Key Points
Question
Is MGMT promoter methylation associated with response to chemotherapy for molecularly classified low-grade and anaplastic gliomas?
Findings
In this cohort study of 411 patients, MGMT promoter methylation was independently associated with progression-free and overall survival among patients who received alkylating chemotherapy, specifically among patients with isocitrate dehydrogenase–wild-type or isocitrate dehydrogenase–mutant and 1p/19q-codeleted tumors.
Meaning
This study suggests that MGMT promoter methylation is a biomarker associated with response to alkylating chemotherapy for low-grade and anaplastic gliomas and may be considered as a stratification factor in future clinical trials.
Abstract
Importance
O6-methylguanine-DNA methyltransferase (MGMT [OMIM 156569]) promoter methylation (mMGMT) is predictive of response to alkylating chemotherapy for glioblastomas and is routinely used to guide treatment decisions. However, the utility of MGMT promoter status for low-grade and anaplastic gliomas remains unclear due to molecular heterogeneity and the lack of sufficiently large data sets.
Objective
To evaluate the association of mMGMT for low-grade and anaplastic gliomas with chemotherapy response.
Design, Setting, and Participants
This cohort study aggregated grade II and III primary glioma data from 3 prospective cohort studies with patient data collected from August 13, 1995, to August 3, 2022, comprising 411 patients: MSK-IMPACT, EORTC (European Organization of Research and Treatment of Cancer) 26951, and Columbia University. Statistical analysis was performed from April 2022 to January 2023.
Exposure
MGMT promoter methylation status.
Main Outcomes and Measures
Multivariable Cox proportional hazards regression modeling was used to assess the association of mMGMT status with progression-free survival (PFS) and overall survival (OS) after adjusting for age, sex, molecular class, grade, chemotherapy, and radiotherapy. Subgroups were stratified by treatment status and World Health Organization 2016 molecular classification.
Results
A total of 411 patients (mean [SD] age, 44.1 [14.5] years; 283 men [58%]) met the inclusion criteria, 288 of whom received alkylating chemotherapy. MGMT promoter methylation was observed in 42% of isocitrate dehydrogenase (IDH)–wild-type gliomas (56 of 135), 53% of IDH-mutant and non-codeleted gliomas (79 of 149), and 74% of IDH-mutant and 1p/19q-codeleted gliomas (94 of 127). Among patients who received chemotherapy, mMGMT was associated with improved PFS (median, 68 months [95% CI, 54-132 months] vs 30 months [95% CI, 15-54 months]; log-rank P < .001; adjusted hazard ratio [aHR] for unmethylated MGMT, 1.95 [95% CI, 1.39-2.75]; P < .001) and OS (median, 137 months [95% CI, 104 months to not reached] vs 61 months [95% CI, 47-97 months]; log-rank P < .001; aHR, 1.65 [95% CI, 1.11-2.46]; P = .01). After adjusting for clinical factors, MGMT promoter status was associated with chemotherapy response in IDH–wild-type gliomas (aHR for PFS, 2.15 [95% CI, 1.26-3.66]; P = .005; aHR for OS, 1.69 [95% CI, 0.98-2.91]; P = .06) and IDH-mutant and codeleted gliomas (aHR for PFS, 2.99 [95% CI, 1.44-6.21]; P = .003; aHR for OS, 4.21 [95% CI, 1.25-14.2]; P = .02), but not IDH-mutant and non-codeleted gliomas (aHR for PFS, 1.19 [95% CI, 0.67-2.12]; P = .56; aHR for OS, 1.07 [95% CI, 0.54-2.12]; P = .85). Among patients who did not receive chemotherapy, mMGMT status was not associated with PFS or OS.
Conclusions and Relevance
This study suggests that mMGMT is associated with response to alkylating chemotherapy for low-grade and anaplastic gliomas and may be considered as a stratification factor in future clinical trials of patients with IDH–wild-type and IDH-mutant and codeleted tumors.
This cohort study evaluates the association of MGMT promoter methylation for low-grade and anaplastic gliomas with chemotherapy response.
Introduction
Epigenetic silencing of MGMT (O6-methylguanine-DNA methyltransferase [OMIM 156569]) is considered the single most important biomarker predictive of response to temozolomide (TMZ) chemotherapy for glioblastoma (GBM).1 The MGMT gene encodes a DNA-repair protein that removes alkyl groups from the O6 position of guanine, an important site of DNA alkylation, and, therefore, is believed to confer resistance to alkylating chemotherapy.2 Conversely, epigenetic silencing of MGMT through promoter methylation (mMGMT) is associated with response to treatment.2,3,4 Despite widespread acceptance and use of MGMT promoter status to guide treatment decisions for GBM,1,5 its role as a biomarker for low-grade and anaplastic gliomas remains unclear.6,7 To our knowledge, no randomized clinical trial has yet demonstrated a role for MGMT promoter status to guide treatment decisions for grade II and III gliomas.8,9,10,11 Post hoc analyses and nonrandomized prospective studies have yielded mixed results (eTable 1 in Supplement 1),12,13,14,15,16,17,18,19,20,21,22,23,24 in part due to sample sizes that were not large enough to distinguish the molecular heterogeneity among low-grade and anaplastic gliomas.
Since 2016, the World Health Organization (WHO) has redefined gliomas along molecular features, rather than tumor morphologic characteristics.25 The molecular classification system is defined principally by the presence of an isocitrate dehydrogenase 1 or 2 (IDH1/2 [IDH1, OMIM 147700; IDH2, OMIM 147650]) mutation, which is present in more than 70% of gliomas and 5% to 7% of primary GBMs,26,27,28 and 1p/19q chromosomal codeletion, which is present in approximately 70% of oligodendrogliomas, 4% of astrocytomas, and 2% of GBMs.29 Therefore, analyses of historical clinical trials that recruited patients based on histologic features represent mixed populations of patients by current standards. Furthermore, the high frequency of co-occurrence between mMGMT and IDH mutation and/or 1p/19q codeletion makes it especially difficult to disentangle the independent association of mMGMT with treatment response.8,9,14,30,31,32 To our knowledge, no prospective study to date has found a significant association of MGMT methylation with treatment response for IDH-mutant glioma after accounting for 1p/19q status.
In this study, we sought to evaluate the association of mMGMT with treatment response for molecularly defined low-grade and anaplastic gliomas. We combined data from 3 prospective glioma cohorts, creating the largest database, to our knowledge, of treated gliomas with both 1p/19q-codeleted and non-codeleted tumors, to date, and stratified analyses based on receipt of alkylating chemotherapy.
Methods
Data Sources
Data sources were identified and selected if the source was derived from a prospective study, included information on mMGMT status and survival status, and contained individual patient-level data. We identified 3 available studies, with patient data collected from August 13, 1995, to August 3, 2022, that met these criteria. The study by Jonsson et al33 (MSK-IMPACT) was a prospective study conducted at Memorial Sloan Kettering Cancer Center designed to integrate genomic data with clinical and treatment phenotypes to assess genetic aberrations in glioma and GBM that are associated with clinical behavior, evolution of therapy, or response to therapy. A randomized clinical trial designed by the European Organization of Research and Treatment of Cancer, EORTC 26951, evaluated the effectiveness of adjuvant alkylating chemotherapy for anaplastic oligodendroglial tumors.34 A third cohort was derived from the Columbia University Irving Medical Center (CUIMC) for patients prospectively enrolled in the Comprehensive Brain Malignancy, Brain Tumor and Brain Radiotherapy Clinical Database. Detailed descriptions of the source data and variable selection and coding are available in the eMethods in Supplement 1. Data on patients treated at CUIMC in the Department of Radiation Oncology were enrolled to the Comprehensive Brain Malignancy, Brain Tumor and Brain Radiotherapy Clinical Database; these patients provided written consent. Data collection on deidentified publicly available databases (MSK-IMPACT and EORTC 26951) were exempt from review by the Columbia University institutional review board. This report followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for observational studies.
Variable Selection and Coding, Patient Selection, and Outcomes
Age, sex, molecular classification, tumor grade, chemotherapy, radiotherapy, and MGMT promoter methylation status were included as covariables. Patients were included in the study if they received a diagnosis of a primary grade II or III glioma (astrocytoma, oligodendroglioma, or oligoastrocytoma histologic characteristics). Patients were excluded if they had unknown MGMT promoter status, could not be classified by IDH and 1p/19q codeletion status, had unknown histologic grade, or were previously treated with chemotherapy or radiotherapy. The primary outcome was progression-free survival (PFS), and the secondary outcome was overall survival (OS).
Statistical Analysis
Statistical analysis was performed from April 2022 to January 2023. All statistical analyses were conducted using RStudio software, version 1.4.1106 (RStudio Inc). For our primary analysis, we measured the association between MGMT promoter status and PFS or OS among patients who received chemotherapy, stratified by molecular classification and adjusted for covariables. Exploratory analyses included PFS and OS for all patients, regardless of treatment, and for patients who did not receive chemotherapy (Figure 1). Descriptive statistics were generated and stratified by MGMT promoter status. Associations between MGMT promoter status and other clinical variables were determined using the Pearson χ2 test and the Wilcoxon rank sum test. The Fisher exact test was substituted for comparisons with expected frequency cell counts less than 5. Kaplan-Meier estimates were obtained for PFS and OS and compared between MGMT promoter status using the log-rank test. Univariable and multivariable Cox proportional hazards regression analyses of both PFS and OS were conducted. Variables that approached significance (using P < .15 as cutoff) were included in the multivariable analysis. Interactions between MGMT promoter status and chemotherapy were tested within the univariable and multivariable Cox proportional hazards regression models. The Schoenfeld test of weighted residuals was used to assess proportional hazard assumptions.35 If the proportional hazards assumptions for a variable were violated, the variable was removed and used as a stratification factor in the Cox proportional hazards regression models (as a sensitivity analysis). The Benjamini-Hochberg method was used to adjust for multiple hypothesis testing in molecular subgroups for the primary and secondary outcomes. All analyses were performed at the .05 significance level based on 2-sided statistical testing.
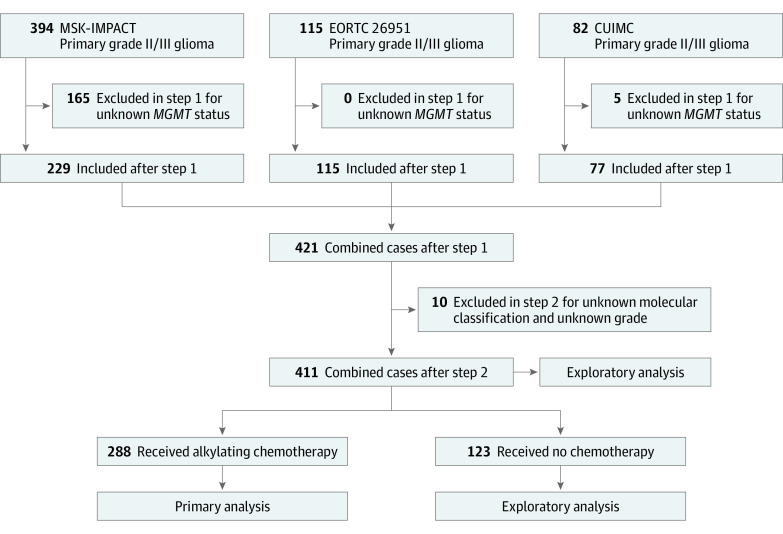
Figure 1. Schema for Patient Selection and Analyses.
CUIMC indicates Columbia University Irving Medical Center; and EORTC, European Organization of Research and Treatment of Cancer.
Results
Demographic and Clinical Characteristics
We identified 591 patients with primary grade II or III glioma, 411 (70%; mean [SD] age, 44.1 [14.5] years; 283 men [58%]) of whom met inclusion criteria with known MGMT promoter status, IDH and 1p/19q codeletion status, and tumor grade (Figure 1). Clinical and demographic characteristics are displayed in Table 1. A total of 288 patients (70%) received chemotherapy as first-line treatment before progression. Of the patients who received chemotherapy, 226 (79%) received TMZ-based regimens; 61 (21%) received procarbazine, lomustine, and vincristine (PCV); and 1 (0.3%) received carmustine (eTable 2 in Supplement 1). The MGMT promoter was methylated in 42% of IDH–wild-type gliomas (56 of 135), 53% of IDH-mutant and non-codeleted gliomas (79 of 149), and 74% of IDH-mutant and codeleted gliomas (94 of 127) (P < .001). Patients with mMGMT were more likely to receive radiotherapy (59% [135 of 229] vs 48% [87 of 182]; P = .02). The median follow-up time was 40 months (IQR, 16-84 months), with 242 progressions (59%) and 177 deaths (43%). A summary of clinical characteristics stratified by molecular class is displayed in eTables 3, 4, and 5 in Supplement 1.
Table 1. Patient Demographic and Clinical Characteristics.
| Characteristic | No. (%) | P valuea | |
|---|---|---|---|
| Methylated MGMT promoter (n = 229) | Unmethylated MGMT promoter (n = 182) | ||
| Age, median (IQR), y | 43 (35-53) | 44 (31-56) | .69 |
| Sex | |||
| Female | 96 (42) | 77 (42) | .94 |
| Male | 133 (58) | 105 (58) | |
| Molecular class | |||
| IDH–wild type | 56 (25) | 79 (43) | <.001 |
| IDH-mutant and non-codeleted | 79 (35) | 70 (39) | |
| IDH-mutant and codeleted | 94 (41) | 33 (18) | |
| Grade | |||
| II | 78 (34) | 72 (40) | .25 |
| III | 151 (66) | 110 (60) | |
| Chemotherapy | |||
| Yes | 157 (69) | 131 (72) | .45 |
| No | 72 (31) | 51 (28) | |
| Radiotherapy | |||
| Yes | 135 (59) | 87 (48) | .02 |
| No or unknownb | 94 (41) | 95 (52) | |
Calculated with the Pearson χ2 test and the Wilcoxon rank sum test.
For patients in the MSK-IMPACT data set, receipt of radiotherapy was inferred from subsequent surgical samples. Therefore, if no further surgeries were conducted, the patient was coded as “No or unknown” (eMethods in Supplement 1).
Survival Analysis of Patients Receiving Alkylating Chemotherapy
Our primary analysis included patients who received alkylating chemotherapy as part of their initial course of treatment before progression (Figure 1). The results are summarized in Table 2. Full univariable and multivariable analyses are available in eTables 6 to 13 in Supplement 1.
Table 2. Summary of Univariable and Multivariable Subgroup Analyses in Patients Who Received Chemotherapya.
| Molecular subgroup | Univariable | Multivariableb | ||||
|---|---|---|---|---|---|---|
| HR of uMGMT vs mMGMT (95% CI) | P value | P value for interaction with treatmentb | Adjusted HR of uMGMT vs mMGMT (95% CI) | P value | P value for interaction with treatment (adjusted)c | |
| Progression-free survival | ||||||
| All cases | 2.29 (1.66-3.17) | <.001 | .004 | 1.95 (1.39-2.75) | <.001d | .004 |
| IDH–wild type | 1.95 (1.15-3.30) | .01 | .004 | 2.15 (1.26-3.66) | .005d | <.001 |
| IDH-mutant and non-codeleted | 1.19 (0.67-2.12) | .56 | .41 | NRe | NRe | .94 |
| IDH-mutant and codeleted | 2.54 (1.24-5.20) | .01 | .39 | 2.99 (1.44-6.21) | .003d | .59 |
| Overall survival | ||||||
| All cases | 2.30 (1.57-3.37) | <.001 | .003 | 1.65 (1.11-2.46) | .01d | .01 |
| IDH–wild type | 1.83 (1.06-3.15) | .03 | .12 | 1.69 (0.98-2.91) | .06 | .02 |
| IDH-mutant and non-codeleted | 1.07 (0.54-2.12) | .85 | .32 | NRe | NRe | .70 |
| IDH-mutant and codeleted | 2.40 (0.81-7.17) | .12 | .21 | 4.21 (1.25-14.2) | .02d | .26 |
Abbreviations: HR, hazard ratio; mMGMT, methylated MGMT promoter; NR, not reported; uMGMT, unmethylated MGMT promoter.
Summary statistics are provided from separate univariable and multivariable analyses in patients who received alkylating chemotherapy and stratified analyses by molecular subgroups. Full univariable and multivariable analyses are included in eTables 6, 7, 8, 9, 10, 11, 12, and 13 in Supplement 1. Multiple hypothesis testing in molecular subgroups was accounted for in the multivariable analyses of progression-free and overall survival.
Variables included in the analysis were age, sex, molecular class, grade, chemotherapy, and radiotherapy. Variables that approached significance on univariable analysis, using P < .15 as a threshold, were included in the multivariable analyses.
Interaction between MGMT promoter status and chemotherapy was derived from analysis for all patients stratified by the chemotherapy and no chemotherapy groups (Figure 1; eTable 17 in Supplement 1).
Significant after adjusting for multiple hypothesis testing.
Variables that were not significant on univariable analysis were not included in the multivariable analysis and therefore not reported.
Among patients who received alkylating chemotherapy, the median PFS was 54 months (95% CI, 40-64 months), and the median OS was 92 months (95% CI, 77-120 months). The median PFS and OS were significantly longer among patients with tumors with mMGMT vs those with tumors with an unmethylated MGMT promoter (uMGMT) (PFS, 68 months [95% CI, 54-132 months] vs 30 months [95% CI, 15-54 months]; P < .001; OS, 137 months [95% CI, 104 months to not reached [NR]] vs 61 months [95% CI, 47-97 months]; P < .001) (Figure 2 and Figure 3). After adjustment for age, sex, molecular class, grade, and receipt of radiotherapy, MGMT promoter status remained significantly associated with survival (adjusted hazard ratio [aHR] of uMGMT for PFS, 1.95 [95% CI, 1.39-2.75]; P < .001; and OS, 1.65 [95% CI, 1.11-2.46]; P = .01) (Table 2).
Figure 2. Kaplan-Meier Curves for Progression-Free Survival Based on MGMT Promoter Methylation Status.
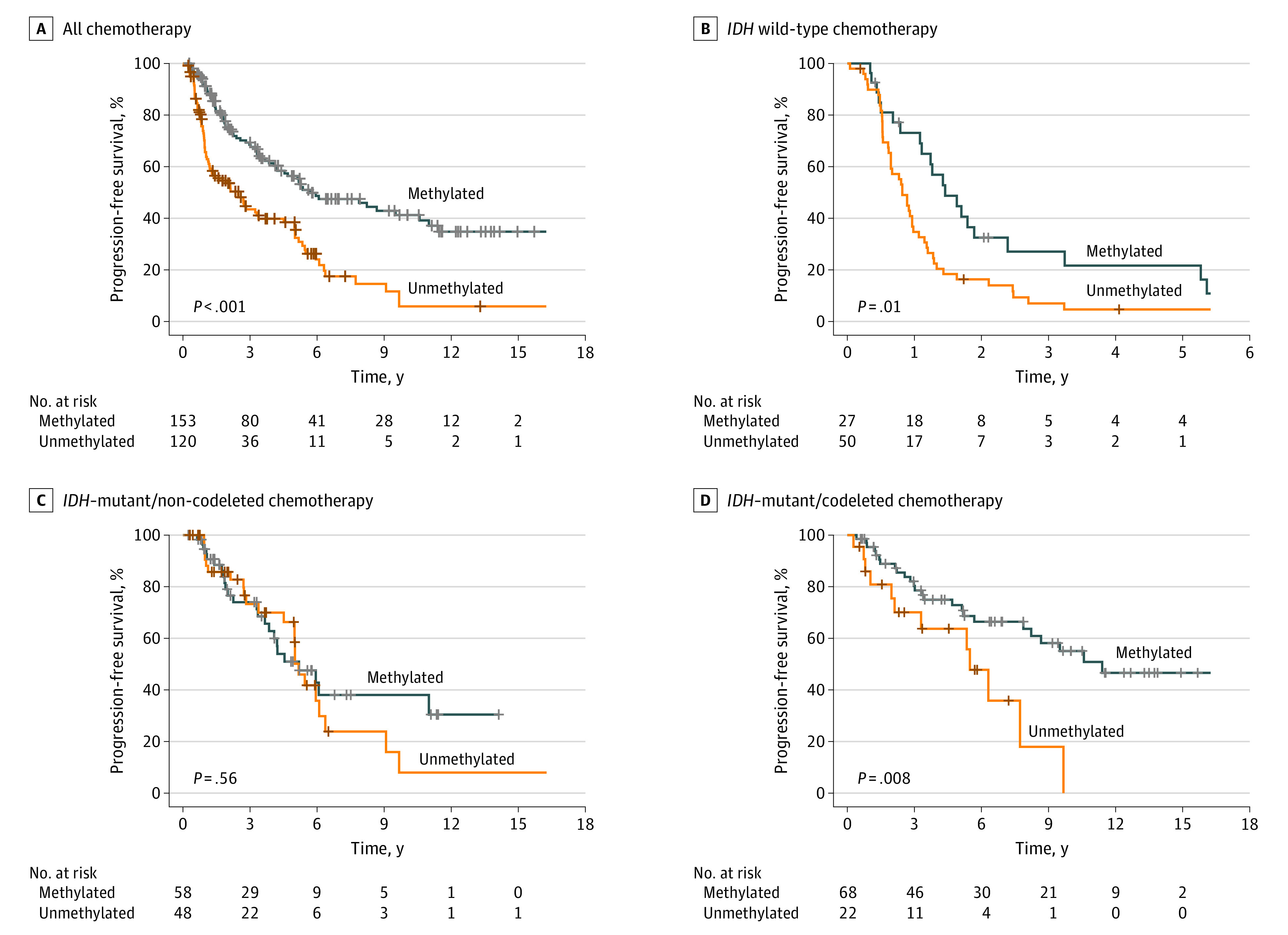
A, Patients who received chemotherapy. B, Patients with IDH–wild type tumors. C, Patients with IDH–mutant and non-codeleted tumors. D, Patients with IDH–mutant and codeleted tumors.
Figure 3. Kaplan-Meier Curves for Overall Survival Based on MGMT Promoter Methylation Status.
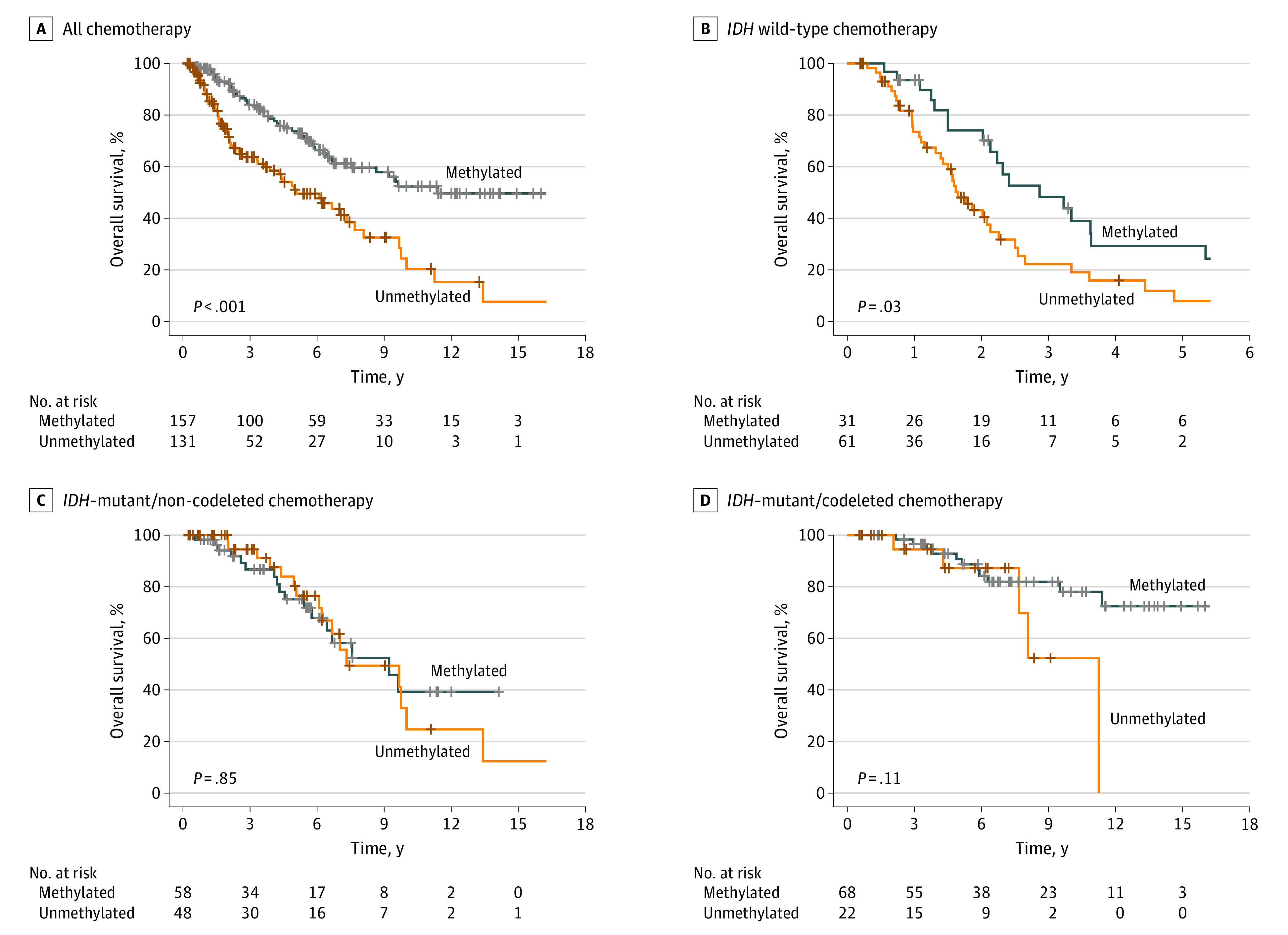
A, Patients who received chemotherapy. B, Patients with IDH–wild-type tumors. C, Patients with IDH–mutant and non-codeleted tumors. D, Patients with IDH–mutant and codeleted tumors.
We further stratified our analysis by molecular class. The median PFS times were 12 months (95% CI, 9.6-15 months) for IDH–wild-type tumors, 62 months (95% CI, 54-76 months) for IDH-mutant and non-codeleted tumors, and 114 months (95% CI, 76 months to NR) for IDH-mutant and codeleted tumors. The median OS times were 25 months (95% CI, 19-30 months) for IDH–wild-type tumor, 111 months (95% CI, 80-NR) for IDH-mutant and non-codeleted tumors, and 253 months (95% CI, 137-NR) for IDH-mutant and codeleted tumors. For IDH–wild-type tumors, mMGMT status was associated with improved median PFS compared with uMGMT status (18 months [95% CI, 13-39 months] vs 9.8 months [95% CI, 7.8-13 months]; P = .01; aHR for uMGMT, 2.15 [95% CI, 1.26-3.66], P = .005) and improved median OS compared with uMGMT status (34 months [95% CI, 26-67 months] vs 20 months [95% CI, 17-27 months]; P = .03). However, the association with OS was not significant on multivariable analysis (aHR for uMGMT, 1.69 [95% CI, 0.98-2.91]; P = .06). For IDH-mutant and codeleted tumors, mMGMT status was independently associated with PFS compared with uMGMT status (median, 11.4 years [95% CI, 8.25-NR] vs 5.5 years [95% CI, 40-NR]; P = .008; aHR for uMGMT, 2.99 [95% CI, 1.44-6.21]; P = .003). In univariable analysis, mMGMT status was not associated with OS for IDH-mutant and codeleted tumors compared with uMGMT status (median, 21.1 years [95% CI NR-NR] vs 11.3 years [95% CI, 7.7-NR]; P = .11), but mMGMT status was independently associated with OS after accounting for other known confounders (aHR for uMGMT, 4.21 [95% CI, 1.25-14.2]; P = .02).
Among IDH-mutant and non-codeleted tumors, mMGMT status was not associated with PFS compared with uMGMT status (median, 62 months [95% CI, 46-NR] vs 62 months [95% CI, 60-109 months; P = .56; aHR for uMGMT, 1.19 [95% CI, 0.67-2.12]; P = .56) or OS (median, 111 months [95% CI, 77-NR] vs 88 months [95% CI, 80-NR]; P = .85; aHR for uMGMT, 1.07 [95% CI, 0.54-2.12]; P = .85).
Interaction With Treatment
We conducted exploratory analyses for all patients, regardless of chemotherapy status, and for patients who did not receive alkylating chemotherapy before progression (Figure 1). Among all patients, the association of methylation was significant but attenuated for PFS (aHR, 1.36 [95% CI, 1.04-1.79]; P = .03) and was no longer significant for OS (aHR, 1.17 [95% CI, 0.86-1.60]; P = .31) (eTable 14 and eFigure 1 in Supplement 1). The effect size of methylation was attenuated for IDH-mutant and codeleted tumors and was no longer significant for IDH–wild-type tumors. Among patients who did not receive chemotherapy, mMGMT was not associated with poorer PFS or OS in any subgroup and was associated with improved PFS in IDH–wild-type tumors (aHR, 0.42 [95% CI, 0.21-0.85]; P = .02) (eTable 15 and eFigure 2 in Supplement 1). There was a significant interaction between mMGMT status and chemotherapy for the entire cohort (Table 2).
Sensitivity Analyses
There were 2 patients with anaplastic oligodendrogliomas in the EORTC 26951 cohort with discordant IDH1 and 1p/19q codeletion status (IDH1–wild type and 1p/19q codeleted).36 IDH2 mutational status, which accounts for approximately 10% of IDH-mutant anaplastic astrocytomas, was not available for this cohort.37 Additional molecular markers, such as ATRX and TP53, were not available to resolve this discordance.38 Because false-positive 1p/19q codeletions detected via fluorescent in situ hybridization are rare in properly worked-up samples,38 we assumed that these 2 cases were IDH-mutant and codeleted tumors. However, we performed a sensitivity analysis in which these 2 cases were coded as IDH–wild-type tumors with similar results observed (eTable 16 in Supplement 1).
In the primary analysis of PFS among patients who received chemotherapy, the proportional hazards assumption was violated for molecular class and grade (eFigures 3 and 4 in Supplement 1). Stratification by molecular class was conducted as a part of the primary analysis (Figure 2; Table 2). After stratification by molecular status, age and grade in the IDH–wild-type subgroup and grade in the IDH-mutant and non-codeleted subgroup violated the proportional hazards assumption. Nonetheless, similar results were found after stratifying our analysis by these factors (eFigure 5 and eTables 17-21 in Supplement 1). In our secondary analysis of OS among patients who received chemotherapy, the proportional hazards assumption was violated for age, molecular class, and grade (eFigures 6 and 7 in Supplement 1). There were no violations of the proportional hazards assumption after stratifying by molecular class.
Because there were only 17 deaths in the IDH-mutant and codeleted group, we performed a sensitivity analysis including only covariables that were statistically significant at the P < .05 threshold, to account for potential overfitting of the model. Similar results were observed (eTable 22 in Supplement 1). Performance status and extent of resection were available for 185 patients in the EORTC and CUIMC cohorts. Methylated MGMT promoter status was not associated with performance status or extent of resection among patients with IDH–wild-type and IDH-mutant and codeleted tumors and was associated with PFS and OS after chemotherapy in a multivariable model that included performance status and extent of resection (eFigure 8 and eTables 23-27 in Supplement 1).
Discussion
In this study, we found that mMGMT was associated with response to alkylating chemotherapy for low-grade and anaplastic gliomas. Specifically, MGMT status was associated with response to treatment for 1p/19q-codeleted and IDH–wild-type tumors in patients who received alkylating chemotherapy, but not for IDH-mutant and non-codeleted tumors, regardless of treatment status. To our knowledge, this is the first study to report an independent association of MGMT methylation with response to treatment for low-grade and anaplastic gliomas after accounting for both IDH and 1p/19q codeletion status, and it is the only study to report the association of MGMT methylation with response to treatment in 1p/19q-codeleted tumors.
Radiotherapy with neoadjuvant or adjuvant PCV is a standard therapy for anaplastic oligodendroglial tumors based on the RTOG 9402 and EORTC 26951 trials, particularly for tumors with IDH mutations and/or 1p/19q codeletions.14,39 Although IDH mutations and/or 1p1/9q codeletions have been proposed as a biomarker for benefit from treatment with PCV, our ability to isolate the association of MGMT status with response to treatment for IDH-mutant and 1p/19q-codeleted tumors suggests that MGMT status may be a better clinical biomarker for oligodendroglial tumors that are defined by modern molecular guidelines.15
This finding is particularly salient in the context of the ongoing CODEL (NCT00887146) and POLCA (NCT02444000) phase 3 clinical trials, which evaluate various alkylating strategies for 1p/19q-codeleted tumors. Given the favorable prognosis of patients with 1p/19q-codeleted gliomas, there is interest in de-escalating therapies, either with substitution of PCV with TMZ or potentially omission of radiotherapy, which are under investigation.
Despite the absence of randomized clinical trials supporting the use of TMZ over PCV and some data suggesting inferior outcomes, TMZ remains commonly used as first-line therapy in clinical practice due to its favorable hematologic toxicity profile.7,11,40,41 The initial study design of the phase 3 CODEL trial randomized patients with anaplastic 1p/19q-codeleted gliomas to receive radiotherapy alone, TMZ alone, or radiotherapy with concomitant and adjuvant TMZ.11 Analysis of the first 36 patients randomized suggested inferior PFS for patients treated with TMZ alone vs either of the radiotherapy groups. The CODEL trial was subsequently modified, and its current design compares radiotherapy plus TMZ in the experimental group with radiotherapy plus PCV in the standard group. Although MGMT status was not included as a stratification factor, correlation between exploratory biomarkers (ie, MGMT status) and survival is a prespecified secondary goal.
The phase 3 POLCA trial, currently enrolling patients in France, similarly seeks to de-escalate therapy for anaplastic 1p/19q-codeleted gliomas, testing whether omission of radiotherapy with PCV alone can improve neurocognitive deterioration without decreasing PFS and OS compared with standard-of-care radiotherapy plus PCV. Although the POLCA trial does not prespecify prospective analysis of MGMT methylation as a study goal, conclusions from our study suggest that MGMT promoter status may be evaluated in a post hoc manner when available. The CODEL and POLCA trials are expected to complete enrollment in 2025 and 2024, respectively; therefore, results will not be available for many years.
IDH–wild-type tumors, in contrast to IDH-mutant tumors, have more consistently demonstrated an association between MGMT methylation and response to treatment.17,18,20,23 Our results largely validate findings from the recently published CATNON trial, which found MGMT status to be prognostic in anaplastic IDH–wild-type gliomas.23 In the CATNON trial, there was no benefit associated with concurrent or adjuvant TMZ to radiotherapy in an unplanned subgroup analysis of patients with IDH–wild-type tumors. When the study group further defined a cohort of molecular GBMs in 157 of 751 patients randomized (defined by telomerase reverse transcriptase promoter mutation, chromosome 7 trisomy and loss of heterozygosity of chromosome 10, or epidermal growth factor receptor amplification), MGMT was prognostic, but concurrent or adjuvant TMZ was not associated with benefit in both the mMGMT and uMGMT subgroups.24 Although this post hoc analysis of the CATNON trial is insufficient to warrant a change in standard-of-care treatment, the authors stated that a well-powered prospective study on the effectiveness of TMZ in IDH–wild-type gliomas is warranted. MGMT status may be considered as a stratification factor, in the same way as it has been done in contemporary GBM trials defined by histologic and molecular characteristics.3,4,42
In the CATNON trial, IDH-mutant and non-codeleted tumors benefited from adjuvant but not concurrent TMZ, which defines a new standard of care.10,23,43 Consistent with our findings, MGMT status was not associated with response to TMZ in IDH-mutant and non-codeleted tumors. The interaction between MGMT and codeletion status that we demonstrated in our study may resolve the discordant results observed from previous studies, which inconsistently found MGMT status to be associated with response to treatment in IDH-mutant tumors but did not simultaneously account for 1p/19q codeletion status (eTable 1 in Supplement 1).9,13,14,15,17,18,19,20,22 Our results emphasize that IDH-mutant and codeleted and non-codeleted tumors may be considered separate entities with regard to the association of mMGMT with response to treatment.
In the most recent update of the WHO Classification of Tumors of the Central Nervous System, published in November 2021, MGMT status does not currently change grading of gliomas or GBMs but may be incorporated into future updates.44 Challenges in the standardization of laboratory assays to detect mMGMT hinders widespread adoption in clinical practice. There is considerable variability between techniques, and it has been shown that specific methylation sites on CpG islands within the MGMT promoter may be more associated with response to treatment than others.15 The most recent clinical trials have used a methylation-specific polymerase chain reaction technique with the STP27 algorithm.9,10,15,16,20,22
Strengths and Limitations
Our study has some strengths, including analysis of prospectively enrolled patients with high-quality data. With the exception of the recently published CATNON second interim analysis, this is one of the largest studies of MGMT methylation in prospectively collected low-grade and anaplastic gliomas and the largest of 1p/19q-codeleted gliomas, to our knowledge.
Our study also has some limitations, including retrospective or post hoc analysis of prospectively enrolled patients. Treatments and follow-up are heterogenous, and thus unmeasured confounders may have affected our results.8,34,45,46 Definitions of progression may have varied slightly across the data sets. Radiotherapy was more commonly delivered to patients with methylated tumors, likely because radiotherapy was the standard group in the EORTC study of anaplastic oligodendroglial tumors. Given that MGMT status is not routinely incorporated into treatment-related decisions for low-grade and anaplastic gliomas, it is unlikely that clinician knowledge of MGMT status, when applicable, was associated with treatment choices. Finally, because deaths were rare for patients with 1p/19q-codeleted tumors, the association of MGMT status with OS depended on the statistical parameters used. Validation with longer follow-up would support that the PFS advantage observed translates into an OS benefit. Because we are the first to report an association of mMGMT with treatment response for 1p/19q-codeleted tumors, our work should be externally validated.
Conclusions
Our cohort study shows that MGMT status was associated with response to alkylating chemotherapy for molecularly classified low-grade and anaplastic gliomas. Specifically, it was associated with response to chemotherapy among patients with IDH–wild-type or IDH-mutant and codeleted tumors. Therefore, MGMT status may be considered a stratification factor in future clinical trials evaluating the effectiveness of alkylating chemotherapy. This may be particularly relevant for IDH–wild-type and IDH-mutant and codeleted tumors, in which the role of TMZ and/or PCV in standard-of-care treatment remains obscure and an area of active investigation.
eMethods
eTable 1. Review of Prospective Studies that Evaluated MGMT Promoter Methylation in Primary Low Grade and Anaplastic Gliomas
eReferences.
eTable 2. Chemotherapy Regimens Used as First-Line Treatment
eTable 3. Patient Demographical and Clinical Characteristics in IDH-Wildtype Tumors
eTable 4. Patient Demographical and Clinical Characteristics in IDH-Mutant/Non-Codeleted Tumors
eTable 5. Patient Demographical and Clinical Characteristics in IDH-Mutant/Codeleted Tumors
eTable 6. Univariable and Multivariable Analysis of Progression-Free Survival in All Patients that Received Chemotherapy
eTable 7. Univariable and Multivariable Analysis of Progression-Free Survival in IDH-wildtype Patients that Received Chemotherapy
eTable 8. Univariable and Multivariable Analysis of Progression-Free Survival in IDH-Mutant/Non-codeleted Patients that Received Chemotherapy
eTable 9. Univariable and Multivariable Analysis of Progression-Free Survival in IDH-Mutant/Codeleted Patients that Received Chemotherapy
eTable 10. Univariable and Multivariable Analysis of Overall Survival in All Patients that Received Chemotherapy
eTable 11. Univariable and Multivariable Analysis of Overall Survival in IDH-Wildtype Patients That Received Chemotherapy
eTable 12. Univariable and Multivariable Analysis of Overall Survival in IDH-Mutant/Non-codeleted Patients That Received Chemotherapy
eTable 13. Univariable and Multivariable Analysis of Overall Survival in IDH-Mutant/Codeleted Patients That Received Chemotherapy
eTable 14. Summary of Univariable and Multivariable Subgroup Analyses in All Patients With Any First-Line Treatment
Table 15. Summary of Univariable and Multivariable Subgroup Analyses in Patients That Received No Chemotherapy During First-Line Treatment
eTable 16. Summary of Sensitivity Analysis of Univariable and Multivariable Subgroup Analyses in Patients That Received Chemotherapy
eTable 17. Univariable and Multivariable Analysis of Progression-Free Survival in Age 0 – 43 IDH-Wildtype Patients That Received Chemotherapy
eTable 18. Univariable and Multivariable Analysis of Progression-Free Survival in Age 44+ IDH-wildtype Patients That Received Chemotherapy
eTable 19. Univariable and Multivariable Analysis of Progression-Free Survival in Grade III IDH-Wildtype Patients That Received Chemotherapy
eTable 20. Univariable and Multivariable Analysis of Progression-Free Survival in Grade II IDH-Mutant/Non-codeleted Patients That Received Chemotherapy
eTable 21. Univariable and Multivariable Analysis of Progression-Free Survival in Grade III IDH-Mutant/Non-codeleted Patients That Received Chemotherapy
eTable 22. Univariable and Multivariable Analysis of Overall Survival in IDH-Mutant/Codeleted Patients That Received Chemotherapy, Using P < .05 as Threshold for Inclusion
eTable 23. Patient Demographic and Clinical Characteristics of IDH-Wildtype and IDH-Mutant/Codeleted Patients in the EORTC/CUIMC Cohorts
eTable 24. Univariable and Multivariable Analysis of Progression-Free Survival in Patients With IDH-Wildtype and IDH-Mutant/Codeleted Tumors That Received Chemotherapy in the EORTC/CUIMC Cohorts, Including Performance Status and Extent of Resection
eTable 25. Univariable and Multivariable Analysis of Progression-Free Survival in Patients With IDH-Wildtype and IDH-Mutant/Codeleted Tumors That Received No Chemotherapy in the EORTC/CUIMC Cohorts, Including Performance Status and Extent of Resection
eTable 26. Univariable and Multivariable Analysis of Overall Survival in Patients With IDH-Wildtype and IDH-Mutant/Codeleted Tumors That Received Chemotherapy in the EORTC/CUIMC Cohorts, Including Performance Status and Extent of Resection
eTable 27. Univariable and Multivariable Analysis of Overall Survival in Patients With IDH-Wildtype and IDH-Mutant/Codeleted Tumors That Received No Chemotherapy in the EORTC/CUIMC Cohorts, Including Performance Status and Extent of Resection
eFigure 1. Kaplan-Meier Curves for Progression-Free (A) and Overall (B) Survival Based on MGMT Promoter Methylation Status in All Patients, Regardless of Treatment Status
eFigure 2. Kaplan-Meier Curves for (A-D) Progression-Free Survival and (E-H) Overall Survival Based on MGMT Promoter Methylation Status in Patients That Did Not Receive Chemotherapy
eFigure 3. Schoenfeld Residual Plots of PFS for MGMT Status
eFigure 4. Schoenfeld Residual Plots of PFS for MGMT Status
eFigure 5. Kaplan-Meier Curves for Progression-Free Survival Based on MGMT Promoter Methylation Status in Patients That Received Chemotherapy
eFigure 6. Schoenfeld Residual Plots of OS for MGMT Status
eFigure 7. Schoenfeld Residual Plots of OS for MGMT Status
eFigure 8. Kaplan-Meier Curves Based on MGMT Promoter Status for Progression-Free Survival (A, B) and Overall Survival (C, D) in Patients With IDH-Wildtype or IDH-Mutant/Codeleted Tumors in the EORTC/CUIMC Cohorts
Data Sharing Statement
References
- 1.Weller M, van den Bent M, Preusser M, et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol. 2021;18(3):170-186. doi: 10.1038/s41571-020-00447-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997-1003. doi: 10.1056/NEJMoa043331 [DOI] [PubMed] [Google Scholar]
- 3.Wick W, Platten M, Meisner C, et al. ; NOA-08 Study Group of Neuro-oncology Working Group (NOA) of German Cancer Society . Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13(7):707-715. doi: 10.1016/S1470-2045(12)70164-X [DOI] [PubMed] [Google Scholar]
- 4.Malmström A, Grønberg BH, Marosi C, et al. ; Nordic Clinical Brain Tumour Study Group (NCBTSG) . Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13(9):916-926. doi: 10.1016/S1470-2045(12)70265-6 [DOI] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology: Central Nervous System Cancers. Version 1.2021. National Comprehensive Cancer Network; 2021. [Google Scholar]
- 6.Mair MJ, Geurts M, van den Bent MJ, Berghoff AS. A basic review on systemic treatment options in WHO grade II-III gliomas. Cancer Treat Rev. 2021;92:102124. doi: 10.1016/j.ctrv.2020.102124 [DOI] [PubMed] [Google Scholar]
- 7.Lassman AB, Cloughesy TF. Early results from the CODEL trial for anaplastic oligodendrogliomas: is temozolomide futile? Neuro Oncol. 2021;23(3):347-349. doi: 10.1093/neuonc/noab006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wick W, Hartmann C, Engel C, et al. NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J Clin Oncol. 2009;27(35):5874-5880. doi: 10.1200/JCO.2009.23.6497 [DOI] [PubMed] [Google Scholar]
- 9.Baumert BG, Hegi ME, van den Bent MJ, et al. Temozolomide chemotherapy versus radiotherapy in high-risk low-grade glioma (EORTC 22033-26033): a randomised, open-label, phase 3 intergroup study. Lancet Oncol. 2016;17(11):1521-1532. doi: 10.1016/S1470-2045(16)30313-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van den Bent MJ, Tesileanu CMS, Wick W, et al. Adjuvant and concurrent temozolomide for 1p/19q non-co-deleted anaplastic glioma (CATNON; EORTC study 26053-22054): second interim analysis of a randomised, open-label, phase 3 study. Lancet Oncol. 2021;22(6):813-823. doi: 10.1016/S1470-2045(21)00090-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaeckle KA, Ballman KV, van den Bent M, et al. CODEL: phase III study of RT, RT + TMZ, or TMZ for newly diagnosed 1p/19q codeleted oligodendroglioma: analysis from the initial study design. Neuro Oncol. 2021;23(3):457-467. doi: 10.1093/neuonc/noaa168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van den Bent MJ, Dubbink HJ, Sanson M, et al. MGMT promoter methylation is prognostic but not predictive for outcome to adjuvant PCV chemotherapy in anaplastic oligodendroglial tumors: a report from EORTC Brain Tumor Group Study 26951. J Clin Oncol. 2009;27(35):5881-5886. doi: 10.1200/JCO.2009.24.1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van den Bent MJ, Dubbink HJ, Marie Y, et al. IDH1 and IDH2 mutations are prognostic but not predictive for outcome in anaplastic oligodendroglial tumors: a report of the European Organization for Research and Treatment of Cancer Brain Tumor Group. Clin Cancer Res. 2010;16(5):1597-1604. doi: 10.1158/1078-0432.CCR-09-2902 [DOI] [PubMed] [Google Scholar]
- 14.van den Bent MJ, Brandes AA, Taphoorn MJB, et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC Brain Tumor Group Study 26951. J Clin Oncol. 2013;31(3):344-350. doi: 10.1200/JCO.2012.43.2229 [DOI] [PubMed] [Google Scholar]
- 15.van den Bent MJ, Erdem-Eraslan L, Idbaih A, et al. MGMT-STP27 methylation status as predictive marker for response to PCV in anaplastic oligodendrogliomas and oligoastrocytomas: a report from EORTC study 26951. Clin Cancer Res. 2013;19(19):5513-5522. doi: 10.1158/1078-0432.CCR-13-1157 [DOI] [PubMed] [Google Scholar]
- 16.Dubbink HJ, Atmodimedjo PN, Kros JM, et al. Molecular classification of anaplastic oligodendroglioma using next-generation sequencing: a report of the prospective randomized EORTC Brain Tumor Group 26951 phase III trial. Neuro Oncol. 2016;18(3):388-400. doi: 10.1093/neuonc/nov182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wick W, Meisner C, Hentschel B, et al. Prognostic or predictive value of MGMT promoter methylation in gliomas depends on IDH1 mutation. Neurology. 2013;81(17):1515-1522. doi: 10.1212/WNL.0b013e3182a95680 [DOI] [PubMed] [Google Scholar]
- 18.Wick W, Roth P, Hartmann C, et al. ; Neurooncology Working Group (NOA) of the German Cancer Society . Long-term analysis of the NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with PCV or temozolomide. Neuro Oncol. 2016;18(11):1529-1537. doi: 10.1093/neuonc/now133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hartmann C, Hentschel B, Wick W, et al. Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas. Acta Neuropathol. 2010;120(6):707-718. doi: 10.1007/s00401-010-0781-z [DOI] [PubMed] [Google Scholar]
- 20.Bell EH, Zhang P, Fisher BJ, et al. Association of MGMT promoter methylation status with survival outcomes in patients with high-risk glioma treated with radiotherapy and temozolomide: an analysis from the NRG Oncology/RTOG 0424 trial. JAMA Oncol. 2018;4(10):1405-1409. doi: 10.1001/jamaoncol.2018.1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fleming JL, Pugh SL, Fisher BJ, et al. Long-term report of a comprehensive molecular and genomic analysis in NRG Oncology/RTOG 0424: a phase II study of radiation and temozolomide in high-risk grade II glioma. JCO Precis Oncol. 2021;5(5):1397-1407. doi: 10.1200/PO.21.00112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bell EH, Zhang P, Shaw EG, et al. Comprehensive genomic analysis in NRG Oncology/RTOG 9802: a phase III trial of radiation versus radiation plus procarbazine, lomustine (CCNU), and vincristine in high-risk low-grade glioma. J Clin Oncol. 2020;38(29):3407-3417. doi: 10.1200/JCO.19.02983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tesileanu CMS, van den Bent MJ, Sanson M, et al. Prognostic significance of genome-wide DNA methylation profiles within the randomized, phase 3, EORTC CATNON trial on non-1p/19q deleted anaplastic glioma. Neuro Oncol. 2021;23(9):1547-1559. doi: 10.1093/neuonc/noab088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tesileanu CMS, Sanson M, Wick W, et al. Temozolomide and radiotherapy versus radiotherapy alone in patients with glioblastoma, IDH-wildtype: post hoc analysis of the EORTC randomized phase III CATNON trial. Clin Cancer Res. 2022;28(12):2527-2535. doi: 10.1158/1078-0432.CCR-21-4283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803-820. doi: 10.1007/s00401-016-1545-1 [DOI] [PubMed] [Google Scholar]
- 26.Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321(5897):1807-1812. doi: 10.1126/science.1164382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765-773. doi: 10.1056/NEJMoa0808710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brennan CW, Verhaak RG, McKenna A, et al. ; TCGA Research Network . The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462-477. doi: 10.1016/j.cell.2013.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kinslow CJ, Canoll P, Cheng SK, Wang TJC. Misclassification of diffuse gliomas—letter. Clin Cancer Res. 2020;26(5):1198. doi: 10.1158/1078-0432.CCR-19-3257 [DOI] [PubMed] [Google Scholar]
- 30.Turcan S, Rohle D, Goenka A, et al. IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature. 2012;483(7390):479-483. doi: 10.1038/nature10866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gan HK, Rosenthal MA, Dowling A, et al. A phase II trial of primary temozolomide in patients with grade III oligodendroglial brain tumors. Neuro Oncol. 2010;12(5):500-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeAngelis LM. Anaplastic glioma: how to prognosticate outcome and choose a treatment strategy. J Clin Oncol. 2009;27(35):5861-5862. doi: 10.1200/JCO.2009.24.5985 [DOI] [PubMed] [Google Scholar]
- 33.Jonsson P, Lin AL, Young RJ, et al. Genomic correlates of disease progression and treatment response in prospectively characterized gliomas. Clin Cancer Res. 2019;25(18):5537-5547. doi: 10.1158/1078-0432.CCR-19-0032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van den Bent MJ, Carpentier AF, Brandes AA, et al. Adjuvant procarbazine, lomustine, and vincristine improves progression-free survival but not overall survival in newly diagnosed anaplastic oligodendrogliomas and oligoastrocytomas: a randomized European Organisation for Research and Treatment of Cancer phase III trial. J Clin Oncol. 2006;24(18):2715-2722. doi: 10.1200/JCO.2005.04.6078 [DOI] [PubMed] [Google Scholar]
- 35.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515-526. doi: 10.1093/biomet/81.3.515 [DOI] [Google Scholar]
- 36.Labussière M, Idbaih A, Wang XW, et al. All the 1p19q codeleted gliomas are mutated on IDH1 or IDH2. Neurology. 2010;74(23):1886-1890. doi: 10.1212/WNL.0b013e3181e1cf3a [DOI] [PubMed] [Google Scholar]
- 37.Brat DJ, Verhaak RG, Aldape KD, et al. ; Cancer Genome Atlas Research Network . Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med. 2015;372(26):2481-2498. doi: 10.1056/NEJMoa1402121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ball MK, Kollmeyer TM, Praska CE, et al. Frequency of false-positive FISH 1p/19q codeletion in adult diffuse astrocytic gliomas. Neurooncol Adv. 2020;2(1):vdaa109. doi: 10.1093/noajnl/vdaa109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cairncross JG, Wang M, Jenkins RB, et al. Benefit from procarbazine, lomustine, and vincristine in oligodendroglial tumors is associated with mutation of IDH. J Clin Oncol. 2014;32(8):783-790. doi: 10.1200/JCO.2013.49.3726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lassman AB, Iwamoto FM, Cloughesy TF, et al. International retrospective study of over 1000 adults with anaplastic oligodendroglial tumors. Neuro Oncol. 2011;13(6):649-659. doi: 10.1093/neuonc/nor040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Panageas KS, Iwamoto FM, Cloughesy TF, et al. Initial treatment patterns over time for anaplastic oligodendroglial tumors. Neuro Oncol. 2012;14(6):761-767. doi: 10.1093/neuonc/nos065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stupp R, Taillibert S, Kanner A, et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA. 2017;318(23):2306-2316. doi: 10.1001/jama.2017.18718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van den Bent MJ, Baumert B, Erridge SC, et al. Interim results from the CATNON trial (EORTC study 26053-22054) of treatment with concurrent and adjuvant temozolomide for 1p/19q non–co-deleted anaplastic glioma: a phase 3, randomised, open-label intergroup study. Lancet. 2017;390(10103):1645-1653. doi: 10.1016/S0140-6736(17)31442-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Louis DN, Perry A, Wesseling P, et al. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol. 2021;23(8):1231-1251. doi: 10.1093/neuonc/noab106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garton ALA, Kinslow CJ, Rae AI, et al. Extent of resection, molecular signature, and survival in 1p19q-codeleted gliomas. J Neurosurg. 2020;134(5):1357-1367. doi: 10.3171/2020.2.JNS192767 [DOI] [PubMed] [Google Scholar]
- 46.Kinslow CJ, Garton ALA, Rae AI, et al. Extent of resection and survival for oligodendroglioma: a U.S. population-based study. J Neurooncol. 2019;144(3):591-601. doi: 10.1007/s11060-019-03261-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
eTable 1. Review of Prospective Studies that Evaluated MGMT Promoter Methylation in Primary Low Grade and Anaplastic Gliomas
eReferences.
eTable 2. Chemotherapy Regimens Used as First-Line Treatment
eTable 3. Patient Demographical and Clinical Characteristics in IDH-Wildtype Tumors
eTable 4. Patient Demographical and Clinical Characteristics in IDH-Mutant/Non-Codeleted Tumors
eTable 5. Patient Demographical and Clinical Characteristics in IDH-Mutant/Codeleted Tumors
eTable 6. Univariable and Multivariable Analysis of Progression-Free Survival in All Patients that Received Chemotherapy
eTable 7. Univariable and Multivariable Analysis of Progression-Free Survival in IDH-wildtype Patients that Received Chemotherapy
eTable 8. Univariable and Multivariable Analysis of Progression-Free Survival in IDH-Mutant/Non-codeleted Patients that Received Chemotherapy
eTable 9. Univariable and Multivariable Analysis of Progression-Free Survival in IDH-Mutant/Codeleted Patients that Received Chemotherapy
eTable 10. Univariable and Multivariable Analysis of Overall Survival in All Patients that Received Chemotherapy
eTable 11. Univariable and Multivariable Analysis of Overall Survival in IDH-Wildtype Patients That Received Chemotherapy
eTable 12. Univariable and Multivariable Analysis of Overall Survival in IDH-Mutant/Non-codeleted Patients That Received Chemotherapy
eTable 13. Univariable and Multivariable Analysis of Overall Survival in IDH-Mutant/Codeleted Patients That Received Chemotherapy
eTable 14. Summary of Univariable and Multivariable Subgroup Analyses in All Patients With Any First-Line Treatment
Table 15. Summary of Univariable and Multivariable Subgroup Analyses in Patients That Received No Chemotherapy During First-Line Treatment
eTable 16. Summary of Sensitivity Analysis of Univariable and Multivariable Subgroup Analyses in Patients That Received Chemotherapy
eTable 17. Univariable and Multivariable Analysis of Progression-Free Survival in Age 0 – 43 IDH-Wildtype Patients That Received Chemotherapy
eTable 18. Univariable and Multivariable Analysis of Progression-Free Survival in Age 44+ IDH-wildtype Patients That Received Chemotherapy
eTable 19. Univariable and Multivariable Analysis of Progression-Free Survival in Grade III IDH-Wildtype Patients That Received Chemotherapy
eTable 20. Univariable and Multivariable Analysis of Progression-Free Survival in Grade II IDH-Mutant/Non-codeleted Patients That Received Chemotherapy
eTable 21. Univariable and Multivariable Analysis of Progression-Free Survival in Grade III IDH-Mutant/Non-codeleted Patients That Received Chemotherapy
eTable 22. Univariable and Multivariable Analysis of Overall Survival in IDH-Mutant/Codeleted Patients That Received Chemotherapy, Using P < .05 as Threshold for Inclusion
eTable 23. Patient Demographic and Clinical Characteristics of IDH-Wildtype and IDH-Mutant/Codeleted Patients in the EORTC/CUIMC Cohorts
eTable 24. Univariable and Multivariable Analysis of Progression-Free Survival in Patients With IDH-Wildtype and IDH-Mutant/Codeleted Tumors That Received Chemotherapy in the EORTC/CUIMC Cohorts, Including Performance Status and Extent of Resection
eTable 25. Univariable and Multivariable Analysis of Progression-Free Survival in Patients With IDH-Wildtype and IDH-Mutant/Codeleted Tumors That Received No Chemotherapy in the EORTC/CUIMC Cohorts, Including Performance Status and Extent of Resection
eTable 26. Univariable and Multivariable Analysis of Overall Survival in Patients With IDH-Wildtype and IDH-Mutant/Codeleted Tumors That Received Chemotherapy in the EORTC/CUIMC Cohorts, Including Performance Status and Extent of Resection
eTable 27. Univariable and Multivariable Analysis of Overall Survival in Patients With IDH-Wildtype and IDH-Mutant/Codeleted Tumors That Received No Chemotherapy in the EORTC/CUIMC Cohorts, Including Performance Status and Extent of Resection
eFigure 1. Kaplan-Meier Curves for Progression-Free (A) and Overall (B) Survival Based on MGMT Promoter Methylation Status in All Patients, Regardless of Treatment Status
eFigure 2. Kaplan-Meier Curves for (A-D) Progression-Free Survival and (E-H) Overall Survival Based on MGMT Promoter Methylation Status in Patients That Did Not Receive Chemotherapy
eFigure 3. Schoenfeld Residual Plots of PFS for MGMT Status
eFigure 4. Schoenfeld Residual Plots of PFS for MGMT Status
eFigure 5. Kaplan-Meier Curves for Progression-Free Survival Based on MGMT Promoter Methylation Status in Patients That Received Chemotherapy
eFigure 6. Schoenfeld Residual Plots of OS for MGMT Status
eFigure 7. Schoenfeld Residual Plots of OS for MGMT Status
eFigure 8. Kaplan-Meier Curves Based on MGMT Promoter Status for Progression-Free Survival (A, B) and Overall Survival (C, D) in Patients With IDH-Wildtype or IDH-Mutant/Codeleted Tumors in the EORTC/CUIMC Cohorts
Data Sharing Statement