Abstract
Cells use transition metal ions as structural components of biomolecules and cofactors in enzymatic reactions, making transition metals vital cellular components. The buildup of a particular metal ion in certain stress conditions becomes harmful to the organism due to the misincorporation of the excess ion into biomolecules, resulting in perturbed enzymatic activity or metal-catalyzed formation of reactive oxygen species. Organisms optimize metal concentration by regulating the expression of proteins that import and export that metal, often in a metal concentration-dependent manner. One such regulation mechanism is via riboswitches, which are 5’-untranslated regions (UTR) of an mRNA that undergo conformational changes to promote or inhibit the expression of the downstream gene, commonly in response to a ligand. The yybP-ykoY family of bacterial riboswitches shares a conserved aptamer domain that binds manganese (Mn2+). In E. coli, the yybP-ykoY riboswitch precedes and regulates the expression of two genes: mntP, which based on extensive genetic evidence encodes an Mn2+ exporter, and alx, which encodes a putative metal ion transporter whose cognate ligand is currently in question. Expression of alx is upregulated by both elevated intracellular concentrations of Mn2+ and alkaline pH. With metal ion measurements and gene expression studies, we demonstrate that the alkalinization of media increases cytoplasmic Mn2+ content, which in turn enhances alx expression. Alx then exports excess Mn2+ to prevent toxic buildup of the metal inside the cell, with the export activity maximal at alkaline pH. Using mutational and complementation experiments, we pinpoint a set of acidic residues in the predicted transmembrane segments of Alx that play a crucial role in its Mn2+ export. We propose that Alx-mediated Mn2+ export provides a primary protective layer that fine-tunes the cytoplasmic Mn2+ levels, especially during alkaline stress.
Introduction
Transition metals are essential in all organisms as structural elements of proteins and RNA and as reactive centers in enzymes. Amongst these metals, Fe2+ acts as a cofactor in many cellular enzymes that are essential for life, e.g., those involved in respiratory pathways. During aerobic growth or in response to oxidizing agents such as hydrogen peroxide (H2O2), cells generate reactive oxygen species (ROS) that can oxidize Fe2+, thereby inactivating iron-dependent enzymes and leading to cytotoxic effects if not treated. To counter such ROS-caused negative consequences, Escherichia coli (E. coli) relies on isoenzymes that use Mn2+ instead of Fe2+ as a cofactor. Such enzymes protect the cell against ROS when the activity of their Fe2+-dependent isoenzymes is compromised (Hopkin et al., 1992). An example of such an Fe2+/Mn2+-dependent isozyme system in E. coli is superoxide dismutase (SOD), an enzyme that converts highly reactive superoxide radicals to molecular oxygen and H2O2: cytosolic Mn2+-dependent SodA takes over in aerobic conditions when the activity of Fe2+-dependent SodB is not sufficient to scavenge superoxide. SodC is another example of a non-iron-dependent SOD enzyme in E. coli that is expressed in the aerobic stationary phase and requires Cu2+Zn2+ as a cofactor to protect against ROS in the periplasmic space (Benov and Fridovich, 1994; Puget and Michelson, 1974; Strohmeier Gort et al., 1999).
To be ready for an impending ROS threat, E. coli maintains a constant cellular pool of Mn2+ (15–21 μM) through the uptake activity of its only known Mn2+ importer, MntH (Anjem et al., 2009; Kaur et al., 2017). MntH uses conserved acidic transmembrane residues to coordinate Mn2+ for import and relies on a proton gradient across the inner membrane of an E. coli cell as a driving force for Mn2+ uptake (Bozzi et al., 2019; Haemig and Brooker, 2004; Kehres et al., 2000; Makui et al., 2000). Notwithstanding its critical role within the cell, Mn2+ ion concentration must be limited as high concentrations of it are toxic to the cell. Excess Mn2+ replaces similarly sized Fe2+ as a cofactor in cellular enzymes and can alter levels of other metal ions (Kaur et al., 2017; Martin et al., 2015). To prevent the toxic buildup of Mn2+, the expression of mntH is repressed by elevated Mn2+ and an Mn2+-dependent transcriptional regulator MntR (Patzer and Hantke, 2001; Waters et al., 2011). As an additional layer of protection, excess Mn2+ is transported out of E. coli by its only exporter characterized to date, MntP (Martin et al., 2015; Waters et al., 2011). Similar to MntH, several conserved acidic residues within the membrane are implicated in the Mn2+ efflux activity of MntP (Zeinert et al., 2018). Like with mntH, the expression of mntP is regulated at the transcriptional and post-transcriptional levels by Mn2+ (Dambach et al., 2015).
One of the mechanisms by which mntP expression is tuned in response to the changing intracellular [Mn2+] is via the riboswitch in the 5’ untranslated region (UTR) of the mntP gene. Riboswitches are cis-acting elements in the UTRs of mRNAs, meaning that they alter transcriptional and/or translational outcomes for that mRNA. Riboswitches do so by shifting their structural ensembles upon binding to a ligand (Serganov and Nudler, 2013). For example, ligand binding might favor folding of the riboswitch RNA into a hairpin structure that terminates transcription to attenuate expression of the downstream gene (“transcriptional riboswitch”, e.g., an Mg2+-sensing M-box riboswitch that controls the expression of bacterial Mg2+ transporters mgtA and mgtE (Cromie et al., 2006; Dann et al., 2007). Alternatively, ligand binding can promote the formation of the mRNA with a single-stranded ribosome binding site (RBS), thus enhancing the translation of that mRNA (“translational riboswitch”). The mntP riboswitch was characterized as a translational riboswitch where the translation is turned on in response to increased intracellular [Mn2+] (Dambach et al., 2015). As a member of the ubiquitous riboswitch family (yybP-ykoY) (Breaker, 2022; Meyer et al., 2011), the mntP riboswitch regulates translation initiation on the mntP mRNA by binding Mn2+ and disfavoring formation of a stem-loop structure that sequesters the RBS of mntP mRNA. In vitro Kd measurements for binding to the mntP riboswitch vary from a low nM for aptamer-only (Bachas and Ferré-D’Amaré, 2018) to μM for a full-length riboswitch (Kalita et al., 2022). A second yybP-ykoY riboswitch in E. coli precedes a gene (alx) that, curiously, is highly induced in response to alkaline pH (Bingham et al., 1990). The expression of both mntP and alx increases in media with elevated [Mn2+] (Dambach et al., 2015). The alx encodes a putative Mn2+ transporter that belongs to the TerC superfamily of proteins (Anantharaman et al., 2012; Zeinert et al., 2018); however, the function of the Alx protein has not been definitively established.
A prior study indicated that overexpression of Alx results in an increase in the intracellular [Mn2+] and suggested that Alx may act as an Mn2+ importer (Zeinert et al., 2018). This proposal, however, is contradicted by the observations from earlier reports that expression of alx and mntP (Mn2+ exporter) are increased by elevated [Mn2+] in the media whereas expression of mntH (Mn2+ importer) is repressed. If Alx were indeed an Mn2+ importer, its expression in response to changing [Mn2+] would have paralleled that of MntH, not MntP. Here, we present evidence that Alx is an exporter of Mn2+ that serves as the first line of defense against the potential buildup of cytoplasmic Mn2+ at alkaline pH. By examining the effect of alkaline intracellular pH and elevated [Mn2+] on alx expression through transcriptional and translational reporters, we establish the link between these two environmental cues. Additionally, we demonstrate that Alx activity is stimulated by alkaline pH and posit involvement of transmembrane acidic residues of Alx in Mn2+ export. Our work expands the repertoire of known metal ion transporters with a transporter (Alx) that displays an alkaline pH-dependent transport activity, which may be paradigmatic of a special class of transporters responsive to multiple environmental signals.
Results
Both alkaline pH and increased intracellular Mn2+ concentration enhance alx expression
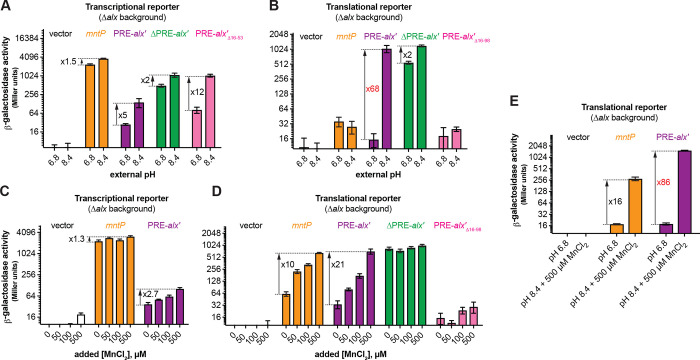
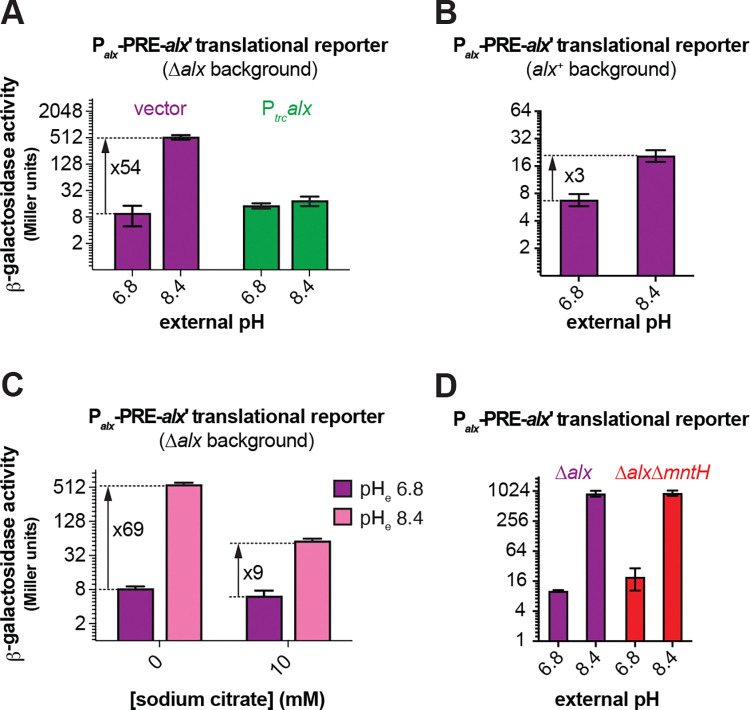
To study the connection between alkaline pH and Mn2+ homeostasis, we employed transcriptional and translational lacZ reporter fusions of alx and mntP cloned with their respective native promoters into single copy plasmids (Fig. 1A). Effects of extracellular alkaline pH and elevated [Mn2+] on gene expression were measured by β-galactosidase assays performed in E. coli strains that lack alx (referred to as Δalx). The Δalx strain was transformed with plasmids containing either alx or mntP transcriptional or translational reporters (Fig. 1A, Table 2). The strains containing reporter plasmids were cultivated in (i) neutral pH (LBK pH 6.8) or alkaline pH (LBK pH 8.4) media to test the effect of pH on alx transcriptional and translational reporters as described in prior work (Nechooshtan et al., 2009), and (ii) LB (pH 6.8) with supplemented MnCl2 to test the effect of Mn2+ on alx transcriptional and translational reporters. These experimental conditions were also tested on mntP transcriptional and translational reporters – a necessary control since the mntP riboswitch is only responsive to elevated [Mn2+]. We observed that alx transcription increased 5-fold at alkaline pH (Fig. 2A), whereas the mntP transcriptional reporter displayed a 1.5-fold induction in alkaline pH (Fig. 2A), consistent with an increase in the rate of nucleotide addition as pH increases (Mishanina et al., 2017; Stephen and Mishanina, 2022). The higher increase in the alx transcriptional reporter activity at alkaline pH compared to mntP can be explained by the proposed intrinsic terminator in hairpin D forming within the 5’UTR of alx in neutral but not alkaline pH (Nechooshtan et al., 2009; Stephen and Mishanina, 2022; Fig. S1). In contrast, the alx translational reporter produced a striking 68-fold higher signal in alkaline pH, whereas the mntP translational reporter was unaffected by alkaline pH (Fig. 2B). These results indicate that alx expression is largely regulated post-transcriptionally in alkaline pH, consistent with previous work (Nechooshtan et al., 2009).
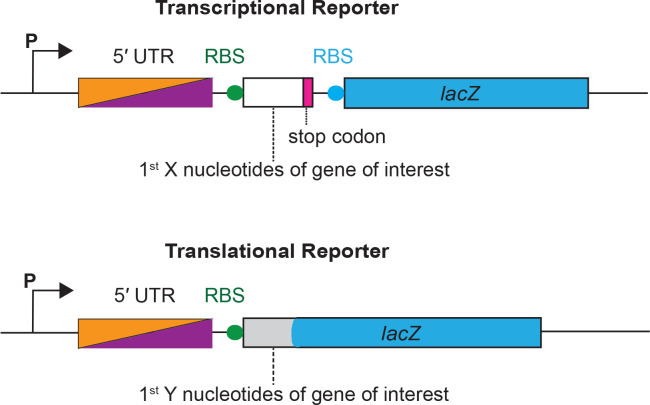
Figure 1.
A schematic illustration of transcriptional and translational reporters of alx and mntP. A transcriptional reporter contains a promoter (P) of aix or mntP, followed by the 5’ UTR (PRE In the case of alx), a ribosome binding site (RBS) of the gene of Interest Indicated by a filled circle in green, the first X nucleotides of the gene of interest (53 and 47 nt for alx and mntP, respectively), and a stop codon indicated by a rectangular box in magenta, followed by lacZ gene with its own RBS indicated by a rectangular box and filled circle respectively in blue. A translational reporter contains a promoter (P) of alx or mntP is followed by a 5’ UTR element (PRE in the case of alx), an RBS of the gene of interest indicated by a filled circle in blue, and first Y nucleotides of the gene of interest (99 and 47 nt for alx and mntP, respectively) fused to in frame with the 8th codon of lacZ gene.
Figure 2.
Regulation of alx expression by PRE in response to alkaline pH and elevated [Mn2+]. Shown in panels A and C are the β-galactosidase activities (Miller units) of mid-log phase grown cultures of Δalx::Kan derivatives of MC4100 strain of E. coli (RAS31) carrying one of the following plasmids: promoter-less vector with lacZ (pMU2385), transcriptional reporter of mntP (Pmntp-5’UTR-mntP’-lacZ, pRA48), transcriptional reporter of alx {Palx-PRE-alx’-locZ, pRA40), ΔPRE derivative of alx transcriptional reporter (P alx-alx’-lacZ, pRA41), or a transcriptional reporter of alx that lacks putative transcriptional pause sites In the vicinity of the first alx codon (alx1 Δ16–53, pRA49). Above cultures were cultivated in LBK media with pH 6.8 and pH 8.4 (panel A) and pH 6.8 LB with and without supplemented MnCP2(panel C). Similarly shown in panels B and D are the β-galactosidase activities of mid-log phase grown cultures of Δalx::Kan derivatives of MC4100 strain of E. coli (RAS31) carrying one of the following plasmids: promoter-less vector with lacZ (pMU2386), translational reporter of mntP [Pmntp-5’UTR-mntP’-lacZ, pRA57), translational reporter of alx (Palx-PRE-alx’-lacZ, pRA54), ΔPRE derivative of alx translational reporter (Palx-alx’-lacZ, pRA55), or translational reporter of alx that lacks putative transcriptional pause sites in the vicinity of the first alx codon (alx’ Δ16–98, pRA56). Above cultures were cultivated In LBK media with pH 6.8 and 8.4 (panel B) and pH 6.8 LB with and without supplemented MnCh (panel D). A combined effect of alkaline pH and supplemented MnCp on translational reporters is illustrated In panel E. The error shown is standard deviation of three repeats of the experiment.
The 5’ UTR of alx mRNA referred to as the pH-responsive RNA element (PRE) regulates alx translation in response to a pH change (Nechooshtan et al., 2009. We observed that the translational reporter of alx that lacks PRE (ΔPRE) exhibits only a 2-fold increase in alkaline pH vs 68-fold increase with PRE present (Fig. 2B). PRE contains two intrinsic transcription terminators (Fig. S1), and their absence is the dominant cause of higher transcription output in the ΔPRE transcriptional reporter. In addition to riboswitch regulation, bacterial translational output also appears to be tuned by the presence of transcriptional pause sites. Specifically, the analysis of cellular nascent elongating transcripts (NET-seq) isolated from immunoprecipitated RNA polymerases (RNAPs) revealed that transcriptional pause sites are generally enriched in the vicinity of the translation start sites in E. coli and B. subtilis (Larson et al 2014). We observed seven prominent transcriptional pauses on analysis of reported NET-seq data for E. coli (Larson et al., 2014) in the vicinity of the translation start site of alx (Fig. S2). Earlier studies that employed translational reporters of alx lacked the last but dominant transcriptional pause sequence. The translational reporter of alx used in this study includes all putative transcriptional pause sequences mentioned in Fig. S2. We observed a much higher fold-change in translational reporter activity (68-fold) in comparison to the translational reporter (7.8-fold) employed in previous report (Nechooshtan et al., 2009), stressing the importance of the translation start-site proximal RNAP pauses for efficient translation initiation. We noted a 12-fold increase for the transcriptional reporter that lacks nucleotides 16–53 of alx’ (alx’Δ16–53) containing putative transcriptional pause sequences (Fig. S2). Absence of PRE or putative transcriptional pause sequences in alx’ increased transcriptional output in both pH conditions (Fig. 2A), suggesting that these sequences are important to slow down alx transcription. In comparison, ΔPRE alx translational reporter displayed high β-galactosidase activity in both pH conditions, with a 2-fold increase in β-galactosidase activity in alkaline vs neutral pH (Fig. 2B), in good agreement with the corresponding 2-fold increase in ΔPRE alx transcription upon alkalinization (Fig. 2A). Taken together, these results suggest that PRE regulates both transcription and translation of alx in a pH-responsive manner. Surprisingly, there was very low reporter activity in case of translational reporter fusion of alx bearing alx’Δ16–98, suggesting that the putative transcriptional pauses proximal to the alx translation start site are critical for alx translation initiation.
Upon supplementation of 500 μM MnCl2 in the LB media (pH 7.2), the alx and mntP transcriptional reporter outputs increased only 2.7-fold and 1.4-fold, respectively (Fig. 2C). In stark contrast, both alx and mntP translational reporters were progressively induced by increasing [Mn2+] in the media, with a 21-fold and 10-fold increase in the reporter activity, respectively, at the highest MnCl2 concentration tested (500 μM) (Fig. 2D). These results suggest that expression of both alx and mntP is post-transcriptionally responsive to elevated extracellular [Mn2+]. The ΔPRE translational reporter of alx was unaffected by the supplemented MnCl2 and displayed high reporter activity throughout (Fig. 2D), likely due to the absence of premature transcription termination within PRE (Fig. S1). Collectively, these observations suggest that PRE tunes alx expression in an Mn2+ concentration-responsive manner. Similar to the pH response experiments above, the translational reporter fusion of alx bearing alx’Δ16–98 displayed very low β-galactosidase activity, reinforcing the notion that translation start-site proximal RNAP pauses are important for successful translation of alx.
We were intrigued to test the combined effect of the two environmental signals, alkaline pH and elevated [Mn2+], on alx and mntP translational reporter fusions. We found that alx and mntP translational fusions are induced 86-fold and 16-fold, respectively, in alkaline media supplemented with 500 μM MnCl2 (Fig. 2E). Altogether, our results (Fig. 2B, 2D, 2E) demonstrate that the effects of elevated extracellular [Mn2+] and alkaline pH on alx expression are additive in nature and may have independent routes to enhance alx expression. On the other hand, mntP expression is induced further if both alkaline pH and extra MnCl2 are provided compared to MnCl2 supplementation alone (Fig. 2E), even though alkaline pH had no significant impact on mntP expression (Figs. 2A and B). These observations indicate that alkaline pH enhances the effect of elevated [Mn2+] on mntP expression, consistent with previously published work (Kalita et al., 2022).
Alx does not participate in maintaining cellular pH or redox homeostasis
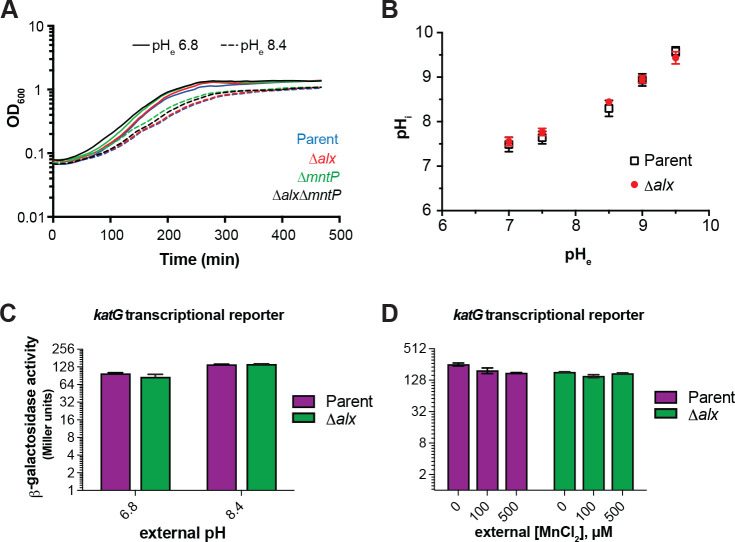
Our study confirms previously published work which demonstrated that the expression of alx is induced in media with alkaline pH and elevated [Mn2+] (Fig. 2, (Bingham et al., 1990; Dambach et al., 2015)). Perhaps the simplest explanation for the pH stress-induced production of Alx would be its direct involvement in bringing the high intracellular pH back into its neutral physiological range. To probe the contribution of Alx to pH homeostasis, we tested the growth of the parent strain (MC4100) and its Δalx derivative (RAS31) in LBK media at pH 6.8 or 8.4. The growth of the ΔmntP mutant and the Δalx ΔmntP double mutant was also tested under these same conditions. In general, the growth of strains slowed in the pH 8.4 media compared to pH 6.8 (Fig. 3A). Absence of Alx did not affect the growth rate in alkaline pH in comparison to its parent strain, suggesting that Alx does not provide a growth advantage in alkaline pH.
Figure 3.
Effect of increased external pH on growth, cytoplasmic pH, and cellular oxidative stress. (A) Growth of the parent strain (MC4100) and Its derivatives (Δalx::Kan derivative, RAS31; AmntP::Kan derivative, RAS32, and Δalx AmntP::Kan derivative, RAS42) in LBK media with pH 6.8 and pH 8.4. (B) Measurements of cytoplasmic pH in the parent strain (MC4100) and its Δalx::Kan derivative (RAS31) expressing pHluorin in M63A media with varying pH. β-galactosidase activity (Miller units) as a reporter of katG transcription was measured for mid-log phase grown cultures of the parent strain (AL441) and its Δalx::Kan derivative (RAS136) cultivated In LBK media with pH 6.8 and 8.4 (panel C) or pH 6.8 LB with and without supplemented MnCh (panel D). The error shown is standard deviation of three repeats of the experiment.
To further rule out the involvement of Alx in pH homeostasis, cytoplasmic pH of the parent strain and its Δalx derivative were measured in M63A media over a range of pH values (7, 7.5, 8.5, 9, and 9.5) using genetically encoded ratiometric pHluorin as a reporter (Martinez et al., 2012). Cytoplasmic pH of the parent strain increased as external pH was elevated (Fig. 3B). Cytoplasmic pH of the Δalx mutant did not display any significant difference compared to its parent strain when external pH was elevated (Fig. 3B). These results indicate that Alx participation in countering alkalization of cytoplasmic pH is unlikely.
Mn2+ protects cells from oxidative damage by serving as a cofactor in enzymes where it can replace oxidized iron in the catalytic center to rescue activity or as a cofactor in enzymes that prevent the buildup of ROS, such as SodA. Indeed, the expression of mntH (Mn2+ importer) increased in the presence of high extracellular or endogenously produced H2O2 (Anjem et al., 2009; Kehres et al., 2002, 2000). MntH becomes vital for growth in aerobic conditions of a strain (ΔkatG ΔkatE ΔahpCF) lacking catalase and peroxidases that would normally clear accumulating H2O2 (Anjem et al). Previously, it was noted that alx expression is sensitive to the presence of an oxidizing agent, paraquat (Pomposiello et al., 2001). Considering that alx expression is induced by alkaline pH, we investigated the connection between alkaline pH and oxidative stress. We assessed the oxidative stress in the parent strain and its Δalx mutant in alkaline pH and in the presence of elevated extracellular [Mn2+], using katG (bifunctional catalase-peroxidase) transcriptional reporter, which is induced in the oxidative environment (Li and Imlay, 2018). We measured the katG transcriptional reporter activity in LBK media with pH 6.8 or 8.4 (Fig. 3C). Low induction (1.5-fold) of the katG transcription reporter was observed in both parent and its Δalx derivative at pH 8.4. The Δalx mutant displayed similar katG transcriptional reporter activity to its parent strain at both pHs. These results indicate mild oxidative stress at alkaline pH. The katG transcriptional reporter activity was somewhat repressed in both parent strain and Δalx mutant in LB (pH 6.8) media upon supplementation of MnCl2 (Fig. 3D), consistent with the role of Mn2+ in alleviating oxidative stress. The difference in the induction of the katG transcriptional reporter activity in the parent vs its Δalx mutant in LB media lessened with increasing Mn2+ concentration in the media. The mild repression of the katG transcriptional reporter activity in the Δalx mutant compared to its parent strain will be elaborated on in the Discussion. Overall, these results suggest that Alx may not be directly participating in the maintenance of a redox state at alkaline pH or elevated extracellular [Mn2+].
Alx mediates the export of Mn2+ in alkaline environment
A previous study reported that mntP encodes an exporter of Mn2+ and its absence makes E. coli growth extremely sensitive to elevated [Mn2+] in the media (Waters et al., 2011). We observed that the growth of the ΔmntP mutant and the Δalx ΔmntP double mutant slowed in LB media (pH 6.8) supplemented with 500 μM MnCl2, as expected (Fig. S3). In contrast, the absence of Alx alone did not lead to any growth retardation of the strains in media with elevated [Mn2+] (Fig. S3), suggesting Alx does not contribute significantly to Mn2+ homeostasis. In testing the combined effect of pH 8.4 and 500 μM MnCl2 supplementation on E. coli growth, we observed that the growth of the ΔmntP mutant and the Δalx ΔmntP double mutant slowed compared to the parent strain, whereas its Δalx derivative’s growth rate was indistinguishable from the parent (Fig. S3).
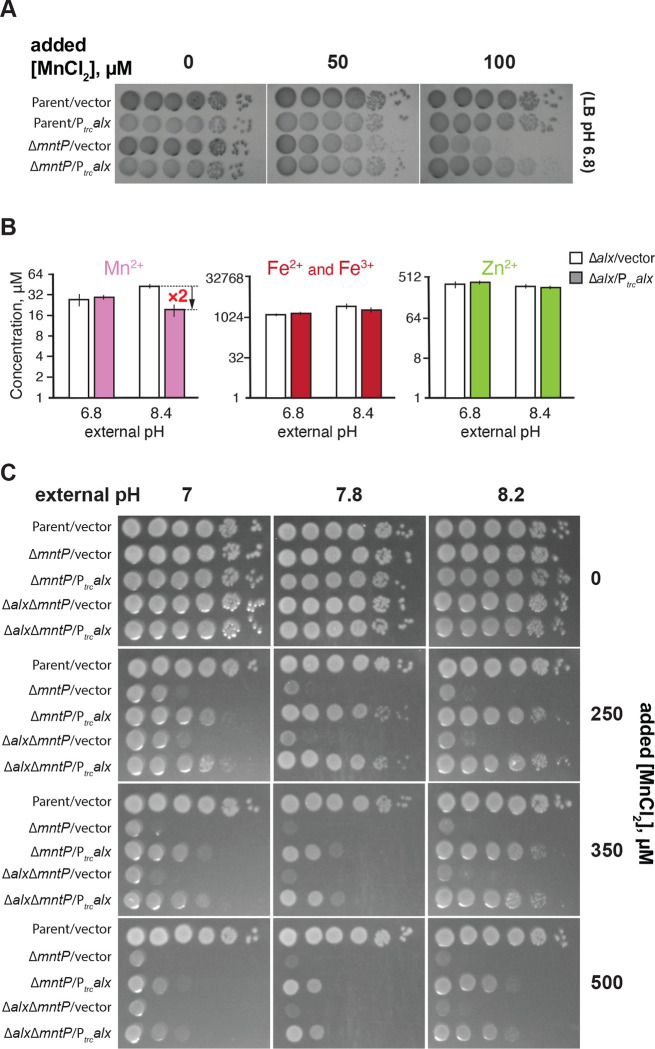
Expression of both alx and mntP is controlled by the riboswitches within their 5’ UTRs, which belong to the yybP-ykoY family of transition metal ion-binding riboswitches (Barrick et al., 2004, (Dambach et al., 2015). Like MntP, Alx is predicted to encode an inner membrane transition metal ion transporter (Daley et al). In light of these similarities and the observed upregulation of both alx and mntP by elevated [Mn2+] (Fig. 2D, (Dambach et al., 2015)), we were curious to test whether heterologous expression of alx would rescue the Mn2+ sensitivity phenotype of the ΔmntP mutant (RAS32 strain). We found that the expression of alx from a trc promoter (Ptrc) indeed partially rescued the growth of ΔmntP mutant in the presence of supplemental Mn2+ (Fig. 4A), whereas growth of the parent strain was not altered by the expression of alx from Ptrc. These results indicate that Alx may mediate the export of Mn2+ in circumstances when cytoplasmic Mn2+ levels are elevated. To test whether the Mn2+ content of the cells increases at alkaline pH when Alx is most expressed, intracellular concentrations of transition metals ions (Mn2+, total iron and Zn2+) were measured in the Δalx mutant (to preclude any transport of metal ions by the Alx prior to the measurement) under neutral and alkaline pH (Fig. 4B). Overall, our measured metal ion concentrations agree with previously published values (Anjem et al., 2009; Kaur et al., 2014). An increase of 1.5-fold in the total intracellular Mn2+ (from 28 to 42 μM) in the Δalx mutant was indeed noted at pH 8.4 when compared with pH 6.8 (Fig 4B). Importantly, we observed that although Ptrc-driven expression of Alx did not change total intracellular Mn2+ levels in the Δalx mutant at pH 6.8, it did reduce the total intracellular Mn2+ 2-fold at pH 8.4 (from 42 to 19 μM). These results suggest that the Alx function may be to prevent the buildup of intracellular Mn2+ specifically under alkaline pH. In the case of the total iron content (Fe2+ and Fe3+), we noted an increase of 2-fold in the total intracellular iron of the Δalx mutant at pH 8.4 vs 6.8. There was no significant change in total intracellular Zn2+ in the Δalx mutant at pH 8.4 vs. 6.8. The Ptrc-driven expression of Alx did not produce a significant change in total intracellular iron or Zn2+ in the Δalx mutant at either pH, suggesting that Alx likely has no role in the transport of iron or Zn2+ and is selective for Mn2+.
Figure 4.
Alx exports Mn2+ in alkaline pH. (A) Tenfold serial dilutions of overnight-grown cultures of parent strain (MC4100) and its ΔmntP::Kan derivative, each bearing an empty vector (pHYD5001) or the same vector expressing Alx from a Ptrc promoter were spotted on the surface of LB agar containing the appropriate concentration of ampicillin, MnCb and IPTG. (B) Intracellular Mn2+, Fe, and Zn2+ concentrations were measured in cultures of A mutant (RAS31) containing a vector (pHYD5001) or a derivative of pHYD5001 expressing Alx from a Ptrc promoter (pRA27), grown to mid-log in LBK media pHs 6.8 and 8.4 supplemented with 1 mM IPTG and ampicillin. The error shown is standard deviation of three repeats of the experiment. (C) Tenfold serial dilutions of overnight-grown cultures of parent strain (MC4100) and its derivatives (ΔmntP::Kan derivative, RAS32, and ΔalxΔmntP::Kan derivative, RAS42), each bearingan empty vector (pHYD5001) or the same vector expressing Alx from a Ptrc promoter were spotted on the surface of LB agar of varying pH and supplemented with appropriate concentration of ampicillin, MnCl2, and IPTG.
The above data indicated that Ptrc-driven expression of Alx decreases intracellular [Mn2+] in the Δalx mutant at pH 8.4, but not pH 6.8, suggesting that Mn2+ transport by Alx is pH-dependent (Fig. 3S). To test the possibility that the mechanism of Mn2+ export by Alx is proton dependent, we performed assays for detecting substrate-induced proton release in inside-out vesicles using published procedures (Dubey et al., 2021). Everted membrane vesicles were prepared for the strain that contains an in-frame deletion of both alx and mntP on the chromosome (RAS42). In place of the chromosomally encoded proteins, a human influenza hemagglutinin (HA)-tagged derivative of Alx or MntP (AlxHA or MntPHA) was expressed in the strain RAS42, from pRA50 or pRA70 plasmid, respectively. Successful expression of each tagged protein was confirmed by anti-HA Western blotting. A pH gradient across the vesicle membrane was generated via F0F1 ATPase activity by the addition of ATP to the vesicle suspension (Fig. S4). To monitor the generation of pH gradient, a pH gradient-sensitive, fluorescent dye 9-amino-6-chloro-2-methoxyacridine (ACMA) was employed. We noted an expected quenching of fluorescence upon the addition of ATP due to the generation of a proton gradient across the membrane suggesting vesicles were active. If Mn2+ transport by Alx or MntP is dependent on proton release, then a dequenching of ACMA fluorescence upon the addition of MnCl2 would be expected. However, we did not observe a significant change in the fluorescence intensity of ACMA upon the addition of MnCl2, suggesting that the transport of Mn2+ by Alx and MntP is unlikely to be accompanied by an H+ antiport.
We observed above that Alx expression from a trc promoter decreased the intracellular [Mn2+] in alkaline pH (Fig. 4B) without an obvious accompanying H+ transport (Fig. S4). Based on these findings, we speculated that alkaline pH may stimulate the Mn2+ export activity of Alx directly, perhaps by altering the protonation state of key Alx residues (see “A set of acidic residues in the transmembrane helices are crucial for Alx-mediated Mn2+ export” section of the Results below). To test this hypothesis, we probed the combined effect of elevated pH and extracellular [Mn2+] on the Mn2+ sensitivity phenotype of the ΔmntP mutant (Fig. 4C). We noted that the Mn2+ sensitivity of the ΔmntP mutant (RAS32 strain) was exacerbated by elevating the concentration of MnCl2 in the media or increasing the pH of the media. This is expected since increasing concentration of MnCl2 in the media correlates with an increase in the cytoplasmic [Mn2+] in the ΔmntP mutant (Martin et al., 2015; Waters et al., 2011), and alkalinization of the media likewise increases in the cytoplasmic Mn2+ (Fig. 4B), leading to Mn2+ toxicity. Surprisingly, we noted a pH-dependent boost in the ability of Ptrc-expressed Alx to rescue the growth of the ΔmntP mutant in media with elevated [Mn2+] (Fig. 4C). These results support the notion that the Mn2+ export activity of Alx is stimulated by alkaline pH. Alx appears to be a low-activity Mn2+ exporter because its rescue ability dropped off at particularly high concentrations of Mn2+ (see 350 and 500 μM MnCl2 panels), in contrast to MntP. The growth of the Δalx ΔmntP double mutant closely resembled that of the ΔmntP mutant at elevated media [Mn2+] and pH (Fig. 4C). Likewise, the rescue of the growth of the Δalx ΔmntP double mutant by overexpression of Alx was similar to that in the ΔmntP mutant (Fig. 4C). These observations suggest that chromosomally encoded Alx mitigates the mild perturbations of Mn2+ levels brought about by alkaline pH, and its Mn2+ export activity appears to be milder in comparison to the chromosomally encoded MntP.
alx expression is post-transcriptionally autoregulated by Mn2+ in alkaline pH
As described above, the alx translational reporter activity significantly increased at alkaline pH in the Δalx strain (RAS31, Fig. 2B). Intriguingly, this induction was reverted by 1) expressing Alx from a heterologous promoter in the Δalx strain (Fig. 5A) or 2) preserving the chromosomally encoded Alx (Fig. 5B). These results suggest that Alx represses its own expression post-transcriptionally in alkaline pH. Similarly, a 2-fold reduction in the induction of alx translational reporter fusion was observed in a strain expressing Alx chromosomally from the native promoter in LB (pH 6.8) supplemented with 500 μM MnCl2 (Fig. S5).
Figure 5.
The induction of alx expression in alkaline pH and its dependence on [Mn2+]. (A) β-galactosidase activity (Miller units) as a reporter of alx translation (Palx-PRE-alx’-locZ, pRA54) was measured in mid-log phase grown cultures of Δalx::Kan derivative (RAS31) bearing an empty vector (pHYD5001) or pHYD5001 expressing Alx from a Ptrc promoter (pRA27). The cultures were cultivated in LBK media with pHs 6.8 and 8.4, supplemented with appropriate concentration of ampicillin, MnCh and IPTG. (B) β-galactosidase activity (Miller units) as a reporter of alx translation (Palx-PRE-alx’-locZ, pRA54) was measured in mid-log phase grown cultures of alx+ strain (MC4100) in LBK media with pHs 6.8 and 8.4. (C) β-galactosidase activity (Miller units) as a reporter of alx translation (Palx-PRE-alx’-lacZ, pRA54) was measured in mid-log phase grown cultures of Δalx::Kan derivative (RAS31) in LBK media with pHs 6.8 and 8.4 supplemented with 10 mM sodium citrate. (D) β-galactosidase activity (Miller units) as a reporter of alx translation (Palx-PRE-alx’-lacZ, pRA54) was measured in mid-log phase grown cultures of Δalx mutant (RAS40) and its ΔmntH::Kan derivative (RAS93) in LBK media with pH 6.8 and 8.4. The error shown is standard deviation of three repeats of the experiment.
We noted a 1.5-fold increase in the total Mn2+ content in the Δalx strain cultivated in LBK media at pH 8.4 compared to pH 6.8, without any additional Mn2+ in the media (Fig. 4B), suggesting that trace Mn2+ in LBK was imported into the cells upon alkalinization. These observations offer a new hypothesis where expression of alx is induced by elevated intracellular Mn2+ brought about by alkaline pH, rather than by alkaline pH directly. To probe this hypothesis, we measured alx expression in the Δalx strain in LBK media at pH 6.8 or 8.4 supplemented with 10 mM sodium citrate (Fig. 5C). Citrate chelates divalent and trivalent metal ions in the media, precluding their import into the cells (Anjem et al., 2009; Tong and Rouault, 2007; Westergaard et al., 2017). We noted a significant drop from 69- to 9-fold in the induction of alx translation by alkaline pH. These results indicate that elevating intracellular [Mn2+] is one of the routes through which alkaline pH induces alx expression. The residual 9-fold increase in alx translation could be due to incomplete Mn2+ chelation by citrate (although 10 mM citrate should be sufficient to chelate 0.25 μM Mn2+ in commercial LB broth (Anjem et al., 2009)), an actual direct effect of alkaline pH, and/or effect of other metal ions on alx expression. Citrate chelates many divalent metal ions besides Mn2+, so this experiment did not completely rule out the involvement of other divalent metal ions in perturbing alx expression at alkaline pH. The potential contribution of divalent metal ions other than Mn2+ to alx expression will be the subject of future work.
One possible mechanism by which alkaline pH may increase the intracellular concentration of Mn2+, thereby increasing alx translational reporter activity, is by enhancing the activity of MntH, a known transporter for Mn2+ uptake in E. coli. To address this possibility, we measured the alx translational reporter activity in a strain that lacks both chromosomally encoded Alx and MntH (RAS93). The absence of MntH had no impact on the pH-induced increase in the activity of alx translation reporter, as the fold induction was the same in the Δalx ΔmntH double mutant as in the Δalx mutant (Fig. 5D). These observations indicated that the induction of alx translational reporter in alkaline pH is independent of Mn2+ uptake by MntH. Similarly, we measured the activity of alx translation in LB (pH 6.8) media supplemented with 500 μM MnCl2 (Fig. S5). We did not observe any change in the fold induction of the activity of alx translation reporter in the Δalx ΔmntH double mutant in comparison to that of the Δalx mutant. These data implicate the presence of an MntH-independent route for Mn2+ uptake.
A set of acidic residues in the transmembrane helices is crucial for Alx-mediated Mn2+ export
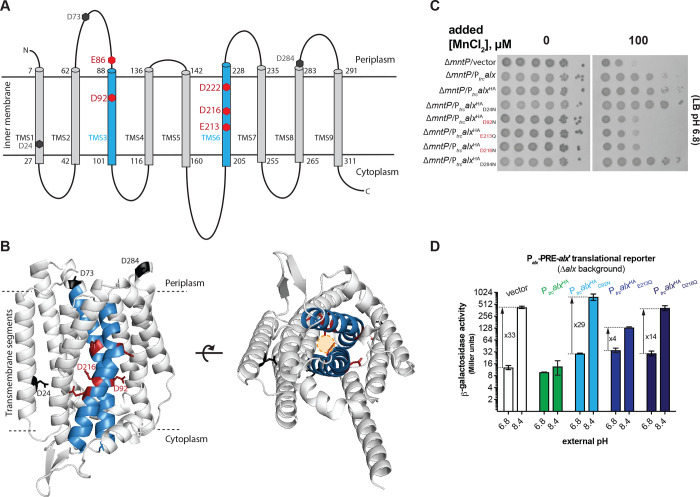
Currently, no experimental three-dimensional (3D) structural information for either Alx or MntP exists. To glean some insight into Alx architecture, its two-dimensional (2D) topology prediction was obtained with multiple web-based tools listed in Table S4. This prediction identified nine Alx transmembrane segments (TMS1–9, Fig. 6A and Table S4), with an overall N-out (periplasmic) and C-in (cytoplasmic) Alx topology. The predicted length of each TMS is similar (~20 amino acids), although there are some differences in the lengths of TMS3, TMS6 and TMS7 depending on the tool used and in the localization of amino acid residues (in the periplasmic or cytoplasmic environment) residing near the ends of each TMS. Regardless of the prediction tool used, we noted the presence of acidic residues in the transmembrane segments, which is unusual and may suggest the functional importance of these side chains, as demonstrated previously for the export of Mn2+ by MntP (Zeinert et al., 2018) (Fig. 6A and Table S4). Two of these residues (D92 and D222) were found to be conserved across members of the TerC family to which Alx belongs (Zeinert et al., 2018). To complement 2D prediction, we also examined an AlphaFold-predicted 3D structure of Alx. Similar to topology predicted arrangement, Alx displayed an N-out C-in conformation in the structure predicted by the AlphaFold server (Fig. 6B). The major difference was in the length of TMS3 in AlphaFold (69 to 103 amino acids) vs topology predicted structure (88 to 101 amino acids (Table S4)). Additional acidic residues localized to the TMS regions of Alx structure predicted by the AlphaFold, e.g., D73 and E86 in TMS3 and D222 in TMS6. The Alx structure predicted by the AlphaFold server was in complete agreement with the RoseTTAFold server prediction (data not shown).
Figure 6.
Structural model of Alx and functional relevance of its negatively charged residues in Mn2+ export. (A) The two-dimensional topological model of Alx structure predicted with DeepTMHMM algorithm and relative positions of negatively charged residues in transmembrane segments (TMS). (B) AlphaFold-predicted structure of Alx and relative positions negatively charged residues in TMS. A hypothetical path for the export of Mn2+ is displayed in the predicted structure. (C) Tenfold serial dilutions of overnight-grown cultures of ΔmntP::Kan mutant bearing one of the following plasmids were spotted on the surface of LB agar containing the appropriate concentration of ampicillin, MnCl2 and IPTG: a vector (pHYD5001), a derivative of pHYD5001 expressing Alx from a P trc promoter (pRA27), a derivative of pHYD5001 expressing AlxHA from a ptrc promoter (pRA50), a derivative of pRA50 expressing AlxHAD24N (pRA61), AlxHAD92N (pRA62), AlxHAE213Q(pRA63), AlxHAD216N (pRA64), and AlxHAD284N (pRA58). (D) β-galactosidase activity (Miller units) as a reporter of alx translation (Palx-PRE-alx’-lacZ, pRA54) was measured in mid-log phase grown cultures of Ao/x::Kan strain (RAS31) bearing vector (pHYD5001) and a derivative of pHYD5001 expressing AlxHA (pRA27), AIxHAD92N (pRA62), AlxHAE213Q (pRA63), AlxHAD216N (pRA64) from a Ptrc promoter. The cultures were grown in LBK media with pH 6.8 and 8.4, supplemented with appropriate concentration of ampicillin and IPTG. The error shown is standard deviation of three repeats of the experiment.
To test the role of the acidic residues predicted to be in the TMS of Alx, a strategy was employed where the effect of Ptrc-driven expression of an HA-tagged Alx bearing conservative (D to N or E to Q) replacements was tested on the growth of the ΔmntP mutant in LB media (pH 7.2) supplemented with MnCl2. With this strategy, expression of the wild-type HA-tagged Alx rescued the growth of the ΔmntP mutant like the tag-less version of Alx, suggesting that the HA tag did not alter the activity of Alx. The Ptrc-driven expression of Alx bearing E86Q, D92N, E213Q, D216N, or D222N replacement (denoted as AlxHAE86Q, AlxHAD92N, AlxHAE213Q, AlxHAD216N, or, AlxHAD222N respectively) did not rescue the growth of the ΔmntP mutant in media supplemented with MnCl2, whereas Alx bearing D24N, D73N or D284N substitution did so (Figs. 6C and S6). The expression of HA-tagged Alx mutants was unchanged in comparison to the wild-type Alx (Fig. S7), ruling out Alx expression defects as a cause for the failure to rescue the ΔmntP mutant. These results indicated that acidic residues E86, D92, E213, D216 and D222 are important for Alx-mediated Mn2+ export.
To experimentally determine compartment-specific positions of Alx acidic residues mentioned above, we followed the substituted cysteine accessibility method (SCAM) as described previously (Butler et al., 2013; Dubey et al., 2021). We substituted residues of interest with a cysteine (Cys, one at a time) and then probed the accessibility of this Cys toward methoxypolyethylene glycol maleimide (Mal-PEG). A DNA sequence encoding N-terminally HA-tagged Alx with an acidic residue-to-Cys mutation was cloned into a plasmid pHYD5001. A culture of the Δalx mutant expressing Cys AlxHA mutant was first separately treated with N-ethylmaleimide (NEM) or sodium (2-sulfonatoethyl)methanethiosulfonate (MTSES), to block solvent-exposed Cys residues and prevent their further reaction with Mal-PEG. NEM is membrane permeable and blocks both cytoplasmic and periplasmic Cys on proteins, whereas MTSES is impermeable to the inner membrane and therefore blocks only periplasmic Cys. A Cys that is not blocked by these reagents forms a covalent adduct with the maleimide moiety of Mal-PEG, producing an ~5 kDa shift in protein mobility on an SDS-PAGE gel. Thus, the reactivity of a particular Cys substitution toward Mal-PEG can infer the topological location of that substituted residue. After blocking the samples with NEM and MTSES, cells were washed, lysed, and then labelled with Mal-PEG. First, we validated the method by investigating the D284 position, which is expected to localize to the periplasmic face of the Alx protein at the end of TMS7 (Fig. 6A). As expected, the D284C mutation did not display reactivity toward Mal-PEG in the presence of MTSES (Fig. S9). Next, we investigated the compartment-specific positions of residues 92, 213, and 216, all of which are predicted to be buried in the inner membrane of the Alx protein (Figs. 6A and B). We observed that Mal-PEG shifted the mobility of Alx in the case of E213C substitution treated with NEM and MTSES (Fig. S9), indicating that E213 is buried in the membrane, as predicted by topology and AlphaFold models. In the case of D92C, Mal-PEG shifted the molecular weight of Alx in the presence of MTSES; however, the bulk of Alx did not display a shift in mobility in the presence of NEM (Fig. S9) suggesting that either D92 is localized to an inner membrane region with solvent accessibility or D92 is in very close proximity to the cytoplasm. Similarly, in the case of D216C, NEM partially blocked the cysteine residues. Thus, Mal-PEG did not shift the molecular weight of Alx completely in the presence of NEM (Fig. S9), suggesting that this residue has limited exposure to the cytoplasmic environment.
To expand upon the functional role of inner membrane acidic residues in the Alx protein, we performed a suite of alx translational reporter fusions. Our prior reporter assays demonstrated that Alx displays negative autoregulation, since a translational reporter of alx was not induced by alkaline pH or elevated [Mn2+] if Alx was expressed from Ptrc, presumably because of Alx-mediated Mn2+ export (Figs. 5A, 2D and S5). We thus took advantage of this behavior and employed Alx mutants defective in Mn2+ export to link the effects of alkaline pH and elevated Mn2+ on alx expression. Specifically, we tested the impact of Ptrc-driven expression of AlxHAD92N, AlxHAE213Q and AlxHAD216N on alx translational reporter activity in LBK pH 6.8 or 8.4 (Fig. 6D) and LB (pH 6.8) supplemented with 500 μM MnCl2 (Fig. S8). Expression of wild-type AlxHA from a plasmid repressed the activity of alx translational reporter at alkaline pH in the Δalx strain (RAS31) as expected. The reason behind the lower (33- vs 68-fold) pH-induced increase in alx translation in this experiment (strain co-transformed with the translational reporter and Alx expression vector) vs earlier experiment (Fig. 2B, strain transformed with translational reporter only) is unclear. Nevertheless, expression of either AlxHAD92N, AlxHAE213Q or AlxHAD216N did not repress the activity of alx translational reporter activity as wild-type AlxHA did. The fold induction of alx translational reporter activity varied (29, 4 and 14 for AlxHAD92N, AlxHAE213Q and AlxHAD216N, respectively), indicating that AlxHAE213Q and AlxHAD216N retain partial activity, whereas AlxHAD92N is inactive. We likewise observed the inability of AlxHAD92N, AlxHAE213Q and AlxHAD216N to repress Mn2+-induced increase in alx translational reporter activity (Fig. S6). However, because this fold increase (~2) is significantly lower than in alkaline pH, no conclusions about the extent of mutant Alx activity loss could be drawn. Overall, negative autoregulation of alx expression in response to alkaline pH is no longer observed when Alx mutants defective in Mn2+ transport are expressed; in other words, alx expression stays “on”. This suggests a connection between the induction of alx expression and Alx-mediated export of Mn2+ in alkaline pH, where the return of intracellular Mn2+ back to its “healthy” levels via Alx export shuts down further production of Alx at alkaline pH.
Alx is monomeric in vivo
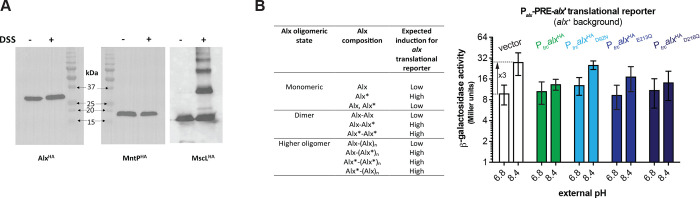
To examine the oligomeric state of Alx and MntP, we employed a non-cleavable membrane permeable crosslinker, disuccinimidyl suberate (DSS). Two N-hydroxysuccinimide (NHS) esters of DSS react with primary amines on proteins (lysines and the N-terminus) resulting in a protein mobility shift on an SDS-PAGE gel. The crude membrane fractions of the Δalx or ΔmntP strain expressing N-terminally HA-tagged Alx or MntP, respectively, from a plasmid were isolated and treated with DSS or DMSO as a solvent control (described in Materials and Methods). The samples were electrophoresed by SDS-PAGE and immunoblotted against the HA tag. We did not observe a mobility shift for either Alx or MntP (Fig. 7A), whereas a positive control of an HA-tagged MscL, a mechanosensitive channel, expressed in a ΔmscL strain displayed multiple oligomeric forms, consistent with earlier reports (Blount et al., 1996; Dubey et al., 2021; Pathania et al., 2016). These results indicated that Alx and MntP likely exist in a monomeric form in vivo.
Figure 7.
Evidence for a monomeric state of Alx and MntP in vivo. (A) Immunoblots probed with anti-HA antibody after electrophoretic mobility of samples containing crude membrane preparations. The crude membranes were harvested for strains RAS31, RAS32, and RAS130 expressing AlxHA, MntPHA, and MscLHA, respectively, and treated with a crosslinker DSS. (B) β-galactosidase activity (Miller units) as a reporter of alx translation (P alx-PRE-alx’-lacZ, pRA54) was measured in mid-log phase grown cultures of alx+ strain (MC4100) bearing a vector (pHYD5001) or a derivative of pHYDSOOl expressing AlxHA (pRA27), AlxHAD92N (pRA62), AIxHAE213Q (pRA63), or AlxHAD216N (pRA64) from a Ptrc promoter. The cultures were grown in LBK media with pH 6.8 and 8.4, supplemented with appropriate concentration of ampicillin and IPTG. The expected outcome of the experiment is summarized in the table. The error shown is standard deviation of three repeats of the experiment.
A limitation of the DSS crosslinking experiment above is that it relies on the spatial proximity of two primary amines to form the crosslink between protein monomers. An alternative experiment was thus performed to further support Alx’s existence as a monomer in vivo. Specifically, we used Alx mutants defective in Mn2+ transport as a tool. As described in an earlier section, expression of AlxHAD92N, AlxHAE213Q or AlxHAD216N from a synthetic promoter 1) did not rescue the growth of the ΔmntP mutant in LB media supplemented with 100 μM MnCl2 (Fig. 6C) and 2) increased the signal from alx translational reporter, in contrast to the decrease in the reporter output upon expression of the wild-type Alx from either a plasmid or the chromosome (Fig. 6D). If Alx is dimeric or multimeric in vivo, then co-expression of the active wild-type Alx from the chromosome and inactive Alx mutants from a plasmid would induce the activity of alx translational reporter. Otherwise, alx translational reporter activity will remain repressed if Alx is monomeric in vivo. In other words, the Ptrc-driven expression of an Alx mutant that is defective in Mn2+ transport will “poison” the activity of Alx expressed from its native promoter on the chromosome if complexes of these proteins are dimeric or form higher-order oligomers. The effect of Ptrc-driven expression of AlxHAD92N, AlxHAE213Q or AlxHAD216N was tested on alx translational reporter fusion in a wild-type strain (MC4100) that encodes Alx chromosomally. The Ptrc-driven expression of Alx and its mutated derivatives left the activity of alx translational reporter repressed in pH 8.4 media (Fig. 7B). These data are indicative of the monomeric Alx state in vivo, consistent with the DSS crosslinking results.
Discussion
In this work, we investigated in depth the effect of extracellular alkaline pH and elevated concentration of Mn2+ on alx expression and provided evidence for Alx export of Mn2+ upon alkalinization of the cytoplasm. Our results with alx transcriptional and translational reporters corroborate earlier findings that alx expression is regulated by both alkaline pH and elevated [Mn2+] (Bingham et al., 1990; Dambach et al., 2015; Nechooshtan et al., 2009; Stancik et al., 2002) in a riboswitch-dependent manner. Using a pH responsive GFP variant (pHluorin, (Martinez et al., 2012)), we confirmed that cytoplasm indeed alkalinizes when cells are grown in media with alkaline pH; therefore, our observed changes in gene expression and intracellular metal ion content are a consequence of alkaline cytoplasmic pH. The absence of Alx had no impact on cytoplasmic alkalinization with increasing media pH (Fig. 3B) and did not affect cellular growth in alkaline media (Fig. 3A), ruling out direct Alx involvement in pH homeostasis. Expression of Alx did, however, lower intracellular [Mn2+] (and not other tested transition metal ions), but only at alkaline pH (Fig. 4B), thus implicating Alx as a Mn2+ exporter in alkaline pH. With this newly uncovered function of Alx, our work points to a connection between the two environmental cues: alkaline pH and elevated [Mn2+]. A recent study demonstrated that cytosol alkalinizes in the presence of excess extracellular Mn2+ due to an increase in ammonia production within an E. coli cell (Kalita et al., 2022); here, we show that the reverse is also true: an alkaline environment promotes the import of Mn2+ into the cell.
Intracellular pH and Mn2+ content are linked
We find that alkalinization of the cytoplasm leads to an increase in the intracellular concentration of Mn2+. Specifically, our intracellular metal ion measurements show a 1.5-fold increase in [Mn2+] at pH 8.4 vs 6.8 in the Δalx strain, from 28 to 42 μM (Fig. 4B). Additional indirect pieces of data support this increase. First, the Mn2+ sensitivity of ΔmntP mutant is exacerbated at alkaline pH (Fig. 4C). Second, even though alkaline pH alone did not impact mntP translation, a combination of alkaline pH and extra Mn2+ in the media led to a greater mntP induction than Mn2+ alone (18 and 11-fold, respectively, Fig. 2E). Because upregulation of mntP translation is directly proportional to [Mn2+] (Fig. 2D), the additional increase is likely due to the additional Mn2+ imported from the medium into the cell at alkaline vs. neutral pH. The fact that alkaline pH alone had no effect on mntP translation suggests that there is a threshold intracellular [Mn2+] needed to begin producing additional MntP (>42 μM). Third, the introduction of a metal chelator (citrate) into the growth medium significantly reduced the alkaline pH-induced increase in alx translation, from 68- to 9-fold (Fig. 5C). Citrate is unlikely to be ingested by E. coli to affect the expression of alx (Ingolia and Koshland, 1979); therefore, the observed drop in alx induction is likely due to citrate chelating trace Mn2+ in the medium and preventing it from being imported. The mechanism of the alkaline pH-induced Mn2+ import is unclear at this point but does not involve the only characterized Mn2+ importer MntH in E. coli K12 (Figs. 5D and S5), implicating a potential alternative path for Mn2+ into the cell.
Why would a cell import Mn2+ upon cytosol alkalinization? Mn2+ acts as a redox center in the superoxide dismutase SodA and other mononuclear metal enzymes where it can replace Fe2+ as a cofactor to prevent protein damage in response to oxidative stress (Anjem et al., 2009; Hopkin et al., 1992; Whittaker et al., 2006). It may thus be an adaptive strategy that cells import Mn2+ in response to elevated ROS in alkaline pH. To test this hypothesis, our study employed the katG transcriptional reporter to measure the oxidative stress in the parent strain and its Δalx derivative. The readout was indicative of mild oxidative stress in alkaline pH (Fig. 3C). This mild oxidative stress in the parent strain was marginally repressed by supplementation of Mn2+ or by the absence of chromosomally encoded Alx (Fig. 3D). An increase in the activity of Mn2+-dependent SodA is likely behind the repression of mild oxidative stress by supplemented Mn2+ (Pugh et al., 1984), whereas accumulation of Mn2+ in the Δalx strain (due to lack of Alx-mediated Mn2+ export) is likely responsible for oxidative stress repression in the Δalx strain. These observations support our finding that Alx exports Mn2+ and is thereby indirectly related to redox homeostasis. Nevertheless, the physiological basis for mild induction of oxidative stress in alkaline pH and its relationship to alx expression is still unclear.
The heterologous expression of MntP (Mn2+ exporter) and Alx slowed the aerobic growth of a sensitized strain that lacks hydrogen peroxide degrading enzymes (AhpCF and KatG) (Zeinert et al., 2018). The effects of MntP overproduction on the growth of ΔahpCF ΔkatG double mutant were explained by reduced intracellular [Mn2+] and corresponding reduced protection from elevated ROS, believed to occur through reduced activity of Mn2+-dependent SodA and reduced protection of mononuclear enzymes where Fe2+ acts as a cofactor (Anjem et al., 2009). However, similar effects of Alx overproduction on the growth of the abovementioned strain were explained differently by (Zeinert et al., 2018) as Alx was viewed as an importer of Mn2+. Alx export of Mn2+ by analogy to MntP, on the other hand, better explains the observed slower growth of the ΔahpCF ΔkatG strain upon Alx overexpression because Ptrc-driven expression of Alx rescues the growth of the ΔmntP mutant in media supplemented with extra Mn2+ and reduces the intracellular Mn2+ levels in the alkaline pH.
The alx translational reporter displayed a 68-fold induction in alkaline pH media and a 22-fold induction in neutral pH media containing 500 μM MnCl2, with an 86-fold induction when two environmental cues (alkalinity and high [Mn2+]) were combined (Fig. 2). These results favor the notion that alkaline pH augments the effects of elevated [Mn2+] on alx expression. The alkaline pH-induced Mn2+ import also provides an alternate explanation to a recent report that elevated cytoplasmic [Mn2+] results in higher activation of mntP riboswitch upon alkalinization in media supplemented with excess Mn2+, in contrast to the proposed tighter interaction between Mn2+ and riboswitch element (Kalita et al., 2022) Differences in the fold induction by the two cues are reflective of a distinct mechanism that may operate for modulation of alx expression in alkaline pH that depends on elevated cytoplasmic [Mn2+]. One of the future directions for deconvoluting the mechanism of pH and Mn2+ control of alx expression will be to examine how pH and Mn2+ differentially affect alx mRNA folding, and specifically folding of its 5’ UTR riboswitch. The second direction will focus on the identification of novel pH-dependent transporters that address the involvement of other metal ions besides Mn2+ at alkaline pH to enhance alx expression.
Mn2+ export by Alx
A previous study proposed that Alx may function as an Mn2+ uptake protein based on the cellular [Mn2+] measurements in the presence of supplemented Mn2+ in the media (Zeinert et al., 2018). Contrary to earlier studies, here we provided multiple pieces of evidence for the Alx-mediated export of Mn2+ in alkaline pH or conditions where cytoplasmic Mn2+ levels are elevated. First, the inability of ΔmntP mutant to grow in the media with elevated [Mn2+] was partially rescued with Ptrc-driven expression of Alx (Fig. 4A). This rescue phenotype was missed in the previous work (Zeinert et al., 2018) likely because of the following differences between our and this prior experimental setup: (i) Alx was expressed from a weaker promoter (PBAD vs stronger Ptrc in our work) and (ii) rescue experiments were performed at neutral pH only. Strikingly, the rescue of ΔmntP mutant’s sensitivity towards Mn2+ by Ptrc-driven expression of Alx becomes more and more pronounced with increasing pH, while Mn2+ sensitivity of ΔmntP mutant becomes exacerbated with increasing pH (Fig. 4C). Secondly, the Ptrc-driven expression of Alx in the Δalx mutant resulted in ~2-fold reduction of intracellular [Mn2+] but only in alkaline pH, returning intracellular [Mn2+] from 42 to 19 μM (Fig. 4B). Therefore, alleviation of the growth of the ΔmntP mutant in media with alkaline pH and extra Mn2+ by Ptrc-driven expression of Alx can be explained by increased activity of Alx in alkaline pH. Overall, our observations corroborate that the Alx export of Mn2+ is stimulated by alkaline pH.
Mn2+ export activity of Alx at alkaline pH is also supported by the observed negative feedback regulation of alx expression. Specifically, the alkaline induction of alx translational reporter (68-fold) was repressed by the presence of Alx encoded chromosomally from a native promoter or expressed from Ptrc (Figs. 2B and 5AB). These results can be explained if Alx exports Mn2+ thereby reducing cytoplasmic [Mn2+] to the levels that no longer stimulate alx translation. In another observation, the expression of Alx chromosomally from a native promoter resulted in only a two-fold reduction in the activity of the alx translational reporter in the pH 6.8 media supplemented with 500 μM MnCl2 (Fig. S5). The mild reporter activity reduction, in this case, can be explained by the lack of alkaline pH-stimulated Mn2+ export activity of Alx.
The driving force and mechanism behind Alx’s export of Mn2+ remain a mystery to us. A proton gradient is unlikely to drive this transport because we do not observe a loss of pH gradient upon Mn2+ introduction to inverted membrane vesicles containing Alx (Fig. S4). This observation rules out the possibility that Alx is an Mn2+/H+ antiporter. In another system, a high concentration of potassium ion (K+) in the media was proposed to stimulate the activity of K+ export proteins and inhibit the activity of K+ uptake proteins (Li et al., 2002; Roe et al., 2000; Sharma et al., 2016). However, in the case of Alx, the stimulation of its activity by alkaline pH is unlikely through just an increase in cellular Mn2+ levels. This reasoning was supported by the observations that the rescue of Mn2+ sensitivity of ΔmntP mutant by Ptrc-driven expression of Alx is absent in the media with high [Mn2+] in comparison to intermediate [Mn2+] where Ptrc-driven expression of Alx rescues the growth of the ΔmntP mutant (Fig. 4A, 4C). The most likely explanation for the stimulation of Alx activity in alkaline pH could be pH-driven structural changes in the Alx protein that affect its Mn2+ export. We identified acidic residues of Alx (E86, D92, E213, D126, and D222) in TMS3 and TMS6 that are crucial for Mn2+ transport, by analogy to MntP and MntH (Fig. 6B, (Haemig and Brooker, 2004; Zeinert et al., 2018)). The interaction of positively charged solute (Mn2+) and acidic side chains of TMS3 and TMS6 of Alx may provide a path for Mn2+ transport as depicted in Fig. 6B. Some of the key side chains point away from each other in the Alphafold structure of Alx. Alkaline pH-induced conformational changes, however, may reorient TMS 3 and 6 of Alx to allow Mn2+ coordination. Such pH-induced conformational change was hypothesized to be the structural basis of alkaline pH-stimulated transport by an E. coli Na+/H+ antiporter, NhaA (Hunte et al., 2005; Taglicht et al., 1991). We speculate alkaline pH-induced conformational changes in the crossed TMS3 and TMS6 of Alx, similar to NhaA (Hunte et al., 2005), where acidic or neutral cytoplasmic pH favors a locked conformation limiting Mn2+ export into the periplasm and alkaline pH orients the two helices into an open conformation stimulating the export of Mn2+.
A Mn2+/Ca2+ exporter MgtA in Streptococcus pneumonia and a Mn2+ exporter YoaB in Lactobacillus lactis are putative P-type ATPases that use ATP hydrolysis to translocate their metal cargo against its concentration gradient. The expression of these exporters is regulated by the yybP-ykoY family of riboswitches (Martin et al., 2019; Price et al., 2015). It would be fascinating to find out whether Alx and MntP, whose expression is likewise regulated by riboswitches from the yybP-ykoY family, employ active transport to mitigate toxic levels of Mn2+ like classical P-type ATPases. However, the cytoplasmic domain of Alx in the AlphaFold structure is much smaller in comparison to P-type ATPases (Farley, 2011). Interestingly, the cytoplasmic loop of Alx contains several positively charged residues that may participate in the binding of ATP, perhaps in concert with a protein partner in the cell and help in the transfer of high-energy acyl phosphate to an aspartate to drive a conformational change needed for Mn2+ translocation across the membrane. In our experiments, we did not observe any growth phenotype in the Δalx mutant indicating that chromosomally encoded Alx does not provide a significant advantage in the tested laboratory conditions of additional Mn2+ in the media and/or alkaline pH. This observation also suggests that a pH-driven increase in intracellular [Mn2+] to 42 μM is not toxic to E. coli. Overexpression of Alx, however, did rescue growth of the ΔmntP mutant in media with elevated [Mn2+] and alkaline pH, suggesting Alx functions as a weak Mn2+ exporter in an alkaline environment, meaning that its rate of Mn2+ export is too low to be consequential to E. coli growth under tested conditions. Curiously, an earlier study reported that alx expression is induced in both neutral and alkaline pH under anaerobic growth conditions (Hayes et al). In anaerobic conditions, the absence of superoxide dismutase enzymes (sodA and sodB double mutant) does not cause a growth defect (Carlioz et al., 1986), implying that ROS stress is minimal. Thus, a cell no longer needs additional Mn2+ during growth in an anaerobic environment and preventing Mn2+ buildup due to its uptake becomes important. This explains why alx is expressed even at neutral pH in anaerobic conditions (Hayes et al., 2006). We speculate that the expression of alx may provide an advantage in environmental niches where E. coli and other enterobacteria are challenged by both alkaline pH and hypoxic conditions, such as the human gut (Litvak et al., 2018; Rogers et al., 2021). The elevation of cellular Mn2+ levels in alkaline conditions (Fig. 4B) favors the expression of alx to get rid of excess Mn2+. To cope with the threat of high [Mn2+] in the environment, the Mn2+ export activity of chromosomally encoded MntP is sufficient to protect the cell. On the other hand, when changes in intracellular [Mn2+] are mild, e.g., as brought about by alkaline pH, Alx fulfills the job of maintaining healthy levels of Mn2+ inside the cell. We thus pose that Alx-mediated Mn2+ export provides a primary protective layer that fine-tunes the cytoplasmic Mn2+ levels, especially during alkaline stress.
Materials
Manganese (II) chloride tetrahydrate and potassium benzoate were purchased from Alfa Aesar. Tris(hydroxymethyl)methyl-3-amino propane sulfonic acid (TAPS) was purchased from Acros Organics. Mal-PEG, N-(1,1-dimethyl-2-hydroxyethyl)-3-amino-2-hydroxy-propane sulfonic acid (AMPSO), nigericin sodium salt and valinomycin were purchased from Sigma-Aldrich. ACMA was purchased from Invitrogen. NEM and o-nitrophenyl-β-D-galactopyranoside were purchased from Thermo Scientific. Isopropyl-β-D-thiogalactopyranoside (IPTG) and 3-(N-morpholino) propane sulfonic acid (MOPS) were purchased from Fisher Scientific. MTSES was purchased from Biotium.
Methods
Tests of the Mn2+ sensitive phenotype and its rescue
Strains were inoculated in LB broth overnight at 37 °C in a shaker. The 5 μl of 10-fold serial dilutions of an overnight culture of an appropriate strain was spotted on LB agar supplemented with MnCl2 as described in the Results. Whenever required, LB broth or agar media were supplemented with an appropriate concentration of antibiotics and IPTG. LB agar plates were imaged after incubation at 37 °C for 14 to 16 hours.
β-galactosidase assays
Overnight cultures of the strains were inoculated in LBK broth with pH 6.8 and 8.4 or in LB broth with or without appropriate concentration of MnCl2 at 37 °C to a mid-log phase. The appropriate concentration of antibiotics (trimethoprim and/or ampicillin) and IPTG (1 mM) were supplemented when needed in the experiments. β-galactosidase assays were carried out by following the method of Miller, and β-galactosidase-specific activity was reported in Miller units (Miller JH, 1992). Each reported value with a standard deviation is the average of three independent experiments.
ICP-MS measurement of cellular metal ions
The total amounts of Mn2+, Fe, and Zn2+ were quantified from 5-ml cultures. Cells were grown overnight in LB broth and then inoculated in LBK pH 6.8, and LBK pH 8.4 media supplemented with 1 mM IPTG and appropriate concentration of ampicillin respectively. After growth to the mid-log phase at 37 °C, cells were harvested using centrifugation at 4000g for 10 minutes. Cell pellets were washed with 10 mM N-2-hydroxyethylpiperazine-N-2-ethane sulfonic acid (HEPES) pH 7.5, containing 2 mM EDTA and then washed twice with 10 mM HEPES as described in (Zeinert et al., 2018). Cell pellets were dried for 1 hour in a centrifuge evaporator. Dried cell pellets were solubilized in 400 μl of 30% (v/v) HNO3 and incubated at 95 °C for 10 min. Then samples were centrifuged at 20,000g for 5 minutes. Samples were prepared for inductively coupled plasma mass spectrometry (ICP-MS) by diluting 300 μl of supernatant of lysed cells into 2.7 ml of 2.5% (v/v) HNO3 and run on an iCAP RQ ICP-MS (Thermo Scientific). Metal concentrations are presented as intracellular levels after correction for mean cell volume determined from total protein content (Martin et al., 2015). The data obtained were presented from three repeats of the experiment.
Cytoplasmic pH measurements
The wild-type strains of Escherichia coli and its Δalx mutant containing expressing pHluorin (pRA46) were grown overnight in LBK medium buffered with 50 mM of MOPS with pH 7.5 and an appropriate concentration of ampicillin. Cells were inoculated and grown to mid-log phase in fresh LBK medium with pH 7.5 with an appropriate concentration of ampicillin and 1mM IPTG at 37 °C. Cells were harvested from appropriate volume of the cultures by spinning at 4000 rpm. Then cells were resuspended in 4 ml of M63A minimal medium [0.4 g/liter KH2PO4, 0.4 g/liter KH2PO4, 2 g/liter (NH4)2SO4, 7.45 g/liter KCl supplemented with 2 g/liter casein hydrolysate) and buffered to the desired pH with 50 mM concentration of the appropriate buffer pHs 7.0 and 7.5, MOPS; 8.5, TAPS; and pHs 9 and 9.5, AMPSO. Due to poor growth in extremely alkaline conditions, the initial A600 for cells growing in M63A media with external pH (pHe) 9 and 9.5 was chosen to be around 0.2, and in M63A medium with pHe 7, 7.5, and 8.5 was chosen to be around 0.05. The cultures were grown for 2 hours at 37 °C with mild shaking. To generate a standard curve, 95 μl volume of the culture of parent strain expressing pHluorin from each buffered media was withdrawn and mixed with potassium benzoate to a final concentration of 40 mM in 96-well plates. The cultures were incubated at room temperature for 3 min. Methanol amine was added to the culture at a final concentration of 20 mM. The cultures were incubated for 3 min at room temperature. The 100 μl of the parent strain and its Δalx mutant expressing pHluorin were withdrawn from each buffered media to 96 well plates and used for the internal pH (pHi) measurements. The measurements with fluorescence intensity at 530 nm were taken for the two excitation (410 and 470 nm) wavelengths for each strain expressing pHluorin as described in (Martinez et al., 2012). The ratio of fluorescence intensity of pHluorin at two excitation wavelengths against pH was plotted to generate a standard curve. The slope of the curve was used to calculate the pHi across different pHe.
In vivo cross-linking with disuccinimidyl suberate
To determine the oligomeric state of Alx and MntP, we performed in vivo cross-linking with disuccinimidyl suberate (DSS). The strains RAS31 (MC4100 Δalx::Kan), RAS32 (MC4100 ΔmntP::Kan), and RAS130 (MC4100 ΔmscL::Kan) bearing the plasmid pRA50, encoding AlxHA, pRA70 encoding MntPHA and the plasmid pRA72 encoding MscLHA (MscL bearing a C-terminal HA tag) respectively, were grown in 100 ml of LB with appropriate concentration of ampicillin. Cells were simultaneously induced with 1 mM IPTG for Ptrc-driven expression of AlxHA, MntPHA, and MscLCHA from the above-mentioned plasmids. Cells were grown to the mid-log phase and normalized for A600 of 50. Cells were harvested by centrifugation and pellets were washed in reaction buffer (30 mM sodium phosphate, pH 7.5 and 100 mM NaCl), and resuspended in 5 ml of the same buffer as described in (Dubey et al., 2021; Pathania et al., 2016). Cells were broken using a QSONICA sonicator, at 6/10 power 15 seconds on, 15 seconds off for 3 min on ice. The lysate was centrifuged at 12,000 rpm for 15 min to remove cell debris and unbroken cells. The supernatant was subjected to an ultracentrifugation step, at 48,000g for 90 min. The recovered crude membrane pellet was resuspended in 4 ml of reaction buffer. Two ml of the membrane suspension was transferred to two tubes. DSS was added into one tube at 1 mM of final concentration and the other received an equal volume of solvent (DMSO). Two tubes were kept on a shaking platform for 30 min at room temperature. The reaction was quenched with the addition of Tris-HCl pH 8 at 100 mM final concentration. The membrane suspension was pelleted by ultracentrifugation at 48,000g for 30 min and solubilized in 100 μl of SDS loading buffer. Samples were loaded and separated in 12% SDS-PAGE, and protein detection was performed by immunoblotting with an anti-HA antibody.
Supplementary Material
Acknowledgements
We would like to thank Drs. Abhijit Sardesai, James Imlay, and Robert Browne for graciously providing plasmids and strains. We thank the members of the Mishanina lab for critical reading and helpful suggestions for improving the manuscript, as well as Drs. Manuel Raffatellu and Daniel Roston. We are grateful to Dr. Neal Arakawa at the Environmental and Complex Analysis Laboratory (ECAL) at UCSD for assistance with ICP-MS measurements and to Dr. Terence Hwa for the pHluorin plasmid and use of the plate reader for intracellular pH and growth rate measurements. We would like to thank Iman Saeed for her assistance with cloning experiments. We also thank Drs. Mark Herzik and Galia Debelouchina for providing access to their lab instruments. This work was funded by the National Institutes of Health/National Institute of General Medical Sciences (ESI; grant no. R35 GM142785), UCSD institutional support, and Yinan Wang Memorial Chancellor’s Endowed Junior Faculty Fellowship to T. V. M.
References
- Anantharaman V, Iyer LM, Aravind L. 2012. Ter-dependent stress response systems: Novel pathways related to metal sensing, production of a nucleoside-like metabolite, and DNA-processing. Mol Biosyst 8:3142–3165. doi: 10.1039/c2mb25239b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anjem A, Varghese S, Imlay JA. 2009. Manganese import is a key element of the OxyR response to hydrogen peroxide in Escherichia coli. Mol Microbiol 72:844–858. doi: 10.1111/j.1365-2958.2009.06699.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachas ST, Ferré-D’Amaré AR. 2018. Convergent Use of Heptacoordination for Cation Selectivity by RNA and Protein Metalloregulators. Cell Chem Biol 25:962–973.e5. doi: 10.1016/j.chembiol.2018.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrick JE, Corbino KA, Winkler WC, Nahvi A, Mandal M, Collins J, Lee M, Roth A, Sudarsan N, Jona I, Kenneth Wickiser J, Breaker RR. 2004. New RNA motifs suggest an expanded scope for riboswitches in bacterial genetic control, PNAS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benov LT, Fridovich I. 1994. Escherichia coli expresses a copper- and zinc-containing superoxide dismutase. Journal of Biological Chemistry 269:25310–25314. doi: 10.1016/s0021-9258(18)47248-1 [DOI] [PubMed] [Google Scholar]
- Bingham RJ, Hall KS, Slonczewski JL. 1990. Alkaline Induction of a Novel Gene Locus, alx, in Escherichia coli Downloaded from, JOURNAL OF BACTERIOLOGY. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blount P, Sukharev SI, Moe PC, Schroeder MJ, Guy HR, Kung C. 1996. Membrane topology and multimeric structure of a mechanosensitive channel protein of Escherichia coli. EMBO Journal 15:4798–4805. doi: 10.1002/j.1460-2075.1996.tb00860.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozzi AT, Zimanyi CM, Nicoludis JM, Lee BK, Zhang CH, Gaudet R. 2019. Structures in multiple conformations reveal distinct transition metal and proton pathways in an Nramp transporter. doi: 10.7554/eLife.41124.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breaker RR. 2022. The Biochemical Landscape of Riboswitch Ligands. Biochemistry. doi: 10.1021/acs.biochem.1c00765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler EK, Davis RM, Bari V, Nicholson PA, Ruiz N. 2013. Structure-function analysis of MurJ reveals a solvent-exposed cavity containing residues essential for peptidoglycan biogenesis in Escherichia coli. J Bacteriol 195:4639–4649. doi: 10.1128/JB.00731-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlioz A, Touati D, Radman M. 1986. Isolation of superoxide dismutase mutants in Escherichia coli: is superoxide dismutase necessary for aerobic life?, The EMBO Journal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromie MJ, Shi Y, Latifi T, Groisman EA. 2006. An RNA Sensor for Intracellular Mg2+. Cell 125:71–84. doi: 10.1016/j.cell.2006.01.043 [DOI] [PubMed] [Google Scholar]
- Dambach M, Sandoval M, Updegrove BT, Anantharaman V, Aravind L, Waters SL, Storz G. 2015. The Ubiquitous yybP-ykoY riboswitch is a manganese-responsive regulatory element. Mol Cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dann CE, Wakeman CA, Sieling CL, Baker SC, Irnov I, Winkler WC. 2007. Structure and Mechanism of a Metal-Sensing Regulatory RNA. Cell 130:878–892. doi: 10.1016/j.cell.2007.06.051 [DOI] [PubMed] [Google Scholar]
- Dubey S, Majumder P, Penmatsa A, Sardesai AA. 2021. Topological analyses of the L-lysine exporter LysO reveal a critical role for a conserved pair of intramembrane solvent-exposed acidic residues. Journal of Biological Chemistry 279. doi: 10.1016/j.jbc.2021.101168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley RA. 2011. Active Ion Transport by ATP-Driven Ion PumpsCell Physiology Source Book: Essentials of Membrane Biophysics. Elsevier. pp. 167–177. doi: 10.1016/B978-0-12-387738-3.00012-3 [DOI] [Google Scholar]
- Haemig HAH, Brooker RJ. 2004. Importance of conserved acidic residues in MntH, the Nramp homolog of Escherichia coli. Journal of Membrane Biology 201:97–107. doi: 10.1007/s00232-004-0711-x [DOI] [PubMed] [Google Scholar]
- Hayes ET, Wilks JC, Sanfilippo P, Yohannes E, Tate DP, Jones BD, Radmacher MD, BonDurant SS, Slonczewski JL. 2006. Oxygen limitation modulates pH regulation of catabolism and hydrogenases, multidrug transporters, and envelope composition in Escherichia coli K-12. BMC Microbiol 6. doi: 10.1186/1471-2180-6-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkin$ KA, Papazian MA, Steinmane HM. 1992. THE JOURNAL OF BIOLOGICAL CHEMISTRY Functional Differences between Manganese and Iron Superoxide Dismutases in Escherichia coli K-lZ*, Nu. [PubMed] [Google Scholar]
- Hunte C, Screpanti E, Venturi M, Rimon A, Padan E, Michel H. 2005. Structure of a Na+/H+ antiporter and insights into mechanism of action and regulation by pH. Nature 435:1197–1202. doi: 10.1038/nature03692 [DOI] [PubMed] [Google Scholar]
- Ingolia TD, Koshland DE. 1979. Response to a Metal Ion-Citrate Complex in Bacterial Sensing, JOURNAL OF BACTERIOLOGY. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalita A, Mishra RK, Kumar V, Arora A, Dutta D. 2022. An Intrinsic Alkalization Circuit Turns on mntP Riboswitch under Manganese Stress in Escherichia coli. Microbiol Spectr 10. doi: 10.1128/spectrum.03368-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur G, Kumar V, Arora A, Tomar A, Ashish, Sur R, Dutta D. 2017. Affected energy metabolism under manganese stress governs cellular toxicity. Sci Rep 7. doi: 10.1038/s41598-017-12004-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur G, Sengupta S, Kumar V, Kumari A, Ghosh A, Parrack P, Dutta D. 2014. Novel MntR-Independent mechanism of manganese homeostasis in escherichia coli by the ribosome-associated protein HflX. J Bacteriol 196:2587–2597. doi: 10.1128/JB.01717-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehres DG, Janakiraman A, Slauch JM, Maguire ME. 2002. Regulation of Salmonella enterica serovar Typhimurium mntH transcription by H2O2, Fe2+, and Mn2+. J Bacteriol 184:3151–3158. doi: 10.1128/JB.184.12.3151-3158.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehres DG, Zaharik ML, Finlay BB, Maguire ME. 2000. The NRAMP proteins of Salmonella typhimurium and Escherichia coli are selective manganese transporters involved in the response to reactive oxygen. Mol Microbiol. doi: 10.1046/j.1365-2958.2000.01922.x [DOI] [PubMed] [Google Scholar]
- Larson MH, Mooney RA, Peters JM, Windgassen T, Nayak D, Gross CA, Block SM, Greenleaf WJ, Landick R, Weissman JS. 2014. A pause sequence enriched at translation start sites drives transcription dynamics in vivo. Science (1979) 344:1042–1047. doi: 10.1126/science.1251871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Imlay JA. 2018. Improved measurements of scant hydrogen peroxide enable experiments that define its threshold of toxicity for Escherichia coli. Free Radic Biol Med 120:217–227. doi: 10.1016/j.freeradbiomed.2018.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Moe PC, Chandrasekaran S, Booth IR, Blount P. 2002. Ionic regulation of MSCk, a mechanosensitive channel from Escherichia coli. EMBO Journal 21:5323–5330. doi: 10.1093/emboj/cdf537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvak Y, Byndloss MX, Bäumler AJ. 2018. Colonocyte metabolism shapes the gut microbiota. Science (1979) 362. doi: 10.1126/science.aat9076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makui H, Roig E, Cole ST, Helmann JD, Gros P, Cellier MFM. 2000. Identification of the Escherichia coli K-12 Nramp orthologue (MntH) as a selective divalent metal ion transporter. Mol Microbiol 35:1065–1078. doi: 10.1046/j.1365-2958.2000.01774.x [DOI] [PubMed] [Google Scholar]
- Martin JE, Le MT, Bhattarai N, Capdevila DA, Shen J, Winkler ME, Giedroc DP. 2019. A Mn-sensing riboswitch activates expression of a Mn2+/Ca2+ ATPase transporter in Streptococcus. Nucleic Acids Res 47:6885–6899. doi: 10.1093/nar/gkz494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JE, Waters LS, Storz G, Imlay JA. 2015. The Escherichia coli Small Protein MntS and Exporter MntP Optimize the Intracellular Concentration of Manganese. PLoS Genet 11. doi: 10.1371/journal.pgen.1004977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez KA, Kitko RD, Mershon JP, Adcox HE, Malek KA, Berkmen MB, Slonczewski JL. 2012. Cytoplasmic pH response to acid stress in individual cells of Escherichia coli and Bacillus subtilis observed by fluorescence ratio imaging microscopy. Appl Environ Microbiol 78:3706–3714. doi: 10.1128/AEM.00354-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer MM, Hammond MC, Salinas Y, Roth A, Sudarsan N, Breaker RR. 2011. Challenges of ligand identification for riboswitch candidates. RNA Biol 8:5–10. doi: 10.4161/rna.8.1.13865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JH. 1992. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory. [Google Scholar]
- Mishanina T V., Palo MZ, Nayak D, Mooney RA, Landick R. 2017. Trigger loop of RNA polymerase is a positional not acid-base, catalyst for both transcription and proofreading. Proc Natl Acad Sci U S A 114:E5103–E5112. doi: 10.1073/pnas.1702383114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nechooshtan G, Elgrably-Weiss M, Sheaffer A, Westhof E, Altuvia S. 2009. A pH-responsive riboregulator. Genes Dev 23:2650–2662. doi: 10.1101/gad.552209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathania A, Gupta AK, Dubey S, Gopal B, Sardesai AA. 2016. The topology of the L-arginine exporter ArgO conforms to an Nin-Cout configuration in Escherichia coli: Requirement for the cytoplasmic N-terminal domain, functional helical interactions, and an aspartate pair for ArgO function. J Bacteriol 198:3186–3199. doi: 10.1128/JB.00423-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzer SI, Hantke K. 2001. Dual repression by Fe2+-Fur and Mn2+-MntR of the mntH gene, encoding an NRAMP-like Mn2+ transporter in Escherichia coli. J Bacteriol 183:4806–4813. doi: 10.1128/JB.183.16.4806-4813.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomposiello PJ, Bennik MHJ, Demple B. 2001. Genome-wide transcriptional profiling of the Escherichia coli responses to superoxide stress and sodium salicylate. J Bacteriol 183:3890–3902. doi: 10.1128/JB.183.13.3890-3902.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price IR, Gaballa A, Ding F, Helmann JD, Ke A. 2015. Mn2+-Sensing Mechanisms of yybP-ykoY Orphan Riboswitches. Mol Cell 57:1110–1123. doi: 10.1016/j.molcel.2015.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puget BK, Michelson AM. 1974. BIOCHEMICAL AND BIOPHYSICAL RESEARCH COMMUNICATIONS ISOLATION OF A NEW COPPER-CONTAINING SUPEROXIDE DISMUTASE. [DOI] [PubMed]
- Pugh SYR, Diguiseppi JL, Fridovich I. 1984. Induction of Superoxide Dismutases in Escherichia coli by Manganese and Iron, JOURNAL OF BACTERIOLOGY. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe AJ, McLaggan D, O’Byrne CP, Booth IR. 2000. Rapid inactivation of the Escherichia coli Kdp K+ uptake system by high potassium concentrations. Mol Microbiol 35:1235–1243. doi: 10.1046/j.1365-2958.2000.01793.x [DOI] [PubMed] [Google Scholar]
- Rogers AWL, Tsolis RM, Bäumler AJ. 2021. Salmonella versus the Microbiome. Microbiology and Molecular Biology Reviews 85. doi: 10.1128/mmbr.00027-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serganov A, Nudler E. 2013. A decade of riboswitches. Cell. doi: 10.1016/j.cell.2012.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Shimada T, Mishra VK, Upreti S, Sardesai AA. 2016. Growth inhibition by external potassium of Escherichia coli lacking PtsN (EIIANtr) is caused by potassium limitation mediated by YcgO. J Bacteriol 198:1868–1882. doi: 10.1128/JB.01029-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stancik LM, Stancik DM, Schmidt B, Barnhart DM, Yoncheva YN, Slonczewski JL. 2002. pH-dependent expression of periplasmic proteins and amino acid catabolism in Escherichia coli. J Bacteriol 184:4246–4258. doi: 10.1128/JB.184.15.4246-4258.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen C, Mishanina T V. 2022. Alkaline pH has an unexpected effect on transcriptional pausing during synthesis of the Escherichia coli pH-responsive riboswitch. Journal of Biological Chemistry 298. doi: 10.1016/j.jbc.2022.102302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohmeier Gort A, Ferber DM, Imlay JA. 1999. The regulation and role of the periplasmic copper, zinc superoxide dismutase of Escherichia coli. Mol Microbiol 32:179–191. doi: 10.1046/j.1365-2958.1999.01343.x [DOI] [PubMed] [Google Scholar]
- Taglicht D, Padan E, Schuldiner S. 1991. Overproduction and purification of a functional Na+/H+ antiporter coded by nhaA (ant) from Escherichia coli. Journal of Biological Chemistry 266:11289–11294. doi: 10.1016/s0021-9258(18)99161-1 [DOI] [PubMed] [Google Scholar]
- Tong WH, Rouault TA. 2007. Metabolic regulation of citrate and iron by aconitases: Role of iron-sulfur cluster biogenesisBioMetals. pp. 549–564. doi: 10.1007/s10534-006-9047-6 [DOI] [PubMed] [Google Scholar]
- Waters LS, Sandoval M, Storz G. 2011. The Escherichia coli MntR miniregulon includes genes encoding a small protein and an efflux pump required for manganese homeostasis. J Bacteriol 193:5887–5897. doi: 10.1128/JB.05872-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westergaard N, Waagepetersen HS, Belhage B, Schousboe A. 2017. Citrate, a Ubiquitous Key Metabolite with Regulatory Function in the CNS. Neurochem Res 42:1583–1588. doi: 10.1007/s11064-016-2159-7 [DOI] [PubMed] [Google Scholar]
- Whittaker MM, Mizuno K, Bächinger HP, Whittaker JW. 2006. Kinetic analysis of the metal binding mechanism of Escherichia coli manganese superoxide dismutase. Biophys J 90:598–607. doi: 10.1529/biophysj.105.071308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeinert R, Martinez E, Schmitz J, Senn K, Usman B, Anantharaman V, Aravind L, Waters LS. 2018. Structure–function analysis of manganese exporter proteins across bacteria. Journal of Biological Chemistry 293:5715–5730. doi: 10.1074/jbc.M117.790717 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.