Abstract
Background
Myelin imaging has increasingly been applied to study the impact of nutrition on brain development in recent years. Although individual dynamics for nutrient intakes and myelin trajectories previously have been investigated across childhood, the longitudinal interaction between both remains unclear in typically developed children.
Objectives
The objective of this work was to explore the developmental dynamics of nutrient-myelin interactions from infancy to early childhood using myelin imaging as a marker for brain maturation.
Methods
Brain neuroimaging (1 scan per child) and dietary nutrient intake data were analyzed for 88 nutrients from 293 children (127 female, 62% White) from a longitudinal cohort study in the United States. A sliding window approach was used to investigate correlations between nutrient intakes and brain myelination over a continuous set of age windows. Image processing techniques (Sobel-filter vertical edge detection) were applied to determine age windows with unique association profiles, providing novel insight into how these relationships change with child age.
Results
We identified 3 nutrient-myelin windows covering the age range of 1–5 y: window 1 from 6 to 20 mo with 60% positive nutrient correlations, window 2 from 20 to 30 mo with 20% positive correlations, and window 3 from 30 to 60 mo with 37% positive correlations. The windows are aligned with reported myelin and white matter dynamics that change in the first 5 y from fast and steep (window 1) to continued but slower growth (window 3), with window 2 possibly representing the inflection period.
Conclusions
To our knowledge, this is the first study in typically developing children demonstrating the developmental dynamics between early life nutrient intakes and brain maturation in toddlerhood. The knowledge can be applied for identifying targeted and brain-stage–appropriate nutritional interventions for this critical stage of brain development.
Keywords: nutrition, dynamics, myelination, brain development, MRI, toddlers
Introduction
Myelin imaging is a viable noninvasive in vivo technique that has helped to map brain structural maturation in infancy and childhood revealing specific developmental dynamics. Myelination of cerebral axons begins in utero and continues into the second and third decades of life [1]. It advances particularly rapidly over the first 2 y of life following a specific posterior-to-anterior pattern and in close interplay with emerging neurobehavioral development [[2], [3], [4], [5], [6], [7]].
Longitudinal myelin imaging studies report myelin trajectories in neurotypical infants and young children that show a steep initial increase in myelin content in the brain throughout infancy, an inflection point around 18–25 mo depending on the brain region, followed by a continuous but slower increase in myelin content throughout the remainder of childhood and adolescence [5, [8], [9], [10]]. Increasing evidence suggests alterations in myelin trajectories based on health and environmental factors, for example, in children with febrile seizures [11], in young adults with autism [12], and differences between formula-fed and breast-fed children [13, 14]. Differences in myelin development may be linked to developmental outcomes, as myelination has been linked to cognitive [5, [15], [16], [17]] and social-emotional development in young children [18].
Understanding the dynamics of brain development in relation to influencing factors is critical for identifying and studying early life interventions, such as nutritional opportunities. Myelin imaging has increasingly been applied to study the impact of nutrition on brain development in recent years, particularly in young, preverbal children as well as in animal models of development [13, 14, [19], [20], [21]]. These studies indicate the relation between nutritional factors and myelin development and suggest the modifiability of brain structural growth with nutritional modification. However, nutrient-brain interactions in development are complex. The high metabolic demand and the high degree of plasticity make early life a sensitive period for nutritional impact. The nonhomogeneous temporal and spatial development of the brain leads to the need for nutritional intervention to be concomitant with age and stage of brain development [22, 23]. However, scientific publications on brain-nutrient findings by age are still scarce for toddlers and preschool children compared with that for infants and school-aged children [24]. For the age group of 1–5 y, for example, observation and intervention studies have mostly explored the impact of nutrients on motor, cognitive, and social-emotional benefits in stunted, autistic, or nutrient-deficient populations, for example, for iron, vitamin D, vitamin B-12, folic acid, spirulina, and polyunsaturated fatty acids [[25], [26], [27], [28], [29], [30], [31], [32], [33], [34]]. No link was made to brain developmental dynamics, nor are the data conclusive for healthy development.
The quantification and mapping of myelin development may, therefore, provide a measurable and developmentally relevant physiologic and empirical link between nutrition and cognitive-behavioral development. Limited knowledge exists of the dynamic interplay and longitudinal association between nutrients and brain growth during development, in a particular comprehensive investigation of multiple nutrients rather than individual compounds. Studies on cognitive development suggest that multinutrient approaches, including multimicronutrient interventions, can yield a positive impact on the brain and cognitive development in young children [35, 36]. To our knowledge, no study has investigated the brain-nutrient dynamics from infancy to early childhood.
The objective of this work was to identify age- and brain stage–dependent changes in nutrient-myelin associations and to describe the dynamic change in these associations from infancy to early childhood using myelin imaging as a measure for brain maturation. We hypothesized that nutrients from food intake data will show differential association patterns with myelin development based on age and brain stage.
Methods
Participants
Participants in this study were drawn from a larger longitudinal prenatal and postnatal study of child health and neurodevelopment in the United States termed RESONANCE, which is part of the environmental influences on child health outcomes program (echochildren.org). The study aimed to understand the relationships between child genomics, nutrition, and home environmental exposures on brain and cognitive development. Infants and young children were recruited between the ages of birth and 5 y, with study visits scheduled biannually from birth through 30 mo of age and annually thereafter. For this study, a single time point was selected from each child that had high-quality neuroimaging and completed dietary nutrient intake information. Informed consent was obtained from all parents and/or legal guardians, and research activities were performed as approved by the Rhode Island Hospital’s ethical review board.
For inclusion in the analysis of the present study, 293 children aged between 6 mo and 5 y were selected who met the following criteria: 1) singleton full-term (>37 wk) pregnancy; 2) uncomplicated pregnancy (i.e., no reports of pre-eclampsia, gestational hypertension, or gestational diabetes mellitus); 3) no reports of abnormalities on fetal ultrasound; 4) infant 5-min appearance, pulse, grimace, activity, and respiration scores of ≥8; 5) infant birth weight greater than 1500 g; 6) no reported history of neurologic trauma in the child; 7) no reported sibling or parental psychiatric history, including autism; and 8) no reports of major depressive disorder requiring medication in the mother during pregnancy or in the 6 mo prior to becoming pregnant. In addition, children had to have a successful MRI scan and time-matched nutrition intake information for at least one 24-h period within a week of MRI. Anthropometric and demographic information is summarized in Table 1.
TABLE 1.
Anthropometric and demographic data of the children studied
| Variable | Value | |
| Biological sex (N) | Male | 165 |
| Female | 128 | |
| Age (mo), mean (SD) | 25.5 (4.5) | |
| Birth weight (kg), mean (SD) | 3.4 (0.5) | |
| Birth length (cm), mean (SD) | 51.3 (4.8) | |
| Maternal education (N) | Professional degree | 105 |
| College graduate | 74 | |
| Partial college | 49 | |
| High school graduate | 21 | |
| Partial high school | 3 | |
| Not reported | 41 | |
| Race (N) | White | 181 |
| Black or African American | 36 | |
| Asian | 13 | |
| Mixed | 22 | |
| Not reported | 41 | |
| Cognitive development composite,1 mean (SD) | MSEL ELC | 101 (18) |
| MSEL VDQ | 99 (22) | |
| MSEL NVDQ | 105 (18) | |
ELC, early learning composite; MSEL, Mullen scales of early learning; NVDQ, nonverbal developmental quotient; VDQ, verbal developmental quotient.
Myelin MRI data acquisition and processing
All MRIs were performed at 3 Tesla (Siemens Tim Trio) during natural and nonsedated sleep. We have previously described our approach to night-time pediatric imaging [37] using noise-derated imaging sequences, sound-insulating MRI bore liners, MiniMuff ear pads, and sound-attenuating ear protectors.
To measure white matter development and myelination, multicomponent-driven equilibrium single pulse observation (mcDESPOT) of T1 and T2 relaxometry data were collected using age-optimized protocols [9] (Table 2). A consistent voxel dimension of ∼1.8 × 1.8 × 1.8 mm3 was used across the age span, with the field of view and imaging matrix adjusted depending on child age and head size. The theoretical basis for DESPOT1 and mcDESPOT has been detailed previously [38, 39] and involves the acquisition of multiflip angle T1-weighted spoiled gradient recalled (SPGR) images (DESPOT1) as well as T1/T2-weighted balanced steady-state free precession (bSSFP) images at 2 differing radiofrequency (RF) phase-cycling patterns. From these data, single-component and multicomponent relaxometry measures are calculated. For multicomponent analysis, a 3-pool tissue model comprising myelin-associated, intracellular and extracellular, and cerebrospinal fluid water pools is fit to the combined SPGR and bSSFP data using an iterative stochastic region approach [40]. The field of view and matrix size were increased with child age to accommodate the growing head size but keeping the voxel size consistent. All other aspects of acquisition were kept constant across the age range.
TABLE 2.
Age-optimized imaging protocols for qT1, qT2, and myelin water fraction imaging
| Sequence | Parameter | Scanning Age | ||||
|---|---|---|---|---|---|---|
| 3–9 mo | 9–16 mo | 16–28 mo | 28–48 mo | >48 mo | ||
| Field of view (cm) | 14 × 14 × 13 | 17 × 17 × 14.4 | 18 × 18 × 15 | 20 × 20 × 15 | 20 × 20 × 15 | |
| SPGR | TE/TR (ms) | 5.8/12 | 5.9/12 | 5.4/12 | 5.2/11 | 4.2/8 |
| Flip angles (degrees) | 2, 3, 4, 5, 7, 9, 11, 14 | 2, 3, 4, 5, 7, 9, 11, 14 | 2, 3, 4, 5, 7, 9, 11, 14 | 2, 3, 4, 5, 7, 9, 12, 16 | 3, 4, 5, 6, 7, 9, 13, 18 | |
| Bandwidth (Hz/pixel) | 350 | |||||
| Image matrix | 80 × 80 × 72 | 96 × 96 × 80 | 104 × 104 × 84 | 112 × 112 × 84 | 112 × 112 × 84 | |
| IR-SPGR | TI/TE/TR (ms) | (600, 950)/5.8/12 | (600, 900)/5.9/12 | (500, 850)/5.4/12 | (500, 800)/5.2/11 | (450, 750)/4.8/10 |
| Flip angle (degrees) | 5 | |||||
| Image matrix | 80 × 80 × 36 | 96 × 96 × 40 | 108 × 104 × 42 | 112 × 112 × 42 | 112 × 112 × 42 | |
| bSSFP | TE/TR (ms) | 5/10 | 5.1/10.2 | 5/10 | 4.4/9.8 | 3.6/7.2 |
| Flip angles (degrees) | 9, 14, 20, 27, 34, 41, 56, 70 | |||||
| Bandwidth (Hz/pixel) | 350 | |||||
| Image matrix | 80 × 80 × 72 | 96 × 96 × 80 | 104 × 104 × 84 | 112 × 112 × 84 | 112 × 112 × 84 | |
| High-resolution IR-SPGR | Field of view (cm) | 20 × 20 × 15 | ||||
| TI/TE/TR (ms) | 950 ms/6.9 ms/16 ms | |||||
| Flip angle (degrees) | 5 | |||||
| Reconstructed image matrix | 224 × 224 × 150 | |||||
bSSFP, balanced steady-state free precession; IR, inversion recovery; SPGR, spoiled gradient recalled; TE, echo time; TI, inversion time; TR, repetition time.
From the acquired mcDESPOT data, the myelin water fraction (MWF) was calculated using a processing pipeline that includes linear alignment of the SPGR, IR-SPGR, and bSSFP images to account for subtle intrascan head movement [41], nonparenchyma voxel removal [42], and correction of flip angle errors and off-resonance inhomogeneities using DESPOT1-HIFI and DESPOT2-FM [43]. A 3-pool tissue model was fit to the multiangle SPGR and bSSFP data to estimate the MWF, a surrogate and noninvasive measure of myelin content [40]. The MWF images (maps) were then nonlinearly aligned to a common analysis space in approximate Montreal Neurological Institute space using a previously described multistep approach [9] that first aligns the subject’s high flip angle T1-weighted SPGR image to 1 of 14 age-specific templates and then to an overall study-specific template using nonlinear 3-dimensional deformation, advanced normalization tools [44]. The calculated transformation matrices are then applied to the quantitative MWF maps.
Nutritional data collection
Nutrient intake data were collected through the automated self-administered 24-h dietary assessment tool (ASA-24) [45]. The mean daily nutrient intake per scan age was calculated, resulting in 293 observations of 88 nutrients. Fifty-six nutrient intakes were directly yielded from the ASA-24 output. Intake values for amino acids and phospholipids, including sphingomyelin, were retrieved from content information in the relevant USDA databases. The USDA choline database contains contents of free choline, glycerophosphocholine, phosphocholine, phosphatidylcholine, and sphingomyelin for ∼630 foods. Ganglioside concentrations for meat and fish were estimated from Khor et al. [46]; for milk concentrations, we assumed 11 mg of ganglioside per liter, as suggested by Vesper et al. [47]. The oligosaccharides 3’SL and 6’SL are found only in milk products; values of 114 mg/100g and 23 mg/100g were used for their respective concentrations in cow’s milk [48]. For the estimation of oligofructose content, we based our calculations on the study by Moshfegh et al. [49]. We then mapped the foods reported in ASA-24 to the corresponding items in the USDA database and calculated the daily totals. These nutrient contents and concentration values were included in addition to the ASA-24 calculated intake levels to have a comprehensive assessment of dietary intakes.
Brain-nutrient associations across windows
With the MWF maps in standard space, regional mean values were calculated across the brain in each child. The combined data from all 293 children were used to identify dynamics and window size and to perform a series of MWF-nutrient correlations using a sliding window approach. For each nutrient of interest and each brain region, we set a window size of 25 myelin imaging measurements corresponding to a step size of 72–162 d and fit the following general linear model to the data:
| MWFi,j = β0,j + β1log(agei,j) + β2NutrientIntakei,j + β3NutrientIntakei,j × log(agei,j) [1]. |
In our model, MWFi,j is the mean regional MWF estimate in child j at time point i; agei,j is the corresponding child age at the time point; and βo, β1, β2, and β3 are the regression coefficients. Once our general linear model was fit for all age-nutrient-brain myelin combinations, we then plotted the resultant P value “map” (unadjusted P < 0.05) associated with the β2 nutrient intake term, with the age-wise P values along the x axis and nutrients along the y axis. We focused on the direct nutrient intake term since the sliding window approach provided information on the relationship with age.
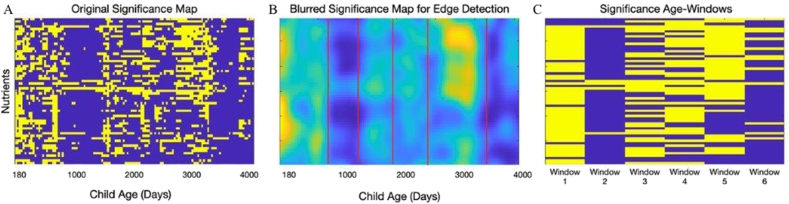
Finally, we used Sobel-filter vertical edge detection with a threshold of 25 to identify age windows with differing patterns of nutrient associations. Nutrients per window were selected on the basis of a threshold of 50% of significance for positive associations in each window (i.e., within the age window, they had an unadjusted P value of <0.05 for at least half the age points).
Potential confounders, such as gender, socioeconomic status, and maternal education were not included in any of the models, as gender and maternal education were not found to be significantly associated with MWF (data not shown).
Nutrient intake dynamics per nutrient-myelin window
The samples were split after window identification according to the following age windows: [6, 20] mo, [20, 30] mo, and [30, 60] mo. For each nutrient, and within each window, a linear model was fit to the data, with the nutrient intake as a response and the scan age as an independent variable. An increasing trend was defined as a positive and a decreasing trend as a negative slope in the linear model, with a P value of <0.05. A nonsignificant slope was considered a “stable trend.” In addition, a linear model was fit to the data for all ages, for each nutrient. In this analysis, the P values were not adjusted, as the objective was to describe the age trend of each nutrient intake, within and between windows, and emphasize the nonlinearity of these trends.
Association of myelin with nutrient intakes across windows
A linear model, such as [1], was fitted to the data set, all ages included, for each nutrient. Linear models with significant and positive coefficients for the nutrient intake terms were considered indicative of a linear positive correlation with myelin. The MWF was Box-Cox transformed to make it more symmetric. In addition, to account for possibly nonlinear associations and for correlations between the nutrients, we performed a regression model using a random forest algorithm [50] and used a feature selection algorithm [51] for finding all relevant variables. We used random forests because of their robustness to collinearity between the predictor variables [52]. They are well-suited to model nonlinear relationships between the outcome and the predictors and are known to deal well with modest sample sizes and high-dimensional feature spaces while being statistically consistent [53].
Results
Brain-nutrient associations (nutrient-myelin windows)
The majority of nutrients assessed (60%) were positively associated with brain maturation over the early developmental period from 6 to 20 mo of age (window 1); then, a period of relative stability, during which only one-fifth of nutrients (20%) had a significant association with brain myelination (20–30 mo, window 2); and, finally, a refractive period of growth during which the 37% of nutrients were positively associated with brain development (30–60 mo, window 3). We note that in all cases, the associations were positive, that is, higher nutrient intakes associated with higher measures of brain myelin. This likely reflects the study samples, which are healthy, neuro-typically developing children living in a high-resource area of the United States. Figure 1 displays the nutrient versus age P value map calculated from our sliding window analysis and summarized into our main age windows. The analysis was initially performed on the full sample up to 12 y of age until the windows were identified that cover the target age range from 1 to 5 y. Lastly, we observed differences in nutrient-myelin dynamics across age windows, for example, amino acids were positively associated with myelin at later ages only (windows 2 and 3), unlike minerals (window 1) or vitamins (windows 1 and 3, but not window 2), see Table 3.
FIGURE 1.
Associations for nutrient intakes and brain myelination in the children studied. Child age (x axis) and nutrient (y axis) P value map based on the sliding window analyses for original significance map (A), blurred significance map for edge detection (B), and summarized into main age windows (C). Yellow represents statistically significant P values; blue indicates nonsignificant values.
TABLE 3.
Nutrient-myelin associations by age1 for the children studied
| Nutrients | Window 1 (N = 56) | Window 2 (N = 20) | Window 3 (N = 35) |
|---|---|---|---|
| Macronutrients | Carbohydrate, protein, dietary fiber, total fat | Carbohydrate | Carbohydrate, protein |
| Micronutrients (vitamins) | Vitamin E as α-tocopherol, folic acid2, folate3, food folate4, niacin, retinol, β-cryptoxanthin, vitamin A, thiamine, vitamin B-12, riboflavin, vitamin B-6, vitamin C, vitamin D, vitamin K, choline | — | Retinol, vitamin C, choline |
| Micronutrients (minerals) | Calcium, iron, magnesium, phosphorus, potassium, selenium, zinc | — | — |
| Other compounds | Gangliosides, oligofructose, sphingomyelin, lycopene, theobromine, cholesterol | Sphingomyelin | Gangliosides, oligofructose, sphingomyelin |
| Fatty acids | Hexadecenoic (palmitoleic, 16:1), octadecenoic (Oleic, 18:1), docosenoic (erucic, 22:1), total monounsaturated fatty acids, octadecadienoic (linoleic, 18:2), octadecatrienoic (α-linolenic acid, 18:3), eicosatetraenoic (eta, 20:4), docosapentaenoic (DPA, 22:5), docosahexaenoic (DHA, 22:6), Total polyunsaturated fatty acids, butanoic acid (4:0), hexanoic acid (6:0), octanoic acid (8:0), decanoic acid (10:0), dodecanoic acid (12:0), tetradecanoic (14:0), hexadecanoic (16:0), octadecanoic (18:0), total saturated fatty acids | Eicosatetraenoic (ETA, 20:4), docosapentaenoic (DPA, 22:5) | Hexadecenoic (palmitoleic, 16:1), eicosenoic (20:1), octadecatetraenoic (18:4), eicosatetraenoic (ETA, 20:4), eicosapentaenoic (EPA, 20:5), docosapentaenoic (DPA, 22:5), docosahexaenoic (DHA, 22:6), total polyunsaturated fatty acids |
| Phospholipids | Phosphatidylcholine, phosphatidylinositol, phosphatidylserine, phosphatidylethanolamine | Phosphatidylcholine, phosphatidylinositol | Phosphatidylcholine, phosphatidylinositol, phosphatidylserine, phosphatidylethanolamine |
| Amino acids | — | Alanine, aspartic acid, cysteine, glutamic acid, glycine, isoleucine, leucine, lysine, methionine, phenylalanine, proline, serine, tyrosine, valine | Alanine, aspartic acid, cysteine, glutamic acid, glycine, isoleucine, leucine, lysine, methionine, phenylalanine, proline, serine, threonine, tyrosine, valine |
Age window 1 from 6 to 20 mo, window 2 from 20 to 30 mo, and window 3 from 30 to 60 mo.
Folic acid is the fully oxidized monoglutamate form of the vitamin that is used in fortified foods and most dietary supplements.
Folate is the generic term for naturally occurring food folates and folates in dietary supplements and fortified foods, including folic acid.
Food folates naturally occur in food and are in the tetrahydrofolate form and usually have additional glutamate residues, making them polyglutamates.
Nutrient intake dynamics for nutrients per window
The pattern of dietary nutrient intake variation per window is displayed in Table 4 for each investigated nutrient. Nutrient intake ranges and medians are summarized in Table 5 [[54], [55], [56], [57], [58], [59]].
TABLE 4.
Nutrient intake patterns1 for the children studied
| Nutrients | Window 1 6–20 mo | Window 2 20–30 mo | Window 3 30–60 mo |
|---|---|---|---|
| Macronutrients: Total Fat,Others: Oligofructose, Phosphatidylinositol, Total PhospholipidsMicronutrients (minerals): Iron,Micronutrients (vitamins): Niacin, Vitamin B-6, Folate, folic acid, Vitamin A, β-carotene, α-carotene, Vitamin DüFatty acids: Butanoic acid (4:0), Octanoic acid (8:0), Decanoic acid (10:0), Dodecanoic acid (12:0), Palmitoleic acid (16:1), Gondoic acid (20:1), Erucic acid (22:1), Stearidonic acid (18:4), Eicosapentaenoic acid [EPA]Amino acids: Threonine, Leucine, Lysine, Methionine, Cystine, Phenylalanine, Valine, Arginine, histidine, Glutamic Acid, Glycine | −→ | −→ | −→ |
| Macronutrients: Energy, Protein,Others: α-lactalbumin, Gangliosides, PhosphatidylserineMicronutrients (minerals): Calcium, Zinc, CopperMicronutrients (vitamins): Vitamin B-12Fatty acids: Saturated Fat, Hexadecanoic acid (16:0), Octadecanoic acid (18:0), Octadecenoic acid (18:1)Amino acids: Isoleucine, Tyrosine, Alanine, Aspartic acid, Proline, Serine | ↑ | −→ | −→ |
| Fatty acids: Arachidonic acid (20:4) | ↓ | −→ | −→ |
| Macronutrients: Moisture, FiberMicronutrients (minerals): Potassium,Micronutrients (vitamins): α-tocopherol,Others: Lycopene, Cholesterol | −→ | ↓ | −→ |
| Fatty acids: Polyunsaturated Fat, Linolelaidic acid (18:2), γ-Linolenic acid 18:3), Docosapentaenoic acid (22:5)Others: Phosphatidylethanolamine | −→ | −→ | ↑ |
| Fatty acids: Monounsaturated Fat,Amino acids: Tryptophan | −→ | −→ | ↓ |
| Micronutrients (vitamins): Vitamin K | ↑ | ↓ | ↑ |
| Others: Choline | ↑ | ↑ | ↓ |
| Micronutrients (minerals): Phosphorus,Others: Sphingomyelin, Phosphatidylcholine | ↑ | −→ | ↑ |
| Macronutrients: CarbohydrateMicronutrients: Vitamin C,Others: Oligosaccharide 6’SLFatty acids: Hexanoic acid (6 :0), Tetradecanoic acid (14:0) | ↑ | −→ | ↓ |
| Micronutrients (minerals): Magnesium, Selenium,Micronutrients (vitamins): Vitamin B-1, Vitamin B-2, Cryptoxanthin | ↑ | ↓ | −→ |
| Micronutrients (minerals): Sodium | −→ | ↓ | ↑ |
| Fatty acids: Docosahexaenoic acid [DHA] | ↓ | −→ | ↑ |
| Others: Oligosaccharide 3’SL | ↑ | ↓ | ↓ |
The pattern indicates the direction of intake changes within the age window, that is, decreasing (↓), stable (−), or increasing (↑) intake for each nutrient.
TABLE 5.
Nutrientintake ranges and medians for nutrient intakes per age window for the children studied
| Nutrients | Window 1 nutrient intake (N = 56) (6–20 mo) |
Window 2 nutrient intake (N = 20) (20–30 mo) |
Window 3 nutrient intake (N = 35) (30–60 mo) |
Dietary reference intakes or adequate intake |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| min | max | median | min | max | median | min | max | median | EAR | AI | UL | |
| Macronutrients | ||||||||||||
| Carbohydrates (g/d) 4 | 25.9 | 255 | 133 | 71.9 | 287 | 178 | 3.1 | 296 | 156 | 1002 | 951 | — |
| Protein (g/d) 4 | 1 | 108 | 37.2 | — | — | — | 0.5 | 82 | 45.1 | — | — | — |
| Total fat (g/d) 4 | 8.8 | 81.3 | 35.3 | — | — | — | — | — | — | — | 301 | — |
| Dietary Fiber (g/d) 4 | 0.3 | 22.7 | 7.8 | — | — | — | — | — | — | — | 193 | — |
| Micronutrients (vitamins) | ||||||||||||
| Vitamin E α- tocopherol (mg/d) 5 | 1.3 | 10.6 | 3.9 | — | — | — | — | — | — | 52 | 51 | 2002 |
| Folic acid (μg/d) | 0.9 | 296 | 97.3 | — | — | — | — | — | — | — | — | — |
| Folate, DFE (μg/d) | 20.9 | 805 | 306 | — | — | — | — | — | — | 1202 | 801 | |
| Food folate (μg/d) | 3.5 | 300 | 132 | — | — | — | — | — | — | — | — | — |
| Niacin (mg/d) 6 | 0.2 | 45.9 | 11.9 | — | — | — | — | — | — | 52 | 41 | — |
| Retinol (mg/d) | 16.7 | 783 | 330 | — | — | — | 0 | 1000 | 342 | — | — | — |
| β-Cryptoxanthin (mg/d) | 0 | 644 | 48.2 | — | — | — | — | — | — | — | — | — |
| Vitamin A, RAE (mg/d) 7 | 30.6 | 2600 | 399 | — | — | — | — | — | — | 2102 | 5001 | — |
| Thiamine (mg/d) 6 | 0 | 1.7 | 0.8 | — | — | — | — | — | — | 0.42 | 0.31 | — |
| Vitamin B-12 (μg/d) 6 | 0.1 | 6.2 | 2.2 | — | — | — | — | — | — | 0.72 | 0.51 | — |
| Riboflavin (mg/d) 6 | 0.3 | 2.6 | 1.3 | — | — | — | — | — | — | 0.42 | 0.41 | — |
| Vitamin B-6 (mg/d) 6 | 0.2 | 4 | 0.9 | — | — | — | — | — | — | 0.42 | 0.31 | — |
| Vitamin C (mg/d) 5 | 0.9 | 154 | 45.4 | — | — | — | 0 | 116 | 31.9 | 132/223 | 501 | 4002/6503 |
| Vitamin D (μg/d) 8 | 0 | 11.5 | 3.4 | — | — | — | — | — | — | 102 | 101 | 381/632 |
| Vitamin K (mg/d) 7 | 0.2 | 443 | 32.9 | — | — | — | — | — | — | — | 2.51/302 | — |
| Choline (mg/d) 7 | 0 | 6.6 | 1.9 | — | — | — | 0 | 8.1 | 2.8 | — | 1501/2002/2503 | 12,3 |
| Micronutrients (minerals) | ||||||||||||
| Calcium (mg/d) 8 | 76.9 | 1100 | 667 | — | — | — | — | — | — | 5002 | 2601 | 15001/25002 |
| Iron (mg/d) 7 | 0.6 | 23 | 8.2 | — | — | — | — | — | — | 6.91/32 | — | 40 1,2 |
| Magnesium (mg/d) 8 | 33.2 | 295 | 143 | — | — | — | — | — | — | 652 | 751 | 652 |
| Phosphorus (mg/d) 8 | 164 | 1350 | 731 | — | — | — | — | — | — | 3802 | 2751 | 30002 |
| Potassium (mg/d) 9 | 131 | 3270 | 1270 | — | — | — | — | — | — | — | 7001/30002 | — |
| Selenium (mg/d) 5 | 6.2 | 150 | 51.6 | — | — | — | — | — | — | 172 | 201 | 601/902 |
| Zinc (mg/d) 7 | 0.2 | 13 | 6 | — | — | — | — | — | — | 2.51,2 | — | 51/72 |
| Other compounds | ||||||||||||
| Gangliosides (mg/d) | 0 | 8.6 | 0.4 | — | — | — | 0.1 | 13 | 2.3 | — | — | — |
| Oligofructose (g/d) | 0 | 25 | 0.4 | — | — | — | 0 | 25.9 | 2.4 | — | — | — |
| Lycopene (mg/d) | 0 | 13.1 | 1.6 | — | — | — | — | — | — | — | — | — |
| Cholesterol (mg/d) | 0 | 404 | 94.6 | — | — | — | — | — | — | — | — | — |
| Sphingomyelin (mg/d) | 0 | 18.5 | 2.4 | 0 | 43.9 | 3.2 | 0.1 | 76.6 | 5.2 | — | — | — |
| Fatty acids | ||||||||||||
| Hexadecenoic acid (Palmitoleic, 16:1) (g/d) | 0 | 1.1 | 0.5 | — | — | — | 0 | 1.5 | 0.4 | — | — | — |
| Eicosenoic acid (20:1) (g/d) | — | — | — | — | — | — | 0 | 0.5 | 0.1 | — | — | — |
| Octadecenoic acid (Oleic 18:1) (g/d) | 1.1 | 23.8 | 12.5 | — | — | — | — | — | — | — | — | — |
| Docosenoic Erucic 22:1) (g/d) | 0 | 0.1 | 0 | — | — | — | — | — | — | — | — | — |
| Total Monounsaturated fatty acids (g/d) | 1.1 | 24.2 | 11.7 | — | — | — | — | — | — | — | — | — |
| Octadecadienoic acid (Linoleic, 18:2) (g/d) | 0.1 | 19.3 | 5.6 | — | — | — | — | — | — | — | 4.61/72/103 | — |
| Octadecatrienoic acid (α-linolenic acid 18:3) (g/d) | 0.1 | 1.9 | 0.8 | — | — | — | — | — | — | — | 0.51/0.72/0.93 | — |
| Octadecatetraenoic (18:4) (g/d) | — | — | — | — | — | — | 0 | 0.1 | 0 | — | — | — |
| Eicosatetraenoic acid (ETA, 20:4) (g/d) | 0 | 0.3 | 0.1 | 0 | 0.3 | 0.1 | 0 | 0.2 | 0.1 | — | — | — |
| Eicosapentaenoic (EPA 20:5) (g/d) | — | — | — | — | — | — | 0 | 0.1 | 0 | — | — | — |
| Docosapentaenoic acid (DPA 22:5) (g/d) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | — | — | — |
| Docosahexaenoic acid (DHA, 22:6) (g/d) | 0 | 0.2 | 0 | — | — | — | 0 | 0.3 | 0 | — | — | — |
| Total polyunsaturated fatty acids (g/d) | 1.3 | 21.8 | 7 | — | — | — | 0.2 | 22.7 | 10.6 | — | — | — |
| Butanoic acid (4:0) (g/d) | 0 | 1.1 | 0.3 | — | — | — | — | — | — | — | — | — |
| Hexanoic acid (6:0) (g/d) | 0 | 0.6 | 0.3 | — | — | — | — | — | — | — | — | — |
| Octanoic acid (8:0) (g/d) | 0 | 2.2 | 0.2 | — | — | — | — | — | — | — | — | — |
| Decanoic acid (10:0) (g/d) | 0 | 1.2 | 0.3 | — | — | — | — | — | — | — | — | — |
| Dodecanoic acid (12:0) (g/d) | 0 | 5 | 0.6 | — | — | — | — | — | — | — | — | — |
| Tetradecanoic acid (14:0) (g/d) | 0.2 | 3.3 | 1.4 | — | — | — | — | — | — | — | — | — |
| Hexadecanoic acid (16:0) (g/d) | 0.6 | 16.4 | 6 | — | — | — | — | — | — | — | — | — |
| Octadecanoic acid (18:0) (g/d) | 0.3 | 8.2 | 2.7 | — | — | — | — | — | — | — | — | — |
| Total Saturated fatty acids (g/d) 4 | 4.2 | 30.8 | 12.7 | — | — | — | — | — | — | As low as possible1,2,3 | ||
| Phospholipids | ||||||||||||
| Phosphatidylcholine (g/d) | 0 | 1.2 | 0.1 | 0 | 3.5 | 0.3 | 0 | 3.2 | 0.4 | — | — | — |
| Phosphatidylinositol (g/d) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.2 | 0 | — | — | — |
| Phosphatidylserine (g/d) | 0 | 0.1 | 0 | — | — | — | 0 | 0.3 | 0 | — | — | — |
| Phosphatidylethanolamine (g/d) | 0 | 0.3 | 0 | — | — | — | 0 | 0.7 | 0.1 | — | — | — |
| Amino acids | ||||||||||||
| Alanine (g/d) | — | — | — | 0 | 1.4 | 0.5 | 0 | 1.4 | 0.5 | — | — | — |
| Aspartic acid (g/d) | — | — | — | 0 | 3.4 | 1.2 | 0 | 3 | 1.2 | — | — | — |
| Cystine (g/d) | — | — | — | 0 | 0.3 | 0.1 | 0 | 0.5 | 0.1 | — | — | — |
| Glutamic acid (g/d) | — | — | — | 0 | 8.7 | 3 | 0 | 8.4 | 2.4 | — | — | — |
| Glycine (g/d) | — | — | — | 0 | 1 | 0.3 | 0 | 1.2 | 0.4 | — | — | — |
| Isoleucine (g/d) | — | — | — | 0 | 2.1 | 0.5 | 0 | 1.7 | 0.5 | — | — | — |
| Leucine (g/d) | — | — | — | 0 | 3.6 | 0.9 | 0 | 2.9 | 1 | — | — | — |
| Lysine (g/d) | — | — | — | 0 | 2.3 | 0.8 | 0 | 2.3 | 0.7 | — | — | — |
| Methionine (g/d) | — | — | — | 0 | 1 | 0.2 | 0 | 0.8 | 0.2 | — | — | — |
| Phenylalanine (g/d) | — | — | — | 0 | 2 | 0.6 | 0 | 1.7 | 0.6 | — | — | — |
| Proline (g/d) | — | — | — | 0 | 4.3 | 1.1 | 0 | 3.3 | 1 | — | — | — |
| Serine (g/d) | — | — | — | 0 | 1.7 | 0.7 | 0 | 1.9 | 0.6 | — | — | — |
| Threonine (g/d) | — | — | — | — | — | — | 0 | 1.4 | 0.4 | — | — | — |
| Tyrosine (g/d) | — | — | — | 0 | 2 | 0.5 | 0 | 1.6 | 0.5 | — | — | — |
| Valine (g/d) | — | — | — | 0 | 2.6 | 0.6 | 0 | 2 | 0.7 | — | — | — |
AI, adequate intake; DFE, dietary folate equivalent; EAR, estimated average requirement; RAE, retinol activity equivalent; UL, tolerable upper intake level.
Recommendations for children aged 7–11 mo.
Recommendations for children aged 1–3 y.
Recommendations for children aged 4–8 y.
All macronutrient Dietary Recommended Intakes are from Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids [54].
Dietary Recommended Intakes are from Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids [55].
Dietary Recommended Intakes are from Dietary reference intakes for thiamine, riboflavin, niacin, vitamin B-6, folate, vitamin B-12, pantothenic acid, biotin, and choline [56].
Dietary Recommended Intakes are from Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc [57].
Dietary Recommended Intakes are from Dietary reference intakes for calcium, phosphorus, magnesium, vitamin D, and fluoride [58].
Dietary Recommended Intakes are from Dietary reference intakes for water, potassium, sodium, chloride, and sulfate [59].
Five nutrients were identified with a positive and significant association with myelin according to the linear model (Table 6). The variables selected by the random forest model and sorted by mean importance for nutrients across ages were as follows: logAge; gangliosides (mg/d); sphingomyelin (mg/d); 18:2, octadecadienoic acid (g/d); 22:6 n-3, docosahexaenoic acid (g/d); phosphatidylcholine (g/d); phosphatidylinositol (g/d); 20:4, eicosatetraenoic acid (g/d); fatty acids, total saturated (g/d); 3'-SL (mg/d); phosphorus (mg/d); fatty acids, total polyunsaturated (g/d); food folate (μg/d); isoleucine (g/d); methionine (g/d); total fat (g/d); total phospholipids (g/d); carotene, β (μg/d); serine (g/d); calcium (mg/d); and phosphatidylserine (g/d).
TABLE 6.
Nutrient intakes across ages in the children studied with a positive and significant myelin association
| Nutrient | Term | Estimate | Standard error | P value |
|---|---|---|---|---|
| Lysine | (Intercept) | −0.168 | 0.012 | <0.001 |
| nutIntake | 0.033 | 0.017 | 0.048 | |
| logAge | 0.036 | 0.002 | <0.001 | |
| nutIntake × logAge | −0.005 | 0.002 | 0.049 | |
| 3’SL | (Intercept) | −0.168 | 0.008 | 0.000 |
| nutIntake | 0.002 | 0.000 | <0.001 | |
| logAge | 0.036 | 0.001 | <0.001 | |
| nutIntake × logAge | 0.000 | 0.000 | <0.001 | |
| 6’SL | (Intercept) | −0.168 | 0.008 | 0.000 |
| nutIntake | 0.009 | 0.002 | <0.001 | |
| logAge | 0.036 | 0.001 | <0.001 | |
| nutIntake × logAge | −0.001 | 0.000 | <0.001 | |
| Oligofructose | (Intercept) | −0.156 | 0.008 | <0.001 |
| nutIntake | 0.004 | 0.002 | 0.039 | |
| logAge | 0.034 | 0.001 | <0.001 | |
| nutIntake × logAge | −0.001 | 0.000 | 0.043 | |
| α-Lactalbumin | (Intercept) | −0.166 | 0.010 | 0.000 |
| nutIntake | 0.097 | 0.040 | 0.015 | |
| logAge | 0.036 | 0.001 | <0.001 | |
| nutIntake × logAge | −0.014 | 0.006 | 0.016 |
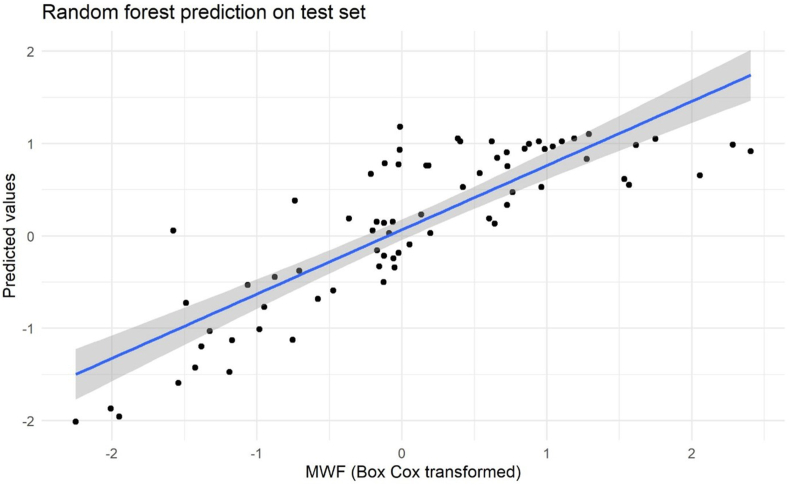
Figure 2 compares the values of the transformed MWF with the values predicted by the random forest on a test set. The Pearson correlation between predictions and original values is 0.88 (95% CI: 0.81, 0.92), indicating that the model predicts myelin values appropriately.
FIGURE 2.
Random forest prediction on the test set for nutrient intakes across ages. Actual myelin water fraction (MWF) versus predicted values for the tested random forest model.
Discussion
To our knowledge, this is the first longitudinal association study describing nutrient-myelin windows and their dynamics in well-nourished young children applying myelin imaging to identifying windows of sensitivity for brain stage–related nutrients. Although 4 of the evaluated nutrients were significantly associated with myelin development at all age windows up to 5 y, most nutrients followed a dynamic association pattern over time. We identified 3 nutrient-myelin windows across the age range of 6–60 mo that follow previously defined brain development dynamics of a steep increase in myelin in the first 1.5 postnatal years, followed by a transition to slower growth around 2 y of age and then continued slower myelin increase at 3–5 y [8, 60, 61].
Relations between brain growth and emerging skills or behaviors [5, [15], [16], [17], [18], [62], [63], [64]] as well as between brain growth and influencing factors [13, 14, 65, 66] have been well described. The dynamic relations between nutrition, brain, and cognitive development have been highlighted by Georgieff et al. [22, 23]. Our findings add to these ideas by supporting new hypotheses and observational data for age and brain-stage–appropriate nutritional opportunities, in alignment with the dynamic needs of the developing brain. Timing is critical for both nutritional deficiencies as well as effective support and nutritional intervention during development [[67], [68], [69], [70]]. Understanding sensitive periods for the specific link between nutrients and brain development are foundational for developing effective nutritional solutions and credible recommendations for brain nutrients. Interestingly, all classes of nutrients showed dynamic patterns and sensitive windows. Micronutrients and fatty acids appear particularly relevant in the first 1.5 y of life during the time of steep myelin increase, whereas both nutrient classes phase out during the brain development window of myelin rate change; fatty acids phase back in after 2.5 y of age when myelin increase continues at a slower rate. This may indicate the relevance of fatty acids in myelin accumulation. The formation of the myelin sheath requires high levels of fatty acid and lipid synthesis as well as the uptake of extracellular fatty acids [71]. Preclinical studies suggest a diet deficient in essential fatty acids during development causes hypomyelination [72] and dietary fatty acids are important in central nervous system myelinogenesis [73]. In contrast, amino acids gain importance in toddlerhood in our study when the myelin spurt slows down and transitions to slower continued growth. Amino acids may be more indirectly relevant for developmental myelination. Amino acid abnormalities have been linked to disturbed protein synthesis, which may affect myelin synthesis [74]. Dietary essential amino acids such as tryptophan and tyrosine may play a role in neurodevelopment, including motor and sensory functions [75]. A study in 2- to 5-y-old Japanese children suggested that ingestion of >800-mg tyrosine or phenylalanine at breakfast results in higher mental health scores than in children who ingested less than 800 mg [76]. The branched-chain amino acids, leucine, isoleucine, and valine, can also influence brain function [77] but have not been investigated yet further for their impact on developmental myelination in normal development.
Of the 52 nutrients significantly associated with myelin development in age window 1, 23 nutrients have dietary intake recommendations for the United States that were met by most of the explored nutrients (18 nutrients). The nutrients that did not meet the recommendations for children aged 6–20 mo were dietary fiber, vitamin E, vitamin D, vitamin A, and choline (Table 5). This is in line with previously reported consumption below dietary recommendations for dietary fiber, vitamin E, and vitamin D among US children aged 12–23.9 mo [78]. For age window 2, only carbohydrates have dietary recommendations (of the 18 nutrient associations) that were met in the study population. In age window 3, of the 33 nutrients associated with myelin, 6 nutrients have dietary recommendations. Nutrient intakes were met in the study population, except for choline and α-linolenic acid. The observed correlations in our study, indicating higher levels of specific nutrients to be associated with higher levels of myelin, must therefore be interpreted taking observed intake ranges as well as nutritional adequacy into account.
For children aged 6–20 mo (window 1), a predominant contribution to higher myelin levels came from fatty acids and micronutrients (vitamins and minerals). In contrast, for children aged 20–30 mo (window 2), mostly amino acids contributed to myelination; for children aged 30–60 mo (window 3), mixed contributions from both fatty acids and amino acids were identified. In addition, age-adjusted models exploring nutrient-myelin associations across age windows suggest the oligosaccharides 3’SL and 6’SL, lysine, α-lactalbumin, and oligofructose to be linked with higher myelin levels. Little is known about the role of these nutrients in developmental myelination. However, 6′SL has been recently shown to impact myelination in a preclinical study, potentially via a reduction in sialylated binding targets for the myelin-associated glycoprotein [79].
Strengths of the study include the sample size with combined nutritional intake and brain imaging data. Furthermore, the longitudinal cohort study design allows for longitudinal associations with more relevance for development than cross-sectional explorations alone. The myelin imaging measure has been previously used in nutrition studies that facilitates the interpretation of the findings. To our knowledge, this is the first analysis of nutrient-myelin associations in relation to age in healthy development.
Limitations of our work include the limitation to a US population. Nutritional intakes vary across geographies; therefore, generalization of the findings to other populations is limited unless intake values are comparable. The novel approach to brain-nutrient dynamics is limited to myelination as a relevant and measurable brain development process. Other markers or behavioral end points were not included in the study and would need further exploration. Future investigations should explore potential interactions or synergistic effects between nutrients on the developing brain.
In conclusion, nutrient-brain development associations are dynamic across child development and suggest the need for age- and brain-stage–appropriate nutritional needs. Although nutrient recommendations are typically targeted at overall health and development, very limited longitudinal data exist for brain benefits during development. The approach described here may provide new insights into nutritional opportunities and their dynamic windows of sensitivity across early life development. Myelin imaging has shown to be an important enabler in fostering the understanding of nutrient-brain interactions.
Author disclosures
NS, FM, SB, and MR are employees of Société des Produits Nestlé SA. SCD received financial compensation from Société des Produits Nestlé SA.
Acknowledgments
NS and SCD conceived and designed the research question and analyses. SD and FM performed the analyses. MR contributed to the analysis and interpretation of the nutritional data. NS, FM, SB, MR, and SCD wrote the paper. All authors: read and approved the final manuscript. We would like to thank Alexandros Kanellopoulos for his constructive input and support of the manuscript.
Funding
Supported by the NIH Office of the Director's Environmental Influences on Child Health Outcomes (ECHO) Program (UG3OD023313) to SCD.
Data Availability
Data described in the manuscript and analytic code will be made available upon request pending application and approval.
References
- 1.Bartzokis G., Lu P.H., Tingus K., Mendez M.F., Richard A., Peters D.G., et al. Lifespan trajectory of myelin integrity and maximum motor speed. Neurobiol Aging. 2010;31(9):1554–1562. doi: 10.1016/j.neurobiolaging.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casey B.J., Tottenham N., Liston C., Durston S. Imaging the developing brain: what have we learned about cognitive development? Trends Cogn Sci. 2005;9(3):104–110. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 3.Fields R.D. Myelination: an overlooked mechanism of synaptic plasticity? Neuroscientist. 2005;11(6):528–531. doi: 10.1177/1073858405282304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fields R.D. White matter in learning, cognition and psychiatric disorders. Trends Neurosci. 2008;31(7):361–370. doi: 10.1016/j.tins.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dai X., Hadjipantelis P., Wang J.-L., Deoni S.C.L., Müller H.-G. Longitudinal associations between white matter maturation and cognitive development across early childhood. Human Brain Mapp. 2019;40(14):4130–4145. doi: 10.1002/hbm.24690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagy Z., Westerberg H., Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. J Cogn Neurosci. 2004;16(7):1227–1233. doi: 10.1162/0898929041920441. [DOI] [PubMed] [Google Scholar]
- 7.van der Knaap M.S., Valk J., Bakker C.J., Schooneveld M., Faber J.A., Willemse J., et al. Myelination as an expression of the functional maturity of the brain. Dev Med Child Neurol. 1991;33(10):849–857. doi: 10.1111/j.1469-8749.1991.tb14793.x. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y., Chen M.H., Baluyot K.R., Potts T.M., Jimenez J., Lin W. MR fingerprinting enables quantitative measures of brain tissue relaxation times and myelin water fraction in the first five years of life. NeuroImage. 2019;186:782–793. doi: 10.1016/j.neuroimage.2018.11.038. [DOI] [PubMed] [Google Scholar]
- 9.Deoni S.C., Dean D.C., III, O'Muircheartaigh J., Dirks H., Jerskey B.A. Investigating white matter development in infancy and early childhood using myelin water faction and relaxation time mapping. NeuroImage. 2012;63(3):1038–1053. doi: 10.1016/j.neuroimage.2012.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deoni S.C. Magnetic resonance relaxation and quantitative measurement in the brain. Methods Mol Biol. 2011;711:65–108. doi: 10.1007/978-1-61737-992-5_4. [DOI] [PubMed] [Google Scholar]
- 11.Moldovan K., Boxerman J.L., O'Muircheartaigh J., Dean D., Eyerly-Webb S., Cosgrove G.R., et al. Myelin water fraction changes in febrile seizures. Clinical neurology and neurosurgery. 2018;175:61–67. doi: 10.1016/j.clineuro.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Deoni S.C., Zinkstok J.R., Daly E., Ecker C., Williams S.C., Murphy D.G. White-matter relaxation time and myelin water fraction differences in young adults with autism. Psychological medicine. 2015;45(4):795–805. doi: 10.1017/S0033291714001858. [DOI] [PubMed] [Google Scholar]
- 13.Deoni S., Dean D., III, Joelson S., O'Regan J., Schneider N. Early nutrition influences developmental myelination and cognition in infants and young children. NeuroImage. 2018;178:649–659. doi: 10.1016/j.neuroimage.2017.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deoni S.C., Dean D.C., III, Piryatinsky I., O'Muircheartaigh J., Waskiewicz N., Lehman K., et al. Breastfeeding and early white matter development: a cross-sectional study. NeuroImage. 2013;82:77–86. doi: 10.1016/j.neuroimage.2013.05.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deoni S.C., O'Muircheartaigh J., Elison J.T., Walker L., Doernberg E., Waskiewicz N., et al. White matter maturation profiles through early childhood predict general cognitive ability. Brain Struct Funct. 2016;221(2):1189–1203. doi: 10.1007/s00429-014-0947-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Muircheartaigh J., Dean D.C., III, Dirks H., Waskiewicz N., Lehman K., Jerskey B.A., et al. Interactions between white matter asymmetry and language during neurodevelopment. J Neurosci. 2013;33(41):16170–16177. doi: 10.1523/JNEUROSCI.1463-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Muircheartaigh J., Dean D.C., III, Ginestet C.E., Walker L., Waskiewicz N., Lehman K., et al. White matter development and early cognition in babies and toddlers. Hum Brain Mapp. 2014;35(9):4475–4487. doi: 10.1002/hbm.22488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schneider N., Greenstreet E., Deoni S.C.L. Connecting inside out: development of the social brain in infants and toddlers with a focus on myelination as a marker of brain maturation. Child Dev. 2022;93:359–371. doi: 10.1111/cdev.13649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schneider N., Hauser J., Oliveira M., Cazaubon E., Mottaz S.C., O'Neill B.V., et al. Sphingomyelin in brain and cognitive development: preliminary data. eNeuro. 2019;6(4) doi: 10.1523/ENEURO.0421-18.2019. ENEURO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fil J.E., Joung S., Hayes C.A., Dilger R.N. Influence of rearing environment on longitudinal brain development, object recognition memory, and exploratory behaviors in the domestic pig (Sus scrofa) Front Neurosci. 2021;15(318) doi: 10.3389/fnins.2021.649536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fanous M., Caputo M.P., Lee Y.J., Rund L.A., Best-Popescu C., Kandel M.E., et al. Quantifying myelin content in brain tissue using color spatial light interference microscopy (cSLIM) PloS One. 2020;15(11) doi: 10.1371/journal.pone.0241084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Georgieff M.K. Nutrition and the developing brain: nutrient priorities and measurement. Am J Clin Nutr. 2007;85(2):614s. doi: 10.1093/ajcn/85.2.614S. 20s. [DOI] [PubMed] [Google Scholar]
- 23.Georgieff M.K., Ramel S.E., Cusick S.E. Nutritional influences on brain development. Acta Paediatr. 2018;107(8):1310–1321. doi: 10.1111/apa.14287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosales F.J., Reznick J.S., Zeisel S.H. Understanding the role of nutrition in the brain and behavioral development of toddlers and preschool children: identifying and addressing methodological barriers. Nutr Neurosci. 2009;12(5):190–202. doi: 10.1179/147683009X423454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Idjradinata P., Pollitt E. Reversal of developmental delays in iron-deficient anaemic infants treated with iron. Lancet. 1993;341(8836):1–4. doi: 10.1016/0140-6736(93)92477-b. [DOI] [PubMed] [Google Scholar]
- 26.Aukett M.A., Parks Y.A., Scott P.H., Wharton B.A. Treatment with iron increases weight gain and psychomotor development. Arch Dis Childh. 1986;61(9):849–857. doi: 10.1136/adc.61.9.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Metallinos-Katsaras E., Valassi-Adam E., Dewey K.G., Lönnerdal B., Stamoulakatou A., Pollitt E. Effect of iron supplementation on cognition in Greek preschoolers. Eur J Clin Nutr. 2004;58(11):1532–1542. doi: 10.1038/sj.ejcn.1602005. [DOI] [PubMed] [Google Scholar]
- 28.Lozoff B. Iron Deficiency and child development. Food Nutr Bull. 2007;28(4_suppl4):S560–S571. doi: 10.1177/15648265070284S409. [DOI] [PubMed] [Google Scholar]
- 29.Saleem J., Zakar R., Zakar M.Z., Belay M., Rowe M., Timms P.M., et al. High-dose vitamin D3 in the treatment of severe acute malnutrition: a multicenter double-blind randomized controlled trial. Am J Clin Nutr. 2018;107(5):725–733. doi: 10.1093/ajcn/nqy027. [DOI] [PubMed] [Google Scholar]
- 30.Kvestad I., Taneja S., Kumar T., Hysing M., Refsum H., Yajnik C.S., et al. Vitamin B12 and folic acid improve gross motor and problem-solving skills in young north Indian children: a randomized placebo-controlled trial. PloS One. 2015;10(6) doi: 10.1371/journal.pone.0129915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masuda K., Chitundu M. Multiple micronutrient supplementation using spirulina platensis and infant growth, morbidity, and motor development: evidence from a randomized trial in Zambia. PloS One. 2019;14(2) doi: 10.1371/journal.pone.0211693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saad K., Abdel-Rahman A.A., Elserogy Y.M., Al-Atram A.A., El-Houfey A.A., Othman H.A., et al. Randomized controlled trial of vitamin D supplementation in children with autism spectrum disorder. J Child Psychol Psychiatry. 2018;59(1):20–29. doi: 10.1111/jcpp.12652. [DOI] [PubMed] [Google Scholar]
- 33.Sun C., Zou M., Zhao D., Xia W., Wu L. Efficacy of folic acid supplementation in autistic children participating in structured teaching: an open-label trial. Nutrients. 2016;8(6):337. doi: 10.3390/nu8060337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olsen M.F., Iuel-Brockdorff A.-S., Yaméogo C.W., Cichon B., Fabiansen C., Filteau S., et al. Early development in children with moderate acute malnutrition: a cross-sectional study in Burkina Faso. Maternal & Child Nutrition. 2020;16(2) doi: 10.1111/mcn.12928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roberts M., Tolar-Peterson T., Reynolds A., Wall C., Reeder N., Rico Mendez G. The effects of nutritional interventions on the cognitive development of preschool-age children: a systematic review. Nutrients. 2022;14(3):532. doi: 10.3390/nu14030532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schneider N., Bruchhage M.M.K., O'Neill B.V., Hartweg M., Tanguy J., Steiner P., et al. A nutrient formulation affects developmental myelination in term infants: a randomized clinical trial. Front Nutr. 2022;9 doi: 10.3389/fnut.2022.823893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dean D.C., III, Dirks H., O'Muircheartaigh J., Walker L., Jerskey B.A., Lehman K., et al. Pediatric neuroimaging using magnetic resonance imaging during non-sedated sleep. Pediatr Radiol. 2014;44(1):64–72. doi: 10.1007/s00247-013-2752-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deoni S.C., Rutt B.K., Arun T., Pierpaoli C., Jones D.K. Gleaning multicomponent T1 and T2 information from steady-state imaging data. Magn Reson Med. 2008;60(6):1372–1387. doi: 10.1002/mrm.21704. [DOI] [PubMed] [Google Scholar]
- 39.Deoni S.C., Mercure E., Blasi A., Gasston D., Thomson A., Johnson M., et al. Mapping infant brain myelination with magnetic resonance imaging. J Neurosci. 2011;31(2):784–791. doi: 10.1523/JNEUROSCI.2106-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deoni S.C., Matthews L., Kolind S.H. One component? Two components? Three? The effect of including a nonexchanging "free" water component in multicomponent driven equilibrium single pulse observation of T1 and T2. Magn Reson Med. 2013;70(1):147–154. doi: 10.1002/mrm.24429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jenkinson M., Bannister P., Brady M., Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 42.Smith S.M. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deoni S.C. Correction of main and transmit magnetic field (B0 and B1) inhomogeneity effects in multicomponent-driven equilibrium single-pulse observation of T1 and T2. Magnetic resonance in medicine. 2011;65(4):1021–1035. doi: 10.1002/mrm.22685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Avants B.B., Tustison N.J., Song G., Cook P.A., Klein A., Gee J.C. A reproducible evaluation of ANTs similarity metric performance in brain image registration. NeuroImage. 2011;54(3):2033–2044. doi: 10.1016/j.neuroimage.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Subar A.F., Kirkpatrick S.I., Mittl B., Zimmerman T.P., Thompson F.E., Bingley C., et al. The Automated Self-Administered 24-hour dietary recall (ASA24): a resource for researchers, clinicians, and educators from the National Cancer Institute. J Acad Nutr Dietetics. 2012;112(8):1134–1137. doi: 10.1016/j.jand.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khor G.L., Shyam S., Misra S., Fong B., Chong M.H.Z., Sulaiman N., et al. Correlation between dietary intake and serum ganglioside concentrations: a cross-sectional study among Malaysian toddlers. BMC Nutr. 2016;2(1):74. [Google Scholar]
- 47.Vesper H., Schmelz E.-M., Nikolova-Karakashian M.N., Dillehay D.L., Lynch D.V., Merrill A.H., Jr. Sphingolipids in Food and the Emerging Importance of Sphingolipids to Nutrition. J Nutr. 1999;129(7):1239–1250. doi: 10.1093/jn/129.7.1239. [DOI] [PubMed] [Google Scholar]
- 48.Kelly V., Davis S., Berry S., Melis J., Spelman R., Snell R., et al. Rapid, quantitative analysis of 3'- and 6'-sialyllactose in milk by flow-injection analysis-mass spectrometry: screening of milks for naturally elevated sialyllactose concentration. J Dairy Sci. 2013;96(12):7684–7691. doi: 10.3168/jds.2013-6972. [DOI] [PubMed] [Google Scholar]
- 49.Moshfegh A.J., Friday J.E., Goldman J.P., Ahuja J.K. Presence of inulin and oligofructose in the diets of Americans. J Nutr. 1999;129(7 Suppl):1407s. doi: 10.1093/jn/129.7.1407S. 11s. [DOI] [PubMed] [Google Scholar]
- 50.Breiman L. Random Forests. Machine Learn. 2001;45(1):5–32. [Google Scholar]
- 51.Kursa M.B., Rudnicki W.R. Feature Selection with the Boruta Package. J Stat Soft. 2010;36(11):1–13. [Google Scholar]
- 52.Genuer R., Poggi J.-M., Tuleau-Malot C. Variable selection using random forests. Pattern Recogn Lett. 2010;31(14):2225–2236. [Google Scholar]
- 53.Scornet E., Biau G., Vert J.-P. Consistency of random forests. Ann Stat. 2015;43(4):1716–1741. [Google Scholar]
- 54.Institute of Medicine . The National Academies Press; Washington, DC: 2005. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. [Google Scholar]
- 55.Institute of Medicine Panel on Dietary A., Related C. National Academies Press; Washington, DC: 2000. Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids. [PubMed] [Google Scholar]
- 56.Institute of Medicine . National Academies Press; Washington, DC: 1998. Standing Committee on the Scientific Evaluation of Dietary Reference I, its Panel on Folate OBV, Choline. The National Academies Collection: reports funded by National Institutes of Health. Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B(6), folate, vitamin B(12), pantothenic acid, biotin, and choline. [PubMed] [Google Scholar]
- 57.Institute of Medicine Panel on M . National Academies Press; Washington, DC: 2002. Dietary reference intakes for vitamin A, Vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, Vanadium, and zinc. [PubMed] [Google Scholar]
- 58.Institute of Medicine . National Academies Press; Washington, DC: 2011. Committee to Review Dietary Reference Intakes for Vitamin D, Calcium. Dietary reference intakes for calcium and vitamin D. [Google Scholar]
- 59.Institute of Medicine . The National Academies Press; Washington, DC: 2005. Dietary reference intakes for water, potassium, sodium, chloride, and sulfate. [Google Scholar]
- 60.Dean D.C., III, O'Muircheartaigh J., Dirks H., Waskiewicz N., Lehman K., Walker L., et al. Modeling healthy male white matter and myelin development: 3 through 60 months of age. NeuroImage. 2014;84:742–752. doi: 10.1016/j.neuroimage.2013.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yu Q., Peng Y., Kang H., Peng Q., Ouyang M., Slinger M., et al. Differential white matter maturation from birth to 8 years of age. Cereb Cortex. 2019;30(4):2674–2690. doi: 10.1093/cercor/bhz268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Johnson M.H., Munakata Y. Processes of change in brain and cognitive development. Trends Cogn Sci. 2005;9(3):152–158. doi: 10.1016/j.tics.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 63.Silbereis J.C., Pochareddy S., Zhu Y., Li M., Sestan N. The Cellular and Molecular Landscapes of the Developing Human Central Nervous System. Neuron. 2016;89(2):248–268. doi: 10.1016/j.neuron.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Casey B.J., Giedd J.N., Thomas K.M. Structural and functional brain development and its relation to cognitive development. Biol Psychol. 2000;54(1–3):241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- 65.Kolb B. Academic Press; Cambridge, MA: 2018. The neurobiology of brain and behavioral development; pp. 51–79. Chapter 3, Overview of factors influencing brain development. [Google Scholar]
- 66.Tooley U.A., Bassett D.S., Mackey A.P. Environmental influences on the pace of brain development. Nat Rev Neurosci. 2021;22(6):372–384. doi: 10.1038/s41583-021-00457-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Knudsen E.I. Sensitive periods in the development of the brain and behavior. J Cogn Neurosci. 2004;16(8):1412–1425. doi: 10.1162/0898929042304796. [DOI] [PubMed] [Google Scholar]
- 68.Colombo J., Gustafson K.M., Carlson S.E. Critical and sensitive periods in development and nutrition. Ann Nutr Metabol. 2019;75(suppl 1):34–42. doi: 10.1159/000508053. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Luby J.L., Baram T.Z., Rogers C.E., Barch D.M. Neurodevelopmental optimization after early-life adversity: cross-species studies to elucidate sensitive periods and brain mechanisms to inform early intervention. Trends Neurosci. 2020;43(10):744–751. doi: 10.1016/j.tins.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wachs T.D., Georgieff M., Cusick S., McEwen B.S. Issues in the timing of integrated early interventions: contributions from nutrition, neuroscience, and psychological research. Ann N Y Acad Sci. 2014;1308:89–106. doi: 10.1111/nyas.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Poitelon Y., Kopec A.M., Belin S. Myelin fat facts: an overview of lipids and fatty acid metabolism. Cells. 2020;9(4):812. doi: 10.3390/cells9040812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Salvati S., Attorri L., Avellino C., Di Biase A., Sanchez M. Diet, Lipids and Brain Development. Dev Neurosci. 2000;22(5–6):481–487. doi: 10.1159/000017479. [DOI] [PubMed] [Google Scholar]
- 73.Di Biase A., Salvati S. Exogenous lipids in myelination and myelination. Kaohsiung J Med Sci. 1997;13(1):19–29. [PubMed] [Google Scholar]
- 74.Perlman J.M., Volpe J.J. Chapter 27, Amino acids. Elsevier; Cambridge, MA: 2018. Volpe's neurology of the newborn; p. 763. 92.e7. [Google Scholar]
- 75.Cohen Kadosh K., Muhardi L., Parikh P., Basso M., Jan Mohamed H.J., Prawitasari T., et al. Nutritional support of neurodevelopment and cognitive function in infants and young children—an update and novel insights. Nutrients. 2021;13(1):199. doi: 10.3390/nu13010199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Akimitsu O., Wada K., Noji T., Taniwaki N., Krejci M., Nakade M., et al. The relationship between consumption of tyrosine and phenylalanine as precursors of catecholamine at breakfast and the circadian typology and mental health in Japanese infants aged 2 to 5 years. J Physiol Anthropol. 2013;32(1):13. doi: 10.1186/1880-6805-32-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fernstrom J.D. Branched-chain amino acids and brain function. J Nutr. 2005;135(6):1539S. doi: 10.1093/jn/135.6.1539S. 46S. [DOI] [PubMed] [Google Scholar]
- 78.Bailey R.L., Catellier D.J., Jun S., Dwyer J.T., Jacquier E.F., Anater A.S., et al. Total usual nutrient intakes of US children (under 48 months): findings from the feeding infants and toddlers study (FITS) 2016. J Nutr. 2018;148(9s):1557s. doi: 10.1093/jn/nxy042. 66s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hauser J., Pisa E., Arias Vásquez A., Tomasi F., Traversa A., Chiodi V., et al. Sialylated human milk oligosaccharides program cognitive development through a non-genomic transmission mode. Mol Psychiatr. 2021;26(7):2854–2871. doi: 10.1038/s41380-021-01054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data described in the manuscript and analytic code will be made available upon request pending application and approval.