Abstract
Background
Evidence regarding dietary phytoestrogens in relation to mortality remains limited.
Objectives
The objective of the study is to examine the associations of intake of isoflavones, lignans, and coumarins with total and cause-specific mortality in US males and females.
Methods
We followed 75,981 females in the Nurses’ Health Study (1984–2018) and 44,001 males in the Health Professionals Follow-up Study (1986–2018), who were free of cardiovascular disease (CVD), diabetes, or cancer at baseline. Their diet was repeatedly assessed using validated food frequency questionnaires every 2–4 y. Associations with mortality were assessed using time-dependent Cox models with adjustments for demographics, dietary and lifestyle factors, and medical history.
Results
During 3,427,156 person-years of follow-up, we documented 50,734 deaths, including 12,492 CVD deaths, 13,726 cancer deaths, and 24,516 other non-CVD and noncancer deaths. After multivariable adjustment, the higher total phytoestrogen intake was associated with lower risk of total CVD and other non-CVD and noncancer mortality: comparing extreme quintiles, the pooled HRs (95% CIs) were 0.89 (0.87, 0.92), 0.90 (0.85, 0.96), and 0.86 (0.82, 0.90), respectively. We did not find a significant association with cancer mortality [0.97 (0.92, 1.03)]. For individual phytoestrogens in relation to total mortality, the pooled HRs (95% CIs) comparing extreme quintiles were 0.90 (0.87, 0.92) for isoflavones, 0.93 (0.90, 0.96) for lignans, and 0.93 (0.90, 0.95) for coumarins. Individual phytoestrogens were also significantly associated with lower risk of CVD mortality and other types of mortality. Primary food sources of phytoestrogens, including tofu, soy milk, whole grains, tea, and flaxseed, were also inversely associated with total mortality.
Conclusions
A higher intake of total phytoestrogens, including isoflavones, lignans, and coumarins, and foods rich in these compounds was associated with lower risk of total and certain cause-specific mortality in generally healthy US adults. These data suggest that these phytochemicals and their dietary sources may be integrated into an overall healthy diet to achieve a longer life span.
Keywords: dietary phytoestrogens, isoflavones, lignans, coumarins, mortality, public health, epidemiology
Introduction
Dietary phytoestrogens are plant-derived nonsteroidal compounds with weak estrogen-like activity. They are found in a wide variety of foods, including soy products, flaxseed, and whole grains [1]. Isoflavones, lignans, and coumarins are the three main classes of phytoestrogens [1]. Dietary phytoestrogens have recently gained considerable attention for their potential health benefits. In particular, the 2015–2020 Dietary Guidelines for Americans recommends three healthy dietary patterns for the prevention of chronic diseases: the Healthy US Eating Pattern, the Healthy Mediterranean Eating Pattern, and the Healthy Vegetarian Eating Pattern, with major common food components being legumes, soy products, and whole grains, all of which are rich in dietary phytoestrogens [2].
Observational and intervention studies have shown that dietary phytoestrogens (e.g., isoflavones and lignans) and their primary food sources (e.g., tofu and whole grains) may improve cardiometabolic risk factors, including blood pressure, obesity, insulin resistance, lipids, and proinflammatory cytokines [[3], [4], [5], [6], [7], [8]], and are associated with a lower risk of cardiovascular disease (CVD) [6, [9], [10], [11], [12]] and breast cancer [13]. These inverse associations may be mainly due to the antioxidant and anti-inflammatory activity of dietary phytoestrogens by binding to estrogen receptors (ER) [1]. However, to date, only a few studies have investigated the role of dietary phytoestrogens and their primary food sources in relation to total and cause-specific mortality in generally healthy populations, and the findings were mixed [[14], [15], [16], [17], [18]]. For example, Nakamoto et al. reported inverse associations between isoflavone intake and total mortality in middle-aged Japanese [19]. In contrast, a prospective study in a Western population observed that the intake of isoflavones was associated with higher total mortality but not CVD mortality [20], whereas other studies showed no associations of isoflavones with total mortality [16, 21]. Furthermore, these prior cohort studies shared some common limitations, including relatively small-to-moderate sample sizes (<32,000 participants and <1250 cases), relatively short duration of follow-up, and lack of repeated measurements of diet and other covariates during the follow-up. In addition, most of these previous studies were conducted among Asian populations, which may limit generalizability to Western populations [[15], [16], [17]]. Taken together, the question of whether dietary phytoestrogens and their primary food sources are beneficial or harmful for premature mortality remains largely unknown.
In the present study, we sought to comprehensively examine the associations of total and subclasses of dietary phytoestrogens (e.g., isoflavones, lignans, and coumarins) and their primary food sources (e.g., soy milk and tofu) with total and cause-specific mortality in two large prospective cohorts of US males and females, with the diet repeatedly measured every 2–4 y for over 30 y of follow-up. We hypothesized that these dietary phytoestrogens and their primary food sources are associated with a lower risk of total and cause-specific mortality.
Methods
Study population
The Nurses’ Health Study (NHS) was established in 1976 among 121,700 US registered female nurses who were aged 30–55 y [22]. The Health Professionals Follow-up Study (HPFS) was established in 1986 among 51,529 US male health professionals aged 40–75 y [23]. The details of the 2 cohorts have been described elsewhere [22, 23]. Briefly, follow-up questionnaires were administered at baseline enrollment and every 2 y thereafter to collect lifestyle and medical information. Diet was assessed using a validated food frequency questionnaire (FFQ) every 4 y. The follow-up rates were 95.4% in the NHS and 95.9% in the HPFS. For the current study, to minimize the reverse causation (e.g., disease diagnosis causing changes in diet), we excluded participants who reported CVD, cancer, or diabetes at baseline (1984 for the NHS and 1986 for the HPFS). We further excluded participants with missing information on phytoestrogen intake or extreme and implausible daily energy intake (<500 or >3500 kcal for females; <800 or >4200 kcal for males). After the exclusions, 119,982 participants (75,981 in the NHS, 44,001 in the HPFS) remained in the final analyses (Supplementary Figure 1).
The study protocol was approved by the institutional review boards of the Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health, and those of participating registries as required.
Assessment of dietary phytoestrogens and food sources
In 1984, a 116-item FFQ was administered to the NHS participants to obtain information on usual intake of food and beverages. Starting from 1986, an expanded 131-item FFQ was administered every 4 y to update their diet. Using a similar FFQ, diet was assessed every 4 y in the HPFS starting from 1986. Since 1998 in the NHS and 2002 in the HPFS, consumption of soy milk was added to the FFQs. Additionally, since 2006 in the NHS and 2010 in the HPFS, consumption of flaxseed and flaxseed oil was added to the FFQs. The average daily intake of isoflavones, lignans, or coumarins was calculated by multiplying the frequency of consumption of each food item that contains phytoestrogens by phytoestrogen content and then summing across from all foods. We further calculated the average daily intake of total phytoestrogens by summing up the average daily intake of isoflavones, lignans, and coumarins. Due to different molecular weights of isoflavones, lignans, and coumarins, we used mole as the unit for total phytoestrogen intake and gram as the unit for the intake level of individual phytoestrogens. We listed the top 10 food sources of each phytoestrogen Supplementary Figure 2, including tofu, soy milk, flaxseed products, whole grains, and tea. The food composition data used to calculate the values of each phytoestrogen were primarily based on the US Department of Agriculture Nutrient Database of phytoestrogen contents of selected foods [24].
The reproducibility and validity of the FFQs used in the NHS and HPFS have been described in detail previously [[25], [26], [27]]. The correlation coefficient for tofu consumption between FFQs and diet records was 0.56 in a validation study conducted in HPFS [28]. In 957 males of the Men’s Lifestyle Validation Study, the Pearson correlation coefficient was 0.53 between total lignan intakes assessed by the FFQ and 7-day diet records (7DDR). Supplementary Figure 3 shows the age-adjusted Spearman correlations between these phytoestrogens in the current study.
Ascertainment of deaths
Deaths were identified by searching the National Death Index or by reports from the next of kin or the US postal authorities. Using these methods, around 98% of the deaths in each cohort were ascertained [29]. The underlying cause of death was assigned by study physicians through the review of death certificates, autopsy reports, and hospital records. For the current analysis, the cause of death was classified according to the International Classification of Diseases Eighth Revision (ICD-8) in the NHS and ICD-9 in the HPFS. Specifically, cardiovascular deaths were determined by ICD-8 codes 390–458 in NHS and ICD-9 codes 390–459 in HPFS, and cancer deaths were determined by ICD-8 codes 140–209 in NHS and ICD-9 codes 140–208 in HPFS.
Assessment of covariates
At baseline, we asked the participants to report their race/ethnicity. Updated information on anthropometric and lifestyle factors, such as body weight, smoking, physical activity, and medication or supplement use, was collected in the follow-up questionnaires administered every 2 y. The menopausal status and postmenopausal hormone use were ascertained in females. We used the alternative healthy eating index (AHEI) score to assess the overall diet quality [30]. The AHEI score summarizes the consumption of 10 foods or nutrients (including the consumption of vegetables, fruits, whole grains, sugar sweetened beverages and fruit juice, nuts and legumes, red and processed meat, trans-fat, long chain n-3 fats, polyunsaturated fats, and sodium) [30]. In the present analysis, the primary food sources of phytoestrogens (Supplementary Figure 2), including tofu, soy milk, and whole grains, were excluded from the AHEI score to avoid overadjustment.
Statistical analyses
We calculated person-years of follow-up for each participant from the return of the baseline questionnaires to the death date, date of last return of a valid follow-up questionnaire, or the end of follow-up (June 30, 2018, in the NHS or January 31, 2018, in the HPFS), whichever occurred first. To minimize random within-person variation and to best represent longterm diet, we calculated the cumulative averages of dietary variables from all FFQs. These averages were derived as the means of all intake assessments and used to represent diet for the next 4-y follow-up. Because participants might change their usual diet after the diagnosis of major chronic diseases, we did not update diet if participants reported a diagnosis of type-2 diabetes, CVD, or cancer. Time-dependent Cox proportional-hazards model was used to estimate the hazard ratios (HRs) for total and cause-specific mortality associated with total phytoestrogens, isoflavones, lignans, and coumarins. The proportional-hazards assumption was tested by including an interaction term between the total phytoestrogen intake and the duration of follow-up, and we did not detect evidence of violations.
In multivariable analyses, in addition to age and calendar time, we further adjusted for ethnicity; smoking status; alcohol intake; physical activity; multivitamin use; aspirin use; history of hypertension and hypercholesterolemia; family history of MI, diabetes, and cancers; body mass index (BMI); total energy intake; and the modified AHEI score, with menopausal status and postmenopausal hormone use only for females. Of note, time-varying covariates were used, except for ethnicity. We tested the significance of linear trends by modeling the median value within each category as a continuous variable and then examining the significance of this variable. In a secondary study, we further examined the HRs for mortality with 3 individual isoflavones—daidzein, genistein, and glycitein—and 4 individual lignans—secoisolariciresinol, matairesinol, pinoresinol, and lariciresinol. To explore the dose–response relationship between each phytoestrogen intake and total mortality, we fitted cubic spline regressions, where the same covariates in the primary analyses were adjusted. We also examined the associations of the primary food sources—tofu, soy milk, whole grains, tea, flaxseed, and flaxseed oil—with total and cause-specific mortality. Furthermore, we explored potential effect modifications by several participant characteristics, including age, BMI, smoking status, alcohol intake, physical activity, modified AHEI score, and intake of animal-based foods. We calculated P values for interaction from the likelihood ratio tests comparing the full model including the product terms between stratifying variables and dietary phytoestrogen (quintiles) with the reduced model with the main terms. A Bonferroni-corrected P value threshold (0.05/6 = 0.008) was used to account for potential multiple comparisons in the interaction tests.
Sensitivity analyses
To test the robustness of our findings, we conducted several sensitivity analyses based on our fully adjusted model (Model 3). First, we conducted a 4-y lagged analysis to minimize the impact of dietary changes made among higher risk individuals, which may lead to reverse causation. For instance, in the NHS, dietary phytoestrogens from 1984 were used to evaluate the mortality after 1988. Second, we also evaluated the associations after applying an 8-y lag. Third, we adjusted for other major dietary variables (e.g., fruits, vegetables, red meat, trans-fat, and ratio of polyunsaturated to saturated fat) or healthy plant-based diet index, a diet score positively rating healthy plant foods and inversely rating less healthy plant-based foods and animal-based foods [31], instead of modified AHEI. Further, we adjusted for the two adapted versions of the modified AHEI that excluded whole grains or soy products instead of the modified AHEI adjusted in the main analysis that excluded whole grains and soy products simultaneously. Fourth, we categorized the intake of phytoestrogens into deciles and explored the associations. Fifth, we mutually adjusted for the three subclasses of phytoestrogens: isoflavones, lignans, and coumarins. Finally, we further explored the associations of total phytoestrogens with more specific other types of mortality (e.g., neurogenerative disease mortality and kidney disease mortality).
The analyses were carried out separately for each cohort, and then, the cohort-specific HRs were pooled. We used the Cochrane Q statistic, the I2 statistic, and the between-study coefficient of variation to assess the heterogeneity between the two cohorts. As we did not observe significant heterogeneity, an inverse variance-weighted fixed-effect meta-analysis was used to pool the results across the two cohorts. We performed the statistical analyses using the SAS statistical package (version 9.4, SAS Institute). P < 0.05 (2-sided) was considered statistically significant unless otherwise specified.
Results
Table 1 shows the age-standardized baseline characteristics of the participants by quintiles of total dietary phytoestrogens. Those who consumed higher total phytoestrogens were more likely to be physically active and had a higher modified AHEI score. Supplementary Table 1 presents the age-standardized baseline characteristics of participants by quintiles of isoflavones, lignans, and coumarins. Similarly, participants with a higher intake of isoflavones, lignans, and coumarins were more likely to engage in physical activity and had higher modified AHEI scores.
Table 1.
Age-standardized baseline characteristics of participants according to quintiles of total phytoestrogen intake
| Nurses’ Health Study |
Health Professionals Follow-Up Study |
|||||
|---|---|---|---|---|---|---|
| Q1 | Q3 | Q5 | Q1 | Q3 | Q5 | |
| Participants, n | 15,196 | 15,197 | 15,196 | 8800 | 8801 | 8800 |
| Total phytoestrogens, umol/d | 1.0 (0.2) | 2.0 (0.2) | 15.5 (68.2) | 1.2 (0.3) | 2.4 (0.2) | 17.6 (45.9) |
| Age1, y | 49.9 (7.4) | 50.5 (7.2) | 50.2 (6.9) | 53.2 (9.9) | 53.9 (9.7) | 52.2 (9.1) |
| White, % | 97.8 | 98.3 | 96.2 | 96.3 | 96.0 | 89.8 |
| Current smoker, % | 16.2 | 22.9 | 33.5 | 6.2 | 10.0 | 10.7 |
| Alcohol intake, g/d | 5.2 (10.1) | 8.0 (12.0) | 6.9 (11.0) | 9.5 (15.3) | 12.8 (15.9) | 10.3 (13.9) |
| Physical activity (metabolic equivalents of task/week) | 13.5 (18.4) | 14.1 (21.4) | 15.4 (25.6) | 20.4 (28.9) | 21.2 (29.0) | 23.3 (34.0) |
| BMI (kg/m2) | 25.0 (5.0) | 25.1 (4.7) | 24.9 (4.6) | 24.8 (4.7) | 25.0 (5.0) | 24.75 (5.1) |
| Family history of myocardial infarction, % | 39.2 | 39.2 | 39.5 | 31.7 | 31.9 | 31.7 |
| Family history of cancer | 45.1 | 44.7 | 44.5 | 25.0 | 25.3 | 24.5 |
| Multivitamin use, % | 37.7 | 36.5 | 38.9 | 41.2 | 41.3 | 45.9 |
| Hypertension, % | 23.4 | 22.1 | 19.1 | 20.6 | 20.1 | 19.5 |
| Hypercholesterolemia, % | 9.1 | 8.4 | 8.5 | 10.0 | 10.5 | 11.5 |
| Total energy intake, kcal/d | 1722 (537) | 1826 (519) | 1675 (533) | 1978 (625) | 2038 (618) | 1976 (642) |
| Red meats (servings/d) | 1.1 (0.7) | 1.2 (0.7) | 1.1 (0.8) | 1.1 (0.8) | 1.2 (0.8) | 1 (0.9) |
| Total animal-based foods (servings/d) | 4.6 (2) | 5.0 (2.1) | 4.7 (2.1) | 4.8 (2.2) | 4.9 (2.2) | 4.5 (2.2) |
| Modified alternative health eating index score | 37.4 (9.0) | 38.0 (8.9) | 41.2 (9.4) | 38.7 (9.3) | 39.9 (9.3) | 42.8 (9.1) |
Values were standardized to the age distribution of the study population.
Data are represented as mean (SD), unless otherwise indicated.
Values were not age adjusted.
During 34 y of follow-up (3,427,156 person-years), we documented 50,734 deaths, including 12,492 CVD deaths, 13,726 cancer deaths, and 24,516 other deaths (other non-CVD and noncancer deaths). In multivariable-adjusted analysis comparing extreme quintiles, higher total phytoestrogen intake was associated with lower risk of total mortality, CVD mortality, and other mortality types with pooled HR and a 95% confidence interval (CI) of 0.89 (0.87, 0.92) for total mortality, a 95% CI of 0.90 (0.85, 0.96) for CVD mortality, and that of 0.86 (0.82, 0.90) for other mortality (all Ptrend < 0.001), whereas the intake of total phytoestrogens was not associated with cancer mortality (Table 2, Table 3).
Table 2.
Associations of dietary phytoestrogens and total mortality
|
Quintiles of dietary phytoestrogen intake | ||||||
|
Total phytoestrogens |
Q1 |
Q2 |
Q3 |
Q4 |
Q5 |
Ptrend |
| Nurses’ Health Study | ||||||
| Median intake, umol/d | 1.2 | 1.8 | 2.3 | 3.0 | 8.1 | |
| Number of case/person y | 7300/451,262 | 6478/453,112 | 6059/453,855 | 5834/454,119 | 4707/456,011 | |
| Model 1 | 1 | 0.88 (0.85, 0.90) | 0.84 (0.81, 0.87) | 0.84 (0.82, 0.87) | 0.71 (0.69, 0.74) | <0.0001 |
| Model 2 | 1 | 0.97 (0.94, 1.00) | 0.94 (0.91, 0.97) | 0.93 (0.90, 0.96) | 0.85 (0.81, 0.88) | <0.0001 |
| Model 3 | 1 | 0.97 (0.94, 1.01) | 0.95 (0.91, 0.98) | 0.94 (0.91, 0.98) | 0.88 (0.84, 0.91) | <0.0001 |
| Health Professionals Follow-Up Study | ||||||
| Median intake, umol/d | 1.4 | 2.1 | 2.7 | 4.0 | 12.2 | |
| Number of case/person ys | 4983/230,161 | 4680/230,932 | 4120/231,888 | 3581/232,449 | 2992/233,368 | |
| Model 1 | 1 | 0.91 (0.87, 0.94) | 0.85 (0.82, 0.89) | 0.82 (0.78, 0.86) | 0.74 (0.71, 0.78) | <0.0001 |
| Model 2 | 1 | 1.02 (0.98, 1.06) | 1.00 (0.96, 1.05) | 0.97 (0.92, 1.01) | 0.87 (0.83, 0.91) | <0.0001 |
| Model 3 | 1 | 1.03 (0.99, 1.07) | 1.02 (0.97, 1.06) | 0.99 (0.95, 1.04) | 0.92 (0.88, 0.97) | <0.0001 |
| Pooled | ||||||
| Model 1 | 1 | 0.89 (0.86, 0.91) | 0.84 (0.82, 0.87) | 0.83 (0.81, 0.85) | 0.72 (0.70, 0.74) | <0.0001 |
| Model 2 | 1 | 0.99 (0.96, 1.01) | 0.96 (0.94, 0.99) | 0.94 (0.92, 0.97) | 0.86 (0.83, 0.88) | <0.0001 |
| Model 3 | 1 | 1.00 (0.97, 1.02) | 0.97 (0.95, 1.00) | 0.96 (0.93, 0.99) | 0.89 (0.87, 0.92) | <0.0001 |
|
Isoflavones |
Q1 |
Q2 |
Q3 |
Q4 |
Q5 |
Ptrend |
| Nurses’ Health Study | ||||||
| Median intake, mg/d | 0.2 | 0.3 | 0.4 | 0.5 | 1.5 | |
| Number of case/person y | 7301/452,782 | 6391/452,305 | 5987/454,122 | 5884/453,456 | 4815/455,693 | |
| Model 1 | 1 | 0.89 (0.86, 0.92) | 0.87 (0.84, 0.90) | 0.89 (0.86, 0.92) | 0.75 (0.73, 0.78) | <0.0001 |
| Model 2 | 1 | 0.97 (0.94, 1.01) | 0.96 (0.93, 1.00) | 0.95 (0.92, 0.99) | 0.86 (0.83, 0.90) | <0.0001 |
| Model 3 | 1 | 0.97 (0.94, 1.00) | 0.96 (0.93, 0.99) | 0.96 (0.92, 0.99) | 0.89 (0.85, 0.92) | <0.0001 |
| Health Professionals Follow-Up Study | ||||||
| Median intake, mg/d | 0.2 | 0.3 | 0.5 | 0.7 | 2.5 | |
| Number of case/person y | 5103/228,812 | 4520/232,517 | 4057/232,035 | 3645/232,363 | 3031/233,070 | |
| Model 1 | 1 | 0.90 (0.87, 0.94) | 0.86 (0.82, 0.89) | 0.86 (0.82, 0.89) | 0.76 (0.73, 0.80) | <0.0001 |
| Model 2 | 1 | 1.01 (0.97, 1.05) | 0.99 (0.95, 1.04) | 0.99 (0.95, 1.04) | 0.88 (0.84, 0.92) | <0.0001 |
| Model 3 | 1 | 1.01 (0.97, 1.05) | 0.99 (0.95, 1.04) | 1.01 (0.96, 1.05) | 0.92 (0.87, 0.96) | <0.0001 |
| Pooled | ||||||
| Model 1 | 1 | 0.90 (0.87, 0.92) | 0.86 (0.84, 0.89) | 0.87 (0.85, 0.90) | 0.76 (0.73, 0.78) | <0.0001 |
| Model 2 | 1 | 0.99 (0.96, 1.01) | 0.98 (0.95, 1.00) | 0.97 (0.94, 1.00) | 0.87 (0.84, 0.89) | <0.0001 |
| Model 3 | 1 | 0.99 (0.96, 1.01) | 0.97 (0.95, 1.00) | 0.98 (0.95, 1.00) | 0.90 (0.87, 0.92) | <0.0001 |
|
Lignans |
Q1 |
Q2 |
Q3 |
Q4 |
Q5 |
Ptrend |
| Nurses’ Health Study | ||||||
| Median intake, ug/d | 169.0 | 210.9 | 244.3 | 283.6 | 361.1 | |
| Number of case/person y | 6836/452,147 | 6106/453,463 | 5856/453,978 | 5758/454,275 | 5822/454,494 | |
| Model 1 | 1 | 0.83 (0.80, 0.86) | 0.76 (0.74, 0.79) | 0.72 (0.70, 0.75) | 0.69 (0.67, 0.72) | <0.0001 |
| Model 2 | 1 | 0.92 (0.89, 0.95) | 0.90 (0.87, 0.94) | 0.90 (0.87, 0.93) | 0.87 (0.84, 0.90) | <0.0001 |
| Model 3 | 1 | 0.94 (0.91, 0.97) | 0.94 (0.90, 0.97) | 0.95 (0.91, 0.98) | 0.94 (0.90, 0.97) | 0.95 |
| Health Professionals Follow-Up Study | ||||||
| Median intake, ug/d | 190.3 | 247.8 | 294.9 | 352.9 | 473.6 | |
| Number of case/person y | 4619/230,515 | 4397/231,170 | 4021/231,930 | 3787/232,351 | 3532/232,832 | |
| Model 1 | 1 | 0.90 (0.86, 0.94) | 0.82 (0.79, 0.86) | 0.76 (0.73, 0.79) | 0.70 (0.67, 0.73) | <0.0001 |
| Model 2 | 1 | 0.99 (0.95, 1.03) | 0.95 (0.91, 0.99) | 0.89 (0.85, 0.93) | 0.84 (0.80, 0.88) | <0.0001 |
| Model 3 | 1 | 1.01 (0.97, 1.06) | 0.99 (0.95, 1.04) | 0.95 (0.91, 1.00) | 0.92 (0.87, 0.96) | 0.005 |
| Pooled | ||||||
| Model 1 | 1 | 0.86 (0.83, 0.88) | 0.79 (0.77, 0.81) | 0.74 (0.72, 0.76) | 0.70 (0.68, 0.72) | <0.0001 |
| Model 2 | 1 | 0.95 (0.92, 0.97) | 0.92 (0.90, 0.95) | 0.90 (0.87, 0.92) | 0.85 (0.83, 0.88) | <0.0001 |
| Model 3 | 1 | 0.97 (0.94, 0.99) | 0.96 (0.93, 0.99) | 0.95 (0.92, 0.98) | 0.93 (0.90, 0.96) | 0.0005 |
|
Coumarins |
Q1 |
Q2 |
Q3 |
Q4 |
Q5 |
Ptrend |
| Nurses’ Health Study | ||||||
| Median intake, ug/d | 0.9 | 1.2 | 1.4 | 1.7 | 2.4 | |
| Number of case/person y | 7162/450,979 | 5849/454,617 | 5631/455,068 | 5635/453,741 | 6101/453,953 | |
| Model 1 | 1 | 0.81 (0.78, 0.84) | 0.75 (0.72, 0.77) | 0.71 (0.69, 0.74) | 0.72 (0.70, 0.75) | <0.0001 |
| Model 2 | 1 | 0.96 (0.92, 0.99) | 0.92 (0.89, 0.95) | 0.88 (0.85, 0.91) | 0.89 (0.86, 0.92) | <0.0001 |
| Model 3 | 1 | 0.97 (0.94, 1.01) | 0.94 (0.91, 0.98) | 0.91 (0.88, 0.95) | 0.92 (0.89, 0.96) | 0.0004 |
| Health Professionals Follow-Up Study | ||||||
| Median intake, ug/d | 1.0 | 1.3 | 1.5 | 1.9 | 2.6 | |
| Number of case/person y | 4484/230,538 | 3753/232,510 | 3723/231,428 | 3800/232,743 | 4596/231,578 | |
| Model 1 | 1 | 0.85 (0.81, 0.88) | 0.80 (0.77, 0.84) | 0.76 (0.73, 0.80) | 0.77 (0.74, 0.80) | <0.0001 |
| Model 2 | 1 | 0.94 (0.90, 0.98) | 0.93 (0.89, 0.97) | 0.90 (0.86, 0.94) | 0.89 (0.85, 0.93) | <0.0001 |
| Model 3 | 1 | 0.96 (0.92, 1.00) | 0.96 (0.92, 1.00) | 0.94 (0.90, 0.98) | 0.93 (0.89, 0.97) | 0.008 |
| Pooled | ||||||
| Model 1 | 1 | 0.82 (0.80, 0.84) | 0.77 (0.75, 0.79) | 0.73 (0.71, 0.75) | 0.74 (0.72, 0.76) | <0.0001 |
| Model 2 | 1 | 0.95 (0.92, 0.98) | 0.92 (0.90, 0.95) | 0.89 (0.86, 0.91) | 0.89 (0.86, 0.91) | <0.0001 |
| Model 3 | 1 | 0.97 (0.94, 0.99) | 0.95 (0.92, 0.98) | 0.92 (0.90, 0.95) | 0.93 (0.90, 0.95) | <0.0001 |
Values are hazard ratios (95% CIs).
Model 1: adjustment for age and race (Caucasians/other races).
Model 2: model 1 + smoking status (never, former, current [1–14, 15–24, or ≥25 cigarettes/d], or missing), alcohol intake (0, 0.1–4.9, 5.0–14.9, and ≥15.0 g/d for females; 0, 0.1–4.9, 5.0–29.9, and ≥30.0 g/d for males; or missing), physical activity (metabolic equivalents of task-hour/week), multivitamin use (yes/no), aspirin use (yes/no), history of hypertension (yes/no) and hypercholesterolemia (yes/no), family history of myocardial infarction (yes/no), family history of cancer (yes/no), family history of diabetes (yes/no), menopausal status and postmenopausal hormone use (premenopause, postmenopause [never, former, or current hormone use], or missing; for females), body mass index (<23, 23–24.9, 25–29.9, 30–34.9, >35 kg/m2, or missing), and total energy intake (kcal/d).
Model 3: model 2 + modified alternative health eating index score.
Pooled results were calculated using the fixed-effects model from Nurses’ Health Study and Health Professionals Follow-Up Study. Ptrend < 0.05 was considered statistically significant.
Table 3.
Pooled associations of dietary phytoestrogens and cause-specific mortality1
| CVD mortality | ||||||
|
Total phytoestrogens |
Q1 (Low) |
Q2 |
Q3 |
Q4 |
Q5 (High) |
Ptrend |
| Number of case/person y | 3144/681,423 | 2808/684,044 | 2461/685,743 | 2238/686,568 | 1841/689,379 | |
| Model 1 | 1 | 0.88 (0.84, 0.93) | 0.82 (0.78, 0.86) | 0.81 (0.77, 0.85) | 0.98 (0.92, 1.04) | <0.0001 |
| Model 2 | 1 | 0.98 (0.93, 1.03) | 0.95 (0.90, 1.00) | 0.93 (0.88, 0.99) | 0.86 (0.81, 0.92) | <0.0001 |
| Model 3 | 1 | 0.98 (0.93, 1.04) | 0.95 (0.90, 1.01) | 0.95 (0.90, 1.01) | 0.90 (0.85, 0.96) | 0.0006 |
|
Isoflavones |
Q1 |
Q2 |
Q3 |
Q4 |
Q5 |
Ptrend |
| Numebr of case/person y | 3211/681,594 | 2736/684,822 | 2403/686,157 | 2273/685,819 | 1869/688,763 | |
| Model 1 | 1 | 0.89 (0.84, 0.93) | 0.82 (0.78, 0.87) | 0.85 (0.81, 0.90) | 0.74 (0.70, 0.79) | <0.0001 |
| Model 2 | 1 | 0.97 (0.92, 1.03) | 0.94 (0.89, 0.99) | 0.96 (0.91, 1.02) | 0.87 (0.82, 0.92) | <0.0001 |
| Model 3 | 1 | 0.97 (0.92, 1.03) | 0.94 (0.89, 0.99) | 0.97 (0.91, 1.02) | 0.90 (0.84, 0.95) | 0.0008 |
|
Lignans |
Q1 |
Q2 |
Q3 |
Q4 |
Q5 |
Ptrend |
| Number of case/person y | 2883/682,662 | 2526/684,633 | 2475/685,908 | 2331/686,626 | 2277/687,326 | |
| Model 1 | 1 | 0.81 (0.77, 0.86) | 0.79 (0.74, 0.83) | 0.71 (0.68, 0.76) | 0.67 (0.63, 0.71) | <0.0001 |
| Model 2 | 1 | 0.90 (0.86, 0.96) | 0.93 (0.88, 0.98) | 0.88 (0.83, 0.93) | 0.84 (0.80, 0.89) | <0.0001 |
| Model 3 | 1 | 0.92 (0.87, 0.98) | 0.96 (0.90, 1.01) | 0.92 (0.87, 0.98) | 0.91 (0.85, 0.97) | 0.002 |
|
Coumarins |
Q1 |
Q2 |
Q3 |
Q4 |
Q5 |
Ptrend |
| Number of case/person y | 2848/681,517 | 2324/687,127 | 2259/686,496 | 2333/686,484 | 2728/685,531 | |
| Model 1 | 1 | 0.82 (0.78, 0.87) | 0.76 (0.72, 0.80) | 0.73 (0.69, 0.77) | 0.74 (0.70, 0.78) | <0.0001 |
| Model 2 | 1 | 0.93 (0.88, 0.98) | 0.89 (0.85, 0.95) | 0.88 (0.83, 0.93) | 0.88 (0.83, 0.93) | <0.0001 |
| Model 3 | 1 | 0.95 (0.89, 1.00) | 0.92 (0.87, 0.97) | 0.91 (0.86, 0.96) | 0.91 (0.86, 0.96) | 0.002 |
|
Cancer mortality | ||||||
|
Total phytoestrogens |
Q1 (Low) |
Q2 |
Q3 |
Q4 |
Q5 (High) |
Ptrend |
| Number of case/person y | 2789/681,423 | 2881/684,044 | 2912/685,743 | 2829/686,568 | 2315/689,379 | |
| Model 1 | 1 | 0.99 (0.94, 1.04) | 1.03 (0.97, 1.08) | 1.04 (0.99, 1.10) | 0.89 (0.84, 0.94) | <0.0001 |
| Model 2 | 1 | 1.04 (0.99, 1.10) | 1.07 (1.01, 1.13) | 1.06 (1.00, 1.12) | 0.95 (0.90, 1.01) | 0.003 |
| Model 3 | 1 | 1.05 (0.99, 1.10) | 1.08 (1.02, 1.13) | 1.07 (1.01, 1.13) | 0.97 (0.92, 1.03) | 0.04 |
|
Isoflavones |
Q1 |
Q2 |
Q3 |
Q4 |
Q5 |
Ptrend |
| Number of case/person y | 2833/681,594 | 2826/684,822 | 2901/686,157 | 2854/685,819 | 2312/688,763 | |
| Model 1 | 1 | 0.99 (0.94, 1.05) | 1.04 (0.99, 1.10) | 1.08 (1.02, 1.14) | 0.91 (0.86, 0.96) | <0.0001 |
| Model 2 | 1 | 1.04 (0.98, 1.09) | 1.08 (1.02, 1.14) | 1.07 (1.02, 1.13) | 0.95 (0.89, 1.00) | 0.001 |
| Model 3 | 1 | 1.04 (0.98, 1.09) | 1.08 (1.02, 1.14) | 1.08 (1.02, 1.14) | 0.96 (0.91, 1.02) | 0.02 |
|
Lignans |
Q1 |
Q2 |
Q3 |
Q4 |
Q5 |
Ptrend |
| Number of case/person y | 2956/682,662 | 2820/684,633 | 2617/685,908 | 2567/686,626 | 2766/687,326 | |
| Model 1 | 1 | 0.91 (0.86, 0.95) | 0.82 (0.78, 0.87) | 0.79 (0.75, 0.83) | 0.81 (0.77, 0.86) | <0.0001 |
| Model 2 | 1 | 0.98 (0.93, 1.03) | 0.93 (0.88, 0.98) | 0.91 (0.86, 0.96) | 0.94 (0.89, 0.99) | 0.003 |
| Model 3 | 1 | 0.99 (0.94, 1.04) | 0.94 (0.89, 1.00) | 0.94 (0.88, 0.99) | 0.98 (0.92, 1.04) | 0.23 |
|
Coumarins |
Q1 |
Q2 |
Q3 |
Q4 |
Q5 |
Ptrend |
| Number of case/person y | 3164/681,517 | 2626/687,127 | 2607/686,496 | 2490/686,484 | 2839/685,531 | |
| Model 1 | 1 | 0.84 (0.80, 0.88) | 0.81 (0.77, 0.85) | 0.74 (0.70, 0.78) | 0.77 (0.73, 0.81) | <0.0001 |
| Model 2 | 1 | 0.95 (0.90, 1.00) | 0.95 (0.90, 1.00) | 0.87 (0.83, 0.92) | 0.90 (0.85, 0.95) | 0.0001 |
| Model 3 | 1 | 0.95 (0.90, 1.00) | 0.95 (0.90, 1.01) | 0.88 (0.84, 0.93) | 0.91 (0.86, 0.96) | 0.001 |
|
Other mortality | ||||||
|
Total phytoestrogens |
Q1 (Low) |
Q2 |
Q3 |
Q4 |
Q5 (High) |
Ptrend |
| Number of case/person y | 6350/681,423 | 5469/684,044 | 4806/685,743 | 4348/686,568 | 3543/689,379 | |
| Model 1 | 1 | 0.85 (0.82, 0.88) | 0.78 (0.75, 0.81) | 0.76 (0.73, 0.79) | 0.66 (0.63, 0.69) | <0.0001 |
| Model 2 | 1 | 0.97 (0.93, 1.01) | 0.93 (0.89, 0.96) | 0.90 (0.86, 0.93) | 0.81 (0.78, 0.85) | <0.0001 |
| Model 3 | 1 | 0.98 (0.94, 1.02) | 0.94 (0.90, 0.97) | 0.92 (0.88, 0.96) | 0.86 (0.82, 0.90) | <0.0001 |
|
Isoflavones |
Q1 |
Q2 |
Q3 |
Q4 |
Q5 |
Ptrend |
| Number of case/person y | 6360/681,594 | 5349/684,822 | 4740/686,157 | 4402/685,819 | 3665/688,763 | |
| Model 1 | 1 | 0.87 (0.84, 0.90) | 0.81 (0.78, 0.84) | 0.80 (0.77, 0.83) | 0.71 (0.68, 0.74) | <0.0001 |
| Model 2 | 1 | 0.98 (0.94, 1.01) | 0.94 (0.91, 0.98) | 0.93 (0.89, 0.97) | 0.84 (0.81, 0.88) | <0.0001 |
| Model 3 | 1 | 0.97 (0.94, 1.01) | 0.94 (0.91, 0.98) | 0.94 (0.90, 0.98) | 0.88 (0.84, 0.92) | <0.0001 |
|
Lignans |
Q1 |
Q2 |
Q3 |
Q4 |
Q5 |
Ptrend |
| Number of case/person y | 5616/682,622 | 5157/684,633 | 4785/685,908 | 4647/686,626 | 4311/687,326 | |
| Model 1 | 1 | 0.86 (0.82, 0.89) | 0.78 (0.75, 0.81) | 0.73 (0.70, 0.76) | 0.66 (0.64, 0.69) | <0.0001 |
| Model 2 | 1 | 0.95 (0.92, 0.99) | 0.92 (0.89, 0.96) | 0.90 (0.87, 0.94) | 0.83 (0.79, 0.86) | <0.0001 |
| Model 3 | 1 | 0.98 (0.94, 1.02) | 0.97 (0.93, 1.01) | 0.97 (0.93, 1.01) | 0.92 (0.87, 0.96) | 0.03 |
|
Coumarins |
Q1 |
Q2 |
Q3 |
Q4 |
Q5 |
Ptrend |
| Number of case/person y | 5634/681,517 | 4652/687,127 | 4488/686,496 | 4612/686,484 | 5130/685,531 | |
| Model 1 | 1 | 0.82 (0.79, 0.85) | 0.76 (0.73, 0.79) | 0.73 (0.71, 0.76) | 0.73 (0.71, 0.76) | <0.0001 |
| Model 2 | 1 | 0.97 (0.93, 1.01) | 0.93 (0.89, 0.97) | 0.91 (0.88, 0.95) | 0.90 (0.86, 0.93) | <0.0001 |
| Model 3 | 1 | 0.99 (0.95, 1.03) | 0.97 (0.93, 1.01) | 0.96 (0.92, 1.00) | 0.95 (0.91, 0.99) | 0.004 |
Values are hazard ratios (95% CIs).
Model 1: adjustment for age and race (Caucasians/other races).
Model 2: model 1 + smoking status (never, former, current [1–14, 15–24, or ≥25 cigarettes/d], or missing), alcohol intake (0, 0.1–4.9, 5.0–14.9, and ≥15.0 g/d for females; 0, 0.1–4.9, 5.0–29.9, and ≥30.0 g/d for males; or missing), physical activity (metabolic equivalents of task-hour/week), multivitamin use (yes/no), aspirin use (yes/no), history of hypertension (yes/no) and hypercholesterolemia (yes/no), family history of myocardial infarction (yes/no), family history of cancer (yes/no), family history of diabetes (yes/no), menopausal status and postmenopausal hormone use (premenopause, postmenopause [never, former, or current hormone use], or missing; for females), body mass index (<23, 23–24.9, 25–29.9, 30–34.9, >35 kg/m2, or missing), and total energy intake (kcal/d).
Model 3: model 2 + modified alternative health eating index score.
Results from Nurses’ Health Study and Health Professionals Follow-Up Study were calculated using the fixed-effects model. Ptrend < 0.05 was considered statistically significant.
2The number of case/person-years was pooled from Nurses’ Health Study and Health Professionals Follow-Up Study.
The median values of total phytoestrogen, isoflavones, lignans, and coumarins within each quintile in Nurses’ Health Study and Health Professionals Follow-Up Study were the same with those in Table 1.
Furthermore, the three subclasses of phytoestrogens—isoflavones, lignans, and coumarins—were all inversely associated with mortality. Specifically, higher intake of isoflavones was associated with lower risk of total mortality [0.90 (0.87, 0.92); Ptrend < 0.0001], CVD mortality [0.90 (0.84, 0.95); Ptrend = 0.0008], and other types of mortality [0.88 (0.84, 0.92); Ptrend < 0.0001], but not cancer mortality. Similarly, higher intake of lignans was associated with lower risk of total mortality, CVD mortality, and other types of mortality only. Higher intake of coumarins was associated with lower risk of total mortality, CVD mortality, cancer mortality, and other types of mortality (Table 2, Table 3).
The associations for individual isoflavones and lignans mirrored those for total intakes. For example, higher intake of daidzein, genistein, and glycitein was associated with lower risk of total mortality, CVD mortality, and other types of mortality. Higher intake of secoisolariciresinol, matairesinol, and pinoresinol was associated with lower risk of total mortality, CVD mortality, cancer mortality, and other types of mortality (Supplementary Table 2).
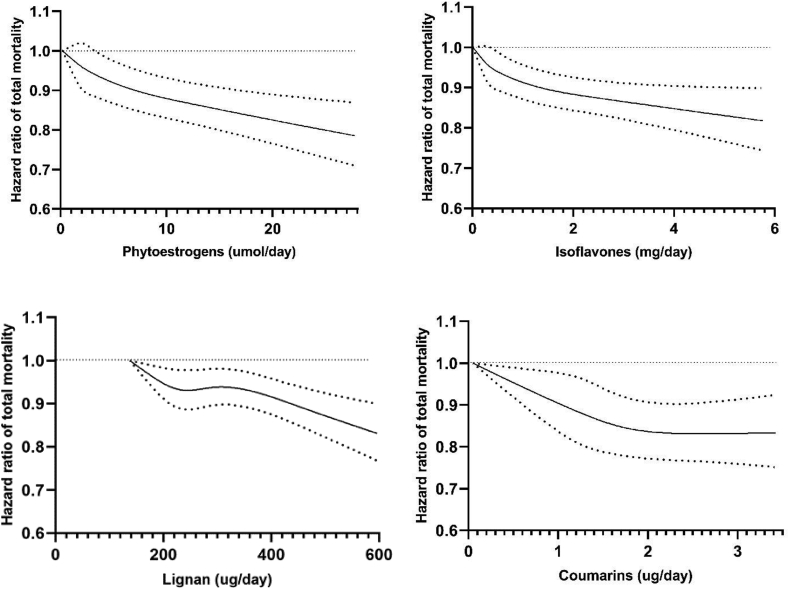
In the cubic spline regression, we found that the total mortality risk reduction flattened between ∼210 ug/d and ∼300 ug/d for lignans (Pnonlinearity = 0.04) and after ∼2 ug/d for coumarins (Pnonlinearity = 0.003), whereas the relationships of total phytoestrogens and isoflavones with total mortality appeared to be more linear (Figure). Supplementary Figure 4 shows inverse linear dose–response associations of daidzein, secoisolariciresinol, pinoresinol, and lariciresinol with total mortality, whereas the dose–response relationships of genistein, glycitein, and matairesinol were significantly nonlinear (all Pnonlinearity < 0.001).
Figure.
Dose–response analyses between phytoestrogen intake and total mortality. Data were truncated at 2.5 and 97.5th percentiles of dietary phytoestrogen intake to limit the impact of extreme values. Dose–response relationships between phytoestrogens and total mortality were estimated by restricted cubic spline Cox proportional-hazards model among 118,618 participants. Multivariable model adjusted for age, ethnicity (Caucasians/other races), smoking status (never, former, current [1–14, 15–24, or ≥25 cigarettes/d], or missing), alcohol intake (0, 0.1–4.9, 5.0–14.9, and ≥15.0 g/d for females; 0, 0.1–4.9, 5.0–29.9, and ≥30.0 g/d for males; or missing), physical activity (metabolic equivalents of task-hour/week), multivitamin use (yes/no), aspirin use (yes/no), history of hypertension (yes/no) and hypercholesterolemia (yes/no), family history of myocardial infarction (yes/no), family history of cancer (yes/no), family history of diabetes (yes/no), menopausal status and postmenopausal hormone use (premenopause, postmenopause [never, former, or current hormone use], or missing; for females), body mass index (<23, 23–24.9, 25–29.9, 30–34.9, >35 kg/m2, or missing), total energy intake (kcal/d), and modified alternative health eating index score. Solid line is point estimate, and dashed lines are 95% CIs. P values for nonlinearity of total phytoestrogens, isoflavones, lignans, and coumarins with total mortality were 0.10, 0.07, 0.04, and 0.003, respectively. P value for nonlinearity <0.05 was considered statistically significant.
Further analyses on food groups showed that tofu, soy milk, whole grains, tea, flaxseeds, and flaxseed oil were all inversely associated with total mortality (Table 4). For instance, compared with the participants who consumed <1 serving/month of tofu or soy milk, the participants who consumed ≥1 serving/week of tofu or soy milk had a 15%–16% lower risk of total mortality. Higher intake of tofu was also inversely associated with CVD mortality and other mortality, and higher intake of soy milk was associated with lower risk of other types of mortality. Furthermore, we observed that higher intake of whole grains and tea was also associated with lower risks of CVD, cancer, and other types of mortality (Table 4).
Table 4.
Associations of main food sources of phytoestrogens and total and cause-specific mortality
| Tofu | ||||||
| <1 serving/month |
<1 serving/week |
≥1 serving/week |
Ptrend |
|||
| Total mortality | 1 | 0.93 (0.90, 0.95) | 0.85 (0.80, 0.89) | <0.0001 | ||
| CVD mortality | 1 | 0.92 (0.87, 0.96) | 0.86 (0.77, 0.96) | 0.001 | ||
| Cancer mortality | 1 | 0.92 (0.88, 0.97) | 0.98 (0.89, 1.07) | 0.42 | ||
| Other mortality | 1 | 0.93 (0.90, 0.97) | 0.78 (0.73, 0.84) | <0.0001 | ||
|
Soy milk | ||||||
|
<1 serving/month |
<1 serving/week |
≥1 serving/week |
Ptrend |
|||
| Total mortality | 1 | 0.73 (0.67, 0.80) | 0.84 (0.79, 0.89) | <0.0001 | ||
| CVD mortality | 1 | 0.97 (0.81, 1.15) | 0.89 (0.78, 1.01) | 0.06 | ||
| Cancer mortality | 1 | 0.75 (0.62, 0.90) | 0.91 (0.80, 1.26) | 0.09 | ||
| Other mortality | 1 | 0.89 (0.79, 1.00) | 0.89 (0.82, 0.97) | 0.005 | ||
|
Whole grains | ||||||
|
Q1 (Low) |
Q2 |
Q3 |
Q4 |
Q5 (High) |
Ptrend |
|
| Total mortality | 1 | 0.93 (0.91, 0.96) | 0.90 (0.87, 0.92) | 0.86 (0.83, 0.88) | 0.81 (0.78, 0.83) | <0.0001 |
| CVD mortality | 1 | 0.86 (0.81, 0.91) | 0.85 (0.80, 0.89) | 0.79 (0.75, 0.83) | 0.76 (0.72, 0.81) | <0.0001 |
| Cancer mortality | 1 | 0.93 (0.88, 0.98) | 0.88 (0.84, 0.93) | 0.84 (0.80, 0.89) | 0.79 (0.75, 0.84) | <0.0001 |
| Other mortality | 1 | 0.97 (0.93, 1.01) | 0.94 (0.90, 0.98) | 0.91 (0.87, 0.95) | 0.85 (0.81, 0.89) | <0.0001 |
|
Tea | ||||||
|
<1 cup/week |
<1 cup/d |
>1 cup/d |
Ptrend |
|||
| Total mortality | 1 | 0.95 (0.93, 0.97) | 0.94 (0.91, 0.96) | <0.0001 | ||
| CVD mortality | 1 | 0.97 (0.94, 1.01) | 0.93 (0.88, 0.98) | 0.01 | ||
| Cancer mortality | 1 | 0.93 (0.90, 0.97) | 0.94 (0.89, 0.98) | 0.01 | ||
| Other mortality | 1 | 0.96 (0.93, 0.99) | 0.95 (0.91, 0.98) | 0.005 | ||
|
Flaxseed1 | ||||||
|
No consumption |
Consumption |
|||||
| Total mortality | 1 | 0.89 (0.83, 0.96) | ||||
| CVD mortality | 1 | 1.05 (0.90, 1.23) | ||||
| Cancer mortality | 1 | 0.85 (0.73, 1.00) | ||||
| Other mortality | 1 | 0.94 (0.86, 1.04) | ||||
|
Flaxseed oil1 | ||||||
|
No consumption |
Consumption |
|||||
| Total mortality | 1 | 0.91 (0.84, 0.99) | ||||
| CVD mortality | 1 | 0.99 (0.83, 1.18) | ||||
| Cancer mortality | 1 | 1.03 (0.86, 1.23) | ||||
| Other mortality | 1 | 0.92 (0.83, 1.02) | ||||
Data were combined from Nurses’ Health Study and Health Professionals Follow-Up Study. Estimates were calculated in Cox proportional-hazards models and adjusted for age, ethnicity (Caucasians/other races), smoking status (never, former, current [1–14, 15–24, or ≥25 cigarettes/d], or missing), alcohol intake (0, 0.1–4.9, 5.0–14.9, and ≥15.0 g/d for females; 0, 0.1–4.9, 5.0–29.9, and ≥30.0 g/d for males; or missing), physical activity (metabolic equivalents of task-hour/week), multivitamin use (yes/no), aspirin use (yes/no), history of hypertension (yes/no) and hypercholesterolemia (yes/no), family history of myocardial infarction (yes/no), family history of cancer (yes/no), family history of diabetes (yes/no), menopausal status and postmenopausal hormone use (premenopause, postmenopause [never, former, or current hormone use], or missing; for females), body mass index (<23, 23–24.9, 25–29.9, 30–34.9, >35 kg/m2, or missing), and total energy intake (kcal/d), and modified alternative health eating index score. Ptrend < 0.05 was considered statistically significant.
The analysis involving flaxseed products only included data after 2006 when they were specifically assessed.
The significant association between total phytoestrogens and total mortality persisted when the analyses were stratified by age, BMI, physical activity, smoking status, alcohol intake, and intake of animal-based foods, and none of the tests for interaction was statistically significant (Supplementary Table 3). However, the association of total phytoestrogens and total mortality was slightly stronger in the participants with higher modified AHEI scores [0.86 (0.83, 0.90)] than those who had lower modified AHEI scores (0.91 (0.87, 0.96); P for interaction = 0.001) (Supplementary Table 3). We also observed that the association of total lignans with total mortality was stronger among the participants with higher modified AHEI scores (Supplementary Table 4).
Our findings remained robust in several sensitivity analyses. We observed similar results when a 4-y lag was added between dietary phytoestrogens and total and cause-specific mortality. For example, comparing extreme quintiles, the HRs (95% CIs) of total mortality were 0.88 (0.85, 0.90) for total phytoestrogens, 0.89 (0.86, 0.91) for isoflavones, 0.94 (0.90, 0.97) for lignans, and 0.92 (0.90, 0.95) for coumarins (Supplementary Table 5). The results were also similar when an 8-y lag was applied. Furthermore, adjustment for other major dietary variables, healthy plant-based diet index, or the two other versions of the modified AHEI, excluding whole grains or soy products, respectively, did not materially change the results (Supplementary Table 5). The results were also similar when phytoestrogen intake was categorized into the deciles (Supplementary Table 6). Moreover, mutual adjustment for isoflavones, lignans, and coumarins did not substantially change the results (Supplementary Table 7). In addition, we observed that the inverse associations of dietary phytoestrogens with other mortality were mainly driven by neurodegenerative disease mortality and kidney disease mortality (Supplementary Table 8).
Discussion
In the present study, we found that higher intake of total phytoestrogens, isoflavones, lignans, and coumarins was significantly associated with lower risks of total, CVD, and other types of mortality, but not cancer mortality. The inverse associations were also observed for individual isoflavones, including daidzein, genistein, and glycitein, as well as secoisolariciresinol, matairesinol, and pinoresinol, whereas lariciresinol was not associated with mortality. Dose–response analyses showed largely linear relationships for total phytoestrogens and isoflavones. Furthermore, higher intake of the primary food sources of phytoestrogens, such as tofu, soy milk, whole grains, tea, flaxseed, and flaxseed oil, was also associated with a lower risk of mortality.
Comparison with previous studies
Our results are consistent with those of several previous studies. A meta-analysis of 5 prospective studies with approximately 50,000 participants showed that compared with those in the lowest category, participants in the highest category of soy isoflavone intake had a 10% lower risk of all-cause mortality [32]. A more recent prospective study reported that higher intake of total isoflavones, daidzein, genistein, and glycitein was associated with a lower risk of total mortality in a middle-aged Japanese population [19]. The aforementioned meta-analysis reported null association of total isoflavones with CVD mortality [32], whereas in our study, total and individual isoflavones were also significantly associated with a lower risk of CVD mortality. For lignans, a meta-analysis of three prospective studies consisting of 48,364 participants showed null associations of lignan intake with total and CVD mortality [33]. Of the three studies, only the study by Milder et al., in the Zutphen Elderly Study of 570 Dutch males aged 64–84 y, examined the associations of 4 individual lignans [18], and the authors observed an inverse association of matairesinol, but not lariciresinol, pinoresinol, or secoisolariciresinol, with total and CVD mortality. In our study, we observed that higher intake of lignans and secoisolariciresinol, matairesinol, and pinoresinol was significantly associated with lower risk of total, CVD, and other types of mortality. The discrepancy between our findings and the prior meta-analysis may be partially due to the sample size, as the meta-analysis included 48,364 participants and 2634 deaths, which may have limited its ability to detect more modest associations. We found no associations of total phytoestrogens, isoflavones, or lignans with cancer mortality. Inverse associations between dietary isoflavones and the incidence of several types of cancers (e.g., breast cancers) have been reported previously [34], and several prospective studies on Asian populations have reported inverse associations of isoflavones and breast cancer mortality, where soy products and isoflavones were more commonly consumed [32]. However, evidence in relation to other cancers has been sparse.
Leveraging the large sample size, long duration of follow-up, and repeated dietary assessments, we made some novel observations in the current analysis. We observed inverse associations not only for lignans but also for coumarins, a relatively minor group of phytoestrogens. We also observed inverse associations of phytoestrogens with neurodegenerative disease mortality and kidney disease mortality. To our knowledge, no previous studies have evaluated these associations. Furthermore, we found linear dose–response relationships for total phytoestrogens and isoflavones with total mortality in our cubic spline regression. A similar dose–response association was also observed when we examined deciles of phytoestrogen intake in relation to total mortality. Finally, the inverse associations of dietary phytoestrogens with mortality were supported by our analyses of phytoestrogen-rich foods and mortality. The inverse associations for tofu, soy milk, whole grains, tea, flaxseeds, and flaxseed oil with mortality are generally in line with those of previous studies [32, [35], [36], [37]]. Furthermore, our previous analysis has also indicated that increased consumption of unhealthy plant-based diets that de-emphasize the phytoestrogen-rich foods (e.g., whole grains, legumes, and tea) was associated with higher mortality [38]. Although our observed associations warrant further investigation in future studies, taken together, the findings overall suggest that phytoestrogens are associated with a lower risk of premature deaths.
Mechanisms
Several mechanisms may explain the beneficial associations of dietary phytoestrogens with mortality. Dietary phytoestrogens are bioactive compounds with weak estrogenic activity and are able to regulate certain gene expressions by binding the ERα and ERβ [3]. This can in turn exert beneficial effects on endothelial function and lipid peroxidation, reduce inflammatory cytokines, and contribute to DNA repair [3]. Isoflavones preferably bind ERβ, leading to subsequent ER-mediated gene transcription [39, 40]. ERβ is highly expressed in coronary vessels [41], and the activation of membrane ERβ initiates a cascade of intracellular mechanisms, such as changes in membrane permeability, expression of endothelial nitric oxide-synthase, ion concentration, and rapid vasodilatation of blood vessels, which can reduce oxidative stress and inflammation in vascular cells [42, 43]. Indeed, our previous analysis reported that higher intake of soy isoflavones and soy products was associated with a lower risk of coronary heart disease [9]. Furthermore, some meta-analyses reported that isoflavones could improve lipid profiles [44]. The mechanisms may involve the regulation of cellular cholesterol efflux from macrophages and hepatic paraoxonase 1 expression and activity [44]. In addition, dietary phytoestrogens may interact with human gut microbiota and produce bioactive compounds that beneficially influence human health. Evidence has indicated that plant lignans can be processed by the human gut microbiome to produce more bioactive enterolignans [45], and higher circulating concentrations of enterolignans have been associated with a lower risk of cardiovascular mortality [46]. Indeed, in the present analysis, we observed the inverse associations of phytoestrogens; especially, the association of lignans with mortality was stronger in participants with a higher AHEI score, which is generally associated with greater gut microbiome diversity [47].
Strengths and limitations
The strengths of our study include the use of data from two large cohort studies with longterm follow-up, repeated assessments of diet and lifestyle, and low rates of loss to follow-up. In addition, along with total dietary phytoestrogens and its three primary classes—isoflavones, lignans, and coumarins, our study also included 3 individual isoflavones, 4 individual lignans, and 6 primary phytoestrogen-rich foods, which together demonstrated consistent associations.
There are also several limitations that are worth discussing. First, the measurement errors for dietary phytoestrogen assessments are inevitable. However, due to the prospective nature of our analysis, measurement error is likely to be nondifferential with respect to the assessments of outcomes of interest, and therefore would tend to bias the risk estimates toward the null. Moreover, the use of cumulatively averaged intake helps reduce random measurement errors and within-person variations. Second, the participants were mainly health professionals of European ancestry, which limits the generalizability of our findings to other racial/ethnic or socioeconomic groups. However, the homogeneity of socioeconomic status helps reduce potential confounding due to socioeconomic status or cultural practices. Third, certain food sources of isoflavones are not widely consumed in Western populations, which may have limited the range of exposure that we were able to assess. However, our results were in line with those of previous studies in Asian populations with high intake of isoflavones. Fourth, it is possible that other potential additive or synergistic effects between dietary phytoestrogens and other nutrients or non-nutrient constituents in phytoestrogen-rich foods have contributed to the observed associations to some extent. Finally, despite extensive statistical adjustments for demographic, lifestyle, and clinical risk factors, residual or unmeasured confounding cannot be ruled out, and hence, we cannot establish a causal relationship, particularly given the modest size of the associations.
Conclusions
Higher consumption of total phytoestrogens, isoflavones, lignans, coumarins, and their primary food sources was significantly associated with a lower risk of total, CVD, and other mortality. Our findings provide further support for the recommendation of adhering to healthy plant-based dietary patterns that emphasize more consumption of phytoestrogen-containing foods such as legumes, whole grains, fruits/vegetables, flaxseed products, and tea for improving health.
Author disclosures
The authors report no conflicts of interest.
Data Availability
Data described in the article, code book, and analytic code will not be made publicly available. Further information including the procedures to obtain and access data from the Nurses’ Health Study and Health Professionals’ Follow-up Study is described at http://www.nurseshealthstudy.org/researchers (email: nhsaccess@channing.harvard.edu) and http://sites.sph.harvard.edu/hpfs/for-collaborators, respectively.
Funding
The NHS and HPFS studies and the current analysis were supported by the National Institutes of Health grants (UM1 CA186107, P01 CA87969, R01 CA49449, R01 HL034594, R01 HL088521, U01 CA176726, R01 CA67262, U01 CA167552, R01 HL035464, R01 HL060712, and R01 DK120870). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgments
The authors thank the participants and staff of the Nurses’ Health Study and Health Professionals Follow-Up Study for their valuable contributions and the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, and WY. The authors assume full responsibility for analyses and interpretation of these data.
The authors’ responsibilities were as follows – ZC, QS, FQ: conceived the study; QS, EBR: were involved in data collection; ZC: analyzed the data; QS, YH: provided statistical expertise; ZC: wrote the first draft of the paper; QS, ZC: are the guarantors; QS, ZC: had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis; and all authors: contributed to the interpretation of the results and revision of the manuscript for important intellectual content, and read and approved the final version of the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajcnut.2022.10.019.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Viggiani M.T., Polimeno L., Di Leo A., Barone M. Phytoestrogens: dietary intake, bioavailability, and protective mechanisms against colorectal neoproliferative lesions. Nutrients. 2019;11(8):1709. doi: 10.3390/nu11081709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Usda HJUDoH . Dietary guidelines for Americans; Washington D, USA: 2015. Services H, US Department of Agriculture; pp. 2015–2020. [Google Scholar]
- 3.Desmawati D., Sulastri D. Phytoestrogens and their health effect. Open Access Maced J Med Sci. 2019;7(3):495–499. doi: 10.3889/oamjms.2019.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Godos J., Bergante S., Satriano A., Pluchinotta F.R., Marranzano M. Dietary phytoestrogen intake is inversely associated with hypertension in a cohort of adults living in the Mediterranean area. Molecules. 2018;23(2):368. doi: 10.3390/molecules23020368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu X., Gao J., Zhang Q., Fu Y., Li K., Zhu S., et al. Soy fiber improves weight loss and lipid profile in overweight and obese adults: a randomized controlled trial. Mol Nutr Food Res. 2013;57(12):2147–2154. doi: 10.1002/mnfr.201300159. [DOI] [PubMed] [Google Scholar]
- 6.Sacks F.M., Lichtenstein A., Van Horn L., Harris W., Kris-Etherton P., Winston M., et al. Soy protein, isoflavones, and cardiovascular health: an American Heart Association Science Advisory for professionals from the Nutrition Committee. Circulation. 2006;113(7):1034–1044. doi: 10.1161/CIRCULATIONAHA.106.171052. [DOI] [PubMed] [Google Scholar]
- 7.Kreijkamp-Kaspers S., Kok L., Bots M.L., Grobbee D.E., van der Schouw Y.T. Dietary phytoestrogens and plasma lipids in Dutch postmenopausal women; a cross-sectional study. Atherosclerosis. 2005;178(1):95–100. doi: 10.1016/j.atherosclerosis.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Pellegrini N., Valtueña S., Ardigò D., Brighenti F., Franzini L., Del Rio D., et al. Intake of the plant lignans matairesinol, secoisolariciresinol, pinoresinol, and lariciresinol in relation to vascular inflammation and endothelial dysfunction in middle age-elderly men and post-menopausal women living in Northern Italy. Nutr Metab Cardiovasc Dis. 2010;20(1):64–71. doi: 10.1016/j.numecd.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Ma L., Liu G., Ding M., Zong G., Hu F.B., Willett W.C., et al. Isoflavone intake and the risk of coronary heart disease in US men and women: results from 3 prospective cohort studies. Circulation. 2020;141(14):1127–1137. doi: 10.1161/CIRCULATIONAHA.119.041306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kokubo Y., Iso H., Ishihara J., Okada K., Inoue M., Tsugane S., et al. Association of dietary intake of soy, beans, and isoflavones with risk of cerebral and myocardial infarctions in Japanese populations: the Japan public health center-based (JPHC) study cohort I. Circulation. 2007;116(22):2553–2562. doi: 10.1161/CIRCULATIONAHA.106.683755. [DOI] [PubMed] [Google Scholar]
- 11.Hu Y., Li Y., Sampson L., Wang M., Manson J.E., Rimm E., et al. Lignan intake and risk of coronary heart disease. J Am Coll Cardiol. 2021;78(7):666–678. doi: 10.1016/j.jacc.2021.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Kleijn M.J., van der Schouw Y.T., Wilson P.W., Grobbee D.E., Jacques P.F. Dietary intake of phytoestrogens is associated with a favorable metabolic cardiovascular risk profile in postmenopausal U.S. Women: the Framingham study. J Nutr. 2002;132(2):276–282. doi: 10.1093/jn/132.2.276. [DOI] [PubMed] [Google Scholar]
- 13.Bedell S., Nachtigall M., Naftolin F. The pros and cons of plant estrogens for menopause. J Steroid Biochem Mol Biol. 2014;139:225–236. doi: 10.1016/j.jsbmb.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Zamora-Ros R., Jiménez C., Cleries R., Agudo A., Sánchez M.J., Sánchez-Cantalejo E., et al. Dietary flavonoid and lignan intake and mortality in a Spanish cohort. Epidemiology. 2013;24(5):726–733. doi: 10.1097/EDE.0b013e31829d5902. [DOI] [PubMed] [Google Scholar]
- 15.Nagata C., Takatsuka N., Shimizu H. Soy and fish oil intake and mortality in a Japanese community. Am J Epidemiol. 2002;156(9):824–831. doi: 10.1093/aje/kwf118. [DOI] [PubMed] [Google Scholar]
- 16.Yamasaki K., Kayaba K., Ishikawa S. Soy and soy products intake, all-cause mortality, and cause-specific mortality in Japan: the Jichi Medical School cohort study. Asia Pac J Public Health. 2015;27(5):531–541. doi: 10.1177/1010539514539545. [DOI] [PubMed] [Google Scholar]
- 17.Katagiri R., Sawada N., Goto A., Yamaji T., Iwasaki M., Noda M., et al. Association of soy and fermented soy product intake with total and cause specific mortality: prospective cohort study. BMJ (Clin Res Ed). 2020;368:m34. doi: 10.1136/bmj.m34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Milder I.E., Feskens E.J., Arts I.C., Bueno-de-Mesquita H.B., Hollman P.C., Kromhout D. Intakes of 4 dietary lignans and cause-specific and all-cause mortality in the Zutphen Elderly Study. Am J Clin Nutr. 2006;84(2):400–405. doi: 10.1093/ajcn/84.1.400. [DOI] [PubMed] [Google Scholar]
- 19.Nakamoto M., Otsuka R., Tange C., Nishita Y., Tomida M., Imai T., et al. Intake of isoflavones reduces the risk of all-cause mortality in middle-aged Japanese. Eur J Clin Nutr. 2021;75(12):1781–1791. doi: 10.1038/s41430-021-00890-w. [DOI] [PubMed] [Google Scholar]
- 20.Ponzo V., Goitre I., Fadda M., Gambino R., De Francesco A., Soldati L., et al. Dietary flavonoid intake and cardiovascular risk: a population-based cohort study. J Transl Med. 2015;13(1):218. doi: 10.1186/s12967-015-0573-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mink P.J., Scrafford C.G., Barraj L.M., Harnack L., Hong C.P., Nettleton J.A., et al. Flavonoid intake and cardiovascular disease mortality: a prospective study in postmenopausal women. Am J Clin Nutr. 2007;85(3):895–909. doi: 10.1093/ajcn/85.3.895. [DOI] [PubMed] [Google Scholar]
- 22.Colditz G.A., Manson J.E., Hankinson S.E. The nurses’ health study: 20-year contribution to the understanding of health among women. J Womens Health. 1997;6(1):49–62. doi: 10.1089/jwh.1997.6.49. [DOI] [PubMed] [Google Scholar]
- 23.Rimm E.B., Giovannucci E.L., Willett W.C., Colditz G.A., Ascherio A., Rosner B., et al. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet. 1991;338(8765):464–468. doi: 10.1016/0140-6736(91)90542-w. [DOI] [PubMed] [Google Scholar]
- 24.Agricultural Research Service. UDoA. Welcome to the United States National Nutrient Database for Standard Reference. [Internet]. Available from: https://ndb.nal.usda.gov/.
- 25.Hu F.B., Rimm E., Smith-Warner S.A., Feskanich D., Stampfer M.J., Ascherio A., et al. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am J Clin Nutr. 1999;69(2):243–249. doi: 10.1093/ajcn/69.2.243. [DOI] [PubMed] [Google Scholar]
- 26.Mullie P., Clarys P., Hulens M., Vansant G. Reproducibility and validity of a semiquantitative food frequency questionnaire among military men. Mil Med. 2009;174(8):852–856. doi: 10.7205/milmed-d-00-1409. [DOI] [PubMed] [Google Scholar]
- 27.Rimm E.B., Giovannucci E.L., Stampfer M.J., Colditz G.A., Litin L.B., Willett W.C. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135(10):1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. discussion 27–36. [DOI] [PubMed] [Google Scholar]
- 28.Feskanich D., Rimm E.B., Giovannucci E.L., Colditz G.A., Stampfer M.J., Litin L.B., et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993;93(7):790–796. doi: 10.1016/0002-8223(93)91754-e. [DOI] [PubMed] [Google Scholar]
- 29.Stampfer M.J., Willett W.C., Speizer F.E., Dysert D.C., Lipnick R., Rosner B., et al. Test of the national death index. Am J Epidemiol. 1984;119(5):837–839. doi: 10.1093/oxfordjournals.aje.a113804. [DOI] [PubMed] [Google Scholar]
- 30.McCullough M.L., Willett W.C. Evaluating adherence to recommended diets in adults: the Alternate Healthy Eating Index. Public Health Nutr. 2006;9(1A):152–157. doi: 10.1079/phn2005938. [DOI] [PubMed] [Google Scholar]
- 31.Chen Z., Drouin-Chartier J.P., Li Y., Baden M.Y., Manson J.E., Willett W.C., et al. Changes in plant-based diet indices and subsequent risk of type 2 diabetes in women and men: three U.S. Prospective cohorts. Diabetes Care. 2021;44(3):663–671. doi: 10.2337/dc20-1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nachvak S.M., Moradi S., Anjom-Shoae J., Rahmani J., Nasiri M., et al. Soy, soy isoflavones, and protein intake in relation to mortality from all causes, cancers, and cardiovascular diseases: A systematic review and dose-response meta-analysis of prospective cohort studies. J Acad Nutr Diet. 2019;119(9):1483–1500. doi: 10.1016/j.jand.2019.04.011. e17. [DOI] [PubMed] [Google Scholar]
- 33.Grosso G., Micek A., Godos J., Pajak A., Sciacca S., Galvano F., et al. Dietary flavonoid and lignan intake and mortality in prospective cohort studies: systematic review and dose-response meta-analysis. Am J Epidemiol. 2017;185(12):1304–1316. doi: 10.1093/aje/kww207. [DOI] [PubMed] [Google Scholar]
- 34.Gómez-Zorita S., González-Arceo M., Fernández-Quintela A., Eseberri I., Trepiana J., Portillo M.P. Scientific evidence supporting the beneficial effects of isoflavones on human health. Nutrients. 2020;12(12):3853. doi: 10.3390/nu12123853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mineharu Y., Koizumi A., Wada Y., Iso H., Watanabe Y., Date C., et al. Coffee, green tea, black tea and oolong tea consumption and risk of mortality from cardiovascular disease in Japanese men and women. J Epidemiol Community Health. 2011;65(3):230–240. doi: 10.1136/jech.2009.097311. [DOI] [PubMed] [Google Scholar]
- 36.Aune D., Keum N., Giovannucci E., Fadnes L.T., Boffetta P., Greenwood D.C., et al. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: systematic review and dose-response meta-analysis of prospective studies. BMJ (Clin Res Ed). 2016;353:i2716. doi: 10.1136/bmj.i2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu J., Liu S., Zhou H., Hanson T., Yang L., Chen Z., et al. Association of green tea consumption with mortality from all-cause, cardiovascular disease and cancer in a Chinese cohort of 165,000 adult men. Eur J Epidemiol. 2016;31(9):853–865. doi: 10.1007/s10654-016-0173-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baden M.Y., Liu G., Satija A., Li Y., Sun Q., Fung T.T., et al. Changes in plant-based diet quality and total and cause-specific mortality. Circulation. 2019;140(12):979–991. doi: 10.1161/CIRCULATIONAHA.119.041014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang Q., Payton-Stewart F., Elliott S., Driver J., Rhodes L.V., Zhang Q., et al. Effects of 7-O substitutions on estrogenic and anti-estrogenic activities of daidzein analogues in MCF-7 breast cancer cells. J Med Chem. 2010;53(16):6153–6163. doi: 10.1021/jm100610w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang Y., Gong P., Madak-Erdogan Z., Martin T., Jeyakumar M., Carlson K., et al. Mechanisms enforcing the estrogen receptor β selectivity of botanical estrogens. FASEB J. 2013;27(11):4406–4418. doi: 10.1096/fj.13-234617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Christian R.C., Liu P.Y., Harrington S., Ruan M., Miller V.M., Fitzpatrick L.A. Intimal estrogen receptor (ER)beta, but not ERalpha expression, is correlated with coronary calcification and atherosclerosis in pre- and postmenopausal women. J Clin Endocrinol Metab. 2006;91(7):2713–2720. doi: 10.1210/jc.2005-2672. [DOI] [PubMed] [Google Scholar]
- 42.Siow R.C.M., Mann G.E. Dietary isoflavones and vascular protection: activation of cellular antioxidant defenses by SERMs or hormesis? Mol Aspects Med. 2010;31(6):468–477. doi: 10.1016/j.mam.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 43.Siow R.C., Li F.Y., Rowlands D.J., de Winter P., Mann G.E. Cardiovascular targets for estrogens and phytoestrogens: transcriptional regulation of nitric oxide synthase and antioxidant defense genes. Free Radic Biol Med. 2007;42(7):909–925. doi: 10.1016/j.freeradbiomed.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 44.Millar C.L., Duclos Q., Blesso C.N. Effects of dietary flavonoids on reverse cholesterol transport, HDL metabolism, and HDL function. Adv Nutr. 2017;8(2):226–239. doi: 10.3945/an.116.014050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heinonen S., Nurmi T., Liukkonen K., Poutanen K., Wähälä K., Deyama T., et al. In vitro metabolism of plant lignans: new precursors of mammalian lignans enterolactone and enterodiol. J Agric Food Chem. 2001;49(7):3178–3186. doi: 10.1021/jf010038a. [DOI] [PubMed] [Google Scholar]
- 46.Rienks J., Barbaresko J., Nöthlings U. Association of polyphenol biomarkers with cardiovascular disease and mortality risk: a systematic review and meta-analysis of observational studies. Nutrients. 2017;9(4):415. doi: 10.3390/nu9040415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramos S., Martín M.Á. Impact of diet on gut microbiota. Curr Opin Food Sci. 2021;37:83–90. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the article, code book, and analytic code will not be made publicly available. Further information including the procedures to obtain and access data from the Nurses’ Health Study and Health Professionals’ Follow-up Study is described at http://www.nurseshealthstudy.org/researchers (email: nhsaccess@channing.harvard.edu) and http://sites.sph.harvard.edu/hpfs/for-collaborators, respectively.