Abstract
Primary retroperitoneal mucinous cystic neoplasms are rare retroperitoneal tumors, which are histologically similar to mucinous cystic neoplasms of the ovaries. Only 31 cases of primary retroperitoneal mucinous cystic neoplasm with borderline malignancy (PRMCN-BM) have been reported (26 in women and five in men). We describe an additional male patient with PRMCN-BM. A 39-year-old man presented to our hospital with back pain. Twelve years earlier, he had undergone an orchiectomy for a germ cell tumor. Computed tomography showed a 6.9- × 4.4-cm cystic mass in the left pararenal space. Laparoscopic mass excision was performed, and a unilocular cystic mass was found in the pararenal space near the lower pole of the left kidney. A histopathological examination showed a cyst lined by atypical mucinous intestinal epithelium without stromal invasion. Targeted next-generation sequencing identified two hotspot mutations, with one each in the KRAS and GNAS genes. Outpatient follow-up 10 months after surgery showed no evidence of tumor recurrence. PRMCNs are extremely rare retroperitoneal neoplasms, especially in men. These neoplasms are rarely considered in the differential diagnosis of retroperitoneal masses, and their preoperative diagnosis is difficult. Evaluation of additional patients is required to better determine the prognosis of PRMCNs and the optimal postoperative follow-up.
Keywords: Primary retroperitoneal mucinous cystic neoplasm, borderline malignancy, retroperitoneum, case report, KRAS, GNAS
Introduction
Primary retroperitoneal mucinous cystic neoplasms (PRMCNs) are rare retroperitoneal tumors that predominantly occur in women. 1 Histologically, PRMCNs resemble ovarian mucinous tumors and are similarly classified into the following three categories: retroperitoneal mucinous cystadenoma, PRMCN with borderline malignancy (PRMCN-BM), and malignant retroperitoneal mucinous cystadenocarcinoma. 2 PRMCN-BM is exceedingly rare, with only 31 (26 in women and 5 in men) cases described in the literature to date. The preoperative diagnosis of PRMCN-BM is challenging, and its pathogenesis, clinical behavior, and management remain unclear because of the small number of registered cases and the absence of pathognomonic clinical and imaging features. 3 The present report describes the sixth case of PRMCN-BM in a male patient and reviews the published data.
Case report
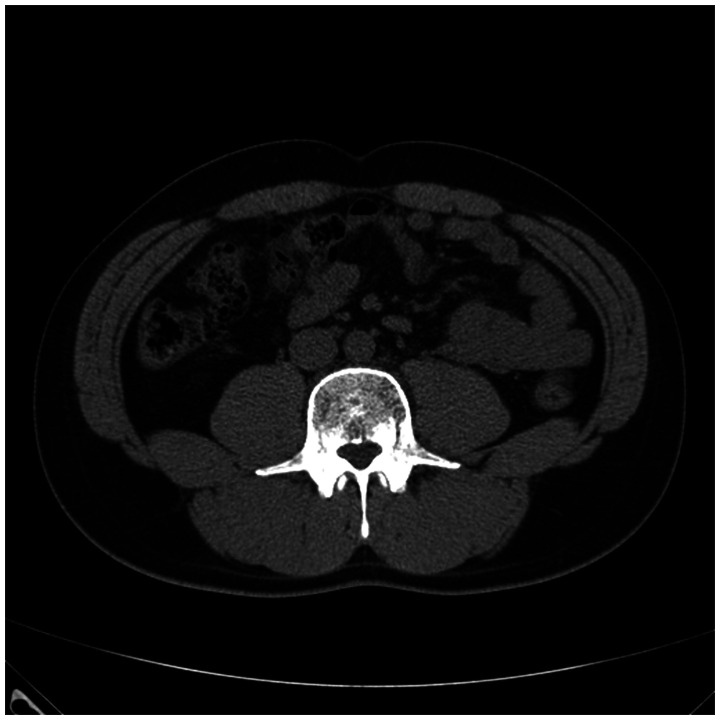
A 39-year-old man presented to our hospital with the complaint of back pain. Twelve years previously, he had undergone an orchiectomy for seminoma at another hospital, and this was followed by four cycles of cisplatin and etoposide chemotherapy. Seven months later, he was transferred to our hospital for follow-up, at which time an abdominal computed tomography (CT) scan was taken. There were no abnormalities, except for the previous orchiectomy site (Figure. 1). He was followed up in our hospital, and there was no recurrence for 12 years.
Figure 1.
Abdominal and pelvic computed tomography scan performed after orchiectomy shows no abnormalities in the retroperitoneum.
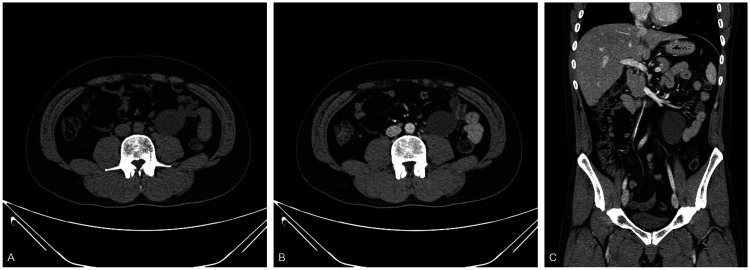
On the basis of the history of the testicular tumor, the patient underwent blood tests for tumor markers, such as alpha-fetoprotein and β-human chorionic gonadotropin, but the results were normal. An abdominal and pelvic CT scan showed a 6.9- × 4.4-cm cystic mass in the left pararenal space (Figure 2a–c). No mural nodules were found on the mass, and there were no signs of peritoneal implants, ascites, or lymphadenopathy.
Figure 2.
Unenhanced computed tomography scan of the abdomen and pelvis in the axial plane (a) contrast-enhanced images in the axial (b) and coronal planes and (c) show a 6.9- × 4.4-cm homogeneous cystic mass with smooth borders in the left pararenal space.
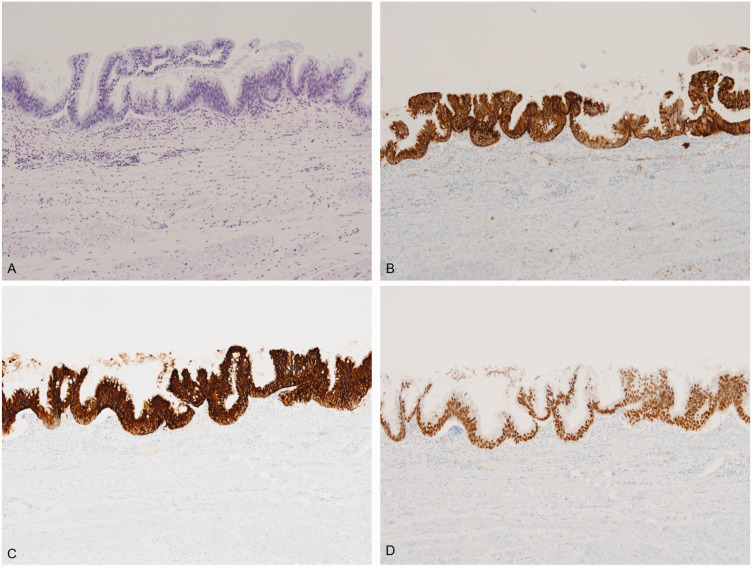
The neoplastic nature of the mass was uncertain. Therefore, the patient underwent laparoscopic excision of the mass. The mass was located in the pararenal space near the lower pole of the left kidney. After careful dissection of the pararenal fat and surrounding structures, the cyst was extracted gently without spillage of contents. No bag was used for retrieval, and the cyst was not aspirated before extraction. A gross examination of the surgically resected specimen showed a previously disrupted unilocular cyst (5.9 × 4.4 × 3.5 cm) containing mucinous material and evidence of hemorrhage. The inner surface of the cyst was smooth with no mural nodules or papillae. The entire cyst wall was tan-yellow to gray in color. A histopathological examination showed that the cyst wall was lined by atypical mucinous epithelium of the intestinal type, consisting of columnar and goblet cells, without stromal invasion. The cyst wall showed focal calcification, with pools of extravasated mucin. The epithelium displayed slightly atypical proliferation with glandular budding, tufting of the epithelium, and stratification two to three cells thick. The tumor cells displayed mild-to-moderate nuclear atypia without mitoses (Figure 3a). The epithelial lining showed an intestinal phenotype with positive staining for cytokeratin (CK)7, CK20, and CDX2 (Figure 3b–d). Immunohistochemical staining for c-kit and D2-40 was negative. Pathologically, this tumor was similar to mucinous borderline tumors observed in the ovaries. A subsequent thorough workup for any primary tumors failed to identify other lesions in this patient. Therefore, he was diagnosed with a PRMCN-BM.
Figure 3.
Histological staining of the tumor sample. (a) Hematoxylin and eosin staining shows the cyst wall with intestinal-type mucinous epithelium and focal nuclear stratification. Papillary tufts with mild cytological atypia can occasionally be seen (original magnification, ×100). (b–d) Staining with antibodies to (b) cytokeratin (CK)7, (c) CK20, and (d) CDX2 shows that the epithelial lining is strongly positive for all three (original magnification, ×100).
The tumor DNA was subjected to targeted next-generation sequencing. Libraries were prepared using Oncomine Comprehensive Assay Plus (Thermo Fisher Scientific, Waltham, MA, USA), and the products were sequenced on the Ion S5 System (Thermo Fisher Scientific). Sequencing data were analyzed using Ion Reporter 5.18. Next-generation sequencing identified two missense mutations, which comprised a c.183A>C (p.Gln61His) missense mutation in exon 3 of the KRAS gene and a c.602G>A (p.Arg201His) missense mutation in exon 8 of the GNAS gene.
We followed the patient with CT examinations at 3 and 10 months after surgery. During this period, the patient remained alive, with no evidence of tumor recurrence.
The reporting of this study conforms to the CARE guidelines. 4
Discussion
PRMCNs are extremely rare retroperitoneal neoplasms, and are histologically similar to mucinous cystic neoplasms of the ovaries. To date, only 31 confirmed cases of PRMCN-BM have been reported (Table 1), with 26 in women and five in men.3,5–24 The first male patient with PRMCN was described in 1994, with only five additional cases of PRMCN-BM in men, including the present patient, reported since this time.7,12,17,18,23
Table 1.
Clinical features of previously reported cases of PRMCN with borderline malignancy
| Case | Year | Reference | Age (years) | Sex | Location | Size (cm) | Clinical symptoms | Preoperative tumor-specific antigen | Imaging features | Gross features | Final diagnosis | Operative method | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1987 | Nagata 18 | 41 | F | ND | 12 × 10 × 9 | Abdominal pain with distension | ND | ND | ND | PRMCN-BM | TR | NED, ND |
| 2 | 1988 | Banerjee 5 | 38 | F | Left lower abdomen | 11 | Abdominal pain and distension | ND | Cyst | Cyst with papillary excrescences < 5 mm tall | PRMCN-BM | TR + resection of the descending colon + left salpingo-oopho-rectomy | Media-stinal lymph node meta-stases 4 years after diagnosis |
| 3 | 1988 | Banerjee 5 | 47 | F | Left upper quadrant mass | 13 | Flu-like symptoms | ND | ND | Cyst with nodular excrescences measuring up to 3.5 × 2.0 cm | PRMCN-BM | TR + splenectomy+ left adrenal-ectomy | ND |
| 4 | 1994 | Motoyama 17 | 42 | F | Right kidney | 6 | Symptoms caused by systemic lupus erythematosus | CEA, 98,000 ng/mL | Cyst | Cyst with papillary lesion | PRMCN with foci of mucinous cystadeno-carcinoma | TR | ND |
| 5 | 1994 | Motoyama 17 | 63 | M | Right kidney | 11 | Abdominal pain | CEA, 15,200 ng/mL | Cyst | Cyst | PRMCN-BM | TR | ND |
| 6 | 1996 | Pearl 20 | 33 | F | Retro-peritoneum of the left flank | ND | Increasing abdominal girth, weight gain, and severe back and abdominal pain | ND | Unilocular cyst with homogenous contents | Cyst with several map-like patches | PRMCN-BM | TR | NED, 10 months |
| 7 | 1997 | Papadogiannakis 19 | 33 | F | Lateral to the descend-ing colon | 13 × 9 | Tiredness, diarrhea and, a feeling of air in the stomach | ND | Cyst with low attenuation | Unilocular cyst with small papillary excrescences measuring 2.0 × 2.0 cm | PRMCN-BM | TR | NED, 12 months |
| 8 | 1998 | Chen 9 | 48 | F | Mesentery of the ascending colon | 15 × 13 × 9 | Postprandial fullness | ND | Homogeneous cyst | Cyst | PRMCN-BM | TR | NED, 8 months |
| 9 | 2003 | Gutsu 12 | 41 | F | Right upper abdominal quadrant | 21 × 16 | Flank pain and abdominal distension | ND | Mosaic structure | Unilocular cyst with some polypoid masses containing multiple mucin-filled cysts | PRMCN-BM | TR | NED, 18 months |
| 10 | 2003 | Song 21 | 31 | F | Right retro-peritoneal space | 21 × 16 × 10 | Abdominal distension | CA 72-4, normal (1.9 /mL) | Multi-septate cyst with calcifications | Multilocular cyst with calcified septa | PRMCN-BM | TR + appendectomy | ND |
| 11 | 2005 | Matsubara 15 | 36 | F | Right lower abdomen | 12 × 8 | Abdominal distension | CA 125 (51.4 U/mL) and CA 19-9 (55.2 U/mL) were elevated (CEA, normal | Cyst with solid portion | Multilocular cyst with solid mass of 5 cm in diameter | PRMCN-BM | TR + appendectomy+ myomectomy | NED, 6 months |
| 12 | 2007 | Bakker 4 | 45 | F | Left lower abdomen | 15 | Abdominal pain | CEA, CA 125, and CA 19-9 were in the normal range | Cyst with nodule | Unilocular cyst with a solid mural nodule of 3.5 × 3.5 × 2.5 cm | PRMCN-BM with a sarcoma-like mural nodule | TR | NED, 12 months |
| 13 | 2007 | Cottrill 10 | 22 | F | ND | 20 × 17.5× 15 | Abdominal pain and distension | ND | Cyst | ND | PRMCN-BM | TR | NED, ND |
| 14 | 2008 | Bifulco 7 | 35 | F | Rear of the cecum and near the last intestinal loop | 28 | Pelvic pain | CA 125, CA 19-9, CA 15-3, tissue polypeptide-specific antigen, and CEA were in the normal range | Cyst with a single septum | Unilocular cyst with a solid area with papillary projections | PRMCN-BM | TR + appendectomy+ partial omentectomy+ multiple biopsies of the peritoneum and ovaries | NED, 24 months |
| 15 | 2009 | Roma 3 | 25 | F | ND | 15 | Kidney mass | ND | ND | Cyst with single papilla | PRMCN-BM | TR | NED, 148 months |
| 16 | 2009 | Roma 3 | 43 | F | ND | 14 | Pelvic pain | ND | ND | Cyst with papilla | PRMCN-BM | TR + SO | NED, 1 month |
| 17 | 2009 | Roma 3 | 35 | F | ND | 3 | Pelvic pain | ND | ND | Multiloculated cyst | PRMCN-BM with microinvasion | TR | NED, 13 months |
| 18 | 2009 | Roma 3 | 48 | F | ND | 7 | Enlarged mass | ND | ND | Thin-walled cyst | PRMCN-BM | TR | NED, 34 months |
| 19 | 2009 | Roma 3 | 47 | F | ND | 21 | Enlarged mass | ND | ND | Cyst with nodules | PRMCN-BM with Ica | TR + SO | NED, 1 month |
| 20 | 2009 | Roma 3 | 24 | F | ND | 18 | Enlarged mass | ND | ND | Thin-walled cyst | PRMCN-BM with ICa | TR | NED, 2 months |
| 21 | 2009 | Roma 3 | 27 | F | ND | 8 | Enlarged mass | ND | ND | Cyst with nodules and papilla | PRMCN-BM with ICa | TR + SO | NED, 11 months |
| 22 | 2009 | Benkirane 6 | 44 | M | Right side | Four portions of 5 × 4 × 3, 4 × 4 × 3, 3 × 3 × 2, and 2 × 2 × 2 | Torsion-like pain and weight loss | ND | Several retroperitoneal masses with heterogenic foci | Multilocular cyst with solid zones | PRMCN-BM | TR | NED, 12 months |
| 23 | 2011 | Falidas 11 | 37 | M | Right abdomen | 22 × 10 | Pain and mass | ND | Cyst with diaphragms | Cysts within the mass, measuring from 1 to 4 cm in diameter | PRMCN-BM | TR | NED, 12 months |
| 24 | 2013 | Mattei 16 | 32 | M | Proximity to the vena cava | 6 × 7.1 × 4.4 | Incidentally found during work-up for a right testicular mass | ND | Cyst with heterogeneous features | ND | PRMCN-BM | TR | ND |
| 25 | 2014 | Haeri 13 | 26 | F | Left lower quadrant | 13 × 10 × 8 | Abdominal distention pain | CEA, AFP, CA 19-9, and CA 125 were in the normal range (3.5 U/mL, 5.3 U/mL, 35.17 U/mL, and 20 U/mL) | Cyst | Cyst with heterogeneous solid and cystic areas | PRMCN-BM | TR | ND |
| 26 | 2015 | Manrai 14 | 65 | F | Left abdominal cavity | 20 | Abdominal distention | CA 125, normal | Multilocular cyst | Multilocular cyst with small papillary areas | PRMCN-BM | TR | NED, 12 months |
| 27 | 2015 | Vargas 22 | 68 | M | Right abdomen | 16 × 4.5 × 11 | Palpable mass | CA 19 was slightly elevated; AFP and CEA were in the normal range | Cyst | Multilocular cyst with calcification | PRMCN-BM | TR | ND |
| 28 | 2019 | Chaves 8 | 62 | F | Right flank | 12 × 8.5 | Abdominal pain | CEA and CA19-9 were elevated (6.7 ng/mL and 122 U/ mL) | Well-defined hypodense mass with heterogeneous structure | Unilocular cyst | PRMCN-BM | TR + laparoscopic ileocecal resection | NED, 18 months |
| 29 | 2020 | Zhang 23 | 65 | F | ND | ND | Mass | ND | ND | Partially septated cyst | PRMCN-BM | TR | NED, 6 months |
| 30 | 2020 | Zhang 23 | 23 | F | ND | ND | Mass | ND | ND | Unilocular cyst | Mucinous and serous PRMCN-BM | TR | ND |
| 31 | 2020 | Zhang 23 | 44 | F | Left lower quadrant | 20 | Abdominal distention and a mass | ND | Cyst with mural nodule | Cyst with mural nodules and solid components | PRMCN-BM with Ica | TR | NED, 2 months |
| 32 | 2022 | Present case | 39 | M | Left pararenal space | 6.9 × 4.4 | Back pain | AFP and β-HCG were in the normal range | Unilocular homogeneous cyst | Unilocular cyst | PRMCN-BM | TR | NED, 10 months |
F, female; ND, not described; PRMCN-BM, primary retroperitoneal mucinous cystic neoplasm with borderline malignancy; TR, tumor resection; NED, no evidence of disease; CEA, carcinoembryonic antigen; PRMCN, primary retroperitoneal mucinous cystic neoplasm; M, male; CA 72-4, carbohydrate antigen 72-4; CA 125, cancer antigen 125; CA 19-9, carbohydrate antigen 19-9; CA 15-3, cancer antigen 15-3; Ica, intraepithelial carcinoma; SO, bilateral salpingo-oophorectomy; AFP, alpha fetoprotein; HCG, human chorionic gonadotropin.
Patients with PRMCN-BM range in age from 22 to 68 years, with a median age of 40 years. The clinical presentation of PRMCN-BM is usually non-specific, with no known specific laboratory tests, tumor markers, or radiological findings. Therefore, the preoperative diagnosis of PRMCN-BM is challenging. 25 Serum tumor markers, such as carcinoembryonic antigen, cancer antigen 125, and carbohydrate antigen 19-9, are elevated in a small proportion of these patients, but these elevations do not have diagnostic or prognostic significance. No pathognomonic imaging features of PRMCN-BM have been described to date. These tumors are mainly cystic, with the greatest dimension of the cysts varying from 3 to 28 cm. The cysts may appear unilocular or multilocular, and imaging showed that cysts in only three previously reported cases11,12,31 had a solid component or mural nodule. These tumors are localized almost exclusively to the lateral retroperitoneal spaces, with 9 located in the left side and 11 in the right side. The treatment of choice is complete tumor resection, without cyst rupture, thereby preventing tumor cell dissemination. 25
The prognosis of patients with these tumors remains uncertain because of their rarity and because most patients were not followed up for ≥ 24 months. Outcomes have been reported in 75% of patients with PRMCN-BM who were followed up for a median of 19 months (range, 1–148 months). Based on published case reports, the overall prognosis of patients with PRMCN-BM and documented disease-free survival was excellent, with little evidence of recurrence, metastasis, or cancer-associated deaths, despite one patient with mediastinal lymph node metastases. 6 Therefore, tumor resection is curative, with no need for adjuvant chemotherapy. No consensus has been reached on the postoperative follow-up strategy for patients with PRMCN-BM. However, long-term follow-up data are required to better determine the prognosis of patients with PRMCN.
The pathogenesis of PRMCNs has not been determined because epithelial cells are usually absent from the retroperitoneum.2,10,26 Several hypotheses regarding the origin of these tumors have been proposed. According to one hypothesis, the histological similarity of PRMCNs to ovarian tumors suggests that they arise from ectopic ovarian tissues. However, this hypothesis cannot explain the occurrence of PRMCNs in men or the absence of ovarian tissue from tumors in women. Alternatively, PRMCNs may originate from retroperitoneal monodermal teratomas in which columnar epithelium dominates. In addition, PRMCNs may be remnants of the embryonal urogenital apparatus. The most widely accepted hypothesis is that PRCMNs are derived from invaginated multipotent mesothelial cells that become entrapped in the retroperitoneum during embryonic development, with subsequent mucinous metaplasia and cyst formation. However, in our case, the tumor was not congenital because it was not present on the CT scan performed after surgery (Figure 1).
Targeted next-generation sequencing of tumor DNA in our patient resulted in the identification of two hotspot mutations, with one in KRAS (c.183A>C p. Gln61His) and the other in GNAS (c.602G>A p.Arg201His). No studies to date have determined the molecular profiles of PRMCNs. Therefore, the results in this patient were compared with results in ovarian neoplasms. KRAS mutations have been reported in 30% to 75% of mucinous borderline tumors of the ovaries. 27 In contrast, activating mutations of GNAS occur in only a small percentage (2/29, 6.9%) of mucinous borderline tumors of the ovaries, although they are more frequent in other pre-malignant or non-aggressive mucinous-type tumors of gastrointestinal origin. 27 The co-occurrence of GNAS and KRAS mutations has been reported in only four mucinous ovarian tumors. 27 Additional molecular studies are required to better understand the molecular characteristics of this neoplasm.
Before diagnosing a patient with PRMCN, alternative diagnoses should be excluded by a careful clinical examination, diagnostic imaging, and thorough macroscopic and microscopic evaluations. The differential diagnosis should include metastatic mucinous tumors from the gastrointestinal tract (including the appendix) and the pancreas, which are more common than PRMCN. The possibility of a monodermal variant of a cystic teratoma arising from an undescended testis should also be considered because the retroperitoneum has no epithelial cells.
We describe the rare occurrence of a PRMCN-BM in a male patient. These tumors are rarely included in the differential diagnosis of retroperitoneal masses, and PRMCN-BM is difficult to diagnose preoperatively. The diagnosis of PRMCN-BM involves the exclusion of other considerations, based on careful clinical, imaging, and microscopic evaluations. Surgical excision is the standard approach for the diagnosis and treatment of PRMCN-BM. Evaluation of additional patients is required to better determine the prognosis and optimal postoperative follow-up of this condition.
Author contributions: Seung-Myoung Son made a substantial contribution to writing the manuscript. Chang Gok Woo and Ok-Jun Lee interpreted the pathological data. Seok Jung Yun analyzed the patient’s data. All authors have read and approved the final manuscript.
The authors declare that there is no conflict of interest.
Funding: This research was supported by the Regional Innovation Strategy (RIS) through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (MOE) (2021RIS-001).
ORCID iDs: Seung-Myoung Son https://orcid.org/0000-0002-1646-4649
Chang Gok Woo https://orcid.org/0000-0002-9131-3779
Ethics statement
This study adhered to the guidelines established by the Declaration of Helsinki and was approved by the Institutional Review Board of Chungbuk National University Hospital (Cheongju, Korea, IRB No: 2022-11-013). The patient consented to publication of the case report.
References
- 1.Wolf B, Kunert C, Horn LC, et al. Management of Primary Retroperitoneal Mucinous Tumors: A Retrospective Meta-Analysis. Int J Gynecol Cancer 2017; 27: 1064–1071. DOI: 10.1097/IGC.0000000000001013. [DOI] [PubMed] [Google Scholar]
- 2.Subramony C, Habibpour S, Hashimoto LA.Retroperitoneal mucinous cystadenoma. Arch Pathol Lab Med 2001; 125: 691–694. DOI: 10.5858/2001-125-0691-RMC. [DOI] [PubMed] [Google Scholar]
- 3.Roma AA, Malpica A.Primary retroperitoneal mucinous tumors: a clinicopathologic study of 18 cases. Am J Surg Pathol 2009; 33: 526–533. DOI: 10.1097/PAS.0b013e3181909018. [DOI] [PubMed] [Google Scholar]
- 4.Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus-based clinical case reporting guideline development. Headache 2013; 53: 1541–1547. DOI: 10.1111/head.12246. [DOI] [PubMed] [Google Scholar]
- 5.Bakker RF, Stoot JH, Blok P, et al. Primary retroperitoneal mucinous cystadenoma with sarcoma-like mural nodule: a case report and review of the literature. Virchows Arch 2007; 451: 853–857. DOI: 10.1007/s00428-007-0479-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banerjee R, Gough J.Cystic mucinous tumours of the mesentery and retroperitoneum: report of three cases. Histopathology 1988; 12: 527–532. DOI: 10.1111/j.1365-2559.1988.tb01972.x. [DOI] [PubMed] [Google Scholar]
- 7.Benkirane A, Mikou A, Jahid A, et al. Primary retroperitoneal mucinous cystadenoma with borderline malignancy in a male patient: a case report. Cases J 2009; 2: 9098. DOI: 10.1186/1757-1626-2-9098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bifulco G, Mandato VD, Giampaolino P, et al. Huge primary retroperitoneal mucinous cystadenoma of borderline malignancy mimicking an ovarian mass: case report and review. Anticancer Res 2008; 28: 2309–2315. [PubMed] [Google Scholar]
- 9.Chaves MM, Castro R, Mota-Vieira L, et al. A rare case of a primary retroperitoneal mucinous cystic tumour with borderline malignancy and literature review. BMJ Case Rep 2019; 12: e230708. DOI: 10.1136/bcr-2019-230708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen JS, Lee WJ, Chang YJ, et al. Laparoscopic resection of a primary retroperitoneal mucinous cystadenoma: report of a case. Surg Today 1998; 28: 343–345. DOI: 10.1007/s005950050137. [DOI] [PubMed] [Google Scholar]
- 11.Cottrill HM, Roberts WS.Primary retroperitoneal mucinous borderline tumor: a case report. Gynecol Oncol 2007; 106: 626–627. DOI: 10.1016/j.ygyno.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 12.Falidas E, Konstandoudakis S, Vlachos K, et al. Primary retroperitoneal mucinous cystadenoma of borderline malignancy in a male patient. Case report and review of the literature. World J Surg Oncol 2011; 9: 98. DOI: 10.1186/1477-7819-9-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gutsu E, Mishin I, Gagauz I.Primary retroperitoneal mucinous cystadenoma. A case report and brief review of the literature. Zentralbl Chir 2003; 128: 691–693. DOI: 10.1055/s-2003-41380. [DOI] [PubMed] [Google Scholar]
- 14.Haeri H, Vosooghi B, Asadi Amoli F. Primary retroperitoneal mucinous tumor of low malignant potential in a Persian woman. Acta Med Iran 2014; 52: 717–720. [PubMed] [Google Scholar]
- 15.Manrai M, Takesita N, Ishida H, et al. Primary retroperitoneal mucinous cystic tumors with borderline malignancy: a case report and literature review. Clin Pract 2015; 5: 722. DOI: 10.4081/cp.2015.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsubara M, Shiozawa T, Tachibana R, et al. Primary retroperitoneal mucinous cystadenoma of borderline malignancy: a case report and review of the literature. Int J Gynecol Pathol 2005; 24: 218–223. DOI: 10.1097/01.pgp.0000161313.30054.1d. [DOI] [PubMed] [Google Scholar]
- 17.Mattei J, Kim FJ, Phillips J, et al. Male primary retroperitoneal mucinous cystadenoma. Urology 2013; 82: e1–e2. DOI: 10.1016/j.urology.2013.03.031. [DOI] [PubMed] [Google Scholar]
- 18.Motoyama T, Chida T, Fujiwara T, et al. Mucinous cystic tumor of the retroperitoneum. A report of two cases. Acta Cytol 1994; 38: 261–266. [PubMed] [Google Scholar]
- 19.Nagata J, Yamauchi M, Terabe K, et al. [A case of retroperitoneal mucinous cystadenoma of borderline malignancy]. Nihon Geka Gakkai zasshi 1987; 88: 489–492. [PubMed] [Google Scholar]
- 20.Papadogiannakis N, Gad A, Ehliar B.Primary retroperitoneal mucinous tumor of low malignant potential: histogenetic aspects and review of the literature. APMIS 1997; 105: 483–486. DOI: 10.1111/j.1699-0463.1997.tb00597.x. [DOI] [PubMed] [Google Scholar]
- 21.Pearl ML, Valea F, Chumas J, et al. Primary retroperitoneal mucinous cystadenocarcinoma of low malignant potential: a case report and literature review. Gynecol Oncol 1996; 61: 150–152. DOI: 10.1006/gyno.1996.0115. [DOI] [PubMed] [Google Scholar]
- 22.Song DE, Kim M, Khang SK, et al. Primary Mucinous Cystic Neoplasm of the Retroperitoneum. The Korean Journal of Pathology 2003; 37: 204–209. [Google Scholar]
- 23.Vargas AC, Lam V, P'Ng CH.A rare case of primary retroperitoneal mucinous neoplasm in a male patient. Pathology 2015; 47: 384–386. DOI: 10.1097/PAT.0000000000000261. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Yang J, Chen Z, et al. Laparoscopic Resection and Pre-Operative Imaging of Primary Retroperitoneal Mucinous Neoplasms: A Retrospective Case Series. Cancer Manag Res 2020; 12: 5451–5460. DOI: 10.2147/CMAR.S254197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dayan D, Abu-Abeid S, Klausner JM, et al. Primary Retroperitoneal Mucinous Cystic Neoplasm: Authors' Experience and Review of the Literature. Am J Clin Oncol 2016; 39: 433–440. DOI: 10.1097/COC.0000000000000298. [DOI] [PubMed] [Google Scholar]
- 26.Tenti P, Romagnoli S, Pellegata NS, et al. Primary retroperitoneal mucinous cystoadenocarcinomas: an immunohistochemical and molecular study. Virchows Arch 1994; 424: 53–57. DOI: 10.1007/BF00197393. [DOI] [PubMed] [Google Scholar]
- 27.Ryland GL, Hunter SM, Doyle MA, et al. Mutational landscape of mucinous ovarian carcinoma and its neoplastic precursors. Genome Med 2015; 7: 87. DOI: 10.1186/s13073-015-0210-y. [DOI] [PMC free article] [PubMed] [Google Scholar]