Abstract
Obesity and related metabolic diseases represent a worldwide health problem. The main factor predisposing to obesity is an unhealthy lifestyle including the lack of physical activity. A pivotal role in the etio-pathogenesis of obesity is carried out by adipose tissue, an endocrine organ secreting several adipokines involved in numerous metabolic and inflammatory processes. Among these, of particular importance is adiponectin, an adipokine involved in the regulation of insulin sensibility and in anti-inflammatory processes. The aim of the study was to determine the effects of 24 weeks of two different training programs polarized (POL) and threshold training (THR) on body composition, physical capacities and adiponectin expression. Thirteen male obese subjects (BMI: 32.0 ± 3.0 kg m-2) followed 24 weeks of two different training programs, POL and THR, consisting of walking or running (or a combination of the two methods) in their normal living conditions. Before (T0) and after the end of the program (T1), the assessment of body composition was assessed by bioelectrical impedance and the concentration of salivary and serum adiponectin was analyzed by enzyme-linked immunosorbent assay and western blotting. Although the results obtained did not show significant differences between the two training programs, body mass and body mass index decreased by a mean of −4.46 ± 2.90 kg and 1.43 ± 0.92 kg m−2 (P < 0.05). Fat mass decreased by −4.47 ± 2.78 kg (P < 0.05). V′O2max increased by a mean of 0.20 ± 0.26 L min−1 (P < 0.05) Also, we observed an increase in saliva and in serum of adiponectin concentrations at T1 compared to T0 by 4.72 ± 3.52 μg mL−1 and 5.22 ± 4.74 ng mL−1 (P < 0.05) respectively. Finally, we found significant correlations between Δ serum adiponectin and Δ Hip (R = −0.686, P = 0.001) and between Δ salivary adiponectin and ΔWaist (R = −0.678, P = 0.011). Our results suggest that a 24 weeks training program, independently from intensity and volume, induces an amelioration of body composition and fitness performance. These improvements are associated with an increase in total and HMW adiponectin expression in both saliva and in serum.
Keywords: Physical activity, Adiponectin, Saliva, Body composition, Obesity
1. Background
The global prevalence of obesity is alarmingly increasing worldwide independently of gender, age and race ([1]). The development of a different but associated variety of adverse sequelae, including metabolic syndrome, type II diabetes (T2D), dyslipidemia, cardiovascular disease, and different cancers, is making obesity more impactful in terms of morbidity, and mortality ([2,3]) worldwide. Among the main factors predisposing to obesity there are unhealthy lifestyle, including the lack of physical activity and a sedentary lifestyle ([4]). On the other hand, there is increasing evidence that physical activity and exercise are broadly as effective as pharmacological interventions in preventing the adverse sequelae (including metabolic syndrome, T2D, cardiovascular diseases, and different cancers) associated with obesity ([3,[5], [6], [7]]). A recent meta-analysis evidenced that lifestyle modification, including physical activity, can lead to significant improvement in obesity and inflammation status among overweight/obese individuals ([8]). Indeed, both endurance and resistance training programs result a common treatment method for improving the health status of individual that are obese or overweight, and/or with metabolic issues ([7]).
The molecular basis underlying physical activity in the control of weight gain are still not completely known and represent an open field of research. Previous studies showed a direct effect of physical activity on adipose tissue metabolism ([9]). Adipose tissue is an active endocrine tissue secreting adipokines involved in regulation of different processes such as energy metabolism, and inflammation ([10]). The endocrine functions of adipose tissue are dysregulated in both overweight and obesity and are crucial in the prevention and/or in pathogenesis of the metabolic diseases ([10]). Physical activity is able to stimulate the production and secretion of adipokines improving the endocrine functions of adipose tissue ([11]). One of the obesity-associated hormones is adiponectin, whose serum levels are inversely related to body mass index (BMI) and insulin resistance. Adiponectin accounts for up to 0.05% of the total serum proteins in circulation, where it can be found in three oligomeric forms, with different molecular weight: trimers of Low Molecular Weight (LMW), hexamers of Medium Molecular Weight (MMW), and oligomers of High Molecular Weight (HMW). These latter elicit the most relevant biological activity of adiponectin, consisting in the homeostatic regulation of glucose levels, lipid metabolism, and insulin sensitivity. Adiponectin levels are highly decreased in obesity and metabolic-related disorders while weight loss leads to increasing adiponectin levels ([12]).
Whether physical exercise exerts its beneficial effects also through the regulation of adiponectin, regardless of modifications in body composition is still a matter of debate. In addition, it was well known that the type of physical exercise (aerobic/anaerobic) differently influences body composition, cardiometabolic health and hormone balance ([13]). Some studies, performed on patients with obesity and involving high intensity interval training (HIIT) and moderate intensity continuous training (MICT), showed positive changes in patient's body composition, maximal oxygen uptake (V’O2max) and adiponectin levels ([[14], [15], [16]]), while others demonstrated that adiponectin concentrations do not change after long-term exercise ([17]). However, to our knowledge, no studies examined the effects of a Polarized (POL) vs a Moderate (threshold training (THR) modified by Veronique Billat) ([18]), volume/intensity program on body composition, and cardiometabolic parameters in relation to adiponectin expression. The POL training program consists of high volume of training at moderate intensity (i.e., approximately 80% of the volume) and the remaining 20% conducted at severe intensity domain ([19]) while the THR training consisted by a higher prevalence of training between heavy and severe intensity domain (i.e., THR with ≥20% of overall volume conducted at intensity between the ventilatory thresholds) ([20]).
Thus, the aim of the present study was to determine the effects of 24 weeks of POL and THR training programs on body composition, physical capacities and adiponectin values in saliva and in serum from male adult obese subjects at T0 (before starting the program) and at T1 (at the end of the program). In addition, the adiponectin oligomeric distribution was related to metabolic and fitness features. The two programs were selected in the light of some studies that emphasized how trainings combining the low volume activity of HIIT, and the high volume of MICT (POL) represent a useful strategy to improve body composition, more effective that HIIT or MICT modalities alone ([3,21,22]). We have analyzed saliva since it constitutes a clinically informative, biological fluid containing specific soluble biomarkers. As a biological fluid, saliva offers several advantages over blood: it is easily collected and stored and therefore stress-free for patients; we therefore analyzed and compared both biological fluids.
2. Methods
2.1. Participants and sample collection
Thirteen male subjects with obesity, part of a previous larger study including twenty subjects (under review) were recruited for the present study from the School of Sport Sciences of the University of Udine (mean age 40.8 ± 6.2 years; mean BMI: 32.0 ± 3.0 kg m−2). All subjects had a full medical history, physical and nutritional examination. Body mass (BM) was stable during the previous two months. The inclusion criteria were: 1) age between 18 and 50 years, 2) BMI ≥30 kg m−2according to ESPEN guidelines (Obes Facts. 2015; 8(6):402-24. https://doi.org/10.1159/000442721), and 3) to be moderately physically active (i.e., perform continuous aerobic activity longer than 20 min at least twice a week) based on International Physical Activity Questionnaire Short Form (IPAQ-SF) ([23]). Conversely, exclusion criteria were the presence of cardiovascular, respiratory, neurologic, muscular-skeletal diseases. All participants were male and Caucasians. The study was approved by the Ethics Committee of the Friuli-Venezia-Giulia Region (Italy) (protocol number 1764) and conducted according to the ethical principles of the Declaration of Helsinki. Informed consent was obtained from all participants before the study began.
Before (T0) and after the end (T1) the training period, blood samples were collected after a 12-h overnight fasting period and centrifuged to collect serum. Serum aliquots were immediately frozen in liquid nitrogen and stored at −80 °C. For saliva samples, participants were asked not to consume food and drinks for at least 2.5 h before 10:30 a.m. Drinking water was allowed before saliva collection began. Subsequently, participants were asked to brush their teeth (new toothbrushes and toothpaste were provided and they were the same for all donors) and finally to rinse their mouth several times with tap water to avoid any contamination from food residues or toothpaste flavourings. After 1 h from tooth brushing, saliva samples were collected, centrifuged at 13.200 rpm and stored at −80 °C for the subsequent analysis. Assessment of anthropometric characteristics together with blood and saliva sample's collection were conducted at T0 and T1 under medical supervision.
During the 24 weeks of the weight management program, each participant received the same nutritional advices to avoid confounding nutritional variables on the outcomes. All participants completed a 4-days dietary record (4-dDR), collecting the food and beverage consumption of 2-week days and 2 weekend days, at T0 and T1.
2.2. Training program
Participants involved in the present study (n: 7 POL and n: 6 THR) performed 3 sessions per week, for 24 consecutive weeks, by walking or running (or a combination of the two methods) in their normal living conditions. The 2 training programs were divided in three 8-week macrocycles, structured as 3 + 1 mesocycles. In both groups, during the first two 8-week macrocycles, training load (TL) increased by ∼30% in the first three weeks of each mesocycles. Between the second and the third 8-week macrocycle, TL increased by ∼10%. The last week of each mesocycle was a recovery week. Every 8-weeks, the three zones model was used for the calculation of the training intensity distribution (TID) using the speed reached at gas exchange threshold (GET), respiratory compensation point (RCP) and V’O2max ([24]): zone 1 (Z1), for intensities below GET; zone 2 (Z2), for intensities between GET and RCP; and zone 3 (Z3), for intensities above RCP. To check the POL TID we used the polarization index (i.e. polarized: Z1 > Z3 > Z2) (25). POL and THR TID programs were matched for the same TL obtained by the training impulse (TRIMP), where each zone has a weighting factor that is multiplied by the duration in this zone ([14]). During the 24 weeks of training, all participants recorded their workouts using an online training diary Polar Flow (Polar Electro Oy, Finland) or Gamin Connect (Garmin, Olathe, USA). The training was checked online registering the training sessions: duration, time spent in each endurance training zone, and rate of perceived exertion (RPE) using the Borg 6–20 Scale ([25]). For maintaining the intensity prescribed, the speed in each zone was increased when the mean HR decreased by 5 bpm for two consecutive training sessions.
2.3. Anthropometric and adiponectin measurements
The anthropometric and biochemical parameters of the study participants are shown in Table 1. Body mass (BM) was measured with a manual weighing scale (approximation 0.1 kg) (Seca 709, Hamburg, 165 Germany). Measurement of stature was performed using a wall-mounted height board. BMI was calculated as BM (kg) × stature (m−2). Measurement of waist circumference (WC) and Hip circumference (HC) was performed using the method of Kagawa ([26]). Body composition assessment was performed using the bioelectrical impedance (BIA, Human IM Plus; DS 171 Dietosystem, Milan, Italy). The values of fat mass (FM) and fat free mass (FFM) were obtained with equations of Gray et al. ([27]).
Table 1.
Anthropometric characteristics and adiponectin values before (T0), and after 24 weeks (T1) of a training program in both groups.
| All (n: 13) |
p value | ||||||
|---|---|---|---|---|---|---|---|
| T0 | T1 | ||||||
| Body mass (kg) | 99.0 | ± | 10.8 | 94.6 | ± | 11.5 | 0.001 |
| BMI (kg m− [2]) | 32.0 | ± | 3.0 | 30.6 | ± | 3.0 | 0.001 |
| Waist (cm) | 103.0 | ± | 6.7 | 102.0 | ± | 7.8 | 0.301 |
| Hip (cm) | 109.0 | ± | 4.7 | 108.2 | ± | 5.7 | 0.480 |
| Waist-to-hip ratio | 0.95 | ± | 0.05 | 0.94 | ± | 0.04 | 0.781 |
| Fat-free mass (kg) | 64.1 | ± | 4.8 | 64.5 | ± | 4.8 | 0.857 |
| Fat Mass (kg) | 34.6 | ± | 7.2 | 30.1 | ± | 7.6 | 0.001 |
| Fat-free mass (%) | 65.2 | ± | 3.9 | 68.6 | ± | 4.2 | 0.001 |
| Fat Mass (%) | 35.0 | ± | 3.9 | 31.5 | ± | 4.2 | 0.001 |
| Serum adiponectin (μg ml−1) | 22.3 | ± | 4.5 | 27.0 | ± | 2.6 | 0.001 |
| Salivary adiponectin (ng ml−1) | 17.6 | ± | 7.5 | 22.8 | ± | 10.0 | 0.005 |
All values are presented as mean ± standard deviation.
BMI: body mass index.
p value obtained with student's test for paired data.
The concentration of total adiponectin in serum and in saliva was measured two times in each individual in triplicate by an enzyme-linked immunosorbent assay (ELISA) using a polyclonal antibody produced in-house versus a human adiponectin amino acid fragment (H2N-ETTTQGPGVLLPLPKG-COOH) as previously described ([28]).
2.4. Graded exercise test (GRAD)
A graded exercise test on a 400 m track was carried out to determine physical capacities of each subject: V’O2max, heart rate max (HRmax), and ventilatory thresholds. During the test, participants followed a researcher on a bike that gave the pace. The duration of each step was 1 min, and the speed increased by 0.5 km/h every minute until the volitional exhaustion. Wearable metabolic unit (K5; Cosmed, Roma, Italy) and a chest strap (Garmin HRMrun, Olathe, USA) were used to measure oxygen uptake (V’O2), carbon dioxide production (V’CO2) and heart rate (HR). We calibrated the volume and gas analyzers before every test using a 3-L calibration syringe and calibration gas (16.00% O2 and 5.00% C’O2), respectively. GET and RCP were determined with the V-slope method. V’O2max was calculated as the average 30-s V’O2 according to the following criteria ([29]): (i) plateau in V’O2 (i.e., increase <150 ml min−1), (ii) Respiratory Exchange Ratio (RER) > 1.1, and (iii) ≥ 90% of theoretical maximal heart rate.
2.5. Western blotting analysis
Serum and saliva samples from all participants were quantified for total proteins by Bradford's method (Bio-Rad, Hercules, CA, USA) and 10 μg of total proteins were treated with 1× Laemmli buffer, heated at 95 °C for 10 min and loaded on 10% SDS-PAGE gel as previously described [30]. The blots were developed by ECL (Amersham Biosciences, Piscataway, NJ, USA) using Kodak BioMax Light film, digitalized with a scanner (1.200 dpi) and analyzed by densitometry with the ImageJ software (http://rsbweb.nih.gov.ij/). All experiments were performed in triplicate.
2.6. Statistical analysis
The data were analyzed using GraphPad Prism (version 9.4.0), with significance set at p < 0.05. The results obtained were expressed as means and standard deviations (SDs). Shapiro–Wilk test was used for evaluation of the normal distribution of the data. Sphericity was verified by Mauchly's test. Greenhouse–Geisser correction was used in cases of sphericity assumption violations. Anthropometric characteristics, body composition, V′O2max, ventilatory thresholds, training characteristics and blood analysis parameters were analyzed with a student's test for paired data. Bivariate associations were determined by Pearson's or Spearman's correlation coefficients (non‐normally distributed data).
3. Results
3.1. Anthropometric characteristics and cardiometabolic parameters of obese subjects
The anthropometric and biochemical parameters of the study participants are shown in Table 1. All participants were considered together in the pre-post analysis independently from the training because the 2-way ANOVA analysis between subjects performing the two programs (POL and THR groups) showed no significant differences in all considered variables.
At T1, BM and BMI statistically decreased by 4.46 ± 2.90 kg (p < 0.001) and 1.43 ± 0.92 kg m−2 (p < 0.001) (Table 1). As well, FFM (%) statistically increased by 3.32 ± 2.12% (p < 0.001) (Table 1). Then, FM (kg) and FM (%) statistically decreased by 4.47 ± 2.78 kg (p < 0.001) and by 3.32 ± 2.12% (p < 0.001) (Table 1). FFM (kg), WC, HC and waist-to-hip ratio did not change significantly (Table 1).
Regarding cardiometabolic parameters, at T1, V’O2max (L min−1) and V’O2max (L kg−1 min−1) statistically increased by 0.20 ± 0.26 L min−1 (p = 0.025) and 3.96 ± 3.48 ml kg−1 min−1 (p = 0.015) (Table 2). HRmax decreased by 4.80 ± 7.37 bpm (p = 0.040). Maximal respiratory exchange ratio (RERmax) did not change significantly (Table 2). V’O2 (ml kg−1 min−1) at RCP statistically increased by mean 3.10 ± 3.54 ml kg−1 min−1 (p = 0.008) (Table 2).
Table 2.
Cardiometabolic Parameters before (T0) and after 24 weeks (T1) of a training program in both groups.
| All (n:13) |
p value | ||||||
|---|---|---|---|---|---|---|---|
| PRE | POST | ||||||
| Maximal oxygen uptake | |||||||
| V'O2 (L min−1) | 3.89 | ± | 0.39 | 4.07 | ± | 0.44 | 0.025 |
| V'O2 (ml kg −1min−1) | 39.6 | ± | 4.4 | 43.3 | ± | 6.4 | 0.015 |
| HRmax (bpm) | 180.2 | ± | 7.7 | 175.4 | ± | 9.6 | 0.040 |
| RER max | 1.10 | ± | 0.06 | 1.09 | ± | 0.04 | 0.567 |
| Respiratory compensation point | |||||||
| V'O2 (L min−1) | 3.50 | ± | 0.35 | 3.62 | ± | 0.44 | 0.186 |
| V'O2 (ml/kg/min) | 35.6 | ± | 4.3 | 38.7 | ± | 6.0 | 0.008 |
| V'O2, %max | 89.9 | ± | 3.3 | 89.3 | ± | 5.4 | 0.743 |
| HR (bpm) | 167.2 | ± | 8.6 | 165.0 | ± | 10.2 | 0.287 |
| HR, %max | 93.0 | ± | 3.6 | 93.8 | ± | 2.6 | 0.405 |
| RER | 1.00 | ± | 0.04 | 0.99 | ± | 0.04 | 0.820 |
| Gas exchange threshold | |||||||
| V'O2 (L min−1) | 2.74 | ± | 0.46 | 3.02 | ± | 0.42 | 0.008 |
| V'O2 (ml/kg/min) | 28.2 | ± | 6.4 | 32.4 | ± | 6.0 | 0.001 |
| V'O2, %max | 70.5 | ± | 10.4 | 75.0 | ± | 8.6 | 0.107 |
| HR (bpm) | 146.5 | ± | 14.0 | 147,4 | ± | 11.6 | 0.822 |
| HR, %max | 81.2 | ± | 6.1 | 84.1 | ± | 6.4 | 0.201 |
| RER | 0.91 | ± | 0.06 | 0.91 | ± | 0.04 | 0.175 |
All values are presented as mean ± standard deviationV’O2: oxygen consumption, HR: heart rate, RER: respiratory exchange ratio, V’O2 %max: percentage of maxima oxygen uptake, HR %max: percentage of heart rate max.
p value obtained with student's test for paired data.
No statistical difference was found for V’O2 (L min−1), HR, Respiratory exchange ratio (RER), % of V’O2max and HRmax under RCP (Table 2). V’O2 (L min−1) and V’O2 (ml kg−1 min−1) at GET statistically increased by 0.28 ± 0.32 L min−1 (p = 0.008) and by 4.22 ± 2.96 ml kg−1min−1 (p < 0.001) (Table 2). In addition, no difference was found for HR, RER, % of V’O2max or HRmax at GET in (Table 2).
Total Adiponectin levels increase in saliva and in serum of obese subjects undergone training programs.
To investigate whether the circulating adiponectin levels are related to the lifestyle modifications, total salivary and serum adiponectin levels were analyzed adiponectin at T0 and at the end of the training period (T1) (Table 1); interestingly, salivary adiponectin were significantly higher in obese subjects at T1 compared to the baseline by +5.22 ± 4.74 ng mL−1 (p < 0.001).
In line with salivary findings, total serum adiponectin levels, as shown in Table 1, are significantly increased by +4.72 ± 3.52 μg mL−1 (p < 0.001) in obese subjects at T1 compared to T0.
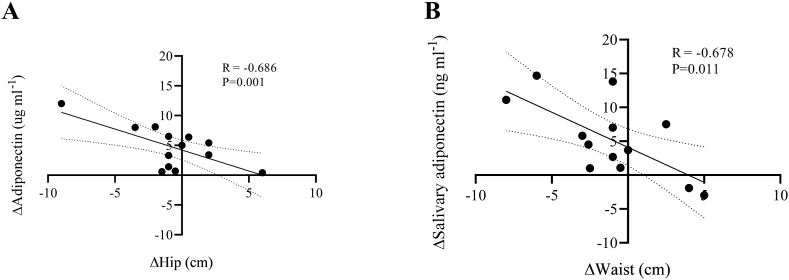
Furthermore, we investigated the potential relationships between the changes in salivary and serum adiponectin and the observed modifications in BM, BMI, FFM, FM, waist, hip, V’O2max (L min−1), and V’O2max (ml kg−1 min−1) (see Table 3). There were significant correlations between Δ serum adiponectin and Δ Hip (Fig. 1, panel A) and between Δ salivary adiponectin and ΔWaist (Fig. 1, panel B).
Table 3.
Correlations between the change (Δ) in serum adiponectin and salivary adiponectin with the change in the measured variables.
| ΔBM (kg) | ΔBMI (kg m−2) | ΔFFM (kg) | ΔFM (kg) | ΔWaist (cm) | ΔHip (cm) | ΔV'O2max (L min−1) | ΔV'O2max (ml kg-1 min−1) | |
|---|---|---|---|---|---|---|---|---|
| ΔSerum adiponectin (μg ml−1) | R = −0.200 P = 0.514 | R = −0.220 P = 0.472 | R = 0.262 P = 0.387 | R = −0.384 P = 0.195 | R = −0.394 P = 0.183 | R = -0.686 P = 0.001 | R = −0.490 P = 0.088 | R = −0.204 P = 0.505 |
| ΔSalivary adiponectin (ng ml−1) | R = −0.442 P = 0.130 | R = −0.432 P = 0.140 | R = −0.111 P = 0.718 | R = −0.497 P = 0.084 | R = -0.678 P = 0.011 | R = −0.215 P = 0.481 | R = 0.363 P = 0.223 | R = 0.451 P = 0.122 |
BM: body mass, BMI: body mass index, FFM: fat free mass, FM: fat mass, V’O2max: maximal oxygen uptake.
R value obtained with Pearson's or Spearman's correlation coefficients (non‐normally distributed data).
Bold text indicates a statistically significant correlation.
Fig. 1.
Correlation between changes in Adiponectin (ΔSerum adiponectin) and Hip (ΔHip) (panel A), and correlation between changes in salivary adiponectin (ΔSalivary adiponectin) and Waist (ΔWaist) (panel B).
3.2. HMW adiponectin oligomers increase in salivary and serum rom obese subjects
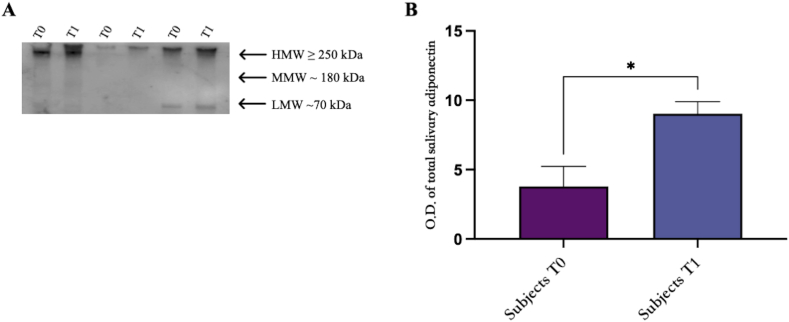
Western blotting analysis performed on saliva samples from all subjects involved in the study confirmed that the adiponectin levels are statistically increased in obese subjects (Fig. 2, panel B).
Fig. 2.
Western blotting analysis of adiponectin oligomers in saliva samples from obese subjects at baseline (T0) and post physical intervention (T1). (A) One representative image of Western blot oligomeric distribution [HMW (≥250 kDa), MMW (180 kDa), and LMW (70 kDa)] of three subjects at baseline (T0) and post physical intervention (T1). (B) Graphical representation of pixel quantization of all analyzed subjects included in the study. Pixel quantization was performed by densitometric analysis with the ImageLab software; p. < 0.01. The uncropped versions of figures [2A] is reported in Supplementary Fig. 1.
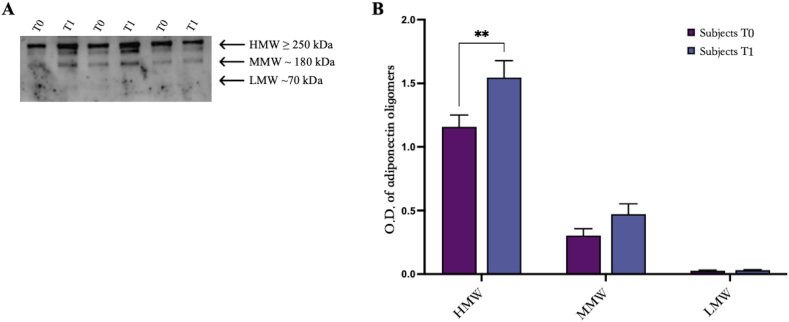
The analysis performed on serum shows three bands corresponding to HMW (≥250 kDa), MMW (≥180 kDa), and LMW (≥70 kDa) oligomers (Fig. 3, A). Also in serum, data confirmed that the adiponectin levels are statistically increased in obese subjects with a specif regard to HMW oligomers (Fig. 3, A) as indicated by the densitometric analysis (Fig. 3, B, p < 0.01).
Fig. 3.
Western blotting analysis of adiponectin oligomers in sera from obese subjects at baseline (T0) and post physical intervention (T1). (A) One representative image of Western blot oligomeric distribution [HMW (≥250 kDa), MMW (180 kDa), and LMW (70 kDa)] of three subjects at baseline (T0) and post physical intervention (T1). (B) Graphical representation of pixel quantization of all analyzed subjects included in the study. Pixel quantization was performed by densitometric analysis with the ImageLab software; p. < 0.01. The uncropped versions of figures [3A] is reported in Supplementary Fig. 1.
4. Discussion
In the present paper we have determined the effects of two different 24-weeks training programs, a high (POL) vs an intermediate (THR modified by Veronique Billat) volume/intensity program to verify the potential beneficial effects on salivary adiponectin expression, cardiometabolic capacities and body composition in male adult obese subjects. Our data shows that a 24-week of POL and THR training program determines in adults with obesity: i. an increase in serum and salivary adiponectin concentrations at T1 compared to T0; ii. an improvement in anthropometric (Fat Free Mass, Fat Mass, BMI, BM) and cardiometabolic parameters (VO2max, HRmax). However, the two different training programs did not determine any difference in terms of improvement of body composition and cardiovascular parameters suggesting that even at lower volume, 24 weeks of training is effective in obese subjects. Indeed, at the end of the six months training programs, the anthropometric parameters (BMI, FFM, FM, FM) and the cardiometabolic values (V’O2max both in absolute values or relative to BM, V’O2 at RCP and GET) improved in both groups. Previously, contrary to our findings, it was found that a POL training induced equal or superior effects on improving V’O2max and body composition in endurance athletes ([3,21]), as well in obese sedentary subjects ([5,22]), compared to MICT and HIIT modalities alone. Although a more recent study confirmed that a high-intensity physical exercise exerts more vigorous effects in the regulation of body composition compared to a moderate exercise ([5]), the high volume in Z1 performed in both groups (i.e., ∼90% for POL vs.∼70% for THR), despite the difference between the two groups, may have led to positive adaptations, such as increased of mitochondrial content and respiratory function, as a marker of an enhanced oxidative metabolism ([31,32]), closely linked to the values of V’O2max ([33]) and V’O2 at the two ventilatory thresholds ([17,22]). Thus, over a long period of time (i.e., ≥24 weeks), may be required a minimum dose of training performing at low intensity for improving body composition and physical capacities in subjects with obesity, in line with studies on endurance athletes ([34]).
However, it is also to notice that the lack of different effects between the two training programs might also be traced to: a) the limited number of participants; b) difference in the volume between the two programs was too low; c) timing of the two programs needs to be longer. In addition, as adiponectin concentrations are related to the adiposity changes, being lower in obese subjects and increasing after weight loss, the extent of weight loss following a training program might represent a confounding factor for the direct effects of physical exercise on adiponectin.
A growing body of research indicates that physical activity exerts beneficial effects also through the regulation of the endocrine functions of the adipose tissue. Indeed, it seems that adiponectin secretion is regulated by physical activity and that such event gives a contribution toward a health status of overfat individuals ([35]). However, whether the involvement of adiponectin is dependent on the type and intensity of exercise is still a matter of debate.
In our population, salivary and serum adiponectin levels are significantly increased in obese subjects at the end of both training programs (POL and THR) without significant differences. Contrary to our data, a study on 148 obese adolescents, reported that only an aerobic plus resistance training is effective in increasing adiponectin concentration compared to a resistance training only ([36]). More recently, Moghadam et al., in accordance with our data, reported that either a 12-week high-intensity interval training (HIIT) or a moderate-intensity continuous training (MICT) increased adiponectin ([30]).
The duration of the training program might also affect adiponectin regulation; Swisher et al. reported an insignificant increase in serum adiponectin following 12 weeks of moderate-intensity aerobic exercise in women ([37]). On the contrary, Cobos-Palacios et al. and Guzel et al. found that adiponectin did not change after a 24-months Mediterranean Diet (MedDiet) program and physical activity training in a pre-pubertal population with obesity ([4,38]). On the other hand, in accordance with our data, previously it has been reported that a combined exercise program (resistance and aerobic physical exercise training) of 12 weeks determines an increase in adiponectin levels, with positive correlations with the percent of lean body and negative correlations with percent body fat ([39]).
Our data outline a correlation between adiponectin expression and both body composition and cardio-metabolic parameters suggesting that the effectiveness of physical exercise in regulating the endocrine functions of adipose tissue are related to these accessory parameters.
Finally, we have evaluated serum and salivary adiponectin in relation to metabolic and fitness parameters. We showed that serum adiponectin did not correlates with VO2%max and inversely correlate with hips circumference; similarly, salivary adiponectin did not directly correlate with VO2%max and inversely correlate with waist circumference. Both models suggest that adiponectin regulation is related to the improvement of body composition in terms of adipose tissue reduction and to the beneficial effects of physical exercise on fitness variables. In line to our data, a very recent paper analyzed adiponectin mRNA expression in gluteal and abdominal adipose tissue of overweight and obese subjects in relation to a 6-months physical exercise program; the authors did not find significantly changes in adiponectin or any correlation to the maximal oxygen consumption ([21]). It is to notice that there are only few papers analysing specifically the effects of a physical exercise program in obese patients, while most of the studies considered a combination of physical activity program together with a diet protocol ([35,40]). On the contrary, Miyatake et al. found that circulating adiponectin levels were associated with peak oxygen uptake although the authors examined normal weight subjects ([41]). The applicability of this study is also limited due to the lack of a detailed nutritional protocol and by unchecked compliance of the patients. However, our data suggest the usefulness of saliva as a non-invasive and easy to collect biological sample to evaluate cardiometabolic status and body composition status of obese subjects undergone a training program.
In conclusion, our results suggest that a 24-weeks physical training is not only an effective tool in the management of weight but also in the physical fitness in relation to the endocrine activity of adipose tissue as suggested by the enhanced adiponectin levels at the end of the training period. In addition, saliva resulted a valid alternative tool to sera for the evaluation of adiponectin secretion by adipose tissue, allowing a less invasive sample collection. Further studies are needed on a larger cohort of patients and for a longer period of time.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the Friuli-Venezia-Giulia Region (Italy) (protocol number 1764) and conducted according to the ethical principles of the Declaration of Helsinki. Informed consent was obtained from all participants.
Availability of data and materials
The datasets used and/or analyzed during the current study are publicly available from the corresponding author on reasonable request.
Competing interests
The authors declare no conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author contribution statement
Ersilia Nigro, Stefano Lazzer, and Aurora Daniele: Conceived and designed the experiments; Wrote the paper.
Marta Mallardo: Performed the experiments; Wrote the paper.
Mattia D'Alleva: Analyzed and interpreted the data; Wrote the paper.
Nicola Giovanelli, Francesco Graniero, Federica Fiori, and Michela Marinoni: Contributed reagents, materials, analysis tools or data; Performed the experiments.
Véronique Billat and Maria Parpinel: Contributed reagents, materials, analysis tools or data; Performed the experiments.
Data availability statement
Data included in article/supplementary material/referenced in article.
Additional information
No additional information is available for this paper.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e15790.
List of abbreviation
- T2D
type II diabetes
- BMI
body mass index
- LMW
low molecular weight
- MMW
medium molecular weight
- HMW
high molecular weight
- HIIT
high intensity interval training
- MICT
moderate intensity continuous training
- V’O2max
maximal oxygen uptake
- POL:
polarized training
- THR
threshold training
- BM
body mass
- IPAQ-SF
international physical activity questionnaire short form
- 4-dDR
4-days dietary record
- TL:
training load
- TID
training intensity distribution
- GET
gas exchange threshold
- RCP
respiratory compensation point
- TRIMP
training impulse
- RPE
rate of perceived exertion
- WC
waist circumference
- HC
hip circumference
- BIA
biolectrical impedance
- FM
fat mass
- FFM
free fat mass
- ELISA
enzyme-linked immunosorbent assay
- HRmax
heart rate max
- V’O2
oxygen uptake
- HR
heart rate
- RER
respiratory exchange ratio
- MedDiet:
Mediterranean diet
Appendix A. Supplementary data
The following is the Supplementary data to this article:
figs1
References
- 1.Boutari C., Mantzoros C.S. A 2022 update on the epidemiology of obesity and a call to action: as its twin COVID-19 pandemic appears to be receding, the obesity and dysmetabolism pandemic continues to rage on. Metabolism. 2022;133 doi: 10.1016/j.metabol.2022.155217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stöggl T., Sperlich B. Polarized training has greater impact on key endurance variables than threshold, high intensity, or high volume training. Front. Physiol. 2014;5:33. doi: 10.3389/fphys.2014.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strissel J.C., Stancheva K., Miyoshi H., Perfield J., 2nd, DeFuria J., Jick Z., Greenberg A., Obin M. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes. 2007;56(12):2910–2918. doi: 10.2337/db07-0767. [DOI] [PubMed] [Google Scholar]
- 4.Guzel Y., Atakan M.M., Areta J.L., Turnagol H.H., Kosar S.H. Ten weeks of low-volume walking training improve cardiometabolic health and body composition in sedentary postmenopausal women with obesity without affecting markers of bone metabolism. Res. Sports Med. 2022:1–13. doi: 10.1080/15438627.2022.2113877. [DOI] [PubMed] [Google Scholar]
- 5.Poon E.T.C., Siu P.M.F., Wongpipit W., Gibala M., Wong S.H.S. Alternating high-intensity interval training and continuous training is efficacious in improving cardiometabolic health in obese middle-aged men. J Exerc Sci Fit. 2022;20(1):40–47. doi: 10.1016/j.jesf.2021.11.003. . Ramadan FA, Bea JA, Garcia DO, Ellingson KD, Canales RA, Raichlen AC, Klimentidis YC. BMJ Open Sport Exerc. Med. 8(3):e001291, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Su L., Pan Y., Chen H. The harm of metabolically healthy obese and the effect of exercise on their health promotion. Front. Physiol. 2022;13 doi: 10.3389/fphys.2022.924649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van der Klaauw A.A., Farooqi I.S. The hunger genes: pathways to obesity. Cell. 2015;161(1):119–132. doi: 10.1016/j.cell.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Mohammad Rahimi G.R., Yousefabadi H.A., Niyazi A., Mohammad Rahimi N., Alikhajeh Y. Effects of lifestyle intervention on inflammatory markers and waist circumference in overweight/obese adults with metabolic syndrome: a systematic review and meta-analysis of randomized controlled trials. Biol. Res. Nurs. 2022;24(1):94–105. doi: 10.1177/10998004211044754. [DOI] [PubMed] [Google Scholar]
- 9.Jiang S., Bae J.H., Wang J., Song W. The potential roles of myokines in adipose tissue metabolism with exercise and cold exposure. Int. J. Mol. Sci. 2022;23(19) doi: 10.3390/ijms231911523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nigro E., Scudiero O., Monaco M.L., Palmieri A., Mazzarella G., Costagliola C., Bianco A., Daniele A. New insight into adiponectin role in obesity and obesity-related diseases. BioMed Res. Int. 2014;2014 doi: 10.1155/2014/658913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Minniti G., Pescinini-Salzedas L.M., Dos Santos Minniti G.A., Fornari Laurindo L., Barbalho S.M., Vargas Sinatora R., Lance Alan Sloan L.A., de Argollo Haber R.S., Cressoni Araújo A., Quesada K., Dos Santos Haber J.F., Dib Bechara M., Portero Sloan K., Organokines Sarcopenia, Repercussions Metabolic. The vicious cycle and the interplay with exercise. Int. J. Mol. Sci. 2022;23(21) doi: 10.3390/ijms232113452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mong Diep Nguyen T. Adiponectin: role in physiology and pathophysiology. Int. J. Prev. Med. 2020;11:136. doi: 10.4103/ijpvm.IJPVM_193_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu Y., Wiltshire H.D., Baker J., Wang Q., Ying S., Li S., Lu Y. Associations between objectively determined physical activity and cardiometabolic health in adult women: a systematic review and meta-analysis. Biology. 2022;11(6):925. doi: 10.3390/biology11060925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Feo P. Is high-intensity exercise better than moderate-intensity exercise for weight loss? Nutr. Metabol. Cardiovasc. Dis. 2013;23(11):1037–1042. doi: 10.1016/j.numecd.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Moghadam B.H., Bagheri R., Ashtary-Larky D., Tinsley G.M., Eskandari M., Wong A. The effects of concurrent training order on satellite cell-related markers, body composition, muscular and cardiorespiratory fitness in older men with sarcopenia. J. Nutr. Health Aging. 2020;24(7):796–804. doi: 10.1007/s12603-020-1431-3. [DOI] [PubMed] [Google Scholar]
- 16.Treff G., Winkert T., Sareban M., Steinacker J.M., Sperlich B. The polarization-index: a simple calculation to distinguish polarized from non-polarized training intensity distributions. Front. Physiol. 2019;10:707. doi: 10.3389/fphys.2019.00707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitchell B.L., Lock M.J., Davison K., Parfitt G., Buckley J.P., Eston R.G. What is the effect of aerobic exercise intensity on cardiorespiratory fitness in those undergoing cardiac rehabilitation? A systematic review with meta-analysis. Br. J. Sports Med. 2019;53(21):1341–1351. doi: 10.1136/bjsports-2018-099153. [DOI] [PubMed] [Google Scholar]
- 18.Molinari C., Edwards J., Billat V. Maximal time spent at VO2max from sprint to the marathon. Int. J. Environ. Res. Publ. Health. 2020;17(24):9250. doi: 10.3390/ijerph17249250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Selier S. What is best practice for training intensity and duration distribution in endurance athletes? Int. J. Sports Physiol. Perform. 2010;5(3):276–291. doi: 10.1123/ijspp.5.3.276. [DOI] [PubMed] [Google Scholar]
- 20.Campos Y., Casado A., Vieira J.G., Guimarães M., Sant'Ana G., Leitão L., da Silva S.F., Silva Marques de Azevedo P.H., Vianna L., Domínguez R. Training-intensity distribution on middle- and long-distance runners: a systematic review. Int. J. Sports Med. 2022;(4):305–316. doi: 10.1055/a-1559-3623. [DOI] [PubMed] [Google Scholar]
- 21.Ryan A.S., Li Guoyan. Adipose and skeletal muscle expression of adiponectin and liver receptor homolog-1 with weight loss and aerobic exercise. J. Endocr. Soc. 2022;6(8):bvac095. doi: 10.1210/jendso/bvac095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou F., Xiaojian Yin X., Phillipe K., Houssein A., Gastinger S., Prioux J. Ventilatory responses at submaximal exercise intensities in healthy children and adolescents during the growth spurt period: a semi-longitudinal study. Eur. J. Appl. Physiol. 2021;121(11):3211–3223. doi: 10.1007/s00421-021-04776-4. [DOI] [PubMed] [Google Scholar]
- 23.Craig C.L., Marshall A.L., Sjöström M., Bauman A.E., Booth M.L., Ainsworth B.E., Pratt M., Ekelund U., Yngve A., Sallis J.F., Oja P. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003;35(8):1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 24.Bellinger P., Arnold B., Minahan C. Quantifying the training-intensity distribution in middle-distance runners: the influence of different methods of training-intensity quantification. Int. J. Sports Physiol. Perform. 2019;11:1–5. doi: 10.1123/ijspp.2019-0298. [DOI] [PubMed] [Google Scholar]
- 25.Borg G. Physical training. 3. Perceived exertion in physical work. Lakartidningen. 1970;67(40):4548–4557. [PubMed] [Google Scholar]
- 26.Kagawa M., Byrne N., Hills A.P. Comparison of body fat estimation using waist: height ratio using different 'waist' measurements in Australian adults. Br. J. Nutr. 2008;100(5):1135–1141. doi: 10.1017/S0007114508966095. [DOI] [PubMed] [Google Scholar]
- 27.Gray D.S., Bray G.A., Gemayel N., Kaplan K. Effect of obesity on bioelectrical impedance. Am. J. Clin. Nutr. 1989;50(2):255–260. doi: 10.1093/ajcn/50.2.255. [DOI] [PubMed] [Google Scholar]
- 28.Nigro E., Piombino P., Scudiero O., Monaco M.L., Schettino P., Chambery A., Daniele A. Evaluation of salivary adiponectin profile in obese patients. Peptides. 2015;63:150–155. doi: 10.1016/j.peptides.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 29.Howley E.T., Bassett D.R., Jr., Welch H.G. Med. Sci. Sports Exerc. 1995;27(9):1292–1301. [PubMed] [Google Scholar]
- 30.Moghadam B.H., Golestani F., Bagheri R., Cheraghloo N., Eskandari M., Wong A., Nordvall M., Suzuki K., Pournemati P. The effects of high-intensity interval training vs. Moderate-intensity continuous training on inflammatory markers, body composition, and physical fitness in overweight/obese survivors of breast cancer: a randomized controlled clinical trial. Cancers. 2021;13(17):4386. doi: 10.3390/cancers13174386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Georgiev A., Granata C., Roden The role of mitochondria in the pathophysiology and treatment of common metabolic diseases in humans. Am. J. Physiol. Cell Physiol. 2022;322(6):C1248–C1259. doi: 10.1152/ajpcell.00035.2022. [DOI] [PubMed] [Google Scholar]
- 32.Bishop D.J., Granata C., Eynon N. Can we optimise the exercise training prescription to maximise improvements in mitochondria function and content? Biochim. Biophys. Acta. 2014;1840(4):1266–1275. doi: 10.1016/j.bbagen.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 33.van der Zwaard S., de Ruiter C.J., Noordhof D., Sterrenburg R., Bloemers F., de Koning J.J., Jaspers R.T., van der Laarse W.J. Maximal oxygen uptake is proportional to muscle fiber oxidative capacity, from chronic heart failure patients to professional cyclists. J. Appl. Physiol. (1985) 2016;121(3):636–645. doi: 10.1152/japplphysiol.00355.2016. [DOI] [PubMed] [Google Scholar]
- 34.Casado A., Tuimil J.L., Iglesias X., Del Olmo M.F., Jiménez-Reyes P., Martín-Acero R., Rodríguez F.A. Maximum aerobic speed, maximum oxygen consumption, and running spatiotemporal parameters during an incremental test among middle- and long-distance runners and endurance non-running athletes. PeerJ. 2022;10 doi: 10.7717/peerj.14035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corbi G., Polito R., Monaco M.L., Cacciatore F., Scioli M., Ferrara N., Daniele A., Nigro E. Adiponectin expression and genotypes in Italian people with severe obesity undergone a hypocaloric diet and physical exercise program. Nutrients. 2019;11(9):2195. doi: 10.3390/nu11092195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.da Silveira Campos R.M., Landi Masquio D., Campos Corgosinho F., de Lima Sanches P., De Piano A., Carnier J., da Silva P., Grotti Clemente A.P., de Castro Ferreira Vicente S.E., Oyama L M., Da Penha Oller do Nascimento C.M., Tock L., Tufik S., de Mello M.T., Dâmaso A.R. Homeostasis model assessment-adiponectin: the role of different types of physical exercise in obese adolescents. J. Sports Med. Phys. Fit. 2017;57(6):831–838. doi: 10.23736/S0022-4707.16.06235-6. [DOI] [PubMed] [Google Scholar]
- 37.Swisher A.K., Abraham J., Bonner D., Gilleland D., Hobbs G., Kurian S., Yanosik M.A., Vona-Davis L. Exercise and dietary advice intervention for survivors of triple-negative breast cancer: effects on body fat, physical function, quality of life, and adipokine profile. Support. Care Cancer. 2015;23(10):2995–3003. doi: 10.1007/s00520-015-2667-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cobos-Palacios L., Ruiz-Moreno M.I., Vilches-Perez A., Vargas-Candela A., Muñoz-Úbeda M., Benítez Porres J., Navarro-Sanz A., Lopez-Carmona M.D., Sanz-Canovas M., Perez-Belmonte L.M., Mancebo-Sevilla J.J., Gomez-Huelgas R., Bernal-Lopez R. Metabolically healthy obesity: inflammatory biomarkers and adipokines in elderly population. PLoS One. 2022;17(6) doi: 10.1371/journal.pone.0265362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jeon J.Y., Han J., Kim H.Y., Park M.S., Seo D.Y., Kwak Y.S. The combined effects of physical exercise training and detraining on adiponectin in overweight and obese children. Integr. Med. Res. 2013;2(4):145–150. doi: 10.1016/j.imr.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Venojärvi M., Lindström J., Aunola S., Nuutila P., Atalay M. Improved aerobic capacity and adipokine profile together with weight loss improve glycemic control without changes in skeletal muscle GLUT-4 gene expression in middle-aged subjects with impaired glucose tolerance. Int. J. Environ. Res. Publ. Health. 2022;19(14):8327. doi: 10.3390/ijerph19148327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miyatake N., Numata T., Murakami H., Kawakami R., Sanada K., Tabata I., Miyachi M., NEXIS Study Group Circulating adiponectin levels are associated with peak oxygen uptake in Japanese. Environ. Health Prev. Med. 2014;19(4):279–285. doi: 10.1007/s12199-014-0390-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are publicly available from the corresponding author on reasonable request.
Data included in article/supplementary material/referenced in article.