Abstract
Summary
Background
Severe anaemia is associated with high in-hospital mortality among young children. In malaria-endemic areas, surviving children also remain at increased risk of mortality for several months after hospital discharge. We aimed to compare the risks of morbidity and mortality among children discharged from hospital after recovery from severe anaemia versus other health conditions in malaria-endemic settings in Africa.
Methods
Following PRISMA guidelines, we searched PubMed, Scopus, Web of Science, and Cochrane Central from inception to Nov 30, 2021, without language restrictions, for prospective or retrospective cohort studies and randomised controlled trials that followed up children younger than 15 years for defined periods after hospital discharge in malaria-endemic countries in Africa. We excluded the intervention groups in trials and studies or subgroups involving children with sickle cell anaemia, malignancies, or surgery or trauma, or those reporting follow-up data that were combined with the in-hospital period. Two independent reviewers extracted the data and assessed the quality and risk of bias using the Newcastle Ottawa Scale or the Cochrane Collaboration’s tool. The coprimary outcomes were all-cause death and all-cause readmissions 6 months after discharge. This study is registered with PROSPERO, CRD42017079282.
Findings
Of 2930 articles identified in our search, 27 studies were included. For children who were recently discharged following hospital admission with severe anaemia, all-cause mortality by 6 months was higher than during the in-hospital period (n=5 studies; Mantel-Haenszel odds ratio 1·72, 95% CI 1·22–2·44; p=0·0020; I2=51·5%) and more than two times higher than children previously admitted without severe anaemia (n=4 studies; relative risk [RR] 2·69, 95% CI 1·59–4·53; p<0·0001; I2=69·2%). Readmissions within 6 months of discharge were also more common in children admitted with severe anaemia than in children admitted with other conditions (n=1 study; RR 3·05, 1·12–8·35; p<0·0001). Children admitted with severe acute malnutrition (regardless of severe anaemia) also had a higher 6-month mortality after discharge than those admitted for other reasons (n=2 studies; RR=3·12, 2·02–4·68; p<0·0001; I2=54·7%). Other predictors of mortality after discharge included discharge against medical advice, HIV, bacteraemia, and hypoxia.
Interpretation
In malaria-endemic settings in Africa, children admitted to hospital with severe anaemia and severe acute malnutrition are at increased risk of mortality in the first 6 months after discharge compared with children admitted with other health conditions. Improved strategies are needed for the management of these high-risk groups during the period after discharge.
Introduction
Substantial progress has been made in reducing all-cause child mortality globally in the past decade, but about 1 in 13 children in sub-Saharan Africa still die before their fifth birthday.1 Severe anaemia and malaria are major contributors to morbidity and mortality in malaria-endemic areas of Africa.2,3 Severe anaemia alone accounts for 2–29% of all paediatric hospital admissions,4–6 and 4–10% of these children die in hospital.7–9 The causes of severe anaemia are multifactorial and include nutritional causes,10,11 and acute and chronic infections such as malaria, tuberculosis, HIV, bacteraemia, and hookworm infestations.12
Studies among children with severe anaemia have focused on interventions to reduce in-hospital mortality.13–15 However, it is increasingly recognised that the high risk of all-cause mortality continues after hospital discharge,16–18 with up to 33% of the children dying or being readmitted within the first 6 months after discharge.7,19 Children admitted with severe anaemia are believed to constitute an especially vulnerable group of seriously ill children that remain at high risk after hospital admission because of a wide range of factors, including clinical, epidemiological, environmental, sociobehavioural, nutritional, and genetic factors.12,17
The period after discharge is a well recognised risk period for children with severe acute malnutrition,20,21 but is less studied in children with severe anaemia. Quantification of the burden of mortality and morbidity after discharge in children recently recovered from severe anaemia is important for the development of post-discharge management strategies. To aid in this process, we did a systematic review and meta-analysis to compare the pooled risks of mortality and readmission after discharge among children admitted with all-cause severe anaemia versus other health conditions without severe anaemia in malaria-endemic areas of Africa. We also compared in-hospital mortality against mortality after discharge.
Methods
Search strategy and selection criteria
This systematic review and meta-analysis was done in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.22 The protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO-CRD42017079282).
We identified eligible studies by doing a literature search using a combination of search terms (appendix p 3) in PubMed, Scopus, Embase, Web of Science, and Cochrane Central from inception to Nov 30, 2021, without language restrictions. We identified other relevant studies by scanning reference lists of all identified articles and searching in Google and Google Scholar. Where necessary, authors of published studies were contacted up to three times for further information.
Eligibility criteria
Prospective or retrospective cohort studies and control groups of individually randomised-controlled trials (RCTs) were eligible for inclusion if they respected the following criteria: presented original data with or without comparator groups; included children younger than 15 years of age admitted with severe anaemia or other health conditions such as malaria, pneumonia, diarrhoea, malnutrition, and HIV, alone or in combination with severe anaemia; defined the duration of the post-discharge follow-up; and were conducted in African countries that are malaria-endemic according to the World Malaria Report 2020.23 The definition of severe anaemia followed the definition used in the source studies (eg, haemoglobin <5 g/dL or <6 g/dL, clinical requirement for blood transfusion). Severe acute malnutrition in children was defined as a mid-upper-arm circumference lower than 115 mm, or a weight-for-height Z score of the WHO Child Growth Standards median of –3 or less.
Studies or subgroups were excluded if they involved admissions for sickle cell anaemia, malignancies, surgery, or road accidents and other trauma cases, or reported follow-up data that were combined with the in-hospital period.
Study selection and data extraction
Two independent reviewers (TKK and ATM) screened titles, abstracts, and full texts of the identified articles and agreed on the final eligibility. Any disagreement between the two reviewers was resolved through consensus or after consultation with a third reviewer (FOtK).
TKK and ATM independently extracted the data using a standardised form and database. Duplicate records were removed. If required, additional information was obtained from the authors. In trials or other comparative studies in which interventions were provided, only the data from the control groups that received the standard of care were included.
Quality assessment
The Cochrane Collaboration’s tool was used to assess the quality and risk of bias of clinical trials.24 For observational and cohort studies with comparison groups, we used the Newcastle Ottawa Scale.25 For cohort studies without comparison groups, we used a modified version of the Newcastle Ottawa Scale that omitted the comparability criteria and the section on selection of the non-exposed cohort in the selection criteria.
Data analysis
Two primary outcome measures were used, comprising all-cause death or all-cause readmissions at 6 months after discharge. Secondary outcomes are defined in the appendix (p 3). The follow-up period after discharge varied from 28 days to 5 years. Follow-up data beyond 1 year was truncated to less than 12 months for analysis. The method used to address loss to follow-up was based on the method provided in the source study. If survival analysis was used, children were censored at the time they were lost before completing 6 months follow-up. In studies reporting risk, children lost to follow-up were excluded from the denominator.
Data were analysed using STATA version 14.0. DerSimonian and Laird random-effects meta-analyses were used to generate pooled relative risks (RRs). The Mantel-Haenszel odds ratio (MHOR) for paired binary outcomes was used to compare in-hospital and post-discharge mortality rates.26 Hazard ratios (HRs) were used to generate pooled effect estimates when the original measures were presented as HR.
Heterogeneity was expressed using the I2 statistic and categorised as low if the I2 values ranged between 0% and 40%, moderate if it ranged between 30% and 60%, substantial if it ranged between 50% and 90%, and considerable if it ranged between 75% and 100%.27 As part of the sensitivity analysis, the pooled effect estimates using fixed-effect models (Mantel-Haenszel) are provided in the forest plots. We intended to do subgroup and sensitivity analyses to establish the influence of study quality, study region, age, and malaria transmission intensity, but these analyses were not possible because of the small number of studies and low heterogeneity in study quality. We aimed to use funnel plots to assess possible publication bias and small-study effects where ten or more studies were included in the meta-analysis. However, this analysis was not possible because of the small number of independent studies that contributed.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
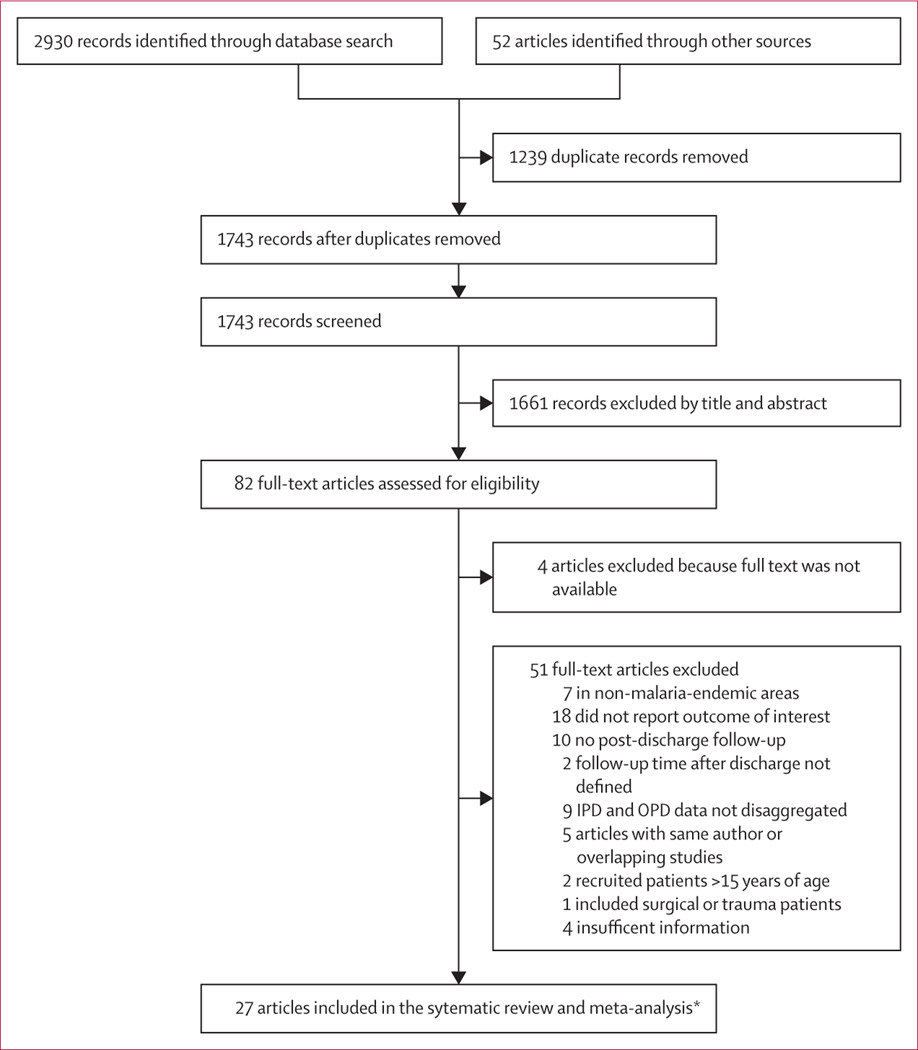
Our search identified 2930 articles. After removing duplicates and screening of titles and abstracts, we further evaluated 82 full-text articles, of which 27 were eligible for inclusion, including 22 cohort studies (with one being our own unpublished data)17,18,21,28–45 and five RCTs46–50 (figure 1; appendix pp 12–14). The studies were published between 1987 and 2021 and conducted in Kenya (nine studies), Malawi (four studies), Uganda (five studies), Guinea-Bissau (two studies), Democratic Republic of Congo (two studies), Tanzania (three studies), The Gambia (one study), Zambia and Zimbabwe (one study), and Mozambique (one study). One of the studies included is our own unpublished data. The main diagnosis on admission, henceforth referred to as the main health condition, included severe anaemia (nine studies), malaria (three studies), pneumonia (four studies), malnutrition (three studies), and diarrhoea (one study); there were ten other studies in which the main health conditions were not specified (unspecified health condition). Mortality after discharge ranged from 1% to 39% on the basis of the total follow-up time after discharge (appendix pp 12–14). The mean and median duration of follow-up for the primary outcome varied between health conditions (appendix p 15). Three RCTs were scored as low risk of bias and two open-label trials as unclear risk of bias (appendix p 16). 21 cohort studies were scored as good quality and one as poor quality (appendix pp 17, 18).
Figure 1: PRISMA flow diagram.
Reasons for exclusion exceed the number of articles excluded because some articles had more than one reason for exclusion. Children in IPD represents admitted children. IPD=in-patient department. OPD=outpatient department.
*Including a prospective follow-up study of children who were initially enrolled in a prospective case-control study.18
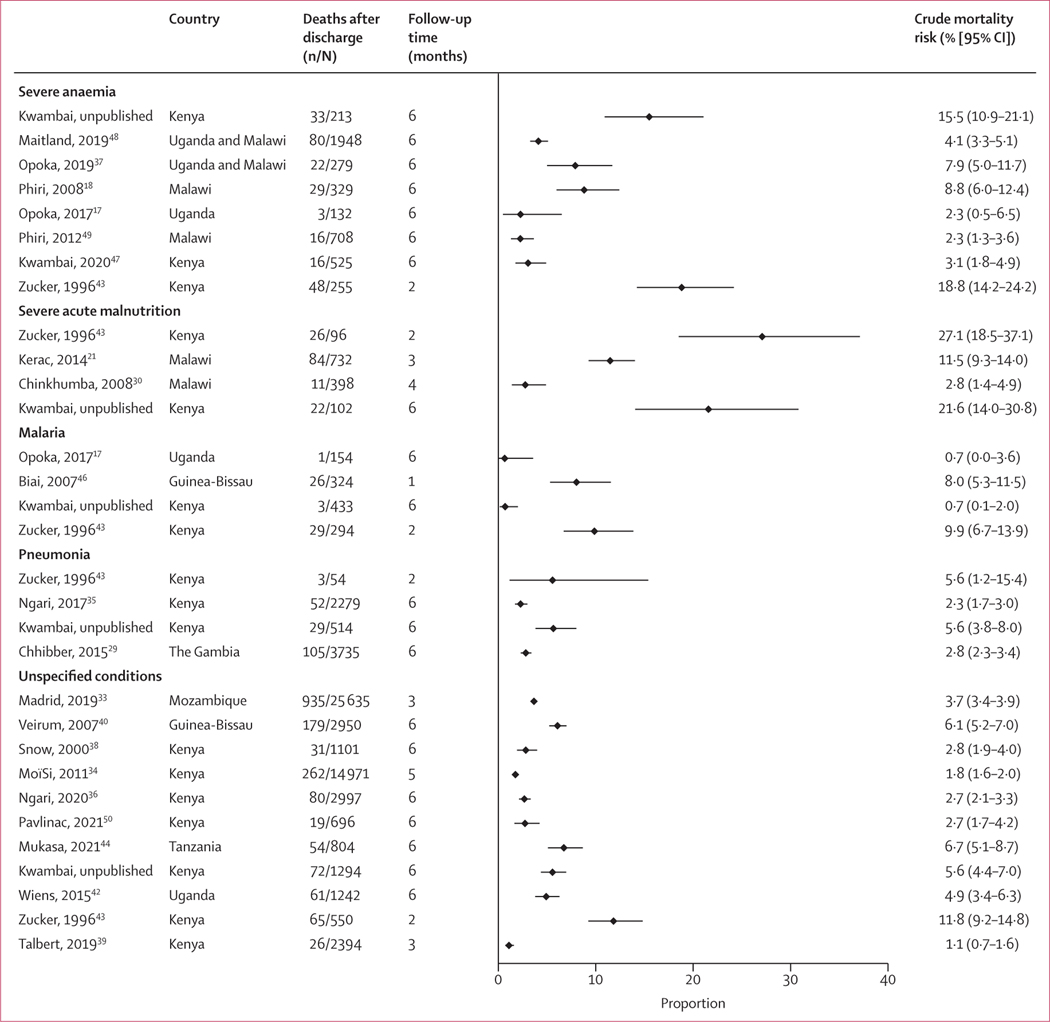
A meta-analysis of mortality after discharge from 20 studies showed that the crude risk of all-cause mortality after discharge by 6 months ranged from 2·3% to 18·8% for severe anaemia, 2·8% to 27·1% for severe acute malnutrition, 0·7% to 9·9% for malaria, 2·3% to 5·6% for pneumonia, and 1·1% to 11·8% for unspecified health conditions (figure 2). The 12-month mortality after discharge by health condition (23 studies) is shown in the appendix (p 4). Because there was considerable heterogeneity in mortality after discharge between (I2=95·4%) and within health-condition groups (I2>70% for all), a pooled summary risk obtained by meta-analysis was not calculated.
Figure 2: Crude mortality risk for any follow-up period in the first 6 months after discharge, all studies.
Data from our unpublished study, Zucker 1996,43 and Opoka, 201617 are included in more than one subgroup, each representing a mutually exclusive group. The malaria studies did not include any post-discharge chemoprophylaxis groups. Summary statistics are not shown because of considerable heterogeneity (overall I2 is 95·5%) between and within admission health-condition groups.
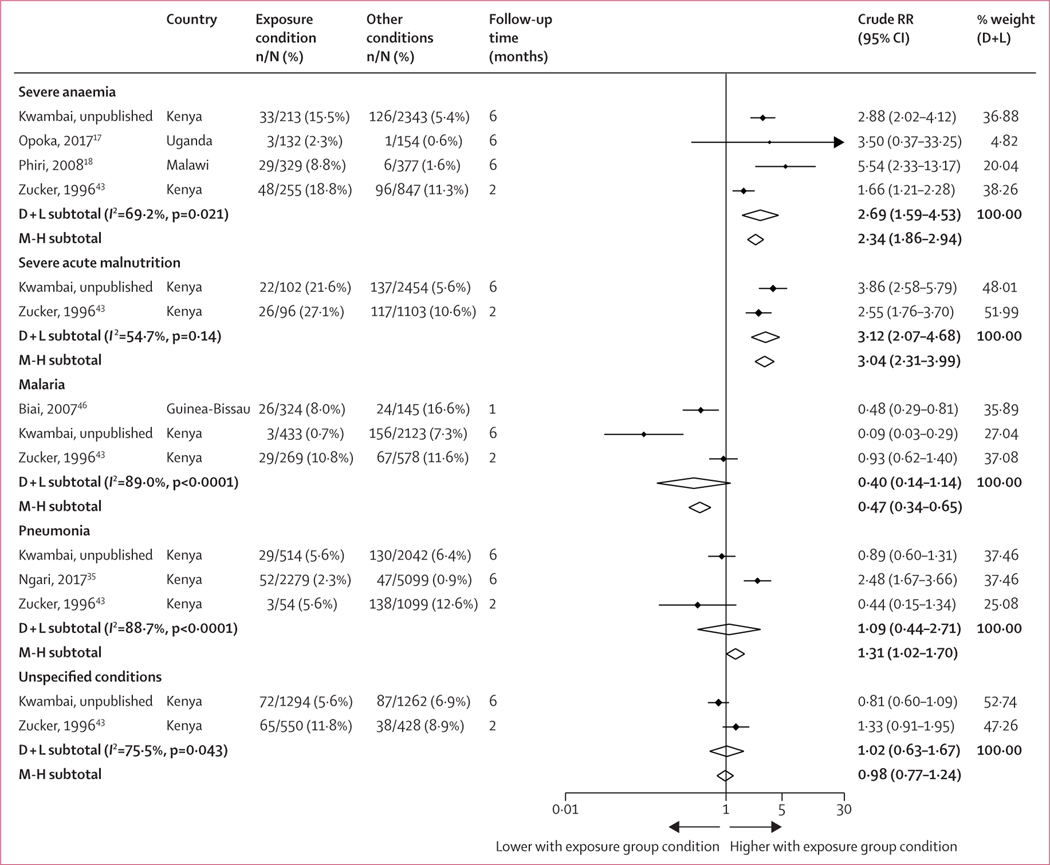
Among these 22 studies, six reported enough detail to allow direct comparisons by admission health condition (figure 3; appendix p 19). 6-month mortality after discharge among children previously admitted with severe anaemia was 2·69 times higher than in children previously admitted without severe anaemia during the same study period and in the same hospitals (n=4 studies; RR 2·69, 95% CI 1·59–4·53; p<0·0001; I2=69·2%). Severe malnutrition was also associated with higher mortality after discharge (n=2 studies; RR 3·12, 2·07–4·68; p<0·0001; I2=54·7%). However, mortality after discharge among children admitted with severe pneumonia, malaria, and unspecified health conditions was similar or lower than in children admitted for other reasons. Similar trends were observed at 12 months after discharge (appendix pp 4–5).
Figure 3: Relative risk of mortality 6 months after discharge among studies reporting results by several health conditions.
Weights are from the random-effects analysis. The figure only includes studies that reported enough detail to allow direct comparisons of mortality after discharge by health condition among children from the same cohort study. Diamond shapes depict pooled effect size. The crude RR is calculated by comparing the exposure condition versus other conditions. For example, in the first section under severe anaemia, the random-effects summary crude RR of 2·69 (95% CI 1·59–4·53) represents the relative risk of mortality after discharge when comparing children who were recently admitted with severe anaemia versus all other children that were recently admitted for any other conditions that excluded severe anaemia (other conditions), such as severe acute malnutrition, severe malaria, severe pneumonia, or other unspecified conditions. Similarly, the second section under malnutrition (summary random-effects RR 3·12) represents the RR of mortality after discharge when comparing children who were recently admitted with severe acute malnutrition versus children that were admitted for any other conditions that excluded severe acute malnutrition. In the case of children that were admitted for any other conditions, other conditions includes children with severe anaemia, severe malaria, severe pneumonia, or other unspecified conditions. D + L=DerSimonian and Laird random effects. M-H=Mantel-Haenszel fixed effect. RR=relative risk.
When the analysis was repeated as a post-hoc analysis, now excluding children with severe acute malnutrition from the comparator group, the RR for 6-month mortality after discharge for children with severe anaemia relative to other children was 2·30 (n=2 studies; 1·11–4·78; p=0·025; I2=88·2%). There were insufficient data for a similar comparison by 12 months. For malnutrition, the RR was three times higher by 6 months when compared to a reference that excluded children with severe anaemia (n=2 studies; RR 3·26, 1·62–6·56; p=0·0010; I2=83·0%). RR was similar by 12 months (n=2 studies; RR 3·26, 2·47–4·30; p<0·0001; I2=0%; appendix p 19).
Children who had both severe anaemia and malaria (ie, severe malarial anaemia) had a lower risk of mortality after discharge than children with severe anaemia without evidence of malaria (n=2 studies; RR 0·71, 0·48–0·94; p<0·0001; I2=0%; appendix p 6).
During the in-hospital period, 2369 (5·6%) of 41 945 children died (n=19 studies), ranging from 0·4% to 13·0% for severe anaemia, and 3·0% to 23·2% for acute malnutrition, 1·0% to 15·1% for malaria, 2·7% to 11·5% for pneumonia, and 1·8% to 12·5% for unspecified health conditions. Because of the high heterogeneity (I2=96·5%), pooled summary risks were not calculated (appendix p 7).
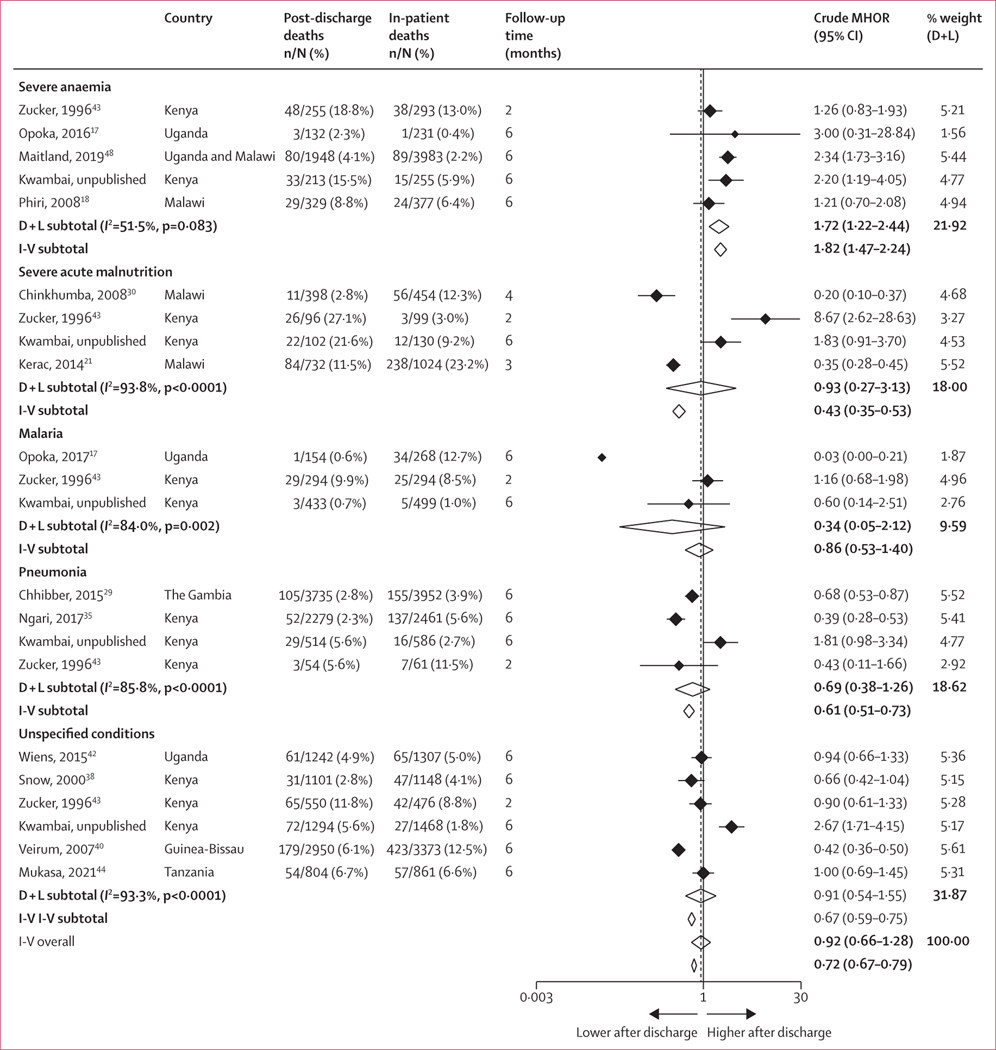
13 cohort studies involving a total of 23 600 admissions reported both in-hospital mortality and mortality after discharge. Overall, across all health conditions pooled, mortality after discharge by 6 months was not different from in-hospital mortality (MHOR 0·92, 95% CI 0·66–1·28; p=0·63; I2=92·1%). However, there was considerable heterogeneity within and between health condition subgroups. Among children admitted with severe anaemia, the odds of mortality were consistently higher in the post-discharge period than during hospital admission (n=5 studies; MHOR 1·72, 1·22–2·44; p=0·0020; I²=51·5%). This finding was not observed for any of the other health conditions (figure 4).
Figure 4: In-patient mortality versus mortality after discharge within 6 months by health condition on admission.
For each study, the MHOR was obtained by comparing the number of deaths after discharge versus in-patient deaths during the initial hospital admission. D + L=DerSimonian and Laird random effects. I-V=inverse-variance fixed effect. MHOR=Mantel-Haenszel odds ratio.
Six prospective follow-up studies showed that mortality during the first 6 months after discharge was nearly five times higher in recently hospitalised children (with any health condition), relative to otherwise healthy children from the community (n=6 studies; RR 4·88, 2·73–7·03; p<0·0001; I2=0%), with the greatest difference among children recently recovered from severe anaemia (appendix p 8).
11 studies analysed other potential risk factors for mortality after discharge. Risk factors significantly associated with mortality after discharge in some of these studies included discharge against medical advice, hypoxia (SPO2 <90%), bacteraemia, low haemoglobin on admission, younger age, previous hospital admission, positive maternal HIV status, poor anthropometric measurements, reduced consciousness, proteinuria, absence of malaria parasites on admission, and admission with a chronic disease. HIV positivity was associated with an increased risk of mortality after discharge in two studies before the widescale introduction of anti-retroviral therapy (ART), and in six studies after the introduction of ART (appendix pp 9, 20–32).
11 studies reported readmissions after discharge for periods ranging between 3 months to 18 months with wide variations in the reported readmission risk (appendix pp 12–14). The crude proportion of children readmitted at least once by 6 months was 897 (17·3%) of 5188 children, ranging from 3·1% to 30·6%, on the basis of seven studies.17,21,37,47–50 Readmisions for severe anaemia at 6 months after discharge was 21·8% and for non-severe anaemia health conditions was 7·2% in these six studies. Only one study allowed for a direct comparison of readmission risk between severe anaemia and other health conditions and reported a three-fold (n=1 study; RR 3·05, 1·12–8·35; p<0·0001) increased risk by 6 months.17
Because of the observed variation in the duration of follow-up between health conditions (appendix p 15), a post-hoc sensitivity analysis was done, excluding four studies that had less than 6 months follow-up. Similar conclusions could be drawn for severe anaemia and severe acute malnutrition from the sensitivity analysis (n=10 studies; appendix p 10) compared with the full dataset (n=13 studies; figure 3) regarding the excess risk of mortality after discharge relative to other health conditions. Similarly, the conclusions remained unaffected for severe anaemia when comparing in-hospital mortality versus mortality after discharge (n=5 studies; figure 4; vs n=4 studies; appendix p 11). However, for severe acute malnutrition, the difference between in-hospital mortality and mortality after discharge was greater when the analysis was restricted to studies with at least 6 months follow-up (n=1 study; MHOR 1·83, 0·91–3·70; p=0·09; appendix p 11) than with the full sample of four studies (n=4 studies; MHOR 0·93, 0·27–3·13; p=0·90; figure 4).
Discussion
To our knowledge, this is the first systematic review and meta-analysis comparing the risk of mortality or readmission after discharge in malaria-endemic areas of Africa by health condition on admission. Children discharged from hospital after recovery from severe anaemia or severe acute malnutrition were 2·7 times and 3·1 times more likely to die from any cause during the first 6 months after discharge, respectively, than children previously admitted with other health conditions. By contrast to children admitted with severe acute malnutrition or other health conditions, children with severe anaemia were also more likely to die in the 6 months after discharge than during hospital admission. Readmissions during this period were also more likely among children previously admitted for severe anaemia than children admitted for other reasons, but this result was based on only one study. Although there was substantial variation in study designs, these findings indicate that in malaria-endemic countries in Africa, children admitted with severe anaemia and severe acute malnutrition remain at a high risk of mortality in the first few months after discharge.
Although mortality after discharge was highest among children admitted with severe anaemia or severe acute malnutrition, this review also showed that any child that was hospitalised, regardless of the health condition on admission, was at a nearly five-times higher increased risk of dying within 6 months after discharge than the apparently healthy, non-hospitalised children from the same community. These findings are consistent with a recent systematic review of all-cause mortality after discharge among the general paediatric population, which indicated a substantial global burden of mortality after discharge, especially in low-income countries.16
The term post-hospital health condition was proposed, which refers to an acquired, transient condition of vulnerability in patients who were recently admitted to hospital, mostly older patients in the USA, resulting in a period of generalised risk for myriad adverse health events not necessarily linked to the original illness. During this period, the patient is not only recovering from the initial acute illness but also has continued physiological and immunological impairment because of the initial insult and other stressors following hospital admission or treatment.51
We were unable to analyse the reasons for the high mortality after discharge or readmission rates observed in children with severe anaemia because of the scarcity of details available from the source studies about the cause of readmissions or deaths after discharge. These factors are likely to be multifactorial, reflecting the complex nature of the causes of anaemia in this setting, which include nutritional, environmental, biological, sociobehavioural, and genetic factors.52 Explanations for high mortality after discharge might include continued exposure to the same risk factors in the community that resulted in the initial admission and possible delays in seeking appropriate care because of local beliefs and perceptions about severe anaemia.53 Although insufficient details were available to allow an analysis of HIV status as a standalone condition on admission, children who were HIV positive were at a 1·4–6·5-times increased risk of mortality after discharge than children who were HIV negative. Recurrent malaria infections have also been reported as risk factors for the recurrence of severe anaemia among these children.17,18,43,47,49
By contrast to severe anaemia, severe acute malnutrition is recognised as a major risk factor for mortality after discharge in resource-poor countries, with guidelines for further follow-up and care at home and periodic monitoring to avoid relapse.20,54,55 Children who were malnourished were not only at increased risk of mortality after discharge compared with other children, but the coexistence of severe undernutrition was also an important contributor to mortality after discharge among children recently admitted with other health conditions, including those with severe anaemia.21,29,34,41 Many children who are undernourished in resource-poor settings have underlying medical or comorbid conditions, either directly causing, contributing to, or complicating malnutrition.14
It was of interest to see that children with severe malaria per se (eg, cerebral malaria without severe anaemia) had lower mortality after discharge than children with severe anaemia. We also found a substantially lower risk of mortality after discharge among children with severe malarial anaemia compared with children with severe anaemia without malaria. It is possible that the attribution of multiple and chronic causes of severe anaemia such as micronutrient deficiencies or chronic infections, such as tuberculosis and HIV, was greater among the children without malaria. If so, they would require multiple and long-term interventions after discharge. This type of care might not be needed for children in whom malaria was the main cause of severe anaemia, in particular if the initial event was successfully treated with blood transfusion and effective anti-malarials. Alternatively, the in-patient population treated for severe malaria might reflect a heterogeneous group of patients, given that in many countries, large numbers of patients with uncomplicated malaria are admitted as in-patients to health facilities.56
Although in-hospital severe malaria was not a major risk factor for death after discharge, in highly endemic areas, malaria can become an important risk factor for readmissions or mortality after discharge in all groups. Malaria infection causes dyserythropoiesis, which can result in bone marrow suppression and might be prolonged in children who become reinfected or in whom the initial infection is not cleared, or not recognised and therefore not treated.3,43,57,58 In children initially admitted with severe anaemia who have prolonged dyserythropoiesis, bone marrow suppression, or both, the benefit of blood transfusions during hospital admission might be too short-lived to allow for full haematological recovery. These children might develop rebound severe anaemia and poor immunological responses to bacterial and other infections.
Other risk factors significantly associated with mortality after discharge irrespective of the initial exposure condition included hypoxia, bacteraemia or sepsis, jaundice, hepatomegaly, splenomegaly, prolonged hospital admission, lower socioeconomic status, reduced consciousness on admission (Blantyre coma scale <5), delay in seeking care, history of previous hospitals admissions, young age, and discharge against medical advice.
These findings support the need for management strategies for recently discharged children, especially those with severe anaemia and severe acute malnutrition in malaria-endemic areas of Africa. Improved diagnosis for the underlying causes of severe anaemia is also merited. Interventions after discharge that have been shown to be effective include preventive zinc supplementation to reduce morbidity and mortality caused by diarrhoea and pneumonia59 and prophylaxis with cotrimoxazole to reduce morbidity and mortality in individuals who are HIV positive.60 More recently, neither 3 months of enhanced supplementation with multivitamin multimineral supplement or cotrimoxazole prophylaxis improved 6-month survival versus iron and folate treatment.48 However, 3 months of malaria chemoprevention with artemether-lumefantrine after discharge among children admitted with severe malaria anaemia prevented 41% of deaths or readmissions during the 12-week intervention period in a trial in Malawi,49 and this was 70% when using dihydroartemisinin-piperaquine in a trial in Kenya and Uganda, which recruited children with all-cause severe anaemia.47 Policy guidelines for the management of severe anaemia after discharge in children living in malaria-endemic areas of Africa are needed urgently.61
There are important limitations to this type of secondary analysis. Some limitations are common to many meta-analyses. First, there was considerable variation in the duration or reporting of follow-up data, or both; for instance, some studies followed children only for 1 month, whereas others followed them for much longer than 6 months but did not report results by 6 months and could thus not contribute to our meta-analysis. For this reason, a post-hoc sensitivity analysis was done that excluded four studies with less than 6 months follow-up, which confirmed the excess post-discharge mortality in children with severe anaemia relative to other health conditions or relative to in-hospital mortality. A second limitation is that variations in the design of observational studies and RCTs and their reporting, and in the prevalence of various background causes of mortality will have contributed to substantial observed heterogeneity. Third, few studies reported both in-hospital mortality and mortality after discharge, and few studies reported mortality after discharge by admission health condition. Those that did had relatively modest sample sizes and events. Fourth, the results of random-effect models based on a small number of studies should be interpreted with caution because reliable estimates of the between-studies variance (and thus the confidence interval), summary point estimates, and the dispersion effect are difficult to obtain.62 Fifth, we could not analyse the causes of deaths after discharge because of insufficient details reported. Sixth, we were unable to assess small-study effects or publication bias because of the small number of studies per health condition and the probable presence of heterogeneity or true small-study effects for meta-analysis of observational studies. Seventh, we were unable to assess the effect of any residual confounding by comparing crude and adjusted meta-analyses because of the limitations of the data reported. Lastly, included studies that recruited children with severe anaemia did not report on the causes of anaemia on admission. Therefore, we could not determine the cause-specific severe anaemia mortality burden in hospital or link the causes to mortality burden after discharge. The variation in reporting the risk factors for mortality after discharge and scarcity of data on the causes of mortality after discharge are important considerations for future research to develop a comprehensive management plan after discharge.
This analysis confirms that children younger than 15 years of age who were recently discharged from hospital after recovery from severe anaemia or severe acute malnutrition are at excess risk of mortality during the first 6 months after discharge compared with children admitted for other causes, such as severe malaria. There is a need to develop post-discharge management strategies for these high-risk groups.
Supplementary Material
Research in context.
Evidence before this study
Severe anaemia is a major cause of morbidity and mortality in children in malaria-endemic sub-Saharan Africa. Post-discharge mortality in children hospitalised for severe anaemia and other health conditions has been shown in some studies to be similar to or higher than in-hospital mortality. The extent of the problem has not been quantified. We searched PubMed, Scopus, Embase, Web of Science, and Cochrane Central from inception to Nov 30, 2021, without language restrictions, for cohort studies and randomised controlled trials. We scanned reference lists of identified articles and requested more information from authors where feasible. There are few studies that have analysed morbidity and mortality after discharge in children younger than 15 years in Africa. We identified one systematic review of the mortality after discharge in developing countries, but this did not include a meta-analysis. The review concluded that studies consistently found mortality after discharge to be similar, or to exceed, in-hospital mortality.
Added value of this study
To our knowledge, this is the first meta-analysis that compares the mortality and morbidity burden after discharge in malaria-endemic Africa for different health conditions. The available information confirms that children who were recently hospitalised with severe anaemia or severe acute malnutrition are at excess risk of mortality during the first 6 months after discharge compared to children admitted for other health conditions. Among the children recently admitted with severe anaemia, mortality is higher during the first 6 months after discharge than during the in-hospital period.
Implications of all the available evidence
The period after discharge is a well recognised risk period for children with severe acute malnutrition, for which appropriate follow-up and management strategies exist. However, it is largely unaddressed for children hospitalised with severe anaemia. Improved strategies after discharge directed at this high-risk paediatric population need to be developed.
Acknowledgments
We appreciate the generosity of the authors who volunteered additional data or information for this review. This study was funded by the Research Council of Norway through the Global Health and Vaccination programme (project number 234487), which is part of the European and Developing Countries Clinical Trials Partnerships programme, supported by the EU; and by the US Centers for Disease Control and Prevention through a cooperative agreement with the Liverpool School of Tropical Medicine. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
Funding
Research Council of Norway and US Centers for Disease Control and Prevention.
Footnotes
Declaration of interests
We declare no competing interests.
Data sharing
All aggregated data collected during this analysis will be made available and access to data provided when a proposal has been approved by the investigators, after consideration of overlap between the proposal and any ongoing efforts. Proposals should be directed to FOtK (feiko.terkuile@lstmed.ac.uk) and TKK (qbb5@cdc.gov) to gain access. Data requesters will need to sign a data access agreement, and the database will be transferred electronically.
References
- 1.UN Inter-agency Group for Child Mortality Estimation. Levels and trends in child mortality: report 2018, estimates developed by the UN Inter-agency Group for Child Mortality Estimation. 2018. https://www.unicef.org/publications/index_103264.html (accessed April 29, 2022).
- 2.WHO. Severe falciparum malaria. 2000. https://www.who.int/malaria/publications/atoz/who-severe-malaria-tmih-supplement-2014.pdf (accessed April 29, 2022).
- 3.Akech SO, Hassall O, Pamba A, et al. Survival and haematological recovery of children with severe malaria transfused in accordance to WHO guidelines in Kilifi, Kenya. Malaria J 2008; 7: 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.English M, Ahmed M, Ngando C, Berkley J, Ross A. Blood transfusion for severe anaemia in children in a Kenyan hospital. Lancet 2002; 359: 494–95. [DOI] [PubMed] [Google Scholar]
- 5.Koram KA, Owusu-Agyei S, Utz G, et al. Severe anemia in young children after high and low malaria transmission seasons in the Kassena-Nankana district of northern Ghana. Am J Trop Med Hyg 2000; 62: 670–74. [DOI] [PubMed] [Google Scholar]
- 6.Obonyo CO, Vulule J, Akhwale WS, Grobbee DE. In-hospital morbidity and mortality due to severe malarial anemia in western Kenya. Am J Trop Med Hyg 2007; 77: 23–28. [PubMed] [Google Scholar]
- 7.Bojang KA, Van Hensbroek MB, Palmer A, Banya WA, Jaffar S, Greenwood BM. Predictors of mortality in Gambian children with severe malaria anaemia. Ann Trop Paed 1997; 17: 355–59. [DOI] [PubMed] [Google Scholar]
- 8.Lackritz EM, Hightower AW, Zucker JR, et al. Longitudinal evaluation of severely anemic children in Kenya: the effect of transfusion on mortality and hematologic recovery. AIDS 1997; 11: 1487–94. [DOI] [PubMed] [Google Scholar]
- 9.Marsh K, Forster D, Waruiru C, et al. Indicators of life-threatening malaria in African children. N Engl J Med 1995; 332: 1399–404. [DOI] [PubMed] [Google Scholar]
- 10.WHO. Iron deficiency anaemia: assessment, prevention and control: a guide for programme managers. 2001. https://www.who.int/nutrition/publications/micronutrients/anaemia_iron_deficiency/WHO_NHD_01.3/en/ (accessed April 29, 2022).
- 11.Stevens GA, Finucane MM, De-Regil LM, et al. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: a systematic analysis of population-representative data. Lancet Glob Health 2013; 1: e16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Hensbroek MB, Jonker F, Bates I. Severe acquired anaemia in Africa: new concepts. Br J Haematol 2011; 154: 690–95. [DOI] [PubMed] [Google Scholar]
- 13.Bojang KA, Palmer A, Boele van Hensbroek M, Banya WA, Greenwood BM. Management of severe malarial anaemia in Gambian children. Trans R Soc Trop Med Hyg 1997; 91: 557–61. [DOI] [PubMed] [Google Scholar]
- 14.Caulfield LE, de Onis M, Blossner M, Black RE. Undernutrition as an underlying cause of child deaths associated with diarrhea, pneumonia, malaria, and measles. Am J Clin Nutr 2004; 80: 193–98. [DOI] [PubMed] [Google Scholar]
- 15.Lackritz EM, Campbell CC, Ruebush T, Hightower AW, Wakube W, Were J. Effect of blood transfusion on survival among children in a Kenyan hospital. Lancet 1992; 340: 524–28. [DOI] [PubMed] [Google Scholar]
- 16.Nemetchek B, English L, Kissoon N, et al. Paediatric postdischarge mortality in developing countries: a systematic review. BMJ Open 2018; 8: e023445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Opoka RO, Hamre KES, Brand N, Bangirana P, Idro R, John CC. High postdischarge morbidity in Ugandan children with severe malarial anemia or cerebral malaria. J Pediatric Infect Dis Soc 2017; 6: e41–e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phiri KS, Calis JC, Faragher B, et al. Long term outcome of severe anaemia in Malawian children. PLoS One 2008; 3: e2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malamba S, Hladik W, Reingold A, et al. The effect of HIV on morbidity and mortality in children with severe malarial anaemia. Malaria J 2007; 6: 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bahwere P, Mtimuni A, Sadler K, Theresa B, Collins S. Long term mortality after community and facility based treatment of severe acute malnutrition: analysis of data from Bangladesh, Kenya, Malawi and Niger. J Pub Health Epidemiol 2012; 4: 215–25. [Google Scholar]
- 21.Kerac M, Bunn J, Chagaluka G, et al. Follow-up of post-discharge growth and mortality after treatment for severe acute malnutrition (FuSAM study): a prospective cohort study. PLoS One 2014; 9: e96030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015; 350: g7647. [DOI] [PubMed] [Google Scholar]
- 23.WHO, Global Malaria Programme. World malaria report 2020: 20 years of global progress & challenges. 2020. https://www.who.int/publications/i/item/9789240015791 (accessed April 29, 2022).
- 24.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2011. http://www.evidencebasedpublichealth.de/download/Newcastle_Ottowa_Scale_Pope_Bruce.pdf. [Google Scholar]
- 26.Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. Int J Epidemiol 2002; 31: 140–49. [DOI] [PubMed] [Google Scholar]
- 27.Higgins J, Green S. The Cochrane handbook for systematic reviews of interventions 4ed. Chichester, UK: John Wiley & Sons, 2006. [Google Scholar]
- 28.Carme B, Bouquety J, Plassart H. Mortality and sequelae due to cerebral malaria in African children in Brazzaville, Congo. Am J Trop Med Hyg 1993; 48: 216–21. [DOI] [PubMed] [Google Scholar]
- 29.Chhibber AV, Hill PC, Jafali J, et al. Child mortality after discharge from a health facility following suspected pneumonia, meningitis or septicaemia in rural Gambia: a cohort study. PLoS One 2015; 10: e0137095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chinkhumba J, Tomkins A, Banda T, Mkangama C, Fergusson P. The impact of HIV on mortality during in-patient rehabilitation of severely malnourished children in Malawi. Trans R Soc Trop Med Hyg 2008; 102: 639–44. [DOI] [PubMed] [Google Scholar]
- 31.Hau DK, Chami N, Duncan A, et al. Post-hospital mortality in children aged 2–12 years in Tanzania: a prospective cohort study. PLoS One 2018; 13: e0202334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hennart P, Beghin D, Bossuyt M. Long-term follow-up of severe protein-energy malnutrition in Eastern Zaire. J Trop Pediatr 1987; 33: 10–12. [DOI] [PubMed] [Google Scholar]
- 33.Madrid L, Casellas A, Sacoor C, et al. Postdischarge mortality prediction in sub-Saharan Africa. Pediatrics 2019; 143: e20180606. [DOI] [PubMed] [Google Scholar]
- 34.Moisi JC, Gatakaa H, Berkley JA, et al. Excess child mortality after discharge from hospital in Kilifi, Kenya: a retrospective cohort analysis. Bull World Health Organ 2011; 89: 725–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ngari MM, Fegan G, Mwangome MK, et al. Mortality after inpatient treatment for severe pneumonia in children: a cohort study. Paediatr Perinat Epidemiol 2017; 31: 233–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ngari MM, Obiero C, Mwangome MK, et al. Mortality during and following hospital admission among school-aged children: a cohort study. Wellcome Open Res 2020; 5: 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Opoka RO, Waiswa A, Harriet N, John CC, Tumwine JK, Karamagi C. Blackwater fever in Ugandan children with severe anemia is associated with poor postdischarge outcomes: a prospective cohort study. Clin Infect Dis 2019; 70: 2247–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Snow RW, Howard SC, Mung’Ala-Odera V, et al. Paediatric survival and re-admission risks following hospitalization on the Kenyan coast. Trop Med Int Health 2000; 5: 377–683. [DOI] [PubMed] [Google Scholar]
- 39.Talbert A, Ngari M, Bauni E, et al. Mortality after inpatient treatment for diarrhea in children: a cohort study. BMC Med 2019; 17: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Veirum JE, Sodeman M, Biai S, Hedegard K, Aaby P. Increased mortality in the year following discharge from a paediatric ward in Bissau, Guinea-Bissau. Acta Paediatr 2007; 96: 1832–38. [DOI] [PubMed] [Google Scholar]
- 41.Villamor E, Misegades L, Fataki MR, Mbise RL, Fawzi WW. Child mortality in relation to HIV infection, nutritional status, and socio-economic background. Int J Epidemiol 2005; 34: 61–68. [DOI] [PubMed] [Google Scholar]
- 42.Wiens MO, Kumbakumba E, Larson CP, et al. Postdischarge mortality in children with acute infectious diseases: derivation of postdischarge mortality prediction models. BMJ Open 2015; 5: e009449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zucker JR, Lackritz EM, Ruebush TK, 2nd, et al. Childhood mortality during and after hospitalization in western Kenya: effect of malaria treatment regimens. Am J Trop Med Hyg 1996; 55: 655–60. [DOI] [PubMed] [Google Scholar]
- 44.Mukasa O, Masanja H, DeSavigny D, Schellenberg J. A cohort study of survival following discharge from hospital in rural Tanzanian children using linked data of admissions with community-based demographic surveillance. Emerg Themes Epidemiol 2021; 18: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bwakura-Dangarembizi M, Dumbura C, Amadi B, et al. Risk factors for postdischarge mortality following hospitalization for severe acute malnutrition in Zimbabwe and Zambia. Am J Clin Nutr 2021; 113: 665–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Biai S, Rodrigues A, Gomes M, et al. Reduced in-hospital mortality after improved management of children under 5 years admitted to hospital with malaria: randomised trial. BMJ 2007; 335: 862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kwambai TK, Dhabangi A, Idro R, et al. Malaria chemoprevention in the postdischarge management of severe anemia. N Engl J Med 2020; 383: 2242–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maitland K, Olupot-Olupot P, Kiguli S, et al. Co-trimoxazole or multivitamin multimineral supplement for post-discharge outcomes after severe anaemia in African children: a randomised controlled trial. Lancet Glob Health 2019; 7: e1435–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Phiri K, Esan M, van Hensbroek MB, Khairallah C, Faragher B, ter Kuile FO. Intermittent preventive therapy for malaria with monthly artemether-lumefantrine for the post-discharge management of severe anaemia in children aged 4–59 months in southern Malawi: a multicentre, randomised, placebo-controlled trial. Lancet Infect Dis 2012; 12: 191–200. [DOI] [PubMed] [Google Scholar]
- 50.Pavlinac PB, Singa BO, Tickell KD, et al. Azithromycin for the prevention of rehospitalisation and death among Kenyan children being discharged from hospital: a double-blind, placebo-controlled, randomised controlled trial. Lancet Glob Health 2021; 9: e1569–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krumholz HM. Post-hospital syndrome: an acquired, transient condition of generalized risk. N Engl J Med 2013; 368: 100–02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Calis JC, Phiri KS, Faragher EB, et al. Severe anemia in Malawian children. Malawi Med J 2016; 28: 99–107. [PMC free article] [PubMed] [Google Scholar]
- 53.Dhabangi A, Idro R, John CC, et al. Community perceptions of paediatric severe anaemia in Uganda. PLoS One 2019; 14: e0209476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O’Sullivan NP, Lelijveld N, Rutishauser-Perera A, Kerac M, James P. Follow-up between 6 and 24 months after discharge from treatment for severe acute malnutrition in children aged 6–59 months: a systematic review. PLoS One 2018; 13: e0202053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ashworth A, Khanum S, Jackson A, C S. Guidelines for the inpatient treatment of severely malnourished children. Geneva: World Health Organization, 2003. [Google Scholar]
- 56.Camponovo F, Bever CA, Galactionova K, Smith T, Penny MA. Incidence and admission rates for severe malaria and their impact on mortality in Africa. Malaria J 2017; 16: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Camacho LH, Gordeuk VR, Wilairatana P, Pootrakul P, Brittenham GM, Looareesuwan S. The course of anaemia after the treatment of acute, falciparum malaria. Ann Trop Med Parasitol 1998; 92: 525–37. [DOI] [PubMed] [Google Scholar]
- 58.Kurtzhals JA, Rodrigues O, Addae M, Commey JO, Nkrumah FK, Hviid L. Reversible suppression of bone marrow response to erythropoietin in Plasmodium falciparum malaria. Br J Haematol 1997; 97: 169–74. [DOI] [PubMed] [Google Scholar]
- 59.Yakoob MY, Theodoratou E, Jabeen A, et al. Preventive zinc supplementation in developing countries: impact on mortality and morbidity due to diarrhea, pneumonia and malaria. BMC Public health 2011; 11: S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.WHO. Co-trimoxazole prophylaxis for malaria and bacterial infections in people with HIV. 2014. https://www.who.int/hiv/topics/arv/cotrimoxazole_factsheet_dec2014/en/ (accessed March 14, 2020).
- 61.WHO. Updates on the management of severe acute malnutrition in infants and children. 2013. https://www.who.int/nutrition/publications/guidelines/updates_management_SAM_infantandchildren/en/ (accessed April 29, 2022). [PubMed]
- 62.Borenstein M, Hedges LS, Rothstein HR. Meta-Analysis: fixed effect vs. random effects: meta-analysis.com. 2007. https://www.meta-analysis.com/downloads/M-a_f_e_v_r_e_sv.pdf. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.