Abstract
Introduction and importance
Primary thyroid sarcomas are very rare tumours, accounting for less than 1 % of all thyroid malignancies. We present the fifth case in the literature of primary thyroid rhabdomyosarcoma and the third in adults with, for the first time, an extensive molecular analysis.
Case presentation
A 61-year-old woman presented with a rapidly progressive neck mass with extensive local invasion of the tumour.
Clinical discussion
Histologically, the neoplasm was composed of sheets of pleomorphic or spindle-shaped cells with eosinophilic cytoplasm and few large and very pleomorphic cells admixed with the spindle cell proliferation, without any thyroid epithelial component. Immunohistochemically, the tumour cells were positive for muscular markers and negative for epithelial and thyroid differentiation markers. Molecular tests revealed the presence of NF1, PTEN and TERT pathogenic mutations. Classifying undifferentiated neoplasm with muscular differentiation into the thyroid is challenging as many more common differential diagnoses could be favoured including anaplastic thyroid carcinoma with rhabdoid phenotype, leiomyosarcoma, and other rare sarcomas.
Conclusion
Primary thyroid rhabdomyosarcoma is extremely rare and can be diagnostically challenging. We emphasize the histological, immunohistochemical and molecular criteria in order to make an accurate diagnosis.
Keywords: Rhabdomyosarcoma, Thyroid, Head and neck, RNA sequencing, Diagnostic Molecular Pathology
Highlights
-
•
Primary sarcomas of the thyroid are uncommon tumours accounting for less than 1% of all thyroid malignancies.
-
•
Rhabdomyosarcoma (RMS) is a malignant skeletal muscle tumour that originates from embryonic mesenchymal tissue and is the most common type of soft-tissue sarcoma in the pediatric age group.
-
•
The frequency of Rhabdomyosarcoma decreases with advancing age and becomes very rare in adults.
-
•
Rhabdomyosarcoma of the thyroid is extremely rare with until our case only four cases reported in the literature.
-
•
The main differential diagnosis of Rhabdomyosarcoma in the thyroid gland is anaplastic carcinoma with rhabdoid phenotype.
-
•
In the presence of an undifferentiated spindle cell tumour with muscular component in the thyroid, the first hypothesis to be raised is that of a thyroid anaplastic carcinoma.
1. Introduction
Primary sarcomas of the thyroid are uncommon tumours, accounting for less than 1 % of all thyroid malignancies [1]. Rhabdomyosarcoma (RMS) is a malignant skeletal muscle tumour that originates from embryonic mesenchymal tissue. RMS is the most common type of soft-tissue sarcoma in the pediatric age group and accounts for 4–6 % of the malignancies among children and young adults [2]. In children, approximately 40 % of the RMSs are seen in the head and neck region [3] but only two cases have been reported in the thyroid [4,5]. The frequency of RMS decreases with advancing age and becomes very rare in adults where the pleiomorphic variant, located at lower limbs, is the more common subtype. In case reported herein we present the fifth case in the literature of primary thyroid rhabdomyosarcoma and the third in adults with, for the first time, an extensive molecular analysis.
This work has been reported in line with the SCARE 2020 criteria [6].
2. Case report
2.1. Clinical presentation
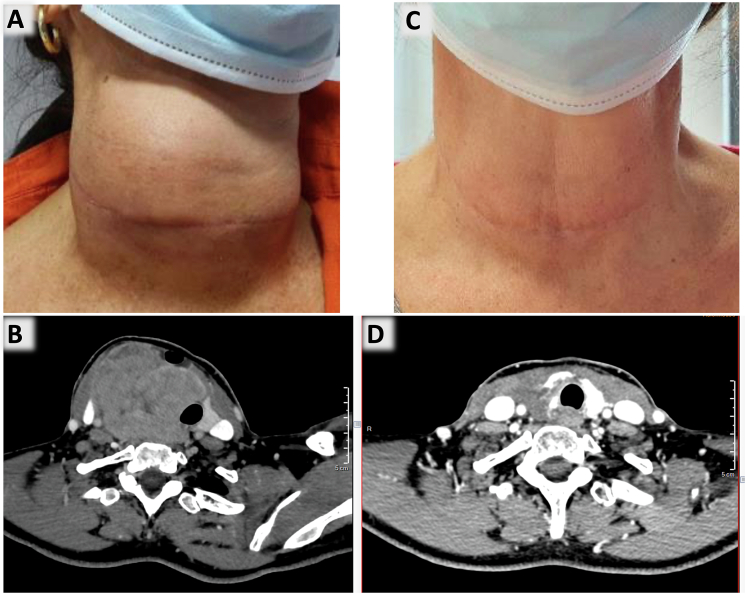
A 61-year-old female with no significant previous medical history (in particular with no history of pre-existing thyroid disease) presented with a progressive right neck mass for six months (Fig. 1A). The results of the thyroid function test showed normal levels, and there were no symptoms reported apart from discomfort caused by the mass effect. The initial thyroid ultrasound revealed a macro-nodule measuring 32 mm in the right lobe, which was classified as EU-TIRADS 4. Fine Needle Aspiration Cytology (FNAC) was performed and diagnosed as nondiagnostic (according to the Bethesda System, second edition). However, there was a rapid progression of the nodule, as confirmed by a second thyroid ultrasound, which showed a significant increase in size to an estimated 46 mm, along with the presence of multiple cervical lymphadenopathies. Neck computed tomography scan revealed a 4.6 cm-long thyroid lobe mass invading cervical strap muscles, carotid and pushing the trachea (Fig. 1B). Positron emission tomography scan revealed a standardised uptake value of the thyroid mass, and there were no enlarged or positron emission tomography-avid lymph nodes. Total thyroidectomy was initially contraindicated due to the extensive local invasion of the tumour; consequently a surgical biopsy was performed.
Fig. 1.
Clinical and radiological images pre (A and B) and post treatment (C and D). A: rapidly progressive right neck mass; B: neck computed tomography scan revealing a 4.6 cm long thyroid lobe mass; C: the neck mass reduced significantly after chemotherapy; D: neck computed tomography scan revealing significant remission of the mass after chemotherapy.
2.2. Pathological examination of the biopsy
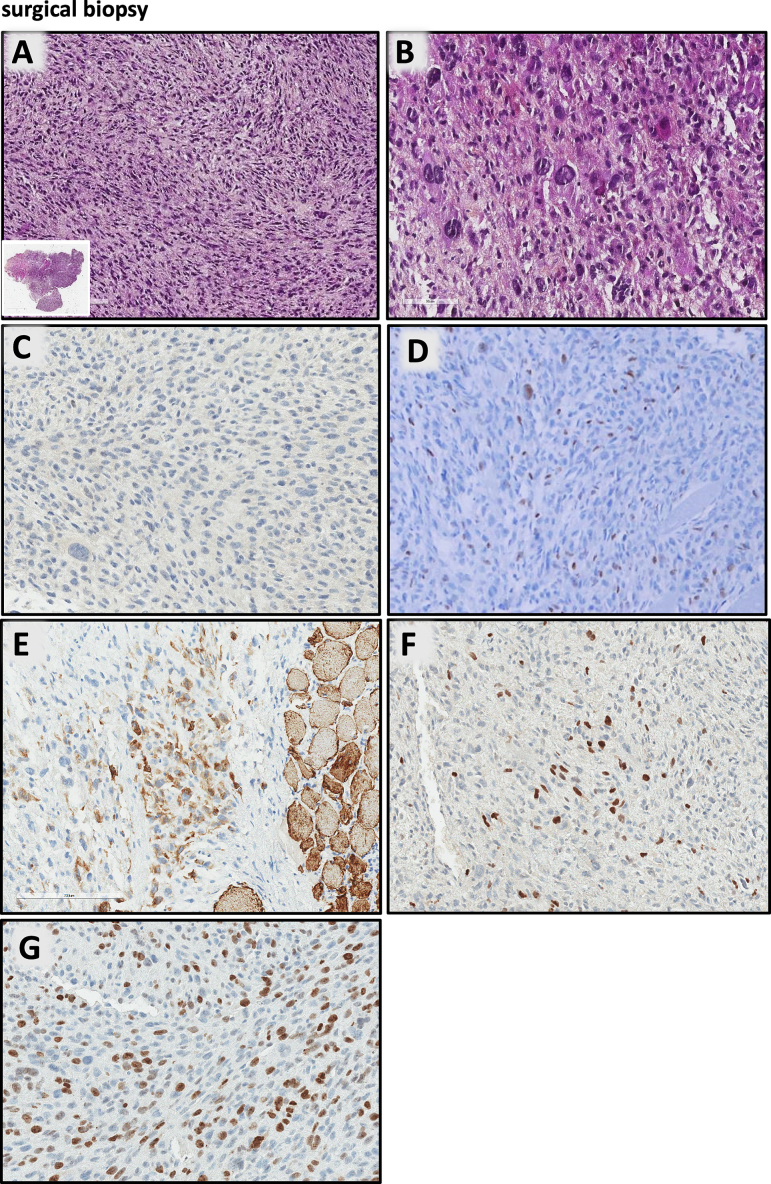
The tumour was composed of sheets of pleomorphic or spindle-shaped cells with eosinophilic cytoplasm and blurred cytoplasmic boundaries (Fig. 2A). The nuclei were small and rounded and sometimes hyperchromatic with coarse chromatin. Numerous mitotic figures (up to 5 mitoses per high-power field) and atypical mitoses were found. Few large and very pleomorphic cells (Fig. 2B) were mixed with the spindle cell proliferation. No significant associated inflammatory infiltrate was observed. In the periphery, flaps of striated muscle were infiltrated by the spindle cells and no thyroid epithelial component was observed.
Fig. 2.
Surgical biopsy A: tumour composed of sheets of pleomorphic or spindle-shaped cells with eosinophilic cytoplasm and blurred cytoplasmic boundaries (inset: low-power whole-mount image); B: large and pleomorphic cells mixed with the spindle cell proliferation; C–G: immunohistochemistry: tumour cells are negative for PAX8 (C); positive for MyoD1 (D); Desmin (E); Myogenin (F); index of proliferation Ki67 estimated as 40 % (G).
Immunohistochemistry showed that the tumour cells were negative for epithelial and thyroid differentiation markers (AE1-AE3, EMA, CK5/6, TTF1, PAX8, thyroglobulin; Fig. 2C) but diffusely positive for muscular markers: MyoD1 (Fig. 2D), desmin (Fig. 2E), myogenin (Fig. 2E). Index of proliferation Ki67 estimated as 40 % (Fig. 2F). A small, targeted NGS analysis including BRAF, HRAS, NRAS, KRAS and TP53 was performed and was negative.
The case was sent to the French pathologist network for soft tissues tumours (Réseau de Référence en Pathologie des Sarcomes des tissus mous et des viscères, RRePS). The proposed diagnosis was pleomorphic tumoral proliferation with rhabdomyosarcoma differentiation, rather in favour of a sarcomatoid anaplastic carcinoma with heterologous mesenchymal skeletal muscle component than a primary RMS in the thyroid location.
The patient had received four courses of neoadjuvant chemotherapy (cisplatin and doxorubicin) and external radiotherapy. The treatment significantly reduced the neck mass (Fig. 1C and D); a total thyroidectomy with right recurrent nerve resection was then possible and performed.
2.3. Pathological examination of the total thyroidectomy
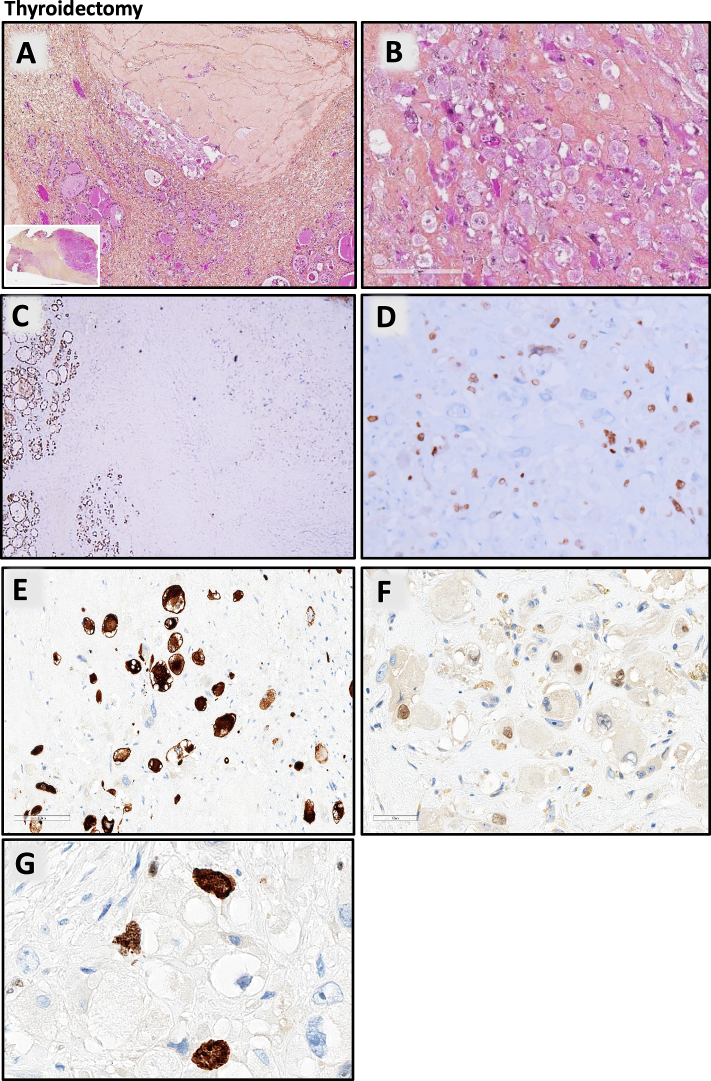
Grossly, a 5 cm-long poorly demarcated whitish nodule of the right thyroid lobe was observed. Microscopically, the thyroid parenchyma was totally fibrotic and atrophic with rare small thyroid follicles enclosed within the fibrosis (Fig. 3A). Few cells arranged in sheets and nests were scattered in the thyroid parenchyma. These cells were large, with a large eosinophilic cytoplasm with intracellular eosinophilic filaments and large nuclei strongly nucleated (Fig. 3B); mitoses were very rare, and no necrosis was found. These atypical cells extended beyond the thyroid into the striated muscle tissue and the adipose tissue. No follicular, papillary or poorly differentiated thyroid carcinoma component was seen on all the thyroid. The margins of resection were free of tumour, and regional lymph nodes were negative.
Fig. 3.
Thyroidectomy. A: thyroid parenchyma totally fibrotic with rare small thyroid follicles enclosed within the fibrosis (inset: low-power whole-mount image); B: large pleomorphic cells with a large eosinophilic cytoplasm and large nuclei strongly nucleated with intra cellular eosinophilic filaments; immunohistochemistry: tumour cells are negative for PAX8 (C); positive for MyoD1 (D); Desmin (E); Myogenin (F); index of proliferation Ki67 estimated as 20 % (G).
Immunohistochemistry showed that the tumour cells were negative for epithelial and thyroid differentiation markers (AE1-AE3, EMA, CEA, CK5/6, TTF1, PAX8, thyroglobulin; Fig. 3C), and strongly and diffusely positive for MyoD1 (Fig. 3D) desmin (Fig. 3E) and myogenin (Fig. 3F). Index of proliferation Ki67 estimated as 20 % (Fig. 3G). Using a large pan-cancer DNA-seq panel (custom panel from SOPHIA GENETICS), we identified inactivating mutations in NF1 (c.6852_6855del p.(Tyr2285Thrfs*5)) and PTEN (c.860C>G p.(Ser287*)) tumour suppressor genes. We also observed an activating mutation in the TERT promoter (c.-146C>T). RNA sequencing (ARCHER Panel FusionPlex) did not detect any fusion.
3. Discussion
Primary thyroid RMS is extremely rare; the present report describes the fifth case of primary thyroid rhabdomyosarcoma, the third in adults and the first with extensive molecular analysis [4,5,7,8]. Table 1 summarises the clinicopathological and molecular features of all primary thyroid RMS reported cases, including the case reported herein.
Table 1.
Clinicopathological and molecular features of primary thyroid RMS cases reported herein and in the literature.
| Case | Age (sex) | Size, cm | Molecular investigations | Tumour extension | Pathological examination |
Treatment | Follow-up | ||
|---|---|---|---|---|---|---|---|---|---|
| Histological description | Immunohistochemical profile | Diagnosis | |||||||
| Furze et al (2005) [5] | 7 months (M) | 5 | NA | Left piriform sinus Clavicule, cricoid cartilage. | Spindle cells. | NA | Embryonal RMS | Lobectomy and chemotherapy | Alive (9 months) |
| Dutta et al (2013) [4] | 7 years (M) | 6.5 | NA | Strap muscles, right SCM, carotid, clavicle | Spindle cells, scattered rhabdomyoblasts. | Desmin, myogenin: + Cytokeratin, CD34, CD31: − |
Embryonal RMS | Lobectomy and chemotherapy | Neck recurrence leading to chemotherapy and radiotherapy. Alive (4 years) |
| Ozaslan et al (2016) [6] | 68 years (M) | 5 | NA | Mediastinum, oesophagus recurrent laryngeal nerve. | Spindle cells, scattered rhabdoid cells. | Desmin: + AE1-AE3, Actin, TTF1: − |
Pleomorphic RMS | Total thyroidectomy | NA |
| Febrero et al (2017) [7] | 67 years (M) | 6 | NA | Intrathoracic, large veins thrombosis, auricular thrombosis | Small cells, scattered rhabdomyoblasts. | Desmin, actin, myogenin: + AE1-AE3, EMA, CEA, TTF1: − |
RMS | Biopsy | Died 48 h after surgery (cardiac insufficiency) |
| Current case | 61 years (F) | 4.6 | NF1 c.6852_6855del, PTEN c.860C>G and TERT promoter (c.-146C>T). | SCM, carotid, recurrent nerve | Spindle cells, scattered rhabdomyoblasts. | Desmin, MyoD1, Myogenin: + AE1-AE3, EMA, CEA, TTF1, PAX8, Tg: − |
Pleomorphic RMS | Biopsy followed by chemotherapy and radiotherapy then total thyroidectomy | Alive (10 months) No recurrence |
Abbreviations: F: Female; M: Male; NA: Not available; RMS: Rhabdomyosarcoma; SCM: sternocleidomastoid muscle; TG: thyroglobulin.
Classifying this undifferentiated neoplasm with muscular differentiation into the thyroid is challenging as many more common differential diagnoses could be favoured [9]. Anaplastic thyroid carcinoma (ATC) with rhabdoid phenotype is the main differential diagnosis; this is also called sarcomatoid carcinoma with heterologous mesenchymal elements, carcinosarcoma, or rhabdoid tumour, although nowadays only the first term should be used. According to the 2022 WHO classification, ATC is a high-grade tumour, composed mainly of spindle and pleomorphic cells, sometimes associated with rhabdoid, angiomatoid, chondroid or osteoid components [1,[9], [10], [11]]. The main clinical and histological features helpful to differentiate sarcomatoid ATC from a true sarcoma are the occurrence in elderly patients with a longstanding history of pre-existing thyroid disease, the identification of associated well-differentiated thyroid carcinoma (present in 30–70 % of ATC), an inflammatory stroma, In immunohistochemistry, positivity for epithelial markers (25–70 % of cases) and PAX8 (50–75 % of cases), whereas TTF1 and thyroglobulin are most frequently negative. The muscular markers (myogenin, MyoD1 and desmin) are negative or patchy in ATC and positive in RMS (often for more than one maker) [1,[10], [11], [12]].
The second differential diagnosis that should be considered is another sarcoma, but which are very rare in the thyroid location. Angiosarcoma is the most frequent thyroid sarcoma, but nowadays, many cases are reclassified as angiomatoid ATC; leiomyosarcoma represent 11 % of thyroid sarcomas, and there are only very rare case reports of other sarcomas (such as malignant peripheral nerve sheath tumours, synovial sarcoma, etc.) [1,11].
RMS thyroid metastases in adults have been reported twice [13,14]. In our case, a PET scan was performed to rule out other neoplastic lesions.
In anaplastic thyroid carcinomas, TERT promoter mutation alongside RAS, BRAF p.V600E, and TP53 mutations are the most frequently found [1,11].
NF1, PTEN, and TERT promoter mutations are not specific to either RMS or ATC. NF1 mutation had been described in 4 % of RMS [15]. TERT promoter mutations are rarely found in RMS. Among RMS, some molecular alterations correlated with distinct new subtypes of rhabdomyosarcomas (RMS with MYOD1 mutations, RMS with TFCP2 fusions, and RMS with VGLL2/NCOA2 fusions subtypes), some RMS are associated with cancer syndromes (loss of function mutations of TP53 with the Li-Fraumeni syndrome, DICER 1 syndrome). In contrast, pleiomorphic and embryonal RMS have no specific molecular alterations [2]. The molecular findings we found in our case are compatible with the diagnosis of pleomorphic RMS.
In conclusion, the present study reports an exceptional case of primary thyroid rhabdomyosarcoma in an adult. Primary thyroid rhabdomyosarcoma is extremely rare and can be diagnostically challenging. Immunohistochemical staining and molecular biology may help establish a diagnosis and distinguish the disease from anaplastic carcinoma and other mimicking differential diagnoses.
Ethical approval
The case report was approved by the department and the university ethical committee.
Funding
None.
CRediT authorship contribution statement
ZA, FC and MDP did the conception, design of the work, and the data collection; ZA, MDP, FD and JL did the data analysis and interpretation; JCL, FD, JL, NZ, CCB, AM and MDP did the critical revision of the article; ZA and MDP did the final approval of the version to be published.
Guarantor
Ziyad Alsugair and Myriam Decaussin-Petrucci.
Research registration number
None.
Patient consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Ethical statement
Ethical approval for this study was provided by the ethics committee of the medical faculty and the state medical board of Lyon 1 university, Lyon Sud hospital, Lyon, France on 15 February 2023.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Conflict of interest statement
No conflicts of interest.
Acknowledgment
The authors wish to express their gratitude to Pr Jonathan Lopez, Dr. Nikolaos Zirganos, and Dr. Céline Charon-Barra for their valuable contributions towards diagnosing and managing this case.
References
- 1.WHO Classification of Tumours Editorial Board . International Agency for Research on Cancer; Lyon: 2022. Endocrine And Neuroendocrine Tumours [Internet]https://tumourclassification.iarc.who.int/chapters/36 [cited 2022 Sep 19]. Available from: [cited 2022 Sep 19]. Available from: [Google Scholar]
- 2.Leiner J., Le Loarer F. Virchows Arch. 2020;476:97. doi: 10.1007/s00428-019-02676-9. [DOI] [PubMed] [Google Scholar]
- 3.Kraus D.H., Saenz N.C., Gollamudi S., Heller G., Moustakis M., Gardiner S., Gerald W.L., Ghavimi F., LaQuaglia M.P. Am. J. Surg. 1997;174:556. doi: 10.1016/s0002-9610(97)00171-2. [DOI] [PubMed] [Google Scholar]
- 4.Dutta M., Chatterjee I., Roy S., Gure P.K. Laryngoscope. 2013;123:2072. doi: 10.1002/lary.23794. [DOI] [PubMed] [Google Scholar]
- 5.Furze A.D., Lehman D.A., Roy S. Int. J. Pediatr. Otorhinolaryngol. 2005;69:267. doi: 10.1016/j.ijporl.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Agha R.A., Franchi T., Sohrab C., Mathew G., Kirwan A., Thomas A., et al. The SCARE 2020 guideline: updating consensus Surgical Case Report (SCARE) guidelines. Int. J. Surg. 2020;84(1):226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 7.Ozaslan E., Berk V., Baldane S., Eker B., Bozkurt O., Senol S., Ocak Duran A., Cubukcu G., Karaca H., Ozkan M. Eurasian J. Med. 2016;48:69. doi: 10.5152/eurasianjmed.2015.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Febrero B., Oviedo I., Ríos A., Rodríguez J.M. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2017;134:49. doi: 10.1016/j.anorl.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 9.Bishop J.A., Thompson L.D.R., Cardesa A., Barnes L., Lewis J.S., Triantafyllou A., Hellquist H., Stenman G., Hunt J.L., Williams M.D., Slootweg P.J., Devaney K.O., Gnepp D.R., Wenig B.M., Rinaldo A., Ferlito A. Head Neck Pathol. 2015;9:507. doi: 10.1007/s12105-015-0624-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abe I., Lam A.K.-Y. Histol. Histopathol. 2021;36:239. doi: 10.14670/HH-18-277. [DOI] [PubMed] [Google Scholar]
- 11.Baloch Z.W., Asa S.L., Barletta J.A., Ghossein R.A., Juhlin C.C., Jung C.K., LiVolsi V.A., Papotti M.G., Sobrinho-Simões M., Tallini G., Mete O. Endocr. Pathol. 2022;33:27. doi: 10.1007/s12022-022-09707-3. [DOI] [PubMed] [Google Scholar]
- 12.Ragazzi M., Ciarrocchi A., Sancisi V., Gandolfi G., Bisagni A., Piana S. Int. J. Endocrinol. 2014;2014 doi: 10.1155/2014/790834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hafez M.T., Hegazy M.A., Abd Elwahab K., Arafa M., Abdou I., Refky B. Head Neck Oncol. 2012;4:27. doi: 10.1186/1758-3284-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walavalkar V., Fischer A.H., Owens C.L. Acta Cytol. 2014;58:288. doi: 10.1159/000358265. [DOI] [PubMed] [Google Scholar]
- 15.Shern J.F., Chen L., Chmielecki J., Wei J.S., Patidar R., Rosenberg M., Ambrogio L., Auclair D., Wang J., Song Y.K., Tolman C., Hurd L., Liao H., Zhang S., Bogen D., Brohl A.S., Sindiri S., Catchpoole D., Badgett T., Getz G., Mora J., Anderson J.R., Skapek S.X., Barr F.G., Meyerson M., Hawkins D.S., Khan J. Cancer Discov. 2014;4:216. doi: 10.1158/2159-8290.CD-13-0639. [DOI] [PMC free article] [PubMed] [Google Scholar]