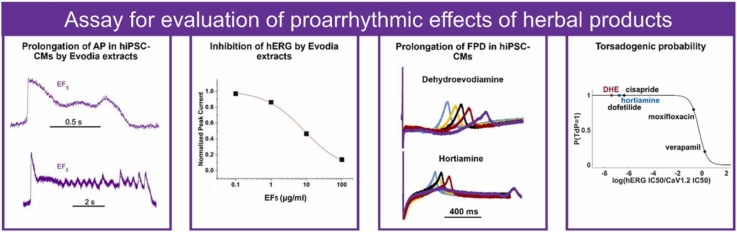
Abstract
Guidelines for preclinical drug development reduce the occurrence of arrhythmia-related side effects. Besides ample evidence for the presence of arrhythmogenic substances in plants, there is no consensus on a research strategy for the evaluation of proarrhythmic effects of herbal products. Here, we propose a cardiac safety assay for the detection of proarrhythmic effects of plant extracts based on the experimental approaches described in the Comprehensive In vitro Proarrhythmia Assay (CiPA). Microelectrode array studies (MEAs) and voltage sensing optical technique on human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) were combined with ionic current measurements in mammalian cell lines, In-silico simulations of cardiac action potentials (APs) and statistic regression analysis. Proarrhythmic effects of 12 Evodia preparations, containing different amounts of the hERG inhibitors dehydroevodiamine (DHE) and hortiamine were analysed. Extracts produced different prolongation of the AP, occurrence of early after depolarisations and triangulation of the AP in hiPSC-CMs depending on the contents of the hERG inhibitors. DHE and hortiamine dose-dependently prolonged the field potential duration in hiPSC-CMs studied with MEAs. In-silico simulations of ventricular AP support a scenario where proarrhythmic effects of Evodia extracts are predominantly caused by the content of the selective hERG inhibitors. Statistic regression analysis revealed a high torsadogenic risk for both compounds that was comparable to drugs assigned to the high-risk category in a CiPA study.
Keywords: Evodia rutaecarpa, Cardiac safety, HERG, TdP, HiPSC-CMs
Graphical Abstract
Highlights
-
•
hiPSC-CMs are suitable for detecting and analysing proarrhythmic effects of Evodia extracts.
-
•
Proarrhythmic effects of 12 Evodia preparations correlate with dehydroevodiamine and hortiamine content.
-
•
In-silico simulations reproduce in-vitro effects of dehydroevodiamine and hortiamine on ventricular action potentials.
-
•
A safety assay for the detection of proarrhythmic effects of plant extracts is proposed.
1. Introduction
In the past, several commercialised drugs such as terfenadine, astemizole, cisapride, dofetilide and sertindole had to be withdrawn from the market or limited in use due to cardiotoxicity mediated by a blockage of the human voltage-sensitive K+ channel hERG [29]. The hERG channel plays a fundamental role in cardiac AP repolarization, effectively controlling the QT interval of the electrocardiogram [25]. Meanwhile, established guidelines for the assessment of drug-induced Torsade de Pointes (TdP) (ICH S7B and ICH E14) and the Comprehensive In vitro Proarrhythmia Assay (CiPA), substantially minimise the proarrhythmic risk of novel drugs already at the preclinical development stage. CiPA studies are based on detailed electrophysiological studies of drug effects on cardiac ionic channels expressed in mammalian cell lines, In-silico reconstruction of drug effects of human ventricular AP, and assessment of drug effects on hiPSC-CMs (Food and Drug Administration, 2017, [3]).
Despite clear evidence for the occurrence of hERG inhibitors and other ion channel blockers in herbal drugs and phytomedicines [14], [33], there is a limited awareness of possible risks and, as a consequence, no consensus on a comprehensive approach for the detection and evaluation of proarrhythmic effects of such products. This is particularly problematic in the case of herbal drugs like Evodia rutaecarpa which contains potent hERG inhibitors, such as dehydroevodiamine (DHE) and hortiamine [2]. These alkaloids inhibit the hERG channel at concentrations comparable to that of drugs like terfenadine and cisapride which had to be withdrawn from the market.
The fruits of Evodia rutaecarpa (A.Juss.) Hook.f. & Thomson (Tetradium ruticarpum (A.Juss.) T.G.Hartley), known in traditional Chinese medicine (TCM) as ”Wu Zhu Yu”, is a frequently used herbal drug to treat, among others, problems of digestion, stomach cramps and other gastrointestinal disorders, and headache [15], [31], [36]. The herbal drug is used alone or, more frequently, in combination with other herbs. The herbal drug itself, and Evodia-containing products such as granules are commercially available via various distribution channels, not only in East Asia, but also in Western countries.
Hortiamine, DHE, and methanolic extracts of Evodia were found to prolong the duration of the AP, and to induce early after depolarisations (EADs) in hiPSC-CMs [2]. The main alkaloid DHE increased the QT interval in anaesthetised rabbits and chronic atrioventricular block dogs, and induced TdP arrhythmias [2]. Pharmacological studies have shown that Evodia fruits exert multiple effects on the cardiovascular system [15]. Several pharmacokinetic studies in rats have been conducted in recent years with Evodia decoctions, DHE, and Evodia-containing TCM formula [17], [24], [32], [35], and the bioavailability of DHE after oral gavage was approx. 15 % [17]. However, to the best of our knowledge no pharmacokinetic studies in humans have been published. Hence, plasma concentrations of DHE and hortiamine in humans after intake of Evodia preparations are currently unknown.
In this study, we implemented essential experimental approaches used in the CiPA assay to herbal products. We combined analytical methods, voltage-sensing optical (VSO) technique, MEA, patch clamp studies on mammalian cell lines expressing NaV1.5, CaV1.2, and hERG, and In-silico simulations of DHE and hortiamine effects on cardiac AP and statistic regression analysis [13], for an evaluation of proarrhythmic effects of Evodia products from different sources. Decoctions of the herbal drug samples were prepared in accordance with TCM principles, to mimic a possible detoxification by boiling or a selective extraction process by the aqueous media (Tang and Eisenbrand, 2011). In recent years, soluble granules containing extracts of TCM herbal drugs have become increasingly available on the market. Proarrhythmic effects of decoctions on APs of hiPSC-CMs correlated with their DHE content. A statistic regression model predicts a torsadogenic potential of DHE and hortiamine comparable to drugs with risk of causing TdP [3]. Simulations of human cardiac APs further supported a scenario where proarrhythmic effects of Evodia extracts are primarily caused by the content of these selective hERG inhibitors. We propose an algorithm for the identification of proarrhythmic herbal preparations, identification of proarrhythmic compounds, and assessment of their torsadogenic risk.
2. Methods
2.1. Chemicals
DHE (≥ 98 % purity) was purchased from Chengdu Biopurify Phytochemicals Ltd., China. Hortiamine trifluoroacetate (> 98 % purity) was obtained via purification from fruits of Evodia rutaecarpa [26].
2.2. Sourcing of Evodia herbal drug and granules
For this study, seven different samples of Evodiae fructus and five different samples of Evodia-containing granules were purchased from various sources, either directly, or via internet commerce. The results are displayed as EF 1–7 and EP 1–5 in an anonymized fashion (see Supplemental Table 16S).
To compare the DHE and hortiamine contents in the fruits to the amounts found in the TCM decoction, one sample of Evodiae fructus was analysed in detail. Powdered Evodia fruits were extracted in triplicates by accelerated solvent extraction (ASE) with methanol (MeOH) at 70 °C and 120 bar in 4 extraction cycles for 5 min each. Afterwards, the residue was extracted in a fifth extraction cycle to assess completion of the extraction via HPLC analysis. All extracts were dried under reduced pressure, dissolved in DMSO at 3.5 mg/ml, and DHE and hortiamine contents were determined by HPLC with UV detection at 365 nm.
2.3. Preparation of decoctions
Decoctions were prepared following a traditional recipe [18].
Ca. 1 g of Evodia fruits or Evodia preparation were accurately weighted and placed in a beaker. After addition of 50 ml of tap water the fruits were allowed to soak for 3 h. The total weight of the beaker was recorded. The mixture was then boiled for 30 min, and evaporated water was replaced up to the initial weight. The supernatant was decanted into a 100 ml volumetric flask and, if needed, centrifuged at 3000 rpm for 5 min, prior to decanting. For a determination of the alkaloid content of the first infusion, an aliquot (1 ml) was taken from the supernatant for HPLC analysis. The solid residue was suspended in 40 ml of tap water for the second infusion and the total weight was noted. The mixture was boiled for additional 20 min. Afterwards, water was replaced up to the initial weight and the supernatant was combined with the first infusion in a 100 ml volumetric flask. The total volume was adjusted to 100 ml using distilled water to yield the final decoction. An aliquot was taken for HPLC analysis (1 ml), and the rest of the decoction was lyophilised to assess the extraction yield and for biological testing. Yield concentrations after each preparation step are summarised in Supplementary Fig. 3S.
2.4. Determination of DHE and hortiamine in Evodia infusions and granules
HPLC-PDA-ELSD-ESIMS data were recorded in the positive and negative mode on a Shimadzu LC-MS/MS 8030 triple quadrupole ESI-MS system connected via a T-splitter (1:10) to a Shimadzu HPLC system consisting of a degasser, binary high-pressure mixing pump, autosampler, column oven, and diode array detector, and via a T-split to an Alltech 3300 ELSD detector. The chromatographic separation was performed on a Waters SunFireTM C18 column (3.5 µm, 150 × 3 mm) with water (containing 0.1 % formic acid (A)) and acetonitrile (containing 0.1 % formic acid (B)) as mobile phase. The following gradient was used: 10 -> 40 % (0–20 min), 40 -> 100 % (20–22 min), 100 % (22–30 min). The flow rate was 0.5 ml/min, and the injection volume was 10 μL. Quantification was performed using UV detection at 365 nm. Trifluoroacetic acid salts of DHE and hortiamine were used as external standards (isolated by [26]). Calibration curves between 10 and 500 μg/ml of DHE, and 0.1 and 100 μg/ml of hortiamine were recorded (Supplementary Fig. 4S). Concentrations were calculated as mg compound per gram Evodiae fructus (or Evodia granules) used.
2.5. AP recordings with VSO technique in hiPSC-CMs
AP measurements with the voltage-sensing optical (VSO) technique were carried out making use of FluoVolt™ (Thermo Fisher, Vienna, Austria) as described previously [2]. Cor .4 U® hiPSC-CMs (Ncardia, Cologne, Germany) were incubated at 37 °C, 5 % CO2 in Cor .4 U® complete human cardiomyocyte culture medium. Before the experiments, the cells were transiently loaded with FluoVolt™ for 30 min at room temperature. Afterwards, the medium containing VSO dye was replaced by fresh serum-free medium (DMEM, Sigma Aldrich, Vienna, Austria). The multi-well plate was placed in an environmentally controlled stage incubator (37 °C, 5 % CO2, water-saturated air atmosphere) (Okolab Inc, Burlingame, CA, USA). The fluorescence signal was recorded from a 0.2 × 0.2 mm area using a 40 × (NA 0.6) objective lens. Excitation wavelength was 470 ± 10 nm using a light-emitting diode (LED), and emitted light was collected by a photomultiplier (PMT) at 510–560 nm. LED, PMT, and associated power supplies and amplifiers were supplied by Cairn Research Ltd (Kent, UK). Fluorescence signal was digitised at 10 kHz. Acute effects of drugs were assessed by exposure to studied drug concentration with matched vehicle controls (DMSO). A 20 s recording was then taken 5 and 30 min after exposure to the extract, or vehicle with only one concentration applied per well. The procedure was repeated five times.
In order to observe changes in the AP repolarization, the time for 30 %, 50 % and 90 % repolarization (APD30, APD50 and APD90) was estimated. APD90 characterises the final phase of AP repolarisation and is therefore a key parameter for potential proarrhythmic effects [3]). AP prolongation at APD90 reflects the nature of the Ikr-current, which is the strongest at the end of the AP (see e.g., Fig. 3 of [9]. Additionally, the time points for repolarization by 30 % and 50 % (APD30 and APD50 accordingly) were estimated and used to calculate a triangulation parameter defined as Triangulation (%) = (APD90 - APD30)/APD90). Fridericia’s formula [6] was used to correct the AP duration for beating rate (APDc, see Supplementary Fig. S14).
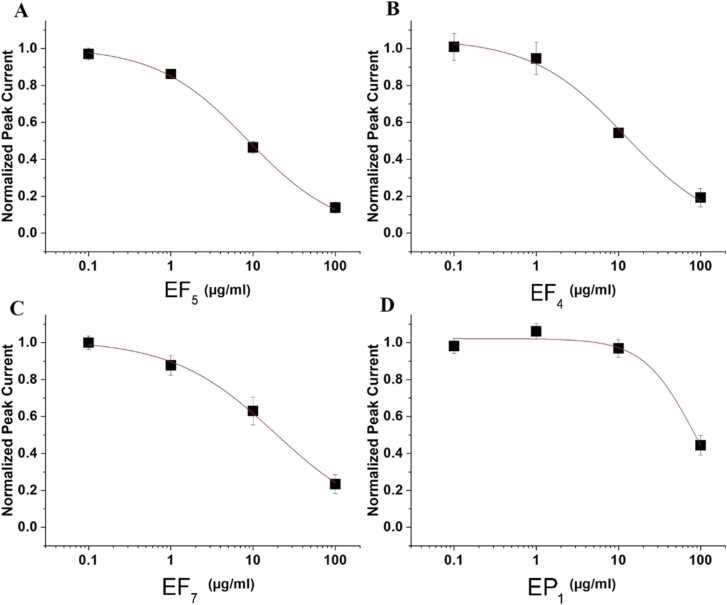
Fig. 3.
hERG inhibition by Evodia extracts
Concentration response curves of hERG inhibition by the high arrhythmogenic extract EF5 (IC50 = 8,7 ± 1,1 µg/ml, A) the intermediate arrhythmogenic extracts EF4 (IC50 = 12,1 ± 3,2 µg/ml, B) and EF7 (IC50 = 18,8 ± 3,6 µg/ml, C) and the low arrhythmogenic EP1 (IC50 = 83,1 ± 16,1 µg/ml, D).
The maximal rate of rise of the AP was estimated to analyse effects on sodium inward currents through NaV1.5. The estimated “time between APs” in a spontaneously beating monolayer sections may reflect effects on ion channels involved in automaticity. For subsequent offline analysis of the AP parameters described above, we made use of a semiautomated algorithm written in MATLAB (MathWorks, Natick, MA, USA).
2.6. Field potential recordings with MEAs in hiPSC-CMs
Human stem cell-derived cardiomyocytes (hiPSC-CMs) (iCell2, Cellular Dynamics International, Madison, WI, USA) were plated according to cell supplier recommendations. In short, the MEA surfaces were coated with 1 % fibronectin and hiPSC-CMs seeded at 1.56 × 105 cells/cm2. The cells were cultured in a cell culture incubator at 37 °C and 5 % CO2 for 4–5 days prior to start of experiment. The experimental endpoints recorded with the MEA2100 system (Multi Channel Systems GmbH, Germany) were: i) spike amplitude (AMP), ii) field potential duration (FPD) and iii) beat period (BP). The AMP provides an indirect measure of drug effects on the AP upstroke. Effects on repolarization were estimated from changes in the field potential duration (FPD, corresponding to the QT interval of clinical ECGs). The FPD was estimated from the interval between the depolarisation spike and the peak of the repolarization feature. The interval between 2 consecutive depolarisation spikes defines the beating period (BP). Fridericia’s formula was used to correct the FPD dependence on beating rate (APDc, FPDc, see Supplementary Table 15S).
Four concentrations of DHE and hortiamine were cumulatively applied to the spontaneously beating, confluent cardiomyocyte monolayer.
An independent vehicle (0.1 % DMSO) control was included on each six-well MEA. Following 10 min equilibration, three minutes of spontaneous activity were recorded as baseline. Immediately thereafter, the first concentration of test compound was added to the well. Five minutes after dose application, the next three-minute recording was acquired. This was repeated until a total of four doses were applied. Cardiomyocyte activity was sampled at 10 kHz and the raw traces were exported and analysed with a custom-made MATLAB program. Traces with sodium peak amplitudes under 200 µV as well as recordings with baseline beating frequency outside the 0.3–1.5 Hz range were excluded from analysis.
2.7. Patch clamp studies on mammalian cell lines expressing hERG, NaV1.5 and CaV1.2 channels
Chinese hamster ovary (CHO) cells stably expressing hERG channels (B’SYS GmbH, Witterswill, Switzerland) were grown in Ham’s F-12 with Glutamine containing 10% heat-inactivated FBS, 1 % Penicillin/Streptomycin, Hygromycin (100 µg/ml) and G418 (100 µg/ml). Human embryonic kidney (HEK) cells stably expressing human NaV1.5 (ChanPharm GmbH, Vienna, Austria) were grown in DMEM containing 10 % heat-inactivated FBS, 1 mM Sodium Pyruvate, 2 mM L-Glutamine and Zeocin (100 µg/ml). CHO cells inducibly expressing human CaV1.2 (α1c, β2 and α2δ) (NMI TT GmbH, Reutlingen, Germany) were grown in Ham's F-12 containing 10 % heat-inactivated FBS, 2 mM L-Glutamine, G418 (600 µg/ml), Hygromycin (600 µg/ml), Doxycycline (10 ng/ml). All cells were grown in culture flasks at 37 °C in a 5 % CO2-humidified incubator and harvested as previously described [2].
Currents through hERG (IhERG) and NaV1.5 (INa) channels were studied within 8 h of harvesting, in the whole-cell configuration of the planar patch clamp technique (NPC-16 Patchliner®, Nanion Technologies GmbH, Munich, Germany) [2], making use of an EPC 10 patch clamp amplifier (HEKA, Lambrecht/Pfalz, Germany). Currents through CaV1.2 (ICa) channels were studied within 8 h of harvesting, in the whole-cell configuration of the planar patch clamp technique (SyncroPatch®, Nanion Technologies GmbH, Munich, Germany).
The extracellular bath solution for IhERG and INa recordings contained (in mM): NaCl 140, KCl 4, CaCl2 2, MgCl2 1, D-Glucose*H2O 5 and HEPES 10 (pH 7.4 with NaOH). The intracellular solution for IhERG recordings contained (in mM): KCl 50, NaCl 10, KF 60, EGTA 20 and HEPES 10 (pH 7.2 with KOH). The intracellular solution for INa recordings contained (in mM): CsCl 50, NaCl 10, CsF 60, EGTA 20 and HEPES 10 (pH 7.2 with CsOH). NPC-16 Patchliner® was used for drug applications.
PatchMaster software v.2.91 (HEKA, Lambrecht/Pfalz, Germany) was used for data acquisition for IhERG, ICa, and INa recordings. The voltage protocol for IhERG recordings was designed to simulate voltage changes during a cardiac AP with a 300 ms depolarisation to + 20 mV (analogous to the plateau phase of the cardiac AP), a repolarization for 300 ms to − 50 mV (inducing a tail current) and a final step to the holding potential of − 80 mV. hERG channel block was estimated as the decrease in the peak tail current amplitude during a pulse train. INa were recorded in response to 10 ms pulses (0.2 Hz) from a holding potential of -120 to 0 mV, and the NaV1.5 channel block was estimated as the decrease in the peak current amplitude during a pulse train.
The extracellular bath solution for ICa recordings contained (in mM): NaCl 80, NMDG 60, KCl 4, CaCl2 2, MgCl2 1, Glucose 5, HEPES 10 (pH 7.4 with HCl). The intracellular solution for ICa recordings contained (in mM): CsF 110, CsCl 10, NaCl 10, EGTA 10, HEPES 10 and Aescin 15 (perforator) (pH 7.2 with CsOH). ICa were measured during 50 ms pulses applied at 0.05 Hz from a holding potential of − 90 mV to + 10 mV. CaV1.2 channel block was estimated as the decrease in the peak current amplitude during a pulse train.
2.8. Estimation of half-maximal ion channel inhibitions
The concentration–inhibition curves of peak ICa, INa or tail currents of IhERG were fitted using the Hill equation:Idrug/Icontrol = (100-A)/(1 +(C/IC50)nH)+A.
where IC50 is the concentration at which current inhibition is half-maximal, C is the applied drug concentration, A is the fraction of current that is not blocked and nH is the Hill coefficient.
2.9. In-silico simulations of DHE effects on AP
In addition to statistical methods such as the MICE model, In-silico approaches to proarrhythmic risk estimation also include the simulation of compound effects on mechanistic models of the cardiac AP [20]. The latter, describes the time course of the transmembrane voltage in dependence on ionic currents and intracellular processes by means of systems of nonlinear differential equations [5]. The ionic current models involve the respective maximum ionic conductance (or permeability) gion, which in the presence of a compound with concentration C is, according to the simple pore block approach, reduced by a factor
Out of the many AP models available, we have chosen the popular O’Hara-Rudy model of the human ventricular cardiomyocyte [21], also featured by the CiPA initiative in an optimised version [4]. Fig. 5 shows a simulation of the model for the mid-myocardial cell type in the absence of a compound (i.e., with C = 0) paced at 1 Hz. All simulations were performed in MATLAB using the mode15s solver for stiff differential equations.
Fig. 5.
Simulated AP of the human ventricular cardiomyocyte at steady state with pacing at 1 Hz according to the O’Hara-Rudy model [21].
2.10. Analysis of torsadogenic probabilities of DHE and hortiamine by means of logistic regression models
According to the ICH S7B guidelines for preclinical cardiac safety testing, a compound is considered to be torsadogenic if its hERG IC50 value and its effective free therapeutic plasma concentration (EFTPC) satisfy IC50/EFTPC < 30. However, negative hERG blocking effects on cardiac repolarization might be compensated by additional blockage of other channels, which may cause false positive signals of the pure hERG assay. In order to take such multichannel effects (MICE) into account, several logistic regression models with hERG IC50, EFTPC, CaV1.2 IC50 and NaV1.5 IC50, as direct features, were trained based on data for 32 torsadogenic (TdP = 1) and 23 non-torsadogenic (TdP = 0) drugs [13]. The model that was found to reduce false positives and false negatives most significantly in comparison with the hERG test is given by:
with β0 = −0.75 and β1 = 3.04, where p = P(TdP = 1) is the probability of being torsadogenic. Given a compound and knowing its ratio between hERG IC50 and CaV1.2 IC50, the logistic regression model predicts the associated probability of belonging to the class with TdP = 1.
2.11. Drugs
DHE hydrochloride and hortiamine trifluoroacetate were dissolved in 100 mM DMSO (dimethylsulphoxide, Sigma Aldrich, Vienna, Austria) and diluted in extracellular solutions to the desired concentration. Maximal DMSO concentrations during VSO electrophysiological recordings were 0.3 % and 0.1 % for MEA recordings.
2.12. Statistics
Data are presented as mean ± SEM. Differences were considered significant at P < 0.05.
3. Results
3.1. DHE content of Evodia extracts correlates with proarrhythmic effects on AP of hiPSC-CMs
Effects of 12 Evodia preparations (EF 1 – 7 and EP 1 – 5) with previously determined DHE and hortiamine contents (Table 1) on the AP duration of spontaneously beating hiPSC-CMs were studied with VSO technique (Fig. 1). Changes in AP repolarization (APD30, APD50 and APD90), the upstroke velocity (dV/dt), the beating intervals (dT) and triangulation were estimated 5- and 30-minutes following application of 0.1, 1, 10 and 100 µg/ml (Fig. 1, Table 1S–12S). The content of DHE (the major proarrhythmic alkaloid of Evodia, [26] ranged from 0.45 mg/g (EP1) to 5.97 mg/g (EF5, Table 1).
Table 1.
Changes in APD90 (ΔAPD90) after 5- or 30-min incubation in 10 µg/ml of the indicated extracts.
| Extract (applied at 10 µg/ml) |
ΔAPD90 (after 5 min), % | ΔAPD90 (after 30 min), % | DHE (mg/g) | Hortiamine (mg/g) |
| Low-arrhythmogenic | ||||
| EP1 | 0 ± 1 | 4 ± 1 | 0.45 | 0.32 |
| EF2 | 7 ± 2 | 11 ± 4 | 2.65 | 0.23 |
| EF1 | -4 ± 1 | 12 ± 1 | 2.5 | 0.97 |
| EP3 | 8 ± 1 | 14 ± 1 | 1.07 | 0.07 |
| EP2 | 10 ± 1 | 18 ± 1 | 2.25 | 0.11 |
| EP5 | 6 ± 1 | 18 ± 1 | 1.31 | 0.21 |
| EF6 | 23 ± 4 | 42 ± 3 | 4.46 | 0.21 |
| Intermediate-arrhythmogenic | ||||
| EF3 | 29 ± 3 | 54 ± 2 | 3.36 | 0.19 |
| EF4 | 41 ± 3 | 68 ± 2 | 4.92 | 0.34 |
| EF7 | 34 ± 2 | 108 ± 38 | 3.54 | 0.35 |
| High-arrhythmogenic | ||||
| EF5 | 182 ± 18 | AP oscillations | 5.97 | 0.96 |
| EP4 | 92 ± 9 | AP oscillations | 5.17 | 0.70 |
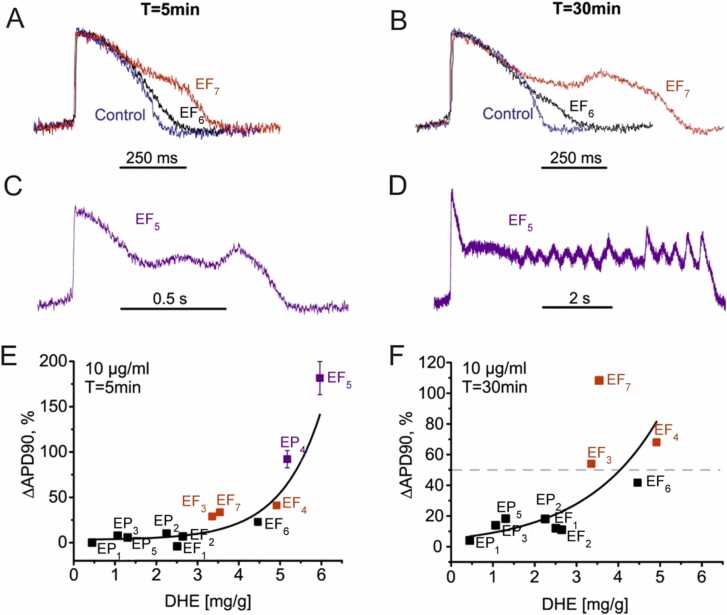
Fig. 1.
Effects of Evodia extracts on AP in hiPSC-CMs A, B Representative APs in control (blue lines) and after 5- or 30-minutes in the presence of EF6 (black lines) or EF7 (red lines). C, D Representative APs after 5- or 30-minutes incubation in EF5 (purple lines). E, Changes in APD90 (in %) after 5 min incubation with indicated extracts plotted versus respective DHE concentrations of the extract. F, Changes in APD90 (in %) after 30 min incubation with indicated extracts plotted versus respective DHE concentrations of the extract. See Supplementary Table 14S for the rate corrected action potential duration (APDc). Number of datapoints in A-F = 4. Dotted line in F marks the 50% threshold to make the division into the low- intermediate- and high-arrhythmogenic categories. Extracts in A – F were applied at a concentration of 10 mg/ml.
Based on the effects of 10 µg/ml on APs the extracts could be divided into low- (ΔAPD90 <50 %, e.g., EF7), intermediate- (ΔAPD90 >50 %, e.g., EF7) or high-arrhythmogenic (AP oscillations, EF5, EF7, Table 1).
With respect to the observed prolongation of APD90 after 30 min incubation time, extracts were assigned to a low-arrhythmogenic (ΔAPD90 <50 %), intermediate-arrhythmogenic (ΔAPD90 >50 %) or high-arrhythmogenic group (AP oscillations after 30 min incubation or cessation of beating). The introduced threshold of 50 % between intermediate- and high-arrhythmogenic activity is illustrated by the dashed line in Fig. 1F. At a higher extract concentration (100 µg/ml) most extracts induced oscillations or cessation of beating (Table 13S in Supplemental materials, see detailed analysis of extract effects at all the tested concentration in Table 1S–12S, Supplemental materials). Low concentrations (0.1 and 1 µg/ml) of most extracts did not induce significant effects on the AP parameters (see Table 1S–12S, Supplemental materials). Significant proarrhythmic effects (increase in APD90 and triangulation) upon application of 10 and 100 µg/ml (see Table 1S–12S) were observed.
Fig. 1 A and B illustrate the high-arrhythmogenic effects of EF5 and EP4. After incubating hiPSC-CMs with 10 µg/ml for 5 min, these extracts prolonged APD90 by about 90% (EF7) (red line AP, Fig. 1A, B) and 180 % (EF5, cyan AP, Fig. 1C, D, see Table 1). After 30 min, APD90 prolongation by EF5 turned into oscillations accompanied by further prolongation of the AP (Fig. 1C, D Table 1). Proarrhythmic effects of intermediated severity are exemplified for EF6 (AP with black lines in Fig. 1A, B, Table 1). This extract induced after 5 min incubation at 10 µg/ml, only moderate APD90 prolongation of 23% (see other extracts with moderate proarrhythmic effects in Table 1 and Table 1S–12S, Supplemental material). Low-arrhythmogenic extracts induced at 10 µg/ml neither after 5 nor after 30 min significant changes in APD90 (e.g., EP1) or only minor prolongation of APD90 (e.g., EF2 by 7% and 11%, Table 2 S and Table 3 S, Supplemental materials).
At 100 µg/ml, however, most extracts induced severe proarrhythmic effects such as oscillations of the membrane potential (EF2, EF5, EP3) or quiescence (i.e., cessation of beating) (EF3, EP2, EF4, EP4, EF6, EF7, see Table 1S–13S, Supplemental materials). A non-linear correlation between DHE content of the studied extracts and APD90 prolongation is illustrated in Fig. 1, E, F.
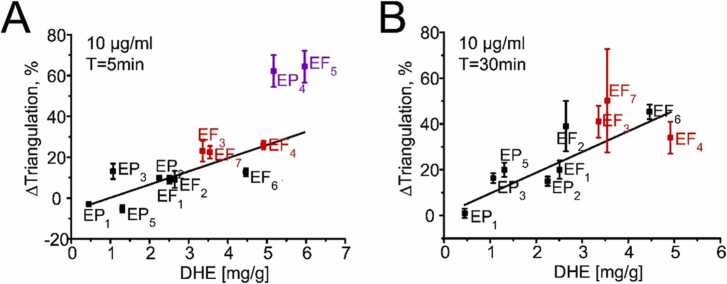
Hondeghem et al. [8] pointed out that drug-induced triangulation of the cardiac AP correlates with the ability to cause TdP. We have, therefore, included this parameter in our AP analysis. Triangulation of the AP occurred usually at similar concentrations and time points as the prolongations of APD90 (Table 1S–12S, Supplemental materials). Triangulation increased with increasing DHE content of the studied extracts. This is illustrated by the linear correlation between DHE content and triangulation induced by 10 µg/ml of the extract after 5 and 30 min (Fig. 2A, B).
Fig. 2.
Changes in AP triangulation in hiPSC-CMs plotted against DHE content of Evodia extracts
Changes in triangulation after 5 min (A) or 30 min (B) incubation with indicated extracts plotted versus their DHE concentration (Fig. 2). Data points are coloured according to the assignment of the extracts to different groups in Table 1 (low-, intermediate- and high-arrhythmogenic). Estimated correlation coefficients R2 were 0.86 (A) and 0.88 (B). Extracts in A and B were applied at a concentration of 10 µg/ml. The most prominent triangulation was induced by EF5 and EP4 after 30 min exposure time (1736 ± 263, Table 7 S). However, oscillations during the plateau of the AP (Fig. 1 B) complicated the estimation of the APD90 prolongation and these data points were therefore not included in (B).
The observed relation between prolongation of APD90 and triangulation, and the DHE content of the extract suggests a key role of hERG inhibition for all studied Evodia extracts (Fig. 1, Fig. 2).
This prompted us to analyse the extract effects on hERG channels with planar patch clamp. All extracts inhibited hERG channels expressed in CHO cells with individual half maximal inhibitory concentrations (IC50) ranging from about 83 µg/ml (EP1) to 9 µg/ml (EF5, Table 2). Fig. 3 exemplifies the corresponding concentration-dependent inhibition of potassium currents through hERG channels by different concentrations of the high-arrhythmogenic extract EF5 (Fig. 1C, D, Table 1), the intermediate arrhythmogenic extracts EF4 and EF7 and the low arrhythmogenic extract EP1.
Table 2.
Half maximal inhibitory concentrations of hERG channels in planar patch clamp experiments by indicated Evodia extracts. Concentration response curves for hERG inhibition by EF5, EF4, EF7 and EP1 are illustrated in Fig. 3 A-D.
| Extract | IC50 (µg/ml) |
|---|---|
| EF1 | 25,9 ± 4,8 (n = 3) |
| EF2 | 16,6 ± 2,9 (n = 4) |
| EP1 | 83,1 ± 16,1 (n = 3) |
| EF3 | 13,5 ± 1,9 (n = 4) |
| EP2 | 52,2 ± 5,9 (n = 3) |
| EF4 | 12,1 ± 3,2 (n = 3) |
| EF5 | 8,7 ± 1,1 (n = 3) |
| EP3 | 43,7 ± 11,1 (n = 3) |
| EP4 | 12,6 ± 5,9 (n = 3) |
| EP5 | 64,3 ± 16,7 (n = 3) |
| EF6 | 22,9 ± 14,7 (n = 3) |
| EF7 | 18,8 ± 3,6 (n = 4) |
3.2. Effects of DHE and hortiamine on field potentials of hiPSC-CMs
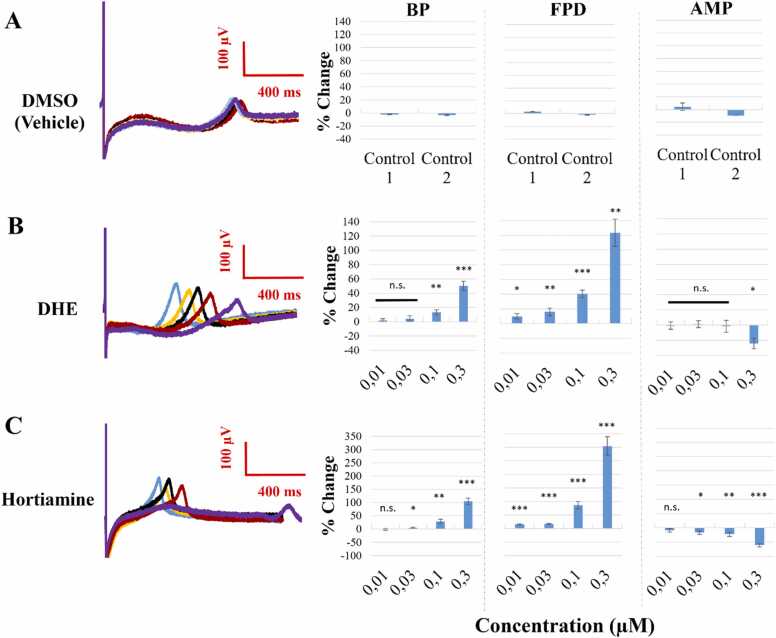
In a preceding study, Baburin et al. [2] illustrated that DHE and hortiamine prolong the AP of hiPSC-CMs in a concentration-dependent manner. Further evidence for drug-induced QT prolongation can be obtained with MEA technology [3]. Fig. 4 A shows example field potential waveforms from before (blue) and after exposure to the vehicle control (0.1 % DMSO) in each of four MEA chips and different concentrations of DHE and hortiamine (blue- control, yellow- 0.01 µM, black- 0.03 µM, red- 0.1 µM, and purple- 0.3 µM), along with the concentration-dependent effects on BP, FPD and AMP. The vehicle control did not induce significant effects on BP, FPD, FPDc, or AMP (Fig. 4, Supplemental Fig. S15). DHE and hortiamine elicited, however, concentration-dependent repolarisation prolongation as evident from the increase of FPD (Fig. 4 B, C). A concentration dependent increase in the beating period up to 50.2 % (DHE) and 104.3 % (hortiamine) was observed while a significant inhibition of AMP occurred only at the highest concentration (300 nM) (Fig. 4).
Fig. 4.
DHE and hortiamine prolong the FPD (A) Averaged traces on the left represent the independent, time matched, DMSO control field potential. The percent change in beat period (BP), field potential duration (FPD), and depolarisation spike amplitude (AMP) for five consecutive recordings is shown on the right. Control 1 combines the controls of the two MEAs treated with DHE, and control 2 – the control wells on the two chips treated with hortiamine. (B) The representative, averaged traces on the left visualise the shift of the t-wave and the prolongation of the FPD following treatment with DHE: blue (baseline), yellow (0.01 µM), black (0.03 µM), red (0.1 µM), and purple (0.3 µM). The relative changes in BP, FPD, and AMP, compared to baseline are shown on the right (n = 10). One-sample t-test was used, n.s. p > 0.05, *p < 0.05, **p < 0.01, ***p < 0.001. (C) The representative traces on the left visualise the FPD following treatment with hortiamine. The percent changes to the three parameters (BP, FPD, AMP) are shown on the right (n = 10). One-sample t-test was used. See Supplementary Table 15 S for the beat rate corrected field potential duration (FPDc).
3.3. In-silico analysis of DHE- and hortiamine-induced deteriorations of AP
The effects of DHE and hortiamine were further analysed by running computer simulations with the O’Hara-Rudy model [21] of the human ventricular AP (Fig. 5). For both DHE and hortiamine we chose concentrations of 1, 3 and 10 µM, which based on the pore block model along with the data from Fig. 1 S and 2 S (Supplemental materials) led to the following reductions (in percent) of the maximum ion channel conductances of the hERG, CaV1.2 and NaV1.5 channels (Table 3).
Table 3.
Calculated reduction of the maximum conductance of hERG, CaV1.2 and NaV1.5 channels at the indicated drug concentrations.
| DHE | Hortiamine | |||||
|---|---|---|---|---|---|---|
| hERG | CaV1.2 | NaV1.5 | hERG | CaV1.2 | NaV1.5 | |
| 1 µM | 88.55 | 0.47 | 1.08 | 88.98 | 0.02 | 0.01 |
| 3 µM | 97.30 | 1.26 | 2.56 | 97.41 | 0.13 | 0.06 |
| 10 µM | 99.49 | 3.62 | 6.44 | 99.51 | 0.88 | 0.43 |
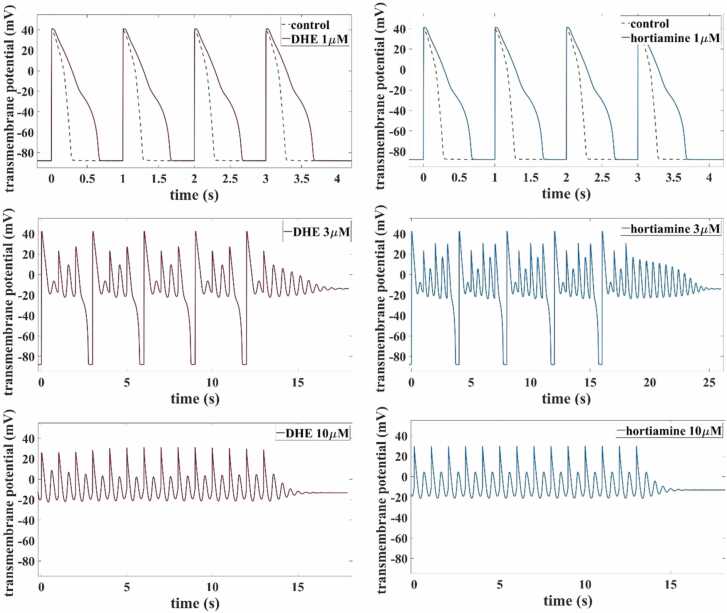
In all six cases, the AP under periodic pacing at 1 Hz was seriously distorted, see Fig. 6 A-C. At the low concentration, AP prolongation of about 140 % was obtained both for DHE and hortiamine (Fig. 5 A). At the medium concentration, EADs appeared with a repolarization failure after termination of pacing (Fig. 5 B). At the high concentration, repolarization did not even take place during pacing, leaving small scale voltage oscillations at an elevated level that subsided after removal of the stimulus (Fig. 5 C).
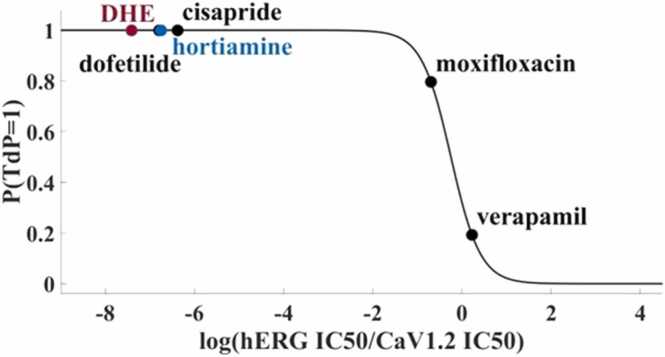
Fig. 6.
Torsadogenic probability of DHE and hortiamine calculated using the MICE model [13].
The nominal parameter setting for the endocardial cell type was chosen (Fig. 5, green dotted line).
Simulations of the impact of DHE and hortiamine on the AP using the O’Hara-Rudy model in combination with the pore block approach. The model was paced at 1 Hz until steady state was reached, before pacing was stopped and a final transient was recorded. Qualitatively similar results were obtained for hortiamine and DHE. At a concentration of 1 µM, an APD prolongation of about 140 % was observed. At a concentration of 3 µM, abnormal AP dynamics with EADs occurred with final repolarization failure. Finally, at 10 µM, small scale EAD-like oscillations were observed without any repolarization during pacing. For comparison, we also run simulations with the Kernik AP model [10] for hiPSC-CMs. While the nominal parameter setting corresponds to regular APs of a spontaneously beating cell, a reduction of ionic conductances according to Table 3 suppresses any beating activity (after a transient of damped small-scale oscillations) already at 1 µM both for DHE and hortiamine (data not shown).
3.4. Logistic regression predicts high torsadogenic probability of DHE and hortiamine
Simultaneous block of depolarising calcium and sodium currents can partially offset the effect of hERG block. We have therefore compared the potencies (IC50 values) of DHE and hortiamine on hERG, CaV1.2 and NaV1.5 inhibition (see concentration-dependent block of CaV1.2, NaV1.5 and hERG by DHE and hortiamine in Fig. 1S and 2S, Supplemental materials). Their probability of being torsadogenic can be estimated by means of the MICE model [13], which features the ratio of hERG IC50 and CaV1.2 IC50 as the only predictor variable. Based on the data from Fig. 1 S and 2 S, the model predicts both DHE and hortiamine as torsadogenic, see Fig. 6. Note that this result is robust against variation of the IC50 values within the intervals determined by the margin of error.
IC50 ratios were calculated from the values given in the subscripts of Fig. 4, Fig. 5. Black dots indicate predictions for some of the reference compounds (from [13], also used for training of the model. Accordingly, DHE and hortiamine have a very high probability of belonging to the class of torsadogenic compounds.
4. Discussion
There is clear evidence for occurrence of hERG inhibitors and other ion channel blockers in herbal products [2], [14], [33]; Dahai et al., 2017; [7]; [34]. The potential risks for arrhythmia are, however, poorly understood. While significant data sets for cardiotoxic drug effects on APs of hiPSC‐CMs have been generated to classify proarrhythmic events [1], [3], [12], such studies for herbal drugs are currently missing.
A step forward was made recently with Evodia alkaloids [2], in which a large number of experimental conditions were applied, ranging from In vitro electrophysiological studies of extract effects on single myocardial cells with VSO technique to In vivo demonstration of TdP tachycardia in animal models.
The herbal drug Evodiae fructus and Evodia-containing products, however, continue to be sold worldwide without restrictions. A likely reason for this is that herbal drugs are considered safe based on empirical knowledge from use over centuries. However, this argument obviously cannot apply to TdP tachycardia, given that drug-induced TdP were described only for the first time in detail in 1964 [27]. Moreover, the diagnosing of TdP tachycardia requires at least an electrocardiogram, and associated ventricular fibrillations can lead to sudden death within minutes which, in turn, complicates diagnosing outside a hospital.
Other reasons for continued use of Evodia preparations are the unknown plasma concentrations of DHE and hortiamine in humans, and the lacking awareness of potentially dangerous proarrhythmic effects of plant extracts.
Here, we propose an assessment of the torsadogenity of DHE and hortiamine which makes no reference to the plasma concentrations (Fig. 6). The electrophysiological characterisation of cardiotoxic drug effects of 12 Evodia extracts on hiPSC‐CMs was performed with VSO, MEA, and patch clamp studies on mammalian cell lines [1], [3], [19], [22], [30].
4.1. hiPSC-CMs are a suitable model for analysing proarrhythmic effects of Evodia extracts
In the first part of the study, we analysed the potential of hiPSC-CMs to detect changes in membrane repolarisation induced by 12 Evodia extracts from different providers containing different amounts of the hERG blockers DHE and hortiamine (content DHE varied from 0.45 to 5.97 mg/g; hortiamine from 0.07 to 0.97 mg/g, Table 1). Extracts were prepared as decoctions according to TCM recipes (Martin and Stöger, 2008, [31].
Our analysis revealed qualitatively similar indicators of proarrhythmic activity as previously observed for torsadogenic drugs such as AP prolongation, EADs, AP oscillations, triangulation, and cessation of beating (Fig. 1, Fig. 2, Table 1S–12S, Supplemental materials) [3].
Fig. 1 together with Table 1 illustrate that the prolongation of APD90 and triangulation of the AP increase with increasing content of the major proarrhythmic compound DHE (Fig. 1 E, F, Fig. 2 A, B). Accordingly, hERG channels were inhibited at lower doses by extracts containing higher amounts of DHE (Fig. 3, Table 3).
It appears that the different proarrhythmic effects observed for the various extracts (Table 1), reflect predominantly the content of hERG blockers.
While effects on AP repolarization and triangulation of the AP were most prominent (e.g., APD90 increased after application of 10 µg/ml EF5 and EP4 by 180 % and 92 % respectively and oscillations at 100 µg/ml, additional smaller effects of extracts on other parameters such as the upstroke velocity of the AP or beating frequency were found to variable extents and warrant further research (Table 1S–12S).
4.2. DHE and hortiamine prolong the field potential of hiPSC-CMs
In a preceding study we reported that DHE and hortiamine prolong APs (APD90) studied with VSO technology [2]. Here we provide evidence for delay in cardiac repolarisation making use of the MEA technique. MEA technology enabled the estimation of DHE- and hortiamine-induced prolongation of field potential durations (FPD, corresponding to the QT interval), spike amplitude and beat period in hiPSC-CMs in the absence of a fluorophore.
The data in Fig. 4 illustrate a concentration-dependent prolongation of FPD by both compounds with first highly significant prolongation occurring at 100 nM which nicely corresponds to data obtained with the VSO technique. As observed by Baburin et al. [2] the effect of hortiamine on repolarisation was slightly stronger which corresponded to the lower IC50 estimated for hERG inhibition (Supplementary Fig. 2S). Effects on depolarisation spike amplitude and beat period at the highest applied concentration of 0.3 µM warrant further investigations of the underlying ionic mechanism.
4.3. In-silico effects of DHE and hortiamine on cardiac AP reproduce proarrhythmic effects of herbal extracts
Established hallmarks of the extract effects on hiPSC-CMs such as prolongation of APD90, triangulation, EADs and cessation of beating (Fig. 1, Fig. 2, Table 1S-S13, Supplemental materials) are caused by their content of the hERG inhibitors, and this should be reflected in simulations of the cardiac AP. This assumption was supported by our In-silico studies in which we applied mechanistic modelling of cardiac AP (Fig. 5). In the latter case, EADs caused by Evodia extract EF5 (Fig. 1D) or DHE [2], are qualitatively reproduced under a selective inhibition of the hERG channel (Fig. 3).
4.4. DHE and hortiamine are highly torsadogenic
The severity of torsadogenic effects is substantially determined by the balance between block of repolarising hERG outward and depolarising CaV1.2 and NaV1.5 channels [1], [3], [19], [22], [30]. Kramer et al. [13] made use of large data sets of compounds and quantified (predicted) their torsadogenic potencies by analysing this balance by means of MICE models.
Significantly higher IC50 values for inhibition of sodium and calcium channels (compared to the block of hERG channels at submicromolar concentrations (Fig. 1S and 2S, Supplemental materials), indicate a high degree of torsadogenity for DHE and hortiamine.
The same conclusion can be drawn from our In-silico studies in which we applied statistical data learning (Fig. 6), as well as mechanistic modelling of cardiac AP (Fig. 5).
5. Conclusions and outlook
A major result of our study is that the content of the hERG inhibitors DHE and hortiamine can depend significantly on the manufacturing technology, which in turn may affect consumer safety (Fig. 1, Fig. 2, Table 1–12 Supplemental materials). Fig. 1, Fig. 2, Fig. 3, Fig. 4 illustrate that differences in DHE content were detected in hiPSC-CMs (Fig. 1, Fig. 2), and that proarrhythmic effects correlate with the content of the major hERG blocking compound DHE (Fig. 1, Fig. 2). Preparing the decoctions according to TCM procedures does not eliminate proarrhythmic activity, given the thermal stability of DHE and hortiamine (data not shown). Furthermore, the traditional TCM decoctions are increasingly replaced by ready-to-use formulations like granules. In such formulations, the alkaloid content is not known, as no specific labelling information is provided, such as a drug-extract ratio (DER) and/or information on the content of pharmacologically active compounds in the product. In the case of a herbal drug like Evodia, containing toxicologically critical compounds like DHE and hortiamine, this is an obvious regulatory gap which stands in contrast to legal requirements for phytomedicines that are registered in Europe with the regulatory authorities.
Studies in rats revealed an oral bioavailability of DHE of approximately 15 %, and a plasma protein binding of 65 % [17], but the plasma concentrations of DHE and hortiamine in humans after intake of an Evodia decoction are currently unknown. In small sized clinical studies in which Evodia preparations were administered, no information on proarrhythmic side effects (Q-T prolongation) was reported [11], [16], [28], [32].
The torsadogenity of DHE and hortiamine can, however, be approximated with regression analysis (MICE, [13] based on the estimated selectivity of the hERG channel blockers (Supplementary Fig. S2). With such an approach the torsadogenity of DHE and hortiamine were found to be comparable to gold standard hERG blockers (Fig. 6). Moreover, oscillations during AP repolarization by Evodia extracts are reproduced by AP simulations based on selective hERG inhibition (Fig. 5).
Based on the results of this and our previous studies [2], a recommendation to restrict the use of Evodia is warranted. In principle, a possible alternative approach would be to eliminate the proarrhythmic alkaloids DHE and hortiamine from Evodia products [26]. However, given that these alkaloids significantly contribute to the pharmacological properties of Evodia [15], [36] this second approach would definitively alter the properties of the resulting preparations and thus would not be practical.
Our study demonstrates that hiPSC-CMs have the potential to become an important human In vitro model for the assessment of TdP risk on herbal products. Data obtained with MEA and/or VSO technologies in hiPSC-CMs should be considered along with patch clamp studies on mammalian cell lines, statistical regression analysis [13], In-silico AP modelling to predict proarrhythmic cardiotoxicity of herbal products ([15]; Passini et al., 2017).
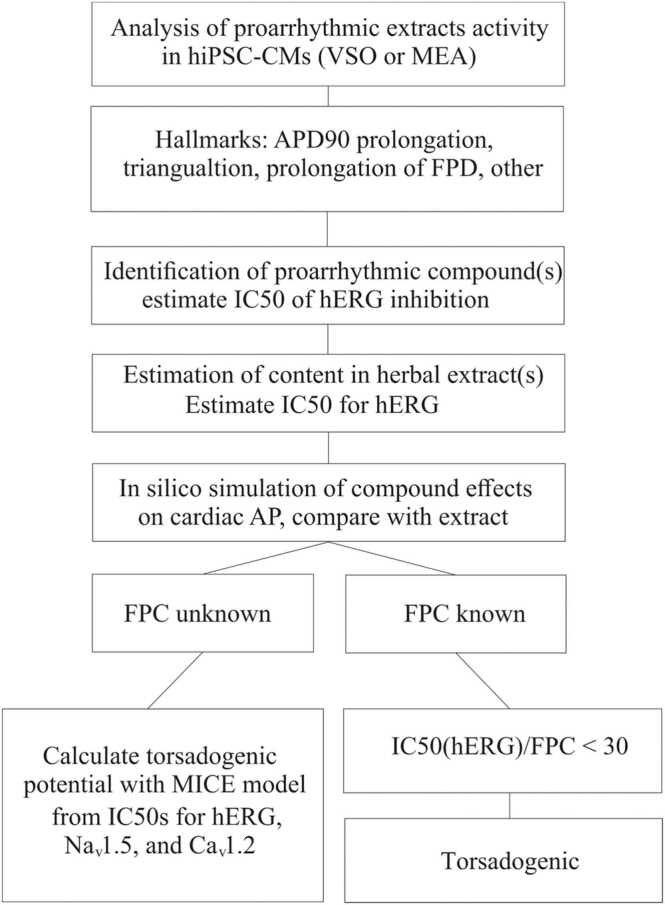
Taken together we propose an In vitro assay for the estimation of the proarrhythmic potential of Evodia and herbal extracts in general (Fig. 7). This approach combines experiments with hiPSC-CMs, identification of hERG inhibitors, estimation of blocker content, or hERG, Nav1.5 and Cav1.2 inhibition, and a calculation of the torsadogenic potential with a MICE model. Its consistent application can help to detect proarrhythmic activity In vitro and thus improve the safety of herbal products.
Fig. 7.
Estimating proarrhythmic activity of herbal products
Arrhythmogenic effects of plant extracts in hiPSC-CMs are detectable with MEA (e.g., by prolongations of the field potential or oscillations) or VSO technology (e.g., by prolongation of the APD90, triangulation or oscillations) (Fig. 1, Fig. 2, Fig. 4) [3].
Subsequent identification of hERG inhibitors e.g., by HPLC-based activity profiling; [23], estimation of their potency for hERG inhibition, and their content enables together with In-silico simulations (Fig. 5), a first judgement on potential proarrhythmic effects. Estimation of IC50 values for hERG, Cav1,.2, Nav1.5 and corresponding simulations of effects on the cardiac AP provide insights into the underlying mechanisms and the role of different ion channels.
If the free plasma concentration of an identified arrhythmogenic compound (e.g., hERG inhibitor) is known, an extract can be considered arrhythmogenic, provided the IC50(hERG) and the FPC of identified hERG inhibitor(s) satisfy IC50(hERG) / FPC < 30 (see ICH S7B). If the free plasma concentration of hERG inhibitors is unknown, estimation of IC50 values for hERG, NaV1.2 and CaV1.2 inhibition enable the calculation of a torsadogenic potential with a MICE model (Fig. 6, [13].
CRediT authorship contribution statement
Writing – original draft: Steffen Hering, Matthias Hamburger, Philipp Kügler. Writing – review & editing: Aleksandra Garifulina, Bozhidar Baltov, Udo Kraushaar. Investigation: Stanislav Beyl, Phillip Szkokan, Bozhidar Baltov, Jakob Reinhardt, Olivier Potterat. Formal Analysis: Stanislav Beyl, Bozhidar Baltov, Philipp Kügler, Eugen Timin, Igor Baburin. Funding acquisition: Steffen Hering, Matthias Hamburger. Software: Stanislav Beyl, Philipp Kügler. Conceptualization: Steffen Hering, Matthias Hamburger, Philipp Kügler, Igor Baburin.
Declaration of Competing Interest
The authors have no competing interests to declare.
Acknowledgements
This study was supported by the doctoral programme “Molecular drug targets” W1232 (B.B., S.H.) of the Austrian Science Fund (FWF) and EU Horizon 2020 grant 964518.
Handling Editor:Prof. L.H. Lash
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.toxrep.2023.04.014.
Appendix A. Supplementary material
Supplementary material.
.
Data availability
Data will be made available on request.
References
- 1.Ando H., Yoshinaga T., Yamamoto W., Asakura K., Uda T., Taniguchi T., Ojima A., Osada T., Hayashi S., Kasai C., Miyamoto N., Tashibu H., Yamazaki D., Sugiyama A., Kanda Y., Sawada K., Sekino Y., Ando H., Yoshinaga T., Asakura K., Osada T., Hayashi S., Kasai C., Tashibu H., Sugiyama A., Sawada K., Sekino Y., Ando H., Uda T., Yoshinaga T., Taniguchi T., Ojima A., Shinkyo R., Kikuchi K., Miyamoto N., Sawada K., Yamamoto W., Asakura K., Hayashi S., Osada T., Kasai C., Tashibu H., Yamazaki D., Kanda Y., Sekino Y., Sugiyama A. A new paradigm for drug-induced torsadogenic risk assessment using human iPS cell-derived cardiomyocytes. J. Pharmacol. Toxicol. Methods. 2017;84:111–127. doi: 10.1016/J.VASCN.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Baburin I., Varkevisser R., Schramm A., Saxena P., Beyl S., Szkokan P., Linder T., Stary-Weinzinger A., van der Heyden M.A.G., Houtman M., Takanari H., Jonsson M., Beekman J.H.D., Hamburger M., Vos M.A., Hering S. Dehydroevodiamine and hortiamine, alkaloids from the traditional Chinese herbal drug Evodia rutaecarpa, are I Kr blockers with proarrhythmic effects In vitro and In vivo. Pharmacol. Res. 2018;131:150–163. doi: 10.1016/J.PHRS.2018.02.024. [DOI] [PubMed] [Google Scholar]
- 3.Blinova K., Dang Q., Millard D., Smith G., Pierson J., Guo L., Brock M., Lu H.R., Kraushaar U., Zeng H., Shi H., Zhang X., Sawada K., Osada T., Kanda Y., Sekino Y., Pang L., Feaster T.K., Kettenhofen R., Stockbridge N., Strauss D.G., Gintant G. International multisite study of human-induced pluripotent stem cell-derived cardiomyocytes for drug proarrhythmic potential assessment. Cell Rep. 2018;24:3582. doi: 10.1016/J.CELREP.2018.08.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dutta S., Chang K.C., Beattie K.A., Sheng J., Tran P.N., Wu W.W., Wu M., Strauss D.G., Colatsky T., Li Z. Optimization of an in silico cardiac cell model for proarrhythmia risk assessment. Front. Physiol. 2017;8 doi: 10.3389/FPHYS.2017.00616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fink M., Niederer S.A., Cherry E.M., Fenton F.H., Koivumäki J.T., Seemann G., Thul R., Zhang H., Sachse F.B., Beard D., Crampin E.J., Smith N.P. Cardiac cell modelling: observations from the heart of the cardiac physiome project. Prog. Biophys. Mol. Biol. 2011;104:2–21. doi: 10.1016/J.PBIOMOLBIO.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Fridericia L.S. The duration of systole in an electrocardiogram in normal humans and in patients with heart disease. Ann. Noninvasive Electrocardiol. 2003 doi: 10.1046/j.1542-474X.2003.08413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamburger M. HPLC-based activity profiling for pharmacologically and toxicologically relevant natural products–principles and recent examples. Pharm. Biol. 2019 doi: 10.1080/13880209.2019.1606261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hondeghem L.M., Carlsson L., Duker G. Instability and triangulation of the action potential predict serious proarrhythmia, but action potential duration prolongation is antiarrhythmic. Circulation. 2001;103:2004–2013. doi: 10.1161/01.CIR.103.15.2004. [DOI] [PubMed] [Google Scholar]
- 9.Jæger K.H., Charwat V., Charrez B., Finsberg H., Maleckar M.M., Wall S., Healy K.E., Tveito A. Improved computational identification of drug response using optical measurements of human stem cell derived cardiomyocytes in microphysiological systems. Front. Pharmacol. 2020;10:1–24. doi: 10.3389/fphar.2019.01648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kernik D.C., Morotti S., Wu H., Di, Garg P., Duff H.J., Kurokawa J., Jalife J., Wu J.C., Grandi E., Clancy C.E. A computational model of induced pluripotent stem-cell derived cardiomyocytes incorporating experimental variability from multiple data sources. J. Physiol. 2019;597:4533–4564. doi: 10.1113/JP277724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim H.J., Park J.M., Kim J.A., Ko B.P. Effect of herbal Ephedra sinica and Evodia rutaecarpa on body composition and resting metabolic rate: a randomized, double-blind clinical trial in Korean premenopausal women. J. Acupunct. Meridian Stud. 2008;1:128–138. doi: 10.1016/S2005-2901(09)60033-9. [DOI] [PubMed] [Google Scholar]
- 12.Kopljar I., Lu H.R., Van Ammel K., Otava M., Tekle F., Teisman A., Gallacher D.J. Development of a human iPSC cardiomyocyte-based scoring system for cardiac hazard identification in early drug safety de-risking. Stem Cell Rep. 2018;11:1365–1377. doi: 10.1016/j.stemcr.2018.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kramer J., Obejero-Paz C.A., Myatt G., Kuryshev Y.A., Bruening-Wright A., Verducci J.S., Brown A.M. MICE models: superior to the HERG model in predicting Torsade de Pointes. Sci. Rep. 2013;3:1–7. doi: 10.1038/srep02100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kratz J.M., Grienke U., Scheel O., Mann S.A., Rollinger J.M. Natural products modulating the hERG channel: heartaches and hope. Nat. Prod. Rep. 2017 doi: 10.1039/c7np00014f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li M., Wang C. Traditional uses, phytochemistry, pharmacology, pharmacokinetics and toxicology of the fruit of Tetradium ruticarpum: a review. J. Ethnopharmacol. 2020 doi: 10.1016/j.jep.2020.113231. [DOI] [PubMed] [Google Scholar]
- 16.Liang X., Li B., Wu F., Li T., Wang Y., Ma Q., Liang S. Bitterness and antibacterial activities of constituents from Evodia rutaecarpa. BMC Complement. Altern. Med. 2017;17:1–7. doi: 10.1186/s12906-017-1701-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin L.C., Li S.H., Wu Y.T., Kuo K.L., Tsai T.H. Pharmacokinetics and urine metabolite identification of dehydroevodiamine in the rat. J. Agric. Food Chem. 2012;60:1595–1604. doi: 10.1021/JF204365M. [DOI] [PubMed] [Google Scholar]
- 18.Martin, J., Stoger, E.A., 2010. Praxisleitfaden TCM-Drogen. Wissenschaftliche Verlagsgesellschaft Stuttgart.
- 19.Millard D., Dang Q., Shi H., Zhang X., Strock C., Kraushaar U., Zeng H., Levesque P., Lu H.R., Guillon J.M., Wu J.C., Li Y., Luerman G., Anson B., Guo L., Clements M., Abassi Y.A., Ross J., Pierson J., Gintant G. Cross-site reliability of human induced pluripotent stem cell-derived cardiomyocyte based safety assays using microelectrode arrays: results from a blinded cipa pilot study. Toxicol. Sci. 2018;164:550–562. doi: 10.1093/toxsci/kfy110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mirams G.R., Cui Y., Sher A., Fink M., Cooper J., Heath B.M., McMahon N.C., Gavaghan D.J., Noble D. Simulation of multiple ion channel block provides improved early prediction of compounds’ clinical torsadogenic risk. Cardiovasc. Res. 2011;91:53–61. doi: 10.1093/CVR/CVR044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Hara T., Virág L., Varró A., Rudy Y. Simulation of the undiseased human cardiac ventricular action potential: model formulation and experimental validation. PLOS Comput. Biol. 2011;7 doi: 10.1371/JOURNAL.PCBI.1002061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel D., Stohlman J., Dang Q., Strauss D.G., Blinova K. Assessment of proarrhythmic potential of drugs in optogenetically paced induced pluripotent stem cell-derived cardiomyocytes. Toxicol. Sci. 2019;170:167–179. doi: 10.1093/TOXSCI/KFZ076. [DOI] [PubMed] [Google Scholar]
- 23.Potterat O., Hamburger M. Concepts and technologies for tracking bioactive compounds in natural product extracts: generation of libraries, and hyphenation of analytical processes with bioassays. Nat. Prod. Rep. 2013 doi: 10.1039/c3np20094a. [DOI] [PubMed] [Google Scholar]
- 24.Qian P., Zhang Y.B., Yang Y.F., Xu W., Yang X.W. Pharmacokinetics studies of 12 alkaloids in rat plasma after oral administration of Zuojin and Fan-Zuojin formulas. Molecules. 2017;22:214. doi: 10.3390/molecules22020214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanguinetti M.C., Tristani-Firouzi M. HERG potassium channels and cardiac arrhythmia. Nature. 2006;440:463–469. doi: 10.1038/nature04710. [DOI] [PubMed] [Google Scholar]
- 26.Schramm A., Hamburger M. Gram-scale purification of dehydroevodiamine from Evodia rutaecarpa fruits, and a procedure for selective removal of quaternary indoloquinazoline alkaloids from Evodia extracts. Fitoterapia. 2014;94:127–133. doi: 10.1016/J.FITOTE.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 27.Selzer A., Wray H.W. Quinidine syncope. Circulation. 1964;30:17–26. doi: 10.1161/01.CIR.30.1.17. [DOI] [PubMed] [Google Scholar]
- 28.Shih Y.S., Tsai C.H., Li T.C., Yu C.J., Chou J.W., Feng C.L., Wang K.T., Lai H.C., Hsieh C.L. Effect of wu chu yu tang on gastroesophageal reflux disease: randomized, double-blind, placebo-controlled trial. Phytomedicine. 2019;56:118–125. doi: 10.1016/j.phymed.2018.09.185. [DOI] [PubMed] [Google Scholar]
- 29.Stockbridge N., Morganroth J., Shah R.R., Garnett C. Dealing with global safety issues: was the response to QT-liability of non-cardiac drugs well coordinated. Drug Saf. 2013 doi: 10.1007/s40264-013-0016-z. [DOI] [PubMed] [Google Scholar]
- 30.Strauss D.G., Gintant G., Li Z., Wu W., Blinova K., Vicente J., Turner J.R., Sager P.T. Comprehensive In vitro proarrhythmia assay (CiPA) update from a cardiac safety research consortium / health and environmental sciences institute / FDA meeting. Ther. Innov. Regul. Sci. 2019;53:519–525. doi: 10.1177/2168479018795117. [DOI] [PubMed] [Google Scholar]
- 31.Tang W., Eisenbrand G. John Wiley & Sons; 2010. Handbook of Chinese Medicinal Plants. [DOI] [Google Scholar]
- 32.Yan R., Wang Y., Shen W., Liu Y., Di X. Comparative pharmacokinetics of dehydroevodiamine and coptisine in rat plasma after oral administration of single herbs and Zuojinwan prescription. Fitoterapia. 2011;82:1152–1159. doi: 10.1016/j.fitote.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 33.Yu D., Lv L., Fang L., Zhang B., Wang J., Zhan G., Zhao L., Zhao X., Li B. Inhibitory effects and mechanism of dihydroberberine on hERG channels expressed in HEK293 cells. PLOS One. 2017;12 doi: 10.1371/journal.pone.0181823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhan G., Wang F., Ding Y. Qi, Li X. Hua, Li Y. Xin, Zhao Z. Rong, Li J. Xin, Liu Y., Zhao X., Yan C. Chuan, Li B. Xin. Rutaecarpine targets hERG channels and participates in regulating electrophysiological properties leading to ventricular arrhythmia. J. Cell. Mol. Med. 2021;25:4938–4949. doi: 10.1111/jcmm.16292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao J., Han X., Zhao X., Wang C., Li Q., Chen X., Bi K. A sensitive liquid chromatographic-mass spectrometric method for simultaneous determination of dehydroevodiamine and limonin from Evodia rutaecarpa in rat plasma. Anal. Bioanal. Chem. 2011;401:289–296. doi: 10.1007/S00216-011-5072-6. [DOI] [PubMed] [Google Scholar]
- 36.Zhao Z., He X., Han W., Chen X., Liu P., Zhao X., Wang X., Zhang L., Wu S., Zheng X. Genus Tetradium L.: a comprehensive review on traditional uses, phytochemistry, and pharmacological activities. J. Ethnopharmacol. 2019 doi: 10.1016/j.jep.2018.11.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.
Data Availability Statement
Data will be made available on request.