Abstract
We have previously shown that the Pseudomonas aeruginosa toxA regulatory protein PtxS autoregulates its own synthesis by binding to a 52-bp fragment. The 3′ end of the 52-bp fragment is located 58 bp 5′ of the ptxS translation start site. We have identified a 14-bp palindromic sequence (TGAAACCGGTTTCA) within the 52-bp fragment. In this study, we used site-directed mutagenesis and promoter fusion experiments to determine if PtxS binds specifically to this palindromic sequence and regulates ptxS expression. We have also tried to determine the roles of specific nucleotides within the palindromic sequence in PtxS binding and ptxS expression. Initial promoter fusion experiments confirmed that the 52-bp fragment does not overlap with the region that carries the ptxS promoter activity. PtxS binding was eliminated upon the deletion of the 14-bp palindromic sequence from the 52-bp fragment. In addition, the deletion of the 14-bp sequence caused a significant enhancement in ptxS expression in the P. aeruginosa strain PAO1 and the ptxS isogenic mutant PAO::ptxS. Mutation of specific nucleotides within the 14-bp sequence eliminated, reduced, or had no effect on PtxS binding. However, mutations of several of these nucleotides produced a significant increase in ptxS expression in both PAO1 and PAO::ptxS. These results suggest that (i) the 14-bp palindromic sequence and specific nucleotides within it play a role in PtxS binding and (ii) deletion of the palindromic sequence or changing of certain nucleotides within it interferes with another mechanism that may regulate ptxS expression.
Pseudomonas aeruginosa is a gram-negative opportunistic pathogen that causes serious infections in burned patients and immunocompromised hosts (4, 22). Damage caused by P. aeruginosa is due to the production of several extracellular and cell-associated virulence factors (7, 23). Exotoxin A, which is the most toxic of the extracellular virulence factors, catalyzes the transfer of an ADP-ribosyl moiety onto elongation factor 2 in eukaryotic cells (6). This results in inhibition of protein synthesis and cell death (6, 21). It is known that exotoxin A production by P. aeruginosa is controlled by both positive and negative regulatory genes (17). We have previously described two P. aeruginosa genes, ptxR and ptxS, that regulate exotoxin A synthesis (3, 5). The ptxR gene codes for a protein that belongs to the LysR family of transcriptional activators and enhances exotoxin A synthesis at the transcriptional level (5). The ptxS gene codes for a protein that belongs to the GalR-LacI family of transcriptional repressors (3). Available evidence suggests that PtxS interferes with the enhancement of exotoxin A synthesis by ptxR (3).
Despite previous analyses, the mechanism(s) through which the expression of ptxS and ptxR is regulated is not known. We have recently provided evidence which suggests that ptxS negatively autoregulates its own synthesis in P. aeruginosa (15). The level of β-galactosidase activity produced by a ptxS-lacZ fusion plasmid in the ptxS isogenic mutant PAO1::ptxS was four- to fivefold higher than that produced by its parent strain, PAO1 (15). DNA gel shift experiments showed that PtxS specifically binds to a 52-bp fragment within the ptxS upstream region (15). In addition, DNase protection experiments localized PtxS binding to a 20-bp fragment (15). This 20-bp fragment contains a 14-bp sequence with complete dyad symmetry (a palindromic sequence). In this study, we used site-directed mutagenesis to determine if the 14-bp sequence constitutes a putative PtxS operator site. We have also tried to determine the role of specific nucleotides within the 14-bp sequence in PtxS binding and ptxS expression.
Methods.
For site-directed mutagenesis, the oligonucleotides that were used to construct a 14-bp deletion within the ptxS upstream region were 5′-CCTTCTGGTATTTT-3′–deletion of 14 bp–5′-AACTCCTGGCATCC-3′ and its complement (accession number AF012100 [3]). The 3′ end of the 14-bp palindrome is located 71 bp 5′ of the ptxS translation initiation codon. Different oligonucleotides that were used in the synthesis of specific nucleotide mutations corresponded to the region 5′-GGTATTTTCTGAAACCGGTTTCAACTCCTGGC-3′ of the ptxS upstream region. Each oligonucleotide was modified to incorporate the required mutations in specific nucleotides. Plasmid pBS9 was used as a template for the site-directed mutagenesis. Plasmid pBS9 is a pKS vector in which the 722-bp BamHI/KpnI ptxR-ptxS intergenic fragment was cloned (15). Site-directed mutagenesis experiments were accomplished using a Quikchange site-directed mutagenesis kit (Stratagene, La Jolla, Calif.) according to the manufacturer's recommendations. The deletion of the 14-bp sequence and the incorporation of specific mutations were confirmed by nucleotide sequence analysis. DNA gel shift experiments were performed as previously described (2, 15) using specific primers to amplify the 38- and 52-bp fragments from the pBS9 derivatives by PCR (20). The 52-bp fragment was used as a probe in previous DNA gel shift experiments (15). The 3′ end of the 52-bp fragment is located 58 bp 5′ of the ptxS translation start site (3). The 38-bp fragment is the 52-bp fragment from which the 14-bp sequence was deleted. The 38- and 52-bp fragments were end-labeled with [γ-32P]ATP (Amersham, Arlington Heights, Ill.) using T4 polynucleotide kinase (2). The source of the PtxS protein for DNA binding experiments was the lysate of the Escherichia coli strain K38/pJAC17, in which ptxS was overexpressed as previously described (15).
For the expression experiments, DNA fragments that carry nested deletions from the 5′ region of ptxS were generated by PCR. Synthesized fragments were then cloned in the previously described lacZ translational fusion vector pSW205 (14). The synthesis of the correct fragments was confirmed by nucleotide sequence analysis. The construction of the ptxS-lacZ fusion clones that carry the 14-bp deletion or specific nucleotide changes was done using the previously described ptxS-lacZ fusion plasmid pBS8-4 (15). For conformity of nomenclature, this plasmid is referred to here as pBS8 instead of pBS8-4. The sources of the DNA fragments that contain either the deletion of the 14-bp sequence or specific mutations were plasmid pBS9 and its derivatives. The 722-bp BamHI/KpnI fragment (which carries the ptxS upstream region) (Fig. 1A) was removed from pBS8 and replaced by either the 708-bp BamHI/KpnI fragment that was isolated from pBS9 or the 722-bp BamHI/KpnI fragments that were isolated from pBS9 derivatives. In all recombinant plasmids that carry the nested deletions or the specific mutations, the ptxS upstream region plus the region that codes for the first five amino acids of ptxS was fused in frame with the β-galactosidase gene. The previously described ptxS-lacZ translational fusion plasmid pBS8 (15) was used as a control. Recombinant plasmids were introduced into the P. aeruginosa strains PAO1 and PAO::ptxS by electroporation (13). Cells were grown in the iron-deficient medium TSB-DC (11) for 14 h, and the level of β-galactosidase activity was determined as previously described (10).
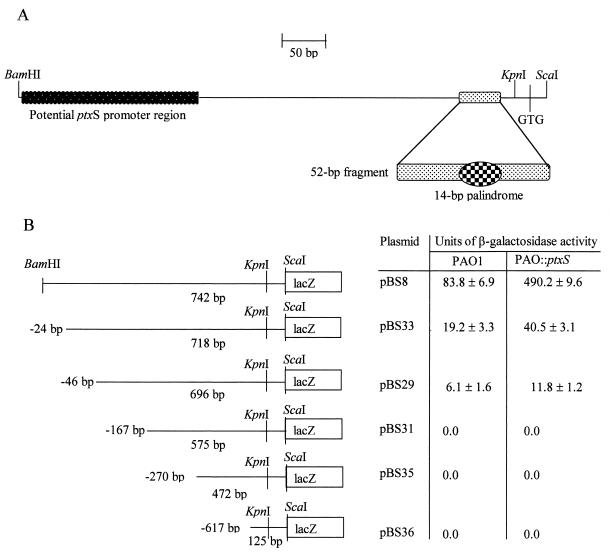
FIG. 1.
(A) Schematic diagram of the ptxS upstream region. The locations of the potential promoter region, the 52-bp fragment, the 14-bp palindromic sequence, and the essential restriction sites are indicated. The 5′ end of the ScaI site is located 9 bp 3′ of the ptxS translation start codon, while the 3′ end of the KpnI site is located 7 bp 5′ of the ptxS translation start codon. (B) Localization of the ptxS promoter activity. DNA fragments that carry the indicated deletions from the 5′ end of the ptxS upstream region were synthesized by PCR and cloned into the lacZ fusion vector pSW205 (14). Recombinant plasmids were introduced into the P. aeruginosa strains PAO1 and PAO::ptxS, and the level of β-galactosidase activity was determined as previously described (10). Values are the averages of results of three independent experiments ± the standard errors of the means.
Identification of the region that carries the ptxS promoter.
The 3′ end of the 52-bp fragment is located 58 bp 5′ of the ptxS translation start codon (Fig. 1A). Prior to the mutagenesis experiments, we tried to determine if the 52-bp fragment to which PtxS binds carries a ptxS promoter. Based on our computer analysis of the ptxS upstream region, we had identified a possible ptxS promoter region that overlaps with the PtxS binding site and carries most of the conserved nucleotides within the ς70 promoters (15). Despite several attempts, we were not successful in determining the ptxS transcriptional start site. Therefore, we tried to localize the ptxS promoter using the previously described lacZ translational fusion vector pSW205 (14). DNA fragments that carry different nested deletions from the 5′ end of the 742-bp BamHI/ScaI fragment (which contains the entire ptxS upstream region) were synthesized by PCR (Fig. 1A). Plasmids pBS33, pBS29, pBS31, pBS35, and pBS36 carry 718, 696, 575, 472, and 125 bp of the ptxS upstream region, respectively (Fig. 1B). In comparison with plasmid pBS8 (which carries the intact 742-bp BamHI/ScaI fragment), plasmids pBS33 and pBS29 produced significantly lower levels of β-galactosidase activity (Fig. 1B). Whether this difference in the levels of β-galactosidase activity is due to the presence of either more than one ptxS promoter or binding sites for a positive regulatory factor is not known at this time. However, plasmids pBS31, pBS35, and pBS36 produced no detectable β-galactosidase activity (Fig. 1B). These results indicate that, despite its observed homology to the ς70 promoters (15), at least the 500-bp region immediately 5′ of the ptxS translation start site does not carry a ptxS promoter. Therefore, it is unlikely that the ptxS promoter overlaps with the PtxS binding site. We have also excluded the possibility that the detected levels of β-galactosidase activity were influenced by PtxS. Higher levels of β-galactosidase activity were obtained when the above-described recombinant plasmids were introduced into the ptxS isogenic mutant PAO::ptxS (due to the derepression of ptxS expression) (Fig. 1B).
Analysis of PtxS binding to the 14-bp palindromic sequence.
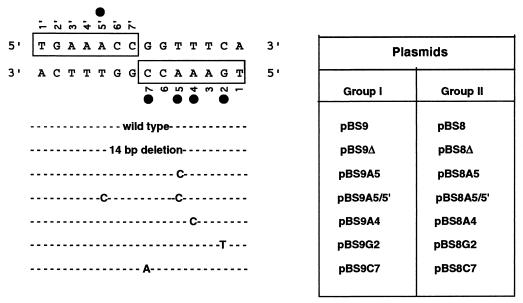
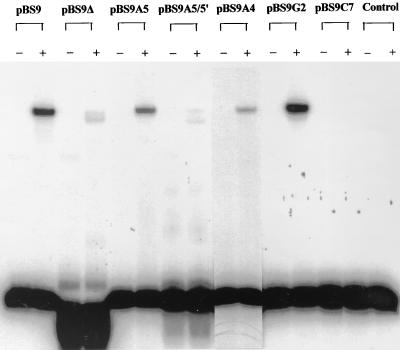
Computer analysis revealed that the 20-bp ptxS upstream fragment to which PtxS binds contains a 14-bp sequence with complete dyad symmetry (palindrome) that may constitute a potential PtxS operator site (Fig. 2). This palindromic sequence is located 71 bp 5′ of the ptxS initiation codon (Fig. 1A). The 14-bp sequence contains the three most conserved nucleotides (5′-AA–C-3′) within the operator sites of the GalR-LacI repressors (18). Therefore, in this study, we tried to determine if PtxS binds to this palindromic sequence and the roles of the specific nucleotides within the palindromic sequence in PtxS binding. The deletion of the palindrome, as well as specific mutations, was accomplished using plasmid pBS9, which carries the 722-bp BamHI/KpnI fragment of the ptxS upstream region (15) (Fig. 1A). As shown in Fig. 3, PtxS did not bind to the 38-bp deletion fragment rather than the 52-bp probe, which supports the possibility that the 14-bp sequence is the PtxS binding site. The first conserved nucleotide that we mutated was the adenine at position 5 within the right half of the palindrome (a transition of A to C; plasmid pBS9A5) (Fig. 2). This mutation reduced PtxS binding (Fig. 3). Quantitative analysis revealed that in comparison to PtxS binding to the intact 52-bp fragment, PtxS binding to the mutated 52-bp fragment was significantly reduced (five- to sixfold, data not shown). In addition to mutating this base, we changed the other adenine at the same position within the left half of the symmetrical sequence (a transition of A to C; plasmid pBS9A5/5′) (Fig. 2). As shown in Fig. 3, changing the adenine in both halves of the dyad symmetrical sequence eliminated PtxS binding to the 52-bp fragment. We then determined the effects of mutating the two other conserved nucleotides at positions 4 (a transition of A to C; plasmid pBS9A4) and 7 (a transition of C to A; plasmid pBS9C7) within the right half of the palindrome (Fig. 2). While the mutation of A at position 4 caused a reduction in PtxS binding, the mutation of C at position 7 eliminated PtxS binding (Fig. 3). Furthermore, we examined the effect of a mutation in a nucleotide within the palindromic sequence other than the conserved AA–C nucleotides. We synthesized a 52-bp fragment in which the G at position 2 within the right half of the palindromic sequence was replaced with T (plasmid pBS9G2) (Fig. 2). However, this mutation had no effect on PtxS binding to the 52-bp fragment (Fig. 3).
FIG. 2.
DNA sequence of the potential PtxS operator (dyad symmetry) site within the ptxS upstream region. Boxed regions indicate the right (bottom, 3′ sense sequence) and left (top, 5′ antisense sequence) halves of the dyad symmetry. The nucleotides are numbered (1 to 7) for the right half and (1′ to 7′) for the left half. Filled circles indicate the positions of the nucleotides that were mutated in this study. The types of the mutations are indicated. Plasmids that carry either deletion of the dyad symmetrical sequence or specific nucleotide mutations were divided into two groups. In group I, the deletion and individual mutations were generated in plasmid pBS9 (which carries the 722-bp fragment) by site-directed mutagenesis. For expression experiments, the 722-bp BamHI/KpnI fragment was deleted from pBS8 and replaced by either the 708-bp BamHI/KpnI fragment from pBS9 or the 722-bp BamHI/KpnI fragments from pBS9 derivatives (group II). Plasmid pBS8 is the same as the previously described plasmid pBS8-4 (15).
FIG. 3.
DNA gel shift experiments to examine the effects of different mutations on the binding of the 52-bp fragment to the partially purified PtxS. The lysate of the E. coli strain K38/pJAC17 was used as a source of the PtxS protein as previously described (15). We used the 38-bp deletion fragment and the 52-bp fragments that carried different mutations that were synthesized from pBS9 or its derivatives by PCR in the DNA gel shift assay as described in the text. Each DNA binding mixture contained 105 cpm of the labeled 52-bp fragment and approximately 2 μg of the cell lysate. With the exception of the reaction mixture with pBS9Δ, all DNA binding reaction mixtures contained the 52-bp fragments that were obtained from their respective plasmids. The binding reaction mixture with pBS9Δ contained the 38-bp deletion fragment. In the control lane, the 50-bp fragment was incubated with the lysate of the E. coli strain K38/pT7-5 only. −, the DNA binding reaction mixtures containing the probe only; +, the DNA binding reaction mixtures in which the probe was incubated with cell lysate. The faint band in pBS9Δ was not detected in several repeated experiments (data not shown). Similar faint bands that migrated at the same lower distance were also detected in pBS9A5/5′ and pBS9C7 (in some but not all experiments), even though these reaction mixtures contained the 52-bp fragment and not the 38-bp deletion fragment.
These results indicate that the deletion of, or specific changes within, the 14-bp sequence either eliminated PtxS binding, significantly reduced its binding, or had no effect on PtxS binding (Fig. 3). The loss of PtxS binding upon the deletion of the 14-bp palindromic sequence suggests that this sequence is the PtxS operator site. Several repressors of the GalR-LacI family are known to bind to specific operator sites (19a). However, two noticeable differences exist between the PtxS potential operator site and the operator sites of the other regulators. First, whereas the potential PtxS operator site contains complete dyad symmetry (Fig. 2), the operator sites of these proteins contain only partial symmetry (the left and right halves of the symmetrical sequence share conserved residues) (18). Second, with the exception of the galactose transport gene mgl (19a), many of these repressors have two operator sites (one within the upstream region of the target gene and another within the upstream region or the protein-coding regions of the target genes) (9, 19b). Binding of both operator sites by the repressor is essential for the complete repression of these genes (12, 19b). Previous analyses suggested that the occupation of both operator sites by their respective repressors causes looping of the intervening DNA sequence that may block the binding of the RNA polymerase and interfere with the transcription of these genes (1). Computer analysis revealed no other PtxS operator site (14-bp palindromic sequence) within the ptxS upstream region (data not shown). We have recently identified another PtxS operator site, which is located 3′ of ptxS and is involved in regulating the genes downstream of ptxS (16). PtxS binding to both operator sites causes looping of the DNA fragment that carries the ptxS open reading frame (but not the ptxS upstream region). The potential ptxS promoter is located 5′ of both operator sites (Fig. 1). Therefore, blocking the binding of the RNA polymerase to the ptxS promoter may not be the mechanism through which PtxS autoregulates its synthesis.
As stated above, mutation of individual nucleotides within the 14-bp palindromic sequence produced various effects on PtxS binding. Based on the comparison of these results with those from previous studies, the following comments regarding the specific nucleotides can be made. First, changing either of the two adenines at positions 4 and 5 reduces but does not eliminate PtxS binding (Fig. 3). The two conserved adenines, which are located within the central part of the GalR-LacI operator sites, are thought to be directly involved in binding the repressors (18). Previous studies suggested that the two adenines contact the first two amino acids within the recognition helix of GalR (valine and alanine) (1). The second amino acid within the recognition region of PtxS is alanine (3). Our results suggest that efficient binding of PtxS to its operator site may depend on the adenine at position 5 within both halves of the palindrome. Such a possibility is supported by the finding that the 52-bp probe that carries a mutation in the A at position 5 within both halves of the 14-bp sequence did not bind PtxS (Fig. 3). We have not tested the effect of the single mutation at position 5′ on PtxS binding. Therefore, we cannot rule out the possibility that PtxS binds to the two halves of the palindrome with different efficiencies. However, since the nucleotide sequences of both halves are the same, it is more likely that the combined mutations in both adenines interfered with the binding of the two PtxS subunits to either half. The complete dyad symmetry of the 14-bp sequence suggests that PtxS binds to this sequence as a dimer (each subunit of the dimer binds to one-half of the dyad symmetry). Such a mechanism was suggested for many repressors of the GalR-LacI family, including GalR, GalS, and PurR (19a). Both GalS and PurR are also known to autoregulate their own synthesis by binding to the operator sites within the upstream regions of their genes (9, 19b). Alternatively, similar to LacI, PtxS may function as a homotetramer (1). Each of the two subunits of the tetramer may bind to one operator site and the individual subunits may bind to each other (1).
Second, the guanine at position 2 does not seem to play a role in PtxS binding (Fig. 3). As with the PtxS operator, this guanine exists at the same position within both halves of the GalR operator sites OI and OE (19a). Majumdar and Adhya (8) have previously shown that GalR retards the methylation of the N7 position of the guanine by dimethyl sulfate, which indicates that this nucleotide participates in the direct contact of GalR with its operators. This nucleotide is located within the peripheral region of the GalR-LacI operators (18). Unlike the nucleotides within the central region of the operators, nucleotides within the peripheral regions are thought to play a role in defining the specificities of binding of their respective repressors (18). Thus, the failure of the mutation in this base pair to affect PtxS binding may be related to the specificity of PtxS binding. Whether other nucleotides within the peripheral region of the 14-bp sequence contribute to the specificity of PtxS binding is yet to be determined.
Third, among the tested nucleotides, the one that appears to be essential for PtxS binding is the cytosine residue at position 7 (Fig. 2), which is a conserved nucleotide in GalR-LacI operators (18). Unlike the mutations in the two conserved adenines, the mutation at this cytosine eliminated PtxS binding (Fig. 3). This cytosine is located at the junction of the two halves of the 14-bp palindromic sequence (Fig. 2). The mutation of C to A at this position may either interfere with PtxS binding or alter the DNA structure at the palindromic sequence and interfere with the interaction with PtxS.
The effects of different mutations on ptxS expression.
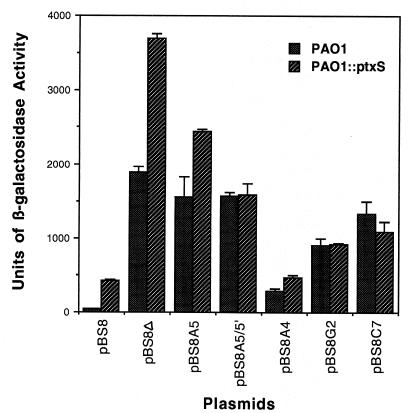
The expression of ptxS that is carried on fragments that contain different mutations in PAO1 was determined using the previously described lacZ translational fusion vector pSW205 (14). As shown in Fig. 4, levels of β-galactosidase activity by plasmids carrying either a deletion of the 14-bp sequence or specific mutations within the 14-bp sequence were significantly higher than that produced by pBS8, which contains the putative PtxS operator. The highest level of ptxS expression (increase in β-galactosidase activity) was detected with plasmid pBS8Δ, which carries a deletion of the 14-bp sequence (48-fold increase) (Fig. 4). Plasmids pBS8A5, pBS8A5/5′, and pBS8C7, in which the A, A and A′, and C at positions 5, 5 and 5′, and 7, respectively, were mutated, produced comparable increases in ptxS expression (an approximately 38-fold increase in β-galactosidase activity) (Fig. 4). The increase in the level of ptxS expression by plasmid pBS8G2, which carries the transition of G to T at position 2, was relatively smaller than that produced by other plasmids (23-fold) (Fig. 4). The smallest increase in ptxS expression was detected with plasmid pBS8A4 (a transition of A to C at position 4) (7.5-fold) (Fig. 4). These increases in the level of ptxS expression are higher than the four- to fivefold increase that we usually detect when ptxS is expressed in the ptxS isogenic mutant PAO::ptxS (15). To determine if this enhancement in ptxS expression varies in the absence of PtxS, the fusion plasmids were introduced in the PAO::ptxS strain. As shown in Fig. 4, with the exception of the pBS8A4 plasmid, all other fusion plasmids produced higher levels of β-galactosidase activity than that produced by PAO::ptxS/pBS8. The levels of β-galactosidase activity produced by pBS8A5/5′, pBS8A4, pBS8G2, and pBS8C7 in both PAO1 and PAO::ptxS were comparable (Fig. 4). However, the levels of β-galactosidase activity produced by pBS8Δ and pBS8A5 in PAO::ptxS were higher than those produced in PAO1 (Fig. 4).
FIG. 4.
Effects of the deletion and different base pair mutations on ptxS expression. Fusion plasmids containing different mutations were introduced into the P. aeruginosa strains PAO1 and PAO::ptxS by electroporation. Cells were grown in TSB-DC medium, and the level of β-galactosidase activity was determined as previously described (10). The description of each plasmid is given in Fig. 2. Values are the averages of results of three independent experiments ± the standard errors of the means. For the sake of conformity, the previously described plasmid pBS8-4 is referred to here as pBS8.
Results of the gel shift experiments confirmed that the 14-bp palindromic sequence and specific nucleotides within it are essential for PtxS binding (Fig. 3). However, analysis of the expression experiments suggest that the enhancement in ptxS expression produced by these changes is not due to the interference with PtxS binding alone (Fig. 4). For example, if the observed increase in ptxS expression is due to the loss of PtxS binding, the level of β-galactosidase activity produced by PAO/pBS8Δ would parallel that produced by PAO::ptxS/pBS8 (in pBS8Δ the entire palindromic sequence is deleted, while in PAO::ptxS no PtxS is produced). However, the level of β-galactosidase activity produced by PAO/pBS8Δ was significantly lower than that produced by PAO::ptxS/pBS8 (Fig. 4). Similarly, the effects of specific nucleotide changes on ptxS expression do not correlate with the effects of these changes on PtxS binding. With the exception of pBS8A4, plasmids that carry other nucleotide changes within the palindromic sequence produced higher levels of ptxS expression (higher than that produced by PAO::ptxS/pBS8) (Fig. 4). In contrast, PtxS binding to the DNA fragments that carry some of these changes was either reduced or not affected (Fig. 3). This pattern of increase in ptxS expression was detected even when the fusion plasmids were introduced into the PAO::ptxS strain (Fig. 4). Based on these results, it is difficult to correlate the effects of the deletion and other mutations on PtxS binding with their effects on ptxS expression. We have already eliminated the possibility that these changes alter the ptxS promoter region (the ptxS promoter region does not overlap with the 14-bp palindromic sequence) (Fig. 1B). At least two possible scenarios can be suggested to explain the high level of ptxS expression in both ptxS+ and ptxS mutant backgrounds. One is that the presence of a ptxS negative regulatory protein represses ptxS expression more efficiently than PtxS and binds to a region that overlaps with the 14-bp palindromic sequence. Deleting the 14-bp sequence or changing its nucleotides may, therefore, interfere with the binding of this protein. However, our previous gel shift experiments do not support this possibility (15). We have previously shown that the PAO1 lysate produces one protein (which is PtxS) that specifically binds to the 103-bp fragment immediately 5′ of the ptxS translation start codon (15). The second possibility is that the deletion or the mutations may produce a crucial change in the DNA structure within the ptxS upstream region. Such a change may either interfere with the binding of other regulatory proteins or facilitate the processivity of the RNA polymerase. Recent analysis of the ptxS upstream region produced some support for this possibility. We have identified at least two potential proteins within the PAO1 lysate that specifically bind to other parts of the ptxS upstream region (other than the 103-bp fragment) (data not shown). In addition, we have provided evidence that indicates that ptxS and four other genes constitute an operon that is involved in the utilization of 2 keto-gluconate and that ptxS is the first gene in that operon (16). Such an operon may be regulated by several proteins.
Acknowledgments
The authors graciously acknowledge Jane A. Colmer.
This study was supported by a Public Health Service grant (AI-33386) to Abdul Hamood. Britta Swanson was supported by a research fellowship from the Cystic Fibrosis Foundation.
REFERENCES
- 1.Adhya S. The lac and gal operons today. In: Lin E C, Simon Lynch A, editors. Regulation of gene expression in Escherichia coli. Austin, Tex: R. G. Lane Company; 1996. pp. 181–194. [Google Scholar]
- 2.Ausubel F, Brent R, Kingston R, Moor D, Seidman J, Smith J, Strauhle K. Current protocols in molecular biology. New York, N.Y: Wiley Intersciences; 1988. [Google Scholar]
- 3.Colmer J A, Hamood A N. Characterization of ptxS, a Pseudomonas aeruginosa gene which interferes with the effect of the exotoxin A positive regulatory gene, ptxR. Mol Gen Genet. 1998;258:250–259. doi: 10.1007/s004380050729. [DOI] [PubMed] [Google Scholar]
- 4.Doring G. Pseudomonas aeruginosa infection in cystic fibrosis patients. In: Campa M, Bendinelli M, Friedman H, editors. Pseudomonas aeruginosa as an opportunistic pathogen. New York, N.Y: Plenum Press; 1993. pp. 245–273. [Google Scholar]
- 5.Hamood A N, Colmer J A, Ochsner U A, Vasil M L. Isolation and characterization of a Pseudomonas aeruginosa gene, ptxR, which positively regulates exotoxin A production. Mol Microbiol. 1996;21:97–110. doi: 10.1046/j.1365-2958.1996.6251337.x. [DOI] [PubMed] [Google Scholar]
- 6.Iglewski B H, Kabat D. NAD-dependent inhibition of protein synthesis by Pseudomonas aeruginosa toxin. Proc Natl Acad Sci USA. 1975;2:2284–2288. doi: 10.1073/pnas.72.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu P V. The roles of various fractions of Pseudomonas aeruginosa in its pathogenesis. III. Identity of the lethal toxins produced in vivo. J Infect Dis. 1966;16:481–489. doi: 10.1093/infdis/116.4.481. [DOI] [PubMed] [Google Scholar]
- 8.Majumdar A, Adhya S. Probing the structure of Gal operator-repressor complexes/conformation change in DNA. J Biol Chem. 1987;262:13258–13262. [PubMed] [Google Scholar]
- 9.Meng L M, Kilstrup M, Nygaard P. Autoregulation of PurR repressor synthesis and involvement of purR in the regulation of purB, purC, purL, purMN, and guaBA expression in Escherichia coli. Eur J Biochem. 1990;187:373–379. doi: 10.1111/j.1432-1033.1990.tb15314.x. [DOI] [PubMed] [Google Scholar]
- 10.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 11.Ohman D C, Sadoff J C, Iglewski B H. Exotoxin A-deficient mutants of Pseudomonas aeruginosa PA103: isolation and characterization. Infect Immun. 1980;28:899–908. doi: 10.1128/iai.28.3.899-908.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rolfes R J, Zalkin H. Autoregulation of Escherichia coli purR requires two control sites downstream of the promoter. J Bacteriol. 1990;172:5758–5766. doi: 10.1128/jb.172.10.5758-5766.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith A, Iglewski B H. Transformation of Pseudomonas aeruginosa by electroporation. Nucleic Acids Res. 1989;17:10509. doi: 10.1093/nar/17.24.10509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Storey D G, Raivio T, Frank D W, Wick M J, Kaye S, Iglewski B H. Effect of regB on expression from P1 and P2 promoters of the Pseudomonas aeruginosa regAB operon. J Bacteriol. 1990;173:6088–6094. doi: 10.1128/jb.173.19.6088-6094.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swanson B L, Colmer J A, Hamood A N. The Pseudomonas aeruginosa exotoxin A regulatory gene, ptxS: evidence for negative autoregulation. J Bacteriol. 1999;181:4890–4895. doi: 10.1128/jb.181.16.4890-4895.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swanson, B. L., P. Hager, P. Phibbs, U. A. Ochsner, M. L. Vail, and A. N. Hamood. Characterization of the 2-ketogluconate utilization operon in Pseudomonas aeruginosa PAO1. M. L., Mictobiol., in press. [DOI] [PubMed]
- 17.Vasil M L, Ochsner U A. The response of Pseudomonas aeruginosa to iron: genetics, biochemistry, and virulence. Mol Microbiol. 1999;34:399–413. doi: 10.1046/j.1365-2958.1999.01586.x. [DOI] [PubMed] [Google Scholar]
- 18.Weickert M J, Adhya S. A family of bacterial regulators homologous to Gal and Lac repressors. J Biol Chem. 1992;267:15869–15874. [PubMed] [Google Scholar]
- 19a.Weickert M J, Adhya S. The galactose regulon of Escherichia coli. Mol Microbiol. 1993;10:245–251. doi: 10.1111/j.1365-2958.1993.tb01950.x. [DOI] [PubMed] [Google Scholar]
- 19b.Weickert M J, Adhya S. Control of transcription of the Gal repressor and isorepressor gene in Escherichia coli. J Bacteriol. 1993;175:251–258. doi: 10.1128/jb.175.1.251-258.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White B A. PCR protocols: current methods and applications. Totowa, N.J: Humana Press; 1993. [Google Scholar]
- 21.Wick M J, Frank D W, Storey D G, Iglewski B H. Structure, function, and regulation of Pseudomonas aeruginosa exotoxin A. Annu Rev Microbiol. 1990;44:335–363. doi: 10.1146/annurev.mi.44.100190.002003. [DOI] [PubMed] [Google Scholar]
- 22.Woods D E. Pseudomonas: the compromised host. Hosp Pract. 1976;11:91–100. doi: 10.1080/21548331.1976.11706983. [DOI] [PubMed] [Google Scholar]
- 23.Woods D E, Iglewski B H. Toxins of Pseudomonas aeruginosa: new perspectives. Rev Infect Dis. 1983;5:S715–S722. doi: 10.1093/clinids/5.supplement_4.s715. [DOI] [PubMed] [Google Scholar]