Abstract
Background
Vitamins, minerals, and natural product (NP)-derived dietary supplements are commonly used among women with breast cancer, where interactions with treatments and the disease are possible, emphasizing the importance for health care providers to be aware of supplement use.
Objectives
The study aimed to investigate current vitamin/mineral (VM) and NP supplement use among those diagnosed with breast cancer, including usage based on tumor type or concurrent breast cancer treatments and primary information sources for specific supplements.
Methods
Social media recruiting to complete an online questionnaire self-reporting current VM and NP use and breast cancer diagnosis and treatment information primarily attracted US participants. Analyses, including multivariate logistic regression, were performed on 1271 women who self-reported breast cancer diagnosis and completed the survey.
Results
Most participants reported current VM (89.5%) and NP (67.7%) use, with 46.5% (VM) and 26.7% (NP) using at least 3 products concurrently. Top-reported (>15% prevalence) products were vitamin D, calcium, multivitamin, and vitamin C for VM and probiotics, turmeric, fish oil/omega-3 fatty acids, melatonin, and cannabis for NP. Overall, VM or NP use was higher among those with hormone receptor-positive tumors. Although overall NP use did not differ according to current breast cancer treatments, VM use was significantly less common among those currently undergoing chemotherapy or radiation, but higher with current endocrine therapy. Among current chemotherapy users, specific VM and NP supplements with possible adverse effects were still used by 23% of respondents. Medical providers were the primary information source for VM, whereas NP information sources were more varied.
Conclusions
Because women diagnosed with breast cancer commonly reported concurrent use of multiple VM and NP supplements, including those with known or underexplored risks (or benefits) in breast cancer, it is important for health care providers to inquire about and facilitate discussions regarding supplement use in this population.
Keywords: botanical, breast cancer, chemotherapy, dietary supplement, endocrine therapy, hormone receptor, mineral, natural product, vitamin
Introduction
Breast cancer accounts for almost one-third of cancer diagnoses in women in the United States and is a leading cause of cancer death in women [1]. One in 8 women will develop breast cancer in their lifetime, mostly after the age of 50 y [1, 2]. US population-based surveys have demonstrated that use of dietary supplements (DSs), which include vitamins, minerals, and natural product (NP)-derived supplements (e.g., fish oil), is higher in women than men, increases with age, and is higher in adult cancer survivors as compared with those without cancer [[3], [4], [5]]. Among survivors of all cancer types, DS use is highest in women with breast cancer [6].
Because of the high incidence of supplement use by women with breast cancer, it is important to consider potential population-specific risk/benefit profiles associated with supplement use due to breast cancer itself, as well as associated treatments. Evidence of efficacy and safety in breast cancer is sparse for many products [7, 8], particularly for nonvitamin, nonmineral, NP-derived DSs. However, population-based associations with risk have been characterized for some supplements. For example, concurrent use of certain vitamin/mineral (VM) DSs, including antioxidants, during breast cancer chemotherapy has been reported to be associated with increased breast cancer recurrence and/or decreased disease-free or overall survival [9].
Thus, because of unclear benefits and potential risks, it is important for health care providers to be aware of supplement use by their patients to enable counseling and monitoring for possible effects on disease and treatment outcomes. Unfortunately, communication between providers and patients regarding supplement use is often inadequate, such that providers are often unaware of their patients’ DS use, with breast cancer populations being no exception [[10], [11], [12], [13], [14], [15], [16], [17]]. Supplement use can also be dynamic, including during the course of breast cancer diagnosis and treatment, where usage patterns have been documented to change within months of diagnosis [18], including supplement use to treat cancer-related symptoms [19]. Possibly consistent with this observation, none of the breast cancer patients in a recent study had a DS list within their medical record that accurately reflected their current use [12]. Adding to this information gap, published population-based data reflecting current supplement use by those diagnosed with breast cancer has primarily focused on VM, such that comprehensive information on current NP use is not readily available [3, 5].
Increased awareness of health care providers regarding use of both VM and NP supplements among women with breast cancer would clearly benefit patient care; thus, this study was developed to evaluate current population-based patterns of VM and NP DS use by those with breast cancer, including primary source of information when choosing to use a supplement and whether usage patterns differed depending on tumor type or concurrent treatments.
Methods
Survey administration
This study, which complies with the Helsinki Declaration, was approved by the Institutional Review Board at the University of Arizona (IRB # 1710898912). The questionnaire was completed anonymously online via a custom link through Research Electronic Data Capture, a software platform used for survey development and data management [20]. Participants were provided with information to allow for implied consent before beginning the online survey. The criteria for initiating survey completion were prior diagnosis of breast cancer and first attempt at completing the survey. The survey was administered solely online, thus geography did not limit recruitment.
Recruitment
Recruitment efforts were mainly through social media including Facebook advertisements, Twitter posts, and online posts in breast cancer support or interest groups. A Facebook page was also created and available to provide additional information about the survey. Facebook advertisements were developed to target individuals with breast cancer specifically. Initially, we targeted women living in the United States aged >40 y who had interests in nonprofit organizations, breast cancer care, or health. After noting a limited response from non-White participants, we further refined our advertisements to target a more diverse group of participants. For example, some advertisements targeted the same parameters as previously mentioned with an added interest in Hispanic culture, including Spanish-language advertisements that linked to a Spanish-language version of the online survey, translated by a translation service (CyraCom International) with additional feedback from Spanish-speaking health care providers. We also had support from the University of Arizona, which posted information about our survey on its various platforms.
Survey design
An online questionnaire, similar in design to a survey previously used to assess DS use in rheumatoid arthritis [11], was developed to capture information related to supplement use for participants with a previous breast cancer diagnosis. Before launch, the survey was reviewed by women who were health care providers and/or breast cancer survivors for layout, clarity, and interpretation, with modifications made based on feedback. The survey queried current use of specific itemized supplements that were curated based on prior publications related to recommended breast cancer supplement use, particularly for NP supplements [7, [21], [22], [23]], as well as interviews with several Arizona-based breast cancer health care providers (i.e., university cancer center-based breast cancer oncologist, primary care physician involved in integrative oncology, registered dietitian, and breast cancer nurse navigator; community-based naturopaths; and health food store “health specialists”). In addition, the survey allowed for write-in responses to account for all supplements currently in use. For supplements with multiple known modalities of administration, participants were asked to select oral, injectable, infusion, and/or diet/culinary. In addition, participants were asked to identify a primary source of information when choosing to take a specific supplement, selecting health care provider (e.g., doctor, nurse, and dietitian), alternative medicine practitioner (e.g., naturopath, acupuncturist), family/friends, internet/social media, or other. Information on the respondents’ breast cancer diagnosis and treatment (initial and current) was also collected. All information, including breast cancer diagnosis, was self-reported.
Study sample
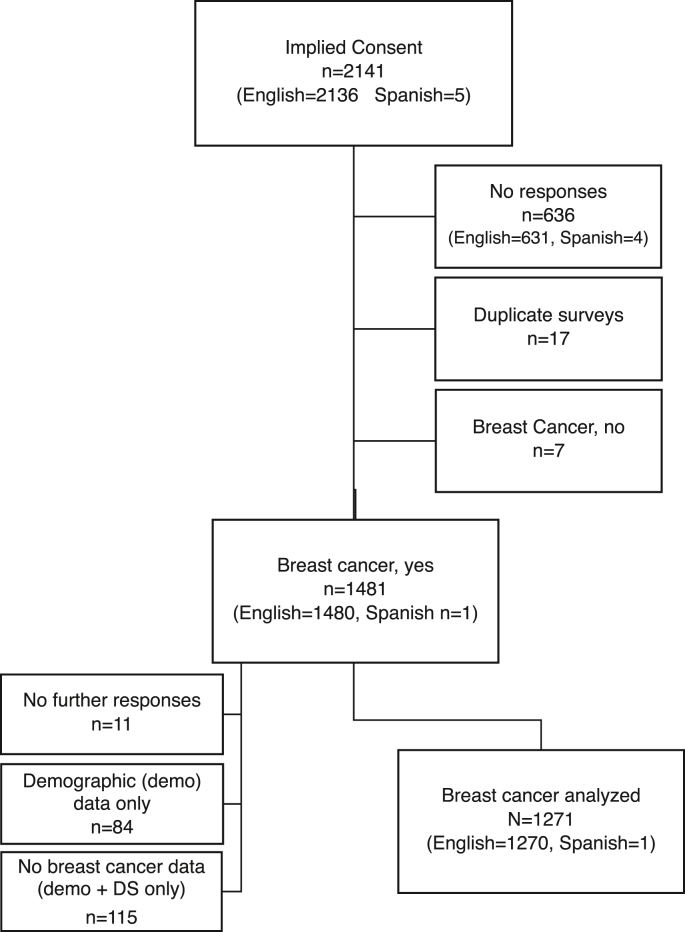
A total of 2141 responses with implied consent were collected over 12 mo (June 2020 to May 2021; Spanish language survey was available from January 2021 to May 2021), as defined by advancing beyond initial implied consent information (Figure 1). Incomplete surveys lacking data beyond implied consent (n = 636), duplicate surveys (n = 17), and those not reporting a breast cancer diagnosis (n = 7) were excluded from analysis. In addition, participants self-reporting a breast cancer diagnosis, but providing: 1) no further responses (n = 11), 2) demographic data without information related to clinical breast cancer or DS use (n = 84), or 3) demographic and supplement use information but no clinical breast cancer data (n = 115) were also excluded from analysis. Among those reporting demographic data only, 8% of participants were Hispanic and 15% were non-White/non-Hispanic. There were 5 total responses to the Spanish version of the survey, four of which were excluded due to incomplete responses. In total, 870 responses were excluded, with a final analyzed sample of 1271 surveys from participants who self-reported a breast cancer diagnosis and provided responses to demographic, clinical, and supplement usage queries.
FIGURE 1.
Study Consolidated Standards of Reporting Trials (CONSORT) diagram. The chart indicates the disposition of all participants who advanced beyond implied consent to initiate the survey (n = 2141). Of those self-reporting a breast cancer diagnosis (n = 1481), 210 (14%) were eliminated because of incomplete demographic (demo), clinical breast cancer, and/or dietary supplement (DS) information, yielding 1271 participants for analysis.
Data analysis and statistical methods
When compiling data for analyses, geographic residence was categorized as non-US compared with US, with US data further reported by region as defined by the US Census Bureau (West, South, Midwest, Northeast) [24]. Hormone receptor-positive (HR+) breast cancer was defined according to self-report of estrogen receptor-positive (ER+) and/or progesterone receptor–positive (PR+) subtype, for which ER−/PR+ is rare (1%) [25], and/or endocrine therapy treatment (initial and/or current). Advanced disease was defined as initially presenting with stage IV cancer or subsequent recurrence. Prevalence of overall VM or NP supplement use was determined by including all itemized and write-in supplements. Data for any write-in product with >5% prevalence of use were compiled and specifically reported.
Participant characteristics were summarized using mean ± SD or n (%). Associations between participant characteristics and individual supplements were tested using logistic regression. Multivariate models adjusted for age, geographic region, and race as confounders. Associations between source of information and current chemotherapy status were tested using chi-square tests. Source of information for VMs compared with NPs overall was tested using logistic regression, clustered on participant. No imputation was performed for missing data, and no corrections were made for multiple comparisons. All statistical analyses, with significance set at the p < 0.05 level, were performed using Stata 17.0 (StataCorp).
Results
Participant characteristics
All participants were female with an average age of 62 y and the majority being non-Hispanic White (Table 1). Most participants resided in the US (96.1%), with a regional distribution generally consistent with United States census data for adults over the age of 18 y [24]. Most (79.8%) individuals reported HR+ tumors, and 19.4% reported HER2+ tumors. Only 6.5% reported a known breast cancer gene 1 or 2 (BRCA1/2) mutation. Lymph node involvement at initial presentation was reported by 22.6%, with metastatic disease at initial diagnosis reported by 2.4% and subsequent recurrence by 13.5%, of whom only 29.7% reported local recurrence limited to the breast. Many participants reported ever-treatment with radiation (58.3%), chemotherapy (50.7%), and/or endocrine therapy (63.0%). Current radiation treatment at the time of survey was reported by only 7.3%. Current chemotherapy was reported by 14.6%, with current endocrine therapy reported by 52.0%, consistent with the relatively short average time since diagnosis (4.4 y). Among those currently using endocrine therapy, 81.9% reported aromatase inhibitor (AI) use. Only 13.8% reported current use of an antiresorptive bone protective drug; among those currently on endocrine therapy, concurrent use of an antiresorptive drug was reported by 19.3%.
TABLE 1.
Demographic and clinical characteristics1 of survey respondents with self-reported breast cancer
| Characteristic | Mean ± SD or n (%) |
|---|---|
| Demographic | |
| Age at time of survey (y) | 62.0 ± 10.1 |
| Female sex | 1262 (100) |
| Hispanic ethnicity | 67 (5.3) |
| Race | |
| White | 1100 (87.5) |
| African American | 66 (5.3) |
| Native American | 12 (1.0) |
| Asian/Pacific Islander | 18 (1.4) |
| Other | 61 (4.9) |
| Geographic region | |
| West | 257 (23.9) |
| South | 395 (36.7) |
| Midwest | 204 (18.9) |
| Northeast | 174 (16.2) |
| Other USA | 5 (0.5) |
| Outside USA | 42 (3.9) |
| Clinical | |
| Age at diagnosis (y) | 57.5 ± 10.4 |
| Time since diagnosis (y) | 4.4 ± 6.3 |
| HR+ | 960 (79.8) |
| HER2+ | 203 (19.4) |
| BRCA1/2 mutation | 82 (6.5) |
| Lymph nodes involved at diagnosis | 283 (22.6) |
| Metastatic at diagnosis | 30 (2.4) |
| Recurrence | 165 (13.5) |
| Radiation (ever) | 704 (58.3) |
| Chemotherapy (ever) | 610 (50.7) |
| Current chemotherapy | 178 (14.6) |
| Endocrine therapy (ever) | 758 (63.0) |
| Current endocrine therapy | 636 (52.0) |
| Current bone protective drug | 169 (13.8) |
Missing data: age (4.2%), sex (0.7%), ethnicity (0.9%), race (1.1%), geographic region (15.3%), age at diagnosis (2.2%), time since diagnosis (5.0%), hormone receptor-positive (HR+) (5.4%), human epidermal growth factor receptor 2-positive (HER2+) (17.6%), breast cancer gene 1 or 2 [BRCA1/2] mutation (0.6%), surgery (4.5%), radiation (5.0%), chemotherapy (5.3%), current chemotherapy (3.9%), endocrine therapy (5.4%), current endocrine therapy (3.9%), and current bone protective drug (3.9%).
Current DS products used
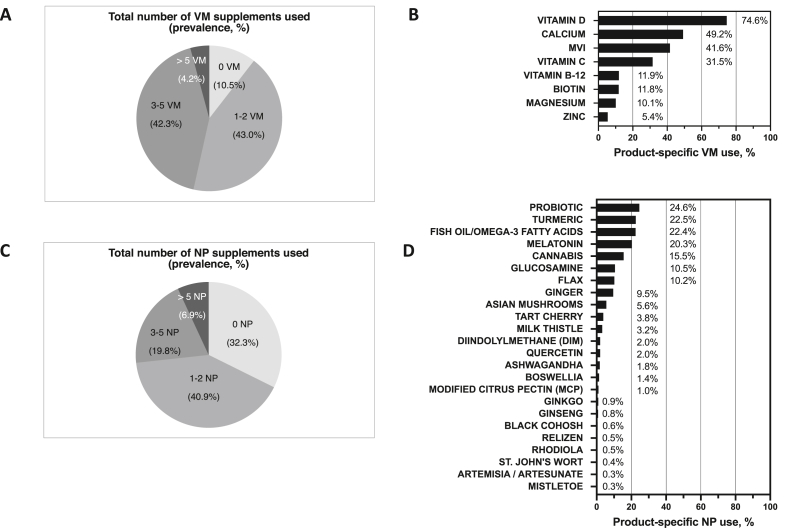
Current VM use was reported by most participants (89.5%), with 46.5% using ≥3 VM products (Figure 2A). The 8 most reported VM products (prevalence > 5%) were vitamin D, calcium, multivitamin (MVI), vitamin C, vitamin B12, biotin, magnesium, and zinc (Figure 2B). Only 14 participants reported injectable VM treatment (n = 11, B12 only; n = 2, vitamin C only; n = 1, vitamin C, B12, and B6). Overall, VM use did not vary by geographic region or by ethnicity, but use increased with age and differed by race, being significantly lower in African American participants (Table 2). In particular, calcium use was significantly lower among African Americans as compared with White participants (ORadj: 0.5; P = 0.02) (Supplemental Table 1).
FIGURE 2.
Dietary supplement use by breast cancer survivors by product type (n = 1271 participants). (A) Total number of vitamin/mineral (VM) supplements used and (B) prevalence of product-specific VM use, including products itemized in survey or write-in products with >5% prevalent use (product-specific missing data ranged from 0.6% to 2.6%). (C) Total number of non-VM, natural product (NP) supplements used and (B) prevalence of product-specific NP use, including products itemized in survey or write-in products with >5% prevalent use (product-specific missing data ranged from 0.1% to 1.8%).
TABLE 2.
Association between participant characteristics and vitamin/mineral (VM) or nonvitamin/mineral, natural product (NP) supplement use
| Characteristic | Any VM supplements |
Any NP supplements |
||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age (per 5 y) | 1.20 (1.10–1.32) | < 0.001 | 0.99 (0.94–1.05) | 0.847 |
| Time since dx (per y) | 1.01 (0.98–1.04) | 0.600 | 0.98 (0.97–1.00) | 0.117 |
| USA region | ||||
| West | 1.0 (ref.) | 1.0 (ref.) | ||
| South | 0.76 (0.45–1.30) | 0.320 | 0.55 (0.39–0.78) | < 0.001 |
| Midwest | 0.74 (0.40–1.35) | 0.322 | 0.71 (0.47–1.08) | 0.109 |
| Northeast | 0.91 (0.47–1.75) | 0.773 | 0.50 (0.33–0.76) | 0.001 |
| Race | ||||
| White | 1.0 (ref.) | 1.0 (ref.) | ||
| African American | 0.47 (0.25–0.92) | 0.026 | 0.56 (0.34–0.93) | 0.025 |
| Native American | 0.32 (0.08–1.19) | 0.088 | 0.45 (0.14–1.41) | 0.171 |
| Asian/Pacific Islander | 0.84 (0.19–3.72) | 0.823 | 1.58 (0.52–4.83) | 0.423 |
| Other | 0.54 (0.27–1.09) | 0.086 | 0.75 (0.44–1.27) | 0.280 |
| Ethnicity | ||||
| Not Hispanic/Latina | 1.0 (ref.) | 1.0 (ref.) | ||
| Hispanic/Latina | 0.99 (0.44–2.20) | 0.971 | 0.84 (0.50–1.40) | 0.502 |
| HR+ | ||||
| Negative | 1.0 (ref.) | 1.0 (ref.) | ||
| Positive | 2.50 (1.66–3.76) | < 0.001 | 1.53 (1.14–2.05) | 0.005 |
| HER2+ | ||||
| Negative | 1.0 (ref.) | 1.0 (ref.) | ||
| Positive | 0.71 (0.43–1.15) | 0.163 | 0.87 (0.63–1.21) | 0.421 |
| Chemotherapy (ever) | ||||
| No | 1.0 (ref.) | 1.0 (ref.) | ||
| Yes | 0.71 (0.48–1.04) | 0.078 | 0.91 (0.71–1.15) | 0.425 |
| Current chemotherapy | ||||
| No | 1.0 (ref.) | 1.0 (ref.) | ||
| Yes | 0.42 (0.27–0.65) | < 0.001 | 0.75 (0.54–1.04) | 0.086 |
| Endocrine therapy (ever) | ||||
| No | 1.0 (ref.) | 1.0 (ref.) | ||
| Yes | 2.90 (1.97–4.27) | < 0.001 | 1.44 (1.12–1.85) | 0.004 |
| Current endocrine therapy | ||||
| No | 1.0 (ref.) | 1.0 (ref.) | ||
| Yes | 2.60 (1.75–3.86) | < 0.001 | 1.25 (0.99–1.59) | 0.066 |
| Radiation (ever) | ||||
| No | 1.0 (ref.) | 1.0 (ref.) | ||
| Yes | 1.08 (0.74–1.57) | 0.706 | 0.92 (0.72–1.17) | 0.481 |
| Advanced disease | ||||
| No | 1.0 (ref.) | 1.0 (ref.) | ||
| Yes | 1.35 (0.77–2.37) | 0.296 | 1.06 (0.76–1.48) | 0.731 |
1 HER2+, human epidermal growth factor receptor 2-positive; HR+, hormone receptor-positive (HR+).
Current NP use was also reported by most participants (67.7%), with 26.7% using ≥3 NP products (Figure 2C). The nine most reported NP products (prevalence > 5%) were probiotics, turmeric, fish oil/omega-3 fatty acids, melatonin, cannabis, glucosamine and/or chondroitin, flax, ginger, and Asian mushrooms (Figure 2D). Cannabis content (e.g., tetrahydrocannabinol [THC] compared with cannabidiol [CBD]) was not queried, and 8.0% of cannabis users reported topical use only (no ingestion). Only two participants reported injectable NP treatment (mistletoe). Overall, NP use did not vary by age or ethnicity, but, as with VM products, was significantly less common among African American participants (Table 2). In particular, probiotics (ORadj: 0.4; P = 0.046) and melatonin (ORadj: 0.3; P = 0.009) were less commonly used by African Americans, whereas ginger use was significantly higher (ORadj: 3.5; P < 0.001), compared with White participants (Supplemental Table 1). Unique to NP (compared with VM) were regional differences in overall use, which was less commonly reported among participants living in the South (ORadj: 0.55; P < 0.001) or Northeast (ORadj: 0.50, P = 0.001), as compared with the West (Table 2). Use of turmeric and fish oil/omega-3 fatty acids, specifically, was significantly lower in the South and Northeast, adjusted for age and race (Supplemental Table 2). Southern participants reported lower use of 4 additional “top-nine” NP products (cannabis, flax, ginger, and Asian mushrooms), whose use was also significantly lower in the Midwest (except flax). The only top-nine NPs without regional differences were probiotics, melatonin, and glucosamine.
Supplement use by clinical breast cancer characteristics and treatment
Overall, VM use was 2.5 times higher (P < 0.001) among those with HR+ tumors, but VM use did not differ by HER2 tumor status (Table 2). In particular, use of vitamin D, calcium, and biotin was all significantly higher in participants with HR+ breast cancer (Table 3). Overall, VM use did not vary with time since breast cancer diagnosis, advanced disease, or ever-treatment with chemotherapy or radiation (Table 2). However, VM use was significantly less common among those currently undergoing chemotherapy or radiation and significantly more common for those currently on endocrine therapy (Table 2), with vitamin D and calcium use, specifically, being highest among those with HR+ tumors and concurrent endocrine therapy (Table 4). Use of vitamin D, calcium, MVI, and vitamin C or other antioxidants was significantly less common among participants currently undergoing chemotherapy compared with those not currently on chemotherapy (Table 5). Among those currently undergoing radiation treatment, vitamin D use was significantly less (ORadj: 0.4; P < 0.001), whereas calcium use was marginally lower (ORadj: 0.6; P = 0.055), compared with those not currently undergoing radiation treatment.
TABLE 3.
Supplement use according to hormone receptor (HR) tumor status
| Supplement | HR–, n (%) | HR+, n (%) | Crude OR (95% CI) | Adjusted1 OR (95% CI) | P value |
|---|---|---|---|---|---|
| Vitamin/mineral (VM) | |||||
| Vitamin D | 156 (65.0) | 744 (78.2) | 1.9 (1.4–2.6) | 2.0 (1.4–2.8) | <0.001 |
| Calcium | 82 (34.2) | 516 (54.4) | 2.3 (1.7–3.1) | 2.2 (1.6–3.2) | <0.001 |
| Multivitamin | 97 (40.1) | 404 (42.5) | 1.1 (0.8–1.5) | 1.1 (0.8–1.5) | 0.692 |
| Vitamin C | 68 (28.8) | 301 (32.1) | 1.2 (0.9–1.6) | 1.3 (0.9–1.9) | 0.181 |
| Vitamin B12 | 22 (9.1) | 123 (12.9) | 1.5 (0.9–2.4) | 1.4 (0.8–2.4) | 0.196 |
| Biotin | 18 (7.4) | 128 (13.4) | 1.9 (1.2–3.2) | 2.0 (1.1–3.7) | 0.023 |
| Magnesium | 16 (6.6) | 111 (11.6) | 1.9 (1.1–3.2) | 1.7 (0.9–3.1) | 0.087 |
| Zinc | 11 (4.5) | 57 (6.0) | 1.3 (0.7–2.6) | 1.0 (0.5–2.1) | 0.987 |
| Natural Product (NP) | |||||
| Probiotic | 40 (16.6) | 256 (26.9) | 1.8 (1.3–2.7) | 1.9 (1.2–2.9) | 0.003 |
| Turmeric | 60 (25.0) | 211 (22.1) | 0.9 (0.6–1.2) | 0.8 (0.6–1.2) | 0.297 |
| Fish oil/omega-3 fatty acids | 49 (20.2) | 225 (23.7) | 1.2 (0.9–1.7) | 1.1 (0.8–1.6) | 0.595 |
| Melatonin | 38 (15.8) | 207 (21.8) | 1.5 (1.0–2.2) | 1.5 (1.0–2.3) | 0.067 |
| Cannabis | 30 (12.6) | 156 (16.4) | 1.4 (0.9–2.1) | 1.2 (0.8–2.0) | 0.393 |
| Glucosamine 2 | 20 (8.3) | 108 (11.4) | 1.4 (0.9–2.3) | 1.6 (0.9–3.0) | 0.128 |
| Flax | 13 (5.4) | 110 (11.6) | 2.3 (1.3–4.1) | 2.6 (1.3–5.1) | 0.007 |
| Ginger | 25 (10.4) | 88 (9.2) | 0.9 (0.5–1.4) | 1.0 (0.6–1.7) | 0.998 |
| Asian mushrooms | 9 (3.8) | 59 (6.2) | 1.7 (0.8–3.5) | 2.0 (0.8–4.8) | 0.140 |
HR+, hormone receptor-positive.
Adjusted for age, geographic region, and race.
With or without chondroitin.
TABLE 4.
Supplement use according to current endocrine therapy (ET) among participants with hormone receptor (HR)+ tumor status
| Supplement | No current ET, n (%) | Current ET, n (%) | Crude OR (95% CI) | Adjusted1 OR (95% CI) | P value |
|---|---|---|---|---|---|
| Vitamin/mineral (VM) | |||||
| Vitamin D | 217 (72.1) | 512 (81.3) | 1.7 (1.2–2.3) | 1.8 (1.3–2.7) | 0.001 |
| Calcium | 126 (42.0) | 379 (60.4) | 2.1 (1.6–2.8) | 2.4 (1.7–3.3) | < 0.001 |
| Multivitamin | 129 (42.7) | 265 (42.3) | 1.0 (0.7–1.3) | 1.1 (0.8–1.6) | 0.442 |
| Vitamin C | 99 (33.3) | 194 (31.2) | 0.9 (0.7–1.2) | 0.9 (0.7–1.3) | 0.722 |
| Vitamin B12 | 41 (13.5) | 80 (12.7) | 0.9 (0.6–1.4) | 1.0 (0.6–1.6) | 0.977 |
| Biotin | 32 (10.6) | 93 (14.7) | 1.5 (1.0–2.2) | 1.4 (0.9–2.3) | 0.173 |
| Magnesium | 28 (9.2) | 79 (12.5) | 1.4 (0.9–2.2) | 1.4 (0.8–2.2) | 0.223 |
| Zinc | 18 (5.9) | 39 (6.2) | 1.0 (0.6–1.9) | 1.0 (0.5–1.9) | 0.946 |
| Natural Product (NP) | |||||
| Probiotic | 85 (28.1) | 166 (26.3) | 0.9 (0.7–1.2) | 0.9 (0.6–1.3) | 0.611 |
| Turmeric | 70 (23.1) | 136 (21.6) | 0.9 (0.7–1.3) | 0.9 (0.6–1.3) | 0.523 |
| Fish oil/omega-3 fatty acids | 60 (19.8) | 161 (25.7) | 1.4 (1.0–2.0) | 1.6 (1.1–2.4) | 0.016 |
| Melatonin | 66 (21.8) | 136 (21.7) | 1.0 (0.7–1.4) | 1.1 (0.8–1.7) | 0.491 |
| Cannabis | 60 (19.8) | 93 (14.8) | 0.7 (0.5–1.0) | 0.6 (0.4–0.9) | 0.012 |
| Glucosamine2 | 28 (9.3) | 78 (12.4) | 1.4 (0.9–2.2) | 1.7 (1.0–2.9) | 0.065 |
| Flax | 46 (15.2) | 59 (9.4) | 0.6 (0.4–0.9) | 0.5 (0.3–0.8) | 0.006 |
| Ginger | 31 (10.2) | 57 (9.0) | 0.9 (0.6–1.4) | 0.7 (0.4–1.2) | 0.162 |
| Asian mushrooms | 24 (8.0) | 34 (5.4) | 0.7 (0.4–1.1) | 0.6 (0.3–1.1) | 0.116 |
Adjusted for age, geographic region, and race.
With or without chondroitin.
TABLE 5.
Supplement use according to current chemotherapy treatment
| Supplement | No current chemo, n (%) | Current chemo, n (%) | Crude OR (95% CI) | Adjusted1 OR (95% CI) | P Value |
|---|---|---|---|---|---|
| Vitamin/mineral (VM) | |||||
| Vitamin D | 791 (76.7) | 113 (63.5) | 0.5 (0.4–0.7) | 0.5 (0.4–0.8) | <0.001 |
| Calcium | 531 (51.6) | 68 (38.4) | 0.6 (0.4–0.8) | 0.6 (0.4–0.8) | 0.002 |
| Multivitamin | 446 (43.2) | 60 (33.9) | 0.7 (0.5–0.9) | 0.7 (0.4–0.9) | 0.025 |
| Vitamin C5 | 336 (33.1) | 34 (19.3) | 0.5 (0.3–0.7) | 0.5 (0.3–0.8) | 0.002 |
| Other antioxidant2,5 | 84 (8.0) | 6 (3.4) | 0.4 (0.2–0.9) | 0.4 (0.1–0.9) | 0.031 |
| Any antioxidant3,5 | 381 (36.5) | 40 (22.5) | 0.5 (0.3–0.7) | 0.5 (0.3–0.8) | 0.002 |
| Vitamin B125 | 130 (12.5) | 17 (9.6) | 0.7 (0.4–1.3) | 0.8 (0.4–1.4) | 0.358 |
| Biotin | 126 (12.1) | 17 (9.6) | 0.8 (0.4–1.3) | 0.7 (0.4–1.3) | 0.298 |
| Magnesium | 105 (10.1) | 19 (10.7) | 1.1 (0.6–1.8) | 0.9 (0.5–1.7) | 0.849 |
| Zinc | 56 (5.4) | 12 (6.7) | 1.3 (0.7–2.4) | 1.2 (0.6–2.4) | 0.690 |
| Iron5 | 17 (1.6) | 5 (2.8) | 1.7 (0.6–4.8) | 1.9 (0.7–5.2) | 0.241 |
| Natural Product (NP) | |||||
| Probiotic | 255 (24.6) | 44 (24.9) | 1.0 (0.7–1.5) | 1.0 (0.7–1.5) | 0.991 |
| Turmeric | 246 (23.7) | 29 (16.4) | 0.6 (0.4–1.0) | 0.6 (0.4–1.0) | 0.037 |
| Fish oil/omega-3 fatty acids5 | 254 (24.5) | 22 (12.4) | 0.4 (0.3–0.7) | 0.5 (0.3–0.8) | 0.003 |
| Any omega-3 fatty acids4,5 | 310 (29.7) | 27 (15.2) | 0.4 (0.3–0.7) | 0.4 (0.3–0.7) | < 0.001 |
| Melatonin | 215 (20.8) | 32 (18.1) | 0.8 (0.6–1.3) | 1.0 (0.6–1.5) | 0.927 |
| Cannabis | 150 (14.5) | 39 (22.2) | 1.7 (1.1–2.5) | 1.5 (1.0–2.4) | 0.060 |
| Glucosamine6 | 122 (11.8) | 8 (4.5) | 0.4 (0.2–0.7) | 0.4 (0.2–0.9) | 0.020 |
| Flax5 | 110 (10.6) | 11 (6.2) | 0.6 (0.3–1.1) | 0.5 (0.2–1.0) | 0.058 |
| Ginger | 97 (9.3) | 20 (11.4) | 1.2 (0.7–2.1) | 1.3 (0.8–2.3) | 0.305 |
| Asian mushrooms | 60 (5.8) | 8 (4.6) | 0.8 (0.4–1.7) | 0.8 (0.4–1.9) | 0.630 |
Adjusted for age, geographic region, and race.
Vitamin A, vitamin E, carotenoids, and/or coenzyme Q10.
Vitamin C and/or other antioxidants.
Fish oil/omega-3 fatty acids, and/or flax.
Concurrent use with breast cancer chemotherapy associated with decreased survival and/or increased recurrence.
With or without chondroitin.
As with VM, overall NP use was higher (1.5-fold) among those with HR+ breast cancer, but it did not differ by HER2 tumor status (Table 2). In particular, use of both probiotics and flax was significantly higher in participants with HR+ tumors (Table 3). Overall, NP use did not differ with time since breast cancer diagnosis, advanced disease, or first-ever use of chemotherapy or radiation (Table 2). However, among those with HR+ tumors currently using endocrine therapy, fish oil/omega-3 fatty acids usage was more prevalent, whereas flax and cannabis use was significantly lower, than among participants with HR+ tumors without current endocrine therapy (Table 4). Use of certain NP products was also less prevalent in current chemotherapy users, including turmeric, omega-3 fatty acid-containing products, and glucosamine (Table 5). Interestingly, use of cannabis or ginger, two products with potential anti-nausea effects, was not significantly higher in current chemotherapy users (Table 5). Among those currently undergoing radiation treatment, cannabis use was marginally higher (ORadj: 1.8; P = 0.050), and among those with advanced disease, use of both cannabis (ORadj: 2.1; P < 0.001) and ginger (ORadj: 1.8; P = 0.041) was significantly increased.
Concurrent use of certain VM and NP supplements (antioxidants, including vitamin C; omega-3 fatty acid-containing products, including fish oil and flax; vitamin B12; and iron) with breast cancer chemotherapy has been reported to be associated with increased recurrence and/or decreased survival [9]. In the current study, among those reporting current chemotherapy, antioxidant or omega-3 fatty acid-containing product use were significantly lower, whereas B12 and iron use was no different (Table 5). Use of turmeric, an NP product reported to have potential adverse effects when taken concurrently with breast cancer chemotherapy [26, 27], was also significantly lower in those currently reporting chemotherapy (Table 5). Of note, however, 23% of those undergoing chemotherapy still reported concurrent usage of supplements with possible adverse outcomes when combined with chemotherapy.
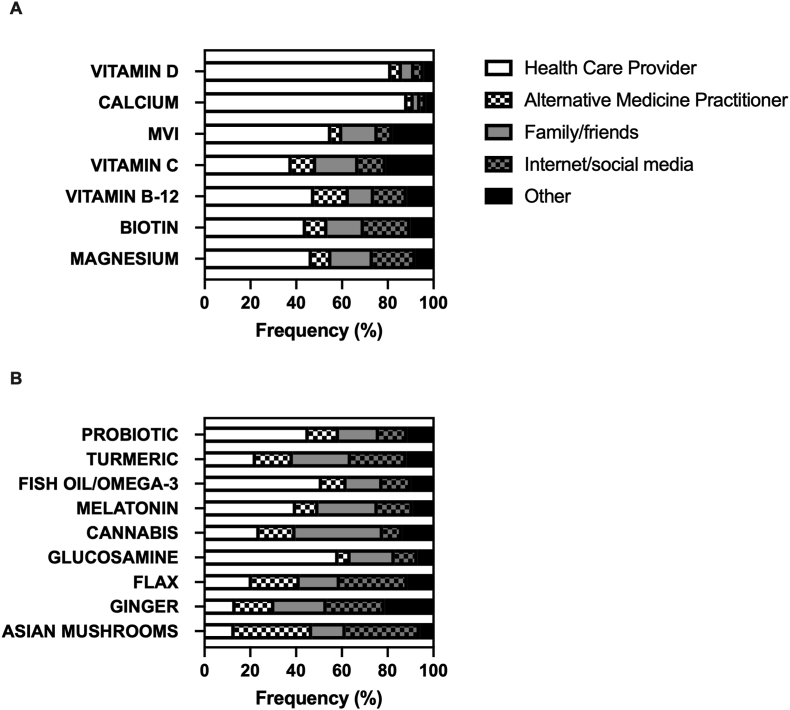
Primary information sources for supplement use by women with breast cancer Medical providers, including alternative medicine practitioners, were the most common source of information reported for each of the top VM (48.7%–91.4%) (Figure 3A). Family/friends and/or social media/internet were significantly more likely to be cited as a primary information source when choosing to use an NP, with an average prevalence (39.0%; 95% CI: 36.7%, 41.4%) almost double that reported for VM (20.7%; 95% CI: 19.3%, 22.0%; P < 0.001). Among the top-nine NPs (Figure 3B), nonmedical information sources were the most common for turmeric, cannabis, flax, ginger, or Asian mushrooms; however, health care providers were the most common reported source of information for the remaining four NP products: glucosamine (58.5%), fish oil/omega-3 fatty acids (51.4%), probiotics (45.5%), and melatonin (40.0%). Asian mushrooms were notable for having alternative medicine practitioners (33.8%) or internet/social media (32.4%) as a primary information source. Internet/social media was the most common source of information when deciding to use ginger (25.4%) or flax (29.6%). Cannabis was notable as the NP most likely to have family/friends as a primary information source (38.2%), with health care providers being the second-leading source (24.1%). Turmeric was notable for having information sources similarly distributed across all reported categories (health care providers, 22.5%; alternative medicine providers, 16.1%; family/friends 25.4%; internet/social media, 24.3%). Interestingly, among VM supplements whose concurrent use with chemotherapy has reported adverse associations [9], health care providers remained the most reported primary information source for vitamin C (48.5%), vitamin B12 (56.2%), and fish oil/omega-3 fatty acids (50.0%) for participants currently undergoing chemotherapy. Similarly, health care providers as a primary information source did not differ among participants currently undergoing chemotherapy for turmeric (27.6%), an NP with possible adverse effects when combined with taxanes [26, 27].
FIGURE 3.
Primary information source when choosing to take a dietary supplement. Product-specific distribution of the reported primary information source for supplement use, including health care providers (e.g., doctor, nurse, and dietitian) (white box); alternative medicine practitioners (e.g., naturopath and acupuncturist) (white box with checkers); family/friends (grey box); internet/social media (grey box with checkers); or other (black box). Note: “other” category frequently included reference to “self” and included other information sources, such as health food stores or books.
Discussion
Elucidation of population-based patterns of VM and NP supplement use in women diagnosed with breast cancer is clinically relevant given well-documented barriers to communication on this topic between individual patients and their providers [6, 28]. Most published data from the United States examining DS use in breast cancer are now a decade old and/or exclude detailed information regarding NP supplement use [5, 6, 9, 18]; thus, information from this study provides insights relevant to current practice.
For VM products, the best-documented supplement class in breast cancer populations, the high prevalence of overall use documented here (90%), which exceeds that of US adult women (63.8%) [4], and the identity of top VM products (including vitamin D, calcium, MVI, and vitamin C) largely agree with previous studies, albeit with differences in the current prevalence of product-specific use. Most notable was the high prevalence of vitamin D use reported here (75%), which exceeded other VMs, including MVI. This finding sharply contrasts with pre-2007 data, in which vitamin D is not even mentioned in a systematic review of VM supplement use in breast cancer [6] and data from a more recent 2014 study reporting a 44% prevalence of vitamin D use [18]. Although differences in study design could account for some variation, the totality of available data suggests a secular trend for increased vitamin D use in women diagnosed with breast cancer, as the data here are also consistent with results from a recent but much smaller (n = 50) breast cancer study by Silver et al. (82% prevalence) [12]. Conversely, MVI use by women diagnosed with breast cancer appears to have decreased over time, with pre-2007 and 2014 MVI use reported as ∼60% [6, 18], as compared with 42% prevalence reported here or 16% prevalence reported by Silver et al. [12]. When compared with recent National Health and Nutrition Examination Survey (NHANES) data for US adults over age 60 y, where MVI, vitamin D, and calcium are the most commonly reported VM supplements [4], prevalence of MVI use in breast cancer, as reported here, is similar to the general US population, whereas vitamin D and calcium use was more than twice as common.
Perhaps the most clinically important findings in this study relate to NP use because little has been published on this topic in the last decade, a period when NP sales in the US have changed dramatically, and available data on safe and effective NP use in breast cancer remain sparse [7, 11, 22]. The prevalence of fish oil/omega-3 fatty acids (22.4%) and glucosamine (10.5%) product use found here is like that reported in a similarly sized 2008 study of breast cancer patients enrolled in a chemotherapy trial (23% and 13%, respectively) [9]. In contrast, probiotic (24.6%) and melatonin (20.3%) use is much higher in the current study (compared with 3.2% or 2.6% prevalence, respectively, in 2008) [9], even among those currently on chemotherapy. Use of NP products not previously appearing in population-wide breast cancer supplement use surveys [9], including turmeric (22.5%), ginger (9.5%), and Asian mushrooms (5.6%), is now more frequent.
One strength of the current study was the detailed listing of specific NP products of possible relevance to those diagnosed with breast cancer to query use and provide examples, followed by open-ended queries to elicit information on additional products. Though this method could have simply generated a more comprehensive summary of supplement use, it is more likely that differences in top NP products used by women diagnosed with breast cancer, as reported here, reflect secular changes, since these same trends are also reflected in other populations. For example, US turmeric sales have increased exponentially over the past decade such that turmeric is now a top selling botanical product [11, 29], a subclass of NP supplements, and, analogous to its position as the most frequently used botanical NP in breast cancer documented here (23%), turmeric was also the most common botanical NP (30%) in a recent survey of supplement use in rheumatoid arthritis [11]. Similarly, probiotic use has been increasing over the past decade, quadrupling between 2007 and 2012, in parallel with increasing interest and information regarding the microbiome [30].
Simultaneous detailed reporting of VM and NP supplement use in a breast cancer population as well as associations of supplement use with tumor type and treatment are both unique features of this study; however, the study also has notable limitations. First, while self-reported supplement use questionnaires have been demonstrated previously to agree well with actual products used [31] and the questionnaire used here was vetted for clarity and format by women who were health care providers and/or breast cancer survivors, the survey instrument used here was not validated. Similarly, while other population-based surveys of supplement use in cancer survivors, such as NHANES [3], rely on self-reported cancer diagnoses, a similar reliance on self-reported diagnosis and additional clinical breast cancer information in this survey is a limitation. However, clinical breast cancer information reported by participants was detailed and consistent with a breast cancer diagnosis in those participants included in the analysis. In addition, clinical breast cancer characteristics reported here were generally reflective of US breast cancer populations; all participants were female, which appropriately represents the US breast cancer population as men account for less than 1% of cases, with a median age at diagnosis of 58 y, which is slightly younger than the national average of 62 y [32]. With respect to staging, self-reported metastatic disease at initial diagnosis was lower than the national average (2% compared with 6%, respectively), possibly reflecting survivor bias, whereas rates of local lymph node involvement were similar to national averages (23% compared with 27%). Prevalence of self-reported breast cancer subtypes for HR+ (79.8%) and HER2+ (19.4%) tumors was slightly higher than national averages (78% and 14%, respectively) [33], suggesting the possibility that women with more aggressive, less treatment-responsive, triple-negative breast cancer were underrepresented.
The self-selected nature of the cohort could have overrepresented individuals with an interest in VM and NP supplements. At the same time, using a freely available online questionnaire in the study design allowed for a simultaneous comparison of supplement use across the US, identifying regional differences in NP use that were also product specific, with participants residing in the West reporting the highest NP use and those in the South, the lowest. This study had low enrollment of Hispanic and non-White participants, despite attempts to better target these groups via Facebook advertisements, our primary recruitment modality, and similar reported rates of social media use by non-White or Hispanic US adults [34, 35]. Although many potential factors could have adversely influenced study recruitment, including higher breast cancer mortality rates among Hispanics and African Americans [36], it is notable that the proportion of Hispanic or non-White participants with breast cancer electing not to complete the survey (after starting) were similar to those that did. In addition, despite lower levels of recruitment of participants other than non-Hispanic Whites, significant differences in supplement use were still seen for African American participants, who were less-frequent users of VM supplements (specifically, calcium) and NP supplements, including probiotics and melatonin, whereas ginger use was significantly higher. These finding are consistent with lower reported VM and NP use by African Americans in the general US population and also, interestingly, with prevalent ginger use in a 1999 survey of supplement use by African American women diagnosed with breast cancer (fifth most commonly reported NP compared with second in this study) [37].
While reasons for supplement use were not queried here, the fact that vitamin D and calcium use was highest among current endocrine therapy users with HR+ tumors and primarily recommended by medical professionals suggests that mitigation of adverse treatment effects on bone was one likely reason for use, as recommended by professional organizations such as the American Society of Clinical Oncology and the National Comprehensive Cancer Network [38, 39]. In this regard, it is also interesting to note that a minority of those on current endocrine therapy reported concurrent treatment with an antiresorptive bone protective drug. In addition, as most current endocrine therapy users reported AI use [39], limited evidence of vitamin D amelioration of AI-associated arthralgias and myalgias may have also driven higher use in this subgroup [8, 39]. Since both symptom management and recurrence reduction are cited as common reasons for complementary and alternative medicine and/or supplement use in breast cancer [40, 41], increased use of certain NPs, such as cannabis, among those with advanced disease or currently undergoing radiation, may be attributable to symptom management. Reasons for differential use of other NP by tumor type and/or treatment (e.g., greater use of fish oil/omega-3 fatty acids among current endocrine therapy users with HR+ tumors) are less clear and require further investigation. Although previous studies have documented changes in supplement use after breast cancer diagnosis (e.g., initiation of calcium and vitamin D supplement use) [3, 18], it should be noted that the cross-sectional nature of the current study prevents analysis of diagnosis-driven changes in supplement use.
Lastly, product-specific primary information sources, as reported here, are of interest, particularly for supplements lacking a strong evidence base for safe use in a breast cancer population. As noted above, use of certain VM products, such as vitamin D, is recommended by expert panels, whereas existing evidence for other VM products suggests possible harm. It is thus encouraging that reported use of some, but not all, VM products with possible adverse effects when combined with chemotherapy (i.e., antioxidants and omega-3 fatty acid-containing products, but not iron or vitamin B12) was significantly lower in participants currently receiving chemotherapy. However, the prevalence of health care providers reported as a primary source of information for this potentially harmful combined use was not diminished in this group. Similarly, the importance of nonmedical sources—including friends, family, social media, and the internet—as a primary source of information for use of NP for which efficacy and safety information is often sparse or inconclusive is also a striking finding that emphasizes the importance of comprehensive communication between patients and providers, especially for products commonly used but less likely to have been provider recommended. For example, reports of hepatitis associated with turmeric, the most common botanical used by almost one-quarter of survey participants, have been emerging [42, 43], with ∼ 5% of participants in turmeric clinical trials reporting abnormalities in liver function testing [42]. Given the importance of liver function testing in surveillance for breast cancer recurrence, a thorough knowledge of patient supplement use and familiarity with basic information on commonly used supplements (e.g., turmeric in this case) is thus clearly needed to deliver optimal patient care.
In summary, given the high prevalence of use of VM and NP supplements, including those with possible adverse effects, open communication between providers and patients regarding supplement use is paramount to maximize benefit and minimize harm. Although beyond the scope of this study, systematic reviews of possible risks and benefits of commonly used NP (and VM) products identified here, as well as additional research on supplement interactions with standard breast cancer treatments and/or possible contributions to breast cancer therapy, would also help to optimize health outcomes for women diagnosed with breast cancer who choose to use supplements, particularly when combined with standard treatment regimens. Although much remains to be learned on best practice use of supplements in breast cancer, routine discussion of DS use and consideration of its possible effects should be a standard of care.
Funding
Funding provided by the NIH (CA023074 to JLF and JWB and AR078424 to JLF) and the University of Arizona Honors College (to AMR).
Author disclosures
The authors report no conflicts of interest.
Data Availability
Data described in the manuscript will be made available upon request pending application and approval.
Acknowledgments
The authors’ responsibilities were as follows – MH, AMR, HK, JLF: designed the research; MH, AMR, JLF: conducted the research; BCW analyzed data; MH, AMR, JLF: wrote the paper, with significant input from BCW, HK, JWB; JLF: had primary responsibility for the final content; and all authors read and approved the final manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tjnut.2022.12.007.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. 2022. [DOI] [PubMed] [Google Scholar]
- 2.Ellington T.D., Miller J.W., Henley S.J., Wilson R.J., Wu M., Richardson L.C. Trends in breast cancer incidence, by race, ethnicity, and age among women aged ≥20 years - United States, 1999-2018. MMWR Morb Mortal Wkly Rep. 2022;71(14):43–47. doi: 10.15585/mmwr.mm7102a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Du M., Luo H., Blumberg J.B., Rogers G., Chen F., Ruan M., et al. Dietary supplement use among adult cancer survivors in the United States. J Nutr. 2020;150(1):1499–1508. doi: 10.1093/jn/nxaa040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mishra S., Stierman B., Gahche J.J., Potischman N. 1–8. NCHS Data Brief; 2021. (Dietary supplement use among adults: United States, 2017-2018). [PubMed] [Google Scholar]
- 5.Li C., Hansen R.A., Chou C., Calderón A.I., Qian J. Trends in botanical dietary supplement use among US adults by cancer status: The National Health and Nutrition Examination Survey, 1999 to 2014. Cancer. 2018;124(15):1207–1215. doi: 10.1002/cncr.31183. [DOI] [PubMed] [Google Scholar]
- 6.Velicer C.M., Ulrich C.M. Vitamin and mineral supplement use among US adults after cancer diagnosis: a systematic review. J Clin Oncol. 2008;26(1):665–673. doi: 10.1200/JCO.2007.13.5905. [DOI] [PubMed] [Google Scholar]
- 7.Lyman G.H., Greenlee H., Bohlke K., Bao T., DeMichele A.M., Deng G.E., et al. Integrative therapies during and after breast cancer treatment: ASCO endorsement of the SIO Clinical Practice Guideline. J Clin Oncol. 2018;36(1):2647–2655. doi: 10.1200/JCO.2018.79.2721. [DOI] [PubMed] [Google Scholar]
- 8.Roberts K.E., Adsett I.T., Rickett K., Conroy S.M., Chatfield M.D., Woodward N.E. Systemic therapies for preventing or treating aromatase inhibitor-induced musculoskeletal symptoms in early breast cancer. Cochrane Database Syst Rev. 2022;1(10) doi: 10.1002/14651858.CD013167.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ambrosone C.B., Zirpoli G.R., Hutson A.D., McCann W.E., McCann S.E., Barlow W.E., et al. Dietary supplement use during chemotherapy and survival outcomes of patients with breast cancer enrolled in a Cooperative Group Clinical Trial (SWOG S0221) J Clin Oncol. 2020;38(10):804–814. doi: 10.1200/JCO.19.01203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zirpoli G.R., Brennan P.M., Hong C.C., McCann S.E., Ciupak G., Davis W., et al. Supplement use during an intergroup clinical trial for breast cancer (S0221) Breast Cancer Res Treat. 2013;137:903–913. doi: 10.1007/s10549-012-2400-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skiba M.B., Hopkins L.L., Hopkins A.L., Billheimer D., Funk J.L. Nonvitamin, Nonmineral dietary supplement use in individuals with rheumatoid arthritis. J Nutr. 2020;150(1):2451–2459. doi: 10.1093/jn/nxaa197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silver J., Goldenberg A., Moore A. Dietary supplement use and documentation in a breast cancer survivorship clinic. Breast Cancer Res Treat. 2022;191:385–388. doi: 10.1007/s10549-021-06454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeSalvo J.C., Skiba M.B., Howe C.L., Haiber K.E., Funk J.L. Natural Product dietary supplement use by individuals with rheumatoid arthritis: a scoping review. Arthritis Care Res (Hoboken). 2019;71:787–797. doi: 10.1002/acr.23696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saxe G.A., Madlensky L., Kealey S., Wu D.P., Freeman K.L., Pierce J.P. Disclosure to physicians of CAM use by breast cancer patients: findings from the Women's Healthy Eating and Living Study. Integr Cancer Ther. 2008;7:122–129. doi: 10.1177/1534735408323081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neuhouser M.L., Smith A.W., George S.M., Gibson J.T., Baumgartner K.B., Baumgartner R., et al. Use of complementary and alternative medicine and breast cancer survival in the Health, Eating, Activity, and Lifestyle Study. Breast Cancer Res Treat. 2016;160:539–546. doi: 10.1007/s10549-016-4010-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roumeliotis G.A., Dostaler G., Boyd K.U. Complementary and alternative medicines and patients with breast cancer: a case of mortality and systematic review of patterns of use in patients with breast cancer. Plast Surg (Oakv) 2017;25:275–283. doi: 10.1177/2292550317716126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yildirim Y. Patterns of the use of complementary and alternative medicine in women with metastatic cancer. Cancer Nurs. 2010;33:194–200. doi: 10.1097/NCC.0b013e3181c295ac. [DOI] [PubMed] [Google Scholar]
- 18.Greenlee H., Kwan M.L., Ergas I.J., Strizich G., Roh J.M., Wilson A.T., et al. Changes in vitamin and mineral supplement use after breast cancer diagnosis in the pathways study: a prospective cohort study. BMC Cancer. 2014;14(29):382. doi: 10.1186/1471-2407-14-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaur H., Hoenemeyer T., Parrish K.B., Demark-Wahnefried W. Dietary supplement use among older cancer survivors: socio-demographic associations, supplement types, reasons for use, and cost. Nutrients. 2022;14(18) doi: 10.3390/nu14163402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Informat. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lemanne D., Maizes V. Advising women undergoing treatment for breast cancer: a narrative review. J Altern Complement Med. 2018;24:902–909. doi: 10.1089/acm.2018.0150. [DOI] [PubMed] [Google Scholar]
- 22.Greenlee H., DuPont-Reyes M.J., Balneaves L.G., Carlson L.E., Cohen M.R., Deng G., et al. Clinical practice guidelines on the evidence-based use of integrative therapies during and after breast cancer treatment. CA Cancer J Clin. 2017;67(6):194–232. doi: 10.3322/caac.21397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Standish L.J., Dowd F., Sweet E., Dale L., Weaver M., Osborne B., et al. Breast cancer integrative oncology care and its costs. Integr Cancer Ther. 2017;16:85–95. doi: 10.1177/1534735416649034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.United States Census Bureau 2022. https://www2.census.gov/geo/pdfs/maps-data/maps/reference/us_regdiv.pdf [cited September 11, 2022]; Available from:
- 25.Ozguzer A., Ozguzer E. The smallest subtype in the SEER Database: estrogen receptor negative progesterone receptor positive breast cancer. World Cancer Res J. 2021;8:e1848. [Google Scholar]
- 26.Abdel-Razeq R., Iweir S., Awabdeh T., Barakat F., Abdel-Razeq H. Prolonged neutropenia and yellowish discoloration of the skin, but not the sclera, following excessive turmeric raw root ingestion. Cureus. 2021;13(29) doi: 10.7759/cureus.14754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalluru H., Mallayasamy S.R., Kondaveeti S.S., Chandrasekhar V., Kalachaveedu M. Effect of turmeric supplementation on the pharmacokinetics of paclitaxel in breast cancer patients: a study with population pharmacokinetics approach. Phytother Res. 2022;36:1761–1769. doi: 10.1002/ptr.7412. [DOI] [PubMed] [Google Scholar]
- 28.Escudero-Vilaplana V., Collado-Borrell R., Gómez Martínez-Sagrera P., Villanueva-Bueno C., Revuelta-Herrero J.L., Gonzalez-Haba E., et al. Complementary and alternative medicine in cancer patients: characteristics of use and interactions with antineoplastic agents. J Cancer Res Clin Oncol. 2022 doi: 10.1007/s00432-022-04172-1. Available from: [DOI] [PubMed] [Google Scholar]
- 29.Smith T., Eckl V., Morton Reynolds C. Herbal supplement sales in US increase by record-breaking 17.3% in 2020. Herbalgram. 2021:52–65. [Google Scholar]
- 30.Laborda-Illanes A., Sanchez-Alcoholado L., Dominguez-Recio M.E., Jimenez-Rodriguez B., Lavado R., Comino-Mendez I., et al. Breast and gut microbiota action mechanisms in breast cancer pathogenesis and treatment. Cancers (Basel) 2020;12(31) doi: 10.3390/cancers12092465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greenlee H., White E., Patterson R.E., Kristal A.R. Supplement use among cancer survivors in the Vitamins and Lifestyle (VITAL) study cohort. J Altern Complement Med. 2004;10:660–666. doi: 10.1089/acm.2004.10.660. [DOI] [PubMed] [Google Scholar]
- 32.American Cancer Society. Breast Cancer Facts & Figures [cited 9/13/2022]; Available from: https://www.cancer.org/research/cancer-facts-statistics/breast-cancer-facts-figures.html.
- 33.Surveillance, Epidemiology, and End Results Program (SEER), National Cancer Institute, National Institutes of Healh. Cancer Stat Facts: Female Breast Cancer Subtypes. [cited 9/16/2022]; Available from: https://seer.cancer.gov/statfacts/html/breast-subtypes.html.
- 34.Hudnut-Beumler J., Po'e E., Barkin S. The use of social media for health promotion in hispanic populations: a scoping systematic review. JMIR Public Health Surveill. 2016;2(11):e32. doi: 10.2196/publichealth.5579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pechmann C., Phillips C., Calder D., Prochaska J.J. Facebook Recruitment using zip codes to improve diversity in health research: longitudinal observational study. J Med Internet Res. 2020;22(5) doi: 10.2196/17554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Azin A., Tahmasebi H., Brar A., Azin S., Ko G., Covelli A., et al. Racial, ethnic and socioeconomic disparities in diagnosis, treatment, and survival of patients with breast cancer. Am J Surg. 2023;225:154–161. doi: 10.1016/j.amjsurg.2022.07.003. [DOI] [PubMed] [Google Scholar]
- 37.Bright-Gbebry M., Makambi K.H., Rohan J.P., Llanos A.A., Rosenberg L., Palmer J.R., et al. Use of multivitamins, folic acid and herbal supplements among breast cancer survivors: the black women's health study. BMC Complement Altern Med. 2011;11(15):30. doi: 10.1186/1472-6882-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Datta M., Schwartz G.G. Calcium and vitamin D supplementation and loss of bone mineral density in women undergoing breast cancer therapy. Crit Rev Oncol Hematol. 2013;88:613–624. doi: 10.1016/j.critrevonc.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hadji P., Aapro M.S., Body J.J., Gnant M., Brandi M.L., Reginster J.Y., et al. Management of Aromatase inhibitor-associated bone loss (AIBL) in postmenopausal women with hormone sensitive breast cancer: Joint position statement of the IOF, CABS, ECTS, IEG, ESCEO IMS, and SIOG. J Bone Oncol. 2017;7:1–12. doi: 10.1016/j.jbo.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weis J., Gschwendtner K., Güthlin C., Holmberg C., Horneber M. Utilisation of complementary medicine in cancer patients and survivors: Expected benefits and its association to psychosocial factors. Eur J Cancer Care (Engl). 2022;17 doi: 10.1111/ecc.13690. [DOI] [PubMed] [Google Scholar]
- 41.Conway R.E., Rigler F.V., Croker H.A., Lally P.J., Beeken R.J., Fisher A. Dietary supplement use by individuals living with and beyond breast, prostate, and colorectal cancer: a cross-sectional survey. Cancer. 2022;128(15):1331–1338. doi: 10.1002/cncr.34055. [DOI] [PubMed] [Google Scholar]
- 42.Lukefahr A.L., McEvoy S., Alfafara C., Funk J.L. Drug-induced autoimmune hepatitis associated with turmeric dietary supplement use. BMJ Case Rep. 2018;10:2018. doi: 10.1136/bcr-2018-224611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lombardi N., Crescioli G., Maggini V., Ippoliti I., Menniti-Ippolito F., Gallo E., et al. Acute liver injury following turmeric use in Tuscany: an analysis of the Italian Phytovigilance database and systematic review of case reports. Br J Clin Pharmacol. 2021;87:741–753. doi: 10.1111/bcp.14460. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript will be made available upon request pending application and approval.