Abstract
Borrelia burgdorferi, the Lyme disease spirochete, possesses a surface protein, VlsE, which undergoes antigenic variation. VlsE contains two invariable domains and a variable one that includes six variable and six invariable regions (IRs). Five of the IRs are conserved among strains and genospecies of B. burgdorferi sensu lato. IR6 is conserved, immunodominant, and exposed at the VlsE surface but not at the spirochete surface, as assessed in vitro. In the present study, the remaining conserved IRs (IR2 to IR5) were investigated. Antisera to synthetic peptides based on each of the IR2 to IR5 sequences were produced in rabbits. Antipeptide antibody titers were similarly high in all antisera. Native VlsE was immunoprecipitable with antibodies to IR2, IR4, and IR5 but not to IR3, indicating that the first three sequences were exposed at the VlsE surface. However, negative surface immunofluorescence and in vitro antibody-mediated killing results indicated that none of the IRs were accessible to antibody at the spirochetal surface in vitro.
Lyme disease, which is caused by the spirochete Borrelia burgdorferi, affects multiple tissues and organs in humans and in animals (19). In untreated individuals, Lyme disease spirochetes can persist for extended periods, even in the presence of a vigorous and persistent immune response (18). Chronic infection may be achieved through a variety of mechanisms, including limited exposure of antigenic targets (6), seclusion of the organisms into immune-privileged sites (14), local and/or systemic suppression of harmful immune responses (8), and antigenic variation (23).
Antigenic variation is an effective strategy evolved by pathogenic microorganisms to evade the host immune system (4). Antigens such as the variant surface glycoprotein of African trypanosomes (5, 22), the variable major protein of the spirochete Borrelia hermsii (13, 20), and the variable surface antigen (VlsE) of B. burgdorferi (23) contain both invariable and variable domains. Antigenic variation affects only the variable domains. Even within variable domains, short invariable regions may be present. The invariable domains and regions are important in maintaining the functional structure of the molecule (4). The variable domains are highly immunogenic and serve as the major target of the host immune response (4, 5, 13, 20, 22, 23). Invariable portions of the variant surface glycoprotein and variable major protein antigens have not been found to be antigenic during natural infections, although antibodies directed to these conserved sequences may be produced by immunization (1, 3, 7).
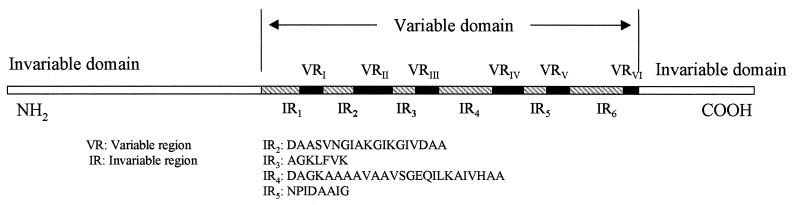
Among molecules that undergo antigenic variation, VlsE is unusual in that more than 75% of its primary structure is invariable. This invariable portion of the molecule is composed of two domains at the amino and carboxyl termini, respectively, which together encompass approximately half of the molecule's length, and six small invariable regions (IR1 to IR6) that are interspersed within the central variable domain (Fig. 1) (11, 23). Any such invariable portion could be explored as a possible target for a protective immune response and vaccine development.
FIG. 1.
Diagrammatic illustration of the VlsE structure. VlsE consists of two invariable domains at the amino and carboxyl termini and a variable one at the center. The variable domain contains six variable regions, VRI to VRVI, and six invariable ones, IR1 to IR6. The sequences of the invariable regions are based on those of the variable domain of VlsE expressed by strain IP90 of B. garinii (11).
To avoid immune responses harmful to the organism, invariable portions may be (i) not exposed on the surface of the molecule, (ii) exposed on the surface of the molecule but not at that of the spirochete, or (iii) nonantigenic, either because of an intrinsic lack of antigenicity in a given host species or because other regions of the molecule are immunodominant. We have already demonstrated that one of the invariable regions of VlsE, namely IR6, is strongly antigenic, exposed at the molecule's surface but not accessible to antibody at the surface of the spirochete in vitro (11).
In the present study, we investigated the exposure, at the surface both of the VlsE molecule and of the spirochete, of four additional invariable regions, IR2 to IR5. To address this issue, we generated antibodies to these four regions by immunizing rabbits with synthetic peptides conjugated to keyhole limpet hemocyanin (KLH). Rabbit antisera were used in immunoprecipitation experiments with native VlsE to determine exposure of invariable regions at the VlsE surface. Exposure of IR2 to IR5 at the spirochete's surface was assessed by indirect immunofluorescence and by antibody-dependent, complement-mediated killing (ADCK) assays using the rabbit antipeptide antibodies.
Borrelia garinii strain IP90 (low passage) was obtained from the Centers for Disease Control and Prevention (Fort Collins, Colo.). Spirochetes were cultivated in Barbour-Stoenner-Kelly (BSK-H) medium supplemented with 10% rabbit or human serum (Sigma Chemical Co., St. Louis, Mo.), as described previously (16).
Reactivity of rabbit antipeptide antibody with the VlsE protein.
To generate antibody to invariable regions of VlsE, peptides were prepared using the fluorenylmethoxycarbonyl synthesis protocol (2) according to the sequences listed in Fig. 1. The synthetic peptides C2, C3, C4, and C5 represented the sequences of IR2, IR3, IR4, and IR5, respectively. A cysteine residue was included at the NH2 terminus of each synthetic peptide and used as a conjugation site when KLH was used as a carrier. Conjugation of the synthetic peptides to KLH was performed by the N-succinimidyl maleimide carboxylate method. The maleimide reagent was from Molecular Probes (Eugene, Oreg.), and the protocol suggested by the manufacturer was followed. The synthetic peptides were also conjugated to biotin and used as peptide-based enzyme-linked immunosorbent assay (ELISA) antigens. Six-month-old New Zealand White rabbits were given three injections at biweekly intervals of 200 μg of peptide-KLH conjugate emulsified with Freund's complete (first injection) or incomplete adjuvant (remaining injections). Ten days after the last injection, the antibody reactivity was determined by peptide-based ELISA and immunoblotting.
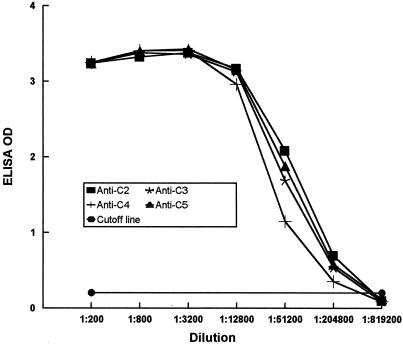
The peptide-based ELISA was performed as previously described (11). Ninety-six-well ELISA plates were coated with 100 μl of streptavidin (4 μg/ml) per well (Pierce Chemical Company, Rockford, Ill.) in coating buffer (0.1 M carbonate buffer [pH 9.2]) and incubated at 4°C overnight. The remaining steps were conducted in a rotatory shaker at room temperature. After two 3-min washes with 200 μl of phosphate-buffered saline-Tween 20 (PBS-T; PBS containing 0.1% Tween 20 [pH 7.4]) per well at 200 rpm, 200 μl of biotinylated peptide (5 μg/ml) dissolved in blocking solution (PBS-T supplemented with 5% nonfat dry milk) was applied to each well. The plate was shaken at 150 rpm for 2 h. After three washes with PBS-T, 50 μl of serial dilutions of rabbit serum with blocking solution was added to each well. The plate was incubated at 150 rpm for 1 h and then washed three times with PBS-T. Each well then received 100 μl of 0.2-μg/ml goat anti-rabbit immunoglobulin G (IgG) (heavy- and light-chain specific [Sigma])–horseradish peroxidase conjugate dissolved in blocking solution. The plate was incubated for 1 h while shaking. After four washes with PBS-T each for 3 to 6 min, the antigen-antibody reaction was probed using the TMB microwell peroxidase substrate system (Kirkegaard & Perry Laboratories, Gaithersburg, Md.), and color was allowed to develop for 10 min. The enzyme reaction was stopped by addition of 100 μl of 1 M H3PO4. Optical density (OD) was measured at 450 nm. Peptide-based ELISAs revealed that antibodies to all of the peptides had similar titers of 1:204,800 (Fig. 2). To confirm the specificity of antipeptide antibodies, rabbit antiserum to each of the peptides was allowed to react with all of the other peptides using the ELISA described above. No signals were detected above background, indicating that the antipeptide antibodies were specific (data not shown).
FIG. 2.
ELISA titers of rabbit antisera to synthetic peptides. Rabbit antipeptide antisera were serially diluted fourfold and reacted with the corresponding peptide bound to an ELISA plate, following the procedure described in the text. Titer was defined as the highest serum dilution at which the ELISA OD was larger than the mean OD value of the preimmune sera of all of the rabbits plus 3 standard deviations.
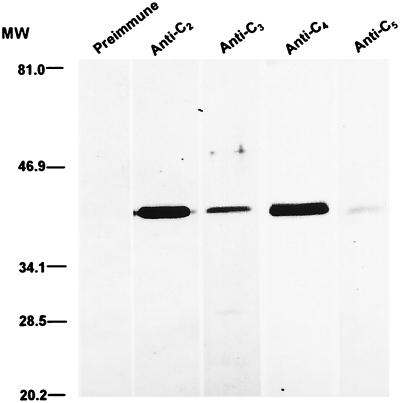
To assess if antipeptide antibodies react appropriately with the original antigen VlsE, whole-spirochete cell lysate immunoblots were conducted. IP90 spirochetes grown to stationary phase in BSK-H medium were harvested and washed twice with PBS by centrifugation at 10,000 × g for 10 min. Resultant pellets were dissolved in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer (125 mM Tris, 3% SDS, 5% β-mercaptoethanol, 10% glycerol, 0.01% bromophenol blue [pH 6.8]) at a concentration of 108 organisms per ml and incubated at 95°C for 5 min. Approximately 10 μl of such preparation was applied to each lane of a 10-well minigel of 12% acrylamide. Resolved proteins were transferred onto nitrocellulose in Towbin transfer buffer (21). The blot was shaken in blocking solution for 2 h and then in the same solution supplemented with a 1:500 dilution of preimmune or immune rabbit serum for an additional 1 h. After three washes with PBS-T, the blot was incubated in 0.2 μg of goat anti-rabbit IgG–horseradish peroxidase conjugate per ml for 1 h. After three more washes with PBS-T, the blot was developed in PBS-T supplemented with 0.05% 4-chloro-naphthol, 0.015% H2O2, and 17% methanol. Although immunization resulted in similar ELISA titers for all of the immune sera, rabbit antisera to peptides C2 and C4 reacted with VlsE more intensely than anti-C3 and -C5 antisera (Fig. 3). The single immunoblot band revealed by each individual rabbit antiserum indicated that antibodies raised against synthetic peptides reacted only with VlsE and that the reactivity was specific. However, our experiment cannot rule out the possibility that antipeptide antibodies also bind other regions of VlsE, such as variable regions and invariable domains. We believe that this is very unlikely.
FIG. 3.
Reactivity of rabbit antipeptide antibodies with VlsE. A whole-cell lysate of B. garinii IP90 spirochetes was separated by SDS-PAGE and transferred onto nitrocellulose. The blot was allowed to react with preimmune or rabbit antipeptide antiserum. MW, molecular weight (values are in thousands).
IR2, IR4, and IR5 but not IR3 are exposed at the surface of VlsE.
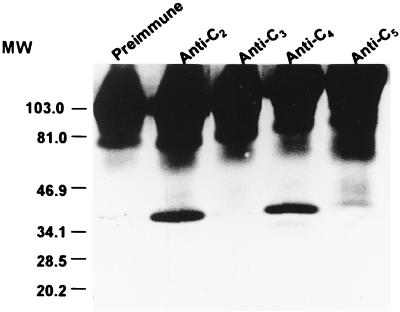
Immunoprecipitation was used to determine if invariable regions are exposed at the VlsE surface. Immunoprecipitation was conducted at 4°C. Approximately 2.5 × 1010 IP90 spirochetes harvested at stationary growth phase were washed twice with PBS and extracted in 7.5 ml of solubilization buffer (50 mM Tris-HCl, 1% Triton X-100, 1 mM EDTA [pH 7.6]) for 30 min. The mixture was centrifuged at 13,000 × g for 30 min, and the supernatant was collected. Each sample of 1.5 ml of this supernatant was mixed with 30 μl of preimmune or immune rabbit serum and incubated for 30 min. Fifty microliters of drained ImmunoPure immobilized protein G beads (Pierce) preequilibrated in solubilization buffer was then added and incubated for an additional 30 min. The beads were washed twice with excess volumes of this buffer by centrifugation at 3,000 × g for 20 min and resuspended in 150 μl of nonreducing SDS-PAGE sample buffer (125 mM Tris-HCl, 3% SDS, and 20% glycerol [pH 6.8]). The suspension was incubated at room temperature for 30 min and then centrifuged at 16,000 × g for 30 min. Ten microliters of supernatant was loaded onto each of 10 lanes of a SDS–12% acrylamide minigel. Separated proteins were electrotransferred to nitrocellulose in Towbin transfer buffer (21). The blot was then processed as described above and was developed with rabbit anti-C6 antiserum. The sequence of the synthetic peptide C6 was based on IR6 (11). This antiserum reacted strongly with VlsE. Rabbit antisera to C2, C4, and C5 were able to precipitate VlsE, indicating that IR2, IR4, and IR5 were exposed at the surface of this antigen (Fig. 4). In contrast, VlsE was not precipitated with anti-C3 antibody. Preimmune serum was also negative.
FIG. 4.
Exposure of IR2, IR4, and IR5 at the VlsE surface. VlsE from B. garinii strain IP90 spirochetes was extracted with solubilization buffer and immunoprecipitated with rabbit antipeptide antiserum or preimmune serum and protein G-agarose. Solubilized immunoprecipitates were separated by SDS-PAGE and blotted onto nitrocellulose. VlsE was visualized with rabbit anti-C6 antiserum and goat anti-rabbit IgG–peroxidase conjugate. In addition to VlsE (approximately 39.5 kDa), precipitated rabbit IgG is visible at the top of each lane. MW, molecular weight (values are in thousands).
IR2, IR4, and IR5 are not accessible to antibody at the surface of intact spirochetes.
To assess if IR2, IR4, and IR5 are exposed at the spirochete's surface, immunofluorescence experiments were conducted. Both unfixed and fixed spirochetes were used in this study. For immunofluorescence with unfixed spirochetes, approximately 108 IP90 spirochetes that were harvested from 1.0 ml of stationary-phase culture (10% rabbit serum–BSK) by centrifugation at 4,000 × g for 20 min were gently resuspended in 100 μl of PBS supplemented with 0.2 μl of preimmune or immune rabbit serum and incubated for 1 h at room temperature. B. burgdorferi-infected mouse antiserum and monkey anti-OspA (outer surface protein A) antiserum were used as positive controls. Mice were infected with spirochetes of the B31 strain by tick inoculation. Anti-OspA antiserum was raised by immunization with the OspA vaccine (17). After two washes by centrifugation at 4,000 × g for 8 min with excess volumes of PBS, the spirochetes were resuspended in 100 μl of PBS containing 2 μg of goat anti-rabbit (Pierce), -monkey, or -mouse (Kirkegaard & Perry) IgG antibody conjugated to fluorescein and incubated for an additional 1 h at room temperature. Spirochetes were washed three times with PBS and resuspended in 500 μl of PBS. Approximately 5 μl of spirochete suspension was applied to a microscope slide, glass covered, and observed under a fluorescence microscope. For immunofluorescence with fixed spirochetes, IP90 spirochetes grown in 10% human serum–BSK medium were harvested by centrifugation and washed once with PBS. Spirochetes were fixed in acetone for 30 min and recovered by centrifugation. Fixed organisms were resuspended in PBS containing 5% human serum and incubated at 37°C for 30 min. After one wash with PBS-human serum, spirochetes were processed as for immunofluorescence for unfixed spirochetes, except for the following modifications: PBS was replaced with PBS-human serum, rabbit serum was diluted at 1:1,000 instead of 1:500, and goat anti-rabbit IgG fluorescein conjugate (Sigma) was absorbed with human serum protein and diluted at 1:150 instead of 1:50. None of the antipeptide antibodies were able to make unfixed spirochetes visible under a fluorescence microscope, while the control mouse anti-B. burgdorferi antibody labeled the bacteria under the same conditions (Fig. 5). Like the mouse antiserum, monkey anti-OspA antibody lit up both fixed and unfixed spirochetes (data not shown), evidence that our gentle treatment did not significantly remove outer surface proteins from the spirochete's surface. This indicates that the invariable regions that are exposed at the VlsE surface are inaccessible to antibody on the intact spirochete. As expected, IR3 also was inaccessible to antibody at the spirochetal surface (Fig. 5). In contrast, acetone-fixed spirochetes were readily labeled with anti-C2, -C4, and -C5 antibodies (Fig. 5). Anti-C3 antibody was essentially negative, although spotted labeling on some of the organisms was visible. Preimmune rabbit serum was negative (Fig. 5).
FIG. 5.
IR2 to IR5 are not accessible to antibody at the surface of spirochetes. B. garinii strain IP90 spirochetes either were fixed with acetone or left unfixed. Unfixed spirochetes were resuspended in PBS containing preimmune or immune rabbit antipeptide antiserum or positive control mouse antiserum, while fixed spirochetes were incubated in PBS-human serum supplemented with the same antiserum samples. Sensitized spirochetes were probed with goat anti-rabbit or -mouse IgG–fluorescein conjugate.
The failure of anti-C2 to -C5 antibodies to access IR2 to IR5 on the intact spirochetes was underscored by the results of the ADCK experiments. These experiments were performed by a procedure described previously (15). No significant killing was observed with any of the antipeptide antibodies at a 1:10 dilution, whereas monkey anti-OspA antibodies readily killed 100% of spirochetes at a 1:25 dilution (data not shown).
VlsE is a surface-exposed lipoprotein of the Lyme disease spirochete (23). Absence of the linear plasmid lp28-1, which contains the VlsE gene, has been shown to correlate with infectivity of B. burgdorferi in a mouse model (23). More recent studies indicate that lp28-1-deficient mutants have a reduced infectivity (S. J. Norris, Abstr. VIII Int. Conf. Lyme Borr. Other Emerg. Tick-Borne Dis., abstr. 37, p. 13, 1999). Immunization of mice with recombinant VlsE protects the animals from a challenge infection with a spirochete clone that expresses the same VlsE that was used for immunization (M. B. Lawrenz, J. M. Hardham, R. T. Owens, and S. J. Norris, Abstr. 99th Gen. Meet. Am. Soc. Microbiol., abstr. D/B-264, p. 260, 1999). Taken together, these data suggest that VlsE is a target of a host protective immune response. The VlsE variable domain contains six invariable regions (Fig. 1), five of which (IR2 to IR6) are conserved among strains and genospecies of B. burgdorferi sensu lato (11). These conserved sequences could serve as targets of protective antibody if they were exposed at the surface of the spirochete. One invariable region, IR6, is immunodominant in both humans and nonhuman primates (11) and may serve as a global probe for the serodiagnosis of Lyme disease (12). Since this sequence is exposed at the surface of VlsE but not of the spirochete (11), antibody to it is likely not protective in vivo.
In the present study we investigated the accessibility of IR2 to IR5 to specific antibodies at both the VlsE and spirochetal surfaces. For this purpose, four peptides reproducing the sequences of IR2 to IR5 were synthesized and conjugated to KLH; the conjugates were used to immunize rabbits. Similarly high ELISA titers of antibody to all of the synthetic peptides were obtained. In spite of this similarity in antipeptide antibody titers, the antibodies did not react equally well with VlsE on immunoblots. The antibody raised against the two longer peptides, C2 (19-mer) and C4 (25-mer), showed strong bands, while antibodies to the two shorter peptides, C3 (7-mer) and C5 (8-mer), did not bind as strongly to VlsE on the immunoblot. It is possible that the sequences that flank the shorter IR3 and IR5 may sterically interfere with the binding of antibody to these regions on the immunoblot more easily than with the antibody binding to the longer IR2 and IR4. Partial or complete re- or denaturation of the SDS-treated VlsE blotted onto nitrocellulose may also help to diminish exposure of certain epitopes on the VlsE surface. In fact, immunoprecipitation revealed that IR2, IR4, and IR5 are probably exposed at the VlsE surface, whereas IR3 is not. Alternatively, the limited length of IR3 may also explain the failure to immunoprecipitate VlsE with the anti-C3 antibody. In addition, immunoprecipitation with the anti-C5 antibody was less efficient than with the anti-C2 and -C4 antibodies, suggesting that IR5 might be only partially exposed at the VlsE surface. Our conclusions on surface exposure, as determined by immunoprecipitation of Triton X-100-solubilized VlsE, stand on the reasonable premise that this detergent does not modify the conformation of VlsE.
Molecularly surface-exposed sequences of a surface protein may not be exposed at the bacterium's surface after the protein is inserted into the spirochetal outer membrane. To address this issue, two different experimental procedures were conducted: immunofluorescence and ADCK. The results of the immunofluorescence experiments indicate that none of the invariable regions are accessible to antibody at the surface of intact (unfixed) spirochetes. In contrast, acetone-fixed spirochetes readily bound anti-C2, -C4, and -C5 antibodies. Very weak fluorescence was at times observed with the anti-C3 antibody. Acetone fixation may modify the spirochetal membrane architecture so as to expose epitopes otherwise inaccessible to antibody and, moreover, modify the VlsE conformation as well. It is interesting that the anti-C5 antibody showed a more intense fluorescence than the anti-C4 antibody, a result opposite to that obtained by immunoblot and immunoprecipitation. It is possible that acetone treatment partially denatured VlsE and thus made IR5 more accessible to the anti-C5 antibody.
The results of the immunofluorescence experiments are entirely consistent with our interpretation of the ADCK assay, as absence of killing is most likely due to failure of the antibodies to bind to the spirochete's surface in vitro. Complement-mediated killing of B. burgdorferi is facilitated by antibody to surface antigens regardless of the complement-activating properties of the antibody. B. burgdorferi spirochetes are able to activate complement through an antibody-independent mechanism (9, 10). Taken together, our immunofluorescence and ADCK results indicate that all of the invariable regions that were investigated herein are cryptic on the spirochetal surface, at least as demonstrated in vitro. It is possible that the display of these regions may be different in vivo.
Our previous and present studies together reveal that none of the more conserved invariable regions of VlsE are accessible to antibody at the surface of the spirochete. We were unable to investigate the surface localization of IR1, which is less conserved (11), as immunization of both rabbits and mice with the corresponding synthetic peptide-KLH conjugate failed to generate an antibody response to this invariable region. A question raised by our studies is which portions of VlsE are exposed at the surface of the spirochete. The recent finding by Lawrenz and colleagues that immunization with recombinant VlsE provides protection against B. burgdorferi expressing the same but not a different VlsE variant suggests that at least some of the variable regions of VlsE are exposed at the surface of the spirochete (Lawrenz et al., Abstr. 99th Gen. Meet. Am. Soc. Microbiol.). It remains to be clarified whether the amino- and carboxyl-terminal invariable domains of VlsE are exposed at the surface of the bacterium. We are currently investigating this issue.
Acknowledgments
This work was supported by grants AI35027 and RR00164 from the National Institutes of Health and by a grant from SmithKline Beecham Biologicals.
REFERENCES
- 1.Barbet A F, McGuire T C. Crossreacting determinants in variant-specific surface antigens of African trypanosomes. Proc Natl Acad Sci USA. 1978;75:1989–1993. doi: 10.1073/pnas.75.4.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barony G, Merrifield R B. The peptides: analysis, synthesis, & Biology. New York, N.Y: Academic Press; 1980. pp. 3–285. [Google Scholar]
- 3.Barstad P A, Coligan J E, Raum M G, Barbour A G. Variable major proteins of Borrelia hermsii. Epitope mapping and partial sequence analysis of CNBr peptides. J Exp Med. 1985;161:1302–1314. doi: 10.1084/jem.161.6.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borst P. Molecular genetics of antigenic variation. Immunol Today. 1991;12:A29–A33. doi: 10.1016/S0167-5699(05)80009-X. [DOI] [PubMed] [Google Scholar]
- 5.Borst P, Cross G A M. Molecular basis for trypanosome antigenic variation. Cell. 1982;29:291–303. doi: 10.1016/0092-8674(82)90146-5. [DOI] [PubMed] [Google Scholar]
- 6.Cox D L, Akins D R, Bourrel K W, Lahdenne P, Norgard M W, Radolf J D. Limited surface exposure of Borrelia burgdorferi outer surface lipoproteins. Proc Natl Acad Sci USA. 1996;93:7973–7978. doi: 10.1073/pnas.93.15.7973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cross G A M. Crossreacting determinants in the C-terminal region of trypanosome variant surface antigens. Nature. 1979;277:310–312. doi: 10.1038/277310a0. [DOI] [PubMed] [Google Scholar]
- 8.Giambartolomei G, Dennis V A, Philipp M T. Borrelia burgdorferi stimulates the production of interleukin-10 in peripheral blood mononuclear cells from uninfected humans and rhesus monkeys. Infect Immun. 1998;66:2691–2697. doi: 10.1128/iai.66.6.2691-2697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kochi S K, Johnson R C, Dalmasso A P. Complement-mediated killing of the Lyme disease spirochete Borrelia burgdorferi: role of antibody in formation of an effective membrane attack complex. J Immunol. 1991;146:3964–3970. [PubMed] [Google Scholar]
- 10.Kochi S K, Johnson R C, Dalmasso A P. Facilitation of complement-dependent killing of the Lyme disease spirochete, Borrelia burgdorferi, by specific immunoglobulin G Fab antibody fragments. Infect Immun. 1993;61:2532–2536. doi: 10.1128/iai.61.6.2532-2536.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang F T, Alvarez A L, Gu Y, Nowling J M, Ramamoorthy R, Philipp M T. An immunodominant conserved region within the variable domain of VlsE, the variable surface antigen of Borrelia burgdorferi. J Immunol. 1999;163:5566–5573. [PubMed] [Google Scholar]
- 12.Liang F T, Steere A C, Marques A R, Johnson B J B, Miller J N, Philipp M T. Sensitive and specific serodiagnosis of Lyme disease by enzyme-linked immunosorbent assay with a peptide based on an immunodominant conserved region of Borrelia burgdorferi VlsE. J Clin Microbiol. 1999;37:3990–3996. doi: 10.1128/jcm.37.12.3990-3996.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meier J T, Simon M I, Barbour A G. Antigenic variation is associated with DNA rearrangements in a relapsing fever Borrelia. Cell. 1985;41:403–409. doi: 10.1016/s0092-8674(85)80013-1. [DOI] [PubMed] [Google Scholar]
- 14.Nocton J J, Dressler F, Rutledge B J, Rys P N, Persing D H, Steere A C. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N Engl J Med. 1994;330:229–234. doi: 10.1056/NEJM199401273300401. [DOI] [PubMed] [Google Scholar]
- 15.Nowling J M, Philipp M T. Killing of Borrelia burgdorferi by antibody elicited by OspA vaccine is inefficient in the absence of complement. Infect Immun. 1999;67:443–445. doi: 10.1128/iai.67.1.443-445.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Philipp M T, Aydintug M K, Bohm R P, Jr, Cogswell F B, Dennis V A, Lanners H N, Lowrie R C, Jr, Roberts E D, Conway M D, Karaçorlu M, Peyman G A, Gubler D J, Johnson B J B, Piesman J, Gu Y. Early and early disseminated phases of Lyme disease in the rhesus monkey: a model for infection in humans. Infect Immun. 1993;61:3047–3059. doi: 10.1128/iai.61.7.3047-3059.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Philipp M T, Lobet Y, Bohm R P, Jr, Roberts E D, Dennis V A, Gu Y, Lowrie R C, Jr, Desmons P, Duray P H, England J D, Hauser P, Piesman J, Xu K. The outer surface protein A (OspA) vaccine against Lyme disease: efficacy in the rhesus monkey. Vaccine. 1997;15:1872–1887. doi: 10.1016/s0264-410x(97)00133-3. [DOI] [PubMed] [Google Scholar]
- 18.Seiler K P, Weis J J. Immunity to Lyme disease: protection, pathology and persistence. Curr Opin Immunol. 1996;8:503–509. doi: 10.1016/s0952-7915(96)80038-0. [DOI] [PubMed] [Google Scholar]
- 19.Steere A C. Lyme disease. N Engl J Med. 1989;321:586–596. doi: 10.1056/NEJM198908313210906. [DOI] [PubMed] [Google Scholar]
- 20.Stoenner H G, Dodd T, Larsen C. Antigenic variation of Borrelia hermsii. J Exp Med. 1982;156:1297–1311. doi: 10.1084/jem.156.5.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van der Ploeg L H T, Gottesdiener K, Lee M G S. Antigenic variation in African trypanosomes. Trends Genet. 1992;8:452–457. doi: 10.1016/0168-9525(92)90330-7. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J R, Hardham J M, Barbour A G, Norris S J. Antigenic variation in Lyme disease Borreliae by promiscuous recombination of VMP-like sequence cassettes. Cell. 1997;89:275–285. doi: 10.1016/s0092-8674(00)80206-8. [DOI] [PubMed] [Google Scholar]