Abstract
The use of PARP inhibitors (PARPi) has transformed the care of advanced high-grade serous/endometrioid ovarian cancer. PARPi are now available to patients in both the first-line and recurrent platinum-sensitive disease settings; therefore, most patients will receive PARPi at some point in their treatment pathway. The majority of this expanding population of patients eventually acquire resistance to PARPi, in addition to those with primary PARPi resistance. We discuss the rationale behind developing combination therapies, to work synergistically with PARPi and overcome mechanisms of resistance to restore drug sensitivity, and clinical evidence of their efficacy to date.
Keywords: angiogenesis, combination therapy, epithelial ovarian cancer, homologous recombination deficiency, PARP inhibitor
Introduction
Ovarian cancer is the eighth most common cause of cancer-related death amongst women, responsible for over 200,000 deaths worldwide each year. 1 Survival for advanced ovarian cancer (Stage III and IV) is poor, with a 5-year overall survival of 26.9% and 13.4%, respectively (UK data from 2019). 1 Unfortunately, there are still no effective tools for general population screening and more than two-thirds of patients present with late-stage disease.2–4 Therefore, the management of advanced ovarian cancer poses a major clinical challenge.
For many decades, the standard of care for first-line treatment of advanced ovarian cancer consisted of cytoreductive surgery with platinum- and taxane-based chemotherapy. 5 The introduction of poly(ADP-ribose) polymerase inhibitors (PARPi) has transformed the management of patients with advanced high-grade serous and high-grade endometrioid ovarian cancer, primary peritoneal and fallopian tube cancers. (These tumour types will subsequently be collectively referred to as HGSOC.)
PARPi work via the principle of synthetic lethality, exhibiting selective toxicity in homologous recombination repair deficient (HRD) cancer cells (see Figure 1). 6 Inhibition of PARP activity leads to an accumulation of unrepaired single strand breaks that result in replication fork collapse during DNA replication, thus leading to double strand breaks (DSBs). In cells which are HRD, these DSBs cannot be repaired, resulting in cell death via apoptosis due to mitotic catastrophe. 7 Approximately 50% of HGSOC tumours have defective homologous recombination, making it an important therapeutic target. 8 This HRD results in chromosomal instability, leading to high levels of aneuploidy and copy number variation characteristic of HGSOC.9,10 Most commonly, this HRD phenotype is secondary to a germline or somatic mutation in either BRCA1 or BRCA2 (20% HGSOC patients), but can also be due to non-mutational changes (such as BRCA1 promoter methylation) and mutations in other homologous recombination (HR) repair-associated genes (e.g. BRIP1 and RAD51C/D, which collectively account for 2% HGSOC cases).11–13
Figure 1.
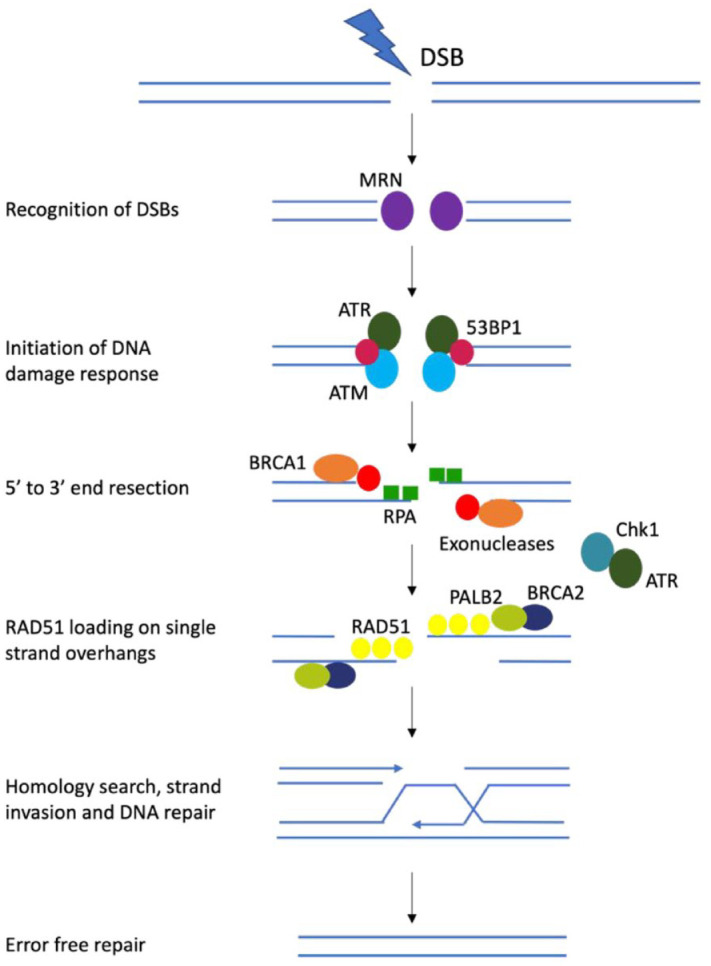
Simplified schematic of homologous recombination (HR) repair pathway.
MRE-11-RAD50-NBS1 (MRN) complex recognizes double strand breaks (DSBs). ATM, ataxia telangiectasia and Rad3-related protein (ATR) and 53BP1 also sense DSBs. The MRN complex activates ATM kinase, which in turns initiates the DNA damage response pathway. BRCA1 is attracted to the DNA ends, displaces 53BP1 and stimulates 5′ to 3′ end resection via exonucleases, creating single-strand DNA (ssDNA) overhangs. The exposed ssDNA is coated with DNA replication protein A (RPA), which activates the ATR response to initiate HR repair and the ATR-Chk1 DNA damage checkpoint, which arrests the cell cycle and protects stalled replication forks. Then RAD51, facilitated by BRCA2 and PALB2, replaces RPA and performs homology sequence searching and strand invasion. DSBs are restored by DNA synthesis, ligation and resolution of Holliday junctions.
PARPi were first shown to display selective killing in BRCA-deficient cell lines and mouse models, hence they were first trialled in patients with germline BRCA1/2 mutations.14–16 In platinum-sensitive recurrent HGSOC, the response rate to PARPi monotherapy was approximately 30–45% in BRCA1/2 mutation carriers. 17
The focus of clinical trials then shifted from PARPi monotherapy to maintenance treatment following response to platinum chemotherapy, to extend the time to progression. Study 19 was a key initial study, carried out in HGSOC patients with platinum-sensitive relapse. As expected, the improvement in progression-free survival (PFS) with olaparib versus placebo was more pronounced in patients with germline/somatic BRCA1/2 mutations [11.2 months versus 4.3 months, hazard ratio (HR): 0.18] compared to all comers (8.4 months versus 4.8 months, HR: 0.35) and BRCA1/2 wild-type patients (7.4 months versus 5.5 months, HR: 0.54). 18
Results from the NOVA study (niraparib, platinum-sensitive recurrent disease) showed that patients with no detectable mutations in the HR pathway still derived significant clinical benefit from PARPi. 19 The largest PFS benefit was seen in the cohort with germline BRCA1/2 (gBRCA1/2) mutations [21.0 months versus 5.5 months in the placebo group, HR: 0.27; 95% confidence interval (CI): 0.17–0.41], but unexpectedly there was also an increase in PFS seen in the HRD test negative subgroup (6.9 months versus 3.8 months, hazard ratio, 0.58; 95% CI 0.36–0.92). On the basis of this study, all patients with platinum-sensitive recurrent HGSOC are eligible to receive maintenance treatment with niraparib following a response to platinum chemotherapy, regardless of their HRD status. Similar results were observed with rucaparib as maintenance in platinum-sensitive relapsed-disease setting in the ARIEL3 trial. 20
Clinical trials then shifted into the first-line maintenance treatment setting. First-line management with cytoreductive surgery and platinum-containing chemotherapy had remained unchanged for several decades, but following the results of the SOLO-1, PRIMA and PAOLA-1 studies, all patients that have had a response to first-line chemotherapy have been shown to benefit from maintenance PARPi.21–23 For example, in the SOLO-1 study, patients with BRCA1/2 mutations receiving first-line maintenance olaparib demonstrated an unprecedented improvement in the median PFS to 56.0 months (95% CI: 41.9–not reached) versus 13.0 months in those receiving placebo (95% CI: 11.1–18.2, HR: 0.30). 24 Similar to the NOVA study, in the PRIMA study an incremental benefit of niraparib versus placebo first-line maintenance treatment was seen based on HRD status; PFS: 22.1 months versus 10.9 months in patients with BRCA1/2 mutations, 19.6 months versus 8.2 months in patients with BRCA1/2 wild-type HRD tumours, and 8.1 months versus 5.4 months in patients with HRD test negative tumours. 22 The PAOLA-1 study showed a benefit with the addition of olaparib to bevacizumab maintenance in patients with HRD tumours. 23 This study is discussed in more detail below. Based on these studies, optimal first-line maintenance strategies are determined by BRCA1/2 mutation and HRD status. Patients with a BRCA1/2 mutation (whether germline or somatic) can receive olaparib, whereas any patient may receive niraparib, regardless of their BRCA1/2 mutation status. Patients with an HRD tumour, defined as having a positive genomic instability score (GIS) and/or a BRCA1/2 mutation, may receive olaparib plus bevacizumab. 12
Multiple trials (see Table 1) have shown increased PFS with the use of PARPi in both the first-line maintenance and recurrent platinum-sensitive ovarian cancer settings. This review will discuss the rationale behind developing combination therapies, to work synergistically with PARPi and overcome mechanisms of resistance to restore drug sensitivity, and the clinical evidence of their efficacy to date.
Table 1.
Important clinical studies leading to the use of PARPi as monotherapy in the clinical setting.
| Study name (NCT number) | Treatment setting | Patient population | Drug name | PFS PARPi versus placebo (months) | HR (95% CI) |
|---|---|---|---|---|---|
| SOLO-1 (NCT01844986) 1 | First-line maintenance | BRCA1/2 mut | Olaparib | BRCA1/2 mut: 56.0 versus 13.8 | 0.33 (0.25–0.43) |
| PRIMA (NCT02655016) 2 | First-line maintenance | All comers | Niraparib | Overall: 13.8 versus 8.2 HRD: 21.9 versus 10.4 HRD test neg: 8.1 versus 5.4 |
0.62 (0.50–0.76) 0.43 (0.31–0.59) 0.68 (0.49–0.94) |
| VELIA (NCT0247058) 3 | In combo with first-line chemo then maintenance | All comers | Veliparib | Overall: 23.5 versus
17.3 BRCA1/2 mut: 34.7 versus 22 HRD: 31.9 versus 20.5 |
0.68 (0.56–0.83) 0.44 (0.28–0.68) 0.57 (0.43–0.76) |
| Study 19 (NCT00753545) 4 | PSR maintenance | All comers | Olaparib | Overall: 10.8 versus
5.4 BRCA1/2 mut: 11.2 versus 4.3 BRCA1/2 wt: 7.4 versus 5.5 |
0.35 (0.25–0.49) 0.18 (0.34–0.85) 0.54 (0.34–0.85) |
| SOLO-2 (NCT01874353) 5 | PSR maintenance | BRCA1/2 mut | Olaparib | BRCA1/2 mut: 19.1 versus 5.5 | 0.33 (0.24–0.44) |
| NOVA (NCT01847274) 6 | PSR maintenance | All comers | Niraparib | gBRCA1/2 mut: 21.0 versus
5.5 HRD & gBRCA1/2 wt: 12.9 versus 3.8 HRD test neg: 6.9 versus 3.8 |
0.27 (0.17–0.41) 0.38 (0.24–0.59) 0.58 (0.36–0.92) |
| ARIEL3 (NCT01968213) 7 | PSR maintenance | All comers | Rucaparib | Overall: 10.8 versus
5.4 BRCA1/2 mut: 16.6 versus 5.4 HRD: 13.6 versus 5.4 |
0.36 (0.30–0.45) 0.23 (0.16–0.34) 0.32 (0.24–0.42) |
BRCA1/2 mut, BRCA1/2 mutation carriers; CI, confidence interval; g, germline; HR, hazard ratio; HRD, homologous recombination deficient; PARPi, PARP inhibitors; PFS, progression-free survival; PSR, platinum-sensitive relapse; wt, wild-type.
Rationale for combination therapy
In most fields of oncology, combination therapies are trialled to prolong PFS and overall survival (OS). The main rationale behind these combination strategies are (i) the additive effect of drugs with different mechanisms of action, (ii) the synergistic effect of drugs that potentiate each other’s effects and (iii) the reversal of resistance mechanisms.
Increased DNA damage
The mainstay of treatment in HGSOC remains platinum-based chemotherapy. 4 Platinum-induced DNA adducts lead to DSBs. Therefore, in HRD tumours that rely on PARP to repair such breaks, PARPi should potentiate the cytotoxic effect of platinum chemotherapy. Unfortunately, despite the appealing rationale behind this combination, the use of PARPi and chemotherapy has not been taken beyond Phase II clinical studies, with increased toxicity (particularly myelosuppression) being a limiting factor (discussed below).25–27
By the same basis, combining PARPi with other drugs that cause DNA damage (e.g. MEK inhibitors) or prevent DNA damage repair (DDR, e.g. Chk1/ATR inhibitors) should lead to the accumulation of DNA damage and subsequent cell death.28,29 Hypoxia induced by inhibiting hypoxia inducible factor (HIF-1) and vascular endothelial growth factor (VEGF) also leads to increased DNA damage and, thereby, increases susceptibility to the effects of PARP inhibition. 30 In hypoxic conditions, hydroxylation of the HIF-1α subunit is restricted, enabling it to combine with the HIF-1β subunit and transcribe hypoxia-survival genes such as VEGF and erythropoietin (EPO). 31
Modulation of homologous recombination
HR is modulated by other cellular pathways, including the MET/PI3K/AKT pathway and VEGF pathways. 32 For example, VEGF receptor-3 (VEGFR-3) inhibition leads to downregulation of BRCA1 and BRCA2 gene expression, and restores chemosensitivity in chemo-resistant cancer cell lines. 33 Hypoxia induced by angiogenesis inhibitors also downregulates RAD51 expression, another key factor in homologous recombination repair (HRR). 34 Angiogenesis inhibitors could therefore induce an HRD state, and sensitize tumours that are usually HR-proficient or have undergone a mutation which has restored HR proficiency to the effects of PARPi.
The RAS/RAF/MEK/ERK and MET/PI3K/AKT pathways are frequently dysregulated in malignancies. Pre-clinical data suggest that PI3K, mTOR and MEK inhibitors can all also downregulate HRR gene expression and induce HRD.35–37
Increased neoantigen production and PD-L1 expression
Conversely, the combination of PARPi with checkpoint inhibitors should potentiate the effect of immunotherapy. Defects in DDR induce increased genomic instability, leading to an increased burden of mutations. These are subsequently presented by major histocompatibility complex proteins as neoantigens and trigger T cell activation. It is known that ovarian carcinomas with HR deficiency exhibit a higher mutational load, and therefore produce more neoantigens in comparison to those that are HR proficient. 38 In this same study by Strickland et al. it was shown that BRCA1/2-mutated tumours had increased numbers of CD3+ and CD8+ tumour infiltrating lymphocytes, and amplified expression of programmed death-1 (PD-1) and programmed death-ligand 1 (PD-L1) in tumour-associated immune cells compared to HR-proficient tumours. Similarly, PARPi have been shown to upregulate PDL-1 expression, via inactivation of GSK3β, a known mediator in cellular processes including inflammation. 39 In addition, BRCA deficiency triggers a STING-dependent innate immune response, by inducing type I interferon and pro-inflammatory cytokine production, which is increased by treatment with PARPi.40,41 Therefore, HRD tumours may be more sensitive to immune checkpoint blockade, and PARPi may act synergistically with immune checkpoint inhibitors.
Furthermore, in pre-clinical studies PARPi have been shown to upregulate PD-L1 expression in cell lines and ex-vivo tumour cells, thus making those tumours more sensitive to PD-L1 blockade.39,42 The combination of PARPi and anti-PD-L1 therapy significantly increased anti-tumour activity in murine models compared with each agent alone. 39
Reversal of PARP inhibitor resistance
Despite being a success story, particularly in certain patient groups, innate and acquired resistance to PARPi is observed. Understanding the mechanisms of resistance, facilitated by dissecting molecular signalling pathways and analysing adaptive responses to therapy in pre-clinical studies, has enabled the development of strategies for combination therapies.
An incremental benefit with PARPi therapy is seen: patients with germline or somatic mutations in BRCA1 or BRCA2 gain most benefit, followed by patients with non-BRCA-related HR deficiency, and then patients with HR-proficient tumours. 19 Those patients with HR-proficient tumours that see minimal benefit with PARPi monotherapy have an innate resistance to PARPi, and agents that induce an HRD phenotype may induce sensitivity to PARPi. Furthermore, not all patients with HRD tumours respond to PARPi, and these strategies may also be relevant for them.
PARPi have two main effects: (i) catalytic inhibition of PARP1 [preventing the formation of poly(ADP)-ribose] chains (PARylation), which recruit further DNA repair proteins and (ii) PARP1 trapping (preventing its release from damaged DNA, thus stalling the progression of replication forks). 43 Multiple mechanisms of acquired resistance have been described in pre-clinical and clinical studies. 44 These can be categorized into two main groups: those that restore HRR, and those that work by other mechanisms, including replication fork stabilization, decreased PARP trapping and increased drug efflux. 43 Platinum-resistant tumours are seen to have increased capability of DNA repair capacity. 45 Therefore, in tumours previously exposed to platinum agents, one can expect some cross-resistance with PARPi.
Reversion mutations which restore the function of HRR genes such as BRCA1/2 and RAD51C/D are linked with resistance to PARP inhibition.46,47 For example, the PEO1 and PEO4 ovarian cancer cell lines were derived from a BRCA2 mutation carrier when the patient was platinum-sensitive and platinum-resistant, respectively. PEO4 cells have a secondary mutation in the BRCA2 gene, restoring BRCA proficiency. 48 Similarly, reversion of epigenetic silencing via loss of BRCA1 and RAD51C promoter methylation and mRNA re-expression is associated with the development of resistance to PARPi. 49
When a DSB initially occurs, 53BP1 localizes to the area of DNA damage and blocks the initiation of DNA end resection. In normal cells, BRCA1 subsequently displaces 53BP1 and activates DNA end resection. When 53BP1 is depleted, however, DNA end resection can occur in the absence of functional BRCA1, thus leading to PARPi resistance. 50
An alternative mechanism for PARPi resistance is the protection of stalled replication forks, a function usually performed by BRCA1, BRCA2 and PARP1. In the absence of these proteins, replication forks are degraded, leading to cell death. Several pre-clinical studies have reported increased stability of replication forks in BRCA-mutant cell lines via loss of MLL3/4 and CHD4 proteins. 51 It has been demonstrated that replication fork stabilization can be disrupted by both Chk1 inhibitors and ATR inhibitors.52,53
To allow time for repair of accumulated DNA damage, cells must pause the cell cycle. This genotoxic stress-induced cell cycle arrest is implemented by checkpoint kinases Chk1 and Chk2, which are activated by short-term and chronic replication stress, respectively. DNA lesions such as stalled replication forks activate ATR, which phosphorylates Chk1. This in turn phosphorylates Cdc25 A phosphatase, targeting it for proteasomal degradation. The subsequent reduction in Cdk2/Cyclin A complex activity leads to G2 checkpoint arrest. 54 Chk1 also phosphorylates BRCA2 and RAD51, thus recruiting the recombinase RAD51 to DSBs and facilitating HR.
It is known that ovarian tumours are more reliant on the G2 checkpoint for DDR, as the ubiquitous loss of p53 in HGSOC renders the S phase checkpoint ineffective. 55 Inhibition of ATR, Chk1 and Wee1 proteins annuls G2 arrest, preventing DNA repair via HRR from occurring. 55 The importance of ATR in PARPi resistance was demonstrated by a study that showed that PARPi-resistant BRCA-1 deficient cells were dependent on ATR for survival. 53
Innate PARPi resistance may be overcome by inducing HRD. In pre-clinical studies, the Chk1 inhibitor prexasertib downregulated BRCA1 and RAD51 expression, which led to a 55% reduction in HR proficiency. 56 BET inhibitors have also been shown to suppress DDR genes and induce an HRD phenotype in HR-proficient cell lines. 57 Furthermore, the combination of BET inhibitors and PARP inhibitors showed increased anti-tumour activity compared to either monotherapy in cell lines with different mechanisms of PARPi resistance, including those that were BRCA1/2 wild-type, or deficient in 53BP1 or PARP1. 57
Thus, by multiple mechanisms, inhibition of the ATR/Chk1/Wee1 axis can re-sensitize tumours to PARPi. Pre-clinical studies, both in cell lines and patient-derived xenograft models, have demonstrated a synergistic effect of Chk1 inhibition and PARPi.56,58 Furthermore, prexasertib sensitized previously resistant cell lines to PARP inhibition. 58
Mechanisms of resistance that are not associated with HR or replication stress include increased expression of drug efflux pumps and decreased PARP1 trapping. The MDR1 (multi-drug resistance 1) efflux pump, whose substrates include olaparib, has been seen to be upregulated in PARPi-resistant ovarian cancer cell lines and recurrent disease primary samples.47,59 In pre-clinical models, the addition of verapamil (a substrate of the MDR1 drug transporter pump) has been employed to increase intracellular concentrations of chemotherapy drugs (e.g. doxorubicin) that are usually removed by the same pump. 60 This approach has not been used with PARPi to date. Below we will discuss the most relevant clinical studies exploring the use of combination therapies with PARPi in HGSOC.
PARP inhibitors in combination with anti-angiogenic agents
Both PARPi and anti-angiogenic agents have shown promising efficacy as monotherapies, and their combination is of particular interest given that they have minimal overlapping toxicities.19,21,22,61,62 The results of the practice-changing PAOLA-1/ENGOT-ov25 study were released in 2019. In this study, 806 patients were randomized in a 2:1 ratio to receive bevacizumab (a monoclonal antibody that binds circulating VEGF) plus olaparib or placebo as first-line maintenance treatment following a response to chemotherapy plus bevacizumab. The addition of maintenance olaparib resulted in a significant PFS benefit (22.1 months versus 16.6 months, HR: 0.59, 95% CI: 0.49–0.72). Subgroup analysis demonstrated that the PFS benefit was incremental based upon HRD and BRCA1/2 mutation status. The largest benefit was seen in patients with a germline or somatic BRCA1/2 mutation (37.2 months versus 17.7 months, HR: 0.33, 95% CI: 0.25–0.45). This was followed by patients with HRD-positive tumours that did not have BRCA1/2 mutations (28.1 months versus 16.6 months, HR: 0.43, 95% CI: 0.28–0.66). HRD test negative patients were found to have no benefit from the addition of a PARP inhibitor (median PFS: 16.9 months versus 16.0 months). 23 An increase in grades 3 and 4 adverse effects (AEs) such as fatigue, diarrhoea and cytopaenias was seen in the combination arm of this study. 23
Unfortunately, there was no olaparib-only arm of this study, as bevacizumab maintenance therapy was standard of care (SOC) throughout much of Europe. Therefore, it is unclear what the additional benefit of bevacizumab to olaparib is in this setting, particularly in patients with BRCA1/2 mutations who are known to gain large benefit for PARPi monotherapy.
The AVANOVA2 study was a small randomized Phase II study of 97 patients, comparing niraparib and bevacizumab versus niraparib alone as definitive treatment for platinum-sensitive recurrent ovarian cancer. 63 The combination arm showed a significantly improved PFS compared with niraparib alone (median PFS: 11·9 months versus 5·5 months, HR: 0·35, 95% CI: 0·21–0·57).
This combination has subsequently been taken forward into the first-line maintenance setting in the single-arm Phase II OVARIO study. 64 The median PFS was 19.6 months (95% CI: 16.5–25.1) in the overall population, with 28.3 months (95% CI: 19.9–NE) and 14.2 months (95% CI: 8.6–16.8) for the HRD and HR test-negative subgroups, respectively. With no comparator arm, it is difficult to interpret this study, but the PFS values for niraparib monotherapy in the PRIMA study were 13.8, 21.9 and 8.1 months for overall population, HRD tumours and HR test-negative tumours, respectively, suggesting minimal benefit from the addition of bevacizumab to niraparib. 22
PARPi have also been trialled in combination with the oral tyrosine kinase inhibitor cediranib, a potent inhibitor of all 3 VEGF receptors (VEGFR-1, -2 and -3). This is following the results of the ICON6 study, which showed an effect of cediranib in recurrent ovarian cancer. 65 A Phase II study of 90 patients (NCT01116648) showed a PFS advantage with the addition of cediranib to olaparib for the treatment of platinum-sensitive relapsed HGSOC (16.5 months versus 8.2 months, HR: 0.50, 95% CI: 0.30–0.83, compared to olaparib alone). 66 Strikingly, those with gBRCA1/2 mutations gained no benefit with the addition of cediranib to olaparib (16.4 months versus 16.5 months, HR: 0.76, 95% CI: 0.38–1.49). The significant prolongation of PFS was therefore driven by those patients without gBRCA1/2 mutation (median PFS: 23.7 months versus 5.7 months, HR: 0.31, 95% CI: 0.15–0.66). This suggests that inhibition of angiogenesis may be promoting a HRD phenotype, increasing the benefit derived from PARPi in this cohort.
In the ICON 9 Phase III study, which is currently in the recruitment phase, patients with recurrent platinum-sensitive ovarian cancer are randomized to receive maintenance therapy with olaparib monotherapy or olaparib in combination with cediranib, following a response to platinum-based chemotherapy. 67 It is hoped that the first results from this study will be presented in 2025.
Table 2 summarizes the trials of combinations of PARPi and anti-angiogenic agents to date.
Table 2.
Trials using a combination of PARPi and anti-angiogenic agents.
| Study name (NCT number) | Treatment setting | Patient population | Drug name | PFS PARPi versus placebo (months) | HR (95% CI) |
|---|---|---|---|---|---|
| PAOLA-1 (NCT02477644) 8 | First-line maintenance | All comers | Olaparib plus bevacizumab | Overall: 22.1 versus
16.6 gBRCA1/2 mut: 37.2 versus 17.7 HRD & BRCA1/2 wt: 28.1 versus 16.6 HRD test neg: 16.9 versus 16.0 |
0.59 (0.49–0.72) 0.33 (0.25–0.45) 0.43 (0.28–0.66) 0.92 (0.72–1.17) |
| AVANOVA-2 (NCT02354131) 9 | PSR maintenance | All comers | Niraparib plus bevacizumab | 11.9 versus 5.5 | 0.35 (0.21–0.57) |
| OVARIO (NCT03326193) 10 | First-line maintenance | All comers | Niraparib plus bevacizumab | Overall: 19.6 (no placebo arm) HRD: 28.3 HRD test neg: 14.2 |
n/a |
| NCT0111664811 | PSR maintenance | All comers | Olaparib plus cedirinib | Overall: 16.5 versus
8.2 gBRCA1/2 mut: 16.4 versus 16.5 gBRCA1/2 wt: 23.7 versus 5.7 |
0.50 (0.30–0.83) 0.76 (0.38–1.49) 0.31 (0.15–0.66) |
| ICON9 (NCT03278717) 12 | PSR maintenance | All comers | Olaparib plus cedirinib | Trial ongoing | n/a |
BRCA1/2 mut, BRCA1/2 mutation carriers; CI, confidence interval; g, germline; HR, hazard ratio; HRD, homologous recombination deficient; PARPi, PARP inhibitors; PFS, progression-free survival; PSR, platinum-sensitive relapse; wt, wild-type.
PARP inhibitors in combination with checkpoint blockade
Immunotherapy is a rapidly expanding area within cancer treatment and has transformed patient outcomes in multiple tumour types, including melanoma and non-small cell lung cancer.68–70 Programmed death-1 (PD-1), a co-inhibitory signal receptor expressed on T cells, and its ligand (PD-L1) regulate anti-tumour immunity. Multiple monoclonal antibodies (checkpoint inhibitors) have been developed to target these molecules and reduce the cancer’s ability to evade host immune recognition. HGSOC is known to have an intermediate mutational load (approximately two mutations per megabase), which usually correlates with response to checkpoint inhibitor therapy. 71 Clinical data from the use of immunotherapy in HGSOC, both as definitive and maintenance treatment, have not been convincing to date.
Early Phase I and II data showed minimal clinical benefit of anti-PD-1 and anti-PDL-1 therapies in HGSOC, with a response rate of approximately 10%.72,73 The Phase III JAVELIN 100 and JAVELIN 200 studies showed no benefit with avelumab (an anti-PD-L1 monoclonal antibody) as monotherapy or in combination with chemotherapy in the frontline and relapsed-disease settings.74,75
Despite these disappointing results, the combination of PARPi with checkpoint blockade is considered a promising strategy, based on the rationale described above. The approach has been tested clinically in two Phase II studies. In the non-randomized Phase I/II MEDIOLA trial, patients with recurrent platinum-sensitive ovarian cancer were stratified depending on gBRCA1/2 mutation status. Those patients with gBRCA1/2 mutations were treated with olaparib plus the PD-L1 inhibitor durvalumab (doublet cohort), whereas patients with non-gBRCA1/2 mutated ovarian cancer were given either doublet therapy or olaparib plus durvalumab and bevacizumab (triplet cohort). 76 In the gBRCA1/2-mutated cohort, the overall response rate (ORR) was 71.9% (95% CI: 53.25–85.25%), with an unexpectedly high complete response (CR) rate of 21.8% (7/32 patients) using a chemotherapy-sparing regimen. 77 Grade 3 and above AEs included anaemia, neutropenia and lymphopenia. In the gBRCA1/2 wild-type patients, the triplet therapy cohort showed promising efficacy data, with an ORR of 77.4% (95% CI: 58.9–90.4) in comparison to 31.3% in the doublet cohort (95% CI: 16.1–50.0). 78
In contrast, the single-arm Phase I/II TOPACIO/Keynote-162 study investigated the safety and efficacy of niraparib in combination with pembrolizumab in patients with recurrent platinum-resistant HGSOC 79 ; 48% had platinum-resistant disease, 27% had platinum-refractory disease and 24% were not eligible for further platinum treatment. The majority of the patients (79%) were BRCA1/2 wild-type. Interim analysis showed that out of the 60 evaluable patients with HGSOC, the ORR was 18% (5% complete response (CR) and 13% partial response (PR)), with a disease control rate of 65%, which are high given the platinum-resistant status of the patients. 80
Due to these promising results, the approach has been taken forward into multiple ongoing Phase III clinical trials, whose characteristics are summarized in Table 3. The ANITA/ENGO-OV41 study is exploring whether the addition of the PD-L1 inhibitor atezolizumab to carboplatin-based chemotherapy followed by maintenance niraparib will improve PFS in patients with platinum-sensitive recurrent HGSOC. 81 A further four Phase III studies are for the first-line treatment of Stage III/IV HGSOC. Three out of these four studies are for all patients, with planned subgroup analysis of response to treatment based on HRD status, whereas the Keylynk-001 study is specifically for non-BRCA1/2 mutated tumours.
Table 3.
Ongoing Phase III clinical trials investigating the combination of PARPi and immune checkpoint inhibitors.
| Study name (NCT number) | Treatment setting | Patient population | Arms of study |
|---|---|---|---|
| ANITA (NCT03598270) 13 | Platinum-sensitive relapse | All comers | (A) Atezolizumab + chemo, followed by maintenance
niraparib + placebo (B) Atezolizumab + chemo, followed by maintenance niraparib + atezolizumab |
| Keylynk-001 (NCT03740165) 14 | First-line maintenance | BRCA1/2 wt | (A) Bevacizumab + placebo + placebo (B) Bevacizumab + pembrolizumab + placebo C)Bevacizumab + pembrolizumab + olaparib |
| FIRST (NCT03602859) 15 | First-line maintenance | All comers | (A) Bevacizumab + placebo + placebo (B) Bevacizumab + niraparib + placebo (C) Bevacizumab + niraparib + dostarlimab |
| ATHENA (NCT03522246) 16 | First-line maintenance | All comers | (A) Placebo + placebo (B) Nivolumab + placebo (C) Placebo + rucaparib (D) Nivolumab + rucaparib |
| DUO-O (NCT03737643) 17 | First-line maintenance | All comers | (A) Bevacizumab + placebo + placebo (B) Bevacizumab + durvalumab + placebo (C)Bevacizumab + durvalumab + olaparib |
BRCA1/2 wt, BRCA1/2 wild-type; PARPi, PARP inhibitors.
The Keylynk-001/Engot-ov43 trial is evaluating the use of chemotherapy with or without concurrent and maintenance pembrolizumab, followed by maintenance treatment with olaparib or placebo. 82 Patients may receive bevacizumab with each cycle of chemotherapy/pembrolizumab at the investigator’s discretion. In the FIRST/Engot-ov44 study, patients are randomized to receive either chemotherapy plus concurrent and maintenance bevacizumab (SOC), SOC plus niraparib maintenance or SOC plus niraparib and the PD-1 inhibitor dostarlimab (commenced with cycle 2 chemotherapy and continued up to 3 years maintenance treatment). 83 Similarly, in the DUO-O/Engot-ov46 study, patients may receive SOC (chemotherapy plus bevacizumab), SOC plus concurrent and maintenance durvalumab or SOC plus durvalumab and maintenance olaparib. 84
ATHENA-COMBO/Engot-ov45 is a study evaluating whether the addition of nivolumab (a PD-1 inhibitor) to rucaparib maintenance therapy, following a response to first-line platinum-based chemotherapy, increases its clinical benefit. 85 The results of these trials are awaited with interest.
PARP inhibitors in combination with chemotherapy
Although the synergistic effect of giving PARPi and chemotherapy together is appealing, bringing this combination to clinical practice has proven challenging due to overlapping toxicities, principally myelosuppression. A study of olaparib combined with carboplatin and paclitaxel in advanced ovarian cancer determined that continuous olaparib led to excessive treatment delays secondary to neutropenia or thrombocytopenia (myelosuppression seen in 71% of patients). 26 In another study of olaparib combined with pegylated liposomal doxorubicin in recurrent HGSOC, 61% of patients had grade 3 or above toxicities, including stomatitis, nausea and neutropenia. 27
In a further Phase II trial, patients with platinum-sensitive recurrent HGSOC were randomized to receive either olaparib combined with platinum-based chemotherapy followed by maintenance olaparib until disease progression or chemotherapy alone. Despite a reduction in carboplatin dose (from AUC 6 to AUC 4) to avoid myelotoxicity, there was a 10% increase in neutropenia in the combination therapy arm (8% increase in grade 3 or 4 neutropenia). 25 Furthermore, there was no change in OS, and the late separation in PFS curves implied that most benefit was derived from the maintenance rather than the combination therapy stage. Therefore, the combined PARPi and chemotherapy approach has been abandoned.
PARP inhibitors in combination with other small molecule inhibitors
Inhibitors of DNA damage response
Several ongoing early phase trials are investigating the use of inhibitors of DNA damage response in ovarian cancer (see Table 4). The EFFORT study (NCT03579316) has shown an increase in ORR in PARPi-resistant HGSOC with the addition of olaparib to the Wee1 inhibitor adavosertib (29% combination versus 23% adavosertib monotherapy). 86 This combination was associated with high levels (76%) of grade 3 or 4 toxicity (mostly haematological and diarrhoea), however, with 88% patients requiring at least one dose interruption and 10% patients not being able to restart due to toxicity. Animal studies have suggested that sequential rather than concurrent administration of the two drugs has a similar efficacy, and the STAR trial is testing this approach.87,88
Table 4.
Trials using a combination of PARPi and other small molecule inhibitors.
| Study name (NCT number) | Phase | PARPi | Combination drug | Treatment setting | Arms of study |
|---|---|---|---|---|---|
| EFFORT (NCT03579316) 18 |
II | Olaparib | Adavosertib (Wee1 inhibitor) | PARPi-resistant recurrent HGSOC | (A) Adavosertib (B) Adavosertib + olaparib |
| STAR (NCT04197713) 19 | I | Olaparib | Adavosertib (Wee1 inhibitor) | PARPi-resistant recurrent HGSOC | Sequential olaparib and adavosertib (alternate weeks) |
| NCT03057145 20 | I | Olaparib | Prexasertib (Chk1 inhibitor) | gBRCA1/2 mutant recurrent HGSOC | Olaparib + prexasertib |
| CAPRI (NCT03462342) 21 | II | Olaparib | Ceralasertib (ATR inhibitor) | Platinum-sensitive recurrent/platinum-resistant/PARPi-resistant cohorts | Olaparib + ceralasertib |
| NCT0426793922 | Ib | Niraparib | Elimusertib (ATR inhibitor) | HRD solid tumours (PARPi-naïve/ PARPi-resistant cohorts) | Niraparib + elimusertib |
| NCT0162334923 | Ib | Olaparib | Alpelisib (PI3Kalpha inhibitor) | gBRCA1/2 mutant recurrent HGSOC | Olaparib + alpelisib |
| EPIK-0 (NCT04729387) 24 | III | Olaparib | Alpelisib (PI3Kalpha inhibitor) |
Platinum-resistant/refractory HGSOC | (A) Alpelisib + Olaparib (B) SOC chemotherapy |
| NCT0220837525 | Ib/II | Olaparib | Vistusertib (mTOR inhibitor) or Capivasertib (AKT inhibitor) |
Recurrent HGSOC | (A) Olaparib + continuous vistusertib (B) Olaparib + intermittent vistusertib (C) Olaparib + intermittent capivasertib |
| ComPAKT (NCT02338622) 26 | I | Olaparib | Capivasertib (AKT inhibitor) | Recurrent HGSOC with somatic DDR or PI3K-AKT pathway alterations | Olaparib + capivasertib |
| NCT0316262727 | I/II | Olaparib | Selumetinib (MEK inhibitor) | PARPi-resistant recurrent HGSOC | Olaparib + selumetinib |
| NCT0320517628 | I | Olaparib | AZD5153 (BET inhibitor) | Platinum-resistant/refractory HGSOC | (A) AZD51513 (B) AZD5153 + olaparib |
DDR, DNA damage response; gBRCA1/2, germline BRCA1/2; HGSOC, high-grade serous ovarian cancer; HRD, homologous recombination deficient; PARPi, PARP inhibitors.
The Chk1 inhibitor prexasertib has shown evidence of clinical activity when trialled in combination with olaparib, including in patients with PARPi-resistant HGSOC. 89 Prexasertib has also been used as a monotherapy in platinum- and PARPi-resistant patients in a recent Phase II study (NCT03414047), with some durable activity seen in a subset of patients, although there was no clear clinical parameter that predicted response.90,91 The most common AEs included thrombocytopenia, neutropenia, anaemia, fatigue and nausea.
Inhibitors of the PI3K/AKT/mTOR signalling pathway
The PI3Kalpha inhibitor alpelisib was combined with olaparib in patients with recurrent, mostly platinum-resistant/refractory HGSOC (92.8% patients) in a Phase I study (NCT01623349). 92 Thirty-six percent patients achieved a PR and 50% had stable disease. The ORR was similar for gBRCA1/2-mutant and gBRCA1/2 wild-type patients, suggesting that PI3K inhibition may induce HRD and sensitize HR-proficient tumours to PARPi. The most common toxicities included hyperglycaemia and nausea. Alpelisib plus olaparib is also being studied in comparison to single-agent chemotherapy in patients with platinum-resistant/refractory HGSOC in an ongoing Phase III trial (EPIK-O/ENGOT-OV61). 93
A further study (NCT02208375) evaluated olaparib with either the mTOR inhibitor vistusertib (AZD2014) or the AKT inhibitor capivasertib (AZD 5363) in various solid tumours, including recurrent ovarian cancer. 94 The combination with vistusertib was well tolerated and showed an ORR of 20%, with 37% achieving a clinical benefit (complete/partial response or stable disease for over 4 months). 95 In the capivasertib arm, out of the 16 patients with ovarian cancer, 87% were platinum-resistant/refractory, and 43% derived a clinical benefit from the combination therapy. 96 Interestingly, analysis of tumour samples from this study revealed that tumours that derived a benefit had decreased levels of mTOR activity, whereas those that were resistant had high levels of mTOR activation. In the ComPAKT study (NCT02338622), a comparable 44.6% patients achieved a clinical benefit, with anti-tumour activity observed in both BRCA1/2-mutant and wild-type tumours. 97
Inhibitors of the RAS/RAF/MEK pathway
The combination of the MEK inhibitor selumetinib and olaparib in solid tumours is currently under investigation in the ongoing Phase I/II NCT03162627 study, which has a cohort for PARPi-resistant ovarian cancer. 98
BET inhibitors
Early phase clinical trials combining PARPi and BET inhibitors are currently underway. For example, the NCT03205176 study is combining olaparib and AZD5153 in advanced solid tumours, including HGSOC. 99
Discussion
Until recently, treatment for patients with advanced ovarian cancer was restricted to a combination of cytoreductive surgery and platinum-containing chemotherapy. Optimal timing of surgical cytoreduction and chemotherapy has been extensively investigated. Two international Phase III trials found that survival with neoadjuvant chemotherapy and interval debulking surgery was non-inferior to primary surgery for women with stage III or IV ovarian cancer, whilst surgical morbidity was lower in the neoadjuvant arms.100,101 More recent studies have demonstrated that amongst women with no residual disease, survival was improved in those that underwent primary debulking surgery rather than neoadjuvant chemotherapy.102,103 Neoadjuvant chemotherapy should therefore be reserved for those patients that will not achieve complete cytoreduction at upfront surgery.
The introduction of PARPi has transformed outcomes in many patients with HGSOC, but benefit is mostly confined to patients with HRD tumours, and the majority of patients relapse with PARPi-resistant disease. Upon relapse, treatment options remain limited. In clinical trials to date, there has been promising efficacy data that suggest combining PARPi with other therapies can enhance their efficacy, with improved ORR and PFS. Furthermore, combination therapy offers the potential to overcome both innate and acquired PARPi resistance.
The most established combination therapy is that of PARPi and anti-angiogenic agents. Following the results of the PAOLA-1 study, in many countries the combination of olaparib and bevacizumab in the first-line maintenance setting is now SOC for patients with a positive GIS. 23 The benefit of adding bevacizumab to a PARPi in those patients with HRD tumours (particularly BRCA1/2-mutated tumours) remains a controversial question, however, given the significant clinical benefit seen with olaparib maintenance in the SOLO-1 study. 24 The Phase II MITO-25 study aims to address this question, with patients randomized 1:1:1 to three treatment arms: chemotherapy plus bevacizumab maintenance, chemotherapy plus rucaparib maintenance and chemotherapy plus bevacizumab and rucaparib maintenance. 104
In addition to the GIS score, loss of RAD51 foci in response to DNA damage has been proposed as a biomarker of HRD.12,105 Both may be useful in predicting those patients who derive the greatest benefit form PARPi and highlighting those patients that may require combination strategies.
Now that PARPi and bevacizumab are established in the first-line maintenance setting, it is unknown whether their repeated use is of clinical benefit in the relapsed-disease setting. The Phase III OreO study is examining the efficacy of olaparib re-treatment in patients with relapsed HGSOC who have had previous PARPi maintenance therapy, and several ongoing early phase studies are investigating the use of PARPi combination therapy in PARPi-resistant disease.89,98,106 As nearly all HGSOC patients will now receive PARPi in the first-line maintenance setting, study populations for clinical trials evaluating PARPi combinations in recurrent ovarian cancer will need to include patients with prior PARPi exposure.
PARPi have also been shown to work synergistically with checkpoint inhibitors, with encouraging ORR results in early phase studies.77,78,80 Results from ongoing randomized Phase III studies are awaited with interest.81–85 Other classes of drugs that, when combined with PARPi in pre-clinical studies, have improved PARPi efficacy and induced or restored sensitivity to PARPi, include ATR inhibitors, Chk1 inhibitors and BET inhibitors.53,56,57 These combinations are still in early phase clinical studies and it remains poorly understood which patients derive clinical benefit. It is important that we utilize translational research to establish predictive biomarkers that identify those patients that benefit from monotherapy versus combination treatment and aid selection of which combination approach to use. Candidate biomarkers could be screened for using tumours specimens or cell free DNA (cfDNA) samples from previous studies and subsequently validated in future prospective clinical trials.
A newly discovered repair pathway of microhomology-mediated end-joining utilizing DNA polymerase theta (POLθ) has been identified as being key to the survival of HRD cells. 107 Studies have shown that POLθ is upregulated in HRD tumours, particularly in those with mutations in other DNA repair genes such as BRCA1 and BRCA2. 107 This upregulation may represent a compensatory mechanism to maintain DNA repair in the absence of functional HR. POLθ depletion synergizes with PARPi in synthetic lethality of HRD tumours. 108 Furthermore, pre-clinical studies have shown that in addition to synergy with PARPi in HRD cells, POLθ inhibitors are cytotoxic in PARPi-resistant cells. 109 Thus, POLθ inhibitors are a promising new drug, both used alone and in combination with PARPi, and Phase 1 studies are eagerly awaited.
A consideration when utilizing combination therapies is whether drugs should be combined from the start of treatment or alternated/sequenced to avoid overlapping toxicities. Similarly, it is unknown whether it is equally effective to add in drugs that target resistance mechanisms on development of PARPi resistance rather than from the beginning of that line of treatment. Furthermore, it is concerning that if we combine all drugs within the first-line maintenance setting, our options for treatment within the relapsed-disease setting may be limited. Findings from ongoing studies will help improve our understanding of how to schedule treatments to optimize outcomes.
As with all combination therapies, one needs to balance the improvement in outcomes versus the potential increase in adverse events. Combination treatment with PARPi and bevacizumab is well tolerated, with similar discontinuation rates due to treatment-related AEs in the olaparib plus bevacizumab and olaparib monotherapy arms of the PAOLA-1 and SOLO-1 studies (25% and 28%, respectively).21,23 Interestingly, in the PAOLA-1 study the rate of grade three-fourths hypertension was reduced in the combination arm compared to bevacizumab monotherapy. The early phase studies of other PARPi combinations show that their combination is tolerable in terms of toxicities, and results of larger studies are awaited to assess the incidence of AEs.
It is important to note the increased cost associated with combination therapies. This is not only due to the actual cost of the drugs themselves, but the resources required to deliver treatment and the cost of managing any treatment-related toxicity. Who is responsible for this financial burden is dependent on local health authorities/reimbursement procedures in each county, but these escalating costs may be prohibitive for many patients/health authorities and should be factored into decision making.
As the number of treatment options continues to expand, it is increasingly important that we develop predictive biomarkers to stratify patients for combination therapy and define the optimal sequencing of treatments. As data from ongoing and future clinical studies mature, we hope to answer these outstanding questions, thus enabling PARPi to further improve HGSOC patient outcomes.
Acknowledgments
None.
Footnotes
ORCID iD: Rowan E. Miller  https://orcid.org/0000-0002-2400-1716
https://orcid.org/0000-0002-2400-1716
Contributor Information
Helen Hockings, Department of Medical Oncology, St Bartholomew’s Hospital, London, UK.
Rowan E. Miller, Department of Medical Oncology, St Bartholomew’s Hospital, London, UK; Department of Medical Oncology, University College London Hospital, 250 Euston Road, London, NW1 2BU, UK.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contribution(s): Helen Hockings: Conceptualization; Writing – original draft; Writing – review & editing.
Rowan E. Miller: Conceptualization; Writing – original draft; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declare that there is no conflict of interest.
Availability of data and materials: Not applicable.
References
- 1.International WCRF. Ovarian cancer statistics | World Cancer Research Fund International [Internet]. https://www.wcrf.org/cancer-trends/ovarian-cancer-statistics/ (2022, accessed 23 February 2023)
- 2.JacobsMenon IJU, MenonGentry-Maharaj UA, RyanBurnell AM, et al. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet 2016; 387: 945–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buys SS, Partridge E, Black A, et al. Effect of screening on ovarian cancer mortality: the Prostate, Lung, Colorectal and Ovarian (PLCO) cancer screening randomized controlled trial. J Am Med Assoc 2011; 305: 2295–2302. [DOI] [PubMed] [Google Scholar]
- 4.Colombo N, Sessa C, Bois A. Du, et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: Pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Int J Gynecol Cancer 2019; 29: 728–760. [DOI] [PubMed] [Google Scholar]
- 5.Ledermann JA, Raja FA, Fotopoulou C, et al. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013; 24(Suppl. 6): vi24–vi32. [DOI] [PubMed] [Google Scholar]
- 6.Dedes KJ, Wilkerson PM, Wetterskog D, et al. Synthetic lethality of PARP inhibition in cancers lacking BRCA1 and BRCA2 mutations. Cell Cycle 2011; 10: 1192–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ashworth A.A synthetic lethal therapeutic approach: poly(ADP) ribose polymerase inhibitors for the treatment of cancers deficient in DNA double-strand break repair. J Clin Oncol 2008; 26: 3785–3790. [DOI] [PubMed] [Google Scholar]
- 8.Konstantinopoulos PA, Ceccaldi R, Shapiro GI, et al. Homologous recombination deficiency: Exploiting the fundamental vulnerability of ovarian cancer. Vol. 5, Cancer Discovery. American Association for Cancer Research Inc, 2015;5 (11): 1137–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bell D, Berchuck A, Birrer M, et al. Integrated genomic analyses of ovarian carcinoma. Nature 2011; 474: 609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciriello G, Miller ML, Aksoy BA, et al. Emerging landscape of oncogenic signatures across human cancers. Nat Genet 2013; 45: 1127–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Norquist BM, Harrell MI, Brady MF, et al. Inherited mutations in women with ovarian carcinoma. JAMA Oncol 2016; 2: 482–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller RE, Leary A, Scott CL, et al. ESMO recommendations on predictive biomarker testing for homologous recombination deficiency and PARP inhibitor benefit in ovarian cancer. Ann Oncol 2020; 31: 1606–1622. [DOI] [PubMed] [Google Scholar]
- 13.Shah S, Cheung A, Kutka M, et al. Epithelial ovarian cancer: providing evidence of predisposition genes. Int J Environ Res Public Health 2022; 19: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bryant HE, Schultz N, Thomas HD, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 2005; 434: 913–917. [DOI] [PubMed] [Google Scholar]
- 15.Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005; 434: 917–921. [DOI] [PubMed] [Google Scholar]
- 16.Audeh MW, Carmichael J, Penson RT, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet 2010; 376: 245–251. [DOI] [PubMed] [Google Scholar]
- 17.Kaye SB, Lubinski J, Matulonis U, et al. Phase II, open-label, randomized, multicenter study comparing the efficacy and safety of olaparib, a poly (ADP-ribose) polymerase inhibitor, and pegylated liposomal doxorubicin in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer. J Clin Oncol 2012; 30: 372–379. [DOI] [PubMed] [Google Scholar]
- 18.Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol 2014; 15: 852–861. [DOI] [PubMed] [Google Scholar]
- 19.Mirza MR, Monk BJ, Herrstedt J, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. New Engl J Med 2016; 375: 2154–2164. [DOI] [PubMed] [Google Scholar]
- 20.Coleman RL, Oza AM, Lorusso D, et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017; 390: 1949–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore K, Colombo N, Scambia G, et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N Engl J Med 2018; 379: 2495–2505. [DOI] [PubMed] [Google Scholar]
- 22.González-Martín A, Pothuri B, Vergote I, et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. New Engl J Med 2019; 381: 2391–2402. [DOI] [PubMed] [Google Scholar]
- 23.Ray-Coquard I, Pautier P, Pignata S, et al. Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. New Engl J Med 2019; 381: 2416–2428. [DOI] [PubMed] [Google Scholar]
- 24.Banerjee S, Moore KN, Colombo N, et al. Maintenance olaparib for patients with newly diagnosed advanced ovarian cancer and a BRCA mutation (SOLO1/GOG 3004): 5-year follow-up of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2021; 22: 1721–1731. [DOI] [PubMed] [Google Scholar]
- 25.Oza AM, Cibula D, Benzaquen AO, et al. Olaparib combined with chemotherapy for recurrent platinum-sensitive ovarian cancer: a randomised phase 2 trial. Lancet Oncol 2015; 16: 87–97. [DOI] [PubMed] [Google Scholar]
- 26.van der Noll R, Ang JE, Jager A, et al. Phase I study of olaparib in combination with carboplatin and/or paclitaxel in patients with advanced solid tumors. J Clin Oncol 2013; 31: 2579–2579. [Google Scholar]
- 27.Del Conte G, Sessa C, von Moos R, et al. Phase I study of olaparib in combination with liposomal doxorubicin in patients with advanced solid tumours. Br J Cancer 2014; 111: 651–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim H, George E, Ragland R, et al. Targeting the ATR/CHK1 axis with PARP inhibition results in tumor regression in BRCA-mutant ovarian cancer models. Clin Cancer Res 2017; 23: 3097–3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun C, Fang Y, Yin J, et al. Rational combination therapy with PARP and MEK inhibitors capitalizes on therapeutic liabilities in RAS mutant cancers. Sci Transl Med 2017; 9(392):eaal5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bristow RG, Hill RP.Hypoxia and metabolism: hypoxia, DNA repair and genetic instability. Nat Rev Cancer 2008; 8: 180–192. [DOI] [PubMed] [Google Scholar]
- 31.Loizzi V, Del Vecchio V, Gargano G, et al. Biological pathways involved in tumor angiogenesis and bevacizumab based anti-angiogenic therapy with special references to ovarian cancer. Int J Mol Sci 2017; 18: 1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller RE, El-Shakankery KH, Lee JY.PARP inhibitors in ovarian cancer: overcoming resistance with combination strategies. J Gynecol Oncol 2022; 33: e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lim J, Yang K, Taylor-Harding B, et al. VEGFR3 inhibition chemosensitizes ovarian cancer stem like cells through down-regulation of BRCA1 and BRCA2. Neoplasia 2014; 16: 343–353.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bindra RS, Schaffer PJ, Meng A, et al. Down-regulation of rad51 and decreased homologous recombination in hypoxic cancer cells. Mol Cell Biol 2004; 24: 8504–8518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ibrahim YH, García-García C, Serra V, et al. PI3K inhibition impairs BRCA1/2 expression and sensitizes BRCA-proficient triple-negative breast cancer to PARP inhibition. Cancer Discov 2012; 2: 1036–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mo W, Liu Q, Lin CC, et al. MTOR inhibitors suppress homologous recombination repair and synergize with PARP inhibitors via regulating SUV39H1 in BRCA-proficient triple-negative breast cancer. Clin Cancer Res 2016; 22: 1699–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vena F, Jia R, Esfandiari A, et al. MEK inhibition leads to BRCA2 downregulation and sensitization to DNA damaging agents in pancreas and ovarian cancer models. Oncotarget 2018; 9: 11592–11603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strickland KC, Howitt BE, Shukla SA, et al. Association and prognostic significance of BRCA1/2-mutation status with neoantigen load, number of tumor-infiltrating lymphocytes and expression of PD-1/PD-L1 in high grade serous ovarian cancer. Oncotarget 2016; 7: 13587–13598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiao S, Xia W, Yamaguchi H, et al. PARP inhibitor upregulates PD-L1 expression and enhances cancer-associated immunosuppression. Clin Cancer Res 2017; 23: 3711–3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reisländer T, Lombardi EP, Groelly FJ, et al. BRCA2 abrogation triggers innate immune responses potentiated by treatment with PARP inhibitors. Nat Commun 2019; 10: 3143–3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ding L, Kim HJ, Wang Q, et al. PARP inhibition elicits STING-Dependent antitumor immunity in brca1-deficient ovarian cancer. Cell Rep 2018; 25: 2972–2980.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chabanon RM, Muirhead G, Krastev DB, et al. PARP inhibition enhances tumor cell-intrinsic immunity in ERCC1-deficient non-small cell lung cancer. J Clin Investig 2019; 129: 1211–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mateo J, Lord CJ, Serra V, et al. A decade of clinical development of PARP inhibitors in perspective. Ann Oncol 2019; 30: 1437–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lord CJ, Ashworth A.Mechanisms of resistance to therapies targeting BRCA-mutant cancers. Nat Med 2013; 19: 1381–1388. [DOI] [PubMed] [Google Scholar]
- 45.McMullen M, Karakasis K, Madariaga A, et al. Overcoming platinum and parp-inhibitor resistance in ovarian cancer. Multidisciplinary Digital Publishing Institute, 2020. Vol. 12, pp.1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blagden SP, Hamilton AL, Mileshkin L, et al. Phase IB dose escalation and expansion study of AKT inhibitor afuresertib with carboplatin and paclitaxel in recurrent platinum-resistant ovarian cancer. Clin Cancer Res 2019; 25: 1472–1478. [DOI] [PubMed] [Google Scholar]
- 47.Patch AM, Christie EL, Etemadmoghadam D, et al. Whole–genome characterization of chemoresistant ovarian cancer. Nature 2015; 521: 489–494. [DOI] [PubMed] [Google Scholar]
- 48.Sakai W, Swisher EM, Jacquemont C, et al. Functional restoration of BRCA2 protein by secondary BRCA2 mutations in BRCA2-Mutated ovarian carcinoma. Cancer Res 2009; 69: 6381–6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kondrashova O, Topp M, Nesic K, et al. Methylation of all BRCA1 copies predicts response to the PARP inhibitor rucaparib in ovarian carcinoma. Nat Commun 2018; 9(1): 3970 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nacson J, Krais JJ, Bernhardy AJ, et al. BRCA1 mutation-specific responses to 53BP1 loss-induced homologous recombination and PARP inhibitor resistance. Cell Rep 2018; 24: 3513–3527.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chaudhuri AR, Callen E, Ding X, et al. Erratum: Replication fork stability confers chemoresistance in BRCA-deficient cells. Nature2016; 539: 456–457. [DOI] [PubMed] [Google Scholar]
- 52.Haynes B, Murai J, Lee JM.Restored replication fork stabilization, a mechanism of PARP inhibitor resistance, can be overcome by cell cycle checkpoint inhibition. Cancer Treat Rev 2018; 71: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yazinski SA, Comaills V, Buisson R, et al. ATR inhibition disrupts rewired homologous recombination and fork protection pathways in PARP inhibitor-resistant BRCA-deficient cancer cells. Genes Dev 2017; 31: 318–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith J, Mun Tho L, Xu N, et al. The ATM-Chk2 and ATR-Chk1 pathways in DNA damage signaling and cancer. Advances in Cancer Research. 2010; 108: 73–112. [DOI] [PubMed] [Google Scholar]
- 55.Rundle S, Bradbury A, Drew Y, et al. Targeting the ATR-CHK1 axis in cancer therapy. Cancers 2017; 9: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mani C, Jonnalagadda S, Lingareddy J, et al. Prexasertib treatment induces homologous recombination deficiency and synergizes with olaparib in triple-negative breast cancer cells. Breast Cancer Res 2019; 21: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun C, Yin J, Fang Y, et al. BRD4 inhibition is synthetic lethal with PARP inhibitors through the induction of homologous recombination deficiency. Cancer Cell 2018; 33: 401–416.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parmar K, Kochupurakkal BS, Lazaro JB, et al. The CHK1 inhibitor prexasertib exhibits monotherapy activity in high-grade serous ovarian cancer models and sensitizes to PARP inhibition. Clin Cancer Res 2019; 25: 6127–6140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vaidyanathan A, Sawers L, Gannon AL, et al. ABCB1 (MDR1) induction defines a common resistance mechanism in paclitaxel- and olaparib-resistant ovarian cancer cells. Br J Cancer 2016; 115: 431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Silva AS, Kam Y, Khin ZP, et al. Evolutionary approaches to prolong progression-free survival in breast cancer. Cancer Res 2012; 72: 6362–6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Perren TJ, Swart AM, Pfisterer J, et al. A Phase 3 trial of bevacizumab in Ovarian Cancer. New Engl J Med 2011; 365: 2484–2496. [DOI] [PubMed] [Google Scholar]
- 62.Coleman RL, Brady MF, Herzog TJ, et al. Bevacizumab and paclitaxel–carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 2017; 18: 779–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mirza MR, Åvall Lundqvist E, Birrer MJ, et al. Niraparib plus bevacizumab versus niraparib alone for platinum-sensitive recurrent ovarian cancer (NSGO-AVANOVA2/ENGOT-ov24): a randomised, phase 2, superiority trial. Lancet Oncol 2019; 20: 1409–1419. [DOI] [PubMed] [Google Scholar]
- 64.Hardesty MM, Krivak TC, Wright GS, et al. OVARIO phase II trial of combination niraparib plus bevacizumab maintenance therapy in advanced ovarian cancer following first-line platinum-based chemotherapy with bevacizumab. Gynecol Oncol 2022; 166: 219–229. [DOI] [PubMed] [Google Scholar]
- 65.Ledermann JA, Embleton AC, Raja F, et al. Cediranib in patients with relapsed platinum-sensitive ovarian cancer (ICON6): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2016; 387: 1066–1074. [DOI] [PubMed] [Google Scholar]
- 66.Liu JF, Barry WT, Birrer M, et al. Overall survival and updated progression-free survival outcomes in a randomized phase II study of combination cediranib and olaparib versus olaparib in relapsed platinum-sensitive ovarian cancer. Ann Oncol 2019; 30: 551–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Elyashiv O, Ledermann J, Parmar G, et al. ICON 9-an international phase III randomized study to evaluate the efficacy of maintenance therapy with olaparib and cediranib or olaparib alone in patients with relapsed platinum-sensitive ovarian cancer following a response to platinum-based chemotherapy. Int J Gynecol Cancer 2021; 31: 134–138. [DOI] [PubMed] [Google Scholar]
- 68.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. New Engl J Med 2010; 363: 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (checkmate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2015; 16: 375–384. [DOI] [PubMed] [Google Scholar]
- 70.Vokes EE, Ready N, Felip E, et al. Nivolumab versus docetaxel in previously treated advanced non-small-cell lung cancer (CheckMate 017 and CheckMate 057): 3-year update and outcomes in patients with liver metastases. Ann Oncol 2018; 29: 959–965. [DOI] [PubMed] [Google Scholar]
- 71.Alexandrov LB, Nik-Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature 2013; 500: 415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hamanishi J, Mandai M, Konishi I.Immune checkpoint inhibition in ovarian cancer. Int Immunol 2016; 28: 339–348. [DOI] [PubMed] [Google Scholar]
- 73.Hamanishi J, Mandai M, Ikeda T, et al. Safety and antitumor activity of Anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol 2015; 33: 4015–4022. [DOI] [PubMed] [Google Scholar]
- 74.Ledermann JA, Colombo N, Oza AM, et al. Avelumab in combination with and/or following chemotherapy vs chemotherapy alone in patients with previously untreated epithelial ovarian cancer: results from the phase 3 javelin ovarian 100 trial. Gynecol Oncol 2020; 159: 13–14.32771275 [Google Scholar]
- 75.Pujade-Lauraine E, Fujiwara K, Ledermann JA, et al. Avelumab alone or in combination with chemotherapy versus chemotherapy alone in platinum-resistant or platinum-refractory ovarian cancer (JAVELIN Ovarian 200): an open-label, three-arm, randomised, phase 3 study. Lancet Oncol 2021; 22: 1034–1046. [DOI] [PubMed] [Google Scholar]
- 76.NIH. A Phase I/II Study of MEDI4736 in Combination With Olaparib in Patients With Advanced Solid Tumors [Internet]. clinicaltrials.gov, https://clinicaltrials.gov/ct2/show/NCT02734004
- 77.Drew Y, Kaufman B, Banerjee S, et al. Phase II study of olaparib + durvalumab (MEDIOLA): updated results in germline BRCA-mutated platinum-sensitive relapsed (PSR) ovarian cancer (OC). Ann Oncol 2019; 30: v485–v486. [Google Scholar]
- 78.Drew Y, Penson RT, O’Malley DM, et al. 814MO Phase II study of olaparib (O) plus durvalumab (D) and bevacizumab (B) (MEDIOLA): initial results in patients (pts) with non-germline BRCA-mutated (non-gBRCAm) platinum sensitive relapsed (PSR) ovarian cancer (OC). Ann Oncol 2020; 31: S615–S616. [Google Scholar]
- 79.NIH. Niraparib in combination with pembrolizumab in patients with triple-negative breast cancer or ovarian cancer [Internet]. clinicaltrials.gov, https://clinicaltrials.gov/ct2/show/NCT02657889 (accessed 8 May 2023)
- 80.Konstantinopoulos PA, Waggoner S, Vidal GA, et al. Single-Arm Phases 1 and 2 trial of niraparib in combination with pembrolizumab in patients with recurrent platinum-resistant ovarian carcinoma. JAMA Oncol 2019; 5: 1141–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gonzalez Martin A, Sanchez Lorenzo L, Colombo N, et al. A phase III, randomized, double blinded trial of platinum based chemotherapy with or without atezolizumab followed by niraparib maintenance with or without atezolizumab in patients with recurrent ovarian, tubal, or peritoneal cancer and platinum treatment free interval of more than 6 months: ENGOT-Ov41/GEICO 69-O/ANITA Trial. Int J Gynecol Cancer 2021; 31: 617–622. [DOI] [PubMed] [Google Scholar]
- 82.NIH. Study of chemotherapy with Pembrolizumab (MK-3475) followed by maintenance with Olaparib (MK-7339) for the first-line treatment of women with BRCA non-mutated advanced epithelial ovarian cancer (EOC) (MK-7339-001/KEYLYNK-001/ENGOT-ov43/GOG-3036) [Internet]. clinicaltrials.gov, https://clinicaltrials.gov/ct2/show/NCT03740165?term=keylynk&draw=2&rank=3 (2021)
- 83.NIH. A Phase 3 comparison of platinum-based therapy With TSR-042 and Niraparib versus standard of care (SOC) platinum-based therapy as first-line treatment of stage III or IV nonmucinous epithelial ovarian cancer [Internet]. clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT03602859 (accessed 8 May 2023)
- 84.NIH. Durvalumab treatment in combination with chemotherapy and bevacizumab, followed by maintenance durvalumab, bevacizumab and olaparib treatment in advanced ovarian cancer patients [Internet]. clinicaltrials.gov. 2022. https://clinicaltrials.gov/ct2/show/NCT03737643
- 85.NIH. A Study in ovarian cancer patients evaluating rucaparib and nivolumab as maintenance treatment following response to front-line platinum-based chemotherapy [Internet]. clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT03522246
- 86.Westin SN, Coleman RL, Fellman BM, et al. EFFORT: Efficacy of Adavosertib in PARP Resistance: A randomized two-arm non-comparative phase II study of Adavosertib with or without olaparib in women with PARP-resistant ovarian cancer. J Clin Oncol 2021; 39: 5505–5505. [Google Scholar]
- 87.Fang Y, McGrail DJ, Sun C, et al. Sequential therapy with PARP and WEE1 inhibitors minimizes toxicity while maintaining efficacy. Cancer Cell 2019; 35: 851–867.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Testing the Sequential Combination of the Anti-cancer Drugs Olaparib Followed by Adavosertib (AZD1775) in Patients With Advanced Solid Tumors With Selected Mutations and PARP Resistance, STAR Study [Internet]. https://clinicaltrials.gov/ct2/show/NCT04197713 (accessed 8 May 2023)
- 89.Do KT, Kochupurakkal B, Kelland S, et al. Phase 1 combination study of the chk1 inhibitor prexasertib and the PARP inhibitor olaparib in high-grade serous ovarian cancer and other solid tumors. Clin Cancer Res 2021; 27: 4710–4716. [DOI] [PubMed] [Google Scholar]
- 90.ClinicalTrials.gov. A Study of Prexasertib (LY2606368) in Platinum-Resistant or Refractory Recurrent Ovarian Cancer – Full Text View [Internet]. clinicaltrials.govhttps://clinicaltrials.gov/ct2/show/NCT03414047 (2018)
- 91.Konstantinopoulos PA, Lee JM, Gao B, et al. A Phase 2 study of prexasertib (LY2606368) in platinum resistant or refractory recurrent ovarian cancer. Gynecol Oncol 2022; S0090-8258(22)01839-X. DOI: 10.1016/j.ygyno.2022.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Konstantinopoulos PA, Barry WT, Birrer M, et al. Olaparib and α-specific PI3K inhibitor alpelisib for patients with epithelial ovarian cancer: a dose-escalation and dose-expansion phase 1b trial. Lancet Oncol 2019; 20: 570–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Konstantinopoulos PA, Gonzalez-Martin A, Cruz FM, et al. EPIK-O/ENGOT-OV61: alpelisib plus olaparib vs cytotoxic chemotherapy in high-grade serous ovarian cancer (phase III study). Future Oncol 2022; 18: 3481–3492. [DOI] [PubMed] [Google Scholar]
- 94.ClinicalTrials.gov. mTORC1/2 Inhibitor AZD2014 or the Oral AKT Inhibitor AZD5363 for recurrent endometrial and ovarian cancer. clinicaltrials.gov [Internet], 2014, pp. 2014–20. https://clinicaltrials.gov/show/NCT02208375 (2014, accessed 8 May 2023).
- 95.Westin SN, Litton JK, Williams RA, et al. Phase I trial of olaparib (PARP inhibitor) and vistusertib (mTORC1/2 inhibitor) in recurrent endometrial, ovarian and triple negative breast cancer. J Clin Oncol 2018; 36: 5504–5504. [Google Scholar]
- 96.Westin SN, Labrie M, Litton JK, et al. Phase Ib dose expansion and translational analyses of olaparib in combination with capivasertib in recurrent endometrial, triple-negative breast, and Ovarian Cancer. Clin Cancer Res 2021; 27: 6354–6365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yap TA, Kristeleit R, Michalarea V, et al. Phase I trial of the parp inhibitor olaparib and AKT inhibitor capivasertib in patients with brca1/2- and non–BRCA1/2-mutant cancers. Cancer Discov 2020; 10: 1528–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.ClinicalTrials.gov. Selumetinib and Olaparib in Solid Tumors [Internet]. clinicaltrials.gov, https://clinicaltrials.gov/show/NCT03162627 (accessed 8 May 2023)
- 99.AZD5153 in patients with relapsed or refractory solid tumors, including lymphomas [Internet]. clinicaltrials.gov, https://clinicaltrials.gov/ct2/show/NCT03205176
- 100.Kehoe S, Hook J, Nankivell M, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet 2015; 386: 249–257. [DOI] [PubMed] [Google Scholar]
- 101.Vergote I, Tropé CG, Amant F, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. New Engl J Med 2010; 363: 943–953. [DOI] [PubMed] [Google Scholar]
- 102.May T, Comeau R, Sun P, et al. Comparison of survival outcomes in advanced serous ovarian cancer patients treated with primary debulking surgery versus neoadjuvant chemotherapy. Int J Gynecol Cancer 2017; 27: 668–674. [DOI] [PubMed] [Google Scholar]
- 103.Rosen B, Laframboise S, Ferguson S, et al. The impacts of neoadjuvant chemotherapy and of debulking surgery on survival from advanced ovarian cancer. Gynecol Oncol 2014; 134: 462–467. [DOI] [PubMed] [Google Scholar]
- 104.NIH. Carboplatin-Paclitaxel-Bevacizumab vs Carbo-Pacli-Beva-Rucaparib vs Carbo-Pacli-Ruca, Selected According to HRD Status, in patients with advanced ovarian, primary peritoneal and fallopian tube cancer, Preceded by a Phase I Dose Escalation Study on Rucapar [Internet]. clinicaltrials.gov, https://clinicaltrials.gov/ct2/show/NCT03462212 (accessed 8 May 2023)
- 105.Cruz C, Castroviejo-Bermejo M, Gutiérrez-Enríquez S, et al. RAD51 foci as a functional biomarker of homologous recombination repair and PARP inhibitor resistance in germline BRCA-mutated breast cancer. Ann Oncol 2018; 29: 1203–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.NIH. A study to examine olaparib maintenance retreatment in patients with Epithelial ovarian cancer [Internet]. clinicaltrials.gov, https://clinicaltrials.gov/ct2/show/NCT03106987 (accessed 8 May 2023)
- 107.Ceccaldi R, Liu JC, Amunugama R, et al. Homologous-recombination-deficient tumours are dependent on Polθ-mediated repair. Nature 2015; 518: 258–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mateos-Gomez PA, Gong F, Nair N, et al. Mammalian polymerase θ promotes alternative NHEJ and suppresses recombination. Nature 2015; 518: 254–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhou J, Gelot C, Pantelidou C, et al. A first-in-class polymerase theta inhibitor selectively targets homologous-recombination-deficient tumors. Nat Cancer 2021; 2: 598–610. [DOI] [PMC free article] [PubMed] [Google Scholar]


