Abstract
The energy-dependent electron transfer pathway involved in the reduction of pyridine nucleotides which is required for CO2 fixation to occur in the acidophilic chemolithotrophic organism Thiobacillus ferrooxidans was investigated using ferrocytochrome c as the electron donor. The experimental results show that this uphill pathway involves a bc1 and an NADH-Q oxidoreductase complex functioning in reverse, using an electrochemical proton gradient generated by ATP hydrolysis. Based on these results, a model is presented to explain the balance of the reducing equivalent from ferrocytochrome c between the exergonic and endergonic electron transfer pathways.
Thiobacillus ferrooxidans is the main bacterium used in the industrial extraction of copper and uranium from ores with the microbial leaching technique (18). The energy required for its growth and cell maintenance is provided by the oxidation of reduced sulfur compounds or ferrous ions under acidic conditions, using O2 as the oxidant (23, 29, 31). When this bacterium is grown on Fe2+, little energy is available as a result of the oxidative reaction. Nevertheless, the bacterium fixes its own CO2, and Fe2+ oxidation must therefore be coupled to reduction of the NAD(P) required for this fixation and other anabolic processes; since the midpoint potential (Em) of the couple Fe2+-Fe3+ (770 mV) is much more positive than that of the NAD(P)-NAD(P)H couple (−305 mV at pH 6.5, the cytoplasmic pH of T. ferrooxidans [14]), the reduction of NAD(P) from ferrous ions requires energy. It has been suggested that an uphill electron transfer, established at the expense of the energy derived from the oxidation of Fe2+ by oxygen, may be involved in the reduction of NAD(P)+ from Fe2+ (20). The electron transfer chain from Fe2+ to O2 is now thought to involve a Fe2+ cytochrome c oxidoreductase (8, 19), rusticyanin (12, 13, 28), at least one cytochrome c (the 14-kDa soluble cytochrome c [30] and/or the c4 cytochrome [9], which shows a strong affinity for the membrane), and a cytochrome c oxidase (22). In addition, two membrane-bound c-type cytochromes with apparent molecular masses of 46 and 30 kDa have been isolated from T. ferrooxidans (15). Ingledew has put forward the idea that the uphill electron transfer from Fe2+ to NAD+ may involve a putative bc1 complex, according to the chemiosmotic mechanism, possibly via a Q-cycle mechanism operating in reverse (20). Members of our group recently provided evidence for the presence of a bc1 complex in T. ferrooxidans and characterized this complex in detail (16, 17). This was the first and only evidence so far for the existence of a bc1 complex in an acidophilic proteobacterium; a mechanism possibly accounting for the electron transfer in this complex has also been described (17).
One of the first reports on the existence of a reverse electron transfer concerned the electron transfer mechanism from succinate to NAD+ in animal mitochondria (10, 11). Since then, numerous cases of reverse electron transfer in chemoautotrophic bacteria have been reported (1–3, 24, 25). However, the reverse electron transfer between Fe2+ and NAD+ which is required to allow CO2 fixation via the Calvin cycle in chemoautotrophic bacteria has received little attention so far (1, 35).
In the present study, we present evidence for the existence of an uphill electron transfer through the bc1 and NADH-Q oxidoreductase complex (NDH-1) in T. ferrooxidans. Since the classical bc1 complexes exhibit thermodynamically favorable DBH2 cytochrome c reductase activity, the electron donor involved in the reverse reaction has to be a ferrocytochrome c. Here we examined the pathways taken by the electrons arising from exogenous cytochrome c in T. ferrooxidans. Based on the experimental results, a model describing the balance between the exergonic and endergonic electron transfer chains is presented.
T. ferrooxidans was kindly supplied by D. Morin (Bureau des Recherches Géologiques et Minières, Orléans, France). The strain was isolated from drainage water at the Salsigne sulfur mine (France). Large-scale growth of the organism was performed at pH 1.6 in the 9 K medium described by Silverman and Lundgren (33) supplemented with 1.6 mM CuSO4 · 5H2O, using a homemade polypropylene fermentor with a capacity of 400 liters. Cells were harvested according to the method of Bodo and Lundgren (7) and stored as pellets at −70°C. About 12 g (wet weight) of cells was obtained from 300 liters of cell culture.
Membrane fragments and spheroplasts were prepared as described in references 15 and 17, respectively. Protein concentrations were measured using Lowry's method (26). Oxygen uptake occurring in the presence of ferrocytochrome c (ascorbate [50 mM] + cytochrome c [100 μM]) alone or in the presence of uncouplers or potassium cyanide (1 mM KCN) was measured polarographically with a Clark electrode (Gilson oxygraph). The oxidation rate of ferrocytochrome c (50 μM) was monitored spectrophotometrically in a dual mode at 550 nm, using 540 nm as a reference, in 20 mM β-alanine–H2SO4 buffer (pH 3.5) in the presence or absence of KCN (1 mM). Inhibitors of the bc1 and NDH-1 complexes were added from an ethalonic solution at the final concentration indicated: antimycin A, 9 μM; myxothiazol, 35 μM; stigmatellin, 8 μM; 2.5-dibromo-3-methyl-5-isopropylbenzoquinone (DBMIB), 10 μM; 2-hydroxy-3-undecyl-1,4-naphthoquinone (UHDBT), 35 μM; funiculosin, 8 μM; rotenone, 30 μM; amobarbital (Amytal), 2 mM; and atabrine, 0.7 mM. Uncouplers were used at a final concentration of 15 μM (carbonyl cyanide m-chlorophenylhydrazone [CCCP] and 2,4-dinitrophenol [DNP]), oligomycin was used at a 9 μM concentration, and ATP was used at a 9 mM concentration. The ethanol concentration never exceeded 1%.
Absorbance changes and spectra were recorded with an Aminco DW 2A dual-wavelength spectrophotometer using 1-cm light path cuvettes. The heme content was determined from the reduced spectrum minus the oxidized-difference spectrum, using the following extinction coefficients for the α-peak: 18 mM−1 cm−1 (cytochrome c), 24 mM−1 cm−1 (cytochrome b), and 21 mM−1 cm−1 (cytochrome oxidase).
The electron transfer between exogenous ferrocytochrome c and oxygen.
The oxygen uptake induced by adding ferrocytochrome c (ascorbate in the presence of ferricytochrome c) to a spheroplast suspension of T. ferrooxidans was measured polarographically at acidic pH. This cytochrome c oxidase activity (1.8 nmol of O2/min/mg of protein) increased by 20% when the protonophore CCCP (or DNP) was added and was completely inhibited in the presence of 1 mM KCN. It was insensitive to the classical inhibitors of the bc1-type complexes (antimycin, funiculosin, 2-heptyl-4-hydroxyquinoline N-oxide (HQNO), diuron, stigmatellin, DBMIB, myxothiazol, and UHDBT) and to salicylhydroxamic acid (SHAM), a classical inhibitor of alternative oxidases (results not shown).
Existence of an electron transfer between exogenous ferrocytochrome c and NAD+.
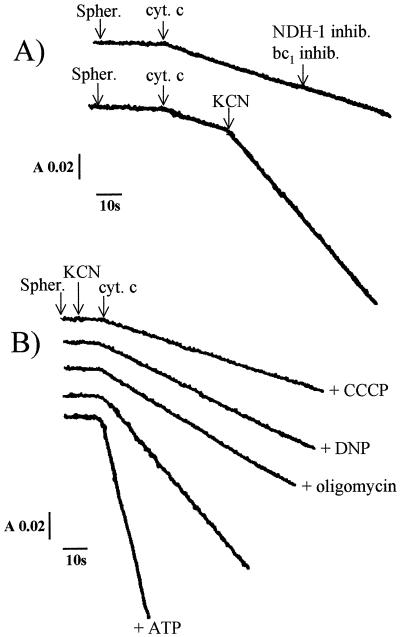
The oxidation of exogenous ferrocytochrome c was monitored spectrophotometrically in order to determine whether the electrons arising from this cytochrome might take a pathway other than that via cytochrome oxidase. When added to a spheroplast suspension at acidic pH, exogenous ferrocytochrome c was slowly oxidized (Fig. 1A); at the end of the reaction, when external ferrocytochrome c was completely oxidized and when anaerobiosis was reached, cytochromes c and cytochrome oxidase of the bacteria were reduced 80 and 95%, respectively, in comparison with dithionite-reduced cytochromes (results not shown). This oxidation process was weakly accelerated upon addition of CCCP and was insensitive to all the inhibitors of the bc1 and NDH-1-type complexes (Fig. 1A). Unexpectedly, adding KCN either before or after ferrocytochrome c considerably enhanced the rate of oxidation of this cytochrome (Fig. 1A); once all the external ferrocytochrome c became oxidized, the T. ferrooxidans cytochrome c552 was 30% reduced, whereas the cytochrome oxidase was completely reduced and exhibited an absorption maximum at 593 nm, indicating the formation of a cyanide-oxidase complex. This oxidation of ferrocytochrome c induced by KCN was found to be sensitive to the uncouplers CCCP and DNP and to oligomycin, leading to inhibition rates of 75, 65, and 55%, respectively; ATP accelerated this oxidative activity, and the resulting activity was inhibited by oligomycin (Fig. 1B). These results suggest that this ferrocytochrome c oxidative process is energy dependent and that it is driven by the proton motive force resulting from ATP hydrolysis.
FIG. 1.
(A) Oxidation of ferrocytochrome c (cyt. c) (10 μM) measured at 550-540 nm with T. ferrooxidans spheroplasts (spher.). Effects of the various bc1 (antimycin, HQNO, funiculosin, myxothiazol, and stigmatellin) and NDH-1 (rotenone, amobarbital, and atabrine) complex inhibitors (inhib.) and of KCN addition are shown. (B) Oxidation of ferrocytochrome c (10 μM) measured at 550-540 nm with T. ferrooxidans spheroplasts in the presence of KCN. Effects of protonophores (CCCP and DNP), oligomycin, and ATP are shown. The protein (4 mg/ml) was suspended in 20 mM β-alanine–H2SO4 buffer, pH 3.5. See the text for inhibitor, ATP, and uncoupler concentrations. A, absorbance; s, seconds.
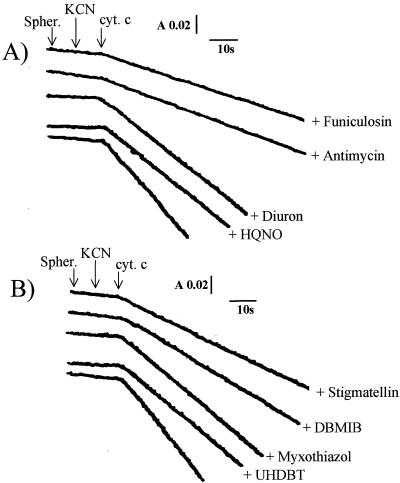
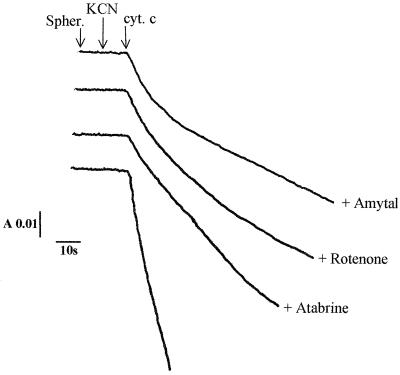
In other respects, ferrocytochrome c oxidation in the presence of KCN (and in either the absence or presence of ATP) was found to be sensitive to all the inhibitors of the bc1-type complexes: the inhibition was 70% with antimycin, 75% with funiculosin, 30% with diuron, and 30% with HQNO, the classical inhibitors of the Qn site of the bc1 complexes (Fig. 2A); with the inhibitors of the Qp site, the inhibitory effects amounted to 65% with stigmatellin, 55% with DBMIB, and 30% with myxothiazol and UHDBT (Fig. 2B). The Qn and Qp sites are quinone-binding sites in cytochrome bc-type complexes located close to the cytoplasmic (quinone reduction site) and periplasmic (quinol oxidation site) sides of the membrane, respectively. Antimycin, funiculosin, and stigmatellin are the most potent inhibitors of this electron transfer pathway. The residual activities measured in the presence of the various bc1 complex inhibitors were found to be SHAM insensitive. In addition, this ferrocytochrome c oxidative activity was inhibited by the classical inhibitors of the NDH-1-type complexes, amobarbital, rotenone, and atabrine, the inhibition effects of which were found to be 75, 85, and 65%, respectively (Fig. 3). These findings indicate the existence of an electron transfer pathway from ferrocytochrome c to NAD+ which proceeds through the bc1- and NDH-1-like complexes in T. ferrooxidans.
FIG. 2.
The uphill electron transfer chain in T. ferrooxidans. Effects of the various bc1 complex inhibitors on the oxidation of ferrocytochrome c (cyt. c) in spheroplasts (spher.), in the presence of KCN, are shown. (A) Qn site inhibitors; (B) Qp site inhibitors. Experimental conditions are as described in the legend to Fig. 1 and in the text. Protein concentration, 5 mg/ml. A, absorbance; s, seconds.
FIG. 3.
The uphill electron transfer chain in T. ferrooxidans. Effects of the various NDH-1 complex inhibitors on the oxidation of ferrocytochrome c (cyt. c) in spheroplasts (spher.), in the presence of KCN, are shown. Experimental conditions are as described in the legend to Fig. 1 and in the text. Protein concentration, 5 mg/ml. A, absorbance; s, seconds.
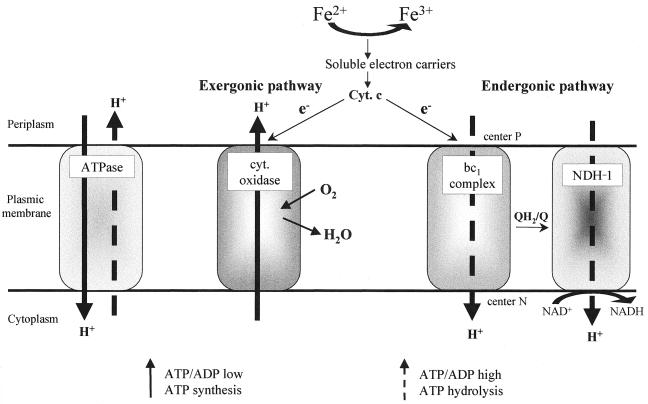
In the acidophilic chemolithotrophic bacterium T. ferrooxidans, the energy-dependent reversal of the electron transfer involved in the reduction of pyridine nucleotides is a physiologically important phenomenon, since the CO2 fixation is coupled to this process. In the present study, evidence was obtained that this process involves a bc1 complex and a NDH-1 complex functioning in reverse: the experimental results show that at acidic pH, electrons arising from ferrocytochrome c take two pathways, the first one being the classical energy-producing cytochrome c-cytochrome oxidase-oxygen pathway and the second one corresponding to a proton motive force-dependent pathway via the bc1 complex and a NDH-1-type complex (since amobarbital, rotenone, and atrabine caused a marked inhibition of this electron transfer process). Is this energy-dependent electron transfer process driven directly by the proton motive force established through cytochrome oxidase or indirectly via ATP? Under our experimental conditions, this uphill electron transfer pathway via the bc1 and NDH-1 complexes was found to occur in the presence of KCN. In mitochondria and in neutrophilic bacteria, adding inhibitors led to the collapse of the proton motive force. In the case of the acidophilic bacterium Thiobacillus acidophilus, Matin et al. (26a) have observed that at pH 3, adding KCN or azide, which inhibited the oxygen uptake by 95%, decreased the protein motive force, while the remaining force (35 mV) contributed to opposing the entry of H+ into the cell. On the other hand, it has been established that ATP synthesis is reversible and works in the direction of net ATP synthesis only when the proton motive force is continuously regenerated and when the cell needs and uses ATP. If the respiratory chain is inhibited and if there is enough endogenous or supplied ATP in the cell, the ATP synthase functions like an ATPase, generating a proton motive force comparable to that produced by the respiratory chain. Since the energy-requiring electron transfer pathway via the bc1 and NDH-1 complexes was detected under low-proton-motive-force conditions (in the presence of KCN) and was found to be inhibited by oligomycin and accelerated by ATP, it can be concluded that at least part of the proton motive force required for this energy-dependent process is produced by ATP hydrolysis. It is worth noting that uphill electron transfers in mitochondria (10, 11) and in chemoautotrophic bacteria (1–3, 24, 25, 35) have always been observed so far under strictly anaerobic conditions or in the presence of KCN, i.e., under low-proton-motive-force conditions. However, what may happen in vivo under physiological conditions? The controlled bifurcation of electrons from the exergonic electron transfer chain towards the endergonic uphill electron flow is of major importance, and the question arises as to how the balance of reducing equivalents between these two pathways is detected and controlled in vivo. The results of the present experiments show that in the absence of KCN, the oxidation of cytochrome c is insensitive to the bc1 and NDH-1 complex inhibitors (Fig. 1) and slightly increased by addition of uncouplers; this indicates that under the usual functional conditions for cytochrome oxidase (proton motive force = 256 mV, at pH 2, the optimal pH for growth [14]), none of the electrons arising from ferrocytochrome c took the reverse electron transfer pathway but only the cytochrome oxidase pathway; they favored the uphill pathway only in the presence of KCN, which establishes one of the appropriate initial conditions (low proton electrochemical gradient) for the ATP synthase to be able to function like an ATPase; in the presence of sufficient endogenous ATP, a drop in the proton motive force is therefore required to trigger ATP hydrolysis. Based on these data, we tentatively propose the following in vivo model (Fig. 4): electrons arising from Fe2+ and reducing oxygen via cytochrome oxidase establish a proton motive force which, when the ATP/ADP ratio is low, is used by ATP synthase to synthesize ATP (as in the classical respiratory chains). As long as ATP is used (in protein synthesis), this ratio is low and the ATP synthase synthesizes ATP. But when no carbon is available, ATP is no longer used and the ATP/ADP ratio increases. In mammalian mitochondria, the ATP/ADP ratio is known to regulate the cytochrome c oxidase activity via allosteric inhibition of the enzyme by ATP, which binds to the matrix domain of subunit IV (5, 6). Eucaryotic cytochrome c oxidases are composed of up to 13 different subunits, while cytochrome oxidase from the majority of prokaryotes contains only two or three subunits. However, a fourth subunit has been found to exist in the oxidase from Paracoccus denitrificans (21, 27), the thermophilic bacterium PS3 (34), and T. ferrooxidans strain ATCC 33020 (4). On the other hand, Shoji et al. (32) have established that the activity of the dimeric cytochrome c oxidase of Thiobacillus novellus (which consists of only two subunits per monomer) is regulated by ATP, which dissociates the dimeric form of the enzyme to the less active monomeric form. We hypothesize that ATP also regulates the activity of T. ferrooxidans cytochrome oxidase by decreasing it when the ATP/ADP ratio is high. When ATP accumulates, the proton motive force established via cytochrome oxidase therefore decreases, and ATP synthase functions like an ATPase, generating a proton motive force; this proton electrochemical gradient will then be used for the reverse electron transfer through the bc1 and NDH-1 complexes, leading to the formation of NAD(P)H required for CO2 fixation. The ATP/ADP ratio will then decrease, cytochrome oxidase will be activated, and ATP synthase again will synthesize ATP, as long as there is some carbon available. In this model, the ATP/ADP ratio controls the balance of the reducing equivalents from Fe2+ in favor of either cytochrome oxidase or the uphill electron transfer. This study opens up new prospects for understanding how the reducing equivalents are balanced between the two thermodynamically opposite electron pathways in T. ferrooxidans.
FIG. 4.
Model for the balance of reducing equivalents from ferrocytochrome c between the exergonic cytochrome oxidase and the endergonic bc1 and NDH-1 pathways. The Qp (center P) and Qn (center N) sites are defined in the text.
Acknowledgments
We thank René Toci (Fermentation Plant Unit, Laboratoire de Chimie Bactérienne, Marseille, France) for growing T. ferrooxidans and Jessica Blanc for revising the English manuscript.
REFERENCES
- 1.Aleem M I H, Lees H, Nicholas D J D. ATP dependent reduction of NAD+ by ferrocytochrome c in chemoautotrophic bacteria. Nature. 1963;200:759–761. doi: 10.1038/200759a0. [DOI] [PubMed] [Google Scholar]
- 2.Aleem M I H. Generation of reducing power in chemosynthesis. II. Energy linked reduction of pyridine nucleotides in chemoautotroph Nitrosomonas europaea. Biochim Biophys Acta. 1966;113:216–224. doi: 10.1016/s0926-6593(66)80062-0. [DOI] [PubMed] [Google Scholar]
- 3.Aleem M I H. Coupling of energy with electron transfer reactions in chemolithotrophic bacteria. Symp Soc Gen Microbiol. 1977;27:351–381. [Google Scholar]
- 4.Appia-Ayme C, Guiliani N, Ratouchniak J, Bonnefoy V. Characterization of an operon encoding two c-type cytochromes, an aa3-type cytochrome oxidase, and rusticyanin in Thiobacillus ferrooxidans ATCC 33020. Appl Environ Microbiol. 1999;65:4781–4787. doi: 10.1128/aem.65.11.4781-4787.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnold S, Kadenbach B. Cell respiration is controlled by ATP, an allosteric inhibitor of cytochrome-c oxidase. Eur J Biochem. 1997;249:350–354. doi: 10.1111/j.1432-1033.1997.t01-1-00350.x. [DOI] [PubMed] [Google Scholar]
- 6.Arnold S, Kadenbach B. The intramitochondrial ATP/ADP-ratio controls cytochrome c oxidase activity allosterically. FEBS Lett. 1999;443:105–108. doi: 10.1016/s0014-5793(98)01694-9. [DOI] [PubMed] [Google Scholar]
- 7.Bodo C, Lundgren D G. Iron oxidation by cell envelopes of Thiobacillus ferrooxidans. Can J Microbiol. 1974;20:1647–1652. doi: 10.1139/m74-256. [DOI] [PubMed] [Google Scholar]
- 8.Cavazza C, Guigliarelli B, Bertrand P, Bruschi M. Biochemical and EPR characterization of a high potential iron-sulfur protein in T. ferrooxidans. FEMS Microbiol Lett. 1995;130:193–200. [Google Scholar]
- 9.Cavazza C, Giudici-Orticoni M T, Nitschke W, Appia C, Bonnefoy V, Bruschi M. Characterization of a soluble cytochrome c4 isolated from T. ferrooxidans. Eur J Biochem. 1996;242:308–314. doi: 10.1111/j.1432-1033.1996.0308r.x. [DOI] [PubMed] [Google Scholar]
- 10.Chance B, Hollunger G. The interaction of energy and electron transfer reactions in mitochondria. I. General properties and nature of the products of succinate-linked reduction of pyridine nucleotide. J Biol Chem. 1961;236:1534–1543. [PubMed] [Google Scholar]
- 11.Chance B. The interaction of energy and electron transfer reactions in mitochondria. II. General properties of adenosine triphosphate-linked oxidation of cytochrome and reduction of pyridine nucleotide. J Biol Chem. 1961;236:1544–1554. [PubMed] [Google Scholar]
- 12.Cobley J G, Haddock B A. The respiratory chain of Thiobacillus ferrooxidans: the reduction of cytochromes by Fe2+ and the preliminary characterization of rusticyanin, a novel 'blue' copper protein. FEBS Lett. 1975;60:29–33. doi: 10.1016/0014-5793(75)80411-x. [DOI] [PubMed] [Google Scholar]
- 13.Cox J C, Boxer D H. The purification and some properties of rusticyanin, a blue copper protein involved in iron(II) oxidation from Thiobacillus ferrooxidans. Biochem J. 1978;174:497–502. doi: 10.1042/bj1740497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cox J C, Nicholls D G, Ingledew W J. Transmembrane electrical potential and transmembrane pH gradient in the acidophile Thiobacillus ferrooxidans. Biochem J. 1979;178:195–200. doi: 10.1042/bj1780195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elbehti A, Lemesle-Meunier D. Identification of membrane-bound c-type cytochromes in an acidophilic ferrous ion oxidizing bacterium Thiobacillus ferrooxidans. FEMS Microbiol Lett. 1996;136:51–56. doi: 10.1111/j.1574-6968.1996.tb08024.x. [DOI] [PubMed] [Google Scholar]
- 16.Elbehti A, Asso M, Guigliarielli B, Lemesle-Meunier D. International Biohydrometallurgy Symposium. Biomine, The Australian Mineral Foundation; 1997. Characterization of the three membrane bound metallo enzymes required to postulate the existence of a bc type complex in T. ferrooxidans; pp. 1–10. [Google Scholar]
- 17.Elbehti A, Nitschke W, Tron P, Michel C, Lemesle-Meunier D. Redox components of cytochrome bc-type enzymes in acidophilic prokaryotes. I. Characterisation of the cytochrome bc1-type complex of the acidophilic ferrous ion-oxidizing bacterium T. ferrooxidans. J Biol Chem. 1999;274:16766–16772. doi: 10.1074/jbc.274.24.16760. [DOI] [PubMed] [Google Scholar]
- 18.Ewart D K, Hugues N H. The extraction of metals from ores using bacteria. Adv Inorg Chem. 1991;36:103–135. [Google Scholar]
- 19.Fukumori Y, Yano T, Sato A, Yamanaka T. Fe(II)-oxidizing enzyme purified from Thiobacillus ferrooxidans. FEMS Microbiol Lett. 1988;50:169–172. [Google Scholar]
- 20.Ingledew W J. T. ferrooxidans, the bioenergetics of an acidophilic chemolithotrophic bacteria. Biochim Biophys Acta. 1982;683:89–117. doi: 10.1016/0304-4173(82)90007-6. [DOI] [PubMed] [Google Scholar]
- 21.Iwata S, Ostermeier C, Ludwig B, Michel H. Structure at 2.8 Å resolution of cytochrome c oxidase from Paracoccus denitrificans. Nature. 1995;376:660–669. doi: 10.1038/376660a0. [DOI] [PubMed] [Google Scholar]
- 22.Kai M, Yano T, Tamagai H, Fukumori Y, Yamanaka T. Thiobacillus ferrooxidans cytochrome c oxidase: purification, molecular and enzymatic features. J Biochem. 1992;112:816–821. doi: 10.1093/oxfordjournals.jbchem.a123982. [DOI] [PubMed] [Google Scholar]
- 23.Kelly D P. Autotrophy: concepts of lithotrophic bacteria and their organic metabolism. Annu Rev Microbiol. 1971;25:177–210. doi: 10.1146/annurev.mi.25.100171.001141. [DOI] [PubMed] [Google Scholar]
- 24.Kiesow L. On the assimilation of energy from inorganic sources in autotrophic forms of life. Proc Natl Acad Sci USA. 1964;52:980–988. doi: 10.1073/pnas.52.4.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiesow L. Energy-linked reactions in chemoautotrophic organisms. Curr Top Bioenerg. 1967;2:195–233. [Google Scholar]
- 26.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 26a.Matin A, Wilson B, Zychlinsky E, Matin M. Proton motive force and the physiological basis of delta pH maintenance in Thiobacillus acidophilus. J Bacteriol. 1982;150:582–591. doi: 10.1128/jb.150.2.582-591.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicoletti F, Witt H, Ludwig B, Brunori M, Malatesta F. Paracoccus denitrificans cytochrome c oxidase: a kinetic study on the two- and four-subunit complexes. Biochim Biophys Acta. 1998;1365:393–403. doi: 10.1016/s0005-2728(98)00092-9. [DOI] [PubMed] [Google Scholar]
- 28.Nunzi F, Woudstra M, Campese D, Bonicel J, Morin D, Bruschi M. Amino-acid sequence of rusticyanin from Thiobacillus ferrooxidans and its comparison with other blue copper proteins. Biochim Biophys Acta. 1993;1162:28–34. doi: 10.1016/0167-4838(93)90123-9. [DOI] [PubMed] [Google Scholar]
- 29.Peck H D. Energy-coupling mechanisms in chemolithotrophic bacteria. Annu Rev Biochem. 1968;22:489–518. doi: 10.1146/annurev.mi.22.100168.002421. [DOI] [PubMed] [Google Scholar]
- 30.Sato A, Fukumori Y, Yano T, Kai M, Yamanaka T. T. ferrooxidans cytochrome c-552: purification and some of its molecular features. Biochim Biophys Acta. 1989;976:129–134. doi: 10.1016/s0005-2728(89)80221-x. [DOI] [PubMed] [Google Scholar]
- 31.Shafia F, Wilkinson R F. Growth of Ferrobacillus ferrooxidans on organic matter. J Bacteriol. 1969;97:256–260. doi: 10.1128/jb.97.1.256-260.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shoji K, Tanigawa M, Hori K, Tomozawa Y, Yamanaka T. The effects of several nucleotides on the molecular state and catalytic activity of Thiobacillus novellus cytochrome c oxidase. ATP affects the oxidase uniquely. Eur J Biochem. 1999;264:960–964. doi: 10.1046/j.1432-1327.1999.00703.x. [DOI] [PubMed] [Google Scholar]
- 33.Silverman M P, Lundgren D G. Studies on the chemoautotrophic iron bacterium Ferrobacillus ferrooxidans. I. An improved medium and a harvesting procedure for securing high cell yields. J Bacteriol. 1959;77:642–647. doi: 10.1128/jb.77.5.642-647.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sone N, Shimada S, Ohmori T, Souma Y, Gonda M, Ishizuka M. A fourth subunit is present in cytochrome c oxidase from the thermophilic bacterium PS3. FEBS Lett. 1990;262:249–252. doi: 10.1016/0014-5793(90)80202-t. [DOI] [PubMed] [Google Scholar]
- 35.Tikhonova G V, Lisenkova L L, Doman N G, Skulachev V P. Electron transport pathways in T. ferrooxidans. Biokhimiya. 1967;32:725–733. [PubMed] [Google Scholar]