Graphical abstract
Keywords: Antioxidant, Disease prevention, Elaeagnus umbellata, Phytochemicals, Therapeutics
Abstract
Medicinal plants encompassing a series of bioactive compounds have gained significant importance for use in the treatment of different diseases. Of them, Elaeagnus umbellata Thunb. (Deciduous shrub found in dappled shade, and sunny hedge) exhibits high medicinal value, with a widespread distribution across the Pir Panjal region of the Himalayas. Fruits serve as an excellent source of vitamins, minerals, and other essential compounds that exhibits hypolipidemic, hepatoprotective, and nephroprotective effects. The phytochemical fingerprint of berries revealed them to have a high content of polyphenols (with major proportion of anthocyanins), followed by monoterpenes and vitamin C. Extract of fruits help in regulating the digestion and absorption of glucose and reduces inflammation and oxidative stress. The phytosterols upholding anticoagulant activity serve the purpose of causing decrease in angina and the blood cholesterol levels. Phytochemicals such as eugenol, palmitic acid, and methyl palmitate exhibit potent antibacterial activity against broad range of disease-causing agents. Additionally, a high percentage of essential oils attribute it with the property of being effective against heart ailments. The present study highlights the importance of E. umbellata in traditional medicinal practices, and summarizes the knowledge of its bioactive constituents and a snapshot vision of remarkable biological activities like antimicrobial, antidiabetic, antioxidant, etc towards understanding its role in the development of efficient drug regimens for use in the treatment of different diseases. It also underlines the need to explore the plant on nutritional aspects to strengthen the existing knowledge pertaining to health promoting potential of E. umbellata.
1. Introduction
Plants (especially medicinal ones) with history to different civilizations serve the purpose of medicine since ancient times. They were inducted to human diet not only for nutritional value but for use as prophylactics and therapeutic agent in the treatment of different diseases. With a major portion of medicinal plants demarked in the Himalayan belt, in India, it fulfills a major proportion (∼80%) of its demand for use in Ayurvedic medicine, followed by Unani medicine (46%) and a sufficient amount (∼33%) of the allopathic drugs (Samant et al., 2007, Neeta et al., 2011). Though 80% of the drugs are synthesized from plants, their utilization as part of the traditional medical system occurs in the area where they grow (Bauer and Brönstrup, 2014, Pešić and Stanković, 2015). The medicinal value attributed to plants owe to the production of secondary metabolites or bioactive moieties commonly referred to as phytochemicals, which includes a series of flavonoids, alkaloids, terpenoids, essential oils, etc (Tariq et al., 2015, AlSheikh et al., 2020). These phytochemicals unfold important medicinal properties such as analgesic, antioxidant, and others, with a definite physiological action within the complex system of the human body (Zglińska et al., 2021). World Health Organization (WHO) in its report suggested that about 80% of the world’s population still follows the practice of traditional medicine for use in the treatment of different diseases (Sen and Chakraborty, 2017). Lately, a major shift was observed in studying bioactive compounds produced by common dietary items (Fruits, vegetables, herbs, etc) in connection with their isolation followed by identification using advanced methodologies towards the development of potent drug alternatives.
Plants with unique medicinal properties are considered as important source to indigenous system of medicine. In consideration of this, resurgence in following the traditional treatment strategies has led to increase in studies on plants with respect to their wide range of bioactive compounds with strong correlation for use in the treatment of disease or in providing the structural active molecules for use in the development of different drugs (Zglińska et al., 2021). Despite the popularity of plant-based secondary metabolites in protecting plants from different stresses, their use as prophylactics and therapeutic agents in the Indian folk medicine has led to decline of their population in natural habitats that often proceeds with the imposition of restrictions on their use and exploitation as a source of medicine. Herein, we studied the occurence and distribution of an important medicinal plant Elaeagnus umbellata in terms of habitat, morphology, traditional importance and importantly production of large number of bioactive compounds in context to their use in the treatment of different human diseases.
2. Elaeagnus umbellata Thunb.
The Elaeagnus family (Elaeagnaceae; native to Northern Hemisphere) comprises 3 genera with 45–64 species. Of the different members, Elaeagnus umbellata Thunb. (also referred to as Autumn Olive) exhibits high medicinal value, with a widespread distribution across the Pir Panjal region of the Himalayas (Dirr, 1990, Ahmad et al., 2005). Its cultivation fulfills the seasonal food shortage for wildlife, besides being used as ornamental, fuel wood, and in making baskets, shelter belts, etc (Munger, 2003). E. umbellata is found at 1200–2100 m a.m.s.l and grows at varying temperatures ranging from 43 to 55 °C (Ahmad and Kamal, 2002) and pH range of 5.5–9.5 (Ahmad et al., 2005). It exhibits remarkable differences in traits such as branches size per plant, size and number of thorns on stem, surface area of leaves, number of leaves and fruits per branch, fruit per bunch and their pulp weight, besides showing differences in biochemical profile such as vitamin C, pulp, and measure of seed oil (Sabir et al., 2003). It is planted in arid settings as a protective hedge around farms, homes, and gardens. It exhibits the property to grow at low temperatures and has a tolerance for pruning. It possesses root nodules that attribute it with the property to fix atmospheric nitrogen (Sternberg, 1982, Paschke et al., 1989). It has the ability to prevent soil erosion, besides serving as an attractant to wildlife (Ahmad et al., 2005, Love, 2020). Its ability to withstand high salt concentration and drought conditions makes it a perfect fit to repair sand mounds along the shore and support vegetation along degraded parts of mountainous terrain (Love, 2020, Gardner, 1958, Kim et al., 1993). E. umbellata maximize photosynthetic rates despite reduced stomatal conductance and xylem pressure potentials. It continues its photosynthesis even under dry conditions where stomata open early in the day and reach their peak rate before noon throughout the summer (Ahmad et al., 2005, Klich, 2000). E. umbellata has an evident benefit in increasing the carbon uptake even under water scarcity conditions, which contributes to its total invasive potential.
3. Morphological features of E. Umbellata
E. umbellata is a deciduous spiny branched shrub of about 2–5 m tall and a diameter of 10 cm with clusters of leaves that are elliptic, oblong, ovate, and alternate (Ahmad et al., 2005, Sather and Eckardt, 1987). It exhibits structural resemblance with plants that grow in shade. For the enhancement of solar capture, the leaves are oriented at horizontal angles (Brantley and Young, 2009). Leaves have smooth margins and blunt tips with sizes of 4–8 cm long and 1–2.5 cm wide. The lower surface is dense, while the upper surface is sparsely white leptitode. Apex is acute or obtuse. Petioles are relatively short dense white leptitode of 0.5–1.0 cm long and densely covered with silvery scales. The leaves have trichomes on both surfaces with the abaxial side being more pubescent (Olson, 2017). Pubescence reduces the leaf absorptance by increasing reflectance (Ahmad et al., 2005, Olson, 2017), thereby lowering the stress induced by the bright light. Representing an adaptive trait to survive in xeric conditions, it protects leaves in a variety of ways during times of stress as is found in the related invasive species E. angustifolia (Klich, 2000). Additionally, the presence of trichomes on stem, leaves, etc acts as an adaptive strategy in avoiding high temperature of hot sunny days by maintaining an optimal temperature for optimal photosynthesis (Ahmad et al., 2005, Olson, 2017).
The flowers are cream to white colored with four spreading lobes. The calyx is shorter than its tubular base. The flowers bloom in the spring and with a warm spice fragrance. The fruits referred to as pseudodropes are spherical in shape with a length of 7 mm. Acting as an attractant, fruits are silver-white (at the young stage) in color that changes to crimson red once it matures (Sather and Eckardt, 1987) (Fig. 1). The fruits have the ability to retain their properties even after 15 days of storage at room temperature (Parmar and Kaushal, 1982, Kim et al., 2016). The pigment responsible for red color of the fruit is lycopene (Fordham et al., 2001). The fruits abundant on growing branches are delicious with a sweet to acidic flavor. The fruits are eaten raw or processed into sauces, and fruit rolls, or used as a tomato substitute. The fruits rich in lycopene are effective in exerting protection against chronic diseases such as myocardial infarction (Kohlmeier et al., 1997, Patel, 2015) and prostate and other types of cancers (Giovannucci et al., 1995, Clinton, 1998). The plant is used as an adornment because of its lovely blooms and silvery foliage leaves.
Fig. 1.
The figure summarizes different stages of the growth of Elaeagnus umbellata. A) Flowering, B) Immature, and C) Ripened fruits.
4. Phytochemistry of E. umbellata
Phytochemicals, in principle the secondary metabolites produced by plants, exert health-promoting benefit that helps in preventing the occurrence of chronic diseases. They are gaining relevance as health-promoting agents that extends numerous benefits in humans following a phytochemical-rich diet (He et al., 2007, Kavanaugh et al., 2007, Christen et al., 2008). The phytochemicals produced by E. umbellata include phenolics, flavonoids, carotenoids, tannins, alkaloids, and saponins, besides being a source of essential minerals and vitamins particularly vitamin C (Fig. 2). Regular consumption of nutrient-rich diet helps in preventing production of free radicals and as such in reducing or delaying oxidative stress (Liu, 2004). Nearly, 84 bioactive constituents have been isolated from E. umbellata of which eugenol, 4-methoxy anisole, 2-nonenal, palmitic acid, 3-hexenylacetate, fatty acid methyl ester, phenylacetaldehyde, 4-methyl phenol, 2-hexanal, and methyl palmitate are the major ones observed in the extract of floral volatiles (Potter, 1995). It also serves as source of heterocyclic alkaloids having indole skelton and have great importance as anticancer, antihypertensive, antiarrhythmic, antimalarial, and sedative for use in biological and therapeutic purposes (Gamba et al., 2020, Massagetov, 1946).
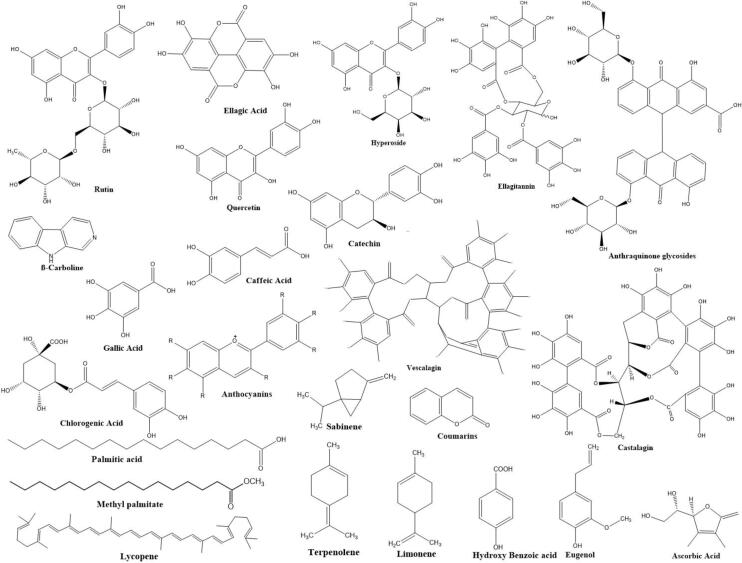
Fig. 2.
Structures of bioactive compounds reported in Elaeagnus umbellata.
The fruits of E. umbellata is promoted as beneficial ingredient in Western Chinese, Koreans, and Japanese diet owing to its ability in being effective against cancer, hepatitis and liver disorders, fractures, injuries, and diarrhoea (Gamba et al., 2020, Pinto et al., 2013). In a study, a series of seven novel tannins (elaeagnatins A-G) were observed in the leaf extract along with fifteen other known tannins (Ito et al., 1999, Ito et al., 1999). While studying autumn olives, Perkins-Veazie et al. (Perkins-Veazie et al., 2005) found them to have a high phenolic content and suggested correlation of the biological activity of phytochemicals with their concentration and structure attributes. Screening of the different E. umbellata genotypes revealed them to have a high phenolic (168.9 to 258.1 mg/100 g) and carotenoid (43.4 to 59.3 mg/100 g) content (Wang and Fordham, 2007). E. umbellata being rich in saponins are known to reduce the risk for cancer development, besides offering a regulatory check of lipids, and blood glucose levels. A diet high in saponins can help prevent dental cavities and platelet aggregation, as well as act as a lead poisoning antidote (Shi et al., 2004). Supplementation of alcoholic extract of E. umbellata with magnesium oxide was found effective in the treatment of athlete’s foot disease (Potter, 1995). Limonene - the most represented compound present in E. umbellata; is used to treat breast cancer (Liu, 2004).
The berries of E. umbellata being rich in phenolics, anthocyanins, flavonoids, and tannins exert beneficial effects through reduction in the macromolecular oxidation via, decrease in the oxidative stress (Veberic et al., 2015). The quantity of organic acid such as citric, malic, and oxalic acid varies significantly during different stages of ripening and was found dependent on the climatic conditions, soil, and cultivar choice (Gamba et al., 2020). Exerting a strong antioxidative effect, anthocyanins effectively help in reducing the occurrence of diseases including cancer, diabetes, etc (Yousuf et al., 2016). The berries of E. umbellata also have a rich content of carotenoids such as lycopene, lutein, β-carotene, etc. Lycopene from E. umbellata has been shown to be 17 times more abundant than in fresh tomatoes and may be responsible for the treatment of cancer and myocardial infarction (Fordham et al., 2001, Kohlmeier et al., 1997, Patel, 2015, Giovannucci et al., 1995). The berries are rich in proteins, pectin, carbohydrates, and acids, besides being a good source of vitamin A, C, and E (Gamba et al., 2020). E. umbellata fruits (100 g) contain moisture content of 64.9 g, soluble solids (14.5 g), acids (1.51 g), total sugar (8.34 g; with reducing (8.13 g) & non-reducing (0.23 g)), and a high content of vitamin C (12.04 mg) (Ahmad et al., 2005). It is also a rich source of phosphorus (0.054), calcium (0.049), magnesium (0.033), potassium (0.346), and iron (0.007) (Ahmad et al., 2005, Gamba et al., 2020). The fruits of this plant are used to treat diarrhoea, itch, and foulness, besides being used in making fruit rolls, juices, and condiments (Pei et al., 2015). The berries can be used raw or cooked. The flowers of E. umbellata are not showy and exhibit a strong characteristic odor which is due to phenolics (Pei et al., 2015). Having a high content of sugar, and fatty acids, leaves of E. umbellata are used to treat bowel disorders in China and Japan.
5. Pharmacological properties of E. Umbellata
E. umbellata shows production of different classes of secondary metabolites, prominent being alkaloids, polyphenols, terpenes, and others (Uddin and Rauf, 2012). Studies on the pharmacological front have revealed them to have properties of treating hepatic dysfunctioning and in exerting potent antibacterial, antiproliferative, antioxidant, and phytotoxic effects (Khattak, 2012, Rafique et al., 2016, Wang et al., 2019, Nazir et al., 2020, Nazir et al., 2018, Zulfiqar et al., 2022). The pharmacological properties of the plant are summarized in Table 1.
Table 1.
Table summarizes information of the bioactive compounds, their origin and application in the treatment of different diseases.
| Bioactive compound | Class | Parts used | Medicinal uses | References |
|---|---|---|---|---|
| Ellagic acid | Benzoic acid | Berries | Inhibits cancer cell progression and improves efficacy of drugs against cancer | (Gamba et al., 2020, La Vignera et al., 2022) |
| Gallic acid | Benzoic acid | Berries | Anti-oxidant, anti-inflammatory and anti-neoplastic activity | (Gamba et al., 2020) |
| Ascorbic acid | Vitamin C | Berries | It helps to heal wounds and enhance the absorption of iron from plant foods. It also supports the immune system. | (Gamba et al., 2020, Zulfiqar et al., 2022) |
| Terpenolene | Monoterpenes | Berries | It is used to treat anxiety and insomnia | (Gamba et al., 2020) |
| Chlorogenic acid | Cinnamic acids | Berries | It reduces the inflammation and modulate inflammatory and neuropathic pain in animal models. | (Gamba et al., 2020) |
| Limonene | Monoterpenes | Berries | It has anticancer, anti-oxidant, antiviral, anti-inflammatory, and gastroprotective | (Gamba et al., 2020) |
| Sabinene | Monoterpenes | Berries | It has anti-fungal and anti-inflammatory properties | (Gamba et al., 2020, Cao et al., 2018) |
| Caffeic acid | Cinnamic acids | Berries | It has the anticancer, anti-oxidant and anti-inflammatory potential | (Gamba et al., 2020, Ishaq et al., 2015) |
| Rutin | Flavanols | Berries | It is commonly used for autism, aging skin, airways infections caused by exercise. It has also the anti-oxidant and anti-inflammatory properties | (Gamba et al., 2020, Nazir et al., 2021) |
| Quercetin | Flavanols | Berries | It helps to protect against heart disease and cancer | (Zglińska et al., 2021, Gamba et al., 2020) |
| Castalagin | Catechins | Berries | It promotes bacterial cell wall disruption via, modulation in the assembly of peptidoglycans | (Gamba et al., 2020, Araújo et al., 2021) |
| Catechin | Catechins | Berries | It is used in the treatment of chronic diseases in humans, such as inflammatory bowel disease (IBD). It has also anticancer, antioxidant, antimicrobial, antidiabetic, and anti-inflammatory properties. | (Gamba et al., 2020, Azeem et al., 2022) |
| Hydroxybenzoic acid | Benzoic acid | Berries | It possesses anti-inflammatory, antimutagenic, antioxidant, hypoglycemic and antimicrobial properties | (Gamba et al., 2020, Xu et al., 2021, Kalinowska et al., 2021) |
| β-carboline | Alkaloid | Bark | It has sedative, anxiolytic, hypnotic, anticonvulsant, anticancer, and antimicrobial, activities. | (Paudel et al., 2020, Tolkachev et al., 2008) |
| Coumarins | Alkaloid | Whole plant | It has anti-inflammatory, anticancer, anticoagulant, antihypertensive, antimicrobial, anti-tuberculous, anticonvulsant, antiadipogenic, antihyperglycemic, and neuroprotective activities | (Paudel et al., 2020, Sharifi-Rad et al., 2021, 2021., 2021.; Minhas et al., 2013) |
| Anthraquinone glycosides | Glycoside | Aerial parts | It is antifungal in nature. It promotes normal kidney function and modulates inflammation via, inhibition of the enzyme cyclooxygenase | (Paudel et al., 2020, George et al., 2014, Rawat et al., 2002) |
| Ellagitannins | Tannins | Leaves | It has anti-inflammatory, anticancer, antioxidant, prebiotic, and cardioprotective properties | (Ozen et al., 2018, Ito et al., 1999, Ito et al., 1999) |
| Eugenol | Allylbenzene | Flower | It is used to treat toothache and more rarely be taken orally to treat gastrointestinal and respiratory complaints | (Gamba et al., 2020, Nazir et al., 2020, Nazir et al., 2018, Nazir et al., 2021) |
| Palmitic acid | Fatty acid | Flower | It acts as anti-inflammatory, promotes metabolic activities, and supports skin health | (Zglińska et al., 2021) |
| Methyl palmitate | Fatty acid methyl ester | Flower | It prevents development of fibrosis via, inhibition of NF-κB in rats |
(Zglińska et al., 2021) |
| Lycopene | Carotenoid | Berries | It is a powerful antioxidant, antidiabetic, and possess neuroprotective and anticancer properties | (Gamba et al., 2020, Kaur et al., 2011) |
| Anthocyanins | Flavonoids | Berries | Reduce risk of cancers, beneficial to improve brain functions, protect from UV rays and also have antimicrobial activity | (Gamba et al., 2020, Ozen et al., 2018) |
| Kaempferol-O-hexidose | Polyphenol | Berries | It possesses strong anti-inflammatory and anticancer properties | (Spínola et al., 2019) |
| Vescalagin | Tannins | Berries | It promotes bacterial cell wall disruption via, modulation in the assembly of peptidoglycans | (Gamba et al., 2020, Araújo et al., 2021) |
| Hyperoside | Cinnamic acids | Berries | It is commonly used as an anti-inflammatory. It also has antioxidant and antimicrobial properties. | (Gamba et al., 2020, Mahomoodally et al., 2018) |
5.1. Antioxidant activity
Most diseases in humans have direct or indirect involvement of free radicals. The free radicals are kept in check by antioxidants that regulate their production within the system (Jan et al., 2011). E. umbellata is an excellent source of nutrients and antioxidants, and as such attribute beneficial effects to the health of an individual. In E. umbellata, a diverse variety of antioxidants found in fruits (berries) exerts synergistic effects in countering free radicals and avoiding damage caused by oxidative stress (Wang et al., 2019, Zulfiqar et al., 2022, Steinberg, 1991). The methanolic extract of berries was found to exert an antioxidant effect in a dose-dependent i.e., a dose of 20, 40, 60, 80, 100, and 120 μg/ml showed scavenging activity of 10.7, 26.7, 49.0, 69.3, 84.9 and 90.2%, respectively (Ahmad et al., 2005). Though acetone and aqueous extracts showed a similar pattern of scavenging activity, however, it was found that the methanolic extract exhibited a lower EC50 value (97.3 μg/ml) compared with the acetone extract showing an EC50 value of 188.0 μg/ml (Khanzadi, 2012). Zglinska et al. (Zglińska et al., 2022) explore the effect of methanol-acetone extract from fruits on the antioxidant capacity of healthy human cells that had undergone oxidative stress. They found the extract effective in boosting cell viability rather than exhibiting any toxicity. On examining its effect on fibroblasts, the extract was found effective in decreasing the expression of chemokine and as such H2O2-induced cell death (Iannuzzi et al., 2020). On studying the effect of essential oils through ABTS and DPPH assays, it was found that a concentration of 1000 µg/ml with IC50 values of 105 and 70 µg/ml shows a free radical scavenging capacity of 88.30 ± 0.81 and 85.24 ± 0.63 against citric acid used as a positive control (Nazir et al., 2021). Of the different extracted essential oils; Octadecanoic acid exhibits a strong antioxidant potential (Wang et al., 2007). The others in the category of essential oils such as linoleic acid, phytol, p-vinyl guaiacol, stearic acid, decanoic acid, etc, displayed reasonable antioxidant activity (Khanzadi, 2012, Cai et al., 2004). Regardless of the reagent used, the lyophilized berries were distinguished by higher antioxidant activity than dried fruit (Zglińska et al., 2022). Despite the fact that aqueous extract exhibits better antioxidant impact in the liver and brain of mice (Ishaq et al., 2015), antioxidant status and FRAP test of the dried berries revealed higher activity for methanolic extract than aqueous one (Zglińska et al., 2022, Ozen et al., 2017). Zulfiqar et al. (Zulfiqar et al., 2022) reported that silver nanoparticles synthesized for fruit extract showed to have an antioxidant potential of 43.38 µg/ml (IC50 value) in DPPH assay and an inhibition of 69%. Compared with fruits, leaves having a good proportion of acids (fumaric, 4-hydroxybenzoic acid, etc), and flavonols (rutin, neohesperdin, and hesperdin) also exhibit strong antioxidant properties (Zglińska et al., 2021, Zglińska et al., 2022). In the screen of antioxidant potential in DPPH and ABTS assay, IC50 values of 40, 45, and 60 g/ml and 57, 70, and 120 g/ml were observed for the leaf extract prepared in chloroform, ethyl acetate, and butanol (Nazir et al., 2021) (Fig. 3).
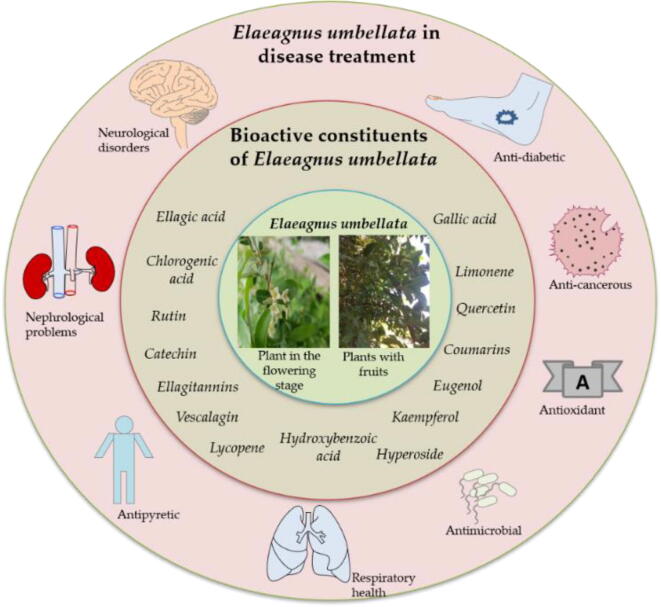
Fig. 3.
Figure depicting the potential of a wide range of bioactive compounds of Elaeagnus umbellata in exerting benefits towards the treatment of different diseases.
5.2. Anticancer activity
Cancer is the second largest cause of mortality worldwide. Its development starts in a multistage process from a normal cell to a precancerous lesion and finally to a malignant tumor (Chan et al., 2019, 2019.). In 2018, it was estimated that around 9.6 million people died from cancer alone (Chan et al., 2019, 2019.). As phytonutrients are considered beneficial in preventing the incidences of diseases such as heart disease, cancer, and others, an increased intake of fruits and vegetables was observed among the diet of consumers (WCRF, AICR., 2007). Of them, antioxidants from plant sources are well recognized as effective in avoiding arteriosclerosis, cancers, cardiovascular diseases, cellular injuries, inflammations, neurological problems, and dysregulation of the immune system (Steinberg, 1991, Ozen et al., 2017). Besides having a good percentage of lutein, α-carotene, and other similar compounds, Fordham et al. (Fordham et al., 2001) reported that E. umbellata fruits contain 7–17 times more lycopene than tomatoes with its role in extending protection against a wide range of malignancies. Ellagic acid extracted from the fruits of E. umbellata prevents cancer cell growth and as such improves the efficacy of anticancer drugs (La Vignera et al., 2022). Catechin belonging to the class catechins was extracted from the berries of the plant which shows anticancer properties (Azeem et al., 2022). It was studied that Anthraquinone glycosides (a glycoside extracted from the whole plant) inhibits excessive renal tubular cell proliferation (Paudel et al., 2020, Rawat et al., 2002). In another study, it was shown that Caffeic acid extracted from the berries exhibit higher anticancer potential (Gamba et al., 2020, Kim et al., 2005).
5.3. Anticholinesterase activity
Alzheimer’s disease (a neurodegenerative disorder) is characterized by an increase in oxidative stress, β-amyloid build-up, low levels of the neurotransmitter acetylcholine (ACh), and a decrease in cholinergic transmission (Perry et al., 1998, Vickers et al., 2016, Niranjan, 2018). According to the World Alzheimer's Organization's 2015 estimate, there are approximately 46.85 million people worldwide who are affected by Alzheimer's disease and other kinds of dementia (Kuca et al., 2016, Kumar et al., 2018). They also anticipate that by 2030, this number will have doubled, and by 2050, it will be three times than its current level. The decrease in cholinergic transmission occurs due to the breakdown of the neurotransmitter acetylcholine (ACh) by acetylcholinesterase (AChe) and butyrylcholinesterase (BChe). In this process, inhibition of the AChe and BChe enzymes helps in maintaining the Ach levels, thereby relieving the body of symptoms pertaining to dementia, Parkinson, and Alzheimer diseases (Rahman and Choudhary, 2001, Mangialasche et al., 2010). The secondary metabolites such as alkaloids, found in the E. umbellata extracts exhibit considerable cholinesterase activity; thereby serving as a source of cholinesterase inhibitors (Mehta et al., 2012, Murray et al., 2013). Phyto-constituents of the E. umbellata fruits including different acids (chlorogenic, ellagic acid, and gallic acid) and phloroglucinol were found attributing anti-cholinesterase activity both under in vitro and in vivo conditions (Mehta et al., 2012, Murray et al., 2013, Ghias and Rauf, 2012). Treatment of animal models with ellagic and chlorogenic acids showed positive effects in terms of reducing oxidative stress and a subsequent enhancement of the cognitive effects that attribute neuroprotection against scopolamine-induced amnesia (Kwon et al., 2010, Jha et al., 2018). The chloroform extract helps in restoring the cholinergic activities via, inhibition of the acetylcholinesterase enzyme, leading to the retention of higher levels of acetylcholine levels in the brain which is considered crucial for cognitive impairment. Similarly, Nazir et al. (Nazir et al., 2021) found that essential oils of E. umbellata effectively inhibit the enzymes AChe and BChe, thus claiming that essential oils of E. umbellata hold potential for use as a medication in the treatment of neurological diseases. The well-known alkaloids Galantamine and rivastigmine obtained from E. umbellata are used to treat Alzheimer's disease (Ahmad et al., 2005, Murray et al., 2013). Galantamine, donepezil, and rivastigmine are the only FDA-approved acetylcholinesterase inhibitors for Alzheimer's disease (Mangialasche et al., 2010). As antioxidant therapy has shown positive effects in improving cognitive impairment and behavioral patterns, supplementation of natural products rich in antioxidants is believed to be effective in reducing the chances of neuronal cell death and as such delaying the early onset of the disease (Erejuwa et al., 2012, Vina et al., 2011).
5.4. Anti-inflammatory activity
The fruit extract of E. umbellata exhibits anti-inflammatory property owing to its ability in controlling inflammation; thereby increase in its usage as a functional additive (Zglińska et al., 2021). The extract was found effective in increasing the production of metalloproteinases such as TIMP-1 (Tissue inhibitor of metalloproteinases-1), and MMP-9 (Matrix metalloproteinase-9), suggesting that collagen may be guarded against breakdown under oxidative stress. These traits, along with increase in the expression of brain-derived neurotrophic factor (BDNF) highlight the possibility of its use in the management of neuro-inflammatory conditions concerned to Alzheimer's and Parkinson's diseases (Zglińska et al., 2021). While studying LPS-stimulated cells, Kang et al. (Kang et al., 2020) observed that gallic acid from the leaves of E. umbellata is capable of inhibiting nitric oxide production, thereby exhibiting a strong anti-inflammatory activity. Kaempferol present in the leaves of the E. umbellata shows strong anti-inflammatory activity and inhibits the T- cell proliferation at the concentration of 100 µM (Kang et al., 2020, Wang et al., 2018). Phytoene extracted from the E. umbellata is also found to have anti-inflammatory properties (Paudel et al., 2020). Another component adiponectin has been found effective in reducing the level of pro-inflammatory cytokines, thus functioning as an important anti-inflammatory factor (Polyzos et al., 2010). In another study methyl palmitate extracted from the flower of the plant reduce the chances of fibrosis in rats following treatment with CCl4 and bleomycin via, inhibition of the NF-κB (Zglińska et al., 2021).
5.5. Anti-diabetic activity
Diabetes (mostly Type 2) caused by less production of insulin and insulin resistance show 2–10 times higher mortality risk owing to vascular (both macro and micro) diseases (Manson et al., 1991, King et al., 1998, Cheng, 2005). As α-amylase causes the breakdown of starch to disaccharides, it is acted upon by α-glucosidase that hydrolyzes disaccharides into simple sugars like glucose for absorption at the intestinal surface; thereby resulting in postprandial hyperglycemia (Dhital et al., 2013). Diabetes representing a postprandial hyperglycemic condition is often associated with increase in the oxidative stress (Brownlee, 2001) and risk for the development of cardiovascular complications (Bonora and Muggeo, 2001, Ceriello, 2005). Postprandial hyperglycemia is controlled by inhibiting the enzyme α-glucosidase that impairs digestion of the dietary carbohydrates in the intestines (Standl et al., 1999). Affecting the absorption of monosaccharides, inhibition of α-glucosidase flattens the postprandial increase of the blood glucose; thereby results in the alleviation of postprandial hyperglycemia condition (Miura et al., 1998, Heo et al., 2009). Acarbose - a potent hypoglycemic agent, lowers the incidences of hyperglycemia and hyperinsuliminea via, improved insulin sensitivity that proceeds with full control over hyperglycemia (Standl et al., 1999, Breuer, 2003). Despite bringing benefits of controlling the hyperglycemic condition, oral intake of acarbose was found to cause gastrointestinal problems such as abdominal discomfort, flatulence, and diarrhoea (Bressler and Johnson, 1992).
Diabetes serve as an important risk factor in the development of cardiovascular complications. In such a scenario, plant polyphenolics such as flavonoids, etc are often correlated positively with inhibition of the α-glucosidase activity (Miura et al., 1998, Heo et al., 2009). As the antidiabetic effect includes a regulatory check of glucose absorption, reduction in inflammation, protein glycation, and oxidative stress, consumption of E. umbellata (often referred to as Autumn olive berries, AOB) fruits is considered a safe and cost-effective approach in reducing the hyperglycemic condition (Nazir et al., 2018, Harris et al., 2014, Edirisinghe and Burton-Freeman, 2016). Consumption of AOB was found effective in inhibiting α-glucosidase, besides having a significant effect on the expression of adiponectin that plays important role in sensitizing insulin action (Nigro et al., 2014). As diabetes often increases inflammation and as such diabetic associated complications (Alexandraki et al., 2008), adiponectin was found effective in reducing the levels of pro-inflammatory cytokines and as such is capable of exerting an anti-inflammatory effect (Polyzos et al., 2010). Together, AOB was found effective to overcome insulin sensitivity via, inhibition of the α-glucosidase and a subsequent increase in the expression of hormone, adiponectin (Nigro et al., 2014). AOB serves as a potent hypoglycemic agent in flattening the glucose levels towards achieving the goal of preventing the occurrence of diabetes and diabetes-associated complications (Kim et al., 2019).
The essential oil of fruits in E. umbellata also exhibits antidiabetic potential (Nazir et al., 2021). α-linolenic acid reported in the fruit essential oil through GC–MS was found to have antidiabetic activities (Dhital et al., 2013, Jovanovski et al., 2017). Phytol (3, 7, 11, 15-tetramethyl-2-hexadecen-1-ol) also shows strong antidiabetic activity (Elmazar et al., 2013). Similarly, other components of the essential oils of E. umbellata like stearic acid, humulene epoxide, and ascorbic acid were reported to possess antidiabetic potential (Zhao et al., 2017, Lalitha et al., 2015). Proanthocyanidins present in berries improve insulin sensitivity via, enhancement in adiponectin expression in rats fed with a high fructose diet (Meeprom et al., 2011). Lycopene in berries exhibits strong antioxidant potential; thereby is effective against the development of oxidative stress (Veazie et al., 2005). The methanolic, chloroform, and ethylacetate extract of berries exhibits a strong inhibitory effect against different enzymes such as α-amylase, α-glucosidase, etc (Wang et al., 2000). The antihyperglycemic effect of the extract also includes reduction in triglycerides (TGS), low-density lipoproteins (LDL), and cholesterol level. Additionally, the extract was found to exhibit an anti-hepatoprotective effect among the diabetic control group demarked by reduction SGOT, SGPT, and ALP levels.
5.6. Antimicrobial activity
Berries and leaves of E. umbellata contain a high amount of phenolic compounds such as hydroxybenzoic acids, besides having a good proportion of flavanols that attribute it with strong antibacterial activity (Chopra et al., 1986,, Gairola et al., 2021). The seeds contain a good percentage of acids such as chlorogenic acid, gallic acid, etc. Their presence attributes E. umbellata with the property of being effective in the treatment of bacterial infections. The essential oils extracted from E. umbellata are effective in destroying the cell membrane and cell wall, besides causing impairment in the membrane permeability that causes release of their intracellular contents, disturbance of the nutrient uptake and electron transport system, and overall hindrance with the membrane function (Minhas et al., 2013). The antimicrobial activity of E. umbellata extracts (chloroform, n-hexane, ethyl acetate, and methanol) against varied pathogenic strains (S. epidermis, S. aureus, B. subtilis, E. coli, and K. pneumonia), shows a different level of inhibition. Gram-positive bacteria were shown to be resistant to all fractions, whereas gram-negative bacteria were completely inert. Among all the fractions ethyl acetate were exhibiting the highest activity against S. epidermis. The fraction of ethyl acetate and methanol also showed significant effectiveness against S. epidermis, S. aureus, and other bacterial strains (Ghias and Rauf, 2012). Similarly, the activity of the extract prepared from the flower in ether was found effective against broad range of pathogenic strains (B. subtilis, E. coli, S. aureus and others) (Minhas et al., 2013, Mubasher et al., 2007). The ethanolic extract of leaves shows strong activity against strains belonging to both gram +ve and gram -ve bacteria, while the aqueous extract of berries against B. subtilis showed a relatively small zone of inhibition but strongly inhibits the growth of S. aureus and E. coli (Minhas et al., 2013, Mubasher et al., 2007). The aqueous extract of berries was shown to have no effect on multi-drug-resistant P. aeruginosa, while growth was inhibited by acetone extract (Nazir et al., 2021).
6. Conclusion and future perspectives
Plants comprising a large proportion of the human diet fulfill the daily caloric requirements (both organic and inorganic) needed to perform biological activities on a routine basis. With varied composition and nutritional value, they contribute significantly as a source of proteins, vitamins, minerals, etc, that defend the body from chronic illnesses and varied human diseases. Of the different plants, E. umbellata rich in bioactive constituents (flavonoids, phenolics, etc), vitamins (A, C & E), and minerals (zinc, iron, calcium, and others), is considered indispensable to the life of different organisms including humans. Besides acting as a source of energy, it fulfills the nutritional requirements for maintaining normal health by having active participation in the growth, maintenance, and routine functions of the body. In the recent past, plant-based phytochemicals are gaining popularity for use in the treatment of different diseases. The bioactive constituents of E. umbellata have been well-researched for their use in the treatment of different diseases, besides having considerable antioxidant, anticancer, anti-inflammatory, anticholinesterase, and antimicrobial potential. It not only helps in controlling blood glucose levels via, inhibition of α-glucosidase and alteration in the expression of hormone, adiponectin, but also plays an active role in the amelioration of cognitive dysfunction and in the restoration of acetylcholine levels. In short, the consumption of a phytochemical-rich diet fulfilling the daily requirements ascertains how significant these nutrients are to the health and well-being of humans.
In the current scenario of an increase in the emergence of drug resistance that has raised serious concerns pertaining to human health, there is an urgent need to strategize the policies regarding the exploration of plant species as a possible therapeutic option. With less production of new drugs and increased cases of drug resistance, there is an urgent need for possible exploration of medicinal plants as a possible source of drugs to combat the menace of drug resistance. Considering the economic burden and substantial health concerns, medicinal plants exhibiting potent drug properties need exploration for new bioactive moieties and their synthesis pathways with a possible trigger towards manipulation for enhancing their production and perpetuation as a possible drug molecule.
7. Methods adopted for search of the literature
The bibliometric research for the article was carried out on a broader range; starting with medicinal plants of the family Elaeagnaceae, the health benefits of Elaeagnus umbellata, its role in different diseases (1950–2022) across different databases (PubMed, Scopus, Web of Sciences, and others). The data collected was differently classified and explored based on origin, role in traditional medicine, structural properties, bioactive compounds, and their pharmacological properties.

Author contributions
All authors contributed equally to the writing of the contents present under different sub-headings of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Author Arif Tasleem Jan would like to thank DST-SERB (CRG/2019/004106) and J&K Science Technology and Innovation Council (JK ST&IC/SRE/996–998), India for support in terms of the grant.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
Authors are highly thankful to their colleagues for their positive criticism that helped in improving the contents of the manuscript. Authors extend sincere thanks to Dr. Ommer Bashir for help in developing structures using ChemDraw and Dr. Asif Adil for help in the development of word cloud.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Mujtaba Aamir Bhat, Email: mujtaba@bgsbu.ac.in.
Awdhesh Kumar Mishra, Email: awdhesh@ynu.ac.kr.
Mohammad Azhar Kamal, Email: ma.kamal@psau.edu.sa.
Safikur Rahman, Email: safikur.rahman@mscollege.ac.in.
Arif Tasleem Jan, Email: atasleem@bgsbu.ac.in.
References
- Ahmad S.D., Kamal M. Morpho-molecular characterization of local genotypes of Hyppophae rhamnoides L. ssp. turkestanica a multipurpose plant from Northern areas of Pakistan. J. Biol. Sci. 2002;2(5):351–354. [Google Scholar]
- Ahmad S.D., Sabir M.S., Juma M., Asad H.S. Morphological and biochemical variations in Elaeagnus umbellata Thunb. from mountains of Pakistan. Acta Bot. Croat. 2005;64(1):121–128. [Google Scholar]
- Alexandraki K.I., Piperi C., Ziakas P.D., Apostolopoulos N.V., Makrilakis K., Syriou V., Diamanti-Kandarakis E., Kaltsas G., Kalofoutis A. Cytokine secretion in long-standing diabetes mellitus type 1 and 2: associations with low-grade systemic inflammation. J. Clin. Immunol. 2008;28:314–321. doi: 10.1007/s10875-007-9164-1. [DOI] [PubMed] [Google Scholar]
- AlSheikh H.M.A., Sultan I., Kumar V., Rather I.A., Al-Sheikh H., Jan A.T., Haq Q.M.R. Plant-based phytochemicals as possible alternative to antibiotics in combating bacterial drug resistance. Antibiotics. 2020;9(8):480. doi: 10.3390/antibiotics9080480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araújo A.R., Araújo A.C., Reis R.L., Pires R.A. Vescalagin and castalagin present bactericidal activity toward methicillin-resistant bacteria. ACS Biomater. Sci. Engineer. 2021;7(3):1022–1030. doi: 10.1021/acsbiomaterials.0c01698. [DOI] [PubMed] [Google Scholar]
- Azeem M., Hanif M., Mahmood K., Ameer N., Chughtai F.R.S., Abid U. An insight into anticancer, antioxidant, antimicrobial, antidiabetic and anti-inflammatory effects of quercetin: a review. Polym. Bull. 2022:1–22. doi: 10.1007/s00289-022-04091-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer A., Brönstrup M. Industrial natural product chemistry for drug discovery and development. Nat. Prod. Rep. 2014;31(1):35–60. doi: 10.1039/c3np70058e. [DOI] [PubMed] [Google Scholar]
- Bonora E., Muggeo M. Postprandial blood glucose as a risk factor for cardiovascular disease in type II diabetes: the epidemiological evidence. Diabetologia. 2001;44:2107–2114. doi: 10.1007/s001250100020. [DOI] [PubMed] [Google Scholar]
- Brantley, S.T., Young, D.R., 2009. Linking light attenuation, sunflecks and canopy architecture in mesic shrub thickets.
- Bressler R., Johnson D. New pharmacological approaches to therapy of NIDDM. Diabetes Care. 1992;15:792–805. doi: 10.2337/diacare.15.6.792. [DOI] [PubMed] [Google Scholar]
- Breuer H.W. Review of acarbose therapeutic strategies in the long-term treatment and in the prevention of type 2 diabetes. Int. J. Clin. Pharmacol. Ther. 2003;41:421–440. doi: 10.5414/cpp41421. [DOI] [PubMed] [Google Scholar]
- Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- Cai Y., Luo Q., Sun M., Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004;74(17):2157–2184. doi: 10.1016/j.lfs.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Zhang H., Liu H., Liu W., Zhang R., Xian M., Liu H. Biosynthesis and production of sabinene: current state and perspectives. Appl. Microbiol. Biotechnol. 2018;102(4):1535–1544. doi: 10.1007/s00253-017-8695-5. [DOI] [PubMed] [Google Scholar]
- Ceriello A. Postprandial hyperglycemia and diabetes complications: is it time to treat? Diabetes. 2005;54:1–7. doi: 10.2337/diabetes.54.1.1. [DOI] [PubMed] [Google Scholar]
- Chan C.K., Aimagambetova G., Ukybassova T., Kongrtay K., Azizan A. Human papillomavirus infection and cervical cancer: epidemiology, screening, and vaccination-review of current perspectives. J. Oncol. 2019 doi: 10.1155/2019/3257939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng D. Prevalence, predisposition and prevention of type II diabetes. Nutr. Metab. (Lond.) 2005;2:29. doi: 10.1186/1743-7075-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra R.N., Nayar S.L., Chopra L.C. council of scientific and industrial research; New Delhi: 1986. Glossary of Indian medicinal plants; pp. 238–240. [Google Scholar]
- Christen W.G., Liu S., Glynn R.J., Gaziano J.M., Buring J.E. Dietary carotenoids, vitamins C and E, and risk of cataract in women: a prospective study. Arch. Ophthalmol. 2008;126:102–109. doi: 10.1001/archopht.126.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton S.K. Lycopene chemistry, biology, and implications for human health and disease. Nutr. Rev. 1998;56:35–51. doi: 10.1111/j.1753-4887.1998.tb01691.x. [DOI] [PubMed] [Google Scholar]
- Dhital S., Lin A.H., Hamaker B.R., Gidley M.J., Muniandy A. Mammalian mucosal α-glucosidases coordinate with α-amylase in the initial starch hydrolysis stage to have a role in starch digestion beyond glucogenesis. PLoS One. 2013;8(4):e62546. doi: 10.1371/journal.pone.0062546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirr, M.A., 1990 Manual of woody landscape plants: their identification, ornamental characteristics, culture, propagation and uses (No. Ed. 4). Stipes Publishing Co.
- Edirisinghe I., Burton-Freeman B. Anti-diabetic actions of Berry polyphenols - Review on proposed mechanisms of action. J. Berry Res. 2016;6:237–250. [Google Scholar]
- Elmazar M.M., El-Abhar H.S., Schaalan M.F., Farag N.A. Phytol/Phytanic acid and insulin resistance: potential role of phytanic acid proven by docking simulation and modulation of biochemical alterations. PLoS One. 2013;8(1):e45638. doi: 10.1371/journal.pone.0045638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erejuwa O., Sulaiman S., Wahab A.B. Honey: a novel antioxidant. Molecules. 2012;17(4):4400–4423. doi: 10.3390/molecules17044400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fordham I.M., Clevidence B.A., Wiley E.R., Zimmerman R.H. Fruit of autumn olive; A rich source of lycopene. Hort. Sci. 2001;36:1136–1137. [Google Scholar]
- Gairola S., Singh K., Kumar P., Kumar B., Sharma Y.P. The Wild Edible Plants of Paddar Valley, Jammu division, Jammu and Kashmir, India. Ethnobotany Res. Appl. 2021;22:1–21. [Google Scholar]
- Gamba G., Donno D., Mellano M.G., Riondato I., De Biaggi M., Randriamampionona D., Beccaro G.L. Phytochemical characterization and bioactivity evaluation of autumn olive (Elaeagnus umbellata Thunb.) pseudodrupes as potential sources of health-promoting compounds. Appl. Sci. 2020;10:4354. [Google Scholar]
- Gardner Nitrogen fixation in elaeagnus root nodules. Nature. 1958;181:717–718. [Google Scholar]
- George D.R., Finn R.D., Graham K.M., Sparagano O.A. Present and future potential of plant-derived products to control arthropods of veterinary and medical significance. Parasit. Vectors. 2014;7(1):1–12. doi: 10.1186/1756-3305-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghias U., Rauf A. Phytochemical screening and biological activity of the aerial parts of Elaeagnus umbellata. Sci. Res. Essays. 2012;7(43):3690–3694. [Google Scholar]
- Giovannucci E., Ascherio A., Rimm E.B., Stampfer M.J., Colditz G.A., Willett W.C. Intake of carotenoids and retinol in relation to risk of prostate cancer. J. Natl Cancer Inst. 1995;87:1767–1776. doi: 10.1093/jnci/87.23.1767. [DOI] [PubMed] [Google Scholar]
- Harris C.S., Cuerrier A., Lamont E., Haddad P.S., Arnason J.T., Bennett S.A.L., Johns T. Investigating wild berries as a dietary approach to reducing the formation of advanced glycation endproducts: Chemical correlates of in vitro antiglycation activity. Plant Foods Hum. Nutr. 2014;69(1):71–77. doi: 10.1007/s11130-014-0403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F.J., Nowson C.A., Lucas M., MacGregor G.A. Increased consumption of fruit and vegetables is related to a reduced risk of coronary heart disease: meta-analysis of cohort studies. J. Hum. Hypertens. 2007;21:717–728. doi: 10.1038/sj.jhh.1002212. [DOI] [PubMed] [Google Scholar]
- Heo S.J., Hwang J.Y., Choi J.I., Han J.S., Kim H.J., Jeon Y.J. Diphlorethohydroxycarmalol isolated from Ishige okamurae, a brown algae, a potent α-glucosidase and α-amylase inhibitor, alleviates postprandial hyperglycemia in diabetic mice. Eur. J. Pharmacol. 2009;615:252–256. doi: 10.1016/j.ejphar.2009.05.017. [DOI] [PubMed] [Google Scholar]
- Iannuzzi A.M., Giacomelli C., De Leo M., Pietrobono D., Camangi F., De Tommasi N., Martini C., Trincavelli M.L., Braca A. Antioxidant activity of compounds isolated from Elaeagnus umbellata promotes human gingival fibroblast well-being. J. Nat. Prod. 2020;83(3):626–637. doi: 10.1021/acs.jnatprod.9b01030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishaq S., Rathore H.A., Sabir S.M., Maroof M.S. Antioxidant properties of Elaeagnus umbellata berry solvent extracts against lipid peroxidation in mice brain and liver tissues. Food Sci. Biotechnol. 2015;24(2):673–679. [Google Scholar]
- Ito H., Miki K., Yoshida T. Tannins and related polyphenols from Elaeagnaceous plants. Part 3. Elaeagnatins A-G, C-Glucosidic ellagitannins from Elaeagnus umbellata. Chem. Pharm. Bull. 1999;47:536–542. [Google Scholar]
- Ito H., Miki K., Yoshida T. Elaeagnatins A-G, C-Glucosidic Ellagitannins from Elaeagnus umbellata. Chem. Pharm. Bull. 1999;47:536–542. [Google Scholar]
- Jan A.T., Ali A., Haq Q.M.R. Glutathione as an antioxidant in inorganic mercury induced nephrotoxicity. J. Postgrad. Med. 2011;57(1):72. doi: 10.4103/0022-3859.74298. [DOI] [PubMed] [Google Scholar]
- Jha A., Panchal S., Shah A. Ellagic acid: insights into its neuroprotective and cognitive enhancement effects in sporadic Alzheimer's disease. Pharmacol. Biochem. Behav. 2018;175:33–46. doi: 10.1016/j.pbb.2018.08.007. [DOI] [PubMed] [Google Scholar]
- Jovanovski E., Li D., Ho H.V.T., Djedovic V., Marques A.D.C.R., Shishtar E., et al. The effect of alpha-linolenic acid on glycemic control in individuals with type 2 diabetes: a systematic review and meta-analysis of randomized controlled clinical trials. Medicine. 2017;96(21):e6531. doi: 10.1097/MD.0000000000006531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinowska M., Gołębiewska E., Świderski G., Męczyńska-Wielgosz S., Lewandowska H., Pietryczuk A., et al. Plant-derived and dietary hydroxybenzoic acids-A comprehensive study of structural, anti-/pro-oxidant, lipophilic, antimicrobial, and cytotoxic activity in MDA-MB-231 and MCF-7 cell lines. Nutrients. 2021;13(9):3107. doi: 10.3390/nu13093107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H.R., Jung J.K., Park S.H., Lee J.Y., Yang S.A. Anti-oxidant, anti-inflammatory, and anti-bacterial effects of extracts from Elaeagnus umbellata leaves obtained using different extract conditions. Korean J. Food Preserv. 2020;27(3):374–384. [Google Scholar]
- Kaur H., Chauhan S., Sandhir R. Protective effect of lycopene on oxidative stress and cognitive decline in rotenone induced model of Parkinson’s disease. Neurochem. Res. 2011;36(8):1435–1443. doi: 10.1007/s11064-011-0469-3. [DOI] [PubMed] [Google Scholar]
- Kavanaugh C.J., Trumbo P.R., Ellwood K.C.T. The U.S. Food and Drug Administration's evidence-based review for qualified health claims: tomatoes, lycopene, and cancer. J. Natl Cancer Inst. 2007;99:1074–1085. doi: 10.1093/jnci/djm037. [DOI] [PubMed] [Google Scholar]
- Khanzadi F.K. Free radical scavenging activity, phytochemical composition and nutrient analysis of Elaeagnus umbellata berry. J. Med. Plants Res. 2012;6(39):5196–5203. [Google Scholar]
- Khattak K.F. Free radical scavenging activity, phytochemical composition, and nutrient analysis of Elaeagnus umbellata berry. J. Med. Plants Res. 2012;6:5196–5203. [Google Scholar]
- Kim J.I., Baek H.J., Han D.W., Yun J.A. Autumn olive (Elaeagnus umbellata Thunb.) berry reduces fasting and postprandial glucose levels in mice. Nutr. Res. Prac. 2019;13(1):11–16. doi: 10.4162/nrp.2019.13.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.C., Kim C.H., An C.S., Ku C.D., Park M.C., Song S.D. Isolation of symbiotic Frankia Eulk 1 strain from root nodules of E. umbellata. Korean J. Bot. 1993;36:177–182. [Google Scholar]
- Kim M.J., Lim J.S., Yang S.A. Component Analysis and Anti-Proliferative Effects of Ethanol Extracts of Fruits, Leaves, and Stems from Elaeagnus umbellata in HepG2 Cells. Korean J. Food Nutr. 2016;45(6):828–834. [Google Scholar]
- Kim S.S., Park R.Y., Jeon H.J., Kwon Y.S., Chun W. Neuroprotective effects of 3, 5- dicaffeoylquinic acid on hydrogen peroxide-induced cell death in SH-SY5Y cells. Phytother. Res. 2005;19:243–245. doi: 10.1002/ptr.1652. [DOI] [PubMed] [Google Scholar]
- King H., Aubert R.E., Herman W.H. Global burden of diabetes, 1995–2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998;21:1414–1431. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- Klich M.G. Leaf variations in Elaeagnus angustifolia related to environmental heterogeneity. Environ. Exp. Bot. 2000;44:171–183. doi: 10.1016/s0098-8472(00)00056-3. [DOI] [PubMed] [Google Scholar]
- Kohlmeier L., Kark J.D., Gomez-Garcia E., Martin B.C., Steck S.E., Kardinaal A.F., Ringstad J., Thamm M., Masaev V., Riemersma R., Martin-Moreno J.M., Huttunen J.K., Kok F.J. Lycopene and myocardial infarction risk in the EURAMIC study. Am. J. Epidemiol. 1997;146:618–626. doi: 10.1093/oxfordjournals.aje.a009327. [DOI] [PubMed] [Google Scholar]
- Kuca K., Soukup O., Maresova P., Korabecny J., Nepovimova E., Klimova B., et al. Current approaches against Alzheimer's disease in clinical trials. J. Braz. Chem. Soc. 2016;27(4):641–649. [Google Scholar]
- Kumar K., Kumar A., Keegan R.M., Deshmukh R. Recent advances in the neurobiology and neuropharmacology of Alzheimer’s disease. Biomed. Pharmacother. 2018;98:297–307. doi: 10.1016/j.biopha.2017.12.053. [DOI] [PubMed] [Google Scholar]
- Kwon S.H., Lee H.K., Kim J.A., Hong S.I., Kim H.C., Jo T.H., et al. Neuroprotective effects of chlorogenic acid on scopolamine-induced amnesia via antiacetylcholinesterase and anti-oxidative activities in mice. Eur. J. Pharmacol. 2010;649(1–3):210–217. doi: 10.1016/j.ejphar.2010.09.001. [DOI] [PubMed] [Google Scholar]
- La Vignera S., Basile L., Aversa A., Calogero A.E., Grillo A., Cannarella R., et al. The Use of Ellagic Acid and Annona Muricata Improves Semen Quality in Men with High-Risk Papillomavirus Infection. J. Clinic. Med. 2022;11(16):4691. doi: 10.3390/jcm11164691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalitha V., Korah M., Sengottuvel S., Sivakumar T. Antidiabetic and antioxidant activity of resveratrol and vitamin-C combination on streptozotocin induced diabetic rats. Int. J. Pharm. Pharm. Sci. 2015;7:455–458. [Google Scholar]
- Liu R.H. Potential synergy of phytochemicals in cancer prevention: Mechanism of action. J. Nutr. 2004;134:3479S–3485S. doi: 10.1093/jn/134.12.3479S. [DOI] [PubMed] [Google Scholar]
- Love V.L. University of Sheffield; 2020. A Study on the Effects of Species, Plant Density, Population Size and Species Mixture on the Morphological, Physiological and Thermal Performance of Six Woody Shrubs Growing on Rooftops in Sheffield, UK. Doctoral dissertation. [Google Scholar]
- Mahomoodally M.F., Atalay A., Picot M.C.N., Bender O., Celebi E., Mollica A., Zengin G. Chemical, biological and molecular modelling analyses to probe into the pharmacological potential of Antidesma madagascariense Lam.: a multifunctional agent for developing novel therapeutic formulations. J. Pharma Biomed. Anal. 2018;161:425–435. doi: 10.1016/j.jpba.2018.09.002. [DOI] [PubMed] [Google Scholar]
- Mangialasche F., Solomon A., Winblad B., Mecocci P., Kivipelto M. Alzheimer’s disease: clinical trials and drug development. Lancet Neurol. 2010;9:702–716. doi: 10.1016/S1474-4422(10)70119-8. [DOI] [PubMed] [Google Scholar]
- Manson J.E., Colditz G.A., Stampfer M.J., Willett W.C., Krolewski A.S., Rosner B., Arky R.A., Speizer F.E., Hennekens C.H. A prospective study of maturity-onset diabetes mellitus and risk of coronary heart disease and stroke in women. Arch. Intern. Med. 1991;151:1141–1147. [PubMed] [Google Scholar]
- Massagetov P.S. Alkaloids of plants of the family Elaeagnaceae. Zh. Obshch. Khim. 1946;16:139. [Google Scholar]
- Meeprom A., Sompong W., Suwannaphet W., Yibchok-anun S., Adisakwattana S. Grape seed extract supplementation prevents high-fructose diet-induced insulin resistance in rats by improving insulin and adiponectin signalling pathways. Br. J. Nutr. 2011;106:1173–1181. doi: 10.1017/S0007114511001589. [DOI] [PubMed] [Google Scholar]
- Mehta M., Adem A., Sabbagh M. New acetylcholinesterase inhibitors for Alzheimer’s disease. Int. J. Alzheimer’s Dis Article. 2012;1:1–8. doi: 10.1155/2012/728983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minhas F.A., Aziz S., ur Habib R., Irshad M., Ahmed M.N., Yasin K.A. Antiplasmodial activity of compounds isolated from Elaeagnus umbellata. J. Med. Plants Res. 2013;7:277–283. [Google Scholar]
- Minhas F.A., Rehaman H.U., Yasin A., Awan Z.I., Ahmed N. Antimicrobial activities of the leaves and roots of Elaeagnus umbellata Thunb. Afr. J. Biotechnol. 2013;12(48):6754–6760. [Google Scholar]
- Miura T., Koide T., Ohichi R., Kako M., Usami M., Ishihara E., Yasuda N., Ishida H., Seino Y., Tanigawa K. Effect of acarbose (α-glucosidase inhibitor) on disaccharase activity in small intestine in KK-Ay and ddY mice. J. Nutr. Sci. Vitaminol. (Tokyo) 1998;44:371–379. doi: 10.3177/jnsv.44.371. [DOI] [PubMed] [Google Scholar]
- Mubasher S.S., Dilnawaz S.A., Imtiaz M.H., Kaleem M.T. Antibacterial activity of Elaeagnus umbellata (Thunb.) a medicinal plant from Pakistan. Saudi Med. J. 2007;28(2):259–263. [PubMed] [Google Scholar]
- Munger, G.T., 2003. Elaeagnus umbellata. Fire Effects Information System. US Department of Agriculture. www. feis-crs. org/feis.
- Murray A., Faraoni M., Castro M., Alza N., Cavallaro V. Natural AChE inhibitors from plants and their contribution to Alzheimer’s disease therapy. Curr. Neuropharmacol. 2013;11:388–413. doi: 10.2174/1570159X11311040004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazir N., Zahoor M., Nisar M., Khan I., Karim N., Abdel-Halim H., Ali A. Phytochemical analysis and antidiabetic potential of Elaeagnus umbellata (Thunb.) in streptozotocin-induced diabetic rats: pharmacological and computational approach. BMC Complement. Altern. Med. 2018;18:1–16. doi: 10.1186/s12906-018-2381-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazir N., Zahoor M., Nisar M., Karim N., Latif A., Ahmad S., Uddin Z. Evaluation of neuroprotective and anti-amnesic effects of Elaeagnus umbellata Thunb. On scopolamine-induced memory impairment in mice. BMC Complement. Med. Ther. 2020;20(1):1–17. doi: 10.1186/s12906-020-02942-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazir N., Zahoor M., Uddin F., Nisar M. Chemical composition, in vitro antioxidant, anticholinesterase, and antidiabetic potential of essential oil of Elaeagnus umbellata Thunb. BMC Complement Med. Therap. 2021;21(1):1–13. doi: 10.1186/s12906-021-03228-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeta S.R., Jyoti B., Anjuvan S., Prabhjot K. Antibacterial potential of Achyranthus aspera Linn Procured from Himachal Pradesh, Punjab and Haryana, India. Res. J. Chem. Sci. 2011 [Google Scholar]
- Nigro E., Scudiero O., Monaco M.L., Palmieri A., Mazzarella G., Costagliola C., Bianco A., Daniele A. New insight into adiponectin role in obesity and obesity-related diseases. Biomed Res. Int. 2014;658913 doi: 10.1155/2014/658913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niranjan R. Recent advances in the mechanisms of neuroinflammation and their roles in neurodegeneration. Neurochem. Int. 2018;120:13–20. doi: 10.1016/j.neuint.2018.07.003. [DOI] [PubMed] [Google Scholar]
- Olson C. Vol. 52. Government Printing Office; 2017. (A guide to nonnative invasive plants inventoried in the north by forest inventory and analysis). [Google Scholar]
- Ozen T., Yenigun S., Altun M., Demirtas I. Phytochemical Constituents, ChEs and Urease Inhibitions, Antiproliferative and Antioxidant Properties of Elaeagnus umbellata Thunb. Comb. Chem. High Throughput Screen. 2017;20(6):559–578. doi: 10.2174/1386207320666170127161837. [DOI] [PubMed] [Google Scholar]
- Ozen T., Yildirim K., Toka M. The impacts of Elaeagnus umbellata Thunb. leaf and fruit aqueous extracts on mice hepatic, extrahepatic antioxidant and drug metabolizing enzymes related structures. Braz J Pharmaceut Sci. 2018:53. [Google Scholar]
- Parmar C., Kaushal M.K. Kalyani Publishers; India, New Delhi: 1982. Elaeagnus: A Widely Distributed Temperate Nitrogen Fixer. Wild Fruits; pp. 23–25. [Google Scholar]
- Paschke M.W., Dawason J.O., David M.B. Soil nitrogen mineralization under black walnut interplanted with Elaeagnus umbellata or Black Alder. J. Plant Soil. 1989;118:33–42. [Google Scholar]
- Patel S. Plant genus Elaeagnus: underutilized lycopene and linoleic acid reserve with permaculture potentia. Fruits. 2015;70(4):191–199. [Google Scholar]
- Paudel S.B., Han A.R., Choi H., Nam J.W. Phytochemical constituents of leaves and twigs of Elaeagnus umbellata. Biochem Systemat Ecol. 2020;93 [Google Scholar]
- Pei R., Yu M., Bruno R., Bolling B.W. Phenolic and tocopherol content of autumn olive (Elaeagnus umbellata) berries. J. Funct. Foods. 2015;16:305–314. [Google Scholar]
- Perkins-Veazie P.M., Black B.L., Fordham I.M., Howard L.R. Lycopene and total phenol content of autumn olive (Elaeagnus umbellata) selections. Hort. Sci. 2005;40:883–893. [Google Scholar]
- Perry E.K., Pickering A.T., Wang W.W., Houghton P., Perry N.S. Medicinal plants and Alzheimer's disease: integrating ethnobotanical and contemporary scientific evidence. J. Alternat. Complement Med. 1998;4(4):419–428. doi: 10.1089/acm.1998.4.419. [DOI] [PubMed] [Google Scholar]
- Pešić M., Stanković S. Development of natural product drugs in a sustainable manner. Brief for United Nations Global Sustainable Development Report. 2015 [Google Scholar]
- Pinto C., Rodríguez-Galdón B., Cestero J.J., Macías P. Hepatoprotective effects of lycopene against carbon tetrachloride-induced acute liver injury in rats. J. Funct. Foods. 2013;5(4):1601–1610. [Google Scholar]
- Polyzos S.A., Kountouras J., Zavos C., Tsiaousi E. The role of adiponectin in the pathogenesis and treatment of non-alcoholic fatty liver disease. Diabetes Obes. Metab. 2010;12:365–383. doi: 10.1111/j.1463-1326.2009.01176.x. [DOI] [PubMed] [Google Scholar]
- Potter T.L. Floral volatiles of Elaeagnus umbellata Thunb. J. Essent. Oil Res. 1995;7:347–354. [Google Scholar]
- Rafique N., Khan T., Shah A.J. Calcium entry blocking activity of the Elaeagnus umbellata fruit extract explains its use in diarrhea and gut spasm. Bangladesh J. Pharmacol. 2016;11:585–592. [Google Scholar]
- Rahman A., Choudhary M. Bioactive natural products as a potential source of new pharmacophores a theory of memory. Pure Appl. Chem. 2001;73:555–560. [Google Scholar]
- Rawat U., Sati S.C., Sati O.P., Takeda Y. Anthraquinone glycoside from Elaeagnus umbellata Thunb. J. Indian Chem. Soc. 2002;79:383–384. [Google Scholar]
- Sabir S.M., Ahmad S.D., Lodhi N. Morphological and biochemical variation in Sea buckthorn Hippophae rhamnoides L. ssp turkestanica, a multipurpose plant for fragile mountains of Pakistan. S African J Bot. 2003;69:587–592. [Google Scholar]
- Samant P., Singh M., Lal M., Singh A., Sharma A., Bhandari S. Medicinal plants in Himachal Pradesh, north western Himalaya, India. Intern. J. Biodiver. Sci. Manage. 2007;3(4):234–251. [Google Scholar]
- Sather, N., Eckardt, N., 1987. Element stewardship abstract for Elaeagnus umbellata.
- Sen S., Chakraborty R. Revival, modernization and integration of Indian traditional herbal medicine in clinical practice: Importance, challenges and future. J. Trad. Complement Med. 2017;7(2):234–244. doi: 10.1016/j.jtcme.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifi-Rad J., Cruz-Martins N., López-Jornet P., Lopez E.P.F., Harun N., Yeskaliyeva B., et al. Natural coumarins: exploring the pharmacological complexity and underlying molecular mechanisms. Oxidative Med. Cellul. Longevity. 2021 doi: 10.1155/2021/6492346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J., Arunasalam K., Yeung D., Kakuda Y., Mittal G., Jiang Y. Saponins from edible legumes: chemistry, processing and health benefits. J. Med. Food. 2004;7:67–78. doi: 10.1089/109662004322984734. [DOI] [PubMed] [Google Scholar]
- Spínola V., Pinto J., Llorent-Martínez E.J., Castilho P.C. Changes in the phenolic compositions of Elaeagnus umbellata and Sambucus lanceolata after in vitro gastrointestinal digestion and evaluation of their potential anti-diabetic properties. Food Res. Int. 2019;122:283–294. doi: 10.1016/j.foodres.2019.04.030. [DOI] [PubMed] [Google Scholar]
- Standl E., Baumgartl H.J., Füchtenbusch M., Stemplinger J. Effect of acarbose on additional insulin therapy in type 2 diabetic patients with late failure of sulphonylurea therapy. Diabetes Obes. Metab. 1999;1:215–220. doi: 10.1046/j.1463-1326.1999.00021.x. [DOI] [PubMed] [Google Scholar]
- Steinberg D. Antioxidants and atherosclerosis, a current assessment. Circulation. 1991;84:1420–1425. doi: 10.1161/01.cir.84.3.1420. [DOI] [PubMed] [Google Scholar]
- Sternberg G. Elaeagnus umbellata in Illinois conservation practice. Report.111. Dept. of conservation. Virginiana. 1982:251–278. [Google Scholar]
- Tariq A., Mussarat S., Adnan M. Review on ethnomedicinal, phytochemical and pharmacological evidence of Himalayan anticancer plants. J. Ethnopharmacl. 2015;164:96–119. doi: 10.1016/j.jep.2015.02.003. [DOI] [PubMed] [Google Scholar]
- Tolkachev O.N., Abizov E.A., Abizova E.V., Mal’Tsev S.D. Phytochemical study of the bark of some plants of the Elaeagnaceae family as a natural source of β-carboline indole alkaloids. Pharmaceut Chem J. 2008;42(11):630–632. [Google Scholar]
- Uddin G., Rauf A. Phytochemical screening and biological activity of the aerial parts of Elaeagnus umbellata. Sci. Res. Essays. 2012;7(43):3690–3694. [Google Scholar]
- Veazie P., Black B., Fordham I., Howard L. Lycopene and total phenol content of autumn olive (Elaeagnus umbellata) selections. Hortic. Sci. 2005;40(3):883–884. [Google Scholar]
- Veberic R., Slatnar A., Bizjak J., Stampar F., Mikulic-Petkovsek M. Anthocyanin composition of different wild and cultivated berry species. LWT Food Sci. Technol. 2015;60:509–517. [Google Scholar]
- Vickers J.C., Mitew S.A., Woodhouse C.M., Fernandez M., Kirkcaldie M.T., Canty A.J., et al. Defining the earliest pathological changes of Alzheimer’s disease. Curr. Alzheimer Res. 2016;13(3):281–287. doi: 10.2174/1567205013666151218150322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vina J., Lloret A., Giraldo E., Badia M., Alonso M. Antioxidant pathways in Alzheimer’s disease: possibilities of intervention. Curr. Pharm. Des. 2011;17(35):3861–3864. doi: 10.2174/138161211798357755. [DOI] [PubMed] [Google Scholar]
- Wang C.Y., Chen Y.W., Hou C.Y. Antioxidant and antibacterial activity of seven predominant terpenoids. Int. J. Food Prop. 2019;22:229–237. [Google Scholar]
- Wang J., Fang X., Ge L., Cao F., Zhao L., Wang Z., Xiao W. Antitumor, antioxidant and anti-inflammatory activities of kaempferol and its corresponding glycosides and the enzymatic preparation of kaempferol. PLoS One. 2018;13(5):e0197563. doi: 10.1371/journal.pone.0197563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.Y., Fordham I.M. Differences in chemical composition and antioxidant capacity among different genotypes of autumn olive (Elaeagnus umbellata Thunb) Food Technol. Biotechnol. 2007;45:402–409. [Google Scholar]
- Wang Z.J., Liang C.L., Li G.M., Yu C.Y., Yin M. Stearic acid protects primary cultured cortical neurons against oxidative stress 4. Acta Pharmacol. Sin. 2007;28(3):315–326. doi: 10.1111/j.1745-7254.2007.00512.x. [DOI] [PubMed] [Google Scholar]
- Wang T., Shankar K., Ronis M.J., Mehendale H.M. Potentiation of thioacetamide liver injury in diabetic rats is due to induced CYP2E1. J. Pharmacol. Exp. Ther. 2000;294(2):473–479. [PubMed] [Google Scholar]
- WCRF, AICR, 2007. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. World Cancer Research Fund, American Institute for Cancer Research, Washington DC, pp. 1-394.
- Xu X., Luo A., Lu X., Liu M., Wang H., Song H., Wei C., Wang Y., Duan X. p-Hydroxybenzoic acid alleviates inflammatory responses and intestinal mucosal damage in DSS-induced colitis by activating ERβ signaling. J. Funct. Foods. 2021;87 [Google Scholar]
- Yousuf B., Gul K., Wani A.A., Singh P. Health benefits of anthocyanins and their encapsulation for potential use in food systems: A review. Crit. Rev. Food Sci. Nutr. 2016;56:2223–2230. doi: 10.1080/10408398.2013.805316. [DOI] [PubMed] [Google Scholar]
- Zglińska K., Galewska A.R., Bryś J., Koczoń P., Borek K., Roguski M., Niemiec T. Elaeagnus umbellata fruit-chemical composition, bioactive compounds, and kinetic of DPPH inhibition compared to standard antioxidants. Emirates J. Food Agric. 2021:639–646. [Google Scholar]
- Zglińska K., Niemiec T., Łozicki A., Matusiewicz M., Szczepaniak J., Puppel K., Kutwin M., Jaworski S., Rygało-Galewska A., Koczoń P. Effect of Elaeagnus umbellata (Thunb.) fruit extract on H2O2-induced oxidative and inflammatory responses in normal fibroblast cells. PeerJ. 2021;9:e10760. doi: 10.7717/peerj.10760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zglińska K., Rygało-Galewska A., Niemiec T. Antioxidative properties of autumn olive (Elaeagnus umbellata) fruits according to the method of fruit preservation and solvent used in extraction. Polish Technical Rev. 2022 [Google Scholar]
- Zhao L., Ni Y., Yu H., Zhang P., Zhao A., Bao Y., et al. Serum stearic acid/ palmitic acid ratio as a potential predictor of diabetes remission after rouxen- Y gastric bypass in obesity. FASEB J. 2017;31(4):1449–1460. doi: 10.1096/fj.201600927R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zulfiqar H., Amjad M.S., Mehmood A., Mustafa G., Binish Z., Khan S., Arshad H., Proćków J., Pérez de la Lastra J.M. Antibacterial, Antioxidant, and Phytotoxic Potential of Phytosynthesized Silver Nanoparticles Using Elaeagnus umbellata Fruit Extract. Molecules. 2022;27(18):5847. doi: 10.3390/molecules27185847. [DOI] [PMC free article] [PubMed] [Google Scholar]
Further Reading
- Abizov E.A., Tolkachev O.N. Dynamics of accumulation and distribution of β-carboline alkaloids in Elaeagnus species cultivated in Moscow Region. Pharmaceut. Chem. J. 2012;45(10):632–635. [Google Scholar]