Abstract
Background
Food allergy has become an increasingly important public health problem. However, information regarding epidemiological studies of food allergy among Chinese adults is very limited. This study aims to estimate the prevalence of self-reported food allergy among adults in China.
Method
A population-based cross-sectional study was administered to estimate the prevalence of self-reported food allergy on the basis of a face-to-face questionnaire survey. The participants were recruited by cluster random sampling from three prefectures in Jiangxi Province, China.
Results
A total of 12 082 questionnaires were distributed, and 11 935 (98.8%) of completed ones were collected. The prevalence of self-reported food allergy was 4.0% (3.1% in men and 4.8% in women), self-reported doctor-diagnosed food allergy accounted for 1.4%. The most common allergic symptom was skin reaction showing in 63.9% of the participants with self-reported food allergy. The main allergic foods were shrimp, mollusks, and mango, accounting for the prevalence of 39.8%, 20.8%, and 18.7%, respectively. The self-reported food allergy was significantly linked with gender, age group, body height and other allergic conditions.
Conclusions
The prevalence of self-reported food allergy is about 4.0% among adults in China. The three most common allergenic foods were shrimp, mollusks and mango. Gender, age, and other allergic diseases could be contributing factors associated with food allergy in adults. These findings will provide scientific basis for the further research and prevention of food allergy in adults.
Keywords: Food allergy, Prevalence, Adult, China
Introduction
Food allergy (FA) represents a potentially life-threatening health problem that has affected public health, quality of life, and economic burden in both developed and developing countries over the past few decades.1,2 Food allergy is also an abnormal immune response condition due to the exposure to certain food components with a series of clinical symptoms.3 Commonly, these clinical symptoms can occur within minutes after intake and last for several days. These abnormal reactions mainly manifested in skin, gastrointestinal tract, respiratory and cardiovascular system.4
Food allergy is usually diagnosed by clinical history, combining skin prick test (SPT) or serum allergen-specific IgE test (sIgE), and if indicated, oral food challenge test (OFC).5 However, understanding of the prevalence of FA is a population-based study, and it is often a challenge to estimate the prevalence in the absent clinical diagnosis and various experiments. Most survey studies only assessed the FA prevalence by self-reported food allergies.6 For instance, the research that carried out among European adults found that the prevalence of self-reported FA ranged from 1.7% to 37.3% to any food and from 0.5% to 18.9% to at least 1 priority food.7
Population-based studies on the prevalence of food allergies in adults have been extensively reported in developed countries, and the reported prevalence was approximately 10%, approaching to an alarming rate.8 The most common allergic foods in adults were shellfish, milk, peanuts, tree nuts, and fish.8, 9, 10, 11, 12 However, many studies on the prevalence of food allergies in developing nations were mainly focused on children, and data on food allergies in adults are sparse.13,14 Thus, the main purpose of this study is to investigate the prevalence, the common causative foods, and manifestation of FA among adults in China.
Materials and methods
Sampling and population
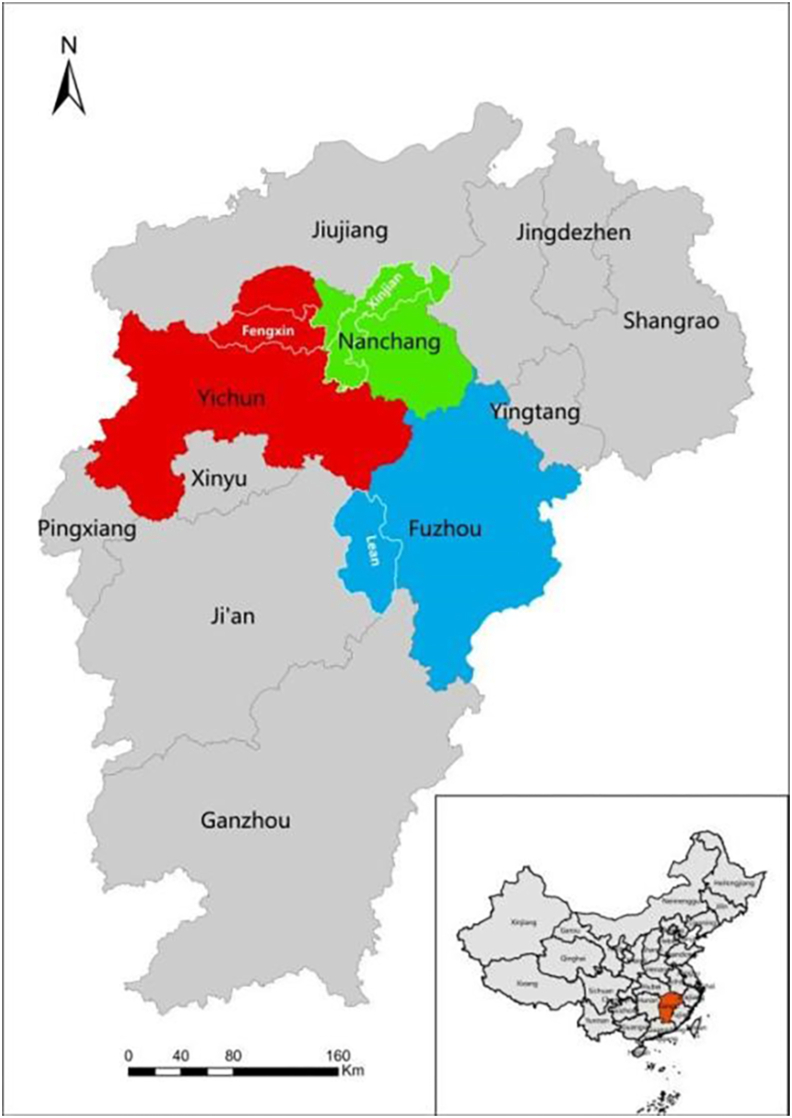
This cross-sectional study was performed via self-administered questionnaire survey. Participants were recruited by stratified cluster random sampling in Jiangxi, China. We obtained more than 12 000 adult samples aged 18–70 years from 3 regions as 3 survey centers (Fig. 1), they were Fengxin County (Yichun Prefecture) (4032 samples), Lean County (Fuzhou Prefecture) (4015 samples), and Xinjian District (Nanchang Prefecture) (4035 samples), respectively.
Fig. 1.
Distribution map of survey centers in Jiangxi Province
Exclusion criteria
We excluded populations in the study if they met the following exclusion criteria: (1) under 18 years old or beyond 70 years old; (2) Absence at home; (3) Who is difficult to complete the study because of severe diseases; and (4) Refusal to participate or refusal to sign the consent form.
Questionnaire and data collection
This questionnaire was designed for adults mainly on the basis of standard EuroPrevell FA questionnaire, including demographic information, FA, common symptoms, allergenic food, and allergic disease history as well as height, weight, and family size, etc. Trained and qualified investigators distributed the questionnaires to the adults; the participants completed the questionnaire by themselves on site. Then the staff collected and reviewed the questionnaires. Unqualified questionnaires would be returned for supplement or improvement. Written informed consents were obtained from each subject. This study was approved by the Ethics Committee of the National Food Safety Risk Assessment Center.
Definition of self-reported food allergy
The participants were asked, “Have you ever suffered from illness or physical discomfort caused by eating certain foods?” in the questionnaire. The abnormal problems included oral allergy symptoms (itching, tingling or swelling in the mouth, lips or throat), skin symptoms (rash, nettle sting or itchy skin), gastrointestinal symptoms (diarrhea or vomiting), respiratory symptoms (breathlessness), cardiovascular symptoms (fainting or dizziness), runny or stuffy nose or red, sore or running eyes, swallowing difficulty, stiffness in joints, headache, and other unpleasant symptoms; if answered “yes”, they were regarded as self-reported FA.
Statistical analysis
The data were double-entered to a database using Epidata 3.0, and the continuous variables were expressed as mean and standard deviation; the comparison between groups was made by two-sample T test and analysis of variance (ANOVA). The categorical variables were presented in percentage, and chi-square test was performed for comparing the difference between groups. Logistic regression analysis was applied to analyze the factors related to self-reported FA. The value of P less than 0.05 was statistically significant. SPSS22.0 software was used to statistically analyze the data in this study.
Results
Food allergy and demographics
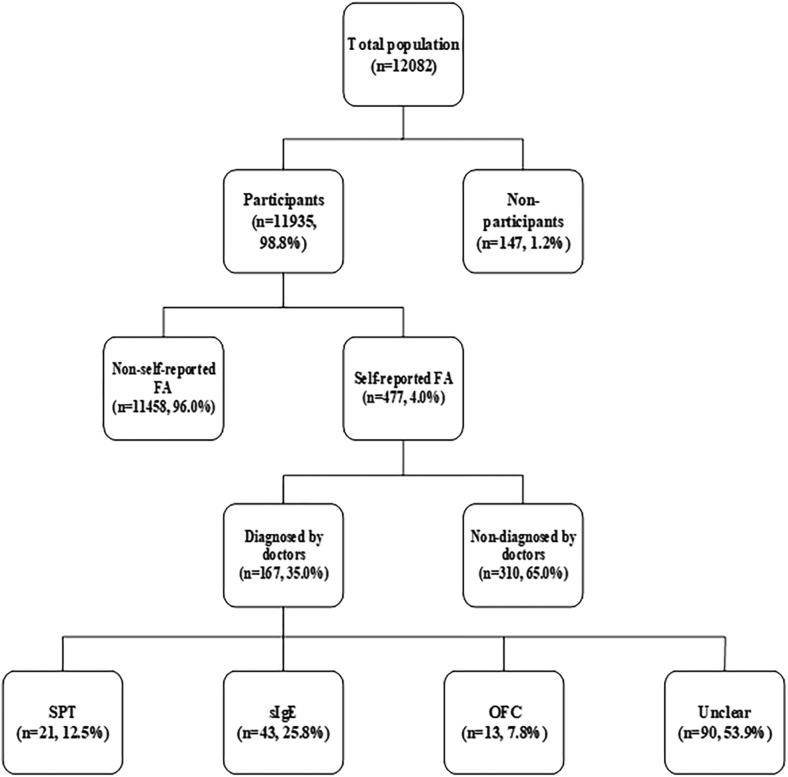
We recruited 12 082 adults aged 18–70 years from 3 survey centers, and collected 11 935 questionnaires, with 98.8% effective response rate. Among these participants, 477 (4.0%) reported FA, and 167 (1.4%) reported a doctor-diagnosed FA. The reported main methods of doctor-diagnosed FA were sIgE test, less than 8.0% of participants with doctor-diagnosed food allergies were confirmed the FA condition by OFC. Fig. 2 showed the distribution of respondents. Table 1 presented the demographic characteristics of the respondents. The proportion of the FA between men and women was very similar (49.1% vs 50.9%) in the participants. The prevalence of self-reported FA in women was 4.8% that was greater than 3.1% reported in men (P < 0.001). The mean age in participants with self-reported FA was 43.38 ± 14.00 years, mildly younger compared with 44.78 ± 12.86 years in those without FA. The prevalence of self-reported FA decreased slightly with age increase based on two-sample T test (P = 0.021). The participants were also divided into 3 (youth, middle, and old) age groups according to the age classification criteria of World Health Organization (WHO). The FA prevalence in the middle age group was 3.5%, which was lower than 4.2% or 4.5% detected for youth or old age group; however, the difference is not statistically significant (P = 0.086). The mean height of the participants with self-reported FA was 1.61 m, which was shorter than 1.62 m of the participants without FA (P = 0.001). Among 3 study centers, the prevalence of self-reported FA was highest in Fengxin county (5.1%) and lowest in Xinjian district (2.6%) (P < 0.001).
Fig. 2.
Distribution of participants
Table 1.
Demographic characteristics of the adults.
| Variablesa | Total |
Self-reported FA |
Non-self-reported FA |
Pb-value | |
|---|---|---|---|---|---|
| (N = 11,935) | (N = 477) | (N = 11,458) | |||
| Age, mean | 44.72 (12.91) | 43.38 (14.00) | 44.78 (12.86) | 0.021 | |
| Age groupc | Youth | 601c | 254 (4.2) | 5764 (95.8) | 0.086 |
| Middle age | 4229 | 147 (3.5) | 4082 (96.5) | ||
| Old age | 1688 | 76 (4.5) | 1612 (95.5) | ||
| Gender | Men | 5859 | 183 (3.1) | 5676 (96.9) | <0.001 |
| Women | 6076 | 294 (4.8) | 5782 (95.2) | ||
| Ethnicity | Han | 11,929 | 477 (4.0) | 11,452 (96.0) | 0.998 |
| Elsed | 6 | 0 | 6 (100d0) | ||
| Residence | Rural | 6046 | 230 (3.8) | 5816 (96.2) | 0.277 |
| Urban | 5889 | 247 (4.2) | 5642 (95.8) | ||
| Height | 1.62 (0.08) | 1.61 (0.07) | 1.62 (0.08) | 0.001 | |
| BMI, mean | 22.59 (2.75) | 22.54 (3.09) | 22.59 (2.73) | 0.727 | |
| BMIe | Lean | 561 | e3 (5.9) | 528 (94.1) | 0.063 |
| Normal | 9222 | 358 (3.9) | 8864 (96.1) | ||
| Overweight or obesity | 2152 | 86 (4.0) | 2066 (96.0) | ||
| Family size | 1 | 184 | 7 (3.8) | 177 (96.2) | 0.545 |
| 2 | 1281 | 44 (3.4) | 1237 (96.6) | ||
| ≥3 | 10,470 | 426 (4.1) | 10,044 (95.9) | ||
| Regions | Fengxin county | 3910 | 199 (5.1) | 3711 (94.9) | <0.001 |
| Lean county | 3996 | 173 (4.3) | 3823 (95.7) | ||
| Xinjian district | 4029 | 105 (2.6) | 3924 (97.4) | ||
| Other allergies | 323 | 129 (39.9) | 194 (60.1) | <0.001 | |
| Allergic dermatitis | 192 | 89 (46.4) | 103 (53.6) | <0.001 | |
| Allergic rhinitis | 124 | 43 (34.7) | 81 (65.3) | <0.001 | |
| Allergic asthma | 13 | 6 (46.2) | 7 (53.8) | <0.001 | |
| Elsef Allergies | 22 | 7 (31,8) | 15 (68.2) | <0.001 | |
Qualitative variables were expressed by composition ratio while quantitative variables were expressed by mean and standard deviation (SD).
P-value less than 0.05 is statistically significant.
Age was divided into three groups according to the criteria of the WHO: with age <45 years defined as youth, age between 45 and 59.9 years defined as middle age, and age ≥60 years defined as old age.
Else included Mongolian, Miao, Yao, She, Yi.
BMI was categorized according to the criteria of the World Health Organization, with BMI <18.5 kg/m2 defined as lean, BMI between 18.5 and 25 kg/m2 defined as normal, and BMI ≥25 kg/m2 defined as overweight and obesity.
Else included pollen allergy, drug allergy etc.
The prevalence of self-reported FA in Han nationality was 4.0%; no adverse food reactions were reported in ethnic minorities. Self-reported FA was not significantly associated with ethnicity and family size ((P = 0.998, P = 0.545, respectively). There was no significant difference for the FA prevalence among the participants who lived in rural areas compared with those who lived in urban areas (P = 0.277).
Body Mass Index (BMI) is an internationally unified obesity classification standard considering both weight and height recommended by the WHO. The mean and standard deviation of BMI in the participants was 22.59 ± 2.75 kg/m2. Two methods were employed for data analysis on BMI, which was taking BMI as continuous variable presented mean value and categorical variable divided into 3 groups according to the WHO classification criteria, such as BMI <18.5 kg/m2 (lean); BMI between 18.5 and 25 kg/m2 (normal), and BMI ≥25 kg/m2 (overweight or obesity). Regardless of the analysis method, there was no significant difference between BMI and self-reported FA (P = 0.727, P = 0.063, respectively), although the prevalence of FA in the normal BMI group was lower than that in lean or overweight group.
Common symptoms and foods
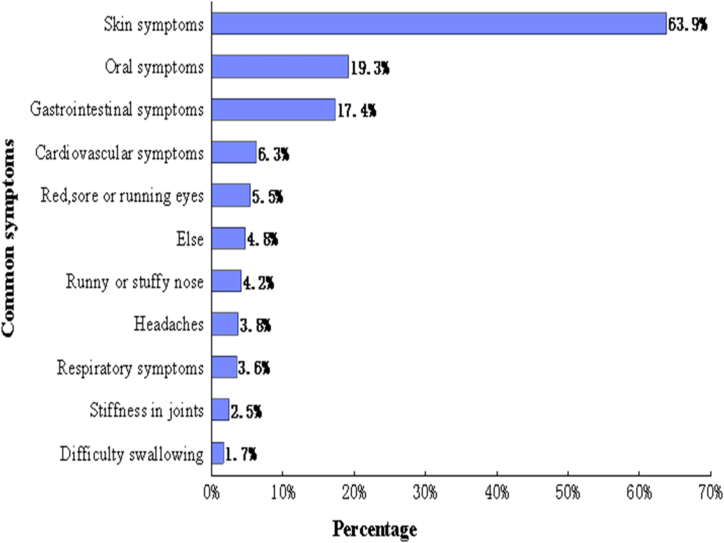
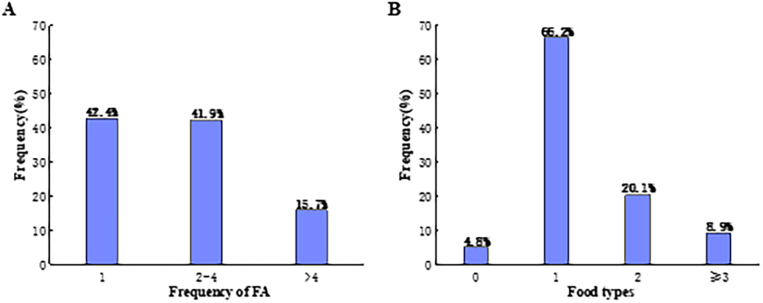
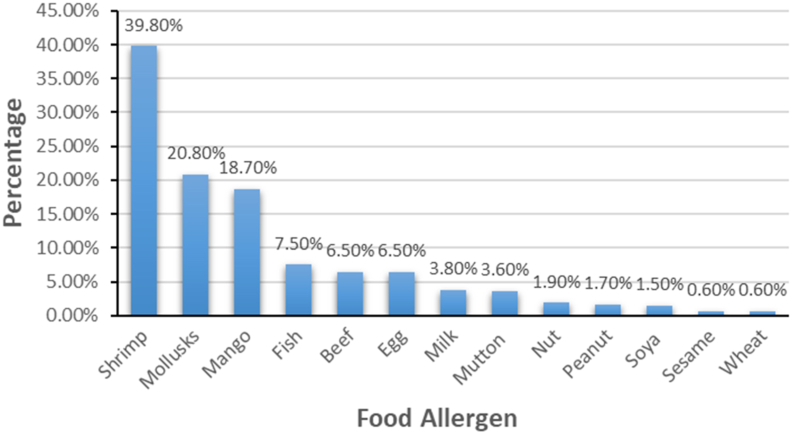
The most reported FA reactions were skin symptoms (63.9%), such as a rash, nettle sting, or itchy skin, followed by oral allergy symptoms (19.3%), such as itching, or tingling or swelling of the mouth, lips, or throat. The distribution of common symptoms of self-reported FA were shown in Fig. 3. Among the participants with self-reported FA, 42.4% appeared only once allergic reaction, 41.9% suffered 2 to 4 times, 15.7% had at least 5 times, and 95.2% were allergic to at least 1 of the common causative foods listed in this questionnaire (Fig. 4). The 8 common allergic foods were shrimp, mollusks, mango, fish, egg, beef, milk, and mutton. Nearly 40.0% adults with FA reported that they had adverse reactions after consuming shrimp and 20.8% mentioned their symptoms related to the mollusks (Fig. 5).
Fig. 3.
Common symptoms of FA. Oral allergy symptoms = itching, tingling or swelling of the mouth, lips or throat; Skin symptoms = rash, nettle sting or itchy skin; Gastrointestinal symptoms = diarrhea or vomiting (other than food poisoning); Respiratory symptoms = breathlessness; Cardiovascular symptoms = fainting or dizziness; Else = stomachache, abdominal distention, cough, limb numbness, palpitation and fatigue or hypoglycemic shock
Fig. 4.
(A) Frequency of adverse reactions after consuming certain food. (B) Types of foods causing adverse reactions
Fig. 5.
List of the main food allergens
Other allergy diseases
This study analyzed other allergic diseases besides FA. A total of 351 adults (2.9%) mentioned that they had at least 1 other allergic disease including the most frequently implicated allergic dermatitis (1.6%), rhinitis (1.0%), and asthma (0.1%). In addition, the individuals with comorbid allergies had a significantly higher likelihood of having self-reported FA (P < 0.001) (Table 1).
Multivariate regression analysis
The possible influence factors of FA were analyzed by multivariate logistic regression including age, gender, height, regions, and comorbid diseases. The associations between these variables and the prevalence of self-reported FA were shown in Table 2. Women were more likely to report food allergies than men [OR = 1.573, 95% confidence interval (CI), 1.226–2.019]. Age was significantly associated with the prevalence of FA (P = 0.009, OR = 0.0990). Populations from different regions had also different FA prevalence, FA prevalence in Fengxin (OR = 2.269) and Lean (OR = 1.826) was higher than Xinjian. Comorbid diseases, including allergic dermatitis, allergic rhinitis and atopic asthma were related to the increased prevalence of FA. There was no significant difference between height and the risk of FA after adjusted some factors (p = 0.674).
Table 2.
Related factors of food allergy were analyzed by multivariate logistic regression analysis.
| Variables | Wald χ2 | P-value | OR | 95% confidence interval |
||
|---|---|---|---|---|---|---|
| Low | Upper | |||||
| Gendera | 12.649 | .000 | 1.573 | 1.226 | 2.019 | |
| Age | 6.876 | .009 | 0.990 | 0.982 | 0.997 | |
| Height | .176 | .674 | 1.369 | .316 | 5.935 | |
| Other allergic diseases | Allergic dermatitis | 374.231 | .000 | 24.357 | 17.625 | 33.660 |
| Allergic rhinitis | 98.281 | .000 | 9.601 | 6.139 | 15.016 | |
| Allergic asthma | 5.617 | .018 | 6.189 | 1.371 | 27.947 | |
| Regions | Xinjian | 1 [reference] | ||||
| Fengxin | 38.657 | .000 | 2.269 | 1.752 | 2.937 | |
| Lean | 19.987 | .000 | 1.826 | 1.402 | 2.378 | |
male as reference.
Discussion
This study showed that the prevalence of self-reported FA was about 4.0% in adults of Jiangxi Province, which is lower than 19.0% reported in the United States and 19.7% in another nationwide survey on self-reported FA in Saudi Arabia.15,16 Irani et al assessed that the self-reported FA prevalence among Lebanese adults in the Middle East was 3.2%,17 which was also lower than the rate of this study. Compared with some other developing countries, the prevalence of self-reported FA from Jiangxi was lower than that reported from Brazil (10.8%) and Turkey (9.5%).18,19 Our study also demonstrated a significant difference on prevalence of self-reported FA in 3 survey centers, which may be attributed by the fact that the distinction of epidemic situation in these regions such as different cultures, daily diet and genetic background.
Our research suggested that the prevalence of FA did not always increase with age, showing the lowest rate in middle age and the highest rate in old age. Our finding was consistent with the report by De Martinis et al who concluded that the functions of the immune and gastrointestinal system in the elderly were significantly weaken, which triggered a higher rate of food allergies in the elderly than the middle-aged.20
The 3 common causative foods detected in this study were shrimp, mollusks, and mango. The finding of the allergenic foods was quite different from those reported in Europe, the United States, and Canada, where the commonly reported food allergens were peanuts, tree nuts, shellfish, fish, and milk.12,15,21, 22, 23 However, our list of common allergic foods is highly similar to those reported from India, Thailand, Taiwan, and other regions of China.24, 25, 26 Previous studies also showed that food allergies are likely to be influenced by ethnicity and geographic position; those were probably related to dietary habit, environment, and genetic difference.27 Furthermore, 63.9% of respondents with self-reported FA exhibited skin symptoms, which was consistent with the results reported by Salah et al from an online survey in 5 countries.28
Our survey demonstrated that there was no obvious association in the prevalence of self-reported FA between rural residents and urban residents (3.8% vs 4.2%, P = 0.277), which is inconsistent with previous studies. Park et al reported that urban residents were more likely to occur FA than rural residents (OR = 1.293), Sakai-Bizmark et al also reported that urban had a higher risk in food-related anaphylaxis.29,30 This inconsistent result was probably because of the development of urbanization in Jiangxi, the continuous improvement of living standards, and the accessibility of food types are basically similar between urban and rural. We did not find relationship between the prevalence of self-reported FA and ethnicity; it was possibly due to a small number of minority participants (6 adults) in this study.
Most previous studies showed that obese adults have a higher risk of food allergies than normal ones. A study in 2019 showed that obesity affected the immune system, which disrupted the intestinal barrier and increased the risk of food allergies.31 Another survey reported that obesity caused an imbalance of intestinal microbiota, which might increase susceptibility to food allergens.32 Conversely, our findings showed a higher prevalence of food allergies in the lean population. Weight in school children could be affected by multiple factors, one of them may be restrictive diet for avoiding food allergens, resulting in impairing growth and nutritional deficiencies as described previously.33
The results of this study also suggested there is direct relation between self-reported FA and other allergic diseases, such as 18.7% of allergic dermatitis, 9.0% of allergic rhinitis, and 1.3% of allergic asthma among participants with self-reported FA. These results are consistent with the findings reported by Wang et al34 Meanwhile, a population-based study of FA in the United States showed 56% participants had not only food allergies but also allergic dermatitis.35 Althumiri et al reported a significant association between food allergies and asthma.16 A recent study has indicated that a partial coexistence of FA and allergic diseases such as dermatitis, rhinitis and asthma may be associated with general genetic risk variations which would influence the related-gene expression.36 This association may be explained by the allergic march proposed by Yang et al., which refers to the presence of AD and FA in the initial stage, gradually increasing risk of allergic rhinitis and asthma later.37
This study was conducted with a large sample size, very high response rate, and face to face collection promoted high compliance with questionnaires. However, there were also several limitations. We determined the prevalence of FA by the self-reported FA and did not use objective indicator to confirm FA; and did not differentiate IgE mediated food allergy and non-IgE mediated symptoms. In addition, the questionnaire survey was possibly influenced by the subjective factors of participants, such as recall bias. In view of the above reasons, the actual prevalence of FA in adults needs be further examined with the use of allergen test, such as SPT and sIgE, even OFC if indicated.
Conclusion
The prevalence of self-reported food allergy was about 4.0% among adults in Jiangxi Province, China. Shrimp, mollusks, and mango were the most common food allergens. Among adults with FA, 63.9% experienced adverse skin reactions and 19.3% experienced oral symptoms. Gender, age, regions, and comorbid diseases were associated with the occurrence of FA. More future studies are underway by following up the participants who reported FA to confirm whether they are truly allergic to food or foods by clinical diagnostic tests such as SPT, sIgE, and OFC, and explore the influence factors of FA in the further study.
Abbreviations
ARF, Adverse reactions to food; FA, Food allergy; SPT, Skin prick test; sIgE, Specific immunoglobulin E; OFC, Oral food challenge; SD, Standard Deviation; OR, Odds ratio.
Acknowledgements
The authors thank the staff at Center for Disease Control and Prevention in Fengxin, Jiangxi province; Center for Disease Control and Prevention in Xinjian, Jiangxi province; and Center for Disease Control and Prevention in Le'an, Jiangxi province, China, for their support throughout this investigation.
Funding
Supported by CAMS Innovation Fund for Medical Science (CIFMS 2019-I2M-5-024); TCM Science and Technology Project of Jiangxi Province in 2021 (2021B696); Health Commission of Jiangxi Province, Science and Technology Program in 2022 (SKJP220229266).
Availability of data and materials
The data that support the findings of this study are available from the corresponding author (Y.N.W.) upon reasonable request.
Author contributions
H.F., J.D.Z., Y.N.W. and Y.C., were responsible for study conception, design, implementation logistics, and research team recruitment and subsequent supervision. H.F., X.J.X. and N.L., conducted survey administration, data collection, and data management. J.D.Z assisted in data management and conducted data analysis. H.F., A.L.Y., Z.F.Y. and Q.Z. was responsible for initial drafting of the manuscript; all authors provided critical feedback and edits to assist in the writing of the manuscript.
Ethics approval
This study was approved by this study was approved by the Ethical Committees of China Center for Food Safety Risk Assessment (2019027).
Author consent for publication
All authors have reviewed and approve the manuscript for publication; they certify that data was collected under appropriate ethical guidelines and regulatory approval and that the work on this manuscript is original. The authors have no conflicts of interest to disclose. There were no sources of financial assistance for this study. This study has not been previously published.
Declaration of competing interest
The authors have no conflicts of interest to disclose.
Footnotes
Full list of author information is available at the end of the article
Contributor Information
Yan Chen, Email: chenyan@cfsa.net.cn.
Lianglu Wang, Email: wanglianglu@sina.com.
Yongning Wu, Email: wuyongning@cfsa.net.cn.
References
- 1.Warren C.M., Jiang J., Gupta R.S. Epidemiology and burden of food allergy. Curr Allergy Asthma Rep. 2020;20(2):6. doi: 10.1007/s11882-020-0898-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leung A.S.Y., Wong G.W.K., Tang M.L.K. Food allergy in the developing world. J Allergy Clin Immunol. 2018;141(1):76–78.e71. doi: 10.1016/j.jaci.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 3.Valenta R., Hochwallner H., Linhart B., Pahr S. Food allergies: the basics. Gastroenterology. 2015;148(6):1120–1131.e1124. doi: 10.1053/j.gastro.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muraro A., Roberts G., Worm M., et al. Anaphylaxis: guidelines from the European academy of allergy and clinical immunology. Allergy. 2014;69(8):1026–1045. doi: 10.1111/all.12437. [DOI] [PubMed] [Google Scholar]
- 5.Macchia D., Melioli G., Pravettoni V., et al. Guidelines for the use and interpretation of diagnostic methods in adult food allergy. Clin Mol Allergy. 2015;13:27. doi: 10.1186/s12948-015-0033-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verrill L., Bruns R., Luccioli S. Prevalence of self-reported food allergy in U.S. adults: 2001, 2006, and 2010. Allergy Asthma Proc. 2015;36(6):458–467. doi: 10.2500/aap.2015.36.3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lyons S.A., Burney P.G.J., Ballmer-Weber B.K., et al. Food allergy in adults: substantial variation in prevalence and causative foods across Europe. J Allergy Clin Immunol Pract. 2019;7(6):1920–1928.e1911. doi: 10.1016/j.jaip.2019.02.044. [DOI] [PubMed] [Google Scholar]
- 8.Savage J., Johns C.B. Food allergy: epidemiology and natural history. Immunol Allergy Clin. 2015;35(1):45–59. doi: 10.1016/j.iac.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sicherer S.H., Warren C.M., Dant C., Gupta R.S., Nadeau K.C. Food allergy from infancy through adulthood. J Allergy Clin Immunol Pract. 2020;8(6):1854–1864. doi: 10.1016/j.jaip.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nachshon L., Schwartz N., Elizur A., et al. The prevalence of food allergy in young Israeli adults. J Allergy Clin Immunol Pract. 2019;7(8):2782–2789.e2784. doi: 10.1016/j.jaip.2019.05.046. [DOI] [PubMed] [Google Scholar]
- 11.Iweala O.I., Choudhary S.K., Commins S.P. Food allergy. Curr Gastroenterol Rep. 2018;20(5):17. doi: 10.1007/s11894-018-0624-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGowan E.C., Keet C.A. Prevalence of self-reported food allergy in the national health and nutrition examination survey (NHANES) 2007-2010. J Allergy Clin Immunol. 2013;132(5):1216–1219.e1215. doi: 10.1016/j.jaci.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J., Ogorodova L.M., Mahesh P.A., et al. Comparative study of food allergies in children from China, India, and Russia: the EuroPrevall-INCO surveys. J Allergy Clin Immunol Pract. 2020;8(4):1349–1358.e1316. doi: 10.1016/j.jaip.2019.11.042. [DOI] [PubMed] [Google Scholar]
- 14.Wong G.W., Mahesh P.A., Ogorodova L., et al. The EuroPrevall-INCO surveys on the prevalence of food allergies in children from China, India and Russia: the study methodology. Allergy. 2010;65(3):385–390. doi: 10.1111/j.1398-9995.2009.02214.x. [DOI] [PubMed] [Google Scholar]
- 15.Gupta R.S., Warren C.M., Smith B.M., et al. Prevalence and severity of food allergies among US adults. JAMA Netw Open. 2019;2(1) doi: 10.1001/jamanetworkopen.2018.5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Althumiri N.A., Basyouni M.H., AlMousa N., AlJuwaysim M.F., BinDhim N.F., Alqahtani S. A prevalence of self-reported food allergies and their association with other health conditions among adults in Saudi arabia. Int J Environ Res Publ Health. 2021;18(1) doi: 10.3390/ijerph18010347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Irani C., Maalouly G. Prevalence of self-reported food allergy in Lebanon: a middle-eastern taste. Int Sch Res Notices. 2015;2015 doi: 10.1155/2015/639796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gelincik A., Büyüköztürk S., Gül H., et al. Confirmed prevalence of food allergy and non-allergic food hypersensitivity in a Mediterranean population. Clin Exp Allergy. 2008;38(8):1333–1341. doi: 10.1111/j.1365-2222.2008.03019.x. [DOI] [PubMed] [Google Scholar]
- 19.Silva L.A., Silva A.F., Ribeiro  C., Silva A.O., Vieira F.A., Segundo G.R. Adult food allergy prevalence: reducing questionnaire bias. Int Arch Allergy Immunol. 2016;171(3-4):261–264. doi: 10.1159/000453036. [DOI] [PubMed] [Google Scholar]
- 20.De Martinis M., Sirufo M.M., Viscido A., Ginaldi L. Food allergies and ageing. Int J Mol Sci. 2019;20(22) doi: 10.3390/ijms20225580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shek L.P., Cabrera-Morales E.A., Soh S.E., et al. A population-based questionnaire survey on the prevalence of peanut, tree nut, and shellfish allergy in 2 Asian populations. J Allergy Clin Immunol. 2010;126(2):324–331. doi: 10.1016/j.jaci.2010.06.003. 331.e321-327. [DOI] [PubMed] [Google Scholar]
- 22.Burney P.G., Potts J., Kummeling I., et al. The prevalence and distribution of food sensitization in European adults. Allergy. 2014;69(3):365–371. doi: 10.1111/all.12341. [DOI] [PubMed] [Google Scholar]
- 23.Ben-Shoshan M., Harrington D.W., Soller L., et al. A population-based study on peanut, tree nut, fish, shellfish, and sesame allergy prevalence in Canada. J Allergy Clin Immunol. 2010;125(6):1327–1335. doi: 10.1016/j.jaci.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 24.Lertnawapan R., Maek-a-nantawat W. Anaphylaxis and biphasic phase in Thailand: 4-year observation. Allergol Int. 2011;60(3):283–289. doi: 10.2332/allergolint.10-OA-0256. [DOI] [PubMed] [Google Scholar]
- 25.Mahesh P.A., Wong G.W., Ogorodova L., et al. Prevalence of food sensitization and probable food allergy among adults in India: the EuroPrevall INCO study. Allergy. 2016;71(7):1010–1019. doi: 10.1111/all.12868. [DOI] [PubMed] [Google Scholar]
- 26.Wu T.C., Tsai T.C., Huang C.F., et al. Prevalence of food allergy in Taiwan: a questionnaire-based survey. Intern Med J. 2012;42(12):1310–1315. doi: 10.1111/j.1445-5994.2012.02820.x. [DOI] [PubMed] [Google Scholar]
- 27.Hua Feng Nan L., Chen Fang, Li Xinyu. Etc.: self-reported food allergy prevalence among elementary school children — Nanchang city, Jiangxi province, China, 2021. China CDC Weekly. 2022;4:761–765. doi: 10.46234/ccdcw2022.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salah S., Taieb C., Demessant A.L., Haftek M. Prevalence of skin reactions and self-reported allergies in 5 countries with their social impact measured through quality of life impairment. Int J Environ Res Publ Health. 2021;18(9) doi: 10.3390/ijerph18094501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park H.J., Kim E.J., Yoon D., et al. Prevalence of self-reported allergic diseases and IgE levels: a 2010 KNHANES analysis. Allergy Asthma Immunol Res. 2017;9(4):329–339. doi: 10.4168/aair.2017.9.4.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sakai-Bizmark R., Friedlander S.M.I., Oshima K., et al. Urban/rural residence effect on emergency department visits arising from food-induced anaphylaxis. Allergol Int. 2019;68(3):316–320. doi: 10.1016/j.alit.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 31.Guo X., Cheng L., Yang S., Che H. Pro-inflammatory immunological effects of adipose tissue and risk of food allergy in obesity: focus on immunological mechanisms. Allergol Immunopathol (Madr) 2020;48(3):306–312. doi: 10.1016/j.aller.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 32.Singh M., Benencia F. Inflammatory processes in obesity: focus on endothelial dysfunction and the role of adipokines as inflammatory mediators. Int Rev Immunol. 2019;38(4):157–171. doi: 10.1080/08830185.2019.1638921. [DOI] [PubMed] [Google Scholar]
- 33.Meyer R. Nutritional disorders resulting from food allergy in children. Pediatr Allergy Immunol. 2018;29:689–704. doi: 10.1111/pai.12960. [DOI] [PubMed] [Google Scholar]
- 34.Wang X.Y., Zhuang Y., Ma T.T., Zhang B., Wang X.Y. Prevalence of self-reported food allergy in six regions of Inner Mongolia, Northern China: a population-based survey. Med Sci Mon Int Med J Exp Clin Res. 2018;24:1902–1911. doi: 10.12659/msm.908365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Willits E.K., Park M.A., Hartz M.F., Schleck C.D., Weaver A.L., Joshi A.Y. Food allergy: a comprehensive population-based cohort study. Mayo Clin Proc. 2018;93(10):1423–1430. doi: 10.1016/j.mayocp.2018.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sugita K., Akdis C.A. Recent developments and advances in atopic dermatitis and food allergy. Allergol Int. 2020;69(2):204–214. doi: 10.1016/j.alit.2019.08.013. [DOI] [PubMed] [Google Scholar]
- 37.Yang L., Fu J., Zhou Y. Research progress in atopic March. Front Immunol. 2020;11:1907. doi: 10.3389/fimmu.2020.01907. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author (Y.N.W.) upon reasonable request.