Abstract
The purpose is to discuss abdominal tuberculosis mimicking malignancy involving the abdominal viscera. TB of the abdominal viscera is common, especially in countries where tuberculosis is endemic and in pockets of non-endemic countries. Diagnosis is challenging as clinical presentations are often non-specific. Tissue sampling may be necessary for definitive diagnosis. Awareness of the early and late disease imaging appearances of abdominal tuberculosis involving the viscera that can mimic malignancy can aid detecting TB, providing a differential diagnosis, assessing extent of spread, guiding biopsy, and evaluating response.
Graphical abstract
Keywords: Tuberculosis, Cancer, Ultrasonography, CT, MRI, Abdominal viscera
Introduction
Tuberculosis (TB) remains endemic in many countries and pockets in various non-endemic countries still experience TB or are seeing a resurgence. The latter may be related to socioeconomic conditions such as congregated living, homelessness, and addiction; immigration from endemic areas; and delayed diagnosis due to the COVID-19 pandemic [1]. It is a global health challenge with increasing prevalence due to secondary immunosuppression and development of multi-drug-resistant strains. It often presents a conundrum to radiologists because it has an extensive spectrum of imaging manifestations and frequently mimics malignancy. TB most commonly infects the lungs and can spread to any organ in the body via the bloodstream, lymphatics from infected nodes, direct contiguous spread from infected foci, and ingestion of infected sputum. The abdomen is the most common extrapulmonary site for tuberculosis resulting in infection of the gastrointestinal tract, peritoneum, viscera and nodes by Mycobacterium tuberculosis (M. tuberculosis) [2, 3]. Isolated visceral tuberculosis is less common, seen in 15%-20% of cases [4]. Route of spread is usually haematogenous and, less often, by direct extension from infected foci. The genitourinary system is often involved. Fifteen percent of liver, spleen, and pancreas TB is found with concomitant lung involvement [5].
Diagnosis of visceral TB poses a challenge because it usually presents without any specific symptoms. Moreover, it is difficult to make a prospective diagnosis because TB can mimic neoplastic conditions, such as lymphoma and malignancy of various abdominal organs. Distinguishing these is clinically significant because the therapy for one may negatively impact or make the other worse. For example, resulting in dissemination of TB or metastases or resulting in inappropriate upstaging of malignancy. Moreover, TB and malignancy may coexist, and patients with cancer have a higher incidence of TB due to immunosuppression [6, 7]. A detailed medical history, potential exposure to TB, classic radiologic features, serum markers, and acid-fast bacilli (AFB) on biopsy specimens with, or sometimes without, successful mycobacterial culture can help differentiate this disease [8]. A high index of suspicion is necessary in the appropriate clinical setting supplemented by cross sectional imaging and tissue sampling for diagnosis of abdominal visceral TB.
Generally, abdominal TB is rare, particularly if isolated, and is more commonly due to hematologic spread in the setting of disseminated disease. It often presents with coexisting lymphadenopathy and its appearance depends on chronicity. Specific organ-based features are described below. Abdominal TB is usually diagnosed by a combination of radiologic and histopathologic studies. Radiology can aid detecting the disease, providing a differential diagnosis, assessing extent of spread, guiding biopsy, and evaluating response to therapy. At biopsy, caseating necrosis in granulomas is the histologic hallmark of TB [9, 10]. Staining for AFB is often negative [10]. Polymerase chain reaction (PCR)-based assays can be more sensitive and specific for diagnosing TB [11, 12]. If there is a high degree of suspicion, patients may be treated empirically with anti-tuberculosis therapy and imaging used to assess response. A paper on TB and malignancies it may mimic in lymph nodes, gastrointestinal tract, and peritoneum has been recently published [7]. In this article, we discuss the various cross-sectional imaging features of visceral abdominal tuberculosis and malignancy mimics.
Hepatic tuberculosis
The prevalence of hepatic TB is variable in the reported literature. An estimate of the incidence is around 1% of all active TB cases [13, 14]. The incidence of extrapulmonary TB, including hepatic TB, is higher in HIV-infected populations. In a study of 164 patients with HIV infected with disseminated TB, hepatic TB was seen in 17.4%. Hepatic TB is typically seen with other features of disseminated abdominal disease. Isolated hepatic TB represents less than 1% of all cases [8]. Clinical features of hepatic TB are nonspecific, resulting in late diagnosis. The most common signs of hepatic TB include hepatomegaly, abdominal pain, fever, and weight loss. Rare manifestations include splenomegaly and jaundice [15–18]. Liver function tests can show elevated transaminases, alkaline phosphatase (ALP) and gamma-glutamyl transferase (GGT) whereas these tend to be preserved in low burden metastatic disease. An inverted albumin/globulin ratio favors the diagnosis of hepatic TB, particularly in endemic areas. [19].
Morphologically, hepatic TB can manifest as parenchymal involvement with focal or diffuse, micronodular (miliary) and/or macronodular involvement. A very rare variant is the serohepatic type that involves the connective tissue beneath the serosa of liver. It manifests as a thickened liver capsule with subcapsular nodular lesions and is referred to as “sugar-coated” or “frosted” liver [20]. Haematogenous dissemination from lungs is the most common mode of spread to the liver [21] and occurs via the hepatic artery. Local spread from gastrointestinal tract or abdominal lymph nodes is uncommon.
Micronodular hepatic TB refers to diffuse, randomly arranged nodules 0.5–2 mm in diameter [22]. It occurs due to haematogenous spread of the disease, usually in the setting of disseminated disease. On ultrasound, these appear as multiple discrete echogenic lesions. Sometimes, if the nodules are too small to be detected, the liver can show a diffusely echogenic appearance with hepatomegaly. On CT, these nodules typically appear hypodense to background liver parenchyma. Differential diagnosis includes fungal infections, sarcoidosis, and rarely, leukemia and lymphoma and other granulomatous infections such as brucellosis [23, 24]. Clinical, biochemical correlation as well as histopathologic examination may be necessary to reach a diagnosis in certain cases but PCR is more sensitive. Finding AFB or culture of M. tuberculosis is the most specific for diagnosing hepatic TB. In cases with high index of suspicion, especially in endemic settings, empirical anti-tubercular therapy (ATT) is usually started [25].
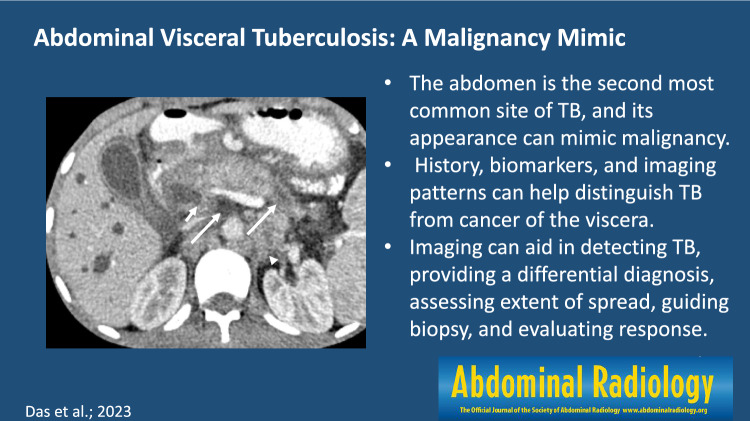
The macronodular, or pseudotumoral, form may present either as a localized focal tuberculoma or multiple large lesions. This is primarily acquired via the portal vein from the bacilli in the gastrointestinal tract. Compared to micronodular hepatic TB, macronodular TB usually lacks associated pulmonary infection and is characterized by > 2 mm nodules located near the portal triads [26]. The pathological feature of hepatic TB is granulomatous inflammation that occurs as a result of cell-mediated immunological response, consisting of aggregates of macrophages that may coalesce to form Langerhans giant cells associated with surrounding lymphocytes [27]. These undergo progressive central caseous necrosis with peripheral granulation tissue that eventually undergo fibrosis and calcification. Many of these granulomas will coalesce forming a large tuberculoma; liquefaction necrosis within a tuberculoma can result in a tubercular abscess [11]. Imaging appearances vary based on the stage of the disease, from solid homogeneously enhancing nodules in the granulomatous stage, rim-enhancing nodules when they develop caseous necrosis (Fig. 1), to calcified nodules in the chronic stage. On magnetic resonance imaging (MRI), these lesions appear hypointense on T1-weighted sequences and hyperintense on T2-weighted sequences relative to the background parenchyma. On post gadolinium images, enhancement pattern of the lesions varies from rim-enhancement to heterogeneous patterns of enhancement [28, 29]. Macronodular lesions can show heterogeneous enhancement with central necrosis that can have an appearance like a necrotic hepatic malignancy or metastases (Fig. 2).
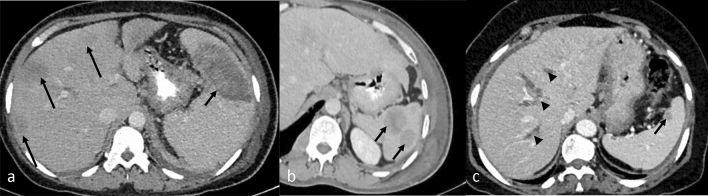
Fig. 1.
CECT axial images of abdomen show different pattern of liver involvement in various cases of TB (arrow) as (a) large focal low attenuation lesion, (b) few hypodense nodules and, (c, d) hepatomegaly with multiple hypodense nodules, including 0.5–2 mm nodules that can be seen with the micronodular type (arrowhead)
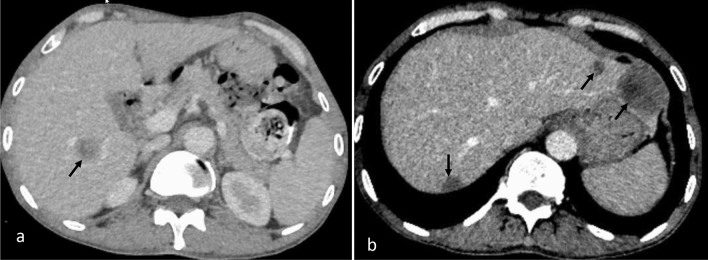
Fig. 2.
CECT abdomen axial image (a) shows a solitary hypoenhancing focal lesion (arrow) in liver with “target” appearance of peripheral enhancement in a known case of rectal cancer suggesting liver metastasis. CECT axial abdomen image (b) shows similar multiple hypoenhancing lesions (arrows) in a patient with colorectal carcinoma, suggesting liver metastases
Imaging features that favor a diagnosis of TB include the presence of “target sign,” used to describe the rim enhancement of tuberculomas with caseous necrosis, and “cluster sign,” which represents the coalescence of smaller tuberculomas to form an abscess [20, 28]. The target sign is suggestive of, but not specific for, TB as this sign can also be seen in liver metastasis (Fig. 2a) and other liver abscesses. The cluster sign is commonly seen with a liver abscess and rarely with a liver tumor. These signs in disseminated TB can suggest hepatic involvement, otherwise biopsy may be needed.
Hepatic tuberculomas tend to calcify when a normal immune response is mounted, and the presence of calcified liver and/or splenic lesions, particularly in endemic areas, strongly favors TB. In non-endemic areas, calcified granulomas in the liver and spleen can suggest fungal infection.
Splenic tuberculosis
Like liver, splenic involvement in TB is usually a part of disseminated disease. Isolated splenic TB has been rarely reported [19]. Manifestations include splenomegaly as well as micronodular and/or macronodular nodules [30]. Micronodular lesions may or may not be visualized on ultrasound or CT except as splenomegaly. Macronodular disease can appear as solitary or multifocal hypoechoic nodules on ultrasound, and most commonly hypodense lesions with peripheral enhancement on CT (Fig. 3). Differential considerations include leukemia or lymphoma, metastases (Fig. 4), sarcoidosis, hemangioma, cyst, and fungal infection [31]. Fungal abscesses, most commonly Candida, are a greater consideration in immunosuppressed populations. Among malignancies, the most common to the spleen are melanoma, lymphoma, and breast cancer. Splenic involvement of these is almost invariably commonly part of disseminated stage IV cancer. In this setting, TB is difficult to diagnose and often a high suspicion of TB is needed [32, 33] with confirmatory biopsy.
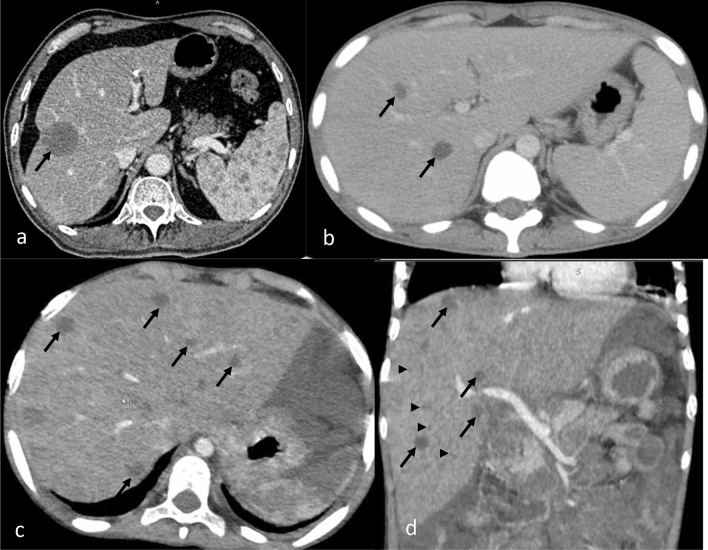
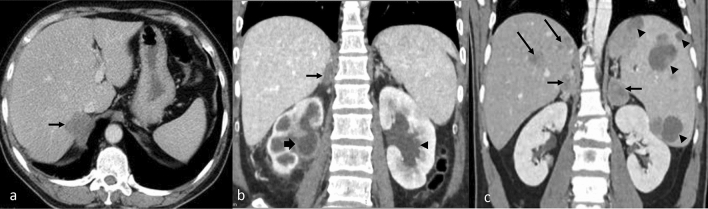
Fig. 3.
CECT axial images of abdomen show splenic involvement in various cases of TB as (a) large focal lesion with rim enhancement (arrow), splenomegaly with multiple small focal lesions (arrow) (b), and large splenic abscesses (c). Note retroperitoneal adenopathy (thick arrow) in (b) and liver lesions (long arrow) in (c)
Fig. 4.
CECT abdomen images showing various forms of splenic metastases which can mimic TB involvement: (a) large low attenuation splenic metastasis (arrow) and liver metastases (long arrow) from breast cancer, (b) enhancing lesions in the spleen due to non-Hodgkin’s lymphoma, (c) solitary low attenuation splenic metastasis from breast cancer. Intrahepatic ductal dilatation is also seen (arrowheads)
Gallbladder tuberculosis
Tuberculosis of the gallbladder (GB) is rare and formulating a prospective diagnosis is not easy. Cholelithiasis or obstructed cystic duct are considered risk factors for GB TB. It may present as a nodule, mass or circumferential thickening of the GB, with homogeneous or heterogeneous enhancement. The differential diagnoses include carcinoma, chronic cholecystitis, and xanthogranulomatous cholecystitis. The most important feature that may help to prospectively diagnose TB is the presence of calcific foci within the GB wall lesion, as well as associated necrotic rim-enhancing mesenteric nodes and concomitant liver involvement [34].
Biliary tuberculosis
Tuberculosis involving the biliary ducts is a rare cause of biliary stricture and is very difficult to diagnose preoperatively in isolation. Biliary TB most commonly occurs due to spread from the portal tracts to biliary radicles; rarely it may spread from periportal tubercular lymph nodes or ascending infection via the Ampulla of Vater [14, 35]. The biliary system can also be involved via direct spread from the hepatic granulomas or by the haematogenous route. Both large and small caliber bile ducts can be affected, however involvement of the extrahepatic bile ducts is rare. This form of TB can present with obstructive jaundice, morphological features similar to cholangiocarcinoma, or inflammatory biliary stricture (Fig. 5). In the presence of other manifestations of abdominal TB, endoscopic or ultrasound-guided fine needle aspiration biopsy or brush cytology can yield the diagnosis pre-operatively, avoiding unnecessary complex surgery. Biliary involvement is often best evaluated by magnetic resonance cholangiopancreaticography (MRCP).Wall thickening and dilatation may be seen when large ducts are involved. Multiple strictures at various segments with intervening sections of dilatation can mimic primary sclerosing cholangitis [36]. Ancillary findings of hepatic granulomas, periportal lymph nodes or calcification along the bile ducts favor biliary tuberculosis [24, 37]. Segmental parenchymal atrophy of the involved segments may be seen [38]. Other differential diagnoses include inflammatory/sclerosing cholangiopathies, IgG4-related strictures, AIDS cholangiopathy, recurrent pyogenic cholangitis and malignant strictures secondary to cholangiocarcinoma or carcinoma of the gallbladder.
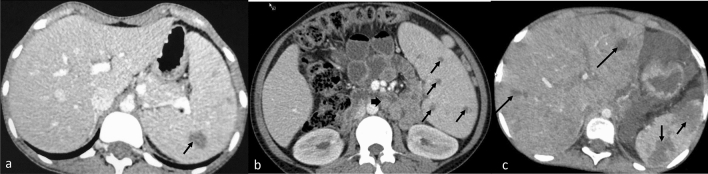
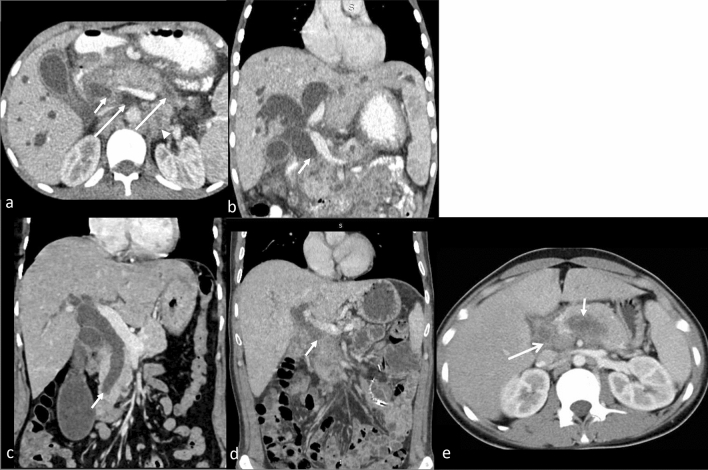
Fig. 5.
CECT abdomen images (a axial, b cor) in a case of TB reveal dilated bilateral intrahepatic biliary radicles and CBD with its abrupt cut off (arrow). Pancreas is bulky with hypodense area in tail and uncinated process (long arrow). Necrotic retroperitoneal nodes (arrowhead) are noted. Similar appearance can be seen with (c, cor) distal cholangiocarcinoma (arrow) and (d, cor) pancreatic adenocarcinoma showing abrupt cut off of the dilated common bile duct. Axial CT (e) image showing a hypodense lesion (short arrow) in pancreatic body with necrotic peri pancreatic node (long arrow) highly suspicious for pancreatic malignancy. On biopsy, it was found to be pancreatic tuberculosis
Pancreatic tuberculosis
Few cases of isolated pancreatic involvement of TB have been reported. It has been postulated that pancreatic enzymes confer a protective effect from tubercular bacilli [39]. Pancreatic TB is most frequently noted in immunocompromised patients, with the haematogenous route being the most common mode of spread. The pancreatic body is most commonly involved, followed by head and tail [39]. There can be diffuse involvement of the pancreas or there may be multifocal or solitary focal lesions that mimic pancreatic cancer (Fig. 5d and e). The most common morphology is that of a hypodense, peripherally enhancing necrotic mass that can be difficult to distinguish from adenocarcinoma. Biomarkers such as CA-19-9 may aide distinction. Associated prominent peripancreatic necrotic lymphadenopathy is common with TB, in up to 75% of cases [40], but uncommon in pancreatic adenocarcinoma. The main pancreatic duct may be normal or mildly dilated with TB, but is usually dilated with pancreatic carcinoma. Although vascular invasion is not expected in TB, it may cause vasculitis and mimic vascular invasion, as can be seen with adenocarcinoma [41]. The presence of calcifications in both the pancreatic mass and adjacent lymph nodes suggests the diagnosis of TB rather than malignancy. Calcification is rare in pancreatic cancer, although calcification may be seen in the presence of chronic pancreatitis [42].
Kidney ureter bladder (KUB) tuberculosis
The kidneys are affected in 4–17% of patients with TB, with genitourinary TB commonly affecting patients15–40 years old. Often there is a long latent period between the primary infection and dissemination to the kidneys [43]. Transmission is via the haematogenous route, with bacilli lodging in the glomeruli and peritubular capillaries. Caseous necrosis and subsequent calcifications occur resulting in calcified granulomas. If the disease progresses, the tubercular granulomas coalesce and subsequent capillary rupture results in spread to collecting tubules, renal pelvis, ureter, and urinary bladder. Clinical manifestations are non-specific, often leading to a delay in diagnosis. Increased urinary frequency, dysuria and flank pain are the most common presenting symptoms and tend to occur once the lower urinary tract is involved. On routine laboratory investigations, sterile pyuria is commonly found. Twenty-four hour urine AFB analysis is positive in ~ 80–90% of cases. At least 6–8 weeks are required for urine culture to become positive. The false-negative rate is approximately 10–20% [44]. Radiologic manifestations in the kidneys depend on the stage of the disease and may appear as focal pyelonephritis, focal tubercular abscess, or parenchymal scarring.
Intravenous pyelography may detect the early findings of mucosal irregularity and edema. Abdominal radiographs may show calcifications along the genitourinary tract or lymph nodes in late stages. Renal calcifications may be granular or amorphous in cases of focal involvement. Ring-like calcifications may be seen with papillary necrosis. In the end-stage, there may be homogeneous or nodular often dense calcifications of the entire kidney, called putty kidney (Fig. 6). Ultrasound and CT scan may demonstrate parenchymal lesions in the stage of evolving tuberculomas in the parenchyma and CT may show inflammation within the collecting system in the form of irregular asymmetrical urothelial enhancement. In the end-stage, due to the presence of fibrosis, there may be strictures within the infundibulum, resulting in focal caliectasis or partial opacification of the calyx called a “phantom calyx.” Renal pelvis involvement may appear as a smooth, angulated kink, also referred to as “Kerr’s kink,” with diffuse pelvic narrowing or asymmetrical hydronephrosis. The differential diagnosis of parenchymal renal TB includes other pyogenic or fungal renal infections. Chronic infection can mimic xanthogranulomatous pyelonephritis. Solitary renal tuberculoma, although rare, can be difficult to distinguish from renal cell carcinoma, metastases and lymphoma. Metastasis to the kidneys tends to occur in the setting of disseminated stage IV cancer, and solitary renal lymphoma is rare.
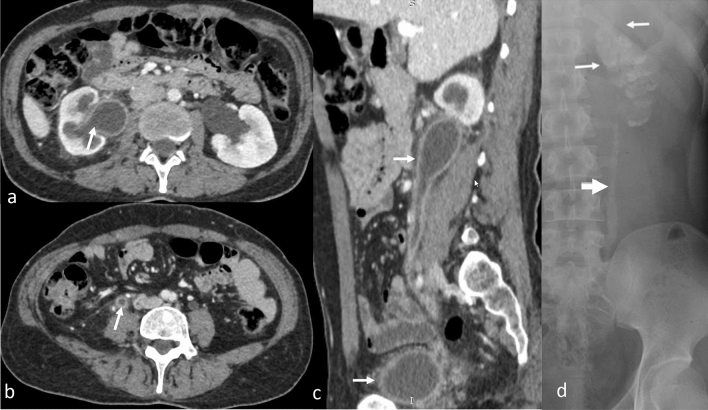
Fig. 6.
Genitourinary TB (arrow), CECT axial (a, b), and sagittal (c) images depict dilatation, irregular thickening and enhancement of (a) right pelvicalyceal system, (b, c) ureter, and (c) urinary bladder. These findings can be confused with urothelial carcinoma. Plain radiograph (d) shows lobar-pattern of calcification of the entire left kidney signifying putty kidney (white arrows) and calcification of the left upper ureter (block arrow) in an advanced case of TB
In the early stages, ureteral involvement may manifest as irregular urothelial enhancement with hydronephrosis (Fig. 6). Later, multi-level ureteral strictures can form resulting in a beaded appearance due to alternate segments of dilatation and constriction. In advanced cases, extensive fibrosis can result in short and straightened ureter, referred to as a “pipe stem ureter” and parenchymal calcifications associated with putty kidney [45]. Findings may mimic urothelial carcinoma (Fig. 7).
Fig. 7.
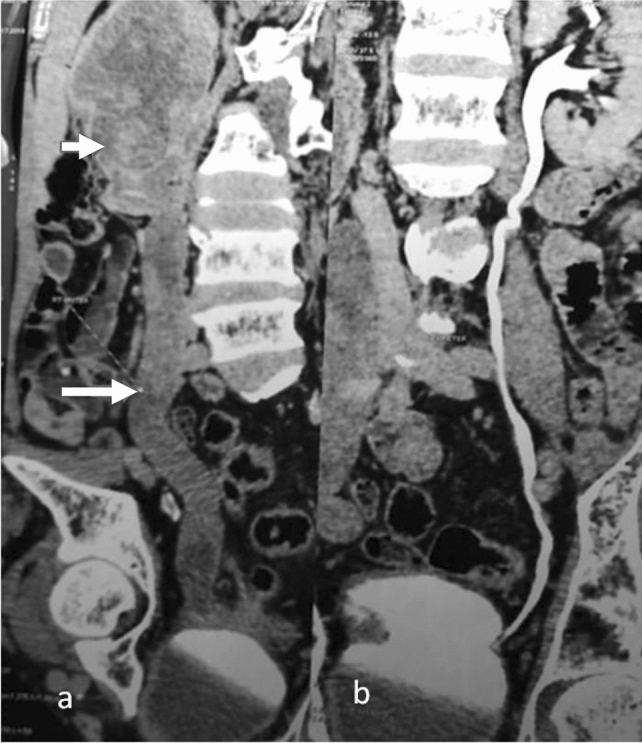
CECT coronal images of the abdomen in a patient with urothelial carcinoma shows (a) a mass in right pelvis (arrow) and ureter with no contrast excretion (long arrow) whereas (b) normal contrast excretion is seen in the pelvicalyceal system and ureter on the opposite side
Urinary bladder tuberculosis is nearly always secondary to renal TB and is seen in about 33% of cases [46]. In the early stage, there may be no imaging findings. Later, asymmetrical wall thickening may mimic transitional cell carcinoma on imaging (Fig. 8). Transurethral biopsy may help in distinguishing from malignancy. In the chronic form, a small capacity, thick walled bladder known as a “thimble bladder” may develop and vesicoureteral reflux may be seen. Thimble bladder usually presents with voiding symptoms such as dysuria, frequency and incontinence [47].
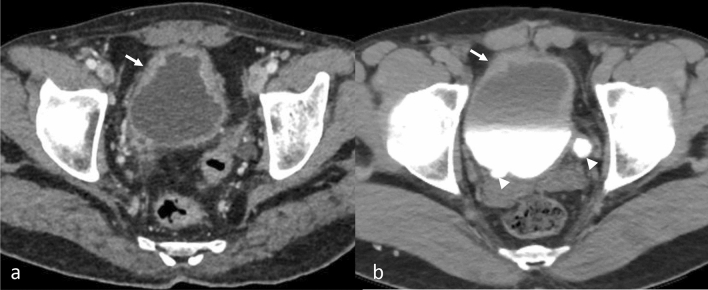
Fig. 8.
CECT pelvis axial images (a) show irregular wall thickening of the urinary bladder (arrow) in a patient suspected for urothelial carcinoma who underwent cystoscopy and biopsy. Biopsy showed caseating granuloma suggestive of genitourinary TB. Small bladder capacity is due to thimble bladder. CT urography (b) showing irregular wall thickening with proximal dilatation of bilateral ureters (arrowheads) in a case of urothelial carcinoma of the urinary bladder
Adrenal tuberculosis
Although TB involving the adrenal glands can present as unilateral or bilateral adrenal mass(es), approximately 90% are bilateral, thought to be due to haematogenous mode of spread [48]. The morphology depends on the stage of involvement. In the early stage of caseous granulomatous necrosis, adrenal glands increase in size, with or without preservation of the contour, and may include peripheral rim-enhancement and central hypodensity corresponding to caseous necrosis (Fig. 9). In the late fibrotic stage, calcification is found within the masses, followed by atrophy at the end stages. Differential diagnoses include metastases, which are more commonly seen in the setting of known malignancy and more often unilateral; lymphoma, which rarely involves the adrenal glands; adrenal haemorrhage, which tends to evolve, may calcify or resolve, and does not enhance with intravenous contrast; as well as fungal infection (most commonly Histoplasma), which is rare. Other differential considerations of primary adrenal carcinoma and pheochromocytoma are most commonly unilateral. Features favoring adrenal tuberculosis from an adrenal neoplasm include bilateral involvement, calcification, peripheral enhancement, and central hypodensity [48], although central hypodensity also may be seen with adrenal carcinoma or metastases. Blood biochemical markers (metanephrins) are commonly elevated with pheochromocytoma. The radiological features of adrenal TB and histoplasmosis are fairly similar but, as stated, adrenal fungal involvement is rare in TB. Taking into consideration the prevalence of TB in the area may further aid in distinguishing between TB and histoplasmosis. Tuberculosis remains the most common cause of Addison’s disease or primary adrenal insufficiency in endemic countries [49, 50].
Fig. 9.
CECT abdomen axial images show (a) enlarged, hypoenhancing and nodular right adrenal gland in a patient with adrenal TB involvement, (b) coronal image shows enlarged right adrenal gland (arrow) with dilatated and thickened pelvicalyceal system (thick arrow) of the right kidney consistent with renal and adrenal TB as well as left hydronephrosis (arrowhead). These appearances can mimic malignancy. CECT coronal image (c) showing bilateral low attenuation adrenal metastasis (arrow) from melanoma with associated liver (long arrow) and splenic (arrowhead) metastases
Visceral TB summary
Visceral TB (Table 1) can mimic many diseases and may not be considered in areas where it is thought to be of low prevalence. In areas of high prevalence, it may be mistaken for other diseases such as cancer, thus both a high index of suspicion and knowledge of local demographics are important. Imaging features that favor TB in abdominal viscera are systemic TB, associated caseating lymphadenopathy (i.e. non-enhancing, low attenuation center) in early TB or calcified lymph nodes in later disease, micronodules or macronodules in early disease, and architectural distortion in later disease. Tumor markers may be elevated in correlated cancers but not with TB. Imaging can guide biopsy. PCR, rather than simply AFB staining, may be needed for confirmation. Imaging may assess response to anti-TB therapy. CT and MRI can be used both for the diagnosis as well as follow up of these patients after antitubercular therapy.
Table 1.
Summary of visceral TB
| Type | Clinical features | Imaging findings | Differential diagnoses |
|---|---|---|---|
| Hepatic tuberculosis | Hepatomegaly, abdominal pain, jaundice, fever, and weight loss |
Focal/ diffuse, miliary/macrnodular Serohepatic variant (rare) Late: calcifications, architectural distortion |
Primary malignancy or metastatic disease, fungal infections, sarcoidosis, and rarely, leukemia and lymphoma and other granulomatous infections such as brucellosis |
| Splenic tuberculosis |
Splenomegaly Abdominal pain |
Micronodular and/or macronodular nodules, sometimes splenomegaly Late: calcifications, architectural distortion |
Metastatic disease, leukemia or lymphoma, sarcoidosis, hemangioma, cyst, and fungal infection |
| Gallbladder tuberculosis | Right upper quadrant pain, mass, anaemia |
Nodule, mass or circumferential thickening of the GB Late: calcifications, architectural distortion |
Carcinoma, chronic cholecystitis, and xanthogranulomatous cholecystitis |
| Biliary tuberculosis | Abdominal pain, hepatomegaly, jaundice, fever, and chills |
Multifocal biliary strictures with/without intervening sections of dilatation Extrahepatic biliary duct involvement is rare Late: calcifications |
Cholangiocarcinoma, primary sclerosing cholangitis, inflammatory/sclerosing cholangiopathies, IgG4-related strictures, AIDS cholangiopathy, recurrent pyogenic cholangitis |
| Pancreatic tuberculosis | Epigastric pain, nausea, vomiting, mass, diarrhea, jaundice |
Diffuse involvement of the pancreas or multifocal or solitary focal lesions, peripancraetic necrosis Late: calcifications, architectural distortion |
Pancreatic carcinoma, pseuodocyst, chonic pancreatitis |
| Kidney ureter bladder (KUB) tuberculosis | Increased urinary frequency, dysuria and flank pain |
Parenchymal tuberculoma in kidney, papillary necrosis, renal calcifications, phantom calyx, Kerr’s kink, irregular asymmetrical urothelial enhancement Pipe stem ureter Asymmetrical wall thickening of UB, thimble bladder Additional late: calcifications and further architectural distortion |
Xanthogranulomatous pyelonephritis, renal cell carcinoma, metastases, lymphoma, urothelial carcinoma |
| Adrenal TB | Symptoms of adrenal insufficiency- fatigue and abdominal pain | Unilateral or bilateral adrenal masses, early stage-adrenal glands increase in size, with or without preservation of the contour, peripheral rim-enhancement and central hypodensity corresponding to caseous necrosis. In the late fibrotic stage, calcification within the masses, followed by atrophy at the end stages | Metastasis, lymphoma, fungal infection, primary adrenal carcinoma and pheochromocytoma |
Caseating or calcified lymphadenopathy may be seen. Visceral TB is often in the setting of systemic disease
Conclusion
In conclusion, abdominal visceral tuberculosis is common, especially in countries where tuberculosis is endemic. Diagnosis is challenging as clinical presentations are often non-specific and imaging appearances may mimic other diseases, especially cancer. Tissue sampling may be necessary for definitive diagnosis. Radiology can aid detecting TB, providing a differential diagnosis, assessing extent of spread, guiding biopsy, and evaluating response.
Author contributions
CJD: contributed to the concept of the manuscript, wrote the manuscript, contributed images and captions, and provided final approval of the manuscript. NR, ZV, AA, SHC: contributed to writing of the manuscript and provided final approval of the manuscript. VK: contributed to the concept of the manuscript, helped write the manuscript, contributed images and captions, oversaw the process, and provided final approval of the manuscript.
Data availability
Not applicable.
Declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Ethics approval
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chandan J. Das, Email: dascj@yahoo.com
Vikas Kundra, Email: vkundra@som.umaryland.edu.
References
- 1.Trends in Tuberculosis, 2021 [Internet]. Centers for Disease Control and Prevention; [cited 2023 Apr 18]. Available from: https://www.cdc.gov/tb/publications/factsheets/statistics/tbtrends.htm
- 2.Sheer TA, Coyle WJ. Gastrointestinal tuberculosis. Curr Gastroenterol Rep. 2003;5(4):273–278. doi: 10.1007/s11894-003-0063-1. [DOI] [PubMed] [Google Scholar]
- 3.Donoghue HD, Holton J. Intestinal tuberculosis. Curr Opin Infect Dis. 2009;22(5):490–496. doi: 10.1097/QCO.0b013e3283306712. [DOI] [PubMed] [Google Scholar]
- 4.Tirumani SH, Ojili V, Gunabushanam G, Shanbhogue AKP, Nagar A, Fasih N, et al. Imaging of tuberculosis of the abdominal viscera: beyond the intestines. J Clin Imaging Sci. 2013;3:17. doi: 10.4103/2156-7514.111234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Padma V, Anand NN, Rajendran SM, Gurukal S. Primary tuberculosis of stomach. J Indian Med Assoc. 2012;110(3):187–188. [PubMed] [Google Scholar]
- 6.Cheng MP, Abou Chakra CN, Yansouni CP, Cnossen S, Shrier I, Menzies D, et al. Risk of Active Tuberculosis in Patients with Cancer: A Systematic Review and Meta-Analysis. Clin Infect Dis. 2017;64(5):635–644. doi: 10.1093/cid/ciw838. [DOI] [PubMed] [Google Scholar]
- 7.Dobler CC, Cheung K, Nguyen J, Martin A. Risk of tuberculosis in patients with solid cancers and haematological malignancies: a systematic review and meta-analysis. Eur Respir J. 2017;50(2):1700157. doi: 10.1183/13993003.00157-2017. [DOI] [PubMed] [Google Scholar]
- 8.Das CJ, Vora Z, Sharma R, Addula D, Kundra V. Tuberculosis of abdominal lymph nodes, peritoneum, and GI tract: a malignancy mimic. Abdom Radiol (NY). 2022;47(5):1775–1787. doi: 10.1007/s00261-022-03472-x. [DOI] [PubMed] [Google Scholar]
- 9.Sharma MP, Bhatia V. Abdominal tuberculosis. Indian J Med Res. 2004;120(4):305–315. [PubMed] [Google Scholar]
- 10.Cheng W, Zhang S, Li Y, Wang J, Li J. Intestinal tuberculosis: clinico-pathological profile and the importance of a high degree of suspicion. Trop Med Int Health. 2019;24(1):81–90. doi: 10.1111/tmi.13169. [DOI] [PubMed] [Google Scholar]
- 11.Agarwala R, Dhooria S, Khaire NS, Mishra S, Verma S, Shah J, et al. Xpert MTB/RIF for diagnosis of tubercular liver abscess. A case series. Infez Med. 2020;28(3):420–424. [PubMed] [Google Scholar]
- 12.Kohli M, Schiller I, Dendukuri N, Dheda K, Denkinger CM, Schumacher SG, et al. Xpert® MTB/RIF assay for extrapulmonary tuberculosis and rifampicin resistance. Cochrane Database Syst Rev. 2018 Aug 27;8:CD012768. [DOI] [PMC free article] [PubMed]
- 13.Essop AR, Posen JA, Hodkinson JH, Segal I. Tuberculosis hepatitis: a clinical review of 96 cases. Q J Med. 1984;53(212):465–477. [PubMed] [Google Scholar]
- 14.Tai WC, Kuo CM, Lee CH, Chuah SK, Huang CC, Hu TH, et al. Liver tuberculosis in Southern Taiwan: 15-years clinical experience. J Intern Med Taiwan - Search Results [Internet]. PubMed. [cited 2022 Oct 18]. Available from: https://pubmed.ncbi.nlm.nih.gov/?term=Tai+WC%2C+Kuo+CM%2C+Lee+CH%2C+Chuah+SK%2C+Huang+CC%2C+Hu+TH%2C+et+al.+Liver+tuberculosis+in+Southern+%09++Taiwan%3A+15-years+clinical+experience.+J+Intern+Med+Taiwan
- 15.Wang JY, Hsueh PR, Wang SK, Jan IS, Lee LN, Liaw YS, et al. Disseminated tuberculosis: a 10-year experience in a medical center. Medicine (Baltimore). 2007;86(1):39–46. doi: 10.1097/MD.0b013e318030b605. [DOI] [PubMed] [Google Scholar]
- 16.Mert A, Ozaras R, Tabak F, Ozturk R, Bilir M. Localized hepatic tuberculosis. Eur J Intern Med. 2003;14(8):511–512. doi: 10.1016/j.ejim.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Vilaichone R, Mahachai V. Hepatic Tuberculosis : A Clinico-Pathological Study - Search Results [Internet]. PubMed. [cited 2022 Oct 18]. Available from: https://pubmed.ncbi.nlm.nih.gov/?term=Vilaichone+R%2C+Mahachai+V.+Hepatic+Tuberculosis%E2%80%AF%3A+A+Clinico-Pathological+Study
- 18.Kok KY, Yapp SK. Isolated hepatic tuberculosis: report of five cases and review of the literature. J Hepatobiliary Pancreat Surg. 1999;6(2):195–198. doi: 10.1007/s005340050106. [DOI] [PubMed] [Google Scholar]
- 19.Alvarez SZ. Hepatobiliary tuberculosis. J Gastroenterol Hepatol. 1998;13(8):833–839. doi: 10.1111/j.1440-1746.1998.tb00743.x. [DOI] [PubMed] [Google Scholar]
- 20.Yu RS, Zhang SZ, Wu JJ, Li RF. Imaging diagnosis of 12 patients with hepatic tuberculosis. World J Gastroenterol. 2004;10(11):1639–1642. doi: 10.3748/wjg.v10.i11.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hersch C. Tuberculosis of the liver. A study of 200 cases. S Afr Med J. 1964 Oct 31;38:857–63. [PubMed]
- 22.Chien RN, Lin PY, Liaw YF. Hepatic tuberculosis: comparison of miliary and local form. Infection. 1995;23(1):5–8. doi: 10.1007/BF01710049. [DOI] [PubMed] [Google Scholar]
- 23.Lee WK, Van Tonder F, Tartaglia CJ, Dagia C, Cazzato RL, Duddalwar VA, et al. CT appearances of abdominal tuberculosis. Clin Radiol. 2012;67(6):596–604. doi: 10.1016/j.crad.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Karaosmanoglu AD, Onur MR, Sahani DV, Tabari A, Karcaaltincaba M. Hepatobiliary Tuberculosis: Imaging Findings. AJR Am J Roentgenol. 2016;207(4):694–704. doi: 10.2214/AJR.15.15926. [DOI] [PubMed] [Google Scholar]
- 25.Maharaj B, Leary WP, Pudifin DJ. A prospective study of hepatic tuberculosis in 41 black patients. Q J Med. 1987;63(242):517–522. [PubMed] [Google Scholar]
- 26.Kawamori Y, Matsui O, Kitagawa K, Kadoya M, Takashima T, Yamahana T. Macronodular tuberculoma of the liver: CT and MR findings. AJR Am J Roentgenol. 1992;158(2):311–313. doi: 10.2214/ajr.158.2.1729789. [DOI] [PubMed] [Google Scholar]
- 27.Turhan N, Kurt M, Ozderin YO, Kurt OK. Hepatic granulomas: a clinicopathologic analysis of 86 cases. Pathol Res Pract. 2011;207(6):359–365. doi: 10.1016/j.prp.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 28.Burrill J, Williams CJ, Bain G, Conder G, Hine AL, Misra RR. Tuberculosis: a radiologic review. Radiographics. 2007;27(5):1255–1273. doi: 10.1148/rg.275065176. [DOI] [PubMed] [Google Scholar]
- 29.Chen Y, Wu N, Xue T, Hao Y, Dai J. Comparison of contrast-enhanced sonography with MRI in the diagnosis of complex cystic renal masses. J Clin Ultrasound. 2015;43(4):203–209. doi: 10.1002/jcu.22232. [DOI] [PubMed] [Google Scholar]
- 30.Singh B, Ramdial PK, Royeppen E, Moodley J, Chetty R. Isolated splenic tuberculosis. Trop Doct. 2005;35(1):48–49. doi: 10.1258/0049475053001976. [DOI] [PubMed] [Google Scholar]
- 31.Sharma SK, Smith-Rohrberg D, Tahir M, Mohan A, Seith A. Radiological manifestations of splenic tuberculosis: a 23-patient case series from India. Indian J Med Res. 2007;125(5):669–678. [PubMed] [Google Scholar]
- 32.D P, Ss H, Se M, A C, Nk S, M H. Tuberculosis--the great mimicker. Seminars in ultrasound, CT, and MR [Internet]. 2014 Jun [cited 2022 Oct 18];35(3). Available from: https://pubmed.ncbi.nlm.nih.gov/24929261/ [DOI] [PubMed]
- 33.Ozgüroğlu M, Celik AF, Demir G, Aki H, Demirelli F, Mandel N, et al. Primary splenic tuberculosis in a patient with nasal angiocentric lymphoma: mimicking metastatic tumor on abdominal CT. J Clin Gastroenterol. 1999;29(1):96–98. doi: 10.1097/00004836-199907000-00024. [DOI] [PubMed] [Google Scholar]
- 34.Xu XF, Yu RS, Qiu LL, Shen J, Dong F, Chen Y. Gallbladder tuberculosis: CT findings with histopathologic correlation. Korean J Radiol. 2011;12(2):196–202. doi: 10.3348/kjr.2011.12.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inal M, Aksungur E, Akgül E, Demirbaş O, Oğuz M, Erkoçak E. Biliary tuberculosis mimicking cholangiocarcinoma: treatment with metallic biliary endoprothesis. Am J Gastroenterol. 2000;95(4):1069–1071. doi: 10.1111/j.1572-0241.2000.01944.x. [DOI] [PubMed] [Google Scholar]
- 36.Wenzel JS, Donohoe A, Ford KL, Glastad K, Watkins D, Molmenti E. Primary biliary cirrhosis: MR imaging findings and description of MR imaging periportal halo sign. AJR Am J Roentgenol. 2001;176(4):885–889. doi: 10.2214/ajr.176.4.1760885. [DOI] [PubMed] [Google Scholar]
- 37.Kakkar C, Polnaya AM, Koteshwara P, Smiti S, Rajagopal KV, Arora A. Hepatic tuberculosis: a multimodality imaging review. Insights Imaging. 2015;6(6):647–658. doi: 10.1007/s13244-015-0440-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chong VH. Hepatobiliary tuberculosis: a review of presentations and outcomes. South Med J. 2008;101(4):356–361. doi: 10.1097/SMJ.0b013e318164ddbb. [DOI] [PubMed] [Google Scholar]
- 39.Nagar AM, Raut AA, Morani AC, Sanghvi DA, Desai CS, Thapar VB. Pancreatic tuberculosis: a clinical and imaging review of 32 cases. J Comput Assist Tomogr. 2009;33(1):136–141. doi: 10.1097/RCT.0b013e31816c82bc. [DOI] [PubMed] [Google Scholar]
- 40.Saluja SS, Ray S, Pal S, Kukeraja M, Srivastava DN, Sahni P, et al. Hepatobiliary and pancreatic tuberculosis: a two decade experience. BMC Surg. 2007;24(7):10. doi: 10.1186/1471-2482-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gupta D, Patel J, Rathi C, Ingle M, Sawant P. Primary Pancreatic Head Tuberculosis: Great Masquerader of Pancreatic Adenocarcinoma. Gastroenterology Res. 2015;8(2):193–196. doi: 10.14740/gr650w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liang X, Huang X, Yang Q, He J. Calcified peripancreatic lymph nodes in pancreatic and hepatic tuberculosis mimicking pancreatic malignancy: A case report and review of literature. Medicine (Baltimore). 2018;97(36):e12255. doi: 10.1097/MD.0000000000012255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Merchant S, Bharati A, Merchant N. Tuberculosis of the genitourinary system-Urinary tract tuberculosis: Renal tuberculosis-Part II. Indian J Radiol Imaging. 2013;23(1):64–77. doi: 10.4103/0971-3026.113617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gibson MS, Puckett ML, Shelly ME. Renal tuberculosis. Radiographics. 2004;24(1):251–256. doi: 10.1148/rg.241035071. [DOI] [PubMed] [Google Scholar]
- 45.Muttarak M, ChiangMai WN, Lojanapiwat B. Tuberculosis of the genitourinary tract: imaging features with pathological correlation. Singapore Med J. 2005 Oct;46(10):568–74; quiz 575. [PubMed]
- 46.Hemal AK, Aron M. Orthotopic neobladder in management of tubercular thimble bladders: initial experience and long-term results. Urology. 1999;53(2):298–301. doi: 10.1016/S0090-4295(98)00504-4. [DOI] [PubMed] [Google Scholar]
- 47.Nzerue C, Drayton J, Oster R, Hewan-Lowe K. Genitourinary tuberculosis in patients with HIV infection: clinical features in an inner-city hospital population. Am J Med Sci. 2000;320(5):299–303. doi: 10.1097/00000441-200011000-00001. [DOI] [PubMed] [Google Scholar]
- 48.Yang ZG, Guo YK, Li Y, Min PQ, Yu JQ, Ma ES. Differentiation between tuberculosis and primary tumors in the adrenal gland: evaluation with contrast-enhanced CT. Eur Radiol. 2006;16(9):2031–2036. doi: 10.1007/s00330-005-0096-y. [DOI] [PubMed] [Google Scholar]
- 49.Mayo-Smith WW, Boland GW, Noto RB, Lee MJ. State-of-the-art adrenal imaging. Radiographics. 2001;21(4):995–1012. doi: 10.1148/radiographics.21.4.g01jl21995. [DOI] [PubMed] [Google Scholar]
- 50.Guo YK, Yang ZG, Li Y, Ma ES, Deng YP, Min PQ, et al. Addison’s disease due to adrenal tuberculosis: contrast-enhanced CT features and clinical duration correlation. Eur J Radiol. 2007;62(1):126–131. doi: 10.1016/j.ejrad.2006.11.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.