Abstract
Background
Previous studies have confirmed the higher risk of bladder cancer (BC) and rectal cancer (RC) development among prostate cancer (PCa) patients receiving radiotherapy. In this study, we intend to explore the long-term trend in second BC and RC incidence among PCa patients undergoing radiotherapy.
Method
We identified first primary PCa patients diagnosed between 1975 and 2014 from the Surveillance, Epidemiology, and End Results (SEER)-9 cancer registries. Standardized incidence ratios (SIRs) were calculated by calendar year of diagnosis among PCa patients receiving radiotherapy and not. P trends were evaluated using Poisson regression. 10-year cumulative incidence of BC and RC was calculated utilizing competing risk regression model.
Result
Of PCa patients treated with radiotherapy, SIRs of BC increased from .82 (95% CI: .35- 1.61) in 1980–1984 to 1.58 (95% CI: 1.48–1.68) in 2010-2014 (Ptrend=.003). SIRs of RC increased from 1.01 (95% CI: .27-2.58) in 1980–1984 to 1.54 (95% CI: 1.31–1.81) in 2010-2014 (Ptrend=.025). No statistically significant change in both BC and RC incidence was observed. The 10-year cumulative incidence of BC increased from 1975–1984 (.04%) to 2005–2014 (.15%) among PCa treated with radiotherapy. Simultaneously, the 10-year cumulative incidence of RC was demonstrated to range from 1975–1984 (.02%) to 2005–2014 (.11%).
Conclusion
we have observed an increasing trend in second BC and RC incidence in PCa patients receiving radiotherapy. There was no significant change in the incidence of second BC and RC in PCa without radiotherapy. These results reflect the increasing clinical burden of second malignant tumors in PCa patients undergoing radiotherapy.
Keywords: prostate cancer, radiotherapy, second malignant tumor, bladder cancer, rectal cancer, SEER
Introduction
It is estimated that more than 2 million men in the United States are diagnosed with prostate cancer (PCa). 1 Ionizing radiation is an established treatment option recommended by most guidelines for PCa, and its long-term prognosis is similar to that of radical prostatectomy. 2 In addition to the acute side effects during and after radiotherapy (RT), such as irritating genitourinary system and gastrointestinal symptoms, radiologists have paid increasing attention to the long-term side effects like the second malignant tumor induced by RT in recent decades due to excellent progress has been made in the long-term survival of prostate cancer. 3 Studies reporting second malignancies after RT for PCa have yielded conflicting results, both positive and negative.4,5 Until recently, a meta-analysis including 26 retrospective studies came to a convincing conclusion which suggested RT was associated with higher risks of bladder cancer and rectal cancer development for PCa patients. 6
Radiation therapy involves inevitably irradiating normal organs and tissues outside the target area, and the bladder and rectum inevitably receive relatively more radiation as the pelvic organs. 7 It seems not surprised that RT for the prostate increases the risk of bladder cancer and rectal cancer based on the idea that ionizing radiation has long been considered to have carcinogenic potential. 5 To solve this problem, radiologists are committed to improving the RT technology to accurately provide the specified target dose and minimize the dose to the surrounding key organs. For instance, using modern therapeutic techniques like brachytherapy or intensity-modulated radiation therapy (IMRT) was suggested not to significantly increase the risk of bladder cancer and rectal cancer development. 8 However, some inconsistencies have indicated that advances in RT technology are insufficient to reduce the risk of second primary pelvic tumors, although they may be possible by reducing the possibility of pelvic organs being exposed to high-dose radiation. 9
This improvement in RT and the awareness of radiation carcinogenicity should affect the incidence rate of second pelvic malignancies in prostate cancer survivors. If American men who receive modern forms of prostate cancer RT have an increased risk of bladder or rectal cancer, it may have an impact on public health, cancer surveillance, and patient counseling. 1 Up to our knowledge, the changes in second bladder and rectal cancer in prostate cancer survivors receiving RT in the United States have not been well described. In this study, we explored the long-term trends in second bladder cancer and rectal cancer incidence among men PCa patients utilizing data from the Surveillance, Epidemiology, and End Results (SEER)-9 cancer registries.
Methods
Data Source and Study Population
This retrospective, population-based cohort study was based on the SEER-9 database (1975–2019), which covered 9 registries in the United States, accounting for about 9.4% of the population of the United States. 10 Cancer registration data are used to characterize cancer incidence, treatment, and survival. Incidence data in the United States were maintained by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention (CDC).
We identified men patients diagnosed with first primary prostate cancer since the treatment information in SEER was limited to the first course of treatment. The study period was from January 1, 1975, to December 31, 2014. Then we excluded patients who met the following terms: (1) age less than 20 years and (2) unknown survival month or survival month less than 60 months (Figure 1). The data from SEER was publicly available and de-identified. Consent was not requested.
Figure 1.
Flow-chart showing the procedure used to identify patients with prostate cancer registered in the SEER database from 1975 to 2014 287x125 mm (600 x 600 DPI).
Treatment Information
We divided the study population into cohorts of receiving RT and non-RT (recorded as no/unknown). The RT strategy in receiving RT cohort included external beam radiotherapy (ERBT), brachytherapy (BT), or a combination of external beam radiotherapy and brachytherapy (ERBT + BT). Patients in the non-RT cohort were recorded as no/unknown radiotherapy or refusing radiotherapy. Additionally, we separately included a group of patients who underwent surgical treatment in the population that did not receive radiotherapy.
Definition of Outcome
The primary outcome of this study was the occurrence of BC or RC more than 5 years after PCa patients received RT considering the incubation period of at least 5 years from radiation exposure to a solid tumor. The SEER program followed the guidelines of the third edition of the International Classification of Oncological Diseases to distinguish between second malignant tumors and recurrent diseases. The case list of “Sequence number” recorded the order of cancer history in which all the primary tumors can be reported in the patient’s life.
Statistical Analyses
Patients were followed beginning 5-year PCa diagnosis to the end of diagnosis of BC or RC, all-cause death, or date of the last contact, whichever occurred first. To evaluate the risk of BC or RC development relative to the general population, we calculated standardized incidence ratios (SIRs) and corresponding 95% confidence intervals (CIs). SIRs were calculated by dividing the observed number of BC or RC cases among prostate cancer survivors by the expected number in the US male general population, adjusted for age at diagnosis (5-year groups), race (White/Black/other), and year of diagnosis. The SIRs were calculated with SEER*Stat 8.4.0(ID: 20 420-Nov2020). We explored the trend in SIRs of bladder cancer or rectal cancer by calendar year of PCa diagnosis among a cohort of PCa patients treated with RT, non-RT, or undergoing surgery, respectively. Further examination was performed by stratifying the study population by age at diagnosis (<60 years; 60-74 years and >74 years) and RT strategy (ERBT and ERBT + BT or BT).
We performed Poisson regression model with expected events as the offset to test the trend of SIRs of BC or RC by calendar year of PCa diagnosis (adjust for age at diagnosis and race). To evaluate the clinical burden of BC or RC incidence among PCa treated with RT, the cumulative incidence of BC or RC was calculated by calendar year of PCa diagnosis using Fine-Gray competing risk regression analysis. Experiencing the death from all-cause were taken into account as competing events. We calculated the 10-year cumulative incidence of BC and RC by the calendar year of PCa diagnosis.
Two-sample t-tests and Chi-square test were applied for continuous and categorical variables to verify heterogeneity between receiving and without RT cohorts. All statistical analysis was performed by R software (version4.1.3). All tests were 2-sided with statistical significance set at P < .05.
Results
Descriptive Characteristics
Finally, we enrolled 400,243 patients diagnosed with prostate cancer from 1975 to 2014 (Figure 1). Of these patients, 149 065 were recorded as receiving RT, and 251,178 recoded with non-RT (Table 1). In a cohort receiving RT, there were 103 406 (69.37%), 18 834 (12.63%), and 26,825 (18%) treated with ERBT, ERBT + BT, and BT, respectively. Patients treated with RT had a longer survival time than those without RT. Of patients treated with RT, there were 3191 (1.9%) developing BC and 701 (.42%) developing RC, respectively. In the cohort of non-RT, there were 3234 (.69%) developing BC and 700 (.15%) developing RC, respectively. The latency of BC in receiving RT cohort was statistically shorter when compared with that in the non-RT cohort (mean: 167 months vs 178 months; median: 158 months vs 172 months). Similarly, the mean and median latency of RC in PCa patients with RT was shorter than in those without.
Table 1.
Overall Characteristics of Prostate Cancer Survivors, 1975–2014.
| Characteristic | Receiving radiation | Non or Unknown Receiving radiation | P-value |
|---|---|---|---|
| (N = 149,065) | (N = 251,178) | ||
| Age(year) | <.001 | ||
| <60 | 23,685 (15.9%) | 61,212 (24.4%) | |
| 60-74 | 95,086 (63.8%) | 140,575 (56.0%) | |
| >74 | 30,294 (20.3%) | 49,391 (19.7%) | |
| Stage | <.001 | ||
| Localized/regional | 106,422 (71.4%) | 163,048 (64.9%) | |
| Distant | 931 (.6%) | 2786 (1.1%) | |
| Unknown | 41,712 (28.0%) | 85,344 (34.0%) | |
| Year of diagnosis | <.001 | ||
| 1975–1984 | 10,058 (6.7%) | 21,990 (8.8%) | |
| 1985–1994 | 30,372 (20.4%) | 58,497 (23.3%) | |
| 1995–2004 | 56,581 (38.0%) | 82,143 (32.7%) | |
| 2005–2014 | 52,054 (34.9%) | 88,548 (35.3%) | |
| Radiation recode | NA | ||
| ERBT | 103,406 (69.37%) | 0 (0%) | |
| ERBT + BT | 18,834 (12.63%) | 0 (0%) | |
| BT | 26,825 (18%) | 0 (0%) | |
| Survival month | <.001 | ||
| Mean (SD) | 115 (74.8) | 105 (82.2) | |
| Median | 108 | 89 | |
| Developed BC | 3191 (1.9%) | 3234 (.69%) | <.001 |
| Latency(month) | |||
| Mean (SD) | 167 (58.9) | 178 (65.8) | |
| Median | 158 | 172 | |
| Developed RC | 701 (.42%) | 700 (.15%) | |
| Latency(month) | <.001 | ||
| Mean (SD) | 170 (57.9) | 179 (68.4) | |
| Median | 162 | 170 |
BRT: External beam radiotherapy; EBRT+BT: interstitial brachytherapy or a combination of external beam radiotherapy; BT: brachytherapy; SD standard deviation; BC: bladder cancer; RC: rectal cancer.
Trend in SIRs of Bladder Cancer and Rectal Cancer in Prostate Cancer Patients
Overall, bladder cancer incidence in PCa patients treated with RT increased significantly relative to the general population by the calendar year of prostate cancer diagnosis, from an SIR of .82 (95% CI: .35–1.61) in 1980–1984 to 1.58 (95% CI: 1.48–1.68) in 2010–2014 (Ptrend=.003) (Table 2). Simultaneously, we observed an increasing trend in the incidence of rectal cancer, from an SIR of 1.01 (95% CI: .27–2.58) in 1980–1984 to 1.54 (95% CI: 1.31–1.81) in 2010–2014, in cohort of receiving RT (Ptrend=.025). Of PCa patients who did not receive RT, there was no statistically significant change in both bladder cancer and rectal cancer incidence (Ptrend=.25 for BC; Ptrend=.38 for RC). Similarly, no statistical trends in BC and RC SIRs were observed among PCa patients undergoing surgery (all Ptrend>.05).
Table 2.
Bladder Cancer or Rectal Cancer Sirs by Calendar Year of Diagnosis and Radiation.
| Receiving Radiation | ||||||
| Bladder Cancer | Rectal Cancer | |||||
| Calendar year of diagnosis | Observed | SIR | 95%CI | Observed | SIR | 95%CI |
| 1975–1979 | NA | NA | NA | NA | NA | NA |
| 1980–1984 | 8 | 0.82 | 0.35–1.61 | 4 | 1.01 | 0.27-2.58 |
| 1985–1989 | 49 | 1.33 | 0.98–1.76 | 18 | 1.36 | 0.81-2.15 |
| 1990–1994 | 101 | 1.37 | 1.11–1.66 | 29 | 1.21 | 0.81-1.74 |
| 1995–1999 | 218 | 1.34 | 1.17–1.53 | 58 | 1.22 | 0.93-1.58 |
| 2000–2004 | 382 | 1.40 | 1.26–1.54 | 96 | 1.35 | 1.09-1.65 |
| 2005–2009 | 607 | 1.4 | 1.29–1.51 | 144 | 1.56 | 1.31-1.83 |
| 2010-2014 | 902 | 1.58 | 1.48–1.68 | 151 | 1.54 | 1.31-1.81 |
| P-trend | 0.003 | 0.025 | ||||
SIRs: Standardized incidence ratios; NA: Not applicable; CI: confidence interval.
The bold part is statistically significant.
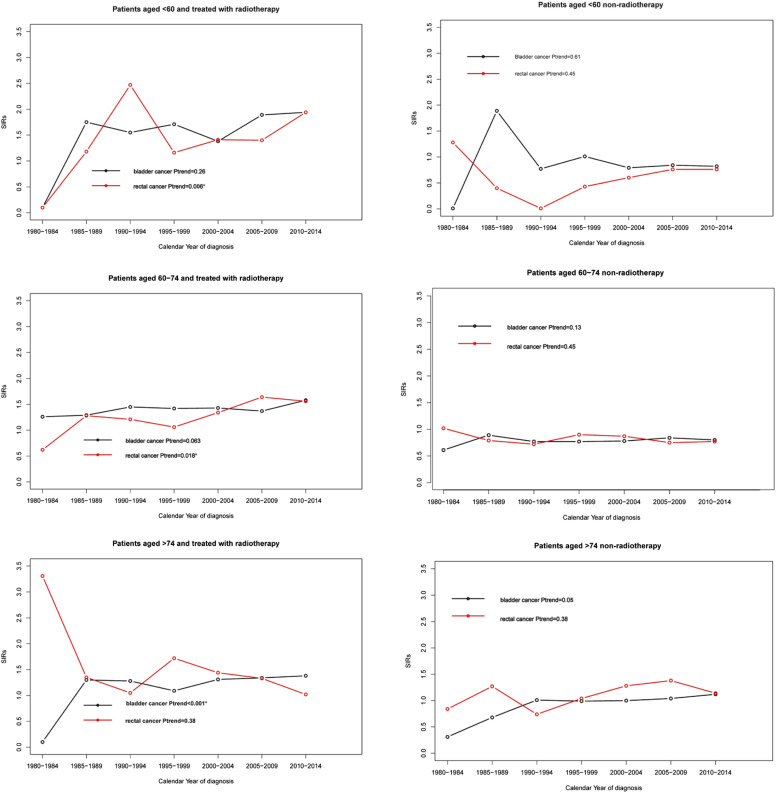
In the cohort receiving RT, we only observed a significantly rising incidence of bladder cancer in PCa patients aged over 74 years (Ptrend<.001) (Figure 2(C)), and no statistical trend was obtained in the age group of <60 and 60–74 years (all Ptrend>.05) (Figure 2AB). There was a significantly increased incidence of rectal cancer among PCa patients aged <60 (Ptrend = .003) (Figure 2(A)) and 60–74 years (Ptrend=.018) (Figure 2(B)), and we failed to observe any change in the age group of >74 (Ptrend=.38). Of PCa patients without RT, there was no statistical trend in both bladder cancer and rectal cancer incidence when we stratified the study population by age (all Ptrend >.05) (Figure 2DEF).
Figure 2.
Trends in second bladder cancer and rectal cancer SIRs among patients with prostate cancer by age at diagnosis. 249x249 mm (600 x 600 DPI).
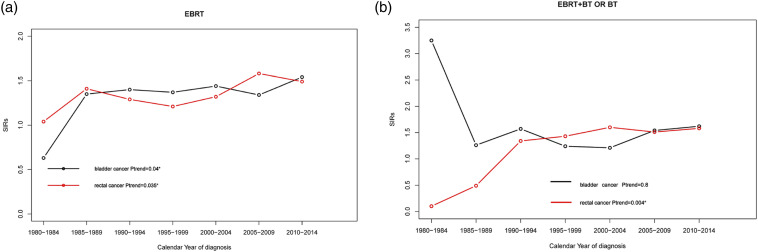
Finally, we examined the trend of BC and RC incidence among PCa patients treated with RT by radiotherapy strategy. For PCa patients receiving ERBT, we found an ascendant trend in both BC and RC incidence (Ptrend=.04 for BC; Ptrend=.035 for RC) (Figure 3(A)). For PCa patients treated with ERBT + BT or BT, this increasing incidence was only observed in RC (Ptrend=.004) (Figure 3(B)).
Figure 3.
Trends in second bladder cancer and rectal cancer SIRs among prostate cancer patients treated with radiotherapy by radiotherapy strategy 350x120 mm (600 x 600 DPI).
Cumulative Incidence of Bladder Cancer and Rectal Cancer
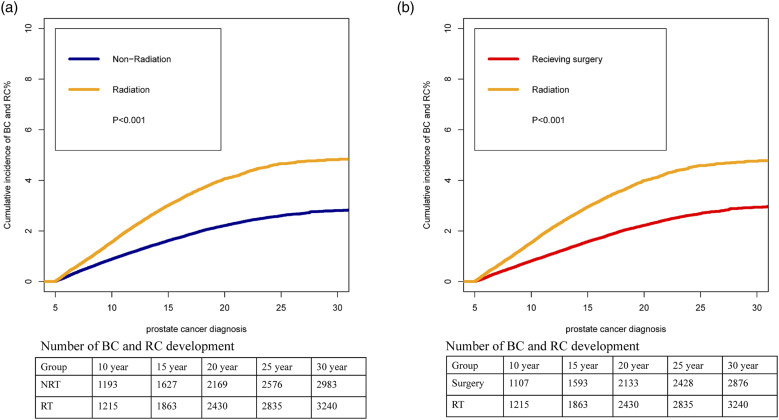
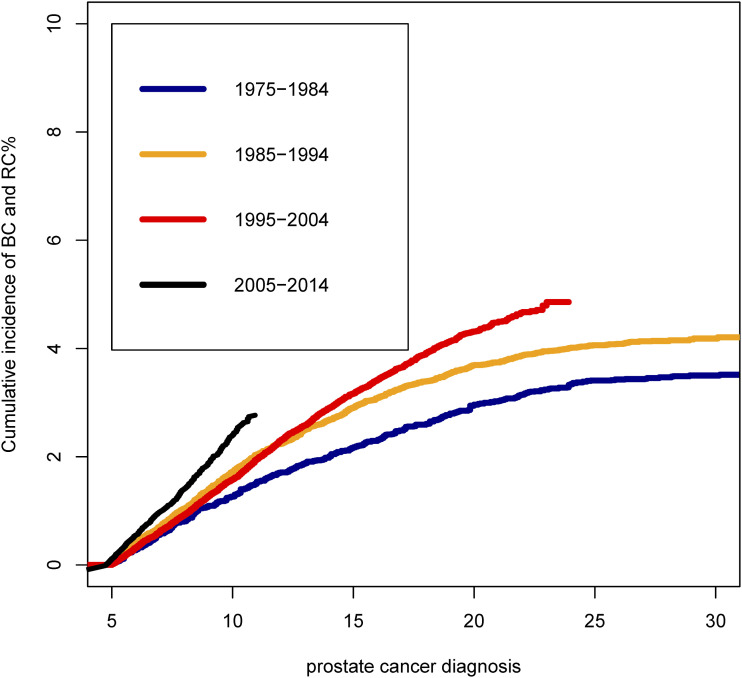
Cumulative mortality analyses showed the varying clinical burden of BC and RC among PCa patients treated with RT by the calendar year of PCa diagnosis (Figure 4). The cumulative incidence of BC and RC in PCa patient treated with RT were significantly higher than that with non-RT (Figure 4(A)). Simultaneously, the cumulative incidence of bladder and rectal cancer was significantly higher in PCa patients receiving RT than those undergoing surgery (Figure 4(B)). We observed that the cumulative incidence of BC and RC increased with the progress of the calendar year of PCa diagnosis among patients treated with RT (Figure 5). The 10-year cumulative incidence of BC and RC increased from 1975–1984 (1.03%) to 2005–2014 (2.01%) among PCa treated with RT (P < .001).
Figure 4.
Cumulative incidence of second bladder cancer and rectal cancer among prostate cancer patients: (A) radiotherapy vs non-radiotherapy (B) radiotherapy vs surgery 127x95 mm (600 x 600 DPI).
Figure 5.
Cumulative incidence of second bladder cancer and rectal cancer among prostate cancer patients treated with radiotherapy by calendar year of prostate cancer diagnosis. 177x177 mm (600 x 600 DPI).
Discussion
To the best of our knowledge, this is the first descriptive study that provides a new perspective on the trend of second bladder and rectal cancer among prostate cancer survivors by comparing with the general population in the United States through a 45-year follow-up. In this study, we found a significant increase in the incidence rate of second bladder cancer and rectal cancer among prostate cancer patients receiving RT in the United States compared with the general population. Simultaneously, in prostate cancer patients who did not receive RT, we did not observe a statistically significant change in the incidence of second bladder cancer and rectal cancer.
Previous studies had clearly explained the association between RT for PCa and the high risk of second bladder cancer and rectal cancer.1,6,7 Although there was a lack of extensive prospective studies to provide a higher level of evidence, such trials seemed unlikely in the near future for logical reasons. However, the trend of second bladder and rectal cancer in patients with prostate cancer receiving RT has not been well explored. Alan M. Nieder et al. utilizing data from SEER (1988–2003) found the excessive incidence of bladder cancer relative to the general population among PCa patients treated with RT, and they did not observe a similar result in rectal cancer. 1 Nevertheless, they did not examine the incidence rate trend, and the data were relatively remote, so these results could not provide updated information to reflect the modern results. In this study, this relatively high incidence than the general population was only observed for bladder cancer after 1990 and for rectal cancer after 2000 among PCa patients treated with RT.
Overall, this study not only found that PCa patients undergoing RT had a relatively higher incidence of bladder cancer and rectal cancer than the general population but also that the incidence rate was increasing by the calendar year of PCa diagnosis. Normally, the improved RT technique is transmitted to the diseased tissue more accurately through the radiation dose and, simultaneously, reduces the radiation from the surrounding normal tissue. 11 For example, modern improved ERBT techniques include three-dimensional conformal RT, intensity-modulated RT, volumetric modulated arc therapy and stereotactic body radiation therapy. 11 They were proved to have improved in terms of tumor identification and description, patient location accuracy, and dose delivery accuracy. Meanwhile, brachytherapy was suggested to save more normal tissues by placing the radiation sources inside the prostate or tumor. 8 Data showed that the improved ERBT technique and BT could diminish the average bladder radiation dose and the volume of the bladder exposed to low dose radiation to reduce the risk of bladder cancer development.12,13
In some older SEER data, most PCa patients received 60Co treatment with a large pelvic field of view, which would provide higher doses to pelvic organs compared to modern RT technology. 11 Therefore, it should normally get improvement in bladder and rectal cancer development among PCa patients treated with modern RT. Instead, we observed an increase in the incidence of bladder and rectal cancer with the year of PCa diagnosis. A possible important explanation for this result is the relatively long incubation period from RT to second malignant tumors. Previous studies have defined the latency from PCa diagnosis to second malignant tumor as 5 to 10 years.6,14,15 Aryeh Keehn et al. observed a significantly increased risk of bladder cancer development after 10 years after RT for PCa patients based on data of SEER from 1973 to 2011. 16 Meanwhile, some previous studies have suggested that the risk of radiation-related second malignant tumors in patients with prostate cancer increased over time. 6 This relatively long latency made the improvement brought about by modern RT exhibit a significant lag effect. In addition, with the progress of the treatment of prostate cancer, the number of long-term cancer survivors has increased significantly. The 10-year cancer-specific survival rate of PCa patients was reported to be 93%, and the 15-year survival rate was 79%. 8 Similarly, due to the widespread use of PSA, more and more PCa patients were detected earlier, which improved the survival time of PCa. 17 Therefore, there will be more PCa patients in later periods to meet the standard that the long-term side effects of this kind of RT could be observed. Another important explanation is the improvement of clinical detection methods and the increase in frequency of bladder and rectal cancer. For instance, in the surveillance of PCa patients, imaging examination of pelvic organs would be increased, which was conducive to the early detection of bladder cancer and rectal cancer.
It was still controversial that modern RT can improve second bladder and rectal cancer in patients with prostate cancer.8,12 The main explanation was that such results often came from single-center studies, which were limited by the number of patients and follow-up time, so there was significant heterogeneity between the results of studies. In this study, we confirmed the increasing trend in the incidence of both bladder and rectal cancer among PCa patients receiving ERBT and an ascendant trend in rectal cancer incidence in PCa patients with BT or ERBT + BT. In general, we did not obtain a trend of improvement in bladder and rectal cancer development regardless of the RT strategy. SEER data sources cover multiple registrations, and the RT information was homogenized, so our results represented the average level of RT in the United States. 18 Large prospective studies are needed in the future to examine the association between modern RT techniques and second malignant tumors in PCa patients.
Second malignant tumors were a severe clinical burden for PCa patients, especially those with low risk because the prognosis of bladder cancer and rectal cancer was relatively poor compared with PCa.19,20 According to contemporary data results, more serious attention should be paid to the long-term complications caused by prostate cancer RT, when we were not sure whether modern RT could significantly reduce the risk of bladder and rectal cancer development. Meanwhile, our results further supported the development of more targeted precisely radiation technologies. Perhaps most importantly, this study confirmed the belief that for patients with low-risk prostate cancer who do not need treatment at all, a second malignant tumor should be added to the already long list of avoidable risks associated with treatment. 21
There were several limitations in this study. First, although a large number of patients could be studied using SEER data, the lack of detailed information about treatment, radiation techniques, or other potential confounding factors limited furtherly analysis of the results. In addition, we had to admit that there were some patients with missing follow-up in the SEER program who did not record whether they had developed malignant tumors, so there should be more PCa patients with second malignant tumors than we obtained. However, this limitation would not affect our results. Lastly, we cannot obtain more direct indicators of changes in the incidence rate of BC and RC among PCa patients, such as crude incidence rates and age-standardized rates. Therefore, we used the SIR index.
Conclusion
In conclusion, we have observed an increasing trend in the incidence of second bladder and rectal cancer in PCa patients receiving RT compared with the general population in the United States. Meanwhile, there was no significant change in the incidence of second bladder or rectal cancer in PCa patients who did not receive RT. This study reflected the increasing clinical burden of second malignant tumors in PCa patients undergoing RT. In the future, more work should be spent on optimizing RT technology and selecting PCa patients suitable for undergoing RT.
Footnotes
Author Contributions: JinFang Lin, Xiangpeng Zhan, and Ru Chen contributed equally to this work and should be considered co-first authors.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Project of Natural Science Foundation of Jiangxi Province (20202BABL206032), Grant 2021QNA070 for Youth Research Fund from Fujian Provincial Health Commission, China, Key Project of Natural Science Foundation of Jiangxi Province (20212ACB206013), and Youth Project of Natural Science Foundation of Jiangxi Province (20212BAB216037).
Ethical Approval: The data from SEER is publicly available and de-identified. Consent is not requested. The study was conducted in accordance with the Declaration of Helsinki. This study used previously collected deidentified data, which was deemed exempt from review by The First Affiliated Hospital of Nanchang University.
ORCID iD
References
- 1.Nieder AM, Porter MP, Soloway MS. Radiation therapy for prostate cancer increases subsequent risk of bladder and rectal cancer: a population based cohort study. J Urol. 2008;180(5):2005-2009. discussion 9-10. [DOI] [PubMed] [Google Scholar]
- 2.Greenberger BA, Zaorsky NG, Den RB. Comparison of radical prostatectomy versus radiation and androgen deprivation therapy strategies as primary treatment for high-risk localized prostate cancer: a systematic review and meta-analysis. Eur Urol Focus. 2020;6(2):404-418. [DOI] [PubMed] [Google Scholar]
- 3.Bohmer D, Wirth M, Miller K, Budach V, Heidenreich A, Wiegel T. Radiotherapy and hormone treatment in prostate cancer. Dtsch Arztebl Int. 2016;113(14):235-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall EJ. Intensity-modulated radiation therapy, protons, and the risk of second cancers. Int J Radiat Oncol Biol Phys. 2006;65(1):1-7. [DOI] [PubMed] [Google Scholar]
- 5.Müller AC, Ganswindt U, Bamberg M, Belka C. Risk of second malignancies after prostate irradiation? Strahlenther Onkol. 2007;183(11):605-609. [DOI] [PubMed] [Google Scholar]
- 6.Wallis CJ, Mahar AL, Choo R, Herschorn S, Kodama RT, Shah PS, et al. Second malignancies after radiotherapy for prostate cancer: systematic review and meta-analysis. BMJ (Clinical research ed). 2016;352:i851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moon K, Stukenborg GJ, Keim J, Theodorescu D. Cancer incidence after localized therapy for prostate cancer. Cancer. 2006;107(5):991-998. [DOI] [PubMed] [Google Scholar]
- 8.Huang J, Kestin LL, Ye H, Wallace M, Martinez AA, Vicini FA. Analysis of second malignancies after modern radiotherapy versus prostatectomy for localized prostate cancer. Radiother Oncol. 2011;98(1):81-86. [DOI] [PubMed] [Google Scholar]
- 9.Maddams J, Parkin DM, Darby SC. The cancer burden in the United Kingdom in 2007 due to radiotherapy. Int J Cancer. 2011;129(12):2885-2893. [DOI] [PubMed] [Google Scholar]
- 10.Vo JB, Ramin C, Barac A, Berrington de Gonzalez A, Veiga L. Trends in heart disease mortality among breast cancer survivors in the US, 1975-2017. Breast Cancer Res Treat. 2022;192(3):611-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Podder TK, Fredman ET, Ellis RJ. Advances in radiotherapy for prostate cancer treatment. Adv Exp Med Biol. 2018;1096:31-47. [DOI] [PubMed] [Google Scholar]
- 12.Filippi AR, Franco P, Ricardi U. Is stereotactic ablative radiotherapy an alternative to surgery in operable stage I non-small cell lung cancer? Rep Pract Oncol Radiother. 2014;19(4):275-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murray L, Henry A, Hoskin P, Siebert FA, Venselaar J, PROBATE group of GEC ESTRO . Second primary cancers after radiation for prostate cancer: a systematic review of the clinical data and impact of treatment technique. Radiother Oncol. 2014;110(2):213-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murray EM, Werner D, Greeff EA, Taylor DA. Postradiation sarcomas: 20 cases and a literature review. Int J Radiat Oncol Biol Phys. 1999;45(4):951-961. [DOI] [PubMed] [Google Scholar]
- 15.Sale KA, Wallace DI, Girod DA, Tsue TT. Radiation-induced malignancy of the head and neck. Otolaryngol Head Neck Surg. 2004;131(5):643-645. [DOI] [PubMed] [Google Scholar]
- 16.Keehn A, Ludmir E, Taylor J, Rabbani F. Incidence of bladder cancer after radiation for prostate cancer as a function of time and radiation modality. World J Urol. 2017;35(5):713-720. [DOI] [PubMed] [Google Scholar]
- 17.Davis EJ, Beebe-Dimmer JL, Yee CL, Cooney KA. Risk of second primary tumors in men diagnosed with prostate cancer: a population-based cohort study. Cancer. 2014;120(17):2735-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guan X, Wei R, Yang R, Lu Z, Liu E, Zhao Z, et al. Association of radiotherapy for rectal cancer and second gynecological malignant neoplasms. JAMA Netw Open. 2021;4(1):e2031661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilkinson N. Management of rectal cancer. Surg Clin North Am. 2020;100(3):615-628. [DOI] [PubMed] [Google Scholar]
- 20.Kirkali Z, Chan T, Manoharan M, Algaba F, Busch C, Cheng L, et al. Bladder cancer: epidemiology, staging and grading, and diagnosis. Urology. 2005;66(6 suppl 1):4-34. [DOI] [PubMed] [Google Scholar]
- 21.Eyler CE, Zietman AL. A (relatively) risky business: the link between prostatic radiotherapy and second malignancies. BMJ (Clinical research ed). 2016;352:i1073. [DOI] [PMC free article] [PubMed] [Google Scholar]