Abstract
Background
Preterm birth is one of the key causes of morbidity and mortality among neonates in low-income countries. In Rwanda, at least 35,000 babies are born prematurely each year, and 2600 children under the age of five die due to direct complications of prematurity each year. A limited number of studies have been conducted locally, many of which are not nationally representative. Thus, this study determined the prevalence as well as the maternal, obstetric, and gynecological factors associated with preterm birth in Rwanda at the national level.
Methods
A longitudinal cohort study was conducted from July 2020 to July 2021 among first-trimester pregnant women. A total of 817 women from 30 health facilities in 10 districts were included in the analysis. A pre-tested questionnaire was used to collect data. In addition, medical records were reviewed to extract relevant data. Ultrasound examination was used to assess and confirm gestational age on recruitment. A multivariable logistic regression analysis was performed to determine the independent maternal, obstetric, and gynecological factors associated with preterm birth.
Results
The prevalence of preterm births was 13.8%. Older maternal age- 35 to 49 years [Adjusted odds ratio (AOR) = 2.00; 95% Confidence Interval (CI) = 1.13–3.53)], secondhand smoke exposure during pregnancy (AOR = 1.91; 95% CI = 1.04–3.51), a history of abortion (AOR = 1.89; 95% CI = 1.13–3.15), premature membrane rupture (AOR = 9.30; 95% CI = 3.18–27.16), and hypertension during pregnancy (AOR = 4.40; 95% CI = 1.18–16.42) were identified as independent risk factors for preterm birth.
Conclusion
Preterm birth remains a significant public health issue in Rwanda. The associated risk factors for preterm birth were advanced maternal age, secondhand smoke, hypertension, history of abortion, and preterm membrane rupture. This study therefore recommends routine antenatal screening to identify and closely follow-up of those high-risk groups, in order to avoid the short- and long-term effects of preterm birth.
Keywords: First-trimester, Longitudinal cohort study, Preterm birth, Risk factors
Background
Preterm birth is the delivery of the fetus before 37 weeks of gestation. According to estimates from the World Health Organization, 15 million (or one in ten) babies are born early each year [1]. African and South Asian countries account for more than 80% of these preterm births [2]. On average, 12% of babies are born prematurely in low-income nations, compared to 9% in high-income countries [3] with differences in incidence among countries. According to different studies, this incidence is reported to vary from 5.0% in Sweden [4], 16.8% in Nigeria [5], 18.3% in Kenya [6], and between 4.4 and 25.9% in Ethiopia [7–10].
Preterm birth is the primary cause of perinatal illness and mortality worldwide [2]. It is one of the biggest healthcare challenges as it is associated with long-term disability and financial strain from the costs of care especially in underdeveloped nations [11]. According to Wagura et al. [6], it accounts for around one-third of all neonatal deaths as a result of increased risk of infection [12]. It also has long-term adverse effects, such as poor neurodevelopment leading to learning disabilities, cerebral palsy, and vision abnormalities among others [13].
Studies have found a number of risk factors for preterm birth, which can be categorized as: (1) Maternal risk factors, such as maternal age, education level, low socioeconomic status, marital status, maternal malnutrition, substance use, inadequate antenatal care (ANC), and stress, among others [14–17], (2) gynecological risk factors such as intrauterine infection, urinary tract infection, pregnancy induced hypertension, sexually transmitted infections, premature rupture of membranes, uterine malformations, and uterine adhesions [18–21] and (3) Obstetric risk factors include: primiparity, short birth interval, antenatal care, multiparity, history of preterm birth, history of abortion, history of stillbirth or miscarriage, and multiple pregnancies [22–24]. However, the factors from various studies are uneven, with some factors showing direct association in some studies but having an inverse association or no association in others.
In Rwanda, despite the improvement of maternal and child health services, at least 35,000 babies are born too soon each year, and 2600 children under five die due to direct preterm complications [25]. The factors causing increased preterm birth in Rwanda are currently unknown. Some small-scale studies representing specific locality or hospital have been done. For instance, Nwankwo et al. demonstrated preterm birth prevalence of 17.5% with husband’s smoking, low ANC attendance and low maternal MUAC as independent predictors of preterm birth [26]. This was a single center facility based cross-sectional study whose findings cannot be generalized. However, as far as we are aware, there is no publication of a longitudinal study that attempt to investigate risk factors associated with preterm birth at a national level. Hence, this study aimed at determining the maternal, obstetric, and gynecological factors associated with preterm birth in Rwanda.
Methods
Study design and setting
Rwanda is a landlocked country located in central-eastern part of Africa. It is a low-income country with a population of about 13 million people. The country ‘health system is a pyramid with the top of pyramid, being the Ministry of health responsible for sector coordination and oversight, and setting health policies and strategies; Rwanda Biomedical Centre responsible of implementation of health programs such as Tuberculosis, Malaria, HIV and Maternal and child health; Rwanda Food and Drugs Authority for regulation of human and veterinary medicines, vaccines and other biological products, processed food, poisons, medical cosmetics, medical devices, and other products; and Rwanda Medical Supply with the mandate of ensuring availability of medicines, medical supplies and consumables to the health facilities. Currently, Rwanda has 565 public health facilities including 504 Health centers, 7 Medicalised Health Centers, 40 District, 4 Provincial, 4 Specialized and 8 Referral hospitals. Health posts are entities working at lowest level and operating under public-private partnership models; there are 1222 Health Posts (HPs) in Rwanda. Private health facilities are 317 and distributed as follow: 115 private dispensaries, 113 general clinics, 33 specialized clinics, 28 polyclinics, 9 dental clinics, 6 nutrition cabinets, 4 Private Hospitals, 4 Specialized hospitals, 3 Psychology clinics, and 2 laboratories [27].
A health facility follow-up study using a longitudinal single baseline cohort design among first-trimester pregnant women was conducted from July 2020 to July 2021. The study was conducted in 30 health facilities including hospitals and health centers located in 10 districts around the country. The sampling unit was hospital and then convenience sampling was used to select one health center in urban area and one health center in rural area under each selected hospital. Therefore, pregnant women in the first trimester from 10 hospitals and 20 health centers were recruited in the study.
Population, sample size and sampling technique
Participants in this study were pregnant women in their first trimester. Being in the first trimester of pregnancy (within the 13 weeks gestation), intending to stay in the study for the duration of the study, and being able to sign or thumbprint informed consent were the inclusion criteria. However, pregnant women with chronic diseases such as hypertension, heart disease, and renal disease were excluded.
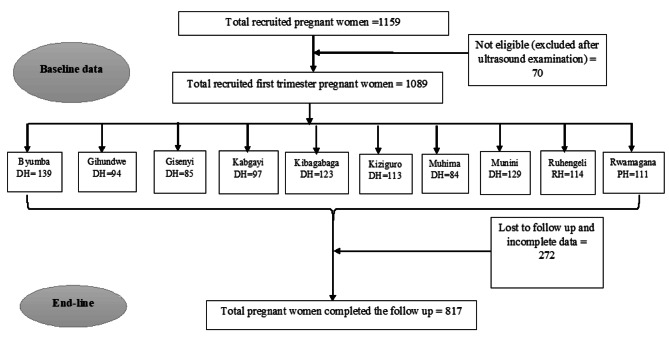
A multi-stage sampling strategy was adopted, with the first stage consisting of the simple random selection of 30 health facilities (acting as clusters) from around the nation (Fig. 1). Then, pregnant women who attended the antenatal care department in the selected healthcare facilities and were determined to be in their first trimester were included in the study. Between July and December 2020, all eligible women from each selected health facility were enrolled in the study. A total of 1159 pregnant women in their first trimester took part in the study after taking into account all inclusion criteria and 817 pregnant women were included in the analysis (Fig. 1).
Fig. 1.
Flowchart displaying participant enrollment and follow-up
Data collection procedure and tools
30 qualified nurses and midwives collected the data under the direct supervision of 10 team leaders from Mount Kenya University, and the study team. Team leaders, nurses and midwives received one-day training on the study protocol, inclusion criteria, sampling, and research tools, and data collection procedures. The questionnaire was pretested among 50 pregnant women who attended two health centers in Kigali City for ANC services. With the results of this pilot study, the study tool was adjusted as necessary. Pregnant women were told about the goal of the study during the data collection procedure, and they consented to participate.
Face-to-face interviews were used to gather the data using a structured questionnaire, and antenatal care records were examined to gather some additional medical information on maternal conditions. The questionnaire was adopted from the literature and contextualized according to Rwandan context. The questionnaire was initially prepared in English, and then translated into Kinyarwanda, then back into English. At the base line (first trimester) as well as in the end line (shortly after delivery) factors related to maternal characteristics (age, residence marital status, level of education, employment, socio-economic status, alcohol use and smoking during pregnancy and exposure to passive smoking), obstetric (parity, birth spacing, mode of delivery ANC use, preterm rupture of membrane, amniotic fluid volume HIV status Blood group and history of still birth, abortion and previous Cesarean section) and gynecologic (hospitalization during pregnancy, gestational diabetes, hypertension in pregnancy, malaria during pregnancy, hepatitis B, urethritis infection, cystitis infection, pyelonephritis infection and vaginitis infection) were collected.
Measurement of the primary outcome (pre-term birth)
Preterm delivery, which is defined as a live birth at less than 37 weeks of gestation, was the main outcome in this study. The latest menstrual period (LMP) date was used to estimate the gestational age, and an ultrasound examination was used to confirm this. Since ultrasounds are not typically performed at health centers, the pregnant women recruited from the health centers were directed to the nearest hospital for ultrasound examination. Ultrasound examination was done by the obstetrician or general practitioner at hospital.
Data analysis
Using IBM SPSS Statistics 25, data was entered and examined. Counts and percentages were used to describe the characteristics of respondents. Tables and text were used to present the results. For each categorical independent variable, a Chi-square assumption was made. Then, binary logistic regression using bivariate and multivariate analysis was used to identify the risk factors related to preterm birth. For the multivariable logistic regression analysis, variables having a p-value of less than 0.2 in the bivariate logistic regression were taken into account. The Adjusted Odds Ratio (AOR) with a 95% confidence interval was determined in the multivariable logistic regression analysis. Finally, factors were deemed significant if their p-value was less than 0.05. The Hosmer-Lemeshow test was used to gauge the model’s fitness.
Ethical consideration
With reference No. 131/RNEC/2020, the Rwanda National Ethics Committee granted its approval, and each of the chosen hospitals provided a letter of permission. Participants in the study were given a thorough description of the study’s objectives before providing their written informed consent. Legally authorized representatives of illiterate participants provided informed consent for the study. Informed consent was obtained from a parent and/or legal guardian for participants aged under 16.
Participants in the study had the freedom to decline or leave at any moment without incurring any penalties. Furthermore, the participants received assurances regarding the confidentiality of the data, and no personal identifying information was included on the questionnaires.
Results
Socio-demographic and lifestyle factors stratified by preterm
A total of 817 women and new-born pairs were included in the study to achieve the objectives. The prevalence of preterm birth was 13.83%, with a 95% confidence interval of 11.54% and 16.39%. The majority of new-born babies (63.2%) were female; however, this was not significantly associated with preterm birth. Similarly, there was no statistically significant association observed between risk of preterm birth and residence, marital status, level of education, occupation, and social class, as well as alcohol consumption and smoking during pregnancy. However, a significant association was observed between maternal age, partners’ smoking status, and the risk of preterm birth. The proportion of mothers who delivered preterm babies was significantly higher in the 35–49 age group (23% vs. 13.4%, p = 0.018) (Table 1). Mothers with preterm deliveries were more likely to be exposed to secondhand smoke compared to mothers with full-term births (p = 0.017) (Table 1).
Table 1.
Socio-demographic and lifestyle factors stratified by preterm
| Attributes | Pre-term | Full-term | Total | p value | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Gender of the newborn baby | |||||||
| Male | 36 | 31.9 | 265 | 37.6 | 301 | 36.8 | 0.237 |
| Female | 77 | 68.1 | 439 | 62.4 | 516 | 63.2 | |
| Maternal age [years] | |||||||
| 15 to 24 | 44 | 38.9 | 340 | 48.3 | 384 | 47.0 | 0.018 |
| 25 to 34 | 43 | 38.1 | 270 | 38.4 | 313 | 38.3 | |
| 35 to 49 | 26 | 23.0 | 94 | 13.4 | 120 | 14.7 | |
| Residence | |||||||
| Rural | 84 | 74.3 | 525 | 74.6 | 609 | 74.5 | 0.957 |
| Urban | 29 | 25.7 | 179 | 25.4 | 208 | 25.5 | |
| Maternal marital status | |||||||
| Married | 62 | 54.9 | 307 | 43.6 | 369 | 45.2 | 0.082 |
| Cohabiting | 43 | 38.1 | 338 | 48.0 | 381 | 46.6 | |
| Single | 8 | 7.1 | 59 | 8.4 | 67 | 8.2 | |
| Maternal level of education | |||||||
| No formal education | 8 | 7.1 | 64 | 9.1 | 72 | 8.8 | 0.410 |
| Primary | 64 | 56.6 | 438 | 62.2 | 502 | 61.4 | |
| Secondary | 37 | 32.7 | 180 | 25.6 | 217 | 26.6 | |
| Tertiary | 4 | 3.5 | 22 | 3.1 | 26 | 3.2 | |
| Occupation | |||||||
| Government employed | 8 | 7.1 | 49 | 7.0 | 57 | 7.0 | 0.404 |
| Business | 14 | 12.4 | 87 | 12.4 | 101 | 12.4 | |
| Farmer | 72 | 63.7 | 392 | 55.7 | 464 | 56.8 | |
| House wife | 16 | 14.2 | 155 | 22.0 | 171 | 20.9 | |
| Others | 3 | 2.7 | 21 | 3.0 | 24 | 2.9 | |
| Socio-class category | |||||||
| Social category 1x | 21 | 18.6 | 116 | 16.5 | 137 | 16.8 | 0.678 |
| Social category 2y | 57 | 50.4 | 386 | 54.8 | 443 | 54.2 | |
| Social category 3z | 35 | 31.0 | 202 | 28.7 | 237 | 29.0 | |
| Maternal alcohol use during pregnancy | |||||||
| Yes | 27 | 23.9 | 147 | 20.9 | 174 | 21.3 | 0.468 |
| No | 86 | 76.1 | 557 | 79.1 | 643 | 78.7 | |
| Maternal smoking during pregnancy | |||||||
| Yes | 2 | 1.8 | 3 | 0.4 | 5 | 0.6 | 0.089 |
| No | 111 | 98.2 | 701 | 99.6 | 812 | 99.4 | |
| Partner’s smoking status | |||||||
| Yes | 17 | 15.0 | 57 | 8.1 | 74 | 9.1 | 0.017 |
| No | 96 | 85.0 | 647 | 91.9 | 743 | 90.9 | |
xCategory 1: Citizens who were homeless, extremely vulnerable, and unable to provide for their basic needs.
yCategory 2: Citizens who could afford to eat once or twice a day but were unemployed and could only afford some type of low-class owned or rented housing.
zCategory 3: Citizens who had a paying job or even employed people. Small farmers who went beyond subsistence farming or owners of small and medium-sized businesses were under this category.
Obstetric factors associated with preterm birth
The distribution of maternal obstetric factors according to gestational age is summarized in Table 2. Mothers with preterm deliveries attended fewer than four ANC visits than mothers with full-term deliveries (p = 0.021). Other obstetric factors associated with preterm birth that were statistically significant were history of abortion (p = 0.018), history of cesarean section (p = 0.042), and preterm membrane rupture (p < 0.001).
Table 2.
Obstetric factors associated with preterm birth
| Attributes | Pre-term | Full-term | Total | p value | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| ANC visits | |||||||
| < 4 | 43 | 38.1 | 193 | 27.4 | 236 | 28.9 | 0.021 |
| >=4 | 70 | 61.9 | 511 | 72.6 | 581 | 71.1 | |
| Parity | |||||||
| Primi-gravida | 45 | 39.8 | 352 | 50.0 | 397 | 48.6 | 0.123 |
| 1 to 2 | 49 | 43.4 | 241 | 34.2 | 290 | 35.5 | |
| 2 to 3 | 15 | 13.3 | 73 | 10.4 | 88 | 10.8 | |
| 5+ | 4 | 3.5 | 38 | 5.4 | 42 | 5.1 | |
| Birth spacing (n = 389) | |||||||
| < 24 | 13 | 19.7 | 74 | 22.9 | 87 | 22.4 | 0.568 |
| >=24 | 53 | 80.3 | 249 | 77.1 | 302 | 77.6 | |
| History of still birth | |||||||
| Yes | 5 | 4.4 | 29 | 4.1 | 34 | 4.2 | 0.880 |
| No | 108 | 95.6 | 675 | 95.9 | 783 | 95.8 | |
| History of abortion | |||||||
| Yes | 25 | 22.1 | 96 | 13.6 | 121 | 14.8 | 0.018 |
| No | 88 | 77.9 | 608 | 86.4 | 696 | 85.2 | |
| History of previous Cesarean section | |||||||
| Yes | 16 | 14.2 | 58 | 8.2 | 74 | 9.1 | 0.042 |
| No | 97 | 85.8 | 646 | 91.8 | 743 | 90.9 | |
| Became pregnant while using contraceptive | |||||||
| Yes | 11 | 9.7 | 45 | 6.4% | 56 | 6.9 | 0.192 |
| No | 102 | 90.3 | 659 | 93.6% | 761 | 93.1 | |
| Taking medicine in pregnancy | |||||||
| Yes | 31 | 27.4 | 231 | 32.8% | 262 | 32.1 | 0.255 |
| No | 82 | 72.6 | 473 | 67.2% | 555 | 67.9 | |
| Preterm rupture of membrane | |||||||
| Yes | 10 | 8.8 | 6 | 0.9 | 16 | 2.0 | < 0.001 |
| No | 103 | 91.2 | 698 | 99.1 | 801 | 98.0 | |
| Amniotic fluid volume | |||||||
| Normal | 105 | 92.9 | 663 | 94.2 | 768 | 94.0 | 0.602 |
| Polyhydramnios | 8 | 7.1 | 41 | 5.8 | 49 | 6.0 | |
| Mode of delivery | |||||||
| Normal | 98 | 86.7 | 628 | 89.2 | 726 | 88.9 | 0.437 |
| CS | 15 | 13.3 | 76 | 10.8 | 91 | 11.1 | |
| Type of CS (n = 91) | |||||||
| Emergency | 5 | 33.3 | 29 | 38.2 | 34 | 37.4 | 0.724 |
| Elective | 10 | 66.7 | 47 | 61.8 | 57 | 62.6 | |
| HIV status | |||||||
| Positive | 2 | 1.8 | 17 | 2.4 | 19 | 2.3 | 0.673 |
| Negative | 111 | 98.2 | 687 | 97.6 | 798 | 97.7 | |
| Blood group | |||||||
| A | 9 | 8.0 | 100 | 14.2 | 109 | 13.3 | 0.223 |
| AB | 3 | 2.7 | 25 | 3.6 | 28 | 3.4 | |
| B | 13 | 11.5 | 79 | 11.2 | 92 | 11.3 | |
| O | 31 | 27.4 | 215 | 30.5 | 246 | 30.1 | |
| Not done | 57 | 50.4 | 285 | 40.5 | 342 | 41.9 | |
Gynecological factors associated with preterm birth
Table 3 presents factors related to gynecological conditions according to the gestational age at delivery. Hospitalization during pregnancy was significantly higher among mothers with preterm birth compared to those mothers with full term birth (p = 0.002). Similarly, the risk of preterm birth was significantly higher among mothers with hypertension (p = 0.002) and mothers with vaginal infections (p = 0.029).
Table 3.
Gynecological factors associated with preterm birth
| Attributes | Pre-term | Full-term | Total | p value | |||
|---|---|---|---|---|---|---|---|
| n | % | N | % | n | % | ||
| Hospitalization during pregnancy | |||||||
| Yes | 8 | 7.1 | 14 | 2.0 | 22 | 2.7 | 0.002 |
| No | 105 | 92.9 | 690 | 98.0 | 795 | 97.3 | |
| Any chronic diseases | |||||||
| Yes | 5 | 4.4 | 25 | 3.6 | 30 | 3.7 | 0.647 |
| No | 108 | 95.6 | 679 | 96.4 | 787 | 96.3 | |
| Gestational Diabetes | |||||||
| Yes | 0 | 0.0 | 1 | 0.1 | 1 | 0.1 | 0.689 |
| No | 113 | 100.0 | 703 | 99.9 | 816 | 99.9 | |
| Hypertension during pregnancy | |||||||
| Yes | 5 | 4.4 | 6 | 0.9 | 11 | 1.3 | 0.002 |
| No | 108 | 95.6 | 698 | 99.1 | 806 | 98.7 | |
| Malaria during pregnancy | |||||||
| Yes | 1 | 0.9 | 5 | 0.7 | 6 | 0.7 | 0.840 |
| No | 112 | 99.1 | 699 | 99.3 | 811 | 99.3 | |
| Hepatitis B | |||||||
| Yes | 0 | 0.0 | 1 | 0.1 | 1 | 0.1 | 0.689 |
| No | 113 | 100.0 | 703 | 99.9 | 816 | 99.9 | |
| Urethritis infection | |||||||
| Yes | 7 | 6.2 | 51 | 7.2 | 58 | 7.1 | 0.687 |
| No | 106 | 93.8 | 653 | 92.8 | 759 | 92.9 | |
| Cystitis infection | |||||||
| Yes | 24 | 21.2 | 135 | 19.2 | 159 | 19.5 | 0.607 |
| No | 89 | 78.8 | 569 | 80.8 | 658 | 80.5 | |
| Pyelonephritis infection | |||||||
| Yes | 5 | 4.4 | 40 | 5.7 | 45 | 5.5 | 0.587 |
| No | 108 | 95.6 | 664 | 94.3 | 772 | 94.5 | |
| Vaginitis infection | |||||||
| Yes | 18 | 15.9 | 65 | 9.2 | 83 | 10.2 | 0.029 |
| No | 95 | 84.1 | 639 | 90.8 | 734 | 89.8 | |
Bivariate and multivariable logistic regression analysis of factors predicting preterm birth
On bivariate logistic regression analysis, maternal age, secondhand smoking, ANC visits, history of abortion, preterm rupture of membranes, hospitalization, hypertension, and vaginal infections were significantly associated with preterm delivery at a p value less than 0.05. After considering all these variables together in multivariable logistic regression by specifying the backward conditional method, maternal age, second-hand smoking, history of abortion, preterm rupture of membrane, and hypertension were predicting preterm birth (Table 4).
Table 4.
Bivariate and multivariable logistic regression analysis of factors predicting preterm birth
| Variables | Unadjusted OR (95%CI) | Adjusted OR (95%CI) |
|---|---|---|
| Maternal age [years] | ||
| 15 to 24 | 1.00 | 1.00 |
| 25 to 34 | 1.23(0.78–1.93) | 1.27(0.79–2.03) |
| 35 to 49 | 2.14(1.25–3.65) | 2.00(1.13–3.53) |
| Partner’s smoking status | ||
| Yes | 2.01(1.12–3.60) | 1.91(1.04–3.51) |
| No | 1.00 | 1.00 |
| ANC visits | ||
| < 4 | 1.63(1.08–2.46) | 1.51(0.98–2.34) |
| >=4 | 1.00 | 1.00 |
| History of abortion | ||
| Yes | 1.80(1.10–2.95) | 1.89(1.13–3.15) |
| No | 1.00 | 1.00 |
| Preterm rupture of membrane | ||
| Yes | 11.29(4.02–31.73) | 9.30(3.18–27.16) |
| No | 1.00 | 1.00 |
| Hospitalization during pregnancy | ||
| Yes | 3.75(1.54–9.17) | 2.23(0.75–6.60) |
| No | 1.00 | 1.00 |
| Hypertension in Pregnancy | ||
| Yes | 5.39(1.62–17.95) | 4.40(1.18–16.42) |
| No | 1.00 | 1.00 |
| Vaginal infection | ||
| Yes | 1.86(1.06–3.28) | 1.39(0.75–2.57) |
| No | 1.00 | 1.00 |
Older mothers aged 35 to 49 years were 2 times more likely to deliver preterm babies compared to younger mothers aged 15 to 24 years (AOR = 2.00; 95%CI = 1.13–3.53). Preterm birth was 1.9 times more likely to occur among mothers exposed to secondhand smoke (AOR = 1.91; 95% CI = 1.04–3.51). Mothers who had previously had an abortion had a 1.9-fold increased risk of preterm birth (AOR = 1.89; 95% CI = 1.13–3.15; Preterm birth was about nine fold more common among mothers who experienced preterm membrane rupture (AOR = 9.30; 95CI = 3.18–27.16) and 4.4 times more common among hypertensive mothers (AOR = 4.40; 95% CI = 1.18–16.42).
Discussion
The purpose of this study was to establish maternal, obstetric, and gynecological factors associated with preterm birth in Rwanda. In our study, the prevalence of preterm birth was found to be 13.8%. After adjusting for potential confounders using a multivariable logistic regression model, the identified independent risk factors associated with preterm birth were advanced pregnancy age, secondhand smoke, abortion history, preterm membrane rupture, and pregnancy-induced hypertension.
The prevalence of preterm birth in Rwanda was higher than studies done in multi-center health facilities in Iran (5.1%) [28] and in Australia (6.8%) [29], as well as the WHO estimates for sub-Saharan Africa (9.5%) and lower-income countries (12.0%) [30]. However, it was similar to the pooled prevalence from metal-analysis in Ethiopia (11.4%) [31] as well as findings from multi-center study in Brazil (12.3%) [32] and from a referral hospital in Tanzania (14.2%) [33]. Further, it was slightly lower than a study conducted in Kenya (18.3%) [6] and in one District Hospital in Rwanda (17.5%) [26].
However, this prevalence is lower than recent cross-sectional studies done in teaching hospitals in Ghana, whereby it was reported at 37.3% [34], and 25.9% in Ethiopia [10]. These disparities in the prevalence of preterm birth among the studies could be attributed to small sample sizes in a small-scale study setting [9, 26, 34]. It could also be the method used to estimate the gestational age, as most studies depend on the last menstrual period reported retrospectively by the mothers, which could result in a biased prevalence [6, 26, 35]. Moreover, most studies recruited respondents from a single hospital, which could possibly overestimate the prevalence of preterm birth [9, 34, 36]. Our study, on the other hand, used a sufficient sample size from the community and the gold standard approach of ultrasonography to determine gestational age.
Our study showed that advanced maternal age was associated with preterm birth. Several studies have also found that advanced maternal age is a risk factor for preterm birth [16, 17, 37–39]. This increased risk of preterm birth among women of advanced age (> 35 years) could be attributed to the fact that reproductive organs and fertility decrease after 35 years. Moreover, complications related to pregnancy are more common in this advanced age group. However, other research from the United Kingdom [40] failed to detect an association, which may be related to socio-economic differences.
It is acknowledged that the primary risk factor for preterm birth is premature membrane rupture [41]. According to our research, women who experienced premature membrane rupture had a 9.3 times higher risk of having a preterm birth. This is consistent with several other studies [6, 36, 42–46]. This may be as the result of endogenous prostaglandins released during membrane rupture, which start the uterine contraction and lead to preterm birth [31]. This implies that pregnant women who have a history of PROM should receive additional care and management.
In concordance with other previous studies, [34, 43, 47, 48], this current study observed that hypertension during pregnancy was an independent risk factor for preterm birth. In East Africa, studies conducted in Kenya and Tanzania found that pregnancy induced hypertension is a risk factor for preterm birth [6, 33]. The poor pregnancy outcomes linked to hypertensive diseases during pregnancy, including premature delivery, are plausibly explained by uteroplacental ischemia [49, 50], despite the biology of this illness still not being fully understood. Thus, early detection of pregnancy-induced hypertension during antenatal care visits and screening is critical for appropriate treatment and management.
The current study found that women with a history of abortion were significantly more likely to give birth prematurely. This is in agreement with other studies as well as a systematic review and a meta-analysis [9, 10, 45, 51, 52]. According to the literature, the risk of abortion in early pregnancy is associated with undesirable birth outcomes, including preterm birth. The risk of infection associated with recurrent abortion may be the biological mechanism causing this connection. It has been reported that women who have had abortions before are more likely to get intra-amniotic infections [53]. Preterm delivery has a known risk factor known as intra-amniotic infection [54]. Women and medical professionals (midwives and obstetricians) should also be made aware of the potential link between abortion and premature delivery.
Our study also revealed that pregnant women exposed to secondhand smoking had significantly higher odds of delivering premature babies. There has been evidence that pregnant women who smoke cigarettes are at increased risk of preterm birth [9, 10, 29, 55]. In our study, the number of women who smoked during pregnancy was not significant (p = 0.089). However, the link between secondhand smoke exposure and preterm birth is still up for debate. Two recent studies done in Vietnam and the USA depicted a significant association between preterm birth and secondhand smoking [56, 57]. The pathway and connection between secondhand smoke and preterm delivery must be better understood in order to guide policymakers in putting preventative initiatives in place.
Although UTI and ANC attendance were significant in bivariate analysis, they were not significant in multivariate analysis. This finding is similar to that of a study conducted in Kenya which reported no significant association between preterm birth and number of ANC visits [6]. In contrast, a systematic and meta-analyses study found that women who had less than four ANC visits had a higher risk of preterm birth [45], which could be due to a failure to identify preterm birth risk factors during ANC visits. Other studies and reviews have found that maternal urinary tract infection (UTI) is linked to preterm birth [6, 20, 45], with infections weakening the baby’s amniotic sac, leading to PROM and preterm birth [58].
One of the study’s strengths is its large representative sample drawn from 30 health facilities selected in 10 districts. The findings thus represent the prevalence of preterm births in Rwanda with reasonable certainty. This study does have some drawbacks, though. The many independent variables and preterm birth do not necessarily have a cause-and-effect connection. Additionally, there may be a chance of recollection bias because the data were obtained through interviews. Loss to follow-up (LTFU) among recruited persons was another constraint (24.97%), but after comparing the baseline characteristics of the LTFU and those of participants included in the data analysis, there were no significant discrepancies, ensuring the internal validity of the study.
Conclusion
The magnitude of preterm birth was slightly higher than in the most recent Rwandan demographic health survey. It was independently affected by advanced age during pregnancy, secondhand smoke, a history of abortion, preterm membrane rupture, and pregnancy-induced hypertension. Therefore, it is preferable to take into account pregnant women who are older, exposed to secondhand smoke, have had abortions before, and have hypertension when screening and intervening in order to avoid the short- and long-term effects of premature birth.
Acknowledgements
This work was carried out with financial support from the Government of Rwanda through National Council for Science and Technology under Excellence Research Grant with grant Number: NCST-NRIF/ERG-BATCH1/P05/2019. We gratefully acknowledge the contribution of participating mothers, nurses, midwives, hospitals, and health centers.
List of Abbreviations
- AOR
Adjusted odds ratio
- ANC
Antenatal care
- COR
Crude odds ratio
- HIV
Human Immunodeficiency Virus
- LMP
Latest menstrual period
- PROM
Premature membrane rupture
- SPSS
Statistical package for social sciences
- USA
United States of America
- UTI
Urinary tract infection, WHO:World Health Organization
- WHO
World Health Organization
Authors’ contributions
The authors’ responsibilities were as follows- ER led the design of research protocol, follow-up data collection, conducted data analysis and led the manuscript writing. MH and MM contributed from the inception of the research protocol, supervision of data collection and during the manuscript. CN and JNU contributed in supervision of data collectors, data analysis, and manuscript writing. All authors read and approved the final manuscript.
Funding
This research was funded by the Rwanda National Council of Science and Technology (NCST). The funding body had no involvement in any part of the study design, implementation, or analysis.
Data Availability
The data and materials used in this study are available from the corresponding author upon request.
Declarations
Ethical approval and consent to participate
With reference No. 131/RNEC/2020, the Rwanda National Ethics Committee granted its approval, and each of the chosen hospitals provided a letter of permission. Informed consent was obtained from all participants. Legally authorized representatives of illiterate participants provided informed consent for the study. Informed consent was obtained from a parent and/or legal guardian for participants aged under 16. We confirm that all methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors confirm that they have no conflicts of interest in this research.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Quinn J-A, Munoz FM, Gonik B, Frau L, Cutland C, Mallett-Moore T, et al. Preterm birth: case definition & guidelines for data collection, analysis, and presentation of immunisation safety data. Vaccine. 2016;34:6047–56. doi: 10.1016/j.vaccine.2016.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chawanpaiboon S, Vogel JP, Moller A-B, Lumbiganon P, Petzold M, Hogan D, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health. 2019;7:e37–46. doi: 10.1016/S2214-109X(18)30451-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang HH, Larson J, Blencowe H, Spong CY, Howson CP, Cairns-Smith S, et al. Preventing preterm births: analysis of trends and potential reductions with interventions in 39 countries with very high human development index. Lancet. 2013;381:223–34. doi: 10.1016/S0140-6736(12)61856-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cnattingius S, Villamor E, Johansson S, Edstedt Bonamy A-K, Persson M, Wikström A-K, et al. Maternal obesity and risk of preterm delivery. JAMA. 2013;309:2362–70. doi: 10.1001/jama.2013.6295. [DOI] [PubMed] [Google Scholar]
- 5.Butali A, Ezeaka C, Ekhaguere O, Weathers N, Ladd J, Fajolu I, et al. Characteristics and risk factors of preterm births in a tertiary center in Lagos, Nigeria. Pan Afr Med J. 2016;24:1. doi: 10.11604/pamj.2016.24.1.8382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wagura P, Wasunna A, Laving A, Wamalwa D. Ng’ang’a P. Prevalence and factors associated with preterm birth at kenyatta national hospital. BMC Pregnancy Childbirth. 2018;18:107. doi: 10.1186/s12884-018-1740-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gebreslasie K. Preterm Birth and Associated Factors among Mothers Who Gave Birth in Gondar Town Health Institutions. Advances in Nursing. 2016;2016:1–5. Available from: https://www.hindawi.com/journals/anurs/2016/4703138/. [cited 2021 Jul 6].
- 8.Mengesha HG, Lerebo WT, Kidanemariam A, Gebrezgiabher G, Berhane Y. Pre-term and post-term births: predictors and implications on neonatal mortality in Northern Ethiopia. BMC Nurs. 2016;15:48. doi: 10.1186/s12912-016-0170-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelkay B, Omer A, Teferi Y, Moges Y. Factors Associated with Singleton Preterm Birth in Shire Suhul General Hospital, Northern Ethiopia, 2018. J Pregnancy. 2019;2019:4629101. doi: 10.1155/2019/4629101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bekele I, Demeke T, Dugna K. Prevalence of Preterm Birth and its Associated Factors among Mothers Delivered in Jimma University Specialized Teaching and Referral Hospital, Jimma Zone, Oromia Regional State, South West Ethiopia. J Women’s Health Care. 2017;06. Available from: https://www.omicsgroup.org/journals/prevalence-of-preterm-birth-and-its-associated-factors-among-mothers-deliveredin-jimma-university-specialized-teaching-and-referra-2167-0420-1000356.php?aid=86087. [cited 2021 Jul 7].
- 11.Newnham JP, Kemp MW, White SW, Arrese CA, Hart RJ, Keelan JA. Applying Precision Public Health to prevent Preterm Birth. Front Public Health. 2017;5:66. doi: 10.3389/fpubh.2017.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collins A, Weitkamp J-H, Wynn JL. Why are preterm newborns at increased risk of infection? Arch Dis Child Fetal Neonatal Ed. 2018;103:F391–4. doi: 10.1136/archdischild-2017-313595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blencowe H, Lee ACC, Cousens S, Bahalim A, Narwal R, Zhong N, et al. Preterm birth-associated neurodevelopmental impairment estimates at regional and global levels for 2010. Pediatr Res. 2013;74(Suppl 1):17–34. doi: 10.1038/pr.2013.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lilliecreutz C, Larén J, Sydsjö G, Josefsson A. Effect of maternal stress during pregnancy on the risk for preterm birth. BMC Pregnancy Childbirth. 2016;16:5. doi: 10.1186/s12884-015-0775-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kondracki AJ, Hofferth SL. A gestational vulnerability window for smoking exposure and the increased risk of preterm birth: how timing and intensity of maternal smoking matter. Reprod Health. 2019;16:43. doi: 10.1186/s12978-019-0705-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuchs F, Monet B, Ducruet T, Chaillet N, Audibert F. Effect of maternal age on the risk of preterm birth: a large cohort study. PLoS ONE. 2018;13:e0191002. doi: 10.1371/journal.pone.0191002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uwambaye P, Munyanshongore C, Rulisa S, Shiau H, Nuhu A, Kerr MS. Assessing the association between periodontitis and premature birth: a case-control study. BMC Pregnancy Childbirth. 2021;21:204. doi: 10.1186/s12884-021-03700-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alfirevic Z, Stampalija T, Medley N. Cervical stitch (cerclage) for preventing preterm birth in singleton pregnancy. Cochrane Database Syst Rev. 2017;6:CD008991. doi: 10.1002/14651858.CD008991.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reekie J, Roberts C, Preen D, Hocking JS, Donovan B, Ward J, et al. Chlamydia trachomatis and the risk of spontaneous preterm birth, babies who are born small for gestational age, and stillbirth: a population-based cohort study. Lancet Infect Dis. 2018;18:452–60. doi: 10.1016/S1473-3099(18)30045-8. [DOI] [PubMed] [Google Scholar]
- 20.Zini ME, Omo-Aghoja LO. Clinical and sociodemographic correlates of preterm deliveries in two tertiary hospitals in southern Nigeria. Ghana Medical Journal. 2019;53:20. Available from: https://www.ajol.info/index.php/gmj/article/view/185455. [cited 2022 Dec 14]. [DOI] [PMC free article] [PubMed]
- 21.Mulualem G, Wondim A, Woretaw A. The effect of pregnancy induced hypertension and multiple pregnancies on preterm birth in Ethiopia: a systematic review and meta-analysis. BMC Res Notes. 2019;12:91. doi: 10.1186/s13104-019-4128-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halimi Asl AA, Safari S, Parvareshi Hamrah M. Epidemiology and related risk factors of Preterm Labor as an obstetrics emergency. Emerg (Tehran) 2017;5:e3. [PMC free article] [PubMed] [Google Scholar]
- 23.Shachar BZ, Mayo JA, Lyell DJ, Baer RJ, Jeliffe-Pawlowski LL, Stevenson DK, et al. Interpregnancy interval after live birth or pregnancy termination and estimated risk of preterm birth: a retrospective cohort study. BJOG. 2016;123:2009–17. doi: 10.1111/1471-0528.14165. [DOI] [PubMed] [Google Scholar]
- 24.Fernandez Turienzo C, Sandall J, Peacock JL. Models of antenatal care to reduce and prevent preterm birth: a systematic review and meta-analysis. BMJ Open. 2016;6:e009044. doi: 10.1136/bmjopen-2015-009044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.USAID USA for ID, Rwanda. Profile of preterm and low birth weight prevention and care. (2017). https://www.healthynewbornnetwork.org/hnn-content/uploads/Rwanda20171-min.pdf. 2017.
- 26.Nwankwo HC, Habtu M, Rutayisire E, Kalisa R. Prevalence and factors associated with preterm birth in a rural district hospital, Rwanda. Pan Afr Med J. 2022;43:173. doi: 10.11604/pamj.2022.43.173.34113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ministry of Health, Rwanda. Health sector annual Performance Report 2020–2021. Available from https://www.moh.gov.rw/index.php?eID=dumpFile&t=f&f=36820&token=2e1aac6615a585b990697f58e2915ee3ee2c6f9a. 2021
- 28.Alijahan R, Hazrati S, Mirzarahimi M, Pourfarzi F, Ahmadi Hadi P. Prevalence and risk factors associated with preterm birth in Ardabil, Iran. Iran J Reprod Med. 2014;12:47–56. [PMC free article] [PubMed] [Google Scholar]
- 29.Xu XK, Wang YA, Li Z, Lui K, Sullivan EA. Risk factors associated with preterm birth among singletons following assisted reproductive technology in Australia 2007–2009–a population-based retrospective study. BMC Pregnancy Childbirth. 2014;14:406. doi: 10.1186/s12884-014-0406-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.WHO. World health organization updates fact sheet on Preterm birth. https://communitymedicine4asses.wordpress.com/2017/11/18/who-updates-fact-sheet-on-preterm-birth-16-november-2017/. 2017.
- 31.Sendeku FW, Beyene FY, Tesfu AA, Bante SA, Azeze GG. Preterm birth and its associated factors in Ethiopia: a systematic review and meta-analysis. Afr Health Sci. 2021;21:1321–33. doi: 10.4314/ahs.v21i3.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Souza RT, Cecatti JG, Passini R, Tedesco RP, Lajos GJ, Nomura ML, et al. The Burden of Provider-Initiated Preterm Birth and Associated factors: evidence from the brazilian Multicenter Study on Preterm Birth (EMIP) PLoS ONE. 2016;11:e0148244. doi: 10.1371/journal.pone.0148244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Temu TB, Masenga G, Obure J, Mosha D, Mahande MJ. Maternal and obstetric risk factors associated with preterm delivery at a referral hospital in northern-eastern Tanzania. Asian Pacific Journal of Reproduction. 2016;5:365–70. Available from: http://linkinghub.elsevier.com/retrieve/pii/S2305050016300768. [cited 2021 Jul 6].
- 34.Anto EO, Ofori Boadu WI, Opoku S, Senu E, Tamakloe VCKT, Tawiah A, et al. Prevalence and risk factors of Preterm Birth among pregnant women admitted at the Labor Ward of the Komfo Anokye Teaching Hospital, Ghana. Front Glob Womens Health. 2022;3:801092. doi: 10.3389/fgwh.2022.801092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alamneh TS, Teshale AB, Worku MG, Tessema ZT, Yeshaw Y, Tesema GA, et al. Preterm birth and its associated factors among reproductive aged women in sub-saharan Africa: evidence from the recent demographic and health surveys of sub-sharan african countries. BMC Pregnancy Childbirth. 2021;21:770. doi: 10.1186/s12884-021-04233-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adugna DG. Prevalence and associated risk factors of preterm birth among neonates in referral hospitals of Amhara Region, Ethiopia. PLoS ONE. 2022;17:e0276793. doi: 10.1371/journal.pone.0276793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Londero AP, Rossetti E, Pittini C, Cagnacci A, Driul L. Maternal age and the risk of adverse pregnancy outcomes: a retrospective cohort study. BMC Pregnancy Childbirth. 2019;19:261. doi: 10.1186/s12884-019-2400-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soltani M, Tabatabaee HR, Saeidinejat S, Eslahi M, Yaghoobi H, Mazloumi E, et al. Assessing the risk factors before pregnancy of preterm births in Iran: a population-based case-control study. BMC Pregnancy Childbirth. 2019;19:57. doi: 10.1186/s12884-019-2183-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mehari M-A, Maeruf H, Robles CC, Woldemariam S, Adhena T, Mulugeta M, et al. Advanced maternal age pregnancy and its adverse obstetrical and perinatal outcomes in Ayder comprehensive specialized hospital, Northern Ethiopia, 2017: a comparative cross-sectional study. BMC Pregnancy Childbirth. 2020;20:60. doi: 10.1186/s12884-020-2740-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khalil A, Syngelaki A, Maiz N, Zinevich Y, Nicolaides KH. Maternal age and adverse pregnancy outcome: a cohort study. Ultrasound Obstet Gynecol. 2013;42:634–43. doi: 10.1002/uog.12494. [DOI] [PubMed] [Google Scholar]
- 41.Robinson JN, Norwitz ER. Preterm birth: Risk factors, interventions for risk reduction, and maternal prognosis. UpToDate[(accessed on 20 January 2022)] Available online: https://www.uptodate.com/contents/preterm-birth-risk-factors-interventions-for-risk-reduction-and-maternal-prognosis. 2018.
- 42.Shetty B, K MBM, Malyala M, Swarup A, Pathadan DS, Pocha S. Preterm birth: associated risk factors and outcome in tertiary care center. Int J Reprod Contracept Obstet Gynecol. 2017;6:3271. Available from: http://www.ijrcog.org/index.php/ijrcog/article/view/3156. [cited 2022 Dec 14].
- 43.Sureshbabu RP, Aramthottil P, Anil N, Sumathy S, Varughese SA, Sreedevi A, et al. Risk factors Associated with Preterm Delivery in Singleton pregnancy in a Tertiary Care Hospital in South India: a Case Control Study. Int J Womens Health. 2021;13:369–77. doi: 10.2147/IJWH.S282251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Phillips C, Velji Z, Hanly C, Metcalfe A. Risk of recurrent spontaneous preterm birth: a systematic review and meta-analysis. BMJ Open. 2017;7:e015402. doi: 10.1136/bmjopen-2016-015402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laelago T, Yohannes T, Tsige G. Determinants of preterm birth among mothers who gave birth in East Africa: systematic review and meta-analysis. Ital J Pediatr. 2020;46:10. doi: 10.1186/s13052-020-0772-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aseidu EK, Bandoh DA, Ameme DK, Nortey P, Akweongo P, Sackey SO et al. Obstetric determinants of preterm delivery in a regional hospital, Accra, Ghana 2016. BMC Pregnancy Childbirth. 2019;19:248. Available from: https://bmcpregnancychildbirth.biomedcentral.com/articles/10.1186/s12884-019-2404-6. [cited 2023 Mar 24]. [DOI] [PMC free article] [PubMed]
- 47.Jamal S, Srivastava R. A retrospective analytical study of the epidemiology and causes of preterm birth. Int J Reprod Contracept Obstet Gynecol. 2017;6:5453. Available from: http://www.ijrcog.org/index.php/ijrcog/article/view/3800. [cited 2022 Dec 14].
- 48.Davies EL, Bell JS, Bhattacharya S. Preeclampsia and preterm delivery: A population-based case–control study. Hypertension in Pregnancy. 2016;35:510–9. Available from: https://www.tandfonline.com/doi/full/10.1080/10641955.2016.1190846. [cited 2022 Dec 14]. [DOI] [PubMed]
- 49.Mustafa R, Ahmed S, Gupta A, Venuto RC. A comprehensive review of hypertension in pregnancy. J Pregnancy. 2012;2012:105918. doi: 10.1155/2012/105918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Berry C, Atta MG. Hypertensive disorders in pregnancy. World J Nephrol. 2016;5:418–28. doi: 10.5527/wjn.v5.i5.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Malacova E, Regan A, Nassar N, Raynes-Greenow C, Leonard H, Srinivasjois R, et al. Risk of stillbirth, preterm delivery, and fetal growth restriction following exposure in a previous birth: systematic review and meta-analysis. BJOG. 2018;125:183–92. doi: 10.1111/1471-0528.14906. [DOI] [PubMed] [Google Scholar]
- 52.Ghelichkhani S, Masoumi SZ, Shirzadeh AA, Khazaei S, Shahbazi F. Evaluation of maternal risk factors for preterm delivery in Fatemieh Hospital of Hamadan, Iran, 2019: a case-control study. J Family Med Prim Care. 2021;10:3832–7. doi: 10.4103/jfmpc.jfmpc_1032_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gomez R, Romero R, Edwin SS, David C. Pathogenesis of preterm labor and preterm premature rupture of membranes associated with intraamniotic infection. Infect Dis Clin North Am. 1997;11:135–76. doi: 10.1016/s0891-5520(05)70347-0. [DOI] [PubMed] [Google Scholar]
- 54.Krohn MA, Germain M, Mühlemann K, Hickok D. Prior pregnancy outcome and the risk of intraamniotic infection in the following pregnancy. Am J Obstet Gynecol. 1998;178:381–5. doi: 10.1016/s0002-9378(98)80029-x. [DOI] [PubMed] [Google Scholar]
- 55.He J-R, Ramakrishnan R, Lai Y-M, Li W-D, Zhao X, Hu Y, et al. Predictions of Preterm Birth from early pregnancy characteristics: born in Guangzhou Cohort Study. J Clin Med. 2018;7:E185. doi: 10.3390/jcm7080185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rang NN, Hien TQ, Chanh TQ, Thuyen TK. Preterm birth and secondhand smoking during pregnancy: a case-control study from Vietnam. PLoS ONE. 2020;15:e0240289. doi: 10.1371/journal.pone.0240289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hoyt AT, Canfield MA, Romitti PA, Botto LD, Anderka MT, Krikov SV et al. Does Maternal Exposure to Secondhand Tobacco Smoke During Pregnancy Increase the Risk for Preterm or Small-for-Gestational Age Birth? Matern Child Health J. 2018;22:1418–29. Available from: http://link.springer.com/10.1007/s10995-018-2522-1. [cited 2022 Dec 14]. [DOI] [PubMed]
- 58.Devlieger R, Millar LK, Bryant-Greenwood G, Lewi L, Deprest JA. Fetal membrane healing after spontaneous and iatrogenic membrane rupture: A review of current evidence. American Journal of Obstetrics and Gynecology. 2006;195:1512–20. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0002937806001463. [cited 2022 Dec 14]. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data and materials used in this study are available from the corresponding author upon request.