Abstract
The present study evaluated the impact of rac-GR24 on biomass and astaxanthin production under phenol stress coupled with biodiesel recovery from Haematococcus pluvialis. Phenol supplementation showed negative impact on growth, where the lowest biomass productivity of 0.027 g L-1 day−1 was recorded at 10 µM phenol, while 0.4 µM rac-GR24 supplementation showed the highest recorded biomass productivity of 0.063 g L-1 day−1. Coupling 0.4 µM rac-GR24 at different phenol concentrations confirmed the potential of rac-GR24 to mitigate the toxic effect of phenol by enhancing yield of PSII yield, RuBISCo activity, and antioxidant efficiency, which resulted in improved phenol phycoremediation efficiency. In addition, results suggested a synergistic action by rac-GR24 supplementation under phenol treatment where rac-GR24 enhanced lipid accumulation, while phenol enhanced astaxanthin production. Dual supplementation of rac-GR24 and phenol showed the highest recorded FAMEs content, which was 32.6% higher than the control, with improved biodiesel quality. The suggested approach could enhance the economic feasibility of triple-purpose application of microalgae in wastewater treatment, astaxanthin recovery, and biodiesel production.
Keywords: Biodiesel, Green energy, Value-added chemicals, Wastewater treatment, Zero-waste
1. Introduction
Phenolic compounds are one of the main classes of secondary metabolites in plants, where phenol is the simplest member of this group composed of hydroxyl group (–OH) attached to benzene ring. It has high solubility in a wide range of aqueous and organic solvents, and can be detected at elevated concentrations, as high as 4.5 g L-1, in industrial wastewater streams generated from pharmaceutical, oil refinery, coke, and floral foam industry (Ebaid et al., 2022, Estrada-Arriaga et al., 2016). Phenol can also reach the ecosystem due to natural processes such as forest fires or natural degradation of organic wastes (ATSDR, 2023). It is readily absorbed through multiple routes of exposure including ingestion, dermal, and inhalational, then distributes widely through the body within minutes. Untreated dermal exposure to phenol may result in necrotic soft tissue damage or partial/full-thickness chemical burns (Ahmed and Kazmi, 2000). In addition, systemic complications of severe phenol toxicity are myriad, including acute kidney injury, acute hepatotoxicity, and methemoglobinemia (Downs and Wills, 2022). Thus, phenol removal from wastewater has received increasing attention in recent years for proper wastewater treatment process.
Microalgae have several characteristics that make them well-suited for efficient phenol removal and wastewater treatment. They are highly efficient photosynthetic microbes with the potential to remove a wide range of pollutants from water through biodegradation and/or adsorption (Abomohra and Hanelt, 2022). They are able to grow on non-arable land using seawater or waste streams such as agricultural runoff and municipal wastewater, making them a potentially sustainable and environmentally friendly candidate for phenol removal (Abomohra et al., 2016b, Faisal et al., 2022). Despite these advantages, there are several challenges for efficient application of microalgae for phenol removal. The main challenge is contamination of biomass with phenol which prevents their direct use for human or animal feed without prior costly treatment. Thus, using microalgal biomass as biofuel feedstock seems one of the promising routes. In that context, biofuels have several potential advantages over fossil fuels, including reduction of greenhouse gas emissions and the reliance on non-renewable resources (Abomohra et al., 2020a). Although biofuel production from microalgae has been suggested as a promising approach, there are still several challenges, where one of the main challenges is the high production cost of the final product due to high investment in microalgae cultivation using synthetic growth medium and the need of large amounts of freshwater. Thus, coupling wastewater treatment with biofuel production could provide a potential route for effective microalgae cultivation (Abomohra and Hanelt, 2022). In case of phenol removal, microalgal growth is inhibited at elevated levels of phenols, which requires further research and development to optimize microalgal growth and applications at relatively high phenol concentrations.
Strigolactones (SLs) represent a class of phytohormones that regulate the plant growth under varied nutrient availability and environmental changes (Shen et al., 2020). They are obtained naturally from root exudates as they stimulate root hair elongation and also facilitate seedling recruitment by activation of arbuscular mycorrhizae (J. Wang et al., 2019). In recent years, SLs have gained much interest to stimulate particular parasitic weeds and control the plant development (Shen et al., 2020). Regarding microalgae, SLs showed the potential to enhance the levels of nitric oxide (NO) and cytosolic Ca2+ in Monoraphidium sp., which further stimulated the cellular growth (Song et al., 2019). Among SLs, synthetic strigolactone GR24 was reported to improve the removal efficiency of chemical oxygen demand (COD) and enhance the resistance of plant cell against varied aquatic environment (Kramna et al., 2019). However, no systematic research evaluated the effect of synthetic GR24 on algal biomass production under phenol stress coupled with astaxanthin recovery from Haematococcus pluvialis and biodiesel production. Therefore, the present study aimed to initially evaluate the individual impact of different rac-GR24 and phenol concentrations on microalgal growth. Afterwards, the impact of optimum rac-GR24 supplementation on algal growth under different phenol concentrations was studied. In addition, the mechanism of possible recovery of photosynthetic activity at different phenol concentrations due to rac-GR24 supplementation was suggested. Moreover, astaxanthin and biodiesel recovery from the biomass grown under the optimized conditions of phenol and rac-GR24 was evaluated.
2. Materials and methods
2.1. Algal growth and photosynthetic activity
Haematococcus pluvialis SAG 192.80 was cultivated axenically in 2-L glass bottles (DURAN, GL45, Germany) containing 1.5 L/each BG11 synthetic growth medium (Stanier et al., 1971) at average initial cell density of 11.52 × 103 cell mL−1. Cultures were incubated at 25 °C under light conditions at light intensity of 100 μmol photons m−2 s−1 and photoperiod of 16:8h light/dark cycle as described in the previous study (Touliabah and Almutairi, 2021) with minor modifications. A sterile filtered air was continuously supplied from the tube bottom and the cellular growth was monitored by cell count using Neubauer hemocytometer at 2 days interval. The algal cellular dry weight (dw) was determined at the start and end of exponential phase (SoE and EoE, respectively) by overnight oven drying of a filtered sample at 100 °C, then biomass productivity was calculated using Eq. (1) (Abomohra et al., 2013);
| (1) |
where dwE and dwS refer to the dry weight (g L-1) at EoE and SoE, respectively, within a time interval .
The growth of H. pluvialis on BG11 supplemented with 1 mL L-1 DMSO (as a solvent for rac-GR24) was compared with the typical BG11. A stock solution (1 M) of rac-GR24 (CAS 76974–79-3, purity > 98.0%, Solarbio, China) was prepared in DMSO. In order to initially determine the optimum rac-GR24 concentration for growth enhancement, it was supplemented to the sterilized medium at final concentrations of up to 1.0 µM. Based on preliminary experiments and in order to avoid phenol stress during the lag growth phase, phenol was added to the culture at early exponential phase (on the 4th day) at final concentrations up to 10 µM.
Photosynthetic activity was determined by measuring the yield of photosystem II (Fv/Fm) using chlorophyll fluorometer (Phyto-PAM, Heinz Walz GmbH, Germany). In addition, the activity of RuBisCO was measured according to the standard protocol of Gerard and Driscoll (1996). Briefly, 50 mL of H. pluvialis culture was centrifuged for 10 min at 3000 × g. The algal cells were harvested and ground for 5 min at −20 °C, and enzymes were extracted from the pulverized sample by a mixture of magnesium chloride (20 mM), sodium bicarbonate (10 mM), ethylenediaminetetraacetic acid (1 mM), β-mercaptoethanol (10 mM), and 4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid (50 mM). RuBisCO activity in the obtained extract was determined spectrophotometrically at 340 nm (UV − vis spectrophotometer, Shimadzu, Japan).
2.2. Phenol and astaxanthin assay
At 2 days intervals, algal cells were separated from 5 mL of algal culture by centrifugation at 3000 × g for 10 min, then the supernatant was filtered through 0.45 µm syringe filter to ensure algal cells separation. The concentration of residual phenol in the supernatant was determined according to Emerson (1943). Filtrate (1 mL) was mixed with 1 mL/each of Na2CO3/NaHCO3 buffer (60 mM:40 mM, pH 10.0), Na2CO3 (0.1 M), and 4-amino-antipyrine (0.06%), then 0.24% w/v potassium ferricyanide was added, consecutively. The absorbance of the mixture was measured at 510 nm against the corresponding blank without phenol.
Cellular astaxanthin content was determined in the freeze dried microalgal cells as described by Liyanaarachchi et al. (2020). Briefly, pigment extraction was performed using 5 mL DMSO followed by heat treatment for 10 min in 70 °C shaking water bath. The supernatant was collected by centrifugation of the mixture at 4000 × g for 5 min. The aforementioned extraction step was repeated until a colorless supernatant was obtained, then each collected extract was diluted to a final volume of 25 mL using DMSO. The absorbance of the extracted astaxanthin solution was determined at 530 nm, and a purified astaxanthin (Sigma-Aldrich) was used as a reference standard to calculate astaxanthin concentration (Ac, mg L-1), then cellular astaxanthin content (Adw, mg g−1 dw) was calculated on the dw (g L-1) basis using Eq. (2);
| (2) |
2.3. Oxidative stress
Intracellular reactive oxygen species (ROS) of H. pluvialis were measured using DCFH-DA (Rajneesh et al., 2017). DCFH-DA was added to 2 mL of H. pluvialis culture at a mixture ratio of 100:1 (v/v), followed by dark incubation for 30 min at 25 °C. After incubation, cells were separated by centrifugation at 3000 × g for 10 min, which were further resuspended in saline solution. The fluorescence of the suspended cells was detected on a microplate reader (Bio-Tek Instruments, Winooski, USA) at excitation and emission wavelengths of 488 and 550 nm, respectively. The level of ROS was expressed as fold change in correspondence to 1 g dry biomass at the 4th day as a control. Antioxidant enzymes activity including catalase (CAT), superoxide dismutase (SOD), and peroxidase (POD) were determined using commercial kits (Beyotime, China) following the manufacturer’s manual.
2.4. Lipid extraction and FAMEs analysis
Cells were collected from a certain volume of culture (15–30 mL) by centrifugation at 3000 × g for 10 min. Lipids were extracted from algal cells using 10 mL of chloroform/methanol (2/1, v/v) by 2 h incubation at room temperature on a shaker at 120 rpm (Folch et al., 1957). In order to enhance the separation of water-soluble compounds, 3 mL of 0.9% NaCl were added to the mixture and vigorously vortexed for 30 s, then it was centrifuged for 2 min at 200 × g for phase separation. After solvent evaporation from the lower organic phase, it was dried for 30 min at 80 °C and total lipids were measured gravimetrically. Lipid productivity was further calculated on volume basis using the modified Eq. (1).
Lipids extracted from 10 mL of culture were transesterified to fatty acid methyl esters (FAMEs) according to the modified method of Christie (1993). Before lipid extraction, cells were treated by microwave for 1 min to deactivate lipases (Abomohra et al., 2016a). Transesterification was carried out using 167 µL of 0.5 M sodium methoxide and 333 µL of methanol:toluene (1:1, v/v), followed by 20 min incubation at room temperature. Afterwards, 50 µL of 37% HCl and 500 µL of 1 M NaCl were vigorously mixed with vortex for 30 s. FAMEs were extracted by adding 1.5 mL of hexane with vigorous vortex, followed by centrifugation at 200 × g for 2 min. Hexane phase was separated and hexane was evaporated using a stream of argon, then FAMEs were dissolved in 40 µL hexane and analyzed by GC-FID (Varian 3900 GC) equipped with 50 m × 0.25 mm capillary column. Based on fatty acid profile, the main biodiesel characteristics including unsaturation degree (US), kinematic viscosity (KV), cloud point (CP), cetane number (CN), iodine value (IV), specific gravity (SG), higher heating value (HHV), CFPP and LCSF as previously described (Abomohra et al., 2020b). In addition, the content of fatty acids containing ≥ 4 double bonds (Db ≥ 4) were directly determined from fatty acid profile.
2.5. Statistical analysis
The experiments were carried out in three biological replicates, and data were calculated as the mean ± SD. Statistical analysis was done using SPSS (v.20, IBM), where differences between treatments were analyzed by one-way ANOVA followed by LSD test at P ≤ 0.05 as indicated by the different letters on series or mean values.
3. Results and discussion
3.1. Microalgal growth
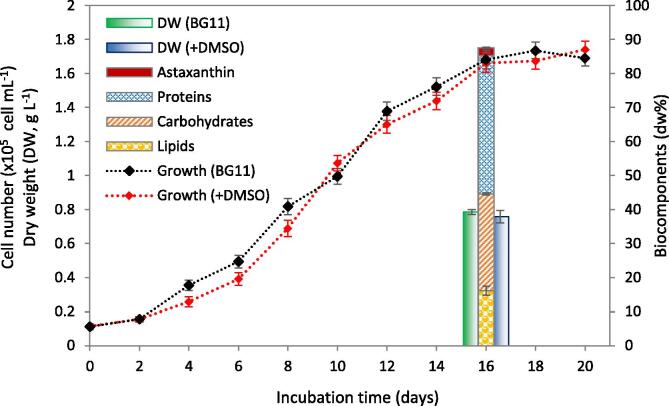
rac-GR24 is one of synthetic SLs that is soluble in ethanol, N, N-dimethylformamide (DMF), methanol, and DMSO. In the present study, DMSO was used because it was reported as a suitable carrier non-toxic solvent for green microalgae. In that context, Abou-Waly (2007) confirmed insignificant differences in the Chl-a content of Scenedesmus quadricauda over 10 days incubation in synthetic medium containing up to 7.29 mL L-1 DMSO. In addition, comparative 96-hour toxicity tests of different organic solvents (acetone, ethanol, methanol, DMF, and DMSO) were applied on microalgae, where DMSO was reported as the best solvent for microalgal growth (Okumura et al., 2001). However, DMSO levels higher than 1.0% (v/v) resulted in significant growth inhibition of Chlamydomonas eugametos, while concentrations above 50% showed complete inhibition (Hess, 1980). Interestingly, Wang et al. (2022) recorded higher biomass yield in H. pluvialis culture under treatment with 1 μM DMSO compared to the control. In the present study, using 1 mL L-1 of DMSO showed insignificant changes in cellular growth and biomass yield of H. pluvialis cultivated in BG11 (Fig. 1). In both studied growth media, the exponential growth phase started after 2 days of growth and ended at the 16th day. At the end of exponential phase (EoE), biomass yield showed insignificant difference between BG11 and that supplemented with DMSO (0.786 and 0.759 g L-1, respectively). Biomass of H. pluvialis showed 23.3 mg g−1 dw astaxanthin content, with a relatively high proportion of protein (40.6 dw%), suggesting the whole biomass of H. pluvialis as a potential feeding feedstock.
Fig. 1.
Growth of Haematococcus pluvialis in a typical BG11 medium (BG11) and under 1 mL L-1 DMSO supplementation (+DMSO), showing the macromolecules content of biomass grown in DMSO-supplemented medium at the end of exponential phase.
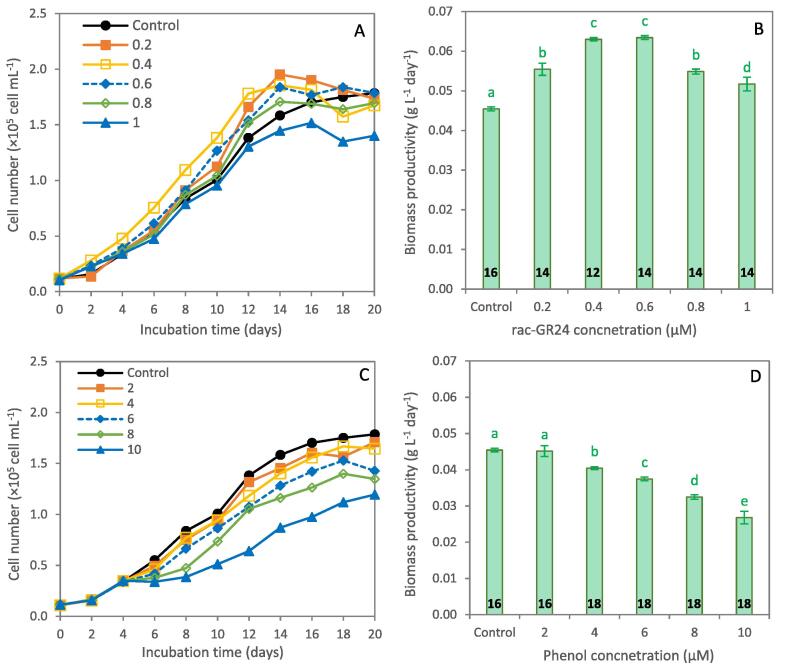
There was a significant impact on growth and biomass productivity of H. pluvialis at different concentrations of rac-GR24 and phenol (Fig. 2). Increasing of rac-GR24 up to 0.8 µM enhanced the microalgal growth comparing to the control, with shorter exponential phase, while further increase resulted in growth retardation (Fig. 2A). The maximum biomass productivity of 0.063 g L-1 day−1 was recorded in the 0.4 µM rac-GR24-supplemented culture, which was insignificant with that of 0.6 µM DMSO and 40% over the control (Fig. 2B). In that context, SLs have been identified as a new class of phytohormones with high potential to regulate plant development under various environmental changes (Wang et al., 2019). In a recent study, the highest cell density of 3.31 × 105 cells mL−1 was recorded in H. pluvialis treated by 1 μM rac-GR24, with the maximum biomass yield of 0.53 g L-1 at end of the macrozooid stage at 12th day and 0.54 g L-1 at the end of the hematocyst stage at 16th day (Wang et al., 2022). In addition, treatment of Chlorella vulgaris with 0.1 μM GR24 showed the maximum biomass productivity of 0.097 g L-1 day−1 (Shen et al., 2020). In plants, treatment with rac-GR24 was reported to interferes with the genes involved in cell division and, therefore, regulating the root elongation of tall fescue (Hu et al., 2018). In addition, treatment with rac-GR24 could alleviate the oxidative stress and increase the photosynthetic efficiency towards improved plant growth (Lu et al., 2019), which was evaluated further in Section 3.2.
Fig. 2.
Growth curves and biomass productivity of Haematococcus pluvialis grown in a typical BG11 medium (Control) and by supplementation with different concentrations of rac-GR24 (A, B) and phenol (C, D). rac-GR24 was added at the start of growth, while phenol was added at early exponential phase (4th day). Numbers on the columns represent the time (days) at which biomass productivity was calculated. Columns with the same letter showed insignificant differences (at P ≤ 0.05).
On the other hand, supplementation of phenol showed negative effect on growth and biomass yield of H. pluvialis, where lowest growth profile was recorded at the highest applied phenol dose (Fig. 2C). However, biomass productivity showed insignificant difference with the control at the lowest applied phenol dose of 2 µM (Fig. 2D). Further increase of phenol showed significant decrease in biomass productivity, showing the minimum recorded value of 0.027 g L-1 day−1 at 10 µM phenol, which was 39.9% lower than the control. It was reported that microalgae can utilize phenol and/or its derivatives heterotrophically, while microalgal phenol biodegradation is photo-dependent (Fawzy et al., 2022). For instance, the chlorophyte Chlorella pyrenoidosa showed the ability to remove 81.56% of phenol under light/dark cycle, compared to only 7% under dark conditions (Das et al., 2015). Similarly, S. obliquus showed 5% reduction in phenol removal efficiency under dark incubation (Papazi and Kotzabasis, 2007), which confirms the higher efficiency of phenol removal under light/dark cultivation. Based on these findings, 0.4 µM rac-GR24 was considered as the optimum value for enhanced microalgal growth and was applied further at different phenol concentrations.
3.2. Mitigation of phenol impact using rac-GR24
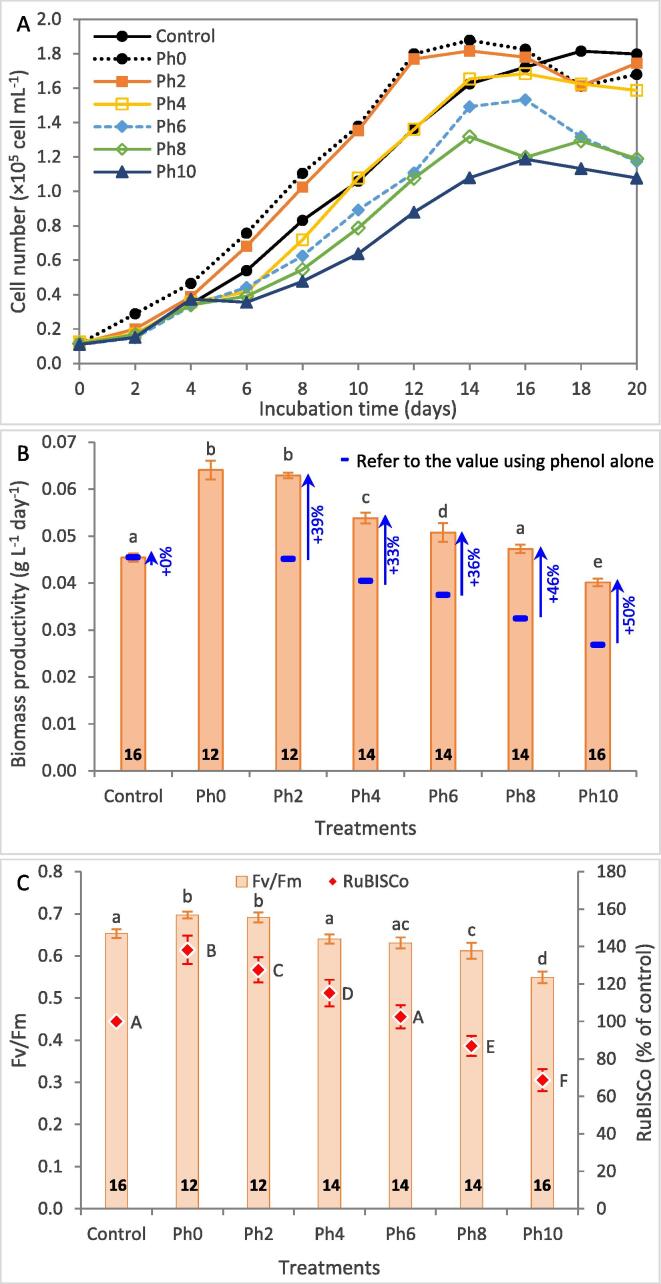
Fig. 3 shows the growth pattern and photosynthetic activity of H. pluvialis grown in BG11 supplemented with 0.4 µM rac-GR24 at different phenol concentrations. Compared to the control, rac-GR24 supplementation enhanced the growth at the lowest phenol concentration (2 µM) (Fig. 3A). It can be noted that biomass productivity enhanced at all phenol treatments under rac-GR24 supplementation compared to solo phenol treatment (Fig. 3B). The enhancement of biomass productivity after rac-GR24 supplementation ranged between 33 and 50%, which confirms its potential to mitigate the toxic effect of phenol. In addition, significant higher biomass productivity compared to the control was recorded at low phenol doses (2–6 μM) due to rac-GR24 supplementation. The highest recorded biomass productivity was 0.063 g L-1 day−1, which was 40% higher than the control and insignificant with that supplemented with rac-GR24 without phenol. Interestingly, at 4 and 6 µM phenol, biomass productivity was also enhanced by 20.0% and 13.3%, respectively over the control.
Fig. 3.
Growth curve (A), biomass productivity (B), and photosynthetic activity (C) of Haematococcus pluvialis grown in a typical BG11 medium (Control) and that supplemented with 0.4 µM rac-GR24 with different concentrations of phenols (0, 2, 4, 6, 8, and 10 µM). rac-GR24 was added at the start of growth, while phenol was added at early exponential phase (4th day). Numbers on the columns represent the time (days) at which the corresponding parameter was measured. Columns of the same series with the same letter showed insignificant differences (at P ≤ 0.05).
The photosynthetic efficiency of H. pluvialis under 0.4 μM rac-GR24 supplementation at different phenol concentrations was examined. As shown in Fig. 3C, photosynthetic activity (Fv/Fm and RuBISCo activity) of H. pluvialis treated with 0.4 μM rac-GR24 (Ph0) was significantly higher than that of the control (6.7% and 38.2%, respectively), which confirms that rac-GR24 at very low doses significantly increase the photosynthesis activity of H. pluvialis at the macrozooid stage, which increases the biomass yield during hematocyst stage. Similarly, supplementation of rac-GR24 showed positive impact on the cellular development of C. vulgaris by enhancing the photosynthetic activity, intracellular activity of carbonic anhydrase, and Chl-a content (Shen et al., 2020). At low phenol doses of 2–6 μM, rac-GR24 was completely able to mitigate the toxic effect of phenol to achieve higher or similar photosynthetic activity as the control (Fig. 3C).
CO2 is a crucial raw material for the dark reaction of microalgal photosynthesis, and thus high efficiency of CO2 utilization is a prerequisite for enhanced microalgal growth and biomass yield. RuBisCO is the key enzyme tangled in carbon fixation pathway and is responsible for CO2 capture to support the microalgal growth (Huang et al., 2017). rac-GR24 supplementation showed the highest RuBisCO activity, which is in consistence with the findings of other studies (Wang et al., 2022). However, addition of phenol significantly reduced RuBisCO activity, reaching the lowest recorded value at 10 μM phenol (31.3% lower than the control). Interestingly, RuBisCO activity at low phenol doses (2 and 4 μM) was still significantly higher than the control (27.6% and 15.2%, respectively). Therefore, it can be concluded that rac-GR24 supplementation could enhance the CO2 utilization efficiency even in phenol-rich wastewater up to 4 μM by enabling more CO2 to enter Calvin − Benson − Bassham (CBB) cycle through enhanced RuBisCO activity, which increases the photosynthetic rate. Therefore, biomass yield increased from 0.779 g L-1 in the control to 0.812 g L-1 under rac-GR24 supplementation with reduction of EoE from 16 to 12 days (Table 1). It can be noted that phenol addition at 2 and 4 μM with rac-GR24 supplementation showed significantly higher biomass yield than the control.
Table 1.
The end of exponential phase (EoE), dry weight (DW), lipid production, and astaxanthin production (Asta) from Haematococcus pluvialis grown in a typical BG11 medium (Control) and that supplemented with 0.4 µM rac-GR24 under different concentrations of phenols (0, 2, 4, 6, 8, and 10 µM). rac-GR24 was added at the start of growth, while phenol was added at early exponential phase (4th day).
| Treatment | EoE (days) | DW (g L-1) | Content (mg g−1 dw) |
Productivity (mg L-1 day−1) |
|||
|---|---|---|---|---|---|---|---|
| Lipids* | Asta* | Lipids | Asta | ||||
| Control | 16 | 0.776 ± 0.011a | 165.8 ± 9.4a | 25.6 ± 3.9a | 7.47 ± 0.34a | 1.16 ± 0.20a | |
| Ph0 | 12 | 0.812 ± 0.009b | 198.9 ± 8.8b | 34.2 ± 4.1b | 12.70 ± 0.71b | 2.20 ± 0.30b | |
| Ph2 | 12 | 0.797 ± 0.002b | 201.5 ± 9.9b | 35.3 ± 1.3b | 12.66 ± 0.63b | 2.23 ± 0.09bc | |
| Ph4 | 14 | 0.803 ± 0.007b | 210.5 ± 10.2b | 42.5 ± 3.1c | 11.44 ± 0.68c | 2.34 ± 0.15bc | |
| Ph6 | 14 | 0.756 ± 0.015a | 209.6 ± 9.7b | 48.6 ± 4.1d | 10.67 ± 0.69 cd | 2.53 ± 0.07de | |
| Ph8 | 14 | 0.713 ± 0.016d | 211.5 ± 8.9b | 52.4 ± 2.3de | 10.13 ± 0.63d | 2.57 ± 0.17e | |
| Ph10 | 16 | 0.687 ± 0.013e | 215.6 ± 10.8b | 54.5 ± 2.2e | 8.68 ± 0.57e | 2.25 ± 0.13b | |
Values in the same columns with the same letter showed insignificant differences (at P ≤ 0.05).
*Lipids and astaxanthin were measured 2 days after the EoE.
3.3. Lipids and astaxanthin production
H. pluvialis grown in typical synthetic medium showed 165.8 and 25 mg g−1 dw of lipids and astaxanthin, respectively (Table 1). rac-GR24 supplementation enhanced lipid and astaxanthin contents by 20.0% and 33.6%, respectively. Phenol treatment at different doses under rac-GR24 supplementation significantly increased lipid content up to 215.6 mg g−1 dw at the highest applied phenol concentration. However, all treatments with phenol showed insignificant differences with that of rac-GR24 alone. This finding suggests that phenol has no significant effect on lipid accumulation. It was stated that rac-GR24 supplementation enhances the carbon precursors to be directed towards lipogenesis during stationary phase (Wang et al., 2021). Therefore, both lipids and astaxanthin showed significant increase under the combination of rac-GR24 and phenol. In that context, astaxanthin was reported to be deposited together with triacylglycerols (TAG) in liposomes of H. pluvialis (Holtin et al., 2009). Thus, astaxanthin accumulation can be correlated to lipid biosynthesis in H. pluvialis, where astaxanthin production at the hematocyst stage is usually accompanied by fatty acid synthesis, which leads to accumulation of lipids (Ota et al., 2018). Consequently, rac-GR24 supplementation (Ph0) enhanced astaxanthin content by 33.6% over the control (Table 1). In addition, phenol supplementation showed gradual significant increase in astaxanthin content up to 54.5 mg g−1 dw in the highest applied phenol dose, which represented 1.12-time higher than the control. These findings suggest that supplementation of rac-GR24 under phenol treatment results in synergistic action where rac-GR24 enhances the lipid accumulation, while phenol enhances astaxanthin production. Considering the effect of both factors on biomass yield and macromolecules accumulation, a highest lipid productivity of 12.66 mg L-1 day−1 was recorded using 2 μM phenol under rac-GR24 supplementation, while the highest astaxanthin productivity of 2.53 mg L-1 day−1 was detected using 6 μM phenol under rac-GR24 supplementation (Table 1). Based on astaxanthin productivity and due to its higher economic value compared to lipids (Li et al., 2011), a combined treatment of 0.4 µM rac-GR24 and 6 µM phenol was further compared with the control, 0.4 µM rac-GR24, and 6 µM phenol.
3.4. Antioxidant potential and phenol removal
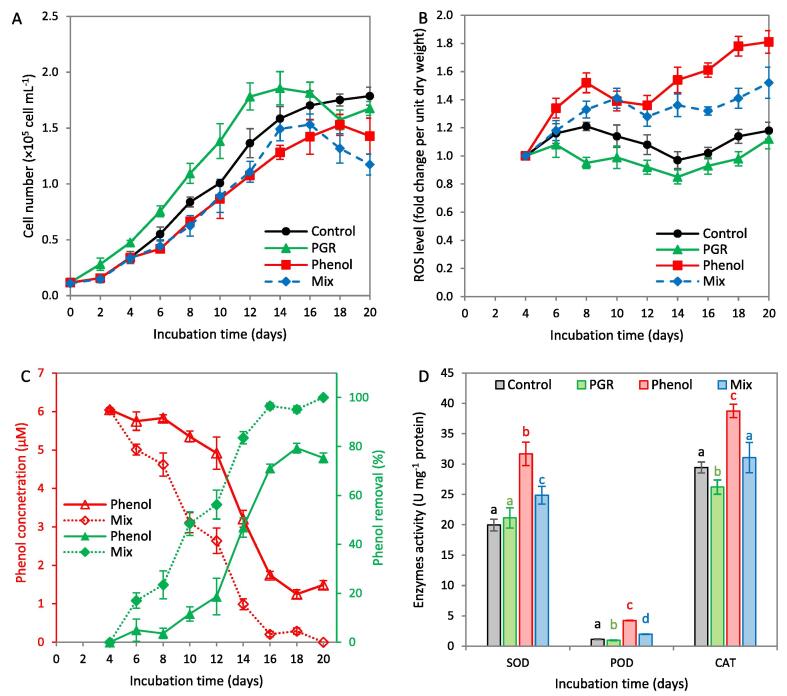
Growth pattern, ROS levels, and phenol removal of the four studied treatments were compared (Fig. 4). The highest growth was recorded with rac-GR24 (1.78 × 105 cell mL−1) on day 12, which represented 30.6% higher than the control at the same time point (Fig. 4A). Phenol treatment showed pronounced reduction in the growth at all points compared to the control, which reached the maximum value of 1.53 × 105 cell mL−1 at day 18, representing 12.6% lower than the control at the same day. Dual supplementation of phenol and rac-GR24 showed the highest growth on day 14 (1.49 × 105 cell mL−1), which was insignificant with the control at the same time point (P = 0.267). It can be noted that phenol supplementation at the 4th day, alone or with rac-GR24, results in growth retardation for a couple of days (Fig. 4A), which might be attributed to the adaptation of the culture to the applied stress.
Fig. 4.
Growth curve (A), reactive oxygen species (ROS) levels (B), phenol removal efficiency (C), and oxidation enzymes activity (D) including superoxide dismutase (SOD), peroxidase (POD) and catalase (CAT) of Haematococcus pluvialis grown in a typical BG11 medium (Control) and that supplemented with 0.4 µM rac-GR24 (PGR), 6 µM phenol (Phenol), or mixture of both PGR and phenol (Mix). rac-GR24 was added at the start of growth, while phenol was added at early exponential phase (4th day).
ROS levels in the culture treated with rac-GR24 showed a reduction trend compared to the control, which reached the lowest level of 0.85-fold change at day 14 (Fig. 4B). However, individual phenol supplementation showed the highest ROS, reaching 1.81-fold change at the 20th day. Supplementation of rac-GR24 to the phenol-treated culture reduced the ROS level by 16.0% below that of phenol at day 20. These results suggest that rac-GR24 might reduce the excessive ROS concentrations in H. pluvialis, leading to significant reduction in the oxidative damage. In addition, it can reduce the oxidative damage resulting from the stress with phenolic compounds, which results in better phenol phycoremediation efficiency (Fig. 4C). In phenol-treated culture, a sharp reduction in phenol concentration was recorded from day 8, reaching a maximum phenol removal efficiency of 79.3% at day 18. However, rac-GR24 supplementation enhanced phenol reduction from the day 4, which was completely removed at day 20. This demonstrates that rac-GR24 does not only alleviate the oxidative response of H. pluvialis under phenol stress, but also enhances phenol phycoremediation due to growth enhancement. Previous reports confirmed that exogenous SLs could alleviate the oxidative stress in plants by upregulating the antioxidant system (Min et al., 2019). Therefore, the activity of key antioxidant enzymes including SOD, CAT, and POD were examined in the present study (Fig. 4D). Compared to the control, SOD, POD, and CAT activities were significantly increased under phenol treatment (58.8%, 2.8-time, and 31.6%, respectively, higher than the control), which indicates the high oxidative stress. rac-GR24 supplementation to phenol-treated culture resulted in reduction of the aforementioned enzymes to levels close to the control, which confirms that it mitigated the oxidative stress resulting from phenol treatment. Combined with the results of astaxanthin (Section 3.3) and shortening the exponential phase duration, it can be concluded that rac-GR24 stimulate astaxanthin ROS-scavenging capacity of H. pluvialis cultures as a defense mechanism against phenol toxicity. These results agree with previous findings where GR24 significantly reduced the lipid peroxidation levels without affecting the total antioxidant capacities, while phenolic contents and radical scavenging activity were diminished (Omoarelojie et al., 2021).
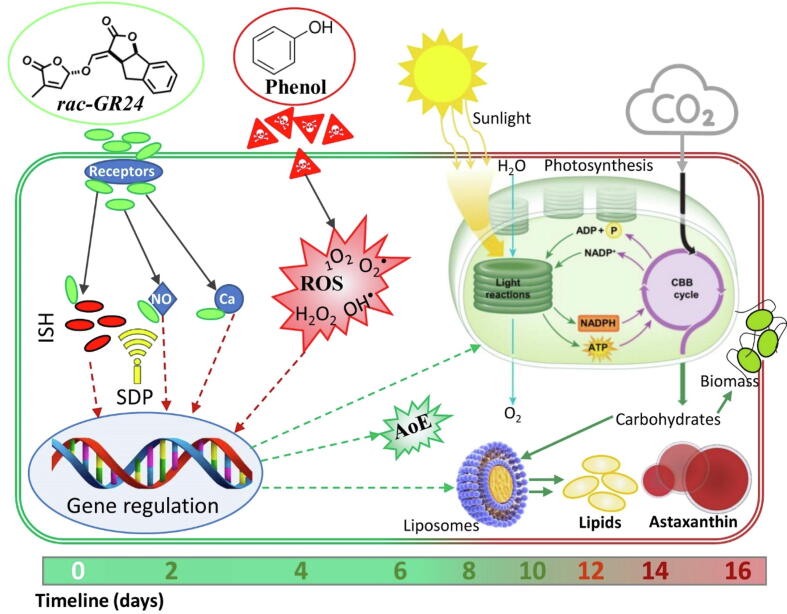
3.5. Suggested mechanism of action
A hypothetical suggested model of how rac-GR24 regulates the growth, lipid accumulation, and astaxanthin synthesis by H. pluvialis under the oxidative stress caused by phenol is summarized in Fig. 5. Plant hormones are specific where each hormone plays a unique role to regulate a variety of cellular processes. Despite the marginal knowledge on the effect of rac-GR24 on algal growth, the present results suggested that it promotes the growth through enhancement of cell division and photosynthesis. It can be confirmed from the significant increase in cell number and photosynthetic activity after rac-GR24 supplementation. In particular, rac-GR24 upregulate the expression level of RuBisCO, which could enhance CO2 sequestration efficiency towards biomass production. Previous studies confirmed that rac-GR24 elicits the genetic push-and-pull response towards cellular astaxanthin overproduction (Wang et al., 2022), which was confirmed in the present study to be used as a defense mechanism towards the oxidative stress due to phenol treatment.
Fig. 5.
The suggested hypothesis model on regulation of growth, astaxanthin synthesis, and lipid accumulation in Haematococcus pluvialis by rac-GR24 supplementation under the oxidative stress caused by phenol. ROS, AoE, ISH, and SDP refer to reactive oxygen species, antioxidant enzymes, intracellular stress hormones, and signal transduction pathway, respectively.
As shown in Fig. 5, plant hormones are accompanying signal transduction pathways, including those of Ca2+, NO, and intracellular stress hormones (ISH) which results in a complex signaling network that control stress tolerance, cellular growth and metabolism (Zhao et al., 2019). It was also reported that phytohormones can promote cellular growth in microalgae by regulating cell division, the expression of carbon fixation-related genes, and stress hormone levels. In addition, phytohormones can increase lipid accumulation in microalgae by manipulating various factors, including oxidative stress, antioxidant enzyme activities, GSH content, and signal transduction, which ultimately lead to the activation of lipid biosynthetic genes (Zhao et al., 2019). Moreover, ROS have been shown to play a role in activating defense responses against abiotic stresses and enhancing metabolite activity in microalgae (Almarashi et al., 2020, Barati et al., 2021). To counteract the potentially harmful effects of ROS, microalgae utilize an array of antioxidants and antioxidases to maintain redox balance (Zhao et al., 2018). Numerous studies have shown that exogenous phytohormones can induce metabolite accumulation in microalgae under stress conditions and enhance their antioxidant defenses, which helps to reduce the ROS levels and improve cellular tolerance. The results of the present study suggest that rac-GR24 acts as an antioxidant that not only directly alleviates the impact of ROS but also activates the antioxidant system of H. pluvialis, leading to production of antioxidases and astaxanthin under phenol treatment, resulting in suppression of oxidative damage. Therefore, addition of rac-GR24 and phenol further enhanced lipid and astaxanthin coaccumulation by stimulating the expression levels of carotenogenesis and lipogenesis-related gene to overcome the oxidative stress of phenol treatment. In addition, rac-GR24 and phenol supplementation upregulated the antioxidant enzymes expression levels, which are involved in the oxidative stress modulation.
3.6. FAMEs profile and characteristics
H. pluvialis grown in BG11 (control) showed 147.5 mg g−1 dw FAMEs content, which represents 90.2% of total lipids (Table 2). However, rac-GR24 or phenol supplementation enhanced FAMEs content by 18.5% and 14.4%, respectively, over the control. Therefore, dual supplementation of rac-GR24 and phenol showed the highest recorded FAMEs content, which was 32.6% higher than the control, which is consistent with the notable upsurge of astaxanthin under these conditions. There were also significant changes in the fatty acid profile due to rac-GR24 and/or phenol supplementation. The dominant fatty acids in the BG11 were C16:0, C18:3, C18:2, and 18:1, representing 32.4%, 23.1%, 17.7%, and 15.1%, respectively, of total FAMEs (Table 2). It can be noted that C18:1 significantly increased from 15.1% in the control to 19.0% after rac-GR24 supplementation. In that context, increasing of C18:1 content was attributed to astaxanthin esterification level, where C18:1 and astaxanthin contents were both improved at the same time after treatment with melatonin and Ca2+ (Cui et al., 2020). Thus, C18:1 was found to contribute to the astaxanthin production in microalgae (Yu et al., 2022). PUFAs represented the highest proportion of 42.5%, while SFAs and MUFAs showed 38.0% and 19.6%, respectively. rac-GR24 supplementation showed significant changes in fatty acid proportions, where MUFAs proportion significantly increased to 27.7%, with simultaneous reduction of PUFAs to 31.8%, mainly due to reduction in mainly C18:2 and C18:3. In this context, supplementation of extracellular abscisic acid enhanced SFAs content of S. quadricauda up to 11.17% over that of nitrogen-deficient culture (Sulochana and Arumugam, 2016), and extracellular ABA addition also improved the culture tolerance to higher salinity and osmotic stress (Yoshida et al., 2004). Phenol treatment showed higher impact on fatty acid profile, where SFAs increased to 62.2%, while MUFAs and PUFAs decreased to 17.6% and 20.3%, respectively (Table 2). It is in consistent with previous results where saturation reactions of fatty acids increased under the oxidative stress (Almarashi et al., 2020). In addition, significant increase of SFAs in favor of PUFAs was recorded due to the oxidative stress at high salinity (Abomohra et al., 2020b, Matos et al., 2015).
Table 2.
Fatty acid methyl esters (FAMEs, mg g−1 dw) profile of lipids from Haematococcus pluvialis grown in a typical BG11 medium (Control) and that supplemented with 0.4 µM rac-GR24 (PGR), 6 µM phenol (Phenol), or mixture of both PGR and phenol (Mix). rac-GR24 was added at the start of growth, while phenol was added at early exponential phase (4th day).
| FAMEs | Control | PGR | Phenol | Mix |
|---|---|---|---|---|
| C12:0 | 0.00a (0.0) | 2.03 ± 0.36b (1.2) | 2.45 ± 0.39b (1.5) | 4.15 ± 0.46c (2.1) |
| C14:0 | 0.98 ± 0.14a (0.7) | 4.70 ± 0.44b (2.7) | 11.56 ± 1.40c (6.9) | 15.88 ± 1.04d (8.1) |
| C14:1 | 0.23 ± 0.06a (0.2) | 0.19 ± 0.03a (0.1) | 1.63 ± 0.19b (1.0) | 0.81 ± 0.06c (0.4) |
| C16:0 | 47.71 ± 5.13a (32.4) | 58.12 ± 6.80ab (33.3) | 65.28 ± 6.04b (38.7) | 79.21 ± 8.89c (40.5) |
| C16:1 | 3.88 ± 0.35a (2.6) | 10.52 ± 0.83b (6.0) | 2.09 ± 0.17c (1.2) | 4.23 ± 0.48a (2.2) |
| C16:2 | 1.81 ± 0.19a (1.2) | 4.60 ± 0.39b (2.6) | 1.62 ± 0.17a (1.0) | 3.83 ± 0.37c (2.0) |
| C18:0 | 6.02 ± 0.64a (4.1) | 3.32 ± 0.20b (1.9) | 12.68 ± 1.09c (7.5) | 10.66 ± 0.76d (5.5) |
| C18:1 | 22.33 ± 2.82a (15.1) | 33.13 ± 4.35b (19.0) | 23.82 ± 2.56a (14.1) | 31.41 ± 2.87b (16.1) |
| C18:2 | 26.08 ± 2.40a (17.7) | 21.75 ± 3.30b (12.5) | 14.25 ± 1.20c (8.5) | 10.02 ± 0.69d (5.1) |
| C18:3 | 34.09 ± 3.41a (23.1) | 28.05 ± 3.66b (16.1) | 17.31 ± 1.28c (10.3) | 16.44 ± 1.15c (8.4) |
| C20:0 | 0.71 ± 0.15a (0.5) | 2.20 ± 0.45a (1.3) | 10.88 ± 1.19b (6.5) | 13.83 ± 1.09c (7.1) |
| C20:1 | 2.40 ± 0.35a (1.6) | 4.60 ± 0.95b (2.6) | 2.11 ± 0.25a (1.3) | 1.88 ± 0.29a (1.0) |
| C20:2 | 0.69 ± 0.13a (0.5) | 1.14 ± 0.14b (0.7) | 1.02 ± 0.09b (0.6) | 1.60 ± 0.28c (0.8) |
| C24:0 | 0.56 ± 0.12a (0.4) | 0.39 ± 0.06a (0.2) | 2.01 ± 0.34b (1.2) | 1.68 ± 0.16b (0.9) |
| SFAs | 55.98 ± 6.18Aa (38.0) | 70.76 ± 8.31Aa (40.5) | 104.84 ± 10.46Ab (62.2) | 125.41 ± 12.40Ac (64.1) |
| MUFAs | 28.85 ± 3.58Ba (19.6) | 48.42 ± 6.16Ab (27.7) | 29.64 ± 3.16Ba (17.6) | 38.32 ± 3.70Bc (19.6) |
| PUFAs | 62.66 ± 6.14Aa (42.5) | 55.54 ± 7.48Aa (31.8) | 34.20 ± 2.73Bb (20.3) | 31.87 ± 2.49Bb (16.3) |
| Total FAMEs (mg/g dw) | 147.49 ± 15.90Ca | 174.72 ± 21.96Ba | 168.69 ± 16.36Ca | 195.61 ± 18.60Cb |
Values in brackets refer to the proportion of each individual FAME in relation to total FAMEs (%).
The same capital letter in the same column, and the same small letter in the same raw indicate insignificant differences (at P ≤ 0.05).
High saturation degree is desirable feature for enhanced biodiesel quality. The estimated biodiesel features from the four studied treatment are presented in Table 3 comparing to international standards. Application of rac-GR24, phenol, or both resulted in reduction of the unsaturation degree by 17.6%, 47.2%, and 53.6%, respectively, below the control. Generally, specific gravity, kinematic viscosity, cetane number, and Db ≥ 4 for all studied treatments complied to the recommended ranges of international standards. However, the highest cetane number of 59.01 was recorded by rac-GR24 supplementation together with phenol treatment, which provides advantageous characteristics for better combustion and reduced emissions. Therefore, the suggested approach is not only important from a quantitative aspect, but also provides added value for enhanced biodiesel quality.
Table 3.
The main estimated characteristics of fatty acid methyl esters produced from Haematococcus pluvialis grown in a typical BG11 medium (Control) and that supplemented with 0.4 µM rac-GR24 (PGR), 6 µM phenol (Phenol), or mixture of both PGR and phenol (Mix). rac-GR24 was added at the start of growth, while phenol was added at early exponential phase (4th day).
| Characteristics |
Haematococcus pluvialis |
International standards |
|||||
|---|---|---|---|---|---|---|---|
| Control | PGR | Phenol | Mix | US | Europe | ||
| Unsaturation degree | 1.25 | 1.03 | 0.66 | 0.58 | – | – | |
| Kinematic viscosity (mm2 s−1) | 4.42 | 4.55 | 4.79 | 4.84 | 1.9–6.0 | 3.5–5.0 | |
| Specific gravity | 0.88 | 0.88 | 0.88 | 0.88 | 0.85–0.9 | – | |
| Cloud point (°C) | 3.29 | 6.18 | 11.19 | 12.25 | – | – | |
| Cetane number | 54.54 | 55.98 | 58.48 | 59.01 | Min. 47 | Min. 51 | |
| Iodine value (g I2 per 100 g) | 105.73 | 89.62 | 61.74 | 55.84 | – | Max. 120 | |
| HHV (MJ kg−1) | 40.74 | 40.35 | 39.69 | 39.55 | – | – | |
| Db ≥ 4 (wt %) | 0.00 | 0.00 | 0.00 | 0.00 | ≤1 | – | |
| LCSF | 0.065 | 0.060 | 0.165 | 0.156 | |||
| CFPP | −16.27 | −16.29 | −15.96 | −15.99 | ≤5/≤−20 | ||
| Reference | This study | (ASTM D6751-08, 2008) | (EN 14214, 2008) | ||||
HHV higher heating value; Db ≥ 4 the content of fatty acids with 4 or more double ponds.
4. Conclusions
rac-GR24 showed the potential to mitigate the toxic effect of phenol, where biomass productivity was enhanced by up to 20.0% at low phenol doses. In addition, photosynthetic activity significantly increased during the macrozooid stage by rac-GR24 supplementation, resulting in high biomass accumulation during hematocyst stage. At low phenol doses of 2–6 μM, rac-GR24 was completely able to mitigate the toxic effect of phenol to achieve higher or similar photosynthetic activity as the control by reducing the oxidative stress. Results suggested that rac-GR24 supplementation under phenol treatment results in synergistic action where rac-GR24 enhances lipid accumulation, while phenol enhances astaxanthin production. Future studies are needed to evaluate the economic feasibility of phenol removal and biofuel production under rac-GR24 supplementation in large-scale, which could support the Saudi Green Initiative of Vision 2030.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
Author thank King Abdulaziz University for providing facilities to perform the experiments in the present study.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abomohra, A., Hanelt, D., 2022. Recent Advances in Micro-/Nanoplastic (MNPs) Removal by Microalgae and Possible Integrated Routes of Energy Recovery. Microorg. 2022, Vol. 10, Page 2400 10, 2400. https://doi.org/10.3390/MICROORGANISMS10122400. [DOI] [PMC free article] [PubMed]
- Abomohra, Elsayed, M., Esakkimuthu, S., El-Sheekh, M., Hanelt, D., 2020a. Potential of fat, oil and grease (FOG) for biodiesel production: A critical review on the recent progress and future perspectives. Prog. Energy Combust. Sci. 81, 100868. https://doi.org/10.1016/j.pecs.2020.100868
- Abomohra, Zheng, X., Wang, Q., Huang, J., Ebaid, R., 2020b. Enhancement of biodiesel yield and characteristics through in-situ solvo-thermal co-transesterification of wet microalgae with spent coffee grounds. Bioresour. Technol. 124640 [DOI] [PubMed]
- Abomohra A.-E.-F., Wagner M., El-Sheekh M., Hanelt D. Lipid and total fatty acid productivity in photoautotrophic fresh water microalgae: Screening studies towards biodiesel production. J. Appl. Phycol. 2013;25:931–936. doi: 10.1007/s10811-012-9917-y. [DOI] [Google Scholar]
- Abomohra A.-E.-F., Jin W., El-Sheekh M. Enhancement of lipid extraction for improved biodiesel recovery from the biodiesel promising microalga Scenedesmus obliquus. Energy Convers. Manag. 2016;108:23–29. [Google Scholar]
- Abomohra A.-E.-F., Jin W., Tu R., Han S.-F., Eid M., Eladel H. Microalgal biomass production as a sustainable feedstock for biodiesel: current status and perspectives. Renew. Sustain. Energy Rev. 2016;64:596–606. [Google Scholar]
- Abou-Waly, H.F., 2007. Effect of organic solvents on growth of freshwater algae. http://dx.doi.org/10.1080/00207230008711285 57, 411–418. https://doi.org/10.1080/00207230008711285.
- Ahmed I., Kazmi S.A.H. Phenol toxicity. J. Coll. Physicians Surg. Pakistan. 2000;10:344–345. [Google Scholar]
- Almarashi J.Q.M., El-Zohary S.E., Ellabban M.A., Abomohra A.E.F. Enhancement of lipid production and energy recovery from the green microalga Chlorella vulgaris by inoculum pretreatment with low-dose cold atmospheric pressure plasma (CAPP) Energy Convers. Manag. 2020;204 doi: 10.1016/j.enconman.2019.112314. [DOI] [Google Scholar]
- ATSDR, 2023. PHENOL: POTENTIAL FOR HUMAN EXPOSURE
- Barati B., Zeng K., Baeyens J., Wang S., Addy M., Gan S.Y., El-Fatah Abomohra A. Recent progress in genetically modified microalgae for enhanced carbon dioxide sequestration. Biomass and Bioenergy. 2021;145 doi: 10.1016/J.BIOMBIOE.2020.105927. [DOI] [Google Scholar]
- Christie W. In: Advances in Lipid Methodology – Two. Adlof R., editor. Oily Press; Dundee: 1993. Preparation of ester derivatives of fatty acids for chromatographic analysis; pp. 69–111. [Google Scholar]
- Cui, J., Yu, C., Zhong, D. bo, Zhao, Y., Yu, X., 2020. Melatonin and calcium act synergistically to enhance the coproduction of astaxanthin and lipids in Haematococcus pluvialis under nitrogen deficiency and high light conditions. Bioresour. Technol. 305, 123069. https://doi.org/10.1016/J.BIORTECH.2020.123069 [DOI] [PubMed]
- Das B., Mandal T.K., Patra S. A comprehensive study on Chlorella pyrenoidosa for phenol degradation and its potential applicability as biodiesel feedstock and animal feed. Appl. Biochem. Biotechnol. 2015;176:1382–1401. doi: 10.1007/S12010-015-1652-9/FIGURES/6. [DOI] [PubMed] [Google Scholar]
- Downs, J., Wills, B., 2022. Phenol Toxicity [WWW Document]. Treasure Isl. StatPearls Publ. URL https://www.ncbi.nlm.nih.gov/books/NBK542311/ (accessed 2.9.23)
- Ebaid R., Wang Q., Faisal S., Li L., Abomohra A. Valorization of floral foam waste via pyrolysis optimization for enhanced phenols recovery. Chemosphere. 2022;136758 doi: 10.1016/J.CHEMOSPHERE.2022.136758. [DOI] [PubMed] [Google Scholar]
- Emerson E. The condensation of aminoantipyrine. II. A new color test for phenolic compounds. J. Org. Chem. 1943;8:417–428. doi: 10.1021/jo01193a004. [DOI] [Google Scholar]
- Estrada-Arriaga E.B., Zepeda-Aviles J.A., García-Sánchez L. Post-treatment of real oil refinery effluent with high concentrations of phenols using photo-ferrioxalate and Fenton’s reactions with membrane process step. Chem. Eng. J. 2016;285:508–516. doi: 10.1016/j.cej.2015.10.030. [DOI] [Google Scholar]
- Faisal, S., Zaky, A., Wang, Q., Huang, J., Abomohra, A., 2022. Integrated Marine Biogas: A Promising Approach towards Sustainability. Ferment. 2022, Vol. 8, Page 520 8, 520. https://doi.org/10.3390/FERMENTATION8100520.
- Fawzy M.A., El-Naeb E.H., Hifney A.F., Adam M.S., Gomaa M. Growth behavior, phenol removal and lipid productivity of microalgae in mixotrophic and heterotrophic conditions under synergistic effect of phenol and bicarbonate for biodiesel production. J. Appl. Phycol. 2022;34:2981–2994. doi: 10.1007/S10811-022-02845-5/TABLES/2. [DOI] [Google Scholar]
- Folch J., Lees M., Stanley G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957;226:495–509. [PubMed] [Google Scholar]
- Gerard V.A., Driscoll T. A spectrophotometric assay for rubisco activity: application to the Kelp Laminaria Saccharina and implications for radiometric assays1. J. Phycol. 1996;32:880–884. doi: 10.1111/j.0022-3646.1996.00880.x. [DOI] [Google Scholar]
- Hess F.D. A chlamydomonas algal bioassay for detecting growth inhibitor herbicides. Weed Sci. 1980;28:515–520. doi: 10.1017/S0043174500061130. [DOI] [Google Scholar]
- Holtin K., Kuehnle M., Rehbein J., Schuler P., Nicholson G., Albert K. Determination of astaxanthin and astaxanthin esters in the microalgae Haematococcus pluvialis by LC-(APCI)MS and characterization of predominant carotenoid isomers by NMR spectroscopy. Anal. Bioanal. Chem. 2009;395:1613–1622. doi: 10.1007/S00216-009-2837-2. [DOI] [PubMed] [Google Scholar]
- Hu Q., Zhang S., Huang B. Strigolactones and interaction with auxin regulating root elongation in tall fescue under different temperature regimes. Plant Sci. 2018;271:34–39. doi: 10.1016/J.PLANTSCI.2018.03.008. [DOI] [PubMed] [Google Scholar]
- Huang Y., Cheng J., Lu H., He Y., Zhou J., Cen K. Transcriptome and key genes expression related to carbon fixation pathways in Chlorella PY-ZU1 cells and their growth under high concentrations of CO 2. Biotechnol. Biofuels. 2017;10:1–10. doi: 10.1186/s13068-017-0868-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramna B., Prerostova S., Vankova R. Strigolactones in an experimental context. Plant Growth Regul. 2019 doi: 10.1007/s10725-019-00502-5. [DOI] [Google Scholar]
- Li J., Zhu D., Niu J., Shen S., Wang G. An economic assessment of astaxanthin production by large scale cultivation of Haematococcus pluvialis. Biotechnol. Adv. 2011;29:568–574. doi: 10.1016/J.BIOTECHADV.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Liyanaarachchi V.C., Nishshanka G.K.S.H., Premaratne R.G.M.M., Ariyadasa T.U., Nimarshana P.H.V., Malik A. Astaxanthin accumulation in the green microalga Haematococcus pluvialis: Effect of initial phosphate concentration and stepwise/continuous light stress. Biotechnol. Reports. 2020;28 doi: 10.1016/j.btre.2020.e00538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T., Yu H., Li Q., Chai L., Jiang W. Improving plant growth and alleviating photosynthetic inhibition and oxidative stress from low-light stress with exogenous GR24 in tomato (Solanum lycopersicum l.) seedlings. Front. Plant Sci. 2019;10:490. doi: 10.3389/FPLS.2019.00490/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matos, Â.P., Feller, R., Moecke, E.H.S., Sant’Anna, E.S., 2015. Biomass, lipid productivities and fatty acids composition of marine Nannochloropsis gaditana cultured in desalination concentrate. Bioresour. Technol. 197, 48–55 [DOI] [PubMed]
- Min Z., Li R., Chen L., Zhang Y., Li Z., Liu M., Ju Y., Fang Y. Alleviation of drought stress in grapevine by foliar-applied strigolactones. Plant Physiol. Biochem. 2019;135:99–110. doi: 10.1016/J.PLAPHY.2018.11.037. [DOI] [PubMed] [Google Scholar]
- Okumura Y., Koyama J., Takaku H., Satoh H. Influence of organic solvents on the growth of marine microalgae. Arch. Environ. Contam. Toxicol. 2001;41:123–128. doi: 10.1007/S002440010229. [DOI] [PubMed] [Google Scholar]
- Omoarelojie L.O., Kulkarni M.G., Finnie J.F., Van Staden J. Strigolactone analog (rac-GR24) enhances chilling tolerance in mung bean seedlings. South African J. Bot. 2021;140:173–181. doi: 10.1016/J.SAJB.2021.03.044. [DOI] [Google Scholar]
- Ota, S., Morita, A., Ohnuki, S., Hirata, A., Sekida, S., Okuda, K., Ohya, Y., Kawano, S., 2018. Carotenoid dynamics and lipid droplet containing astaxanthin in response to light in the green alga Haematococcus pluvialis. Sci. Reports 2018 81 8, 1–10. https://doi.org/10.1038/s41598-018-23854-w. [DOI] [PMC free article] [PubMed]
- Papazi A., Kotzabasis K. Bioenergetic strategy of microalgae for the biodegradation of phenolic compounds—Exogenously supplied energy and carbon sources adjust the level of biodegradation. J. Biotechnol. 2007;129:706–716. doi: 10.1016/J.JBIOTEC.2007.02.021. [DOI] [PubMed] [Google Scholar]
- Rajneesh, ., Pathak, J., Chatterjee, A., Singh, S., Sinha, R., 2017. Detection of Reactive Oxygen Species (ROS) in Cyanobacteria Using the Oxidant-sensing Probe 2’,7’-Dichlorodihydrofluorescein Diacetate (DCFH-DA). BIO-PROTOCOL 7. https://doi.org/10.21769/bioprotoc.2545 [DOI] [PMC free article] [PubMed]
- Shen X., Xue Z., Sun L., Zhao C., Sun S., Liu J., Zhao Y., Liu J. Effect of GR24 concentrations on biogas upgrade and nutrient removal by microalgae-based technology. Bioresour. Technol. 2020;312 doi: 10.1016/J.BIORTECH.2020.123563. [DOI] [PubMed] [Google Scholar]
- Song X., Zhao Y., Li T., Han B., Zhao P., Xu J.W., Yu X. Enhancement of lipid accumulation in Monoraphidium sp. QLY-1 by induction of strigolactone. Bioresour. Technol. 2019;288 doi: 10.1016/J.BIORTECH.2019.121607. [DOI] [PubMed] [Google Scholar]
- Stanier R.Y., Kunisawa R., Mandel M., Cohen-Bazire G. Purification and properties of unicellular blue-green algae (order Chroococcales) Bacteriol. Rev. 1971;35:171. doi: 10.1128/BR.35.2.171-205.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulochana S.B., Arumugam M. Influence of abscisic acid on growth, biomass and lipid yield of Scenedesmus quadricauda under nitrogen starved condition. Bioresour. Technol. 2016;213:198–203. doi: 10.1016/j.biortech.2016.02.078. [DOI] [PubMed] [Google Scholar]
- Touliabah H.E.S., Almutairi A.W. Effect of phytohormones supplementation under nitrogen depletion on biomass and lipid production of nannochloropsis oceanica for integrated application in nutrition and biodiesel. Sustain. 2021;13:1–12. doi: 10.3390/su13020592. [DOI] [Google Scholar]
- Wang, J.Y., Haider, I., Jamil, M., Fiorilli, V., Saito, Y., Mi, J., Baz, L., Kountche, B.A., Jia, K.P., Guo, X., Balakrishna, A., Ntui, V.O., Reinke, B., Volpe, V., Gojobori, T., Blilou, I., Lanfranco, L., Bonfante, P., Al-Babili, S., 2019. The apocarotenoid metabolite zaxinone regulates growth and strigolactone biosynthesis in rice. Nat. Commun. 2019 101 10, 1–9. https://doi.org/10.1038/s41467-019-08461-1. [DOI] [PMC free article] [PubMed]
- Wang X., Mou J.H., Qin Z.H., Hao T.B., Zheng L., Buhagiar J., Liu Y.H., Balamurugan S., He Y., Lin C.S.K., Yang W.D., Li H.Y. Supplementation with rac-GR24 facilitates the accumulation of biomass and Astaxanthin in two successive stages of Haematococcus pluvialis cultivation. J. Agric. Food Chem. 2022;70:4677–4689. doi: 10.1021/ACS.JAFC.2C00479/ASSET/IMAGES/LARGE/JF2C00479_0009.JPEG. [DOI] [PubMed] [Google Scholar]
- Wang J., Zhang J., Yao Z., Yang X., Sun R., Zhao Y. Nonlinear characteristics of a multi-degree-of-freedom spur gear system with bending-torsional coupling vibration. Mech. Syst. Signal Process. 2019;121:810–827. doi: 10.1016/J.YMSSP.2018.12.002. [DOI] [Google Scholar]
- Wang X., Zhang M.M., Liu S.F., Xu R.L., Mou J.H., Qin Z.H., Zhou Z.G., Li H.Y., Lin C.S.K., Sun Z. Synergistic bioconversion of lipids and carotenoids from food waste by Dunaliella salina with fulvic acid via a two-stage cultivation strategy. Energy Convers. Manag. 2021;234 doi: 10.1016/J.ENCONMAN.2021.113908. [DOI] [Google Scholar]
- Yoshida K., Igarashi E., Wakatsuki E., Miyamoto K., Hirata K. Mitigation of osmotic and salt stresses by abscisic acid through reduction of stress-derived oxidative damage in Chlamydomonas reinhardtii. Plant Sci. 2004;167:1335–1341. doi: 10.1016/J.PLANTSCI.2004.07.002. [DOI] [Google Scholar]
- Yu C., Wang H.P., Yu X. The associative induction of succinic acid and hydrogen sulfide for high-producing biomass, astaxanthin and lipids in Haematococcus pluvialis. Bioresour. Technol. 2022;358 doi: 10.1016/J.BIORTECH.2022.127397. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Li D., Xu J.-W., Zhao P., Li T., Ma H., Yu X. Melatonin enhances lipid production in Monoraphidium sp. QLY-1 under nitrogen deficiency conditions via a multi-level mechanism. Bioresour. Technol. 2018;259:46–53. doi: 10.1016/j.biortech.2018.03.014. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Wang H.P., Han B., Yu X. Coupling of abiotic stresses and phytohormones for the production of lipids and high-value by-products by microalgae: a review. Bioresour. Technol. 2019;274:549–556. doi: 10.1016/J.BIORTECH.2018.12.030. [DOI] [PubMed] [Google Scholar]