Abstract
Using immunofluorescence microscopy, we have examined the dependency of localization among three Bacillus subtilis division proteins, FtsZ, DivIB, and DivIC, to the division site. DivIC is required for DivIB localization. However, DivIC localization is dependent on DivIB only at high growth temperatures, at which DivIB is essential for division. FtsZ localization is required for septal recruitment of DivIB and DivIC, but FtsZ can be recruited independently of DivIB. These localization studies suggest a more specific role for DivIB in division, involving interaction with DivIC.
It is very likely that a large protein complex forms at the division site in bacteria (20, 23, 25). To elucidate the molecular mechanism of cell division, an understanding of how this complex forms and functions is necessary. In Escherichia coli, morphogenetic evidence suggests that the various division proteins are required at different stages of septation: for example, FtsZ and FtsW act early, while FtsA, FtsQ, and FtsI are required for septal elongation and closure (21). Consistent with the order of action of division proteins, FtsZ localizes first, then FtsA and ZipA localize independently of each other (2, 10, 19), and the remaining membrane-bound proteins assemble in the following order: FtsK (26), FtsQ (6), FtsL (9), PBP 3 (FtsI) (28), and FtsN (1). It is unclear precisely when FtsW localizes in this sequence. In these localization dependency studies, no two division proteins were interdependent for septal recruitment, strongly suggesting that in E. coli, proteins are recruited to the division site in a strict linear sequence (28).
In Bacillus subtilis there is no ZipA or FtsN homolog. FtsZ and three membrane-bound division proteins, DivIB (FtsQ homolog) (11), DivIC (FtsL-like) (14, 16), and PBP 2B (PBP 3 homolog) (29), have been shown to localize to the division site (7, 13, 14, 17, 27). All of these proteins appear to be required early in septation (5, 7, 8, 12, 16), although PBP 2B is additionally required for the duration of septal ingrowth (7). As in E. coli, FtsZ probably assembles early since Z rings can form at midcell in the absence of detectable localization of FtsL, DivIC, DivIB, or PBP 2B at 37°C (7, 8, 17, 18). However, the septal recruitment pathway for the membrane-bound division proteins in B. subtilis appears to be different from that in E. coli. FtsL depletion at 37°C results in rapid degradation of DivIC, while DivIB and PBP 2B localizations are undetectable, suggesting that all three proteins localize either after FtsL or together with FtsL (7, 8). Such division protein instability in the absence of another division protein has not been demonstrated in E. coli, suggesting that the septation process, or at least the assembly of the division complex, is regulated somewhat differently in the two organisms. Surprisingly, when PBP 2B is depleted at 37°C, DivIB and DivIC localizations are significantly decreased and occur only at midfilament positions, probably at incomplete septa (7). In contrast, in E. coli the PBP 2B homolog, PBP 3, appears to be required only for the recruitment of FtsN (1). PBP 2B also fails to localize in divIB and divIC temperature-sensitive mutants at the nonpermissive temperature, 48°C (7). The interdependency of DivIB, DivIC, and PBP 2B localization suggests that these three proteins localize together. However, an alternative possibility is that at a lower temperature (30°C) PBP 2B localization assembles earlier than that of DivIB and DivIC, but at 48°C the latter two proteins are needed to maintain PBP 2B at the septal site.
To further elucidate how division proteins of B. subtilis are incorporated into the septal complex, we examined for the first time the dependency of localization of DivIB and DivIC on each other and the dependency of DivIB and DivIC localization on FtsZ. Our observations suggest a role for DivIB in cell division, involving interaction with DivIC.
The B. subtilis strains used are derivatives of B. subtilis 168, which is designated SU5 in our collection. Tryptose blood agar plates containing appropriate antibiotics were used for colony growth. Isopropyl-β-d-thiogalactopyranoside (IPTG) was added to 1 mM, and chloramphenicol, phleomycin, and spectinomycin were used at 5, 2, and 100 μg/ml, respectively. Growth in liquid medium was performed in L broth (without glucose) (22). FtsZ depletion in BB11 cells (Pspac-ftsZ) (4) was achieved by growth (in IPTG-containing medium) at 37°C to an A590 of 0.4 and then resuspension in IPTG-lacking medium to an A590 of 0.1, after filtering and washing with L broth (in IPTG-lacking medium). The resuspended culture was incubated for 1 h at 37°C and then diluted fourfold in IPTG-lacking medium and grown for a further 30 min at 37°C. Samples for immunofluorescence microscopy (IFM) and Western blot analysis were taken prior to and 20, 40, 60, and 90 min after resuspension. Mid-exponential-phase cultures of the divIB null strain, SU321 (13), and its congenic parent, SU5, were obtained by growth at 30°C to an A590 of 0.4. Aliquots were then diluted eightfold into fresh prewarmed media and shifted to 49°C for 1 h. Samples for IFM and Western blotting were harvested prior to and 20, 40, and 60 min after the shift. The temperature-sensitive divIC mutant strain, SU391 (Pspac-RRC), and its parent strain, SU392 (Pspac-divIC) (15), were grown at 30°C to an A590 of 0.4. Aliquots of both cultures were filtered, washed in IPTG-lacking medium, resuspended in medium lacking or containing IPTG to an A590 of ∼0.07, and then incubated at 30 or 45°C for 45 min. Samples of each culture were collected for IFM and Western blot analysis just prior to and 45 min after resuspension. DivIB, DivIC, and FtsZ IFM was performed as described previously (8), with the following minor modifications. Treatment of microscope slides with poly-l-lysine before and after the addition of lysozyme-treated cells to the slide was essential for DivIB and DivIC detection in SU391 and SU392. For FtsZ detection, slides were treated with poly-l-lysine prior to the addition of lysozyme-treated cells to the slide. The cells for DivIC IFM were, in some cases, incubated in 2% bovine serum albumin–0.05% Tween 20–5% whole goat serum (Jackson Immunoresearch) prior to incubation with anti-DivIC antibody. Affinity-purified anti-DivIB, -DivIC, and -FtsZ antibodies were used at 1/10, 1/50 to 1/200, and 1/400 dilutions, respectively. Microscopy and image analysis have been described previously (13). For each experiment, more than 100 cells or approximately 50 filaments (long nonseptate cells) were scored for the presence or absence of localizations. For Western blot analysis, samples (10 ml) of cells were collected at the times listed above and cell lysates were prepared as described previously (12). Western blotting and immunodetection were performed using an alkaline phosphatase detection system (13, 14). Rabbit anti-DivIB, -DivIC, and -FtsZ antisera were used at dilutions of 1/500, 1/1,000, and 1/4,000, respectively.
Z rings are required for targeting of DivIB and DivIC to the division site.
Although it is likely that Z-ring assembly is required for the recruitment of the membrane-bound division proteins DivIB and DivIC, this has not been demonstrated in B. subtilis. To test the effect of FtsZ depletion on targeting of DivIB and DivIC, strain BB11 (Pspac-ftsZ) (4) was grown in the presence of IPTG and then resuspended in IPTG-lacking medium for up to 90 min. Localization of DivIB, DivIC, and FtsZ was examined by IFM. Prior to resuspension, 19, 29, and 86% of cells contained DivIB, DivIC, and FtsZ localizations, respectively. Average cell length began to increase approximately 20 min following resuspension in IPTG-lacking medium. By 90 min following resuspension, the mean cell length was 31.9 μm and very few Z rings were observed (9% of the t0 level). The number of both DivIB and DivIC localizations also decreased in a similar manner, with 5 and 6% of the t0 level present at 90 min, respectively. In control experiments when strain BB11 was resuspended in IPTG-containing medium, the frequency of septal localizations remained high for all three proteins (50 to 140% of the t0 level).
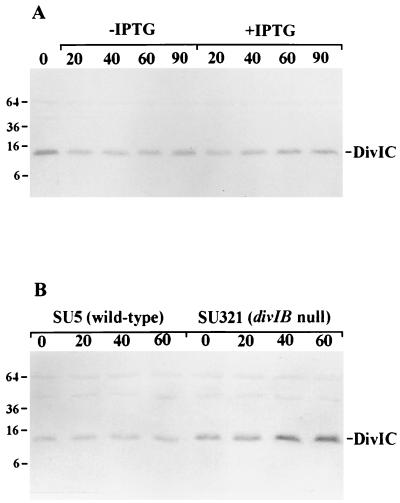
The inability of DivIB and DivIC to target to the septum in FtsZ-depleted cells was not a consequence of decreased levels of these proteins. Western blot analysis showed that DivIB levels remained unaltered under all conditions described above (not shown). There appeared to be a slight drop in DivIC signal 20 min after resuspension in either IPTG-containing or IPTG-lacking medium (Fig. 1A). The reason for this is unknown, but it is not specific to the depletion of FtsZ. No change in DivIC levels was observed at later times in either medium. As expected, cellular FtsZ levels dropped with time in the absence of IPTG, with about a fourfold reduction 90 min after resuspension in IPTG-lacking medium (not shown). We conclude that although DivIB and DivIC levels are unchanged in the absence of Z rings, they are not targeted to the division site, at least at detectable levels. A previous study showed that when B. subtilis cells are depleted of FtsL, DivIC is degraded, raising the possibility that DivIC is only stable if it is targeted to the division site (8). However, our present observations demonstrate that localization of DivIC is not essential for its stability under all conditions. Furthermore, our data suggest that if FtsL stabilizes DivIC (8), then it does so in the absence of FtsZ.
FIG. 1.
Western blots showing cellular levels of DivIC in BB11 (Pspac-ftsZ) (A) and in the divIB null mutant, SU321, and its wild-type parent strain, SU5 (B). (A) BB11 was grown at 37°C in IPTG-containing medium (0 min) and resuspended in either IPTG-lacking or IPTG-containing medium for 20, 40, 60, and 90 min. (B) SU321 and SU5 were shifted from 30°C at mid-exponential phase to 49°C for 1 h. Samples were collected prior to (0 min) and 20, 40, and 60 min after the shift. A590 equivalents were loaded in each case. Standard protein positions are expressed in kilodaltons.
Localization of DivIB at the division site requires DivIC.
The dependency of DivIB localization on DivIC has not been examined previously. DivIC is essential for growth (16). However, a strain (SU392) containing a Pspac-divIC gene can readily divide in the absence of IPTG because wild-type levels of DivIC are not required for division (see below) (15). We have therefore also examined DivIB localization in a temperature-sensitive divIC mutant strain, SU391, which contains the tolR-divIC hybrid gene under Pspac control (RRC) (15). This hybrid gene encodes a protein comprising the cytoplasmic and membrane domains of the E. coli TolR protein fused to the external C-terminal domain of DivIC (RRC) (15). At 30 and 45°C (IPTG-containing medium), wild-type levels of RRC are present in SU391. However, the strain is temperature sensitive, since division is inhibited at 45°C in the presence or absence of IPTG. These two strains thus allowed us to monitor DivIB localization while altering the levels of mutant or wild-type DivIC. SU391 and SU392 were grown at 30°C with IPTG. At mid-exponential growth phase, the cultures were washed and resuspended in media with or without IPTG and incubated at 30 or 45°C. Samples were taken prior to and 45 min after resuspension for cell length measurements and IFM. In SU392 cells (Pspac-divIC) in the presence of IPTG, DivIB localizations were present in 15% of cells at 30°C and in 21% of those shifted to 45°C for 45 min (examples of the latter short cells are shown in Fig. 2). Due to the increase in frequency of DivIB immunostaining at 45°C, which always occurred, the relative staining frequencies for all samples were normalized to that of SU392 grown with IPTG at the same temperature (Table 1).
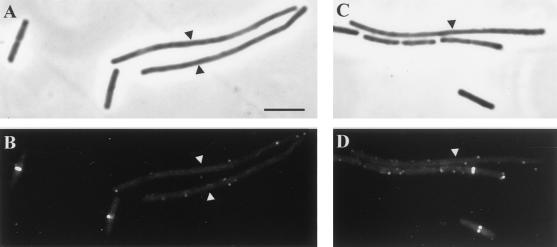
FIG. 2.
DivIB localization in SU391 (Pspac-RRC) and SU392 (Pspac-divIC) in the presence of IPTG at 45°C. All panels show both SU391 and SU392 cells (long and short, respectively) that were mixed prior to IFM processing. (A and C) Phase-contrast images; (B and D) fluorescein isothiocyanate-immunostained images of the same field. Cells were grown at 30°C to mid-exponential phase and then shifted to 45°C for 45 min. Arrowheads indicate the long SU391 cells. Bar = 5 μm (A).
TABLE 1.
Effect of varying the level of mutant or wild-type DivIC protein on DivIB localization
| Strain | Growth conditions | Relative cellular level of DivICa | Avg cell length (μm)b | Relative frequency (%) of DivIB localizationsc |
|---|---|---|---|---|
| SU392 (Pspac-divIC) | 30°C, +IPTG | ++++ | 4.04 ± 0.05 | 100 |
| 30°C, −IPTG | + | 4.23 ± 0.06 | 47 | |
| 45°C, +IPTG | ++++ | 3.92 ± 0.09 | 100 | |
| 45°C, −IPTG | + | 8.12 ± 0.28 | 35 | |
| SU391 (Pspac-RRC) | 30°C, +IPTG | ++++ | 4.63 ± 0.08 | 77 |
| 30°C, −IPTG | None | 14.0 ± 0.58 | 22 | |
| 45°C, +IPTG | +++ | 22.72 ± 1.24 | 5 | |
| 45°C, −IPTG | None | 30.40 ± 1.56 | 6 |
For ease of reference, the relative levels of DivIC (either wild-type or fusion) present in both SU391 and SU392 are indicated. These values were determined by Western blot analysis performed on cell lysates harvested at the same time as the IFM samples and are essentially the same as those reported previously (15). ++++, +++, and +, wild-type level, 50 to 75% of the wild-type level, and less than 10% of the wild-type level of DivIC, respectively.
The error given is the standard error of the mean.
The percentages given were normalized to the number of localizations per micrometer for SU392 grown at 30 or 45°C in IPTG-containing medium (for comparison against other 30 and 45°C cultures, respectively).
At 30°C in the absence of IPTG, SU392 (Pspac-divIC) cells contained very little DivIC and were slightly (5%) longer than those grown in the presence of IPTG (Table 1). Under these conditions, DivIB localization decreased to 47%. At 30°C in the presence of IPTG, SU391 cells (Pspac-RRC) were slightly (15%) longer than SU392 and DivIB localizations decreased to 77%. This localization decrease was consistently observed, as was the slight increase in cell length (see also reference 15). In the absence of IPTG at 30°C, SU391 cells were significantly longer than wild-type cells (three to four times) and DivIB localizations further decreased to 22%. A significant proportion (35%) of these localizations occurred where septa were visible, suggesting that DivIB can assemble only if enough DivIC is present at the division site to allow septation. Interestingly, we also noticed that in SU391 cells growing at 30°C in the presence of IPTG, DivIB localizations were extremely faint, much fainter than those in SU392 grown under the same conditions (not shown). It has been shown previously that at 30°C, in the presence of IPTG, DivIC midcell staining was significantly reduced in SU391 compared to SU392, although the cellular level of the hybrid protein was at least as high as that of the wild type (15). These observations strongly suggest that at 30°C, DivIB requires DivIC for its localization and that the efficiency of DivIB localization depends on the level of DivIC actually assembled at the division site.
At 45°C, SU392 cells (Pspac-divIC) remained short after the temperature shift in IPTG-containing medium but became slightly filamentous in IPTG-lacking medium at 45°C, averaging 8.12 μm (Table 1). In these longer cells, DivIC is barely detectable by Western analysis. DivIB localization decreased to 35%, and these localizations were fainter than those observed in the presence of IPTG at the same temperature. In contrast, SU391 (Pspac-RRC) cells became extremely filamentous when shifted to 45°C, regardless of whether IPTG was added (22.72 μm in IPTG-containing medium and 30.40 μm in IPTG-lacking medium), indicative of a rapid block in cell division under both conditions. Furthermore, at 45°C in both the presence and absence of IPTG, essentially no DivIB localized in the long filaments of SU391 (long cells [IPTG-containing medium] in Fig. 2B and D) (Table 1). So DivIB localization is also dependent on DivIC at 45°C. Furthermore, the level of DivIB localization at 45°C depends on both the cellular level of wild-type DivIC and the ability of DivIC to function. To determine whether the inability of the RRC protein to support cell division at high temperatures occurs at the level of its assembly at the division site, it was of interest to examine whether the RRC protein can localize efficiently in SU391 at 45°C in the presence of IPTG. Although the RRC protein was not detected at the division site under these conditions, for unknown reasons even normal levels of wild-type DivIC are barely detected in strains SU391 and SU392 using IFM at these high temperatures, making it impossible to draw any such conclusions.
Western blot analysis of cell lysates of SU391 and SU392 revealed that DivIB levels were unaltered in all samples (data not shown). This is consistent with the previous finding that DivIB is stable under conditions in which DivIC is degraded (8).
Localization of DivIC depends on DivIB only at high temperatures, but FtsZ localization is independent of DivIB at all temperatures.
FtsZ and DivIC are essential for division at all temperatures (4, 16). Since DivIB is essential only at high temperatures (3), FtsZ and DivIC must be able to localize to the division site in the absence of DivIB at low (permissive) temperatures. Can FtsZ and DivIC localize in the complete absence of DivIB at the nonpermissive temperature? We examined FtsZ and DivIC localization in the divIB null strain, SU321, at 49°C. Although it has been reported previously that divIB is essential for cell division at temperatures of >30°C (3), when the originally constructed divIB null mutation from KU608 (3) is present in our laboratory 168 strain (SU5), it is significantly less temperature sensitive. In strain SU321, at temperatures of up to 37°C, cell division is normal and a complete division block is only observed at 49°C (our unpublished observations). The strain-dependent temperature sensitivity of the divIB null mutation has been observed by others (P. Levin and R. Losick, personal communication).
Exponentially growing cells of SU321 were shifted from 30 to 49°C. DivIC and FtsZ IFM was performed on cells taken prior to the temperature shift and at every 20 min after the shift, up to 1 h. At 30°C, the average cell length of the divIB null strain was 4.42 μm (Table 2), marginally longer than that of the congenic wild-type strain, SU5, grown under identical conditions (not shown). Prior to the temperature shift, many bright DivIC and FtsZ localizations were observed at the division site (midcell) in both SU5 and SU321, with 37 and 85% of cells containing DivIC and FtsZ localizations, respectively. As expected, DivIB is not required for Z-ring formation at the permissive temperature. It is also concluded that DivIC localization does not require DivIB at the lower temperature of 30°C, at which DivIB is not essential. These findings are consistent with the fact that FtsZ and DivIC are essential for division at all temperatures (4, 16).
TABLE 2.
FtsZ and DivIC localization in the absence of DivIB
| SU321 growth temp (°C) | Min after temp shift | Avg cell length (μm)a | No. of localizations/μm as % of t0b
|
|
|---|---|---|---|---|
| FtsZ | DivIC | |||
| 30 | 0 | 4.42 ± 0.07 | 100 | 100 |
| 49 | 20 | 9.67 ± 0.32 | 86 | 15 |
| 40 | 24.71 ± 1.32 | 61 | 5 | |
| 60 | 45.11 ± 2.50 | 52 | 2 | |
The error given is the standard error of the mean.
t0 is the time at which a portion of the culture growing at 30°C was used to inoculate media prewarmed at 49°C.
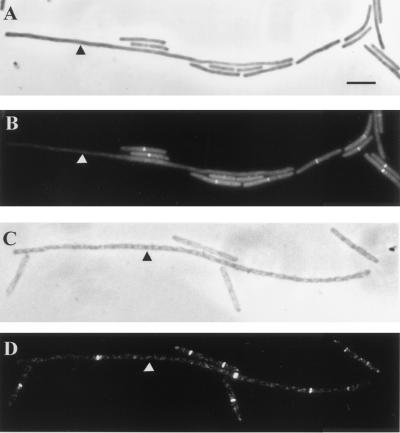
At 49°C the wild-type parent strain, SU5, was able to grow and divide normally, and FtsZ and DivIC localization frequencies never varied more than twofold. However, division was completely blocked in SU321 cells at 49°C. Cell length doubled approximately every generation, and cells were extremely long after 60 min, averaging 45.11 μm (Table 2). The frequency of FtsZ localization did decrease in these filaments to 52% of the level observed at 30°C (Table 2). Nonetheless, multiple Z rings formed in the filaments (one filament is indicated in Fig. 3C and D), with an average of ∼3.8 rings per cell, and these were positioned at potential division sites (1/2, 1/4, and 1/8, etc., positions) (data not shown). In contrast, the frequency of DivIC localizations fell dramatically after the temperature shift, and by 60 min after the shift, virtually none were observed (Table 2) (long filament in Fig. 3B).
FIG. 3.
DivIC and FtsZ localization in SU321 (divIB null) grown at 30°C to mid-exponential phase and then shifted to 49°C for 1 h. (B and D) Cells immunostained with fluorescein isothiocyanate; (A and C) phase-contrast image of the same field. DivIC localization is shown in panel B, while FtsZ localization is shown in panel D. Cells grown at 30°C (short) were mixed with long cells (indicated by arrowheads) that had been shifted to 49°C for 1 h. Bar = 5 μm (A).
In light of the unexpected finding that extreme temperatures were required to block septation in SU321, a shift to 45°C was performed on one of the originally constructed divIB null strains, KU121, that does not grow on plates at 37°C (3). At 45°C in KU121 cells, a complete block to cell division occurred (data not shown). The results obtained with respect to both division ability and localization of DivIC and FtsZ were the same as for SU321 shifted to 49°C (not shown).
Western blot analysis of whole-cell lysates of SU321 (divIB null) and the wild-type parent strain, SU5, showed that FtsZ levels were very similar in the strains at both temperatures (not shown). Surprisingly however, at 30°C DivIC levels were consistently two- to threefold higher in SU321 than in SU5 (Fig. 1B). These levels were further enhanced when SU321 was grown at 49°C for 60 min. Similar results were obtained with KU121 (divIB null) and its congenic parent strain, tms12+, both prior to and after a shift to 45°C (not shown).
In conclusion, midcell Z-ring assembly does not depend on DivIB at 30°C or even at 49°C, when DivIB is essential for division. DivIC localization to the division site does not depend on DivIB at 30°C; however, at 49°C, DivIC is unable to localize in the absence of DivIB. Thus, DivIC is recruited to the septum site independently of DivIB at 30°C, but at 49°C, DivIB is required to maintain DivIC at the division site, perhaps by direct interaction. Increased cellular levels of DivIC observed in the absence of DivIB raise the possibility that DivIB negatively influences DivIC synthesis. However, this is not likely to be direct since the only essential domain of DivIB (80% C-terminal portion) is external to the cytoplasm (15). Alternatively, DivIB might somehow decrease the stability of the DivIC protein.
Our present findings concerning division protein localization in B. subtilis can be summarized as follows. (i) Z rings can assemble at midcell independently of DivIB, even at higher temperatures at which DivIB is essential for division. (ii) Septal recruitment of both DivIB and DivIC requires FtsZ localization. (iii) DivIB localization requires DivIC at both 30 and 45°C. (iv) DivIC can be recruited to the septum site in the absence of DivIB at 30°C but not at 49°C, when DivIB is essential for division. In other words, DivIB and DivIC are codependent for localization only at higher temperatures. This suggests that DivIC is recruited to the septum site earlier than or together with DivIB at 30°C, but at 49°C, DivIB is required to maintain DivIC at the division site, perhaps by direct interaction. A role for DivIB in promoting division complex assembly at higher temperatures has been proposed previously (12, 24). It has been proposed that FtsL and DivIC interact directly (8). Perhaps all three proteins interact directly at the septum site. The finding that DivIB localization efficiency depends on the level of DivIC assembled at the division site is consistent with the suggestion that these two proteins interact directly in a fixed stoichiometric ratio at the site of septum formation. In previous work, the dependency of PBP 2B localization on DivIB and DivIC was examined only at 48°C (7), so at higher temperatures at least, all three proteins are interdependent for localization. At lower growth temperatures, like 30°C, a linear order of localization is still conceivable, with FtsL localizing first, followed by PBP 2B, DivIC, and DivIB. Intriguingly, both scenarios are distinctly different from the division protein assembly pathway in E. coli (28). What remains to be established in B. subtilis is whether FtsL localization requires the other three membrane-bound proteins, presuming FtsL itself is targeted.
Acknowledgments
This work was supported by the Australian Research Council.
REFERENCES
- 1.Addinall S G, Cao C, Lutkenhaus J. FtsN, a late recruit to the septum in Escherichia coli. Mol Microbiol. 1997;25:303–309. doi: 10.1046/j.1365-2958.1997.4641833.x. [DOI] [PubMed] [Google Scholar]
- 2.Addinall S G, Lutkenhaus J. FtsA is localized to the septum in an FtsZ-dependent manner. J Bacteriol. 1996;178:7167–7172. doi: 10.1128/jb.178.24.7167-7172.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beall B, Lutkenhaus J. Nucleotide sequence and insertional inactivation of a Bacillus subtilis gene that affects cell division, sporulation, and temperature sensitivity. J Bacteriol. 1989;171:6821–6834. doi: 10.1128/jb.171.12.6821-6834.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beall B, Lutkenhaus J. FtsZ in Bacillus subtilis is required for vegetative septation and for asymmetric septation during sporulation. Genes Dev. 1991;5:447–455. doi: 10.1101/gad.5.3.447. [DOI] [PubMed] [Google Scholar]
- 5.Callister H, Wake R G. Characterization and mapping of temperature-sensitive division initiation mutations of Bacillus subtilis. J Bacteriol. 1981;145:1042–1051. doi: 10.1128/jb.145.2.1042-1051.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J C, Weiss D S, Ghigo J-M, Beckwith J. Septal localization of FtsQ, an essential cell division protein in Escherichia coli. J Bacteriol. 1999;181:521–530. doi: 10.1128/jb.181.2.521-530.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daniel R A, Harry E J, Errington J. Role of penicillin-binding protein PBP 2B in assembly and functioning of the division machinery of Bacillus subtilis. Mol Microbiol. 2000;35:299–311. doi: 10.1046/j.1365-2958.2000.01724.x. [DOI] [PubMed] [Google Scholar]
- 8.Daniel R A, Harry E J, Katis V L, Wake R G, Errington J. Characterization of the essential cell division gene ftsL (yllD) of Bacillus subtilis and its role in the assembly of the division apparatus. Mol Microbiol. 1998;29:593–604. doi: 10.1046/j.1365-2958.1998.00954.x. [DOI] [PubMed] [Google Scholar]
- 9.Ghigo J-M, Weiss D S, Chen J C, Yarrow J C, Beckwith J. Localization of FtsL to the Escherichia coli septal ring. Mol Microbiol. 1999;31:725–737. doi: 10.1046/j.1365-2958.1999.01213.x. [DOI] [PubMed] [Google Scholar]
- 10.Hale C A, de Boer P A J. Recruitment of ZipA to the septal ring of Escherichia coli is dependent on FtsZ and independent of FtsA. J Bacteriol. 1999;181:167–176. doi: 10.1128/jb.181.1.167-176.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harry E J, Partridge S R, Weiss A S, Wake R G. Conservation of the 168 divIB gene in Bacillus subtilis W23 and B. licheniformis, and evidence for homology to ftsQ of Escherichia coli. Gene. 1994;147:85–89. doi: 10.1016/0378-1119(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 12.Harry E J, Stewart B J, Wake R G. Characterization of mutations in divIB of Bacillus subtilis and cellular localization of the DivIB protein. Mol Microbiol. 1993;7:611–621. doi: 10.1111/j.1365-2958.1993.tb01152.x. [DOI] [PubMed] [Google Scholar]
- 13.Harry E J, Wake R G. The membrane-bound cell division protein DivIB is localized to the division site in Bacillus subtilis. Mol Microbiol. 1997;25:275–283. doi: 10.1046/j.1365-2958.1997.4581822.x. [DOI] [PubMed] [Google Scholar]
- 14.Katis V L, Harry E J, Wake R G. The Bacillus subtilis division protein DivIC is a highly abundant membrane-bound protein that localizes to the division site. Mol Microbiol. 1997;26:1047–1055. doi: 10.1046/j.1365-2958.1997.6422012.x. [DOI] [PubMed] [Google Scholar]
- 15.Katis V L, Wake R G. Membrane-bound division proteins DivIB and DivIC of Bacillus subtilis function solely through their external domains in both vegetative and sporulation division. J Bacteriol. 1999;181:2710–2718. doi: 10.1128/jb.181.9.2710-2718.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levin P A, Losick R. Characterization of a cell division gene from Bacillus subtilis that is required for vegetative and sporulation septum formation. J Bacteriol. 1994;176:1451–1459. doi: 10.1128/jb.176.5.1451-1459.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levin P A, Losick R. Transcription factor SpoOA switches the localization of the cell division protein FtsZ from a medial to a bipolar pattern in Bacillus subtilis. Genes Dev. 1996;10:478–488. doi: 10.1101/gad.10.4.478. [DOI] [PubMed] [Google Scholar]
- 18.Levin P A, Losick R, Stragier P, Arigoni F. Localization of the sporulation protein SpoIIE in Bacillus subtilis is dependent upon the cell division protein FtsZ. Mol Microbiol. 1997;25:839–846. doi: 10.1111/j.1365-2958.1997.mmi505.x. [DOI] [PubMed] [Google Scholar]
- 19.Liu Z, Mukherjee A, Lutkenhaus J. Recruitment of ZipA to the division site by interaction with FtsZ. Mol Microbiol. 1999;31:1853–1861. doi: 10.1046/j.1365-2958.1999.01322.x. [DOI] [PubMed] [Google Scholar]
- 20.Lutkenhaus J. The regulation of bacterial cell division: a time and a place for it. Curr Opin Microbiol. 1999;1:210–215. doi: 10.1016/s1369-5274(98)80013-1. [DOI] [PubMed] [Google Scholar]
- 21.Lutkenhaus J, Addinall S G. Bacterial cell division and the Z ring. Annu Rev Biochem. 1997;66:93–116. doi: 10.1146/annurev.biochem.66.1.93. [DOI] [PubMed] [Google Scholar]
- 22.Morrison D A. Transformation and preservation of competent bacterial cells by freezing. Methods Enzymol. 1979;68:326–331. doi: 10.1016/0076-6879(79)68023-0. [DOI] [PubMed] [Google Scholar]
- 23.Rothfield L, Justice S, Garcia-Lara J. Bacterial cell division. Annu Rev Genet. 1999;33:423–448. doi: 10.1146/annurev.genet.33.1.423. [DOI] [PubMed] [Google Scholar]
- 24.Rowland S L, Katis V L, Partridge S R, Wake R G. DivIB, FtsZ and cell division in Bacillus subtilis. Mol Microbiol. 1997;23:295–302. doi: 10.1046/j.1365-2958.1997.2141580.x. [DOI] [PubMed] [Google Scholar]
- 25.Vicente M, Errington J. Structure, function and controls in microbial division. Mol Microbiol. 1996;20:1–7. doi: 10.1111/j.1365-2958.1996.tb02482.x. [DOI] [PubMed] [Google Scholar]
- 26.Wang L, Lutkenhaus J. FtsK is an essential cell division protein that is localized to the septum and induced as part of the SOS response. Mol Microbiol. 1998;29:731–740. doi: 10.1046/j.1365-2958.1998.00958.x. [DOI] [PubMed] [Google Scholar]
- 27.Wang X, Lutkenhaus J. The FtsZ protein of Bacillus subtilis is localized at the division site and has GTPase activity that is dependent upon FtsZ concentration. Mol Microbiol. 1993;9:435–442. doi: 10.1111/j.1365-2958.1993.tb01705.x. [DOI] [PubMed] [Google Scholar]
- 28.Weiss D S, Chen J C, Ghigo J-M, Boyd D, Beckwith J. Localization of FtsI (PBP3) to the septal ring requires its membrane anchor, the Z ring, FtsA, FtsQ, and FtsL. J Bacteriol. 1999;181:508–520. doi: 10.1128/jb.181.2.508-520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yanouri A, Daniel R A, Errington J, Buchanan C E. Cloning and sequencing of the cell division gene pbpB, which encodes penicillin-binding protein 2B in Bacillus subtilis. J Bacteriol. 1993;175:7604–7616. doi: 10.1128/jb.175.23.7604-7616.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]