
| Female | 26 (36.11) | 9 (37.50) | 17 (35.42) | |
| BMI | 23.01 [21.65, 26.16] | 22.22 [21.65, 27.15] | 23.61 [21.82, 25.88] | 0.477 |
| Chief complaint | 0.255 | |||
| Examination | 47 (65.28) | 13 (54.17) | 34 (70.83) | |
| Symptom | 25 (34.72) | 11 (45.83) | 14 (29.17) | |
| Laterality | 0.677 | |||
| Left | 35 (48.61) | 13 (54.17) | 22 (45.83) | |
| Right | 37 (51.39) | 11 (45.83) | 26 (54.17) | |
| Diameter (cm) | 4.50 [2.88, 6.10] | 5.95 [4.00, 8.72] | 3.90 [2.77, 5.38] | 0.011 * |
| T | 0.335 | |||
| T1 | 55 (76.39) | 17 (70.83) | 38 (79.17) | |
| T2 | 8 (11.11) | 4 (16.67) | 4 (8.33) | |
| T3 | 8 (11.11) | 2 (8.33) | 6 (12.50) | |
| T4 | 1 (1.39) | 1 (4.17) | 0 (0.00) | |
| N | 0.203 | |||
| N0 | 68 (94.44) | 21 (87.50) | 47 (97.92) | |
| N1 | 4 (5.56) | 3 (12.50) | 1 (2.08) | |
| M | 0.333 | |||
| M0 | 71 (98.61) | 23 (95.83) | 48 (100.00) | |
| M1 | 1 (1.39) | 1 (4.17) | 0 (0.00) | |
| AJCC group | 0.488 | |||
| Stage I | 55 (76.39) | 17 (70.83) | 38 (79.17) | |
| Stage II | 7 (9.72) | 3 (12.50) | 4 (8.33) | |
| Stage III | 9 (12.50) | 3 (12.50) | 6 (12.50) | |
| Stage IV | 1 (1.39) | 1 (4.17) | 0 (0.00) | |
| WHO ISUP grade | 0.161 | |||
| G1 | 2 (3.08) | 0 (0.00) | 2 (4.17) | |
| G2 | 37 (56.92) | 7 (41.18) | 30 (62.50) | |
| G3 | 23 (35.38) | 8 (47.06) | 15 (31.25) | |
| G4 | 3 (4.62) | 2 (11.76) | 1 (2.08) | |
| Surgery | 0.140 | |||
| RN | 26 (36.11) | 12 (50.00) | 14 (29.17) | |
| nonRN | 46 (63.89) | 12 (50.00) | 34 (70.83) | |
| Drug therapy | 1.000 | |||
| Yes | 9 (13.24) | 3 (15.00) | 6 (12.50) | |
| No | 59 (86.76) | 17 (85.00) | 42 (87.50) | |
| NLR | 2.21 [1.66, 3.14] | 2.74 [2.13, 3.42] | 2.01 [1.59, 2.85] | 0.017 * |
| Scr | 80.50 [67.00, 94.25] | 81.50 [71.75, 90.25] | 80.50 [67.00, 96.25] | 0.820 |
| LVI | 1.000 | |||
| Yes | 6 (8.45) | 2 (8.70) | 4 (8.33) | |
| No | 65 (91.55) | 21 (91.30) | 44 (91.67) | |
| IHC_TFE3 | <0.001*** | |||
| + | 24 (33.33) | 24 (100.00) | 0 (0.00) | |
| - | 48 (66.67) | 0 (0.00) | 48 (100.00) | |
| Ki67 | 8.00 [3.00, 10.00] | 10.00 [5.00, 15.00] | 6.50 [3.00, 10.00] | 0.111 |
| Recurrence | 0.839 | |||
| Yes | 4 (5.71) | 2 (8.70) | 2 (4.26) | |
| No | 66 (94.69) | 21 (91.30) | 45 (95.74) | |
| Metastasis | 0.022* | |||
| Yes | 6 (8.57) | 5 (21.74) | 1 (2.13) | |
| No | 64 (91.43) | 18 (78.26) | 46 (97.87) | |
| Survival status | 0.325 | |||
| Alive | 65 (90.28) | 20 (83.33) | 45 (93.75) | |
| Dead | 7 (9.72) | 4 (16.67) | 3 (6.25) |
TFE3(+) ccRCC, ccRCC with positive TFE3 immunohistochemistry; TFE3(−) ccRCC, ccRCC with negative TFE3 immunohistochemistry; BMI, body mass index; AJCC, American Joint Committee on Cancer; WHO, World Health Organization; ISUP, International Society of Urological Pathology; RN, partial nephrectomy; NLR, neutrophil/lymphocyte ratio; Scr, serum creatinine; LVI, lymphovascular invasion; IHC, immunohistochemistry. *, 0.01 < P < 0.05; ***, P < 0.001.
The survival analysis suggested that compared with TFE3(+) ccRCC, TFE3(−) ccRCC had significantly better OS [P = 0.043, HR = 4.089, 95% CI = (0.732, 22.841)] and PFS [(P = 0.016, HR = 4.616, 95% CI = (1.113, 19.147)] (Fig. 4C and D). Specifically, the OS for TFE3(−) ccRCC patients was 97.9% (95% CI, 94.0%–100.0%) at both 1 and 2 years and 95.5% (95% CI, 89.5%–100.0%) at 3 years, and the PFS was 95.3% (95% CI, 89.3%–100.0%) at 1, 2 and 3 years. For TFE3(+) ccRCC patients, the survival probability was described above, with 1-, 2- and 3-year OS rates of 100%, 85.2% (95% CI, 70.9%–100.0%), and 77.4% (95% CI, 59.6%–100.0%) and 1-, 2- and 3-year PFS rates of 83.1% (95% CI, 69.3%–99.7%), 77.9% (95% CI, 62.5%–97.2%), and 69.3% (95% CI, 50.3%–95.4%), respectively. Overall, TFE3(+) ccRCC patients had a larger tumor diameter, higher NLR and metastatic potential, and correspondingly worse survival probability than TFE3(−) ccRCC patients.
3.4. TFE3 gene rearrangement and protein expression contribute to poor prognosis in RCC
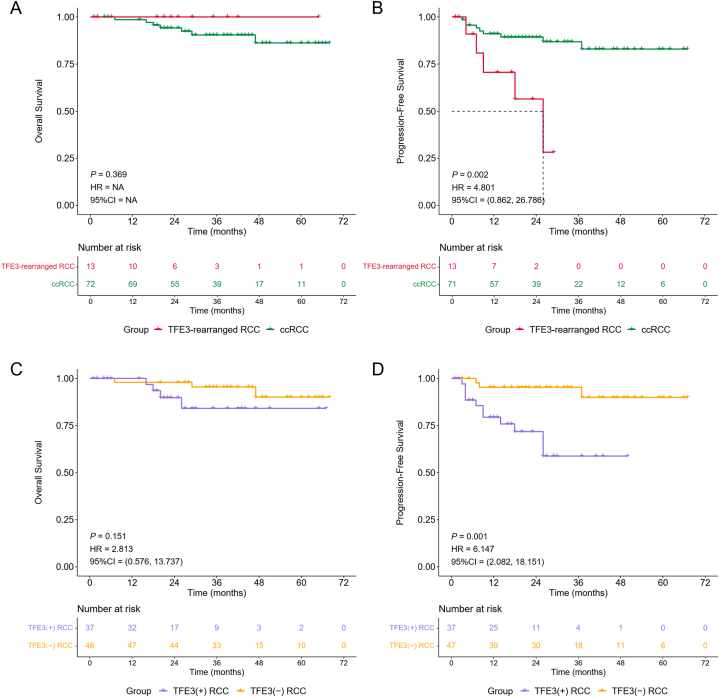
TFE3(+) ccRCC and TFE3(−) ccRCC were combined to form ccRCC, and survival analysis between TFE3-rearranged RCC and ccRCC was carried out to compare their prognosis. It was difficult to compare the OS between TFE3-rearranged RCC and ccRCC [P = 0.369, HR =NA, 95% CI = NA], since that of TFE3-rearranged RCC was maintained at 100% during the follow-up period. However, we found that the PFS of TFE3-rearranged RCC patients was worse than that of ccRCC patients [P = 0.002, HR = 4.804, 95% CI = (0.862, 26.786)], indicating that TFE3 gene rearrangement contributes to a poor prognosis in RCC (Fig. 5A and B). The OS (maintained at 100%) and PFS of TFE3-rearranged RCC were 70.7% (95% CI, 47.6%–100.0%), 70.7% (95% CI, 47.6%–100.0%) and 56.6% (95% CI, 31.3%–100.0%) at 1, 2 and 3 years, respectively. For ccRCC at 1, 2 and 3 years, the OS was 98.6% (95% CI, 95.8%–100.0%), 94.2% (95% CI, 88.8%–99.9%), and 90.5% (95% CI, 83.5%–98.1%), respectively, and the PFS was 91.1% (95% CI, 84.5%–98.2%), 89.4% (95% CI, 82.3%–97.2%), and 86.9% (95% CI, 78.7%–96.1%), respectively.
Fig. 5.
Subgroup analysis of Kaplan‒Meier survival curves stratified by cancer type: TFE3-rearranged RCC vs ccRCC (A–B) and TFE3(+) RCC vs TFE3(−) RCC (C–D).
Meanwhile, survival analysis between TFE3(−) RCC and TFE3(+) RCC, which included TFE3-rearranged RCC and TFE3(+) ccRCC, was performed to explore whether TFE3 protein expression affected prognosis (Fig. 5C and D). The OS of TFE3-rearranged RCC was not significantly different between TFE3(+) ccRCC and TFE3(−) RCC; however, it still showed a trend that TFE3(+) ccRCC had a worse OS [P = 0.151, HR = 2.813, 95% CI = (0.576, 13.737)]. In terms of PFS, TFE3(+) ccRCC showed a worse prognosis than TFE3(−) RCC, with a statistically significant difference [P = 0.001, HR = 6.147, 95% CI = (2.082, 18.151)], indicating that positive TFE3 protein expression contributes to a poor prognosis in RCC. While the prognosis data of TFE3(−) RCC were presented above, the OS and PFS of TFE3(+) RCC were as follows: the OS at 1, 2 and 3 years was 96.8% (95% CI, 90.8%–100.0%), 89.8% (95% CI, 79.5%–100.0%), and 84.2% (95% CI, 70.6%–100.0%), respectively, and the PFS was 79.4% (95% CI, 66.9%–94.3%), 71.8% (95% CI, 57.6%–89.6%), and 58.8% (95% CI, 41.2%–83.9%), respectively. In summary, TFE3 gene rearrangement and protein expression contribute to a poor prognosis in RCC.
3.5. The combination of TFE3 and LVI may be a new risk stratification system for RCC
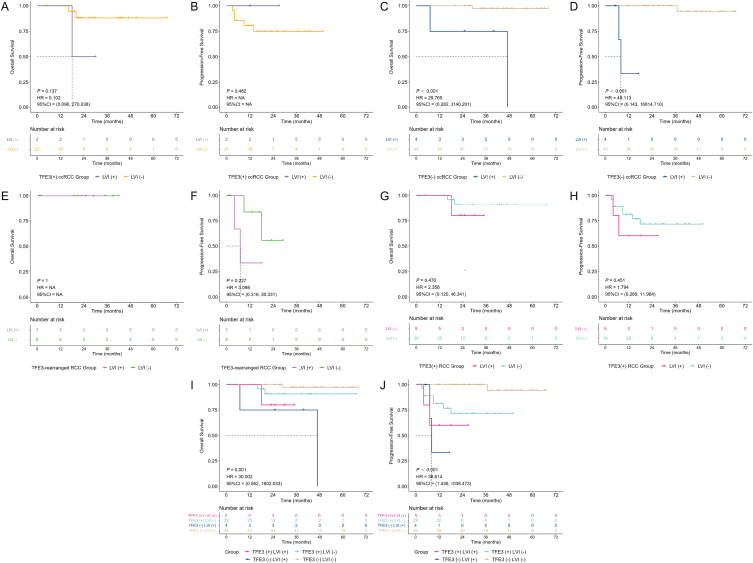
Since previous studies have shown that LVI is a marker indicating a poor prognosis of RCC [25,26], we were interested in the results of survival analysis of patients in the current study stratified by LVI. For TFE3(+) ccRCC patients, there was no statistically significant difference in either OS [P = 0.137, HR = 5.102, 95% CI = (0.096, 270.038)] or PFS [P = 0.482, HR = 4. NA, 95% CI = NA] between LVI(+) and LVI(−), in which the LVI(+) patients tended to have a worse OS, while LVI(−) patients tended to have a worse PFS (Fig. 6A and B). This may be because there were only 2 cases of LVI(+) TFE3(+) ccRCC patients, leading to the insignificant role of LVI in TFE3(+) ccRCC. Among TFE3(−) ccRCC patients, the OS [P < 0.001, HR = 29.765, 95% CI = (0.282, 3140.201)] and PFS [P < 0.001, HR = 49.113, 95% CI = (0.143, 16814.710)] of LVI(+) patients were worse than those of LVI(−) patients, both with statistically significant differences, consistent with the trend of previous studies (Fig. 6C and D).
Fig. 6.
Subgroup analysis of Kaplan‒Meier survival curves stratified by lymphovascular invasion (LVI) of TFE3(+) ccRCC (A–B), TFE3(−) ccRCC (C–D), TFE3-rearranged RCC (E–F), and TFE3(+) RCC (G–H) and stratified by the combination of TFE3 and LVI (I–J).
The prognostic role of LVI in TFE3-rearranged RCC is not clear, and in the current study, in which the number of LVI(+) and LVI(−) TFE3-rearranged RCC patients was 3 and 8, no significant difference was found for both OS and PFS between them, and the important role LVI plays may be masked by the insufficient number of cases (Fig. 6E and F). As all TFE3-rearranged RCC patients survived, survival analysis for OS stratified by LVI was not meaningful [P = 1.000, HR = NA, 95% CI = NA] (Fig. 6E). In regard to PFS, LVI(+) TFE3-rearranged RCC patients seemed to have a worse prognosis [P = 0.227, HR = 3.098, 95% CI = (0.316, 30.331)] (Fig. 6F). When TFE3(+) ccRCC and TFE3-rearranged RCC were combined into TFE3(+) RCC for analysis, there was no significant difference in OS [P = 0.470, HR = 2.358, 95% CI = (0.120, 46.341)] and PFS [P = 0.451, HR = 1.794, 95% CI = (0.269, 11.964)] between LVI(+) and LVI(−) patients, although there was a trend of worse prognosis for LVI(+) TFE3(+) RCC (Fig. 6G and H).
Furthermore, we attempted to perform survival analysis for all patients according to the combination of TFE3 with LVI and found that there was a statistically significant difference in both OS [P = 0.001, HR = 30.002, 95% CI = (0.562, 1602.033)] and PFS [P < 0.001, HR = 38.614, 95% CI = (1.436, 1038.473)], in which the TFE3(−) LVI(−) patients had the best prognosis, followed by TFE3(+) LVI(−) patients, then TFE3(+) LVI(+) patients, and finally the TFE3(−) LVI(+) patients had the worst prognosis, showing that the prognosis of LVI(+) patients was worse than that of LVI(−) patients (Fig. 6I and J). The prognosis of TFE3(+) patients was worse than that of TFE3(−) patients among LVI(−) patients (Fig. 6I and J). However, limited by the small number of cases, TFE3(−) patients showed a worse prognosis than TFE3(+) patients in LVI(+) patients. According to our previous conclusion that TFE3 protein expression contributes to a poor prognosis in RCC, we speculated that as the number of cases increases, the prognosis of TFE3(+) patients may also show a worse trend than that of TFE3(−) patients among LVI(+) patients; however, this needs further research. Specifically, for OS, TFE3(−) LVI(−) patients decreased from 100% to 97.4% (95% CI, 92.4%–100.0%) at 29 months, TFE3(+) LVI(−) patients decreased from 100% to 95.8% (95% CI, 88.2%–100.0%) at 16 months and then to 90.8% (95% CI, 79.3%–100.0%) at 20 months, TFE3(+) LVI(+) patients decreased from 100% to 80.0% (95% CI, 51.6%–100.0%) at 18 months, and TFE3(−) LVI(+) patients decreased from 100% to 75% (95% CI, 42.6%–100.0%) at 7 months. For PFS, TFE3(−) LVI(−) patients decreased from 100% to 94.4% (95% CI, 84.4%–100.0%) at 37 months, TFE3(+) LVI(+) patients decreased from 100% to 80.0% (95% CI, 51.6%–100.0%) at 4 months and then to 60.0% (95% CI, 29.3%–100.0%) at 7 months, TFE3(−) LVI(+) patients decreased from 100% to 66.7% (95% CI, 30.0%–100.0%) at 7 months and then to 33.3% (95% CI, 6.7%–100.0%) at 8 months, and the PFS of 6-, 12-, and 18-month for TFE3(+) LVI(−) patients was 88.9% (95% CI, 77.8%–100.0%), 81.2% (95% CI, 67.5%–97.5%) and 71.5% (95% CI, 55.5%–92.2%), respectively. In summary, our current results suggest that the combination of TFE3 and LVI may be a new risk stratification system for RCC.
3.6. Two case reports of TFE3-rearranged RCC and TFE3(+) ccRCC with poor prognosis
We revealed the poor prognostic impact of TFE3 gene rearrangement and protein expression above, and here, we provide two case reports of TFE3-rearranged RCC and TFE3(+) ccRCC to facilitate a deeper understanding of these two subtypes of RCC.
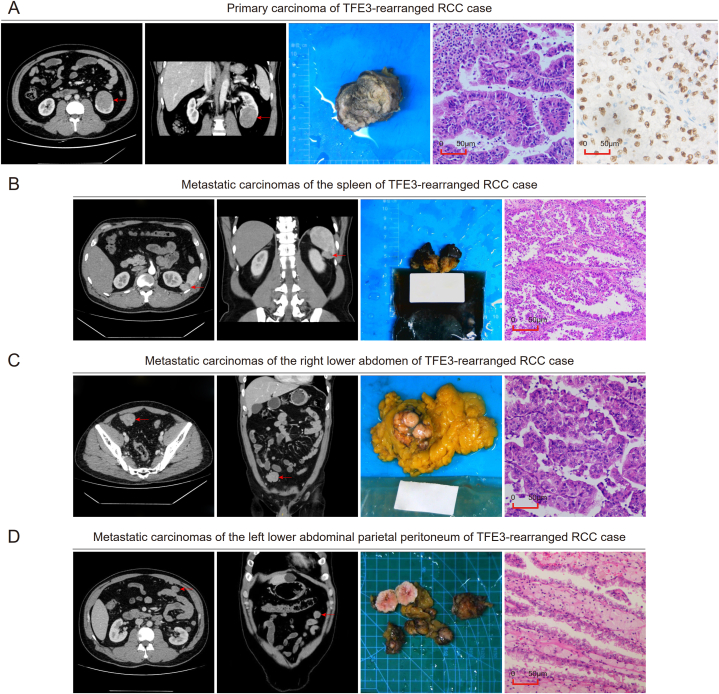
A 40-year-old male was admitted to the hospital in July 2019 with a chief complaint of “left lumbago for more than 10 days”, and the CT examination indicated “a 43 mm*43 mm*53 mm round-like tumor in the lower pole of the left kidney, considered a malignant tumor”. The postoperative pathology of subsequent robot-assisted laparoscopic partial nephrectomy of the left kidney showed left renal cell carcinoma, and TFE3-rearranged RCC was not excluded (Fig. 7A). With the help of later FISH detection, the FISH separation ratio accounted for approximately 85% of the tumor cells, and the patient was finally diagnosed with TFE3-rearranged RCC, T1bN0M0. The patient underwent regular re-examination, and the tumors were found to metastasize in the spleen (Fig. 7B), the right lower abdomen (Fig. 7C), and the left lower abdominal parietal peritoneum (Fig. 7D) 9, 12, and 19 months after the operation, respectively. The corresponding tumor resection was performed, and all pathology revealed tumors as TFE3-rearranged RCC metastases. The patient was once treated with sunitinib 11 months after the initial operation, 50 mg once a day; however, the patient stopped taking the drug one month later due to an intolerable hand-foot reaction, and no other drug treatment was taken later. Since then, the patient was re-examined in the outer hospital, and on 31 October 2022, the last follow-up time, the patient was still alive, providing information that there was no tumor recurrence or new metastasis.
Fig. 7.
Case report of TFE3-rearranged RCC. (A) Horizontal imaging, coronal imaging, gross specimen, hematoxylin-eosin staining imaging, and TFE3 immunohistochemical imaging of the primary renal carcinoma. (B–D) Horizontal imaging, coronal imaging, gross specimen, and hematoxylin-eosin staining imaging of metastatic carcinomas of the spleen (B), right lower abdomen (C), and left lower abdominal parietal peritoneum (D).
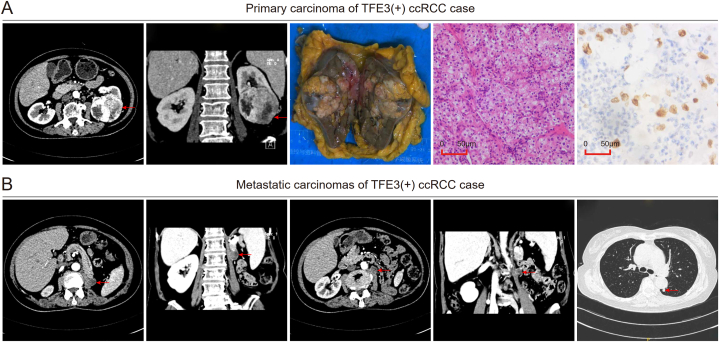
A 68-year-old female with a history of hypertension was hospitalized in June 2017 because of repeated heart palpitations for 4 months, and a soft tissue mass approximately 70 mm*62 mm in size in the lower pole of the left kidney was found in the subsequent general examination, which was considered renal cancer. Laparoscopic radical left nephrectomy was performed, with postoperative pathology in accordance with left renal cell carcinoma, and TFE3-rearranged RCC was not excluded (Fig. 8A). The patient was finally diagnosed with TFE3(+) ccRCC, T1bN0M0, because of the negative result of the later FISH detection, with the FISH separation ratio accounting for less than 15% of the tumor cells. Two masses at the L1 and L2 levels in the outer margin of the left psoas major and scattered masses in both lungs were found 4 months after the operation (Fig. 8B), all considered metastatic carcinomas. The patient did not undergo surgery or drug treatment, and she died with a survival time of 20 months.
Fig. 8.
Case report of TFE3(+) ccRCC. (A) Horizontal imaging, coronal imaging, gross specimen, hematoxylin-eosin staining imaging, and TFE3 immunohistochemical imaging of the primary renal carcinoma. (B) Horizontal and coronal imaging of the left psoas major metastatic carcinoma at the L1 and L2 levels and horizontal imaging of the pulmonary metastatic carcinoma.
4. Discussion
TFE3-rearranged RCC is a special kind of tumor with a low incidence [7,8], with no consensus about whether it has a worse prognosis than ccRCC [7,17,19,22]. In recent years, second-generation sequencing was used for the diagnosis of TFE3-rearranged RCC in some studies [[27], [28], [29]], because it can overcome the false negatives caused by FISH [15,30]. However, second-generation sequencing has not been included in the Chinese guidelines to diagnose TFE3-rearranged RCC, so it is an unreachable technology for the study. Because FISH is still the current gold standard for the diagnosis of TFE3-rearranged RCC [[14], [15], [16]], we used FISH for diagnosis in this study. By identifying patients diagnosed with FISH confirmed TFE3-rearranged RCC at SYSMH, we investigated TFE3-rearranged RCC and the role of TFE3 in renal cell carcinoma. We found that even if the tumor stage of TFE3-rearranged RCC patients is early at the first diagnosis, recurrence and new metastasis are relatively common. After dividing 37 patients suspected of TFE3-rearranged RCC into 13 TFE3-rearranged RCC and 24 TFE3(+) ccRCC by FISH, we compared these two groups and found that there was no significant difference in tumor characteristics and clinical prognosis between them, but TFE3-rearranged RCC patients tended to be younger and have a smaller preoperative NLR. This may be due to the small number of patients, so the differences between the two groups are not reflected. However, these results may also suggest that TFE3-rearranged RCC is very similar to TFE3(+) ccRCC in tumor characteristics and clinical prognosis.
Considering that some studies have mentioned that the prognosis of TFE3-rearranged RCC was worse than that of non-TFE3-rearranged RCC [17,22], including ccRCC, while there was no difference in prognosis between TFE3-rearranged RCC and TFE3(+) ccRCC in our study, we suspected that TFE3(−) ccRCC may be one of the reasons for this phenomenon. The comparison between TFE3(+) ccRCC and TFE3(−) ccRCC revealed that TFE3(+) ccRCC patients tended to have larger tumor diameters and higher NLR and metastatic potential; correspondingly, TFE3(+) ccRCC had a worse OS and PFS than TFE3(−) ccRCC, both with significant differences. By analyzing the expression of the TFE3 gene in kidney renal clear cell carcinoma (KIRC) patients in The Cancer Genome Atlas (TCGA) database, Lee et al. found that the OS and PFS of TFE3-positive patients were worse than those of TFE3-negative patients, which was similar to our results [31]. The cancer-promoting effect of TFE3 in RCC has been revealed at the cellular and molecular levels. Based on experiments in cells and human specimens, Guo et al. found that TFE3 promoted the proliferation of ccRCC cells and mediated immune evasion by positively regulating the expression of PD-L1 [32]. In addition, Li et al. confirmed the key role TFE3 plays in acquiring resistance by constructing sunitinib-resistant RCC cell lines in vivo [33]. All this indicates that ccRCC can be divided into two subgroups according to TFE3 IHC staining, in which the subgroup with positive TFE3 protein expression on IHC has a higher degree of malignancy, revealing that TFE3 protein expression may be a marker of poor prognosis for ccRCC.
When we analyzed the prognosis of TFE3-rearranged RCC and ccRCC, which combined TFE3(+) ccRCC and TFE3(−) ccRCC together, and the prognosis of TFE3(+) RCC, which combined TFE3-rearranged RCC and TFE3(+) ccRCC together, and TFE3(−) RCC, which was TFE3(−) ccRCC exactly, it was found that the PFS of TFE3-rearranged RCC was worse than that of ccRCC, and the PFS of TFE3(+) ccRCC was worse than that of TFE3(−) RCC, both with significant difference, indicating that FISH confirmed TFE3 gene rearrangement and IHC confirmed positive TFE3 protein expression would contribute a poor prognosis to RCC. Previous studies have revealed results consistent with ours. Choo et al. performed PSM at a ratio of 3 to match 61 cases of TFE3-rearranged RCC and 183 cases of ccRCC, and later survival analysis showed that there was no difference in OS between the two groups [34]. Lee et al. compared 32 TFE3(+) RCC with 271 TFE3(−) RCC [31], Dong et al. compared 91 TFE3(+) RCC with 705 TFE3(−) RCC [29], and they consistently found poor PFS in TFE3(+) RCC. Although the prognosis of ccRCC is better than that of TFE3-rearranged RCC, the prognosis of TFE3(+) ccRCC is worse than that of TFE3(−) ccRCC and similar to that of TFE3-rearranged RCC; therefore, it seems wise to take more active subsequent treatment when renal tumors are immunohistochemically positive for TFE3, regardless of whether they can be distinguished as TFE3-rearranged RCC or ccRCC.
Considering that some studies have shown that LVI is an indicator of poor prognosis in renal tumors [24,25], combined with our findings that TFE3 protein expression contributes to a poor prognosis in RCC, we were quite interested in the impact of the combination of TFE3 and LVI on the prognosis of RCC. We found that the order of good prognosis was TFE3(−) LVI(−), TFE3(+) LVI(−), TFE3(+) LVI(+) and TFE3(−) LVI(+), with statistically significant differences for both OS and FPS. In the current study, the prognosis of LVI(+) patients was worse than that of LVI(−) patients. The prognosis of TFE3(+) patients was worse than that of TFE3(−) patients among LVI(−) patients. Limited by the small number of cases, TFE3(−) patients show worse prognosis than TFE3(+) patients in LVI(+) patients; however, we speculated that the prognosis of TFE3(+) patients may also show a worse trend than TFE3(−) patients among LVI(+) patients as the number of cases increases, as TFE3 protein expression would contribute a poor prognosis to RCC. No research has mentioned the prognostic role of the combination of TFE3 and LVI, and we are the first to propose that it may be a new risk stratification system for RCC; however, further validation of a larger cohort is needed.
There are still some limitations in our study. First, all patients involved came from SYSMH, and patients from a single center may lead to a certain selection bias. Second, limited by objective factors, we used FISH, rather than second-generation sequencing, to diagnose TFE3-rearranged RCC in the current study, which may cause FISH-negative TFE3-rearranged RCC to be undetectable. Third, the number of patients included in our study was small, causing our analysis results to provide only a certain amount of reference and early mining. Finally, since the date of hospital visit we identified ranged from October 2016 to September 2022, combined with the cutoff date of 31 October 2022, we failed to provide long-term follow-up information.
5. Conclusions
Overall, we found that both FISH confirmed TFE3 gene rearrangement RCC (TFE3-rearranged RCC) and TFE3-positive [TFE3(+)] ccRCC have a worse prognosis than TFE3-negative [TFE3(−)] ccRCC, suggesting that active treatment and surveillance are needed when the TFE3 protein is positive in renal tumors. In addition, we found that the combination of TFE3 and LVI may be a new risk stratification system for RCC; however, further research is needed.
Ethical approval and consent to participate
The studies were conducted in accordance with recognized ethical guidelines and approved by the Ethics Committees of Sun Yat-sen Memorial Hospital of Sun Yat-sen University (approval number: SYSKY-2023-247-01).
Author contribution statement
Junyi Lin; Zhuang Tang; Chengjunyu Zhang: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Wen Dong; Yeqing Liu; Hao Huang; Hao Liu: Contributed reagents, materials, analysis tools or data.
Jian Huang; Tianxin Lin; Xu Chen: Conceived and designed the experiments.
Funding statement
This study was supported by the National Key Research and Development Program of China (Grant No. 2022YFC2408300), the National Natural Science Foundation of China (Grant Nos. 82273421, 81825016, 82072827, U21A20383), Guangdong Basic and Applied Basic Research Foundation (Grant Nos. 2021B1515020009, 2020A1515010888), Key Research and Development Program of Guangdong (Grant Nos. 2018B010109006), Science and Technology Program of Guangzhou (Grant No. 202102010002), Guangdong Provincial Clinical Research Center for Urological Diseases (Grant No. 2020B1111170006), Guangdong Science and Technology Department (Grant Nos. 2020B1212060018, 2018B030317001, 2017B030314026), and Fundamental Research Funds for the Central Universities, Sun Yat-sen University (23ykbj002 for Xu Chen).
Data availability statement
Data will be made available on request.
Additional information
No additional information is available for this paper.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors thank Prof. Jianming Li and Dr. Xinxin He (Department of Urology, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University) for providing technical assistance.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e16076.
Contributor Information
Jian Huang, Email: huangj8@mail.sysu.edu.cn.
Tianxin Lin, Email: lintx@mail.sysu.edu.cn.
Xu Chen, Email: chenx457@mail.sysu.edu.cn.
Abbreviations
- TFE3-rearranged RCC
TFE3-rearranged renal cell carcinoma
- ccRCC
clear cell renal cell carcinoma
- TFE3(+) ccRCC
ccRCC with positive TFE3 protein expression on immunohistochemistry
- TFE3(−) ccRCC
ccRCC with negative TFE3 protein expression on immunohistochemistry
- pRCC
papillary renal cell carcinoma
- WHO
World Health Organization
- ISUP
International Society of Urological Pathology
- SYSMH
Sun Yat-sen Memorial Hospital
- OS
overall survival
- PFS
progression-free survival
- IHC
immunohistochemistry
- FISH
fluorescence in situ hybridization
- PSM
propensity score matching
- LVI
lymphovascular invasion
- BMI
body mass index
- AJCC
American Joint Committee on Cancer
- RN
partial nephrectomy
- NLR
neutrophil/lymphocyte ratio
- Scr
serum creatinine
- AGR
albumin/globulin ratio
- IQR
interquartile range
- CI
confidence interval
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Argani P., et al. A distinctive pediatric renal neoplasm characterized by epithelioid morphology, basement membrane production, focal HMB45 immunoreactivity, and t(6;11)(p21.1;q12) chromosome translocation. Am. J. Pathol. 2001;158(6):2089–2096. doi: 10.1016/S0002-9440(10)64680-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Argani P., et al. Primary renal neoplasms with the ASPL-TFE3 gene fusion of alveolar soft part sarcoma: a distinctive tumor entity previously included among renal cell carcinomas of children and adolescents. Am. J. Pathol. 2001;159(1):179–192. doi: 10.1016/S0002-9440(10)61684-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Argani P., et al. PRCC-TFE3 renal carcinomas: morphologic, immunohistochemical, ultrastructural, and molecular analysis of an entity associated with the t(X;1)(p11.2;q21) Am. J. Surg. Pathol. 2002;26(12):1553–1566. doi: 10.1097/00000478-200212000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Inamura K. Translocation renal cell carcinoma: an update on clinicopathological and molecular features. Cancers. 2017;9(9) doi: 10.3390/cancers9090111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moch H., et al. The 2022 World Health organization classification of tumours of the urinary system and male genital organs-part a: renal, penile, and testicular tumours. Eur. Urol. 2022;82(5):458–468. doi: 10.1016/j.eururo.2022.06.016. [DOI] [PubMed] [Google Scholar]
- 6.Alaghehbandan R., Siadat F., Trpkov K. What's new in the WHO 2022 classification of kidney tumours? Pathologica. 2023 doi: 10.32074/1591-951x-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sukov W.R., et al. TFE3 rearrangements in adult renal cell carcinoma: clinical and pathologic features with outcome in a large series of consecutively treated patients. Am. J. Surg. Pathol. 2012;36(5):663–670. doi: 10.1097/PAS.0b013e31824dd972. [DOI] [PubMed] [Google Scholar]
- 8.Zhong M., et al. Dual-color, break-apart FISH assay on paraffin-embedded tissues as an adjunct to diagnosis of Xp11 translocation renal cell carcinoma and alveolar soft part sarcoma. Am. J. Surg. Pathol. 2010;34(6):757–766. doi: 10.1097/PAS.0b013e3181dd577e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun H., Wei X., Zeng C. Autophagy in Xp11 translocation renal cell carcinoma: from bench to bedside. Mol. Cell. Biochem. 2021;476(12):4231–4244. doi: 10.1007/s11010-021-04235-w. [DOI] [PubMed] [Google Scholar]
- 10.Argani P., et al. Aberrant nuclear immunoreactivity for TFE3 in neoplasms with TFE3 gene fusions: a sensitive and specific immunohistochemical assay. Am. J. Surg. Pathol. 2003;27(6):750–761. doi: 10.1097/00000478-200306000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Martignoni G., et al. Differential expression of cathepsin K in neoplasms harboring TFE3 gene fusions. Mod. Pathol. 2011;24(10):1313–1319. doi: 10.1038/modpathol.2011.93. [DOI] [PubMed] [Google Scholar]
- 12.Argani P., et al. Xp11 translocation renal cell carcinoma (RCC): extended immunohistochemical profile emphasizing novel RCC markers. Am. J. Surg. Pathol. 2010;34(9):1295–1303. doi: 10.1097/PAS.0b013e3181e8ce5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X.M., et al. TRIM63 is a sensitive and specific biomarker for MiT family aberration-associated renal cell carcinoma. Mod. Pathol. 2021;34(8):1596–1607. doi: 10.1038/s41379-021-00803-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rao Q., et al. TFE3 break-apart FISH has a higher sensitivity for Xp11.2 translocation-associated renal cell carcinoma compared with TFE3 or cathepsin K immunohistochemical staining alone: expanding the morphologic spectrum. Am. J. Surg. Pathol. 2013;37(6):804–815. doi: 10.1097/PAS.0b013e31827e17cb. [DOI] [PubMed] [Google Scholar]
- 15.Tretiakova M.S. Chameleon TFE3-translocation RCC and how gene partners can change morphology: accurate diagnosis using contemporary modalities. Adv. Anat. Pathol. 2022;29(3):131–140. doi: 10.1097/pap.0000000000000332. [DOI] [PubMed] [Google Scholar]
- 16.Simonaggio A., et al. MiTF/TFE translocation renal cell carcinomas: from clinical entities to molecular insights. Int. J. Mol. Sci. 2022;23(14) doi: 10.3390/ijms23147649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng J., et al. Computational analysis of pathological images enables a better diagnosis of TFE3 Xp11.2 translocation renal cell carcinoma. Nat. Commun. 2020;11(1):1778. doi: 10.1038/s41467-020-15671-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyer P.N., et al. Xp11.2 translocation renal cell carcinoma with very aggressive course in five adults. Am. J. Clin. Pathol. 2007;128(1):70–79. doi: 10.1309/lr5g1vmxpy3g0cuk. [DOI] [PubMed] [Google Scholar]
- 19.Ellis C.L., et al. Clinical heterogeneity of Xp11 translocation renal cell carcinoma: impact of fusion subtype, age, and stage. Mod. Pathol. 2014;27(6):875–886. doi: 10.1038/modpathol.2013.208. [DOI] [PubMed] [Google Scholar]
- 20.Song H.C., et al. Biological characteristics of pediatric renal cell carcinoma associated with Xp11.2 translocations/TFE3 gene fusions. J. Pediatr. Surg. 2014;49(4):539–542. doi: 10.1016/j.jpedsurg.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Caliò A., et al. MiT family translocation renal cell carcinoma: from the early descriptions to the current knowledge. Cancers. 2019;11(8) doi: 10.3390/cancers11081110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu L., et al. Xp11.2 translocation renal cell carcinomas in young adults. BMC Urol. 2015;15:57. doi: 10.1186/s12894-015-0055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parola R., et al. Trauma risk score matching for observational studies in orthopedic trauma dataset and code. Data Brief. 2022;40 doi: 10.1016/j.dib.2022.107794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brookman-May S., et al. Features associated with recurrence beyond 5 years after nephrectomy and nephron-sparing surgery for renal cell carcinoma: development and internal validation of a risk model (PRELANE score) to predict late recurrence based on a large multicenter database (CORONA/SATURN Project) Eur. Urol. 2013;64(3):472–477. doi: 10.1016/j.eururo.2012.06.030. [DOI] [PubMed] [Google Scholar]
- 25.Bedke J., et al. Microvascular and lymphovascular tumour invasion are associated with poor prognosis and metastatic spread in renal cell carcinoma: a validation study in clinical practice. BJU Int. 2018;121(1):84–92. doi: 10.1111/bju.13984. [DOI] [PubMed] [Google Scholar]
- 26.Belsante M., et al. Lymphovascular invasion in clear cell renal cell carcinoma–association with disease-free and cancer-specific survival. Urol. Oncol. 2014;32(1) doi: 10.1016/j.urolonc.2012.11.002. 30.e23-8. [DOI] [PubMed] [Google Scholar]
- 27.Tretiakova M.S., et al. Gene fusion analysis in renal cell carcinoma by FusionPlex RNA-sequencing and correlations of molecular findings with clinicopathological features. Genes Chromosomes Cancer. 2020;59(1):40–49. doi: 10.1002/gcc.22798. [DOI] [PubMed] [Google Scholar]
- 28.Di Mauro I., et al. RBM10-TFE3 fusions: a FISH-concealed anomaly in adult renal cell carcinomas displaying a variety of morphological and genomic features: comprehensive study of six novel cases. Genes Chromosomes Cancer. 2021;60(11):772–784. doi: 10.1002/gcc.22985. [DOI] [PubMed] [Google Scholar]
- 29.Dong X., et al. Clinicopathological features and prognosis of TFE3-positive renal cell carcinoma. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.1017425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X.T., et al. RNA sequencing of Xp11 translocation-associated cancers reveals novel gene fusions and distinctive clinicopathologic correlations. Mod. Pathol. 2018;31(9):1346–1360. doi: 10.1038/s41379-018-0051-5. [DOI] [PubMed] [Google Scholar]
- 31.Lee H.J., et al. TFE3 translocation and protein expression in renal cell carcinoma are correlated with poor prognosis. Histopathology. 2018;73(5):758–766. doi: 10.1111/his.13700. [DOI] [PubMed] [Google Scholar]
- 32.Guo X., et al. TFE3-PD-L1 axis is pivotal for sunitinib resistance in clear cell renal cell carcinoma. J. Cell Mol. Med. 2020;24(24):14441–14452. doi: 10.1111/jcmm.16066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li L., et al. Sunitinib treatment promotes metastasis of drug-resistant renal cell carcinoma via TFE3 signaling pathway. Cell Death Dis. 2021;12(2):220. doi: 10.1038/s41419-021-03511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choo M.S., et al. Clinicopathologic characteristics and prognosis of Xp11.2 translocation renal cell carcinoma: multicenter, propensity score matching analysis. Clin. Genitourin. Cancer. 2017;15(5):e819–e825. doi: 10.1016/j.clgc.2017.04.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.