Highlights
-
•
Opportunistic fungal pathogens along with the resistance to drugs represent serious problem in health.
-
•
Probiotics, one of the well studied biological products, are safe upon consumption and are explored to treat various fungal infections such as Aspergillus, Mucor, Rhizopus, Candida, Fusarium, Penicillium and Dermatophytes.
-
•
The antifungal potency of major groups of probiotics such as Lactobacillus spp, Leuconostoc spp, Saccharomyces etc. and their postbiotics such as organic acids, bacteriocin like metabolites, Hydrogen peroxide, cyclic dipeptides etc. to inhibit these fungal pathogens which is most common to cause diseases in humans have been discussed in this report.
Keywords: Antifungal agents, Aspergillus. Candida, Mucor, Opportunistic fungal infections, Postbiotics, Probiotics
Abstract
During past twenty years the opportunistic fungal infections have been emerging, causing morbidity and mortality. The fungi belonging to Aspergillus, Mucor, Rhizopus, Candida, Fusarium, Penicillium, Dermatophytes and others cause severe opportunistic fungal infections. Among these Aspergillus and Candida spp cause majority of the diseases. The continuum of fungal infections will prolong to progress in the surroundings of the growing inhabitants of immunocompromised individuals. Presently many chemical-based drugs were used as prophylactic and therapeutic agents. Prolonged usage of antibiotics may lead to some severe effect on the human health. Also, one of the major threats is that the fungal pathogens are becoming the drug resistant. There are many physical, chemical, and mechanical methods to prevent the contamination or to control the disease. Owing to the limitations that are observed in such methods, biological methods are gaining more interest because of the use of natural products which have comparatively less side effects and environment friendly. In recent years, research on the possible use of natural products such as probiotics for clinical use is gaining importance. Probiotics, one of the well studied biological products, are safe upon consumption and are explored to treat various fungal infections. The antifungal potency of major groups of probiotic cultures such as Lactobacillus spp, Leuconostoc spp, Saccharomyces etc. and their metabolic byproducts which act as postbiotics like organic acids, short chain fatty acids, bacteriocin like metabolites, Hydrogen peroxide, cyclic dipeptides etc. to inhibit these opportunistic fungal pathogens have been discussed here.
1. Introduction
The emergence of opportunistic fungi which infect immunosuppressed individuals is a growing health concern [1], presenting a massive problem and confront for treatment and diagnosis to health care professionals causing significant mortality and morbidity. These evolving fungal infections are increasingly affecting patients with predisposing circumstances such as complex HIV infection, cancer, granulocytopenia, organ transplantation, severe burn, diabetes, trauma, malnutrition and other issues leading to low immunity [2].
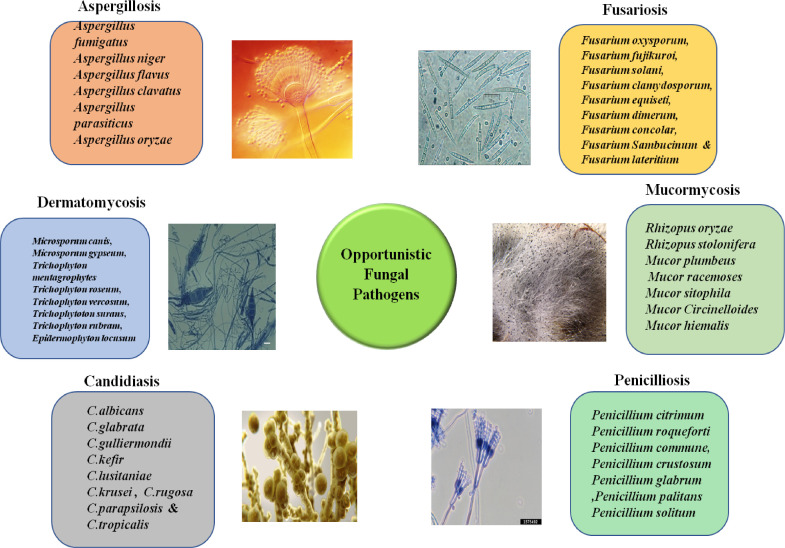
The appearance and re-appearance of opportunistic fungal infections like Candidiasis, Cryptococcosis, Zygomycosis, Mucormycosis and Pneumocytosis are fairly common. In a nationwide surveillance study done in US hospitals, one of the most common nosocomial pathogens causing bloodstream infections was Candida spp. Of the different species isolated from 1890 cases in this study, C albicans tops the list responsible for 54% of cases, followed by Candida glabrata (19%), Candida parapsilosis (11%), Candida tropicalis (11%), and Candida krusei (5%) [3]. Several studies have stated that, candidiasis, specifically candidemia, was the most common mycotic infection of hospitalized patients and is associated with significant mortality and prolonged hospital stay [4]. Similarly, Aspergillus spp such as Aspergillus fumigatus, Aspergillus terreus, Aspergillus flavus, Aspergillus nidulans and Aspergillus niger are opportunistic moulds which cause invasive and allergic infections such as aspergillosis can affect about more than 45% of immunocompromised patients. It is alarming to observe that among the patients hospitalized in ICU due to invasive fungal infections there is a mortality rate of 67% [5]. Like Aspergillus species, Zygomycetes are common nosocomial pathogens causing systemic Zygomycosis, and they are widespread among people with uncontrolled diabetes mellitus, burns, metabolic acidosis and malignant hematological disorders all around the globe [6] (Fig.1).
Fig 1.
Different species of fungi which cause opportunistic fungal infections such as Aspergillosis, Fusariosis, Mucormycosis, Penicilliosis, Candidiasis and Dermatomycosis.
For a few decades local and systemic antifungal agents like nystatin, amphotericin B and fluconazole have been effectively used as prophylactic and therapeutic agents to preclude colonization of invasive opportunistic infections of fungi [7]. Though, their effectiveness is compromised because for thier frightening raise in the appearance of antibiotic resistant fungal strains globally [8]. Therefore, alternative therapies have been implicated for opportunistic fungal diseases/infections together with the usage of natural products like oils, phytochemicals and peptides [9]. Although promising, their bio-tolerance and toxicities of these compounds are of apprehension. Hence, they are until now in the investigational period of improvement [10]. Hence, for these concerns, the need of biocontrol agent such as probiotic bacteria and its postbiotics has been anticipated as an substitute approach of treatment against opportunistic human fungal infections [11].
According to Food and Agriculture Organization of the United Nations (FAO) and the World Health Organization (WHO), Probiotics and beneficial microorganisms, that when administered in sufficient quantity provide health advantage on host [12]. The mainly common probiotic microorganisms are strains from the genera Lactobacillus (i.e., Lactobacillus acidophilus, Lactobacillus rhamnosus, Lactiplantibacillus plantarum, L actobacillus delbrueckii subsp. bulgaricus, Lactobacillus casei, etc.) and Bifidobacterium (i.e., Bifidobacterium animalis subsp. lactis, Bifidobacterium infantis, Bifidobacterium longum, etc.). Other probiotic bacteria include Lactococcus lactis subsp. lactis, Pediococcus acidilactici, Bacillus subtilis, Leuconostoc mesenteroides, Enterococcus faecium, Escherichia coli and Streptococcus thermophilus etc. [13]. Certain yeasts like Saccharomyces boulardii as well proved to be probiotics [14,15]. These organisms have been proposed to control many human pathogens including fungi.
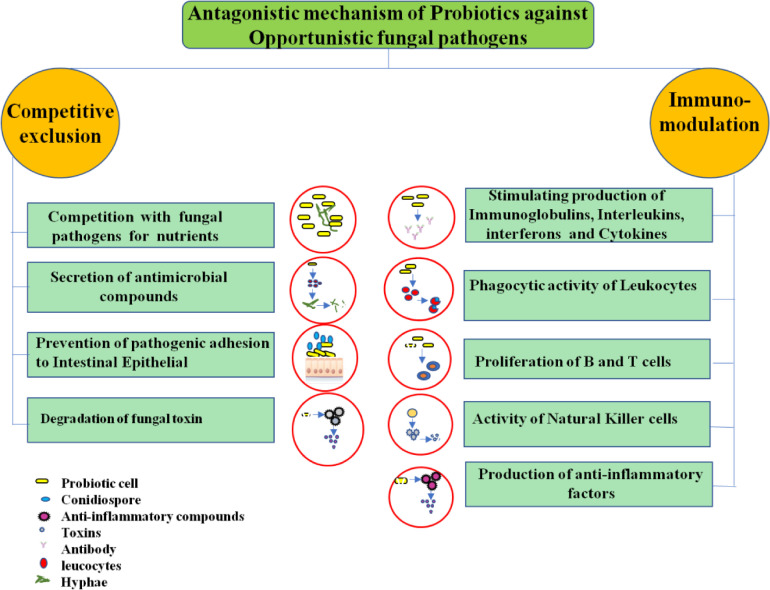
Antagonism is most significant probiotic management method caused by immune modulation through stimulating the host defense systems and competitive exclusion involves the production of secondary metabolites (antimicrobial compounds), prevention of pathogen adhesion to epithelial cells and toxin bioavailability reduction. In addition, signaling molecules also triggers gene expression changes. Major antimicrobial compound produced by probiotic microorganisms include formic acid, lactic acid, phenyllactic acid, benzoic acid, acetic acid and also organic acids that lower pH, hydrogen peroxide, short chain fatty acids, diacetyl, acetoin, carbon dioxide, acetaldehyde, bacteriocins and bacteriocin like protienaceous compounds [13] (Fig. 2).
Fig 2.
Antagonistic mechanism of probiotics for the prevention of opportunistic fungal pathogens in humans.
2. Challenges
Challenges in the management of opportunistic fungal diseases are major and multiple. Firstly, it is difficult in making an early diagnosis of most of the opportunistic fungal infections. Second, the antifungal agents are effective invitro often for the management of the fungal diseases is not as effective in vivo conditions. Third, problems related to appropriate and sufficient amount of drug doses for the treatment, and uncertainty in making the decision of when to stop antifungal therapy [15]. These problems are most apparent in the management of opportunistic fungal diseases with chemical drugs. Therefore, the extensive use of antifungal agents may also lead to several health issues [16] with patients undertaking solid-organ transplantation, neoplastic disease, blood and bone marrow transplantation as well as major surgery, immunosuppressive therapy and those with AIDS, advanced age, or premature birth [17].
The exploitation of antifungal drugs and antibiotics can effortlessly escort to the progress of drug resistance. This not only contradicts the outcome of the existing antifungal drugs but also guide to the variation in microbe flora of human. Moreover, a reduction in the immunity of body, creating invasive opportunistic fungal diseases more difficult to control [18]. Every year, exact analysis and the successful practice of suitable antimycotic remedy are tough, which conduct a high death rate in immunosuppressed patients with invasive fungal infections (IFI) [31]. The epidemiology of opportunistic pathogens has altered accompanying with the extensive use of antifungal prophylaxis [32]. Non-fumigatus Aspergillus, Non-albicans Candida, and other molds have turned out to be more frequent opportunistic pathogens instigating invasive infections, and the majority of these incipient invasive fungi are less susceptible or resistant to standard antifungals [17]. Therefore, opportunistic fungal infections owing to this formerly erratic fungus are further difficult to treat and prevent. Advances in additional compelling and fewer noxious antifungal agents like fluconazole, amphotericin B, flucytosine, itraconazole, echinocandins and triazoles, may potentially progress the outcomes of these invasive fungal infections [16]. Hence, it is indispensable to develop more effective and also safe chemical drug alternatives to treat opportunistic fungal infections. Therefore, use of biocontrol agents from probiotics as alternative therapeutic mode of treatment against opportunistic fungal pathogens has been explored.
Worldwide, probiotics are currently available in a variety of food supplements. With the GRAS status, they tend to supply as enhancement/supplement to the microflora of host and are not as obvious considered pathogenic. Probiotics are well-known for promising results like enhanced gut barrier function; adding up to their sole ability to battle with pathogenic microorganisms for adhesion to the gut epithelial cells and develop their colonization [19], [20], [21]. Consumption of potential probiotics/postbiotics is connected with a series of health benefits including protection against diarrheal diseases, lowering of cholesterol, stimulation/modulation of the immune system (Fig. 3), nosocomial and respiratory tract infections, reduction of immune inflammatory disorders [22]. Therefore the use of probiotics to prevent and treat a variety of disease conditions has procured approve in the past ten years. This is relatively, due to a requirement to find alternative to conventional therapies such as antifungal agents/antibiotics for opportunistic fungal infections and disease.
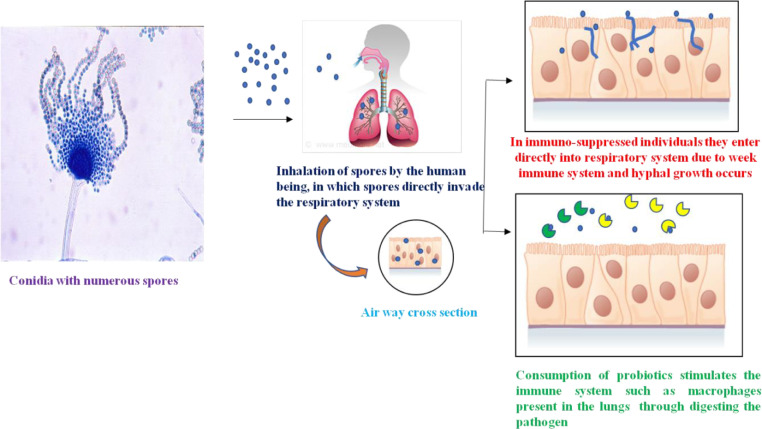
Fig 3.
Fungal agents enter the respiratory tract and cause invasive fungal infections in immunosuppressed patients, whereas consumption of probiotics stimulates the immune system to fight against fungal agents.
In this review the opportunistic fungal diseases and their negative impacts caused to human health are underscored. To overcome this impact the role of Probiotics/Postbiotics to control the growth of such fungi is discussed in this review.
3. Probiotics used against Candida species
Candida species are implicated as the major opportunistic yeast infections in the globe. however among the species of this genus, C. albicans endures to be the mainly widespread which is accountable for almost 50–90% of candidiasis in human [23]. Furthermore, with the hasty rise in candidiasis prevalence, species of Candida other than Candida albicans have been concerned in such infections [24], [25], [26]. The most general species are Candida glabrata, Candida metapsilosis, Candida dubliniensis, Candida tropicalis, Candida parapsilosis, Candida famata, Candida orthopsilosis, Candida kruseri, Candida guillermondii and Candida lusitaniae [27], [28], [29], [30]. Depending on the locality on the body, candidiasis classified as intrauterine candidiasis, Genital candidiasis, nail candidiasis, anal and oral candidiasis. In addition, the giantism of Candida albicans is an imperative source of an extensive range of indications that influence straightly to the welfare of individuals, consequently there is a crucial necessity to diagnose candidiasis as a multifaceted medical syndrome and appraise the degree of related problem concerning prevention and treatment, which passes throughout the prevention of the risk factor.
Applying probiotics to treat and prevent candida fungal infections is derived from the evidence that assured probiotic strains employ a defensive outcome in vivo by hindering the epithelial cells adhesion and colonization by the infectious fungus to the mucosa, secretion of metabolites and also increasing epithelial cell immune defense mechanisms [33]. The different Lactobacillus spp which have demonstrated potential antifungal activity against C. albicans include L. plantarum, L. fermentum [34,35], L. reuteri, L. rhamnosus, L. johnsonii [36], L. acidophilus [37], Lactobacillus paracasei [38], Lactobacillus pentosus [39], L. crispatus, L. gasseri and L. vaginalis [40]. In addition, many of these species have also shown activity against non-albicans Candida species like Candida crusie, Candida glabrata, Candida lusitaniae, Candida tropicalis, C. paropsilosis etc., [39], [40], [41], [42]. The two standard probiotic ATCC cultures L. reuteri RC-14 and L. rhamnosus GR-1 have been repeatedly used to demonstrate their antifungal activity against different Candida species. One such study with these two cultures has been tested against C. albicans causing vulvovaginal candidiasis (VVC). Transcriptome analysis of C. albicans chromosome revealed increased gene expression related to stress and under expression of fluconazole resistance related genes which asserted the effect of probiotic cultures on C albicans survival [36]. Various probiotic lactobacillus demonstrating potential anticandidal activity against different pathogenic strains has been shown in Table 1. Apart from different Lactobacillus species, other probiotic cultures that have exhibited anticandidal activity include lactic acid bacteria like Pediococcus pentosaceous, Weisella confusa [34], Pediococcus acidilactici [43], Bifidobacterium bifidum [44], Bifidobacterium breve and yeasts like Saccharomyces boulardii [45], S cerevisiae [46] etc.,
Table 1.
Antifungal activity of probiotic isolates against Candida species.
| Sl No | Probiotic isolate | Pathogen | Results | References |
|---|---|---|---|---|
| 1 |
L. acidophilus ATCC 4356 |
C. albicans ATCC 18,804 | L. acidophilus cell free supernatant efficiently reduced growth of C. albicans cells by 45.1% | Vilela et al. [37] |
| 2 | L. rhamnosus GR-1ATCC 55,826 and L. reuteri RC-14ATCC 55,845 |
C. glabrata ATCC 2001 C.glabrata (vaginal isolates) namely C. glabrata 95,670, 91,152, 94,885, 98,328 |
Lactobacilluscells and their culture filtrate increased the candidicidal activity against C. glabrata. - in addition, Both Lactobacillus exhibited strong coaggregation and autoaggregation in the presence of Candida |
Chew et al. [50] |
| 3 | L. rhamnosus GR-1 and L. reuteri RC-14 Lactobacillus johnsonii PV016 | C. albicans SC5314 | Lactobacillus showed visible zones of candida growth inhibition in agar plates and suppressed the biofilm formation in broth culture. | Köhler et al. [36] |
| 4 |
L. rhamnosus IMC 501 - L. paracasei IMC 502 Combination of both SYNBIO(1:1 combination) |
C. albicans ATCC 10,261,ISS2,ISS7,C.albicans resistant ISS1, Candida Glabrata ISS3, Candida kruseiISS4,Candida parapsilosis ISS5, and Candida tropicalis ISS6 |
L. rhamnosus - Inhibitory activity against two C. albicans strains (ATCC 10,261 and ISS7). All Candida spp were inhibited except C. glabrata and C. tropicalis. SYNBIO- Inhibitory activity especially C. albicans and C. krusei. SYNBIO gave a high antagonistic activity against all pathogens with a percentage of antagonistic effectiveness between 75% and 100%. |
Coman et al. [123] |
| 5 | L. paracasei subsp paracasei 303, L. plantarum 319, L. fermentum 404, L. rhamnosus IMC 501, and L. paracasei IMC 502 |
C. albicans ISS2, C. glabrata ISS1,C. krusei ISS4, C. parapsilosis ISS5, C.tropicalis ISS6 (clinical isolates) |
All lactobacillus had the potential to inhibit the candida and able to produce antimicrobial compounds such as hydrogen peroxide. All lactobacillus able to coaggregate well with candida species in different degree followed by SYNBIO. |
Verdenelli et al. [41] |
| 6 |
L. plantarum (ATCC 8014) and L. johnsonii (clinical isolate) |
C. albicans (ATCC 14,053) | Conventional hole-plate diffusion: lactobacillus cell free supernatant combination with selenium dioxide showed anti-candida activity; whereas supernatant without selenium did not showed antifungal activity. | Kheradmand et al. [124] |
| 7 | CFS of Lactic acid bacteria | C. albicans | Eight of the 41 fractions excibited antifungal effects against C.albicans. among these eight fractions (A8, A10, B8 and B9 exhibited a complete growth inhibitory effect (100%) in the broth microdilution assay when incubated with C. albicans for 48 h or more. | Seneviratne et al. [125] |
| 8 | L. paracasei 28.4, L. rhamnosus5.2 and L. fermentum 20.4 | C. albicans ATCC 18,804,60(CA60) and CA230S | The most significant reduction in the number of recovered fungal CFUs was attributed to L. paracasei 28.4 that reduced fungal cells by 0.72 Log (p = 0.0001). Lactobacillus supernatant decreased the C. albicans growth by 0.4 Logfor L. rhamnosus 5.2 (p = 0.0001), 0.6 Log for L. fermentum 20.4 (p = 0.0001) and 0.6 Log for L. paracasei 28.4 (p = 0.0001). |
Rossoni et al. [38] |
| 9 | Lactobacillus rhamnosus GG | C.albicans SC5314 | LGG had a significant impact on major virulence attributes, including adhesion, invasion, and hyphal extention, whose reduction consequently prevented epithelial damage. | Mailänder-Sánchez et al. [51] |
| 10 | S. cerevisiae CNCM I −3856 | C.albicans (CA-6) | Affected the expression of virulence traits of C . albicans such as aspartyl proteinases as well as hyphae-asso3ciated proties Hwp 1 and Ece 1 in the vaginal cavity. | Gabrielli et al. [46] |
| 11 | LAB 1 CFS (Lactobacillus pentosus strain LAP 1) | C.albicans, C. tropicalis and C. krusei | - The viability of C. albicans was found to be slightly reduced at 0.5 × MIC of CFNS C. albicans was killed after 8 h and 4 h at MIC and 2 × MIC values, - the killing of C. tropicalis was observed within 8 h and 4 h at MIC and 2 × MIC values C. krusei was killed after 8 h and 6 h at MIC and 2 × MIC values of CFNS. |
Aarti et al. [39] |
| 12 | Lactobacillus fermentum MG901 and L. plantarum MG 989 | C.albicans |
C.albicans cells lost metabolic activity and eventually killed . -high surface hydrophobicity that enhanced its adhesion ability to epithelial cell and showed coaggregation with C.albicans to affect their adhesion and colonization, |
Kang et al. [35] |
| 13 | L. gasseri and L. rhamnosus | C.tropicalis BF,C.krusei BF and C. parapsilosis BF | 64.66%, 67.83% and 33.03% reduction were observed when the biofilms treated with L. gasseri. (CFS) 66.84%,70.56% and 41.33% reduction were observed when the biofilms treated with L. rhamnoses(CFS) | Tan et al. [42] |
| 14 | L.crispatus B1-BC8, L.gasseri BC9-BC14 and L.vaginalis BC15-BC17 |
C.albicans, C.glabrata, C.krusei, C.tropicalis, C.lusitaniae |
Braod spectrum activity was observed for L.crispatus BC1, BC4, BC5 and L.vaginalis BC 15, demonstrating fungicidal activity against all isolates of C.albicans and C.lusitaniae and reduced pathogen adhesion. | Parolin et al. [40] |
| 15 |
L.fermentum,L. rhamnosus, L.plantarum, L.acidophilus |
C. albicans and C.pseudotropicalis | A small proportion of the lactobacilli tested adhered strongly to cultured Vaginal epithelial cells and Inhibited the growth of C. albicans but not of C. pseudotropicalis | Strus et al. [126] |
Biofilm formation among Candida spp is one of the most important factors that contributes towards virulence [47]. Numerous studies with probiotic isolates and their culture filtrates (CFS) have shown efficient antibiofilm activity towards Candida spp. A study conducted using Lactobacillus pentosus (LAP1 strain) manifested significant antibiofilm property against Candida tropicalis, Candida albicans and Candida krusei. Additionally, In the time killing assay, these three Candida spp were completely killed at 8 hrs with the culture filtrate [39]. In another study, mature biofilm formed by single species as well as consortium with Candida non-albicans along with Candida tropicalis, Candida krusie and C parapsilosis was also disrupted by cell free supernatants of lactobacilli in 24 hrs. This has been examined and certified by scanning electron microscopy (SEM) and confocal laser scanning microscopy (CLSM) [48]. One more study by Hager et al. [45] proved that culture filtrates of probiotic strains L. acidophilus, Lactobacillus rhamnosus, Saccharomyces boulardii, and Bifidobacterium breve prevented polymicrobial biofilm formation by Candida tropicalis along with the combination of Serratia marcescens and E. coli.
With the intention of better understanding the anticandidal activity of potential probiotic cultures, vaginal epithelial cell line of human such as VK2/E6E7 has been used as an experiment model [49]. This study has shown that Lactobacillus reuteri RC-14 alone and in conjunction with Lactobacillus rhamnosus GR-1 have the ability to hinder Candidal growth and their cell free supernatant may upregulate interleukin secretion by epithelial cell line which could play a role in clearing the yeast growth in vivo. The same group has done a clinical trial in 2009 demonstrating the efficacy of these two strains as a therapeutic and prophylactic adjuvant in the prevention or treatment of vulvovaginal candidiasis (VVC) [49]. Further, the antifungal outcome of these two strains was experimented against Candida glabrata by plate based microtitre method, spot agar overlay method and live/dead yeast viability method using CLSM. The metabolic actions of all the 4 strains of C. glabrata clinical isolates were found to be hindered by the probiotics exhibiting strong coaggregation and autoaggregation traits [50].
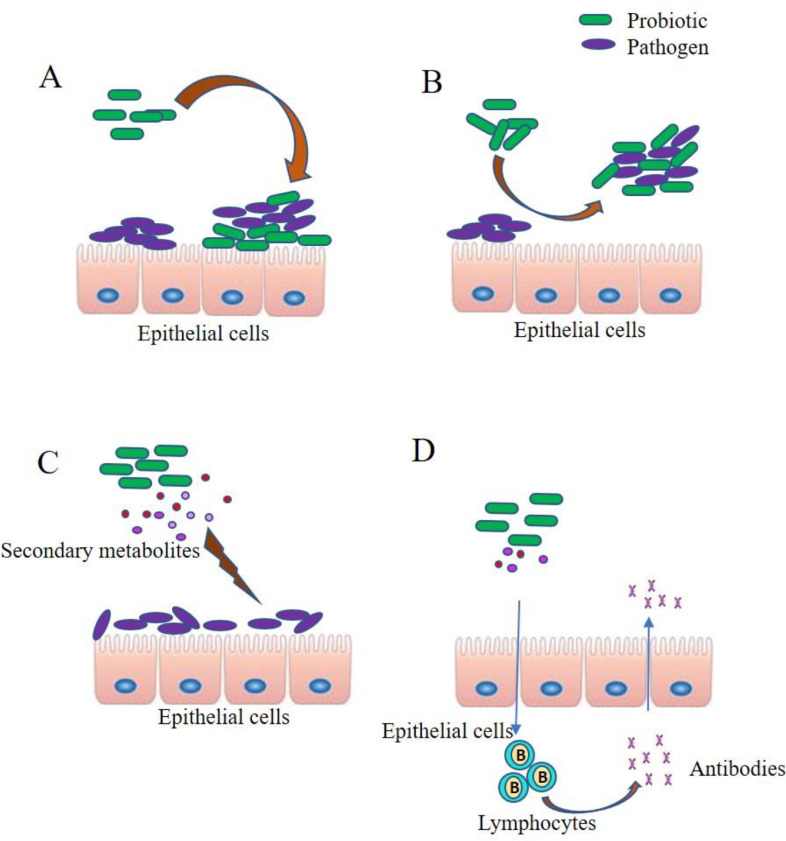
An interesting study establishing the protective cause of probiotic strain Lactobacillus rhamnosus GG (LGG) on the epithelial tissue of oral cavity from the damage caused by Candida albicans was published in 2017 [51] in which an in vitro representation of reconstructed human oral epithelial multilayers (RHOEs) and human keratinocytes (TR146 monolayers) were used. This study not only proved the protective action of probiotic by inhibiting fungal adhesion, invasion and hyphal extension, but also indicated the metabolic reprogramming in Candida due to nutrient depletion. Many Lactobacillus species are robust at inhibiting Candida infection. The anticandidal activity of potential probiotics is represented in the Fig. 4.
Fig 4.
Anticandidal activity of probiotic isolates by different mode of actions. A) Anticandidal activity competition adhesion by the probiotics to the epithelial cells, B) Anticandidal activity by Coaggregation of pathogens and probiotics, C) Anticandidal activity by the production of secondary metabolites that kills the pathogen, D) Anticandidal activity by immunomodulation.
4. Probiotic bacteria against Mucor and Rhizopus species
The second most recurrent mold infection seen in immunosuppressed patients is Mucormycosis. This infection can progress expeditiously in both immunocompetent and immunocompromised patients [52]. Rhizopus species (34%), Mucor species (12%), Leichteimia species (19%) and Rhizomucor (23%) are the most common agents that cause mucormycosis [53]. These moulds enter the body of the human through skin or respiratory tract and less usually by the means of gastrointestinal tract, evoking an response of acute inflammatory [54] (Fig.2). Under suitable circumstances like in immunocompromised individuals, these moulds plague the blood vessels and causing substantial thrombosis of vessels as well as ischemic tissue necrosis [6]. Even after active management of these infections, they are quickly/rapidly increasing and resulting in high rate of death [55]. The antifungals, Amphotericin B (AMB) and their lipid formulations, and newly introduced antifungal agent isavuconazole have been considered as initial treatment for mucormycosis [56]. The pro-drug isavuconazonium sulfate derive from the biologically active antifungal agent such as new broad spectrum triazole and isavuconazole [57]. Some other antifungal agents such as caspofungin, micafungin or anidulafungin, deferasirox and echinocandins also been used for the treatment of mucormycosis. However, these antifungal treatments are nephrotoxic, dose dependent and do not seem to propose an increased chance of survival [58]. Moreover, Rhino orbital mucormycosis reports have been increased in populace among coronavirus disease 2019 particularly in India. In addition, Diabetes mellitus (DM) is one more an autonomous threat factor for both mucormycosis and severe COVID-19 [59]. Incidence of Mucormycosis is apparent typical in the context of immunosuppression like, Solid organ transplantation, diabetes mellitus, hematopoietic stem cell transplantation and hematologic malignancy [60]. Subsequent angioinvasion by hyphae onsets with an accurate interaction with endothelial cells and can bring about systemic proliferation of the disease [58]. Diverse clinical syndromes can emerge in liable hosts such as rhino orbito-cerebral, gastrointestinal, pulmonary cutaneous, disseminated and uncommon appearances [61].
Numerous evidence of the probiotics antifungal effects in vitro against Mucor and Rhizopous species has proved probiotics and their postbiotics as potential biocontrol strategies. Lactobacillus lactis, Pedicoccus pentosaceus, Lactobacillus plantarum and LactoBacillus brevis expressed significant antifungal activity against Rhizopus stolonifer [62]. Sodium caseinate fermentate from Lactobacillus fermentum NCDC141 depicted the inhibitory effect against mold culture Rhizopous oryzae NCDC52 [63]. In another report Rhizopus stolonifer populaces were totally repressed by the use of Lactobacillus plantarum A6 coating. This outcome validates the Lactobacillus platarum A6 strains antagonistic activity and confirms the constructive effects of edible coating applications [64]. Mucor plumbeus was inhibited by Lactobacillus species like L. casei, L. reuteri, L. plantarum and L. bucheneri in the invitro antifungal assays [65]. In another study, Lactobacillus rhamnosus strain 2002 showed significant antifungal activity against Mucor plumbeus [66]. Lactobacillus harbinansis L172, Lactobacillus plantarum L244, Lactobacillus plantarum CIRM-BIA1108, Lactobacillus casei L142, LactoB. brevis L128, Lactobacillus mesenteroides L126 and Lactobacillus citreum CIRM-BIA1456 exhibited good antagonistic activity against Mucor racemoses with strong zone of inhibition [67]. Lactobacillus plantarum TF10 and Lactobacillus plantarum IT10 showed good antifungal activity against Mucor sitophila MD6 with 57.33±4.50 mm and 44.66±4.50 mm of zone of inhibition respectively. CFS characterization of these LAB proved the role of low molecular weight peptides as antifungal compounds [68]. Another report reveals that Mucor circinelloides 01,180,023 and 3 strains of Mucor plumbeus 01,180,036, 01,180,037, 0,110,010 were strongly inhibited by probiotic bacteria such as Lactobacillus rhamnosus LRH01, LRH05, LRH14, LRH16, LRH43, Lactobaillus plantarum LP01, LP37, LP48, Lactobacillus paracasei LPC44, LPC46, Lactobacillus parabuchneri LPB02, LPB04 [69].The antimicrobial peptides produced by Lactobacillus plantarum LR/14 has been shown to have potential activity against Rhizopus stolonifer, and Mucor racemosus [70].
5. Use of probiotic bacteria for the control of dermatomycosis causing fungi
Dermatophytes are most filamentous keratinophylic fungi usually from the genera Trichophyton, Epidermophyton, Microsporum, and Nannizzia, which have an effect on skin, nails and hair. The main significant is dermatophytic fungi are slow in growing and as well the time, probably to get the ultimate result of culture is commonly 2–3 weeks. Therefore, initially the infections are commonly treated by practical administration of potential antifungal agents. Moreover, the only antifungal drugs that the Food and Drug Administration (FDA) of the United States(US) has permitted for the better treatment and prevention of superficial mycoses is terbinafine, griseofulvin, itraconazole and ciclopirox [71]. Though many such antifungal drugs have been developed in current years for dermatomycoses, they are restricted to a small number of chemical groups. Additionally, the incidence of resistance to these drugs has been observed in clinical strains results in failure in the treatment [72], [73], [74]. Apart from this various side effects such as affecting estrogen levels, liver-damage and allergic reactions are also found inpatients. For example, the azole group of drugs causes anaphylaxis [75]. The azole antifungals like ketoconazole and itraconazole able to act as both inhibitors and substrates of glycoprotein, that which eliminates toxins into the intestines [76]. These azole drugs also block steroid synthesis in humans [77].
Probiotics are well-known to hinder the development of dermatophytes and some reports indicate that they can used to prevent and treat the dermatophytic fungal infections as they are effective antifungal agents as well as safe upon consumption. A significant study was carried out by Guo et al., [78] in which among the 5 strains that showed strong antidermatophytic activity, the strain Lactobacillus reuteri R2 has effective inhibitory activity against T tonsurans and when the freeze dried supernatant of the LAB culture was incorporated at >1% concentration the mycelial growth and the conidial germination were inhibited completely. characterization of the CFS demonstrated the non proteinaceous nature of the antifungal compound found in it. Further the research from the same laboratory has shown that the antidermatophyte strain L reuteri ee1p exhibited activity against Microsporum gypseum, M canis and E floccosum. LCMS analysis of the CFS resulted in the detection of atleast 10 antifungal compounds including Hydroxyisocapric acid, hydrocinnamic acid, phenyllactic acid, azelaic acid, vanillic acid, p coumaric acid, 4-hydroxybenzoic acid, hydroxyphenyl lactic acid and also 3-hydroxydecanoic acid [78]. Lactobacillus plantarum KCC-10 inhibited Epidermophyton floccosum (KACC 44,918), Trichophyton roseum (KACC 40,956) and Trichophyton mentagrophytes (KACC 45,479) and 3-phenyl lactic acid was the antifungal agent produced by the isolate [79]. L. acidophilus and Saccharomyces cerivisiae expressed good antifungal potential against Candida albicans, and Trichophyton mentagrophytes. The in vitro study with the potential probiotics with 0.25, 0.5, 0.75, 1 and 1.5% (w/v) concentrations inhibited the Trichophyton mentagrophtyes growth with inhibition percentage 76%, 79%, 82.8%, 86% and 87% [80]. Lactobacillus casei (PTCC 1608), the microorganisms which is freeze dried and sealed glass ampoules that are used to inhibit the growth of Tricophyton rubrum (PTCC 5143), Tricophyton verocosum (PTCC 5056), Microsporum canis (PTCC 5069) and Microsporum gypseum (PTCC 5070). The utmost average inhibition zone was measured as 34 mm against Tricophyton rubrum. The Minimum Inhibitory Concentration assay showed that stabilized extract of probiotic had additional antidermatophyte activities compared to cell free supernatant [81]. There are several cases nearly 101 in which the potential probiotics are administered in combination with conventional drugs clinically for more than 102 fungal infections like vulvovaginal candidiasis. Moreover, 103 patents have been obtained for these probiotic formulations for topical applications [143] . The selective probiotic isolates with their specific antidermatophytic activity has been given in Table 2.
Table 2.
Antifungal activity of probiotic isolates against dermatomycosis.
| Sl No | Probiotic isolate | Pathogen | Results | References |
|---|---|---|---|---|
| 1. | L. acidophilus (108 cfu /g) Bacillus subtilis (109 cfu/g; Lactobacillus spp. (108 cfu /g) and Saccharomyces cerevisiae (109 cfu/g)-Iraqi probiotic | Trichophyton mentagrophyte | Mean inhibition percentage of T. mentagrophytes in 0.25%, 0.5%, 0.75%, 1% and 1.5% concentration of probiotic is 76%, 79%, 82.8, 86% and 87% compared with control. | Ajah et al. [80] |
| 2. | 165 LAB isolates from sourdough, cereals, cheese, intestines of human, pig and cow. |
T. tonsurans DSMZ12285 |
Five strains showed anti dermatophytic activity.L reuteri R2 and its CFS at >1% conc had strong inhibitory effect. Antifungal compound was of non proteinaceous in nature. |
Guo et al. [78] |
| 3. | 220 LAB isolates from sourdough, cheese, cereals, intestines of human infants, cow, pig, mice |
M. canis DSM10708, M. gypseum DSM3824 and E. floccosum DSM10709 | 4 strains showed strong inhibitory activity.L. reuteri ee1p and its CFS at >2% conc exhibited maximuminhibition. 10 antifungal metabolites were detected. |
Guo et al. [78] |
| 4. |
Lactobacillus casei PTCC 1608 |
Microsporum canis PTCC 5069, Microsporum gypseum PTCC 5070, Trichophyton rubrum PTCC 5143, Tricophyton verrucosum PTCC5056. |
Greatest zone of inhibition was seen against T.rubrum. Stabilized probiotic extract had more antidermatophyte effect compared to supernatant (P < 0.01). |
Alamderloo et al. [81] |
| 5. | Fermentation product of herb by LAB (FHL), Enterococcus faecalis |
T rubrum T mentagrophytes |
The antifungal activity of FHL at a concentration of 34.6 mg/ml was as high as that of the synthetic fungicide. FHL had a higher level of antifungal activity under the low-pH conditions. | Kuwaki et al. [127] |
6. Probiotic bacteria for the control of Aspergillus species
Aspergillosis is the utmost habitual mold infection in human beings, accounting for ˃85% of invading mold disease [82]. In immuno suppressed patients, Aspergillus species proceed to be a major source of life intimidating infection [83]. Morbidity and mortality are caused significantly due to the infections occurred by the Aspergillus species. Among 250 species of Aspergillus, only about 40 are revised as clinically important but the index is now growing [84]. The majority of species causing aspergillosis in humans are the Aspergillus fumigates, Aspergillus flavus and Aspergillus terreus. Some of the most commonly used drugs for the treatment are prednisone, prednisolone, caspofungin, voricanazole, methylprednisolone and itraconazole. Though these antifungal medications are generally used to treat infection, their efficacy is compromised due to the serious side effects including nephrotoxicity, hypersensitivity, electrolyte disturbances, kidney and liver damage, visual disturbances, described as photophobia, blurred vision, and altered color perception[85]. Hence there is a necessity for an alternative drug. Use of probiotics and postbiotics has opened a new avenue for the prevention and treatment of opportunistic infections such as aspergillosis which can be supplemented in the diet or added to medical formulations.
Numerous studies have reported on probiotics isolates for the control of Aspergillus growth. Probiotics strains isolated from cereal gruels [86], vegetables [87], kimchi- a soy based fermented food [88] and thai food [89] showed complete inhibition of growth of A. flavus. In addition, the reduction in fungal mat by Lactobacillus species also reported by numerous studies. Coculture of the probiotic isolates from Egyptian fermented food such as Lactobacillus rhamnosus, Lactobacillus paracasei, L. plantarum and L. acidophilus demonstrated efficient antifungal effect against different Aspergillus species i.e. A. niger, A. flavus and A. fumigatus. Among these, L. rhamnosus exhibited strong inhibition of all the fungi used for the study. Whereas L. paracasei partially inhibited A. fumigatus and minimal inhibition was obtained with A. flavus and A. niger. L. plantarum and L. acidophilus had shown minimal to partial inhibition with all the fungal pathogens [90]. A study done by Pundir et al. [91] out of the 26 Lactic Acid Bacteria isolates from different fresh foods, eight isolates showed potential antifungal activity against human pathogenic strains A. fumigatus and C. albicans.
Additionally, the concentrated Cell Free Supernatant (cCFS) of the strain Lactiplantibacillus plantarum 16 strain isolated from steep water during malt production completely controlled the spore germination and hyphal development in A. fumigatus and R. stolonifer [92]. Trancriptomic analysis of the above pathogen revealed many genes with altered transcription suggesting total metabolic shutdown resulting in cell death. The Lactobacillus delbrueckii and Lactobacillus. brevis exhibited significant effects on Aspergillus biomass growth as reported by Bayankaram et al. [93] with 67.43% and 69.38% reduction in biomass of Aspergillus parasiticus and Aspergillus flavus respectively. The antagonistic activity of Lactobacillus species isolated from a diversity of sources is due to the production of antifungal postbiotics [94,95]. These secondary metabolites have been recognized as diverse phenyl lactic acids, organic acids, phenolic acid, hydrogen peroxide, hydroxyl fatty acids, cyclic dipeptides and proteinaceous secondary metabolites [96].
The work done by Ström et al. [97] demonstrated the antifungal effect of Lactobacillus plantarum strain (MiLAB 393) isolated from source grass silage against A. fumigatus. The antifungal cyclic dipeptides, cyclo(L-Phe-trans-4-OH-l-Pro) and cyclo(L-Phe-l-Pro) production by the LAB has been reported in this study for the first time. Another antifungal compound that was identified in this study was 3 - phenyllactic acid. According to Arasu et al. [79] in their work have demonstrated the antifungal efficiency of a novel isolate L. plantarum K46 and L. plantarum KCC-10 against 24 fungal strains including Aspergillus clavatus (KACC 40,071), Aspergillus fumigatus (KACC 40,080), Aspergillus niger (KACC 40,280) and Aspergillus pullulans (KACC 41,291).The NMR spectral analysis of the purified antifungal compound was identified as 3-phenyllactic acid. The Minimum Inhibitory Concentration of the antimicrobial compound against Aspergillus oryzae and Aspergillus clavatus was 2.5 mg/ml and with respect to Aspergillus fumigatus and Aspergillus niger were 5.0 mg/ml.
Antifungal metabolites from two Lactobacillus species Lactiplantibacillus plantarum BCH-1 and L coryniformis BCH-4 which showed remarkable inhibition of A. fumigatus and A. flavus were identified by HPLC and GC–MS analysis. Citric acid and Lactic acid are seen as foremost organic acids produced from L coryniformis and Lactiplantibacillus plantarum respectively. In addition, these two species also produced hexadecanoic acid and 9, 12-otadecadienoic acid (Z, Z)-methyl ester as main fatty acids and also found as potential secondary metabolite against these filamentous fungi from these two species [98]. The study directed by Yang et al. [88] discovered the presence of another low molecular weight antifungal compound such as δ-dodecalactone from L. plantarum AF1isolated from Kimchi showing strong antifungal activity against A. fumigatus, A. flavus, A. ochraceus, A. petrakii, and A. nidulans. Therefore, some of the Lactobacillus species used to control the growth of Aspergillus mold is listed in the Table 3.
Table 3.
Antifungal activity of Lactobacillus against Aspergillus species.
| Sl no | Probiotic isolate | Fungal pathogen | Results | References |
|---|---|---|---|---|
| 1 |
Lactobacillus plantarum 62 L. plantarum 16 |
A. fumigatus | Inhibited the growth of pathogen by the production of Antifungals and organic acids | Crowley et al. [92] |
| 2. |
L. plantarum LB-1 L.plantarum F-3 L. plantarum F-50 |
A. ochraceous | Exhibited stronger antifungal activity with 20 mm diameter of inhibition zone | Sun et al. [128] |
| 3 |
L. plantarum Lp MYS44 |
A. parasiticus | Suppressed the germination and growth of the spores and reduced the toxin by 34.2% | Poornachandra Rao et al. [129] |
| 4. |
L. cellobiosus L. rhamnosus P. pentosaceus |
A. flavus A. repens |
Bacteriocins produced by the Lactobacillus expressed good antifungal activity against the aspergillus species | Adesina et al. [130] |
| 5 |
L. plantarum CH1 L. paracasei L. mesenteroides |
A. tubingensis | Complete inhibition of the pathogen was observed | Ouiddir et al. [131] |
| 6 |
L. plantarum L. rhamnosus L. paracasei and acidophillus |
A. niger A. flavus A. fumigatus |
L. rhamnosus inhibited all the pathogens L. plantarum inhibited A. flavus, strong inhibition was seen by Lactibacillus acidophillus against A. niger, Aspergillus fumigatus was inhibited by L paracasei. The inhibition is due to the bacteriocins produced |
Ali et al. [132] |
| 7 | L. plantarum UT9121 | A. flavus | Probiotic modulate the mold growth and inhibited the fungal growth | Russo et al. [133] |
| 8. | L. plantarum | A. flavus | Peptide mixture as the biocontrol agent reduce the spore formation | Muhialdin et al. [134] |
| 9 |
L. mesenteroides DU15 L. plantarum TE10 L. plantarum IT10 L. plantarum IS10 |
A. niger | The CFS with low molecular peptides inhibited the pathogen by 94%, 93%, 94% respectively | Muhialdin et al. [68] |
| 10 | L. kefir FR7 |
A. flavus A. carboneras |
Inhibited A flavus by 51.67% and A carbonarius by 45.56%. Reduced the% of AFB1,AFB2,OTA by 97.22%, 76.26%, 75.2% respectively | Ben Taheur et al. [135] |
| 11 |
L. pentosaceus L. planatrum |
A. niger A. carneus |
84% 0f OTA was reduced by P pentosaceus 94% of OTA by L plantarum |
Taroub et al. [136] |
| 12 | Bifidobacterium bifidum (DSM 20,082), L. acidophilus (DSM 20,079) and Lactobacillus plantarum (DSM 20,174) | Aspergillus flavus strain (EMCC 274) and Aspergillus parasiticus (EMCC 886T) | Probiotic culture supernatant (PCS) at 1% concentration achieved high inhibition of AFB1 production by Aspergillus flavus by percentage reached to 76%. But this percentage was increased up to 77% in case of Aspergillus parasiticus. | Hamad et al. [137] |
| 13. | Lactobacillus plantarum KCC-10 |
Aspergillus clavatus (KACC 40,071), A. fumigates (KACC 40,080), A. niger (KACC 40,280), A. oryzae (KACC 44,823),A. pullulans (KACC 41,291), |
3-phenyl lactic acid was found as antifungal agent. The minimum inhibitory concentration of the compound against Aspergillus clavatus, A. oryzae was 25 mg/ ml and against A. fumigatus, A. niger was 50 mg/ ml, respectively. | Arasu et al. [79] |
7. Probiotic bacteria against Fusarium and Penicillium species
Fusariosis is a contagion that affects animals, plants as well as humans and are brought about by several fungi of the genera Fusarium [99]. In therapeutic arena, various Fusarium species have been correlated to systemic or local invasive infections in both immune competent personalities and immune depressed individuals [100]. Furthermore, it is probable that ecological isolates from the Fusarium species attain resistance owed to earlier contact to antifungals that are used in agronomic practices and these Fusarium species may spread and therefore, infect human biengs [101,102]. Fusarium species reveal worldwide distribution and it is assumed that nearly 10 species were associated to human pathogens including, Fusarium oxysporum, Fusarium fujikuroi, Fusarium solani, Fusarium clamydosporum, Fusarium incarnateum-equiseti, Fusarium dimerum, Fusarium concolar, Fusarium Sambucinum and Fusarium lateritium. Among these species, members of Fusarium solani are quite common and infectious, afterward Fusarium oxysporum, Fusarium fujikori and Fusarium moniliforms [103]. Fusarium species are source for a diverse range of human infections, extending from superficial, localised to disseminate with the most predominant being onychomycosis, keratitis and skin infections [104,105].
The most used antifungal agents comprise voriconazole, natamycin, parconazole and amphotericin B. Fusarium species exhibit inherent resistance to antibiotics echinocandins and some species show resistance to antibiotic azoles [106]. Moreover, minimum inhibitory concentration (MIC) and minimum fungicidal concentrations (MFC) have also not been well-known for Fusarium spp [107]. Aspects that subsidize to the fusariosis severity include amplified occurrence of multidrug resistance to species and the dearth of the research concerning to expansion of novel therapeutic options for prevention and treatment [104]. Therefore, biological control strategies are being increasingly explored. Several invitro studies have proved Fusarium species are sensitive to different strains of lactic acid bacteria. Two LAB isolates L. plantarum LPLUV10 and L. paracasei LPAUV12 are shown to have growth inhibitory effect on Fusarium oxysporum by 76% and 55% respectively [108]. In another study, 14 probiotic strains of Lactobacillus (Lactobacillus pentosus, Lactobacillus plantarum, Lactobacillus. brevis) and their CFS showed good antifungal activity against Fusarium oxysporum including biomass inhibition and mycelial growth inhibition. The antifungal nature of the compound found in the CFS was investigated and some of them were found to be proteinaceous in nature suggesting the presence of bacteriocin like compounds or peptides [109]. The antifungal effect of another strain L. salivarius and its culture filtrate was determined with F solani and it was found that mycelial growth and conidial germination of the pathogenic fungi was significantly inhibited by the culture filtrate. Characterization of CFS showed a synergistic effect of pH and proteinaceous substance for their antifungal activity [110]. The work of Zebboudj et al. [111] revealed the antifusarial effect of Lactococcus lactis subsp.lactis biovar diacetylactis and Leoconostoc mesenteroides subsp mesenteroides biovar dextranicum against 12 strains F. oxysporum showing inhibitory percentage ranging from 13.5% to 100%.The CFS of the selected strains showed 49.41% inhibition. The antagonistic compounds identified include organic acids [112], bacteriocins, hydrogen peroxide, compounds with low molecular weight (cyclic dipeptides, reuterin, phenyllactic acid, methylhydantoin, benzoic acid, mevalonolactone and hydroxylated fatty acids) [113]. Evidence of the probiotics antifungal effects in vitro against Fusarium species are listed in the Table 4.
Table 4.
Antifungal activity of Lactobacillus species against Fusarium species.
| Sl No | Probiotic isolate | Fungal pathogen | Results | References |
|---|---|---|---|---|
| 1 | Lactbacillus sakei KTUO5–7 |
F. culmoruml-2 F. avenaceum F poe F. solani |
Probiotic inhibited fusarium species with 13.5 ± 1.4 mm zone of inbition for F. culmoruml-2, 11.8 ± 0.5 mm for F. avenaceum, 9.3 ± 1.0 mm for F. poe, 13.8 ± 0.5 mm for F. solani. |
Juodeikiene et al. [138] |
| 2 |
L. plantarum LPLUV10 L. paracasei LPAUV7 |
F. oxysporum f.ssp.lycoperici |
L. plantarum LPLUV10inhibited fusarium by 55% and about 76% of inhibition was observed by L. paracasei LPAUV7 |
López-Seijas et al. [108] |
| 3. | Four strains of Lactobacillus strains of L. plantarum, L Leuconostoc and L brevis | Fusarium oxysporum | All the isolated inhibited the growth of pathogen with 20.15–22.8 mm of inhibition zone | Abouloifa et al. [109] |
| 4 |
L. salivarius ssp. Salivarius JCM1231 |
F. solani | Conidial germination and mycelia growth of F. solani was significantly inhibited by the Lactobacillus salivarius culture filterate | Hu et al. [110] |
| 5 | L. paracasei ssp. Tolerans |
F. proliferatum M5689,M5991 F. graminearum R4053 |
The probiotic isolated completely inhibited the mycelial growth of the fusarium species | Hassan and Bullerman, [139] |
| 6. | L. planatrum 108 and 121 | F. culmorum | CFs of the probiotic isolate inhibited the pathogen by 62% and 60% respectively | Russo et al. [140] |
| 7 |
L. plantarum KCC 37 L. plantarum KCC-38 |
F. oxysporum | Showed intense antifungal activity with inhibition zone of 35.03±0.33 mm and 30.72±1.28 mm respectively | Muthusamy et al. [141] |
| 8 | Leuconostoc mesenteroides ssp dextranicium |
F. oxysporum F. redulene F. solani |
All the fusarium species are inhibited by the LAB isolate between 4.3 and 19.7% after 72 hour incubation on PDA plates | Zebboudj et al. [111] |
| 9 | L. plantarum TR71 | F. verticilloides | Yellow mustard fermented extract with L. plantarum TR71 reduced the Fuminosin B1 by 92.6% and the antifungal metabolites produced are lactic acid, #- phenyl acetic acid, benzoic acid. | Torrijos et al. [142] |
Penicilliosis is a fungal infection instigated by Penicillium marnaffei, a dimorphic fungus thermally. In humans, Penicillium marneffei is an opportunistic mold that infects immunocompromised patients and also HIV positive patients. Incorporation of fungus conidia might be the approach of transmission to human host. Even though the most frequent forms of disease appearance are non-specific as well as constitutes of anemia, low-grade fever and weight loss and the distinctive lesion in skin (central umbilicated papule) and even respiratory infections may also occur [114].
Some of the probiotic bacteria showed antifungal activity against various Penicillium species. Lactobacillus lactis, Pediococcus pentosaceus, LactoB. brevis and Lactiplantibacillus plantarum inhibited the growth of penicillium citrimum with 26.50±1.50 mm, 2.50±3.50 mm, 29.00±1.00 mm,20.00±0.00 mm of inhibition zone [62]. In another study, Penicillium roqueforti was inhibited by the Lactobacillus plantarum TE10 and IT5 with 72.33±1.52 mm and 71.00±2.64 mm diameter of inhibition zone respectively [68]. Lactobacillus Rhamnosus R-2002 endowed significant antifungal activity against Penicillium aurantioviolaceum [66]. Probiotic bacteria such as Lactobacillus Rhamnosus LRH01, LRH05, LRH14, LRH16, LRH43, Lactobaillus plantarum LP01, LP37, LP48, Lactobacillus paracasei LPC44, LPC46, Lactobacillus parabuchneri LPB02, LPB04 strongly inhibited Penicillium commune 01,180,002, 01,180,014, 01,180,015, Penicillium crustosum 01,180,001, Penicilium glabrum IS13, Penicillium palitans PPao1, Penicilium solitum IS15 [69].
8. Use of postbiotics from probiotics
Commensal bacteria produce byproducts of metabolites to maintain their perseverance in the host and award a survival benefit over invading fungal pathogens [115]. Lactic Acid Bacteria generate various short chain aliphatic organic acids like acetic acid and lactic acid, H2O2 and other compounds. Production and release of H2O2 is an important probiotic attribute of Lactobacillus species to fight against fungal diseases [116]. Other antifungal products formed by bacteria are minute molecules like biosurfactants and bacteriocins [117]. Bacteriocins are proteinaceous substances produced by bacteria, mainly by lactic acid bacteria, that show antimicrobial activity against closely related species. However when they are not fully characterised, they can be called bacteriocin like inhibitory subtsances and often hinder a broader range of species such as gram positive, gram negative bacteria and infection causing fungi [118]. Lactobacillus plantarum BCH-1 produced tartaric acid, pyruvic acid, lactic acid, citric acid, malic acid, formic and succinic acid. Among these acids the concentration of Citric acid and Lactic acid is more than other acids. Lactobacillus coryniformis BCH-4 secreted pyruvic acid, tartaric acid, citric acid, malonic acid, malic acid, lactic acid and succinic acid. Furthermore, among them, tartaric and lactic acid were found more in concentration [98]. Lactobacillus plantarum produced tetra deconoic acid, 1-methyl ethyl ester, pentadeconoic acid, hexadeconoic acid, 12-hydroxydeconoic acids and are found to have antifungal properties. In addition, Lactobacillus casei AST18 expressed good antifungal activity due to the assembly of some antifungal agents such as lactic acid (93.70 mg/ml) acetic acid (2.42 mg/ml), citric acid (1.29 mg/ml), tartaric acid (9.59 mg/ml) and reported as lactic acid is the major antifungal compound. Also cyclo(Leu-pro):5,10diethoxy-2,3,7,8-tetrahydro-1H-dipyrrolo[1,2-a:1′,2′-d] pyrazine:2,6-diphenyl-piperidine was found as a good antifungal compound by GCMS [119]. Lactobacillus plantarum produced bioactive compounds from which 3 purified peptides were presented with amino acid sequences LVGKKVQTF, SGADTTFLTK, and GTLIGQDYK as identified from bioinformatics program. Among these SGADTTFLTK inhibited Penicillium expansum by 58% and Aspergillus parasiticus by 73% [120]. The most copious antifungal compounds found in Lactobacillus rhamnosus derived fermentatives corresponded to lactic acid and acetic acid. Other organic acids, volatile organic compounds, free fatty acids were also found at lower levels. In addition, 9-amino acid peptides resulting from άs2-casein from the Lactobacillus rhamnosus resultant fermentate inhibited Mucor racemoses and Rhizopus mucilaginosa [121]. Similarly twelve organic compounds have been reported from liquid-liquid extraction of CFS-Lactobacillus plantarum MYS6 [112]. The purified active antifungal compounds of Lactobacillus plantarum EM were identified as 3‑hydroxy-5-dodecenoic acid, lactic acid and acetic acid. Combine usage of these 3 acids cause severe damage to Aspergillus mycelial conidia cells in conjunction with aggregation of cells that are damaged, consequently, in fungicidal activity against Aspergillus fumigatus [122].
9. Conclusion and future perspectives
Opportunistic fungal pathogens together with the antifungal drugs resistance symbolize serious human health problem. The treatment and prevention with probiotics restores the natural microbiota with reward over conventional antifungals because they are non toxic and do not persuade microbial resistance that when administered in sufficient quantity, and as a result probiotics do not create adverse side effects, and further modulate the immune system. Therefore, the properties of probiotics have made it a subject of interest for various fields. Hence, there is a requirement for further elaborate assays, especially in vivo assays which would better characterize the complex interactions among the probiotics/postbiotics and the pathogenic fungi to realize the consequences of the antimicrobials production of the microorganisms. Even though all these specifics make research on antagonistic activity of potential probiotics organisms even more complex, nonetheless it presents an enormous opportunity for research in future.
CRediT authorship contribution statement
The author contributions are as follows: S. Divyashree: Conceptualization, Writing- original draft, Data curation, Software, Validation, Vizualization, Investigation, Methodology B. Shruthi and P R. Vanitha: Formal analysis, Resources, Coordinating, Editing, Correcting. M.Y. Sreenivasa: Conceptualization, Supervision, Funding acquisition, Project administration, Validation, Visualization, Review, and Editing. All authors contributed to the study conception and design. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no conflict of interest for the possible consideration for publication in the reputed journal “Biotechnology Reports”.
Acknowledgements
DS (FBO/Fell/1/2022-ECD-II) and SB (Myco/Fell/8/2022-ECD-II) are grateful to the Indian Council for Medical Research (ICMR; New Delhi) for a SRF fellowship.
Data availability
No data was used for the research described in the article.
References
- 1.Dixon F., Michael Genta Robert, John Yardley, Pelayo Correa. The participants in the international workshop on the histopathology of Gastritis, Houston 1994 classification and grading of gastritis. Am. J. Surg. Pathol. 1996;20:1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Ravikant K.Tanveer, Satish G., Mandeep K. A review on emerging fungal infections and their significance. J. Bacteriol. Mycol. 2015;1:39–41. doi: 10.15406/jbmoa.2015.01.00009. [DOI] [Google Scholar]
- 3.Wisplinghoff H., Bischoff T., Tallent S.M., Seifert H., Wenzel R.P., et al. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 2004;39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 4.Pfaller M.A., Diekema D.J. Role of sentinel surveillance of candidemia: trends in species distribution and antifungal susceptibility. J. Clin. Microbiol. 2002;40:3551–3557. doi: 10.1128/JCM.40.10.3551-3557.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maschmeyer G., Haas A., Cornely O.A. Invasive aspergillosis: epidemiology, diagnosis and management in immune compromised patients. Drugs. 2007;67:1567–1601. doi: 10.2165/00003495-200767110-00004. [DOI] [PubMed] [Google Scholar]
- 6.Chakrabarti A., Chatterjee S.S., Das A., Panda N., Shivaprakash M.R., Kaur A., et al. Invasive zygomycosis in India: experience in a tertiary care hospital. Postgrad. Med. J. 2009;85:573–581. doi: 10.1136/pgmj.2008.076463. [DOI] [PubMed] [Google Scholar]
- 7.Pappas P.G. Antifungal clinical trials and guidelines: what we know and do not know. Cold Spring Harb. Perspect. Med. 2014;4(11) doi: 10.1101/cshperspect.a019745. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanguinetti M., Posteraro B., Lass-Flörl C. Antifungal drug resistance among Candida species: mechanisms and clinical impact. Mycoses. 2015;58:2–13. doi: 10.1111/myc.12330. [DOI] [PubMed] [Google Scholar]
- 9.Sardi J.C., Scorzoni L., Bernardi T., Fusco-Almedia A.M., Mendes Giannini M.J. Candida Species: current epidemiology, Pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J. Med. Microbiol. 2013;62:10–24. doi: 10.1099/jmm.0.045054-0. [DOI] [PubMed] [Google Scholar]
- 10.Nett J.E. Future directions for anti-biofilm therapeutics targeting Candida. Expert Rev. Anti Infect. Ther. 2014;12:375–382. doi: 10.1586/14787210.2014.885838. [DOI] [PubMed] [Google Scholar]
- 11.Matsubra V.H., Bandara H.M., Mayer M.P., Samaranayake L.P. Probiotics as antifungals in mucosal candidiasis. Clin. Infect. Dis. 2017;1(62):1143–1153. doi: 10.1093/cid/ciwo38. [DOI] [PubMed] [Google Scholar]
- 12.FAO/WHO . 2002. Guidelines For the Evaluation of Probiotics in Food; pp. 1–11. [Google Scholar]
- 13.Divyashree S., Anjali P.G., Somashekaraiah R., Sreenivasa M.Y. Probiotic properties of Lactobacillus casei – MYSRD 108 and Lactobacillus plantarum-MYSRD 71 with potential antimicrobial activity against Salmonella paratyphi. Biotechnol. Reports. 2021;32:e00672. doi: 10.1016/j.btre.2021.e00672. Elsevier B.V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fijan S. In: Probiotics and Prebiotics in Human Nutrition and Health. Rao V., Rao & L.G., editors. IntechOpen; 2016. Antimicrobial Effect of Probiotics against Common Pathogens. [DOI] [Google Scholar]
- 15.Shruthi B., Deepa N., Somashekaraiah R., Adithi G., Divyashree S., Sreenivasa M.Y. Exploring biotechnological and functional characteristics of probiotic yeasts: a review. Biotechnol. Reports. 2022;34:e00716. doi: 10.1016/j.btre.2022.e00716. Elsevier B.V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.C.C. Lai, C.K. Tan, Y.T. Huang, P.L. Shao, P.R. Hsueh, Current challenges in the management of invasive fungal infections. 14(2008), pp. 77–85. https://doi.org/10.1007/s10156-007-0595-7. [DOI] [PubMed]
- 17.T.J. Walsh, A. Groll, J. Hiemenz, R. Fleming, E. Roilides, E. Anaissie, Infections due to emerging and uncommon medically important fungal pathogens. 2004, pp. 48–66. https://doi.org/10.1111/j.1470-9465.2004.00839.x. [DOI] [PubMed]
- 18.Meng L., Hua F., Bian Z. Coronavirus disease 2019 (COVID-19): emerging and future challenges for dental and oral medicine. J. Dental Res. 2020;99:481–487. doi: 10.1177/0022034520914246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McFarland L.V. Meta-analysis of probiotics for the prevention of traveler's diarrhea. Travel Med. Infect. Dis. 2007;5:97–105. doi: 10.1016/j.tmaid.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Sazawal S., Hiremath G., Dhingra U., Malik P., Deb S., Black R.E. Efficacy of probiotics in prevention of acute diarrhoea: a meta-analysis of masked, randomised, placebo-controlled trials. Lancet Infect. Dis. 2006;6:374–382. doi: 10.1016/S1473-3099(06)70495-9. [DOI] [PubMed] [Google Scholar]
- 21.Adithi G., Somashekaraiah Rakesh, Divyashree S., Shruthi B., Sreenivasa M.Y. Assessment of probiotic and antifungal activity of Lactiplantibacillus plantarum MYSAGT3 isolated from locally available herbal juice against mycotoxigenic Aspergillus species. Food Biosci. 2022;50 doi: 10.1016/j.fbio.2022.102118. [DOI] [Google Scholar]
- 22.Britton R.A., Versalovic J. Probiotics and Gastrointestinal Infections. Interdiscip Perspect. Infect. Dis. 2008:1–10. doi: 10.1155/2008/290769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martins N., Ferreira I.C.F.R., Barros L., Silva S., Henriques M. Candidiasis: predisposing factors, prevention, diagnosis and alternative treatment. Mycopathologia. 2014;177:223–240. doi: 10.1007/s11046-014-9749-1. [DOI] [PubMed] [Google Scholar]
- 24.Deepa N., Achar P.N., Sreenivasa M.Y. Current perspectives of biocontrol agents for management of Fusarium verticillioides and Its Fumonisin in cereals—A Review. J. Fungi. 2021;7 doi: 10.3390/jof7090776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eggimann P., Garbino J., Pittet D. Epidemiology of Candida species infections in critically ill non-immunosuppressed patients. Lancet Infect. Dis. 2003;3:685–702. doi: 10.1016/s1473-3099(03)00801-6. [DOI] [PubMed] [Google Scholar]
- 26.Lott T.J., Fundyga R.E., Kuykendall R.J., Arnold J. The human commensal yeast, Candida albicans, has an ancient origin. Fungal Genet Biol. 2005;42:444–451. doi: 10.1016/j.fgb.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 27.Brunke S., Hube B. Two unlike cousins: candida albicans and C.glabrata infection strategies. Cell Microbiol. 2013;15:701–708. doi: 10.1111/cmi.12091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mayer F.L., Wilson D., Hube B. Candida albicans pathogenicity mechanisms. Virulence. 2013;4:119–128. doi: 10.4161/viru.22913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsai P.W., Chen Y.T., Hsu P.C., Lan C.Y. Study of Candida albicans and its interactions with the host: a mini review. Biomed. 2013;3:51–64. doi: 10.1016/j.biomed.2012.12.004. [DOI] [Google Scholar]
- 30.Ferreira A.V., Prado C.G., R.R.Carvalho K.S.T.Dias, Dias A.L.T. Candida albicans and Non-C. albicans Candida species: comparison of biofilm production and metabolic activity in biofilms, and putative virulence properties of isolates from hospital environments and infections. Mycopathologia. 2013;175:265–272. doi: 10.1007/s11046-013-9638-z.31. [DOI] [PubMed] [Google Scholar]
- 31.Develoux M., Bretagne S. Candidiasis and yeast infections. EMC - Mal Infect. 2005;2:119–139. doi: 10.1016/j.emcmi.2005.04.001. [DOI] [Google Scholar]
- 32.Rakesh S., Walid M., Adithi G., Udith J., Riad H., Sreenivasa M.Y. Probiotic and antifungal attributes of levilactobacillus brevis MYSN105, isolated from an Indian traditional fermented food Pozha. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.696267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang C., Wang H., Chen T. Interactions between Intestinal Microflora/Probiotics and the Immune System. Biomed. Res. Int. 2019:1–8. doi: 10.1155/2019/676491934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bamidele T.A., Adeniyi B.A., Smith S.I. In vitro, acidic, non-proteinaceous antifungal activities of lactic acid bacteria isolated from salad vegetables against human pathogenic Candida albicans. Afr. J. Clin. Exp. Microbiol. 2019;2:20. doi: 10.4314/ajcem.v20i2.7. 137. [DOI] [Google Scholar]
- 35.Kang C.H., Kim Y.G., Han S.H., Kim J.S., Paek N.S., So J.S. In vitro probiotic properties of vaginal Lactobacillus fermentum MG901 and Lactobacillus plantarum MG989 against Candida albicans. Eur. J. Obstet Gynecol. Reprod. Biol. 2018:228:232. doi: 10.1016/j.ejogrb.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 36.Kohler G.A., Assefa S., Reid G. Probiotic interference of Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 with the opportunistic fungal pathogen Candida albicans. Infect. Dis. Obstet Gynecol. 2012;636474 doi: 10.1155/2012/636474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vilela S.F.G., Barbosa J.O., Rossoni R.D., Santos J.D., Prata M.C.A., Anbinder A.L., et al. Lactobacillus acidophilus ATCC 4356 inhibits biofilm formation by C. Albicans and attenuates the experimental candidiasis in Galleria mellonella. Virulence. 2015;6:29–39. doi: 10.4161/21505594.2014.981486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rossoni R.D., de Barros P.P., de Alvarenga J.A., Ribeiro F de C., Velloso M dos S., Fuchs B.B., et al. Antifungal activity of clinical Lactobacillus strains against Candida albicans biofilms: identification of potential probiotic candidates to prevent oral candidiasis. Biofouling. Taylor & Francis. 2018;34:212–225. doi: 10.1080/08927014.2018.1425402. [DOI] [PubMed] [Google Scholar]
- 39.Aarti C., Khusro A., Varghese R., Arasu M.V., Agastian P., Al-Dhabi N.A., et al. In vitro investigation on probiotic, anti-Candida, and antibiofilm properties of Lactobacillus pentosus strain LAP1. Arch. Oral Biol. Elsevier. 2018;89:99–106. doi: 10.1016/j.archoralbio.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 40.Parolin C., Marangoni A., Laghi L., Foschi C., Palomino R.A.N., Calonghi N., et al. Isolation of vaginal lactobacilli and characterization of anti-candida activity. PLoS ONE. 2015;10:1–17. doi: 10.1371/journal.pone.0131220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Verdenelli M.C., Coman M.M., Cecchini C., Silvi S., Orpianesi C., Cresci A. Evaluation of antipathogenic activity and adherence properties of human Lactobacillus strains for vaginal formulations. J. Appl. Microbiol. 2014;116:1297–1307. doi: 10.1111/jam.12459.42. [DOI] [PubMed] [Google Scholar]
- 42.Tan Y., Leonhard M., Moser D., Ma S., Schneider-Stickler B. Inhibitory effect of probiotic lactobacilli supernatants on single and mixed non-albicans Candida species biofilm. Arch. Oral Biol. 2018;85:40–45. doi: 10.1016/j.archoralbio.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 43.Bulgasem B.Y., Lani M.N., Hassan Z., Wan Yusoff W.M., Fnaish S.G. Antifungal activity of lactic acid bacteria strains isolated from natural honey against Pathogenic Candida species. Mycobiology. 2016;44:302–309. doi: 10.5941/MYCO.2016.44.4.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Denkova R., Yanakieva V., Denkova Z., Nikolova V., Radeva V. In vitro inhibitory activity of bifidobacterium and lactobacillus strains against Candida Albicans. Bulg. J. Vet. Med. 2013;16:186–197. [Google Scholar]
- 45.Hager C.L., Isham N., Schrom K.P., Chandra J., McCormick T., Miyagi M., et al. Effects of a novel probiotic combination on pathogenic bacterial-fungal polymicrobial biofilms. MBio. 2019;10:1–11. doi: 10.1128/mBio.00338-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gabrielli E., Pericolini E E., Ballet N., Roselletti E., Sabbatini S., Mosci P., et al. Saccharomyces cerevisiae-based probiotic as novel anti-fungal and anti-inflammatory agent for therapy of vaginal candidiasis. Benef. Microbes. 2018;9:219–230. doi: 10.3920/BM2017.0099. [DOI] [PubMed] [Google Scholar]
- 47.Rakesh S., Shruthi S., Deepthi B.V., Sreenivasa M.Y. Probiotic properties of lactic acid bacteria isolated from Neera: a naturally-fermenting coconut palm nectar. Front. Microbiol. 2019 doi: 10.3389/fmicb.2019.01382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tan Y., Leonhard M., Moser D., Ma S., Schneider-Stickler B. Inhibitory effect of probiotic lactobacilli supernatants on single and mixed non-albicans Candida species biofilm. Arch. Oral Biol. 2018;85:40–45. doi: 10.1016/j.archoralbio.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 49.Martinez P.C.R., Seney S.L., Summers K.L., Nomizo A., De Martinis E.C.P., Reid G. Effect of Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 on the ability of Candida albicans to infect cells and induce inflammation. Microbiol. Immunol. 2009;53:487–495. doi: 10.1111/j.1348-0421.2009.00154.x. [DOI] [PubMed] [Google Scholar]
- 50.Chew S.Y., Cheah Y.K., Seow H.F., Sandai D., Than L.Y.L. Probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 exhibit strong antifungal effects against vulvovaginal candidiasis-causing Candida glabrata isolates. J. Appl. Microbiol. 2015;118:1180–1190. doi: 10.1111/jam.12772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mailänder-Sánchez D., Braunsdorf C., Grumaz C., Müller C., Lorenz S., Stevens P., et al. Antifungal defense of probiotic Lactobacillus rhamnosus GG is mediated by blocking adhesion and nutrient depletion. PLoS ONE. 2017;12:1–19. doi: 10.1371/journal.pone.0184438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hopkins R.J., Rothman M., Fiore A. Cerebral mucormycosis associated with intravenous drug use : three case reports and review. Clin. Infect. Dis. 1994;19:1133–1137. doi: 10.1093/clinids/19.6.1133. [DOI] [PubMed] [Google Scholar]
- 53.Gomes M.Z.R., Lewis R.E., Kontoyiannis D.P. Mucormycosis caused by unusual mucormycetes, non-Rhizopus, -Mucor, and -Lichtheimia species. Clin. Microbiol. Rev. 2011;24:411–445. doi: 10.1128/CMR.00056-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mantadakis E., Samonis G. Clinical presentation of zygomycosis. Clin. Microbiol. Infect. 2009;15:15–20. doi: 10.1111/j.1469-0691.2009.02974.x. [DOI] [PubMed] [Google Scholar]
- 55.Chakrabarti A., Singh R. Mucormycosis in India: unique features. Mycoses. 2014;57:85–90. doi: 10.1111/myc.12243. [DOI] [PubMed] [Google Scholar]
- 56.Marty F.M., Ostrosky-Zeichner L., Cornely O.A., Mullane K.M., Perfect J.R., Thompson G.R., et al. Isavuconazole treatment for mucormycosis: a single-arm open-label trial and case-control analysis. Lancet Infect. Dis. 2016;16:828–837. doi: 10.1016/S1473-3099(16)00071-2. [DOI] [PubMed] [Google Scholar]
- 57.Groll A.H., Desai A., Han D., Howieson C., Kato K., Akhtar S., et al. Pharmacokinetic assessment of drug-drug interactions of isavuconazole with the immunosuppressants cyclosporine, mycophenolic acid, prednisolone, sirolimus, and tacrolimus in healthy adults. Clin Pharmacol Drug Dev. 2017;6:76–85. doi: 10.1002/cpdd.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu M., Spellberg B., Phan Q.T., Fu Y., Lee A.S., et al. The endothelial cell receptor GRP78 is required for mucormycosis pathogenesis in diabetic mice. J. Clin. Invest. 2010;120:1914–1924. doi: 10.1172/JCI42164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Singh A.K., Singh R., Joshi S.R., Misra A. Mucormycosis in COVID-19: a systematic review of cases reported worldwide and in India. Diabetes Metab. Syndr. Clin. Res. Rev. 2021;15 doi: 10.1016/j.dsx.2021.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Serris A., Danion F., Lanternier F. Disease entities in mucormycosis. J. Fungi. 2019;5:23. doi: 10.3390/jof5010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jeong W., Keighley C., Wolfe R., Lee W.L., Slavin M.A., Kong D.C.M., et al. The epidemiology and clinical manifestations of mucormycosis: a systematic review and meta-analysis of case reports. Clin. Microbiol. Infect. 2019;25(2019):26–34. doi: 10.1016/j.cmi.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 62.Nwozor N.C., et al. Antagonistic activity of lactic acid bacteria bioactive molecules against fungi isolated from onion (Allium cepa) EC Microbiol. 2019;15:318–327. [Google Scholar]
- 63.Bachanti P.R., Vij S. Antimicrobial activity of sodium caseinate fermentate of Lactobacillus fermentum NCDC 141. Asian J. Dairy Food Res. 2015;34:265–269. doi: 10.18805/ajdfr.v34i4.6875. [DOI] [Google Scholar]
- 64.Álvarez A., Manjarres J.J., Ramírez C., Bolívar G. Use of an exopolysaccharide-based edible coating and lactic acid bacteria with antifungal activity to preserve the postharvest quality of cherry tomato. Lwt. 2021;151 doi: 10.1016/j.lwt.2021.112225. [DOI] [Google Scholar]
- 65.Delavenne E., Mounier J., Déniel F., Barbier G., Blay G.Le. Biodiversity of antifungal lactic acid bacteria isolated from raw milk samples from cow, ewe and goat over one-year period. Int. J. Food Microbiol. 2012;155:185–190. doi: 10.1016/j.ijfoodmicro.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 66.Toplaghaltsyan A., Bazukyan I., Trchounian A. The effects of different carbon sources on the antifungal activity by lactic acid bacteria. Curr. Microbiol. 2017;74:168–174. doi: 10.1007/s00284-016-1168-8. [DOI] [PubMed] [Google Scholar]
- 67.Salas M.L., Thierry A., Lemaître M., Garric G., Harel-Oger M., Chatel M., et al. Antifungal activity of lactic acid bacteria combinations in dairy mimicking models and their potential as bioprotective cultures in pilot scale applications. Front. Microbiol. 2018;9:1–18. doi: 10.3389/fmicb.2018.01787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Muhialdin B.J., Hassan Z., Saari N. In vitro antifungal activity of lactic acid bacteria low molecular peptides against spoilage fungi of bakery products. Ann. Microbiol. 2018;68:557–567. doi: 10.1007/s13213-018-1363-x. [DOI] [Google Scholar]
- 69.Shi C., Knøchel S. Sensitivity of molds from spoiled dairy products towards bioprotective lactic acid bacteria cultures. Front. Microbiol. 2021 doi: 10.3389/fmicb.2021.631730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gupta R., Srivastava S. Antifungal effect of antimicrobial peptides (AMPs LR14) derived from Lactobacillus plantarum strain LR/14 and their applications in prevention of grain spoilage. Food Microbiol. 2014;42:1–7. doi: 10.1016/j.fm.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 71.Rosso J.Q.D.E.L. The role of topical antifungal therapy for onychomycosis and the emergence of newer agents. J. Clin. Aesthetic Dermatol. 2014;7:10–18. [PMC free article] [PubMed] [Google Scholar]
- 72.Singh A., Masih A., Monroy-nieto J., Kumar P., Bowers J., Travis J., et al. A unique multidrug-resistant clonal Trichophyton population distinct from Trichophyton mentagrophytes/Trichophyton interdigitale complex causing an ongoing alarming dermatophytosis outbreak in India : genomic insights and resistance profile. Fungal Genet Biol. 2019;133 doi: 10.1016/j.fgb.2019.103266. [DOI] [PubMed] [Google Scholar]
- 73.Méndez-tovar L.J., Manzano-gayosso P., Velásquez-hernández V., Millan-chiu B., Hernández-hernández F., Mondragón-gonzález R., et al. Resistencia a compuestos azólicos de aislamientos clínicos de Trichophyton spp . Trichophyton spp . strains. Rev. Iberoam Micol. 2007;24:320–322. doi: 10.1016/S1130-1406(07)70065-7. [DOI] [PubMed] [Google Scholar]
- 74.Salehi Z., Shams-ghahfarokhi M., Razzaghi-abyaneh M. Antifungal drug susceptibility profile of clinically important dermatophytes and determination of point mutations in terbinafine-resistant isolates. Eur. J. Clin. Microbiol. Infect. Dis. 2018;12–5(37):1841–1846. doi: 10.1007/s10096-018-3317-4. [DOI] [PubMed] [Google Scholar]
- 75.J. Chen, X. Song, P. Yang, J. Wang, Appearance of anaphylactic shock after long-term intravenous itraconazole treatment. 43(2015), pp. 537–41. https://doi.org/10.1345/aph.1L343. [DOI] [PubMed]
- 76.Tapaninen T., Backman J.T., Kurkinen K.J., Neuvonen P.J., Niemi M. Itraconazole, a P-glycoprotein and CYP3A4 inhibitor, markedly raises the plasma concentrations and enhances the renin-inhibiting effect of aliskiren. J. Clin. Pharm. 2011;51:359–367. doi: 10.1177/0091270010365885. [DOI] [PubMed] [Google Scholar]
- 77.K.Venkatakrishnan, Moltke LL Von, D.J. Greenblatt, Effects of the antifungal agents on oxidative drug metabolism clinical relevance. 38(2000), pp.111–80. https://doi.org/10.2165/00003088-200038020-00002. [DOI] [PubMed]
- 78.Jiahui Guo, Brosnan Brid, Furey Ambrose, Arendt Elke, Murphy Padraigin, Coffey Aidan. Antifungal activity of lactobacillus against microsporum canis, microsporum gypseum and epidermophyton floccosum. Bioeng Bugs. 2012;3:102–111. doi: 10.4161/bbug.19624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Arasu V.M., Jung M.W., Ilavenil S., Jane M., Kim D.H., Lee K.D., et al. Isolation and characterization of antifungal compound from L actobacillus plantarum KCC-10 from forage silage with potential beneficial properties. J. Appl. Microbiol. 2013;115:1172–1185. doi: 10.1111/jam.12319. [DOI] [PubMed] [Google Scholar]
- 80.Ajah H.A. In vitro and in vivo studies on the antifungal activity of probiotics and Seaweed extract (Ascophyllum nodosum) Int. J. Innovative Sci., Eng. Technol. 2016;3:306–312. [Google Scholar]
- 81.Alamdarloo M.S., Ameri A., Moghimipour E., Gholipour S., Saadatzadeh A. Formulation development of a topical probiotic gel for antidermatophytosis effect. Jundishapur J. Nat. Pharm. Prod. 2016;11 doi: 10.17795/jjnpp-35893. [DOI] [Google Scholar]
- 82.Alastruey-Izquierdo A., Mellado E., Peláez T., Pemán J., Zapico S., Alvarez M., et al. Population-based survey of filamentous fungi and antifungal resistance in Spain (FILPOP study) Antimicrob. Agents Chemother. 2013;57:3380–3387. doi: 10.1128/AAC.00383-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Patterson T.F., Thompson G.R., Denning D.W., Fishman J.A., Hadley S., Herbrecht R., et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the infectious diseases society of America. Clin. Infect. Dis. 2016;63:e1–60. doi: 10.1093/cid/ciw326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Klich M.A. Identification of clinically relevant aspergilli. Med. Mycol. 2006;44:127–131. doi: 10.1080/13693780600796546. [DOI] [PubMed] [Google Scholar]
- 85.Cornely O.A., Maertens J., Winston D.J., Perfect J., Ullmann A.J., Walsh T.J., Angulo-Gonzalez D. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N. Engl. J. Med. 2007;356:348–359. doi: 10.1056/NEJMoa061094. [DOI] [PubMed] [Google Scholar]
- 86.Onilude A.A., Fagade O.E., Bello M.M., Fadahunsi I.F. Inhibition of aflatoxin producing Aspergilli by lactic acid bacteria isolates from indigenously fermented cereal gruels. Afr. J. Biotechnol. 2005;4:1404–1408. [Google Scholar]
- 87.Sathe G.B., Mandal S. Fermented products of india and its implication: a review. Asian J. Dairy and Food Res. 2019;35:1–9. doi: 10.18805/ajdfr.v35i1.9244. [DOI] [Google Scholar]
- 88.Yang E.J., Kim Y.S., Chang H.C. Purification and characterization of antifungal δ-dodecalactone from lactobacillus plantarum AF1 isolated from kimchi. J. Food Prot. 2011;74:651–657. doi: 10.4315/0362-028X.JFP-10-512. [DOI] [PubMed] [Google Scholar]
- 89.Sangmanee P., Hongpattarakere T. Inhibitory of multiple antifungal components produced by Lactobacillus plantarum K35 on growth, aflatoxin production and ultrastructure alterations of Aspergillus flavus and Aspergillus parasiticus. Food Control. 2014;40:224–233. doi: 10.1016/j.foodcont.2013.12.005. [DOI] [Google Scholar]
- 90.Shehata M.G., Badra A.N., ElSohaimy S.A., Asker D., Awad T.S. Characterization of antifungal metabolites produced by novel lactic acid bacterium and their potential application as food biopreservatives. Ann. Agricultural Sci. 2019;64:71–78. doi: 10.1016/j.aoas.2019.05.002. [DOI] [Google Scholar]
- 91.Pundir R.K., Rana S., Kashyap N., Kaur A. Probiotic potential of lactic acid bacteria isolated from food samples: an in vitro study. J. Appl. Pharm. Sci. 2013;3:85–93. doi: 10.7324/JAPS.2013.30317. [DOI] [Google Scholar]
- 92.Crowley S., Mahony J., van Sinderen D. Broad-spectrum antifungal-producing lactic acid bacteria and their application in fruit models. Folia Microbiol. (Praha) 2013;58:291–299. doi: 10.1007/s12223-012-0209-3. [DOI] [PubMed] [Google Scholar]
- 93.Bayankaram P.P., Sellamuthu P.S. Antifungal and anti-aflatoxigenic effect of probiotics against Aspergillus flavus and Aspergillus parasiticus. Toxin Rev. 2016;35:10–15. doi: 10.1080/15569543.2016.1178147. [DOI] [Google Scholar]
- 94.Arasu M.V., Jung M.W., Ilavenil S., Jane M., Kim D.H., Lee K.D., Park H.S., Hur T.Y., et al. Isolation and characterization of antifungal compound from Lactobacillus plantarum KCC-10 from forage silage with potential beneficial properties. J. Appl. Microbiol. 2013;115:1172–1185. doi: 10.1111/jam.12319. [DOI] [PubMed] [Google Scholar]
- 95.Falguni P., Shilpa V.I.J., Mann B. Production of proteinaceous antifungal substances from Lactobacillus brevis NCDC 02. Int J Dairy Technol. 2010;63:70–76. doi: 10.1111/J.1471-0307.2009.00553.X. [DOI] [Google Scholar]
- 96.Rouse S., Harnett D., Vaughan A., Sinderen D.V. Lactic acid bacteria with potential to eliminate fungal spoilage in foods. J. Appl. Microbiol. 2008;104:915–923. doi: 10.1111/j.1365-2672.2007.03619.x. [DOI] [PubMed] [Google Scholar]
- 97.Ström K., Sjögren J., Broberg A., Schnürer J. Lactobacillus plantarum MiLAB 393 produces the antifungal cyclic dipeptides cyclo(L-Phe-l-Pro) and cyclo(L-Phe-trans-4-OH-l-Pro) and 3-phenyllactic acid. Appl. Environ. Microbiol. 2002;68:4322–4327. doi: 10.1128/AEM.68.9.4322-4327.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bukhari S.A., Salman M., Numan M., Javed M.R., Zubair M., Mustafa G. Characterization of antifungal metabolites produced by Lactobacillus plantarum and Lactobacillus coryniformis isolated from rice rinsed water. Mol. Biol. Rep. 2020;47:1871–1881. doi: 10.1007/s11033-020-05281-1. [DOI] [PubMed] [Google Scholar]
- 99.Al-Hatmi A.M.S., Bonifaz A., Ranque S., Sybren de Hoog G., Verweij P.E., Meis J.F. Current antifungal treatment of fusariosis. Int. J. Antimicrob. Agents. 2018;51:326–332. doi: 10.1016/j.ijantimicag.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 100.Van Diepeningen A.D., Brankovics B., Iltes J., van der Lee T.A.J., Waalwijk C. Diagnosis of fusarium infections: approaches to identification by the clinical mycology laboratory. Curr. Fungal Infect. Rep. 2015;9:135–143. doi: 10.1007/s12281-015-0225-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ribas e Ribas A.D., Spolti P., Del Ponte E.M., Donato K.Z., Schrekker H., Fuentefria A.M. Is the emergence of fungal resistance to medical triazoles related to their use in the agroecosystems? A mini review. Brazilian J. Microbiol. 2016;47:793–799. doi: 10.1016/j.bjm.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang N., O'Donnell K., Sutton D.A., Nalim F.A., Summerbell R.C., Padhye A.A., et al. Members of the Fusarium solani species complex that cause infections in both humans and plants are common in the environment. J. Clin. Microbiol. 2006;44:2186–2190. doi: 10.1128/JCM.00120-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Van Diepeningen A.D., Al-Hatmi A.M.S., Brankovics B., de Hoog G.S. Taxonomy and clinical spectra of fusarium species: where do we stand in 2014? Curr. Clin. Microbiol. Reports. 2014;1:10–18. doi: 10.1007/s40588-014-0003-x. [DOI] [Google Scholar]
- 104.Batista B.G., de Chaves M.A., Reginatto P., Saraiva O.J., Fuentefria A.M. Human fusariosis: an emerging infection that is difficult to treat. Rev. Soc. Bras Med. Trop. 2020;53:1–7. doi: 10.1590/0037-8682-0013-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Guarro J. Fusariosis, a complex infection caused by a high diversity of fungal species refractory to treatment. Eur. J. Clin. Microbiol. Infect. Dis. 2013;32:1491–1500. doi: 10.1007/s10096-013-1924-7. [DOI] [PubMed] [Google Scholar]
- 106.Homa M., Galgóczy L., Manikandan P., Narendran V., Sinka R., árpád Csernetics, et al. South Indian Isolates of the Fusarium solani species complex from clinical and environmental samples: identification, antifungal susceptibilities, and virulence. Front. Microbiol. 2018 doi: 10.3389/fmicb.2018.01052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Al-Hatmi A.M.S., Curfs-Breuker I., de Hoog G.S., Meis J.F., Verweij P.E. Antifungal susceptibility testing of fusarium: a practical approach. J. Fungi. 2017;3:19. doi: 10.3390/jof3020019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.López-Seijas J., García-Fraga B., da Silva A.F., Sieiro C. Wine lactic acid bacteria with antimicrobial activity as potential biocontrol agents against fusarium oxysporum f. Sp. Lycopersici. Agronomy. 2020;10 doi: 10.3390/agronomy10010031. [DOI] [Google Scholar]
- 109.Abouloifa H H., Gaamouche S S., Rokni Y Y., Hasnaoui I I., Bellaouchi R R., Ghabbour N., et al. Antifungal activity of probiotic Lactobacillus strains isolated from natural fermented green olives and their application as food bio-preservative. Biol. Control. 2021;152 doi: 10.1016/j.biocontrol.2020.104450. [DOI] [Google Scholar]
- 110.Hu J., Chen F., Kan T., Zhuang H., Zhang J., Han X. Inhibition of fusarium solani infection in Murine Keratocytes by lactobacillus salivarius ssp. salivarius JCM1231 culture filtrate in vitro. Curr Eye Res. Taylor & Francis; 2017;42:1339–1347. doi: 10.1080/02713683.2017.1317816. [DOI] [PubMed] [Google Scholar]
- 111.Zebboudj N., Yezli W., Hamini-kadar N. Antifungal activity of lactic acid bacteria against Fusarium species responsible for tomato crown and root rots. Environ. Exp. Biol. 2020;18 doi: 10.22364/eeb.18.02. [DOI] [Google Scholar]
- 112.B.V. Deepthi, K.P. Rao, G. Chennapa, M.K. Naik, K.T. Chandrashekara, Antifungal attributes of lactobacillus plantarum MYS6 against Fumonisin producing fusarium proliferatum associated with poultry feeds. 11(2016), pp. 1–22. https://doi.org/10.1371/journal.pone.0155122.1–22. [DOI] [PMC free article] [PubMed]
- 113.Schnürer J., Magnusson J. Antifungal lactic acid bacteria as biopreservatives. Trends Food Sci. Technol. 2005;16:70–78. doi: 10.1016/j.tifs.2004.02.014. [DOI] [Google Scholar]
- 114.Boonsarngsuk V., Eksombatchai D., Kanoksil W., Tantrakul V. Airway obstruction caused by penicilliosis: a case report and review of the literature. Arch. Bronconeumol. 2015;51:e25–e28. doi: 10.1016/j.arbr.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 115.Lebeer S., Vanderleyden J., De Keersmaecker S.C.J. Genes and molecules of lactobacilli supporting probiotic action. Microbiol. Mol. Biol. Rev. 2008;72:728–764. doi: 10.1128/MMBR.00017-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mijač V.D., Dukić S.V., Opavski N.Z., Dukić M.K., Ranin L.T. Hydrogen peroxide producing lactobacilli in women with vaginal infections. Eur. J. Obstet. Gynecol. Reprod. Biol. 2006;129:69–76. doi: 10.1016/j.ejogrb.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 117.Peleg A.Y., Hogan D.A., Mylonakis E. Medically important bacterialg-fungal interactions. Nat. Rev. Microbiol. 2010;8:340–349. doi: 10.1038/nrmicro2313. [DOI] [PubMed] [Google Scholar]
- 118.Rodrigues L., Banat I.M., Teixeira J., Oliveira R. Biosurfactants: potential applications in medicine. J. Antimicrob. Chemother. 2006;57:609–618. doi: 10.1093/jac/dkl024. [DOI] [PubMed] [Google Scholar]
- 119.Li H., Liu L., Zhang S., Cui W., Lv J. Identification of antifungal compounds produced by Lactobacillus casei AST18. Curr. Microbiol. 2012;65:156–161. doi: 10.1007/s00284-012-0135-2. [DOI] [PubMed] [Google Scholar]
- 120.C. Luz Mínguez, Tesis doctoral internacional vitro and in food of lactic acid bacteria (2020).
- 121.Garnier L., Penland M., Thierry A., Maillard M.B., Jardin J., Coton M. Antifungal activity of fermented dairy ingredients: identification of antifungal compounds. Int. J. Food Microbiol. 2020;322 doi: 10.1016/j.ijfoodmicro.2020.108574. [DOI] [PubMed] [Google Scholar]
- 122.Mun S.Y., Kim S.K., Woo E.R., Chang H.C. Purification and characterization of an antimicrobial compound produced by Lactobacillus plantarum EM showing both antifungal and antibacterial activities. Lwt. 2019;114 doi: 10.1016/j.lwt.2019.108403. [DOI] [Google Scholar]
- 123.Coman M.M., Verdenelli M.C., Cecchini C., Silvi S., Orpianesi C., Boyko N., et al. In vitro evaluation of antimicrobial activity of Lactobacillus rhamnosus IMC 501®, Lactobacillus paracasei IMC 502® and SYNBIO® against pathogens. J. Appl. Microbiol. 2014;117:518–527. doi: 10.1111/jam.12544. [DOI] [PubMed] [Google Scholar]
- 124.E. Kheradmand, F. Rafii, M.H. Yazdi, A.A. Sepahi, A.R. Shahverdi, M.R. Oveisi, The antimicrobial effects of selenium nanoparticle-enriched probiotics and their fermented broth against Candida albicans 22(2014), pp. 48. https://doi.org/10.1186/2008-2231-22-48. [DOI] [PMC free article] [PubMed]
- 125.Seneviratne C.J., Samaranayake L.P., Ohshima T., Maeda N., Jin L.J. Identification of antifungal molecules from novel probiotic Lactobacillus bacteria for control of Candida infection. Hong Kong Med. J. 2016;22:34–36. [PubMed] [Google Scholar]
- 126.Strus M., Kucharska A., Kukla G., Brzychczy-Włoch M., Maresz K., Heczko P.B. The in vitro activity of vaginal Lactobacillus with probiotic properties against Candida. Infect. Dis. Obstet Gynecol. 2005;13:69–75. doi: 10.1080/10647440400028136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kuwaki S., Ohhira I., Takahata M., Murata Y., Tada M. Antifungal activity of the fermentation product of herbs by lactic acid bacteria against tinea. J. Biosci. Bioeng. 2002;94:401–405. doi: 10.1016/S1389-1723(02)80216-X. [DOI] [PubMed] [Google Scholar]
- 128.Sun L., Li X., Zhang Y., Yang W., Ma G., Ma N., et al. A novel lactic acid bacterium for improving the quality and shelf life of whole wheat bread. Food Control. 2020;109 doi: 10.1016/j.foodcont.2019.106914. [DOI] [Google Scholar]
- 129.Poornachandra Rao K., Deepthi B.V., Rakesh S., T.Ganesh P.Achar, Sreenivasa M.Y. Antiaflatoxigenic Potential of Cell-Free Supernatant from Lactobacillus plantarum MYS44 Against Aspergillus parasiticus. Probiotics Antimicrob Proteins. Probiotics and Antimicrobial Proteins. 2019;11:55–64. doi: 10.1007/s12602-017-9338-y. [DOI] [PubMed] [Google Scholar]
- 130.Adesina I., Ojokoh A., Arotupin D. Inhibitory properties of lactic acid bacteria against moulds associated with spoilage of bakery products. J. Adv. Microbiol. 2017;4:1–8. doi: 10.9734/JAMB/2017/31386. [DOI] [Google Scholar]
- 131.Ouiddir M., Bettache G., Salas M.Leyva, Pawtowski A., Donot C., Brahimi S., et al. Selection of Algerian lactic acid bacteria for use as antifungal bioprotective cultures and application in dairy and bakery products. Food Microbiol. 2019;82:160–170. doi: 10.1016/j.fm.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 132.Ali F.S., Zayed G., Saad O.A.O., Salwa A.H. Gharib, antimicrobial activity and probiotic properties of lactic acid bacteria isolated from traditional fermented dairy products. J. Modern Res. 2020:40–48. doi: 10.21608/jmr.2020.22931.1015. [DOI] [Google Scholar]
- 133.Russo P., Arena M.P., Fiocco D., Capozzi V., Drider D., Spano G. Lactobacillus plantarum with broad antifungal activity: a promising approach to increase safety and shelf-life of cereal-based products. Int. J. Food Microbiol. 2017;247:48–54. doi: 10.1016/j.ijfoodmicro.2016.04.027. [DOI] [PubMed] [Google Scholar]
- 134.Muhialdin B.J., Algboory H.L., Kadum H., Mohammed N.K., Saari N., Hassan Z., et al. Antifungal activity determination for the peptides generated by Lactobacillus plantarum TE10 against Aspergillus flavus in maize seeds. Food Control. 2020;109 doi: 10.1016/j.foodcont.2019.106898. [DOI] [Google Scholar]
- 135.Ben Taheur F., Mansour C., Kouidhi B., Chaieb K. Use of lactic acid bacteria for the inhibition of Aspergillus flavus and Aspergillus carbonarius growth and mycotoxin production. Toxicon. 2019;166:15–23. doi: 10.1016/j.toxicon.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 136.Taroub B., Salma L., Manel Z., Ouzari H.I., Hamdi Z., Moktar H. Isolation of lactic acid bacteria from grape fruit: antifungal activities, probiotic properties, and in vitro detoxification of ochratoxin A. Ann. Microbiol. Ann. Microbiol. 2019;69:17–27. doi: 10.1007/s13213-018-1359-6. [DOI] [Google Scholar]
- 137.Hamad G.M., Abu-Serie M.M., Ali S.H., Hafez E.E. Combination probiotic supernatants reduce growth and aflatoxin production by Aspergillus spp in food contamination. Am. J. Food Sci. Technol. 2018;6:57–67. doi: 10.12691/ajfst-6-2-1. [DOI] [Google Scholar]
- 138.Juodeikiene G., Bartkiene E., Cernauskas D., Cizeikiene D., Zadeike D., Lele V., et al. Antifungal activity of lactic acid bacteria and their application for Fusarium mycotoxin reduction in malting wheat grains. Lwt. 2018;89:307–314. doi: 10.1016/j.lwt.2017.10.061. [DOI] [Google Scholar]
- 139.Divyashree S., Anjali P.G., Deepthi B.V., Somashekaraiah Rakesh, Walid M., Hammami Riad, Sreenivasa M.Y. Black cherry fruit as a source of probiotic candidates with antimicrobial and antibiofilm activities against Salmonella. South Afr. J. Botany. 2022;150:861–872. doi: 10.1016/j.sajb.2022.08.045. [DOI] [Google Scholar]
- 140.Russo P., Fares C., Longo A., Spano G., Capozzi V. Lactobacillus plantarum with broad antifungal activity as a protective starter culture for bread production. Foods. 2017;6:110. doi: 10.3390/foods6120110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Muthusamy K., Soundharrajan I., Srisesharam S., Kim D., Kuppusamy P., Lee K.D., et al. Probiotic characteristics and antifungal activity of Lactobacillus plantarum and its impact on fermentation of Italian 00ryegrass at low moisture. Appl. Sci. 2020;10 doi: 10.3390/app10010417. [DOI] [Google Scholar]
- 142.Torrijos R., de Melo Nazareth T., Vila-Donat P., Mañes J., Meca G. Use of mustard extracts fermented by lactic acid bacteria to mitigate the production of fumonisin B1 and B2 by fusarium verticillioides in corn ears. Toxins (Basel) 2022;14:80. doi: 10.3390/toxins14020080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Russo R., Edu A., De Seta F. Study on the effects of an oral lactobacilli and 761 lactoferrin complex in women with intermediate vaginal microbiota. Arch. Gynecol. 762 Obstet. 2018;298:139–145. doi: 10.1007/s00404-018-4771-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.