Abstract
Background
Glyphosate, a herbicide marketed under the trade name Roundup, is now widely used, in part because genetically modified organism plants that are resistant to this agent have been developed. Environmental or dietary exposure to glyphosate is omnipresent and there are concerns this exposure could impair cognitive function in addition to carcinogenicity.
Methods
Using hippocampal slices from juvenile male rats, we investigated whether glyphosate alters synaptic transmission and induction of long-term potentiation (LTP), a cellular model of learning and memory. Our hypothesis is that glyphosate alters neuronal function and impairs LTP induction via activation of pro-inflammatory processes, because increases in pro-inflammatory cytokines and neuroinflammation have been reported following glyphosate exposure. LTP was induced by delivery of 100 Hz x 1 sec high frequency stimulation (HFS) of the Schaffer collateral pathway and excitatory synaptic potentials (EPSPs) were monitored 60 min after HFS.
Resulsts:
We first tested effects of Roundup on basal synaptic function and LTP induction. Roundup depressed EPSPs in a dose-dependent manner. Basal synaptic transmission was completely suppressed by 2000 ppm. At concentrations ≤ 20 ppm Roundup did not affect basal transmission, but 4 ppm Roundup administered 30 min before HFS inhibited LTP induction. We also observed that acute administration of 10–100 μM glyphosate inhibits LTP induction. Minocycline, an inhibitor of microglial activation, and TAK-242, an inhibitor of toll-like receptor 4 (TLR4), both overcame the inhibitory effects of 100M glyphosate. Similarly, lipopolysaccharide from Rhodobacter sphaeroides (LPS-RS) overcame the inhibitory effects. In addition, ISRIB (integrated stress response inhibitor) and quercetin, an inhibitor of endoplasmic reticulum stress, allowed LTP induction in the presence of glyphosate. We also observed that in vivo glyphosate injection (16.9 mg/kg i.p.) impaired one-trial inhibitory avoidance learning. This learning deficit was overcome by TAK-242.
Conclusion
While Roundup inhibits LTP induction, these observations indicate that glyphosate alone, the major ingredient of Roundup, can impair cognitive function through pro-inflammatory signaling in microglia. Manipulation of pro-inflammatory signaling could be a useful strategy to prevent cognitive impairment after exposure to a glyphosate-based herbicide (GBH).
Keywords: Roundup, glyphosate, microglia, neuroinflammation, NLRP3-independence, TLR4 receptors, cognitive impairment
Background
Glyphosate, which was first developed in 1950, was originally used as a descaling agent to clean pipes in the 1960’s. Because glyphosate inhibits the plant enzyme 5-enolpyruvylshikimate-3-phosphate synthase in the aromatic amino acid biosynthetic pathway, it was patented as a herbicide in 1970 and brought to market under the trade name Roundup. The market for glyphosate expanded exponentially as genetically modified plants were developed in the 1990’s. Accordingly, human exposure to glyphosate has become routine across populations. In the US, glyphosate is commonly detected in stream water samples (Medalie et al., 2020). In France, it was detected in over 99% of human urine samples (Grau et al., 2022), while in central India, glyphosate was detected in 93% of the urine samples with a mean (SD) concentration of 3.4(1.2)μg/l (Parvez et al., 2018).
Given this vast exposure, a critical question is whether glyphosate is toxic. In addition to its potential carcinogenicity including non-Hodgkin’s lymphoma and hepatic cancer, some studies have linked glyphosate with autism spectrum disorder (ASD) (Samsel & Seneff, 2013). In mice, maternal glyphosate exposure results in abnormal behaviors and growth retardation in offspring (Coullery et al., 2020; Pu et al., 2020), implying that exposure to glyphosate alters neuronal function directly or indirectly. Additionally, acute exposure of rats to glyphosate decreases monoamine levels in brain (Hernández-Plata et al., 2015; Martínez et al., 2018), supporting a possible link to Parkinson’s disease (PD) (Wang et al., 2011). In mice, oral exposure to glyphosate is reported to cause depression-like behaviors (Aitbali et al., 2018; Cattani et al., 2017). Moreover, glyphosate exposure may diminish memory formation in mice (Ait Bali et al., 2017). Although it is plausible that these neuronal sequelae are at least partially induced indirectly by intestinal microbial degradation (Rueda-Ruzafa et al., 2019), it is also possible that the herbicide directly impairs neuronal function because glyphosate passes the blood brain barrier (BBB) (Martinez & Al-Ahmad, 2019) and infiltrates the brain to induce neuroinflammation (Astiz et al., 2012; Sato et al., 2011; Winstone et al., 2022).
Using ex vivo rat hippocampal slices, we investigated whether glyphosate administered directly onto brain tissue alters synaptic transmission and long-term potentiation (LTP), a form of synaptic plasticity thought to contribute to learning and memory. We also examined whether activation of neural pro-inflammatory processes contributes to effects of glyphosate on hippocampal function.
Methods
Animals
Sprague-Dawley albino rats were offspring of pregnant female rats obtained from Charles River Laboratories (Indianapolis IN) and were housed in approved facilities at Washington University. Animal use followed National Institute of Health (NIH) guidelines and was approved by the Washington University Institutional Animal Care and Use Committee (IACUC). The reporting in this manuscript follows recommendations in the ARRIVE guidelines.
Hippocampal slice preparation and physiology
Hippocampal slices were prepared from postnatal day (P) 28-32 male albino rats using previously described methods (Izumi, Cashikar, et al., 2021; Izumi et al., 2022). Dissected hippocampi were pinned on an agar base in ice-cold artificial cerebrospinal fluid (ACSF) containing (in mM): 124 NaCl, 5 KCl, 2 MgSO4, 2 CaCl2, 1.25 NaH2PO4, 22 NaHCO3, 10 glucose, bubbled with 95% O2-5% CO2 at 4-6°C. The dorsal two-thirds of the hippocampus was cut into 500 μm slices using a rotary slicer (Stein et al., 2017). Acutely prepared slices were kept in an incubation chamber containing gassed ACSF for at least 1 hour at 30°C before experiments.
For electrophysiological studies, slices were transferred to a submersion-recording chamber at 30°C with ACSF and perfused continuously at 2 ml/min. Extracellular recordings were obtained from the apical dendritic layer (stratum radiatum) of area CA1 for monitoring excitatory postsynaptic potentials (EPSPs) with electrodes filled with 2 M NaCl (5-10 MΩ resistance).
Because LTP is a synaptic phenomenon, we focused on recordings of EPSP slope. EPSPs were evoked using 0.1 ms constant current pulses through a bipolar stimulating electrode in the Schaffer collateral (SC) pathway. Responses were monitored by applying single stimuli every 60 sec at half-maximal intensity based on a control input-output (IO) curve. After obtaining stable baseline recordings for at least 10 min, LTP was induced by a single 100 Hz x 1 s high frequency stimulation (HFS) using the same intensity stimulus. Following HFS, responses were monitored by single stimuli once per minute during the period of post-tetanic potentiation (PTP) and then every five minutes for the remainder of an experiment. For display purposes, graphs show data every 5 minutes except during initial post-tetanic potentiation.
In vivo injection of glyphosate and behavioral studies
Rats were tested for memory acquisition in a one-trial inhibitory avoidance learning task (Izumi, Mennerick, et al., 2021; Izumi & Zorumski, 2020; Whitlock et al., 2006). This task reflects explicit-declarative fear memories and has been associated with hippocampal LTP; the task is relatively simple to administer with high reliability and clear behavioral endpoints (Izumi, Cashikar, et al., 2021; Izumi et al., 2022; Parent et al., 1994; Tokuda et al., 2010). The testing apparatus consists of two chambers, only one of which is lit. Both compartments have a floor of stainless steel rods (4 mm diameter, spaced 10 mm apart) through which an electrical shock could be delivered in the dark chamber (12 x 20 x 16 cm). The adjoining lit compartment (30 x 20 x 16 cm) was illuminated with four 13 W lights. Light intensity in the lit chamber was 1000 lux while that in the dark chamber was < 10 lux. On the first day of testing, rats were brought to the lab for vehicle injection, placed in the lit chamber, and allowed to habituate to the apparatus by freely moving between chambers for 10 min without any foot shocks being administered. On the next day, rats were administered glyphosate (16.9 mg/kg ip) or vehicle (saline) 1 hour prior to training. TAK-242 (3 mg/kg i.p.) was injected 24 hours and 2 hours before glyphosate administration. At the time of training, animals were initially placed in the lit compartment and allowed to explore the apparatus freely for up to 300 s (5 min). When rats completely entered the dark chamber, they were immediately given a foot shock. After each 300 s session, rats were removed from the apparatus and returned to their home cages. On the next day of testing, rats were placed in the lit chamber without any drug treatment and the latency to enter the dark compartment was recorded over a 300s trial.
Chemicals
TAK-242 (CAS 243984-11-4 Cat 6587) was purchased from R&D Systems (Minneapolis MN). Lipopolysaccharide from Rhodobacter sphaeroides (LPS-RS) (Catalog # tlrl-rslps) and MCC950 (CAS 210826-40-7, Catalog # inh-mcc) were purchased from InvivoGen (San Diego CA). Trans-ISRIB (CAS 1597403-47-8, Cat 5284) was from Tocris (Ellisville MO). Other chemicals, including glyphosate (CAS 1071-83-6), minocycline (CAS 13614-98-7, Cat# M2280000) and IL1-Ra (Cat# SRP 3084), quercetin (CAS 849061-97-8, PHR1488) and salts were obtained from Millipore Sigma Chemical Company. Roundup, a herbicide containing glyphosate, was purchased from a local store. Drugs were prepared as stock solutions in either ACSF or DMSO and diluted to final concentration at the time of experiment. The concentrations of TAK-242, LPS-RS and minocycline are based on our previous studies using those inhibitors against lipopolysaccharide (LPS) and acrylamide (Izumi, Cashikar, et al., 2021; Izumi et al., 2022). The concentrations of MCC950 were also based on our previous paper (Izumi et al., 2022). The dose of TAK-242 in the behavioral study followed a proceeding report by Ono et al. (Ono et al., 2020).
Statistical analysis
Physiological data were collected and analyzed using PClamp software (Molecular Devices, San Jose CA). Data are expressed as mean ± SEM 60 min following HFS, and are normalized with respect to initial baseline recordings (taken as 100%). Statistical comparisons in physiological studies were based on IO curves at baseline and sixty minutes following HFS to determine the degree of change in EPSP slope at the 50% maximal point with p < 0.05 considered significant. Data in figures for physiological studies are from continuous monitoring of EPSPs at low frequency during the course of experiments and thus may differ from numerical results described in the text, which represent analyses based on comparison of input-output curves. Statistics were performed using commercial software (GraphPad Prism 9.2.0, GraphPad Software, La Jolla California). For comparisons of LTP results among 0 ppm, 0.4 ppm and 4 ppm Roundup, data were analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. For comparisons of LTP results with 100 μM glyphosate, data were analyzed by one-way analysis of variance (ANOVA) followed by Dunnet’s multiple comparison test. For non-normally distributed data analysis of one-trial learning after in vivo injection of glyphosate, Kruskal-Wallis test followed by Dunn’s multiple comparison test was used.
Results
Glyphosate inhibits hippocampal LTP
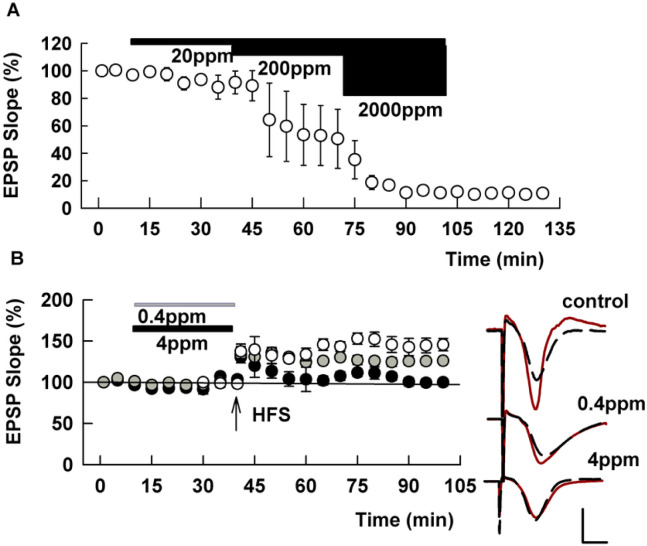
In initial experiments, we exposed hippocampal slices to increasing doses of Roundup (a glyphosate-based herbicide, GBH) to determine whether it affects basal synaptic transmission in the CA1 region. When GBH was perfused in increasing concentrations every 30 min, EPSPs were suppressed by high concentrations of GBH and the suppression was irreversible after wash out of the herbicide (N=3, Fig. 1A). Although 20 ppm or less of GBH did not affect baseline EPSPs, administration of a 100 Hz x 1 sec HFS failed to induce LTP in slices pretreated with 4 ppm GBH for 2-4 hours (97.1 ± 2.2%, N=5, Fig. 1B). This is statistically smaller than matching control LTP in the absence of GBH (146.5 ± 11.6%, N=5, P=.0006). The degree of LTP induced in slices pretreated with 0.4 ppm GBH for 2-4 hours (124.2 ± 2.6%, N=5, Fig. 1B) is not statistically different from control LTP (P=0.851) but is larger than changes observed at 4 ppm (P=0.0351).
Figure 1.
Roundup suppresses basal synaptic transmission and LTP in the CA1 region of hippocampal slices. A. In 3 slices, the concentration of Roundup was raised stepwise every 30 minutes. EPSPs, evoked by stimulation of the Schaffer collateral pathway, were not altered by 20 ppm Roundup but were completely suppressed at 2000 ppm, which is equivalent to 840 ppm of glyphosate. The suppression was irreversible after 30 min washout. B. In control slices (open circles) and slices preincubated with 0.4 ppm GBH (gray circles), but not in slices preincubated with 4 ppm GBH for 2-4 hours (closed circles), LTP was observed after a single 100 Hz x 1 s HFS (arrow). N=5 for each concentration. Traces to the right of the graph in this and subsequent figures show representative EPSPs during baseline recordings (black dashed traces) and 60 min following HFS (red traces). Calibration bar: 1 mV, 5 ms.
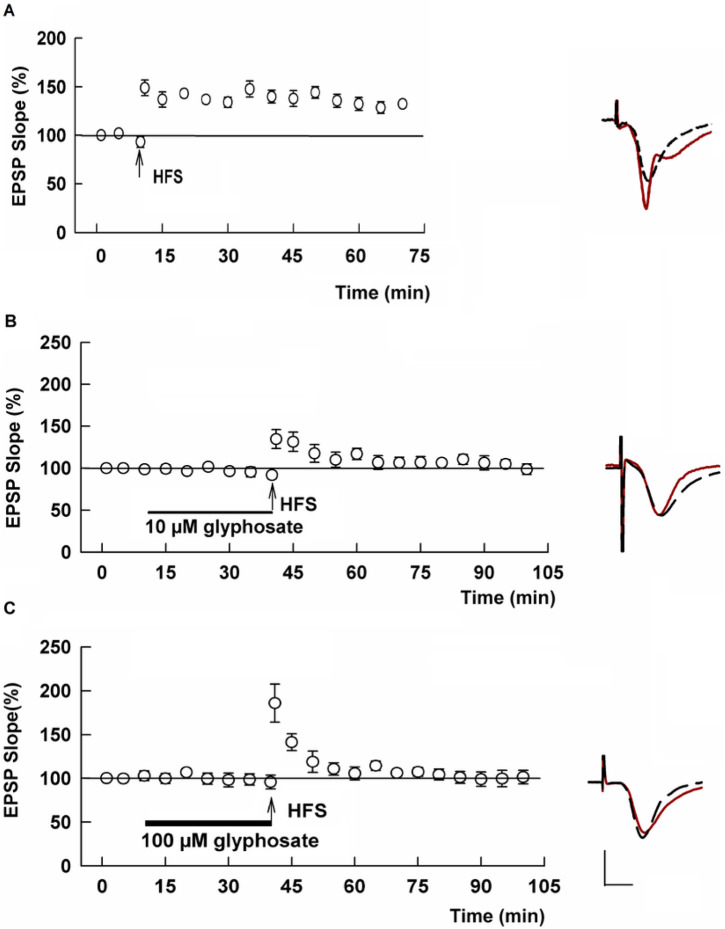
Because glyphosate is the main ingredient in GBH, we next examined whether glyphosate itself alters basal transmission or LTP induction. In the absence of glyphosate, HFS consistently induced LTP in control slices (Control LTP: 135.0 ± 2.8% of baseline measured 60 min after HFS, N=5, Fig. 2A).
Figure 2.
Glyphosate acutely inhibits LTP in the CA1 region. A. In the absence of glyphosate, HFS (arrow) successfully induced LTP. B. Acute administration of 10 μM glyphosate (black bar) produced variable suppression of LTP induction. C. One hundred μM glyphosate (black bar) inhibited LTP induction completely and reliably. Traces to the right of this graph show representative EPSPs as in Figure 1. Calibration bar (Panel B): 1 mV, 5 ms.
When administered for 30 min, neither 10 mM nor 100 mM glyphosate had a significant effect on basal synaptic responses. However, 30 min administration of 10 μM glyphosate dampened LTP induction with some variability among slices (113.8 ± 6.2 %, N=8, Fig. 2B). At 100 μM, glyphosate completely and reliably suppressed LTP induction (100.7 ± 4.5 %, N=5, P=.0002 vs control LTP, Fig. 2B). We also observed that a lower concentration of glyphosate inhibited LTP when slices were pretreated with 1 μM glyphosate for 2-4 hours (101.7 ± 4.7%, N=7), though LTP induction was not altered by similar administration of 0.1 μM glyphosate (128.0 ± 2.7 %, N=5, Supplemental Figure 1). In subsequent experiments, we focused on acute administration of 100 μM glyphosate to elucidate mechanisms underlying LTP inhibition.
Glyphosate inhibits LTP & learning via pro-inflammatory signaling
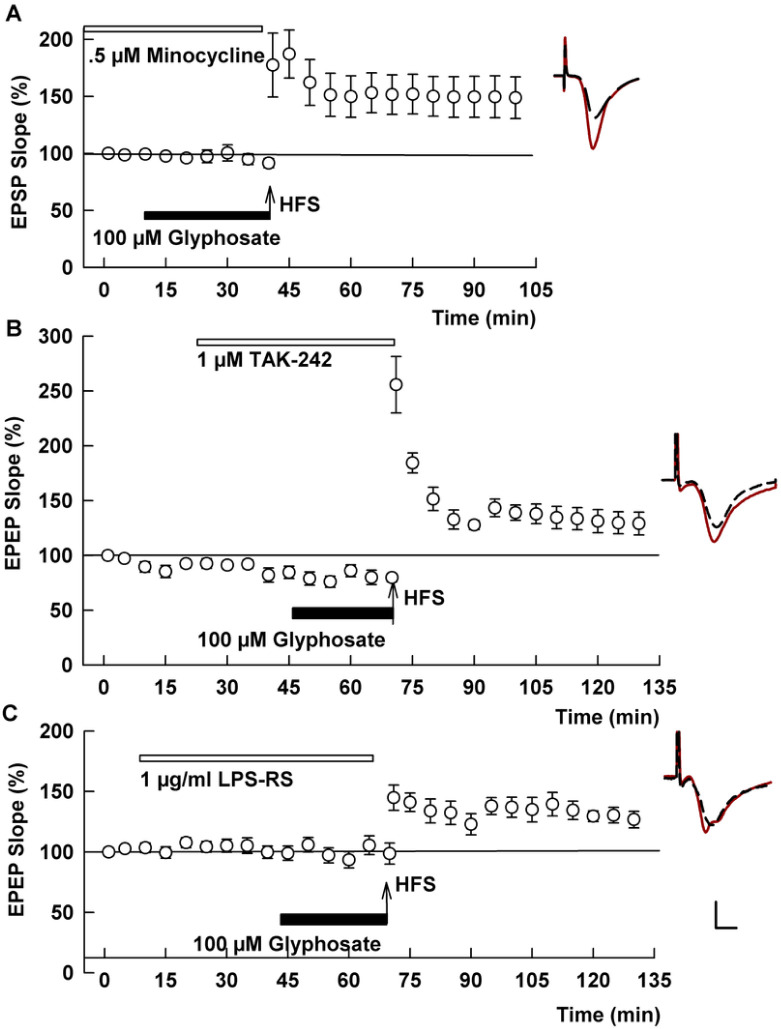
Based on a recent study indicating that glyphosate evokes inflammatory responses (Winstone et al., 2022), we examined whether microglial activation is involved in the adverse effects on LTP. For these studies, we used minocycline, an agent that is known to inhibit microglial activation and to have anti-inflammatory effects (Tikka & Koistinaho, 2001; Wu et al., 2015). We found that pre-treatment with minocycline overcame the inhibitory action of glyphosate. In slices pre-incubated with 0.5 μM minocycline, HFS readily induced LTP in the presence of 100 μM glyphosate (131.0 ± 4.7%, N=7, P=0.0004 vs 100 μM glyphosate alone, Fig. 3A), supporting a role for microglia in the acute effects of glyphosate.
Figure 3.
Modulators of microglial neuroinflammation overcome effects of 100 μM glyphosate on LTP. A. Prolonged administration of minocycline (white bar), an inhibitor of microglia, allows LTP induction after HFS (arrow) in spite of the presence of 100 μM glyphosate (black bar). B. TAK 242 (white bar), a TLR4 antagonist, also allowed LTP induction C. Similarly, LPS-RS (white bar), another TLR4 antagonist, overcomes inhibitory effects of 100 μM glyphosate on LTP induction. Traces show representative EPSPs. Calibration: 1 mV, 5 ms.
Because the toll-like receptor 4 (TLR4) signaling complex plays a key role in activation of microglia by pro-inflammatory stimuli (Buchanan et al., 2010), we next examined a role for TLR4 in the effects of glyphosate using inhibitors of this receptor. We found that the specific TLR4 antagonist, TAK-242 (1 mM) completely prevented the effects of 100 uM glyphosate on LTP induction (130.7 ± 4.5%, N=5, P=0.0013 vs. 100 uM glyphosate alone, Fig. 3C). A structurally distinct TLR4 inhibitor that also antagonizes TLR4 via two distinct mechanisms (Coats et al., 2007; Golenbock et al., 1991), LPS-RS, also overcame the inhibitory effects of glyphosate on LTP at a concentration of 1 μg/ml (128.3 ± 1.4 %, N=5, P=0.0034 vs. glyphosate alone, Fig. 3B).
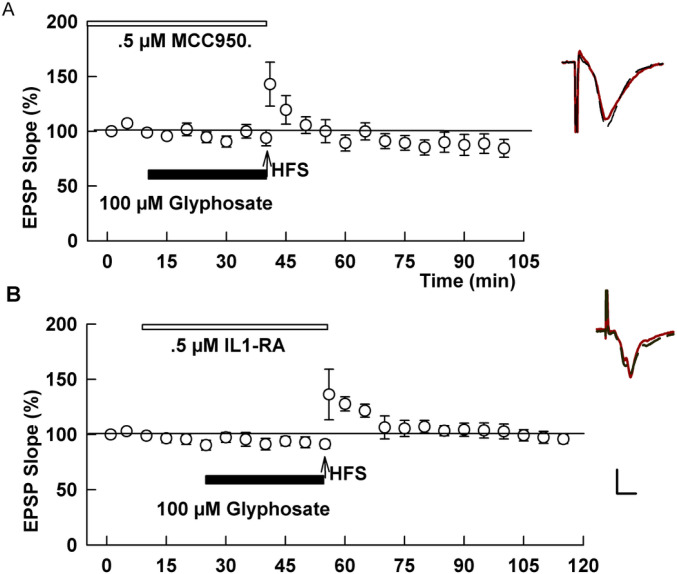
One of the major consequences of TLR4 activation is stimulation of the NLRP3 inflammasome and release of the pro-inflammatory cytokine, interleukin-1 (IL-1) (Fitzpatrick et al., 2020; Liu et al., 2020). However, we observed that 100 μM glyphosate still inhibits LTP induction in slices pre-incubated with 0.5 μM MCC950, an inhibitor of NLRP3 (93.4.8 ± 3.0, N=5, P=0.8957 vs. glyphosate alone, Fig. 4A). Similarly, 100 μM glyphosate still inhibits LTP in the presence of 100 ng/ml interleukin-1 receptor antagonist (IL-1Ra) (106.5 ± 3.3%, N=5, P>0.999 vs. glyphosate alone, Fig. 4B). These negative results suggest that glyphosate dampens synaptic plasticity independently from NLRP3 activation
Figure 4.
An NLRP3 inhibitor and IL-1 receptor antagonist failed to overcome effects of glyphosate on LTP. A. Administration of 0.5 μM MCC950, an inhibitor of NLRP3 for 2-4 hours (white bar) prior to HFS (arrow) did not prevent LTP inhibition in the presence of 100 μM glyphosate (black bar). B. Similarly, in the presence of 100 ng/ml interleukin-1 receptor antagonist (white bar), glyphosate still blocked LTP induction. Traces show representative EPSPs. Calibration: 1 mV, 5 ms.
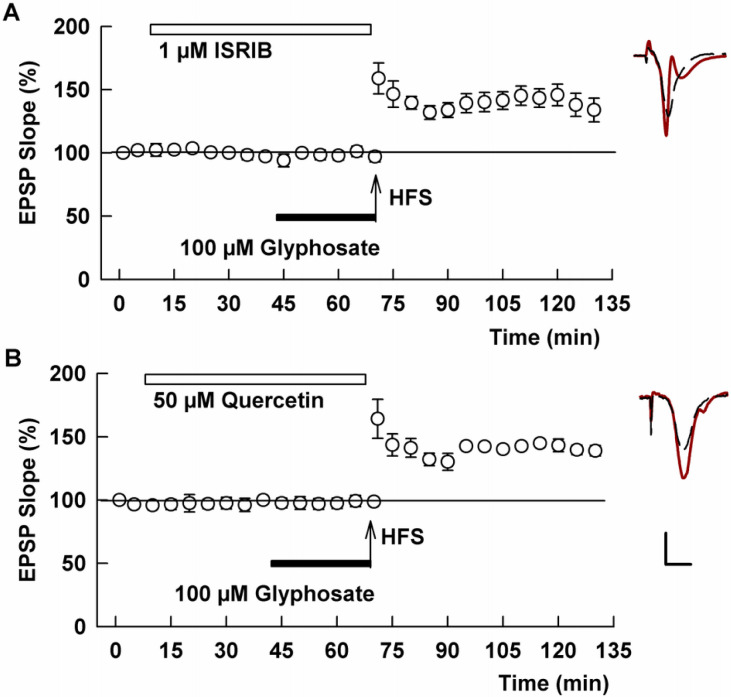
TLR4 signaling and microglial activation are also known to stimulate intracellular stress responses, which, in turn, can adversely modulate induction of synaptic plasticity (Izumi, Cashikar, et al., 2021; Izumi et al., 2022). To test this possibility, we used ISRIB, a specific inhibitor of the integrated stress response that reverses the effects of eIF2 phosphorylation and preserves memory functions (Tsai et al., 2018). In the presence of 1 μM ISRIB, glyphosate failed to inhibit LTP induction (132.0 ± 7.7%, N=5, P=0.0008 vs. glyphosate alone, Fig. 5A). We also examined the effects of quercetin, a flavonoid that attenuates inflammatory processes through inhibition of endoplasmic reticulum stress (Feng et al., 2019). At 50 μM, quercetin allowed robust LTP induction in the presence of 100 μM glyphosate (146.6 ± 5.4%, N=5, P <0.0001, Fig. 5B).
Figure 5.
Inhibitors of cellular stress responses overcome the effects of glyphosate on LTP. A. Administration of 1 μM ISRIB (white bar) prior to HFS (arrow) allowed LTP induction in spite of the presence of 100 μM glyphosate (black bar). B. Similarly, in the presence of 50 μM quercetin (white bar), glyphosate failed to block LTP induction. Traces show representative EPSPs. Calibration: 1 mV, 5 ms.
To determine whether effects observed in ex vivo hippocampal slices translate into changes in learning and memory, we also examined the effects of glyphosate on a one-trial inhibitory avoidance task that has been linked previously to hippocampal LTP (Whitlock et al., 2006). Glyphosate was injected at a dose of 16.9 mg/kg, i.p. 24 hours before conditioning. This dose of glyphosate produced no noticeable changes in gait, coordination or body weight. However, glyphosate treatment had marked acute effects on performance in one-trial learning compared to saline-treated controls when tested 24 h after conditioning. The glyphosate-induced defect in learning was manifest by rats more readily entering the dark chamber where they had been shocked during training, whereas saline-treated controls remained in the lit compartment for the full duration of the 300 second trial (P = 0.0048 by Dunn’s test, N=8, Fig. 6). The adverse effects of glyphosate on learning were completely prevented by pretreatment with the TLR4 antagonist, TAK-242. In rats treated with TAK-242 (3mg/kg i.p. twice), glyphosate had no effect on one-trial learning (P=0.0048 vs. glyphosate alone by Dunn’s test, N=5, Fig. 6).
Figure 6.
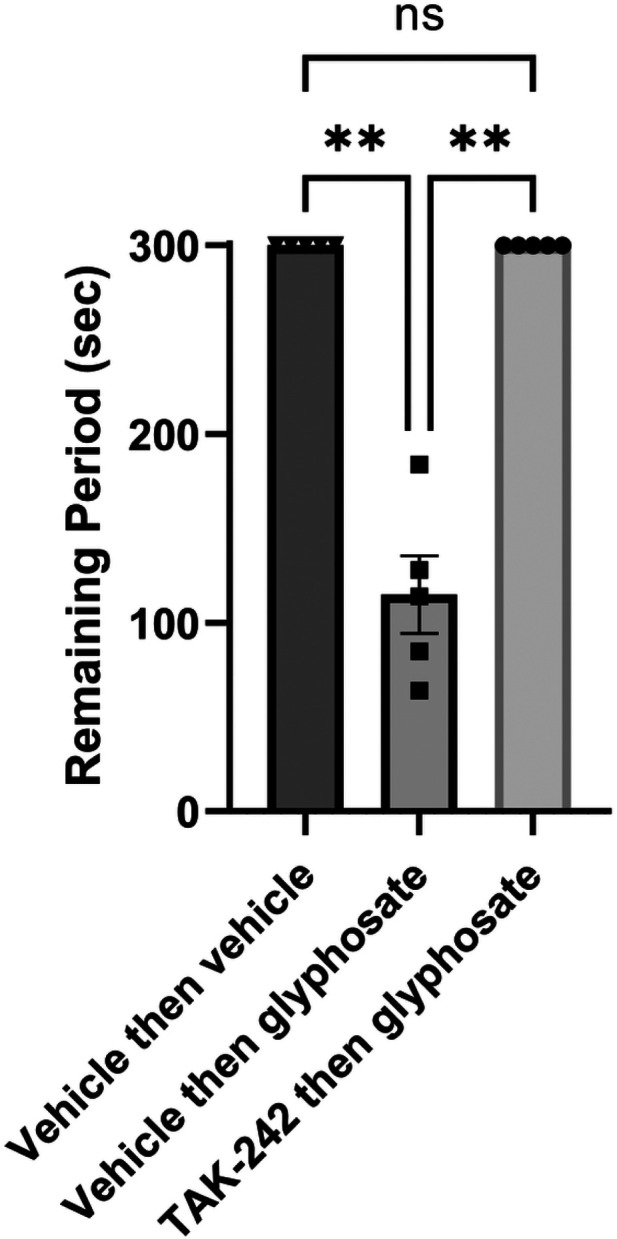
Effects of glyphosate in vivo. Intraperitoneal injection of glyphosate (16.9 mg/kg ip) one day prior to inhibitory avoidance training results in a defect in learning as manifest by rats more readily leaving the lit chamber to enter the dark chamber where they had received a foot shock one day previously. Injection of TAK-242 (3 mg/kg, 24 hours and 2 hours before glyphosate administration) prevented the learning deficit. ** p = 0.0048 by Dunn’s multiple comparison test.
Discussion
The primary mechanism of glyphosate in plants involves disruption of the shikimate pathway. Because this pathway is absent in animal cells, it has long been held that glyphosate is not harmful to animals. However, in 2015, the World Health Organization’s International Agency for Research on Cancer identified glyphosate as a probable human carcinogen (Guyton et al., 2015). Although the carcinogenicity of glyphosate is still debated (Benbrook, 2019), there are other concerns with this environmental agent. In particular, there are now concerns that the CNS is one of the targets of glyphosate (Costas-Ferreira et al., 2022). Parkinson’s disease (PD) was observed in a woman after chronic occupational exposure to GBH (Wang et al., 2011), and in rats even shorter exposure to glyphosate alters dopaminergic systems (Hernández-Plata et al., 2015; Martinez & Al-Ahmad, 2019). Additionally, a possible link with autism is speculated based on epidemiological data (von Ehrenstein et al., 2019), and maternal exposure to glyphosate results in autism-spectrum disorder (ASD)-like behaviors in murine offspring (Pu et al., 2020, 2020; von Ehrenstein et al., 2019). Excitotoxicity in the CNS is also possible because CSF levels of aspartate and glutamate double within a day after a single oral dose of GBH in rats (Limberger et al., 2020). In mice, intranasal exposure to GBH results in anxiogenic behaviors (Baier et al., 2017). Moreover, oral administration of 250–500 mg/kg GBH results in memory impairment in mice observed as decreased latency in a passive avoidance test (Bali et al., 2019).
In the present study, we first tested if GBH itself alters basal synaptic function and found that EPSPs were acutely depressed by 200 ppm GBH, a level that is equivalent to 82 ppm or about 500 μM glyphosate. We also found that LTP, a mechanism of learning and memory, was completely disrupted by 4 ppm GBH. This level is higher than concentrations detected in healthy human fluids. For example the maximal concentration in urine from young individuals in Germany is 11.1 μg/L (Lemke et al., 2021). However, the levels we examined in hippocampal slices may be observed in individuals who are exposed to Roundup accidentally or following a suicide attempt. Sato et al. (2011) described a semi-comatose woman in whom glyphosate was 122.5 μg/ml in CSF (and 1294.5 μg/ml in the serum) two days after ingestion. Given that 4 ppm GBH inhibits LTP induction and 200 ppm depresses synaptic transmission (Fig. 1), this level in the CSF is likely sufficient to impair consciousness and cognitive function.
In our studies, we hypothesized that glyphosate in GBH causes neuroinflammation to impair cognitive function. It has been recently shown that oral administration of glyphosate to mice (125, 250 and 500 mg/kg for 14 days) elevates glyphosate levels (10–50 ng/mg) and tumor necrosis factor- (TNFα) in the brain (Winstone et al., 2022). This study has two important implications: orally administered glyphosate infiltrates the CNS and elevates pro-inflammatory cytokines in the CSF. The aforementioned case of GBH ingestion (Sato et al., 2011) also suggests that GBH may trigger inflammation in the CNS. Microglia are major contributors to neuroinflammation. Consistent with this, LTP was successfully induced in the presence of glyphosate when hippocampal slices were pretreated with minocycline, an inhibitor of microglia. The inhibitory effect of glyphosate on LTP induction at least partially shares mechanisms with LPS and acrylamide, both of which induce neuroinflammation (Izumi, Cashikar, et al., 2021; Izumi et al., 2022). The ability of LPS-RS to overcome effects of glyphosate suggests that glyphosate behaves like LPS in the CNS. Moreover, we observed that TAK-242, a selective TLR4 antagonist, clearly overcomes the effects of glyphosate in both LTP and behavioral experiments, suggesting that activation of TLR4 is pivotal for glyphosate to disrupt the CNS.
Although LTP induction was impaired by acute administration of 10 μM glyphosate, we used 100 μM glyphosate for our experiments because it consistently and completely blocked LTP induction, allowing us to determine mechanisms underlying its neurotoxicity. With this experimental paradigm, we observed that TAK-242 efficiently allows LTP induction in the presence of glyphosate. However, 100 μM glyphosate could be excessive and obscure other possible effective treatments. Consistent with this, we were surprised that MCC-950, a reliable NLRP3 inhibitor, failed to overcome the inhibitory effect of glyphosate on LTP induction because MCC-950 effectively overcomes the LTP inhibiting effects of acrylamide, another environmental toxin (Izumi, Cashikar, et al., 2021; Izumi et al., 2022). The failure of MCC-950 does not necessarily preclude a role for NLRP3 but the discrepancy may imply that glyphosate activates pro-inflammatory pathways in a manner different from other toxins. For example, proteasome inhibitors, which are used for the treatment of multiple sclerosis, induce inflammasome activation independent of NLRP3 (Ullrich et al., 2022). Similarly, in gout-related arthritis triggered by phagocytosis of monosodium urate crystals, caspase-1 activation, which is a fundamental mechanism for IL-1β secretion, occurs independently of NLRP3 (Chuang et al., 2020).
Even if the activation of pro-inflammation is independent from NLRP3, it can be attenuated by other cellular stress inhibitors. Because the ISR contributes to the pathogenesis of memory impairment and neurodegeneration accompanied by inflammation, systemic inhibition of ISR by ISRIB can reverse memory deficits (Chou et al., 2017). Consistent with this, ISRIB successfully overcame the inhibitory effect of glyphosate on LTP induction in the current study. Quercetin, a flavonoid, attenuates inflammation by inhibition of endoplasmic reticulum stress (Feng et al., 2019). Interestingly, hepatotoxicity induced by sub-chronic administration of glyphosate in rats is reportedly attenuated by simultaneous administration of quercetin (Soudani et al., 2019). Moreover, it has been reported that quercetin overcame the decrease of reduced glutathione levels and increase in reactive oxygen species in the mouse hippocampus after sub-chronic exposure to a GBH (Bicca et al., 2021). Consistent with these reports, quercetin was effective in allowing LTP in the presence of glyphosate in our study. Regular dietary intake of quercetin in vegetables such as onions could help prevent neuroinflammation triggered by GBH if these vegetables are not contaminated with the herbicide. Although it is difficult to prevent GBH exposure as evidenced by that observation that glyphosate is detected in the urine of nearly all (99.8%) of the French population in one study (Grau et al., 2022), it is important to identify measures to prevent its neurotoxcity. These measures may include inhibitors of integrated stress responses, or modulators of endoplasmic reticulum stress.
In this study, we focused on direct neurotoxic aspects of glyphosate and found that glyphosate activates microglia via TLR4 and triggers cellular stress to impair hippocampal plasticity and learning. However, the neurotoxicity of GBH may not be limited to the direct actions of glyphosate. The GBH, Roundup, uses polyethoxylated tallow amine (POEA) as a surfactant and POEA can also contribute to toxicity (Brausch et al., 2007) because POEA is a strong inducer of ER stress (Mesnage et al., 2022). Gut microbiota dysbiosis by glyphosate also may result in neuronal impairment (Samsel & Seneff, 2013) because block of the shikimate pathway impacts microbiota. Furthermore, aminomethylphosphonic acid (AMPA), one of glyphosate’s main metabolites, may have additional actions. Thus, the neurotoxicity of GBH is likely more complicated and perhaps more severe than the results observed in the present study.
Funding:
Work in the authors’ laboratory is funded by the Taylor Family Institute for Innovative Psychiatric Research and MH122379 from the National Institute of Mental Health.
Abbreviations
- ACSF
artificial cerebrospinal fluid
- AMPA
aminomethylphosphonic acid
- ANOVA
analysis of variance
- ASD
autism spectrum disorder
- BBB
blood brain barrier
- CNS
central nervous system
- EPSP
excitatory postsynaptic potentials
- GBH
a glyphosate-based herbicide
- HFS
high frequency stimulation
- IACUC
Institutional Animal Care and Use Committee
- IL-1
Interleukin-1
- IO
input-output
- i.p.
intraperitoneally
- LPS
Lipopolysaccharide
- LPS-RS
Lipopolysaccharide from Rhodobacter sphaeroidesIR-Ira: Interleukin-1 receptor antagonist
- ISR
integrated stress response
- ISRIB
integrated stress response inhibitor
- LTP
long-term potentiation
- mtROS
mitochondrial reactive oxygen species
- NIH
National Institute of Health
- NLRP3
NOD-, LRR- and pyrin domain-containing protein 3
- P
postnatal day
- PD
Parkinson’s disease
- TNF-α
tumor necrosis factor-
Footnotes
Competing interests: CFZ serves on the Scientific Advisory Board of Sage Therapeutics and has equity in the company. Sage Therapeutics was not involved in this work. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Ait Bali Y., Ba-Mhamed S., & Bennis M. (2017). Behavioral and Immunohistochemical Study of the Effects of Subchronic and Chronic Exposure to Glyphosate in Mice. Frontiers in Behavioral Neuroscience, 11, 146. 10.3389/fnbeh.2017.00146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aitbali Y., Ba-M’hamed S., Elhidar N., Nafis A., Soraa N., & Bennis M. (2018). Glyphosate based-herbicide exposure affects gut microbiota, anxiety and depression-like behaviors in mice. Neurotoxicology and Teratology, 67, 44–49. 10.1016/j.ntt.2018.04.002 [DOI] [PubMed] [Google Scholar]
- 3.Astiz M., de Alaniz M. J. T., & Marra C. A. (2012). The oxidative damage and inflammation caused by pesticides are reverted by lipoic acid in rat brain. Neurochemistry International, 61(7), 1231–1241. 10.1016/j.neuint.2012.09.003 [DOI] [PubMed] [Google Scholar]
- 4.Baier C. J., Gallegos C. E., Raisman-Vozari R., & Minetti A. (2017). Behavioral impairments following repeated intranasal glyphosate-based herbicide administration in mice. Neurotoxicology and Teratology, 64, 63–72. 10.1016/j.ntt.2017.10.004 [DOI] [PubMed] [Google Scholar]
- 5.Bali Y. A., Kaikai N.-E., Ba-M’hamed S., & Bennis M. (2019). Learning and memory impairments associated to acetylcholinesterase inhibition and oxidative stress following glyphosate based-herbicide exposure in mice. Toxicology, 415, 18–25. 10.1016/j.tox.2019.01.010 [DOI] [PubMed] [Google Scholar]
- 6.Benbrook C. M. (2019). How did the US EPA and IARC reach diametrically opposed conclusions on the genotoxicity of glyphosate-based herbicides? Environmental Sciences Europe, 31(1), 2. 10.1186/s12302-018-0184-7 [DOI] [Google Scholar]
- 7.Bicca D. F., Spiazzi C. C., Ramalho J. B., Soares M. B., & Cibin F. W. S. (2021). A subchronic low-dose exposure of a glyphosate-based herbicide induces depressive and anxious-like behavior in mice: Quercetin therapeutic approach. Environmental Science and Pollution Research International, 28(47), 67394–67403. 10.1007/s11356-021-15402-3 [DOI] [PubMed] [Google Scholar]
- 8.Brausch J. M., Beall B., & Smith P. N. (2007). Acute and Sub-Lethal Toxicity of Three POEA Surfactant Formulations to Daphnia magna. Bulletin of Environmental Contamination and Toxicology, 78(6), 510–514. 10.1007/s00128-007-9091-0 [DOI] [PubMed] [Google Scholar]
- 9.Buchanan M. M., Hutchinson M., Watkins L. R., & Yin H. (2010). Toll-like Receptor 4 in CNS Pathologies. Journal of Neurochemistry, 114(1), 13–27. 10.1111/j.1471-4159.2010.06736.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cattani D., Cesconetto P. A., Tavares M. K., Parisotto E. B., De Oliveira P. A., Rieg C. E. H., Leite M. C., Prediger R. D. S., Wendt N. C., Razzera G., Filho D. W., & Zamoner A. (2017). Developmental exposure to glyphosate-based herbicide and depressive-like behavior in adult offspring: Implication of glutamate excitotoxicity and oxidative stress. Toxicology, 387, 67–80. 10.1016/j.tox.2017.06.001 [DOI] [PubMed] [Google Scholar]
- 11.Chou A., Krukowski K., Jopson T., Zhu P. J., Costa-Mattioli M., Walter P., & Rosi S. (2017). Inhibition of the integrated stress response reverses cognitive deficits after traumatic brain injury. Proceedings of the National Academy of Sciences, 114(31), E6420–E6426. 10.1073/pnas.1707661114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chuang J.-P., Kao C.-Y., Lee J.-C., Ling P., Maa M.-C., & Leu T.-H. (2020). EPS8 regulates an NLRP3 inflammasome-independent caspase-1 activation pathway in monosodium urate crystal-treated RAW264.7 macrophages. Biochemical and Biophysical Research Communications, 530(3), 487–493. 10.1016/j.bbrc.2020.05.084 [DOI] [PubMed] [Google Scholar]
- 13.Coats S. R., Do C. T., Karimi-Naser L. M., Braham P. H., & Darveau R. P. (2007). Antagonistic lipopolysaccharides block E. coli lipopolysaccharide function at human TLR4 via interaction with the human MD-2 lipopolysaccharide binding site. Cellular Microbiology, 9(5), 1191–1202. 10.1111/j.1462-5822.2006.00859.x [DOI] [PubMed] [Google Scholar]
- 14.Costas-Ferreira C., Durán R., & Faro L. R. F. (2022). Toxic Effects of Glyphosate on the Nervous System: A Systematic Review. International Journal of Molecular Sciences, 23(9), Article 9. 10.3390/ijms23094605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coullery R. P., Ferrari M. E., & Rosso S. B. (2016). Neuronal development and axon growth are altered by glyphosate through a WNT non-canonical signaling pathway. Neurotoxicology, 52, 150–161. 10.1016/j.neuro.2015.12.004 [DOI] [PubMed] [Google Scholar]
- 16.Coullery R., Pacchioni A. M., & Rosso S. B. (2020). Exposure to glyphosate during pregnancy induces neurobehavioral alterations and downregulation of Wnt5a-CaMKII pathway. Reproductive Toxicology, 96, 390–398. 10.1016/j.reprotox.2020.08.006 [DOI] [PubMed] [Google Scholar]
- 17.Feng K., Chen Z., Pengcheng L., Zhang S., & Wang X. (2019). Quercetin attenuates oxidative stress-induced apoptosis via SIRT1/AMPK-mediated inhibition of ER stress in rat chondrocytes and prevents the progression of osteoarthritis in a rat model. Journal of Cellular Physiology, 234(10), 18192–18205. 10.1002/jcp.28452 [DOI] [PubMed] [Google Scholar]
- 18.Fitzpatrick S., King A., & Ryan S. (2020). Blockage of TLR4 or NLRP3 Inhibits IL-1β Activation in Response to Intermittent Hypoxia. European Respiratory Journal, 56(suppl 64). 10.1183/13993003.congress-2020.4734 [DOI] [Google Scholar]
- 19.Golenbock D. T., Hampton R. Y., Qureshi N., Takayama K., & Raetz C. R. (1991). Lipid A-like molecules that antagonize the effects of endotoxins on human monocytes. Journal of Biological Chemistry, 266(29), 19490–19498. 10.1016/S0021-92580855023-7 [DOI] [PubMed] [Google Scholar]
- 20.Grau D., Grau N., Gascuel Q., Paroissin C., Stratonovitch C., Lairon D., Devault D. A., & Di Cristofaro J. (2022). Quantifiable urine glyphosate levels detected in 99% of the French population, with higher values in men, in younger people, and in farmers. Environmental Science and Pollution Research, 29(22), 32882–32893. 10.1007/s11356-021-18110-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guyton K. Z., Loomis D., Grosse Y., El Ghissassi F., Benbrahim-Tallaa L., Guha N., Scoccianti C., Mattock H., Straif K., & International Agency for Research on Cancer Monograph Working Group, IARC, Lyon, France. (2015). Carcinogenicity of tetrachlorvinphos, parathion, malathion, diazinon, and glyphosate. The Lancet. Oncology, 16(5), 490–491. 10.1016/S1470-2045(15)70134-8 [DOI] [PubMed] [Google Scholar]
- 22.Hernández-Plata I., Giordano M., Díaz-Muñoz M., & Rodríguez V. M. (2015). The herbicide glyphosate causes behavioral changes and alterations in dopaminergic markers in male Sprague-Dawley rat. Neurotoxicology, 46, 79–91. 10.1016/j.neuro.2014.12.001 [DOI] [PubMed] [Google Scholar]
- 23.Izumi Y., Cashikar A., Krishnan K., Paul S. M., Covey D. F., Mennerick S. J., & Zorumski C. F. (2021). A Pro-inflammatory Stimulus Disrupts Hippocampal Plasticity and Learning via Microglial Activation and 25-Hydroxycholesterol. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, JN-RM-1502-21. 10.1523/JNEUROSCI.1502-21.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Izumi Y., Fujii C., O’Dell K. A., & Zorumski C. F. (2022). Acrylamide inhibits long-term potentiation and learning involving microglia and pro-inflammatory signaling. Scientific Reports, 12(1), Article 1. 10.1038/s41598-022-16762-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Izumi Y., Mennerick S. J., Doherty J. J., & Zorumski C. F. (2021). Oxysterols Modulate the Acute Effects of Ethanol on Hippocampal N-Methyl-d-Aspartate Receptors, Long-Term Potentiation, and Learning. The Journal of Pharmacology and Experimental Therapeutics, 377(1), 181–188. 10.1124/jpet.120.000376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Izumi Y., & Zorumski C. F. (2020). Inhibitors of cellular stress overcome acute effects of ethanol on hippocampal plasticity and learning. Neurobiology of Disease, 141, 104875. 10.1016/j.nbd.2020.104875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lemke N., Murawski A., Schmied-Tobies M. I. H., Rucic E., Hoppe H.-W., Conrad A., & Kolossa-Gehring M. (2021). Glyphosate and aminomethylphosphonic acid (AMPA) in urine of children and adolescents in Germany – Human biomonitoring results of the German Environmental Survey 2014–2017 (GerES V). Environment International, 156, 106769. 10.1016/j.envint.2021.106769 [DOI] [PubMed] [Google Scholar]
- 28.Limberger C., Ferreira P. C. L., Fontella F. U., Oliveira A. C. L. J., Salles G. B., Souza D. O., Zimmer E. R., & de Souza D. G. (2020). Glyphosate-based herbicide alters brain amino acid metabolism without affecting blood-brain barrier integrity. Alzheimer’s & Dementia, 16(S2), e043847. 10.1002/alz.043847 [DOI] [Google Scholar]
- 29.Liu Y., Dai Y., Li Q., Chen C., Chen H., Song Y., Hua F., & Zhang Z. (2020). Beta-amyloid activates NLRP3 inflammasome via TLR4 in mouse microglia. Neuroscience Letters, 736, 135279. 10.1016/j.neulet.2020.135279 [DOI] [PubMed] [Google Scholar]
- 30.Martinez A., & Al-Ahmad A. J. (2019). Effects of glyphosate and aminomethylphosphonic acid on an isogeneic model of the human blood-brain barrier. Toxicology Letters, 304, 39–49. 10.1016/j.toxlet.2018.12.013 [DOI] [PubMed] [Google Scholar]
- 31.Martínez M.-A., Ares I., Rodríguez J.-L., Martínez M., Martínez-Larrañaga M.-R., & Anadón A. (2018). Neurotransmitter changes in rat brain regions following glyphosate exposure. Environmental Research, 161, 212–219. 10.1016/j.envres.2017.10.051 [DOI] [PubMed] [Google Scholar]
- 32.Medalie L., Baker N. T., Shoda M. E., Stone W. W., Meyer M. T., Stets E. G., & Wilson M. (2020). Influence of land use and region on glyphosate and aminomethylphosphonic acid in streams in the USA. Science of The Total Environment, 707, 136008. 10.1016/j.scitotenv.2019.136008 [DOI] [PubMed] [Google Scholar]
- 33.Mesnage R., Ferguson S., Brandsma I., Moelijker N., Zhang G., Mazzacuva F., Caldwell A., Halket J., & Antoniou M. N. (2022). The surfactant co-formulant POEA in the glyphosate-based herbicide RangerPro but not glyphosate alone causes necrosis in Caco-2 and HepG2 human cell lines and ER stress in the ToxTracker assay. Food and Chemical Toxicology, 168, 113380. 10.1016/j.fct.2022.113380 [DOI] [PubMed] [Google Scholar]
- 34.Ono Y., Maejima Y., Saito M., Sakamoto K., Horita S., Shimomura K., Inoue S., & Kotani J. (2020). TAK-242, a specific inhibitor of Toll-like receptor 4 signalling, prevents endotoxemia-induced skeletal muscle wasting in mice. Scientific Reports, 10(1), Article 1. 10.1038/s41598-020-57714-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parent M. B., West M., & McGaugh J. L. (1994). Memory of rats with amygdala lesions induced 30 days after footshock-motivated escape training reflects degree of original training. Behavioral Neuroscience, 108(6), 1080–1087. 10.1037/0735-7044.108.6.1080 [DOI] [PubMed] [Google Scholar]
- 36.Parvez S., Gerona R. R., Proctor C., Friesen M., Ashby J. L., Reiter J. L., Lui Z., & Winchester P. D. (2018). Glyphosate exposure in pregnancy and shortened gestational length: A prospective Indiana birth cohort study. Environmental Health, 17, 23. 10.1186/s12940-018-0367-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pu Y., Yang J., Chang L., Qu Y., Wang S., Zhang K., Xiong Z., Zhang J., Tan Y., Wang X., Fujita Y., Ishima T., Wan D., Hwang S. H., Hammock B. D., & Hashimoto K. (2020). Maternal glyphosate exposure causes autism-like behaviors in offspring through increased expression of soluble epoxide hydrolase. Proceedings of the National Academy of Sciences, 117(21), 11753–11759. 10.1073/pnas.1922287117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rueda-Ruzafa L., Cruz F., Roman P., & Cardona D. (2019). Gut microbiota and neurological effects of glyphosate. NeuroToxicology, 75, 1–8. 10.1016/j.neuro.2019.08.006 [DOI] [PubMed] [Google Scholar]
- 39.Samsel A., & Seneff S. (2013). Glyphosate’s Suppression of Cytochrome P450 Enzymes and Amino Acid Biosynthesis by the Gut Microbiome: Pathways to Modern Diseases. Entropy, 15(4), Article 4. 10.3390/e15041416 [DOI] [Google Scholar]
- 40.Sato C., Kamijo Y., Yoshimura K., & Ide T. (2011). Aseptic meningitis in association with glyphosate-surfactant herbicide poisoning. Clinical Toxicology, 49(2), 118–120. 10.3109/15563650.2011.552065 [DOI] [PubMed] [Google Scholar]
- 41.Soudani N., Chaâbane M., Ghorbel I., Elwej A., Boudawara T., & Zeghal N. (2019). Glyphosate disrupts redox status and up-regulates metallothionein I and II genes expression in the liver of adult rats. Alleviation by quercetin. General Physiology and Biophysics, 38(2), 123–134. 10.4149/gpb_2018043 [DOI] [PubMed] [Google Scholar]
- 42.Stein L. R., Zorumski C. F., & Izumi Y. (2017). Dissection method affects electrophysiological properties of hippocampal slices. Oruen: The CNS Journal, 3(2), 94–101. [PMC free article] [PubMed] [Google Scholar]
- 43.Tikka T. M., & Koistinaho J. E. (2001). Minocycline provides neuroprotection against N-methyl-D-aspartate neurotoxicity by inhibiting microglia. Journal of Immunology (Baltimore, Md.: 1950), 166(12), 7527–7533. 10.4049/jimmunol.166.12.7527 [DOI] [PubMed] [Google Scholar]
- 44.Tokuda K., O’Dell K. A., Izumi Y., & Zorumski C. F. (2010). Midazolam Inhibits Hippocampal Long-Term Potentiation and Learning through Dual Central and Peripheral Benzodiazepine Receptor Activation and Neurosteroidogenesis. Journal of Neuroscience, 30(50), 16788–16795. 10.1523/JNEUR0SCI.4101-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsai J. C., Miller-Vedam L. E., Anand A. A., Jaishankar P., Nguyen H. C., Renslo A. R., Frost A., & Walter P. (2018). Structure of the nucleotide exchange factor eIF2B reveals mechanism of memory-enhancing molecule. Science (New York, N.Y.), 359(6383), eaaq0939. 10.1126/science.aaq0939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ullrich F., Verhoeven T., Andryka K., Schmitz S., Soler S. B., Zillinger T., Hartmann G., & Bartok E. (2022). NLRP3-Independent Inflammasome Activation By Proteasome Inhibitors. Blood, 140(Supplement 1), 3125. 10.1182/blood-2022-169718 [DOI] [Google Scholar]
- 47.von Ehrenstein O. S., Ling C., Cui X., Cockburn M., Park A. S., Yu F., Wu J., & Ritz B. (2019). Prenatal and infant exposure to ambient pesticides and autism spectrum disorder in children: Population based case-control study. BMJ (Clinical Research Ed.), 364, 1962. 10.1136/bmj.1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang G., Fan X.-N., Tan Y.-Y., Cheng Q., & Chen S.-D. (2011). Parkinsonism after chronic occupational exposure to glyphosate. Parkinsonism & Related Disorders, 17(6), 486–487. 10.1016/j.parkreldis.2011.02.003 [DOI] [PubMed] [Google Scholar]
- 49.Whitlock J. R., Heynen A. J., Shuler M. G., & Bear M. F. (2006). Learning induces long-term potentiation in the hippocampus. Science (New York, N.Y.), 313(5790), 1093–1097. 10.1126/science.1128134 [DOI] [PubMed] [Google Scholar]
- 50.Winstone J. K., Pathak K. V., Winslow W., Piras I. S., White J., Sharma R., Huentelman M. J., Pirrotte P., & Velazquez R. (2022). Glyphosate infiltrates the brain and increases pro-inflammatory cytokine TNFα: Implications for neurodegenerative disorders. Journal of Neuroinflammation, 19(1), 193. 10.1186/s12974-022-02544-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu Y., Dissing-Olesen L., MacVicar B. A., & Stevens B. (2015). Microglia: Dynamic Mediators of Synapse Development and Plasticity. Trends in Immunology, 36(10), 605–613. 10.1016/j.it.2015.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]