Abstract
In this work, the aqueous leaf extracts of three Ophiorrhiza genus species, namely Ophiorrhiza mungos (Om), Ophiorrhiza harrisiana (Oh) and Ophiorrhiza rugosa (Or), have been used as the reducing and capping agents to control the size of AgNPs, Om-AgNPs, Oh-AgNPs and Or-AgNPs, respectively and found to be an effective antimicrobial agent against a wide range of bacteria and fungi. The biosynthesized AgNPs were studied by UV–Visible spectrophotometer, powder X-ray diffraction (XRD), scanning electron microscopy (SEM), energy dispersive X-ray, transmission electron microscopy (TEM) and Fourier transform infrared spectrometer (FTIR). The average particle sizes of Om-AgNPs, Oh-AgNPs and Or-AgNPs were measured as 17 nm, 22 nm and 26 nm, respectively, and observed to be spherical and face-centered cubic crystals. The antibacterial test of synthesized AgNPs was performed against Staphylococcus aureus, Bacillus cereus, Escherichia coli, and Vibrio cholerae where the maximum antibacterial activity was observed by reducing the nano-size and increasing the silver content of AgNPs. The antifungal effect of these three types of AgNPs on Penicillium notatum and Aspergillus niger was also evaluated and their growth with AgNPs concentrations of 450 μg/mL was inhibited up to 80–90% and 55–70%, respectively. The size-control synthesis of AgNPs using the Ophiorrhiza genus species is presented here for the first time where the synthesized AgNPs showed higher stability and antimicrobial activities. Therefore, this study might lead to synthesize AgNPs with different morphologies using plant extracts of the same genus but from different species and provide strong encouragement for future applications in treating infectious diseases.
Keywords: Green synthesis, Silver nanoparticles, Ophiorrhiza genus, Plant extract, Antibacterial activity, Antifungal activity
Graphical abstract
Biosynthesis of stable silver nanoparticles having various sizes in range of 17–26 nm using aqueous leaf extracts of three Ophiorrhiza genus species, namely Ophiorrhiza mungos, Ophiorrhiza harrisiana and Ophiorrhiza rugosa are described for the first time with excellent antibacterial and antifungal properties.
1. Introduction
Nanotechnology is used to improve the properties of the material by manipulating its structure at the nanoscale for developing innovative materials and technologies with distinctive features [1]. These particles have unique characteristics that are entirely different from those of the bulk because they are tiny and have a high surface area-to-volume ratio [2,3]. Nanomaterials offer answers to numerous technological and environmental problems because of their various magnetic, electrical, and optical capabilities [4]. Because of their unique properties, they can be applied in case of drugs, manufacturing and/or tailoring of materials, electronics, mechanics, antimicrobials, optics, catalysis, electronics, sensing, energy harvesting and environment and so on [3,5]. Specially, noble metals like Au, Ag, Pt etc. And their oxides, sulfides are most applied because of their extrinsic applications but among them, silver nanoparticles (AgNPs) are more advantageous in the case of biomedical applications [1,6]. AgNPs act at the membrane level because they can pass through the outer membrane and accumulate in the inner membrane [7]. According to another proposed mechanism, AgNPs can affect the structure of a cell's membrane in addition to breaking through it [8,9]. Furthermore, In addition to being highly effective against both Gram-negative and Gram-positive bacterial strains, AgNPs are also used as photocatalysts, biopesticides, and biosensors [10]. AgNPs act as adsorbents to remove dye, antioxidant, anti-inflammatory, anticoagulant, anticoagulant, wound healing, anticancer etc. purposes [[11], [12], [13]]. Again, AgNPs can be considered one of the widely studied nanomaterials and therefore have been chosen for our study.
However, the conventional physical (e.g., mechanical milling, laser ablation, sputtering) and chemical (e.g., chemical reduction, electrochemical precipitation, vapor deposition, pyrolysis, atomic condensation) methods of synthesizing AgNPs involve the use of toxic solvents and hazardous chemicals posing a great threat on the environment [[14], [15], [16], [17]]. Besides, these synthesis procedures are time-consuming and require sophisticated instrumental set up which may not be cost-effective [18]. Stabilization of the synthesized AgNPs is a great concern. Thus, researchers developed unique “green chemistry” methodologies for producing nanomaterials in biological systems like bacteria, fungi, and plant extract in response to the escalating environmental concerns [[19], [20], [21]]. Moreover, using microorganisms in the synthesis of AgNPs is time-consuming, consists of several preparative steps, and is subsequently expensive [22]. Accounting for all aspects of readily scalable, less expensive, and rapid procedure, green synthesis using plant extract was the most prominent method for AgNPs synthesis [23,24]. Plant extract contains a wide range of biomolecules, viz., phenolic acids, flavonoids, xanthones, and phloroglucinols, which can act as reducing, capping, and stabilizing agents [25,26]. Noteworthy that, the overall characteristics of the nanoparticles are influenced by the chemical composition, size, shape and surface charge [27]. Especially, biomedical applications of AgNPs are notably size and shape-dependent [28]. Smaller AgNPs can exhibit the largest dose- and time-dependent antibacterial activity [29,30]. The size and shape can be controlled by varying pH, synthesis temperature, and reaction time [31,32]. In plant extract-based methods, different plants have different reducing, capping, and stabilizing agents to generate an enormous range of shapes and sizes, which are significant features for several biomedical applications [33,34]. Hence, the synthesis of AgNPs with variable sizes using plant extract under an eco-friendly route is highly desirable.
Genus Ophiorrhiza belonged to the Rubiaceae family is commonly found in the wet forests of Asia, Australia, New Guinea and the Pacific Islands. This genus containing 321 species, 5 varieties and 1 subspecies, is used in both traditional and modern medicine for its medicinal properties (antioxidant, antitussive and analgesic agent) and to treat snakebite, stomatitis, ulcers, inflammation, pain, cancer, wounds and bacterial and viral infections [26,[35], [36], [37]]. As mentioned above, the Ophiorrhiza plant extract is full of various biomolecules [38]. The aim of this study is to prepare various sizes of AgNPs using plant extract under eco-friendly route. The presence of various bio-chemicals in plant species of the Ophiorrhiza genus encouraged us to test the usefulness of functional groups of bio-chemicals for the green synthesis of AgNPs as a potential antimicrobial agent in treating infectious diseases. In this work, we have selected the three plant species of the Ophiorrhiza genus viz., Ophiorrhiza mungos (Om), Ophiorrhiza harrisiana (Oh) and Ophiorrhiza rugosa (Or) (Fig. 1a–c), which having different biomolecules, may be responsible for the formation of different size of AgNPs [26,35,38]. These three plant species are commonly available in the hilly area of University of Chittagong (Chattogram, Bangladesh). We describe the synthesis of highly stable AgNPs at different sizes from the aqueous leaf extracts of three Ophiorrhiza species, Om-AgNPs, Oh-AgNPs and Or-AgNPs. At the same time, we also highlighted its action as the effective antibacterial and antifungal agents against a wide range of bacteria and fungi namely Staphylococcus aureus, Bacillus cereus, Escherichia coli, Vibrio cholera, Penicillium notatum and Aspergillus niger which usually responsible for human diseases. To our knowledge, the formation of different sizes of AgNPs using the same plants of Ophiorrhiza genus and their antimicrobial activities has not been previously reported. Thus, we aimed to use the aqueous leaf extract of three Ophiorrhiza genus species (Om, Oh and Or) which have been used for the first time as the reducing and capping agents to control the size of AgNPs. In addition, their antimicrobial potential against a wide range of bacteria and fungi have been investigated.
Fig. 1.
Image of (a) Ophiorrhiza mungos, (b) Ophiorrhiza harrisiana and (c) Ophiorrhiza rugosa leaf along with its flower and stem.
2. Material and methods
2.1. Materials
AgNO3 and Trypan blue stain solution (4%) were obtained from Merck, Germany and Bio Rad, Hercules (CA, USA), respectively, and used as received. Bactotrypton, bacto agar and yeast extract were obtained from Difco Laboratories (Detroit, MI, USA). Sodium chloride was acquired from Wako (Osaka, Japan). Fresh leaves of the plants (Ophiorrhiza rugosa, Ophiorrhiza mungos and Ophiorrhiza harrisiana) were obtained from the hilly area of the University of Chittagong (Chattogram, Bangladesh). These plants were identified and acknowledged by Farhana Rumzum Bhuiyan, Assistant Professor, Dept. of Botany, University of Chittagong, Bangladesh. Analytical-grade reagents were utilized throughout.
2.2. Leaf extracts preparation
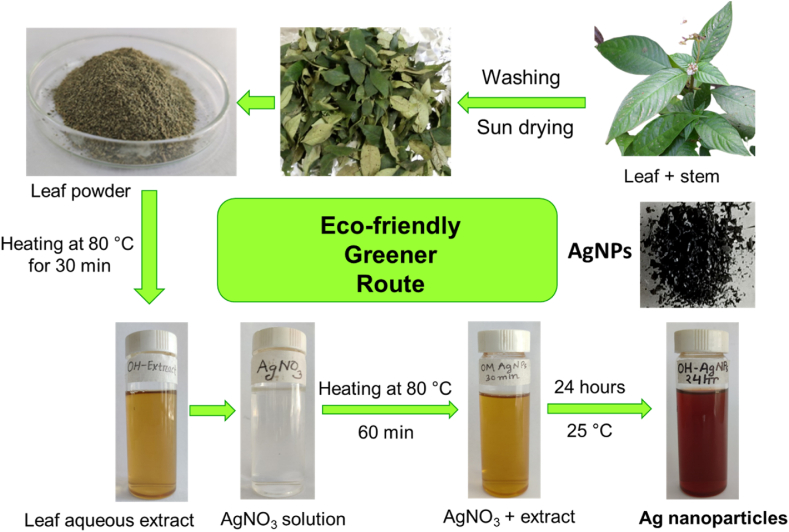
The extraction procedure was performed following the literature with slight modifications [39]. A scheme of the biosynthesis of AgNPs is shown in Fig. 2. Each of the plant leaves was carefully washed with tap water and double distilled water, and placed for drying under sunlight, then powdered. Individually, 1.0 g of dried leaf powder with 100 mL deionized water blended under continuous stirring at 80 °C for 30 min. Whatman No. 1 filter paper was then used to separate and filter the supernatant, which was then preserved in a sealed container and maintained in a cooler until use.
Fig. 2.
Schematic representation of the synthesis of AgNPs from the aqueous leaf extracts of three Ophiorrhiza species.
2.3. Synthesis of AgNPs
AgNPs were synthesized using the methodology reported by Ullah et al. [40]. Briefly, the AgNPs were successfully synthesized using the leaf extracts of three different Ophiorrhiza species separately. Briefly, 20 mL of each plant extract was added in a drop-wise manner (20 min) to the 100 mL aqueous solution of AgNO3 (1 mM) with continuous stirring for 60 min at 80 °C. For complete bio-reduction, the resulting liquor was preserved for 24 h at room temperature. After 24 h, the solution was centrifuged at 5000 rpm for 20 min. The paste obtained was washed three times to eliminate any contaminants and dried at 60 °C for 12 h. The product (AgNPs) was then stored in a sealed glass vial, and reserved in a desiccator until needed. The samples are denoted as Om-AgNPs, Oh-AgNPs and Or-AgNPs for the Om-, Oh- and Or-mediated plant extract, respectively.
2.4. Characterization of AgNPs
For optical characterization, UV–visible spectrophotometer (UV-1800, Shimadzu, Japan) was used. X-ray diffraction (XRD) analysis was conducted to determine the AgNPs' crystalline phase using Rigaku Ultima IV diffractometer with Cu-Kα (λ = 1.5406 Å) radiation at 0.2 min−1. Fourier Transform Infrared Spectrophotometer (FTIR) spectra were measured as KBr discs on an IR Prestige (Shimadzu) spectrometer to identify the presence of the functional group. Using Scanning Electron Microscope (SEM) (JSM-6490 L A), the surface morphology of AgNPs was investigated, while the average particle size and distribution were determined by Transmission Electron Microscope (TEM) (FEI, Bellaterra Spain). To check the elemental composition, the Energy-dispersive X-ray (EDX) was employed using a BRUKER system attached to the SEM.
2.5. Antibacterial and antifungal test
The disc diffusion method was applied to identify the antibacterial activity of the synthesized AgNPs following the standard protocol [40]. Staphylococcus aureus (ATCC 12228), Bacillus cereus (ATCC 14579), Escherichia coli (ATCC 25922), and Vibrio cholerae (ATCC 39315) were assessed for the antibacterial activity of the AgNPs. On the other hand, the antifungal activity was tested employing the poisoned food technique using potato dextrose agar (PDA) as the culture medium [41]. Briefly, a sterilized pipette was used to transfer 0.5 mL of each solution into separate sterile petri dishes after Om-AgNPs, Oh-AgNPs and Or-AgNPs had been dispersed in deionized water. Then, the medium (20 mL) was added to each Petri dish and let to set. The middle of each petri-dish was then injected with a 5 mm mycelium block of each fungus which was positioned in an upside-down posture. Three days after incubation at 27 ± 2 °C the mycelial growth was assessed. The measurements were carried out three times and the average values were plotted.
3. Results and discussion
3.1. Visual examination and UV–visible spectral analysis
The green synthesis of AgNPs was performed using aqueous leaf extract of three Ophiorrhiza species and 1 mM of AgNO3 at 80 °C. The aqueous leaf extract of Om, Oh and Or exhibited a slightly acidic nature of pH at 6.1, 5.7, and 5.8, respectively. The pH of the colloidal AgNPs solution was reached at ∼4.7 after 1 h reaction between plant extract and AgNO3, and then remained virtually the same afterward. To examine the formation of nanoparticles, a visual examination and UV–visible spectroscopy analysis were carried out. The visual color change of the plant extract solution from yellow to deep brown during synthesis (Fig. 3 i) owing to a surface plasmon resonance (SPR) of the bio-reduction of Ag + to Ag0 [42].
Fig. 3.
(I) Color change during the formation of AgNPs. Plant extract without AgNO3 solution Om, Oh and Or. Plant extract with AgNO3 solution after 30 min, 1 h, 2 h, 4 h and 24 h. (ii) UV–Visible absorption spectra of the synthesized AgNPs (a) Om-AgNPs, (b) Oh-AgNPs and (c) Or-AgNPs formation at different time intervals.
UV–visible spectroscopy analysis is commonly applied to assess the structural characterization of nanoparticle. The reaction profile for Om-, Oh- and Or-mediated AgNPs as a function of time is shown in Fig. 3 ii (a - c). The SPR bands were observed at 413, 424 and 451 nm for Om-, Oh- and Or-mediated AgNPs, respectively, and the absorption intensity increased gradually with increasing reaction time up to 24 h incubation. The reaction was continued further and the Ag peak's intensity did not change. The peak position is unchanged during the reaction time, indicating that nucleation of AgNPs starts with time and the size remains unaffected during the reaction. UV–visible spectra of synthesized AgNPs were found to be coherent with Mie theory. A single SPR band was found at 413 nm, 424 nm and 451 nm for Om-AgNPs, Oh-AgNPs and Or-AgNPs, respectively, where average particle sizes were calculated as 18 nm, 22 nm 26 nm, respectively. Another group of researchers made identical assignments for the formation AgNPs using the surface plasmon resonance peak of AgNPs [43,44].
Literature surveys showed that the three species of plant extracts contain various phytochemicals [26,35]. The size-control synthesis of AgNPs may be due to different biomolecules, which play a vital role as reducing, capping and stabilizing agents for the growth of various sizes of AgNPs. Previous work demonstrated that the shape, size and stability of AgNPs also depend on the pH value during the reaction [45]. The present results indicated that the different pH values of different species of Ophiorrhiza genus might also be provided some additional features to the generation of various size AgNPs using aqueous leaf extract of Om, Oh and Or.
3.2. X-ray diffraction analysis
Fig. 4 shows the XRD patterns of Or-AgNPs, Om-AgNPs and Oh-AgNPs synthesized nanoparticles by three Ophiorrhiza species extract. The diffraction peaks of the Or-AgNPs, Om-AgNPs and Oh-AgNPs were observed close to 28°, 32°, 38°, 44°, 46°, 55°, 57°, 64° and 77° in the 2θ range of 20°–80°, which can be ascribed to the (210), (122), (111), (200), (231), (142), (241), (220) and (311) silver crystalline planes of face-centered cubic structure (JCPDS, file No. 04–0783).
Fig. 4.
XRD patterns of the synthesized AgNPs.
The crystallite size of AgNPs has been calculated using the Debye-Scherrer equation [46]:
where, D is the average crystallite size, k is the geometric factor (0.9), λ is the wavelength of X-ray radiation source (0.15406 nm) and β is the FWHM (full-width at half maximum) of the XRD peak at the diffraction angle θ and the results are presented in Table 1. The average crystallite size of Om-AgNPs, Oh-AgNPs and Or-AgNPs were 16 nm, 20 nm and 28 nm, respectively.
Table 1.
X-ray powder diffraction results of synthesized AgNPs.
| Sample | Plane (hkl) | 2 Theta (°) | FWHM | Crystallite size, D (nm) | Average size (nm) |
|---|---|---|---|---|---|
| Om-AgNPs | [210] | 27.8 | 0.34 | 25.4 | 16.3 |
| [122] | 32.2 | 0.32 | 27.1 | ||
| [111] | 38.0 | 0.68 | 12.9 | ||
| [200] | 44.2 | 1.13 (7) | 7.9 | ||
| [231] | 46.2 | 0.54 | 16.7 | ||
| [142] | 54.7 | 0.48 | 19.5 | ||
| [241] | 57.4 | 0.56 | 16.9 | ||
| [220] | 64.4 | 0.87 | 11.3 | ||
| [311] | 77.1 | 1.18 | 9.0 | ||
| Oh-AgNPs | [210] | 27.79 | 0.33 | 25.8 | 20.0 |
| [122] | 32.22 | 0.29 | 29.9 | ||
| [111] | 38.14 | 0.54 | 16.3 | ||
| [200] | 44.34 | 1.07 | 8.4 | ||
| [231] | 46.27 | 0.36 | 25.0 | ||
| [142] | 54.81 | 0.48 | 19.5 | ||
| [241] | 57.50 | 0.47 | 20.2 | ||
| [220] | 64.48 | 0.38 | 25.8 | ||
| [311] | 77.19 | 1.19 | 8.9 | ||
| Or-AgNPs | [210] | 27.79 | 0.29 | 29.5 | 27.7 |
| [122] | 32.19 | 0.25 | 34.5 | ||
| [111] | 38.08 | 0.41 | 21.2 | ||
| [200] | 44.39 | 0.90 | 9.9 | ||
| [231] | 46.22 | 0.25 | 36.3 | ||
| [142] | 54.78 | 0.27 | 34.3 | ||
| [241] | 57.43 | 0.41 | 23.1 | ||
| [220] | 64.44 | 0.31 | 32.1 | ||
| [311] | 77.38 | 0.37 | 28.8 |
3.3. SEM, TEM, EDX and SAED analysis
The morphology and size of synthesized AgNPs were identified using SEM and TEM analysis. Spherical agglomerates with variable sizes were found during SEM analysis (Fig. 5a–c). TEM images of synthesized AgNPs are shown in Fig. 6 a. The images also show spherical shapes with variable sizes. The particle size distribution graph (Fig. 6 b) shows the average size of Om-AgNPs, Oh-AgNPs and Or-AgNPs to be approximately 17 ± 5 nm, 22 ± 9 nm and 26 ± 10 nm, respectively, which is in well agreement with the calculated size from XRD (16 nm, 20 nm and 28 nm) and SPR band (18 nm, 22 nm 26 nm) analysis. The SAED pattern (Fig. 6 c) showed that the diffraction rings might be indexed as (210), (122), (111), (200), (231), (142), (241), (220), and (311) reflections, which correspond to face-centered cubic polycrystalline silver from inner to outer with minimal variations in d-spacing.
Fig. 5.
Scanning electron micrographs of (a) Om-AgNPs, (b) Oh-AgNPs and (c) Or-AgNPs.
Fig. 6.
(a) Transmission electron micrographs, (b) particle size distribution and (c) SAED pattern of Om-AgNPs, Oh-AgNPs and Or-AgNPs.
Energy Dispersive X-ray spectra (EDX) of synthesized AgNPs are shown in Fig. 7 (a - c) and the percentage of elemental compositions is summarized in Table 2. All synthesized AgNPs showed an intense sharp signal at 3 keV indicating the formation of AgNPs as silver elements typically exhibits a characteristic optical absorption peak owing to surface plasmon resonance [47]. The percentage of the silver elemental composition of Om-AgNPs (88.74%) and Oh-AgNPs (91.96%) is much higher than that of Or-AgNPs (64.91%). EDX profile also showed other weak signals due to oxygen and carbon (Table 2), indicating the presence of oxygen and carbon-containing biomolecules on the surface of the AgNPs [48,49]. Our findings are in line with the other reports [50,51].
Fig. 7.
EDX spectra of (a) Om-AgNPs, (b) Oh-AgNPs and (c) Or-AgNPs.
Table 2.
Chemical analysis of synthesized AgNPs using EDX analysis.
| Sample | Weight (%) |
||
|---|---|---|---|
| Ag | O | C | |
| Om-AgNPs | 64.91 | 22.46 | 12.63 |
| Oh-AgNPs | 88.74 | 7.64 | 3.62 |
| Or-AgNPs | 91.96 | 5.71 | 2.33 |
3.4. FTIR analysis
The FTIR spectra of leaf extracts and synthesized AgNPs are shown in Fig. 8a–c. The spectra in all samples exhibited bands at ca. 1720 cm−1 due to the stretching vibration of the carbonyl group (C=O) [52]. The peaks were observed at ca. 1620 cm−1, 1535 cm−1, 1380 cm−1 and 1065 cm−1 due to C=C aromatic rings, C=C alkene, germinal methyls and ether linkages, respectively, indicating that the surface of synthesized AgNPs may contain terpenoids or flavanones [53,54]. On the other hand, the EDX profile of synthesized AgNPs confirmed the presence of oxygen within AgNPs, suggesting that the oxygen-containing biomolecules such as alcohol, carboxylic acid and ester groups, are encapsulated on the surface of as-synthesized AgNPs, confirmed by FTIR also earlier in this report. These results further confirmed the presence of phytoconstituents like flavanones or polyphenols or alkaloids on the surface of synthesized AgNPs which assisted in the reduction of Ag+ along with the stabilization of the surface of the AgNPs.
Fig. 8.
FTIR spectra of leaf extracts of Ophiorrhiza genus species and synthesized AgNPs: (a) Om-AgNPs, (b) Oh-AgNPs and (c) Or-AgNPs.
3.5. Antibacterial activity
To investigate the antibacterial activity of the AgNPs, three different sizes of AgNPs, Or-AgNPs, Om-AgNPs and Oh-AgNPs, where organic biomolecules like Om-, Oh- and Or-capped around the AgNPs were tested for antibacterial activity against 2 g-positive (Staphylococcus aureus and Bacillus cereus) and 2 g-negative (Escherichia coli and Vibrio cholerae) bacteria by varying concentration of AgNPs (100 μg/mL, 200 μg/mL and 300 μg/mL). As shown in Table 3, no clear zone of inhibition was found against Staphylococcus aureus while applying 100 μg/mL of AgNPs. With an increase in AgNPs concentration, the antibacterial activity increased. However, when compared in antibacterial activity of Oh-AgNPs and Or-AgNPs, the former afforded the broadest zone of inhibition against all four bacteria than the latter due to the highest content of silver in Oh-AgNPs (91.96% Ag) than Or-AgNPs (64.91% Ag). The antibacterial activity was also found to be influenced by the size of AgNPs, the larger size Or-AgNPs (26 nm) showed a lower zone of inhibition than lower size AgNPs, Om-AgNPs (18 nm) and Oh-AgNPs (22 nm) (Table 3), suggesting the concentration-dependent nature of AgNPs mediated bacterial death. A similar trend in concentration-dependent activity was observed by groups of researchers [55]. All three types of AgNPs showed the highest rate of inhibition against Vibrio cholera at a concentration of 100 μg/mL.
Table 3.
Zone of Inhibition (mm) for Or-AgNPs, Om-AgNPs and Oh-AgNPs tested against different gram-positive and gram-negative bacteria.
| Microorganisms | Antibacterial agent | Zone of inhibition (mm) [for different concentrations of AgNPs Suspension] |
Negative Controla | Positive Controla | ||
|---|---|---|---|---|---|---|
| 100 μg/mL | 200 μg/mL | 300 μg/mL | ||||
| Staphylococcus aureus | Or-AgNPs | 0 | 7 | 7 | 0 | 31 |
| Om-AgNPs | 0 | 7 | 7 | |||
| Oh-AgNPs | 0 | 8 | 9 | |||
| Bacillus cereus | Or-AgNPs | 0 | 6 | 7 | 0 | 26 |
| Om-AgNPs | 0 | 6 | 6 | |||
| Oh-AgNPs | 7 | 7 | 7 | |||
| Escherichia coli | Or-AgNPs | 0 | 6 | 7 | 0 | 26 |
| Om-AgNPs | 0 | 6 | 6 | |||
| Oh-AgNPs | 7 | 7 | 7 | |||
| Vibrio cholerae | Or-AgNPs | 7 | 7 | 10 | 0 | 32 |
| Om-AgNPs | 9 | 9 | 11 | |||
| Oh-AgNPs | 8 | 8 | 11 | |||
Chloramphenicol and deionized water were used as positive and negative control, respectively.
3.6. Antifungal activity
The antifungal activity of all three types of nano-size silver from 250 μg/mL to 400 μg/mL was examined against Penicillium notatum and Aspergillus niger using antifungal drug Nystatin as a positive control under similar control conditions. The synthesized AgNPs exhibited more activity than the standard antibiotic. The growth of Penicillium notatum was inhibited 60–80% with 250 μg/mL AgNPs concentration, whereas the inhibition (%) was observed to be less than 50% for Aspergillus niger. As the concentration of AgNPs increased, the inhibitory activities steadily increased (Fig. 9). However, the Oh-AgNPs exhibited higher antifungal activity against Penicillium notatum with 250 μg/mL AgNPs concentration than the other two synthesized AgNPs. Among them, Om-AgNPs showed minimum activity against these two fungi even though the size of the Om-AgNPs were less among the three AgNPs which is somewhat opposite to other reports [56,57]. This is probably due to the fact that biomolecules present on the surface of Om-AgNPs might be responsible for their minimum antifungal action. However, the results suggest that, AgNPs have the potential to be employed as antifungal agents possibly due to their ability to impair membrane integrity and cause cellular and organelle structural degradation, all of which are necessary for fungal growth and survival [58]. Similar studies have also been reported on fungal species, probably due to the solution's ability to saturate and adhere to the fungal hyphae with great intensity [59]. In addition, according to previous research, AgNPs are likely to cause damage to the transport system, resulting in the efflux of intracellular ions and the accumulation of silver ions, as well as the inhibition of metabolic and respiratory activities [60,61]. Although the mechanism is not clear in our study, the present results imply that AgNPs have significant antifungal efficacy, deserving further study for clinical applications.
Fig. 9.
Antifungal activity of the synthesized AgNPs.
4. Conclusions
In the work, using leaf extracts of Ophiorrhiza mungos, Ophiorrhiza harrisiana and Ophiorrhiza rugosa, we described a simple, efficient and eco-friendly greener route to synthesize different particle sizes of AgNPs. The biosynthesized AgNPs were spherical in shape and highly stable. The mean sizes of AgNPs were 17 ± 5 nm, 22 ± 9 nm and 26 ± 10 nm for Om-AgNPs, Oh-AgNPs and Or-AgNPs, respectively. Size-dependent antibacterial activity was observed. Though high antibacterial activity was found in all three types of AgNPs, the highest antibacterial activity was observed in the case of Vibrio cholera. In addition, antifungal activity was evident with notable inhibition for all three types of AgNPs where Oh-AgNPs were most lethal to the fungus used in this study. This study describes the nano-size-control synthesis of AgNPs using aqueous plant extracts for the first time from the same genus of Ophiorrhiza species. These synthesized AgNPs demonstrated potential biological applications and warrant further study of the role of biomolecules in the synthesis of nano-sized AgNPs and their antimicrobial potential via multifaceted mechanisms and binding sites. Furthermore, size-dependent activities like catalytic, optical, electrical and sensing activity can also be the potential subject to be explored. It can also be concluded that the present study might be taken as a baseline study for the size-controlled synthesis by varying species within the same genus. We believe this plant extract based synthesis of metal nanoparticles of a controlled size will be promising synthesis route for various fields, including biomedicine.
Author contribution statement
Sumon Ganguli: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Sabbir Howlader: Conceived and designed the experiments; Performed the experiments.
A.K.M. Atique Ullah, Farhana Rumzum Bhuiyan: Contributed reagents, materials, analysis tools or data.
Aklima A Akhia, Abid Hasan, Kamol Dey: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Saiful Islam, Ferdousi Ali, Ashok Kumar Chakraborty: Performed the experiments; Analyzed and interpreted the data.
Samiran Bhattacharjee, Benu Kumar Dey: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Data availability statement
Data included in article/supp. Material/referenced in article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
Acknowledgments
The authors gratefully acknowledge the financial support by the BUGC postdoctoral fellowship program from the Bangladesh University Grants Commission (BUGC). We also acknowledge the support from Centre for Advanced Research in Sciences (University of Dhaka), Research and Publication Cell (University of Chittagong) and Ministry of Education (Bangladesh). In addition, the authors acknowledge Bithi Mojumder, Assistant Professor Department of English, Noakhali Science and Technology University, Bangladesh for her assistance in English language editing.
Contributor Information
Samiran Bhattacharjee, Email: s.bhattacharjee@du.ac.bd.
Benu Kumar Dey, Email: benudey@yahoo.com.
References
- 1.Huang J., Tao Y., Ran S., Yang Y., Li C., Luo P., Chen Z., Tan X. A hydroxy-containing three dimensional covalent organic framework bearing silver nanoparticles for reduction of 4-nitrophenol and degradation of organic dyes. New J. Chem. 2022;46:17153–17160. doi: 10.1039/D2NJ02437C. [DOI] [Google Scholar]
- 2.Ettlinger R., Lachelt U., Gref R., Horcajada P., Lammers T., Serre C., Couvreur P., Morris R.E., Wuttke S. Toxicity of metal–organic framework nanoparticles: from essential analyses to potential applications. Chem. Soc. Rev. 2022;51:464–484. doi: 10.1039/D1CS00918D. [DOI] [PubMed] [Google Scholar]
- 3.Khan I., Saeed K., Khan I. Nanoparticles: properties, applications and toxicities, arab. J. Chem. 2019;12:908–931. doi: 10.1016/J.ARABJC.2017.05.011. [DOI] [Google Scholar]
- 4.Baig N., Kammakakam I., Falath W., Kammakakam I. Nanomaterials: a review of synthesis methods, properties, recent progress, and challenges, Mater. Advisor. 2021;2:1821–1871. doi: 10.1039/D0MA00807A. [DOI] [Google Scholar]
- 5.Mousavi S.M., Hashemi S.A., Ghasemi Y., Atapour A., Amani A.M., Savar Dashtaki A., Babapoor A., Arjmand O. Green synthesis of silver nanoparticles toward bio and medical applications: review study. Artif. Cells, Nanomed. Biotechnol. 2018;46 doi: 10.1080/21691401.2018.1517769. S855–S872. [DOI] [PubMed] [Google Scholar]
- 6.Hossain N., Islam M.A., Chowdhury M.A. Synthesis and characterization of plant extracted silver nanoparticles and advances in dental implant applications. Heliyon. 2022;8 doi: 10.1016/J.HELIYON.2022.E12313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruna T., Maldonado-Bravo F., Jara P., Caro N. Silver nanoparticles and their antibacterial applications. Int. J. Mol. Sci. 2021;22 doi: 10.3390/IJMS22137202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seil J.T., Webster T.J. Antimicrobial applications of nanotechnology: methods and literature. Int. J. Nanomed. 2012;7:2767–2781. doi: 10.2147/IJN.S24805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ivask A., Elbadawy A., Kaweeteerawat C., Boren D., Fischer H., Ji Z., Chang C.H., Liu R., Tolaymat T., Telesca D., Zink J.I., Cohen Y., Holden P.A., Godwin H.A. Toxicity mechanisms in Escherichia coli vary for silver nanoparticles and differ from ionic silver. ACS Nano. 2014;8:374–386. doi: 10.1021/NN4044047. [DOI] [PubMed] [Google Scholar]
- 10.Moorthy K., Chang K.C., Yu P.J., Wu W.J., Liao M.Y., Huang H.C., Chien H.C., Chiang C.K. Synergistic actions of phytonutrient capped nanosilver as a novel broad-spectrum antimicrobial agent: unveiling the antibacterial effectiveness and bactericidal mechanism. New J. Chem. 2022;46:15301–15312. doi: 10.1039/D2NJ02469A. [DOI] [Google Scholar]
- 11.Sankar Sana S., Haldhar R., Parameswaranpillai J., Chavali M., Kim S.C. Silver nanoparticles-based composite for dye removal: a comprehensive review. Clean. Mater. 2022;6 doi: 10.1016/J.CLEMA.2022.100161. [DOI] [Google Scholar]
- 12.Park Y. New paradigm shift for the green synthesis of antibacterial silver nanoparticles utilizing plant extracts. Toxicol. Res. 2014;30:169. doi: 10.5487/TR.2014.30.3.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nandhini S.N., Sisubalan N., Vijayan A., Karthikeyan C., Gnanaraj M., Gideon D.A.M., Jebastin T., Varaprasad K., Sadiku R. Recent advances in green synthesized nanoparticles for bactericidal and wound healing applications. Heliyon. 2023;9 doi: 10.1016/J.HELIYON.2023.E13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eltaweil A.S., Abdelfatah A.M., Hosny M., Fawzy M. Novel biogenic synthesis of a Ag@Biochar nanocomposite as an antimicrobial agent and photocatalyst for methylene blue degradation. ACS Omega. 2022;7:8046–8059. doi: 10.1021/ACSOMEGA.1C07209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu D., Yang X., Chen W., Feng Z., Hu C., Yan F., Chen X., Qu D., Chen Z. Rhodiola rosea Rhizome extract-mediated green synthesis of silver nanoparticles and evaluation of their potential antioxidant and catalytic reduction activities. ACS Omega. 2021;6:24450–24461. doi: 10.1021/ACSOMEGA.1C02843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmed F., Ali I., Ali H.S., Yasmeen S., Ullah S., Burki S., Adil M., Nisar J., Shah M.R. Synthesis and characterization of a plant growth regulator based silver nanoparticles for the ultrasensitive detection of environmentally toxic Hg2+ ions in tap water. New J. Chem. 2021;45:18039–18047. doi: 10.1039/D1NJ03393J. [DOI] [Google Scholar]
- 17.Mikhailova E.O. Silver nanoparticles: mechanism of action and probable bio-Application. J. Funct. Biomater. 2020;11 doi: 10.3390/JFB11040084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei X., Cheng F., Yao Y., Yi X., Wei B., Li H., Wu Y., He J. Facile synthesis of a carbon dots and silver nanoparticles (CDs/AgNPs) composite for antibacterial application. RSC Adv. 2021;11:18417–18422. doi: 10.1039/D1RA02600C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma N.K., Vishwakarma J., Rai S., Alomar T.S., Almasoud N., Bhattarai A. Green route synthesis and characterization techniques of silver nanoparticles and their biological adeptness. ACS Omega. 2022;7:27004–27020. doi: 10.1021/ACSOMEGA.2C01400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vanlalveni C., Lallianrawna S., Biswas A., Selvaraj M., Changmai B., Rokhum S.L. Green synthesis of silver nanoparticles using plant extracts and their antimicrobial activities: a review of recent literature. RSC Adv. 2021;11:2804–2837. doi: 10.1039/D0RA09941D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nawabjohn M.S., Sivaprakasam P., Anandasadagopan S.K., Begum A.A., Pandurangan A.K. Green synthesis and characterisation of silver nanoparticles using Cassia tora seed extract and investigation of antibacterial potential. Appl. Biochem. Biotechnol. 2022;194:464–478. doi: 10.1007/S12010-021-03651-4/FIGURES/8. [DOI] [PubMed] [Google Scholar]
- 22.Chugh D., Viswamalya V.S., Das B. Green synthesis of silver nanoparticles with algae and the importance of capping agents in the process. J. Genet. Eng. Biotechnol. 2021;191(19):1–21. doi: 10.1186/S43141-021-00228-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alharbi N.S., Alsubhi N.S., Felimban A.I. Green synthesis of silver nanoparticles using medicinal plants: characterization and application. J. Radiat. Res. Appl. Sci. 2022;15:109–124. doi: 10.1016/J.JRRAS.2022.06.012. [DOI] [Google Scholar]
- 24.Ahmad S., Munir S., Zeb N., Ullah A., Khan B., Ali J., Bilal M., Omer M., Alamzeb M., Salman S.M., Ali S. Green nanotechnology: a review on green synthesis of silver nanoparticles — an ecofriendly approach. Int. J. Nanomed. 2019;14:5087–5107. doi: 10.2147/IJN.S200254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamedi S., Shojaosadati S.A. Rapid and green synthesis of silver nanoparticles using Diospyros lotus extract: evaluation of their biological and catalytic activities. Polyhedron. 2019;171:172–180. doi: 10.1016/J.POLY.2019.07.010. [DOI] [Google Scholar]
- 26.Taher M., Shaari S.S., Susanti D., Arbain D., Zakaria Z.A. Genus Ophiorrhiza: a Review of its distribution, traditional uses, phytochemistry, biological activities and propagation. Mol. 2020;25:2611. doi: 10.3390/MOLECULES25112611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pajerski W., Chytrosz-Wrobel P., Golda-Cepa M., Pawlyta M., Reczynski W., Ochonska D., Brzychczy-Wloch M., Kotarba A. Opposite effects of gold and silver nanoparticle decoration of graphenic surfaces on bacterial attachment. New J. Chem. 2022;46:13286–13295. doi: 10.1039/D2NJ00648K. [DOI] [Google Scholar]
- 28.Ma J., Li K., Gu S., Wu Y., Zhang J., Wu J., Zhao L., Li X. Antimicrobial carbon-dot–stabilized silver nanoparticles. New J. Chem. 2022;46:2546–2552. doi: 10.1039/D1NJ05798G. [DOI] [Google Scholar]
- 29.Dong Y., Zhu H., Shen Y., Zhang W., Zhang L. Antibacterial activity of silver nanoparticles of different particle size against Vibrio Natriegens. PLoS One. 2019;14 doi: 10.1371/JOURNAL.PONE.0222322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skomorokhova E.A., Sankova T.P., Orlov I.A., Savelev A.N., Magazenkova D.N., Pliss M.G., Skvortsov A.N., Sosnin I.M., Kirilenko D.A., Grishchuk I.V., Sakhenberg E.I., Polishchuk E.V., Brunkov P.N., Romanov A.E., Puchkova L.V., Ilyechova E.Y. Size-Dependent bioactivity of silver nanoparticles: antibacterial properties, influence on copper status in mice, and whole-body turnover. Nanotechnol. Sci. Appl. 2020;13:137–157. doi: 10.2147/NSA.S287658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramos R.M.C.R., Regulacio M.D. Controllable synthesis of bimetallic nanostructures using biogenic reagents: a green perspective. ACS Omega. 2021;6:7212–7228. doi: 10.1021/ACSOMEGA.1C00692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hartati Y.W., Topkaya S.N., Gaffar S., Bahti H.H., Cetin A.E. Synthesis and characterization of nanoceria for electrochemical sensing applications. RSC Adv. 2021;11:16216–16235. doi: 10.1039/D1RA00637A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Javed R., Zia M., Naz S., Aisida S.O., ul Ain N., Ao Q. Role of capping agents in the application of nanoparticles in biomedicine and environmental remediation: recent trends and future prospects. J. Nanobiotechnol. 2020;18:1–15. doi: 10.1186/S12951-020-00704-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nene A., Galluzzi M., Hongrong L., Somani P., Ramakrishna S., Yu X.F. Synthetic preparations and atomic scale engineering of silver nanoparticles for biomedical applications. Nanoscale. 2021;13:13923–13942. doi: 10.1039/D1NR01851E. [DOI] [PubMed] [Google Scholar]
- 35.Adnan M., Chy M.N.U., Kamal A.T.M.M., Azad M., Paul A., Uddin S., Barlow J., Faruque M., Park C., Cho D. Investigation of the biological activities and characterization of bioactive constituents of Ophiorrhiza rugosa var. prostrata (D.Don) & amp; Mondal Leaves through in Vivo, in Vitro, and in Silico Approaches. Molecules. 2019;24:1367. doi: 10.3390/molecules24071367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang C.D., He X.Z., Gou G.Q. Ophiorrhiza guizhouensis (Rubiaceae), a new species from Guizhou Province, southwestern China. PhytoKeys. 2018:121. doi: 10.3897/PHYTOKEYS.95.22506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krishnakumar G., Dintu K.P., Varghese S.C., Nair D.S., Gopinath G., Rameshkumar K.B., Satheeshkumar K., Krishnan P.N., Ophiorrhiza A promising herbaceous source of the anticancer compound camptothecin. Plant Sci. Today. 2020;7:240–250. doi: 10.14719/PST.2020.7.2.660. [DOI] [Google Scholar]
- 38.Gopinath G., Jose B., Ravichandran P., Satheeshkumar K. Tissue culture of Ophiorrhiza mungos L., a prospective method for the production of an anticancer drug, camptothecin. Plant Sci. Today. 2018;5:1–8. doi: 10.14719/PST.2018.5.1.359. [DOI] [Google Scholar]
- 39.Malik M., Iqbal M.A., Malik M., Raza M.A., Shahid W., Choi J.R., Pham P.V. Biosynthesis and characterizations of silver nanoparticles from Annona squamosa leaf and fruit extracts for size-dependent biomedical applications. Nanomaterials. 2022;12 doi: 10.3390/NANO12040616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ullah A.K.M.A., Kabir M.F., Akter M., Tamanna A.N., Hossain A., Tareq A.R.M., Khan M.N.I., Kibria A.K.M.F., Kurasaki M., Rahman M.M. Green synthesis of bio-molecule encapsulated magnetic silver nanoparticles and their antibacterial activity. RSC Adv. 2018;8:37176–37183. doi: 10.1039/C8RA06908E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Durval I.J.B., Meira H.M., de Veras B.O., Rufino R.D., Converti A., Sarubbo L.A. Green synthesis of silver nanoparticles using a biosurfactant from Bacillus cereus UCP 1615 as stabilizing agent and its application as an antifungal Agent. Fermentatio. 2021;7:233. doi: 10.3390/FERMENTATION7040233. [DOI] [Google Scholar]
- 42.Priya S., Murali A., Preeth D.R., Dharanibalaji K.C., Jeyajothi G. Green synthesis of silver nanoparticle-embedded poly(methyl methacrylate-co-methacrylic acid) copolymer for fungal-free leathers. Polym. Bull. 2022;79:4607–4626. doi: 10.1007/S00289-021-03714-W/FIGURES/12. [DOI] [Google Scholar]
- 43.Cruz D., Falé P.L., Mourato A., Vaz P.D., Luisa Serralheiro M., Lino A.R.L. Preparation and physicochemical characterization of Ag nanoparticles biosynthesized by Lippia citriodora (Lemon Verbena) Colloids Surf., B. 2010;81:67–73. doi: 10.1016/J.COLSURFB.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 44.Saion E., Gharibshahi E., Naghavi K. Size-controlled and optical properties of monodispersed silver nanoparticles synthesized by the radiolytic reduction method. Int. J. Mol. Sci. 2013;14:7880. doi: 10.3390/IJMS14047880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Birla S.S., Gaikwad S.C., Gade A.K., Rai M.K. Rapid synthesis of silver nanoparticles from Fusarium oxysporum by optimizing physicocultural conditions. Sci. World J. 2013;2013 doi: 10.1155/2013/796018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kumar B., Vizuete K.S., Sharma V., Debut A., Cumbal L. Ecofriendly synthesis of monodispersed silver nanoparticles using Andean Mortiño berry as reductant and its photocatalytic activity. Vacuum. 2019;160:272–278. doi: 10.1016/J.VACUUM.2018.11.027. [DOI] [Google Scholar]
- 47.Alamu G.A., Adedokun O., Bello I.T., Sanusi Y.K. Plasmonic enhancement of visible light absorption in Ag-TiO2 based dye-sensitized solar cells. Chem. Phys. Impact. 2021;3 doi: 10.1016/J.CHPHI.2021.100037. [DOI] [Google Scholar]
- 48.Baran M.F., Keskin C., Baran A., Hatipoglu A., Yildiztekin M., Kucukaydin S., Kurt K., Hosgoren H., Sarker M.M.R., Sufianov A., Beylerli O., Khalilov R., Eftekhari A. Green synthesis of silver nanoparticles from Allium cepa L. Peel extract, their antioxidant, antipathogenic, and anticholinesterase activity. Molecules. 2023;28:2310. doi: 10.3390/MOLECULES28052310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sidhu A.K., Verma N., Kaushal P. Role of biogenic aapping agents in the synthesis of metallic nanoparticles and evaluation of their therapeutic potential. Front. Nanotechnol. 2022;3:105. doi: 10.3389/FNANO.2021.801620/BIBTEX. [DOI] [Google Scholar]
- 50.Femi-Adepoju A.G., Dada A.O., Otun K.O., Adepoju A.O., Fatoba O.P. Green synthesis of silver nanoparticles using terrestrial fern (Gleichenia Pectinata (Willd.) C. Presl.): characterization and antimicrobial studies. Heliyon. 2019;5:1543. doi: 10.1016/J.HELIYON.2019.E01543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hasan K.M.F., Xiaoyi L., Shaoqin Z., Horvath P.G., Bak M., Bejo L., Sipos G., Alpar T. Functional silver nanoparticles synthesis from sustainable point of view: 2000 to 2023 ‒ A review on game changing materials. Heliyon. 2022;8 doi: 10.1016/J.HELIYON.2022.E12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taheri S., Heravi M.M., Mohammadi P. Synthesis of Ag nanoparticles by Celery leaves extract supported on magnetic biochar substrate, as a catalyst for the reduction reactions. Sci. Rep. 2022;12:1–11. doi: 10.1038/s41598-022-18131-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Logaranjan K., Raiza A.J., Gopinath S.C.B., Chen Y., Pandian K. Shape- and size-controlled synthesis of silver nanoparticles using Aloe vera plant extract and their antimicrobial activity. Nanoscale Res. Lett. 2016;11 doi: 10.1186/S11671-016-1725-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dada A.O., Adekola F.A., Dada F.E., Adelani-Akande A.T., Bello M.O., Okonkwo C.R., Inyinbor A.A., Oluyori A.P., Olayanju A., Ajanaku K.O., Adetunji C.O. Silver nanoparticle synthesis by Acalypha wilkesiana extract: phytochemical screening, characterization, influence of operational parameters, and preliminary antibacterial testing. Heliyon. 2019;5 doi: 10.1016/J.HELIYON.2019.E02517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Asimuddin M., Shaik M.R., Adil S.F., Siddiqui M.R.H., Alwarthan A., Jamil K., Khan M. Azadirachta indica based biosynthesis of silver nanoparticles and evaluation of their antibacterial and cytotoxic effects. J. King Saud Univ. Sci. 2020;32:648–656. doi: 10.1016/J.JKSUS.2018.09.014. [DOI] [Google Scholar]
- 56.Duran N., Duran M., de Jesus M.B., Seabra A.B., Favaro W.J., Nakazato G. Silver nanoparticles: a new view on mechanistic aspects on antimicrobial activity. Nanomed. Nanotechnol. Biol. Med. 2016;12:789–799. doi: 10.1016/J.NANO.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 57.Matras E., Gorczyca A., Przemieniecki S.W., Ocwieja M. Surface properties-dependent antifungal activity of silver nanoparticles. Sci. Rep. 2022;121(12):1–17. doi: 10.1038/s41598-022-22659-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li L., Pan H., Deng L., Qian G., Wang Z., Li W., Zhong C. The antifungal activity and mechanism of silver nanoparticles against four pathogens causing kiwifruit post-harvest rot. Front. Microbiol. 2022;13 doi: 10.3389/FMICB.2022.988633/FULL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim K.J., Sung W.S., Suh B.K., Moon S.K., Choi J.S., Kim J.G., Lee D.G. Antifungal activity and mode of action of silver nano-particles on Candida albicans. Biometals. 2009;22:235–242. doi: 10.1007/S10534-008-9159-2. [DOI] [PubMed] [Google Scholar]
- 60.Morones J.R., Elechiguerra J.L., Camacho A., Holt K., Kouri J.B., Ramirez J.T., Yacaman M.J. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16:2346–2353. doi: 10.1088/0957-4484/16/10/059. [DOI] [PubMed] [Google Scholar]
- 61.Du H., Lo T.M., Sitompul J., Chang M.W. Systems-level analysis of Escherichia coli response to silver nanoparticles: the roles of anaerobic respiration in microbial resistance. Biochem. Biophys. Res. Commun. 2012;424:657–662. doi: 10.1016/J.BBRC.2012.06.134. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. Material/referenced in article.