Abstract
In the early days of assisted reproductive technology (ART), the main target was achieving gestation. Success rates were low, and multiple embryo transfers became common practice, with multiple pregnancies being 20 times higher than in natural conception. Multiple pregnancy is associated with a higher risk of complications for the mother and the baby than a singleton pregnancy. Added to healthcare costs, multiple pregnancy also involves other costs and psychosocial risks, with a high social and health costs. At present, success rates of assisted human reproduction (AHR) have improved dramatically, partially due to advances in laboratory techniques such as culture of blastocyst-stage embryos and vitrification. Additionally, there is a wide range of counseling, health and economic policies that have demonstrated being effective in increasing single-embryo transfer (SET) practices and reducing multiple pregnancies, which ensures satisfactory success rates. Therefore, single-embryo transfer emerges as the approach of choice for AHR to result in a full-term healthy newborn.
Keywords: assisted reproduction techniques, elective single-embryo transfer, infertility, in vitro fertilization, multiple delivery, multiple pregnancy
Introduction
Since the first assisted reproductive technology (ART)-conceived infant was born in 1978, ART have developed and gained popularity worldwide. Thus, ART have increased notably the chances of pregnancy of subfertile couples [1]. At present, 2.6% of newborns in Europe [2], and more than 9% in Spain [3] are conceived by AHR.
In the early days of assisted reproductive technology, several fertilized eggs were transferred to increase the chances of pregnancy. Thus, the transfer of two, three or even four fertilized eggs was common practice [4]. This was routine practice in numerous countries in the 80s and 90s, as it was permitted by law. In Spain, Law 35/1988 on AHR did not establish a limit to the number of fertilized eggs to transfer, a decision that was left at physician’s discretion. This situation changed in 2003 with Law 45/2003, which only authorized the transfer of a maximum of three fertilized eggs per cycle. Since then, numerous studies have alerted on the dramatic increase in the rate of multiple pregnancies associated with ART, especially twin pregnancies, which accounted for 26% of multiple pregnancies [5]. Multiple pregnancies are the most frequent iatrogenic complications of ART and are a risk factor, as compared to singleton pregnancy. Thus, multiple pregnancy is associated with higher maternal mortality and morbidity rates and perinatal problems such as preterm birth, and low birthweight [6].
Materials and methods
A literature search was performed on Entrez Pubmed (US National Library of Medicine, National Institute of Health; http://www.ncbi.nlm.nih.gov/pubmed/). The search terms used were infertility, in vitro fertilization (IVF), multiple embryo transfer, single-embryo transfer (SET), elective single-embryo transfer (eSET), multiple pregnancy, twin pregnancy, embryo cleavage blastocyst, vitrification, time-lapse technology, preimplantation genetic test and ART. After the primary search, other additional relevant articles were identified. A peer-review was performed of the 85 publications that met our inclusion criteria.
European Union (EU) legal framework for embryo transfer
There is a common European legal framework for the use (procurement, storage, distribution and traceability) and testing of reproductive tissue and cells. Notably, a common legal framework has not been developed in the EU for the procedures performed in fertility centers [7], [8]. Therefore, each member state apply their own regulations which are regularly updated based on technical progress and the public or private coverage of treatment. In countries such as India, Japan or USA, ART are not regulated [9]. Nevertheless, most countries provide best practice guidelines designed with National or International Scientific Societies that complement national regulations.
With regard to embryo transfer, EU Members have established a limit to the number of embryos to transfer per attempt, although with a diversity of scenarios. In some countries, only one embryo can be transferred per cycle (Austria or Belgium in women younger than 36), whereas in other countries a maximum of three embryos can be transferred based on patient-physician preferences (Spain and Germany, although a maximum of two embryos is recommended in women younger than 37). In the majority of countries, although SET is recommended, there are age-dependent limits. In other countries such as France and Sweden, transferring more than two embryos is not permitted by law. In some countries such as Belgium, public coverage is contingent on embryo transfer policy [10]. In Bulgaria, the law establishes specific criteria including the age of the mother, number of failed attempts and embryo stage. At the far end is the Czech Republic, where a maximum number of embryos is not established by law, although most clinics recommend transferring one or two embryos.
Scientific Society recommendations on the number of embryos to transfer
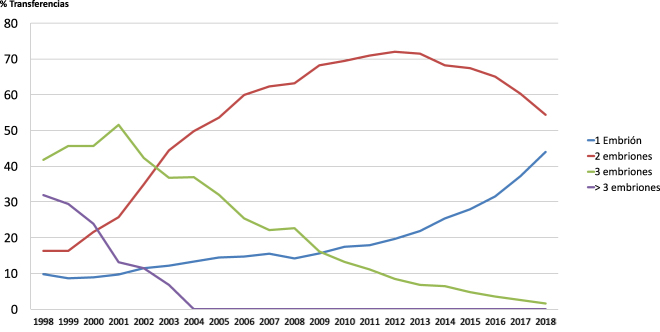
With the aim of reducing the rate of ART-related multiple pregnancies, the most relevant scientific societies have launched information campaigns to raise awareness on the effects of multiple embryo transfer. In 2002, the Spanish Society of Human Reproduction and Embryology (ESHRE) reviewed ART-associated risks and concluded that the purpose of in vitro fertilization (IVF) treatment is the birth of a single healthy infant; therefore, multiple pregnancy is but a complication of these treatments [11]. The Society for Assisted Reproductive Technology (SART) and the American Society for Reproductive Medicine (ASRM) started to raise awareness about the need to reduce the number of embryos to transfer. Indeed, SET accounted only for 1% of the total of embryo transfers performed in USA in 2002 [12], [13]. The same year, in Spain, fresh three-embryo transfer accounted for 42.3%, two-embryo represented 34.9%, and single-embryo transfer accounted for 11.4%, whereas these rates changed to 1.6, 54.4 and 44%, respectively in 2018 (Figure 1) [3], [5].
Figure 1:
Evolution of fresh embryo transfer policies in Spain between 1998 and 2018, according to the 2018 National Registry of Activity—SEF Registry.
In relation to patients, the literature shows that twin pregnancy is widely accepted among patients, especially as infertility time and fertilization failures increase. Some studies, however, demonstrate that patient preference for multiple pregnancy decreases as their understanding of the associated risks increases, and acceptance of SET improves [14]. At present, awareness-raising campaigns and advances in laboratory technologies have resulted in eSET being offered as the best option in most fertility centers [15].
Improvements and advances in eSET technologies
Blastocyst culture
The best quality embryo is selected for eSET. Transfer can be performed at cell stage (2–3 days of development), morule stage (day 4), or blastocyst stage (days 5–6–7). However, laboratories increasingly opt for blastocyst transfer, as it guarantees the selection of the best embryo [16]. Blastocyst is the embryo structure with a highest cellularity and complexity obtained after laboratory culture fertilization and before it is transferred to the uterus. It is an advanced stage that has proven to be able to develop in vitro and differentiate into an inner cell mass that will give rise to the embryo and a trophectoderm that will develop into the placenta. This level of development, along with a qualitative evaluation of the embryo by morphology allow for the selection of the embryo with the best implantation potential [17]. Hence, culture of the embryo until the blastocyst phase, commonly known as long culture, is becoming more frequent. The implementation of this technique requires close control and monitoring of laboratory conditions and procedures. This is made possible by improvements in culture media and quality controls, and the development of time-lapse incubators [18].
The incubators traditionally used in the 80s regulated temperature and CO2, but they were only partially compartmentalized. This way, when incubators were opened, the conditions of all slices were altered. Temperature is set at 37 °C to simulate in vivo conditions, whereas CO2 control is essential to maintain an adequate pH in the culture medium (pH: 7.2–7.4) and ensure the correct development of the embryo [18], [19]. At present, the most widespread type of incubator is the so-called time-lapse incubators, where three gases are employed: CO2, O2 and N2. N2 reduces O2 concentrations to hypoxia levels (5%), as it occurs in the uterus, which has been proven to be essential for the development of the blastocyst [20]. In addition, in these incubators each slide is stored in a separate compartment, which minimizes that culture conditions are disturbed when the incubator is opened. These incubators, also known as benchtop incubators, provide optimal control and monitoring of gas concentrations and temperature.
Embryo cryopreservation: vitrification
Vitrification/devitrification has also been a key to the spread of eSET programs. This procedure involves ultrarapid freezing/thawing by the exchange of water and cryoprotective substances, thereby preventing the formation of ice crystals, reaching survival rates of 78–100% [21]. This technique represents a turning point in ART, as slow freezing, which induces the formation of ice crystals inside cells, was the only technique available so far. This phenomenon causes embryo degeneration, with a survival rate of only 60%. On the other hand, the process is time-consuming and requires the use of more sophisticated equipment as compared to vitrification [22]. The high chances of live birth associated with vitrification facilitate eSET choice. Moreover, vitrification has allowed the spread of deferred frozen-instead of fresh-embryo transfer, which allows optimization of endometrial preparation and uterine environment, and increases the chances of success [23].
These technological advances help create optimal conditions for embryo development and cryopreservation in vitro and facilitate the selection of the embryo with the best implantation potential. This increases the chances of success of eSET, while multiple pregnancy rates are reduced. After the introduction of these changes in the laboratory, the criterion to calculate live birth rates is no longer based on the pregnancy rate per transfer but on “cumulative pregnancy rates”. The latter is based on all transfers of fresh or frozen embryos obtained from a single cycle of ovarian stimulation [23].
Factors influencing eSET choice
Medical contraindications to multiple embryo transfer
Although multiple pregnancy is also a risk factor for healthy women, the term mSET (medical SET) is reserved to women with total or relative contraindication to multifetal gestation for known medical conditions that may interfere with a good outcome, rather than for reasons associated with the fetus [6]. Elective SET is only mandatory in these circumstances. Women should be informed about these risks [6], [24]:
Absolute contraindications:
-
–
Congenital uterine Müllerian anomaly, associated with a high risk for preterm birth.
-
–
History of ruptured uterus.
-
–
Cervical incompetence.
-
–
Turner syndrome.
-
–
Severe systemic disease.
-
–
Severe psychiatric disease.
-
–
Early multiple pregnancy loss.
-
–
Insulin-dependent diabetes.
Relative contraindications:
-
–
History of preterm labor in a singleton/twin pregnancy.
-
–
Women without a couple.
-
–
Couples formed by two women.
-
–
Women of advanced age.
-
–
Paraplegic women.
-
–
Moderate/mild systemic disease.
-
–
Moderate/mild psychiatric disease.
-
–
Thromboembolic disease.
Age of the patient
According to ASRM recommendations, eSET is the approach of choice in women with a good pregnancy prognosis. Thus, eSET is recommended for women younger than 35 on first or second cycle of ovarian stimulation, with a pregnancy in the previous cycle or egg receptors. Elective SET can also be considered in 35–40-year-old women with good quality blastocysts [25]. In women >40 years of age, the decision to transfer one or two blastocysts will depend on whether there are more than two transferrable/vitrificable blastocysts. Although the live birth rate doubles in elective two-blastocyst transfer, the rate of multiple birth increases from 0 to 22% [26]. In the 40–43 age range, age is not a predictive factor of the live birth rate, with a similar cumulative live birth rate in the two cases, but with a dramatic increase in the rate of multiple births (0 vs. 14.9%) [27]. However, previous recommendations about the number of embryos to transfer should also be adapted to the actual ovarian age, which may be enhanced or reduced [24].
In some countries, the number of embryos to transfer is regulated by national laws according to the age of the patient. Thus, we find legally enforced SET in Belgium, which regulates the number of embryos to transfer according to the age of the woman and the number of cycles completed. Multiple fresh embryo transfer is not permitted to women ≤36 years of age in the first or second cycle, except if low quality embryos are obtained in the second cycle. The government reimburses the costs of each cycle, but compliance is mandatory for all women undergoing a fertility treatment in Belgium even if they pay the treatment themselves [28].
Embryo stage: Day 3 (D3) vs. Day 5 (D5)
The rationale for extending the time of culture and performing fresh blastocyst-stage transfer vs. D3 is that it improves uterine/embryo synchrony and allows for viable embryo self-selection, which results in better pregnancy and live birth rates [17, 29, 30]. However, there are no significant differences in multiple pregnancy rates, pregnancy loss rates, or cumulative pregnancy rates per cycle performed with blastocysts vs. D3 cleavage-stage embryos [17]. Additionally, high-quality cleavage-stage embryos (7–8 cells, minimal fragmentation, without multinucleation) show high implantation rates [31]. Thus, clinical pregnancy rates after the transfer of two good-quality cleavage-stage embryos are similar to that associated with elective single blastocyst transfer, but with the former being associated with higher twin pregnancy rates (43.26 vs. 0.6%) [32]. Therefore, the optimal day of transfer is contingent on the number and quality of embryos in D3.
Elective SET should also be considered in frozen cleavage-stage embryo transfer, especially after thawed blastocyst culture, as the rate of multiple pregnancy is significant, although lower than that of fresh embryo transfer.
In summary, eSET can be considered in the two embryo stages, taking into account couple sterility and embryo quality, and the performance of the cyropreservation program of the clinic in case of frozen embryo transfer [25]. Patients who desire a D5 transfer should be aware that they have more chances of no embryo being eventually available for transfer, and of a lower number of embryos being available for cryopreservation, since not all D3 embryos reach the blastocyst stage. Similarly, patients should be familiar with evidence of epigenetic alterations in long embryo culture in animal studies caused by the modulation of DNA methylation, which results in genetic imprinting defects [23].
Number of good quality embryos
According to ASRM guidelines, eSET should be performed only in women with more than one high-quality embryo available and those with additional embryos for cryopreservation [25]. Double embryo transfer with one high-quality embryo plus one poor-quality embryo, at the blastocyst stage, does not increase the live birth rate but increases multiple births when compared with SET with a high-quality embryo during fresh or frozen embryo transfer treatment [33]. These results are consistent with previous studies and support the use of eSET when there is at least a high-quality embryo available [23], [34].
Controlled randomized studies demonstrate that fresh single top-quality blastocyst transfer is associated with a significantly higher ongoing pregnancy rate per cycle as compared to D3 embryo transfer [35], [36].
Conventional morphologic embryo assessment vs. “Time-lapse”
The most widespread method for the selection of embryos is based on morphological evaluation and categorization according to factors such as cell number and symmetry, multinucleation, fragmentation and growth rate, to name a few. In Spain, the Association for the Study of Reproduction Biology (ASEBIR) has established a morphological classification system based on scientific evidence, expert opinion, external quality controls, and multicentric surveys and studies. This system associates a set of specific morphologic characteristics with an estimated likelihood of implantation [37]. On the other hand, assessment of embryo morphology could only be performed by direct observation under an inverted microscope. Although this method is simple, it only provides information of the embryo at the time of observation and is subject to interobserver variability, therefore, embryo evaluation is subjective [38]. The emergence of time-lapse ART technology represents a milestone in assisted reproduction. Time-lapse ART technology is based on a microscope equipped with a camera that allows continuous, noninvasive observation of embryos. This technology allows observation without having to take embryos out of the incubator, which disturbs culture conditions and may alter their development. Time-lapse incubators have made it possible to develop a more objective and reliable method for assessing embryo quality. Thus, the large amount of pictures taken provides thorough information about the morphological development of the embryo [39]. In addition, it offers the advantage that embryos can be selected on the basis of morphological, dynamic or morphokinetic criteria [19]. Therefore, time-lapse is especially useful for eSET, as it provides valuable data from continuous observation of embryonic development [40].
Numerous studies have demonstrated that embryo culture in time-lapse incubators does not affect their development as compared to conventional incubators [41]. In contrast, this new technology provides safe and stable culture conditions that favor embryonic development. A drawback to time-lapse incubator technology is the higher culture cost. However, the information obtained helps embryologist select the top-quality embryo with a higher probability of implantation by the use of predictive programs and algorithms based on more than 70 morphokinetic parameters. Only time-lapse technology enables observation of specific developmental events such as multinucleation, direct division (division from one to three cells) and cell fusion. Observing these events is crucial, as they are associated with a low implantation potential [42]. A plethora of studies have been performed to compare outcomes after embryo selection by morphokinetic criteria vs. specific morphologic observation. According to some authors, the morphokinetic approach improves implantation, pregnancy and live birth rate [43]. Other authors, however, have not found any significant relationship between improved outcomes and the use of time-lapse incubators [44]. There is not strong evidence that live birth rates are significantly higher in time-lapse culture and assessment vs. conventional methods. Therefore, the use of time-lapse technology to opt for eSET cannot be recommended [45].
Preimplantation genetic testing for aneuploidy (PGT-A)
The rate of oocyte aneuploidy increases with maternal age and affects pregnancy and miscarriage rates [46]. PGT-A enables the selection of embryos with a correct set of chromosomes and exclude those with aneuploidy. This way, the rate of healthy live birth increases with the transfer of a single euploid embryo [47]. PGT-A requires the use of a safe technology that does not damage the embryo. The most extended technique today is blastocyst-stage trophectoderm biopsy. By this strategy, a higher number of cells are available for genetic testing, which improves the sensitivity of the technique. Thus, this technique significantly reduces the number of undiagnosed embryos (<5%) and does not require inner cell mass handling, which reduces risks. It also allows for the selection of euployd embryos with a better implantation potential even after the blastocyst is biopsied or vitrified one or two times [48]. Pregnancy rates do decrease with vitrification after a second embryo biopsy [48]. All this said, the guidelines where a specific number of embryos to be transferred are recommended are no longer based on maternal age, the number of embryos available or embryo stage, but also on the ploidy of the embryo [9], [46]. A variety of studies advocate for the use of PGT-A to increase the use of eSET in patients undergoing IVF [47], as the combination of the two approaches increases the live birth rate and reduces the multiple pregnancy rates [49]. There is evidence that PGT-A testing in women 35–40 years of age improves clinical and live birth rates, and mitigates the negative effects of maternal age on outcomes. However, it does not seem to improve cumulative live birth rates [50], [51]. Additionally, it has been observed that implantation, clinical pregnancy and live birth rates remain stable regardless of maternal age at eSET combined with PGT-A, and no significant differences have been observed with double embryo transfer [52]. Finally, it is important to consider that culture to blastocyst in time-lapse incubators combined with PGT-A-based embryo selection vs. conventional incubators significantly improves clinical pregnancy rates [53]. However, although PGT-A may benefit some groups of patients, there is no conclusive evidence available supporting its routine use as it increases treatment cost notably, especially in young women, and it is an invasive technique.
SET live birth rates
Comparative model: 2xeSET vs. 1xDET (Double Embryo Transfer)
Live birth rates associated with SET—whether it is elective or not-are usually lower (10–40%) than those expected after multiple-embryo transfer [54], [55]. In contrast, some authors report similar live birth rates for eSET to those obtained after nonelective multiple-embryo transfer [56]. There is also evidence that similar outcomes can be obtained in women of advanced age with a poorer prognosis [27]. Some authors, however, dispute the efficacy of SET in this group of patients [57]. On the other hand, there is enough evidence that the ongoing pregnancy and cumulative live birth rate are similar when a single cycle of double embryo transfer is compared with repeated SET [58]. Likewise, many studies show that SET helps reduce the high multiple pregnancy rates associated with multiple-embryo transfer (20–50%) to spontaneous multiple pregnancy rates (3%) [58], [59]. In conclusion, the efficacy of two SETs equals, or even improves with eSET, that of DET while avoiding he risks associated with multiple pregnancy.
Cost-effectiveness of eSET
Health costs of multiple pregnancy for the mother and her offspring
Multiple pregnancy is considered the main iatrogenic complication of ART, as it is associated with the occurrence of adverse events, which may affect both the mother and her offspring [13]. The main negative effects of multiple pregnancy are associated with preterm birth, fetal growth restriction, jaundice or respiratory complications. The probability of preterm labor is 5–9 times higher in multiple pregnancy as compared to singleton pregnancy [60]. An increase has been described in the incidence of maternal complications, including pre-eclampsia, gestational diabetes, placenta previa, placental detachment, premature rupture of membranes and caesarean section [60], [61], [62]. These complications may have more severe effects in women of advanced age [63]. Thus, multiple pregnancy is a known risk factor of maternal and fetal morbidity and mortality.
Economic, social and health costs of multiple pregnancy
According to the literature, SET requires a higher number of embryo transfers to achieve pregnancy, as compared to multiple embryo transfer [58]. This involves a higher cost of ART: more hormone stimulation, embryo transfers and vitrification/thawing cycles. In addition, the costs of multiple pregnancy and delivery are 2–7 higher than those of singleton ones [64]. If pediatric care during the first year of life is included, the economic cost of multiple delivery is 20 times higher than singleton delivery [65].
ART costs are borne either by the national health system or by the patients themselves, which influences the choice for an embryo transfer strategy. Multiple pregnancies also involve additional costs in the long-term including clothing, car seats, food, and schooling, to name but a few [55]. After an analysis of costs and outcomes of DET vs. eSET, Fiddelers et al. [66] concluded that DET involves a higher cost from the economic point of view. Therefore, eSET emerges as the most cost-effective strategy, when more than one embryo transfer cycle is considered [66].However, it is worth noting that multiple pregnancy rate after multiple-embryo transfer decreases as the age of the mother increases, so this approach can be considered cost-effective in women of advanced age [67].
In conclusion, multiple pregnancies have a high social and health cost, which can be reduced with repeated SET without it affecting ART effectiveness, if we consider cumulative live birth rates [68].
Psychosocial costs of multiple pregnancy for patients
There are some psycho-social factors associated with pregnancy and delivery that should be considered. According to some population-based studies, women are more likely to have depression and episodes of postpartum stress after multiple delivery, as compared to singleton delivery. Nevertheless, no significant differences were observed in the probability to experience these symptoms following assisted and natural conception [69].
Likewise, some authors associate multiple birth with decreased marital satisfaction, loss of productivity, and a lower probability of the mother to have a paid job [55], [64]. These data support the need to reinforce psychological support to parents of multiples.
Elective SET implementation
Education and counseling
Despite the risks associated with multiple pregnancy and delivery, up to 80% of patients’ desire a multiple-embryo transfer as the first option [55]. The main reasons include unawareness of the associated risks, desire to maximize the chances of success, or reduce the cost of ART, or the idea to achieve a multiple birth in a single attempt. Informing patients on cumulative live birth rate, health risks, and economic and emotional implications of multiple births has been proven to be effective in increasing the choice of SET [14]. The provision of information from personalized prediction tools that estimate the chances of IVF success has also demonstrated to be useful in helping patients make decisions about IVF, as they are perceived as an objective source of information [55].
Economic and social incentive policies
Some countries such as Turkey, Sweden, Denmark, Belgium, New Zealand or Canada have established or promoted a national SET policy. The purpose of these policies is twofold: to avoid health risks for the mother and the newborn, and reduce the high health costs associated with multiple births. Measures range from restrictions to multiple-embryo transfers for most patients to limitations to the number of embryos transferred for patients to the ART process to be partially or totally borne by the national health system. These countries have achieved to increase SET rates and reduce multiple births to 80 and 5%, respectively, in the case of Australia, whereas cumulative clinical pregnancy and live birth rates have remained stable [55].
This evidence demonstrates that educational interventions along with SET incentive policies in women with a good prognosis and public funding are effective in promoting the choice for SET in countries as culturally distant as USA, Japan and New Zealand [14]. Economic stimuli include public funding, total/partial coverage of ART procedures by insurance providers, or promotional campaigns of AHR centers, which may include cryopreservation and/or frozen embryo transfer at free cost. However, it is worth noting that SET incentive measures do not seem to be as effective in countries where public funding is available for ART treatments, as it is the case of Denmark and Sweden [14].
Conclusions
More than seven million children have been born in the world as a result of assisted conception. Since the early days of ART, high multiple pregnancy rates have posed a difficult dilemma due to the higher risk for preterm labor and its higher associated maternal and fetal morbidity and mortality rates.
In the recent years, significant advances have been made in the field of reproductive medicine, which implementation has resulted in a significant increase in live birth rates. Special attention needs to be paid on AHR laboratory innovations such as culture to blastocyst stage, cryopreservation by vitrification, time-lapse technology or PGT-A. These techniques favor the SET application, even in patients with a poor prognosis, without it affecting live birth rates.
Despite continuous progress in the field of ART, potentially significant differences will be observed between SET and multiple-embryo transfer in pregnancy and live birth rates as long as outcomes are expressed as rates per embryo transfer. Cumulative rates are more precise, as they include fresh and frozen embryo transfers of embryos obtained from the same cycle.
This review is aimed at raising awareness among ART professionals about the impact that AHR-related multiple pregnancy has on public health and promoting SET as the strategy of choice for having a healthy full-term newborn at home.
Footnotes
Article Note: The original article can be found here: https://doi.org/10.1515/almed-2020-0095.
Research funding: Not applicable.
Author contributions: All authors have contributed equitably to this work, assume full responsibility for the final version of the manuscript and approve its submission.
Competing interests: Authors state no conflict of interest.
References
- 1.Steptoe PC, Edwards RG. Birth after the reimplantation of a human embryo. Lancet. 1978;312:366. doi: 10.1016/s0140-6736(78)92957-4. [DOI] [PubMed] [Google Scholar]
- 2.De Geyter C, Calhaz-Jorge C, Kupka MS, Wyns C, Mocanu E, Motrenko T, et al. ART in Europe, 2015: results generated from European registries by ESHRE†. Hum Reprod Open. 2020;2020:hoz038. doi: 10.1093/hropen/hoaa038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Registro Nacional de Actividad 2018-Registro SEF Ministerio de Sanidad, Consumo y Bienestar Social (MSCBS) [17 Nov 2020]. https://www.registrosef.com/public/docs/sef2018_IAFIVm.pdf Disponible en. Fecha de consulta.
- 4.De Geyter C, Calhaz-Jorge C, Kupka MS, Wyns C, Mocanu E, Motrenko T, et al. ART in Europe, 2014: results generated from European registries by ESHRE: the European IVF-monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE) Hum Reprod. 2018;33:1586–601. doi: 10.1093/humrep/dey242. [DOI] [PubMed] [Google Scholar]
- 5.Registro FIV-ICSI de la Sociedad Española de Fertilidad . Sociedad Española de Fertilidad (SEF) Año; 2002. [17 Nov 2020]. https://www.registrosef.com/public/docs/sef2002_FIV.pdf Disponible en. Fecha de consulta. [Google Scholar]
- 6.Gerris J, Adamson GD, De Sutter P, Racowsky C, editors. Single embryo transfer. Cambridge: Cambridge University Press; 2008. [Google Scholar]
- 7.Kreyenfeld M, Konietzka D, editors. Childlessness in Europe: contexts, causes, and consequences. Springer International Publishing; 2017. [Google Scholar]
- 8.Comparative analysis of medically assisted reproduction in the EU: regulation and technologies (SANCO/2008/C6/051) [29 Oct 2020]. https://ec.europa.eu/health//sites/health/files/blood_tissues_organs/docs/study_eshre_en.pdf Available at.
- 9.Präg P, Mills MC. Assisted Reproductive technology in Europe: usage and Regulation in the Context of Cross-border Reproductive Care. In: Kreyenfeld M, Konietzka D, editors. Childlessness in Europe: contexts, causes, and consequences. Springer International Publishing; 2017. pp. 289–309. [Google Scholar]
- 10.Landuyt LV, Verheyen G, Tournaye H, Camus M, Devroey P, Steirteghem AV. New Belgian embryo transfer policy leads to sharp decrease in multiple pregnancy rate. Reprod BioMed Online. 2006;13:765–71. doi: 10.1016/s1472-6483(10)61022-x. [DOI] [PubMed] [Google Scholar]
- 11.Land JA, Evers JL. Risks and complications in assisted reproduction techniques: report of an ESHRE consensus meeting. Hum Reprod. 2003;18:455–7. doi: 10.1093/humrep/deg081. [DOI] [PubMed] [Google Scholar]
- 12.2009 Assisted Reproductive technology success rates: national summary and fertility clinic reports. Centers for Disease Control and Prevention; American Society for Reproductive Medicine; Society for Assisted Reproductive Technology. Atlanta: U.S. Department of Health and Human Services; [17 Nov 2020]. https://www.cdc.gov/art/Archived-PDF-Reports/ART_2009_Full.pdf Available at. [Google Scholar]
- 13.Practice Committee of the American Society for Reproductive Medicine Electronic address: ASRM@asrm.org, Practice Committee of the Society for Assisted Reproductive Technology. Guidance on the limits to the number of embryos to transfer: a committee opinion. Fertil Steril. 2017;107:901–3. doi: 10.1016/j.fertnstert.2017.02.107. [DOI] [PubMed] [Google Scholar]
- 14.Sunderam S, Boulet SL, Jamieson DJ, Kissin DM. Effects of patient education on desire for twins and use of elective single embryo transfer procedures during ART treatment: a systematic review. Reprod Biomed Soc Online. 2018;6:102–19. doi: 10.1016/j.rbms.2018.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heino A, Gissler M, Hindori-Mohangoo AD, Blondel B, Klungsøyr K, Verdenik I, et al. Variations in multiple birth rates and impact on perinatal outcomes in Europe. PLoS ONE. 2016;11:e0149252. doi: 10.1371/journal.pone.0149252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang X, Gao Y, Liu W, Liu J, Wu L, Xiong S, et al. Frozen blastocyst embryo transfer vs. frozen cleavage-stage embryo transfer in couples with recurrent implantation failure: a cohort study. Hum Fertil (Camb) 2019;5:1–6. doi: 10.1080/14647273.2019.1633021. [DOI] [PubMed] [Google Scholar]
- 17.Glujovsky D, Farquhar C, Quinteiro Retamar AM, Alvarez Sedo CR, Blake D. Cleavage stage versus blastocyst stage embryo transfer in assisted reproductive technology. Cochrane Database Syst Rev. 2016:CD002118. doi: 10.1002/14651858.CD002118.pub5. [DOI] [PubMed] [Google Scholar]
- 18.Kalleas D, McEvoy K, Horne G, Roberts SA, Brison DR. Live birth rate following undisturbed embryo culture at low oxygen in a time-lapse incubator compared to a high-quality benchtop incubator. Hum Fertil (Camb) 2020;26:1–7. doi: 10.1080/14647273.2020.1729423. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 19.Kemper JM, Wang R, Vuong LN, Mol BW. Single embryo transfer with frozen transfer of all remaining embryos without further embryonic testing should be the standard of care in IVF. BJOG. 2019;126:142–4. doi: 10.1111/1471-0528.15486. [DOI] [PubMed] [Google Scholar]
- 20.Kirkegaard K, Hindkjaer JJ, Ingerslev HJ. Effect of oxygen concentration on human embryo development evaluated by time-lapse monitoring. Fertil Steril. 2013;99:738–44.e4. doi: 10.1016/j.fertnstert.2012.11.028. [DOI] [PubMed] [Google Scholar]
- 21.Rienzi L, Gracia C, Maggiulli R, LaBarbera AR, Kaser DJ, Ubaldi FM, et al. Oocyte, embryo and blastocyst cryopreservation in ART: systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum Reprod Update. 2017;23:139–55. doi: 10.1093/humupd/dmw038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liebermann J. Vitrification of human blastocysts: an update. Reprod Biomed Online. 2009;19:4328. doi: 10.1016/s1472-6483(10)61073-5. [DOI] [PubMed] [Google Scholar]
- 23.Wei D, Liu JY, Sun Y, Shi Y, Zhang B, Liu JQ, et al. Frozen versus fresh single blastocyst transfer in ovulatory women: a multicentre, randomised controlled trial. Lancet. 2019;393:1310–8. doi: 10.1016/s0140-6736(18)32843-5. [DOI] [PubMed] [Google Scholar]
- 24.Gleicher N, Barad D. The relative myth of elective single embryo transfer. Hum Reprod. 2006;21:1337–44. doi: 10.1093/humrep/del026. [DOI] [PubMed] [Google Scholar]
- 25.Practice Committee of Society for Assisted Reproductive Technology; Practice Committee of American Society for Reproductive Medicine Elective single-embryo transfer. Fertil Steril. 2012;97:835–42. doi: 10.1016/j.fertnstert.2011.11.050. [DOI] [PubMed] [Google Scholar]
- 26.Tannus S, Son WY, Dahan MH. Elective single blastocyst transfer in advanced maternal age. J Assist Reprod Genet. 2017;34:741–8. doi: 10.1007/s10815-017-0906-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tannus S, Cohen Y, Son WY, Shavit T, Dahan MH. Cumulative live birth rate following elective single blastocyst transfer compared with double blastocyst transfer in women aged 40 years and over. Reprod Biomed Online. 2017;35:733–8. doi: 10.1016/j.rbmo.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 28.Van Landuyt L, Verheyen G, Tournaye H, Camus M, Devroey P, Van Steirteghem A. New Belgian embryo transfer policy leads to sharp decrease in multiple pregnancy rate. Reprod Biomed Online. 2006;13:765–71. doi: 10.1016/s1472-6483(10)61022-x. [DOI] [PubMed] [Google Scholar]
- 29.Papanikolaou EG, Kolibianakis EM, Tournaye H, Venetis CA, Fatemi H, Tarlatzis B, et al. Live birth rates after transfer of equal number of blastocysts or cleavage-stage embryos in IVF. A systematic review and meta-analysis. Hum Reprod. 2008;23:91–9. doi: 10.1093/humrep/dem339. [DOI] [PubMed] [Google Scholar]
- 30.Practice Committee of the American Society for Reproductive Medicine; Practice Committee of the Society for Assisted Reproductive Technology Electronic address: asrm@asrm.org. Blastocyst culture and transfer in clinically assisted reproduction: a committee opinion. Fertil Steril. 2018;110:1246–52. doi: 10.1016/j.fertnstert.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 31.Dennis SJ, Thomas MA, Williams DB, Robins JC. Embryo morphology score on day 3 is predictive of implantation and live birth rates. J Assist Reprod Genet. 2006;23:171–5. doi: 10.1007/s10815-006-9027-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li M, Wang H, Ma C, Shi J. Transferring two grades I cleavage-stage embryo might not be a good protocol. Gynecol Endocrinol. 2017;33:557–9. doi: 10.1080/09513590.2017.1302420. [DOI] [PubMed] [Google Scholar]
- 33.Dobson SJA, Lao MT, Michael E, Varghese AC, Jayaprakasan K. Effect of transfer of a poor quality embryo along with a top quality embryo on the outcome during fresh and frozen in vitro fertilization cycles. Fertil Steril. 2018;110:655–60. doi: 10.1016/j.fertnstert.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 34.Mancuso A, Kapfhamer J. With a good quality blastocyst, single embryo transfer remains the best choice. Fertil Steril. 2018;110:631. doi: 10.1016/j.fertnstert.2018.06.024. [DOI] [PubMed] [Google Scholar]
- 35.Papanikolaou EG, Camus M, Kolibianakis EM, Van Landuyt L, Van Steirteghem A, Devroey P. In vitro fertilization with single blastocyst-stage versus single cleavage-stage embryos. N Engl J Med. 2006;354:1139–46. doi: 10.1056/nejmoa053524. [DOI] [PubMed] [Google Scholar]
- 36.Zech NH, Lejeune B, Puissant F, Vanderzwalmen S, Zech H, Vanderzwalmen P. Prospective evaluation of the optimal time for selecting a single embryo for transfer: day 3 versus day 5. Fertil Steril. 2007;88:244–6. doi: 10.1016/j.fertnstert.2006.11.070. [DOI] [PubMed] [Google Scholar]
- 37.Cuevas Saiz I, Carme Pons Gatell M, Vargas MC, Delgado Mendive A, Rives Enedáguila N, Moragas Solanes M, et al. The Embryology Interest Group: updating ASEBIR’s morphological scoring system for early embryos, morulae and blastocysts. MEDRE. 2018;5:42–54. doi: 10.1016/j.medre.2017.11.002. [DOI] [Google Scholar]
- 38.Van Royen E, Mangelschots K, De Neubourg D, Valkenburg M, Van de Meerssche M, Ryckaert G, et al. Characterization of a top quality embryo, a step towards single-embryo transfer. Hum Reprod. 1999;14:2345–9. doi: 10.1093/humrep/14.9.2345. [DOI] [PubMed] [Google Scholar]
- 39.Kirkegaard K, Ahlström A, Ingerslev HJ, Hardarson T. Choosing the best embryo by time lapse versus standard morphology. Fertil Steril. 2015;103:323–32. doi: 10.1016/j.fertnstert.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Armstrong S, Bhide P, Jordan V, Pacey A, Farquhar C. Time-lapse systems for embryo incubation and assessment in assisted reproduction. Cochrane Database Syst Rev. 2018;5:CD011320. doi: 10.1002/14651858.CD011320.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kirkegaard K, Hindkjaer JJ, Grøndahl ML, Kesmodel US, Ingerslev HJ. A randomized clinical trial comparing embryo culture in a conventional incubator with a time-lapse incubator. J Assist Reprod Genet. 2012;29:565–72. doi: 10.1007/s10815-012-9750-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barrie A, Homburg R, McDowell G, Brown J, Kingsland C, Troup S. Preliminary investigation of the prevalence and implantation potential of abnormal embryonic phenotypes assessed using time-lapse imaging. Reprod Biomed Online. 2017;34:455–62. doi: 10.1016/j.rbmo.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 43.Adamson GD, Abusief ME, Palao L, Witmer J, Palao LM, Gvakharia M. Improved implantation rates of day 3 embryo transfers with the use of an automated time-lapse–enabled test to aid in embryo selection. Fertil Steril. 2016;105:369–75.e6. doi: 10.1016/j.fertnstert.2015.10.030. [DOI] [PubMed] [Google Scholar]
- 44.Goodman LR, Goldberg J, Falcone T, Austin C, Desai N. Does the addition of time-lapse morphokinetics in the selection of embryos for transfer improve pregnancy rates? A randomized controlled trial. Fertil Steril. 2016;105:275–85.e10. doi: 10.1016/j.fertnstert.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 45.Armstrong S, Bhide P, Jordan V, Pacey A, Marjoribanks J, Farquhar C. Time-lapse systems for embryo incubation and assessment in assisted reproduction. Cochrane Database Syst Rev. 2019;5:CD011320. doi: 10.1002/14651858.CD011320.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hassold T, Hunt P. Maternal age and chromosomally abnormal pregnancies: what we know and what we wish we knew. Curr Opin Pediatr. 2009;21:703–8. doi: 10.1097/mop.0b013e328332c6ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ubaldi FM, Capalbo A, Colamaria S, Ferrero S, Maggiulli R, Vajta G, et al. Reduction of multiple pregnancies in the advanced maternal age population after implementation of an elective single embryo transfer policy coupled with enhanced embryo selection: pre- and post-intervention study. Hum Reprod. 2015;30:2097–106. doi: 10.1093/humrep/dev159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bradley CK, Livingstone M, Traversa MV, McArthur SJ. Impact of multiple blastocyst biopsy and vitrification-warming procedures on pregnancy outcomes. Fertil Steril. 2017;108:999–1006. doi: 10.1016/j.fertnstert.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 49.Penzias A, Bendikson K, Butts S, Coutifaris C, Falcone T, Fossum G, et al. The use of preimplantation genetic testing for aneuploidy (PGT-A): a committee opinion. Fertil Steril. 2018;109:429–36. doi: 10.1016/j.fertnstert.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 50.Munné S, Kaplan B, Frattarelli JL, Child T, Nakhuda G, Shamma FN, et al. Preimplantation genetic testing for aneuploidy versus morphology as selection criteria for single frozen-thawed embryo transfer in good-prognosis patients: a multicenter randomized clinical trial. Fertil Steril. 2019;112:1071–9.e7. doi: 10.1016/j.fertnstert.2019.07.1346. [DOI] [PubMed] [Google Scholar]
- 51.Kang HJ, Melnick AP, Stewart JD, Xu K, Rosenwaks Z. Preimplantation genetic screening: who benefits? Fertil Steril. 2016;106:597–602. doi: 10.1016/j.fertnstert.2016.04.027. [DOI] [PubMed] [Google Scholar]
- 52.Simon AL, Kiehl M, Fischer E, Proctor JG, Bush MR, Givens C, et al. Pregnancy outcomes from more than 1,800 in vitro fertilization cycles with the use of 24-chromosome single-nucleotide polymorphism–based preimplantation genetic testing for aneuploidy. Fertil Steril. 2018;110:113–21. doi: 10.1016/j.fertnstert.2018.03.026. [DOI] [PubMed] [Google Scholar]
- 53.Yang Z, Zhang J, Salem SA, Liu X, Kuang Y, Salem RD, et al. Selection of competent blastocysts for transfer by combining time-lapse monitoring and array CGH testing for patients undergoing preimplantation genetic screening: a prospective study with sibling oocytes. BMC Med Genom. 2014;7:38. doi: 10.1186/1755-8794-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baruffi RL, Mauri AL, Petersen CG, Nicoletti A, Pontes A, Oliveira JB, et al. Single-embryo transfer reduces clinical pregnancy rates and live births in fresh IVF and Intracytoplasmic Sperm Injection (ICSI) cycles: a meta-analysis. Reprod Biol Endocrinol. 2009;7:36. doi: 10.1186/1477-7827-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tobias T, Sharara FI, Franasiak JM, Heiser PW, Pinckney-Clark E. Promoting the use of elective single embryo transfer in clinical practice. Fertil Res Pract. 2016;2:1. doi: 10.1186/s40738-016-0024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Freeman MR, Hinds MS, Howard KG, Howard JM, Hill GA. Guidance for elective single-embryo transfer should be applied to frozen embryo transfer cycles. J Assist Reprod Genet. 2019;36:939–46. doi: 10.1007/s10815-019-01433-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cutting R. Single embryo transfer for all. Best Pract Res Clin Obstet Gynaecol. 2018;53:30–7. doi: 10.1016/j.bpobgyn.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 58.Kamath MS, Mascarenhas M, Kirubakaran R, Bhattacharya S. Number of embryos for transfer following in vitro fertilisation or intra-cytoplasmic sperm injection. Cochrane Database Syst Rev. 2020;8:CD003416. doi: 10.1002/14651858.CD003416.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Monteleone PA, Petersen PG, Peregrino PF, Miorin J, Gomes AP, Fujii MG, et al. Should single embryo transfer be used in patients with any kind of infertility factor? Preliminary outcomes. JBRA Assist Reprod. 2019;23:200–4. doi: 10.5935/1518-0557.20190006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine ACOG Practice Bulletin No. 144: multifetal gestations: twin, triplet, and higher-order multifetal pregnancies. Obstet Gynecol. 2014;123:1118–32. doi: 10.1097/01.AOG.0000446856.51061.3e. [DOI] [PubMed] [Google Scholar]
- 61.Sazonova A, Källen K, Thurin-Kjellberg A, Wennerholm U-B, Bergh C. Neonatal and maternal outcomes comparing women undergoing two in vitro fertilization (IVF) singleton pregnancies and women undergoing one IVF twin pregnancy. Fertil Steril. 2013;99:731–7. doi: 10.1016/j.fertnstert.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 62.La Sala GB, Morini D, Gizzo S, Nicoli A, Palomba S. Two consecutive singleton pregnancies versus one twins pregnancy as preferred outcome of in vitro fertilization for mothers and infants: a retrospective case–control study. Curr Med Res Opin. 2016;32:687–92. doi: 10.1185/03007995.2015.1136602. [DOI] [PubMed] [Google Scholar]
- 63.Avnon T, Ovental A, Many A. Twin versus singleton pregnancy in women ≥ 45 years of age: comparison of maternal and neonatal outcomes. J Matern Fetal Neonatal Med. 2019;18:1–6. doi: 10.1080/14767058.2019.1602115. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 64.Chambers GM, Ledger W. The economic implications of multiple pregnancy following ART. Semin Fetal Neonatal Med. 2014;19:254–61. doi: 10.1016/j.siny.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 65.Lemos EV, Zhang D, Voorhis BJV, Hu XH. Healthcare expenses associated with multiple vs singleton pregnancies in the United States. Am J Obstet Gynecol. 2013;209:586.e1–11. doi: 10.1016/j.ajog.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 66.Fiddelers AAA, Severens JL, Dirksen CD, Dumoulin JCM, Land JA, Evers JLH. Economic evaluations of single- versus double-embryo transfer in IVF. Hum Reprod Update. 2007;13:5–13. doi: 10.1093/humupd/dml053. [DOI] [PubMed] [Google Scholar]
- 67.Scotland GS, McLernon D, Kurinczuk JJ, McNamee P, Harrild K, Lyall H, et al. Minimising twins in in vitro fertilisation: a modelling study assessing the costs, consequences and cost-utility of elective single versus double embryo transfer over a 20-year time horizon. BJOG. 2011;118:1073–83. doi: 10.1111/j.1471-0528.2011.02966.x. [DOI] [PubMed] [Google Scholar]
- 68.Crawford S, Boulet SL, Mneimneh AS, Perkins KM, Jamieson DJ, Zhang Y, et al. Costs of achieving live birth from assisted reproductive technology: a comparison of sequential single and double embryo transfer approaches. Fertil Steril. 2016;105:444–50. doi: 10.1016/j.fertnstert.2015.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Van den Akker O, Postavaru GI, Purewal S. Maternal psychosocial consequences of twins and multiple births following assisted and natural conception: a meta-analysis. Reprod BioMed Online. 2016;33:1–14. doi: 10.1016/j.rbmo.2016.04.009. [DOI] [PubMed] [Google Scholar]