Abstract
Background
Anthocyanins and carotenoids are phytochemicals that may benefit health through provitamin A carotenoid (PAC), antioxidant, and anti-inflammatory activities. These bioactives may mitigate chronic diseases. Consumption of multiple phytochemicals may impact bioactivity in synergistic or antagonistic manners.
Objectives
Two studies in weanling male Mongolian gerbils assessed the relative bioefficacy of β-carotene equivalents (BCEs) to vitamin A (VA) with simultaneous consumption of the non-PAC lycopene or anthocyanins from multicolored carrots.
Methods
After 3-wk VA depletion, 5–6 gerbils were killed as baseline groups. The remaining gerbils were divided into 4 carrot treatment groups; the positive control group received retinyl acetate and the negative control group was given vehicle soybean oil (n = 10/group; n = 60/study). In the lycopene study, gerbils consumed feed varying in lycopene sourced from red carrots. In the anthocyanin study, gerbils consumed feed varying in anthocyanin content sourced from purple-red carrots, and positive controls received lycopene. Treatment feeds had equalized BCEs: 5.59 ± 0.96 μg/g (lycopene study) and 7.02 ± 0.39 μg/g (anthocyanin study). Controls consumed feeds without pigments. Serum, liver, and lung samples were analyzed for retinol and carotenoid concentrations using HPLC. Data were analyzed by ANOVA and Tukey’s studentized range test.
Results
In the lycopene study, liver VA did not differ between groups (0.11 ± 0.07 μmol/g) indicating no effect of varying lycopene content. In the anthocyanin study, liver VA concentrations in the medium-to-high (0.22 ± 0.14 μmol/g) and medium-to-low anthocyanin (0.25 ± 0.07 μmol/g) groups were higher than the negative control (0.11 ± 0.07 μmol/g) (P < 0.05). All treatment groups maintained baseline VA concentrations (0.23 ± 0.06 μmol/g). Combining studies, serum retinol had 12% sensitivity to predict VA deficiency, defined as 0.7 μmol/L.
Conclusions
These gerbil studies suggested that simultaneous consumption of carotenoids and anthocyanins does not impact relative BCE bioefficacy. Breeding carrots for enhanced pigments to improve dietary intake should continue.
Keywords: anthocyanins, β-carotene, bioefficacy, carotenoids, carrot (Daucus carota), lycopene, vitamin A
Introduction
Fruit and vegetables provide essential nutrients and complex assortments of bioactive phytochemicals, especially in multicolored carrots [1,2], which may be crucial for optimal health and protection against chronic diseases [3]. Common phytochemicals include anthocyanins and carotenoids, which likely play a significant role in purported health benefits due to antioxidant, anti-inflammatory, and anticancer properties [3,4]. Carotenoids are responsible for the red, orange, and yellow pigmentation of plants. The most prominently studied, that is, β-carotene (BC) and lycopene, are potent free radical scavengers [5]. More importantly, in the human diet, provitamin A carotenoids (PACs) (e.g., BC, α-carotene, β-cryptoxanthin) can be cleaved during digestion resulting in 1 or 2 molecules of vitamin A (VA) [6]. VA is needed for vision, epithelial cell regeneration, immune competence, reproduction, and retinoid-target genes [7]. WHO considers VA deficiency (VAD) a paramount problem affecting an estimated 210 million children and women [8]. VAD leads to xerophthalmia, infertility, and increased morbidity and mortality. One way to combat VAD is to consume plant foods that contain PACs, which provide up to 80% of dietary VA in low- and middle-income countries [1,9]. It is essential to better understand PAC’s relationship with other bioactives to inform biofortification strategies and dietary patterns to maximize health benefits.
Anthocyanins, the most abundant flavonoids in fruits and vegetables, are water-soluble phytochemicals that confer red, blue, and purple pigmentation to plants [10,11]. Anthocyanins and carotenoids released from the food matrix follow a similar digestion pathway, providing opportunities for interaction during metabolism with the majority of absorption occurring in the small intestine. Limited studies have investigated the interactions between carotenoids, especially lycopene and BC, or between anthocyanins and carotenoids. To exert health benefits, they must be bioavailable, that is, released from the food matrix, absorbed, and available to be utilized or stored. BC is bioavailable from multicolored carrots, and lycopene is bioavailable from red carrots, but the extent of interactions among anthocyanins, BC, and lycopene from different varieties of orange, red, and purple-red carrots is undetermined [1,[12], [13], [14], [15], [16]). Some evidence suggests that anthocyanins increase BC absorption and that lycopene can either increase or decrease BC absorption, but the impact that carrot-derived lycopene and anthocyanins have on BC bioaccessibility and bioavailability has not been thoroughly investigated [[17], [18], [19], [20]).
Many chronic diseases stem from a combination of oxidative stress and inflammation [21]. Due to antioxidant and anti-inflammatory activities, anthocyanins have increased in dietary popularity for consumers with heightened dietary awareness because many studies link their consumption to decreased risk of chronic disease. Efforts to breed carrots focus on color through increases in anthocyanins and carotenoids. Carrots are a popular vegetable, widely available, and a great source of bioactives. Approximately 41 million tons of carrots and turnips were produced globally in 2020 [22]. Historically, multicolored carrots, such as purple-yellow and purple-white, were cultivated. Over time, a few single-colored carrots were domesticated through breeding and cultivation, for example, orange in Europe and the United States and red in India and China. In recent years, dual-colored carrots, such as purple-red varieties, have been selected to provide a more phytochemically enriched alternative to their single-colored counterparts [1,2,12,23]. In theory, the increased phytochemical content could lead to increased health benefits.
The complex array of bioactives in fruits and vegetables, when ingested simultaneously, may interact during absorption and metabolism, and it is essential to understand these interactions. The mechanisms by which gerbils absorb, convert, and store BC are similar to that of humans [12,24]. The combination of lycopene and BC from red carrots and the combination of anthocyanins and carotenoids from purple carrots were studied in Mongolian gerbils (Meriones unguiculatus) to determine the potential influence of lycopene on BC bioefficacy in the first study, and the influence of anthocyanins on BC bioefficacy in the second. The primary outcomes included evaluation of the serum, liver, and lung carotenoid and retinol concentrations. Secondary outcomes included the evaluation of serum, liver, and lung retinol concentrations based on initial body weights. We hypothesized that higher concentrations of lycopene would negatively impact BC absorption and the anthocyanins would protect the carotenoids during digestion and perhaps improve bioavailability.
Methods
Animals and study design
Mongolian gerbils (n = 66/study) were obtained from Charles River Laboratories. Gerbils were 28–35 and 35–42 days old in the lycopene and anthocyanin studies, respectively. Upon arrival, gerbils were weighed and pair housed in plastic cages. Initial body weights were lower in the lycopene study group (12.3–42.3; 26.3 ± 7.3 g) than in the anthocyanin study group (25.9–45.4; 33.8 ± 4.0 g). Gerbils consumed ad libitum an anthocyanin-, carotenoid-, and preformed VA-free feed with or without added carrot powder for the study duration. Gerbils were weighed daily and monitored for health until killed by exsanguination under isofluorane anesthesia. One gerbil in the lycopene study died before allocation to treatment. Blood samples were centrifuged 3000 x g for 15 min to isolate serum in BD Vacutainer Gel and Clot Activator tubes. The liver and lungs were excised, placed in bags, and immediately put on dry ice or submerged in liquid nitrogen. Liver samples from the anthocyanin study were fixed in formalin for histological evaluation. Prepared slides were stained with hematoxylin and eosin or Masson’s trichrome to evaluate fibrosis or cirrhosis. Slides were evaluated by a trained pathologist not involved in the study, and a report was generated. Tissues were stored at −80°C until analysis. All procedures were approved by and performed in compliance with the guidelines of the Animal Care and Use Committee of the University of Wisconsin-Madison College of Agricultural and Life Sciences.
Basal-purified feeds used carotenoid-free ingredients and were VA-deficient (Teklad Custom Diet TD.06632) with carrots added to the treatment feeds (Table 1), which used a published mineral mix [36]. For the VA-depletion phases, the basal feed was consumed for 3 wk. Feed was stored at −20°C and mixed each week to maintain equal concentrations. After depletion, a baseline group was killed (n = 5 or 6), and tissue and serum VA concentrations were established before the commencement of treatments. In the lycopene study, gerbils with extremely high and low weights were included in the baseline group. In the anthocyanin study, the baseline group was random because the gerbils did not have extreme weights. The remaining gerbils were sorted into 6 weight-matched treatment groups (n = 10/group) and placed on their respective treatments for 4 wk (Table 2). For the treatment phase, freeze-dried carrot powder was added to the feed at 3.5% in the lycopene study and 1.4% in the anthocyanin study. In the lycopene study, gerbils were assigned to 1 of 4 treatments and fed high (H), medium-to-high (MH), medium-to-low (ML), or low (L) lycopene content feed with equalized BC content based on BC equivalents (BCEs), which was 5.59 ± 0.96 μg/g. In the anthocyanin study, anthocyanin content was varied between 4 groups (i.e., HA, high anthocyanin; MHA, medium-to-high anthocyanin; MLA, medium-low anthocyanin; LA, low anthocyanin). In the anthocyanin study, BCE (7.02 ± 0.39 μg/g) and lycopene (15.9 ± 0.95 nmol/g) contents were equalized among the 4 treatment feeds. In both studies, when needed to maintain the amount of carrot, the white carrot was used to equalize concentrations among the feeds.
TABLE 1.
Composition of vitamin A-deficient feed used to determine the impact of lycopene and anthocyanin content on the bioefficacy of provitamin A carotenoids (α- and β-carotene) from multicolored carrots in male Mongolian gerbils1
| Feed component | Feed (g/kg) |
|---|---|
| Casein (vitamin-free) | 200.0 |
| L-cystine | 3.0 |
| Sucrose | 360.5 |
| Maltodextrin | 120.0 |
| Corn starch | 150.0 |
| Cottonseed oil | 60.0 |
| Cellulose | 60.0 |
| Mineral mix2 | 35.0 |
| Magnesium oxide | 1.75 |
| Calcium phosphate, dibasic | 2.0 |
| Vitamin mix (without choline, A, D, E)3 | 5.0 |
| Choline bitartrate | 2.5 |
| Vitamin D3, cholecalciferol (500,000 IU/g) | 0.0044 |
| Vitamin E, DL-α-tocopheryl acetate (500 IU/g) | 0.242 |
| Dehydrated carrot4,5 | Varied |
Provided by Harlan Teklad, Madison, WI, USA.
AIN-93M-MX (94049) mineral mix [36].
The vitamin mix (83171) provided the following (feed [g/kg]): biotin, 0.08; calcium pantothenate, 13.22; folic acid, 0.4; inositol, 22.02; menadione, 9.92; niacin, 19.82; p-aminobenzoic acid, 22.02; pyridoxine–HCl, 4.4; riboflavin, 4.4; thiamin (81%), 4.4; vitamin B12 (0.1% in mannitol), 5.94; ascorbic acid (97.5 %), 203.32.
In the lycopene study, dehydrated carrot powder from red carrots was added as fortificant (3.5%) to the feeds fed to the 4 treatment groups, which provided 5.59 μg BCE/g feed and the mean lycopene content (nmol/g) of each treatment group’s feed were as follows: high lycopene, 25.7; medium-high lycopene, 19.6; medium-to-low lycopene, 15.3; and low lycopene 9.42.
In the anthocyanin study, dehydrated carrot powder from purple-red carrots was added as a fortificant (1.4%) to the feeds fed to the 4 treatment groups, which provided 7.02 μg BCE/g and 15.9 nmol lycopene/g of feed and the mean anthocyanin contents (μmol/g) of each treatment group’s feed were as follows: high anthocyanin, 0.098; medium-to-high anthocyanin, 0.083; medium-to-low anthocyanin, 0.056; and low anthocyanin, 0.038.
TABLE 2.
Treatment feeds consumed for 28 d by male Mongolian gerbils in 2 studies. Gerbils were fed a provitamin A carotenoid (PAC), lycopene, and anthocyanin-free feed for 21 d before treatments and baseline measurements 1
| Lycopene study | Treatments | Controls | ||||
|---|---|---|---|---|---|---|
| Group (n) | 1 (10) | 2 (10) | 3 (10) | 4 (10) | Positive (10) | Negative (10) |
| Treatment feed2 | ||||||
| PAC3 | + | + | + | + | — | — |
| Lycopene | H | MH | ML | L | — | — |
| (nmol/g) | 25.7 ± 5.88 | 19.6 ± 2.78 | 15.3 ± 4.11 | 9.42 ± 2.67 | — | — |
| Oil dose | Vehicle | Vehicle | Vehicle | Vehicle | VA4 + | Vehicle |
| Anthocyanin study | ||||||
| Group (n) | 1 (10) | 2 (10) | 3 (10) | 4 (10) | Positive (10) | Negative (10) |
| Treatment feed2 | ||||||
| PAC3 | + | + | + | + | — | — |
| Lycopene4 | + | + | + | + | — | — |
| Anthocyanins | HA | MHA | MLA | LA | — | — |
| (μmol/g) | 0.098 ± 0.18 | 0.083 ± 0.19 | 0.056 ± 0.15 | 0.038 ± 0.005 | — | — |
| Oil dose | Vehicle | Vehicle | Vehicle | Vehicle | VA5 +/Lyc6 + | Vehicle |
H, high lycopene; HA, high anthocyanin; L, low lycopene; LA, low anthocyanin; Lyc, lycopene; MH, medium-to-high lycopene; MHA, medium-to-high anthocyanin; ML, medium-to-low lycopene; MLA, medium-to-low anthocyanin; PAC, provitamin A carotenoid; VA, vitamin A
Baseline group measurements were taken in n = 5 and 6 for the lycopene and anthocyanin studies, respectively.
Treatment feeds fortified with freeze-dried red (the lycopene study) and purple-red carrot (the anthocyanin study).
Equalized as BCE = β-carotene equivalents, 1 μg = 1 μg β-carotene or 2 μg α-carotene. BCE content of the 4 treatment feeds was 5.59 ± 0.96 μg/g in the lycopene study and 7.02 ± 0.39 μg/g in the anthocyanin study.
Lycopene content was 15.9 ± 0.95 nmol/g.
Provided as retinyl acetate in vehicle soybean oil, equalized to theoretical VA intake the prior day. The retinyl acetate concentrations were 0.874 and 2.03 nmol/μL for the lycopene and anthocyanin studies, respectively.
Provided as purified lycopene extracted from commercial supplements and dissolved in vehicle soybean oil with a final concentration of 2.19 nmol/μL.
Carrot preparation and analysis
Carrots were shipped from the University of California Desert Research and Extension Center at 2°C and refrigerated upon arrival. Each genotype was prepared separately by washing and scrubbing to remove soil, but peels were retained. In the lycopene study, carrots were cut into 1-in discs and blanched for 1.5 min in boiling water, submerged in cold water, and allowed to air dry. In the anthocyanin study, the carrot discs were blanched for 1 min to limit the amount of exposure of the anthocyanins to high temperatures but long enough to inactivate enzymes. All carrots were freeze-dried, ground to a powder, and stored at −80°C.
Fresh and freeze-dried carrots were analyzed in triplicate for carotenoid content using modified published methods [12] [Table 3). Briefly, 0.03 g powder or 0.1 g fresh carrot macerate was weighed into 50 mL glass tubes and combined with 50:50 acetone:dichloromethane and β-apo-8′-carotenal as an internal standard. The samples were mixed with a vortex, sonicated, and centrifuged (3000 x g). The supernatants were filtered into 25-mL volumetric flasks, and the remaining sample was extracted 3 more times with 50:50 acetone:dichloromethane. From the collected supernatants, 2 mL aliquots were dried under nitrogen and reconstituted in 50:50 methanol:dichloromethane. Each sample was analyzed on HPLC. HPLC-purified standards and absorption spectra were used for identification. The lycopene from the carrots is approximately 98% in the all-trans configuration.
TABLE 3.
Concentrations of α-carotene, β-carotene, lycopene, and anthocyanins in fresh and lyophilized carrots that were incorporated into Mongolian gerbil feed
| Carrot | α-Carotene | β-Carotene | Lycopene | Anthocyanins |
|---|---|---|---|---|
| Fresh red, nmol/g | 12.6 ± 8.61 | 76.8 ± 29.5 | 186 ± 91.2 | — |
| Lyophilized red, nmol/g | 49.3 ± 20.2 | 362 ± 135 | 570. ± 261 | — |
| Fresh purple, nmol/g | 17.8 ± 8.68 | 50.2 ± 19.5 | 69.2 ± 38.2 | 61.3 ± 56.1 |
| Lyophilized purple, nmol/g | 104 ± 70.9 | 332 ± 89.8 | 533 ± 166 | 7480 ± 5950 |
Values are mean ± SD, n = 3
Anthocyanins were assessed in triplicate in fresh and freeze-dried samples using a modified method [25] (Table 2). Briefly, 1 g fresh carrot macerate was placed in a 50-mL test tube wrapped in foil; 10 mL 90:10 methanol:formic acid was added. The tubes were capped, mixed for 1 min, sonicated for 10 min, and centrifuged for 10 min at 3000 x g. The supernatant was collected into 40-mL brown glass vials. Two more extractions were conducted with 15 mL 90:10 methanol:formic acid. From the combined supernatants, 1 mL aliquots were poured into a 10-mL brown glass vial and mixed with 3 mL water. A 500 μL aliquot of the diluted sample was used for analysis.
Freeze-dried carrot powder (0.1 g) was weighed into 5-mL brown glass vials, and 1 mL 90:10 methanol:formic acid was added. The samples were mixed for 2 min and centrifuged for 5 min at 3000 x g. The supernatant was pipetted into a 5-mL brown glass vial, the extraction was repeated, and supernatants were combined. A 200 μL aliquot was taken in a 10-mL brown glass vial, diluted with 600 μL water, and mixed by vortex for 30 sec.
Aliquots of the diluted samples were injected and analyzed on a Waters HPLC with a Zorbax SB-C18 column (5 μm, 250 mm × 4.6 mm), and guard cartridge (Agilent Technologies). The solvent system consisted of 10% aqueous formic acid (solvent A) and methanol (solvent B). The gradient was linear from 5% to 55% solvent B over 20 min, then 100% solvent B for 5 min to flush the column, and back to 5% solvent B for 10 min for equilibration. The flow rate was 1 mL/min, and the injection volume was 50 μL.
Feed analysis
Feeds were analyzed for carotenoid concentrations using a modified method in triplicate [12]. Briefly, ∼0.5 g was weighed, combined with ethanol (0.1% butylated hydroxytoluene (BHT) as an antioxidant), mixed by vortex, and placed into a hot water bath (85°C) for 5 min. Samples were removed and 500 μL 80% KOH:H2O was added. After mixing, the samples were placed into the bath, mixed, and heated for 5 min. At completion, pure water and β-apo-8′-carotenal as an internal standard were added. Samples were mixed and extracted 3 times with hexanes. Extracts were combined, dried under nitrogen, reconstituted in 500 μL 50:50 dichloroethane:methanol, and 50 μL was injected into an HPLC system (Waters Corporation). HPLC-purified standards were used for quantification.
For anthocyanin analysis, 1.5 g feed was placed into 15 mL glass test tubes. Each sample was extracted 3 times with 4 mL, 3 mL, and 3 mL methanol:formic acid (90:10, v:v), mixed for 1 min, and centrifuged for 8 min at 3000 x g. The supernatants were transferred into 10 mL brown glass vials and kept on ice. A 700 μL aliquot was dried under nitrogen and reconstituted in 100 μL of 1:3 methanolic formic acid:water. Samples were injected onto the HPLC using the same conditions as for carrots.
Oil doses
In the lycopene study, the positive control dose was solely retinyl acetate for comparison with the BC intake from the red carrots. For the anthocyanin study, a mixed supplement was prepared that contained retinyl acetate (Sigma–Aldrich) and lycopene dissolved in soybean oil, which were first quantified separately by spectrophotometry before mixing. Five 20 mg lycopene capsules (Whole Foods tomato sourced gelatin capsules) were dissolved in dichloromethane and concentrated by rotary evaporation. The lycopene was purified on an open column with 2% water-deactivated alumina and eluted with hexanes. To ensure that the resulting supplement was predominantly in the all-trans configuration (>95%), the lycopene fractions that had the all-trans spectra were combined and evaporated, soybean oil was added, and the remaining hexanes were removed under vacuum. The working solutions were sonicated to dissolve all crystals and prepared to administer approximately 35 nmol VA and 54 nmol lycopene each day by mixing 2.87 mL lycopene and 1.72 mL VA oils. The supplements were kept at −20°C. The positive control group represented the intake of the treatment groups by calculating how much was consumed by the 4 carrot treatment feeds the previous day. All other gerbils were administered a dose of soybean oil in the same amount as the supplemental oil to eliminate dietary influences. The gerbils were dosed daily with 29–43 μL/d in the lycopene study and 46–58 μL/d in the anthocyanin study.
Serum and tissues
Modified procedures were used for VA and carotenoid analyses of serum and tissues [12,13]. For all samples, C-23 β-apo-carotenol was added as an internal standard to determine extraction efficiency. Briefly, serum (500 μL) was denatured with ethanol (0.1% BHT) and extracted with hexane 4 times (2 mL and 3 × 1 mL). Supernatants were pooled, dried under nitrogen, and reconstituted in 200 μL 75:25 methanol:dichloroethane. For tissues, 0.5 g liver and 0.1 g lung were weighed, ground with anhydrous sodium sulfate, and extracted with dichloromethane into 50-mL and 25-mL volumetric flasks, respectively. Liver extract (5 mL) was dried under nitrogen and mixed with ethanol (0.1% BHT) and 50:50 KOH:H2O for saponification at 45°C for 1 h; 1 mL water was added and 2 mL hexanes (3×) was used for extraction. The extract was dried and reconstituted in 150 μL 75:25 methanol:dichloroethane. For the lung, 1 mL aliquots were dried under nitrogen and reconstituted in 100 μL 75:25 methanol:dichloroethane. Serum and tissue extracts were run on a Waters UPLC system.
Statistical analysis and calculations
Data are presented as means (± SD) and analyzed using Statistical Analysis System Studio software (SAS Institute Inc., 3.7 Enterprise Edition). ANOVA using the PROC GLM command and Tukey’s studentized range test were used at α < 0.05 to evaluate all outcomes of interest in all groups including the baseline concentrations. Effect sizes were determined using the Eta2 test where values > 0.14 are considered large. Data were considered significant at P < 0.05. Pearson correlation was used to compare 2 variables of interest. Grubb’s test was used to evaluate outliers. Specificity and sensitivity of serum retinol concentrations were determined relative to liver VA concentrations, using the following equations:
| % Specificity = [True Negative / (False Positive + True Negative)] × 100 |
| % Sensitivity = [True Positive / (False Negative + True Positive)] × 100 |
The serum retinol concentration to define VA deficiency was 0.7 μmol/L as recommended by WHO [26]. A liver VA concentration of 0.10 μmol/g was used as the reference test for VA deficiency. t-Tests were applied when testing combined data above and below reference values. This value was suggested by an expert panel [7] and recently reviewed against published biological evidence [27].
Results
Gerbil weights and intakes
In both studies, final body weights did not differ between groups, that is, 73.8 ± 8.5 and 73.9 ± 5.8 in the lycopene and anthocyanin studies, respectively. The starting body weights in the lycopene study were low (26.1 ± 7.4 g); therefore, gerbils with a range of weights at baseline kill were selected (56.6 ± 20.3 g). In the anthocyanin study, the initial weights were similar to previous studies (33.8 ± 4.0 g) and the baseline group’s body weight was 62.7 ± 4.1 g. Daily feed intake was not different between groups. In the lycopene study, intake was 4.9 ± 0.5 g/d per gerbil in treatment groups and 5.1 ± 0.5 g/d in controls. In the anthocyanin study, intake was 4.7 ± 0.4 g/d in treatment groups and 4.3 ± 0.4 g/d in controls.
Over the 28-day treatment period in the lycopene study, each gerbil consumed 817 ± 35.0 μg BCE. During the anthocyanin study, each gerbil consumed 924 ± 51.5 μg BCE and 2090 ± 125 nmol lycopene. The carrot-treated groups, from HA to LA, consumed 12.9 ± 2.4, 10.9 ± 2.6, 7.4 ± 1.8, and 5.0 ± 0.6 μmol anthocyanins.
Liver and lung lycopene, β-carotene, and retinol concentrations
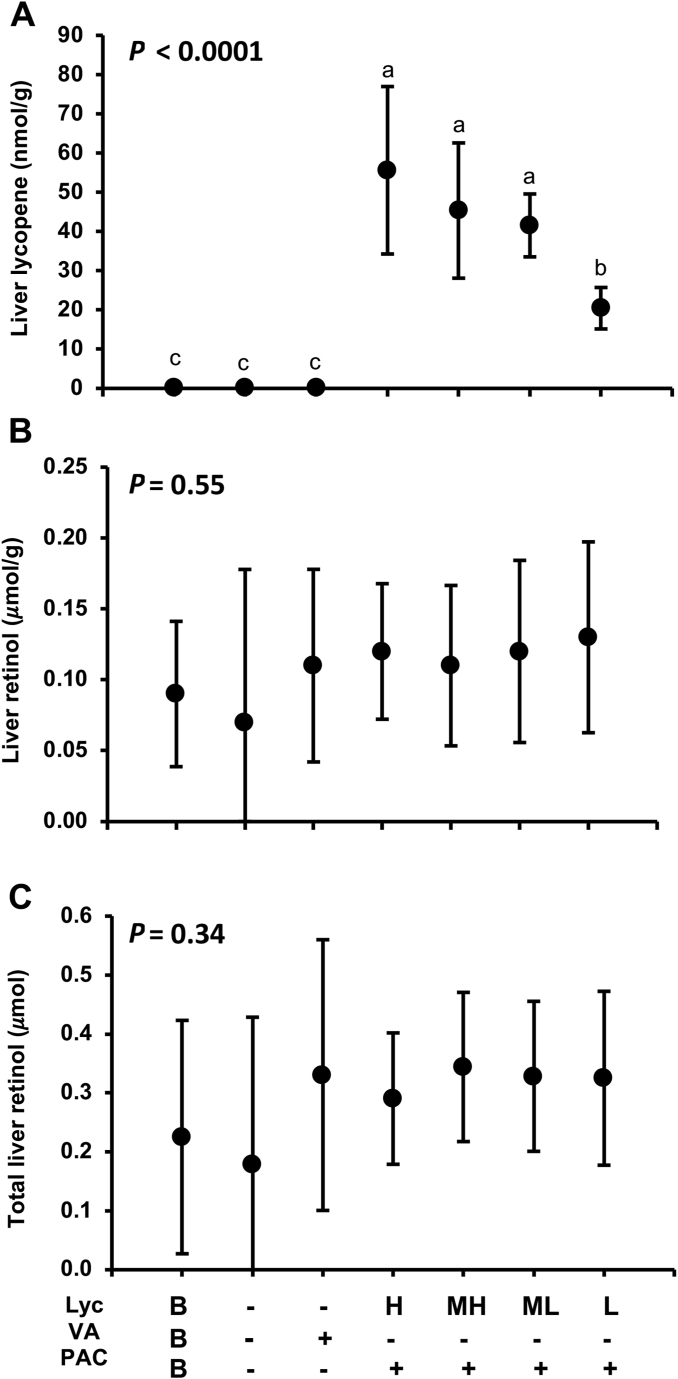
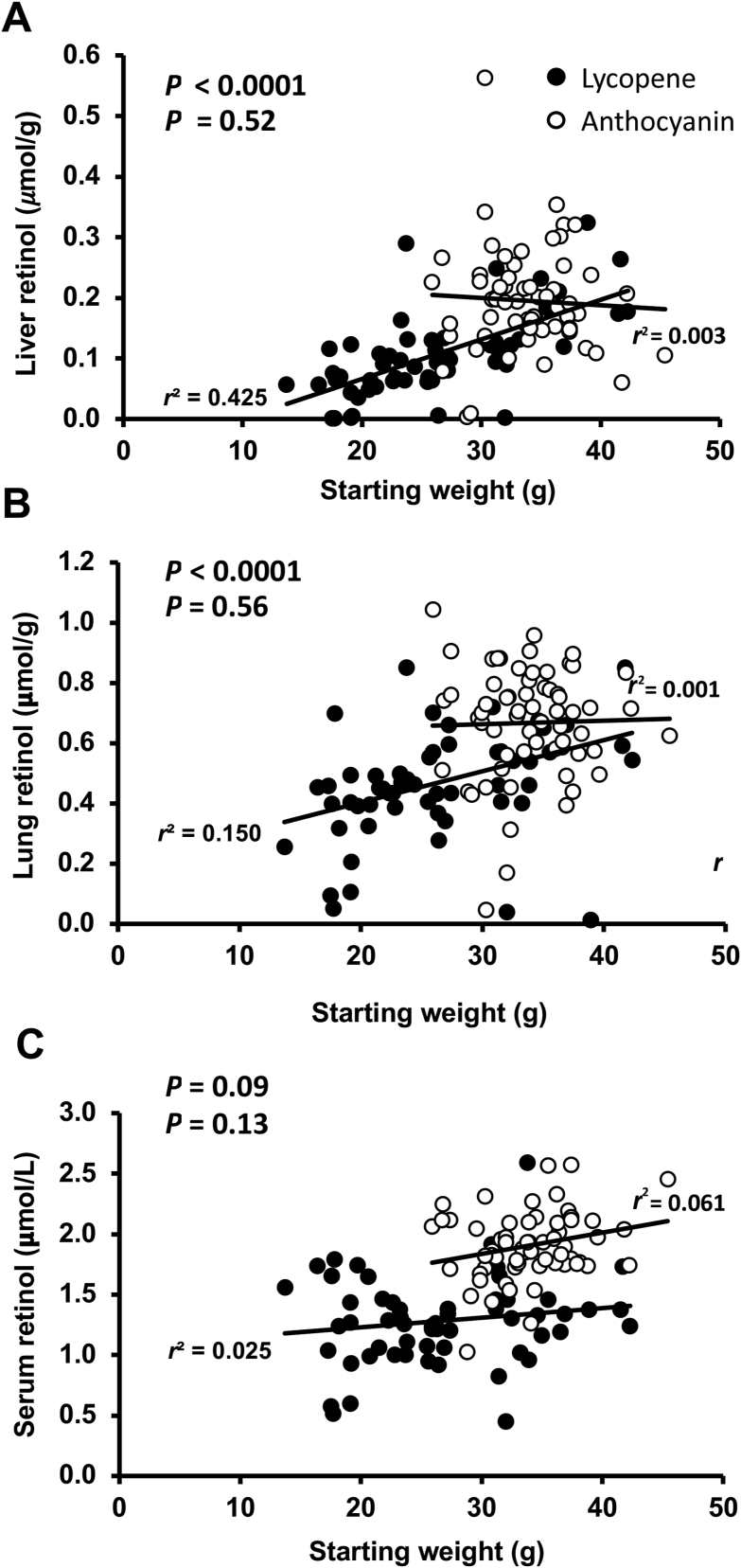
Total liver lycopene and lycopene/g liver were significantly different between the treatment groups and negative controls in both studies. In the lycopene study, all 4 treatment group’s liver lycopene concentrations significantly differed from the baseline, positive control, and negative control groups (P ≤ 0.05) (Figure 1A). The effect size for lycopene concentration and total lycopene were ≥0.70. Total liver VA content and liver retinol concentrations did not differ between groups (Figure 1B and C). All carrot-fed groups did not differ from the positive control. Large ranges were observed in hepatic VA, and almost two-thirds of the gerbils were VA deficient (≤0.1 μmol/g) at the end of the study between groups. The unanticipated range in starting weights in the lycopene study was significantly correlated to liver VA concentrations (Figure 2A). The gerbils received were smaller than those in prior studies using the same age range.
FIGURE 1.
Final liver lycopene (A), liver retinol concentrations per gram of the tissue (B), and total liver content (C) for the lycopene study in male Mongolian gerbils that consumed treatment feeds consisting of H, MH, ML, and L concentrations of lycopene that were equalized in PACs, all sourced from red carrots (Lycopene study). The positive control was dosed with preformed vitamin A in soybean oil. All values are group means ± SD (n = 10/group; baseline n = 5) and groups labeled with different letters were significantly different (P < 0.05). H, high lycopene; L, low lycopene; Lyc, lycopene; MH, medium-high lycopene; ML, medium-low lycopene; PAC, provitamin A carotenoids; VA, vitamin A.
FIGURE 2.
Association between liver retinol concentrations (μmol/g) (A) and lung retinol concentrations (μmol/g) (B) versus the starting weight of male Mongolian gerbils (g) in the lycopene study (P < 0.05) and the anthocyanin study (NS). Serum retinol concentrations did not correlate with starting body weights in either study (C).
In the lycopene study, lung retinol concentrations did not differ from the positive control but all treatment groups were higher than the negative control group (P < 0.05) (Supplemental Figure 1). The retinol concentration of the lycopene treatment groups was higher than the baseline (P < 0.05), and the effect size was 0.35. Total lung retinol was maintained from baseline in all treatment groups (P = 0.011). Lycopene was not detected in the lung tissue. BC was not detected in the liver or the lungs, likely due to bioconversion to retinol to meet requirements. Like the liver, lung retinol also significantly correlated with starting weight in the lycopene study (Figure 2B). Unlike the liver and the lungs, serum retinol concentrations were not correlated with starting body weight in either study (Figure 2C). Interestingly, lung retinol (0.53 ± 0.14 μmol/g) and liver lycopene concentrations (40.7 ± 19.1 μmol/g) were significantly correlated (r = 0.282; P = 0.023), but the correlation only trended to significance when adjusted for total tissue concentration of retinol (0.18 ± 0.06 μmol) and lycopene (116 ± 58.1 μmol) (r = 0.217; P = 0.082).
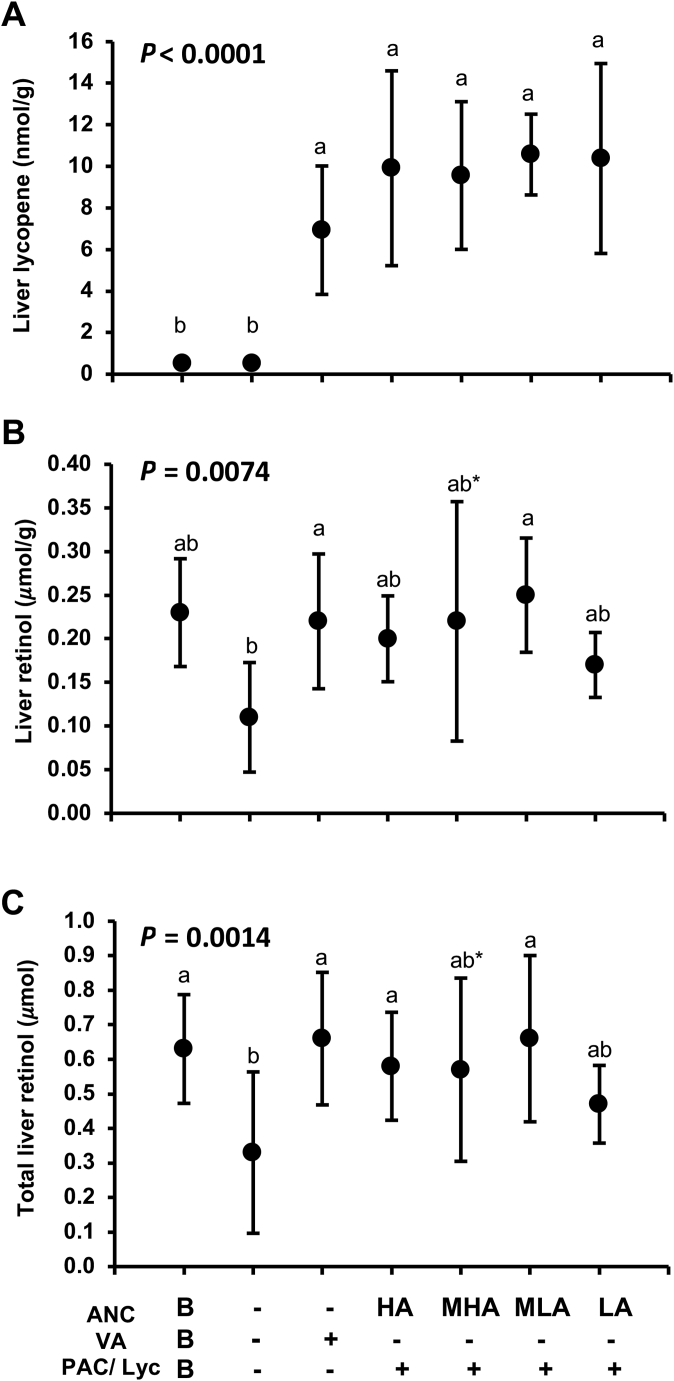
In the anthocyanin study, all purple-red carrot groups and the positive control group had significantly higher liver lycopene concentrations than baseline and negative control groups (P < 0.05) (Figure 3A), and the effect size was 0.60. Total liver retinol content and concentrations in the purple-red carrot groups did not differ from the positive control (Figure 3B and C). However, total liver retinol was significantly higher in the HA and MLA groups than in the negative control group, which resulted in an effect size of 0.30.
FIGURE 3.
Final liver lycopene (A), liver retinol concentrations per gram of the tissue (B), and total liver retinol content (C) for the anthocyanin study in male Mongolian gerbils that consumed treatment feeds consisting of HA, MHA, MLA, and LA concentrations of anthocyanins. Feeds were equalized in PACs and lycopene, all sourced from purple-red carrots. The positive control was dosed with preformed vitamin A in soybean oil. The ∗ denotes P = 0.057 and 0.053 for liver retinol concentration and total retinol being higher than the negative control group. All values are group means ± SD and groups labeled with different letters were significantly different (P < 0.05). ANC, anthocyanin; HA, high anthocyanin; LA, low anthocyanin; Lyc, lycopene; MHA, medium-high anthocyanin; MLA, medium-low anthocyanin; PAC, provitamin A carotenoids; VA, vitamin A.
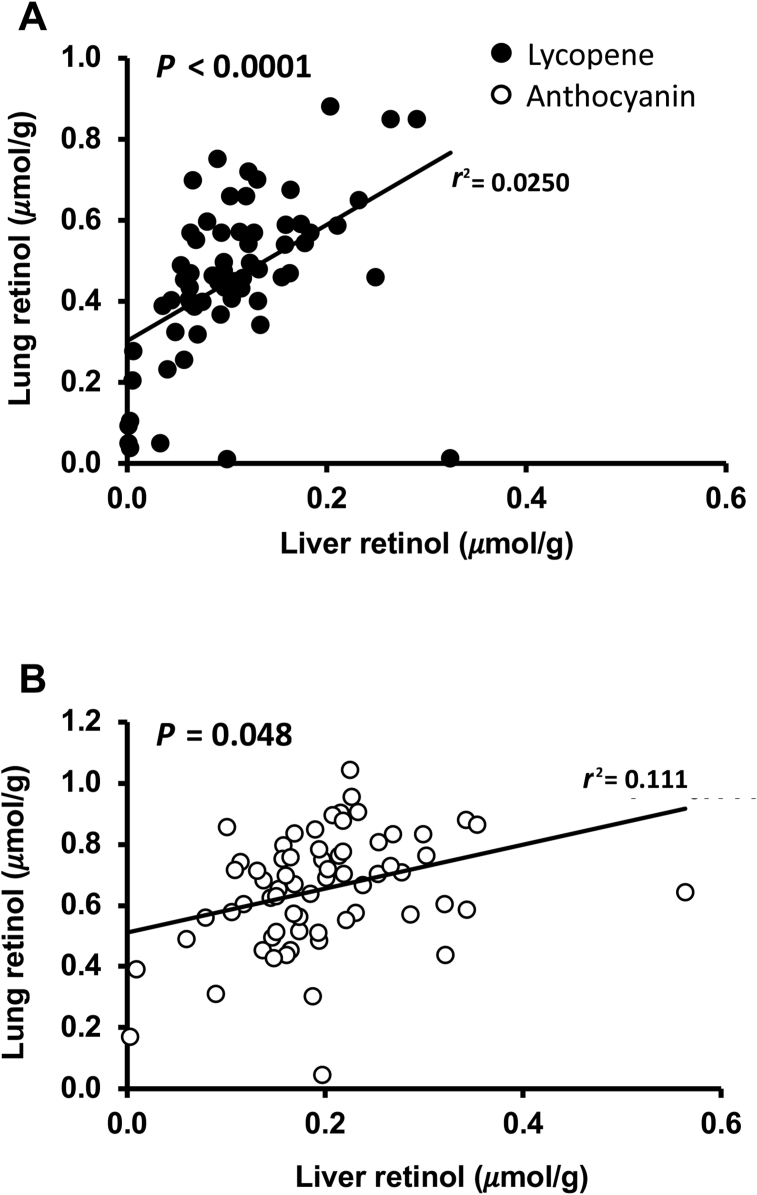
In the anthocyanin study, no differences in lung retinol concentration or total retinol were noted between groups, but concentrations were generally higher than in the lycopene study. In the anthocyanin study, total lung retinol concentration (0.23 ± 0.08 μmol) correlated with total liver lycopene (28.2 ± 12.7 μmol) (r = 0.296; P = 0.016) but was not correlated by retinol concentration (0.66 ± 0.20 μmol/g lung) or liver (10.1 ± 3.72 μmol/g) (r = 0.186; P = 0.13). In both studies, lung retinol concentration was correlated to liver retinol concentration (P < 0.0001 in the lycopene study and P = 0.048 in the anthocyanin study) (Figure 4).
FIGURE 4.
Lung retinol concentrations significantly correlated to liver retinol concentrations in the lycopene study (A) where treatments to male Mongolian gerbils include a variety of red carrots and the anthocyanin study (B) where treatments to male Mongolian gerbils included a variety of purple-red carrots. Liver retinol concentrations were generally lower in the lycopene study.
In the histological report from the evaluation of the livers (n = 4/group) fixed in formalin, liver retinol concentrations were significantly lower (0.14 ± 0.07 μmol/g; P = 0.027) in gerbils that were experiencing degeneration consistent with cirrhosis than in those that were not (0.22 ± 0.13 μmol/g). However, none of the livers had moderate or severe increases in fibrosis as noted in the pathologist’s report.
Serum concentrations
In the lycopene study, there was an outlier in the L group with an extreme serum retinol concentration (7.7 μmol/L) that was detected by Grubb’s test (P < 0.0001), which had a significant influence on the model. With the outlier included, there was no difference in serum retinol concentration between groups. However, when the outlier was removed, there was a significant difference between the negative control group and the MH lycopene group, and the positive control group, whereas the H and ML lycopene groups trended toward being different. In the anthocyanin study, serum retinol concentrations did not differ between groups. Serum carotenoids were not detected in either study.
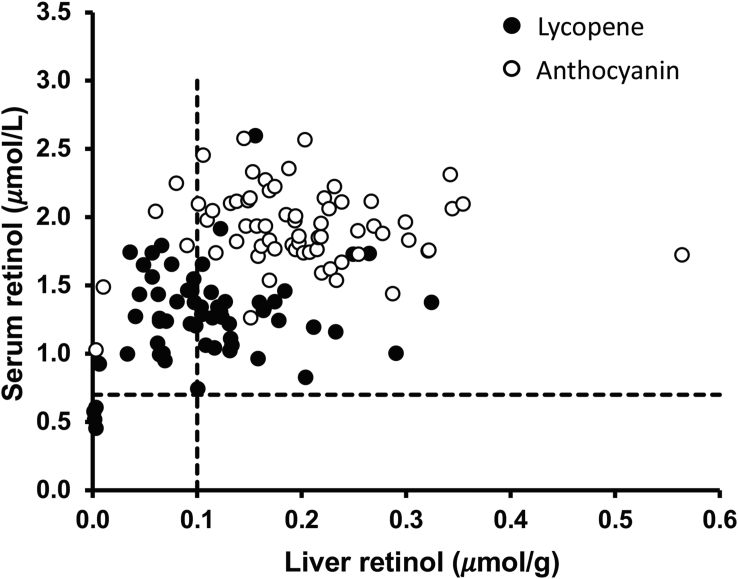
Liver VA reserves are the reference standard for assessing VA status, but for ethical reasons, serum retinol and retinol-binding protein concentrations are commonly accessible biomarkers. In the lycopene study, nearly half of the gerbils had deficient liver VA stores (≤0.10 μmol/g), but only 4 gerbils had serum retinol concentrations indicating VA deficiency (<0.70 μmol/L). These 4 gerbils had severely low liver reserves of 0.0020 ± 0.00092 μmol/g (Figure 5). In the anthocyanin study, 6 gerbils were VA deficient by liver reserves but none of them had deficient serum retinol concentrations (Figure 5).
FIGURE 5.
Comparison of vitamin A deficiency (VAD) based on serum and liver retinol concentrations in 129 male Mongolian gerbils fed a variety of feeds and supplements. The vertical dashed line represents the cutoff for VAD based on 0.10 μmol/g liver. The horizontal line represents the cutoff for VAD based on a serum retinol concentration of 0.70 μmol/L. Data in the top right quadrant are considered to be adequate VA status by both serum and liver retinol concentrations (96 true negative), whereas the ones in the upper left quadrant are considered adequate by serum retinol but deficient by liver retinol concentrations (30 false negative). In the lower left quadrant, the data points are considered deficient by both serum and liver concentrations (4 true positive), whereas those in the lower right quadrant are considered deficient by serum retinol but not by liver retinol concentrations (0 false negative). VAD, vitamin A deficiency.
Of the paired serum and liver samples from both studies (n = 129), there were 29 false negative, 97 true negative, 4 true positive, and 0 false positive cases. The sensitivity of serum retinol to predict VAD was calculated to be 12% [4/(29 ± 4)], indicating that out of the samples in which the liver VA indicated VAD, 12% of the samples had serum retinol concentrations correctly indicating VAD (true positive), whereas 88% had serum retinol concentrations that did not detect VAD (false negative). The specificity of serum retinol was 100% [97/(0 ± 97)], meaning that 100% of the gerbils that had liver reserves above the VAD cutoff (0.1 μmol/g) also had serum retinol concentrations above the VAD cutoff (0.7 μmol/L) (true negatives). In the VA-adequate gerbils from both studies, that is, >0.1 μmol retinol/g liver, serum retinol concentrations were significantly lower in gerbils from the lycopene study (n = 28, 1.33 ± 0.35 μmol/L) than the anthocyanin study (n = 60, 1.94 ± 0.62 μmol/L) (P < 0.0001).
Discussion
Consistent evidence suggests that the consumption of fruits and vegetables decreases the risk of developing chronic diseases, some of which are associated with plant phytochemicals [[28], [29], [30]]. These studies created feeds with multicolored carrots with differing amounts of either lycopene or anthocyanins to assess the impact of simultaneous consumption of lycopene or anthocyanins with PACs on BCE bioefficacy. All groups maintained VA status from a basal state by consuming feeds with carrots as the sole source of VA indicating that BC is bioavailable from red and purple-red carrots with minimal effects of lycopene. A prior study demonstrated reduced lycopene bioavailability from red carrot compared with red tomato paste, which did not have a high BC concentration [13].
In the lycopene study, liver VA concentrations did not differ between groups, but there were large ranges in hepatic VA, and almost two-thirds of the gerbils were deficient (≤0.10 μmol/g). Some gerbils were deficient in every group, including the positive control. This may have been due to the unanticipated range in starting weights, which were significantly correlated to liver VA concentrations at the end of the study. In the anthocyanin study, all groups maintained baseline VA concentrations and total liver content, which suggests that anthocyanins do not have a negative impact on BCE bioavailability and bioefficacy nor a positive impact at high concentrations as hypothesized. These findings are similar to studies conducted by Mills et al. [14] and Arscott et al. [1]. Mills et al. measured the antioxidant potential and VA bioefficacy in Mongolian gerbils of 4 biofortified carrot varieties, including prototype purple-red carrots [14]. Neither the presence of lycopene nor anthocyanins from multicolored carrots influenced the bioconversion of PACs to VA. Arscott et al. found that in women who were fed smoothies containing anthocyanins and carotenoids from purple-orange carrots [1], anthocyanins did not affect the relative bioavailability of BC following an acute feeding. At the time of that study, purple-red carrots were still undergoing breeding to enhance lycopene content. Phan et al. reported an increase in cellular BC absorption when co-present with anthocyanins in isolated Caco-2 cells [19], which may not directly translate to humans.
In the lycopene study, liver lycopene concentrations of all 4 red carrot-treated groups differed from baseline and control groups, which did not receive lycopene. Interestingly, as dietary lycopene increased, the liver lycopene increased reflecting relative intakes, which confirms the differences between the feeds. In the anthocyanin study, lycopene concentrations did not differ between the carrot-fed groups, which confirmed the equalized feed content. These data indicate that lycopene absorption was likely not impacted by the presence of BC in the lycopene study or anthocyanins in the anthocyanin study. Other studies have reported similar findings in that lycopene content did not impact BC bioavailability and bioefficacy. Mills et al. reported in 2 different studies that co-consumption of lycopene and BC in gerbils had no impact on BC bioavailability [13,14] but may have led to a decrease in lycopene bioavailability. In a double-blind study evaluated by area-under-response curve, Johnson et al. reported no significant impact of lycopene on BC but saw an increase in lycopene bioavailability in 10 adult males when 60 mg doses were consumed individually or combined [20]. On the other hand, no BC was detected in the tissues in either of the current studies, indicating that all absorbed BC was metabolized to meet VA requirements.
In both studies, lung VA was correlated with liver VA. The lung contains VA-storing cells similar to hepatic stellate cells [31], and VA is delivered to the lungs by chylomicra [32]. Vitamin A is essential for development both pre- and postnatally [33]. In humans, lung differentiation occurs mainly during the final trimester of pregnancy and continues throughout the first year of life, whereas in rats, this phase does not begin until postpartum, and alveolar structures slowly develop through life [31]. Gerbils likely develop similarly. Comparable to humans, dams pass retinol to rat fetuses in the last third of pregnancy [34]. Although VA accumulates in the liver in the final period before birth, physiological levels of lung and liver retinol are low at birth; however, plasma is not necessarily low in offspring of VA-adequate mothers [33]. It could be that the mothers in the lycopene study were not VA-adequate causing extremely low liver reserves at birth.
Human neonates postpartum have little tissue VA stores to draw from, suggesting that to build reserves, they are highly dependent on getting VA from their mother’s milk, supplements, or food [33]. In rats, retinol stores start to accumulate in the liver after the postnatal 21-day weaning period. This may explain why the small gerbils in the lycopene study had low liver VA stores at baseline. It is possible that they may not have been properly weaned or had not reached the stage of VA accumulation. This may also explain why starting weight was significantly correlated with lung VA, especially in those that started less than 25 g upon arrival. Postweaning, a steady input of VA from the diet is necessary to accumulate and maintain lung retinol. The significant correlation between liver and lung VA in both studies indicates the importance of VA intake postweaning. It is likely that the small gerbils that may have been in their weaning period were consistently using dietary VA for growth and development and did not accumulate tissue stores.
Serum retinol concentrations are under homeostatic control and the current cutoff for VAD is 0.7 μmol/L [7,26]. Although serum retinol is a common method for assessing VA status in humans, it is not a good measure of total liver VA until liver stores are extremely low. Even during VAD, if the animals or humans are without infection and remain otherwise healthy, adequate serum retinol concentration is maintained through increased enterohepatic recycling [27]. This was likely the case in the lycopene study. There were no clinical signs that these gerbils were VA deficient, such as reduced body weight at kill. If serum retinol concentration was the only biomarker assessed, almost all of the gerbils appeared as though they had sufficient VA status when in fact many had depleted liver stores. Poor sensitivity of serum retinol indicates that VA-deficient individuals may be misdiagnosed, supporting previous claims that serum retinol has limited utility as a predictor of VA status. The difference in serum retinol concentrations of the VA-adequate gerbils from both studies indicates that the gerbils in the lycopene study may have had a lower serum retinol set-point due to the initial VA deficiency.
Although a limitation, the unanticipated wide range of starting weights in the lycopene study likely influenced serum and tissue retinol. At the time the gerbils arrived, according to their growth curve, they should have weighed 28–35 g [35], but almost half of them were <28 g, with the smallest weighing 12.6 g. A strong correlation was observed between the starting body weights and final liver retinol concentrations. In the anthocyanin study, all gerbils had typical starting weights, and there was no correlation between serum retinol and liver retinol concentrations, or between starting weight and liver retinol concentration. Both studies add more evidence that serum retinol concentrations have limited utility as predictors of VA status.
Red and purple-red carrots may be considered novel functional foods that provide combinations of different bioactive compounds, such as anthocyanins and carotenoids, which are linked to optimal human health. Future studies should address the relationship of lycopene on BC relative bioavailability in humans from purple-red carrots as well as evaluate the reverse relationship to get a more concise understanding of the impact BC has on lycopene bioavailability. To our knowledge, the anthocyanin study is the first to assess the impact of anthocyanins on the bioavailability of BC from a single food source, that is, purple-red carrots, in an in vivo model. The results from the second study suggest that simultaneous consumption of carotenoids and anthocyanins does not negatively impact the bioefficacy of BC.
Funding
This research was supported by an NIH T32 DK007665 Metabolism and Nutrition Training Program fellowship to MSK; the California Fresh Carrot Advisory Board and the National Institute of Food and Agriculture, U.S. Department of Agriculture, under award numbers 2016-51181-25400 and 2022-51181-38321 (PWS); a Cargill–Benevenga Undergraduate Research Scholarship (JBS); and an endowment entitled “Friday Chair for Vegetable Processing Research” (SAT).
Author disclosures
The authors report no conflicts of interest.
Acknowledgments
We thank Michael Grahn for assistance in the laboratory and Devika Suri for consultation on statistical methods. The authors’ responsibilities were as follows – MSK: carried out the research, fed/cared for the gerbils, analyzed the data, and wrote the first draft of the manuscript; JBS: assisted with gerbil care and sample analysis; CRD: assisted with study design and animal care; PWS: bred and provided carrots for feeds; SAT: designed the research and revised the manuscript; and all authors: read and approved the final manuscript. Data can become available if both senior scientists agree on its use.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tjnut.2022.10.010.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Arscott S.A., Simon P.W., Tanumihardjo S.A. Anthocyanins in purple-orange carrots (Daucus carota L.) do not influence the bioavailability of β-carotene in young women. J Agric Food Chem. 2010;58(5):2877–2881. doi: 10.1021/jf9041326. [DOI] [PubMed] [Google Scholar]
- 2.Surles R.L., Weng N., Simon P.W., Tanumihardjo S.A. Carotenoid profiles and consumer sensory evaluation of specialty carrots (Daucus carota, L.) of various colors. J Agric Food Chem. 2004;52(11):3417–3421. doi: 10.1021/jf035472m. [DOI] [PubMed] [Google Scholar]
- 3.Hung H.C., Joshipura K.J., Jiang R., Hu F.B., Hunter D., Smith-Warner S.A., et al. Fruit and vegetable intake and risk of major chronic disease. J Natl Cancer Inst. 2004;96(21):1577–1584. doi: 10.1093/jnci/djh296. [DOI] [PubMed] [Google Scholar]
- 4.Krebs-Smith S.M., Kantor L.S. Choose a variety of fruits and vegetables daily: understanding the complexities. J Nutr. 2001;131(2S-1) doi: 10.1093/jn/131.2.487S. 487S–501S. [DOI] [PubMed] [Google Scholar]
- 5.Di Mascio P., Kaiser S., Sies H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch Biochem Biophys. 1989;274(2):532–538. doi: 10.1016/0003-9861(89)90467-0. [DOI] [PubMed] [Google Scholar]
- 6.Moran N.E., Mohn E.S., Hason N., Erdman J.W., Johnson E.J. Intrinsic and extrinsic factors impacting absorption, metabolism, and health effects of dietary carotenoids. Adv Nutr. 2018;9(4):465–492. doi: 10.1093/advances/nmy025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanumihardjo S.A., Russell R.M., Stephensen C.B., Gannon B.M., Craft N.E., Haskell M.J., et al. Biomarkers of nutrition for development (BOND) – Vitamin A review. J Nutr. 2016;146(9):1816S. doi: 10.3945/jn.115.229708. 48S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization . WHO; Geneva, Switzerland: 2009. Global prevalence of vitamin A deficiency in populations at risk 1995–2005: WHO Global Database on Vitamin A Deficiency.http://apps.who.int//iris/handle/10665/44110 [Internet] Available from: [Google Scholar]
- 9.FAO–WHO . Report of a joint FAO/WHO expert consultation. FAO; Rome, Italy: 2001. Human vitamin and mineral requirements.https://www.who.int/publications/i/item/9241546123 [Google Scholar]
- 10.Wang L.S., Stoner G.D. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008;269(2):281–290. doi: 10.1016/j.canlet.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prior R.L., Wu X. Anthocyanins: structural characteristics that result in unique metabolic patterns and biological activities. Free Radic Res. 2006;40(10):1014–1028. doi: 10.1080/10715760600758522. [DOI] [PubMed] [Google Scholar]
- 12.Porter Dosti M., Mills J.P., Simon P.W., Tanumihardjo S.A. Bioavailability of β-carotene (βC) from purple carrots is the same as typical orange carrots while high-βC carrots increase βC stores in Mongolian gerbils (Meriones unguiculatus) Br J Nutr. 2006;96(2):258–267. doi: 10.1079/bjn20061562. [DOI] [PubMed] [Google Scholar]
- 13.Mills J.P., Simon P.W., Tanumihardjo S.A. β-Carotene from red carrot maintains vitamin A status, but lycopene bioavailability is lower relative to tomato paste in Mongolian gerbils. J Nutr. 2007;137(6):1395–1400. doi: 10.1093/jn/137.6.1395. [DOI] [PubMed] [Google Scholar]
- 14.Mills J.P., Simon P.W., Tanumihardjo S.A. Biofortified carrot intake enhances liver antioxidant capacity and vitamin A status in Mongolian gerbils. J Nutr. 2008;138(9):1692–1698. doi: 10.1093/jn/138.9.1692. [DOI] [PubMed] [Google Scholar]
- 15.Horvitz M.A., Simon P.W., Tanumihardjo S.A. Lycopene and β-carotene are bioavailable from lycopene ‘red’ carrots in humans. Eur J Clin Nutr. 2004;58(5):803–811. doi: 10.1038/sj.ejcn.1601880. [DOI] [PubMed] [Google Scholar]
- 16.Molldrem K.L., Li J., Simon P.W., Tanumihardjo S.A. Lutein and β-carotene from lutein-containing yellow carrots are bioavailable in humans. Am J Clin Nutr. 2004;80(1):131–136. doi: 10.1093/ajcn/80.1.131. [DOI] [PubMed] [Google Scholar]
- 17.Phan M.A.T., Paterson J., Bucknall M., Arcot J. Interactions between phytochemicals from fruits and vegetables: effects on bioactivities and bioavailability. Crit Rev Food Sci Nutr. 2018;58(5):1310–1329. doi: 10.1080/10408398.2016.1254595. [DOI] [PubMed] [Google Scholar]
- 18.Phan M.A.T., Bucknall M., Arcot J. Interactive effects of β-carotene and anthocyanins on cellular uptake, antioxidant activity and anti-inflammatory activity in vitro and ex vivo. J Func Foods. 2018;45:129–137. [Google Scholar]
- 19.Phan M.A.T., Bucknall M.P., Arcot J. Effects on intestinal cellular bioaccessibility of carotenoids and cellular biological activity as a consequence of co-ingestion of anthocyanin- and carotenoid-rich vegetables. Food Chem. 2019;286:678–685. doi: 10.1016/j.foodchem.2019.02.046. [DOI] [PubMed] [Google Scholar]
- 20.Johnson E.J., Qin J., Krinsky N.I., Russell R.M. Ingestion by men of a combined dose of β-carotene and lycopene do not affect the absorption of β-carotene but improves that of lycopene. J Nutr. 1997;127(9):1833–1837. doi: 10.1093/jn/127.9.1833. [DOI] [PubMed] [Google Scholar]
- 21.Biswas S.K. Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxid Med Cell Longev. 2016 doi: 10.1155/2016/5698931. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.FAO. FAOSTAT Statistical Database. [Internet]. FAO. Available from: https://www.fao.org/faostat/en/#home (Accessed 5 May 2022).
- 23.Simon P.W. Plant pigments for color and nutrition. Hortic Sci. 1997;32(1):12–13. [Google Scholar]
- 24.Lee C.M., Boileau A.C., Boileau T.W.M., Williams A.W., Swanson K.S., Heintz K.A., et al. Review of animal models in carotenoid research. J Nutr. 1999;129(12):2271–2277. doi: 10.1093/jn/129.12.2271. [DOI] [PubMed] [Google Scholar]
- 25.Kurilich A.C., Clevidence B.A., Britz S.J., Simon P.W., Novotny J.A. Plasma and urine responses are lower for acylated vs nonacylated anthocyanins from raw and cooked purple carrots. J Agric Food Chem. 2005;53(16):6537–6542. doi: 10.1021/jf050570o. [DOI] [PubMed] [Google Scholar]
- 26.World Health Organization . Vitamin and Mineral Nutrition Information System. World Health Organization; Geneva, Switzerland: 2011. Serum retinol concentrations for determining the prevalence of vitamin A deficiency in populations.https://apps.who.int/iris/handle/10665/85859 [Internet].Available from: [Google Scholar]
- 27.Tanumihardjo S.A. Biological evidence to define a vitamin A deficiency cutoff using total liver vitamin A reserves. Exp Biol Med. 2021;246(9):1045–1053. doi: 10.1177/1535370221992731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun T., Simon P.W., Tanumihardjo S.A. Antioxidant phytochemicals and antioxidant capacity of biofortified carrots (Daucus carota L.) of various colors. J Agric Food Chem. 2009;57(10):4142–4147. doi: 10.1021/jf9001044. [DOI] [PubMed] [Google Scholar]
- 29.Pan Y., Li H., Zheng S., Zhang B., Deng Z. Implication of the significance of dietary compatibility: Based on the antioxidant and anti-inflammatory interactions with different ratios of hydrophilic and lipophilic antioxidants among four daily agriculture crops. J Agric Food Chem. 2018;66(28):7461–7474. doi: 10.1021/acs.jafc.8b01690. [DOI] [PubMed] [Google Scholar]
- 30.Zheng J., Zhou Y., Li S., Zhang P., Zhou T., Xu D.P., et al. Effects and mechanisms of fruit and vegetable juices on CVD. Int J Mol Sci. 2017;18(3):555–570. doi: 10.3390/ijms18030555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Timoneda J., Rodrígues-Fernándex L., Zaragozá R., Marín M.P., Cabezuelo M.T., Torres L., et al. Vitamin A deficiency and the lung. Nutrients. 2018;10(9):1132. doi: 10.3390/nu10091132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun T., Surles R.L., Tanumihardjo S.A. Vitamin A concentrations in piglet extrahepatic tissues respond differently 10 d after vitamin A treatment. J Nutr. 2008;138(6):1101–1106. doi: 10.1093/jn/138.6.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ross A.C., Li N. Lung retinyl ester is low in young adult rats fed a vitamin A deficient diet after weaning, despite neonatal vitamin A supplementation and maintenance of normal plasma retinol. J Nutr. 2007;137(10):2213–2218. doi: 10.1093/jn/137.10.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chytil F. The lungs and vitamin A. Am J Physiol Lung Cell Mol Physiol. 1992;252:L517–L527. doi: 10.1152/ajplung.1992.262.5.L517. [DOI] [PubMed] [Google Scholar]
- 35.Charles River Laboratories . 2020. Mongolian Gerbil. Charles River Laboratories.https://www.criver.com/products-services/find-model/mongolian-gerbil?region=3611 [Internet]. Available from: [Google Scholar]
- 36.Reeves P.G., Nielsen F.H., Fahey G.C., Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123(11):1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.