Abstract
Background:
Sorafenib,an orally bioavailable, multitarget tyrosine kinase inhibitor, and irinotecan, a topoisomerase I inhibitor, have demonstrated activity in pediatric and adult malignancies. We evaluated the toxicity, pharmacokinetic (PK), and pharmacogenomic (PGX) profile of sorafenib with irinotecan in children with relapsed or refractory solid tumors and assessed the feasibility of incorporating patient-reported outcome (PRO) measures as an adjunct to traditional endpoints.
Methods:
Sorafenib, continuous oral twice daily dosing, was administered with irinotecan, orally, once daily days 1–5, repeated every 21 days (NCT01518413). Based on tolerability, escalation of sorafenib followed by escalation of irinotecan was planned. Three patients were initially enrolled at each dose level. Sorafenib and irinotecan PK analyses were performed during cycle 1. PRO measurements were collected during cycles 1 and 2.
Results:
Fifteen patients were evaluable. Two of three patients at dose level 2 experienced dose-limiting toxicity (DLT), grade 3 diarrhea, and grade 3 hyponatremia. Therefore, dose level 1 was expanded to 12 patients and two patients had DLT, grade 4 thrombocytopenia, grade 3 elevated lipase. Nine of 15 (60%) patients had a best response of stable disease with four patients receiving ≥6 cycles.
Conclusions:
The recommended dose for pediatric patients was sorafenib 150 mg/m2/dose twice daily with irinotecan 70 mg/m2/dose daily × 5 days every 21 days. This oral outpatient regimen was well tolerated and resulted in prolonged disease stabilization. There were no significant alterations in the PK profile of either agent when administered in combination. Patients were willing and able to report their subjective experiences with this regimen.
Keywords: irinotecan, pediatric, phase 1, solid tumors, sorafenib
1 |. INTRODUCTION
1.1 |. Sorafenib
Sorafenib, an orally bioavailable, small-molecule multiprotein kinase inhibitor with targets including CRAF, BRAF, VEGFR-2, VEGFR-3, PDGFR-, RET, FLT3, and c-Kit,1,2 disrupts tumor cell proliferation and angiogenesis, resulting in cellular apoptosis. In vivo and in vitro analyses demonstrated inhibition of tumor growth in adult and pediatric tumor cell lines.3–12 Sorafenib is currently approved by the US Food and Drug Administration (FDA) for the treatment of renal cell carcinoma, hepatocellular carcinoma, and metastatic differentiated thyroid cancer.13–16
In a pediatric phase 1 single-agent trial of sorafenib, administered orally twice daily continuously for a 28-day cycle, the recommended dose was 200 mg/m2/dose for patients with solid tumors (n = 49).17 Fourteen patients demonstrated stable disease (SD) for ≥ 4 cycles. In a subsequent pediatric phase 2 trial utilizing this dose, sorafenib was administered to patients with rhabdomyosarcoma and Wilms tumor (WT). Although responses were not observed, sorafenib was well tolerated without excessive toxicity.18
1.2 |. Irinotecan
Irinotecan, a camptothecin analog that complexes with DNA and topoisomerase I resulting in DNA strand breaks, is a prodrug converted to the potent active metabolite 7-ethyl-10-hydroxy camptothecin (SN-38).19–21
Irinotecan is widely used in pediatric malignancies22–25 and has been evaluated on different dosing schedules utilizing intravenous (IV) and oral formulations.26–29 The maximum-tolerated dose (MTD) of a protracted course of oral irinotecan, daily × 5 days for 2 weeks, in pediatric patients was 60 mg/m2/dose when administered with cefixime.30 When administered as a short course orally daily for 5 days repeated every 21 days in patients with solid tumors, the MTD was 65 mg/m2/dose for patients < 65 years of age.31 All studies demonstrated SN-38 exposure with oral administration to be similar to that documented with IV dosing.
As part of combination therapy, the MTD of a protracted course of oral irinotecan with temozolomide was 60 mg/m2/day in patients with neuroblastoma.32 Oral irinotecan with temozolomide and vincristine was evaluated in patients with solid tumors.33 The irinotecan MTD was 35 mg/m2/daily when administered 5 days for 2 weeks with temozolomide (100 mg/m2/day) or 90 mg/m2/daily × 5 days with temozolomide (150 mg/m2/day).
1.3 |. Sorafenib and irinotecan combination therapy
Preclinical evaluation performed in a colon carcinoma model resulted in a growth delay of 100% with sorafenib therapy, 71% with irinotecan, and 229% with the two drugs in combination.34 In adult clinical studies of combination therapy, there were no significant alterations in the pharmacokinetic (PK) profile of the agents; toxicities experienced were as expected with these agents and did not appear to be increased or exacerbated.35–38 Responses in select tumor types were promising, and there was evidence that the combination might overcome tumor cell-resistance mechanisms.39
Sorafenib with irinotecan is of interest as these agents offer different mechanisms of action with demonstrated efficacy in solid tumor malignancies. An oral regimen adds convenience and cost effectiveness. We conducted a phase 1 multicenter dose-escalation study to describe the toxicity profile and determine the recommended dose of sorafenib administered with irinotecan in pediatric patients with relapsed or refractory solid tumors. We evaluated the plasma PK profile of sorafenib and irinotecan when administered in combination, tested sorafenib exposure in patients expressing a variant UGT1A1 gene and assessed the feasibility of incorporating patient-reported outcome (PRO) measures into a phase 1 trial. Disease response was determined within the confines of a phase 1 trial.
2 |. METHODS
2.1 |. Patients and treatment protocol
Patients 2 to 22 years of age with relapsed or refractory solid tumors and measurable or evaluable disease were eligible for this nonrandomized dose-escalation study. Patients met standard eligibility requirements including performance status and organ function parameters (Supporting Information Table S1). Informed consent was obtained from each patient or guardian (ClinicalTrials.gov identifier: NCT01518413). This study was conducted under an investigator-sponsored IND (IND 112382), cross-referenced to an IND filed with the FDA (IND Number: 60453) by Bayer HealthCare Pharmaceuticals, Inc. Institutional Review Board approval was obtained at all participating institutions: Children’s National Hospital, Children’s Hospital of Philadelphia, Dana-Farber/Boston Children’s Cancer and Blood Disorders Center, and the Pediatric Oncology Branch of the National Cancer Institute, National Institutes of Health (NIH).
Sorafenib (50- and 200-mg tablets), continuous oral administration at a starting dose of 150 mg/m2/dose twice daily, was given with irinotecan 70 mg/m2/dose administered orally once daily days 1–5 of each cycle (Table 1). Cycles were repeated every 21 days. Initial doses represent approximately 75% of the recommended pediatric oral dose of each agent as defined on prior studies.17,33 Cefixime or appropriate equivalent antibiotic was administered beginning two days prior to the first dose of irinotecan and continued through protocol therapy to minimize irinotecan-associated diarrhea. Patients were eligible to continue on protocol therapy for up to 35 cycles (approximately two years) as long as they achieved a response of SD or, better, did not experience dose-limiting toxicity (DLT) and recovered from any treatment-related toxicities.
TABLE 1.
Clinical trial design
| Dose level | Sorafenib dose (mg/m2) | Irinotecan dose (mg/m2) | Initial #of patientsa |
|---|---|---|---|
| 0 | 105 | 70 | |
| 1b | 150 | 70 | 3–6 |
| 2 | 200 | 70 | 3–6 |
| 3 | 200 | 90 | 3–6 |
Abbreviation: MTD, maximum tolerated dose.
Expand at MTD up to 12 patients
Starting dose level.
Sorafenib: twice daily, orally on a continuous schedule days 1–21. Irinotecan: once daily, orally on days 1–5.
Cefixime: once daily, orally beginning day −2 and continued through treatment.
Toxicity was graded according to the CTEP Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 with the exception of hypertension for which CTCAE version 3.0 was utilized. Sorafenib and irinotecan PK analyses were performed during cycle 1 and PRO measurements were collected during cycles 1 and 2 for patients ≤18 years of age. Disease response was assessed according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 guidelines following every second cycle and once off study.40
2.2 |. Dose-limiting toxicity and maximum tolerated dose
Hematological DLT was defined as grade 4 neutropenia or thrombocytopenia on three separate occasions measured over a one-week period or duration of cytopenia > 7 days. Nonhematologic DLT was defined as any ≥ grade 3 nonhematologic toxicity, with the exception of grade 3 nausea, vomiting, or diarrhea controlled within 72 hours, grade 3 ALT elevation that recovered with discontinuation of sorafenib and did not recur and grade 3 hypomagnesemia, hypokalemia, hypocalcemia, or hypophosphatemia, which corrected with supplementation. Grade 3 infection and febrile neutropenia were excluded. Any grade 2 toxicity persisting > 7 days considered intolerable by the patient and/or not controlled with standard supportive care measures, blood pressure > 25 mmHg above the 95th percentile for age, height and gender not controlled within 14 days of initiating or modifying antihypertensive therapy and ≥ grade 4 hypertension or gastrointestinal perforation were considered DLTs.
This phase 1 clinical trial used a 3 + 3 dose-escalation design, three to six patients were enrolled at each dose level and dose escalations proceeded in the absence of DLT attributed to protocol therapy during cycle 1, first with dose escalation of sorafenib and then dose escalation of irinotecan. In the absence of toxicity, both irinotecan and sorafenib doses would be escalated to the recommended dose of each as single agents. The highest dose level at which < 33% of patients (1 of 6) experienced a protocol therapy–related DLT during cycle 1 was considered the MTD. The MTD level was then expanded to up to 12 patients to ensure a representative age distribution for PK analysis.
2.3 |. Pharmacokinetic and pharmacogenomic analysis
Blood samples (3–5 mL in heparinized tubes) were collected, placed on ice, and plasma was obtained by centrifugation (10 000 rpm for 2 minutes). Aliquots were transferred to cryovials then stored, protected from light at −70°C. Blood volume for research purposes remained below NIH pediatric phlebotomy requirements.
Plasma concentrations of irinotecan, its active metabolite SN38, the glucuronic acid metabolite of SN38 (SN38-G), and sorafenib were quantitatively measured using ultra-high-performance liquid chromatography tandem mass spectrometry (HPLC-MS/MS) assays.17,41,42 The lower limits of quantitation were 5 ng/mL for irinotecan, 0.5 ng/mL for SN38 and SN38-G and 50 ng/mL for sorafenib. Cycle 1, day 1 plasma samples were obtained pre-dose, and 1, 2, 4, 7, and 23 hours after administration for irinotecan PK analysis. Samples for sorafenib PK assessment were obtained pre-dose, 1, 2, 5, 8, and 24 hours after dose on day 1 and once between days 5 and 7 of cycle 1. The second dose of sorafenib on day 1 of cycle 1 was held.
A noncompartmental analysis assessed plasma PK parameters using a validated version of Phoenix 6.4 (Certara, Princeton, NJ) based on actual times elapsed post dose. The maximum plasma concentration (CMAX) and the time to CMAX (TMAX) were recorded as observed values, and the area under the concentration-time curve extrapolated to infinity (AUCINF) was calculated using the Linear Up Log Down method. All statistical analyses were performed using GraphPad Prism, v6 (Graph-Pad Software, San Diego, CA).
DNA for the PGX assay was purified from whole blood using the Gentra Puregene Blood Kit (Qiagen). DNA was hybridized to the Pharmacoscan array in a GeneTitan MC instrument (Applied Biosystems) following DNA amplification, purification, and resuspension according to the manufacturer’s instructions. Genotype was determined followed by data analysis using Axiom Analysis Suite v4.0.3.3 software (Thermo Fisher Scientific).
2.4 |. Patient-reported outcomes
The Patient-Reported Outcomes Measurement Information System (PROMIS; www.nihpromis.org) pediatric short forms for mobility, pain interference, fatigue, depressive symptoms, anxiety, and peer relationships were administered to patients 8–18 years of age via computer in a private clinic or hospital room at two time points: time of trial enrollment (T1) and 3 to 4 weeks later (T2). The PROMIS scores were correlated with traditional endpoints of toxicity, PK profile, and tumor response.
3 |. RESULTS
3.1 |. Patient characteristics
Seventeen subjects were enrolled on study and 15 patients were evaluable (Table 2). Two patients were deemed not evaluable and were replaced. One patient was removed from the study due to an allergic reaction probably related to sorafenib, though not meeting criteria for a DLT. Per protocol, in the absence of a DLT, patients must receive ≥ 85% of the prescribed doses during cycle 1 to be evaluable for toxicity assessment. The second patient developed difficulty breathing consistent with disease progression prior to beginning protocol therapy and was removed from the study.
TABLE 2.
Patient demographics
| Total patients Enrolled: Evaluable | 17:15 |
|---|---|
| Age at enrollment | Median: 14.4 years; range: 4.7–20.1 years |
| Male:female | 9:6 |
| Diagnosis | Osteosarcoma (4) Wilms tumor (4) Neuroblastoma (2) Synovial cell sarcoma (1) Anaplastic glioneuronal tumor (1) Desmoplastic small round cell tumor (1) Malignant peripheral nerve sheath tumor (1)Germ cell tumor (1) |
| Prior therapy | Chemotherapy < 3 regimen (12) Chemotherapy ≥ 3 regimen (3) Radiation therapy (7 external beam radiation, 1 MIBG)Autologous stem cell transplant (4) |
Abbreviation: MIBG, metaiodobenzylguanidine.
Of those evaluable, nine were males and six females ranging in age from 4.7 to 20.1 years (median 14.4 years). Diagnoses included osteosarcoma (n = 4), WT (n = 4), neuroblastoma (n = 2), synovial cell sarcoma (n = 1), anaplastic glioneuronal tumor (n = 1), desmoplastic small round cell tumor (n = 1), malignant peripheral nerve sheath tumor (n = 1), and germ cell tumor (n = 1). This was a heavily pretreated population with 67% (10 of 15) of patients having previously received multiple, predominantly cytotoxic chemotherapy regimens (median 2, range, 1–3) coupled with radiation therapy, radiolabeled metaiodobenzylguanidine (MIBG) therapy, and high-dose chemotherapy and autologous stem cell transplant (HD-ASCT) in select patients. A median of four cycles (1–8) were administered with nine patients receiving ≥ 4 cycles. Patients came off protocol therapy due to disease progression (n = 11), DLT (n = 2), and family/physician preference (n = 2).
3.2 |. Safety
There were no DLTs attributed to protocol therapy in the initial three patients treated on dose level 1; therefore, the sorafenib dose was escalated, and three patients were treated on dose level 2 (Table 3). One patient experienced dose-limiting grade 3 diarrhea requiring hospitalization. The patient was eligible to continue on protocol therapy following dose reduction and completed seven additional cycles of therapy. A second patient developed grade 3 dose-limiting hyponatremia. The patient and family elected to withdraw from the study rather than proceed with a dose reduction. With the excessive DLT rate at dose level 2, dose level 1 was expanded to 12 patients. One patient experienced grade 4 thrombocytopenia and came off protocol therapy. One patient developed grade 3 elevated serum lipase, but remained on protocol therapy following dose reduction to complete four cycles. Of the patients with DLT, all were ≥12 years of age at enrollment and two patients had previously received HD-ASCT. The more common adverse events reported, but not meeting criteria for DLT included myelosuppression, anorexia, diarrhea, and nausea (Supporting Information Table S2). Dose level 1, sorafenib 150 mg/m2/dose continuously twice daily with irinotecan 70 mg/m2/dose once daily for 5 days, was determined to be the recommended dose.
TABLE 3.
Dose-limiting toxicity (DLT)
| Dose level | Evaluable patients | Patients with DLT | DLT | Attribution to protocol therapy |
|---|---|---|---|---|
| 1a | 12 | 2 | Grade 3 elevated lipaseGrade 4 thrombocytopenia | ProbablyPossibly |
| 2 | 3 | 2 | Grade 3 diarrheaGrade 3 hyponatremia | DefinitelyPossibly |
| Dose level | Age, years | Evaluable patients | Patients with DLT | |
| 1 | < 12 | 6 | 0 | |
| ≥ 12 | 6 | 2 | ||
| 2 | < 12 | 0 | 0 | |
| ≥ 12 | 3 | 2 |
Abbreviation: MTD, maximum tolerated dose.
17% of patients experience a DLT at the MTD.
3.3 |. Pharmacokinetic and pharmacogenomic analysis
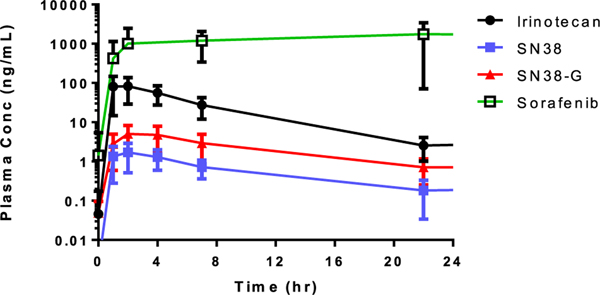
There was sufficient PK data from all 15 evaluable patients to complete noncompartmental analyses (Table 4) and plasma concentration versus time curves (Figure 1) for irinotecan and the metabolites, SN38 and SN38G. The TMAX for orally administered irinotecan, SN38, and SN38G were 1.97 ± 0.85 hours, 2.44 ± 1.32 hours, and 3.17 ± 1.09 hours, respectively. Interpatient variability in TMAX ranged from 60% to 75% coefficient of variation.
TABLE 4.
Irinotecan plasma pharmacokinetic parameters from a noncompartmental analysis
| Irinotecan | SN38 | SN38-G | |
|---|---|---|---|
| CMAX (ng/mL) | 93.9 ± 60.8 (64.8%) | 1.84 ± 1.17 (63.4%) | 5.50 ± 3.38 (61.4%) |
| TMAX (hr) | 1.97 ± 0.85 (43.3%) | 2.44 ± 1.32 (54.0%) | 3.17 ± 1.09 (34.2%) |
| AUCINF (hr•ng/mL) | 594 ± 299 (50.4%) | 16.7 ± 8.81 (52.8%) | 62.1 ± 35.4 (57.0%) |
| t1/2 (hr) | 4.38 ± 0.73 (16.6%) | 7.52 ± 2.85 (37.9%) | 8.03 ± 2.34 (29.1%) |
| CL/F (L/hr/m2) | a 130 ± 64.7 (49.9%) | N/A | N/A |
| Vz/F (L/m2) | a 794 ± 384 (48.3%) | N/A | N/A |
Abbreviations: AUCINF, area under the concentration-time curve extrapolated to infinity; CL/F, oral apparent clearance; CMAX, maximum plasma concentration; N/A, not applicable (SN38 and SN38-G are metabolites and not able to have CL/F and Vz/F properly calculated); TMAX: time to CMAX; Vz/F, volume of distribution.
Denotes one outlier removed from mean calculation (> 2 standard deviations above mean).
FIGURE 1.
Plasma concentration versus time curves for orally administered irinotecan, the metabolites SN38 and SN38G as well as sorafenib. Values are not dose-normalized
The mean CMAX for 70 mg/m2 oral irinotecan was 93.9 ± 60.8 ng/mL with a mean AUCINF value of 594 ± 299 hr•ng/mL. The mean dose-normalized CMAX (CMAX/D) was 1.14 ng/mL/mg (0.18–3.49 ng/mL/mg) and the dose-normalized AUCINF (AUCINF/D) was 7.1 hr•ng/mL/mg (1.7–19.5 hr•ng/mL/mg). Normalized to body surface area, irinotecan exhibited a mean oral apparent clearance (CL/F) of 130 ± 64.7 L/hr/m2 and mean distribution volume (Vz/F) of 794 ± 384 L/m2. The observed half-life (t1/2) in this data set was 4.38 ± 0.73 hours.
SN38-G demonstrated a greater CMAX than the active metabolite SN38. The mean extent of metabolic conversion of irinotecan to SN38 was 0.035 and from SN38 to SN38-G was 3.73.
The PK of sorafenib was evaluated for all 15 patients following the first dose on day 1 of cycle 1. Many 24-hour post-dose time points were sampled after the day 2 morning dose was administered; therefore, these were not true measurements. A noncompartmental analysis was performed to obtain CMAX, TMAX, and AUC from time zero to 8 hours (AUC0–8 hr). There was insufficient elimination during the captured sampling to properly estimate an elimination rate; therefore, t1/2, CL/F, Vz/F, and AUCINF could not be calculated. Sorafenib demonstrated a mean CMAX/D of 8.58 ng/mL/mg (dose range, 100–350 mg), a TMAX of 6.4 hours, and a mean dose-normalized AUC0–8 hr of 38.5 hr•ng/mL/mg. There was no significant age-dependent difference in irinotecan, SN-38, SN-38G, or sorafenib AUC.
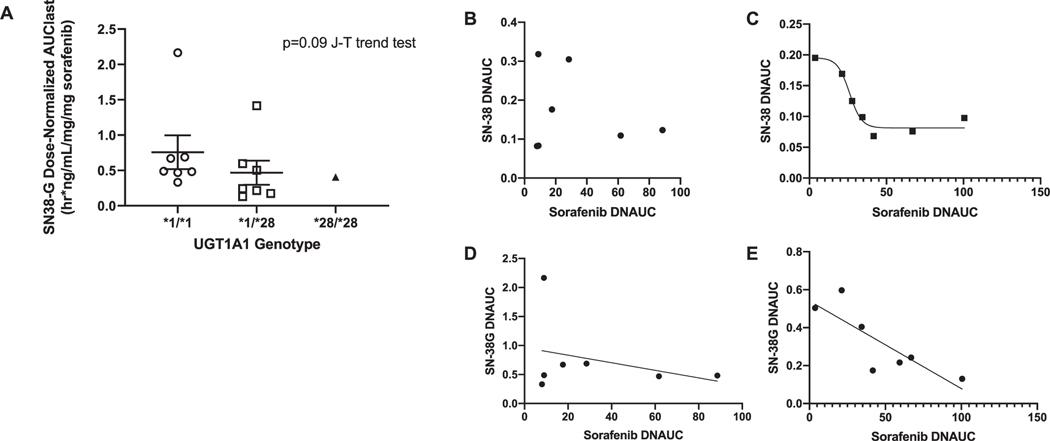
Dose-normalized SN-38G exposure decreased with the number of variant *28 alleles (Figure 2). For patients carrying UGT1A1*1/*1, no association between dose-normalized sorafenib AUC and SN-38 or SN-38G was observed (P > 0.66). Patients harboring at least a single UGT1A1*28 allele, dose-normalized sorafenib AUC fit a four-parameter model with dose-normalized SN-38 AUC (R2 = 0.96), and a linear model of SN-38G AUC (P = 0.022, R2 = 0.68).
FIGURE 2.
Linear regression analysis of (A) dose-normalized SN-38G AUC decreased in relation to decreasing UGT1A1 expression. Regression analysis of sorafenib AUC vs. SN-38 AUC was not possible in (B) those carrying UGT1A1*1/*1, but a relationship between these factors was observed in (C) carriers of a single UGT1A1*28 allele (R2 = 0.96) in a four-parameter model. Regression analysis of sorafenib AUC vs. SN-38G AUC revealed an inversely proportional relationship between these factors in UGT1A1*1/*1 carriers that was not statistically significant (P = 0.48: D) and in UGT1A1*28 carriers that was statistically significant (P = 0.022; R2 = 0.68: E). All AUCs are dose normalized, and the one patient who received the highest dose of sorafenib (350 mg) is excluded from these analyses because this individual was an outlier in all of the above analyses
3.4 |. Patient-reported outcomes
Of the 17 patients, eight were outside the age limit for the PROMIS, one was removed before T1 due to disease progression, and one did not complete PRO measurements. Seven patients completed both T1 and T2 via computer without missing data (by measure or by items). It was noted patients treated on dose level 2 (n = 2) had 13 and 23 total adverse events, whereas those treated on dose level 1 (n = 5) had a median of 7 total adverse events during the study period. Collective patient-reported outcome data, presented separately,43 demonstrated a majority of patients were interested in participating and able to complete the required evaluations. As predicted, as AEs increased, self-reported mobility decreased, and fatigue increased. The willingness to complete the measures and lack of missing data at two time points support the feasibility and acceptability to pediatric oncology patients of embedded PRO measures within a phase 1 trial.
3.5 |. Disease response
Overall, nine of the 15 (60%) evaluable patients had a best response of SD (Supporting Information Table S3). Four patients maintained SD for ≥6 cycles and five additional patients received four cycles of therapy with SD. Two patients with WT were removed from protocol therapy while experiencing disease stabilization. One patient developed central cavitation within previously solid lung nodules after four cycles of treatment (Supporting Information Figure S1). Though this qualified as SD based on RECIST criteria, it was felt to be an excellent response to therapy. Protocol therapy was stopped and the patient underwent surgical resection of known pulmonary lesions. Viable WT was detected on histology, and the patient continued on sorafenib-irinotecan for six additional months off study before eventually developing disease progression. The second patient demonstrated SD after four cycles, but protocol therapy was stopped due to mild (grade 1–2) adverse events and the burden of travel required for follow-up.
Four patients demonstrated disease progression at the first required disease assessment. Two patients came off protocol therapy during cycle 1 due to DLT; a disease assessment was not completed.
4 |. DISCUSSION
The MTD and recommended dose of irinotecan and sorafenib combination therapy was determined to be sorafenib 150 mg/m2/dose by mouth twice daily on a continuous schedule with irinotecan 70 mg/m2/dose orally once daily on days 1–5 of a 21-day cycle. The convenient, oral outpatient regimen was well tolerated with rare moderate or severe toxicities. DLTs included diarrhea, hyponatremia, thrombocytopenia, and elevated serum lipase. The toxicity rate was similar to that observed on trials of single-agent sorafenib in pediatric patients.17,18 It was notable that all DLTs on this study were observed in patients ≥12 years of age, suggesting that the combination was better tolerated in younger patients. Of note, there was no significant difference in irinotecan clearance or dose-normalized AUC0–8 of sorafenib based on a cutoff of 12 years.
More than half (60%) of these heavily pretreated patients demonstrated prolonged disease stabilization, receiving four or more cycles of sorafenib and irinotecan combination therapy. This is in contrast to the SD rate of 29% (14 of 49 patients) on the Children’s Oncology Group phase 1 study of sorafenib in patients with relapsed solid tumors.17 A subsequent phase 2 trial evaluating sorafenib in select disease cohorts demonstrated prolonged SD in 2 of 10 patients (20%) with WT and no responses in the cohort of patients with rhabdomyosarcoma.18 Similarly, single-agent irinotecan therapy evaluated on pediatric phase 1 trials resulted in partial response rates ranging from 0 to 6% and mixed response and/or SD rates of 20–26%.26,27,44 This suggests the combination of sorafenib and irinotecan may offer a therapeutic advantage in patients with solid tumor malignancies.
PRO measures can be a standardized approach to documenting symptoms, function, and quality-of-life experienced by pediatric patients enrolled on early-phase cancer trials. Results utilizing validated outcome measures were reliable such that self-reported outcomes could augment standard toxicity data as a trial endpoint.
It was important to evaluate the PK profile with combination therapy. Many of the irinotecan parameter estimates (e.g., CMAX, AUC, clearance) were consistent with previously reported ranges on a phase 1 study utilizing oral irinotecan in adults.45–47 Overall, CMAX and AUCINF normalized for dose were much lower than observed for adults given IV doses,46 which is consistent with the established absolute oral bioavailability of irinotecan solution of 17%.47
The mean CL/F (dose/AUCINF) was faster than in previous pediatric (2.4–23.4 L/hr/m2)48,49 and adult (mean 17.7 L/hr/m2)46 studies likely due to the lower plasma exposure from decreased absorption/bioavailability with oral administration. For similar reasons, the distribution volume was greater than comparable reports (69–270 L/m2).49 The observed t1/2 in this data set (4.4 hours), was similar to that found in published pediatric and adult studies (4.6–6.5 hours).45,48
Following an irinotecan dose of 70 mg/m2, SN38 exposure levels were lower than expected when compared with oral irinotecan 60 mg/m2 in adults (SN38 CMAX 7.4 ± 4.4 ng/mL).47 The same was true for AUCINF, with a lower mean value than on the adult study (58.7 ± 31 hr*ng/mL). However, the mean extent of metabolic conversion of irinotecan to SN38 was within reported ranges (0.016–0.16).45–47,49 The t1/2 was within reported pediatric ranges (7.3–18.5 hours)48 and consistent with the oral study in adults (11.9 hours). The lower overall exposure of SN38 was likely due to lower exposure of parent irinotecan and not less metabolic conversion to SN38. These data suggest the clearance and elimination mechanisms do not differ between pediatric and adult patients.
SN38-G had a greater mean CMAX than the active metabolite SN38, but still lower than on the oral study in adults (CMAX 21.7 ± 15.2 ng/mL).47 The mean AUCINF was within previously reported range for pediatric patients (30.8–153 hr*ng/mL),49 but lower than in adults (356 ± 348 hr*ng/mL); however, the adult study had a high standard deviation around that mean. The mean extent of metabolic conversion from SN38 to SN38-G was lower than that observed in adults (6.9–13.4),45,46 but comparable to other pediatric reports (1.6–4.1).48,49 The association between sorafenib and SN-38 AUC is derived from a small cohort of patients who had low SN-38 and SN-38G exposure compared with previous studies.47 Sorafenib is a UGT1A1 inhibitor that reduces metabolism of UGT1A1 substrates in carriers of the UGT1A1*28 allele.50–52 We observed that higher sorafenib exposure was associated with lower SN-38G exposure in individuals with at least one UGT1A1*28 allele. By blocking glucuronidation, sorafenib may speed clearance of SN-38, potentially through CYP3A metabolism and reduced enterohepatic recirculation.53,54 Frequency and severity of adverse events did not appear to differ based on allelic status.
The sorafenib PK data, though limited, were consistent with previously reported data using single-agent sorafenib on the same dose regimen (mean CMAX/D 8.33 ng/mL/mg, TMAX 4.98 hours).17
The recommended dose of sorafenib administered with irinotecan was well tolerated at in heavily pretreated patients. Although no objective responses were observed, combination therapy resulted in clinically significant disease stabilization in nine of 15 patients. Overall, there did not appear to be a significant drug interaction altering the PK profile of sorafenib or irinotecan when administered as part of combination therapy. Further evaluation of sorafenib and irinotecan in a phase 2 study in patients with solid malignancies is warranted to assess efficacy of this combination. Evaluating prognostic biomarkers, as has been done in patients with hepatocellular carcinoma treated with sorafenib, might better identify a target population that would benefit from this regimen.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by Bayer and Onyx Pharmaceuticals, Inc., and funding from an American Society of Clinical Oncology Career Development Award, a Childhood Cancer Research Grant from the Pablove Foundation, and a Research Award for Pilot Feasibility Studies/Development of Research Methodologies/Development of New Research Technology from the Clinical and Translational Science Institute-Children’s National.
Funding information
American Society of Clinical Oncology; Pablove Foundation; Clinical and Translational Science Institute-Children’s National
DATA SHARING
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations:
- ALT
alanine transaminase
- AST
aspartate aminotransferase
- AUC0–8 hr
area under the concentration-time curve from time zero to 8 hours
- AUCINF
area under the concentration-time curve extrapolated to infinity
- AUCINF /D
dose-normalized AUCINF
- CL/F
oral apparent clearance
- CMAX
maximum plasma concentration
- CMAX /D
dose-normalized CMAX
- CTCAE
Common Terminology Criteria for Adverse Events
- DLT
dose-limiting toxicity
- FDA
US Food and Drug Administration
- HD-ASCT
high-dose chemotherapy and autologous stem cell transplant
- HPLC-MS/MS
high-performance liquid chromatography tandem mass spectrometry
- IV
intravenous
- MIBG
metaiodobenzylguanidine
- MTD
maximum tolerated dose
- NIH
National Institutes of Health
- PGX
pharmacogenomics
- PK
pharmacokinetic
- PRO
patient-reported outcome
- PROMIS
Patient-Reported Outcomes Measurement Information System
- RECIST
Response Evaluation Criteria in Solid Tumors
- SD
stable disease
- SN-38
7-ethyl-10-hydroxy camptothecin
- SN38-G
glucuronic acid metabolite of SN38
- t1/2
half-life
- TMAX
the time to maximum plasma concentration
- Vz/F
distribution volume
- WT
Wilms tumor
Footnotes
Work previously presented as an abstract at the American Society of Clinical Oncology Annual Meeting. Chicago, IL 2014.
Previous publication: Meany HJ, Hinds PS, Bagatell R, et al: Phase 1 Study of Sorafenib and Irinotecan in Pediatric Patients with Relapsed or Refractory Solid Tumors. American Society of Clinical Oncology Annual Meeting. Chicago, IL 2014.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
CONFLICTS OF INTEREST
The authors do not have relevant conflicts of interest to report.
REFERENCES
- 1.Rini BI. Sorafenib. Expert Opin Pharmacother. 2006;7(4):453–461. [DOI] [PubMed] [Google Scholar]
- 2.Moore M, Hirte HW, Siu L, et al. Phase I study to determine the safety and pharmacokinetics of the novel Raf kinase and VEGFR inhibitor BAY 43–9006, administered for 28 days on/7 days off in patients with advanced, refractory solid tumors. Ann Oncol. 2005;16(10):1688–1694. [DOI] [PubMed] [Google Scholar]
- 3.Wilhelm SM, Carter C, Tang L, et al. BAY 43–9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64(19):7099–7109. [DOI] [PubMed] [Google Scholar]
- 4.Chang YS, Adnane J, Trail PA, et al. Sorafenib (BAY 43–9006) inhibits tumor growth and vascularization and induces tumor apoptosis and hypoxia in RCC xenograft models. Cancer Chemother Pharmacol. 2007;59(5):561–574. [DOI] [PubMed] [Google Scholar]
- 5.Liu L, Cao Y, Chen C, et al. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006;66(24):11851–11858. [DOI] [PubMed] [Google Scholar]
- 6.Sharma A, Trivedi NR, Zimmerman MA, Tuveson DA, Smith CD, Robertson GP. Mutant V599EB-Raf regulates growth and vascular development of malignant melanoma tumors. Cancer Res. 2005;65(6):2412–2421. [DOI] [PubMed] [Google Scholar]
- 7.Yang F, Van Meter TE, Buettner R, et al. Sorafenib inhibits signal transducer and activator of transcription 3 signaling associated with growth arrest and apoptosis of medulloblastomas. Mol Cancer Ther. 2008;7(11):3519–3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chai H, Luo AZ, Weerasinghe P, Brown RE. Sorafenib downregulates ERK/Akt and STAT3 survival pathways and induces apoptosis in a human neuroblastoma cell line. Int J Clin Exp Pathol. 2010;3(4):408–415. [PMC free article] [PubMed] [Google Scholar]
- 9.Pignochino Y, Grignani G, Cavalloni G, et al. Sorafenib blocks tumour growth, angiogenesis and metastatic potential in preclinical models of osteosarcoma through a mechanism potentially involving the inhibition of ERK1/2, MCL-1 and ezrin pathways. Mol Cancer. 2009;8: 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lunenburger H, Lanvers-Kaminsky C, Lechtape B, Fruhwald MC. Systematic analysis of the antiproliferative effects of novel and standard anticancer agents in rhabdoid tumor cell lines. Anticancer Drugs. 2010;21(5):514–522. [DOI] [PubMed] [Google Scholar]
- 11.Hu S, Niu H, Minkin P, et al. Comparison of antitumor effects of multitargeted tyrosine kinase inhibitors in acute myelogenous leukemia. Mol Cancer Ther. 2008;7(5):1110–1120. [DOI] [PubMed] [Google Scholar]
- 12.Keir ST, Maris JM, Lock R, et al. Initial testing (stage 1) of the multitargeted kinase inhibitor sorafenib by the pediatric preclinical testing program. Pediatr Blood Cancer. 2010;55(6):1126–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. [DOI] [PubMed] [Google Scholar]
- 14.Kane RC, Farrell AT, Saber H, et al. Sorafenib for the treatment of advanced renal cell carcinoma. Clin Cancer Res. 2006;12(24):7271–7278. [DOI] [PubMed] [Google Scholar]
- 15.Escudier B, Eisen T, Stadler WM, et al. Sorafenib for treatment of renal cell carcinoma: final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial. J Clin Oncol. 2009;27(20):3312–3318. [DOI] [PubMed] [Google Scholar]
- 16.Brose MS, Nutting CM, Jarzab B, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. 2014;384(9940):319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Widemann BC, Kim A, Fox E, et al. A phase I trial and pharmacokinetic study of sorafenib in children with refractory solid tumors or leukemias: a Children’s Oncology Group Phase I Consortium report. Clin Cancer Res. 2012;18(21):6011–6022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim A, Widemann BC, Krailo M, et al. Phase 2 trial of sorafenib in children and young adults with refractory solid tumors: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2015;62(9):1562–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsiang YH, Lihou MG, Liu LF. Arrest of replication forks by drug-stabilized topoisomerase I-DNA cleavable complexes as a mechanism of cell killing by camptothecin. Cancer Res. 1989;49(18):5077–5082. [PubMed] [Google Scholar]
- 20.Hsiang YH, Hertzberg R, Hecht S, Liu LF. Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. J Biol Chem. 1985;260(27):14873–14878. [PubMed] [Google Scholar]
- 21.Hertzberg RP, Caranfa MJ, Hecht SM. On the mechanism of topoisomerase I inhibition by camptothecin: evidence for binding to an enzyme-DNA complex. Biochemistry. 1989;28(11):4629–4638. [DOI] [PubMed] [Google Scholar]
- 22.Wagner LM. Fifteen years of irinotecan therapy for pediatric sarcoma: where to next?. Clin Sarcoma Res. 2015;5:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palmerini E, Jones RL, Setola E, et al. Irinotecan and temozolomide in recurrent Ewing sarcoma: an analysis in 51 adult and pediatric patients. Acta Oncol. 2018;57(7):958–964. [DOI] [PubMed] [Google Scholar]
- 24.Hol JA, van den Heuvel-Eibrink MM, Graf N, et al. Irinotecan for relapsed Wilms tumor in pediatric patients: SIOP experience and review of the literature—a report from the SIOP Renal Tumor Study Group. Pediatr Blood Cancer. 2018;65(2). doi: 10.1002/pbc.26849. [DOI] [PubMed] [Google Scholar]
- 25.Vassal G, Couanet D, Stockdale E, et al. Phase II trial of irinotecan in children with relapsed or refractory rhabdomyosarcoma: a joint study of the French Society of Pediatric Oncology and the United Kingdom Children’s Cancer Study Group. J Clin Oncol. 2007;25(4):356–361. [DOI] [PubMed] [Google Scholar]
- 26.Blaney S, Berg SL, Pratt C, et al. A phase I study of irinotecan in pediatric patients: a pediatric oncology group study. Clin Cancer Res. 2001;7(1):32–37. [PubMed] [Google Scholar]
- 27.Bomgaars L, Kerr J, Berg S, Kuttesch J, Klenke R, Blaney SM. A phase I study of irinotecan administered on a weekly schedule in pediatric patients. Pediatr Blood Cancer. 2006;46(1):50–55. [DOI] [PubMed] [Google Scholar]
- 28.Bomgaars LR, Bernstein M, Krailo M, et al. Phase II trial of irinotecan in children with refractory solid tumors: a Children’s Oncology Group Study. J Clin Oncol. 2007;25(29):4622–4627. [DOI] [PubMed] [Google Scholar]
- 29.Wagner LM. Oral irinotecan for treatment of pediatric solid tumors: ready for prime time?. Pediatr Blood Cancer;54(5):661–662. [DOI] [PubMed] [Google Scholar]
- 30.Furman WL, Crews KR, Billups C, et al. Cefixime allows greater dose escalation of oral irinotecan: a phase I study in pediatric patients with refractory solid tumors. J Clin Oncol. 2006;24(4):563–570. [DOI] [PubMed] [Google Scholar]
- 31.Drengler RL, Kuhn JG, Schaaf LJ, et al. Phase I and pharmacokinetic trial of oral irinotecan administered daily for 5 days every 3 weeks in patients with solid tumors. J Clin Oncol. 1999;17(2):685–696. [DOI] [PubMed] [Google Scholar]
- 32.Wagner LM, Villablanca JG, Stewart CF, et al. Phase I trial of oral irinotecan and temozolomide for children with relapsed high-risk neuroblastoma: a new approach to neuroblastoma therapy consortium study. J Clin Oncol. 2009;27(8):1290–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wagner LM, Perentesis JP, Reid JM, et al. Phase I trial of two schedules of vincristine, oral irinotecan, and temozolomide (VOIT) for children with relapsed or refractory solid tumors: a Children’s Oncology Group phase I consortium study. Pediatr Blood Cancer;54(4):538–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vincent P, Zhang X, Chen C, et al. Chemotherapy with the raf kinase inhibitor BAY 43–9006 in combination with irinotecan, vinorelbine, or gemcitabine is well tolerated and efficacious in preclinical xenograft models. Proc Am Soc Clin Oncol. 2002;21:23b. [Google Scholar]
- 35.Mross K, Steinbild S, Baas F, et al. Drug-drug interaction pharmacokinetic study with the Raf kinase inhibitor (RKI) BAY 43–9006 administered in combination with irinotecan (CPT-11) in patients with solid tumors. Int J Clin Pharmacol Ther. 2003;41(12):618–619. [DOI] [PubMed] [Google Scholar]
- 36.Mross K, Steinbild S, Baas F, et al. Results from an in vitro and a clinical/pharmacological phase I study with the combination irinotecan and sorafenib. Eur J Cancer. 2007;43(1):55–63. [DOI] [PubMed] [Google Scholar]
- 37.Samalin E, Bouche O, Thezenas S, et al. Sorafenib and irinotecan (NEXIRI) as second- or later-line treatment for patients with metastatic colorectal cancer and KRAS-mutated tumours: a multicentre phase I/II trial. Br J Cancer. 2014;110(5):1148–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takimoto CH, Awada A. Safety and anti-tumor activity of sorafenib (Nexavar) in combination with other anti-cancer agents: a review of clinical trials. Cancer Chemother Pharmacol. 2008;61(4):535–548. [DOI] [PubMed] [Google Scholar]
- 39.Mazard T, Causse A, Simony J, et al. Sorafenib overcomes irinotecan resistance in colorectal cancer by inhibiting the ABCG2 drug-efflux pump. Mol Cancer Ther. 2013;12(10):2121–2134. [DOI] [PubMed] [Google Scholar]
- 40.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. [DOI] [PubMed] [Google Scholar]
- 41.Chen X, Peer CJ, Alfaro R, Tian T, Spencer SD, Figg WD. Quantification of irinotecan, SN38, and SN38G in human and porcine plasma by ultra high-performance liquid chromatography-tandem mass spectrometry and its application to hepatic chemoembolization. J Pharm Biomed Anal. 2012;62:140–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jain L, Gardner ER, Venitz J, Dahut W, Figg WD. Development of a rapid and sensitive LC-MS/MS assay for the determination of sorafenib in human plasma. J Pharm Biomed Anal. 2008;46(2):362–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hinds PS, Wang J, Stern ED, et al. Voices of children and adolescents on phase 1 or phase 2 cancer trials: a new trial endpoint?. Cancer. 2017;123(19):3799–3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vassal G, Doz F, Frappaz D, et al. A phase I study of irinotecan as a 3-week schedule in children with refractory or recurrent solid tumors. J Clin Oncol. 2003;21(20):3844–3852. [DOI] [PubMed] [Google Scholar]
- 45.Younis IR, Malone S, Friedman HS, Schaaf LJ, Petros WP. Enterohepatic recirculation model of irinotecan (CPT-11) and metabolite pharmacokinetics in patients with glioma. Cancer Chemother Pharmacol. 2009;63(3):517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duffy AG, Makarova-Rusher OV, Ulahannan SV, et al. Modulation of tumor eIF4E by antisense inhibition: a phase I/II translational clinical trial of ISIS 183750-an antisense oligonucleotide against eIF4E-in combination with irinotecan in solid tumors and irinotecan-refractory colorectal cancer. Int J Cancer. 2016;139(7):1648–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goff LW, Benson AB, LoRusso PM, et al. Phase I study of oral irinotecan as a single-agent and given sequentially with capecitabine. Invest New Drugs. 2012;30(1):290–298. [DOI] [PubMed] [Google Scholar]
- 48.Kimura T, Kashiwase S, Makimoto A, et al. Pharmacokinetic and pharmacodynamic investigation of irinotecan hydrochloride in pediatric patients with recurrent or progressive solid tumors. Int J Clin Pharmacol Ther. 2010;48(5):327–334. [DOI] [PubMed] [Google Scholar]
- 49.DuBois SG, Chesler L, Groshen S, et al. Phase I study of vincristine, irinotecan, and (1)(3)(1)I-metaiodobenzylguanidine for patients with relapsed or refractory neuroblastoma: a new approaches to neuroblastoma therapy trial. Clin Cancer Res. 2012;18(9):2679–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ma MK, McLeod HL. Lessons learned from the irinotecan metabolic pathway. Curr Med Chem. 2003;10(1):41–49. [DOI] [PubMed] [Google Scholar]
- 51.Peer CJ, Sissung TM, Kim A, et al. Sorafenib is an inhibitor of UGT1A1 but is metabolized by UGT1A9: implications of genetic variants on pharmacokinetics and hyperbilirubinemia. Clin Cancer Res. 2012;18(7):2099–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miners JO, Chau N, Rowland A, et al. Inhibition of human UDP-glucuronosyltransferase enzymes by lapatinib, pazopanib, regorafenib and sorafenib: implications for hyperbilirubinemia. Biochem Pharmacol. 2017;129:85–95. [DOI] [PubMed] [Google Scholar]
- 53.Hanioka N, Ozawa S, Jinno H, Tanaka-Kagawa T, et al. Interaction of irinotecan (CPT-11) and its active metabolite 7-ethyl-10-hydroxycamptothecin (SN-38) with human cytochrome P450 enzymes. Drug Metab Dispos. 2002;30(4):391–396. [DOI] [PubMed] [Google Scholar]
- 54.Rosner GL, Panetta JC, Innocenti F, et al. Pharmacogenetic pathway analysis of irinotecan. Clin Pharmacol Ther. 2008;84(3):393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.