Abstract
This study aimed to investigate the effects of polyherbal mixtures (PHM) on growth performance, antioxidant capacities, immune function, and intestinal health in yellow-feathered broilers. PHM is composed of five traditional Chinese medicine herbs (Portulaca oleracea L., Radix Sophora flavescens, Thalictrum glandulosissimum, Terra flava usta, and Pogostemon cablin). A total of 270 one-day-old yellow-feathered broilers were randomly allotted into 3 treatments for a 42-d feeding trial, each with 6 replicates of 15 birds. The dietary treatments consisted of a basal diet (CON), a basal diet supplemented with 50 mg/kg chlortetracycline (CTC), and a basal diet supplemented with 1000 mg/kg PHM. The results showed that dietary PHM supplementation increased body weight, ADG, and decreased F/G compared to the CON. PHM also increased spleen index and mRNA expression of IL-4 (d 21), and thymus index, serum IgA (d 42) and IgG, IL-4 and sIgA in jejunal mucosa (d 21 and 42), but decreased serum IFN-γ and mRNA expression of IFN-γ (d 21 and 42). In addition, PHM increased serum SOD, GSH-Px (d 21 and 42) and T-AOC (d 42), but decreased the content of serum MDA (d 21), the up-regulated mRNA expression of GSH-Px, CAT (d 21), SOD and CAT (d 42). Furthermore, PHM also improved the intestinal epithelial barrier indicators by the up-regulated mRNA expression of CLDN-1, OCLN (d 21 and 42) and ZO-1 (d 21), and the increased of villus height and villus height to crypt depth in jejunum (d 42). The high-throughput sequencing results showed that dietary PHM supplementation increased the alpha diversity and relative abundance of Oscillospira and Ruminococcus (d 21) and Lactobacillus (d 42), whereas decreasing that of Enterococcus (d 21) compared with CON. PICRUSt analysis revealed that metabolic pathways of carbohydrate, energy, lipid, cofactors, and vitamins were significantly enriched in the PHM group. Spearman's correlation analysis revealed that the genera Lactobacillus, Enterococcus, Ruminococcus, Oscillospira, and Faecalibacterium were related to growth performance, intestinal integrity, immune-related factors, antioxidant indices, and tight junction proteins. In conclusion, the results indicated that dietary PHM supplementation improved growth performance and immune status of yellow-feathered broilers by enhancing antioxidant capacities, barrier function, and modulated jejunal microbial communities. PHM used in our study has the potential to replace prophylactic antibiotic use in poultry production systems.
Key words: polyherbal mixtures, broiler, growth performance, immune status, jejunal microbiota
INTRODUCTION
In recent decades, poultry meat has become one of the most important animal protein sources due to its comparative advantages of good quality of nutrition, short production period, and low production cost (Petracci et al., 2015; Kleyn and Ciacciariello, 2021). The use of antibiotics as growth promoters was banned in the European Union (2005) (Dibner and Richards, 2005) and China (2020) (Melaku et al., 2021) due to the risk of antimicrobial resistance and antibiotic residues. However, the presence of stressful conditions in the intensive poultry production system reduces immune function and growth performance and facilitates the spread of diseases (Abo et al., 2020). Hence, finding effectively antibiotic alternatives for intensive poultry production system is urgent.
Naturally available feed additives are promising antibiotic alternatives in livestock. The positive effects of Chinese herbs on the growth promoting properties (Ghorbanali et al., 2016), immunity (Abdel-Razek et al., 2019), and gut microbiota (Wang et al., 2021) of broilers have been widely reported. Our previous study has shown that a Chinese herbal mixture (Portolaca oleracea L., Radix Sophorae Flavescentis, Thalictrum glandulosissimum, Terra flava usta, and Pogostemon cablin) could to improve the growth performance and prevent disease of yellow-feathered broilers (Li, 2021). Recent studies also indicated that purslane as herbal feed additives has benefit activities (Holleran et al., 2020) and improves immune dysfunction and intestinal flora (Chen et al., 2020). Thalictrum glandulosissimum usually serve all effects on enhancing the appetite of animals, promoting growth performance (Wang et al., 2021), and regulating gut microbiota (Mahmood et al., 2019). The main active ingredients of Radix Sophorae Flavescentis are alkaloids and flavonoids, which exert extensive influence in anti-inflammatory and antioxidant activities (Holleran et al., 2020), and improve immune dysfunction and intestinal flora (Chen et al., 2020). Thalictrum glandulosissimum usually serve as local substitutes for Coptis chinensis Franch., a famous traditional Chinese medicine (TCM), as they have similar active ingredients, such as berberine, which has an effect on antibacterial activity and antirotavirus activity (Hu et al., 2021; Zhou et al., 2022). Terra flava usta mainly contains silicic acid, aluminum oxide, and ferric oxide, which could effectively protect gastrointestinal function, nutritional status, and immune functions of PC patients (Wu and Jin, 2021). The various bioactive compounds of Pogostemon cablin were terpenoids, phytosterols, flavonoids, organic acids, and so on, which had been recommended for the treatment of gastrointestinal diseases, and promote the healthy functioning of organs and tissues (Junren et al., 2021). Previous studies revealed that the coexisting constituents in herbal mixture promoted the intestinal absorption of active constituents by improving solubility and increasing the membrane permeability of enterocytes (Zhao et al., 2020).
The intestinal microbiota of animals regulates nutrient digestion and protects against enteric pathogens bacteria as a means of improving intestinal immunity and performing other physiological functions (Juan et al., 2019). Animal feed additives from natural plants have been reported to potential raise the health benefits and growth-promoting effects by modulating the gut microbiota (Ayalew et al., 2022). However, studies on the effects of dietary supplementation of PHM on the productive performance of yellow-feathered broilers were still limited. In this study, we explored the effects of adding dietary PHM as a substitute for antibiotics on growth performance, antioxidant capacity, immune status, intestinal morphology, intestinal barrier, intestinal microbiota, and microbiota metabolites of yellow-feathered broilers.
MATERIALS AND METHODS
Preparation of PHM
Portolaca oleracea L., Radix Sophorae Flavescentis, Thalictrum glandulosissimum, Terra flava usta, and Pogostemon cablin at a ratio of 1:1:1:1:1, pulverize in a beater and pass through a sieve of 80 mesh. The proximate chemical composition of PHM (ie, crude protein [CP, method 984.13], ether extract [EE, method 920.39], crude fiber [CF, method 978.10], ash [method 942.05], total phosphorus [P, method 946.06], and calcium [Ca, method 968.08]) was measured according to AOAC methods (AOAC, 2006). The total polysaccharides of PHM were determined by phenol-sulfuric acid method with glucose as a standard (Chen and Huang, 2019). The determination of total flavonoids was performed by NaNO2-Al (NO3)3-NaOH colorimetric method with rutin as a standard for estimating the content of total flavonoids (Xiang et al., 2021). The detailed nutritional levels were presented in Table 1.
Table 1.
The effective active ingredients and nutritive composition of PHM powder.
| Nutritive ingredients | Content (%) | Effective active ingredients | Content (mg/g) |
|---|---|---|---|
| Crude protein | 11.34 | Total flavonoids (%) | 8.37 |
| Crude fat | 1.23 | Total polysaccharides (%) | 5.81 |
| Crude fiber | 9.57 | ||
| Ash | 23.45 | ||
| Ca | 1.41 | ||
| P | 0.23 |
Experimental Design and Diets
A total of 270 one-day-old male yellow-feathered broilers with similar body weight (38.7 ± 1.4 g) were purchased from Foshan Nanhai Breeders & Poultry Co. Ltd. (Guangdong, China). The PHM consists of five kinds of Chinese herbal medicines, including Portolaca oleracea L., Radix Sophorae Flavescentis, Thalictrum glandulosissimum, Terra flava usta, and Pogostemon cablin. All Chinese herbal medicines were purchased from Anguo Qi'an Pharmaceutical Co. Ltd. (Hebei, China). All experimental procedures used in this study were approved by the Animal Ethics Committee of the South China Agricultural University (Guangzhou, China). The care and use of all animals were performed according to the Guidelines for Animal Experiments of the South China Agricultural University.
Broilers were randomly assigned to 3 dietary treatments with 6 replications and 15 broilers per replication. The 3 dietary treatments were as follows: 1) CON group (basal diet), 2) CTC group (basal diet supplemented with 50 mg/kg Chlortetracycline), and 3) PHM group (basal diet supplemented with 1000 mg/kg PHM). Chlortetracycline 20% Premix was purchased from Jinhe Biotechnology Co. Ltd. (Inner Mongolia, China). The experimental diets were fed for 42 d, including starter (d 1–21) and finisher (d 22–42) phases. The ingredients and nutrient levels of basal diets are shown in Table 2. All broilers had free access to feed and clean water during the experiment. The temperature of chicken coop was maintained at 33°C at the age of 1 to 4 d and then reduced by 2°C per week to a final temperature of around 24°C. The BW of the broilers was measured at 1, 21, and 42 d. The average body weight (BW), average daily gain (ADG), average daily feed intake (ADFI), and ratio of feed to weight gain (F: G) were calculated for each repetition at 21 and 42 d, respectively.
Table 2.
The ingredient and nutrient composition of the basal diet (% as fed basis).
| Component | Days 1–21 | Days 22–42 |
|---|---|---|
| Ingredient (%) | ||
| Corn | 55.60 | 55.20 |
| Soybean meal (CP, 44%) | 29.00 | 24.00 |
| Cottonseed meal | 2.50 | 3.00 |
| Wheat flour | 4.00 | 4.00 |
| Hydrolyzed feather meal | 1.50 | 1.50 |
| Dicalcium phosphate (16.5%) | 0.90 | 0.80 |
| Limestone powder | 1.50 | 1.50 |
| Bentonite | 1.00 | 1.00 |
| Soy oil | 2.00 | 7.00 |
| Premix1 | 2.00 | 2.00 |
| Total | 100.00 | 100.00 |
| Calculation of nutrients2 | ||
| Metabolizable energy, kcal/kg | 2894 | 3212 |
| Crude protein, % | 21.50 | 19.51 |
| Calcium, % | 0.96 | 0.84 |
| Total phosphorus, % | 0.66 | 0.55 |
| Lysine, % | 1.45 | 1.40 |
| Methionine, % | 0.54 | 0.50 |
| Threonine, % | 0.91 | 0.80 |
The premix provided the following per kilogram of diet: VA, 6,000 IU; VD3, 2,000 IU; VE, 30 mg; VK3, 2 mg; VB1, 3 mg; VB2, 5 mg; pantothenic acid, 800 mg; choline chloride 1,500 mg; nicotinic acid, 30 mg; pyridoxine, 3 mg; folic acid, 500 mg; biotin, 0.2 mg; VB12, 1 mg; Fe, 100 mg; Cu, 8 mg; Mn, 100 mg; Zn, 100 mg; I, 0.42 mg; Se, 0.3 mg.
The nutrient levels were calculated from data provided by Feed Database in China.
Sample Collection and Immune Organ Indexes
On d 21 and 42 of the experiment, one broiler was randomly selected from each replicate. Blood samples were collected from wing veins and centrifuged (3000 rpm, 4°C, 10 min), then the supernatant was collected and stored at −20°C for biochemical analysis. The birds were selected randomly and selected birds were sacrificed by cervical dislocation and exsanguinated. After opening birds, lymphoid organs (spleen, bursa of fabricus, and thymus) were separated, weighed, and calculated the visceral weight index (Formulation: viscera weight index (%) = viscera weight/final body weight ×100%). Subsequently, the jejunum was excised, flushed gently with cold saline solution, and the middle of jejunum (approximately 1 cm) was collected, then immediately immersed in 4% paraformaldehyde solution for histological examination. Similarly, the mucosal tissue was scraped with sterile glass slide from small intestine after being washed with ice-cold saline solution, and stored at −80°C for further analysis. The jejunum contents were quickly collected on ice, snap-frozen in liquid nitrogen, and then stored at −80°C for further microbial analysis.
Determination of Serum Parameters and Intestinal Mucosal sIgA Concentration
Chicken secretory immunoglobulin A (sIgA) kit, chicken immunoglobulin A (IgA) kit, chicken immunoglobulin G (IgG) kit, chicken interferon-γ (IFN-γ) kit, and chicken interleukin-4 (IL-4) kit were purchased from Shanghai Mlbio Co., Ltd. (Shanghai, China). Glutathione peroxidase (GSH-Px), superoxide dismutase (SOD), total antioxidant capacity (T-AOC), and malondialdehyde (MDA) kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). All measurements were performed at least in triplicate following the manufacturer's instructions.
Histological Procedures
The jejunum segment was removed from the 4% paraformaldehyde fixing solution and then embedded in paraffin. Paraffin sections were made by the microtome and stained with hematoxylin-eosin staining. The microscope image processing software (Image-Pro Plus 6.0) was used to measure villus height (VH), crypt depth (CD), and ratio of villus height to crypt depth (V/C).
Tissue RNA Extraction and qRT-PCR Analysis
Total RNA was extracted from the jejunum samples using TRIzol reagent (Vazyme Biotech Co., Ltd., Nanjing, China). Total cDNA was synthesized with total RNA (1 μg) using HiScript III RT SuperMix for qPCR (+gDNA wiper) (Vazyme Biotech Co., Ltd., Nanjing, China) and subjected to real-time quantitative PCR (RT-qPCR) amplification using the ChamQ universal SYBR qPCR Master Mix (Vazyme Biotech Co., Ltd., Nanjing, China) in a QuantStudio5 (Thermo Fisher Scientific, Inc. Waltham, MA). The primers sequences used for PCR are listed in Table S1, and the relative expression of the target gene was analyzed through the 2−ΔΔCt method after normalization against the geometric mean of the expression of β-actin and GAPDH (Livak and Schmittgen, 2001).
16S rRNA Sequencing and Gut Microbiota Analysis
Total microbial DNA from jejunal fecal samples was isolated using a QIAamp DNA Stool Kit (Qiagen, Valencia, CA). The V3–V4 region of the bacterial 16S rRNA gene was amplified by PCR (forward primer: 5′-ACTCCTACGGGAGGCAGCA-3′ and reverse primer: 5′-GGACTACHVGGGTWTCTAAT-3′). PCR products were purified with Vazyme VAHTS DNA Clean Beads (Vazyme Biotech Co., Ltd., Nanjing, China) and quantified using a PicoGreen dsDNA Assay Kit (Invitrogen, Braunschweig, Germany). 16s rRNA sequencing was performed using Illumina Novaseq_PE250 (Illumina, San Diego, CA) sequencing platform, sequencing services were provided by Personal Biotechnology Co., Ltd. (Shanghai, China). Data collection and analysis were conducted with the help of the free online platform, Genescloud Platform (www.genescloud.cn).
Statistical Analysis
The data were analyzed by 1-way ANOVA using SPSS 22.0 software (IBM, Armonk, NY). GraphPad Prism 7.0 software (GraphPad, San Diego, CA) was used to generate graphs. All data are presented as the mean value ± SD of at least 3 independent experiments. Multiple comparisons and significance of differences among groups were performed using the Turkey method followed by unpaired Wilcoxon comparison test, as well as by the nonparametric factorial Kruskal–Wallis test. Differences were considered significantly different at P < 0.05.
RESULTS
Effect of PHM on the Growth Performance Parameters of Broilers
The effects of dietary PHM on the growth performance (BW, ADG, ADFI, and F/G) of broilers are shown in Table 3. Compared with the CON group, PHM increased the BW on d 21 (P = 0.079) and d 42 (P < 0.01), whereas dietary CTC increased the BW on d 42 (P < 0.05). The ADG and ADFI in the PHM group were significantly increased (P < 0.05) compared with CON group during the 22–42 d and 1–42 d phase, whereas the PHM group tended to increase ADG (P = 0.079) compared to CON group during the 1–21 d. In addition, compared to the CON group, the F/G of broilers fed PHM showed a decreasing trend during the 1–21 d and 1–42 d phase (P = 0.068, P = 0.060).
Table 3.
Effect of dietary PHM supplementation on growth performance of 1 to 42-day-old broilers.
| Item | CON | CTC | PHM | P value |
|---|---|---|---|---|
| 1–21 d | ||||
| BW at d 1(g) | 38.28 ± 1.13 | 39.32 ± 1.51 | 38.63 ± 1.52 | 0.439 |
| BW at d 21(g) | 451.66 ± 14.61 | 474.28 ± 21.96 | 479.48 ± 23.75 | 0.073 |
| ADG (g/d) | 19.68 ± 0.66 | 20.71 ± 1.00 | 20.99 ± 1.12 | 0.071 |
| ADFI (g/d) | 29.51 ± 1.30 | 29.88 ± 0.99 | 29.82 ± 0.68 | 0.799 |
| F/G | 1.50 ± 0.05 | 1.44 ± 0.06 | 1.42 ± 0.06 | 0.073 |
| 22–42 d | ||||
| BW at d 42 (g) | 1335.17 ± 12.34b | 1380.00 ± 16.73a | 1402.50 ± 17.82a | <0.01 |
| ADG (g/d) | 42.07 ± 0.23b | 43.13 ± 1.45ab | 43.95 ± 1.28a | 0.035 |
| ADFI (g/d) | 97.20 ± 1.02b | 98.52 ± 1.00ab | 99.31 ± 0.94a | 0.007 |
| F/G | 2.31 ± 0.03 | 2.29 ± 0.09 | 2.26 ± 0.08 | 0.495 |
| 1–42 d | ||||
| ADG (g/d) | 30.88 ± 0.27c | 31.92 ± 0.39b | 32.47 ± 0.42a | <0.01 |
| ADFI (g/d) | 62.94 ± 0.97b | 63.95 ± 0.88ab | 64.43 ± 0.71a | 0.026 |
| F/G | 2.04 ± 0.02 | 2.00 ± 0.04 | 1.98 ± 0.04 | 0.071 |
Abbreviations: ADFI: average daily feed intake; ADG: average daily gain; BW: body weight; CON: control group, basal diet; CTC: basal diet supplemented with 50 mg/kg Chlortetracycline; FCR, feed conversion ratio; PHM: basal diet supplemented with 1000 mg/kg polyherbal mixtures.
Means within a row with no common superscript differ significantly (P < 0.05). Data are presented in mean ± SD (n = 6).
Effect of PHM on the Jejunal Mucosa and Serum Immune Factors
The results of the effects of PHM on immune organ indexes, serum immunoglobulins, intestinal sIgA concentration, and inflammatory cytokines of broilers are displayed in Table 4. At 21 d of age, compared with the CON, supplement with PHM significantly increased the spleen index (P < 0.05), the concentration of serum IgG (P < 0.05), IL-4 (P < 0.01), and sIgA in jejunal mucosa (P < 0.05), but decreased serum IFN-γ (P < 0.05). At 42 d of age, when compared with the CON group, the thymus index in the PHM group was significantly increased (P < 0.05), PHM group also significantly increased the concentration of serum IgA (P < 0.01), IgG (P < 0.01), IL-4 (P < 0.001), and sIgA in the mucosa of jejunum (P < 0.05), whereas decreased the concentration of serum IFN-γ (P < 0.01).
Table 4.
Effects of dietary PHM supplementation on the immune organ indices in broilers.
| Item | CON | CTC | PHM | P value |
|---|---|---|---|---|
| 21 d | ||||
| Spleen index (%) | 0.15 ± 0.01b | 0.17 ± 0.01ab | 0.19 ± 0.02a | 0.004 |
| Thymus index (%) | 0.36 ± 0.05 | 0.40 ± 0.04 | 0.39 ± 0.05 | 0.230 |
| Bursa index (%) | 0.41 ± 0.08 | 0.41 ± 0.07 | 0.45 ± 0.07 | 0.481 |
| IgA (μg/mL) | 294.49 ± 22.17 | 314.28 ± 18.55 | 321.78 ± 19.03 | 0.081 |
| IgG (μg/mL) | 2823.83 ± 76.77b | 2933.56 ± 101.65ab | 3007.92 ± 133.42a | 0.029 |
| IL-4 (pg/mL) | 137.82 ± 3.52b | 140.99 ± 2.72ab | 146.11 ± 4.12a | 0.003 |
| IFN-γ (pg/mL) | 78.63 ± 2.87a | 73.99 ± 3.68ab | 72.33 ± 3.44b | 0.014 |
| sIgA (mg/g protein) | 4.85 ± 0.19b | 5.15 ± 0.32ab | 5.43 ± 0.34a | 0.013 |
| 42 d | ||||
| Spleen index (%) | 0.19 ± 0.02 | 0.18 ± 0.02 | 0.20 ± 0.03 | 0.320 |
| Thymus index (%) | 0.39 ± 0.03b | 0.41 ± 0.07ab | 0.48 ± 0.05a | 0.024 |
| Bursa index (%) | 0.27 ± 0.02 | 0.33 ± 0.06 | 0.34 ± 0.05 | 0.053 |
| IgA (μg/mL) | 307.31 ± 18.68b | 330.64 ± 17.62ab | 350.21 ± 17.23a | 0.003 |
| IgG (μg/mL) | 2674.33 ± 186.82b | 2873.19 ± 70.82ab | 3029.31 ± 164.39a | 0.003 |
| IL-4 (pg/mL) | 127.42 ± 3.68c | 132.83 ± 2.44b | 138.12 ± 2.58a | <0.001 |
| IFN-γ (pg/mL) | 73.28 ± 2.54a | 67.11 ± 3.87b | 64.11 ± 4.17b | 0.002 |
| sIgA (mg/g protein) | 4.99 ± 0.19b | 5.38 ± 0.20a | 5.43 ± 0.22a | 0.004 |
Abbreviations: IgA: immunoglobulin A; IgG: immunoglobulin G; IL-4: interleukin-4; IFN-γ: interferon-γ; sIgA, secreted immunoglobulin; CON: control group, basal diet; CTC: basal diet supplemented with 50 mg/kg Chlortetracycline; PHM: basal diet supplemented with 1000 mg/kg polyherbal mixtures.
Means within a row with no common superscript differ significantly (P < 0.05). Data are presented in mean ± SD (n = 6).
Effect of PHM on the Serum Antioxidant Capacity
The results associated with the effect of dietary PHM supplementation on the antioxidant activity in the serum of broilers were presented in Table 5. At 21 d of age, compared with the CON group, the PHM and CTC groups significantly increased contents of serum SOD (P < 0.01, P < 0.05) and GSH-Px (P < 0.05, P < 0.05), and decreased the content of serum MDA (P < 0.01, P < 0.001). At 42 d of age, the contents of serum SOD, GSH-Px, and T-AOC activity were higher in broilers fed PHM diets compared with the CON group (P < 0.05). Similarly, the PHM group increased the content of serum SOD on d 42 compared with the CTC group (P < 0.05).
Table 5.
Effects of dietary PHM supplementation on the serum antioxidant capacity in broilers.
| Item | CON | CTC | PHM | P value |
|---|---|---|---|---|
| 21 d | ||||
| SOD (U/mL) | 166.57 ± 7.67b | 183.78 ± 8.96a | 189.24 ± 9.59a | 0.001 |
| GSH-Px (U/mL) | 603.26 ± 22.10b | 641.56 ± 25.52ab | 656.72 ± 37.78a | 0.018 |
| T-AOC (U/mL) | 10.41 ± 0.45 | 10.77 ± 0.39 | 10.92 ± 0.53 | 0.183 |
| MDA (nmol/mL) | 3.92 ± 0.15b | 3.35 ± 0.23b | 3.23 ± 0.25a | <0.001 |
| 42 d | ||||
| SOD (U/mL) | 182.48 ± 4.34b | 184.73 ± 6.26b | 194.58 ± 7.75a | 0.010 |
| GSH-Px (U/mL) | 706.91 ± 17.64b | 748.56 ± 35.66ab | 765.05 ± 36.23a | 0.015 |
| T-AOC (U/mL) | 10.56 ± 0.37b | 11.45 ± 0.57ab | 11.86 ± 1.06a | 0.020 |
| MDA (nmol/mL) | 4.51 ± 0.33 | 4.32 ± 0.33 | 4.12 ± 0.40 | 0.197 |
Abbreviations: SOD: superoxide dismutase; GSH-Px: glutathione peroxidase; T-AOC: total antioxidant capacity; MDA: malondialdehyde; CON: control group, basal diet; CTC: basal diet supplemented with 50 mg/kg Chlortetracycline; PHM: basal diet supplemented with 1000 mg/kg polyherbal mixtures.
Means within a row with no common superscript differ significantly (P < 0.05). Data is presented in mean ± SD (n = 6).
Effect of PHM on the Intestinal Morphology
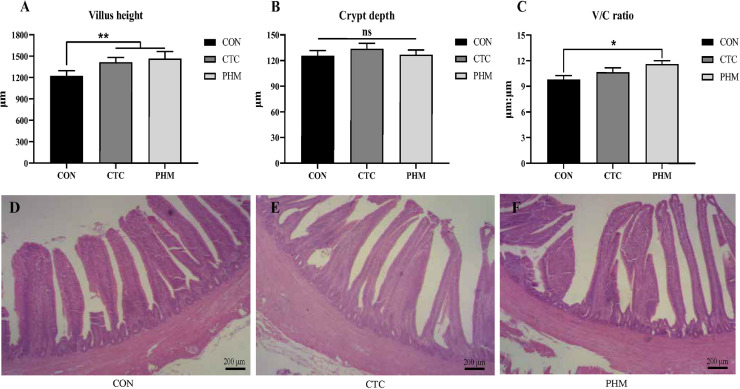
The influence of dietary PHM supplementation on intestinal morphology of broilers at 42 d was shown in Figure 1. The villus height of the jejunum in broilers from CTC and PHM group increased significantly compared to the CON group (P < 0.01). The ratio of jejunum villus height to crypt depth of the jejunum from PHM group had a significant increase compared to the CON group (P < 0.05).
Figure 1.
Effects of dietary PHM supplementation on the jejunum morphology of broilers on d 42. (A) jejunum villus height; (B) jejunum crypt depth; (C) V/C ratio; (D–F) jejunum microvillus morphology of CON, CTC, and PHM groups. Bars represent mean values ± SD (n = 6). *indicates a significant difference at P < 0.05, and ** indicates P < 0.01; ns represents no significant difference.
Effect of PHM on Jejunal Gene Expression Levels
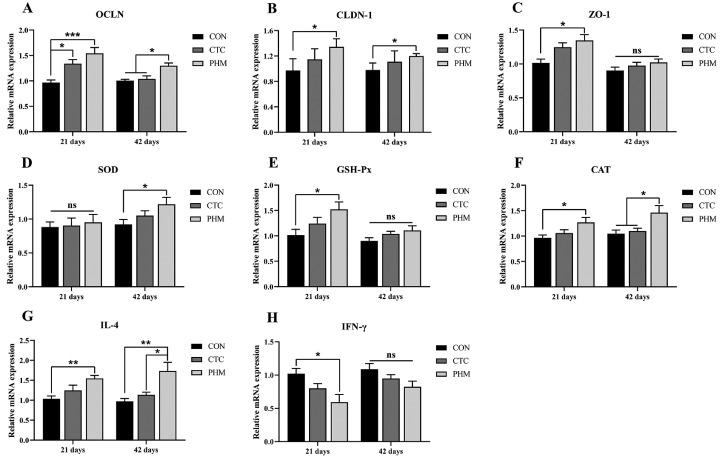
The gene expression data for dietary PHM supplementation on mRNA abundance of antioxidant, jejunum inflammatory cytokines, and tight junction genes in the jejunum were summarized in Figure 2. At 21 d of age, compared with the CON group, dietary supplementation of the PHM significantly up-regulated the mRNA expressions of OCLN (P < 0.001), CLDN-1, ZO-1, GSH-Px, and CAT (P < 0.05), and IL-4 (P < 0.01), whereas decreased the mRNA expression of IFN-γ (P < 0.05). At 42 d of age, compared with the CON group, the CTC and PHM groups increased the mRNA expressions of OCLN and CAT (P < 0.05); however, the PHM group increased the mRNA expressions of IL-4 (P < 0.05) when compared with the CON group. Additionally, the mRNA expressions of CLDN-1 (P < 0.05), SOD (P < 0.05) and IL-4 (P < 0.01) in PHM group were higher than that of the CON group at d 42.
Figure 2.
Effects of dietary PHM supplementation on the gene expression of jejunum cytokines, jejunal barrier genes, and jejunum antioxidant-related factors in broilers. (A–C) Jejunal barrier gene expression levels (OCLN, occludin; CLDN-1, claudin 1; ZO-1, zonula occludens-1); (D–F) jejunum antioxidant-related factors (SOD, superoxide dismutase; GSH-Px, glutathione peroxidase; CAT, catalase); (G, H) jejunum cytokines (IL-4, interleukin-4; IFN-γ, interferon-γ). Bars represent mean values ± SD (n = 6). *indicates a significant difference at P < 0.05, ** indicates P < 0.01 and *** indicates P < 0.001; ns represents no significant difference.
Effect of PHM on Jejunal Microbiota Diversity and Composition
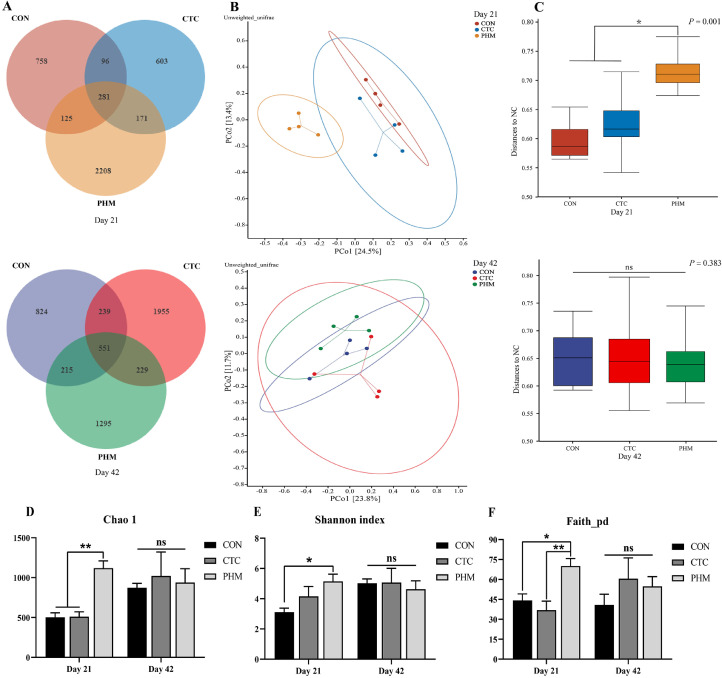
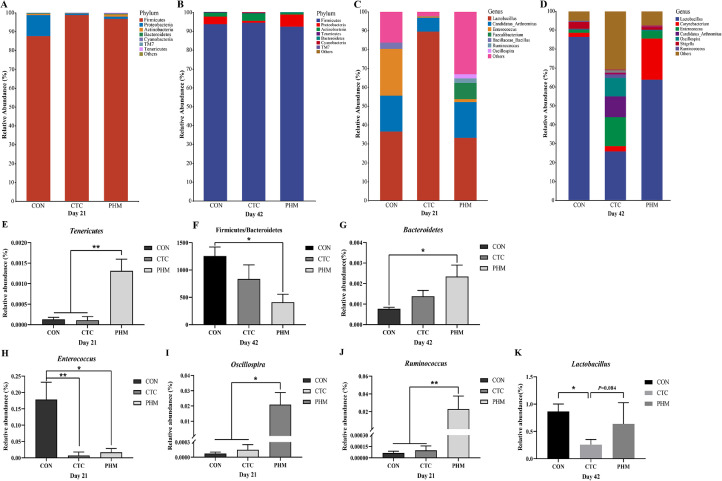
In this study, the generation of a Venn diagram showed that 281 OTUs were found among the 3 groups, and 758, 603, and 2208 specific OTUs were unique to the CON, CTC, and PHM groups on 21-day-old broilers, respectively. On 42 d of age, 824, 1955, and 1295 specific OTUs existed respectively in the CON, CTC, and CHM groups, with 551 OTUs shared (Figure 3A). A principal coordinate analysis (PCoA) was performed to assess similarities and differences among the three groups (Figure 3B). The PCoA results demonstrated that distinct clusters of jejunal microbial composition among the three groups. PERMANOVA analysis based on unweighted unifrac distance was performed to quantify the differences in species diversity. The data revealed that PHM significantly altered the β diversity index compared to the CON group on d 21, whereas there was no obvious difference from 3 groups on d 42 (Figure 3C). We employed Chao 1, Faith_pd, and Shannon indices to assess the alpha diversity of gut microbiota (Figure 3D–F). At 21 d of age, compared with the CON and CTC groups, the PHM group significantly increased Chao 1 index (P < 0.01) and Faith_pd (P < 0.05, P < 0.01); in addition, the PHM group increased Shannon index (P < 0.05) when compared with the CON group. Next, we analyzed the most dominant microbiota at phylum levels at the phylum level to assess the overall gut microbiota composition shift among 3 groups at 21d and 42d (Figures 4A and 4B). The most predominant phyla in jejunal samples of broilers are Firmicutes, Proteobacteria, and Actinobacteria on d 21 and 42. The PHM group has greater relative abundance of Tenericutes (P < 0.05) than the CON and CTC group on 21 d (Figure 4E). At 42 d, the Firmicutes-to-Bacteroidetes ratio was significantly reduced (P < 0.05) in the CHM group on d 42 (Figure 4F). Additionally, on d 42, dietary supplementation with PHM significantly increased the relative abundance of Bacteroidetes (P < 0.05) compared with CON group (Figure 4G). Moreover, the relative abundance of the 7 predominant genera in each group was analyzed to illustrate the specific changes in the microbial taxa at 21 and 42 d (Figures 4C and 4D). At the genus level of 21 d, the abundance of Ruminococcus and Oscillospira in the PHM group was higher (P < 0.05) than the CON and CTC groups; in addition, less Enterococcus in the CTC (P < 0.01) and PHM (P < 0.05) groups were identified when compared with the CON groups (Figure 4H–J). At the genus level of 42 d, there was an increasing tendency in the relative abundance of Lactobacillus from the PHM group compared with the CTC group (P = 0.084) (Figure 4K).
Figure 3.
Dietary PHM altered the composition and community diversity of jejunal microbiota. (A) Venn diagram between treatments on OTUs level. (B) PCoA analysis based on unweighted unifrac distance. (C) PERMANOVA analysis based on unweighted unifrac distance. (D) Alpha-diversity based on indices of Chao 1, Shannon, and Faith_pd. Bars represent mean values ± SD (n = 4). *indicates a significant difference at P < 0.05, and ** indicates P < 0.01; ns represents no significant difference.
Figure 4.
Alteration of jejunal microbiota on the phylum and genus level. (A, B) The top 7 phyla in the relative abundance of each group. (C, D) Relative abundance of top 7 bacterial genera presents in each group. (E) Relative abundance of Tenericutes. (F) The ratio of Firmicutes/Bacteroidetes. (G) Relative abundance of Bacteroidetes. (H–J) The relative abundance of the Enterococcus, Oscillospira, and Ruminococcus differs significantly among groups. (K) Relative abundance of Lactobacillus. n = 4 pens of broilers. *indicates a significant difference at P < 0.05, and ** indicates P < 0.01; ns represents no significant difference.
Functional Prediction of Jejunum Microbes
In order to predict the functional alterations of microbes in jejunum, PICRUSt was performed to analyze the possible levels of KEGG pathways. On 21-day-old broilers, 40 KEGG pathways were obtained at level 2, with the highest proportion being the translation, amino acid metabolism, biosynthesis of other secondary metabolites, carbohydrate metabolism, energy metabolism, lipid metabolism, metabolism of cofactors and vitamins, metabolism of terpenoids and polyketides, xenobiotics biodegradation and metabolism, and metabolism of other amino acids. There were significantly difference in 37 KEGG pathways of the CTC group (Figure 5A) and 9 KEGG pathways of PHM group (Figure 5C) compared to the CON group (P < 0.05). Significant difference was identified in 38 KEGG pathways (Figure 5B) between the CTC group and the PHM group (P < 0.05). On 42-day-old broilers, 14 KEGG pathways were obtained at level 2, with the highest proportion being the carbohydrate metabolism, energy metabolism, lipid metabolism, and metabolism of cofactors and vitamins. There were significantly difference in 15 KEGG pathways of the CTC group (Figure 5D) and 3 KEGG pathways of PHM group (Figure 5F) compared to the CON group (P < 0.05). One KEGG pathway (Figure 5E) was identified to be significantly different between the CTC group and the PHM group (P < 0.05).
Figure 5.
The differences in level 3 KEGG pathway of the jejunal microbiota. (A–C) Comparison of the functional pathways of microbes among 3 groups at the age of 21 d. (D–F) Comparison of the functional pathways of microbes among 3 groups at the age of 42 d. *indicates a significant difference at P < 0.05, ** indicates P < 0.01 and *** indicates P < 0.001; n = 4 pens of broilers.
Correlation Analysis Between Jejunum Microbe and Different Indicators
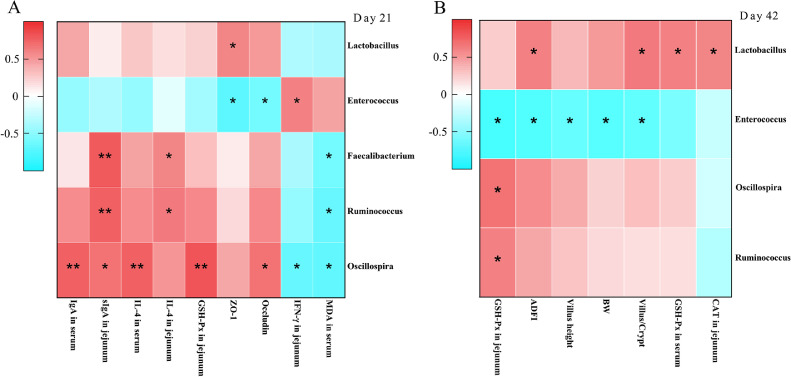
Through Spearman correlation analysis, the relationships of changes in intestinal microflora at the genus level with growth performance, serum biochemistry profile, intestinal integrity, immune-related factors, antioxidant indices, and tight junction proteins were discussed (Figure 6). At 21 d of age (Figure 6A), Correlation analysis revealed that Oscillospira was strongly positively correlated with IgA in serum, IL-4 in serum, and GSH-Px in jejunum (P < 0.01) and sIgA in jejunum and the mRNA expression of occludin (P < 0.05) but negatively correlated with IFN-γ in jejunum and MDA in serum (P < 0.05). The abundance of the genus Faecalibacterium and Ruminococcus were showed highly positive correlations with sIgA in jejunum (P < 0.01) and IL-4 in jejunum (P < 0.05) but negatively correlated with MDA in serum (P < 0.05). The abundance of the genus Enterococcus was positively correlated with IFN-γ in jejunum but negatively correlated with the mRNA expression of occludin, ZO-1 (P < 0.05). The abundance of genus Lactobacillus was showed positive correlation with the mRNA expression of ZO-1 (P < 0.05). At 42 d of age (Figure 6B), the abundance of genus Lactobacillus was showed highly positive correlations with the ADFI, villus/crypt, CAT, and GSH-Px in jejunum (P < 0.05), whereas the abundance of the genus Enterococcus was negatively correlated with ADFI, BW, GSH-Px in jejunum, villus height, and villus/crypt (P < 0.05). In addition, the abundance of genus Ruminococcus and Oscillospira had a positive correlation with the GSH-Px in jejunum (P < 0.05).
Figure 6.
Correlation analysis between jejunal microbiota and different indicators. (A, B) Spearman's correlation analysis of the jejunal microbiota at genus levels with growth performance and intestinal health of broilers. *indicates a significant difference at P < 0.05 and ** indicates P < 0.01; n = 4 pens of broilers.
DISCUSSION
Traditional Chinese herbal medicine contains numerous biologically active compounds, which can provide various nutritional and health benefits to animals, always be used as feed additives in livestock (Abdallah et al., 2019). Accumulating evidence indicates that the herbs and plant extracts in improving the growth performance of poultry and pigs as potential candidates to AGP replacers have been well recognized (Hyun et al., 2018). The PHM (including: Portulaca oleracea L., Radix Sophora flavescens, Thalictrum glandulosissimum, Terra flava usta, and Pogostemon cablin) contain various bioactive compounds, such as flavonoids, polysaccharides, alkaloids, and volatile oils. The synergistic effects of PHM are achieved by applying a pair of herbs with similar therapeutic functions, which have been shown to be more effective in improve the nutrition and well-being of farm animals than single herbal extracts (Xian et al., 2016). Our results showed that broilers fed PHM (Portulaca oleracea L., Radix Sophora flavescens, Thalictrum glandulosissimum, Terra flava usta, and Pogostemon cablin) improved BW, ADG and ADFI, and decreased F/G in broilers. Research evidence has shown that the addition of different proportions of P. oleracea extracts had different effects on the BW and FCR, could promote the growth performance of broilers (Zhao et al., 2013). Isoquinoline alkaloids (IQ), extracted from plant sources, have improved growth rate, feed consumption, and inflammatory status under HS conditions in chickens (Kikusato et al., 2020). Many herbs or their bioactive compounds have been used as supplements and additive, which could promote growth performance in animals (Giannenas et al., 2018). The results we obtained were relatively in agreement with those reported, which also shows that it might be ascribed to these bioactive components from PHM improved overall growth performance in chicken.
As the main immune organs of broilers, the thymus, spleen, and bursa of Fabricius play an important role in host immune response against various pathogenic substances (Seidavi et al., 2017). IgG, IgM, and sIgA could resist the intrusion of a variety of pathogens and toxins. Our results showed that PHM enhanced the immune function indicated by the increased levels of spleen index, the thymus index, serum IgA, IgG, and sIgA in the mucosa of jejunum. Previous studies have reported that Portulaca oleracea L. polysaccharide (POL-P3b) is an immunoregulatory agent, and enhancing the immune efficacy of dendritic cell vaccine for breast cancer (Jia et al., 2021). Berberine has been shown to have antibacterial, anti-inflammatory, and immunoregulatory effects (Anil et al., 2015). Terra flava usta mainly could effectively protect gastrointestinal function and immune functions of PC patients (Wu and Jin, 2021). As before mentioned, commercial CPCMP and traditional Chinese medicine group significantly increased glutathione peroxidase and spleen/bursa indices (Gao et al., 2022). The fish fed 0.5 to 1 mL/kg of herbal essential oils blend had higher blood bio-immunological indices than in control (Magouz et al., 2022). Some studies suggested that sIgA eliminated the pathogens with immune exclusion via nonspecific immunity, and had more extensive protective functions (Blaise, 2013). IgA can activate immune function by regulating the production of TNF and other key cytokines (Hansen et al., 2019). The pleiotropic cytokine IFN-γ exerts antiviral and immunomodulatory functions, which is a potent pro-inflammatory cytokine essential for disease and infection responses (Jorgovanovic et al., 2020). Our study found that PHM supplementation increased serum IL-4, and decreased the levels of serum IFN-γ, and up-regulated the mRNA expression of IL-4 and down-regulated the mRNA expression of IFN-γ. Similar results have been reported in some previous studies, which indicated that PHM could be beneficial to enhance the immunity of broilers. Berberine can regulate the function of macrophages and neutrophils (Yang et al., 2019), coptisine (COP) suppressed the mRNA expression of IFN-γ in the jejunal mucosa (Yuan et al., 2019). Based on the above results, we speculated that PHM could regulate the immune function of broilers. P. oleracea contain organic acids, flavonoids, terpenoids, and alkaloids, and these bioactive compounds may have anti-inflammatory effects that can protect against the generation of the inflammatory response (Jing-Yi et al., 2019). Sophora flavescens was often used alone in the form of raw powder or combined with other herbal medicine and their extracts to treat UC, such as “Kushen tablets” and “Kushen Huanglian wan”, which could inhibit immune-inflammatory response in modern clinical practice (Wu et al., 2021). Based on the above results, we speculated that PHM could regulate the immune function of broilers, which may be attributed to the fact that the synergistic effects of PHM are greater biological efficiency (bioactive compounds) than that of a single herbal medicine.
As is well known, the serum antioxidant indicators like GSH, T-AOC, SOD, GSH-Px, and CAT were regularly applied for playing vital roles in antioxidant defense mechanisms (Pardo et al., 2021). MDA has been shown as a biomarker for oxidative stress, which was a decomposed product of lipid peroxidation (Cheng et al., 2018). In this study, we observed that PHM improved the antioxidative capacities by the increased activity of serum SOD, GSH-Px, and T-AOC (d 42), the decreased content of serum MDA (d 21), the up-regulated mRNA expression of GSH-Px, CAT (d 21), SOD and CAT (d 42). Purslane contains phenolic constituents involving flavonoids, phenolic acids, and alkaloids, which can improve broiler chicken antioxidant status (Ghorbani et al., 2014). This result is in agreement with that of Yang et al. (2019), which reported that berberine inhibits oxidative stress in a variety of tissues by increasing the activity of SOD and GSH-Px but significantly prevented the increase in MDA. A previous study reported that the addition of purslane to the diet significantly increased SOD, catalase (CAT), and glutathione peroxidase activities in the plasma of Nile tilapia (Abdel-Razek et al., 2019). Matrine up-regulated antioxidative enzyme protein expression and enhanced antioxidant activities (Zhang et al., 2013). Therefore, PHM could improve the antioxidant function of broilers due to the five herbs used in PHM contain a variety of active ingredients with antioxidant effects.
The morphological indexes of intestinal villus heights, crypt depths, and V/C ratio at the level of small intestines were commonly used to assess accurately health and functional status in chickens (Wilson et al., 2018). The peripheral membrane protein ZO-1 and the transmembrane protein OCLN were proteins contained in the intestinal barrier, the main mechanism of which was to maintain the integrity of the barrier and mucosal barrier function (Goo et al., 2019). In this study, these results suggested that PHM improved the intestinal epithelial barrier indicated by the up-regulated mRNA expression of CLDN-1, OCLN, and ZO-1, and the increased of villus height and villus height to crypt depth in jejunum (d 42). This is consistent with the results of Giannenas et al. (2018), who found that herbal feed additives could improve the villus height and crypt depth in jejunum. Previous studies have shown that dietary supplementation with 40 mg/kg BBR increased the expression levels of tight junction proteins and the villus height and crypt depth in the jejunum (Tang et al., 2021). Patchouli oil, the major active fraction of Pogostemon cablin, increased the mRNA expression of ZO-1 and occludin, thereby stabilizing intestinal barrier (Gan et al., 2020). COP protects intestinal mucosa by increasing the secretion of mucin and the expression of ZO-1 and occluding (Yuan et al., 2019). Taken together, the above data revealed that PHM supplementation may improve intestinal morphology and regulate barrier function by regulating tight junctions, which may be the reason for the synergistic effects of 5 herbs.
Accumulating evidence indicate that intestinal microbiota contributed to the health status of host animals, which are the first barrier against pathogens from food (Zhu et al., 2002; Zhang et al., 2020). Some studies suggested that diet supplementation with purslane could improve growth performance of broilers by increasing the abundance of Lactobacillus in intestine, modulate the environment of gut microbiota in a beneficial direction, and promoting the metabolism of carbohydrates (Wang et al., 2021). Matrine can increase the relative abundance of beneficial bacteria (Wu et al., 2021). Dietary BBR supplementation improved the growth performance of yellow-feathered broilers, and was closely related to the significant changes in cecal microbiota composition (Zhu et al., 2020). Therefore, we hypothesized that the great significance for the prevention and growth performance of broilers by dietary supplementation with PHM might be related to its modulation of the composition and structure of the gut microbiota. In the present study, the results of altered alpha- and beta-diversity indices showed that PHM significantly changed the diversity of gut microbiota at an early age. Modulatory effects of PHM on beta-diversity were consistent from different studies (Wang et al., 2021), which indicated that dietary PHM may have an effective on the abundance and diversity of the gut microbiota. The results demonstrated that Firmicutes, Proteobacteria, and Actinabactieria were the most predominant phyla in gut bacterial communities of three groups, which was in accordance with previous results (Wei et al., 2013). The Firmicutes/Bacteroidetes ratio in the PHM group was lower than that in the CON group. Modulatory effects of PHM on F/B ratio were consistent from different studies (Sun et al., 2022), which plant polysaccharides decreasing the ratio of F/B, thereby regulating furfural degradation and antibiotic biosynthesis. BBR treatment also altered gut microbiota composition, which reduced the F/B ratio and increased the abundances of Lactobacillus (Wang et al., 2022). Our study found that dietary PHM supplementation increased the relative abundance of Oscillospira and Ruminococcus (d 21) and Lactobacillus (d 42) and decreased the relative abundance of Enterococcus (d 21).At the same time, BBR could inhibit the growth of potential harmful bacteria, such as reducing the counts of Enterococcus spp. (Liu et al., 2018) abundance, while increased the proportion of genera Lactobacillus and Ruminococcus in rats (Chen et al., 2020). Similarly, matrine administration significantly increased the proportion of beneficial bacteria such as Ruminococcaceae (Wu et al., 2021). Ruminococcaceae could be capable of generating butyrate and other SCFA, such as acetic and succinic acids, which could inhibit the growth and reproduction of pathogenic intestinal bacteria (Biasato et al., 2019). Our findings indicated that supplement with PHM to the diet had an inhibitory effect on pathogenic bacteria like Enterococcus while increasing the relative abundance of beneficial bacteria like Lactobacillus, Oscillospira, and Ruminococcus.
PICRUST results showed that the jejunal microbiota of broilers supplemented PHM showed an enriched in metabolic pathways of carbohydrate, energy, lipid, cofactors, and vitamins, which indirectly reflected the microorganisms participate in the digestion and absorption of nutrients in the broiler intestine. These findings are in accordance with the previous reports that bacteria were mainly enriched in the carbohydrate metabolism pathway by purslane (Wang et al., 2021). The present study has identified several key jejunal bacteria such as Ruminococcus, Faecalibacterium, Oscillospira, and Lactobacillus associated with improved growth performance and immunity and antioxidant capacities in yellow-feathered broilers. Furthermore, on d 42, the relative abundance of Ruminococcus and Oscillospira were both positively associated with higher sIgA and IL-4 as improved by dietary PHM. Specially, our studies indicated that the abundance of Lactobacillus were showed highly positive correlations with ZO-1 (d 21), ADFI, and VH/CD (d 42). This was in accordance with a recent demonstration that the proportion of Lactobacillus was positively related to ADFI outcomes in broilers (Chen and Yu, 2020). These results indicated that feeding PHM to broiler chickens enhances growth performance and immune function by modulating jejunal microbiota, which might be related to the improvement of the intestinal health.
CONCLUSIONS
In conclusion, the results in our study demonstrated that dietary supplementation with PHM (Portulaca oleracea L., Radix Sophora flavescens, Thalictrum glandulosissimum, Terra flava usta, and Pogostemon cablin) significantly improved growth performance, antioxidant capacities, immune function, intestinal morphology, and intestinal barrier function of yellow-feathered broilers. The beneficial effects of PHM on broilers may be associated with the increased Oscillospira, Ruminococcus, and Lactobacillus abundance. These findings could provide further evidence that PHM may as an alternative to antibiotics for poultry production, and further studies are needed to maximize these positive effects with herbal formulations.
ACKNOWLEDGMENTS
This work was supported by the R & D Projects in important areas of Guangdong Province, Studies and Applications of Key Technology Biosynthesis in Antibiotic-Free Feeds, 2019B020218003.
DISCLOSURES
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2023.102714.
Appendix. Supplementary materials
REFERENCES
- Abdallah A., Zhang P., Zhong Q., Sun Z. Application of traditional Chinese herbal medicine by-products as dietary feed supplements and antibiotic replacements in animal production. Curr. Drug Metab. 2019;20:54–64. doi: 10.2174/1389200219666180523102920. [DOI] [PubMed] [Google Scholar]
- Abdel-Razek N., Awad S.M., Abdel-Tawwab M. Effect of dietary purslane (Portulaca oleracea L.) leaves powder on growth, immunostimulation, and protection of Nile tilapia, Oreochromis niloticus against Aeromonas hydrophila infection. Fish Physiol. Biochem. 2019;45:1907–1917. doi: 10.1007/s10695-019-00685-8. [DOI] [PubMed] [Google Scholar]
- Abo G.M.M., Abd E.M.E., Othman S.I., Taha A.E., Allam A.A., Eid A.A. Impact of different rearing systems on growth, carcass traits, oxidative stress biomarkers and humoral immunity of broilers exposed to heat stress. Poult. Sci. 2020;99:3070–3078. doi: 10.1016/j.psj.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anil K., Ekavali C.K., Madhurima M., Raghavender P., Dinesh K.D. Current knowledge and pharmacological profile of berberine: an update. Eur. J. Pharmacol. 2015;761:288–297. doi: 10.1016/j.ejphar.2015.05.068. [DOI] [PubMed] [Google Scholar]
- AOAC . International: Official Methods of Analysis of International. 18th Ed. AOAC International; Gaithersburg, MD: 2006. [Google Scholar]
- Ayalew H., Zhang H., Wang J., Wu S., Qiu K., Qi G., Tekeste A., Wassie T., Chanie D. Potential feed additives as antibiotic alternatives in broiler production. Front. Vet. Sci. 2022;9 doi: 10.3389/fvets.2022.916473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biasato I., Ferrocino I., Grego E., Dabbou S., Gai F., Gasco L., Cocolin L., Capucchio M.T., Schiavone A. Gut microbiota and Mucin composition in female broiler chickens fed diets including yellow mealworm (Tenebrio molitor, L.) Animals (Basel) 2019;9:213. doi: 10.3390/ani9050213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaise E. Multi-faceted functions of secretory IgA at mucosal surfaces. Front. Immunol. 2013;4:185. doi: 10.3389/fimmu.2013.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Zhang F., Li R., Liu Y., Wang X., Zhang X., Xu C., Li Y., Guo Y., Yao Q. Berberine regulates fecal metabolites to ameliorate 5-fluorouracil induced intestinal mucositis through modulating gut microbiota. Biomed. Pharmacother. 2020;124 doi: 10.1016/j.biopha.2020.109829. [DOI] [PubMed] [Google Scholar]
- Chen L., Huang G. Antioxidant activities of sulfated pumpkin polysaccharides. Int. J. Biol. Macromol. 2019;126:743–746. doi: 10.1016/j.ijbiomac.2018.12.261. [DOI] [PubMed] [Google Scholar]
- Chen M., Ding Y., Tong Z. Efficacy and safety of Sophora flavescens (Kushen) based traditional Chinese medicine in the treatment of ulcerative colitis: clinical evidence and potential mechanisms. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.603476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.C., Yu Y.H. Bacillus licheniformis-fermented products improve growth performance and the fecal microbiota community in broilers. Poult. Sci. 2020;99:1432–1443. doi: 10.1016/j.psj.2019.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y.F., Chen Y.P., Wen C., Wang W.B., Wang A.Q., Zhou Y.M. Evaluation of dietary palygorskite supplementation on growth performance, mineral accumulations, antioxidant capacities, and meat quality of broilers fed lead-contaminated diet. Biol. Trace Elem. Res. 2018;181:314–322. doi: 10.1007/s12011-017-1047-6. [DOI] [PubMed] [Google Scholar]
- Dibner J.J., Richards J.D. Antibiotic growth promoters in agriculture: history and mode of action. Poult. Sci. 2005;84:634–643. doi: 10.1093/ps/84.4.634. [DOI] [PubMed] [Google Scholar]
- Gan Y., Ai G., Wu J., Luo H., Chen L., Huang Q., Wu X., Xu N., Li M., Su Z., Liu Y., Huang X. Patchouli oil ameliorates 5-fluorouracil-induced intestinal mucositis in rats via protecting intestinal barrier and regulating water transport. J. Ethnopharmacol. 2020;250 doi: 10.1016/j.jep.2019.112519. [DOI] [PubMed] [Google Scholar]
- Gao J., Wang R., Liu J., Wang W., Chen Y., Cai W. Effects of novel microecologics combined with traditional Chinese medicine and probiotics on growth performance and health of broilers. Poult. Sci. 2022;101:4–12. doi: 10.1016/j.psj.2021.101412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghorbanali S., Ahmad K., Foad S., Asaad V., Danial F. The effects of purslane (Portulaca oleracea L.) powder on growth performance, carcass characteristics, antioxidant status, and blood metabolites in broiler chickens. Livest. Sci. 2016;184:35–40. [Google Scholar]
- Ghorbani R.M., Bojarpur M., Mayahi M., Fayazi J., Fatemitabatabaei R., Tabatabaei S., Idrus Z. Effects of purslane extract on performance, immunity responses and cecal microbial population of broiler chickens. Span. J. Agric. Res. 2014;12:4. [Google Scholar]
- Giannenas I., Bonos E., Skoufos I., Tzora A., Stylianaki I., Lazari D., Tsinas A., Christaki E., Florou-Paneri P. Effect of herbal feed additives on performance parameters, intestinal microbiota, intestinal morphology and meat lipid oxidation of broiler chickens. Br. Poult. Sci. 2018;59:545–553. doi: 10.1080/00071668.2018.1483577. [DOI] [PubMed] [Google Scholar]
- Goo D., Kim J.H., Choi H.S., Park G.H., Han G.P., Kil D.Y. Effect of stocking density and sex on growth performance, meat quality, and intestinal barrier function in broiler chickens. Poult. Sci. 2019;98:1153–1160. doi: 10.3382/ps/pey491. [DOI] [PubMed] [Google Scholar]
- Hansen I.S., Baeten D., den Dunnen J. The inflammatory function of human IgA. Cell Mol. Life Sci. 2019;76:1041–1055. doi: 10.1007/s00018-018-2976-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holleran G., Scaldaferri F., Gasbarrini A., Currò D. Herbal medicinal products for inflammatory bowel disease: a focus on those assessed in double-blind randomised controlled trials. Phytother Res. 2020;34:77–93. doi: 10.1002/ptr.6517. [DOI] [PubMed] [Google Scholar]
- Hu Q.F., Wu F., Zhu Y.N., Liu L., Liu M.X., Cai B.B., Li M.F., Miao D., Zhou M., Yang G.Y. Three new anti-rotavirus quinoline alkaloids from the whole plant of Thalictrum glandulosissimum. Heterocycles. 2021;102:1588–1594. [Google Scholar]
- Hyun L., Yanhong L., Sergio C., Mariano E.F., Fang C., Ron L.C., Sungtaek O., Cyril G.G. Phytochemicals as antibiotic alternatives to promote growth and enhance host health. Vet. Res. 2018;49:76. doi: 10.1186/s13567-018-0562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia G., Shao X., Zhao R., Zhang T., Zhou X., Yang Y., Li T., Chen Z., Liu Y. Portulaca oleracea L. polysaccharides enhance the immune efficacy of dendritic cell vaccine for breast cancer. Food Funct. 2021;12:4046–4059. doi: 10.1039/d0fo02522d. [DOI] [PubMed] [Google Scholar]
- Jing-Yi Q., Han-Wei L., Fu-Gang L., Yu-Cheng L., Shuo T., Li-Hua C., Kai H., Xiang-Xiang W., Ming-San M., Chingfeng W. Effects of portulaca oleracea extract on acute alcoholic liver injury of rats. Molecules. 2019;24:2887. doi: 10.3390/molecules24162887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgovanovic D., Song M., Wang L., Zhang Y. Roles of IFN-gamma in tumor progression and regression: a review. Biomark. Res. 2020;8:49. doi: 10.1186/s40364-020-00228-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juan M.D.C., Natalia A.C., Mariano E.F.M. Microbiota, gut health and chicken productivity: what is the connection? Microorganisms. 2019;7:374. doi: 10.3390/microorganisms7100374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junren C., Xiaofang X., Mengting L., Qiuyun X., Cheng P. Pharmacological activities and mechanisms of action of Pogostemon cablin Benth: a review. Chin. Med. UK. 2021;16:1. doi: 10.1186/s13020-020-00413-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikusato M., Xue G., Pastor A., Niewold T.A., Toyomizu M. Effects of plant-derived isoquinoline alkaloids on growth performance and intestinal function of broiler chickens under heat stress. Poult. Sci. 2020;100:957–963. doi: 10.1016/j.psj.2020.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleyn F.J., Ciacciariello M. Future demands of the poultry industry: will we meet our commitments sustainably in developed and developing economies? World Poult. Sci. J. 2021;77:267–278. [Google Scholar]
- Li Y. South China Agricultural University; Guangzhou, China: 2021. Effects of Dietary Supplement With a Chinese Herbal Mixture on Growth Performance, Immune Function, and Gut Microbiota in Yellow-Feathered Broilers. Master's Thesis. [Google Scholar]
- Liu D., Zhang Y., Liu Y., Hou L., Li S., Tian H., Zhao T. Berberine modulates gut microbiota and reduces insulin resistance via the TLR4 signaling pathway. Exp. Clin. Endocrinol. Diabetes. 2018;126:513–520. doi: 10.1055/s-0043-125066. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Magouz F.I., El-Din M., Amer A.A., Gewaily M.S., El-Dahdoh W.A., Dawood M. A blend of herbal essential oils enhanced the growth performance, blood bio-immunology traits, and intestinal health of Nile tilapia (Oreochromis niloticus) Ann. Anim. Sci. 2022;22:751–761. [Google Scholar]
- Mahmood H., Ghorbanali S., Ahmad K. Comparative effects of powder, aqueous and methanolic extracts of purslane (Portulaca oleracea L.) on growth performance, antioxidant status, abdominal fat deposition and plasma lipids in broiler chickens. Anim. Prod. Sci. 2019;59:89–100. [Google Scholar]
- Melaku M., Zhong R., Han H., Wan F., Yi B., Zhang H. Butyric and citric acids and their salts in poultry nutrition: effects on gut health and intestinal microbiota. Int. J. Mol. Sci. 2021;22:10392. doi: 10.3390/ijms221910392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo Z., Fernandez-Figares I., Lachica M., Lara L., Nieto R., Seiquer I. Impact of heat stress on meat quality and antioxidant markers in Iberian pigs. Antioxidants (Basel) 2021;10:1911. doi: 10.3390/antiox10121911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petracci M., Mudalal S., Soglia F., Cavani C. Meat quality in fast-growing broiler chickens. Worlds Poult. Sci. J. 2015;71:363–374. [Google Scholar]
- Seidavi A., Dadashbeiki M., Alimohammadi-Saraei M., van den Hoven R., Payan-Carreira R., Laudadio V., Tufarelli V. Effects of dietary inclusion level of a mixture of probiotic cultures and enzymes on broiler chicken immunity response. Environ. Sci. Pollut. Res. Int. 2017;24:4637–4644. doi: 10.1007/s11356-016-8206-8. [DOI] [PubMed] [Google Scholar]
- Sun Q., Xu W., Liu Y., Zhan S., Shao X., Wu Z., Weng P., Cheng K., Zhang X. Single-cell transcriptomic analysis demonstrates the regulation of peach polysaccharides on circadian rhythm disturbance. Mol. Nutr. Food Res. 2022;66 doi: 10.1002/mnfr.202101170. [DOI] [PubMed] [Google Scholar]
- Tang M., Yuan D., Liao P. Berberine improves intestinal barrier function and reduces inflammation, immunosuppression, and oxidative stress by regulating the NF-kappaB/MAPK signaling pathway in deoxynivalenol-challenged piglets. Environ. Pollut. 2021;289 doi: 10.1016/j.envpol.2021.117865. [DOI] [PubMed] [Google Scholar]
- Wang C., Liu Q., Ye F., Tang H., Xiong Y., Wu Y., Wang L., Feng X., Zhang S., Wan Y., Huang J. Dietary purslane (Portulaca oleracea L.) promotes the growth performance of broilers by modulation of gut microbiota. Amb. Express. 2021;11:31. doi: 10.1186/s13568-021-01190-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Wu F., Zhou Q., Qiu Y., Zhang J., Tu Q., Zhou Z., Shao Y., Xu S., Wang Y., Tao J. Berberine improves vascular dysfunction by inhibiting trimethylamine-N-oxide via regulating the gut microbiota in angiotensin II-induced hypertensive mice. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.814855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S., Morrison M., Yu Z. Bacterial census of poultry intestinal microbiome. Poult. Sci. 2013;92:671–683. doi: 10.3382/ps.2012-02822. [DOI] [PubMed] [Google Scholar]
- Wilson F.D., Cummings T.S., Barbosa T.M., Williams C.J., Gerard P.D., Peebles E.D. Comparison of two methods for determination of intestinal villus to crypt ratios and documentation of early age-associated ratio changes in broiler chickens. Poult. Sci. 2018;97:1757–1761. doi: 10.3382/ps/pex349. [DOI] [PubMed] [Google Scholar]
- Wu H., Chen Q., Liu J., Chen X., Luo H., Ye Z., Liu J. Microbiome analysis reveals gut microbiota alteration in mice with the effect of matrine. Microb. Pathogenesis. 2021;156:104926. doi: 10.1016/j.micpath.2021.104926. [DOI] [PubMed] [Google Scholar]
- Wu H., Chen Q., Wang W., Chu S., Liu X., Liu Y., Tan C., Zhu F., Deng S., Dong Y., Yu T., Gao F., He H., Leng X., Fan H. Compound sophorae decoction enhances intestinal barrier function of dextran sodium sulfate induced colitis via regulating notch signaling pathway in mice. Biomed. Pharmacother. 2021;133 doi: 10.1016/j.biopha.2020.110937. [DOI] [PubMed] [Google Scholar]
- Wu T., Jin T. Prevention and treatment of digestive tract issues induced by postoperative adjuvant chemotherapy for pancreatic cancer using Terra Flava Usta. Am. J. Transl. Res. 2021;13:3182–3189. [PMC free article] [PubMed] [Google Scholar]
- Xian Z., Sai W.S., Dennis C., Hosen K., Hosen K., Hosen K., Valentina R., Kelvin C., Kelvin C., Kelvin C., Alan B. Synergistic effects of Chinese herbal medicine: a comprehensive review of methodology and current research. Front. Pharmacol. 2016;7:201. doi: 10.3389/fphar.2016.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Q., Hu S., Ligaba-Osena A., Yang J., Tong F., Guo W. Seasonal variation in transcriptomic profiling of Tetrastigma hemsleyanum fully developed Tuberous roots enriches candidate genes in essential metabolic pathways and phytohormone signaling. Front. Plant. Sci. 2021;12 doi: 10.3389/fpls.2021.659645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Liu G., Liang X., Wang M., Zhu X., Luo Y., Shang Y., Yang J.Q., Zhou P., Gu X.L. Effects of berberine on the growth performance, antioxidative capacity and immune response to lipopolysaccharide challenge in broilers. Anim. Sci. J. 2019;90:1229–1238. doi: 10.1111/asj.13255. [DOI] [PubMed] [Google Scholar]
- Yuan Z., Yang L., Zhang X., Ji P., Hua Y., Wei Y. Huang-Lian-Jie-Du decoction ameliorates acute ulcerative colitis in mice via regulating NF-kappaB and Nrf2 signaling pathways and enhancing intestinal barrier function. Front. Pharmacol. 2019;10:1354. doi: 10.3389/fphar.2019.01354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Li C.X., S Q., Chen W.B., Ma L., Xu W.H., Li Y.X., Huang S.C., Ma Y.B. Effects of glycyrrhiza polysaccharide in diet on growth performance, serum antioxidant capacity, and biochemistry of broilers. Poult. Sci. 2020;100:3. doi: 10.1016/j.psj.2020.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.F., Shi L.J., Song G.Y., Cai Z.G., Wang C., An R.J. Protective effects of matrine against progression of high-fructose diet-induced steatohepatitis by enhancing antioxidant and anti-inflammatory defences involving Nrf2 translocation. Food Chem. Toxicol. 2013;55:70–77. doi: 10.1016/j.fct.2012.12.043. [DOI] [PubMed] [Google Scholar]
- Zhao Q., Luan X., Zheng M., Tian X.H., Zhao J., Zhang W.D., Ma B.L. Synergistic mechanisms of constituents in Herbal Extracts during intestinal absorption: focus on natural occurring nanoparticles. Pharmaceutics. 2020;12:128. doi: 10.3390/pharmaceutics12020128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X.H., He X., Yang X.F., Zhong X.H. Effect of Portulaca oleracea extracts on growth performance and microbial populations in ceca of broilers. Poult. Sci. 2013;92:1343–1347. doi: 10.3382/ps.2012-02434. [DOI] [PubMed] [Google Scholar]
- Zhou T., Gao Q., Mi Q.L., Yang F.X., Wu F., Zhu Y.N., Liu L., Li J., Liu X., Kong W.S., Yang G.Y., Hu Q.F., Li X.M. Two new benzo[C]azepin-1-ones from whole plants of Thalictrum glandulosissimum and their antibacterial activity. Chem. Nat. Compd. 2022;58:254–257. [Google Scholar]
- Zhu C., Huang K., Bai Y., Feng X., Gong L., Wei C., Huang H., Zhang H. Dietary supplementation with berberine improves growth performance and modulates the composition and function of cecal microbiota in yellow-feathered broilers. Poult. Sci. 2020;100:1034–1048. doi: 10.1016/j.psj.2020.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X.Y., Zhong T., Pandya Y., Joerger R.D. 16S rRNA-based analysis of microbiota from the cecum of broiler chickens. Appl. Environ. Microb. 2002;68:124–137. doi: 10.1128/AEM.68.1.124-137.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.