Abstract
Male C57BL/6N mice exposed to the chronic subordinate colony housing (CSC; 19 days) paradigm, a preclinically validated model of chronic psychosocial stress, are characterized by unaffected basal morning plasma corticosterone (CORT) concentrations despite adrenal and pituitary hyperplasia and increased adrenocorticotropic hormone (ACTH) plasma concentrations, compared with single-housed control (SHC) mice. However, as CSC mice are still able to show an increased CORT secretion towards novel heterotypic stressors, these effects might reflect an adaptation rather than a functional breakdown of general hypothalamus-pituitary-adrenal (HPA) axis functionality. In the present study we used male mice of a genetically modified mouse line, to investigate whether genetically-driven ACTH overexpression compromises adaptational processes occurring at the level of the adrenals during CSC exposure. Experimental mice carried a point mutation in the DNA binding domain of the glucocorticoid (GC) receptor (GR), attenuating dimerization of GR (GRdim), resulting in a congenially compromised negative feedback inhibition at the level of the pituitary.
In line with previous studies, CSC mice in both the wild type (WT; GR+/+) and GRdim group developed adrenal enlargement. Moreover, compared with respective SHC and WT mice, CSC GRdim mice show increased basal morning plasma ACTH and CORT concentrations. Quantitative polymerase chain reaction (qPCR) analysis revealed neither a genotype effect, nor a CSC effect on pituitary mRNA expression of the ACTH precursor proopiomelanocortin (POMC). Finally, CSC increased anxiety-related behavior, active coping and splenocyte in vitro (re)activity in both WT and GRdim mice, while a CSC-induced increase in adrenal lipid vesicles and splenic GC resistance was detectable only in WT mice. Of note, lipopolysaccharide (LPS)-stimulated splenocytes of GRdim mice were resistant to the inhibitory effects of CORT.
Together our findings support the hypothesis that pituitary ACTH protein concentration is negatively controlled by GR dimerization under conditions of chronic psychosocial stress, while POMC gene transcription is not dependent on intact GR dimerization under both basal and chronic stress conditions. Finally, our data suggest that adrenal adaptations during chronic psychosocial stress (i.e., ACTH desensitization), aiming at the prevention of prolonged hypercorticism, are protective only to a certain threshold of plasma ACTH levels.
Keywords: Chronic subordinate colony housing (CSC), Chronic psychosocial stress, Glucocorticoid receptor (GR) dimerization, Negative feedback
1. Introduction
While the underlying aetiology of most stress-associated diseases is not fully understood, chronic stress-induced dysregulation of almost all psycho-neuro-immunological systems, including the hypothalamus-pituitary-adrenal (HPA) axis, are likely to contribute to the complex, and multifactorial, aetiology of such disorders. Interestingly, both hyper- and hypoactivity of the HPA axis have been reported for patients diagnosed with stress-associated disorders, suggesting that stress-induced dysregulations in either direction are able to compromise mental and physical health (Langgartner et al., 2015; Raison and Miller, 2003; Holsboer, 2000, 2001; Heim et al., 2000; Fries et al., 2005; Pariante and Miller, 2001; Reber et al., 2016a). For instance, while major depression is most consistently reported to be associated with a hyperactive HPA axis, posttraumatic stress disorder (PTSD) is rather linked to a reduced HPA axis activity. In detail, patients with major depression exhibit increased concentrations of cortisol in plasma, urine, and cerebrospinal fluid (CSF), an exaggerated cortisol response to adrenocorticotropic hormone (ACTH) challenge and an enlargement of both the pituitary and the adrenal glands, all of which are likely mediated by a compromised glucocorticoid (GC) receptor (GR) sensitivity and negative feedback inhibition of the HPA axis (Holsboer, 1983, 2000, 2001; Pariante and Miller, 2001; Gold et al., 1988; Holsboer and Barden, 1996; Nemeroff, 1996; Owens and Nemeroff, 1993; Bardeleben and Holsboer, 1989). In contrast, PTSD patients typically show decreased baseline and 24h average plasma cortisol concentrations, probably mediated by an increased negative feedback sensitivity of the HPA axis (Lehrner et al., 2016; Morris et al., 2012; de Kloet et al., 2006). The latter is indicated by an enhanced cortisol suppression after administration of the synthetic GC dexamethasone (DEX) in PTSD patients versus healthy controls (de Kloet et al., 2006).
To investigate the psycho-neuro-immunological mechanisms underlying stress-related disorders, in particular PTSD, our laboratory employs the chronic subordinate colony housing (CSC) paradigm, which was extensively characterized over the past 20 years as animal model resembling a PTSD-like phenotype (Reber et al., 2016a). The CSC paradigm is based on chronic subordination (19 days) of four male mice towards a dominant male conspecific (Langgartner et al., 2015; Reber et al., 2016a). Compared to respective single-housed control (SHC) mice, CSC mice develop general- and social anxiety (Amoroso et al., 2020; Langgartner et al., 2017; Slattery et al., 2012), increased alcohol consumption/preference (Peters et al., 2013), hyperactivity (Slattery et al., 2012), spontaneous colitis (Langgartner et al., 2017; Reber et al., 2007, 2011, 2016b), and an aggravated dextran sulfate sodium (DSS)-induced colitis (Reber et al., 2008, 2016b; Amoroso et al., 2019). CSC mice further develop functional splenic in vitro GC resistance as well as splenomegaly when receiving a significant amount of bite wounds during CSC exposure or when undergoing abdominal surgery prior to CSC (Foertsch et al., 2017; Foertsch and Reber, 2020; Kempter et al., 2021).
In addition, CSC mice show a higher pituitary weight, accompanied by increased numbers of ACTH immunoreactive cells, which despite an increased DEX suppression of stress-induced ACTH, results in elevated basal and stress-induced plasma ACTH concentrations (Füchsl et al., 2013). Interestingly, as CSC is associated with unaffected basal plasma morning corticosterone (CORT) concentrations and basal evening hypocorticism, despite significant enlargement of the adrenal glands (Langgartner et al., 2017; Reber et al., 2007), increased basal plasma ACTH concentrations do not translate into increased plasma CORT levels. This is in line with the fact that adrenal explants of CSC mice show a decreased in vitro sensitivity to ACTH (Langgartner et al., 2017; Reber et al., 2007; Uschold-Schmidt et al., 2012). However, as CSC mice show an increased CORT secretion towards novel heterotypic stressors (Uschold-Schmidt et al., 2012), which is in line with what has been reported for PTSD patients (de Kloet et al., 2006), unaffected/lower basal plasma CORT levels in CSC mice and PTSD patients might reflect a beneficial adaptation in the face of adrenal enlargement and increased adrenal ACTH input rather than a functional breakdown of general HPA axis functionality. In support of the latter, CSC mice do not show any signs of depressive-like behavior/passive coping in the tail-suspension test (TST) and the forced-swim test (FST) (Slattery et al., 2012), typically seen in rats as a consequence of chronic CORT administrations (Donner et al., 2011). Importantly, it has not been investigated so far whether 1) the adaptive reduction of adrenal ACTH sensitivity in CSC mice showing a PTSD-like phenotype and no signs of depressive-like behavior is limited to a certain extent of ACTH overstimulation, and 2) whether overcoming these adaptational processes at the levels of the adrenal glands not only translates into increased plasma CORT levels but also development of depressive-like behavior/passive coping.
Glucocorticoids act through nuclear hormone receptors including the GR. The GR acts as a homodimer/monomer that either directly binds to specific DNA motives (Lim et al., 2015; Schiller et al., 2014) or interacts with other transcription factors at enhancer sites, resulting in tethering to other transcription factors (Vettorazzi et al., 2022). Based on an increased POMC mRNA expression and ACTH immunoreactivity in the anterior pituitary (Reichardt et al., 1998) in mice with an attenuated, albeit not completely abrogated GR dimerization (GRdim/dim mice; GRdim), it has been proposed that the negative feedback regulation at the level of the pituitary requires an intact GR dimerization interface (Reichardt et al., 1998).
In the current study we combine the GRdim mouse model with the CSC paradigm to assess whether the CSC-induced increased of pituitary and plasma ACTH concentrations can be further exaggerated by a genetically compromised pituitary negative feedback inhibition, finally resulting in plasma ACTH levels exceeding the adaptive capacities at the level of the adrenal glands and, consequently, resulting in prolonged hypercorticism.
2. Material and methods
2.1. Animals
Male GRDim (Sv129Ev/B6 F1 hybrids) (GVO) mice generated by AG Tuckermann (Ulm University, Ulm, Germany) and characterized by a globally and congenitally attenuated, albeit not completely abrogated GR dimerization (GRdim/dim mice; GRdim) were used as experimental mice. Heterozygous GRdim/WT mice were intercrossed to generate GR+/+ (WT) and GRdim/dim (GRdim) homozygous mutant mice and the offspring was genotyped by PCR on genomic DNA isolated from tail biopsies (as described in (Reichardt et al., 1998) with minor modifications). For the first five weeks after birth mice were kept under standard laboratory conditions (14h light/10h dark cycle, 22 °C, 60% humidity). Afterwards, experimental mice were transferred to an experimental room with a different type of standard laboratory conditions (12h light/12h dark cycle, 22 °C, 60% humidity) and kept single-housed until the start of the experiment. CD-1 mice (30–35g, Charles River, Sulzfeld, Germany) were used as dominant aggressors. All mice were kept in standard polycarbonate mouse cages (16 × 22 × 14 cm) and had free access to tap water and standard mouse diet. The animal study was carried out in accordance with the ARRIVE guidelines 2.0 (Percie du Sert et al., 2020) and was approved by the Federal Animal Care and Use Committee (Regierungspräsidium Tübingen, Germany). All efforts have been made to minimize the number of animals and their suffering.
2.2. Experimental procedures
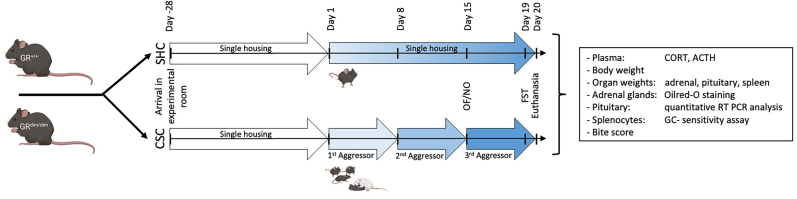
Four weeks after arrival at the experimental room, WT and GRdim mice were either exposed to the CSC paradigm or kept single-housed (SHC). All animals were tested for anxiety-related behavior in the open-field/novel object (OF/NO) test on day 15 and for depressive-like behavior in the forced swim test (FST) on day 19 of CSC between 0700 and 1000 a.m., respectively, before being sacrificed in the morning of day 20 between 0700 and 1000 a.m. following brief CO2 anesthesia. Afterwards, the amount of bite wounds was assessed for bite score analysis. Trunk blood was collected for the assessment of plasma corticosterone (CORT) and adrenocorticotropic hormone (ACTH). The adrenal glands, the pituitary as well as the spleen were removed and weight. Adrenal glands were frozen for the assessment of lipid vesicles by Oil Red-O staining. The pituitary was frozen for the analysis of qPCR analysis of proopiomelanocortin (POMC). Moreover, splenocytes were isolated for in vitro GC sensitivity assay. In total (n = 48) animals divided in four groups (SHC-WT: n = 13; CSC-WT: n = 11; SHC-GRdim: n = 11; CSC-GRdim: n = 13) were used in the present study. For details about the experimental timeline, see Fig. 1.
Fig. 1.
Experimental timeline
After arrival at the experimental room (Day −28), all mice were housed singly for four weeks. At day 1, experimental wild type (WT; GR+/+) and GRdim (GRdim/dim) homozygous mice were weighed and assigned to either the single-housed control (SHC) or the chronic subordinate colony housing (CSC) group. In order to induce chronic psychosocial stress, a group of four CSC mice (two WT and two GRdim mice per cage) was housed together with a dominant male CD-1 mouse for 19 consecutive days. To avoid habituation, CSC mice were exposed to a novel dominant male CD-1 mouse on days 8 and 15. All animals were tested for anxiety-related behavior in the open-field/novel object (OF/NO) test on day 15 and for depressive-like/coping behavior in the forced swim test (FST) on day 19 of CSC, before being euthanized in the morning of day 20. Afterwards, the severity of bite wounds was assessed for bite score analysis. Trunk blood was collected for the assessment of plasma corticosterone (CORT) and adrenocorticotropic hormone (ACTH). The adrenal glands, the pituitary as well as the spleen were removed and weight. Adrenal glands were frozen for the assessment of the adrenal lipid content by Oil Rred-O staining. The pituitary was frozen for quantitative polymerase chain reaction (qPCR) analysis of proopiomelanocortin (POMC). Moreover, splenocytes were isolated for an in vitro glucocorticoid (GC) sensitivity assay. Experimental timeline was partly created with BioRender.com.
2.3. Chronic subordinate colony housing (CSC) procedure
The CSC paradigm was performed as described previously (Langgartner et al., 2015, 2017; Reber et al., 2007, 2008). Briefly, WT and GRdim experimental mice were weighed and assigned to either the SHC or CSC group, matched according to their body weight. During CSC, experimental mice lived in chronic subordination to three different dominant CD-1 aggressor mice between days 1 and 20. In detail, four male mice (2 WT and 2 GRdim mice per cage) were introduced into the home cage of a larger aggressor male CD-1 mouse on day 1 of CSC, resulting in immediate subordination of the four intruder mice. To disturb the established social hierarchy in each CSC cage and to avoid habituation, all four CSC mice of one cage were transferred into the home cage of a novel larger aggressor CD-1 male on days 8 and 15. All resident males were tested before CSC housing for their aggressive behavior and males that were not attacking their opponents or injure their opponents too excessively were not used. SHC mice remained undisturbed for the duration of the experiment, except for changing the bedding once a week and weighing twice a week. Besides being weighed twice a week, SHC and CSC mice were again weighed immediately before being euthanized at day 20.
2.4. Open-field/novel object (OF/NO) test
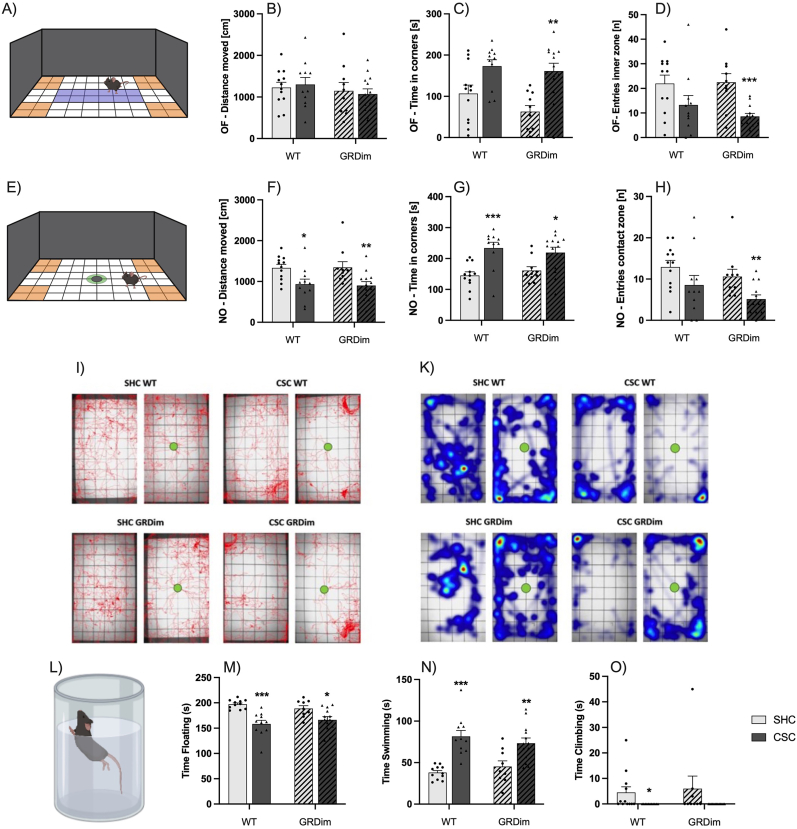
To assess the effects of CSC and an attenuation of GR dimerization on anxiety-related behavior, all mice were exposed to the OF/NO test on day 15 of CSC exposure. The OF/NO was performed as previously described (Langgartner et al., 2017, 2018). Briefly, the arena (45 cm length x 27 cm width x 27 cm height) was subdivided into an inner (27 cm × 9 cm) and an outer zone (Fig. 2A). The arena was cleaned thoroughly before each trial. During each trial, the mouse was placed into the middle of the arena and allowed to explore the arena for 5 min. After 5 min of exploration, a plastic round object (diameter: 3.5 cm; height: 1.5 cm) was placed in the middle of the arena. The mouse was now allowed to explore the arena containing the unfamiliar/novel object for another 5 min (Fig. 2E). During OF exploration the overall distance moved, the number of inner zone entries and the time in the corners of the arena was assessed. During NO exploration the overall distance moved, the number of novel object explorations and the time in the corners of the arena was assessed. All parameters were analyzed using EthoVision XT (Version 11.5, Noldus Information Technology, Wageningen, Netherlands). The test was performed between 0700 and 1000 a.m. under white light conditions (350 lux).
Fig. 2.
Effects on anxiety-related and depressive-like/coping behavior
Chronic subordinate colony housing (CSC; dark-grey bars; triangles) increased anxiety-related behavior during open-field (OF; Fig. 2A) and novel-object (NO; Fig. 2E) exposure in both wild type (WT; GR+/+; unhatched bars) and GRdim (GRdim/dim; hatched bars) mice. In detail, CSC vs. single-housed control (SHC; light-grey bars, circles) mice showed an increased time in corners during NO (Fig. 2G) exposure as well as a decreased distance moved during NO exposure (Fig. 2F), respectively. GRdim but not WT CSC mice showed an increased time in corners during OF exposure (Fig. 2C) and a decreased number of entries into the inner zone of the OF during OF exploration (Fig. 2D) as well as a decreased number of NO explorations (Fig. 2H) when compared to respective GRdim SHC mice. The general locomotion (distance moved during OF exploration) was comparable between all experimental groups (Fig. 2B). Representative track- and time visualizations are shown in (Fig. 2I) and (Fig. 2K), respectively. To assess the effects of CSC and an attenuation of GR dimerization on depressive-like/coping behavior, all mice were exposed to the FST (Fig. 2L) in the morning of day 19 of CSC exposure. In WT and GRdim mice, CSC decreased time of floating (Fig. 2M) and increased the time of swimming (Fig. 2N) during FST exposure, respectively. Moreover, CSC in WT mice decreased the time of climbing during FST exposure (Fig. 2O). Data are presented as bar graphs (mean +SEM) with individual dots. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 vs. respective SHC. Illustrations (Fig. 2A, 2E and 2L) were created with BioRender.com.
2.5. Forced swim test (FST) test
To assess the effects of CSC and an attenuation of GR dimerization on depressive-like/coping behavior, all mice were exposed to the FST test on day 19 of CSC exposure. The FST was performed as previously described (Slattery et al., 2012). Briefly, each mouse was placed into a clear plastic cylinder (diameter: 12 cm; height: 40 cm) filled to 13 cm with 23 ± 2 °C water for 6 min and mouse behavior was recorded (Fig. 2L). The cylinder was filled with fresh water following each trial. The test was performed between 0700 and 1000 a.m. under white light conditions (200 lux). During the last 4 min of the FST, the time spent mobile was analyzed as velocity >2.5 cm/s using EthoVision XT (Version 11.5, Noldus Information Technology, Wageningen, Netherlands). The time spent floating was calculated based on the following formula: 240s - time spent mobile [s]. Moreover, the time spent climbing was quantified by an experienced experimenter blind to treatment and the time spent swimming was calculated based on the following formula: time spent mobile [s] – time spent climbing [s].
2.6. Trunk blood sampling
Within 3 min after removing the cage from the animal room, mice were rapidly euthanized by decapitation following brief CO2-exposure. Trunk blood was collected in ethylenediaminetetraacetic acid (EDTA)-coated tubes (Sarstedt, Nuembrecht, Germany) and stored on ice until centrifugation. Tubes were centrifuged at 4 °C (5000 g, 10 min). Plasma samples were stored at −20 °C until further analysis.
2.7. Assessment of adrenal-, pituitary and spleen weight
Adrenal glands were removed, pruned of fat and stored in ice-cold Dulbecco's Modified Eagle Medium (DMEM/F-12, Life Technologies, Inc, Grand Island, NY, USA; supplemented with 0.1% bovine serum albumin (BSA; Biomol, Hamburg, Germany)), until being weight. Right adrenal glands were subsequently frozen at −80 °C until being used for Oilred-O staining. The pituitary was removed, immediately weight and frozen at −80 °C until being used for qPCR analysis. Spleens were removed, pruned from fat, weighed, and stored in ice-cold Hanks' Balanced Salt solution (HBSS; Sigma-Aldrich) until being used for the isolation of splenocytes.
2.8. Enzyme-linked immunosorbent assay (ELISA) for plasma CORT and ACTH
Singlets of plasma samples were analyzed using a commercially available ELISA for total CORT (intra-assay coefficient of variation (CV) ≤ 7.7%; inter-assay CV ≤ 10.8%; IBL International, Hamburg, Germany) and ACTH (intra-assay CV ≤ 10.3%; inter-assay CV ≤ 7.1%; IBL International, Hamburg, Germany).
2.9. Adrenal Oil Red-O staining
Right adrenal glands were embedded in freezing medium (Tissue-Tek, Sakura Finetek Europe, Zoeterwoude, Netherlands) and frozen at −80 °C. Five μm cryo-sections containing both, adrenal cortex and medulla, were cut using a cryostat (Leica Biosystems, IL, USA) and thaw-mounted onto precoated slides (SuperFrost ®Plus; VWR, Darmstadt, Germany). Adrenal sections were then fixed in paraformaldehyde (3.5–3.7%; Otto Fischar GmbH & Co. KG, Saarbruecken, Germany) for 72h at room temperature (RT). Subsequently, slides were washed in distilled water, rinsed in 60% isopropyl alcohol for 5 min and then stained in freshly filtered Oil Red-O solution (Certistain Oil red-O, Merck, Darmstadt, Germany) for 15 min. Afterwards, the sections were differentiated in 60% isopropyl alcohol and washed in distilled water before being preserved with Roti® Mount Aqua (ROTH, Karlsruhe, Germany) and a microscope cover glass (ROTH, Karlsruhe, Germany).
In order to quantify the amount of lipid vesicles in the adrenal cortex, four images (one image from each quarter of the section) were collected from each section using bright field microscopy (20x magnification (Leica DMI6000B; Leica Biosystems, Nussloch, Germany), camera (Olympus DP73; Olympus GmbH, Hamburg, Germany)). Image analysis was performed using the software Reimage (© Dr. Michael Noll-Hussong). Specifically, within the adrenal cortical zone of each image, a 300 × 300 pixel sized region of interest was defined and analyzed for the amount of Oil Red-O stained pixels. The percentage of stained pixels was calculated for each image. Within each section, the mean percentage of stained pixels was subsequently calculated over all four images. Afterwards, the mean percentage over all sections of an animal was calculated and used for statistics. For further statistical analysis, the mean percentage of stained pixels was multiplied with the weight of the right adrenal gland.
2.10. Quantitative reverse transcriptase PCR for POMC in pituitary mRNA
In order to determine effects of CSC and an attenuation of GR dimerization on pituitary POMC mRNA expression, the pituitary was stored at −80 °C until RNA isolation. RNA was isolated using the PARISKit® (Thermo Fisher Scientific, Waltham, USA) according to the manufacturer's instructions. As Nanodrop 2000 technology (Thermo Fischer Scientific, Waltham, MA, USA) revealed a RNA concentration in all samples <100 ng/μl, a total volume of 10 μl and, thus, less than 1 μg RNA, per sample were used to generate cDNA employing random primers (High-capacity cDNA reverse transcription kit, Applied Biosystems, Thermo Fischer Scientific, Waltham, MA, USA) and additional RNase inhibitor “RNase out” to reduce RNase activity (Invitrogen, Thermo Fischer Scientific, Waltham, MA, USA). Quantitative PCR was performed in triplets using a ViiA7 Real-Time PCR System (Applied Biosystems, Thermo Fischer Scientific, Waltham, MA, USA) and Platinum SYBR Green (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA). The primers (Sigma Aldrich, St. Louis, MO, USA) used were Ribosomal Proteins (RPL; Housekeeper) (forward: 5′ CCTGCTGCTCTCAAGGTT 3’; reverse: 5′ TGGCTGTCACTGCCTGGTACTT 3′) and POMC (forward: 5′ CGGTGAAGGTGTACCCCAACGT 3’; reverse: 5′ GGACCTGCTCCAAGCCTAATGGCC 3′). For the quantification of results, the corresponding Applied Biosystems Software was used and ΔΔ Ct analysis was performed.
2.11. Isolation of splenocytes
The isolation of splenocytes was done as previously described (Foertsch et al., 2017). Briefly, spleens were mechanically disrupted using a nylon cell strainer (70 μm; Corning, USA) and the plunger of a syringe to obtain a single cell suspension. Erythrocytes were lyzed by incubating the cell suspension for 2 min in ASK-lysis buffer (155 mM NH4Cl, 10 mM KHCO3, 10 mM EDTA) and stopped by addition of Hanks’ Balanced Salt solution (HBSS; Sigma-Aldrich)/10% heat-inactivated fetal calf serum (FCS). Following one washing step with HBSS and filtration of the cell suspension through a 70 μM nylon cell strainer, cells were resuspended in ice-cold Roswell Park Memorial Institute Medium (RPMI-1640, Sigma-Aldrich, St. Louis, MO, USA) containing 10% FCS, 50 U/ml penicillin and 50 μg/ml streptomycin (RPMI+) and counted using a cell counter (TC-20, Bio-Rad Laboratories, Munich, Germany).
2.12. Assessment of functional in vitro GC sensitivity of isolated splenocytes
Splenocytes were adjusted to a concentration of 5 × 106 cells/ml in RPMI+. Afterwards all isolated cells were plated in singlets in flat-bottom, 96-well plates in a final total volume of 100 μl/well (splenocytes: 2.5 × 105 cells/well). Cells either remained untreated or were stimulated with LPS (Escherichia coli O111:B4, final concentration 1μg/ml; Sigma-Aldrich, Deisenhofen, Germany). Moreover, corticosterone (CORT; Sigma-Aldrich, Deisenhofen, Germany; final concentration: 0 and 0.1 μM respectively), diluted in 95% ethanol, was added to the wells. Cells were incubated (37 °C, 5% CO2) for 48 h and cell viability was determined using a commercially available colorimetric assay (CellTiter 96 Aqueous One Solution Assay, Promega, Madison, WI). Absorbance at 450 nm was measured using an enzyme-linked immunosorbent assay (ELISA) plate reader (Fluostar Optima, BMG Labtech, Offenburg, Germany). All values were blank-corrected. The absorbance (optical density (OD)) of unstimulated cells was subtracted from the corresponding LPS-stimulated cells and the resulting delta OD was set to 100% for the 0 μM CORT condition. A low cell viability at increasing CORT levels indicates high GC sensitivity and low GC resistance.
2.13. Assessment of bite wound severity
The severity of bite wounds in CSC mice was assessed using a bite score previously established by our group (Foertsch et al., 2017). Briefly, following decapitation, the skin (with fur attached) was removed so that skin and body were still connected at the tail and hind limbs. Afterwards, pictures were taken from each mouse and digitally overlaid with a standardized grid (GIMP 2.6.12), which contained 20 squares covering the skin (dermal wounds) and 20 squares covering the body (subdermal wounds). Each square on the skin was scored according to the affected area (score 0–4), intensity (score 0–4) and the degree of purulence (0–2), whereas each square on the body was scored for the affected area (score 0–3) and the intensity of injury (0–2). The bite score is then represented by the sum of all skin and body injuries and ranges from 0 to 300.
2.14. Statistics
For statistical analysis and graphical illustrations GraphPad Prism (version 9.3.1, GraphPad Software, LCC) was used. Kolmogorov-Smirnov test with Lilliefors’ correction was employed to test for normal distribution. Outliers in normally distributed data sets were identified by Grubbs test and excluded from further analysis. Normally distributed data sets were analyzed by parametric statistics, i.e. two-way ANOVA (two factors, two or more independent samples) followed by post-hoc analysis using Bonferroni pairwise comparison, when a significant main effect was found. Not normally distributed data sets were analyzed by non-parametric statistics, i.e. Mann-Whitney U test (MWU; one factor, two independent samples) and Wilcoxon matched-pairs signed rank test (one factor, two dependent samples). Data are presented as mean +SEM including individual values. The level of significance was set at p ≤ <0.05.
3. Results
3.1. Effects on anxiety-related and depressive-like/coping behavior
To assess the effects of CSC and an attenuated GR dimerization on anxiety-related behavior, all mice were exposed to the OF/NO test (Fig. 2A–K) in the morning of day 15 of CSC exposure (Fig. 1). Both WT and GRdim mice displayed increased anxiety-related behavior in the CSC compared to SHC group. This was indicated by a decreased distance moved during NO exposure (Fig. 2F; WT: P = 0.016 (MWU); GRdim: P = 0.006 (MWU)) and an increased time spent in the corners during NO (Fig. 2G; WT: P < 0.001 (MWU); GRdim: P = 0.021 (MWU)) exposure in CSC vs. SHC mice of both the WT and GRdim group, respectively. GRdim but not WT CSC mice further showed an increased time spent in corners during OF exploration (Fig. 2C; GRdim: P = 0.002 (MWU)), a decreased number of entries into the inner zone during OF exploration (Fig. 2D; P = 0.001 (MWU)), as well as a decreased number of NO exploration (Fig. 2H; P = 0.005 (MWU)) when compared to respective SHC mice. The general locomotion, represented by the distance moved during OF exploration, was comparable between all experimental groups (Fig. 2B). Representative track- and time visualizations are shown in (Fig. 2I) and (Fig. 2K), respectively.
To assess the effects of CSC and an attenuation of GR dimerization on depressive-like/coping behavior, all mice were exposed to the FST (Fig. 2L - O) in the morning of day 19 of CSC exposure. Compared with respective SHC mice, CSC significantly decreased the time floating (Fig. 2M, two-way ANOVA, Main effect for stress: F1,40 = 28.37; P < 0.001) in both, the WT (Bonferroni: P < 0.001) and GRdim (Bonferroni: P = 0.02) group. Furthermore, CSC increased the time spent swimming (Fig. 2N, two-way ANOVA, Main effect for stress: F1,40 = 36.23; P < 0.001) in both the WT (Bonferroni: P < 0.001) and GRdim (Bonferroni: P = 0.004) group compared with respective SHC mice. Finally, CSC significantly decreased the time spent climbing (Fig. 2O) during FST exposure compared with respective SHC mice in the WT (P = 0.014 (MWU)) group.
3.2. Effects on HPA axis parameters and body weight
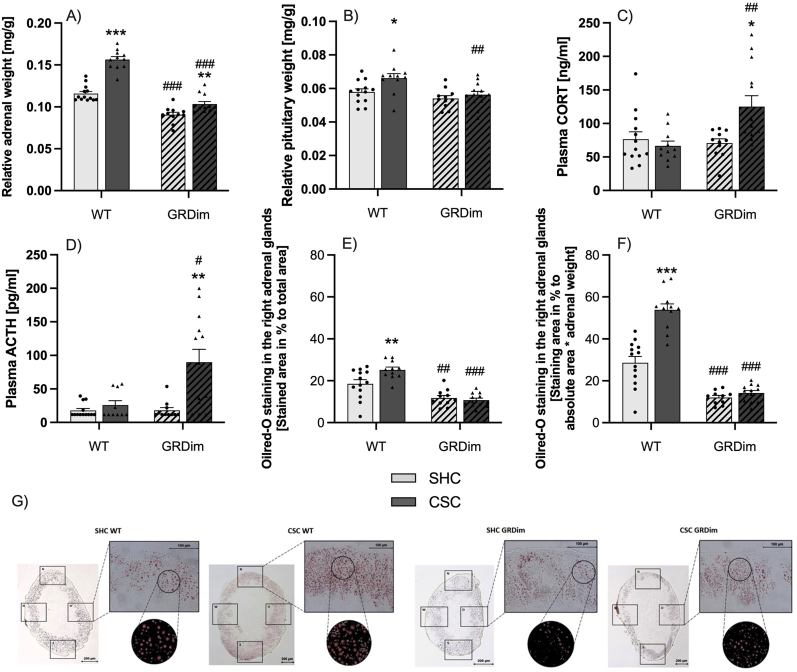
Chronic subordinate colony housing resulted in an increased relative adrenal weight (Fig. 3A) in both the WT (P < 0.001 (MWU)) and GRdim (P = 0.002 (MWU)) group when compared to respective SHC mice. Furthermore, relative adrenal weight of both GRdim SHC (P < 0.001 (MWU)) and CSC (P < 0.001 (MWU)) mice was decreased when compared to respective WT mice. In contrast, relative pituitary weight (Fig. 3B) was only increased in CSC vs. SHC mice of the WT (P = 0.016 (MWU)) but not the GRdim group. Moreover, relative pituitary weight was found to be significantly decreased in GRdim vs. WT CSC mice (P = 0.005 (MWU)). Plasma morning CORT concentrations (Fig. 3C) were significantly increased in GRdim CSC compared with GRdim SHC (P = 0.013 (MWU)) as well as WT CSC (P = 0.003 (MWU)) mice. Similarly, plasma morning ACTH concentrations (Fig. 3D) were significantly increased in GRdim CSC compared with GRdim SHC (P = 0.002 (MWU)) as well as WT CSC (P = 0.015 (MWU)) mice. The availability of adrenal lipids represented by the ratio of Oil Red-O-stained adrenal area to total adrenal area in sections of the right adrenal gland (Fig. 3E, G) was increased in WT (two-way ANOVA: Interaction effect: F1,43 = 6.981; P = 0.011; Main effect for genotype: F1,43 = 53.62; P < 0.001; Bonferroni: P = 0.004) but not GRdim CSC vs. SHC mice. Moreover, adrenal lipid content was significantly lower in both GRdim SHC (Bonferroni: P = 0.003) and GRdim CSC (Bonferroni: P < 0.001) mice compared with the respective WT group. When corrected for differences in adrenal weight (Fig. 3F), adrenal lipids were still increased in CSC vs. SHC mice in the WT (two-way ANOVA: Main effect for stress: F1,42 = 34.03; P < 0.001; Bonferroni: P < 0.001) but not GRdim group, as well as significantly lower in both SHC (two-way ANOVA: Interaction effect: F1,42 = 24.19; P < 0.001; Main effect for genotype: F1,42 = 143.6; P < 0.001; Bonferroni: P < 0.001) and CSC (Bonferroni: P < 0.001) GRdim mice compared with respective WT mice. Fig. 3G shows representative Oil Red staining images of each experimental group. Body weight was comparable between all experimental groups on day 20 of CSC (data not shown).
Fig. 3.
Effects on HPA axis parameters
When compared to respective single-housed control (SHC; light-grey bars; circles) mice, chronic subordinate colony housing (CSC; dark-grey bars; triangles) resulted in an increased relative adrenal weight (Fig. 3A) in both wild type (WT; GR+/+; unhatched bars) and GRdim (GRdim/dim; hatched bars) mice. Relative adrenal weight of both GRdim SHC and CSC mice was decreased when compared to respective WT mice. Relative pituitary weight (Fig. 3B) was increased in WT but not GRdim CSC vs. SHC mice and decreased in GRdim vs. WT CSC mice. Plasma morning corticosterone (CORT; Fig. 3C) and adrenocorticotropic hormone (ACTH; Fig. 3D) concentrations were increased in GRdim CSC compared to GRdim SHC as well as WT CSC mice, respectively. Adrenal lipid content represented by the ratio of Oil Red-O-stained adrenal area to total adrenal area in sections of the right adrenal gland (Fig. 3E) as well as adrenal lipid content per adrenal weight (Fig. 3F) was increased in WT but not GRdim CSC vs. SHC mice and decreased in both GRdim vs. WT SHC and CSC mice, respectively. Representative Oil Red-O staining images of each experimental group are shown in (Fig. 3G). Data are presented as bar graphs (mean +SEM) with individual dots. *P# *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 vs. respective SHC. #P ≤ 0.05, ##P ≤ 0.01, ###P ≤ 0.001 vs. respective WT.
3.3. Effects on relative pituitary POMC mRNA expression
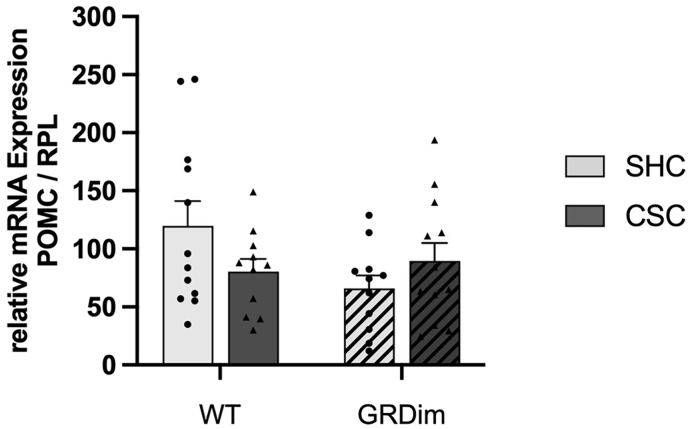
Neither CSC nor the attenuated GR dimerization had a significant effect on the relative POMC mRNA expression in the pituitary (Fig. 4A).
Fig. 4.
Effects on relative pituitary POMC mRNA expression
The relative pituitary proopiomelanocortin (POMC) mRNA expression levels (Fig. 4A) did not differ between wild type (WT; GR+/+; unhatched bars) and GRdim (GRdim/dim; hatched bars) mice exposed to chronic subordinate colony housing (CSC; dark-grey bars; triangles) or single-housing (SHC; light-grey bars; circles). As housekeeping gene, ribosomal proteins (RPL) was used. Data are presented as bar graphs (mean +SEM) with individual dots.
3.4. Effects on spleen weight and functional splenic in vitro GC sensitivity
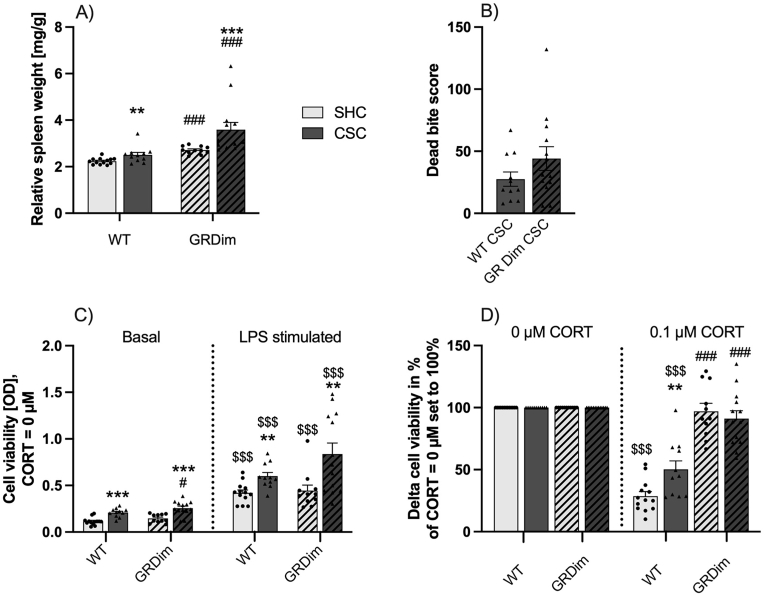
In line with what has been reported by our group for a bite score above 20 (WT: 27.5; GRdim: 44.2; Fig. 5B) (Foertsch et al., 2017), CSC increased relative spleen weight (Fig. 5A) in both WT (P = 0.009 (MWU)) and GRdim (P < 0.001 (MWU)) mice when compared to respective SHC mice. Furthermore, relative spleen weight of both GRdim SHC (P < 0.001 (MWU)) and GRdim CSC (P < 0.001 (MWU)) mice was significantly increased compared to respective WT mice.
Fig. 5.
Effects on spleen weight and functional splenic in vitro GC sensitivity
When compared to respective single-housed control (SHC; light-grey bars; circles) mice, chronic subordinate colony housing (CSC; dark-grey bars; triangles) increased relative spleen weight (Fig. 5A) in both the wild type (WT; GR+/+; unhatched bars) and GRdim (GRdim/dim; hatched bars) group. Furthermore, relative spleen weight of GRdim SHC and CSC mice was increased compared to respective WT mice. Both, WT and GRdim CSC mice had a bite score above 20 (WT: 27.5; GRdim: 44.2; Fig. 5B). Splenic cell in vitro viability in response to lipopolysaccharide (LPS; Fig. 5C) was increased in all groups. Splenocytes from WT and GRdim CSC mice showed a higher basal and LPS-induced cell viability compared to respective SHC mice. Basal cell viability was increased in splenocytes from GRdim vs. WT CSC mice. Delta (LPS - basal) cell viability (0 μM corticosterone (CORT) set to 100%; Fig. 5D) in response to 0.1 μM CORT was reduced in WT SHC and CSC mice when compared to the 0 μM condition. Delta cell viability in response to 0.1 μM CORT was significantly increased in GRdim SHC and CSC vs. respective WT mice, as well as WT CSC vs. SHC mice. Data are presented as bar graphs (mean +SEM) with individual dots. **P ≤ 0.01, ***P ≤ 0.001 vs. respective SHC. #P ≤ 0.05, ###P ≤ 0.001 vs. respective WT. $$$P ≤ 0.001 vs. respective basal or 0 μM condition.
Compared to basal values, splenic cell viability in response to LPS (Fig. 5C) was increased in all groups (WT SHC: P < 0.001; WT CSC: P < 0.001; GRdim SHC: P < 0.001; GRdim CSC: P < 0.001 (all Wilcoxon)). Moreover, splenocytes from WT and GRdim CSC mice showed a higher basal (WT: P < 0.001 (MWU); GRdim: P < 0.001 (MWU)) and LPS-induced (WT: P = 0.002 (MWU); GRdim: P = 0.007 (MWU)) cell viability compared to respective SHC mice. Basal cell viability was further increased in splenocytes from GRdim vs. WT CSC mice (P = 0.047 (MWU)). Delta (LPS - basal) cell viability (0 μM CORT set to 100%; Fig. 5D) in response to 0.1 μM CORT was reduced in the SHC (P < 0.001 (Wilcoxon)) and CSC (P < 0.001 (Wilcoxon)) mice of the WT group when compared to respective groups in the 0 μM condition with the effect being significantly less pronounced in CSC WT mice (CSC vs. SHC WT mice at 0.1 μM CORT: P = 0.007 (MWU)). Of note, delta cell viability in response to 0.1 μM CORT was significantly higher in GRdim SHC (P < 0.001 (MWU)) and CSC (P < 0.001 (MWU)) vs. respective WT mice. Thus, GRdim splenocytes are less sensitive towards the immunosuppressive effects of GCs.
4. Discussion
In the present study we confirm own earlier findings in male C57Bl/6N mice, showing that Sv129Ev/C57Bl/6 (B6) F1 hybrid WT mice exposed to CSC are characterized by increased anxiety-related behavior and no signs of depressive-like/passive coping behavior. Moreover, CSC vs. SHC Sv129Ev/B6 WT mice show unaffected morning plasma CORT levels, despite an increased pituitary and adrenal mass as well as an increased Oil Red-O positive adrenal lipid content in the cortex. In extension, we show that male GRdim mice expressing a GR with a point mutation (A465T) disrupting one of the GR dimerization interfaces and bred as F1 hybrids on a Sv129Ev/B6 background (Sv129Ev/B6 GRdim) have unaffected basal morning plasma ACTH and CORT concentrations compared with respective WT mice. Together with earlier studies (Reichardt et al., 1998) suggesting a compromised negative feedback inhibition at the level of the pituitary in Sv129Ev GRdim mice, these data support the hypothesis that the significant reduction in pituitary and adrenal weight in unstressed Sv129Ev/B6 GRdim in the present study represent a beneficial adaptation to avoid basal hypercorticism and its well-known negative consequences. However, although Sv129Ev/B6 GRdim mice do not develop pituitary enlargement when exposed to CSC, chronic psychosocial stress in Sv129Ev/B6 GRdim mice seems to exceed their capacity of HPA axis adaptation, indexed by increased plasma morning ACTH and CORT levels and adrenal enlargement compared with respective SHC mice. As we neither found a genotype nor CSC effect on pituitary POMC mRNA concentrations, our data support the hypothesis that an affected GR signaling at least indirectly affects plasma ACTH concentrations during chronic psychosocial stress, most likely via regulating the expression of certain factors involved in POMC mRNA translation/post-translational modification.
In contrast to own previous studies in C57Bl/6N mice (Füchsl et al., 2013), CSC in Sv129Ev/B6 WT mice did not result in increased basal morning plasma ACTH concentrations, despite pituitary weight was significantly enhanced. However, given that only a view plasma ACTH values were above the detection limit in both SHC and CSC mice of the Sv129Ev/B6 WT group, this missing CSC effect might be due to overall very low and, thus, undetectable ACTH levels in Sv129Ev/B6 compared with C57Bl/6N mice. Noteworthy, although in Sv129Ev/B6 GRdim SHC mice only a view plasma ACTH concentrations were above the detection limit of the ELISA, comparable plasma ACTH concentrations between Sv129Ev/B6 WT SHC and Sv129Ev/B6 GRdim SHC mice in the present study are in line with an earlier study finding no differences in plasma ACTH between Sv129Ev WT and Sv129Ev GRdim mice (Reichardt et al., 1998). Further different between an earlier study done in mice from a Sv129Ev background (Reichardt et al., 1998) and the current study done in mice from a Sv129Ev/B6 background is the effect of the GR dimerization deficits on pituitary POMC mRNA levels. While non-quantitative in situ hybridization in the earlier study suggested an increased POMC mRNA expression in GRdim vs. WT mice (Reichardt et al., 1998), which was further accompanied by an increased pituitary ACTH immunoreactivity (Reichardt et al., 1998), qPCR done in the current study failed to find a genotype effect. As qPCR analysis of POMC mRNA expression in the present study further failed to detect a CSC effect, we hypothesize that increased plasma ACTH levels in CSC vs. SHC Sv129Ev/B6 GRdim mice to be the consequence of an enhanced POMC mRNA translation/post-translational modification. At the level of the adrenal glands, CSC exposure in both WT and GRdim mice of the Sv129Ev/B6 background caused adrenal enlargement, confirming that the stress procedure worked reliably in both genotypes. The latter is also supported by the CSC-induced increase in anxiety-related behavior in both WT and Sv129Ev/B6 GRdim mice, an effect repeatedly reported for C57Bl/6N mice earlier (Reber et al., 2007, 2016a; Langgartner et al., 2017; Peters et al., 2013). However, while the CSC-induced increase in adrenal mass did not translate into increased plasma CORT concentrations in Sv129Ev/B6 WT mice, it did in Sv129Ev/B6 GRdim mice. Interestingly, unaffected plasma morning CORT levels despite an increased adrenal weight and increased plasma ACTH were previously also reported for CSC-exposed C57Bl/6N mice (Langgartner et al., 2017; Reber et al., 2007; Uschold-Schmidt et al., 2012). Moreover, plasma ACTH levels in the current study were significantly higher in Sv129Ev/B6 GRdim CSC vs. respective WT CSC mice. Therefore, our data supports the hypothesis that a reduction in adrenal ACTH sensitivity during times of chronic stress, adrenal enlargement and increased ACTH levels (Langgartner et al., 2017; Reber et al., 2007; Uschold-Schmidt et al., 2012) represents a beneficial adaptation, protecting the individual at least to a certain threshold of plasma ACTH levels from the negative consequences of stress-induced hypercorticism.
Interestingly, increased basal morning plasma ACTH concentrations in Sv129Ev/B6 GRdim CSC mice resulted in increased plasma CORT concentrations, although the CSC-induced increase in adrenal lipids was only detectable in Sv129Ev/B6 WT but not GRdim mice. The latter supports the hypothesis that while Sv129Ev/B6 GRdim CSC mice are able to translate increased basal morning plasma ACTH concentrations adequately into increased plasma CORT concentrations, this might fail in response to a severe acute heterotypic stressor.
In line with previous studies done in C57Bl/6N mice (Slattery et al., 2012), CSC in the present study did neither increase depressive-like behavior/passive coping in WT nor GRdim mice of the Sv129Ev/B6 background. In contrast, while CSC in C57Bl/6N mice did not affect the time spent immobile during FST (Slattery et al., 2012), CSC in the present study reduced the time floating during FST exposure in both genotypes, suggesting a genotype-independent shift towards active stress coping or development of a hyperactive phenotype. The latter is rather unlikely, as earlier studies by our group revealed that home cage locomotor activity in CSC vs. SHC C57Bl/6N mice is not affected on day 20 of CSC (Slattery et al., 2012). Analysis of the time spent swimming and climbing during FST exposure indicated that the above described effect is predominantly mediated by an increase in swimming but not climbing behavior. One possible explanation for this discrepancy in FST immobility might be that C57Bl/6N mice in the Slattery et al., 2012 study were exposed to the FST 8 days following termination of CSC (Slattery et al., 2012), whereas in the current study the FST was performed on day 19 of CSC. Another possible explanation would be that in the Slattery et al., 2012 study mice exposed to the FST were not exposed to any other behavioral test before, whereas all mice in the present study were tested for their anxiety-related behavior in the OF/NO test on day 15 of CSC.
Strikingly, Sv129Ev/B6 GRdim SHC and CSC mice were totally resistance to the anti-inflammatory effects of CORT on the LPS-induced increase in in vitro splenocyte viability. This is in line with findings showing that anti-inflammatory activity of GCs and the reduction of leukocyte abundance by GCs require intact GR activity, which is strongly hampered in GRdim mice, as demonstrated during LPS-induced acute lung injury (Ichinose et al., 2001), sepsis (Barbee et al., 2010), contact hypersensitivity (Tuckermann et al., 2007), rheumatoid arthritis (Baschant et al., 2011) and TNF-induced inflammation (Vandevyver et al., 2012). Of note, in agreement with own previous findings in C57Bl/6N mice (Foertsch et al., 2017, 2020; Foertsch and Reber, 2020; Kempter et al., 2021), 19 days of CSC in the present study caused an increased spleen weight in both WT and GRdim mice of the Sv129Ev/B6 background, suggesting significant wounding during social stressor exposure in both genotypes (Foertsch et al., 2017, 2020; Foertsch and Reber, 2020). The latter was confirmed by a mean bite score of 27.5 in Sv129Ev/B6 WT and 44.2 in Sv129Ev/B6 GRdim CSC mice. For comparison, C57Bl/6N CSC mice with a bite score above 20 reliably develop splenomegaly (Foertsch et al., 2017). Further in line with own previous data (Foertsch et al., 2017), isolated splenocytes of CSC WT and CSC GRdim mice of the Sv129Ev/B6 background in the absence of CORT showed an increased cell viability when in vitro cultured both in the absence or presence of LPS. Of note, in contrast to Sv129Ev/B6 GRdim mice, respective WT mice in general were sensitive to the anti-inflammatory effects of CORT, with CSC in combination with bite wounds facilitating development of functional splenic in vitro GC resistance, as shown in C57Bl/6N mice (Reber et al., 2007; Foertsch et al., 2017, 2020; Foertsch and Reber, 2020; Kempter et al., 2021).
Together our findings support the hypothesis, that POMC mRNA translation or posttranslational modification into ACTH protein is negatively controlled by intact GR dimerization under conditions of chronic psychosocial stress (summarized in Fig. 6). Moreover, our data suggest that adrenal adaptations during chronic psychosocial stress (i.e, ACTH desensitization), aiming at the prevention of prolonged hypercorticism, are protective only to a certain threshold of plasma ACTH levels.
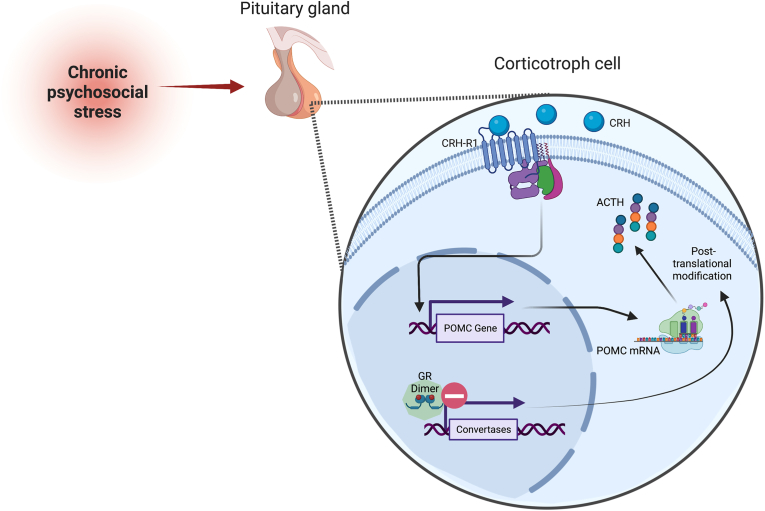
Fig. 6.
Summary Figure
Upon binding of corticotrophin releasing hormone (CRH) to the CRH-receptor (R) 1 on corticotroph cells of the anterior pituitary, proopiomelanocortin (POMC) gene transcription is initiated. POMC mRNA is subsequently translated into POMC and post-transcriptionally cleaved into inter alia adrenocorticotropic hormone (ACTH). During chronic psychosocial stress exposure, glucocorticoid receptor (GR) dimerization is proposed to inhibit the translation of different enzymes/convertases involved in POMC mRNA translation or post-transcriptional processing into ACTH protein (Harno et al., 2018). Figure was created with BioRender.com.
Funding
This study was funded by intramural resources of JT and SOR. MT was supported by the Deutsche Forschungsgemeinschaft (DFG) (Project number: 314061271-TRR 205).
CRediT authorship contribution statement
DL, MK, SK, MT, SV, JT and SOR, planned the study; DL, MK, SK, LG and LK and LGPR performed the experiments; MNH provided the Oil Red-O analysis software; DL and MK did the statistical analysis and created the figures; DL, MK, JT and SOR wrote the manuscript.
Declaration of competing interest
DL, MK, SK, LG, LK, MT, MNH, SV, JT and SOR have nothing to declare and do not have any conflict of interest.
Acknowledgements
The authors thank P. Hornischer and U. Binder for their technical assistance and help in performing the experiments. Furthermore, the authors would also like to thank Dr. S. Ott, E. Merkel and S. Brämisch (local animal research center) for their excellent support in terms of animal housing.
Handling Editor: Prof R Lawrence Reagan
Data availability
Data will be made available on request.
References
- Amoroso M., et al. Intranasal Mycobacterium vaccae administration prevents stress-induced aggravation of dextran sulfate sodium (DSS) colitis. Brain Behav. Immun. 2019;80:595–604. doi: 10.1016/j.bbi.2019.05.005. [DOI] [PubMed] [Google Scholar]
- Amoroso M., et al. Subcutaneous Mycobacterium vaccae promotes resilience in a mouse model of chronic psychosocial stress when administered prior to or during psychosocial stress. Brain Behav. Immun. 2020;87:309–317. doi: 10.1016/j.bbi.2019.12.018. [DOI] [PubMed] [Google Scholar]
- Barbee R.W., Reynolds P.S., Ward K.R. Assessing shock resuscitation strategies by oxygen debt repayment. Shock. 2010;33(2):113–122. doi: 10.1097/SHK.0b013e3181b8569d. [DOI] [PubMed] [Google Scholar]
- Bardeleben U., Holsboer F. Cortisol response to a combined dexamethasone-human corticotrophin-releasing hormone challenge in patients with depression. J. Neuroendocrinol. 1989;1(6):485–488. doi: 10.1111/j.1365-2826.1989.tb00150.x. [DOI] [PubMed] [Google Scholar]
- Baschant U., et al. Glucocorticoid therapy of antigen-induced arthritis depends on the dimerized glucocorticoid receptor in T cells. Proc. Natl. Acad. Sci. U. S. A. 2011;108(48):19317–19322. doi: 10.1073/pnas.1105857108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet C.S., et al. Assessment of HPA-axis function in posttraumatic stress disorder: pharmacological and non-pharmacological challenge tests, a review. J. Psychiatr. Res. 2006;40(6):550–567. doi: 10.1016/j.jpsychires.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Donner N.C., et al. Chronic non-invasive corticosterone administration abolishes the diurnal pattern of tph2 expression. Psychoneuroendocrinology. 2011;37(5):645–661. doi: 10.1016/j.psyneuen.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foertsch S., Reber S.O. The role of physical trauma in social stress-induced immune activation. Neurosci. Biobehav. Rev. 2020;113:169–178. doi: 10.1016/j.neubiorev.2020.02.025. [DOI] [PubMed] [Google Scholar]
- Foertsch S., et al. Splenic glucocorticoid resistance following psychosocial stress requires physical injury. Sci. Rep. 2017;7(1) doi: 10.1038/s41598-017-15897-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foertsch S., Langgartner D., Reber S.O. Abdominal surgery prior to chronic psychosocial stress promotes spleen cell (re)activity and glucocorticoid resistance. Sci. Rep. 2020;10(1):6917. doi: 10.1038/s41598-020-63419-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries E., et al. A new view on hypocortisolism. Psychoneuroendocrinology. 2005;30(10):1010–1016. doi: 10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Füchsl A.M., Langgartner D., Reber S.O. Mechanisms underlying the increased plasma ACTH levels in chronic psychosocially stressed male mice. PLoS One. 2013;8(12) doi: 10.1371/journal.pone.0084161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold P.W., Goodwin F.K., Chrousos G.P. Clinical and biochemical manifestations of depression. Relation to the neurobiology of stress (2) N. Engl. J. Med. 1988;319(7):413–420. doi: 10.1056/NEJM198808183190706. [DOI] [PubMed] [Google Scholar]
- Harno E., et al. POMC: the physiological power of hormone processing. Physiol. Rev. 2018;98(4):2381–2430. doi: 10.1152/physrev.00024.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C., Ehlert U., Hellhammer D.H. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25(1):1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Holsboer F. The dexamethasone suppression test in depressed patients: clinical and biochemical aspects. J. Steroid Biochem. 1983;19(1A):251–257. [PubMed] [Google Scholar]
- Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23(5):477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- Holsboer F. Stress, hypercortisolism and corticosteroid receptors in depression: implicatons for therapy. J. Affect. Disord. 2001;62(1–2):77–91. doi: 10.1016/s0165-0327(00)00352-9. [DOI] [PubMed] [Google Scholar]
- Holsboer F., Barden N. Antidepressants and hypothalamic-pituitary-adrenocortical regulation. Endocr. Rev. 1996;17(2):187–205. doi: 10.1210/edrv-17-2-187. [DOI] [PubMed] [Google Scholar]
- Ichinose F., et al. Attenuation of hypoxic pulmonary vasoconstriction by endotoxemia requires 5-lipoxygenase in mice. Circ. Res. 2001;88(8):832–838. doi: 10.1161/hh0801.089177. [DOI] [PubMed] [Google Scholar]
- Kempter E., et al. Changes in functional glucocorticoid sensitivity of isolated splenocytes induced by chronic psychosocial stress – a time course study. Front. Immunol. 2021;12(4141) doi: 10.3389/fimmu.2021.753822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langgartner D., et al. Chronic subordinate colony housing paradigm: a mouse model to characterize the consequences of insufficient glucocorticoid signaling. Front. Psychiatr. 2015;6:18. doi: 10.3389/fpsyt.2015.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langgartner D., et al. Individual differences in stress vulnerability: the role of gut pathobionts in stress-induced colitis. Brain Behav. Immun. 2017;64:23–32. doi: 10.1016/j.bbi.2016.12.019. [DOI] [PubMed] [Google Scholar]
- Langgartner D., et al. The role of the intestinal microbiome in chronic psychosocial stress-induced pathologies in male mice. Front. Behav. Neurosci. 2018;12:252. doi: 10.3389/fnbeh.2018.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrner A., Daskalakis N., Yehuda R. Posttraumatic Stress Disorder. John Wiley & Sons, Inc.; 2016. Cortisol and the hypothalamic–pituitary–adrenal Axis in PTSD; pp. 265–290. [Google Scholar]
- Lim H.W., et al. Genomic redistribution of GR monomers and dimers mediates transcriptional response to exogenous glucocorticoid in vivo. Genome Res. 2015;25(6):836–844. doi: 10.1101/gr.188581.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris M.C., Compas B.E., Garber J. Relations among posttraumatic stress disorder, comorbid major depression, and HPA function: a systematic review and meta-analysis. Clin. Psychol. Rev. 2012;32(4):301–315. doi: 10.1016/j.cpr.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff C.B. The corticotropin-releasing factor (CRF) hypothesis of depression: new findings and new directions. Mol. Psychiatr. 1996;1(4):336–342. [PubMed] [Google Scholar]
- Owens M.J., Nemeroff C.B. The role of corticotropin-releasing factor in the pathophysiology of affective and anxiety disorders: laboratory and clinical studies. Ciba Found. Symp. 1993;172:296–308. doi: 10.1002/9780470514368.ch15. discussion 308-16. [DOI] [PubMed] [Google Scholar]
- Pariante C.M., Miller A.H. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol. Psychiatr. 2001;49(5):391–404. doi: 10.1016/s0006-3223(00)01088-x. [DOI] [PubMed] [Google Scholar]
- Percie du Sert N., et al. Reporting animal research: explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 2020;18(7):e3000411. doi: 10.1371/journal.pbio.3000411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S., et al. Differential effects of baclofen and oxytocin on the increased ethanol consumption following chronic psychosocial stress in mice. Addiction Biol. 2013;18(1):66–77. doi: 10.1111/adb.12001. [DOI] [PubMed] [Google Scholar]
- Raison C.L., Miller A.H. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am. J. Psychiatr. 2003;160(9):1554–1565. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- Reber S.O., et al. Adrenal insufficiency and colonic inflammation after a novel chronic psycho-social stress paradigm in mice: implications and mechanisms. Endocrinology. 2007;148(2):670–682. doi: 10.1210/en.2006-0983. [DOI] [PubMed] [Google Scholar]
- Reber S.O., et al. Aggravation of DSS-induced colitis after chronic subordinate colony (CSC) housing is partially mediated by adrenal mechanisms. Stress. 2008;11(3):225–234. doi: 10.1080/10253890701733351. [DOI] [PubMed] [Google Scholar]
- Reber S.O., et al. Mucosal immunosuppression and epithelial barrier defects are key events in murine psychosocial stress-induced colitis. Brain Behav. Immun. 2011;25(6):1153–1161. doi: 10.1016/j.bbi.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Reber S.O., et al. Chronic subordinate colony housing paradigm: a mouse model for mechanisms of PTSD vulnerability, targeted prevention, and treatment-2016 Curt Richter Award Paper. Psychoneuroendocrinology. 2016;74:221–230. doi: 10.1016/j.psyneuen.2016.08.031. [DOI] [PubMed] [Google Scholar]
- Reber S.O., et al. Immunization with a heat-killed preparation of the environmental bacterium Mycobacterium vaccae promotes stress resilience in mice. Proc. Natl. Acad. Sci. U. S. A. 2016;113(22):E3130–E3139. doi: 10.1073/pnas.1600324113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichardt H.M., et al. DNA binding of the glucocorticoid receptor is not essential for survival. Cell. 1998;93(4):531–541. doi: 10.1016/s0092-8674(00)81183-6. [DOI] [PubMed] [Google Scholar]
- Schiller B.J., et al. Glucocorticoid receptor binds half sites as a monomer and regulates specific target genes. Genome Biol. 2014;15(7):418. doi: 10.1186/s13059-014-0418-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery D.A., et al. Behavioural consequences of two chronic psychosocial stress paradigms: anxiety without depression. Psychoneuroendocrinology. 2012;37(5):702–714. doi: 10.1016/j.psyneuen.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Tuckermann J.P., et al. Macrophages and neutrophils are the targets for immune suppression by glucocorticoids in contact allergy. J. Clin. Invest. 2007;117(5):1381–1390. doi: 10.1172/JCI28034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uschold-Schmidt N., et al. Chronic psychosocial stress results in sensitization of the HPA axis to acute heterotypic stressors despite a reduction of adrenal in vitro ACTH responsiveness. Psychoneuroendocrinology. 2012;37(10):1676–1687. doi: 10.1016/j.psyneuen.2012.02.015. [DOI] [PubMed] [Google Scholar]
- Vandevyver S., et al. Glucocorticoid receptor dimerization induces MKP1 to protect against TNF-induced inflammation. J. Clin. Invest. 2012;122(6):2130–2140. doi: 10.1172/JCI60006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vettorazzi S., et al. A guide to changing paradigms of glucocorticoid receptor function-a model system for genome regulation and physiology. FEBS J. 2022;289:5718–5743. doi: 10.1111/febs.16100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.