Abstract
Gut microbiota includes a vast collection of microorganisms residing within the gastrointestinal tract. It is broadly recognized that the gut and brain are in constant bidirectional communication, of which gut microbiota and its metabolic production are a major component, and form the so-called gut microbiome–brain axis. Disturbances of microbiota homeostasis caused by imbalance in their functional composition and metabolic activities, known as dysbiosis, cause dysregulation of these pathways and trigger changes in the blood–brain barrier permeability, thereby causing pathological malfunctions, including neurological and functional gastrointestinal disorders. In turn, the brain can affect the structure and function of gut microbiota through the autonomic nervous system by regulating gut motility, intestinal transit and secretion, and gut permeability. Here, we examine data from the CAS Content Collection, the largest collection of published scientific information, and analyze the publication landscape of recent research. We review the advances in knowledge related to the human gut microbiome, its complexity and functionality, its communication with the central nervous system, and the effect of the gut microbiome–brain axis on mental and gut health. We discuss correlations between gut microbiota composition and various diseases, specifically gastrointestinal and mental disorders. We also explore gut microbiota metabolites with regard to their impact on the brain and gut function and associated diseases. Finally, we assess clinical applications of gut-microbiota-related substances and metabolites with their development pipelines. We hope this review can serve as a useful resource in understanding the current knowledge on this emerging field in an effort to further solving of the remaining challenges and fulfilling its potential.
Keywords: gut, intestine, microorganism, bacteria, microbiota, brain, DGBI, metabolite, mental, dysbiosis
Introduction
The Earth microbiome represents the majority of the planet’s biodiversity. Microbial life was the first to inhabit Earth.1 Microbes regulate global nutrient cycles, greenhouse gas exchange, as well as disease transmission and protection, thus providing essential life support to the planet.2 Among many other harbors, including plants, animals, soil, and entire ecosystems, a wide diversity of microorganisms colonize the human body, which are now known to play an essential role in the human host by regulating key physiological functions.
The large collection of microorganisms inhabiting the human body are predominantly bacteria, but also viruses, protozoa, fungi, and archaea. They are collectively known as the human microbiota. Those microorganisms residing in the digestive tracts are known as gut flora or gut microbiota. As a matter of fact, there are more bacterial cells in the human body than human cells—roughly 40 trillion bacterial cells versus only 30 trillion human cells. Together, they function as an extra organ in the human body—a so-called “forgotten organ”—since these microbes have a collective metabolic activity equal to a virtual organ.3 The collective genome of the gut microbes, the gut microbiome, exceeds over 100 times the amount of human genome in the body.4 Considering such enormous genetic potential of the microbiota, it is anticipated that it plays a role in virtually all physiological processes in the human body, including metabolic functions and immune homeostasis.5−11
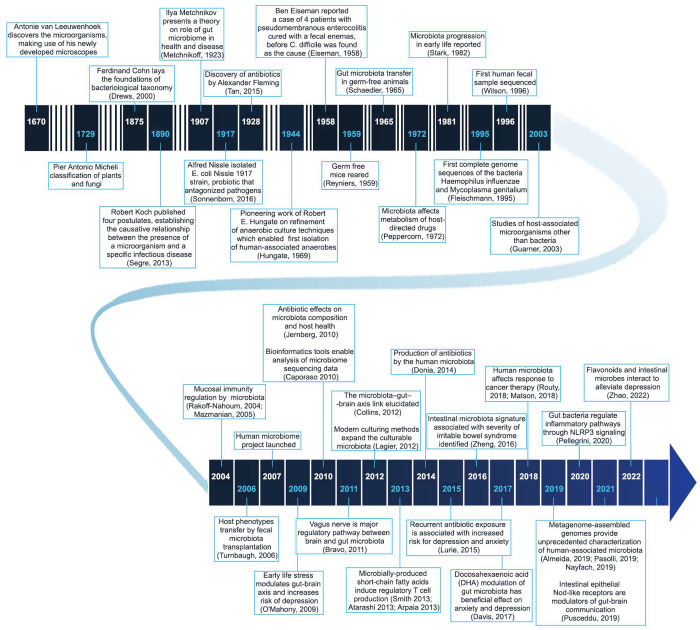
Despite being considered a relatively new field of research, the first reports of human-associated microbiota date back to the 17th century when Antonie van Leeuwenhoek described five different kinds of oral bacteria.12 In the following decades, the foundations of microbiology were laid, and knowledge of the host–microorganism interactions has accumulated (Figure 1).13−22 Despite these early findings, rapid development of the field only started when methods to culture anaerobic organisms were set up in the mid-20th century when representatives of the microbiota were grown and studied in the laboratory.23
Figure 1.
Timeline of major research and development milestones related to the microbiome.12−30,39,49−80
In the 2010s, the gut microbiome field burst into the life sciences research and industry, which prompted Forbes to declare the 2010s “The Decade of the Microbiome.”24 This growth in the field was largely related to the National Institutes of Health’s “The Human Microbiome Project”25 and the MetaHIT project funded by the European Union.26 In 2005, the International Human Microbiome Consortium (IHMC) was founded in a cooperative effort to study the microbiome in human health and disease with the ultimate goal of applying this knowledge to prevent and/or treat diseases, and the abovementioned megaprojects have contributed to fulfilling this goal. They provided significant evidence for the relationship between metabolic, neurological, and autoimmune disorders; allergies, infections, and cancers; and the microorganisms that live on and in humans. Specifically, gastrointestinal diseases/disorders, such as inflammatory bowel diseases that include both Crohn’s disease and ulcerative colitis, irritable bowel syndrome (IBS), functional dyspepsia (FD), constipation, celiac disease, and more, are attracting attention with their close relation to the gut microbiome. Because of these findings and the essential part the gut microbiome plays in drug metabolism, the microbiome has become a popular target in the biotechnology industry. In 2010, the first extensive catalogue of human intestinal microbial genes was published on the basis of the studies of 124 individuals.27 In 2011, the Human Microbiome Project published the sequences of 178 bacterial species.28
Although DNA sequencing has been used for decades, it was only after the development of next-generation sequencing when metagenomic studies became affordable.29 The term metagenomics is used to describe genetic studies of microbial assemblies from environmental samples using sequence-based bioinformatics tools.30 The goal of these studies is to identify the taxonomic diversity of the microbiota and to differentiate the biological roles of the representatives of such samples by performing functional metagenomics.
The human microbiota plays an essential role in human physiology and pathology. It collaborates closely with the digestive tract in several important aspects: (a) it promotes digestion by assisting the absorption of nutrients by gut cells or the fermentation of some food fractions, which generate important metabolites, including short-chain fatty acids;31 (b) it supports the maturation of the digestive tract by participating in the assembly of gastrointestinal mucus and promoting the enzymatic activity of the mucosa;32 (c) it performs a barrier function against pathogens and toxins, where some bacteria release antimicrobial agents that protect from the pathogenic bacteria;33 (d) it plays a protective role in promoting the immune system development; and (e) it supports in the synthesis of essential vitamins like vitamin B: Magnúsdóttir et al. estimated that 86% of the recommended daily allowance (RDA) of vitamin B6, 37% of the RDA of vitamin B9, 31% of the RDA of vitamin B12, and 27% of the RDA of vitamin B3 could be provided by the human gut microbiota.34
Gut microbial disruption (dysbiosis) causes not only gastrointestinal disorders but also disorders in other distal organs and systems. Not long ago, it was found that gut bacteria can affect the central nervous system (CNS) functions.35−38 Indeed, the gut and brain are in constant bidirectional communication, of which the microbiota and its metabolic production are a major component. The gut and brain connect via a neuro-immuno-humoral network of signaling pathways known as the gut microbiome–brain axis, which includes the vagus nerve, the immune system, the hormonal system, and bacterial metabolites and products.39 The digestive system, including the inhabiting microbiota, was even called “the second brain”40 at the time when scientists were beginning to realize that the gut and the brain in humans were involved in constant crosstalk and significantly modulate each other’s function. During disturbance of the microbiota homeostasis caused by an imbalance in their functional composition and metabolic activities, known as dysbiosis, these routes are dysregulated and cause changes in the permeability of the blood–brain barrier (BBB), neuroinflammation, and other pathological malfunctions, including a range of neurodevelopmental and neurodegenerative disorders.41 Disorders of the gut–brain interaction (DGBI) is the recent term proposed by Rome Foundation guidelines for a range of functional gastrointestinal disorders including but not limited to IBS, FD, and functional constipation. This highlights the central role of the miscommunication between the gut and brain in these digestive disorders.42 The gut microorganisms transform and metabolize dietary- and host-derived substances to generate a diverse set of metabolites with important local and systemic outcomes, thereby building a network of immunological, neuronal, and endocrine signaling pathways.
It is generally believed that bacterial colonization begins during birth.43 The neonatal microbiota differs depending on mode of delivery: in vaginally delivered infants, it resembles the maternal vaginal microbiota, while the microbiota in those delivered by cesarean section resembles the maternal skin microbiota.44 Premature birth, feeding method, and perinatal administration of antibiotics are also among the conditions affecting the development of the neonatal microbiome.45 Recently, a new mode of horizontal mother-to-infant microbiome transmission has been revealed where microbes in the maternal gut shared genes with microbes in the infant gut during the perinatal period, which starts shortly before birth and prolongs throughout the first few weeks after birth.46 A major factor of gut microbiota composition during adulthood is diet. Prompt changes in microbiota composition take place in response to dietary style changes. Specific patterns have been reported in plant-based versus animal-based diets.47,48 The development and modifications of the gut microbiota are influenced by multiple other factors, such as exposure to stress, environmental conditions, medications intake, lifecycle, medical disorders, and procedures.
The human gut microbiota is divided into many groups called phyla. The gut microbiota primarily comprises four main phyla, including Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria, with the Firmicutes and Bacteroidetes representing 90% of gut microbiota.81,82 The majority of bacteria reside within the gastrointestinal tract, with most predominantly anaerobic bacteria housed in the large intestine.83
In recent years, sizable technological progress and wealth of knowledge have promoted the advancement of microbiome research, thereby enhancing our understanding of its relationship to human physiology and pathologies. In this paper, we review the advances in the knowledge related to the human gut microbiome, its complexity and functionality, its communication with the central nervous system, and the effect of the gut microbiome–brain axis on mental and digestive health. We examine data from the CAS Content Collection,15 the largest human-curated collection of published scientific information and analyze the publication landscape of recent research in order to provide insights into the scientific advances in the area. We also discuss the correlations between the gut microbiota composition and various diseases, specifically digestive system diseases, mental, and neurodegenerative disorders. We furthermore explore the gut microbiota metabolites with regard to their impact on brain, digestive functions, and their associated diseases. Subsequently, we assess the clinical applications of gut microbiota-related substances and metabolites, their development pipelines, disease categories, development stages, and publication trends. We hope this review can serve as a useful resource in understanding the current state of knowledge in the field of gut microbes and the gut microbiome–brain interactions in an effort to further solve the remaining challenges for fulfilling the potential of the field.
Landscape of Gut Microbiome Research—Insights from the CAS Content Collection
The CAS Content Collection84 is the largest human-curated collection of published scientific knowledge. It is a comprehensive resource to access and remain well-informed on the world’s available scientific literature across disciplines, including chemistry, biomedical sciences, engineering, materials science, agricultural science, and many more, thus allowing quantitative analysis of global scientific publications against variables, such as time, research area, application, disease association, and chemical composition. A search in the CAS Content Collection showed an intense increase of the documents related to microbiome research in the past decade, which overcame other “omics” exploration—for example, the number of proteomics-related documents held up after the initial burst in the early 2000s and were surpassed by the microbiome documents after 2016 (Figure 2, inset). Currently, there are over 250 000 scientific publications (mainly journal articles and patents) in the CAS Content Collection related to gut/intestinal microbiome/microbiota. Nearly 15 000 of them are related to various aspects of mental and gut health. There is a steady, exponential growth of the number of journal articles over time that has been rather explosive from 2021–2022 (Figure 2). The number of patents rapidly grew until 2004, which possibly correlated with the initial accumulation of knowledge and its transfer into patentable applications. Later on, the growth substantially slowed down, perhaps awaiting the forthcoming breakthroughs in the gut microbiome awareness (Figure 2).
Figure 2.
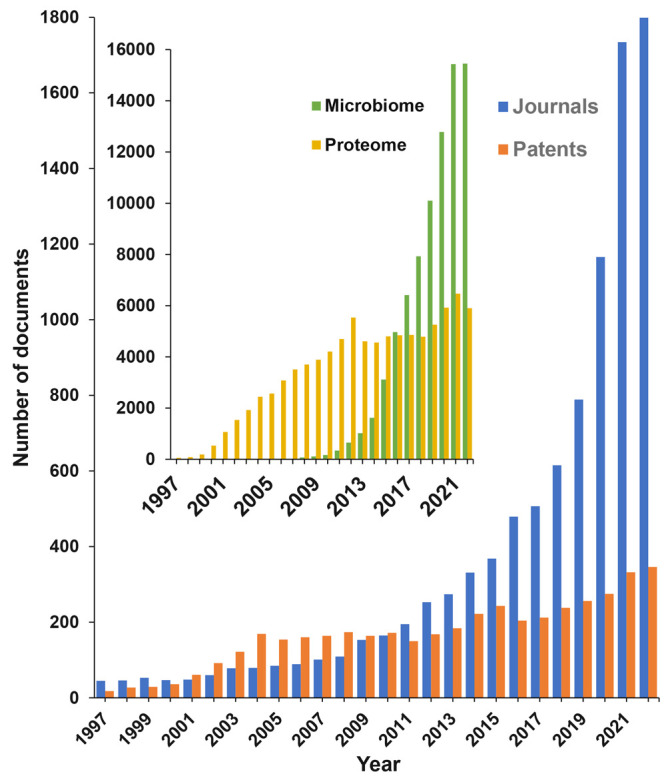
Journal and patent publication trends on gut microbiome research related to mental and gut health according to the CAS Content Collection. Inset: microbiome vs proteome document yearly trends.
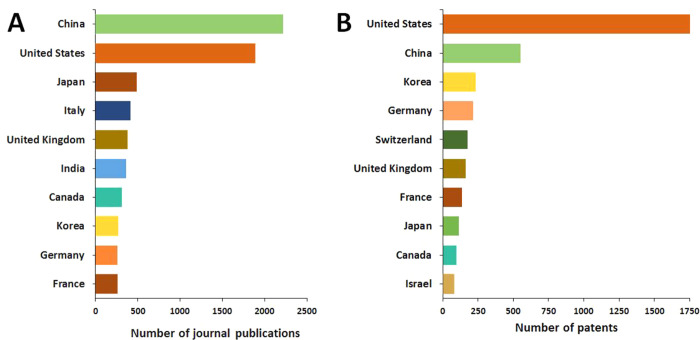
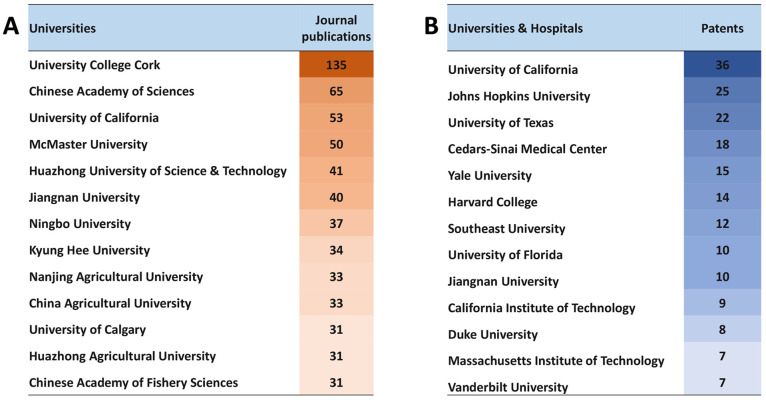
The United States, China, Japan, and Korea are the leaders in the number of published journal articles (Figure 3A) and patents (Figure 3B) related to gut microbiome research in the areas of mental and gut health.
Figure 3.
Top countries publishing journal articles (A) and patents (B) related to gut microbiome research in mental and gut health.
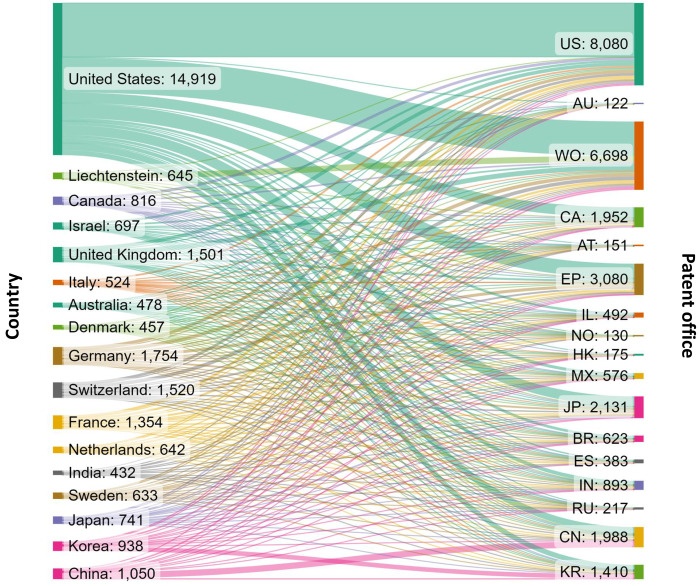
Figure 4 presents the flow of patent filings from different applicant locations to various patent offices. Because patent protection is territorial, and the same invention may be filed for protection in multiple jurisdictions, we looked at all relevant filings on gut microbiome research in mental and gut health. One patent family may have been counted multiple times when it was applied in multiple patent offices. There are diverse patent filing strategies: some patent assignees, such as those from China and Korea, file foremost in their home country patent offices (CN, KR), with a smaller proportion filing through other patent offices or other jurisdictions. Others, for instance United States-based applicants, have a nearly equal number of US and WO filings and a considerable number of filings at other patent offices, such as the European Patent Office (EP) and Canada (CA).
Figure 4.
Flow of patent filings related to gut microbiome research in mental and gut health from different patent assignee locations (left) to various patent offices of filing (right). The abbreviations on the right indicate the patent offices of United States (US), Australia (AU), World Intellectual Property Organization (WO), Canada (CA), Austria (AT), European Patent Office (EP), Israel (IL), Norway (NO), Hong Kong (HK), Mexico (MX), Japan (JP), Brazil (BR), Spain (ES), India (IN), Russian Federation (RU), China (CN), and Korea (KR).
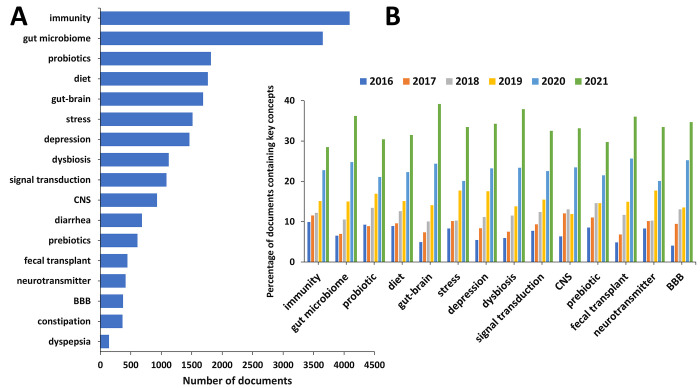
In order to better understand the advance in this research area, we examined the occurrence and trends of certain key concepts in the scientific publications relevant to the gut microbiome research in mental and gut health (Figure 5). With respect to the cumulative number of publications, “immunity” and “gut microbiome” appear as top concepts in the area (Figure 5A), thereby reflecting the rising interest in the relationship between the gut microbiome and systemic immune response pathways and the critical role the gut microbiome plays in training and development of the host’s innate and adaptive immune system. It is noteworthy that the concept concerning the gut–brain relationship exhibits the greatest growth rate in the past two years (Figure 5B), thereby characterizing it as the trendiest concept in the field.
Figure 5.
Key concepts in the scientific publications relevant to the gut microbiome research in mental and gut health. (A) Number of publications exploring key concepts related to gut microbiome research in mental and gut health. (B) Trends in key concepts presented in the articles related to gut microbiome research in mental and gut health during the years 2016–2021. Percentages are calculated with yearly publication numbers for each key concept, normalized by the total number of publications for the same concept in the same time period.
Gut-Microbiota-Participant Bacteria
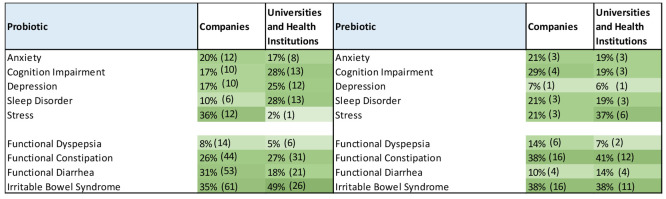
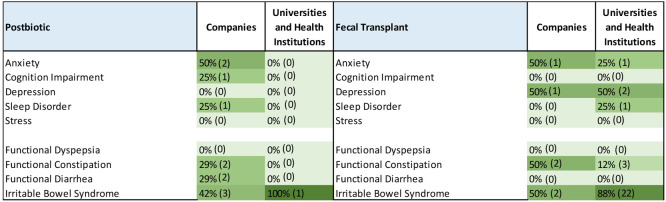
The human gut microbiome, as mentioned above, is a complex mixture of microorganisms, including viruses, archaea, bacteria, yeasts, and fungi, interacting with each other and with their host in complex ways. These interactions at various times involve symbiosis, mutualism, antagonism, and even predation. Not only does the gut microbiome interact directly with the gastrointestinal (GI) tract, but it also interacts with the immune system that is present in the GI tract and with the neurological system through various signaling systems. Gastrointestinal signaling is mediated in part by microbial metabolites and is involved in regulating the gut–brain axis in the host.85 This section focuses on the bacterial microbiome by first discussing the techniques used to identify and study the gut microbiome. Then, we will discuss the human GI tract from an ecological viewpoint. The phyla commonly found in the gut microbiome will be defined and discussed. Finally, we will focus on probiotics and related compositions (prebiotics, postbiotics, synbiotics, and psychobiotics) and present recent examples of each class to illustrate the current state of the art.
Techniques Used to Study the Gut Microbiome
Until recently, about 400 bacterial species were identified in the human microbiome using conventional culturing techniques.86 These techniques, by their nature, underestimate the actual number of species because, to culture a bacterium successfully, one needs to provide the correct nutrients, pH, and redox environment to enable growth. Conventional culture techniques favor fast-growing and nonfastidious species over those present in low concentration, which requires unusual culture conditions and/or complex nutritional requirements.86 Most isolation methods use selective agents, such as bile salts, to enrich the numbers of a desired bacterial type over others in the sample. The choice of the proper selective agent then becomes important. Some gut bacteria depend on other microorganisms in the gut to provide the nutrients they require for growth “cross-feeding.” Devising culture media for these can be a hit-or-miss proposition. Some bacteria will only grow in niches that have a narrow range of pH and/or redox potential, which may be difficult to maintain in vitro. Although there have been methodological advances in anaerobic culturing techniques, they tend to be tedious, time-consuming, and require specialized equipment.31 The successful culture of strict anaerobic bacteria requires training, experience, and careful planning. Additionally, some bacteria might be alive but unculturable. Intercellular adherence may reduce the number of organisms that can give rise to colonies.87
The development of culture-independent metagenomic approaches, such as 16S RNA gene sequencing and high-throughput sequencing, have been an enabling factor in the study of the human gut microbiome. This topic was reviewed in depth by Sankar et al. in 2015.86 The 16S rRNA gene exhibits several advantages, including its distribution in all bacterial species, its absence in eukaryotes, its stability over time, and its size (∼1500 bp), that make it suitable for bioinformatic analyses. High-throughput sequencing methods have given unprecedented access to the analysis of the microbial diversity of complex microbiotas, particularly through metagenomic approaches. Two strategies used are high-throughput sequencing of pooled PCR-amplified 16S rRNA and shotgun sequencing of all DNA fragments present, which enables identification of the microorganisms present and their metabolic genes. With these molecular techniques, it is now estimated that the human GI microbiota comprises more than 2000 species using modern molecular methods.86,88
Recent impressive advances in next generation sequencing technologies, along with the progress and innovations of metagenomics, metabolomics, multiomics, bioinformatics, and artificial intelligence tools, have provided prospects to better characterize the microbial populations and their functions and help in better correlation prediction.
Gastrointestinal Tract
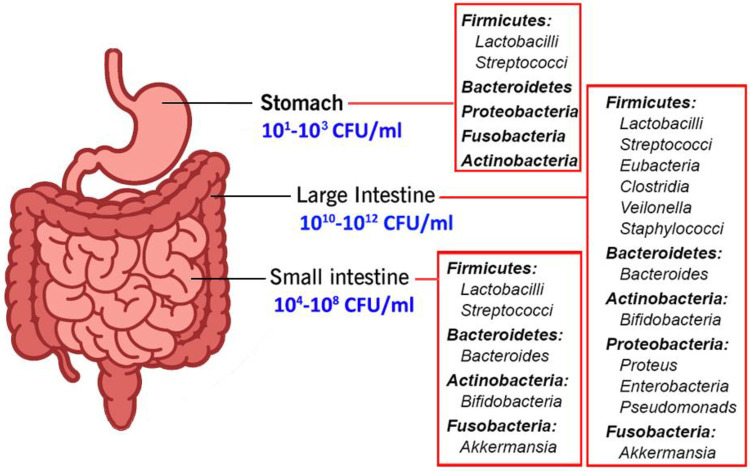
The gut microbiome varies according to the GI anatomy, which varies in terms of physiology, pH and O2 tension, flow rates (rapid from the mouth to the cecum, slower afterward), substrate availability, and host secretions.89Figure 6 presents a representation of the GI tract with some of the bacterial taxa present. The GI tract consists of the stomach, duodenum, jejunum, ilium, cecum, and colon, with each environment ascending in pH and growing progressively more anaerobic from stomach to colon. Each section of the GI tract presents a unique ecological niche that exerts selective pressure on the microbiome. It is an open system with nutrients entering the system intermittently and wastes also leaving intermittently. The microbiome is affected by many factors, including diet, medications (especially antibiotics), ethnicity, age, and general health.82,83
Figure 6.
Gut-microbiota-participant bacteria.
The stomach is an extreme habitat because it is highly acidic (pH = ∼1.5). Once, it was considered sterile because of its acidity until the discovery of Helicobacter pylori in this hostile environment in 1982. The microbial population in the gastric environment is low and in the range of 101–103 cells/mL.87 Investigations since this discovery have revealed that the gastric fluid is predominated by the members of Firmicutes, Bacteroidetes, and Actinobacteria.87 The gastric mucosa was found to have a rich diversity with bacterial members belonging to Firmicutes, Bacteroidetes, Proteobacteria, Fusobacteria, and Actinobacteria. In healthy human stomach, the genera Streptococcus, Prevotella, Veillonella, Rothia, and Haemophilus were found to be predominant; however, the composition of the gastric microbiota is dynamic and affected by such factors as diet, drugs, and diseases.90
The small intestine comprises the duodenum, jejunum, and ileum. The duodenum has a pH of 5–6.8. Bacterial numbers are 103–104 cells/mL where Firmicutes predominate.91 The jejunum and ileum have a higher pH (6–8) with a 104–108 cell/mL density that comprises strict to facultative anaerobic Gram-positive and Gram-negative bacteria. The small intestine is lined with simple columnar epithelial tissue, which is covered by a mucus layer, and has a large surface area because of the villi and microvilli. When food enters the duodenum, the pH and bacterial load are low. These small intestinal mucosae are associated with members of phyla Bacteroidetes and Firmicutes. Food is blended with bile, bicarbonate, and digestive enzymes in the duodenum, and when the intestinal contents reach the large intestine, the food blend has been converted to a neutral to alkaline pH. The small intestine provides a more challenging environment for microbial colonizers given the short transit times (3–5 h) and the high bile concentrations.83,91
The large intestine consists of the cecum and colon and is characterized by slow flow rates and pH varying from 6 to 7.8. It harbors by far the largest microbial community. The large intestine is strictly anaerobic, and the cell density reaches 1012 cells/mL. The large intestine is home to the most complex bacterial diversity in the GI tract because of several factors, such as its larger volume, moderate or less acidic pH, low concentration of bile salts, and the longer retention time caused by slower peristalsis. Five major phyla—Firmicutes, Bacteroidetes, Actinobacteria, Verrucomicrobia, and Proteobacteria—covering a wide range of bacterial genera—Clostridium, Fusobacterium, Bacteroidetes, Actinomyces, and Propionibacterium—are associated with the large intestine. Other Gram-positive cocci—Micrococci, Peptococci, Peptostreptococci, and Ruminococci—have been also reported to play crucial roles in the large intestine. Food that has not been degraded in the upper GI tract reaches the large intestines and supports the microbiota with nutrients and energy. The carbohydrates present are fermented to carbon dioxide, hydrogen, methane, and short-chain fatty acids (SCFA) (primarily acetate, propionate, and butyrate). Most of the SCFA produced in the large intestine are absorbed by the host and provide an energy source. The amount of energy derived from SCFA accounts for 6–9% of the total energy requirement.82,88,91,92
The microbiome composition of the intestinal lumen, known as mucosal and epithelial spaces of the GI tract, is highly diverse and comprises Verrucomicrobia, Fusobacteria, Asteroplasma, Cyanobacteria, Actinobacteria, Lentisphaera, Spirochaetes, Bacteroidetes, Proteobacteria, Bacilli, Clostridia, and Mollicutes. The predominating genera are Escherichia, Klebsiella, Enterococcus, Bacteroides, Ruminococcus, Dorea, Clostridium, Coprococcus, Weisella, and Lactobacillus. Other genera found include Granulicatella, Streptococcus, and Veillonella.83,91
Types of Bacteria Found in the GI Tract
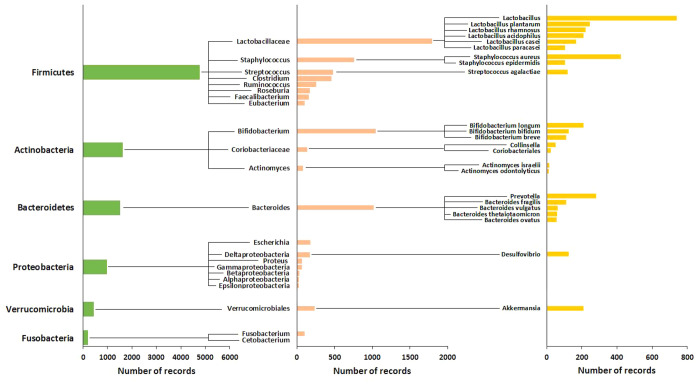
The four dominant phyla residents in the human gut are Firmicutes (which contains lactobacilli), Bacteroidetes, Actinobacteria (which contains Bifidobacteria), and Proteobacteria. Other phyla found in lower numbers are the Fusobacteria and Verrucobacteria. Figure 7 presents the significant phyla, families, and genera of gut bacteria in terms of the number of records that cite them in the CAS Content Collection. This presentation reflects the relative level of research interest in each of these taxonomic groups. Most bacteria belong to the genera Bacteroides, Clostridium, Fusobacterium, Eubacterium, Ruminococcus, Peptococcus, Peptostreptococcus, and Bifidobacterium. Other genera, such as Escherichia and Lactobacillus, are present to a much lesser extent. Twenty-three species from the genus Bacteroides, alone, constitute about 30% of all bacteria in the human gut.93
Figure 7.
Representation (as number of records) of the gut bacteria phyla and species in the CAS Content Collection.
Bacteroidetes
Bacteroidetes are Gram-negative, nonspore-forming, anaerobic or aerobic, rod-shaped bacteria. Bacteroides fragilis, found in the human microbiome, is the type of species for this phylum. The majority of the Bacteroidetes species fall into three genera: Prevotella (bile-sensitive, moderately saccharolytic, with pigmented and nonpigmented species), Porphyromonas (bile-sensitive, pigmented, asaccharolytic species), and Bacteroides (bile-resistant, nonpigmented, saccharolytic species). Other genera in the phyla are Alistipes, Anaerorhabdus, Dichelobacter, Fibrobacter, Megamonas, Mitsuokella, Rikenella, Sebaldella, Tannerella, and Tissierella.94 Some members of the Bacteroides genus, although belonging to the normal gastrointestinal microbiota, can cause opportunistic infections if the integrity of the intestinal mucosal barrier is broken. These infections are usually polymicrobial, but B. fragilis and B. thetaiotaomicron are the most frequent species isolated. Some members of the genera Porphyromonas, Prevotella, and Tannerella are well-known pathogens of the oral cavity, where they can notably cause periodontal disease and dental caries.95 The ability of some members of the Bacteroidetes to degrade polysaccharides explains why they thrive in the GI tract.96
Firmicutes
The Firmicutes phylum comprises Gram-positive bacteria with low G + C DNA content and is composed of more than 200 different genera, such as Lactobacillus, Bacillus, Clostridium, Enterococcus, and Ruminicoccus.82 They can be found in a variety of places, including soil, water, skin, and the GI tract. The phylum includes aerobes, anaerobes, spore-forming, saprophytic, and pathogenic bacteria. Notable among the latter are Clostridium difficile and Listeria monocytogenes. Firmicutes, such as Clostridium botulinum, Clostridium tetani, Clostridium perfringens, and Staphylococcus aureus, can produce proteinaceous toxins. Other important genera are Listeria, Paenibacillus, Staphylococcus, Streptococcus, Pediococcus, and Leuconostoc.97 Some members of Firmicutes are involved in bile acid metabolism in the gut. Accumulating evidence suggests that bile acids play pivotal roles in gut inflammation and the development of intestinal bowel disease (IBD). Patients with IBD exhibit decreased microbial diversity and abnormal microbial composition marked by the depletion of phylum Firmicutes.98
Actinobacteria
The Actinobacteria are Gram-positive bacteria with high G + C DNA content and constitute one of the largest bacterial phyla. They are ubiquitously distributed in both aquatic and terrestrial ecosystems. Many Actinobacteria have a mycelial lifestyle and undergo complex morphological differentiation. They also have an extensive secondary metabolism and produce about two-thirds of all naturally derived antibiotics in current clinical use, as well as many anticancer, anthelmintic, and antifungal compounds. The phylum includes pathogens (species of Corynebacterium, Mycobacterium, Nocardia, and Propionibacterium), soil inhabitants (Micromonospora and Streptomyces species), plant commensals (Frankia spp.), and GI commensals (Bifidobacterium spp.)99 The Bifidobacteria are among the first microbial colonizers of the intestines of newborns and play key roles in the development of their physiology, including maturation of the immune system and use of dietary components. Some Bifidobacterium strains are considered probiotic microorganisms because of their beneficial effects, and they have been included as bioactive ingredients in functional foods, mainly dairy products, as well as in food supplements and pharma products, alone or together with other microbes or microbial substrates.100
Proteobacteria
The name Proteobacteria was first proposed by Stackebrandt et al. in 1988.101 The name was derived from Proteus the ancient Greek god of the sea capable of assuming different shapes, which reflected the high heterogeneity displayed by the bacteria belonging to this phylum. A common trait of Proteobacteria is Gram-negative staining, which indicates the presence of lipopolysaccharide in the outer membrane. On the basis of phylogenetic analysis of the 16S rRNA gene, the Proteobacteria phylum is divided into six classes (previously regarded as subclasses of the phylum): Alphaproteobacteria, Betaproteobacteria, Gammaproteobacteria, Deltaproteobacteria, Epsilonproteobacteria, and Zetaproteobacteria. Considering that the classes division is based on molecular relatedness, it is not surprising that no specific morphological or physiological trait characterizes members within each class.102 Notable genera in the Proteobacteria are Escherichia, Salmonella, Shigella, Desulfovibrio, and Helicobacter. Included in the phyla is the Enterobacteriaceae family, which contains several enteropathogenic bacteria, including Shigella flexneri, Salmonella typhi, and Escherichia coli. Other enteric pathogens in this phylum are Vibrio cholerae and Helicobacter pylori.
Verrucomicrobia
The phylum Verrucomicrobia, like the Proteobacteria, is defined by as a distinct phylogenetic lineage, as determined by 16S rRNA gene sequences. The phylum has been recognized as separate since 1995 but currently counts only a few cultivated microorganisms as members. Verrucomicrobia is a divergent phylum that includes members of the microbial communities of soil and fresh and marine waters. Some extremely acidophilic members from hot springs have been found to oxidize methane.103,104Akkermansia muciniphila is a mucus-degrading member of the Verrucomicrobia found in the human GI tract. A. muciniphila represents from 1 to 4% of the bacterial population in the colon.105A. muciniphila prefers to colonize in the intestinal mucus layer and specifically degrades mucins to produce short-chain fatty acids, thereby providing energy for the host and promoting colonization of the bacterium itself. The degradation of mucins prompts the host to compensate with the production of more mucins, thereby maintaining the dynamics of these proteins.106
Fusobacteria
The phylum Fusobacteria is made up of Gram-negative, nonmotile, facultative aerobic to obligately anaerobic, fermentative, rod-shaped bacteria, which have generally fusiform (spindle-shaped) morphology. Fusobacteria have been known for more than 100 years, but recently, phylogenetic studies have shown that they should be grouped into a distinct phylum. The bacteria from this phylum are commonly associated with the mucous membrane of humans and animals. They are also commonly present in the human and animal GI tract, particularly in the jejunum, the ileum, and the colon.83,107
Gut Microbiome–Disease Correlations
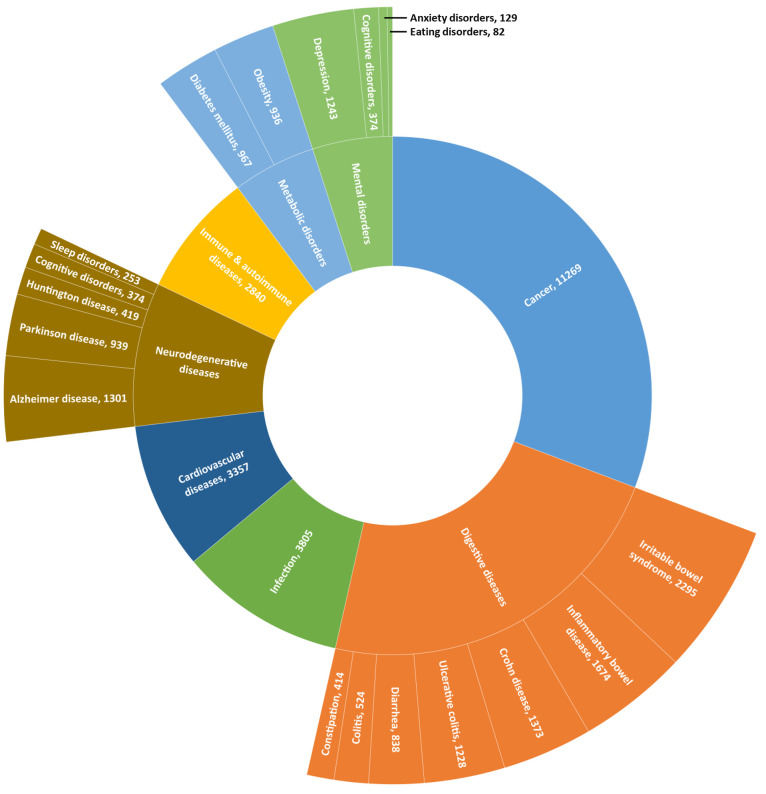
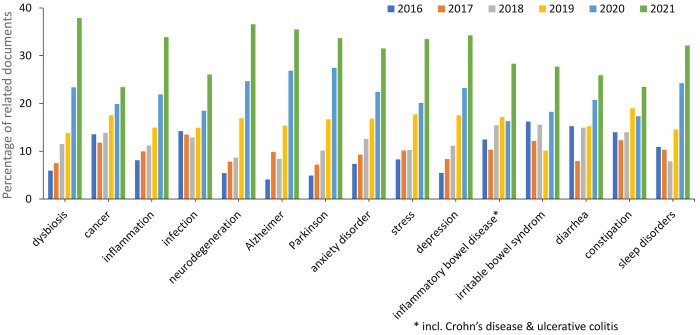
The human microbiome has been recognized as an essential factor for human health.108−110 Specifically, gut microbes contribute directly and/or indirectly to important physiological activities, including immunomodulation and the regulation of various neurotransmitters, hormones, and metabolites. Dysbiosis is a state characterized by distinct alterations in the microbiome that result in an imbalance in the microbiota, modifications in their functional composition and metabolic performance, or a change in their allocation. The impact of the microbiome on human physiology and pathology is so extensive that the microbiome has been considered as an essential organ of the human body.111−113 A search in the CAS Content Collection identified a large collection of studies reporting correlations between gut microbiota and a wide range of diseases, including mental, metabolic, and digestive system disorders; cardiovascular and neurodegenerative diseases; various cancers; and immune and autoimmune diseases (Figure 8). Trends in the number of publications related to various diseases in the recent years (2016–2021) are depicted in Figure 9. The number of documents related to dysbiosis, in general, exhibits the greatest growth rate, thereby characterizing the dominant fundamental approach of the recent studies in the field.
Figure 8.
Distribution of the publications in the CAS Content Collection related to gut microbiome-associated diseases.
Figure 9.
Trends in the number of publications concerning gut microbiome-related diseases during the years 2016–2021. Percentages are calculated with yearly publication numbers for each disease, normalized by the total number of publications for the same disease in the same time period.
Digestive System Diseases and Disorders
Alterations to the gut microbiota composition have been associated with various digestive system disorders and diseases, specifically IBS; IBD, including Crohn’s disease and ulcerative colitis; diarrhea, and constipation (Figure 8).114−116
Irritable bowel syndrome is one of the most prevalent functional gastrointestinal disorders and is considered as the prototype of disorders of the gut–brain interaction. While alterations in gut–brain interactions have clearly been established in IBS, a causative role of the microbiome remains to be determined. Dysbiosis is one of the hallmarks in the miscommunication between gut and brain and could lead to IBS symptoms. The severity of IBS symptoms has been shown to be correlated with dysbiosis.117,118 In IBS cases, the reduction of microbiome diversity, gut barrier deficiency, gut–brain signaling disorders, and immune disorders are significantly related to the abnormal function of the GI tract.119 Modifications in the composition of the normal microbiota and perturbed colonic fermentation in IBS patients are supposed to play a role in the development of IBS, with a considerable, nearly 2-fold, increase in the ratio of Firmicutes to Bacteroidetes.120 Recent studies have reported well-defined distinction between gut microbiota composition in patients with IBS compared with healthy controls. IBS was typified by enhanced quantities of Firmicutes and specifically in Ruminococcus, Clostridium, and Dorea, along with a distinct decrease of beneficial microbes, such as Bifidobacterium and Faecalibacterium spp.121 Moreover, a decrease in probiotic species and an increase in pathogenic species have been reported in patients with IBS, including Proteobacteria, Enterobacteriaceae, Lactobacillaceae, and Bacteroides (Bacteroidetes).122 Fecal transplantation from super donors and microbiome modulation either by pro- or prebiotics have shown beneficial effect in reducing IBS symptoms and improving patients’ quality of life.119,123,124 To date, the guidelines on the treatment of IBS with probiotics remain controversial. The British Society of Gastroenterology guidelines125 on the management of IBS, which were updated in 2021, reported that probiotics may be an effective treatment for improving global symptoms and abdominal pain in patients with IBS, which was consistent with the recommendations of the Canadian Association of Gastroenterology126 and the Japanese Society of Gastroenterology.127 In contrast, the guidelines from the American College of Gastroenterology128 suggest against the use of probiotics for the treatment of global IBS symptoms.129
Like IBS, inflammatory bowel disease-related dysbiosis is associated with a general decrease in richness, diversity, and stability of the microbiota.130 This decline in diversity is concomitant with a weakened immune response and setbacks with the cellular barrier functions that normally block bacterial entry from the gut lumen into gut tissue. These malfunctions trigger complications with antibacterial defense and consequent growth of pathogenic bacteria.131 IBD-related dysbiosis is specifically associated with a comprehensive decrease in the quantity and diversity of Firmicutes and an increase in Proteobacteria.132 The decrease in the numbers of Firmicutes is noteworthy since they produce essential short-chain fatty acids, such as acetic and butyric acids, that are known to exhibit anti-inflammatory properties.133 A common feature of the microbial dysbiosis among IBD patients, especially in Crohn’s disease, is the decreased abundance of Firmicutes bacteria belonging to two families that are important functional members of the human gut microbiota—Ruminococcaceae and Lachnospiraceae—to which most butyrate-producing bacteria in the human gut belong.134,135 Thus, depletion of these bacterial families in IBD is supposedly correlated to the detected disturbances, such as a lower butyrate-producing capacity of the IBD microbiota.136 Butyrate has a significant potential in IBD therapy because it serves as the colonocytes key energy source, enhances the epithelial barrier integrity, and inhibits inflammation. A probiotics treatment, including consumption of butyrate-producing bacteria to increase in situ butyrate production, may restore gut homeostasis.137−139 A recent study reported that an orally delivered cocktail of bacteriophages targeting an IBD-associated strain of the bacterium Klebsiella pneumoniae alleviated intestinal inflammation.131
A growing body of evidence shows that imbalance of the gut microbiota increases susceptibility to various pathogens and causes numerous diseases, including diarrhea.140 At present, the pathogens causing diarrhea are believed to be Escherichia coli, Shigella, Salmonella, Campylobacter, Clostridium difficile, and Aeromonas.141,142 It has been found that microbial intervention can regulate the composition of the intestinal flora to prevent and improve the occurrence of diarrhea.143 Probiotics containing nonpathogenic live bacteria preparations, such as Lactobacillus, Yeast, Bifidobacterium, Enterococcus, and Bacillus, have been demonstrated to treat pathogens-caused diarrhea by preserving or amending the balance of gut microbiota. The mechanisms of the beneficial effect are supposedly related to the inhibitory effect on the colonization of pathogenic bacteria by competing for nutrients and producing antibacterial compounds.144
Accumulating evidence suggests an association between functional constipation and abnormal gut microbiota, with the relationship between gut microbiota and gut transit being likely bidirectional.145 By controlling colonic motility, water content, secretion, and absorption, gut microbiota may promote the development of functional constipation through microbial metabolites, including bile acids, SCFAs, 5-hydroxytryptamine, and methane. Currently, there is no consensus on the gut microbial composition typical for functional constipation patients and the alteration trends of the various microbial classes compared with healthy controls.146−150 However, recent studies showed that changes in the mucosal and fecal microorganisms are linked to functional idiopathic constipation. Taxonomic profiling of intestinal microbiota in constipated adults showed a higher abundance of Bacteroides and other pathogenic microorganisms than in healthy volunteers.151 The increased richness and diversity of the gut microbiomes result in slow colonic transit. In addition, intestinal microbiota in constipated adults have genes involved in pathways that lead to methane, hydrogen, and glycerol production, which can explain the symptoms seen in patients with constipation.151−153 Microbial interventions including probiotics, prebiotics, and synbiotics, which bring about compositional and functional changes of the gut microbiota, have frequently shown beneficial effects on functional constipation that are in favor of the concept of the significant role of gut microbiota in functional constipation.145 This concept is supported also by the reports that many risk factors of functional constipation, including age, diet, obesity, and stress, have a considerable effect on the gut microbiota.154,155
Mental and Neurodegenerative Disorders
Gut microbiota have been reported to affect neurological functions along the so-called gut–brain axis (GBA).156 Gut microbiota communicates with the brain through three major routes: the neural route (vagus nerve, enteric nervous system), the immune route (cytokines), and the endocrine route [hypothalamus–pituitary–adrenal (HPA) axis, gut hormones]. Disturbances in any of these routes can result in mental disorders. Dysbiosis in common intestine microbial species of the phylum Firmicutes and Actinobacteria and the genera Bacteroides and Bifidobacterium are supposedly responsible for mental health disorders.157 Gut microbiota moderate the GBA via various ways, such as preserving gut permeability by controlling the integrity of tight junctions in gut epithelium and producing a large selection of metabolites that include neurotransmitters, SCFAs, and amino acids.
A plethora of research reports have indicated the significance of microbiota in the development of neurodegenerative diseases via a variety of microbial metabolites that transmit from the gut to the brain across the GBA.158,159 Changes in the levels of gut microbial metabolites have been reported to be associated with neurological conditions like Parkinson’s disease,160 anorexia nervosa,161 Alzheimer’s disease,162 autism spectrum disorders,163 and chronic stress and depression.164 It is not clear by now, however, whether these disruptions in mental health are the cause or a result of the changes in gut microbiota. Gut dysbiosis has been associated with increased gut permeability and inflammation and it may also cause enhanced levels of circulating gut microbiota metabolites, such as the neurotoxin β-N-methylamino-l-alanine and microbial amyloids.165,166 β-N-Methylamino-l-alanine is one of the gut cyanobacteria-produced neurotoxins causing neurodegeneration, cognitive impairment, and the accumulation of neurofibrillary tangles.167,168 The hypothesis that Parkinson’s disease starts in the gut and spreads to the brain169 is gaining increasing support, thereby showing that the disease is associated with widespread dysbiosis.170
Alterations in the microbiota composition in patients with Alzheimer’s disease compared with matched healthy controls included a reduction in richness and diversity of gut microbiota with decreased Firmicutes and Bifidobacterium and increased Bacteroidetes.171 Changes in Actinobacteria, Ruminococcus, Lachnospiraceae, and Selenomonadales (1686) have also been reported.172 Cognitively impaired patients exhibited alterations in Bacteroidetes, Firmicutes, Proteobacteria, and Verrucomicrobia compared with age-matched cognitively intact individuals.173
Therapeutic interventions including the administration of pre- and probiotics (psychobiotics) to manage mental disorders and/or their symptoms have been undertaken.174,175 These inventions have included probiotic combinations of lactobacilli and Bifidobacteria, which has resulted in a significant drop in psychological distress,176 enhanced cognition and communication among patients with Alzheimer’s disease177 and autism spectrum disorders,178 and recovering symptoms among patients with Parkinson’s disease.179 On the basis of the promising results of psychobiotics on controlling or modulating the GBA, additional clinical trials are currently being undertaken to identify bacterial strains as promising candidates for the treatment of mental disorders.
Humans are adapted to a circadian rhythm of 24 h associated with the light/dark cycle on earth. The central circadian clock is located in the hypothalamus, which synchronizes information on environmental light and dark signals to peripheral tissues to keep the body functioning in the same rhythm.180 A disruption of circadian rhythms is associated with various diseases, including neurodegenerative diseases, sleep, and psychiatric disorders.181,182 Recent studies have reported that gut microbiota are able to control or be controlled by the circadian clock. The mechanisms of such a relationship requires small molecule gut microbiota metabolites, such as bile acids and SCFAs, to act as intermediaries.180 Thus, the levels of butyrate and propionate show obvious daily oscillations. Moreover, these oscillations are lost under high-fat diets.183 The impacts of gut microbiota metabolites on circadian rhythm are extensive and are connected to other functions of gut microbiota metabolites, such as energy metabolism and immunity. These interconnections between different physiological functions via the link of gut microbiota metabolites are essential for understanding the functions of gut microbiota metabolites and the general role of gut microbes in human health and disease.
Metabolic Disorders
Systemic metabolic diseases that are believed to be strongly affected by gut microbiota status include obesity and diabetes.184 Gut microbial composition is strongly affected by dietary routines. As a result of a high-fat diet, the intestinal microbiome is modified with rising amounts of Firmicutes and Proteobacteria and reduced levels of Bacteroidetes. The Firmicutes/Bacteroides ratio has been correlated to body weight, which means it is larger for obese people.185Clostridium difficile infections can also trigger obesity. Generally, obesity is affected by the inflammatory status induced by gut bacteria or their metabolites, which regulate the GBA.108,185
Diabetes is another metabolic disease that is strongly associated with the gut microbiome. Studies have reported an increased quantity of Villanella, Clostridium, and Bacteroides and a decreased quantity of Lactobacillus, Eubacterium rectale, Blautia coccoides, and Bifidobacterium in children with type 1 diabetes. Besides, negative correlation has been reported between plasma glucose level and Bifidobacterium, Lactobacillus spp., and Firmicutes and Bacteroidetes spp., while there has been positive correlation between Clostridium and plasma glucose level. The ratios of Bacteroidetes to Firmicutes were reported to exhibit a positive connection with plasma glucose levels. The Lactobacillus genus was also in lower quantity in type 2 diabetes patients, and Bifidobacterium was in higher quantity compared with control groups.108,186 Risks for the development of type 2 diabetes have been correlated to the composition of gut microbiota, as well. The alterations in the gut microbiota of individuals with type 2 diabetes have been small compared with the control group, yet a consistent decline in the metabolically beneficial butyrate-producing bacteria was reported.186 Overall, type 2 diabetes was associated with a reduced quantity of SCFAs-producing bacteria, in particular butyric acid, which has been related to insulin sensitivity.187,188 The relation between SCFAs and insulin sensitivity stems from the capacity of SCFAs to stimulate the secretion of GLP-1 by intestinal L-cells via G protein receptors, which has a significant impact on insulin release.189
Close relations between the metabolic and immune systems are now largely supported, and intestinal microbiota is being progressively identified as an important factor connecting genes, environment, and the immune system.190
COVID-19
Recently, correlation has been reported between gut microbiota composition and levels of cytokines and inflammatory markers in patients with COVID-19.191,192 It is suggested that the gut microbiome is involved in the magnitude of COVID-19 symptoms’ severity via modulation of the host’s immune responses. Moreover, gut microbiota dysbiosis could contribute to persistent symptoms even after disease resolution, thereby emphasizing a need to understand how gut microorganisms are involved in inflammation and COVID-19.A recent study demonstrated that SARS-CoV-2 infection indeed disrupts the gut microbiome.193 This boosts secondary bacterial infections both by facilitating pathogenic bacteria to colonize the gut and by modifying gut lining to allow these bacteria to spread from the gut to the bloodstream of COVID-19 patients. These results confirm the direct role of gut microbiome dysbiosis in facilitating grave secondary infections upon COVID-19 malady.193
The alterations to the gut microbiota composition related to digestive system diseases, mental health, and metabolic disorders are summarized in Table 1.
Table 1. Gut Dysbiosis in Digestive System Diseases, Mental Health, and Metabolic Disorders.
| diseases | ↓ decreasing bacteria | ↑ increasing bacteria |
|---|---|---|
| digestive system diseases | ||
| irritable bowel syndrome194−199 | Bifidobacterium | Ruminococcus |
| Faecalibacterium prausnitzii | Dorea | |
| Bacteroides | Enterobacteriaceae | |
| Lactobacillaceae | ||
| Bacteroides | ||
| Firmicutes/Bacteroidetes ratio | ||
| IBD: Crohn’s disease200,201 | Bacteroides | |
| Faecalibacterium prausnitzii | ||
| Bifidobacterium adolescentis | ||
| IBD: ulcerative colitis139,200 | Bifidobacteria | |
| Roseburia hominis | ||
| Faecalibacterium prausnitzii | ||
| Lachnospiraceae | ||
| Ruminococcaceae | ||
| mental health disorders | ||
| anxiety disorder202,203 | Bacteriodetes | Bacteroidaceae |
| Ruminococcus gnavus | Enterobacteriaceae | |
| Fusobacterium | Burkholderiaceae | |
| post-traumatic stress disorder204 | Actinobacteria | |
| Lentisphaerae | ||
| Verrucomicrobia | ||
| depression205−207 | Prevotella | Eggerthella |
| Dialister | Holdemania | |
| Turicibacter | ||
| Paraprevotella | ||
| dementia172 | Actinobacteria | Escherichia |
| Bacteroides | Blautia | |
| Bifidobacterium | ||
| Streptococcus | ||
| Lactobacillus | ||
| Dorea | ||
| metabolic disorders | ||
| diabetes type 1208,209 | Lactobacillus | Clostridium |
| Bifidobacterium | Bacteroides | |
| Blautia coccoides | Veillonella | |
| Eubacterium rectale | Actinobacteria | |
| Prevotella | Proteobacteria | |
| Akkermansia | Lactococcus | |
| Firmicutes | ||
| diabetes type 2199,209,210 | Firmicutes | Betaproteobacteria |
| Clostridia | ||
| Lactobacillus | Bacteroidetes/Firmicutes ratio | |
| Akkermansia muciniphilia | ||
| Roseburia | ||
| obesity199,211 | Bacteroidetes | Enterobacteria |
| Methanobrevibacter smithii | Ruminococcus gnavus | |
| Ruminococcus flavefaciens | Actinobacteria | |
| Bifidobacterium | Prevotellaceae | |
Gut Bacteria–Disease Correlations
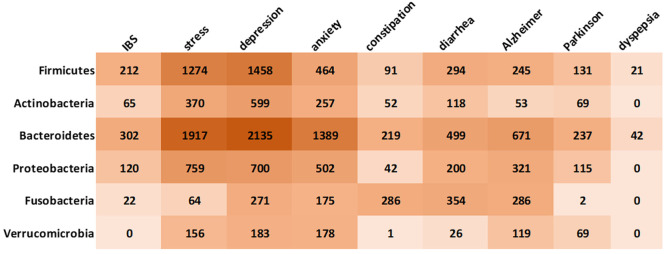
In an effort to get better insight into the gut microbiota impact on well-being, we explored the correlations between the major classes of gut bacteria and certain mental and gastrointestinal disorders, as reflected in the number of records in the CAS Content Collection (Figure 10).
Figure 10.
Correlation between major classes of gut bacteria with mental and gastrointestinal disorders, as reflected in the number of associated records in the CAS Content Collection.
As seen from Figure 10, Bacteroidetes are the most studied class of gut bacteria with relation to gastrointestinal and mental health, specifically with relation to stress, depression, and anxiety. Bacteroidetes are known to have a very broad metabolic potential and are regarded as one of the most stable parts of gastrointestinal microflora that exhibits remarkable nutritional flexibility and an ability to respond to stress.212 However, the exact mechanisms underlying any possible relationship between Bacteroidetes and mental and gastrointestinal health remains unclear, and further research is needed to fully understand this complex relationship.
Firmicutes are the second extensively studied class of gut bacteria with relation to gastrointestinal and mental health, especially in relation to stress and depression (Figure 10). Many members of the Firmicutes phylum, such as Lactobacillus, are probiotic. The relationship between Firmicutes and gastrointestinal disorders may be mediated by a number of different factors, such as the production of SCFAs by Firmicutes, which are an important energy source for the gut epithelium and have anti-inflammatory effects. In addition, some Firmicutes bacteria are involved in the fermentation of complex carbohydrates and the production of beneficial metabolites, such as butyrate, which has been shown to have protective effects against colorectal cancer.213 Like with Bacteroidetes, the exact relationship between Firmicutes and mental and gastrointestinal disorders is still being studied and is not fully understood.
The interest in Fusobacteria with respect to the gastrointestinal and mental health is mainly related to diarrhea and constipation (Figure 10). Recent evidence is emerging that this bacterium may be related to human colon cancer.214
It is noteworthy that recent meta-analysis has reinforced the genetic correlations between Alzheimer’s disease and the gut microbiome genera.215 For example, genus Actinobacterium Collinsella was confirmed to be associated with Alzheimer’s disease, as well as rheumatoid arthritis, atherosclerosis, and type 2 diabetes.215 It is also worth reiterating that the gut microbiota is a complex and diverse community of microorganisms, and changes in any one particular phylum are unlikely to fully explain the development of any particular disorder or condition. Rather, the gut microbiota as a whole are likely to play a role in shaping our physical and mental health, and further research is needed to fully understand the complex relationships between the gut microbiota and overall well-being.
Therapeutic Strategies for the Treatment of Mental and Gastrointestinal Disorders
Imbalances within the gut microbiome–brain axis have been linked to a range of mental and gastrointestinal disorders. Assorted therapeutic interventions aimed at modulating the gut microbiome or the gut–brain axis may be effective in improving outcomes for these conditions.
Dietary Interventions
A high-fiber diet can increase the production of SCFAs, which can help to maintain gut barrier function and reduce inflammation. SCFAs can also promote the growth of beneficial gut bacteria, which can outcompete pathogenic bacteria and reduce inflammation.216
A diet low in fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAP) can reduce symptoms in individuals with irritable bowel syndrome by reducing the fermentation of certain carbohydrates in the gut, which can cause symptoms such as bloating and abdominal pain. However, the low-FODMAP diet can also reduce the diversity of gut bacteria and may have negative long-term effects on gut health.217,218
Probiotics and Prebiotics
Probiotics can improve gut barrier function by enhancing the production of mucus and tight junction proteins, which can prevent the entry of harmful molecules and pathogens into the bloodstream. They can also reduce the production of proinflammatory cytokines and modulate the activity of immune cells in the gut, thereby promoting an anti-inflammatory response.219,220
Prebiotics can increase the production of metabolites, such as SCFAs, which are important energy sources for gut cells and can help to maintain gut barrier integrity. SCFAs can also activate G protein-coupled receptors on immune cells, thereby leading to the production of anti-inflammatory cytokines and the suppression of proinflammatory cytokines.221,222
Antibiotics
Antibiotics can kill harmful bacteria in the gut, which reduces inflammation and restores gut barrier function. However, antibiotics can also have negative effects on the gut microbiome, such as by reducing the diversity of gut bacteria and promoting the growth of antibiotic-resistant bacteria. Antibiotics should be used judiciously and only when necessary.223,224
Fecal Microbiota Transplantation (FMT)
FMT can restore the composition and function of the gut microbiome, which can reduce inflammation and improve gut–brain communication. FMT has been shown to be effective in treating recurrent Clostridium difficile infection and is being investigated for other conditions, such as IBD and autism.225−227
Psychotherapeutic Interventions
Psychotherapeutic interventions can reduce stress and improve gut–brain communication. Stress can disrupt gut microbiome composition and function, which leads to inflammation and the development of diseases related to the gut microbiome–brain axis. The reduction of stress can be an effective way to improve gut health and reduce symptoms in these conditions.228−230
Pharmacological Interventions
Drugs that target the gut–brain axis can modulate gut microbiome composition and function and reduce inflammation. For example, certain drugs that target the serotonin system, such as selective serotonin reuptake inhibitors (SSRIs), can modulate gut–brain communication and have been shown to be effective in treating conditions, such as depression and anxiety.231,232
Figure 11 demonstrates the annual growth in the number of documents in the CAS Content Collection related to various therapeutic interventions applied for the treatment of mental and gastrointestinal disorders. Fecal transplant as a new therapeutic strategy exhibits the fastest growth rate recently.
Figure 11.
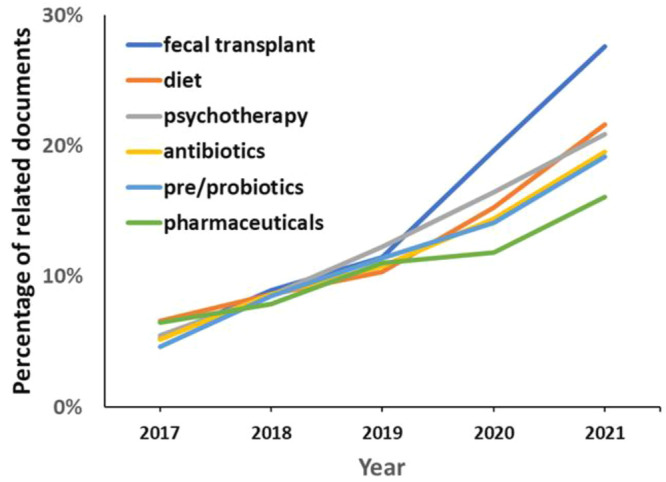
Trends in the therapeutic strategies applied for the treatment of mental and gastrointestinal disorders, as presented in the documents related to gut microbiome research during the years 2017–2021. Percentages are calculated with yearly publication numbers for each type of therapeutic intervention normalized by the total number of publications for the same intervention in the same time period.
The various strategies to treating diseases related to the gut microbiome–brain axis can work through multiple mechanisms, including modulating gut microbiome composition and function, reducing inflammation, and improving gut–brain communication.233 However, the underlying mechanisms are complex and require further research to fully understand.
Gut Microbiota Metabolites
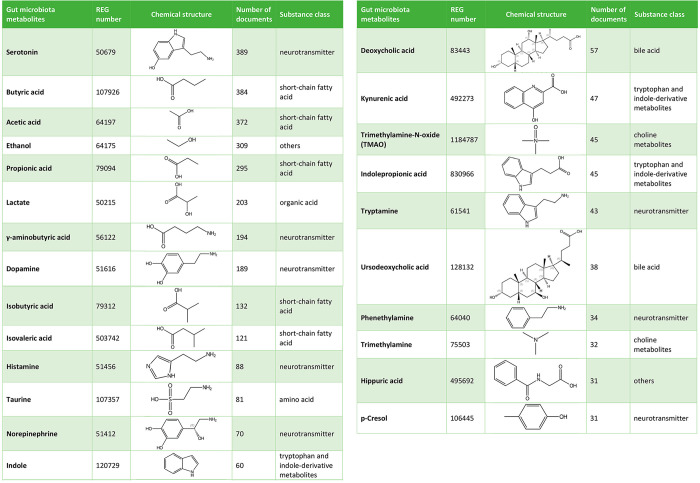
It is estimated that the human gut microbiome contains more than 22 million microbial genes,234 which exceeds the ∼22,000 genes present in the entire human genome.235 These genes enable the gut microbiota in the host to synthesize a myriad of enzymes with versatile capabilities to ferment and degrade a variety of compounds that humans do not have the genetic machinery to metabolize. As a result, the gut microbiota can generate a battery of metabolites with a wide spectrum of bioactivities. The gut microbiota-derived metabolites can be broadly divided into three types according to their origination: (1) metabolites that are produced by gut microbiota directly from diets; (2) metabolites that are generated by the host and modified by gut microbiota; (3) metabolites that are produced de novo.236 A selection of important gut microbiota metabolites related to gut–brain communication is shown in Table 2.
Table 2. Exemplary Gut Microbiota Metabolites, as Represented in the CAS Content Collection.
Gut Microbiota Metabolites with Impact on Brain Function
The gut microbiota provides essential signaling metabolites that are vital for the host’s physiology. While in healthy individuals, gut microbiota metabolites are effective in maintaining the important functions of hosts, perturbations in the production of these metabolites can initiate various diseases, such as digestive system diseases, neurodegenerative and metabolic disorders, and cancer.236
Research has shown that gut microbiota is crucial for normal brain development and function.237,238 For example, administration of 4-ethylphenylsulfate (4-EPS), a tyrosine derivative, to mice at 3–6 weeks postnatal induced anxiety-like behavior. The biosynthetic pathway analysis and mechanisms behind the detrimental effects of 4-EPS showed that 4-EPS interfered with oligodendrocyte maturation, myelination, and brain activity patterns. p-Cresol, another tyrosine derivative and a metabolite, has also been directly associated with neurodevelopmental disorders.239,240 Further, certain bacteria-related metabolites, such as trimethylamine-N-oxide (TMAO), 5-aminovaleric acid (5-AVA), 5-AVA betaine (5-AVAB), imidazolepropionic acid, and hippuric acid have been reported to promote early-life axonogenesis both in vitro and in vivo.241 Moreover, the neurogenic properties of microbe metabolites may not be limited to early life given that indole, a tryptophan metabolite, has been reported to increase neurogenesis in the hippocampus of adult mice.242 Pilot studies using fecal microbiota transplantation in children enabled the assessment of whether early life interventions affecting the gut microbiota composition exerted long-term neurodevelopment effects.243,244 Thus, maternal fecal microbiota transplantation in cesarean-born infants was found to rapidly restore normal gut microbial development.244
Gut microbiota and its metabolites can affect the host metabolism of neuroactive compounds.245 The foremost examples of gut bacteria-derived neurotransmitters are aromatic amino acid derivatives dopamine and norepinephrine and glutamate derivative γ-aminobutyric acid.246,247 Microbiota have been found to extensively contribute to the levels of dopamine and norepinephrine via the activity of β-glucuronidase.248 Kynurenic acid, a tryptophan metabolite, functions as a glutamate modulator to reduce glutamate levels in the glutamatergic signaling in the hippocampus. Thus, enhanced cognitive abilities and memory in model animals have been achieved as a result of enhancing glutamate levels via limiting hippocampal kynurenic supply.249,250 Over 90% of serotonin in the body is known to be produced in the gut in a process in which gut microbes play an important regulatory role.251,252 Tyramine, deoxycholic acid, and 4-aminobenzoic acid have been reported to stimulate serotonin synthesis.252 Furthermore, microbiota-related metabolites, such as norepinephrine, indole, indole-3-aldehyde, isovaleric acid, butyric acid, and isobutyric acid stimulate serotonin release from enterochromaffin cells.253,254 Another gut microbiota metabolite with a likely connection to γ-aminobutyric acid (GABA) expression in brain is lactate.255,256 It has been shown to affect neural plasticity and has a beneficial effect on learning and memory in model animals.257 SCFAs, particularly butyric acid, may also have additional regulatory effects on the signal transduction to the brain via the vagal nerve and by inducing the biosynthesis of neurotransmitters in the CNS.258 Administration of SCFAs, such as butyric, acetic, and propionic acid, has been reported to improve stress response, anxiety, and depression.259 The presence of pipecolic acid in the CNS can be partially derived from the gut microbiota and has been also associated with GABA signaling and release.245,260,261
Gut microbiota has also proven vital for normal BBB function, especially during pre- and postnatal periods.262 In a mice model of traumatic brain injury typified by acute BBB disruption, sodium butyrate administration exhibited an alleviating effect on BBB integrity.263 Propionic acid, another SCFA gut microbiome metabolite, has also been shown to promote BBB integrity by mitigating oxidative and proinflammatory pathways.264 It has been suggested that the effects of SCFAs on BBB integrity may rather be brought about by peripheral signaling instead of direct uptake to the brain, as implied by animal models.258 Secondary bile acids, such as deoxycholic acid and ursodeoxycholic acid, may also modulate BBB integrity.265,266 Trimethylamine, a metabolite of dietary choline, betaine, and l-carnitine, has been reported to exert detrimental impact on the BBB integrity. It is noteworthy that physiologically appropriate doses of the oxidized form of trimethylamine, TMAO, improved the BBB integrity.267
A large portion of the brain’s energy production is consumed by the neurons, the major component of the nervous tissue, in order to maintain the excitability of the synapses.268 Lactate, a major gut microbiota metabolite, is known to augment neural activity as a primary energy source.269 Modulation of brain energy metabolism in the hippocampus along the GBA is suggested to be responsible for improvement in the cognitive function after intermittent fasting in model animals.270 It has been found that fasting considerably increased plasma levels of indolepropionic acid and tauroursodeoxycholic acid and fecal levels of SCFAs. Administrations of indolepropionic acid or tauroursodeoxycholic acid or a SCFAs mixture including acetic, propionic, and butyric acid have been able to reproduce the effects of fasting in cognition, hippocampal mitochondrial biogenesis, and energy metabolism-related gene expression. Findings connecting microbial metabolites to brain bioenergetics are preliminary but show that certain compounds are incorporated in the neuronal energy metabolism.
Gut microbiota metabolites reducing oxidative stress or neurotoxic proteins aggregation are functioning as neuroprotective agents. Metabolites that reduce inflammation or promote neurodevelopment or neurotransmission can also be considered as neuroprotectants. For example, ferulic acid is known to be metabolized by gut microbes.271 It exerts neuroprotective effects by reducing neuronal cell death and recovers memory deficits in a cerebral ischemia and reperfusion injury model.272 It has also ameliorated depressionlike behavior and oxidative stress.273 Dihydroferulic acid, a microbiota metabolite, has also been shown to exhibit neuroprotective antioxidative properties.274
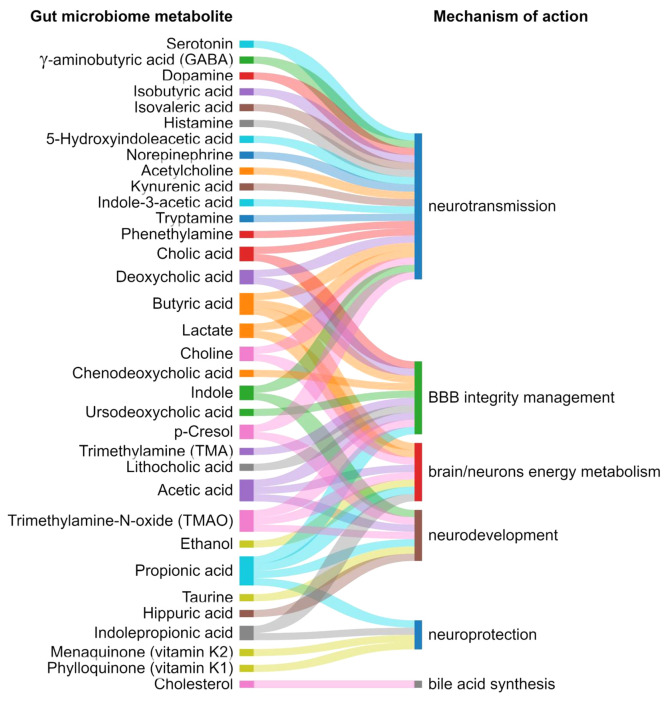
The gut microbiome metabolites and their mechanism of action in mental health and brain development are depicted in Figure 12, and their function and associated diseases are summarized in Table 3.
Figure 12.
Exemplary gut microbiome metabolites and their mechanism of action in gut–brain communications.
Table 3. Gut Microbiota Metabolites, Their Function, and Associated Diseases.
| metabolite class/references | specific functions | associated diseases |
|---|---|---|
| short-chain fatty acids258,285−290 | – gut microbiota composition regulation | – diabetes |
| – gut barrier integrity support | – obesity | |
| – energy homeostasis support | – nonalcoholic fatty liver disease | |
| – gut hormone production | – ulcerative colitis | |
| – circadian rhythm regulation | – Crohn’s disease | |
| – proinflammatory cytokines inhibition | – colorectal cancer | |
| – immunomodulation | – autism spectrum disorder | |
| – water, sodium, calcium, magnesium absorption | – Parkinson’s disease | |
| – regulation of intestinal pH value | – diarrhea | |
| – IBS | ||
| – constipation | ||
| – functional dyspepsia (FD) | ||
| bile acids (BAs)291−295 | – lipid and vitamin absorption regulation | – obesity |
| – gut microbiota composition regulation | – nonalcoholic steatohepatitis | |
| – gut hormones production | – ulcerative colitis | |
| – intestinal immunity | – cancer | |
| – intestinal electrolyte and fluid balance | – multiple sclerosis | |
| – gut motility | – Alzheimer’s disease | |
| – gut barrier integrity | – Parkinson’s disease | |
| – lipid homeostasis | – traumatic brain injury | |
| – glucose homeostasis | – stroke | |
| – amino acid homeostasis | – amyotrophic lateral sclerosis | |
| – circadian rhythm | – IBS | |
| – neurotransmission | ||
| tryptophan and indole derivatives296−300 | – gut microbial spore formation | – ulcerative colitis |
| – drug resistance | – Crohn’s disease | |
| – biofilm formation | – obesity | |
| – intestinal barrier function regulation | – stroke | |
| – gut hormone secretion | – mucosal candidiasis | |
| – gut motility | – autism spectrum disorder | |
| – immunomodulation | – Alzheimer’s disease | |
| – Parkinson’s disease | ||
| – migraine | ||
| – schizophrenia | ||
| – IBS | ||
| choline metabolites301−303 | – bile acid synthesis inhibition | – nonalcoholic fatty liver disease |
| – inflammation promotion | – obesity | |
| – thrombosis | – diabetes | |
| – myocardial hypertrophy and fibrosis | – hypertension | |
| – mitochondrial dysfunction exacerbation | ||
| vitamins304−306 | – cellular metabolism regulation | – vitamin-associated diseases |
| – immunomodulation | – schizophrenia | |
| – cell proliferation | – autism | |
| – vitamins supply | – dementia | |
| – IBS | ||
| – IBD | ||
| neurotransmitters307−309 | – gut motility regulation | – Parkinson’s disease |
| – memory support | – autism spectrum disorder | |
| – stress response | – IBD | |
| – nervous system | – IBS | |
| – immune response | ||
| lipids184,310,311 | – systemic inflammation promotion | – diabetes |
| – hyperinsulinemia regulation | – obesity | |
| – immunomodulation | – nonalcoholic fatty liver disease | |
| – bile acid synthesis | – hyperinsulinemia | |
| – hypercholesterolemia | ||
| – chronic hepatitis C | ||
| gases307,312−316 | – gut motility | – colitis |
| – gut inflammation | – ulcer | |
| – epithelial secretion | – IBS | |
| – mucosal blood flow |
Gut Microbiota Metabolites’ Role in Digestive System
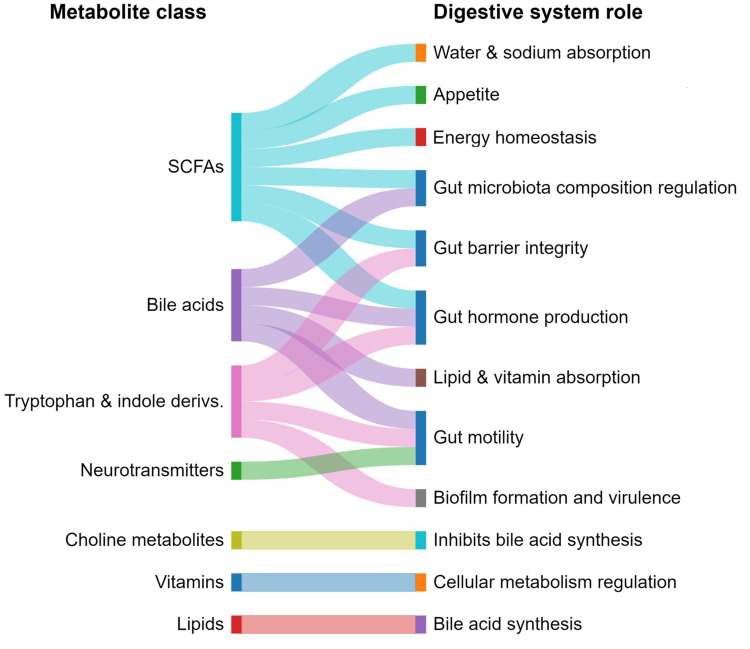
Gut microbiota imparts specific function in the host’s digestive system, in nutrient metabolism, xenobiotic and drug metabolism, in preservation of the integrity of the intestinal mucosal barrier, and in protection against pathogens (Figure 13).
Figure 13.
Gut microbiome metabolite classes and their roles in digestive system functions.
Gut microbiota mostly get nutrients from dietary carbohydrates. Fermentation of the carbohydrates, including indigestible oligosaccharides by the microbes in the colon, such as Bacteroides, Roseburia, Bifidobacterium, Faecalibacterium, and Enterobacter, ends in the synthesis of SCFAs, such as butyric, propionic, and acetic acids, which are important sources of energy for the host.275 Gut microbiota have a positive role in lipid metabolism, as well, by controlling the lipoprotein lipase activity inhibition in adipocytes.31
Intestinal microbiota also exhibit a resourceful protein metabolizing machinery, which operates by means of the microbial proteinases and peptidases in conjunction with the human proteinases. Examples include the conversion of l-histidine into histamine by the bacterial enzyme histamine decarboxylase and glutamate to γ-amino butyric acid by glutamate decarboxylases.276,277
SCFAs can regulate the pH value in the intestine and regulate the absorption of water, sodium, calcium, and magnesium. Furthermore, SCFAs, especially butyrate, provide more than 70% of the energy for the intestinal epithelial cells on top of their abilities to inhibit the multiplication and growth of pathogenic bacteria and the activity of intestinal inflammatory mediators, thus playing an anti-inflammatory role in the intestinal tract.278
Lipid metabolites can affect intestinal permeability and intestinal immunity. The gut microbiota can produce lipopolysaccharides that could stimulate proinflammatory mediators, thereby disrupting the body’s immune system and inducing local and systemic inflammatory responses.279 Sphingolipids can be produced by the intestinal symbiotic bacteria Bacteroidetes and Prevotellaceae. It has been found in animal studies that sphingolipids can also aggravate intestinal inflammation.280
Indole-derived metabolites are produced by fermentation via Clostridium sporogenes and Escherichia coli. Such metabolites are able to participate in the regulation of gastrointestinal disorders by influencing the gut–brain axis and protecting against stress-induced damage in the gastrointestinal tract. Tryptophan is a key monoamine neurotransmitter involved in the regulation of central neurotransmission and intestinal physiological functions, and studies have shown that the gastrointestinal microbiome can regulate the gut–brain axis through tryptophan metabolism.281,282
Gases can be produced by gut microbiota as a result of the fermentation process. These gases include hydrogen (H2), methane (CH4), carbon dioxide (CO2), hydrogen sulfide (H2S), and nitric oxide (NO), which can modulate the gastrointestinal physiology of hosts.236
Xiao et al.283 in a recent review article highlighted the important microbial metabolites in the context of host physiology in patients with different IBS subtypes. The abundance of microorganisms and their corresponding metabolites in constipation-predominant IBS (IBS-C) and diarrhea-predominant IBS (IBS-D) differ, thereby providing a new avenue for the diagnosis and treatment of different IBS subtypes in the future. These microbiota-derived metabolites, such as bile acids (BAs), SCFAs, vitamins, amino acids, 5-HT, and hypoxanthine, can be produced directly by bacteria, or from dietary or relevant substrates. Fluctuations and alterations in the levels of metabolites produced by the host or microbiota provide insights into their interactions during IBS. Moreover, low levels of hypoxanthine may be associated with colonic epithelial energy and capacity for mucosal repair with hypoxia. Purine starvation has been identified as a potential novel mechanism underlying IBS with lower fecal hypoxanthine abundance in IBS-C and IBS-D. Additionally, mucosal biofilms are an endoscopic feature of IBS and are associated with bacterial and BA metabolites dysbiosis. Additionally, deficiency in levels of both vitamins D and B6 have emerged as causative factors in IBS symptoms pathogenesis.283
Microbial dysbiosis and metabolites derived from interaction of the host and gut microbiota have been reported as an intermediate link contributing to the development of functional constipation via various signal pathways, including but not limited to SCFAs, BAs, and methane that occupied a more important position.153 5-HT is also involved in the modulation of gut motility and secretory, as well as sensory, transmission in patients with constipation.284 Altogether, current studies have provided us with a new conception on the microbial mechanisms and therapeutic targets of constipation.
The gut microbiome metabolites and their function and associated digestive diseases/disorders are summarized in Table 3.
Gut Microbiome–Brain Axis
CNS and the human GI tract communicate through the gut–brain axis (GBA). This bidirectional connection involves neuronal, endocrine, and immunological mechanisms. The gut is considered as our “second brain,” because of its hosting the enteric nervous system (ENS), a neural network that allows the gut to work without instructions from the brain. The ENS maintains control of our digestive system; it plays an important role in peristalsis, secretion, and pain perception. There is mounting data that gut microbiota are the source of a number of neuroactive and immunocompetent substances, as shown above, that help to shape the structure and function of brain regions involved in the control of emotions, cognition, and physical activity and contribute to the proper maintenance of gastrointestinal homeostasis. Most GI diseases are associated with altered transmission within the GBA that are influenced by both genetic and environmental factors.317
Functional gastrointestinal disorders (FGIDs) were previously considered as purely functional disorders with no scientific confirmation of a clear pathogenetic mechanism. According to Rome IV, the phenotype of FGIDs results from an altered transmission of nerve and biochemical signals within the gut microbiome–brain axis with mechanisms controlled by both genetic and environmental factors. Consequently, FGIDs were recently renamed into disorders of gut–brain interactions.318 The overlap of DGBI and CNS disorders has been documented, and it has been demonstrated that approximately one-third of IBS patients suffer from depression. It is estimated that psychiatric symptoms occur in at least 36.5% of FGIDs patients. Stasi et al. found that the highest prevalence of mental or spectrum disorders is in patients with functional constipation (60%) compared with patients diagnosed with FD (52.4%), IBS (36.5), and/or functional bloating (47.6%). The most prevalent psychiatric disorders observed in FGIDs were general anxiety disorder and panic.319
Recent advances in this field have enabled us to better understand some of the pathophysiological consequences of an aberrant reciprocal gut–brain network, including exacerbated gut inflammation disorders, altered responses to stress, as well as altered behavioral states. Therefore, the GBA presents an attractive target for the development of novel therapeutics for an ever-growing list of disorders related to mental and digestive health. Improved targeting of the gut microbiome–brain axis, for example through application of biotics, is expected to pave the way for the development of novel disease therapies and self-care products to promote and maintain heathy status.320
Irritable Bowel Syndrome
IBS is one of the most common DGBI worldwide and typically presents in early adulthood with symptoms including abdominal pain, bloating, and altered bowel habits. On the basis of the bowel habits, IBS can be classified into four subtypes: constipation-predominant, diarrhea-predominant, mixed-type (IBS-M), and undefined IBS (IBS-U). Symptom intensity varies over time and between individuals, but IBS has been reported, in severe cases to affect quality of life as much as renal impairment or diabetes.321 IBS represents up to 50% of all referrals to gastroenterologists with a prevalence rate of up to 11% globally.322 The recent consensus view is that IBS results from abnormal gut–brain interactions. Recent epidemiological data has suggested that in individuals developing both IBS and psychological features, the former preceded the latter in two-thirds of cases, and the latter preceded the former in one-third.323 IBS is associated with abnormalities of central pain processing but also increased gut permeability, mast cell activation, disordered motility, and dysbiosis.
A recent genome-wide analysis study for 53 400 IBS patients and 433 201 controls highlights shared genetic pathways between IBS and mood and anxiety disorders. The study identified and confirmed six genetic susceptibility loci for IBS, and four of them are associated with mood and anxiety disorders, thereby suggesting, for example, that shared pathogenic pathways rather than anxiety cause abdominal symptoms.324
5-HT signaling is one particular pathway of importance in IBS pathogenesis. It has been demonstrated that a functional GI tract involves 5-HT signaling between enterochromaffin cells acting as sensory transducers, and the majority of 5-HT is synthesized, stored, and released by these cells, which interact with intrinsic and extrinsic sensory nerve afferents in the mucosal layer of the gut.320 5-HT signaling controls many GI functions, including secretion; vasodilation; peristalsis; and sensory perception, such as pain and nausea.325−327 Moreover, serotonergic function and tryptophan metabolism are known to be altered in IBS patients.328−331
IBS pathophysiology implicates altered gut microbiota composition, impaired intestinal mucosal integrity, and low-grade inflammation. In addition to pathways through the circulatory system, several of these factors may also trigger fluctuations in the activity of the ENS with subsequent effect on the brain.332 Furthermore, the vagus nerve can be modulated by diet-responsive gut microbes and metabolites, such as short-chain fatty acids, or endocrine factors, enzymes, and neurotransmitters, such as serotonin, dopamine, acetylcholine, glutamate, γ-aminobutyric acid, and noradrenaline.333−336 Each of these factors are potentially affected by alterations in microbiota composition and are involved in IBS pathology.337
Identifying a clear IBS microbial signature is not an easy task because of the heterogeneity of the healthy gut microbiota. However, multiple studies have shown differences in the gut microbiota between IBS and healthy controls. A recent systematic review showed that IBS patients have increased levels of the bacterial families Enterobacteriaceae, Lactobacillaceae, and Bacteroidales, whereas Bifidobacterium, Faecalibacterium, and Clostridiales were decreased compared with healthy controls.122 On the contrary, Hugerth et al. recently reported no distinct microbiota signature of IBS in a random Swedish population of 3556 participants.338 Interestingly, intestinal bacterial composition has been reported to be highly dependent on sample type and regional localization. Also, mucosa-associated bacterial composition of the sigmoid colon differs between patients with IBS and healthy controls.339
One of the most consistent findings in brain neuroimaging of IBS patients has been alterations in the structure and function of key regions of the somatosensory network, including the globus pallidus, putamen, and caudate, which composes the basal ganglia.340 Increased gray matter density in the hypothalamus and decreased gray matter density in the prefrontal cortex have been reported in the IBS brain.341 In rectal distention experiments, patients with IBS had a differential brain response in the pain matrix and default mode network.342 IBS patients showed increased engagement of endogenous pain faciliatory pathways and decreased levels of the endogenous pain inhibitory mechanism in the brain regions associated with visceral afferent processing and emotional arousal, including the left dorsal anterior cingulate gyrus and the bilateral anterior insulae.343 A meta-analysis of adult studies that evaluated brain response to rectal balloon distension by functional MRI (fMRI) reported differences between healthy control subjects and patients with IBS in these brain regions.344 IBS patients with a history of abuse reported increased pain and anxiety with rectal distension accompanied by similar fMRI changes.345 The stress and arousal circuit demonstrated in human subjects by fMRI shares significant homology with the stress circuit related to CRF–CRF1 receptor signaling in rodents, thereby potentially implicating the HPA axis as a facilitator of gut–brain axis communication.346
Recently, evidence for disrupted subcortical and cortical regions mediated by gut microbial modulation has been emerging in IBS. An association between brain region-to-region functional connectivity and microbiota has been reported. Labus et al. found a correlation between Clostridia and Bacteroidia with connectivity of the thalamus, the basal ganglia (caudate nucleus, putamen, pallidum, nucleus accumbens), the superior part of the precentral gyrus, the anterior insula, and the ventral prefrontal regions in IBS patients.347 Recently, the same group also reported on fecal metabolites and resting state fMRI where the differences in histidine, cysteine, glycine, glutamate, spermidine, and anserine were significantly associated with the alteration in the left dorsal part of the posterior cingulate gyrus to the left putamen. Also, the changes in histidine, tryptophan, uracil, 2-deoxyuridine, thymidine, and succinate were differentially associated with the alteration in the right superior frontal gyrus to the right putamen. Interestingly, this interaction may be mediated by aberrant tryptophan signaling in IBS, which is important because it is a substrate for serotonin synthesis.348
Previous studies have compared brain differences between IBS-C and IBS-D. These studies have examined task states and have shown group-related differences in brain networks involved in integrating emotions [emotional arousal network, (EAN)], perception [sensorimotor network, (SMN); salience network, (SAL)], visceral functions [central autonomic network, (CAN)], and pain processing [default mode network, (DMN); central executive network, (CEN); SMN; SAL; EAN; and others).349 Prior studies comparing IBS-C, IBS-D, and healthy controls (HCs) undergoing aversive rectal stimuli have identified abnormal connectivity in the SAL350,351 and EAN343,350 in IBS-C and in the occipital network (OCC)351 in IBS-D. This may indicate a greater importance of alterations in sensory, emotional, and autonomic responses associated with the perception of visceral pain and discomfort in IBS-C. A recent study has tested the hypothesis that IBS-C exhibits bowel-habit-specific changes in the brain that reflect altered sensory and emotional regulation processing of visceral inputs from the “top-down,” while IBS-D would have widespread gut microbiome and metabolome changes (e.g., tryptophan, SCFAs), which may translate to “bottom-up” brain changes (e.g., SMN, DMN).349 Indeed, in IBS-D, the study’s findings showed a correlation between high levels of gut metabolites tryptophan and phenylalanine and aberrant connectivity in brain regions involved in processing unpleasant visceral stimuli (SMN) and self-related thoughts (DMN).349 These results suggest that increased tryptophan in the gut may lead to loosened stool, and tryptophan-related signaling may travel to the posterior insula and increase pain perception and emotional salience in IBS-D, thereby suggesting a “bottom-up” signaling direction. However, IBS-C’s microbiome and metabolome resembled HC, and the increased connectivity in the default mode (DMN) and salience (SAL) networks compared with IBS-D may indicate abnormalities in the emotional physiological processing of visceral signals.349 IBS-C’s relatively isolated brain changes may indicate a more “top-down” mechanism to produce the constipation-predominant phenome. That study by Sarnoff et al. has shown a link between the chronicity of IBS symptoms, B. stercoris and F. prausnitzii, and brain connectivity in the caudate nuclei.
Thus, we might be in the mere beginning of understanding how alterations in gut microbiota may lead to the disruption of the intricate host–gut–microbiota interaction: is it a cause or a result of IBS pathology? In the past decade, much knowledge has been gained from clinical microbiota-altering interventions, such as the low-FODMAP diet and fecal microbiota transplantation (FMT), which have emerged as debatably successful treatment strategies. However, their effects on the gut microbiome–brain axis are still far from understood. It has been proposed that probiotic amelioration of IBS symptoms may be acting indirectly through an anti-inflammatory mechanism.352 Such anti-inflammatory mechanisms may also be partially responsible for the positive effects of dietary restrictions, such as those seen in the low-FODMAP (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols) diet in IBS.353−355 An alternate method for IBS treatment involves FMT from non-IBS individuals to patients with IBS.356−359
Functional Dyspepsia
Functional dyspepsia (FD) is a worldwide prevalent DGBI affecting 10%–30% of adults and 3.5%–27% of children worldwide.360 The main clinical symptoms of patients are early satiety, postprandial discomfort, epigastric pain, epigastric distension, epigastric burning, loss of appetite, belching, nausea, and vomiting, which are often accompanied by anxiety and depression.361 FD pathogenesis is linked to GI dysmotility, visceral hypersensitivity, impaired gastric tolerance, disrupted gastrointestinal mucosal integrity, abnormal function of the gut microbiome–brain axis, increased eosinophils in duodenum, dysbiosis, Helicobacter-pylori infection, postgastrointestinal infection, diet, genetics, and mental and psychological factors.278
An increasing number of studies have confirmed the close association between disturbance in the relative abundance and composition of the gastrointestinal microbiota and the occurrence and progression of FD. Actinomyces, Atopobium, Leptotrichia, Prevotella, and Veilonella counts differ between FD and control patients.362 The finding was preceded by an observation that, in FD patients, gut barrier integrity is impaired and expressed as lowered transepithelial resistance; diminished expression of proteins of tight junctions; and lastly, elevated levels of mast cells, eosinophils, and interstitial lymphocytes.363
Surprisingly, FD patients not only had different gastrointestinal microbiota compared with non-FD, but also had different oral microbiota abundance and composition. Proteobacteria were the dominant bacteria in FD patients’ saliva, while Bacteroidetes were the dominant bacteria in healthy controls. The abundance of Spirochaetes in FD patients was higher than that in healthy controls, while the abundance of Fusobacteria, TM7, and Proteobacteria was lower than in healthy controls, and the levels of Kingella and Abiotrophia genus levels were also significantly different.364
Mental illness plays a significant role in the pathogenesis of FD. Anxiety at baseline has been shown to increase the risk of developing FD by almost 8 times after 10 years follow-up.365 Interestingly, multiple studies highlighted that the prevalence of anxiety and depression is significantly increased in patients with FD compared with healthy people. Furthermore, pathophysiological research indicates that psychosocial factors and mental disorders may play a role in FD by modulating both visceral signal processing in the brain366 and the effects of stress hormones on pain perception.367 Furthermore, it is known that psychosocial factors and stress hormones also affect other aspects of the GI tract, such as motility, immune system activation, permeability, and microbiota.
However, FD symptoms are thought to induce anxiety or depression because of a cytokine response in low-grade intestinal inflammation, which plays an important role in the development of psychological distress in patients with FD.368 A growing body of evidence suggests that the gut microbiota communicates with the central nervous system, possibly neuro-immuno-humoral pathways, thereby influencing brain function.369 Furthermore, microbiota release neuroactive compounds, such as GABA, serotonin, dopamine, and acetylcholine, thereby acting locally on the enteric nervous system. Some of these neuroactive substances access the brain through the blood and the circumventricular organs or via the vagus nerve. Therefore, it could be hypothesized that a disturbed microbiome might affect mental health, followed by anxiety and depression. Thus, the mental disorders might be a consequence of the dysbiosis and, therefore, promote the development of FD, which may explain the findings that anxiety increases the risk of FD365 and observations indicating that psychosocial factors and mental disorders may play a role in FD by modulating visceral signal processing in the brain.366,370
Functional Constipation
Functional constipation (FC) is a common DGBI with a global prevalence ranging approximately from 10.1 to 15.3%371 and is characterized by difficult bowel movements and/or a sense of incomplete evacuation, thus influencing quality of life.372 Previous studies showed that the gastrointestinal microbiota composition of constipation is clearly distinct from that of normal individuals. The species diversity of microbiota in the patient samples was lower than that in healthy subjects; it was also accompanied by significantly reduced levels of Bifidobacterium and Lactobacillus and an increased abundance of Desulfovibrionaceae.373 Levels of butyrate-producing bacteria, such as Faecalibacterium and Roseburia, were significantly reduced in patients with FC.373 It has also been confirmed that the relative abundance of methanogenic bacteria is increased in patients with slow transit constipation relative to healthy subjects.373,374 In another study, Chen et al. collected 3056 fecal amplicon sequence data from five research cohorts and used machine-learning methods to construct the constipation discriminant model. The model identified 15 top-ranking biomarkers, particularly inflammation-related pathogenic bacterial genera Serratia, Dorea, and Aeromonas.375 A recent shotgun metagenomics study confirmed the results of previous studies by showing that the relative abundance of Roseburia intestinalis, a prominent butyrate-producing bacterium, was reduced in patients with constipation in comparison with healthy controls, and the microbiome corresponding to constipation was enriched for pathways implicated in methanogenesis.376 In contrast, the microbiome of healthy individuals was characterized by high levels of genes associated with carbohydrate, fatty acid, and lipid metabolism.150
Notably, different intestinal sites harbor certain gut microbiomes, yet the majority of recent research has focused on the analysis of fecal-derived microbiota, which are accessible via noninvasive sampling methods. However, luminal microbiota is generally considered to be representative of the distal large intestinal content. The mucosa-associated microbiota, which live in more intimate contact with the host, cannot be fully replicated by fecal microbiota.377 In patients with constipation, there is even less similarity between fecal- and mucosa-associated microbiota compared with healthy controls and patients with diarrhea.116 These differences may be due to drier stool allowing fewer signaling molecules to enter the mucosa378 or the longer transit time providing more opportunities for the communities to diverge.116 Hence, mucosa-associated microbiota are more likely to affect the host’s epithelial and mucosal function than luminal microbiota.379 Comparative analyses between fecal and mucosal microbiota showed that the colonic mucosal microbiota composition was correlated with constipation (and was accompanied by a significant increase in Bacteroidetes), while the fecal microbial communities were correlated with colonic transit and methane production rather than constipation.380 However, more evidence is needed to prove the relationship between the mucosal profile and constipation.
Slow gut transit has been associated with reduced fecal water content, higher fecal pH, higher microbial cell density and diversity, and a shift in microbial metabolism from saccharolysis toward proteolysis, as reflected by reduced levels of short-chain fatty acids and increased levels of branched-chain fatty acids (BCFA).381 It is likely that once easily accessible carbohydrate sources become scarce in the colon, the gut microbes switch to ferment dietary and mucin-derived proteins. While saccharolysis by the gut microbiota gives rise to SCFA that are beneficial for the host and a source of energy for the colonocytes, proteolysis can lead to the accumulation of compounds such as BCFA, phenols, indoles, ammonium (NH3) and hydrogen sulfide (H2S) that are generally considered detrimental for health. Moreover, hydrogen (H2) with carbon dioxide (CO2) or formate can be converted into methane (CH4) by methanogenic archaea, which are also linked to slower transit time. In addition, the production and circulation of secondary bile acids and hydrolysis of host-derived glucuronides excreted via bile can also be affected by alterations in gut transit time.381
FC in patients would accompany mental disorders like anxiety and varying severity of depression.382 Evidence from recent neuroimaging studies illustrated that FC patients had significant structural and functional alterations in brain regions that are involved in visceral sensorimotor, cognitive control, and emotional regulation,383−389 thereby confirming its reclassification as DGBI. Among these altered brain regions in FC patients is the anterior insula, which is generally regarded as a critical node for its essential role in processing interoceptive signals, modulating visceral activities, and regulating emotions and cognitions.383,385,389−391
Stress and Stress Resilience
Chronic stress is rapidly becoming a global societal challenge. Stress constitutes a state of threatened homeostasis triggered by intrinsic or extrinsic adverse forces (stressors) and is counteracted by an intricate repertoire of physiological and behavioral responses aiming to maintain/reestablish the optimal body equilibrium (eustasis). Stress is a nonspecific response of the body to any demand imposed upon it that disrupts the body homeostasis and manifests with symptoms such as anxiety, depression, or even headache.392 Stress can be hardly avoided in the present-day, modern, competitive life. Although eustress is important for people’s rapid reaction to threats, chronic stress is associated with detrimental effects on physical health and adverse implications on the immune, neuroendocrine, and central nervous systems.392
Acute stress activates the HPA axis, thereby resulting in an immediate release of cortisol. This response prepares the individual to defend against or escape from a threat. After the threat subsides, normal homeostasis should return. However, when that fails to occur, chronic activation of the stress response results in dysregulation of the HPA axis and an increased risk of subsequent diseases/disorders.320 It also acts on the gut to increase the release of proinflammatory mediators, which leads to increased gut permeability.393 Repeated exposure to stress can initiate a vicious cycle of low-grade inflammation and negatively impacts the intestinal barrier and immune signaling within the gut.394,395
A link between stress and the abundance of lactobacilli in mice was discovered for the first time more than 40 years ago.396 Several preclinical studies have documented that stress impacts gut microbial composition in a number of different hosts using different stress models ranging from water avoidance to maternal separation, heat, and acoustic stress and overcrowding.320 These results have shown clinical relevance and translated into human studies, thereby showing the influence of stress on gut microbiota and gut microbiota on stress modulation through different stressors, such as surgical intervention, academic examination, or military training, among others.397−400
Maternal stress during pregnancy displays a distinct fecal microbiota profile, which has generational consequences. The maternal microbiota influences offspring microbiota and correlates with hyper-reactivity of the HPA axis, together with other perinatal factors, as a key determinant of offspring outcomes. These findings have been confirmed in humans in a population-based study whereby infants born to mothers with high cumulative stress during pregnancy exhibited an aberrant microbial composition.401
The effects of early life stress on the microbiota may extend to adulthood.49 It is, therefore, plausible that changes in the gut microbiota due to stress at least partially mediate the onset of stress-related depressive or anxious episodes. Correlational studies have shown that fecal microbiota composition in individuals with anxiety or depression differs from that in healthy controls.202,402,403 Women with a higher fecal Prevotella abundance experienced increased negative emotional response to viewing negative images and lower brain activity in the hippocampus than those with a higher Bacteroides abundance.404
Stress resilience is the ability to experience stressful events without the development of chronic elevated stress (psychological and/or biological) and associated changes in emotional behavior.405,406 Stress susceptibility is related to psychological factors, such as passive coping skills and high emotional reactivity, but is also associated with biological factors such as hypo- or hyper-responsiveness of the stress response system, sex hormones, central and peripheral immune activation, and glucocorticoid resistance.407 The gut microbiome is a biological factor that is emerging as a possible influencer of stress resilience. The broad influence of the gut microbiota on human health, including psychiatric health, has begun to be realized and understood over the past decade. Preclinical studies have reinforced this principle showing a connection between the gut microbiome–brain axis and stress resilience. Li et al. recently reported that certain mice exposed to chronic stress were found to be resilient to stress-induced corticosterone and anxiety-like behavior. These mice contained a relative abundance of Lactobacillus species within their gut microbiome. Subsequent stress-susceptible mice saw decreased anxiety-like behavior and corticosterone levels with Lactobacillus murinus supplementation.408
Modulation of the gut microbiome has emerged as a possible way to improve stress resilience and mental health. In 2013, Dinan et al. coined the term psychobiotics, which refers to live microorganisms when ingested in adequate amounts that produce a health benefit in patients suffering from psychiatric illness; the definition has been expanded to include other interventions that modulate the gut microbiome, such as prebiotic.409 The term psychobiotics has since been widely adopted by neuroscientists conducting research on neurodegenerative diseases and depressive disorders in order to describe the use of different biotics to tackle depression, stress, anxiety, and other mental health complaints through the GBA.
Sleep
Adequate sleep quality and sufficient duration are necessary to support both mental and physical health and overall quality of life.410 Inadequate sleep in either duration and/or quality has been increasingly recognized as a global public health issue. Sleep disturbances are typically characterized by a decrease in one’s ability to initiate and maintain sleep and by a reduced proportion of the deeper, more restorative sleep.411 Increased risk of developing chronic diseases, such as obesity, type 2 diabetes, heart disease, some types of cancer and mental illness, has been associated with inadequate sleep.411,412
Evolving evidence has shown the impact of gut microbiota on sleep. In humans, previous research has shown that partial sleep deprivation can alter the gut microbiome composition in as little as 48 h;413 however, longer periods of sleep deprivation apparently do not have this effect.414 A more recent study showed that high sleep quality was associated with a gut microbiome containing a high proportion of bacteria from the Verrucomicrobia and Lentisphaerae phyla and that this was associated with improved performance on cognitive tasks.415
Microbiome diversity was positively correlated with sleep efficiency and total sleep time and was negatively correlated with the sleep fragmentation, thereby indicating that diversity of the gut microbiome promotes healthier sleep. However, two previous studies in humans suggested that microbiome diversity is insignificantly affected following a period of sleep restriction.413,414 A critical difference between these studies is that the former study measured sleep over an extended period of time (one month), while the latter two studies manipulated sleep by experimentally restricting sleep. Accordingly, it is possible that short-term manipulations to sleep do not influence the gut microbiome diversity, but rather that microbiome diversity can influence sleep in the long term.
Gut microbiota may affect sleep status via degradation products, such as muramyl peptides (MPs), lipopolysaccharide, and melatonin.416 These degradation products could activate immune cells that lead to the release of cytokines, which could affect sleep. Cytokines represent a potential critical interface between sleep physiology and gut microbiome composition. The acute phase pathway, cytokines IL-1β and IL-6, in particular, are strongly associated with sleep physiology. IL-1β is a major somnogenic factor.416 IL-1β administration in human and nonhuman animals increases spontaneous sleep and fatigue, and IL-1β increases with ongoing sleep loss.416 Unlike IL-1β, IL-6 is not a direct somnogenic factor, but sleep loss results in increased IL-6 levels.417 In the gut, IL-6 and IL-1β-mediated inflammation fluctuates in response to stress and disease.418 For example, intestinal mucositis results in an increased expression of IL-6 and-IL-1β in the small intestine419 and in serum and colon tissue420 in mice. In humans, chronic stress, alone, increases IL-6 and-IL-1β.
Alterations in the microbiome have been shown to influence neurotransmission of serotonin in both the peripheral and central nervous system.411 While this may convey a positive impact on mood and psychological well-being,421 it also has the potential to influence sleep422 as serotonin is acetylated and, then, methylated to yield melatonin—the hormone important in helping regulate sleep/wake cycles.423
A recent meta-analysis involving 36 studies showed that sleep disorders were common in IBS, and the prevalence rate was 37.6% (95% confidence interval: 31.4% to 44.3%).424 The pooled odd ratio revealed that sleep disorders were significantly associated with IBS. The reason why sleep disorders are associated with IBS remains unclear; however, the gut microbiome–brain axis could play an important role in the pathogenesis of both. Modification of the autonomic nervous system activity has been observed in cases of sleep deprivation, which indicates that sleep disorder might be associated with autonomic dysregulation.425,426 It has been postulated that sleep inhibits the HPA axis, and sleep disorder may result in a 24 h increased secretion of cortisol.427 Moreover, IBS symptoms, such as abdominal pain, may activate the sympathetic nervous system and, hence, reduce sleep efficiency.428 Microbiome modulation has been shown to influence melatonin production and modulate IBS symptoms in individuals with a normal circadian rhythm.429 Overall, although the reason for sleep disorders seen commonly among IBS patients is obscure, a gut microbiome–brain axis disorder may underlie this association.427
An additional layer of evidence has been shown by the intertwined interactions between the gut microbiome and the central and peripheral circadian rhythms.430 The disruption of the host circadian rhythm alters the gut microbiome equilibrium. In addition, the microbiome is able to mediate host clock gene expression in peripheral organs and the suprachiasmatic nucleus.
Intestinal bacteria have shown inherent circadian rhythms, as shown in previous metagenomic studies.430 Diurnal fluctuations in abundance and activity have been observed in Clostridiales, Lactobacillales, and Bacteroidales, which account for ∼60% of the microbiota.431 Studies on human stool samples of Enterobacter aerogenes have demonstrated responsivity to the circadian hormone melatonin, as well as a daily rhythm.432 The gut epithelium experiences differential bacterial species and metabolites depending on the time of day and expresses toll-like receptors that sense microbiotal metabolites in a rhythmic pattern.433 It has been proposed that the gut microbiome influences the rhythmic expression of the host’s internal clock by signaling molecules, such as butyrate, and by oscillations in microbiotal bacterial content in response to feeding patterns.434
Cognitive Function
Abundant evidence supporting the role of the gut microbiota in modulating cognitive function is mostly based on animal research. However, few studies have examined the influence of gut microbes on human cognition and supported the clinical relevance. One of these studies showed that the gut microbiota composition of obese and nonobese subjects was linked with scores in speed, attention, and cognitive flexibility coupled with alterations in neural activity in the thalamus, hypothalamus, and amygdala, thereby suggesting that obesity affects the microbiota composition and subsequent cognitive performance.435 Additionally, the microbiota composition in 1-year-old babies was associated with cognitive development. Three groups of microbial composition have been identified where better performance was seen in the group with higher levels of Bacteroides.436 This group was also less likely to be born via C-section, which supports the previous observation linking delivery mode with child cognitive development,437 thereby highlighting the importance of gut microbiota colonization in cognitive development and function.436
Microbiome modulation has demonstrated beneficial effects on cognitive performance. Lactobacillus strains have improved cognitive performance in healthy elderly subjects.438 Fermented milk product supplemented with a probiotic has been shown to modulate the activity of brain regions involved in cognitive performance during an emotional attention test in healthy women.439 Also, the modulation of the microbiome via inulin prebiotic has been shown to improve memory and mood in healthy individuals.440
These results taken together indicate the potential role of the gut microbiome–brain axis in regulating cognitive performance and that microbiome modulation could be a promising approach for improving cognitive function in both healthy and vulnerable individuals. However, much more work is needed to understand why specific microbiome-related interventions have the potential to modulate cognition.
Emotional Well-being
Gut microbiota is emerging as a key mechanism for modulating emotional well-being.441 In fact, emotional disorders, such as depression and anxiety, are frequently accompanied by functional gastrointestinal disorders, which suggests an association between gut function and psychiatric diseases.442,443 Recent research reveals the gut–brain axis association with the vagus nerve plays an important role in emotional well-being. The subdiaphragmatic vagus nerve is a major modulatory pathway between the brain and gut microbiota.444 Data suggest that fecal microbiota from depressed mice produce depression-like phenotypes and abnormal gut microbiome composition when transplanted to nondepressed mice via the subdiaphragmatic vagus nerve.445
Gut microbiota is emerging as a key mechanism for the modulation of emotional well-being.441 In fact, emotional disorders, such as depression and anxiety, are frequently accompanied by functional gastrointestinal disorders, which suggests an association between gut function and psychiatric diseases.442,443 Findings from a longitudinal study further suggest that intestinal infections significantly predict the future onset of anxiety disorder.446
Evolving clinical evidence indicates the significant links between the gut and emotion; for example, altered gut microbiota composition447 was reported in patients with depressive disorder in terms of fecal microbial diversity, as well as the level of the genus Faecalibacterium.403 In healthy adults, self-rated higher quality of life and favorable personality types (high in openness and conscientiousness) were associated with the composition of certain gut microbiota (i.e., Faecalibacterium, Coprococcus, and Lachnospiraceae), as well as an enriched diversity of the gut microbiota community.448,449
Recent studies suggest that enterotypes play a role in regulating the association between the gut microbiome and mental health.404,449 Enterotypes refer to robust stratified clusters on the basis of the variation found in the levels of one of three genera in the gut: Bacteroides (enterotype 1), Prevotella (enterotype 2), and Ruminococcus (enterotype 3).450 Individuals’ enterotype cluster depends on, in part, long-term diet—i.e., the amount of ingested animal protein/saturated fats (Bacteroides-type) versus carbohydrates/simple sugars (Prevotella-type)—whereas they are less influenced by the hosts’ body mass, age, and sex.450−452
A recent brain imaging study found distinct patterns of the emotional process and brain connectivity between stratified enterotypes; clusters with a greater abundance of Prevotella show higher levels of emotional response, along with prominence in the connectivity of emotional, attentional, and sensory-processing brain regions when compared with Bacteroides-dominant clusters.404 A large-scale microbiome study also revealed that the Bacteroides-enriched enterotype is significantly associated with a lower score on the subjective feeling of quality of life, as well as a higher score regarding depressive symptoms.
A recent exploratory study revealed that gut microbiome diversity is related to emotional well-being and that enterotypes significantly moderate the links between emotional well-being and gut microbiome diversity.441 The enterotypes did not alter mood status, itself, but moderated the strength of the association between one’s mood and gut microbiome diversity. In the Prevotella-dominant group, emotional status was more closely related to gut microbiota diversity, such that a positive effect was associated with increased gut microbiota diversity. However, in the Bacteroides-dominant group, one’s mood status was not significantly associated with gut microbiome diversity. This finding is in line with the results of Tillisch et al.404 in that only the high-Prevotella group displayed an increased response to affective images in the limbic system. Such findings suggest a significantly tighter connection between emotional well-being and the well-being of the gut microbiota community, particularly in the Prevotella-dominant condition.
There is an evolving body of evidence suggesting that the modulation of the gut microbiome by biotics administration or via food supplements (i.e., psychobiotics) may closely affect one’s mood. Benton et al.421 showed that the consumption of probiotic-containing yogurt improved the self-reported mood of those whose mood was initially poor. Messaoudi et al.176 showed that consumption of the probiotics reduced anxiety and depression scores in subjects with reduced urinary free cortisol. Also, consumption of Lactobacillus helveticas and Bifidobacterium longum reduced somatization, depression, anger and hostility, hospital anxiety, depression-scale global scores, and self-blame scores on coping checklists and increased focus on problem solving, but there was no effect on perceived stress.176 Moreover, the administration of a combination of Lactobacillus acidophilus, Lactobacillus casei, and Bifidobacterium bifidum for 8 weeks improved the depression score.453 In a 2017 systematic review by Wallace and Milev of 10 clinical trials, most of the studies found positive results on measures of depressive symptoms.454 A recent meta-analysis455 included 30 randomized placebo-controlled studies and revealed that most probiotics did not affect mood, anxiety, depression, and psychiatric distress when compared with placebo at the qualitative questionnaire level; however, on the quantitative meta-analysis level, probiotics intervention showed slightly significant effect compared with placebo. In addition, EEG and imaging studies summarized in a recent review proved that probiotics are able to exert effects on CNS function in humans; although, the number of studies is still low.455
Overall, there is emerging evidence on the link between one’s emotional status and gut microbiome diversity and composition. The current evidence suggests that emotional well-being and the feeling of happiness can be associated with gut microbiota profiles in healthy adults, especially when stratified by enterotype. The enterotype-specific links between emotional well-being and gut microbiome diversity suggest that enterotypes may work as an individually tailored intervention. With the expanding interest in the role of the gut microbiome–brain axis in mental health, this nascent field still needs to build up empirical evidence to fill the gap in our understanding of how gut microbiota communicates with the brain to affect emotional well-being and the feeling of happiness.
Prebiotics, Probiotics, Synbiotics, Postbiotics, and Psychobiotics
Probiotics
Probiotics are defined by the International Scientific Association for Probiotics and Prebiotics (ISAPP) as live microorganisms that, when administered in adequate amounts, confer a health benefit on the host.93,456 Probiotics usually comprise bacteria, including, among others, Lactobacillus, Bifidobacterium, and Bacillus, although a few strains of the yeast Saccharomyces have also been included in probiotic cultures. Probiotic microorganisms must have several features, including a demonstrated benefit to the host and the recognition that they are safe for human consumption, i.e., they have generally been recognized as a safe designation. They are resistant to acid and bile salts that are encountered in the GI tract. The ability to adhere to the intestinal epithelium is a useful trait that promotes both the persistence of the probiotic and host interactions. Other useful features are that they are easily cultured and are resistant to drying, freezing, and freeze-drying so that the probiotic may be produced and stored in bulk. Probiotics can enhance immune function, promote fiber assimilation for SCFA production, and help suppress pathogens in the GI tract.93,457,458
Prebiotics
Prebiotics are defined by ISAPP as a substrate that is selectively utilized by host microorganisms conferring a health benefit.31,93 Therefore, such a definition expands beyond the classical prebiotics composed of polysaccharide carbohydrates and could include any other nondigestible microbiome-fermentable ingredients, such as herbal secondary metabolites and flavobiotics. The definition stresses that the effects must be microbiota-mediated and that the beneficial health effects must be documented for a substance to be considered a prebiotic.459 The most well-known products of microbiome-derived fermentation are SCFAs, such as butyric acid, acetic acid, or propionic acid, which have a beneficial effect on the host. Moreover, prebiotics modulate lipid metabolism, increase calcium absorption, have a positive effect on the immune system, and reduce the risk of broad diseases.458
Synbiotics
ISAPP defined synbiotic as a mixture comprising live microorganisms and substrate(s) selectively used by host microorganisms that confer a health benefit on the host. The panel concluded that defining synbiotics as simply mixtures of probiotics and prebiotics could suppress the innovation of synbiotics that are designed to function cooperatively. The ISAPP panel differentiated synbiotics into two groups: complementary synbiotics and synergistic synbiotics. A complementary synbiotic that has not been designed so that its component parts function cooperatively must be composed of a probiotic plus a prebiotic. A synergistic synbiotic is a synbiotic for which the substrate is designed to be selectively utilized by the coadministered microorganisms.460
Postbiotics
Postbiotics are a class of products that has emerged in the last 10 years. Postbiotics are defined by the ISAPP as preparations of inanimate microorganisms and/or their components that confer a health benefit on the host. Effective postbiotics must contain inactivated microbial cells or cell components, with or without metabolites, that contribute to observed health benefits.458,461 Postbiotics, because they are derived from probiotic microorganisms, have many of the same benefits as probiotics. The effects of postbiotics are often more reliable and predictable than probiotics. Postbiotics tend to have a longer shelf life than probiotics and they are more target-specific and safer in terms of their interaction with the human GI tract.93
Psychobiotics
The term psychobiotic was coined to describe bacteria that confer mental health benefits. Psychobiotics have demonstrated the ability to improve mood, reduce anxiety, and enhance cognitive function in both healthy populations and patient groups. While the term psychobiotics originally referred to beneficial live organisms, such as bacteria that are specifically beneficial for mental health,409 the definition has been expanded in recent years to include prebiotics whose effect on the brain is bacteria-mediated.462 It is also worthwhile considering a wider definition of psychobiotics to include any substance that exerts a microbiome-mediated psychological effect or at least possesses psychobiotic properties, such as probiotics, prebiotics, synbiotics, and postbiotics.462,463 Recently a new term “phyto-psychobiotics” has been coined to describe medicinal plants whose mental effects are mediated via gut microbiota modulation by prebiotic-like effects, postbiotic-like effects mediated by the active secondary metabolites produced by the gut microbiome from the nondigestible herbal ingredients, or even by antibiotic-like effects as in the case with some medicinal herbs that have a mental impact by reducing the level of pathogenic bacteria.464
Pre-, Pro-, Postbiotics, and Fecal Microbiota Transplantation in the Development Pipelines
A search of the CAS Content Collection84 reveals the top organizations for research and journal publications related to the gut microbiome and mental and digestive health. All these top players are universities and research institutes, and the University College Cork, the Chinese Academy of Science, the University of California, and McMaster University lead the field (Figure 14A). The lead universities and medical centers related to patenting activity for the gut microbiome in mental and gut health include the University of California, Johns Hopkins University, and the University of Texas (Figure 14B).
Figure 14.
Top universities, research institutes, and hospitals publications (1967–2022) related to gut microbiome research in mental and gastrointestinal health: (A) journal publications and (B) patents.
Private Investment
Researching the overall global private investment activities of the microbiome field provides insight into the commercial interest into this area. Performing a search of prebiotics, probiotics, and the microbiome within PitchBook, an online source for investment data, reveals the overall venture capital activities. The search revealed that both capital raised and deal counts from venture capital investment are rising within this industry.465 From 2014 through 2018, the total capital raised increased from $250 million to over $1 billion. Deal counts followed the same pattern from 2014 to 2018 and increased from 25 to 125. The number of deal counts in 2019 further increased; however, the overall capital raised fell to just under $900 million. Deal counts continued to rise for 2020 and 2021, along with capital raised, which totaled over $2.1 billion, while 2022 ended with a slight decrease in total venture capital investment (Figure 15). The venture capital investment data in this area clearly shows a recent and increasing commercial interest surrounding prebiotics, probiotics, and the microbiome, thereby revealing its potential promise for therapeutic applications.
Figure 15.
Overall capital raised and deal counts of venture capital investment for the prebiotic, probiotic, and the microbiome field ($) (source: pitchbook.com).
Companies and Academic Institutions Investigating Treatment of Mental Disorders and DGBI through Gut Microbiome Modulation
With the GBA being a bidirectional communication network, we herein examine a highlighted selection of global companies and academic institutions researching and producing prebiotics, probiotics, postbiotics, and utilizing fecal microbiota transplantation to treat a variety of consumer health-related mental disorders and DGBI. This global analysis of worldwide companies and academic institutions, while extensive, is not comprehensive and provides insight into both the present and future state of the field.
Probiotics
Companies, along with universities and health institutions, are utilizing probiotics for the treatment of mental disorders at slightly different rates (Figure 16). Companies are focusing more on stress than universities and medical institutions, with all organizations researching anxiety at about the same rate. Universities and medical institutions are researching depression, cognition impairment, and sleep disorders at a higher rate than industry. With universities and medical institutions on the forefront of research, this shows the up-and-coming possibilities for probiotic products for the treatment of depression, cognition impairment, and sleep disorders being produced for consumers in the future. Research in the area of utilizing probiotics for DGBI is much more extensive and historically established (Supplemental Table 1) than mental health disorders (Figure 16). Companies, universities, and medical institutions research most DGBI at a similar rate, with universities and medical institutions having a higher focus on IBS. IBS is more prevalent among those who eat a Western diet. With more of the world’s population adopting a Western diet, the prevalence of IBS is expected to increase.466 The research among universities and medical institutions is shadowing this trend as researchers evaluate probiotic treatment options for this DGBI increasing in prevalence (Figure 16).
Figure 16.
(Left) The percentage and number of analyzed global organizations utilizing probiotics for mental disorders and DGBI treatment. (Right) The percentage and number of analyzed global organizations utilizing prebiotics for mental disorders and DGBI treatment.
Prebiotics
Fewer organizations are researching prebiotics for the treatment of mental disorders and DGBI when compared with probiotics (Figure 16). Prebiotics for DGBI are researched more than mental disorders, with more focus on IBS and functional constipation (Figure 16). This field has many future growth opportunities for both industry and research as it grows and becomes established. Similar to the number of organizations, the number of documents in the CAS Content Collections related to prebiotics is about three times lower compared with those related to probiotics. However, the growth rate in the prebiotics research has increased in the last two years.
Postbiotics
Postbiotics are the least researched therapeutic examined and are mainly limited to a small commercial presence (Figure 16). With such a small commercial and research presence, the opportunities are also abundant for postbiotic therapeutics.
Fecal Microbiota Transplantation
FMT research is found to be rare in comparison with other microbiome modulation strategies in mental disorders and newly established with only a very small number of companies and universities participating in this field (Figure 17). Most of the FMT research in DGBI focuses on IBS treatment (Figure 17). This field is currently showing clinical scientific evidence for the successful treatment of the serious and sometimes deadly condition of Clostridium difficile (C. Diff) infection.467 Recently, Australia’s Therapeutic Goods Administration gave the world’s first regulatory approval to company BiomeBank for its biologic microbiome product called Biomictra for the treatment of C. Diff infection.468 The US FDA followed a few weeks later with its official approval for the biologic drug consisting of live human-derived fecal microbiota, RBX2600 (Rebyota), produced by Ferring Pharmaceuticals, which is also for the treatment of C. Diff infection.469 These first regulatory approvals open this method to endless opportunities for the treatment of many different diseases, and more studies are needed to prove its safety and efficacy.
Figure 17.
(Left) The percentage and number of analyzed global organizations utilizing postbiotics for mental disorders and DGBI treatment. (Right) The percentage and number of analyzed global organizations utilizing fecal transplant for mental disorders and DGBI treatment.
Clinical Trials Landscape for Probiotics in Mental Disorders and DGBI
When examining clinical trials utilizing probiotics for the treatment of the mental disorders discussed within, there are currently a total of 52 clinical trials covering all stages between 2004 and 2022 listed on the US NIH clinical trials website.470 The most studied mental health disorders are stress, followed by depression, anxiety, cognition impairment, and sleep disorders. Clinical trials utilizing probiotics for the treatment of DGBI are the most prolific and historically studied with 174 clinical trials.470 The most studied DGBI are IBS followed by functional constipation, functional diarrhea, and functional dyspepsia.
The most researched probiotics for the treatment of mental disorders are the Lactobacillus species and a combination of Lactobacillus and Bifidobacterium species (Supplemental Table 1). For DGBI, the most widely used probiotic is the Lactobacillus species followed by the Bifidobacterium species, a combination of Lactobacillus and Bifidobacterium species, and finally the Saccharomyces species471 (Supplemental Table 1).
Highlighted clinical trials examining probiotics as a treatment option for mental disorders and DGBI are explored in Tables 4 and 5. A select few are also examined in further detail below to showcase a variety of interventions and targeted conditions in clinical development, along with their status. While experimental data in this area is showing promising results, there are still conflicting results being reported.
Table 4. Highlighted Clinical Trials Utilizing Probiotics for the Treatment of Mental Health Disorders.
| clinical trial identifier | condition | intervention | status |
|---|---|---|---|
| NCT05564767472 | depression, anxiety, stress | Bifidobacterium adolescentis Bif-038, Lacticaseibacillus rhamnosus LGG, Bifidobacterium BB-12 | recruiting |
| NCT03494725477 | stress, anxiety | Lacticaseibacillus paracasei Lpc-37 | complete |
| NCT04767997474 | sleep disorder | undisclosed probiotic formulation | recruiting |
| NCT03601559478 | cognitive impairment | Lactobacillus paracasei Lpc-37 | complete |
| NCT03615651479 | stress, cognition impairment | Lactobacillus helveticus, Bifidobacterium longum, Lactiplantibacillus plantarum | complete |
| NCT05567653473 | stress | Lactobacillus helveticus Rosell-52, Bifidobacterium longum Rosell-175 | recruiting |
| NCT03370458480 | stress | Lactobacillus plantarum DR7 | complete |
Table 5. Highlighted Clinical Trials Utilizing Probiotics for the Treatment of DGBI.
| clinical trial identifier | condition | probiotic intervention | status |
|---|---|---|---|
| NCT02592200485 | functional constipation | Lactobacillus gasseri DSM 27123 | complete |
| NCT04304170486 | functional constipation | Bifidobacterium animalis lactis (LMG P-28145) | complete |
| NCT04662957487 | diarrhea-predominant irritable bowel syndrome | Bifidobacterium breve BB010, Bifidobacterium longum BL020, Bifidobacterium bifidum BF030, Bifidobacterium lactis BL040, Lactobacillus rhamnosus LR110, Lactobacillus paracasei LPC100, Lactobacillus acidophilus LA120, Lactobacillus casei LC130, Lactobacillus plantarum LP140, Streptococcus thermophilus ST25 | complete |
| NCT05566171488 | functional constipation | Bifidobacterium lactis CNCM I-2494, Bifidobacterium lactis DN 173-010 | enrolling by invitation |
| NCT01463293489 | functional constipation | Bifidobacterium lactis HN019 | complete |
| NCT01102036490 | functional constipation | Lactobacillus paracasei F19, Lactobacillus paracasei LA-5, Bifidobacterium lactis BB-12 | complete |
| NCT03721107491 | diarrhea- and constipation-predominant irritable bowel syndrome | Blautia hydrogenotrophica | complete |
| NCT00534170492 | functional diarrhea | Lactobacillus casei Shirota, Bifidobacterium breve Yakult | complete |
| NCT00794924493 | functional diarrhea, functional constipation | Streptococcus thermophilus, Bifidobacterium breve, Bifidobacterium longum, Bifidobacterium infantis, Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus paracasei, Lactobacillus delbrueckii subsp. bulgaricus | complete |
| NCT02213172494 | irritable bowel syndrome | Bifidobacterium longum R0175, Lactobacillus paracasei HA-196 | complete |
| NCT00807326495 | functional diarrhea | Saccharomyces boulardii | complete |
| NCT05054309496 | irritable bowel syndrome | Bifidobacterium longum NCC3001 | recruiting |
| NCT01099696497 | functional dyspepsia | Bifidobacterium infantis 35624 | complete |
| NCT01887834498 | irritable bowel syndrome | Lactobacillus gasseri, Bifidobacterium bifidum, Bifidobacterium longum | complete |
| NCT04605783499 | functional diarrhea | Saccharomyces boulardii CNCM I-745 | not yet recruiting |
| NCT04950296500 | irritable bowel syndrome with diarrhea | Lactobacillus plantarum UALp-05 | complete |
| NCT05149599501 | irritable bowel syndrome | Saccharomyces cerevisiae | complete |
Mental Disorders
Clinical trial number NCT05564767 is currently recruiting participants to assess the treatment of depression and anxiety in adults by utilizing probiotics Bifidobacterium alone and Lacticaseibacillus combined with Bifidobacterium.472 Clinical trial number NCT05567653 is also recruiting for its study researching the treatment of stress in dancers with a probiotic product combination including Lactobacillus and Bifidobacterium.473 Another study (NCT04767997) looking at the effects of probiotics on sleep disorders is also currently recruiting subjects.474 Clinical trial number NCT03615651 researched the effect of a probiotic mixture containing Lactobacillus helveticus, Bifidobacterium longum, and Lactiplantibacillus plantarum on subjects’ functional brain responses during an emotionally stressful attention task.472 Their findings showed a positive effect on brain responses in regions implicated in emotional and cognition processing, which supports the growing evidence that probiotics can help positively influence emotional regulation and brain function.475 Another study (NCT04767997) that is looking at the effects of probiotics on sleep disorders is also currently recruiting subjects. Finally, a recently completed study (NCT03494725) found that supplementation with probiotic Lacticaseibacillus paracasei Lpc-37 significantly reduced perceived stress and anxiety in study participants.476
DGBI
Clinical trial number NCT05566171 is currently recruiting participants to research the treatment of functional constipation with two Bifidobacterium strains. Bifidobacterium was also researched in clinical trial number NCT04304170 and NCT01463293 for the treatment of functional constipation. Lactobaccillus and a combination of Bifidobacterium species and Lactobacillus were also researched for the treatment of functional constipation in completed clinical trials NCT01102036 and NCT02592200, respectively. Utilizing a combination of Streptococcus, Bifidobacterium, and Lactobacillus species, clinical trial number NCT00794924 researched the treatment of functional constipation and diarrhea in elderly hospitalized patients. The study showed positive results with the probiotics reducing days patients suffered from diarrhea or received laxatives, thereby displaying a positive effect on bowel movements. With diarrhea remaining a major public health concern in developing countries, study NCT00534170 researched the use of a probiotic drink containing both Lactobacillus and Bifidobacterium species for the treatment of diarrhea in young children. The study revealed the ingestion of a daily probiotic drink could prevent diarrhea in young children in a community setting within a developing country.481 Another study (NCT00807326) also researched the treatment of functional diarrhea. They compared the treatment results of antigas/antidiarrheal drug combination loperamide/simeticone and probiotic yeast Saccharomyces boulardii. They discovered that while the probiotic did help alleviate symptoms, it was inferior to loperamide/simeticone.482 Clinical trial number NCT01099696 also had discouraging results researching the treatment of functional dyspepsia with probiotic Bifidobacterium infantis 35624. While previous studies showed promise in patients with IBS, Bifidobacterium infantis 35624 did not show significant improvement in symptoms of abdominal discomfort and bloating in that study’s participants. Finally, a study (NCT04662957) researching a multistrain probiotic mixture of four Bifidobacterium, five Lactobacillus, and one Streptococcus species concluded its probiotic could offer benefits for patients with diarrhea-predominant IBS.483 Another study (NCT03721107) showed more positive results for the treatment of both diarrhea and constipation-predominant IBS. Patients reported improvement in bowel symptoms and pain with the use of Blautia hydrogenotrophica.484
Clinical Trials Landscape for Prebiotics in Mental Disorders and DGBI
When examining clinical trials utilizing prebiotics for treatment of the mental disorders discussed within, there are currently a total of 15 clinical trials.470 The most studied mental health disorder is stress, followed by anxiety, depression, sleep disorders, and cognition impairment.470 A total of 50 clinical trials utilize prebiotics for the treatment of DGBI.470 Similar to probiotics, the most studied DGBI using prebiotics is IBS, followed by functional constipation, functional diarrhea, and functional dyspepsia.470 The most commonly used prebiotic in clinical trials is galacto-oligosaccharides (GOS), followed by other sugars such as fructan, glucan, and dextrose, along with various fibers.
Highlighted clinical trials examining the treatment of mental disorders and DGBI with prebiotics are explored in Tables 6 and 7. A select few are also examined in further detail below to showcase the variety of interventions and targeted conditions, along with their status in clinical development. The use of prebiotics for the relief of mental and gastrointestinal disorder symptoms is producing promising results.
Table 6. Highlighted Clinical Trials Utilizing Prebiotics for the Treatment of Mental Health Disorders.
| clinical trial identifier | condition | intervention | status |
|---|---|---|---|
| NCT05372601504 | stress | GOS | complete |
| NCT05239845505 | sleep disorder | polydextrose, GOS | recruiting |
| NCT04324749506 | cognitive impairment, stress | roasted peanuts, peanut butter | complete |
| NCT05528575507 | stress | GOS, inulin, resistant potato starch RS2 | active |
| NCT04616937508 | anxiety, cognitive impairment | GOS | complete |
Table 7. Highlighted Clinical Trials Utilizing Prebiotics for the Treatment of DGBI.
| clinical trial identifier | condition | intervention | status |
|---|---|---|---|
| ISRCTN54052375512 | irritable bowel syndrome | GOS | complete |
| NCT04491734513 | gastresophageal reflux | maltosyl-isomaltooligosaccharides (MIMO) | complete |
| NCT05207618514 | irritable bowel syndrome with diarrhea | chestnut and quebracho tannin extract | complete |
| ACTRN12612001270808510 | functional constipation | green kiwi prebiotic, gold kiwi prebiotic | complete |
| NCT05340712515 | functional constipation | infant formula with lactose (prebiotic) along with probiotics | recruiting |
Mental Disorders
Clinical study NCT04616937 researches the use of GOS for the treatment of anxiety by altering the gut microbiota. GOS increases probiotic bacteria Bifidobacterium abundance in the gut microbiome. The study reports that the supplementation of GOS may improve signs of anxiety and cognition impairment with an increase of reported attention.502 Another recent study (NCT04324749) researched the prebiotic effect of peanut and peanut butter consumption on the cognitive and stress response of college students. The peanut prebiotic fiber and polyphenol content appears to enhance memory function and reduce stress because of the presence of both short-chain and very long-chain saturated fatty acids for healthy young subjects.503
DGBI
A published case study from 2019 showed a new observation that some patients taking a specific prebiotic soluble fiber, maltosyl-isomaltooligosaccharide (MIMO), had their gastroesophageal reflux symptoms resolve.509 Clinical trial NCT04491734 followed about a year later to research this effect. The prebiotic MIMO reduced the severity and frequency of gastroesophageal reflux symptoms and improved the quality of life for participants. Clinical study ACTRN12612001270808 researched the use of a green kiwi prebiotic (inulin) and a gold kiwi prebiotic to treat functional constipation. The study showed a successful increase in bowel movements and also revealed that green kiwi prebiotic (inulin) supports the increase of two probiotic bacteria, Bifidobacteria and Lactobacillus, within the gut microbiome.510 Another research study shows that gold kiwi prebiotic increased the abundance of Faecalibacterium prausnitzii within the gut microbiome, as well.511F. prausnitzii is a butyrate producer and displays anti-inflammatory effects.511 When prebiotic GOS was tested in a clinical trial (ISRCTN54052375), it increased the probiotic bacteria Bifidobacteria within the gut. This helped alleviate symptoms of IBS, thereby showing that GOS is a potential therapeutic agent for this disorder.512
Clinical Trials Landscape for Postbiotics and FMT in Mental Disorders and DGBI
Postbiotic and FMT are the least researched among all clinical trials explored. When examining clinical trials utilizing postbiotics for the treatment of mental disorders, anxiety is the only disorder studied (Supplemental Table 1). Clinical trials utilizing postbiotics for the treatment of DGBI focus on IBS.470 Only three clinical trials are researching FMT for the mental health disorders of depression, anxiety, and sleep disorders. Fecal microbiota transplants researching DGBI is higher with 27 trials researching both IBS and constipation.
Highlighted clinical trials examining the treatment of mental disorders and DGBI with postbiotics and FMT are explored in Tables 8 and 9. A select few are also examined in further detail below to showcase a variety of interventions and targeted conditions, along with their status in clinical development.
Table 8. Highlighted Clinical Trials Utilizing Postbiotics for the Treatment of Mental Disorders and DGBI.
| clinical trial identifier | condition | intervention | status |
|---|---|---|---|
| NCT05475314 (517) | irritable bowel syndrome | microbially fermented postbiotic oat drink | complete |
| NCT05562739 (518) | anxiety | multistrain postbiotic | not yet recruiting |
| NCT05339243 (519) | irritable bowel syndrome with diarrhea | heat-treated Bifidobacterium longum ES1 | recruiting |
Table 9. Highlighted Clinical Trials Utilizing Fecal Microbiota Transplantation for the Treatment of Mental Disorders and DGBI.
| clinical trial identifier | condition | intervention | status |
|---|---|---|---|
| NCT03822299522 | irritable bowel syndrome | fecal microbiota transplantation | complete |
| NCT02092402523 | irritable bowel syndrome | fecal microbiota transplantation | complete |
| NCT05035784524 | functional constipation | fecal microbiota transplantation | recruiting |
| NCT05427331525 | chronic insomnia | fecal microbiota transplantation capsule | recruiting |
Postbiotic Clinical Trials Landscape for the Treatment of Mental Disorders and DGBI
Clinical study NCT05562739 (recruiting) is researching a multistrain postbiotic for anxiety treatment in individuals who were placebo nonresponders from part one of the trial. A recently completed study (NCT05475314) researched the use of a postbiotic fermented oat drink for the treatment of IBS. The study resulted in positive outcomes and showed symptom relief in IBS subjects.516 Lastly, clinical trial NCT05339243 is currently recruiting for their study investigating both Bifidobacterium longum ES1 and the postbiotic heat-treated Bifidobacterium longum ES1 for the treatment of IBS symptoms in subjects with diarrhea-predominant IBS.
FMT Clinical Trials Landscape for the Treatment of Mental Disorders and DGBI
Clinical trial NCT05427331 is currently recruiting for its study examining sleep disorders. FMT through oral capsule administration will be researched to access sleep improvement in patients with insomnia. While fecal transplants are less prevalent for mental disorders, they have shown promise for DGBI with clinical trials by showing favorable results for the treatment of IBS. Clinical trial NCT02092402 showed effective treatment for patients with IBS when using a donor with a diverse microbial gut composition.520 Another study (NCT03822299) also showed success for FMT in IBS. Just as with trial NCT02092402, it also found that the donor’s fecal composition with a favorable microbial signature and diverse gut microbiota was essential for successful treatment.123 Three years after treatment, the study was still experiencing high response rates and long-standing effects.521
Noteworthy Probiotic and Prebiotic Patents
There are a diverse and growing number of patents related to probiotics and prebiotics in the CAS Content Collection. Listed in Table 10 are noteworthy prebiotic and probiotic patents related to the treatment of mental disorders and DGBI.
Table 10. Notable Prebiotic, Probiotic, and Postbiotic Patents.
| patent number | title | summary |
|---|---|---|
| WO2016085356A1 | gold kiwifruit compositions and methods of preparation and use thereof | Prebiotic compositions prepared from gold varieties of Actinidia chinensis. These prebiotic compositions treat or prevent DGBI, such as constipation, and IBS. |
| WO2022191767A1 | GOS preconditioning L. reuteri and GOS in final formulation | Enhancing the survival and activity of probiotic Lactobacillus reuteri strains by preconditioning L. reuteri with GOS. This method produces high synbiotic and beneficial effects of the probiotic bacteria in the gastrointestinal tract, such as boosting calcium and iron solubility, along with enhancing the production of lactic and acetic acid. |
| US20160058808A1 | microbe-based modulation of serotonin biosynthesis | Methods and probiotic compositions that can be used to modulate serotonin levels and adjust the composition of gut microbiota along with adjusting the level of serotonin-related metabolites. |
| WO2022182908A1 | probiotic therapies for social deficit and stress response | Bacterial species, including probiotic Enterococcus faecalis, for use in the treatment of social behavioral deficit symptoms, such as depression, by increasing social behavior and decreasing corticosterone levels along with c-Fos expression in the brain. |
| US9192618B2 | method of treating constipation-predominant irritable bowel syndrome | Prebiotic or probiotic agent that inhibits the growth of methanogenic bacteria or promotes the growth of competing intestinal microbiotia for the treatment of constipation predominant IBS. |
| US10022408B2 | probiotic Bifidobacterium adolescentis strains | Novel isolated strains of probiotic Bifidobacterium adolescentis for the prevention, alleviation of symptoms, or treatment of intestinal inflammatory conditions, such as IBS. |
| WO2005003329A1 | novel GOS composition and the preparation thereof | Novel strains of Bifidobacterium hifidum capable of producing a novel galactosidase enzyme activity that converts lactose to a novel mixture of GOS. The mixture of prebiotic oligosaccharides improves gut health by promoting the growth of bifidobacteria in the gut. |
| US20220040242A1 | modulation of the gut microbiome to treat mental disorders or diseases of the central nervous system | Methods of treating at least one symptom of a mental disorder and the central nervous system by modulating the amount of GABA produced in the gut. Also disclosed are methods of identifying and creating probiotic bacterial strains capable of producing GABA. |
| WO2016029198A1 | process for the production of isomaltooligosaccharide | Provides a method for the production of prebiotic oligosaccharides by the fermentation of dextransucrase-producing microorganisms. |
| WO2022214700A1 | Lacticaseibacillus paracasei EM025-11 and uses thereof | A probiotic strain of Lacticaseibacillus paracasei EM025-11 that adheres to intestinal epithelial cells and has anti-inflammatory activity by upregulating genes associated with immune engagement for the treatment of IBS with constipation. |
| US20220280576A1 | Bifidobacterium longum and functional GI disorders | Methods for treating functional GI disorders with probiotic Bifidobacterium longum ATCC BAA-999. |
| WO2019149941A1 | postbiotic-based composition for the modulation of immune system activation and protection of mucosal barriers | A fermented supernatant of Lactobacillus casei or paracasei species for the promotion of human health and prevention of inflammatory disorders. The postbiotic was shown to stimulate peripheral blood mononuclear cells and protect from endotoxic shock and Salmonella infection. |
| US8551498B2 | solid composition containing Bacillus-type nonpathogenic bacterial spores | Composition of spores of probiotic bacteria Bacillus useful in the pharmaceutical, veterinary, and nutrition fields. |
| ES2824536T3 | use of microbial communities for human and animal health | Mixture of probiotic bacteria belonging to at least six or seven bacterial species to prevent or treat GI disorders. |
| US20220233559A1 | xylooligosaccharide as a multifunctional prebiotic | Prebiotic mixture of xylooligosaccharides derived from sugar cane that were shown to modulate the levels of probiotic bacteria Bifidobacteria and Lactobacillus in the microbiome. |
| WO2022173764A1 | nutritional plant-based foods and beverages, methods of manufacture, and methods of treatment | Formulation of a prebiotic beverage whose ingestion modulates the gut microbiome by enhancing the growth of Bifidobacterium, improving the production of SCFAs, and reducing the levels Escherichia coli. |
| US20220315960A1 | method for producing gamma-aminobutyric acid and fermented culture prepared thereby | Process to produce gamma-aminobutyric acid from glutamic acid and a probiotic composition capable of this biotransformation. Ideally, the composition contains different probiotic bacteria Bifidobacterium and Lactobacillus strains. |
| WO2022208458A1 | inactivated strains of bacteria, such as viable but nonculturable bacteria, compositions, and use thereof | Postbiotic composition of different gamma-irradiated members of bacterial species Lactobacillus, Lacticaseibacillus, Bifidobacterium, and Lactiplantibacillus to treat several gastrointestinal disorders. |
| EP3932415A1 | gut microbiota composition and uses thereof | Probiotic composition for the prevention and/or treatment of a mental disorder with memory impairment by gut microbiome modulation for an increase in memory scores. |
Conclusions and Perspective
Gut microbiota in humans evolving throughout life has been demonstrated to play a key role in health and disease. In healthy individuals, the intestinal microbiota has a multitude of beneficial functions, including metabolic energy utilization, protection from pathogenic attack, and immunomodulation. Furthermore, it is becoming increasingly documented that bidirectional signaling takes place between the gut and the brain and involves gut microbiota. This relationship encompasses various pathways, such as the vagus nerve; the hypothalamic–pituitary–adrenal axis; and immune, hormonal, and metabolic pathways to control various aspects of homeostasis, including the appropriate development and maintenance of digestive and mental functions. A dysbiosis of the intestinal microbiota is becoming documented as a factor in the pathogenesis of various pathological conditions, including a plethora of mental, metabolic, and digestive disorders. The diverse etiology of these disorders has been related to different microbes, although insufficient information is currently available on the causal direction of the association. Recent impressive advances in next generation sequencing technologies, along with the progress and innovations of metagenomics, metabolomics, multiomics, bioinformatics, and artificial intelligence tools, have provided prospects to better characterize the microbial populations and their functions and help in better correlation prediction. Moreover, studies using germ-free animals have provided important knowledge on causality rather than association. The research focus needs to be further shifted from individual microbes and their role in influencing health and disease toward the gut microbiome ecosystem. A better understanding of fundamental rules driving interactions within gut microbial communities and the dynamics through which they are acquired, transmitted, and adapted to single individuals is needed to make further progress.
The gut microbiome–brain axis embodies a sophisticated network of biological constructs that scientists are only beginning to understand. The nutritional and therapeutic approaches to modulate this axis are ultimately aimed at improving human quality of life. Some products are even already on the market, including foods and supplements that promise to improve gut, mood, sleep, or cognitive performance. The scientific background behind some of these promises is, however, still arguable or unknown.
The gut microbiome offers interesting possibilities to enhance therapies. Future studies should focus on identifying if gut microbiome signatures correlate with fecal/luminal metabolites and/or cytokines that might translate into marked differences in patients’ life. Moreover, more studies evaluating the potential of therapeutic modulation of the microbiome by biotics/fecal transplant to enhance therapeutic options for diseases are needed. Nowadays, research has shown that fecal transplants can restore healthy bacteria in the lower intestine, which can help control diseases caused by pathogenic bacteria. As a result, the first approved fecal transplant drugs are already a fact. Australia’s Therapeutic Goods Administration was the first to grant approval to biotechnology company BiomeBank for its microbiome-based therapy product Biomictra for treating infections from Clostridioides difficile bacteria.526,527 Days later, the US FDA also approved its first fecal microbiota product Rebyota produced by Ferring Pharmaceuticals for the prevention of recurrence of C. difficile infection in adults.528
The multitude of relations between the microbiota, gut, and brain are now well-documented. The next step—moving on from correlative analysis toward understanding the mechanisms behind these relations and identifying the best ways to adapt and adjust the microbiota for potential therapeutic approaches—is now the required pathway. Some of the key setbacks in existing knowledge include understanding the immunological function of specific microbes in the human gut microbiota and their role in neurodegenerative and psychiatric disorders and how microbial metabolites influence brain function in tandem with immunological and neurological signaling molecules.
Despite the advances in microbiome knowledge, a lack of standardization significantly complicates and obstructs comparisons across studies, thus hampering insights into the structure and function of microbial populations. Recent efforts in the introduction of standardized protocols and analytical methods to characterize microbiota and explore the relationships and co-occurrences of microbially related metabolites and microbial taxa529 would allow for great improvements in examinations of microbial diversity.
One of the significant challenges in microbiota-based medicine is the delineation of healthy microbiota. Variations in microbiota composition between individuals can be large, i.e., microbiota turn out to be pretty much person-specific, which greatly complicates a “one size fits all” strategy in targeting it. However, it also offers chances since the microbiota might be the outlet for an effective future personalized medicine approach.530 Overall, clinical studies are hampered by the deficiency of specific biomarkers. Yet, recent meta-analyses have validated a positive assessment of the use of psychobiotic interventions for anxiety, schizophrenia, or cognitive performance, which aim at the diversity and complexity of gut microbiota, as well as the various confounding factors that may affect it.462,531−536
Although the prevention of brain disorders still remains out of reach, the knowledge of healthy microbiota and their communication pathways could enable an early prediction of such disorders. Thus, the first signs of neurodegenerative conditions, such as Alzheimer’s and Parkinson’s diseases, are known to develop many years before diagnosis. It might become thinkable to slow down neurodegenerative processes by altering the microbiome. Such possibilities have inspired a growing number of scientists to initiate start-up companies examining therapeutics for the treatment of neurological and other disorders through microbiome modulation. Private investors are showing a strong upward trend to fund such clinical research.
Perfecting the gut microbiota through fecal transplants; pro-, pre-, post-, and syn-biotics; healthy diet; and/or healthy lifestyle to control gut micriobiome–brain axis functions and promote mental and digestive health will be a promising field in the future. Patients suffering from mental and/or digestive disorders will get help through such treatments. Healthy individuals will promote their homeostasis and resilience from these remedies.
Acknowledgments
The authors sincerely appreciate the CAS Data, Analytics & Insights team for their assistance in data extraction. The authors also appreciate Dharmini Patel for project coordination, Manuela Pausan for ideation, and Peter Jap and Cristina Tomeo for insightful discussion. The authors are also grateful to Manuel Guzman, Gilles Georges, Michael Dennis, Dawn Riedel, Dawn George, and Hong Xie for executive sponsorship.
Glossary
Abbreviations
- 4-EPS
4-ethylphenylsulfate
- 5-AVAB
5-aminovaleric acid betaine
- BAs
bile acids
- BBB
blood-brain barrier
- BCFA
branched-chain fatty acids
- CAN
central autonomic network
- CEN
central executive network
- CNS
central nervous system
- DGBI
disorders of gut–brain interaction
- DMN
default mode network
- EAN
emotional arousal network
- ENS
enteric nervous system
- FC
functional constipation
- FCT
fecal microbiota transplantation
- FD
functional dyspepsia
- FGIDs
functional gastrointestinal disorders
- GBA
gut–brain axis
- HC
healthy controls
- HPA
hypothalamus–pituitary–adrenal
- IBS
irritable bowel syndrome
- IHMC
International Human Microbiome Consortium
- MPs
muramyl peptides
- OCC
occipital network
- RDA
recommended daily allowance
- SAL
salience network
- SCFA
short-chain fatty acids
- SMN
sensorimotor network
- TMAO
trimethylamine-N-oxide
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acschemneuro.3c00127.
Prebiotic, probiotic, postbiotic, and fecal transplant therapeutic clinical trial data investigating the treatment of mental disorders and DGBI through gut microbiome modulation (XLSX)
Author Contributions
§ J.M.S., R.M.A., and R.T. contributed equally to this work.
The authors declare the following competing financial interest(s): R.M.A and O.K. are employed by Bayer Consumer Health, Germany. M.G. was an intern in Bayer consumer health, Germany during writing this manuscript.
Supplementary Material
References
- Schopf J. W. Disparate rates, differing fates: tempo and mode of evolution changed from the Precambrian to the Phanerozoic. Proc. Natl. Acad. Sci. U. S. A. 1994, 91, 6735–6742. 10.1073/pnas.91.15.6735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averill C.; Anthony M. A.; Baldrian P.; Finkbeiner F.; van den Hoogen J.; Kiers T.; Kohout P.; Hirt E.; Smith G. R.; Crowther T. W. Defending Earth’s terrestrial microbiome. Nat. Microbiol. 2022, 7, 1717. 10.1038/s41564-022-01228-3. [DOI] [PubMed] [Google Scholar]
- O’Hara A. M.; Shanahan F. The gut flora as a forgotten organ. EMBO reports 2006, 7, 688–693. 10.1038/sj.embor.7400731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckhed F.; Ley R. E.; Sonnenburg J. L.; Peterson D. A.; Gordon J. I. Host-bacterial mutualism in the human intestine. Science 2005, 307, 1915–1920. 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- Cénit M. C.; Matzaraki V.; Tigchelaar E. F.; Zhernakova A. Rapidly expanding knowledge on the role of the gut microbiome in health and disease. Biochim. Biophys. Acta 2014, 1842, 1981–1992. 10.1016/j.bbadis.2014.05.023. [DOI] [PubMed] [Google Scholar]
- Vrancken G.; Gregory A. C.; Huys G. R. B.; Faust K.; Raes J. Synthetic ecology of the human gut microbiota. Nature Reviews Microbiology 2019, 17, 754–763. 10.1038/s41579-019-0264-8. [DOI] [PubMed] [Google Scholar]
- Hasan N.; Yang H. Factors affecting the composition of the gut microbiota, and its modulation. PeerJ. 2019, 7, e7502. 10.7717/peerj.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariente N.; York A.. Milestones in human microbiota research. https://www.nature.com/immersive/d42859-019-00041-z/index.html (accessed October 6, 2022).
- Malla M. A.; Dubey A.; Kumar A.; Yadav S.; Hashem A.; Abd_Allah E. F. Exploring the Human Microbiome: The Potential Future Role of Next-Generation Sequencing in Disease Diagnosis and Treatment. Front. Immunol. 2019, 9, 02868. 10.3389/fimmu.2018.02868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg G.; Rybakova D.; Fischer D.; Cernava T.; Vergès M.-C. C.; Charles T.; Chen X.; Cocolin L.; Eversole K.; Corral G. H.; et al. Microbiome definition re-visited: old concepts and new challenges. Microbiome 2020, 8, 103. 10.1186/s40168-020-00875-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler M. I.; Mörkl S.; Sandhu K. V.; Cryan J. F.; Dinan T. G. The Gut Microbiome and Mental Health: What Should We Tell Our Patients?: Le microbiote Intestinal et la Santé Mentale: que Devrions-Nous dire à nos Patients?. Can. J. Psychiatry. 2019, 64, 747–760. 10.1177/0706743719874168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corliss J. O. Three Centuries of Protozoology: A Brief Tribute to its Founding Father, A. van Leeuwenhoek of Delft. Journal of Protozoology 1975, 22, 3–7. 10.1111/j.1550-7408.1975.tb00934.x. [DOI] [PubMed] [Google Scholar]
- Leidy J.A flora and fauna within living animals. Smithsonian Institution: WA, 1853. [Google Scholar]
- Savage D. C. Microbial biota of the human intestine: a tribute to some pioneering scientists. Curr. Issues Intest. Microbiol. 2001, 2, 1–15. [PubMed] [Google Scholar]
- Pariente N.A field is born. 2019. https://www.nature.com/articles/d42859-019-00006-2.
- Micheli P. A.Nova plantarum genera iuxta Tournefortii methodum disposita; 1729. [Google Scholar]
- Drews G. The roots of microbiology and the influence of Ferdinand Cohn on microbiology of the 19th century. FEMS Microbiol. Rev. 2000, 24, 225–249. 10.1111/j.1574-6976.2000.tb00540.x. [DOI] [PubMed] [Google Scholar]
- Boundless . Koch’s Postulates. https://bio.libretexts.org/Bookshelves/Microbiology/Book%3A_Microbiology_(Boundless)/10%3A_Epidemiology/10.1%3A_Principles_of_Epidemiology/10.1D%3A__Kochs_Postulates (accessed October 6, 2022).
- Metchnikoff E.Prolongation of Life: Optimistic Studies; 1923. [Google Scholar]
- Sonnenborn U. Escherichia coli strain Nissle 1917—from bench to bedside and back: history of a special Escherichia coli strain with probiotic properties. FEMS Microbiol. Lett. 2016, 363, fnw212. 10.1093/femsle/fnw212. [DOI] [PubMed] [Google Scholar]
- Tan S. Y.; Tatsumura Y. Alexander Fleming (1881–1955): Discoverer of penicillin. Singapore Med. J. 2015, 56, 366–367. 10.11622/smedj.2015105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida A.; Mitchell A. L.; Boland M.; Forster S. C.; Gloor G. B.; Tarkowska A.; Lawley T. D.; Finn R. D. A new genomic blueprint of the human gut microbiota. Nature 2019, 568, 499–504. 10.1038/s41586-019-0965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hungate R. E., Chapter IV A Roll Tube Method for Cultivation of Strict Anaerobes. In Methods in Microbiology, Vol. 3; Norris J. R., Ribbons D. W., Eds.; Academic Press, 1969; pp 117–132. [Google Scholar]
- Cat L. A.The Decade Of The Microbiome. https://www.forbes.com/sites/linhanhcat/2019/12/31/decade-of-the-microbiome/?sh=7e621a679961 (accessed October 11, 2022).
- NIH Human Microbiome Project. https://www.hmpdacc.org/overview/ (accessed October 6, 2022).
- MetaHIT. https://www.gutmicrobiotaforhealth.com/metahit/ (accessed October 6, 2022).
- Qin J.; Li R.; Raes J.; Arumugam M.; Burgdorf K. S.; Manichanh C.; Nielsen T.; Pons N.; Levenez F.; Yamada T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor Lita M. The Human Microbiome Project in 2011 and Beyond. Cell Host Microbe 2011, 10, 287–291. 10.1016/j.chom.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Birney E.; Stamatoyannopoulos J. A.; Dutta A.; Guigó R.; Gingeras T. R.; Margulies E. H.; Weng Z.; Snyder M.; Dermitzakis E. T.; Stamatoyannopoulos J. A.; et al. Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature 2007, 447, 799–816. 10.1038/nature05874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesenfeld C. S.; Schloss P. D.; Handelsman J. Metagenomics: Genomic Analysis of Microbial Communities. Annu. Rev. Genet. 2004, 38, 525–552. 10.1146/annurev.genet.38.072902.091216. [DOI] [PubMed] [Google Scholar]
- Jandhyala S. M.; Talukdar R.; Subramanyam C.; Vuyyuru H.; Sasikala M.; Nageshwar Reddy D. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. 10.3748/wjg.v21.i29.8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomas J.; Wrzosek L.; Bouznad N.; Bouet S.; Mayeur C.; Noordine M. L.; Honvo-Houeto E.; Langella P.; Thomas M.; Cherbuy C. Primocolonization is associated with colonic epithelial maturation during conventionalization. FASEB J. 2013, 27, 645–655. 10.1096/fj.12-216861. [DOI] [PubMed] [Google Scholar]
- Caballero S.; Pamer E. G. Microbiota-mediated inflammation and antimicrobial defense in the intestine. Annu. Rev. Immunol. 2015, 33, 227–256. 10.1146/annurev-immunol-032713-120238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnúsdóttir S.; Ravcheev D.; de Crécy-Lagard V.; Thiele I. Systematic genome assessment of B-vitamin biosynthesis suggests co-operation among gut microbes. Frontiers in Genetics 2015, 6, 148. 10.3389/fgene.2015.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer E. A.; Knight R.; Mazmanian S. K.; Cryan J. F.; Tillisch K. Gut microbes and the brain: paradigm shift in neuroscience. J. Neurosci. 2014, 34, 15490–15496. 10.1523/JNEUROSCI.3299-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke G.; Grenham S.; Scully P.; Fitzgerald P.; Moloney R. D.; Shanahan F.; Dinan T. G.; Cryan J. F. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatry 2013, 18, 666–673. 10.1038/mp.2012.77. [DOI] [PubMed] [Google Scholar]
- Bray N.The microbiota–gut–brain axis. https://www.nature.com/articles/d42859-019-00021-3 (accessed October 6, 2022).
- Dinan T. G.; Cryan J. F. The impact of gut microbiota on brain and behaviour: implications for psychiatry. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 552–558. 10.1097/MCO.0000000000000221. [DOI] [PubMed] [Google Scholar]
- Bravo J. A.; Forsythe P.; Chew M. V.; Escaravage E.; Savignac H. M.; Dinan T. G.; Bienenstock J.; Cryan J. F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 16050–16055. 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Sacks H.; Elazar V.; Gao J.; Golomb A.; Adwan H.; Korchov N.; Levy R. J.; Berger M. R.; Golomb G. Delivery and expression of pDNA embedded in collagen matrices. J. Controlled Release 2004, 95, 309–320. 10.1016/j.jconrel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Rutsch A.; Kantsjö J. B.; Ronchi F. The Gut-Brain Axis: How Microbiota and Host Inflammasome Influence Brain Physiology and Pathology. Front. Immunol. 2020, 11, 604179. 10.3389/fimmu.2020.604179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drossman D. A.; Hasler W. L. Rome IV-Functional GI Disorders: Disorders of Gut-Brain Interaction. Gastroenterology 2016, 150, 1257–1261. 10.1053/j.gastro.2016.03.035. [DOI] [PubMed] [Google Scholar]
- Perez-Muñoz M. E.; Arrieta M.-C.; Ramer-Tait A. E.; Walter J. A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: implications for research on the pioneer infant microbiome. Microbiome 2017, 5, 48. 10.1186/s40168-017-0268-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Bello M. G.; Costello E. K.; Contreras M.; Magris M.; Hidalgo G.; Fierer N.; Knight R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. U. S. A. 2010, 107, 11971–11975. 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C. J.; Lynch D. B.; Murphy K.; Ulaszewska M.; Jeffery I. B.; O’Shea C. A.; Watkins C.; Dempsey E.; Mattivi F.; Tuohy K.; et al. Evolution of gut microbiota composition from birth to 24 weeks in the INFANTMET Cohort. Microbiome 2017, 5, 4. 10.1186/s40168-016-0213-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatanen T.; Jabbar K. S.; Ruohtula T.; Honkanen J.; Avila-Pacheco J.; Siljander H.; Stražar M.; Oikarinen S.; Hyöty H.; Ilonen J.; et al. Mobile genetic elements from the maternal microbiome shape infant gut microbial assembly and metabolism. Cell 2022, 185, 4921–4936.e4915. 10.1016/j.cell.2022.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baleato C. L.; Ferguson J. J. A.; Oldmeadow C.; Mishra G. D.; Garg M. L. Plant-Based Dietary Patterns versus Meat Consumption and Prevalence of Impaired Glucose Intolerance and Diabetes Mellitus: A Cross-Sectional Study in Australian Women. Nutrients 2022, 14, 4152. 10.3390/nu14194152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keaver L.; Ruan M.; Chen F.; Du M.; Ding C.; Wang J.; Shan Z.; Liu J.; Zhang F. F. Plant- and animal-based diet quality and mortality among US adults: a cohort study. Br. J. Nutr. 2021, 125, 1405–1415. 10.1017/S0007114520003670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Mahony S. M.; Marchesi J. R.; Scully P.; Codling C.; Ceolho A.-M.; Quigley E. M. M.; Cryan J. F.; Dinan T. G. Early Life Stress Alters Behavior, Immunity, and Microbiota in Rats: Implications for Irritable Bowel Syndrome and Psychiatric Illnesses. Biol. Psychiatry 2009, 65, 263–267. 10.1016/j.biopsych.2008.06.026. [DOI] [PubMed] [Google Scholar]
- Jernberg C.; Löfmark S.; Edlund C.; Jansson J. K. Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology (Reading) 2010, 156, 3216–3223. 10.1099/mic.0.040618-0. [DOI] [PubMed] [Google Scholar]
- Caporaso J. G.; Kuczynski J.; Stombaugh J.; Bittinger K.; Bushman F. D.; Costello E. K.; Fierer N.; Peña A. G.; Goodrich J. K.; Gordon J. I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L.Sequence-based identification of human-associated microbiota. https://www.nature.com/articles/d42859-019-00011-5 (accessed October 24, 2022).
- Collins S. M.; Surette M.; Bercik P. The interplay between the intestinal microbiota and the brain. Nat. Rev. Microbiol. 2012, 10, 735–742. 10.1038/nrmicro2876. [DOI] [PubMed] [Google Scholar]
- Lagier J.-C.; Armougom F.; Million M.; Hugon P.; Pagnier I.; Robert C.; Bittar F.; Fournous G.; Gimenez G.; Maraninchi M.; et al. Microbial culturomics: paradigm shift in the human gut microbiome study. Clinical Microbiology and Infection 2012, 18, 1185–1193. 10.1111/1469-0691.12023. [DOI] [PubMed] [Google Scholar]
- Smith P. M.; Howitt M. R.; Panikov N.; Michaud M.; Gallini C. A.; Bohlooly Y. M.; Glickman J. N.; Garrett W. S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atarashi K.; Tanoue T.; Oshima K.; Suda W.; Nagano Y.; Nishikawa H.; Fukuda S.; Saito T.; Narushima S.; Hase K.; et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 2013, 500, 232–236. 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- Arpaia N.; Campbell C.; Fan X.; Dikiy S.; van der Veeken J.; deRoos P.; Liu H.; Cross J. R.; Pfeffer K.; Coffer P. J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donia M. S.; Cimermancic P.; Schulze C. J.; Wieland Brown L. C.; Martin J.; Mitreva M.; Clardy J.; Linington R. G.; Fischbach M. A. A systematic analysis of biosynthetic gene clusters in the human microbiome reveals a common family of antibiotics. Cell 2014, 158, 1402–1414. 10.1016/j.cell.2014.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurie I.; Yang Y. X.; Haynes K.; Mamtani R.; Boursi B. Antibiotic exposure and the risk for depression, anxiety, or psychosis: a nested case-control study. J. Clin. Psychiatry 2015, 76, 1522–1528. 10.4088/JCP.15m09961. [DOI] [PubMed] [Google Scholar]
- Zheng P.; Zeng B.; Zhou C.; Liu M.; Fang Z.; Xu X.; Zeng L.; Chen J.; Fan S.; Du X.; et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 2016, 21, 786–796. 10.1038/mp.2016.44. [DOI] [PubMed] [Google Scholar]
- Davis D. J.; Hecht P. M.; Jasarevic E.; Beversdorf D. Q.; Will M. J.; Fritsche K.; Gillespie C. H. Sex-specific effects of docosahexaenoic acid (DHA) on the microbiome and behavior of socially-isolated mice. Brain, Behavior, and Immunity 2017, 59, 38–48. 10.1016/j.bbi.2016.09.003. [DOI] [PubMed] [Google Scholar]
- Pasolli E.; Asnicar F.; Manara S.; Zolfo M.; Karcher N.; Armanini F.; Beghini F.; Manghi P.; Tett A.; Ghensi P.; et al. Extensive Unexplored Human Microbiome Diversity Revealed by Over 150,000 Genomes from Metagenomes Spanning Age, Geography, and Lifestyle. Cell 2019, 176, 649–662.e20. 10.1016/j.cell.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayfach S.; Shi Z. J.; Seshadri R.; Pollard K. S.; Kyrpides N. C. New insights from uncultivated genomes of the global human gut microbiome. Nature 2019, 568, 505–510. 10.1038/s41586-019-1058-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusceddu M. M.; Barboza M.; Schneider M.; Stokes P.; Sladek J. A.; Torres-Fuentes C.; Goldfild L. R.; Gillis S. E.; Brust-Mascher I.; Rabasa G. Nod-like receptors are critical for gut-brain axis signaling in mice. J. Physiol. 2019, 597, 5777–5797. 10.1113/JP278640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini C.; Antonioli L.; Calderone V.; Colucci R.; Fornai M.; Blandizzi C. Microbiota-gut-brain axis in health and disease: Is NLRP3 inflammasome at the crossroads of microbiota-gut-brain communications?. Prog. Neurobiol. 2020, 191, 101806. 10.1016/j.pneurobio.2020.101806. [DOI] [PubMed] [Google Scholar]
- Zhao K.; Yao M.; Zhang X.; Xu F.; Shao X.; Wei Y.; Wang H. Flavonoids and intestinal microbes interact to alleviate depression. Journal of the Science of Food and Agriculture 2022, 102, 1311–1318. 10.1002/jsfa.11578. [DOI] [PubMed] [Google Scholar]
- Segre J. A. What does it take to satisfy Koch’s postulates two centuries later? Microbial genomics and Propionibacteria acnes. J. Invest. Dermatol. 2013, 133, 2141–2142. 10.1038/jid.2013.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiseman B.; Silen W.; Bascom G. S.; Kauvar A. J. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery 1958, 44, 854–859. [PubMed] [Google Scholar]
- Reyniers J. A. The pure-culture concept and gnotobiotics. Ann. N.Y. Acad. Sci. 1959, 78, 3–16. 10.1111/j.1749-6632.1959.tb53091.x. [DOI] [Google Scholar]
- Schaedler R. W.; Dubos R.; Costello R. ASSOCIATION OF GERMFREE MICE WITH BACTERIA ISOLATED FROM NORMAL MICE. J. Exp. Med. 1965, 122, 77–82. 10.1084/jem.122.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppercorn M. A.; Goldman P. The Role of Intestinal Bacteria in the Metabolism of Salicylazosulfapyridine. Journal of Pharmacology and Experimental Therapeutics 1972, 181, 555–562. [PubMed] [Google Scholar]
- Stark P. L.; Lee A. The microbial ecology of the large bowel of breast-fed and formula-fed infants during the first year of life. J. Med. Microbiol. 1982, 15, 189–203. 10.1099/00222615-15-2-189. [DOI] [PubMed] [Google Scholar]
- Fleischmann R. D.; Adams M. D.; White O.; Clayton R. A.; Kirkness E. F.; Kerlavage A. R.; Bult C. J.; Tomb J. F.; Dougherty B. A.; Merrick J. M.; et al. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science 1995, 269, 496–512. 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- Wilson K. H.; Blitchington R. B. Human colonic biota studied by ribosomal DNA sequence analysis. Appl. Environ. Microbiol. 1996, 62, 2273–2278. 10.1128/aem.62.7.2273-2278.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarner F.; Malagelada J. R. Gut flora in health and disease. Lancet 2003, 361, 512–519. 10.1016/S0140-6736(03)12489-0. [DOI] [PubMed] [Google Scholar]
- Rakoff-Nahoum S.; Paglino J.; Eslami-Varzaneh F.; Edberg S.; Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 2004, 118, 229–241. 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Mazmanian S. K.; Liu C. H.; Tzianabos A. O.; Kasper D. L. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 2005, 122, 107–118. 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Turnbaugh P. J.; Ley R. E.; Mahowald M. A.; Magrini V.; Mardis E. R.; Gordon J. I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- Routy B.; Le Chatelier E.; Derosa L.; Duong C. P. M.; Alou M. T.; Daillère R.; Fluckiger A.; Messaoudene M.; Rauber C.; Roberti M. P.; et al. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science 2018, 359, 91–97. 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- Matson V.; Fessler J.; Bao R.; Chongsuwat T.; Zha Y.; Alegre M. L.; Luke J. J.; Gajewski T. F. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 2018, 359, 104–108. 10.1126/science.aao3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belizário J. E.; Napolitano M. Human microbiomes and their roles in dysbiosis, common diseases, and novel therapeutic approaches. Front. Microbiol. 2015, 6, 1050. 10.3389/fmicb.2015.01050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinninella E.; Raoul P.; Cintoni M.; Franceschi F.; Miggiano G. A. D.; Gasbarrini A.; Mele M. C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. 10.3390/microorganisms7010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cresci G. A. M.; Izzo K.. Chapter 4 - Gut Microbiome. In Adult Short Bowel Syndrome; Corrigan M. L., Roberts K., Steiger E., Eds.; Academic Press, 2019; pp 45–54. [Google Scholar]
- CAS Content Collection. https://www.cas.org/about/cas-content (accessed January 18, 2023).
- Kho Z. Y.; Lal S. K. The Human Gut Microbiome – A Potential Controller of Wellness and Disease. Front. Microbiol. 2018, 9, 1835. 10.3389/fmicb.2018.01835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankar S. A.; Lagier J. C.; Pontarotti P.; Raoult D.; Fournier P. E. The human gut microbiome, a taxonomic conundrum. Syst. Appl. Microbiol 2015, 38, 276–286. 10.1016/j.syapm.2015.03.004. [DOI] [PubMed] [Google Scholar]
- Manson J. M.; Rauch M.; Gilmore M. S.. The Commensal Microbiology of the Gastrointestinal Tract. In GI Microbiota and Regulation of the Immune System; Huffnagle G. B., Noverr M. C., Eds.; Landes Bioscience and Springer Science+Business Media, LLC: New York, 2008; pp 15–28. [Google Scholar]
- Kim D.-H. Gut Microbiota-Mediated Drug-Antibiotic Interactions. Drug Metab. Dispos. 2015, 43, 1581–1589. 10.1124/dmd.115.063867. [DOI] [PubMed] [Google Scholar]
- Deng P.; Swanson K. S. Gut microbiota of humans, dogs and cats: current knowledge and future opportunities and challenges. Br. J. Nutr. 2015, 113 (Suppl), S6–17. 10.1017/S0007114514002943. [DOI] [PubMed] [Google Scholar]
- Nardone G.; Compare D. The human gastric microbiota: Is it time to rethink the pathogenesis of stomach diseases?. United European Gastroenterol J. 2015, 3, 255–260. 10.1177/2050640614566846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S.; Pramanik S. Structural diversity, functional aspects and future therapeutic applications of human gut microbiome. Arch. Microbiol. 2021, 203, 5281–5308. 10.1007/s00203-021-02516-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leser T. D.; Mo̷lbak L. Better living through microbial action: the benefits of the mammalian gastrointestinal microbiota on the host. Environ. Microbiol. 2009, 11, 2194–2206. 10.1111/j.1462-2920.2009.01941.x. [DOI] [PubMed] [Google Scholar]
- Linares D. M.; Ross P.; Stanton C. Beneficial Microbes: The pharmacy in the gut. Bioengineered 2016, 7, 11–20. 10.1080/21655979.2015.1126015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah H. N.; Olsen I.; Bernard K.; Finegold S. M.; Gharbia S.; Gupta R. S. Approaches to the study of the systematics of anaerobic, Gram-negative, non-sporeforming rods: Current status and perspectives. Anaerobe 2009, 15, 179–194. 10.1016/j.anaerobe.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Thomas F.; Hehemann J.-H.; Rebuffet E.; Czjzek M.; Michel G. Environmental and Gut Bacteroidetes: The Food Connection. Front. Microbiol. 2011, 2, 93. 10.3389/fmicb.2011.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee L. S.; La Rosa S. L.; Westereng B.; Eijsink V. G.; Pope P. B.; Larsbrink J. Polysaccharide degradation by the Bacteroidetes: mechanisms and nomenclature. Environ. Microbiol. Rep. 2021, 13, 559–581. 10.1111/1758-2229.12980. [DOI] [PubMed] [Google Scholar]
- Ludwig W.; Schleifer K.-H.; Whitman W. B.. Revised road map to the phylum Firmicutes. In Bergey’s Manual of Systematic Bacteriology: Vol. Three The Firmicutes; De Vos P., Garrity G. M., Jones D., Krieg N. R., Ludwig W., Rainey F. A., Schleifer K.-H., Whitman W. B., Eds.; Springer New York: New York, 2009; pp 1–13. [Google Scholar]
- Yang M.; Gu Y.; Li L.; Liu T.; Song X.; Sun Y.; Cao X.; Wang B.; Jiang K.; Cao H. Bile Acid–Gut Microbiota Axis in Inflammatory Bowel Disease: From Bench to Bedside. Nutrients 2021, 13, 3143. 10.3390/nu13093143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barka E. A.; Vatsa P.; Sanchez L.; Gaveau-Vaillant N.; Jacquard C.; Klenk H.-P.; Clément C.; Ouhdouch Y.; Wezel G. P. v. Taxonomy, Physiology, and Natural Products of Actinobacteria. Microbiology and Molecular Biology Reviews 2016, 80, 1–43. 10.1128/MMBR.00019-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo-Cantabrana C.; Delgado S.; Ruiz L.; Ruas-Madiedo P.; Sánchez B.; Margolles A. Bifidobacteria and Their Health-Promoting Effects. Microbiol Spectr 2017, 5, 5.3.21. 10.1128/microbiolspec.BAD-0010-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackebrandt E.; Murray R. G. E.; Trüper H. G. Proteobacteria classis nov., a Name for the Phylogenetic Taxon That Includes the “Purple Bacteria and Their Relatives. International Journal of Systematic and Evolutionary Microbiology 1988, 38, 321–325. 10.1099/00207713-38-3-321. [DOI] [Google Scholar]
- Rizzatti G.; Lopetuso L. R.; Gibiino G.; Binda C.; Gasbarrini A. Proteobacteria: A Common Factor in Human Diseases. BioMed. Research International 2017, 2017, 9351507. 10.1155/2017/9351507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.-C.; Webb R. I.; Janssen P. H.; Sangwan P.; Romeo T.; Staley J. T.; Fuerst J. A. Phylum Verrucomicrobia representatives share a compartmentalized cell plan with members of bacterial phylum Planctomycetes. BMC Microbiol. 2009, 9, 5. 10.1186/1471-2180-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappé M. S.; Giovannoni S. J. The Uncultured Microbial Majority. Annu. Rev. Microbiol. 2003, 57, 369–394. 10.1146/annurev.micro.57.030502.090759. [DOI] [PubMed] [Google Scholar]
- Geerlings S. Y.; Ouwerkerk J. P.; Koehorst J. J.; Ritari J.; Aalvink S.; Stecher B.; Schaap P. J.; Paulin L.; de Vos W. M.; Belzer C. Genomic convergence between Akkermansia muciniphila in different mammalian hosts. BMC Microbiol. 2021, 21, 298. 10.1186/s12866-021-02360-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M.-J.; Yang J.-Y.; Yan Z.-H.; Hu S.; Li J.-Q.; Xu Z.-X.; Jian Y.-P. Recent findings in Akkermansia muciniphila-regulated metabolism and its role in intestinal diseases. Clin. Nutr. 2022, 41, 2333–2344. 10.1016/j.clnu.2022.08.029. [DOI] [PubMed] [Google Scholar]
- Gupta R. S.; Sethi M. Phylogeny and molecular signatures for the phylum Fusobacteria and its distinct subclades. Anaerobe 2014, 28, 182–198. 10.1016/j.anaerobe.2014.06.007. [DOI] [PubMed] [Google Scholar]
- Madhogaria B.; Bhowmik P.; Kundu A. Correlation between human gut microbiome and diseases. Infectious Medicine 2022, 1, 180–191. 10.1016/j.imj.2022.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull M. J.; Plummer N. T. Part 1: The Human Gut Microbiome in Health and Disease. Integr. Med. 2014, 13, 17–22. [PMC free article] [PubMed] [Google Scholar]
- Vijay A.; Valdes A. M. Role of the gut microbiome in chronic diseases: a narrative review. Eur. J. Clin. Nutr. 2022, 76, 489–501. 10.1038/s41430-021-00991-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Baquero F.; Nombela C. The microbiome as a human organ. Clin Microbiol Infect 2012, 18 (Suppl 4), 2–4. 10.1111/j.1469-0691.2012.03916.x. [DOI] [PubMed] [Google Scholar]
- Evans J. M.; Morris L. S.; Marchesi J. R. The gut microbiome: the role of a virtual organ in the endocrinology of the host. J. Endocrinol. 2013, 218, R37–R47. 10.1530/JOE-13-0131. [DOI] [PubMed] [Google Scholar]
- Haseeb A.; Shahzad I.; Ghulam H.; Muhammad Naeem F.; Humaira M.; Imtiaz M.; Imran M.; Saima M.; Muhammad Irfan U.. Gut Microbiome: A New Organ System in Body. In Parasitology and Microbiology Research; Gilberto Antonio Bastidas P., Asghar Ali K., Eds.; IntechOpen: Rijeka, Croatia, 2019. [Google Scholar]
- Cani P. D. Human gut microbiome: hopes, threats and promises. Gut 2018, 67, 1716–1725. 10.1136/gutjnl-2018-316723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Price J.; Arze C.; Ananthakrishnan A. N.; Schirmer M.; Avila-Pacheco J.; Poon T. W.; Andrews E.; Ajami N. J.; Bonham K. S.; Brislawn C. J.; et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 2019, 569, 655–662. 10.1038/s41586-019-1237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars R. A. T.; Yang Y.; Ward T.; Houtti M.; Priya S.; Lekatz H. R.; Tang X.; Sun Z.; Kalari K. R.; Korem T.; et al. Longitudinal Multi-omics Reveals Subset-Specific Mechanisms Underlying Irritable Bowel Syndrome. Cell 2020, 182, 1460–1473.e17. 10.1016/j.cell.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinttilä T.; Lyra A.; Krogius-Kurikka L.; Palva A. Real-time PCR analysis of enteric pathogens from fecal samples of irritable bowel syndrome subjects. Gut Pathog. 2011, 3, 6. 10.1186/1757-4749-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer E. A.; Nance K.; Chen S. The Gut-Brain Axis. Annu. Rev. Med. 2022, 73, 439–453. 10.1146/annurev-med-042320-014032. [DOI] [PubMed] [Google Scholar]
- Zhang T.; Zhang C.; Zhang J.; Sun F.; Duan L. Efficacy of Probiotics for Irritable Bowel Syndrome: A Systematic Review and Network Meta-Analysis. Front Cell Infect Microbiol 2022, 12, 859967. 10.3389/fcimb.2022.859967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnusamy K.; Choi J. N.; Kim J.; Lee S. Y.; Lee C. H. Microbial community and metabolomic comparison of irritable bowel syndrome faeces. J. Med. Microbiol. 2011, 60, 817–827. 10.1099/jmm.0.028126-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajilić-Stojanović M.; Biagi E.; Heilig H. G.; Kajander K.; Kekkonen R. A.; Tims S.; de Vos W. M. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology 2011, 141, 1792–1801. 10.1053/j.gastro.2011.07.043. [DOI] [PubMed] [Google Scholar]
- Pittayanon R.; Lau J. T.; Yuan Y.; Leontiadis G. I.; Tse F.; Surette M.; Moayyedi P. Gut Microbiota in Patients With Irritable Bowel Syndrome-A Systematic Review. Gastroenterology 2019, 157, 97–108. 10.1053/j.gastro.2019.03.049. [DOI] [PubMed] [Google Scholar]
- El-Salhy M.; Hatlebakk J. G.; Gilja O. H.; Bråthen Kristoffersen A.; Hausken T. Efficacy of faecal microbiota transplantation for patients with irritable bowel syndrome in a randomised, double-blind, placebo-controlled study. Gut 2020, 69, 859–867. 10.1136/gutjnl-2019-319630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson B.; Rossi M.; Kanno T.; Parkes G. C.; Anderson S.; Mason A. J.; Irving P. M.; Lomer M. C.; Whelan K. β-Galactooligosaccharide in Conjunction With Low FODMAP Diet Improves Irritable Bowel Syndrome Symptoms but Reduces Fecal Bifidobacteria. Am. J. Gastroenterol. 2020, 115, 906–915. 10.14309/ajg.0000000000000641. [DOI] [PubMed] [Google Scholar]
- Vasant D. H.; Paine P. A.; Black C. J.; Houghton L. A.; Everitt H. A.; Corsetti M.; Agrawal A.; Aziz I.; Farmer A. D.; Eugenicos M. P.; et al. British Society of Gastroenterology guidelines on the management of irritable bowel syndrome. Gut 2021, 70, 1214–1240. 10.1136/gutjnl-2021-324598. [DOI] [PubMed] [Google Scholar]
- Moayyedi P.; Andrews C. N.; MacQueen G.; Korownyk C.; Marsiglio M.; Graff L.; Kvern B.; Lazarescu A.; Liu L.; Paterson W. G.; et al. Canadian Association of Gastroenterology Clinical Practice Guideline for the Management of Irritable Bowel Syndrome (IBS). J. Can. Assoc Gastroenterol 2019, 2, 6–29. 10.1093/jcag/gwy071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukudo S.; Okumura T.; Inamori M.; Okuyama Y.; Kanazawa M.; Kamiya T.; Sato K.; Shiotani A.; Naito Y.; Fujikawa Y.; et al. Evidence-based clinical practice guidelines for irritable bowel syndrome 2020. J. Gastroenterol. 2021, 56, 193–217. 10.1007/s00535-020-01746-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy B. E.; Pimentel M.; Brenner D. M.; Chey W. D.; Keefer L. A.; Long M. D.; Moshiree B. ACG Clinical Guideline: Management of Irritable Bowel Syndrome. Am. J. Gastroenterol. 2021, 116, 17. 10.14309/ajg.0000000000001036. [DOI] [PubMed] [Google Scholar]
- Ford A. C.; Harris L. A.; Lacy B. E.; Quigley E. M. M.; Moayyedi P. Systematic review with meta-analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2018, 48, 1044–1060. 10.1111/apt.15001. [DOI] [PubMed] [Google Scholar]
- Caruso R.; Lo B. C.; Núñez G. Host-microbiota interactions in inflammatory bowel disease. Nat. Rev. Immunol. 2020, 20, 411–426. 10.1038/s41577-019-0268-7. [DOI] [PubMed] [Google Scholar]
- Federici S.; Kredo-Russo S.; Valdés-Mas R.; Kviatcovsky D.; Weinstock E.; Matiuhin Y.; Silberberg Y.; Atarashi K.; Furuichi M.; Oka A.; et al. Targeted suppression of human IBD-associated gut microbiota commensals by phage consortia for treatment of intestinal inflammation. Cell 2022, 185, 2879–2898.e24. 10.1016/j.cell.2022.07.003. [DOI] [PubMed] [Google Scholar]
- Nagalingam N. A.; Lynch S. V. Role of the microbiota in inflammatory bowel diseases. Inflamm. Bowel Dis. 2012, 18, 968–984. 10.1002/ibd.21866. [DOI] [PubMed] [Google Scholar]
- Barcenilla A.; Pryde S. E.; Martin J. C.; Duncan S. H.; Stewart C. S.; Henderson C.; Flint H. J. Phylogenetic relationships of butyrate-producing bacteria from the human gut. Appl. Environ. Microbiol. 2000, 66, 1654–1661. 10.1128/AEM.66.4.1654-1661.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfvarson J.; Brislawn C. J.; Lamendella R.; Vázquez-Baeza Y.; Walters W. A.; Bramer L. M.; D’Amato M.; Bonfiglio F.; McDonald D.; Gonzalez A.; et al. Dynamics of the human gut microbiome in inflammatory bowel disease. Nat. Microbiol 2017, 2, 17004. 10.1038/nmicrobiol.2017.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottawea W.; Chiang C.-K.; Mühlbauer M.; Starr A. E.; Butcher J.; Abujamel T.; Deeke S. A.; Brandel A.; Zhou H.; Shokralla S.; et al. Altered intestinal microbiota–host mitochondria crosstalk in new onset Crohn’s disease. Nat. Commun. 2016, 7, 13419. 10.1038/ncomms13419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesi J. R.; Holmes E.; Khan F.; Kochhar S.; Scanlan P.; Shanahan F.; Wilson I. D.; Wang Y. Rapid and noninvasive metabonomic characterization of inflammatory bowel disease. J. Proteome Res. 2007, 6, 546–551. 10.1021/pr060470d. [DOI] [PubMed] [Google Scholar]
- Van Immerseel F.; Ducatelle R.; De Vos M.; Boon N.; Van De Wiele T.; Verbeke K.; Rutgeerts P.; Sas B.; Louis P.; Flint H. J. Butyric acid-producing anaerobic bacteria as a novel probiotic treatment approach for inflammatory bowel disease. J. Med. Microbiol. 2010, 59, 141–143. 10.1099/jmm.0.017541-0. [DOI] [PubMed] [Google Scholar]
- Miquel S.; Martín R.; Rossi O.; Bermúdez-Humarán L. G.; Chatel J. M.; Sokol H.; Thomas M.; Wells J. M.; Langella P. Faecalibacterium prausnitzii and human intestinal health. Curr. Opin. Microbiol. 2013, 16, 255–261. 10.1016/j.mib.2013.06.003. [DOI] [PubMed] [Google Scholar]
- Tamanai-Shacoori Z.; Smida I.; Bousarghin L.; Loreal O.; Meuric V.; Fong S. B.; Bonnaure-Mallet M.; Jolivet-Gougeon A. Roseburia spp.: a marker of health?. Future Microbiol. 2017, 12, 157–170. 10.2217/fmb-2016-0130. [DOI] [PubMed] [Google Scholar]
- Li Y.; Xia S.; Jiang X.; Feng C.; Gong S.; Ma J.; Fang Z.; Yin J.; Yin Y. Gut Microbiota and Diarrhea: An Updated Review. Microbiology 2021, 11, 625210. 10.3389/fcimb.2021.625210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges K.; Gill R. Infectious diarrhea: Cellular and molecular mechanisms. Gut Microbes 2010, 1, 4–21. 10.4161/gmic.1.1.11036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M. Escherichia coli that cause diarrhea: enterotoxigenic, enteropathogenic, enteroinvasive, enterohemorrhagic, and enteroadherent. J. Infect. Dis. 1987, 155, 377–389. 10.1093/infdis/155.3.377. [DOI] [PubMed] [Google Scholar]
- Gallo A.; Passaro G.; Gasbarrini A.; Landolfi R.; Montalto M. Modulation of microbiota as treatment for intestinal inflammatory disorders: An uptodate. World J. Gastroenterol. 2016, 22, 7186–7202. 10.3748/wjg.v22.i32.7186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bron P. A.; Kleerebezem M.; Brummer R. J.; Cani P. D.; Mercenier A.; MacDonald T. T.; Garcia-Ródenas C. L.; Wells J. M. Can probiotics modulate human disease by impacting intestinal barrier function?. Br. J. Nutr. 2017, 117, 93–107. 10.1017/S0007114516004037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S.; Wang R.; Li D.; Zhao L.; Zhu L. Role of gut microbiota in functional constipation. Gastroenterol. Rep. 2021, 9, 392–401. 10.1093/gastro/goab035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoppi G.; Cinquetti M.; Luciano A.; Benini A.; Muner A.; Bertazzoni Minelli E. The intestinal ecosystem in chronic functional constipation. Acta Paediatr. 1998, 87, 836–841. 10.1111/j.1651-2227.1998.tb01547.x. [DOI] [PubMed] [Google Scholar]
- Khalif I. L.; Quigley E. M.; Konovitch E. A.; Maximova I. D. Alterations in the colonic flora and intestinal permeability and evidence of immune activation in chronic constipation. Dig. Liver Dis. 2005, 37, 838–849. 10.1016/j.dld.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Kim S. E.; Choi S. C.; Park K. S.; Park M. I.; Shin J. E.; Lee T. H.; Jung K. W.; Koo H. S.; Myung S. J. Change of Fecal Flora and Effectiveness of the Short-term VSL#3 Probiotic Treatment in Patients With Functional Constipation. J. Neurogastroenterol. Motil. 2015, 21, 111–120. 10.5056/jnm14048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L.; Liu W.; Alkhouri R.; Baker R. D.; Bard J. E.; Quigley E. M.; Baker S. S. Structural changes in the gut microbiome of constipated patients. Physiol. Genomics 2014, 46, 679–686. 10.1152/physiolgenomics.00082.2014. [DOI] [PubMed] [Google Scholar]
- Mancabelli L.; Milani C.; Lugli G. A.; Turroni F.; Mangifesta M.; Viappiani A.; Ticinesi A.; Nouvenne A.; Meschi T.; van Sinderen D.; et al. Unveiling the gut microbiota composition and functionality associated with constipation through metagenomic analyses. Sci. Rep. 2017, 7, 9879. 10.1038/s41598-017-10663-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naseer M.; Poola S.; Uraz S.; Tahan V. Therapeutic Effects of Prebiotics on Constipation: A Schematic Review. Curr. Clin. Pharmacol. 2020, 15, 207–215. 10.2174/1574884715666200212125035. [DOI] [PubMed] [Google Scholar]
- Zhao Y.; Yu Y. B. Intestinal microbiota and chronic constipation. SpringerPlus 2016, 5, 1130. 10.1186/s40064-016-2821-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. K.; Yao S. K. Roles of Gut Microbiota and Metabolites in Pathogenesis of Functional Constipation. Evid. Based Complement. Alternat. Med. 2021, 2021, 5560310. 10.1155/2021/5560310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S. S.; Rattanakovit K.; Patcharatrakul T. Diagnosis and management of chronic constipation in adults. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 295–305. 10.1038/nrgastro.2016.53. [DOI] [PubMed] [Google Scholar]
- Moloney R. D.; Desbonnet L.; Clarke G.; Dinan T. G.; Cryan J. F. The microbiome: stress, health and disease. Mamm. Genome 2014, 25, 49–74. 10.1007/s00335-013-9488-5. [DOI] [PubMed] [Google Scholar]
- Mayer E. A.; Tillisch K.; Gupta A. Gut/brain axis and the microbiota. J. Clin. Invest. 2015, 125, 926–938. 10.1172/JCI76304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado-Contreras A.; Noel S. E.; Ward D. V.; Velez M.; Mangano K. M. Associations between Diet, the Gut Microbiome, and Short-Chain Fatty Acid Production among Older Caribbean Latino Adults. J. Acad. Nutr. Diet. 2020, 120, 2047–2060.e46. 10.1016/j.jand.2020.04.018. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Chen Y.; Wang Z.; Xie G.; Liu M.; Yuan B.; Chai H.; Wang W.; Cheng P. Implications of Gut Microbiota in Neurodegenerative Diseases. Front. Immunol. 2022, 13, 785644. 10.3389/fimmu.2022.785644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy Sarkar S.; Banerjee S. Gut microbiota in neurodegenerative disorders. J. Neuroimmunol. 2019, 328, 98–104. 10.1016/j.jneuroim.2019.01.004. [DOI] [PubMed] [Google Scholar]
- Caspani G.; Kennedy S.; Foster J. A.; Swann J. Gut microbial metabolites in depression: understanding the biochemical mechanisms. Microbial cell (Graz, Austria) 2019, 6, 454–481. 10.15698/mic2019.10.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roubalová R.; Procházková P.; Papežová H.; Smitka K.; Bilej M.; Tlaskalová-Hogenová H. Anorexia nervosa: Gut microbiota-immune-brain interactions. Clin. Nutr. 2020, 39, 676–684. 10.1016/j.clnu.2019.03.023. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Wang Y.; Xiayu X.; Shi C.; Chen W.; Song N.; Fu X.; Zhou R.; Xu Y. F.; Huang L.; et al. Altered Gut Microbiota in a Mouse Model of Alzheimer’s Disease. J. Alzheimers Dis. 2017, 60, 1241–1257. 10.3233/JAD-170020. [DOI] [PubMed] [Google Scholar]
- Wang L.; Christophersen C. T.; Sorich M. J.; Gerber J. P.; Angley M. T.; Conlon M. A. Elevated fecal short chain fatty acid and ammonia concentrations in children with autism spectrum disorder. Dig. Dis. Sci. 2012, 57, 2096–2102. 10.1007/s10620-012-2167-7. [DOI] [PubMed] [Google Scholar]
- Galland L. The gut microbiome and the brain. J. Med. Food 2014, 17, 1261–1272. 10.1089/jmf.2014.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka M. T.; Carson M. J.; El Khoury J.; Landreth G. E.; Brosseron F.; Feinstein D. L.; Jacobs A. H.; Wyss-Coray T.; Vitorica J.; Ransohoff R. M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff R. M. How neuroinflammation contributes to neurodegeneration. Science 2016, 353, 777–783. 10.1126/science.aag2590. [DOI] [PubMed] [Google Scholar]
- Karlsson O.; Roman E.; Berg A. L.; Brittebo E. B. Early hippocampal cell death, and late learning and memory deficits in rats exposed to the environmental toxin BMAA (β-N-methylamino-L-alanine) during the neonatal period. Behav. Brain Res. 2011, 219, 310–320. 10.1016/j.bbr.2011.01.056. [DOI] [PubMed] [Google Scholar]
- Nunes-Costa D.; Magalhães J. D.; G-Fernandes M.; Cardoso S. M.; Empadinhas N. Microbial BMAA and the Pathway for Parkinson’s Disease Neurodegeneration. Front. Aging Neurosci. 2020, 12, 26. 10.3389/fnagi.2020.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H.; Rüb U.; Gai W. P.; Del Tredici K. Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J. Neural Transm (Vienna) 2003, 110, 517–536. 10.1007/s00702-002-0808-2. [DOI] [PubMed] [Google Scholar]
- Wallen Z. D.; Demirkan A.; Twa G.; Cohen G.; Dean M. N.; Standaert D. G.; Sampson T. R.; Payami H. Metagenomics of Parkinson’s disease implicates the gut microbiome in multiple disease mechanisms. Nat. Commun. 2022, 13, 6958. 10.1038/s41467-022-34667-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt N. M.; Kerby R. L.; Dill-McFarland K. A.; Harding S. J.; Merluzzi A. P.; Johnson S. C.; Carlsson C. M.; Asthana S.; Zetterberg H.; Blennow K.; et al. Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 2017, 7, 13537. 10.1038/s41598-017-13601-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang Z. Q.; Shen L. L.; Li W. W.; Fu X.; Zeng F.; Gui L.; Lü Y.; Cai M.; Zhu C.; Tan Y. L.; et al. Gut Microbiota is Altered in Patients with Alzheimer’s Disease. J. Alzheimers Dis. 2018, 63, 1337–1346. 10.3233/JAD-180176. [DOI] [PubMed] [Google Scholar]
- Manderino L.; Carroll I.; Azcarate-Peril M. A.; Rochette A.; Heinberg L.; Peat C.; Steffen K.; Mitchell J.; Gunstad J. Preliminary Evidence for an Association Between the Composition of the Gut Microbiome and Cognitive Function in Neurologically Healthy Older Adults. J. Int. Neuropsychol. Soc. 2017, 23, 700–705. 10.1017/S1355617717000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa R. S. D.; Vieira-Coelho M. A. Probiotics and prebiotics: focus on psychiatric disorders - a systematic review. Nutr. Rev. 2020, 78, 437–450. 10.1093/nutrit/nuz080. [DOI] [PubMed] [Google Scholar]
- Ansari F.; Pourjafar H.; Tabrizi A.; Homayouni A. The Effects of Probiotics and Prebiotics on Mental Disorders: A Review on Depression, Anxiety, Alzheimer, and Autism Spectrum Disorders. Curr. Pharm. Biotechnol. 2020, 21, 555–565. 10.2174/1389201021666200107113812. [DOI] [PubMed] [Google Scholar]
- Messaoudi M.; Lalonde R.; Violle N.; Javelot H.; Desor D.; Nejdi A.; Bisson J. F.; Rougeot C.; Pichelin M.; Cazaubiel M.; et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr. 2011, 105, 755–764. 10.1017/S0007114510004319. [DOI] [PubMed] [Google Scholar]
- Akbari E.; Asemi Z.; Daneshvar Kakhaki R.; Bahmani F.; Kouchaki E.; Tamtaji O. R.; Hamidi G. A.; Salami M. Effect of Probiotic Supplementation on Cognitive Function and Metabolic Status in Alzheimer’s Disease: A Randomized, Double-Blind and Controlled Trial. Front. Aging Neurosci. 2016, 8, 256. 10.3389/fnagi.2016.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimgampalle M.; Kuna Y. Anti-Alzheimer Properties of Probiotic, Lactobacillus plantarum MTCC 1325 in Alzheimer’s Disease induced Albino Rats. J. Clin. Diagn. Res. 2017, 11, Kc01–kc05. 10.7860/JCDR/2017/26106.10428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamtaji O. R.; Taghizadeh M.; Daneshvar Kakhaki R.; Kouchaki E.; Bahmani F.; Borzabadi S.; Oryan S.; Mafi A.; Asemi Z. Clinical and metabolic response to probiotic administration in people with Parkinson’s disease: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2019, 38, 1031–1035. 10.1016/j.clnu.2018.05.018. [DOI] [PubMed] [Google Scholar]
- Frazier K.; Chang E. B. Intersection of the Gut Microbiome and Circadian Rhythms in Metabolism. Trends Endocrinol Metab 2020, 31, 25–36. 10.1016/j.tem.2019.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez Lopez D. E.; Lashinger L. M.; Weinstock G. M.; Bray M. S. Circadian rhythms and the gut microbiome synchronize the host’s metabolic response to diet. Cell Metab. 2021, 33, 873–887. 10.1016/j.cmet.2021.03.015. [DOI] [PubMed] [Google Scholar]
- Leng Y.; Musiek E. S.; Hu K.; Cappuccio F. P.; Yaffe K. Association between circadian rhythms and neurodegenerative diseases. Lancet Neurol. 2019, 18, 307–318. 10.1016/S1474-4422(18)30461-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone V.; Gibbons S. M.; Martinez K.; Hutchison A. L.; Huang E. Y.; Cham C. M.; Pierre J. F.; Heneghan A. F.; Nadimpalli A.; Hubert N.; et al. Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell Host Microbe 2015, 17, 681–689. 10.1016/j.chom.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson J. K.; Holmes E.; Kinross J.; Burcelin R.; Gibson G.; Jia W.; Pettersson S. Host-gut microbiota metabolic interactions. Science 2012, 336, 1262–1267. 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- Moran C. P.; Shanahan F. Gut microbiota and obesity: role in aetiology and potential therapeutic target. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 585–597. 10.1016/j.bpg.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Qin J.; Li Y.; Cai Z.; Li S.; Zhu J.; Zhang F.; Liang S.; Zhang W.; Guan Y.; Shen D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- Donohoe D. R.; Wali A.; Brylawski B. P.; Bultman S. J. Microbial regulation of glucose metabolism and cell-cycle progression in mammalian colonocytes. PLoS One 2012, 7, e46589. 10.1371/journal.pone.0046589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohoe D. R.; Garge N.; Zhang X.; Sun W.; O’Connell T. M.; Bunger M. K.; Bultman S. J. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011, 13, 517–526. 10.1016/j.cmet.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolhurst G.; Heffron H.; Lam Y. S.; Parker H. E.; Habib A. M.; Diakogiannaki E.; Cameron J.; Grosse J.; Reimann F.; Gribble F. M. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 2012, 61, 364–371. 10.2337/db11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vos W. M.; Tilg H.; Van Hul M.; Cani P. D. Gut microbiome and health: mechanistic insights. Gut 2022, 71, 1020–1032. 10.1136/gutjnl-2021-326789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeoh Y. K.; Zuo T.; Lui G. C.-Y.; Zhang F.; Liu Q.; Li A. Y.; Chung A. C.; Cheung C. P.; Tso E. Y.; Fung K. S.; et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut 2021, 70, 698–706. 10.1136/gutjnl-2020-323020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchill E.; Lymberopoulos E.; Menozzi E.; Budhdeo S.; McIlroy J. R.; Macnaughtan J.; Sharma N. The Unique Impact of COVID-19 on Human Gut Microbiome Research. Frontiers in Medicine 2021, 8, 652464. 10.3389/fmed.2021.652464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard-Raichon L.; Venzon M.; Klein J.; Axelrad J. E.; Zhang C.; Sullivan A. P.; Hussey G. A.; Casanovas-Massana A.; Noval M. G.; Valero-Jimenez A. M.; et al. Gut microbiome dysbiosis in antibiotic-treated COVID-19 patients is associated with microbial translocation and bacteremia. Nat. Commun. 2022, 13, 5926. 10.1038/s41467-022-33395-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumpitazi B. P. The gut microbiome as a predictor of low fermentable oligosaccharides disaccharides monosaccharides and polyols diet efficacy in functional bowel disorders. Curr. Opin. Gastroenterol. 2020, 36, 147–154. 10.1097/MOG.0000000000000608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshem A.; Segal E.; Elinav E. The Gut Microbiome and Individual-Specific Responses to Diet. mSystems 2020, 5, e00665–00620. 10.1128/mSystems.00665-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepage P.; Häsler R.; Spehlmann M. E.; Rehman A.; Zvirbliene A.; Begun A.; Ott S.; Kupcinskas L.; Doré J.; Raedler A.; et al. Twin study indicates loss of interaction between microbiota and mucosa of patients with ulcerative colitis. Gastroenterology 2011, 141, 227–236. 10.1053/j.gastro.2011.04.011. [DOI] [PubMed] [Google Scholar]
- Manichanh C.; Borruel N.; Casellas F.; Guarner F. The gut microbiota in IBD. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 599–608. 10.1038/nrgastro.2012.152. [DOI] [PubMed] [Google Scholar]
- Tong M.; Li X.; Wegener Parfrey L.; Roth B.; Ippoliti A.; Wei B.; Borneman J.; McGovern D. P.; Frank D. N.; Li E.; et al. A modular organization of the human intestinal mucosal microbiota and its association with inflammatory bowel disease. PLoS One 2013, 8, e80702. 10.1371/journal.pone.0080702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinane C. M.; Cotter P. D. Role of the gut microbiota in health and chronic gastrointestinal disease: understanding a hidden metabolic organ. Therap. Adv. Gastroenterol. 2013, 6, 295–308. 10.1177/1756283X13482996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassam Z.; Lee C. H.; Yuan Y.; Hunt R. H. Fecal microbiota transplantation for Clostridium difficile infection: systematic review and meta-analysis. Am. J. Gastroenterol. 2013, 108, 500–508. 10.1038/ajg.2013.59. [DOI] [PubMed] [Google Scholar]
- Tang W. H.; Kitai T.; Hazen S. L. Gut Microbiota in Cardiovascular Health and Disease. Circ. Res. 2017, 120, 1183–1196. 10.1161/CIRCRESAHA.117.309715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H. Y.; Zhang X.; Yu Z. H.; Zhang Z.; Deng M.; Zhao J. H.; Ruan B. Altered gut microbiota profile in patients with generalized anxiety disorder. J. Psychiatr. Res. 2018, 104, 130–136. 10.1016/j.jpsychires.2018.07.007. [DOI] [PubMed] [Google Scholar]
- Chen Y. H.; Bai J.; Wu D.; Yu S. F.; Qiang X. L.; Bai H.; Wang H. N.; Peng Z. W. Association between fecal microbiota and generalized anxiety disorder: Severity and early treatment response. J. Affect. Disord. 2019, 259, 56–66. 10.1016/j.jad.2019.08.014. [DOI] [PubMed] [Google Scholar]
- Hemmings S. M. J.; Malan-Müller S.; van den Heuvel L. L.; Demmitt B. A.; Stanislawski M. A.; Smith D. G.; Bohr A. D.; Stamper C. E.; Hyde E. R.; Morton J. T.; et al. The Microbiome in Posttraumatic Stress Disorder and Trauma-Exposed Controls: An Exploratory Study. Psychosom. Med. 2017, 79, 936–946. 10.1097/PSY.0000000000000512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B. R.; Goel R.; Seungbum K.; Richards E. M.; Holbert R. C.; Pepine C. J.; Raizada M. K. Increased human intestinal barrier permeability plasma biomarkers zonulin and FABP2 correlated with plasma LPS and altered gut microbiome in anxiety or depression. Gut 2018, 67, 1555–1557. 10.1136/gutjnl-2017-314759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J. R.; Borre Y.; O'Brien C.; Patterson E.; El Aidy S.; Deane J.; Kennedy P. J.; Beers S.; Scott K.; Moloney G.; et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 2016, 82, 109–118. 10.1016/j.jpsychires.2016.07.019. [DOI] [PubMed] [Google Scholar]
- Lin P.; Ding B.; Feng C.; Yin S.; Zhang T.; Qi X.; Lv H.; Guo X.; Dong K.; Zhu Y.; et al. Prevotella and Klebsiella proportions in fecal microbial communities are potential characteristic parameters for patients with major depressive disorder. J. Affect. Disord. 2017, 207, 300–304. 10.1016/j.jad.2016.09.051. [DOI] [PubMed] [Google Scholar]
- Gough E.; Shaikh H.; Manges A. R. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin. Infect. Dis. 2011, 53, 994–1002. 10.1093/cid/cir632. [DOI] [PubMed] [Google Scholar]
- Craciun C. I.; Neag M. A.; Catinean A.; Mitre A. O.; Rusu A.; Bala C.; Roman G.; Buzoianu A. D.; Muntean D. M.; Craciun A. E. The Relationships between Gut Microbiota and Diabetes Mellitus, and Treatments for Diabetes Mellitus. Biomedicines 2022, 10, 308. 10.3390/biomedicines10020308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nood E.; Vrieze A.; Nieuwdorp M.; Fuentes S.; Zoetendal E. G.; de Vos W. M.; Visser C. E.; Kuijper E. J.; Bartelsman J. F. W. M.; Tijssen J. G. P.; et al. Duodenal Infusion of Donor Feces for Recurrent Clostridium difficile. New England Journal of Medicine 2013, 368, 407–415. 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- Hamilton M. J.; Weingarden A. R.; Sadowsky M. J.; Khoruts A. Standardized frozen preparation for transplantation of fecal microbiota for recurrent Clostridium difficile infection. Am. J. Gastroenterol. 2012, 107, 761–767. 10.1038/ajg.2011.482. [DOI] [PubMed] [Google Scholar]
- Zafar H.; Saier M. H. Jr. Gut Bacteroides species in health and disease. Gut Microbes 2021, 13, 1848158. 10.1080/19490976.2020.1848158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.; Yang Y.; Gu J. Clinical Implications of the Associations Between Intestinal Microbiome and Colorectal Cancer Progression. Cancer Manag. Res. 2020, 12, 4117–4128. 10.2147/CMAR.S240108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullman S.; Pedamallu C. S.; Sicinska E.; Clancy T. E.; Zhang X.; Cai D.; Neuberg D.; Huang K.; Guevara F.; Nelson T.; et al. Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science 2017, 358, 1443–1448. 10.1126/science.aal5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammann D.; Lu Y.; Cummings M. J.; Zhang M. L.; Cue J. M.; Do J.; Ebersole J.; Chen X.; Oh E. C.; Cummings J. L.; et al. Genetic correlations between Alzheimer’s disease and gut microbiome genera. Sci. Rep. 2023, 13, 5258. 10.1038/s41598-023-31730-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva Y. P.; Bernardi A.; Frozza R. L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. 10.3389/fendo.2020.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning L. P.; Yao C. K.; Biesiekierski J. R. Therapy of IBS: Is a Low FODMAP Diet the Answer?. Front. Psychiatry 2020, 11, 865. 10.3389/fpsyt.2020.00865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanayakkara W. S.; Skidmore P. M.; O’Brien L.; Wilkinson T. J.; Gearry R. B. Efficacy of the low FODMAP diet for treating irritable bowel syndrome: the evidence to date. Clin. Exp. Gastroenterol. 2016, 9, 131–142. 10.2147/CEG.S86798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegegne B. A.; Kebede B. Probiotics, their prophylactic and therapeutic applications in human health development: A review of the literature. Heliyon 2022, 8, e09725 10.1016/j.heliyon.2022.e09725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aponte M.; Murru N.; Shoukat M. Therapeutic, Prophylactic, and Functional Use of Probiotics: A Current Perspective. Front. Microbiol. 2020, 11, 562048. 10.3389/fmicb.2020.562048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan S.; Zaveri M.; Macaninch E.; Martyn K. Food & mood: a review of supplementary prebiotic and probiotic interventions in the treatment of anxiety and depression in adults. BMJ. Nutrition, Prevention & Health 2020, 3, 351. 10.1136/bmjnph-2019-000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACFARLANE S.; MACFARLANE G. T.; CUMMINGS J. H. Review article: prebiotics in the gastrointestinal tract. Aliment. Pharmacol. Ther. 2006, 24, 701–714. 10.1111/j.1365-2036.2006.03042.x. [DOI] [PubMed] [Google Scholar]
- Dinan K.; Dinan T. Antibiotics and mental health: The good, the bad and the ugly. J. Int. Med. 2022, 292, 858–869. 10.1111/joim.13543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakan T.; Ozkul C.; Küpeli Akkol E.; Bilici S.; Sobarzo-Sánchez E.; Capasso R. Gut-Brain-Microbiota Axis: Antibiotics and Functional Gastrointestinal Disorders. Nutrients 2021, 13, 389. 10.3390/nu13020389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H. M.; Huang H. L.; Zhou Y. L.; Zhao H. L.; Xu J.; Shou D. W.; Liu Y. D.; Zhou Y. J.; Nie Y. Q. Fecal Microbiota Transplantation: A New Therapeutic Attempt from the Gut to the Brain. Gastroenterol. Res. Pract. 2021, 2021, 6699268. 10.1155/2021/6699268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll J. P. K.; Vázquez-Castellanos J. F.; Schaub A.-C.; Schweinfurth N.; Kettelhack C.; Schneider E.; Yamanbaeva G.; Mählmann L.; Brand S.; Beglinger C. Fecal Microbiota Transplantation (FMT) as an Adjunctive Therapy for Depression—Case Report. Front. Psychiatry 2022, 13, 815422. 10.3389/fpsyt.2022.815422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller K. M. J.; Leong R. W.; Paramsothy S. An update on fecal microbiota transplantation for the treatment of gastrointestinal diseases. J. Gastroenterol. Hepatol. 2022, 37, 246–255. 10.1111/jgh.15731. [DOI] [PubMed] [Google Scholar]
- Hetterich L.; Stengel A. Psychotherapeutic Interventions in Irritable Bowel Syndrome. Front. Psychiatry 2020, 11, 286. 10.3389/fpsyt.2020.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildes J. E.; Bedell A.; Graham A. K.; Kells M. Brain-gut psychotherapies: Promising tools to address gastrointestinal problems in patients with eating disorders. Int. J. Eat. Disord. 2021, 54, 1063–1067. 10.1002/eat.23555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird K. T.; Tanner-Smith E. E.; Russell A. C.; Hollon S. D.; Walker L. S. Comparative efficacy of psychological therapies for improving mental health and daily functioning in irritable bowel syndrome: A systematic review and meta-analysis. Clin. Psychol. Rev. 2017, 51, 142–152. 10.1016/j.cpr.2016.11.001. [DOI] [PubMed] [Google Scholar]
- Colomier E.; Algera J.; Melchior C. Pharmacological Therapies and Their Clinical Targets in Irritable Bowel Syndrome With Diarrhea. Front. Pharmacol. 2021, 11, 629026. 10.3389/fphar.2020.629026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Pharmacological treatment of mental disorders in primary health care. WHO Press: Geneva, Switzerland, 2009. [PubMed] [Google Scholar]
- Fikree A.; Byrne P. Management of functional gastrointestinal disorders. Clin Med. (Lond) 2021, 21, 44–52. 10.7861/clinmed.2020-0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney B. T.; Yang Z.; Luber J. M.; Beaudin M.; Wibowo M. C.; Baek C.; Mehlenbacher E.; Patel C. J.; Kostic A. D. The Landscape of Genetic Content in the Gut and Oral Human Microbiome. Cell Host Microbe 2019, 26, 283–295.e288. 10.1016/j.chom.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Human Genome Sequencing Consortium Finishing the euchromatic sequence of the human genome. Nature 2004, 431, 931–945. 10.1038/nature03001. [DOI] [PubMed] [Google Scholar]
- Liu J.; Tan Y.; Cheng H.; Zhang D.; Feng W.; Peng C. Functions of Gut Microbiota Metabolites, Current Status and Future Perspectives. Aging Dis. 2022, 13, 1106–1126. 10.14336/AD.2022.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijtz R. D.; Wang S.; Anuar F.; Qian Y.; Björkholm B.; Samuelsson A.; Hibberd M. L.; Forssberg H.; Pettersson S. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 3047–3052. 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo N.; Chida Y.; Aiba Y.; Sonoda J.; Oyama N.; Yu X.-N.; Kubo C.; Koga Y. Postnatal microbial colonization programs the hypothalamic–pituitary–adrenal system for stress response in mice. Journal of Physiology 2004, 558, 263–275. 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham B. D.; Funabashi M.; Adame M. D.; Wang Z.; Boktor J. C.; Haney J.; Wu W.-L.; Rabut C.; Ladinsky M. S.; Hwang S.-J.; et al. A gut-derived metabolite alters brain activity and anxiety behaviour in mice. Nature 2022, 602, 647–653. 10.1038/s41586-022-04396-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gacias M.; Gaspari S.; Santos P.-M. G.; Tamburini S.; Andrade M.; Zhang F.; Shen N.; Tolstikov V.; Kiebish M. A.; Dupree J. L.; et al. Microbiota-driven transcriptional changes in prefrontal cortex override genetic differences in social behavior. eLife 2016, 5, e13442. 10.7554/eLife.13442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong H. E.; Pronovost G. N.; Williams D. W.; Coley E. J. L.; Siegler E. L.; Qiu A.; Kazantsev M.; Wilson C. J.; Rendon T.; Hsiao E. Y. The maternal microbiome modulates fetal neurodevelopment in mice. Nature 2020, 586, 281–286. 10.1038/s41586-020-2745-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei G. Z.; Martin K. A.; Xing P. Y.; Agrawal R.; Whiley L.; Wood T. K.; Hejndorf S.; Ng Y. Z.; Low J. Z. Y.; Rossant J.; et al. Tryptophan-metabolizing gut microbes regulate adult neurogenesis via the aryl hydrocarbon receptor. Proc. Natl. Acad. Sci. U. S. A. 2021, 118, e2021091118. 10.1073/pnas.2021091118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D.-W.; Adams J. B.; Gregory A. C.; Borody T.; Chittick L.; Fasano A.; Khoruts A.; Geis E.; Maldonado J.; McDonough-Means S.; et al. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome 2017, 5, 10. 10.1186/s40168-016-0225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpela K.; Helve O.; Kolho K. L.; Saisto T.; Skogberg K.; Dikareva E.; Stefanovic V.; Salonen A.; Andersson S.; de Vos W. M. Maternal Fecal Microbiota Transplantation in Cesarean-Born Infants Rapidly Restores Normal Gut Microbial Development: A Proof-of-Concept Study. Cell 2020, 183, 324–334.e325. 10.1016/j.cell.2020.08.047. [DOI] [PubMed] [Google Scholar]
- Wikoff W. R.; Anfora A. T.; Liu J.; Schultz P. G.; Lesley S. A.; Peters E. C.; Siuzdak G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl. Acad. Sci. U. S. A. 2009, 106, 3698–3703. 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd D.; Spitzer M. H.; Van Treuren W.; Merrill B. D.; Hryckowian A. J.; Higginbottom S. K.; Le A.; Cowan T. M.; Nolan G. P.; Fischbach M. A.; et al. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature 2017, 551, 648–652. 10.1038/nature24661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E.; Ross R. P.; O’Toole P. W.; Fitzgerald G. F.; Stanton C. γ-Aminobutyric acid production by culturable bacteria from the human intestine. J. Appl. Microbiol. 2012, 113, 411–417. 10.1111/j.1365-2672.2012.05344.x. [DOI] [PubMed] [Google Scholar]
- Asano Y.; Hiramoto T.; Nishino R.; Aiba Y.; Kimura T.; Yoshihara K.; Koga Y.; Sudo N. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G1288–1295. 10.1152/ajpgi.00341.2012. [DOI] [PubMed] [Google Scholar]
- Pocivavsek A.; Wu H.-Q.; Potter M. C.; Elmer G. I.; Pellicciari R.; Schwarcz R. Fluctuations in Endogenous Kynurenic Acid Control Hippocampal Glutamate and Memory. Neuropsychopharmacology 2011, 36, 2357–2367. 10.1038/npp.2011.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter M. C.; Elmer G. I.; Bergeron R.; Albuquerque E. X.; Guidetti P.; Wu H.-Q.; Schwarcz R. Reduction of Endogenous Kynurenic Acid Formation Enhances Extracellular Glutamate, Hippocampal Plasticity, and Cognitive Behavior. Neuropsychopharmacology 2010, 35, 1734–1742. 10.1038/npp.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reigstad C. S.; Salmonson C. E.; Rainey J. F. III; Szurszewski J. H.; Linden D. R.; Sonnenburg J. L.; Farrugia G.; Kashyap P. C. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015, 29, 1395–1403. 10.1096/fj.14-259598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano J. M.; Yu K.; Donaldson G. P.; Shastri G. G.; Ann P.; Ma L.; Nagler C. R.; Ismagilov R. F.; Mazmanian S. K.; Hsiao E. Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015, 161, 264–276. 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellono N. W.; Bayrer J. R.; Leitch D. B.; Castro J.; Zhang C.; O’Donnell T. A.; Brierley S. M.; Ingraham H. A.; Julius D. Enterochromaffin Cells Are Gut Chemosensors that Couple to Sensory Neural Pathways. Cell 2017, 170, 185–198.e16. 10.1016/j.cell.2017.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L.; Bae M.; Cassilly C. D.; Jabba S. V.; Thorpe D. W.; Martin A. M.; Lu H. Y.; Wang J.; Thompson J. D.; Lickwar C. R.; et al. Enteroendocrine cells sense bacterial tryptophan catabolites to activate enteric and vagal neuronal pathways. Cell Host Microbe 2021, 29, 179–196.e79. 10.1016/j.chom.2020.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hagan C.; Li J. V.; Marchesi J. R.; Plummer S.; Garaiova I.; Good M. A. Long-term multi-species Lactobacillus and Bifidobacterium dietary supplement enhances memory and changes regional brain metabolites in middle-aged rats. Neurobiol Learn Mem 2017, 144, 36–47. 10.1016/j.nlm.2017.05.015. [DOI] [PubMed] [Google Scholar]
- Mao J.-H.; Kim Y.-M.; Zhou Y.-X.; Hu D.; Zhong C.; Chang H.; Brislawn C. J.; Fansler S.; Langley S.; Wang Y.; et al. Genetic and metabolic links between the murine microbiome and memory. Microbiome 2020, 8, 53. 10.1186/s40168-020-00817-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Hayek L.; Khalifeh M.; Zibara V.; Abi Assaad R.; Emmanuel N.; Karnib N.; El-Ghandour R.; Nasrallah P.; Bilen M.; Ibrahim P.; et al. Lactate Mediates the Effects of Exercise on Learning and Memory through SIRT1-Dependent Activation of Hippocampal Brain-Derived Neurotrophic Factor (BDNF). J. Neurosci. 2019, 39, 2369–2382. 10.1523/JNEUROSCI.1661-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalile B.; Van Oudenhove L.; Vervliet B.; Verbeke K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nature Reviews Gastroenterology & Hepatology 2019, 16, 461–478. 10.1038/s41575-019-0157-3. [DOI] [PubMed] [Google Scholar]
- van de Wouw M.; Boehme M.; Lyte J. M.; Wiley N.; Strain C.; O’Sullivan O.; Clarke G.; Stanton C.; Dinan T. G.; Cryan J. F. Short-chain fatty acids: microbial metabolites that alleviate stress-induced brain–gut axis alterations. Journal of Physiology 2018, 596, 4923–4944. 10.1113/JP276431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernasconi R.; Jones R. S.; Bittiger H.; Olpe H. R.; Heid J.; Martin P.; Klein M.; Loo P.; Braunwalder A.; Schmutz M. Dose pipecolic acid interact with the central GABA-ergic system?. J. Neural Transm. 1986, 67, 175–189. 10.1007/BF01243346. [DOI] [PubMed] [Google Scholar]
- Takahama K.; Hashimoto T.; Wang M. W.; Akaike N.; Hitoshi T.; Okano Y.; Kasé Y.; Miyata T. Pipecolic acid enhancement of GABA response in single neurons of rat brain. Neuropharmacology 1986, 25, 339–342. 10.1016/0028-3908(86)90263-7. [DOI] [PubMed] [Google Scholar]
- Braniste V.; Al-Asmakh M.; Kowal C.; Anuar F.; Abbaspour A.; Tóth M.; Korecka A.; Bakocevic N.; Ng L. G.; Kundu P.; et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, 263ra158–263ra158. 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H.; Sun J.; Wang F.; Ding G.; Chen W.; Fang R.; Yao Y.; Pang M.; Lu Z. Q.; Liu J. Sodium butyrate exerts neuroprotective effects by restoring the blood-brain barrier in traumatic brain injury mice. Brain Res. 2016, 1642, 70–78. 10.1016/j.brainres.2016.03.031. [DOI] [PubMed] [Google Scholar]
- Hoyles L.; Snelling T.; Umlai U.-K.; Nicholson J. K.; Carding S. R.; Glen R. C.; McArthur S. Microbiome–host systems interactions: protective effects of propionate upon the blood–brain barrier. Microbiome 2018, 6, 55. 10.1186/s40168-018-0439-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn M.; McMillin M.; Galindo C.; Frampton G.; Pae H. Y.; DeMorrow S. Bile acids permeabilize the blood brain barrier after bile duct ligation in rats via Rac1-dependent mechanisms. Dig. Liver Dis. 2014, 46, 527–534. 10.1016/j.dld.2014.01.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmela I.; Correia L.; Silva R. F. M.; Sasaki H.; Kim K. S.; Brites D.; Brito M. A. Hydrophilic bile acids protect human blood-brain barrier endothelial cells from disruption by unconjugated bilirubin: an in vitro study. Front. Neurosci. 2015, 9, 80. 10.3389/fnins.2015.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyles L.; Pontifex M. G.; Rodriguez-Ramiro I.; Anis-Alavi M. A.; Jelane K. S.; Snelling T.; Solito E.; Fonseca S.; Carvalho A. L.; Carding S. R.; et al. Regulation of blood–brain barrier integrity by microbiome-associated methylamines and cognition by trimethylamine N-oxide. Microbiome 2021, 9, 235. 10.1186/s40168-021-01181-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistretti P. J.; Allaman I. A cellular perspective on brain energy metabolism and functional imaging. Neuron 2015, 86, 883–901. 10.1016/j.neuron.2015.03.035. [DOI] [PubMed] [Google Scholar]
- Wyss M. T.; Jolivet R.; Buck A.; Magistretti P. J.; Weber B. In Vivo Evidence for Lactate as a Neuronal Energy Source. J. Neurosci. 2011, 31, 7477–7485. 10.1523/JNEUROSCI.0415-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z.; Dai X.; Zhang H.; Shi R.; Hui Y.; Jin X.; Zhang W.; Wang L.; Wang Q.; Wang D.; et al. Gut microbiota mediates intermittent-fasting alleviation of diabetes-induced cognitive impairment. Nat. Commun. 2020, 11, 855. 10.1038/s41467-020-14676-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell W. R.; Hoyles L.; Flint H. J.; Dumas M. E. Colonic bacterial metabolites and human health. Curr. Opin. Microbiol. 2013, 16, 246–254. 10.1016/j.mib.2013.07.002. [DOI] [PubMed] [Google Scholar]
- Ren Z.; Zhang R.; Li Y.; Li Y.; Yang Z.; Yang H. Ferulic acid exerts neuroprotective effects against cerebral ischemia/reperfusion-induced injury via antioxidant and anti-apoptotic mechanisms in vitro and in vivo. Int. J. Mol. Med. 2017, 40, 1444–1456. 10.3892/ijmm.2017.3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeni A. L. B.; Camargo A.; Dalmagro A. P. Ferulic acid reverses depression-like behavior and oxidative stress induced by chronic corticosterone treatment in mice. Steroids 2017, 125, 131–136. 10.1016/j.steroids.2017.07.006. [DOI] [PubMed] [Google Scholar]
- Verzelloni E.; Pellacani C.; Tagliazucchi D.; Tagliaferri S.; Calani L.; Costa L. G.; Brighenti F.; Borges G.; Crozier A.; Conte A.; et al. Antiglycative and neuroprotective activity of colon-derived polyphenol catabolites. Mol. Nutr. Food Res. 2011, 55, S35–S43. 10.1002/mnfr.201000525. [DOI] [PubMed] [Google Scholar]
- Macfarlane S.; Macfarlane G. T. Regulation of short-chain fatty acid production. Proc. Nutr. Soc. 2003, 62, 67–72. 10.1079/PNS2002207. [DOI] [PubMed] [Google Scholar]
- Thomas C. M.; Hong T.; van Pijkeren J. P.; Hemarajata P.; Trinh D. V.; Hu W.; Britton R. A.; Kalkum M.; Versalovic J. Histamine derived from probiotic Lactobacillus reuteri suppresses TNF via modulation of PKA and ERK signaling. PLoS One 2012, 7, e31951. 10.1371/journal.pone.0031951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Biase D.; Pennacchietti E. Glutamate decarboxylase-dependent acid resistance in orally acquired bacteria: function, distribution and biomedical implications of the gadBC operon. Mol. Microbiol. 2012, 86, 770–786. 10.1111/mmi.12020. [DOI] [PubMed] [Google Scholar]
- Zhou L.; Zeng Y.; Zhang H.; Ma Y. The Role of Gastrointestinal Microbiota in Functional Dyspepsia: A Review. Front. Physiol. 2022, 13, 910568. 10.3389/fphys.2022.910568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandrov P. N.; Hill J. M.; Zhao Y.; Bond T.; Taylor C. M.; Percy M. E.; Li W.; Lukiw W. J. Aluminum-induced generation of lipopolysaccharide (LPS) from the human gastrointestinal (GI)-tract microbiome-resident Bacteroides fragilis. J. Inorg. Biochem. 2020, 203, 110886. 10.1016/j.jinorgbio.2019.110886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E. M.; Ke X.; Hitchcock D.; Jeanfavre S.; Avila-Pacheco J.; Nakata T.; Arthur T. D.; Fornelos N.; Heim C.; Franzosa E. A.; et al. Bacteroides-Derived Sphingolipids Are Critical for Maintaining Intestinal Homeostasis and Symbiosis. Cell Host Microbe 2019, 25, 668–680.e67. 10.1016/j.chom.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao K.; Pi Y.; Mu C. L.; Farzi A.; Liu Z.; Zhu W. Y. Increasing carbohydrate availability in the hindgut promotes hypothalamic neurotransmitter synthesis: aromatic amino acids linking the microbiota-brain axis. J. Neurochem. 2019, 149, 641–659. 10.1111/jnc.14709. [DOI] [PubMed] [Google Scholar]
- Gao K.; Mu C.-l.; Farzi A.; Zhu W.-y. Tryptophan Metabolism: A Link Between the Gut Microbiota and Brain. Adv. Nutr. 2020, 11, 709–723. 10.1093/advances/nmz127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L.; Liu Q.; Luo M.; Xiong L. Gut Microbiota-Derived Metabolites in Irritable Bowel Syndrome. Frontiers in Cellular and Infection Microbiology 2021, 11, 729346. 10.3389/fcimb.2021.729346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawe G. M.; Hoffman J. M. Serotonin signalling in the gut--functions, dysfunctions and therapeutic targets. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 473–486. 10.1038/nrgastro.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J.; McKenzie C.; Potamitis M.; Thorburn A. N.; Mackay C. R.; Macia L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014, 121, 91–119. 10.1016/B978-0-12-800100-4.00003-9. [DOI] [PubMed] [Google Scholar]
- Felizardo R. J. F.; Watanabe I. K. M.; Dardi P.; Rossoni L. V.; Câmara N. O. S. The interplay among gut microbiota, hypertension and kidney diseases: The role of short-chain fatty acids. Pharmacol. Res. 2019, 141, 366–377. 10.1016/j.phrs.2019.01.019. [DOI] [PubMed] [Google Scholar]
- Canfora E. E.; Meex R. C. R.; Venema K.; Blaak E. E. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat. Rev. Endocrinol. 2019, 15, 261–273. 10.1038/s41574-019-0156-z. [DOI] [PubMed] [Google Scholar]
- Tahara Y.; Yamazaki M.; Sukigara H.; Motohashi H.; Sasaki H.; Miyakawa H.; Haraguchi A.; Ikeda Y.; Fukuda S.; Shibata S. Gut Microbiota-Derived Short Chain Fatty Acids Induce Circadian Clock Entrainment in Mouse Peripheral Tissue. Sci. Rep. 2018, 8, 1395. 10.1038/s41598-018-19836-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W.; Ao H.; Peng C. Gut Microbiota, Short-Chain Fatty Acids, and Herbal Medicines. Front. Pharmacol. 2018, 9, 1354. 10.3389/fphar.2018.01354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L. L.; Li B. B.; Pan X. H.; Sun J. Gut microbiota in pancreatic diseases: possible new therapeutic strategies. Acta Pharmacol. Sin. 2021, 42, 1027–1039. 10.1038/s41401-020-00532-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perino A.; Demagny H.; Velazquez-Villegas L.; Schoonjans K. Molecular Physiology of Bile Acid Signaling in Health, Disease, and Aging. Physiol. Rev. 2021, 101, 683–731. 10.1152/physrev.00049.2019. [DOI] [PubMed] [Google Scholar]
- Wahlström A.; Sayin S. I.; Marschall H. U.; Bäckhed F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016, 24, 41–50. 10.1016/j.cmet.2016.05.005. [DOI] [PubMed] [Google Scholar]
- Poland J. C.; Flynn C. R. Bile Acids, Their Receptors, and the Gut Microbiota. Physiology (Bethesda) 2021, 36, 235–245. 10.1152/physiol.00028.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindarajan K.; MacSharry J.; Casey P. G.; Shanahan F.; Joyce S. A.; Gahan C. G. M. Unconjugated Bile Acids Influence Expression of Circadian Genes: A Potential Mechanism for Microbe-Host Crosstalk. PLoS One 2016, 11, e0167319 10.1371/journal.pone.0167319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillin M.; DeMorrow S. Effects of bile acids on neurological function and disease. FASEB J. 2016, 30, 3658–3668. 10.1096/fj.201600275R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth W.; Zadeh K.; Vekariya R.; Ge Y.; Mohamadzadeh M. Tryptophan Metabolism and Gut-Brain Homeostasis. Int. J. Mol. Sci. 2021, 22, 2973. 10.3390/ijms22062973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.; Zhu S.; Ma N.; Johnston L. J.; Wu C.; Ma X. Metabolites of microbiota response to tryptophan and intestinal mucosal immunity: A therapeutic target to control intestinal inflammation. Med. Res. Rev. 2021, 41, 1061–1088. 10.1002/med.21752. [DOI] [PubMed] [Google Scholar]
- Modoux M.; Rolhion N.; Mani S.; Sokol H. Tryptophan Metabolism as a Pharmacological Target. Trends Pharmacol. Sci. 2021, 42, 60–73. 10.1016/j.tips.2020.11.006. [DOI] [PubMed] [Google Scholar]
- Roager H. M.; Licht T. R. Microbial tryptophan catabolites in health and disease. Nat. Commun. 2018, 9, 3294. 10.1038/s41467-018-05470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agus A.; Planchais J.; Sokol H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. 10.1016/j.chom.2018.05.003. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Bergeron N.; Levison B. S.; Li X. S.; Chiu S.; Jia X.; Koeth R. A.; Li L.; Wu Y.; Tang W. H. W.; et al. Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur. Heart J. 2019, 40, 583–594. 10.1093/eurheartj/ehy799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Wang Y.; Ke B.; Du J. TMAO: how gut microbiota contributes to heart failure. Transl. Res. 2021, 228, 109–125. 10.1016/j.trsl.2020.08.007. [DOI] [PubMed] [Google Scholar]
- Yang S.; Li X.; Yang F.; Zhao R.; Pan X.; Liang J.; Tian L.; Li X.; Liu L.; Xing Y.; et al. Gut Microbiota-Dependent Marker TMAO in Promoting Cardiovascular Disease: Inflammation Mechanism, Clinical Prognostic, and Potential as a Therapeutic Target. Front. Pharmacol. 2019, 10, 1360. 10.3389/fphar.2019.01360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudzki L.; Stone T. W.; Maes M.; Misiak B.; Samochowiec J.; Szulc A. Gut microbiota-derived vitamins - underrated powers of a multipotent ally in psychiatric health and disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 107, 110240. 10.1016/j.pnpbp.2020.110240. [DOI] [PubMed] [Google Scholar]
- Stacchiotti V.; Rezzi S.; Eggersdorfer M.; Galli F. Metabolic and functional interplay between gut microbiota and fat-soluble vitamins. Crit Rev. Food Sci. Nutr 2021, 61, 3211–3232. 10.1080/10408398.2020.1793728. [DOI] [PubMed] [Google Scholar]
- Masri O. A.; Chalhoub J. M.; Sharara A. I. Role of vitamins in gastrointestinal diseases. World J. Gastroenterol. 2015, 21, 5191–5209. 10.3748/wjg.v21.i17.5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarville J. L.; Chen G. Y.; Cuevas V. D.; Troha K.; Ayres J. S. Microbiota Metabolites in Health and Disease. Annu. Rev. Immunol. 2020, 38, 147–170. 10.1146/annurev-immunol-071219-125715. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Li N.; Yang J. J.; Zhao D. M.; Chen B.; Zhang G. Q.; Chen S.; Cao R. F.; Yu H.; Zhao C. Y.; et al. Probiotics and fructo-oligosaccharide intervention modulate the microbiota-gut brain axis to improve autism spectrum reducing also the hyper-serotonergic state and the dopamine metabolism disorder. Pharmacol. Res. 2020, 157, 104784. 10.1016/j.phrs.2020.104784. [DOI] [PubMed] [Google Scholar]
- Morais L. H.; Schreiber H. L. t.; Mazmanian S. K. The gut microbiota-brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. 10.1038/s41579-020-00460-0. [DOI] [PubMed] [Google Scholar]
- Cani P. D.; Amar J.; Iglesias M. A.; Poggi M.; Knauf C.; Bastelica D.; Neyrinck A. M.; Fava F.; Tuohy K. M.; Chabo C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- Serino M.; Luche E.; Gres S.; Baylac A.; Bergé M.; Cenac C.; Waget A.; Klopp P.; Iacovoni J.; Klopp C.; et al. Metabolic adaptation to a high-fat diet is associated with a change in the gut microbiota. Gut 2012, 61, 543–553. 10.1136/gutjnl-2011-301012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostojic S. M. Inadequate Production of H(2) by Gut Microbiota and Parkinson Disease. Trends Endocrinol Metab 2018, 29, 286–288. 10.1016/j.tem.2018.02.006. [DOI] [PubMed] [Google Scholar]
- Kalantar-Zadeh K.; Berean K. J.; Burgell R. E.; Muir J. G.; Gibson P. R. Intestinal gases: influence on gut disorders and the role of dietary manipulations. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 733–747. 10.1038/s41575-019-0193-z. [DOI] [PubMed] [Google Scholar]
- Pacher P.; Beckman J. S.; Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S. B.; Lin H. C. Hydrogen Sulfide in Physiology and Diseases of the Digestive Tract. Microorganisms 2015, 3, 866–889. 10.3390/microorganisms3040866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen N. Functional and Molecular Insights of Hydrogen Sulfide Signaling and Protein Sulfhydration. J. Mol. Biol. 2017, 429, 543–561. 10.1016/j.jmb.2016.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skonieczna-Żydecka K.; Marlicz W.; Misera A.; Koulaouzidis A.; Łoniewski I. Microbiome—The Missing Link in the Gut-Brain Axis: Focus on Its Role in Gastrointestinal and Mental Health. Journal of Clinical Medicine 2018, 7, 521. 10.3390/jcm7120521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmulson M. J.; Drossman D. A. What Is New in Rome IV. J. Neurogastroenterol. Motil. 2017, 23, 151–163. 10.5056/jnm16214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasi C.; Nisita C.; Cortopassi S.; Corretti G.; Gambaccini D.; De Bortoli N.; Fani B.; Simonetti N.; Ricchiuti A.; Dell’Osso L.; et al. Subthreshold Psychiatric Psychopathology in Functional Gastrointestinal Disorders: Can It Be the Bridge between Gastroenterology and Psychiatry?. Gastroenterol. Res. Pract. 2017, 2017, 1953435. 10.1155/2017/1953435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan J. F.; O’Riordan K. J.; Cowan C. S. M.; Sandhu K. V.; Bastiaanssen T. F. S.; Boehme M.; Codagnone M. G.; Cussotto S.; Fulling C.; Golubeva A. V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. 10.1152/physrev.00018.2018. [DOI] [PubMed] [Google Scholar]
- Gralnek I. M.; Hays R. D.; Kilbourne A.; Naliboff B.; Mayer E. A. The impact of irritable bowel syndrome on health-related quality of life. Gastroenterology 2000, 119, 654–660. 10.1053/gast.2000.16484. [DOI] [PubMed] [Google Scholar]
- O’Keeffe M.; Lomer M. C. Who should deliver the low FODMAP diet and what educational methods are optimal: a review. J. Gastroenterol. Hepatol. 2017, 32, 23–26. 10.1111/jgh.13690. [DOI] [PubMed] [Google Scholar]
- Koloski N. A.; Jones M.; Talley N. J. Evidence that independent gut-to-brain and brain-to-gut pathways operate in the irritable bowel syndrome and functional dyspepsia: a 1-year population-based prospective study. Alimentary pharmacology & therapeutics 2016, 44, 592–600. 10.1111/apt.13738. [DOI] [PubMed] [Google Scholar]
- Eijsbouts C.; Zheng T.; Kennedy N. A.; Bonfiglio F.; Anderson C. A.; Moutsianas L.; Holliday J.; Shi J.; Shringarpure S.; Agee M.; et al. Genome-wide analysis of 53,400 people with irritable bowel syndrome highlights shared genetic pathways with mood and anxiety disorders. Nat. Genet. 2021, 53, 1543–1552. 10.1038/s41588-021-00950-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz E.; Maukonen J.; Hyytiäinen T.; Kieseppä T.; Orešič M.; Sabunciyan S.; Mantere O.; Saarela M.; Yolken R.; Suvisaari J. Analysis of microbiota in first episode psychosis identifies preliminary associations with symptom severity and treatment response. Schizophr. Res. 2018, 192, 398–403. 10.1016/j.schres.2017.04.017. [DOI] [PubMed] [Google Scholar]
- Castro-Nallar E.; Bendall M. L.; Pérez-Losada M.; Sabuncyan S.; Severance E. G.; Dickerson F. B.; Schroeder J. R.; Yolken R. H.; Crandall K. A. Composition, taxonomy and functional diversity of the oropharynx microbiome in individuals with schizophrenia and controls. PeerJ. 2015, 3, e1140. 10.7717/peerj.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkaid Y.; Hand T. W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson G.; Maes M. Bipolar disorder: role of immune-inflammatory cytokines, oxidative and nitrosative stress and tryptophan catabolites. Curr. Psychiatry Rep 2015, 17, 8. 10.1007/s11920-014-0541-1. [DOI] [PubMed] [Google Scholar]
- Clarke G.; Fitzgerald P.; Cryan J. F.; Cassidy E. M.; Quigley E. M.; Dinan T. G. Tryptophan degradation in irritable bowel syndrome: evidence of indoleamine 2,3-dioxygenase activation in a male cohort. BMC Gastroenterol. 2009, 9, 6. 10.1186/1471-230X-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke G.; McKernan D.; Gaszner G.; Quigley E.; Cryan J.; Dinan T. A Distinct Profile of Tryptophan Metabolism along the Kynurenine Pathway Downstream of Toll-Like Receptor Activation in Irritable Bowel Syndrome. Front. Pharmacol. 2012, 3, 90. 10.3389/fphar.2012.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X.; FitzGerald G. A. Timing the Microbes: The Circadian Rhythm of the Gut Microbiome. J. Biol. Rhythms 2017, 32, 505–515. 10.1177/0748730417729066. [DOI] [PubMed] [Google Scholar]
- Enck P.; Aziz Q.; Barbara G.; Farmer A. D.; Fukudo S.; Mayer E. A.; Niesler B.; Quigley E. M.; Rajilić-Stojanović M.; Schemann M.; et al. Irritable bowel syndrome. Nat. Rev. Dis Primers 2016, 2, 16014. 10.1038/nrdp.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalile B.; Vervliet B.; Bergonzelli G.; Verbeke K.; Van Oudenhove L. Colon-delivered short-chain fatty acids attenuate the cortisol response to psychosocial stress in healthy men: a randomized, placebo-controlled trial. Neuropsychopharmacology 2020, 45, 2257–2266. 10.1038/s41386-020-0732-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bercik P.; Park A. J.; Sinclair D.; Khoshdel A.; Lu J.; Huang X.; Deng Y.; Blennerhassett P. A.; Fahnestock M.; Moine D.; et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol. Motil. 2011, 23, 1132–1139. 10.1111/j.1365-2982.2011.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima Y.; Ishihara S. Molecular Mechanisms of Microbiota-Mediated Pathology in Irritable Bowel Syndrome. Int. J. Mol. Sci. 2020, 21, 8664. 10.3390/ijms21228664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeming E. R.; Johnson A. J.; Spector T. D.; Le Roy C. I. Effect of Diet on the Gut Microbiota: Rethinking Intervention Duration. Nutrients 2019, 11, 2862. 10.3390/nu11122862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillestad E. M. R.; van der Meeren A.; Nagaraja B. H.; Bjo̷rsvik B. R.; Haleem N.; Benitez-Paez A.; Sanz Y.; Hausken T.; Lied G. A.; Lundervold A.; et al. Gut bless you: The microbiota-gut-brain axis in irritable bowel syndrome. World J. Gastroenterol. 2022, 28, 412–431. 10.3748/wjg.v28.i4.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugerth L. W.; Andreasson A.; Talley N. J.; Forsberg A. M.; Kjellström L.; Schmidt P. T.; Agreus L.; Engstrand L. No distinct microbiome signature of irritable bowel syndrome found in a Swedish random population. Gut 2020, 69, 1076–1084. 10.1136/gutjnl-2019-318717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundin J.; Aziz I.; Nordlander S.; Polster A.; Hu Y. O. O.; Hugerth L. W.; Pennhag A. A. L.; Engstrand L.; Törnblom H.; Simrén M.; et al. Evidence of altered mucosa-associated and fecal microbiota composition in patients with Irritable Bowel Syndrome. Sci. Rep. 2020, 10, 593. 10.1038/s41598-020-57468-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer E. A.; Labus J.; Aziz Q.; Tracey I.; Kilpatrick L.; Elsenbruch S.; Schweinhardt P.; Van Oudenhove L.; Borsook D. Role of brain imaging in disorders of brain-gut interaction: a Rome Working Team Report. Gut 2019, 68, 1701–1715. 10.1136/gutjnl-2019-318308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminowicz D. A.; Labus J. S.; Bueller J. A.; Tillisch K.; Naliboff B. D.; Bushnell M. C.; Mayer E. A. Regional gray matter density changes in brains of patients with irritable bowel syndrome. Gastroenterology 2010, 139, 48–57.e42. 10.1053/j.gastro.2010.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M.; Grinsvall C.; Ran Q.; Dupont P.; Morishita J.; Muratsubaki T.; Mugikura S.; Ly H. G.; Törnblom H.; Ljungberg M.; et al. Resting state functional connectivity of the pain matrix and default mode network in irritable bowel syndrome: a graph theoretical analysis. Sci. Rep. 2020, 10, 11015. 10.1038/s41598-020-67048-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder-Smith C. H.; Schindler D.; Lovblad K.; Redmond S. M.; Nirkko A. Brain functional magnetic resonance imaging of rectal pain and activation of endogenous inhibitory mechanisms in irritable bowel syndrome patient subgroups and healthy controls. Gut 2004, 53, 1595–1601. 10.1136/gut.2003.028514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillisch K.; Mayer E. A.; Labus J. S. Quantitative meta-analysis identifies brain regions activated during rectal distension in irritable bowel syndrome. Gastroenterology 2011, 140, 91–100. 10.1053/j.gastro.2010.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringel Y.; Drossman D. A.; Leserman J. L.; Suyenobu B. Y.; Wilber K.; Lin W.; Whitehead W. E.; Naliboff B. D.; Berman S.; Mayer E. A. Effect of abuse history on pain reports and brain responses to aversive visceral stimulation: an FMRI study. Gastroenterology 2008, 134, 396–404. 10.1053/j.gastro.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Mayer E. A.; Bradesi S.; Chang L.; Spiegel B. M.; Bueller J. A.; Naliboff B. D. Functional GI disorders: from animal models to drug development. Gut 2008, 57, 384–404. 10.1136/gut.2006.101675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labus J. S.; Osadchiy V.; Hsiao E. Y.; Tap J.; Derrien M.; Gupta A.; Tillisch K.; Le Nevé B.; Grinsvall C.; Ljungberg M.; et al. Evidence for an association of gut microbial Clostridia with brain functional connectivity and gastrointestinal sensorimotor function in patients with irritable bowel syndrome, based on tripartite network analysis. Microbiome 2019, 7, 45. 10.1186/s40168-019-0656-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osadchiy V.; Mayer E. A.; Gao K.; Labus J. S.; Naliboff B.; Tillisch K.; Chang L.; Jacobs J. P.; Hsiao E. Y.; Gupta A. Analysis of brain networks and fecal metabolites reveals brain-gut alterations in premenopausal females with irritable bowel syndrome. Transl Psychiatry 2020, 10, 367. 10.1038/s41398-020-01071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnoff R. P.; Bhatt R. R.; Osadchiy V.; Dong T.; Labus J. S.; Kilpatrick L. A.; Chen Z.; Subramanyam V.; Zhang Y.; Ellingson B. M.; et al. A multi-omic brain gut microbiome signature differs between IBS subjects with different bowel habits. Neuropharmacology 2023, 225, 109381. 10.1016/j.neuropharm.2022.109381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman S. M.; Naliboff B. D.; Suyenobu B.; Labus J. S.; Stains J.; Ohning G.; Kilpatrick L.; Bueller J. A.; Ruby K.; Jarcho J.; et al. Reduced brainstem inhibition during anticipated pelvic visceral pain correlates with enhanced brain response to the visceral stimulus in women with irritable bowel syndrome. J. Neurosci. 2008, 28, 349–359. 10.1523/JNEUROSCI.2500-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guleria A.; Karyampudi A.; Singh R.; Khetrapal C. L.; Verma A.; Ghoshal U. C.; Kumar D. Mapping of Brain Activations to Rectal Balloon Distension Stimuli in Male Patients with Irritable Bowel Syndrome Using Functional Magnetic Resonance Imaging. J. Neurogastroenterol. Motil. 2017, 23, 415–427. 10.5056/jnm16148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ticinesi A.; Milani C.; Lauretani F.; Nouvenne A.; Mancabelli L.; Lugli G. A.; Turroni F.; Duranti S.; Mangifesta M.; Viappiani A.; et al. Gut microbiota composition is associated with polypharmacy in elderly hospitalized patients. Sci. Rep. 2017, 7, 11102. 10.1038/s41598-017-10734-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra K.; Kumar B.; Kuhad A. Pathobiological targets of depression. Expert Opin. Ther. Targets 2011, 15, 379–400. 10.1517/14728222.2011.553603. [DOI] [PubMed] [Google Scholar]
- Dinan T. G.; Cryan J. F. Gut-brain axis in 2016: Brain-gut-microbiota axis - mood, metabolism and behaviour. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 69–70. 10.1038/nrgastro.2016.200. [DOI] [PubMed] [Google Scholar]
- Foster J. A.; McVey Neufeld K. A. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013, 36, 305–312. 10.1016/j.tins.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Halkjær S. I.; Christensen A. H.; Lo B. Z. S.; Browne P. D.; Günther S.; Hansen L. H.; Petersen A. M. Faecal microbiota transplantation alters gut microbiota in patients with irritable bowel syndrome: results from a randomised, double-blind placebo-controlled study. Gut 2018, 67, 2107–2115. 10.1136/gutjnl-2018-316434. [DOI] [PubMed] [Google Scholar]
- Holvoet T.; Joossens M.; Wang J.; Boelens J.; Verhasselt B.; Laukens D.; van Vlierberghe H.; Hindryckx P.; De Vos M.; De Looze D.; et al. Assessment of faecal microbial transfer in irritable bowel syndrome with severe bloating. Gut 2017, 66, 980–982. 10.1136/gutjnl-2016-312513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno S.; Masaoka T.; Naganuma M.; Kishimoto T.; Kitazawa M.; Kurokawa S.; Nakashima M.; Takeshita K.; Suda W.; Mimura M.; et al. Bifidobacterium-Rich Fecal Donor May Be a Positive Predictor for Successful Fecal Microbiota Transplantation in Patients with Irritable Bowel Syndrome. Digestion 2017, 96, 29–38. 10.1159/000471919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoller V.; Laguna A. L.; Prazeres Da Costa O.; Buch T.; Göke B.; Storr M. Fecal microbiota transfer (FMT) in a patient with refractory irritable bowel syndrome. Dtsch. Med. Wochenschr. 2015, 140, 1232–1236. 10.1055/s-0041-103798. [DOI] [PubMed] [Google Scholar]
- Drago L.; Meroni G.; Pistone D.; Pasquale L.; Milazzo G.; Monica F.; Aragona S.; Ficano L.; Vassallo R. Evaluation of main functional dyspepsia symptoms after probiotic administration in patients receiving conventional pharmacological therapies. J. Int. Med. Res. 2021, 49, 300060520982657. 10.1177/0300060520982657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter M. D. E.; Talley N. J. Editorial: new insights into the global prevalence of uninvestigated and functional dyspepsia. Aliment. Pharmacol. Ther. 2020, 52 (8), 1407–1408. 10.1111/apt.16059. [DOI] [PubMed] [Google Scholar]
- Zhong L.; Shanahan E. R.; Raj A.; Koloski N. A.; Fletcher L.; Morrison M.; Walker M. M.; Talley N. J.; Holtmann G. Dyspepsia and the microbiome: time to focus on the small intestine. Gut 2017, 66, 1168–1169. 10.1136/gutjnl-2016-312574. [DOI] [PubMed] [Google Scholar]
- Vanheel H.; Vicario M.; Vanuytsel T.; Van Oudenhove L.; Martinez C.; Keita Å. V.; Pardon N.; Santos J.; Söderholm J. D.; Tack J.; et al. Impaired duodenal mucosal integrity and low-grade inflammation in functional dyspepsia. Gut 2014, 63, 262–271. 10.1136/gutjnl-2012-303857. [DOI] [PubMed] [Google Scholar]
- Liu X. J.; Xie W. R.; Wu L. H.; Ye Z. N.; Zhang X. Y.; Zhang R.; He X. X. Changes in oral flora of patients with functional dyspepsia. Sci. Rep. 2021, 11, 8089. 10.1038/s41598-021-87600-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aro P.; Talley N. J.; Johansson S. E.; Agréus L.; Ronkainen J. Anxiety Is Linked to New-Onset Dyspepsia in the Swedish Population: A 10-Year Follow-up Study. Gastroenterology 2015, 148, 928–937. 10.1053/j.gastro.2015.01.039. [DOI] [PubMed] [Google Scholar]
- Van Oudenhove L.; Aziz Q. The role of psychosocial factors and psychiatric disorders in functional dyspepsia. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 158–167. 10.1038/nrgastro.2013.10. [DOI] [PubMed] [Google Scholar]
- Hannibal K. E.; Bishop M. D. Chronic stress, cortisol dysfunction, and pain: a psychoneuroendocrine rationale for stress management in pain rehabilitation. Phys. Ther. 2014, 94, 1816–1825. 10.2522/ptj.20130597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koloski N. A.; Jones M.; Kalantar J.; Weltman M.; Zaguirre J.; Talley N. J. The brain--gut pathway in functional gastrointestinal disorders is bidirectional: a 12-year prospective population-based study. Gut 2012, 61, 1284–1290. 10.1136/gutjnl-2011-300474. [DOI] [PubMed] [Google Scholar]
- Cryan J. F.; Dinan T. G. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- Browning K. N.; Travagli R. A. Central nervous system control of gastrointestinal motility and secretion and modulation of gastrointestinal functions. Compr Physiol 2014, 4, 1339–1368. 10.1002/cphy.c130055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberio B.; Judge C.; Savarino E. V.; Ford A. C. Global prevalence of functional constipation according to the Rome criteria: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2021, 6, 638–648. 10.1016/S2468-1253(21)00111-4. [DOI] [PubMed] [Google Scholar]
- Vriesman M. H.; Koppen I. J. N.; Camilleri M.; Di Lorenzo C.; Benninga M. A. Management of functional constipation in children and adults. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 21–39. 10.1038/s41575-019-0222-y. [DOI] [PubMed] [Google Scholar]
- Zhuang M.; Shang W.; Ma Q.; Strappe P.; Zhou Z. Abundance of Probiotics and Butyrate-Production Microbiome Manages Constipation via Short-Chain Fatty Acids Production and Hormones Secretion. Mol. Nutr. Food Res. 2019, 63, e1801187. 10.1002/mnfr.201801187. [DOI] [PubMed] [Google Scholar]
- Attaluri A.; Jackson M.; Valestin J.; Rao S. S. Methanogenic flora is associated with altered colonic transit but not stool characteristics in constipation without IBS. Am. J. Gastroenterol. 2010, 105, 1407–1411. 10.1038/ajg.2009.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.; Wu T.; Lu W.; Yuan W.; Pan M.; Lee Y. K.; Zhao J.; Zhang H.; Chen W.; Zhu J. Predicting the Role of the Human Gut Microbiome in Constipation Using Machine-Learning Methods: A Meta-Analysis. Microorganisms 2021, 9, 2149. 10.3390/microorganisms9102149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H.; Ye C.; Yang B.; Cui J.; Zheng Z.; Wu C.; Zhou S.; Lv X.; Qin N.; Qin H.; et al. Gut Metagenome as a Potential Diagnostic and Predictive Biomarker in Slow Transit Constipation. Front. Med. 2022, 8, 777961. 10.3389/fmed.2021.777961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor R. B. Optimal sampling of the intestinal microbiota for research. Nature Reviews Gastroenterology & Hepatology 2015, 12, 253–254. 10.1038/nrgastro.2015.46. [DOI] [PubMed] [Google Scholar]
- Swidsinski A.; Loening-Baucke V.; Verstraelen H.; Osowska S.; Doerffel Y. Biostructure of fecal microbiota in healthy subjects and patients with chronic idiopathic diarrhea. Gastroenterology 2008, 135, 568–579. 10.1053/j.gastro.2008.04.017. [DOI] [PubMed] [Google Scholar]
- Durbán A.; Abellán J. J.; Jiménez-Hernández N.; Salgado P.; Ponce M.; Ponce J.; Garrigues V.; Latorre A.; Moya A. Structural alterations of faecal and mucosa-associated bacterial communities in irritable bowel syndrome. Environ. Microbiol. Rep. 2012, 4, 242–247. 10.1111/j.1758-2229.2012.00327.x. [DOI] [PubMed] [Google Scholar]
- Parthasarathy G.; Chen J.; Chen X.; Chia N.; O’Connor H. M.; Wolf P. G.; Gaskins H. R.; Bharucha A. E. Relationship Between Microbiota of the Colonic Mucosa vs Feces and Symptoms, Colonic Transit, and Methane Production in Female Patients With Chronic Constipation. Gastroenterology 2016, 150, 367–379.e1. 10.1053/j.gastro.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procházková N.; Falony G.; Dragsted L. O.; Licht T. R.; Raes J.; Roager H. M. Advancing human gut microbiota research by considering gut transit time. Gut 2023, 72, 180–191. 10.1136/gutjnl-2022-328166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseinzadeh S. T.; Poorsaadati S.; Radkani B.; Forootan M. Psychological disorders in patients with chronic constipation. Gastroenterol. Hepatol. Bed Bench 2011, 4, 159–163. [PMC free article] [PubMed] [Google Scholar]
- Duan S.; Liu L.; Li G.; Wang J.; Hu Y.; Zhang W.; Tan Z.; Jia Z.; Zhang L.; von Deneen K. M. Altered Functional Connectivity Within and Between Salience and Sensorimotor Networks in Patients With Functional Constipation. Front. Neurosci. 2021, 15, 628880. 10.3389/fnins.2021.628880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C.; Liu L.; Liu L.; Zhang J.; Hu Y.; Zhang W.; Ding Y.; Wang Y.; Zhang Z.; von Deneen K. M.; et al. Cortical morphometry alterations in brain regions involved in emotional, motor-control and self-referential processing in patients with functional constipation. Brain Imaging Behav. 2020, 14, 1899–1907. 10.1007/s11682-019-00133-4. [DOI] [PubMed] [Google Scholar]
- Jin Q.; Duan S.; Li G.; Sun L.; Hu Y.; Hu C.; Zhao J.; von Deneen K. M.; Qian L.; Wang H.; et al. Sex-related differences in resting-state brain activity and connectivity in the orbital frontal cortex and insula in patients with functional constipation. Neurogastroenterol. Motil. 2019, 31, e13566. 10.1111/nmo.13566. [DOI] [PubMed] [Google Scholar]
- Li G.; Zhang W.; Hu Y.; Wang J.; Li J.; Jia Z.; Zhang L.; Sun L.; von Deneen K. M.; Duan S.; et al. Distinct Basal Brain Functional Activity and Connectivity in the Emotional-Arousal Network and Thalamus in Patients With Functional Constipation Associated With Anxiety and/or Depressive Disorders. Psychosom. Med. 2021, 83, 707–714. 10.1097/PSY.0000000000000958. [DOI] [PubMed] [Google Scholar]
- Liu L.; Hu C.; Hu Y.; Zhang W.; Zhang Z.; Ding Y.; Wang Y.; von Deneen K. M.; Sun L.; Wang H.; et al. Abnormalities in the thalamo-cortical network in patients with functional constipation. Brain Imaging Behav. 2021, 15, 630–642. 10.1007/s11682-020-00273-y. [DOI] [PubMed] [Google Scholar]
- Peihong M.; Tao Y.; Zhaoxuan H.; Sha Y.; Li C.; Kunnan X.; Jingwen C.; Likai H.; Yuke T.; Yuyi G. Alterations of White Matter Network Properties in Patients With Functional Constipation. Front. Neurol. 2021, 12, 627130. 10.3389/fneur.2021.627130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q.; Cai W.; Zheng J.; Li G.; Meng Q.; Liu Q.; Zhao J.; von Deneen K. M.; Wang Y.; Cui G.; et al. Distinct resting-state brain activity in patients with functional constipation. Neurosci. Lett. 2016, 632, 141–146. 10.1016/j.neulet.2016.08.042. [DOI] [PubMed] [Google Scholar]
- Mayer E. A. Gut feelings: the emerging biology of gut–brain communication. Nat. Rev. Neurosci. 2011, 12, 453–466. 10.1038/nrn3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio A.; Van Oudenhove L.; Pellissier S.; Ly H. G.; Dupont P.; Lafaye de Micheaux H.; Tack J.; Dantzer C.; Delon-Martin C.; Bonaz B. Uncertainty in anticipation of uncomfortable rectal distension is modulated by the autonomic nervous system--a fMRI study in healthy volunteers. NeuroImage 2015, 107, 10–22. 10.1016/j.neuroimage.2014.11.043. [DOI] [PubMed] [Google Scholar]
- Molina-Torres G.; Rodriguez-Arrastia M.; Roman P.; Sanchez-Labraca N.; Cardona D. Stress and the gut microbiota-brain axis. Behav. Pharmacol. 2019, 30, 187–200. 10.1097/FBP.0000000000000478. [DOI] [PubMed] [Google Scholar]
- Vanuytsel T.; van Wanrooy S.; Vanheel H.; Vanormelingen C.; Verschueren S.; Houben E.; Salim Rasoel S.; Tóth J.; Holvoet L.; Farré R.; et al. Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut 2014, 63, 1293–1299. 10.1136/gutjnl-2013-305690. [DOI] [PubMed] [Google Scholar]
- Yarandi S. S.; Peterson D. A.; Treisman G. J.; Moran T. H.; Pasricha P. J. Modulatory Effects of Gut Microbiota on the Central Nervous System: How Gut Could Play a Role in Neuropsychiatric Health and Diseases. J. Neurogastroenterol. Motil. 2016, 22, 201–212. 10.5056/jnm15146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J. R.; Kennedy P. J.; Cryan J. F.; Dinan T. G.; Clarke G.; Hyland N. P. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front. Cell. Neurosci. 2015, 9, 392. 10.3389/fncel.2015.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock G. W.; Savage D. C. Influences of dietary and environmental stress on microbial populations in the murine gastrointestinal tract. Infect. Immun. 1974, 9, 591–598. 10.1128/iai.9.3.591-598.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H.; Zhao X.; Tang S.; Huang H.; Zhao X.; Ning Z.; Fu X.; Zhang C. Probiotics reduce psychological stress in patients before laryngeal cancer surgery. Asia Pac. J. Clin. Oncol. 2016, 12, e92–96. 10.1111/ajco.12120. [DOI] [PubMed] [Google Scholar]
- Karl J. P.; Margolis L. M.; Madslien E. H.; Murphy N. E.; Castellani J. W.; Gundersen Y.; Hoke A. V.; Levangie M. W.; Kumar R.; Chakraborty N.; et al. Changes in intestinal microbiota composition and metabolism coincide with increased intestinal permeability in young adults under prolonged physiological stress. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G559–g571. 10.1152/ajpgi.00066.2017. [DOI] [PubMed] [Google Scholar]
- Kato-Kataoka A.; Nishida K.; Takada M.; Kawai M.; Kikuchi-Hayakawa H.; Suda K.; Ishikawa H.; Gondo Y.; Shimizu K.; Matsuki T.; et al. Fermented Milk Containing Lactobacillus casei Strain Shirota Preserves the Diversity of the Gut Microbiota and Relieves Abdominal Dysfunction in Healthy Medical Students Exposed to Academic Stress. Appl. Environ. Microbiol. 2016, 82, 3649–3658. 10.1128/AEM.04134-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada M.; Nishida K.; Kataoka-Kato A.; Gondo Y.; Ishikawa H.; Suda K.; Kawai M.; Hoshi R.; Watanabe O.; Igarashi T.; et al. Probiotic Lactobacillus casei strain Shirota relieves stress-associated symptoms by modulating the gut-brain interaction in human and animal models. Neurogastroenterol. Motil. 2016, 28, 1027–1036. 10.1111/nmo.12804. [DOI] [PubMed] [Google Scholar]
- Zijlmans M. A.; Korpela K.; Riksen-Walraven J. M.; de Vos W. M.; de Weerth C. Maternal prenatal stress is associated with the infant intestinal microbiota. Psychoneuroendocrinology 2015, 53, 233–245. 10.1016/j.psyneuen.2015.01.006. [DOI] [PubMed] [Google Scholar]
- Naseribafrouei A.; Hestad K.; Avershina E.; Sekelja M.; Linlo̷kken A.; Wilson R.; Rudi K. Correlation between the human fecal microbiota and depression. Neurogastroenterol. Motil. 2014, 26, 1155–1162. 10.1111/nmo.12378. [DOI] [PubMed] [Google Scholar]
- Jiang H.; Ling Z.; Zhang Y.; Mao H.; Ma Z.; Yin Y.; Wang W.; Tang W.; Tan Z.; Shi J.; et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain. Behav. Immun. 2015, 48, 186–194. 10.1016/j.bbi.2015.03.016. [DOI] [PubMed] [Google Scholar]
- Tillisch K.; Mayer E. A.; Gupta A.; Gill Z.; Brazeilles R.; Le Nevé B.; van Hylckama Vlieg J. E. T.; Guyonnet D.; Derrien M.; Labus J. S. Brain Structure and Response to Emotional Stimuli as Related to Gut Microbial Profiles in Healthy Women. Psychosom. Med. 2017, 79, 905–913. 10.1097/PSY.0000000000000493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfau M. L.; Russo S. J. Peripheral and Central Mechanisms of Stress Resilience. Neurobiol Stress 2015, 1, 66–79. 10.1016/j.ynstr.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin T. B.; Saab B. J.; Mansuy I. M. Neural mechanisms of stress resilience and vulnerability. Neuron 2012, 75, 747–761. 10.1016/j.neuron.2012.08.016. [DOI] [PubMed] [Google Scholar]
- Bear T.; Dalziel J.; Coad J.; Roy N.; Butts C.; Gopal P. The Microbiome-Gut-Brain Axis and Resilience to Developing Anxiety or Depression under Stress. Microorganisms 2021, 9, 723. 10.3390/microorganisms9040723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L. F.; Zou H. W.; Song B. L.; Wang Y.; Jiang Y.; Li Z. L.; Niu Q. H.; Liu Y. J. Increased Lactobacillus Abundance Contributes to Stress Resilience in Mice Exposed to Chronic Social Defeat Stress. Neuroendocrinology 2023, 113, 563. 10.1159/000528876. [DOI] [PubMed] [Google Scholar]
- Dinan T. G.; Stanton C.; Cryan J. F. Psychobiotics: a novel class of psychotropic. Biol. Psychiatry 2013, 74, 720–726. 10.1016/j.biopsych.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Chattu V. K.; Manzar M. D.; Kumary S.; Burman D.; Spence D. W.; Pandi-Perumal S. R. The Global Problem of Insufficient Sleep and Its Serious Public Health Implications. Healthcare 2019, 7, 1. 10.3390/healthcare7010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin C.; McCartney D.; Desbrow B.; Khalesi S. Effects of probiotics and paraprobiotics on subjective and objective sleep metrics: a systematic review and meta-analysis. Eur. J. Clin. Nutr. 2020, 74, 1536–1549. 10.1038/s41430-020-0656-x. [DOI] [PubMed] [Google Scholar]
- Smith R. P.; Easson C.; Lyle S. M.; Kapoor R.; Donnelly C. P.; Davidson E. J.; Parikh E.; Lopez J. V.; Tartar J. L. Gut microbiome diversity is associated with sleep physiology in humans. PLoS One 2019, 14, e0222394 10.1371/journal.pone.0222394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict C.; Vogel H.; Jonas W.; Woting A.; Blaut M.; Schürmann A.; Cedernaes J. Gut microbiota and glucometabolic alterations in response to recurrent partial sleep deprivation in normal-weight young individuals. Molecular Metabolism 2016, 5, 1175–1186. 10.1016/j.molmet.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S. L.; Bai L.; Goel N.; Bailey A.; Jang C. J.; Bushman F. D.; Meerlo P.; Dinges D. F.; Sehgal A. Human and rat gut microbiome composition is maintained following sleep restriction. Proc. Natl. Acad. Sci. U. S. A. 2017, 114, E1564–E1571. 10.1073/pnas.1620673114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. R.; Carroll I.; Azcarate-Peril M. A.; Rochette A. D.; Heinberg L. J.; Peat C.; Steffen K.; Manderino L. M.; Mitchell J.; Gunstad J. A preliminary examination of gut microbiota, sleep, and cognitive flexibility in healthy older adults. Sleep Med. 2017, 38, 104–107. 10.1016/j.sleep.2017.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger J. M.; Opp M. R. Sleep and Microbes. Int. Rev. Neurobiol. 2016, 131, 207–225. 10.1016/bs.irn.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey D. J.; Fleshner M.; Wright K. P. The effects of 40 h of total sleep deprivation on inflammatory markers in healthy young adults. Brain, Behavior, and Immunity 2007, 21, 1050–1057. 10.1016/j.bbi.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Kamada N.; Seo S. U.; Chen G. Y.; Núñez G. Role of the gut microbiota in immunity and inflammatory disease. Nat. Rev. Immunol. 2013, 13, 321–335. 10.1038/nri3430. [DOI] [PubMed] [Google Scholar]
- Yasuda M.; Kato S.; Yamanaka N.; Iimori M.; Matsumoto K.; Utsumi D.; Kitahara Y.; Amagase K.; Horie S.; Takeuchi K. 5-HT3 receptor antagonists ameliorate 5-fluorouracil-induced intestinal mucositis by suppression of apoptosis in murine intestinal crypt cells. Br. J. Pharmacol. 2013, 168, 1388–1400. 10.1111/bph.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H.-L.; Lu L.; Wang X.-S.; Qin L.-Y.; Wang P.; Qiu S.-P.; Wu H.; Huang F.; Zhang B.-B.; Shi H.-L. Alteration of Gut Microbiota and Inflammatory Cytokine/Chemokine Profiles in 5-Fluorouracil Induced Intestinal Mucositis. Frontiers in Cellular and Infection Microbiology 2017, 7, 455. 10.3389/fcimb.2017.00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton D.; Williams C.; Brown A. Impact of consuming a milk drink containing a probiotic on mood and cognition. Eur. J. Clin. Nutr. 2007, 61, 355–361. 10.1038/sj.ejcn.1602546. [DOI] [PubMed] [Google Scholar]
- Parkar S. G.; Kalsbeek A.; Cheeseman J. F. Potential Role for the Gut Microbiota in Modulating Host Circadian Rhythms and Metabolic Health. Microorganisms 2019, 7, 41. 10.3390/microorganisms7020041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D.; Yu Y.; Shen Y.; Liu Q.; Zhao Z.; Sharma R.; Reiter R. J. Melatonin Synthesis and Function: Evolutionary History in Animals and Plants. Front. Endocrinol. 2019, 10, 249. 10.3389/fendo.2019.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B.; Duan R.; Duan L. Prevalence of sleep disorder in irritable bowel syndrome: A systematic review with meta-analysis. Saudi J. Gastroenterol. 2018, 24, 141. 10.4103/sjg.SJG_603_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miglis M. G. Autonomic dysfunction in primary sleep disorders. Sleep Med. 2016, 19, 40–49. 10.1016/j.sleep.2015.10.001. [DOI] [PubMed] [Google Scholar]
- Tobaldini E.; Costantino G.; Solbiati M.; Cogliati C.; Kara T.; Nobili L.; Montano N. Sleep, sleep deprivation, autonomic nervous system and cardiovascular diseases. Neurosci Biobehav Rev. 2017, 74, 321–329. 10.1016/j.neubiorev.2016.07.004. [DOI] [PubMed] [Google Scholar]
- Vgontzas A. N.; Chrousos G. P. Sleep, the hypothalamic-pituitary-adrenal axis, and cytokines: multiple interactions and disturbances in sleep disorders. Endocrinol. Metab. Clin. North Am. 2002, 31, 15–36. 10.1016/S0889-8529(01)00005-6. [DOI] [PubMed] [Google Scholar]
- Schlereth T.; Birklein F. The sympathetic nervous system and pain. Neuromolecular Med. 2008, 10, 141–147. 10.1007/s12017-007-8018-6. [DOI] [PubMed] [Google Scholar]
- Wong R. K.; Yang C.; Song G. H.; Wong J.; Ho K. Y. Melatonin regulation as a possible mechanism for probiotic (VSL#3) in irritable bowel syndrome: a randomized double-blinded placebo study. Dig. Dis. Sci. 2015, 60, 186–194. 10.1007/s10620-014-3299-8. [DOI] [PubMed] [Google Scholar]
- Zhao E.; Tait C.; Minacapelli C. D.; Catalano C.; Rustgi V. K. Circadian Rhythms, the Gut Microbiome, and Metabolic Disorders. Gastro Hep Advances 2022, 1, 93–105. 10.1016/j.gastha.2021.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X.; Bushman F. D.; FitzGerald G. A. Rhythmicity of the intestinal microbiota is regulated by gender and the host circadian clock. Proc. Natl. Acad. Sci. U. S. A. 2015, 112, 10479–10484. 10.1073/pnas.1501305112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulose J. K.; Wright J. M.; Patel A. G.; Cassone V. M. Human Gut Bacteria Are Sensitive to Melatonin and Express Endogenous Circadian Rhythmicity. PLoS One 2016, 11, e0146643 10.1371/journal.pone.0146643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherji A.; Kobiita A.; Ye T.; Chambon P. Homeostasis in intestinal epithelium is orchestrated by the circadian clock and microbiota cues transduced by TLRs. Cell 2013, 153, 812–827. 10.1016/j.cell.2013.04.020. [DOI] [PubMed] [Google Scholar]
- Paschos G. K.; FitzGerald G. A. Circadian Clocks and Metabolism: Implications for Microbiome and Aging. Trends Genet. 2017, 33, 760–769. 10.1016/j.tig.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Real J. M.; Serino M.; Blasco G.; Puig J.; Daunis-i-Estadella J.; Ricart W.; Burcelin R.; Fernández-Aranda F.; Portero-Otin M. Gut Microbiota Interacts With Brain Microstructure and Function. J. Clin Endocrinol Metab 2015, 100, 4505–4513. 10.1210/jc.2015-3076. [DOI] [PubMed] [Google Scholar]
- Carlson A. L.; Xia K.; Azcarate-Peril M. A.; Goldman B. D.; Ahn M.; Styner M. A.; Thompson A. L.; Geng X.; Gilmore J. H.; Knickmeyer R. C. Infant Gut Microbiome Associated With Cognitive Development. Biol. Psychiatry 2018, 83, 148–159. 10.1016/j.biopsych.2017.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polidano C.; Zhu A.; Bornstein J. C. The relation between cesarean birth and child cognitive development. Sci. Rep. 2017, 7, 11483. 10.1038/s41598-017-10831-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y.-C.; Jin H.-M.; Cui Y.; Kim D. S.; Jung J. M.; Park J.-I.; Jung E.-S.; Choi E.-K.; Chae S.-W. Fermented milk of Lactobacillus helveticus IDCC3801 improves cognitive functioning during cognitive fatigue tests in healthy older adults. J. Funct. Foods 2014, 10, 465–474. 10.1016/j.jff.2014.07.007. [DOI] [Google Scholar]
- Tillisch K.; Labus J.; Kilpatrick L.; Jiang Z.; Stains J.; Ebrat B.; Guyonnet D.; Legrain-Raspaud S.; Trotin B.; Naliboff B.; et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology 2013, 144, 1394–1401.e4. 10.1053/j.gastro.2013.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. P.; Sutherland D.; Hewlett P. An Investigation of the Acute Effects of Oligofructose-Enriched Inulin on Subjective Wellbeing, Mood and Cognitive Performance. Nutrients 2015, 7, 8887–8896. 10.3390/nu7115441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.-H.; Yoon S.-H.; Jung Y.; Kim N.; Min U.; Chun J.; Choi I. Emotional well-being and gut microbiome profiles by enterotype. Sci. Rep. 2020, 10, 20736. 10.1038/s41598-020-77673-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros D. F.; Antony M. M.; McCabe R. E.; Swinson R. P. Frequency and severity of the symptoms of irritable bowel syndrome across the anxiety disorders and depression. J. Anxiety Disord. 2009, 23, 290–296. 10.1016/j.janxdis.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Mayer E. A.; Labus J. S.; Tillisch K.; Cole S. W.; Baldi P. Towards a systems view of IBS. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 592–605. 10.1038/nrgastro.2015.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.; Ma L.; Chang L.; Pu Y.; Qu Y.; Hashimoto K. A key role of the subdiaphragmatic vagus nerve in the depression-like phenotype and abnormal composition of gut microbiota in mice after lipopolysaccharide administration. Translational Psychiatry 2020, 10, 186. 10.1038/s41398-020-00878-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu Y.; Tan Y.; Qu Y.; Chang L.; Wang S.; Wei Y.; Wang X.; Hashimoto K. A role of the subdiaphragmatic vagus nerve in depression-like phenotypes in mice after fecal microbiota transplantation from Chrna7 knock-out mice with depression-like phenotypes. Brain, Behavior, and Immunity 2021, 94, 318–326. 10.1016/j.bbi.2020.12.032. [DOI] [PubMed] [Google Scholar]
- Bruch J. D. Intestinal infection associated with future onset of an anxiety disorder: Results of a nationally representative study. Brain. Behav. Immun. 2016, 57, 222–226. 10.1016/j.bbi.2016.05.014. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser J. K.; Wilson S. J.; Shrout M. R.; Madison A. A.; Andridge R.; Peng J.; Malarkey W. B.; Bailey M. T. The gut reaction to couples’ relationship troubles: A route to gut dysbiosis through changes in depressive symptoms. Psychoneuroendocrinology 2021, 125, 105132. 10.1016/j.psyneuen.2021.105132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. N.; Yun Y.; Ryu S.; Chang Y.; Kwon M. J.; Cho J.; Shin H.; Kim H. L. Correlation between gut microbiota and personality in adults: A cross-sectional study. Brain. Behav. Immun. 2018, 69, 374–385. 10.1016/j.bbi.2017.12.012. [DOI] [PubMed] [Google Scholar]
- Valles-Colomer M.; Falony G.; Darzi Y.; Tigchelaar E. F.; Wang J.; Tito R. Y.; Schiweck C.; Kurilshikov A.; Joossens M.; Wijmenga C.; et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nature Microbiology 2019, 4, 623–632. 10.1038/s41564-018-0337-x. [DOI] [PubMed] [Google Scholar]
- Arumugam M.; Raes J.; Pelletier E.; Le Paslier D.; Yamada T.; Mende D. R.; Fernandes G. R.; Tap J.; Bruls T.; Batto J.-M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello E. K.; Lauber C. L.; Hamady M.; Fierer N.; Gordon J. I.; Knight R. Bacterial community variation in human body habitats across space and time. Science 2009, 326, 1694–1697. 10.1126/science.1177486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G. D.; Chen J.; Hoffmann C.; Bittinger K.; Chen Y. Y.; Keilbaugh S. A.; Bewtra M.; Knights D.; Walters W. A.; Knight R.; et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108. 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkasheh G.; Kashani-Poor Z.; Tajabadi-Ebrahimi M.; Jafari P.; Akbari H.; Taghizadeh M.; Memarzadeh M. R.; Asemi Z.; Esmaillzadeh A. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: A randomized, double-blind, placebo-controlled trial. Nutrition 2016, 32, 315–320. 10.1016/j.nut.2015.09.003. [DOI] [PubMed] [Google Scholar]
- Wallace C. J. K.; Milev R. The effects of probiotics on depressive symptoms in humans: a systematic review. Ann. Gen. Psychiatry 2017, 16, 14. 10.1186/s12991-017-0138-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Morvan de Sequeira C.; Hengstberger C.; Enck P.; Mack I. Effect of Probiotics on Psychiatric Symptoms and Central Nervous System Functions in Human Health and Disease: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 621. 10.3390/nu14030621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C.; Guarner F.; Reid G.; Gibson G. R.; Merenstein D. J.; Pot B.; Morelli L.; Canani R. B.; Flint H. J.; Salminen S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nature Reviews Gastroenterology & Hepatology 2014, 11, 506–514. 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- Dahiya D.; Nigam P. S. Clinical Potential of Microbial Strains, Used in Fermentation for Probiotic Food, Beverages and in Synbiotic Supplements, as Psychobiotics for Cognitive Treatment through Gut-Brain Signaling. Microorganisms 2022, 10, 1687. 10.3390/microorganisms10091687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawistowska-Rojek A.; Tyski S. How to Improve Health with Biological Agents-Narrative Review. Nutrients 2022, 14, 1700. 10.3390/nu14091700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson G. R.; Hutkins R.; Sanders M. E.; Prescott S. L.; Reimer R. A.; Salminen S. J.; Scott K.; Stanton C.; Swanson K. S.; Cani P. D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nature Reviews Gastroenterology & Hepatology 2017, 14, 491–502. 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- Swanson K. S.; Gibson G. R.; Hutkins R.; Reimer R. A.; Reid G.; Verbeke K.; Scott K. P.; Holscher H. D.; Azad M. B.; Delzenne N. M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nature Reviews Gastroenterology & Hepatology 2020, 17, 687–701. 10.1038/s41575-020-0344-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen S.; Collado M. C.; Endo A.; Hill C.; Lebeer S.; Quigley E. M. M.; Sanders M. E.; Shamir R.; Swann J. R.; Szajewska H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nature Reviews Gastroenterology & Hepatology 2021, 18, 649–667. 10.1038/s41575-021-00440-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar A.; Lehto S. M.; Harty S.; Dinan T. G.; Cryan J. F.; Burnet P. W. J. Psychobiotics and the Manipulation of Bacteria-Gut-Brain Signals. Trends Neurosci. 2016, 39, 763–781. 10.1016/j.tins.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long-Smith C.; O’Riordan K. J.; Clarke G.; Stanton C.; Dinan T. G.; Cryan J. F. Microbiota-Gut-Brain Axis: New Therapeutic Opportunities. Annu. Rev. Pharmacol Toxicol 2020, 60, 477–502. 10.1146/annurev-pharmtox-010919-023628. [DOI] [PubMed] [Google Scholar]
- Pferschy-Wenzig E. M.; Pausan M. R.; Ardjomand-Woelkart K.; Röck S.; Ammar R. M.; Kelber O.; Moissl-Eichinger C.; Bauer R. Medicinal Plants and Their Impact on the Gut Microbiome in Mental Health: A Systematic Review. Nutrients 2022, 14, 2111. 10.3390/nu14102111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PitchBook. https://www.pitchbook.com/ (accessed March 30, 2023).
- Black C. J.; Ford A. C. Global burden of irritable bowel syndrome: trends, predictions and risk factors. Nature Reviews Gastroenterology & Hepatology 2020, 17, 473–486. 10.1038/s41575-020-0286-8. [DOI] [PubMed] [Google Scholar]
- Fecal Microbiota Transplantation. https://www.idsociety.org/public-health/emerging-clinical-issues/emerging-clinical-issues/fecal-microbiota-transplantation/ (accessed November 28, 2022).
- BiomeBank announces world first regulatory approval for donor derived microbiome drug. https://www.biomebank.com/news/media-release/biomebank-announces-world-first-regulatory-approval-for-donor-derived-microbiome-drug/ (accessed November 22, 2022).
- Ferring Receives U.S. FDA Approval for REBYOTA (fecal microbiota, live-jslm) – A Novel First-in-Class Microbiota-Based Live Biotherapeutic. https://ferringusa.com/?press=ferring-receives-u-s-fda-approval-for-rebyota-fecal-microbiota-live-jslm-a-novel-first-in-class-microbiota-based-live-biotherapeutic (accessed December 6, 2022).
- Clinical Trials. https://www.clinicaltrials.gov/ (accessed January 27, 2023).
- Fijan S. Microorganisms with claimed probiotic properties: an overview of recent literature. Int. J. Environ. Res. Public Health 2014, 11, 4745–4767. 10.3390/ijerph110504745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effect of Two Probiotic Formulations on Mental Health and Mood Biomarkers in Adults With Depressive Symptoms. https://clinicaltrials.gov/ct2/show/NCT05564767?term=NCT05564767&draw=2&rank=1 (accessed November 27, 2022).
- Effects of Probiotics on Gut Microbiota, Endocannabinoid and Immune Activation and Symptoms of Fatigue in Dancers. https://clinicaltrials.gov/ct2/show/NCT05567653?term=NCT05567653&draw=2&rank=1 (accessed November 27, 2022).
- Probiotics on Sleep Among Adults Study. https://clinicaltrials.gov/ct2/show/NCT04767997?term=NCT04767997&draw=2&rank=1 (accessed November 27, 2022).
- Rode J.; Edebol Carlman H. M. T.; König J.; Repsilber D.; Hutchinson A. N.; Thunberg P.; Andersson P.; Persson J.; Kiselev A.; Lathrop Stern L.; et al. Probiotic Mixture Containing Lactobacillus helveticus, Bifidobacterium longum and Lactiplantibacillus plantarum Affects Brain Responses Toward an Emotional Task in Healthy Subjects: A Randomized Clinical Trial. Front Nutr 2022, 9, 827182. 10.3389/fnut.2022.827182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson E.; Griffin S. M.; Ibarra A.; Ellsiepen E.; Hellhammer J. Lacticaseibacillus paracasei Lpc-37® improves psychological and physiological markers of stress and anxiety in healthy adults: a randomized, double-blind, placebo-controlled and parallel clinical trial (the Sisu study). Neurobiol Stress 2020, 13, 100277. 10.1016/j.ynstr.2020.100277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stress & Anxiety Dampening Effects of a Probiotic Supplement Compared to Placebo in Healthy Subjects. https://www.clinicaltrials.gov/ct2/show/NCT03494725?term=NCT03494725&draw=2&rank=1 (accessed November 28, 2022).
- The Cognitive Effects of 6 Weeks Administration With a Probiotic. https://www.clinicaltrials.gov/ct2/show/NCT03601559?term=NCT03601559&draw=2&rank=1 (accessed November 28, 2022).
- Probiotic Effects on the Microbe-brain-gut Interaction and Brain Activity During Stress Tasks in Healthy Subjects. https://www.clinicaltrials.gov/ct2/show/NCT03615651?term=NCT03615651&draw=2&rank=1 (accessed November 28, 2022).
- Lactobacillus Plantarum DR7 for Gut-Brain-Axis Benefits (DR7). https://www.clinicaltrials.gov/ct2/show/NCT03370458?term=NCT03370458&draw=2&rank=1 (accessed November 28, 2022).
- Sur D.; Manna B.; Niyogi S. K.; Ramamurthy T.; Palit A.; Nomoto K.; Takahashi T.; Shima T.; Tsuji H.; Kurakawa T.; et al. Role of probiotic in preventing acute diarrhoea in children: a community-based, randomized, double-blind placebo-controlled field trial in an urban slum. Epidemiology and Infection 2011, 139, 919–926. 10.1017/S0950268810001780. [DOI] [PubMed] [Google Scholar]
- Cottrell J.; Koenig K.; Perfekt R.; Hofmann R.; For the Loperamide–Simethicone Acute Diarrhoea Study T. Comparison of Two Forms of Loperamide–Simeticone and a Probiotic Yeast (Saccharomyces boulardii) in the Treatment of Acute Diarrhoea in Adults: A Randomised Non-Inferiority Clinical Trial. Drugs R&D 2015, 15, 363–373. 10.1007/s40268-015-0111-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrzydło-Radomańska B.; Prozorow-Król B.; Cichoż-Lach H.; Majsiak E.; Bierła J. B.; Kanarek E.; Sowińska A.; Cukrowska B. The Effectiveness and Safety of Multi-Strain Probiotic Preparation in Patients with Diarrhea-Predominant Irritable Bowel Syndrome: A Randomized Controlled Study. Nutrients 2021, 13, 756. 10.3390/nu13030756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley E. M. M.; Markinson L.; Stevenson A.; Treasure F. P.; Lacy B. E. Randomised clinical trial: efficacy and safety of the live biotherapeutic product MRx1234 in patients with irritable bowel syndrome. Aliment. Pharmacol. Ther. 2023, 57, 81–93. 10.1111/apt.17310. [DOI] [PubMed] [Google Scholar]
- Effect of Lactobacillus Gasseri DSM 27123 on Functional Constipation in Healthy Women. https://www.clinicaltrials.gov/ct2/show/NCT02592200?term=NCT02592200&draw=2&rank=1 (accessed November 28, 2022).
- Dietary Supplementation Effects on Bowel Movement Frequency and Intestinal Biological Markers in Seniors Presenting Slowed Intestinal Transit. https://www.clinicaltrials.gov/ct2/show/NCT04304170?term=NCT04304170&draw=2&rank=1 (accessed November 28, 2022).
- Multi Strain Probiotic Preparation in Patients With Irritable Bowel Syndrome. https://www.clinicaltrials.gov/ct2/show/NCT04662957?term=NCT04662957&draw=2&rank=1 (accessed November 28, 2022).
- The Effect of Probiotic Supported Yogurt Consumption on Gastrointestinal Symptoms. https://www.clinicaltrials.gov/ct2/show/NCT05566171?term=NCT05566171&draw=2&rank=1 (accessed November 28, 2022).
- B. Lactis HN019 for Functional Constipation (CTT). https://www.clinicaltrials.gov/ct2/show/NCT01463293?term=NCT01463293&draw=2&rank=1 (accessed November 28, 2022).
- Effects of Cultura Yoghurt in Relation to Transit Time and Digestive Discomfort in Healthy Women and Men. https://www.clinicaltrials.gov/ct2/show/NCT01102036?term=NCT01102036&draw=2&rank=1 (accessed November 28, 2022).
- A Trial for New Treatment of Adult Participants With Irritable Bowel Syndrome. https://clinicaltrials.gov/ct2/show/NCT03721107?term=NCT03721107&draw=2&rank=1 (accessed Feb 16, 2023).
- Randomized Controlled Field Trial of a Probiotics to Assess Its Role in Preventig Diarrhoea (Yakult). https://www.clinicaltrials.gov/ct2/show/NCT00534170?term=NCT00534170&draw=2&rank=1 (accessed November 28, 2022).
- Probiotics and Hospital Outcome in the Elderly (PROAGE). https://www.clinicaltrials.gov/ct2/show/NCT00794924?term=NCT00794924&draw=2&rank=1 (accessed November 28, 2022).
- Evaluation of the Efficacy of Two Probiotic Strains for Irritable Bowel Syndrome (14PIHL). https://www.clinicaltrials.gov/ct2/show/NCT02213172?term=NCT02213172&draw=2&rank=1 (accessed November 28, 2022).
- A Comparison of Three Medications to Treat Diarrhea in Adults. https://www.clinicaltrials.gov/ct2/show/NCT00807326?term=NCT00807326&draw=2&rank=1 (accessed November 28, 2022).
- A Trial to Evaluate the Effects of Bifidobacterium Longum NCC3001 on Intestinal and Psychological Symptoms in Subjects With Irritable Bowel Syndrome. https://www.clinicaltrials.gov/ct2/show/NCT05054309?term=NCT05054309&draw=2&rank=1 (accessed November 28, 2022).
- Study to Evaluate a Probiotic in Healthy Subjects With a History of Abdominal Discomfort and Bloating. https://www.clinicaltrials.gov/ct2/show/NCT01099696?term=NCT01099696&draw=2&rank=1 (accessed November 28, 2022).
- Efficacy of a Multi-strain Probiotic in the Treatment of Irritable Bowel Syndrome (IBS). https://www.clinicaltrials.gov/ct2/show/NCT01887834?term=NCT01887834&draw=2&rank=1 (accessed November 28, 2022).
- Trial to Evaluate Dietary Supplements to Maintain Gut Health During Travel (P3). https://www.clinicaltrials.gov/ct2/show/NCT04605783?term=NCT04605783&draw=2&rank=1 (accessed November 28, 2022).
- To Study the Efficacy and Safety of L. Plantarum UALp-05TM in Diarrhea- Predominant-irritable Bowel Syndrome. https://www.clinicaltrials.gov/ct2/show/NCT04950296?term=NCT04950296&draw=2&rank=1 (accessed November 28, 2022).
- Saccharomyces Cerevisiae for Irritable Bowel Syndrome (IBS). https://www.clinicaltrials.gov/ct2/show/NCT05149599?term=NCT05149599&draw=2&rank=1 (accessed November 28, 2022).
- Johnstone N.; Milesi C.; Burn O.; van den Bogert B.; Nauta A.; Hart K.; Sowden P.; Burnet P. W. J.; Cohen Kadosh K. Anxiolytic effects of a galacto-oligosaccharides prebiotic in healthy females (18–25 years) with corresponding changes in gut bacterial composition. Sci. Rep. 2021, 11, 8302. 10.1038/s41598-021-87865-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parilli-Moser I.; Domínguez-López I.; Trius-Soler M.; Castellví M.; Bosch B.; Castro-Barquero S.; Estruch R.; Hurtado-Barroso S.; Lamuela-Raventós R. M. Consumption of peanut products improves memory and stress response in healthy adults from the ARISTOTLE study: A 6-month randomized controlled trial. Clin. Nutr. 2021, 40, 5556–5567. 10.1016/j.clnu.2021.09.020. [DOI] [PubMed] [Google Scholar]
- Prebiotics and Stress Reduction in Women. https://www.clinicaltrials.gov/ct2/show/NCT05372601?term=NCT05372601&draw=2&rank=1 (accessed November 28, 2022).
- Evaluating the Effects of Prebiotics on Sleep, the Gut Microbiome, Cognition, Immune Function and Stress. https://www.clinicaltrials.gov/ct2/show/NCT05239845?term=NCT05239845&draw=2&rank=1 (accessed November 28, 2022).
- Healthy Prebiotic and Postbiotic Effects of Peanuts and Peanut Butter: College Intervention Trial (ARISTOTLE). https://www.clinicaltrials.gov/ct2/show/NCT04324749?term=NCT04324749&draw=2&rank=1 (accessed November 28, 2022).
- Polyphenols, Prebiotics, the Gut Microbiome and Stress. https://www.clinicaltrials.gov/ct2/show/NCT05528575?term=NCT05528575&draw=2&rank=1 (accessed November 28, 2022).
- Prebiotics and Mental Health: Behavioural. https://www.clinicaltrials.gov/ct2/show/NCT04616937?term=NCT04616937&draw=2&rank=1 (accessed November 28, 2022).
- Selling J.; Swann P.; Madsen L. R. 2nd; Oswald J. Improvement in Gastroesophageal Reflux Symptoms From a Food-grade Maltosyl-isomaltooligosaccharide Soluble Fiber Supplement: A Case Series. Integr. Med. 2018, 17, 40–42. [PMC free article] [PubMed] [Google Scholar]
- Ansell J.; Butts C. A.; Paturi G.; Eady S. L.; Wallace A. J.; Hedderley D.; Gearry R. B. Kiwifruit-derived supplements increase stool frequency in healthy adults: a randomized, double-blind, placebo-controlled study. Nutr. Res. (N.Y.) 2015, 35, 401–408. 10.1016/j.nutres.2015.04.005. [DOI] [PubMed] [Google Scholar]
- Blatchford P.; Stoklosinski H.; Eady S.; Wallace A.; Butts C.; Gearry R.; Gibson G.; Ansell J. Consumption of kiwifruit capsules increases Faecalibacterium prausnitzii abundance in functionally constipated individuals: a randomised controlled human trial. J. Nutr Sci. 2017, 6, e52. 10.1017/jns.2017.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILK D. B. A.; DAVIS A.; VULEVIC J.; TZORTZIS G.; GIBSON G. R. Clinical trial: the effects of a trans-galactooligosaccharide prebiotic on faecal microbiota and symptoms in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2009, 29, 508–518. 10.1111/j.1365-2036.2008.03911.x. [DOI] [PubMed] [Google Scholar]
- Tolerability Study of a Novel Microbiome Therapeutic in Subjects With Gastroesophageal Reflux Disease. https://www.clinicaltrials.gov/ct2/show/NCT04491734?term=NCT04491734&draw=2&rank=1 (accessed November 28, 2022).
- Utility of the Administration of Chesnut and Quebracho Extract for Irritable Bowel Syndrome Diarrhea Predominant. https://www.clinicaltrials.gov/ct2/show/NCT05207618?term=NCT05207618&draw=2&rank=1 (accessed November 28, 2022).
- Evaluation of the Efficacy of a New Specific Infant Formula in Case of Functional Constipation. https://www.clinicaltrials.gov/ct2/show/NCT05340712?term=NCT05340712&draw=2&rank=1 (accessed November 28, 2022).
- ReFerm. https://referm.dk/ (accessed November 27, 2022).
- Effect of Postbiotic Product on Colonic Barriers in IBS. https://www.clinicaltrials.gov/ct2/show/NCT05475314?term=NCT05475314&draw=2&rank=1 (accessed November 28, 2022).
- Pilot Study to Assess the Effect of a Postbiotic Blend on Moderate Self-reported Anxiety (Anx). https://www.clinicaltrials.gov/ct2/show/NCT05562739?term=NCT05562739&draw=2&rank=1 (accessed November 28, 2022).
- Evaluating the Safety and Efficacy of the Probiotic Bifidobacterium Longum ES1 and the Post Biotic Heat-treated Bifidobacterium Longum ES1 (HT-ES1) on IBS Symptom Severity in Patients With Diarrhoea Predominant Irritable Bowel Syndrome. https://www.clinicaltrials.gov/ct2/show/NCT05339243?term=NCT05339243&draw=2&rank=1 (accessed November 28, 2022).
- König J.; Brummer R. J. Faecal microbiota transplantation in IBS — new evidence for success?. Nature Reviews Gastroenterology & Hepatology 2020, 17, 199–200. 10.1038/s41575-020-0282-z. [DOI] [PubMed] [Google Scholar]
- El-Salhy M.; Winkel R.; Casen C.; Hausken T.; Gilja O. H.; Hatlebakk J. G. Efficacy of Fecal Microbiota Transplantation for Patients With Irritable Bowel Syndrome at 3 Years After Transplantation. Gastroenterology 2022, 163, 982–994.e14. 10.1053/j.gastro.2022.06.020. [DOI] [PubMed] [Google Scholar]
- Effects of Faecal Microbiota Transplantation in Patients With IBS. https://www.clinicaltrials.gov/ct2/show/NCT03822299?term=NCT03822299&draw=2&rank=1 (accessed November 28, 2022).
- Fecal Microbiota Transplantation in Patients With Irritable Bowel Syndrome. https://www.clinicaltrials.gov/ct2/show/NCT02092402?term=NCT02092402&draw=2&rank=1 (accessed November 28, 2022).
- RCE With FMT in the Treatment of Childhood Constipation. https://www.clinicaltrials.gov/ct2/show/NCT05035784?term=NCT05035784&draw=2&rank=1 (accessed November 28, 2022).
- FMT Capsules in Treatment of Patients With Insomnia Clinical Research. https://www.clinicaltrials.gov/ct2/show/NCT05427331?term=NCT05427331&draw=2&rank=1 (accessed November 28, 2022).
- Haridy R.Australia gives world-first regulatory approval to fecal transplant therapy. https://newatlas.com/medical/first-approval-fecal-transplant-gut-microbiome-therapy/ (accessed November 22, 2022).
- Shepherd T.A coup for poo: why the world’s first faecal transplant approval matters. The Guardian, November 12, 2022. https://www.theguardian.com/australia-news/2022/nov/13/a-coup-for-poo-why-the-worlds-first-faecal-transplant-approval-matters (accessed November 22, 2022).
- FDA Approves First Fecal Microbiota Product. https://www.fda.gov/news-events/press-announcements/fda-approves-first-fecal-microbiota-product (accessed December 2, 2022).
- Shaffer J. P.; Nothias L.-F.; Thompson L. R.; Sanders J. G.; Salido R. A.; Couvillion S. P.; Brejnrod A. D.; Lejzerowicz F.; Haiminen N.; Huang S. Standardized multi-omics of Earth’s microbiomes reveals microbial and metabolite diversity. Nat. Microbiol. 2022, 7, 2128. 10.1038/s41564-022-01266-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmora N.; Zilberman-Schapira G.; Suez J.; Mor U.; Dori-Bachash M.; Bashiardes S.; Kotler E.; Zur M.; Regev-Lehavi D.; Brik R. B.-Z.; et al. Personalized Gut Mucosal Colonization Resistance to Empiric Probiotics Is Associated with Unique Host and Microbiome Features. Cell 2018, 174, 1388–1405.e21. 10.1016/j.cell.2018.08.041. [DOI] [PubMed] [Google Scholar]
- Zhu G.; Zhao J.; Zhang H.; Chen W.; Wang G. Probiotics for mild cognitive impairment and Alzheimer’s disease: a systematic review and meta-analysis. Foods 2021, 10, 1672. 10.3390/foods10071672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Białecka-Dębek A.; Granda D.; Szmidt M. K.; Zielińska D. Gut microbiota, probiotic interventions, and cognitive function in the elderly: a review of current knowledge. Nutrients 2021, 13, 2514. 10.3390/nu13082514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munawar N.; Ahsan K.; Muhammad K.; Ahmad A.; Anwar M. A.; Shah I.; Al Ameri A. K.; Al Mughairbi F. Hidden role of gut microbiome dysbiosis in schizophrenia: Antipsychotics or psychobiotics as therapeutics?. Int. J. Mol. Sci. 2021, 22, 7671. 10.3390/ijms22147671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Kadosh K.; Basso M.; Knytl P.; Johnstone N.; Lau J. Y. F.; Gibson G. R. Psychobiotic interventions for anxiety in young people: a systematic review and meta-analysis, with youth consultation. Transl Psychiatry 2021, 11, 352. 10.1038/s41398-021-01422-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osadchiy V.; Martin C. R.; Mayer E. A. The Gut-Brain Axis and the Microbiome: Mechanisms and Clinical Implications. Clin. Gastroenterol. Hepatol. 2019, 17, 322–332. 10.1016/j.cgh.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cussotto S.; Strain C. R.; Fouhy F.; Strain R. G.; Peterson V. L.; Clarke G.; Stanton C.; Dinan T. G.; Cryan J. F. Differential effects of psychotropic drugs on microbiome composition and gastrointestinal function. Psychopharmacology (Berl.) 2019, 236, 1671–1685. 10.1007/s00213-018-5006-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.